Highlights
-
•
Developed and validated 4 in-house assays to reliably determine viral load of several different RV-C genotypes in nasal samples.
-
•
Complete homology between assay and target sequence is the most accurate method to determine viral load.
-
•
Three or more mismatches between the probe and target sequence results in substantially inaccurate viral load.
-
•
These assays facilitate accurate investigations into the role of RV-C load in disease outcome.
Abstract
Background
Rhinovirus C (RV-C) is an important respiratory pathogen of children, but little is known about its contribution to disease severity, though viral load appears to be important. Difficulty in RV-C cultivation and target sequence variation has precluded the development of a PCR based quantification method.
Objective
The aim of this study was to develop and validate reverse transcription quantitative PCR (RT-qPCR) assays for a broad range of circulating RV-C genotypes in nasopharyngeal aspirates (NPAs).
Study design
Four assays were designed to quantify a 296 bp region located within the 5′ untranslated region (UTR) of RV-C types. These assays were based on in silico analysis of available RV-C sequences. Probes were designed to provide 100% homology to the corresponding RV-C genotypes.
Results
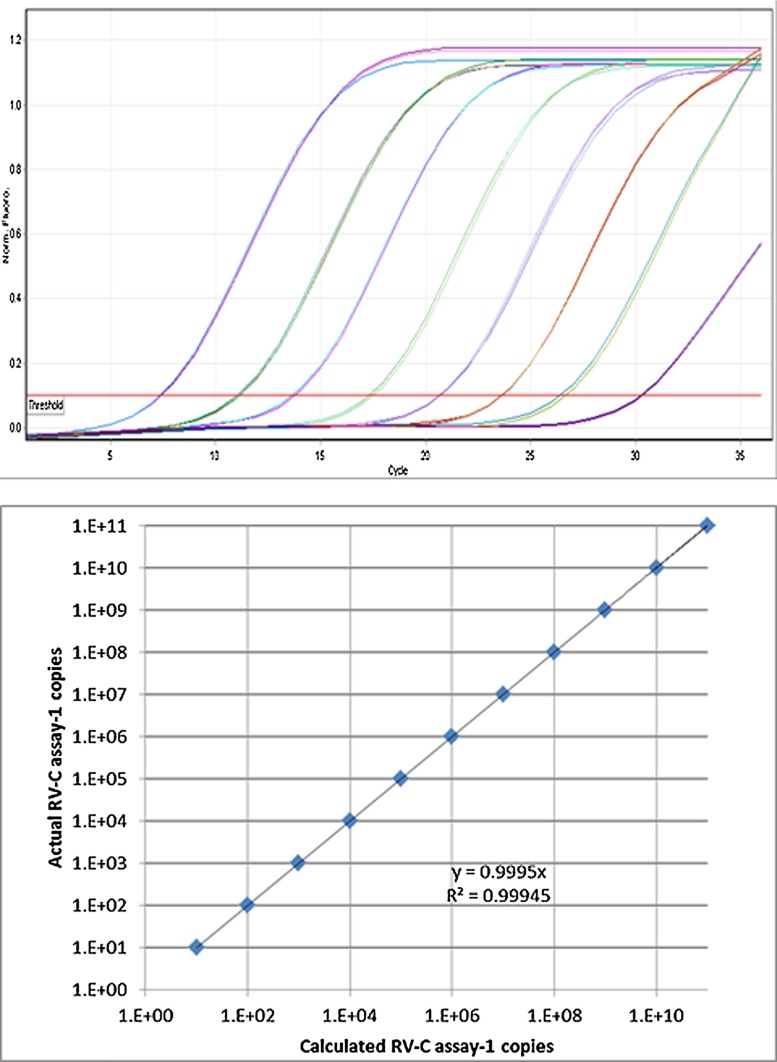
The linear dynamic range of each of the four assays spanned eight orders of magnitude (104–1011 copies/mL). The limit of detection for assays 1–4 was estimated to be 1147 copies/mL, 765 copies/mL, 1138 copies/mL and 1470 copies/mL respectively. Each assay demonstrated a strong linear relationship (r2 = >0.995) and amplification efficiency greater than 95%. Repeatability and reproducibility of the method were shown to be high, with coefficients of variations lower than 8% and 15% respectively.
The assays were tested on a panel of 40 nasopharyngeal aspirate samples. RV-C RNA was detected in all samples with viral load ranging from 3.3 to 9.73 log10 RNA copies/mL.
1. Introduction
Rhinoviruses (RV) are a common cause of acute respiratory infection in people of all ages (Fawkner-Corbett et al., 2015, Puro et al., 2005). RVs are antigenically diverse, thus people can be infected with different RV types over the course of a lifetime (Cooney et al., 1982, Cooney et al., 1975). The spectrum of disease associated with this group of viruses can range from asymptomatic infection, mild upper respiratory tract infection (common cold) to severe lower respiratory tract infections which may include bronchiolitis or pneumonia (Choi et al., 2015, Iwane et al., 2011, Luchsinger et al., 2014). RV has been identified as an important contributor to acute asthma (Bizzintino et al., 2011) and wheezing illness in young children is an important risk factor in the development of asthma later in life (Jackson et al., 2008). Further, RV exacerbates pre-existing airway diseases such as asthma, cystic fibrosis and chronic obstructive pulmonary diseases (Camargo et al., 2012, Kennedy et al., 2014, Luchsinger et al., 2014).
Genome arrangement, capsid properties and conserved sequences are the current basis of RV species classification, of which there are three recognized species (RV-A, RV-B, and RV-C) (McIntyre et al., 2013). RV-C is the most recently described species and to date there are 55 recognised genotypes. Clinical significance of RV-C is still debated as some studies report that RV-C causes more severe disease than the other two species (Bizzintino et al., 2011, Cox et al., 2013, Miller et al., 2009) but others have not found this association (Iwane et al., 2011, Linsuwanon et al., 2009). Inaccurate quantitative methods complicate the evaluation of viral factors that may contribute to disease severity (Schibler et al., 2012). Viral load studies of other viruses have shown that the amount of replicating virus is an important contributor to disease severity (Franz et al., 2010, Jansen et al., 2010), thus an accurate and reliable method of quantifying RV-C load may be an important tool in understanding the contribution of RV-C to disease pathogenesis, disease progression and clinical management.
Conventional culture methods used to measure viral load in clinical samples are not suitable for RV-C types as this virus is non-cultivable using traditional techniques. Molecular methods have overcome this problem, but the inter-genotype sequence variation within the target region prevents the design of a single quantitative assay that quantifies all genotypes at equal efficiencies (Schibler et al., 2012). Furthermore, developing specific primer and probe combinations for each genotype would be impractical in a diagnostic setting. This study aims to develop and validate a minimum set of PCR assays required to quantify circulating RV-C genotypes found in children.
2. Materials and methods
2.1. Clinical specimens
Nasopharyngeal aspirates (n = 40) were collected between June 2013 and April 2015 from children presenting to the Emergency Department of Princess Margaret Hospital for Children in Perth. These samples represented a subset of children with episodic wheeze enrolled in The Prednisolone Response Evaluation in Viral Induced Episodic Wheeze (PREVIEW) study. This study was approved by the Princess Margaret Hospital for Children Ethics Committee (1970/EP).
2.2. RNA extraction
Total nucleic acids were extracted from 200 μL of each respiratory specimen using the MagMAX viral RNA isolation kit (Thermo-Fisher Scientific, Australia) according to the manufacturer’s instructions. Detection of the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) reference mRNA (Gueudin et al., 2003) was utilized to ensure adequate specimen collection, RNA extraction and removal of PCR inhibitors. Good quality samples were considered those with GAPDH quantification cycle values (Cq) no higher than 2 standard deviations (6.4) of the mean Cq value (25.1). Analysis was not performed on samples above the predetermined accepted range.
2.3. Assay design
The primers used in this study were based on previously published primer sequences (Table 1 ) which amplify a 296 bp region within the 5′ untranslated region (UTR) of RV species (Gama et al., 1988, Ireland et al., 1993). Rhinovirus species identification was performed using a published semi nested PCR assay (Ireland et al., 1993) followed by sequencing.
Table 1.
Primers and probes used for RV-C assays.
| Oligonucleotide | Oligonucleotide sequence (Position) |
|---|---|
| IrlonS (Forward Primer) | 5′-GCACTTCTGTTTCCCC-3′ (165–180)a |
| EntA (Reverse Primer) | 5’-GCATTCAGGGGCCGGAG-3’ (461–445)a |
| RV-TYPE-C Probe 1 | 5′-FAM-CCTGCGTGGCTGCC-MGBNFQ-3′ (358–371) |
| RV-TYPE-C Probe 2 | 5′-FAM-CCCGCGTGGCTGCC3′-BHQ-1 (354–367) |
| RV-TYPE-C Probe 3 | 5′-FAM-CCCGCGTGGTGCCC-MGBNFQ-3′ (377–390) |
| RV-TYPE-C Probe 4 | 5′-FAM-CCTGCGTGGTGCCC3′-BHQ-1 (383–396) |
Nucleotide position was determined using this reference sequence [NC_002058.3] from GenBank. LNA bases are underlined.
To select appropriate probe sequences we evaluated 204 RV-C 5′ UTR sequences which represented 34/55 of the currently known RV-C genotypes. These sequences were obtained from our in-house RV-C database (n = 188) and the Picornaviridae study group website (n = 16) (http://www.picornastudygroup.com). Nucleotide sequence alignments were analysed using BioEdit Sequence Alignment Editor Version 7.2.5 (Hall, 1999).
The in silico analysis of the 204 5′ UTR RV-C sequences demonstrated that probe target region of RV-C2 (EF077280), RV-C7 (DQ875932), RV-C9 (GQ223228) and RV-C25 (JF317013) were representative of the overall inter-genotype variation encountered in the probe target region of the assessed RV-C genotypes. Thus, a minimum of 4 distinct probes (Table 1) were designed to overcome the inter-genotype variation in RV-C types. Each probe was determined to be completely homologous to the probe target region of the representative genotypes. Probes were designed using Primer Express v3.0 software (ThermoFisher Scientific, Australia). MGB probes were synthesized by Applied Biosystems (ThermoFisher Scientific, Australia) and Locked Nucleic Acid (LNA) probes were synthesized by Sigma-Aldrich (Sigma-Aldrich, Australia).
2.4. Production and quantification of transcribed RV-C RNA standards
Nucleotide sequences matching the P1-P2 region of the 5′ UTR of RV-C2 (EF077280), RV-C7 (DQ875932), RV-C9 (GQ223228) and RV-C25 (JF317013) were incorporated into individual plasmid constructs manufactured by Integrated DNA Technologies (IDT, Australia). M13 forward (5′-GTA AAA CGA CGG CCA GT-3′) and reverse (CAG GAA ACA GCT ATG ACC) primers were used to amplify the target sequence from each individual plasmid construct. The PCR reaction mix (20 μL) contained PCR buffer (Thermo Fisher Scientific Australia Pty Ltd.), 2 mM MgCl2, 0.2 mM dNTP, 0.2 μM primers (IDT, Australia), 0.5 U AmpliTaq Gold® (ThermoFisher Scientific, Australia) and cycling conditions were as follows: 10 min at 95 °C followed by 45 cycles of 30 s at 94 °C, 45 s at 50 °C and 60 s at 72 °C. Successful amplification was confirmed by the detection of PCR products by agarose gel electrophoresis. Post PCR purification was completed using ExoSAP-It reagent (Affymetrix, Ohio, USA) following the manufacturer’s protocol.
The Megashortscript T7 high yield transcription kit (ThermoFisher Scientific, Australia) was used to synthesize RNA in vitro. All transcription reactions were completed at 37 °C for 16 h followed by TURBO DNA-free™ DNase treatment, DNAse Removal and MEGAclear™ Transcription Clean-Up (ThermoFisher Scientific, Australia) following the manufacturer’s instructions. The RNA transcripts were eluted in THE RNA Storage solution (ThermoFisher Scientific, Australia), and stored in single-use aliquots at −80 °C.
The RNA transcript was quantified using the Qubit RNA Broad Range assay on the Qubit 2.0 fluorometer (Life Technologies, USA). To assess the quantification accuracy of the Qubit 2.0 fluorometer all RNA transcripts were measured in triplicate. The conversion of RNA concentration into RNA copies/μL was done with the following formulae:
-
1.
M.W. of ssRNA = (RNA transcript length (bp) × 320.5) + 159.0.
-
2.
Number of molecules (copies) per ug ssRNA = Avogadros number (6.022 × 1023) × (M.W. of ssRNA).
-
3.
RNA copies/μL = [RNA transcript concentration as per Qubit × number of molecules (copies) per ug ssRNA]/(RNA transcript length).
2.5. Quantitative real time PCR (Viral load)
The qScript XLT One-Step qRT-PCR Toughmix kit (Quanta Biosciences Gaithersburg, USA) was utilized for the qRT-PCR assays. The 20 μL reaction volume contained 8 μL of template, 0.4 μM of the forward primer, 0.8 μM of the reverse primer and 0.2 μM of probe (Table 1). Thermocycling conditions were as follows: 5 min at 50 °C, 1 min incubation at 95 °C then 40 cycles of 20 s at 95 °C and 80 s at 60 °C using the Rotor Gene 6000 real-time thermocycler (Qiagen, Australia). All experiments were performed in triplicate including positive controls and non-template controls. We determined Cq values for each reaction using a manual Cq threshold of 0.10 in the Rotor Gene 6000 application software.
In all experiments, a standard curve was generated by comparing Cq values and the copy number. The reaction efficiencies of the assays were calculated according to the equation: E = 10(−1/M) − 1, where M is the slope of the standard curve. The dynamic range (101–108 copies/μL) of quantification was determined by using tenfold serial dilution of RNA transcript. The RV-C load in clinical samples was determined by interpolation of the quantification cycle value into the appropriate standard curve.
2.6. Analytical performance evaluation
The reliability and reproducibility of RV-C viral load quantification by RT-qPCR was assessed using the Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines (Bustin et al., 2009). Tenfold dilutions of each cRNA transcript were tested in triplicate to assess intra-assay variation. Inter-assay variation of Cq values was determined by analyzing data from five independent assays.
Using the appropriate RNA transcript for each RV-C assay, a tenfold dilution series of twelve concentrations was prepared. The second last dilution of the tenfold dilution series was used to prepare a two-fold dilution series of 10 concentrations. 8 μL of each dilution from the two-fold dilution series was added to a 12 μL PCR reaction mix and run for 50 PCR cycles using the Rotor Gene 6000 real-time thermocycler (Qiagen, Australia). Twenty-four PCR replicates were tested at each concentration. Poisson regression analysis was used to determine the limit for a 95% confidence of detection. To evaluate variability these experiments were repeated on five different occasions.
Analytical specificity was assessed using BLAST searches against other virus families, bacteria and cell sequences from the Genbank nucleotide collection. In addition, an in-house cross reactivity panel was used to assess the specificity of our RV-C assays against other respiratory pathogens.
2.7. Statistical analysis
The standard deviation (SD) and percentage coefficient of variation (%CV) were used to measure intra/inter-assay variability (repeatability and reproducibility) (SPSS v.16, IBM). SPSS (v.16, IBM, USA) was used for Poisson regression analysis.
3. Results
3.1. Validation of real-time PCR assay for RV-C viral load quantification
3.1.1. Analytical sensitivity and specificity
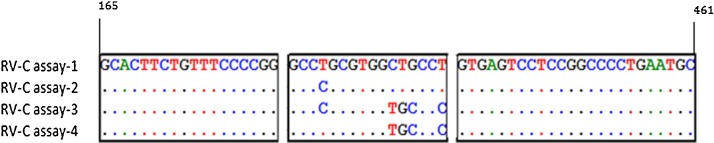
We designed four assays based on RV-C 5′UTR sequences belonging to the 34 RV-C genotypes for which 5′UTR sequences were available. All assays used a common primer pair, but with different specific probe sequences (Fig. 1 ). In silico analysis demonstrated that the probe sequence of assay-1 was homologous to the probe target region of 22 of the 34 RV-C genotypes, while the probe target region of the remaining 12 genotypes aligned completely to the probe in either assay- 2, -3 or -4 (Table A5 ). These assays were unable to be assessed against the other 21 known genotypes because the 5′UTR sequence information was unavailable.
Fig. 1.
A BioEdit sequence alignment of primers and probe sequences targeted by assays one to four. Sequences of forward primer (left box), probe (centre box) and reverse primer (right box). Identical bases at the same position are represented by dots whereas capitalized bases indicate mismatches between sequences.
Table A5.
RV-C genotypes (based on 5′UTR sequences) matched to the appropriate quantification assay.
| Genotype | Assay |
|---|---|
| C-01 | 1 |
| C-02 | 1 |
| C-03 | 1 |
| C-04 | 1 |
| C-05 | 1 |
| C-06 | 1 |
| C-07 | 2 |
| C-08 | 2 |
| C-09 | 3 |
| C-10 | 1 |
| C-11 | 1 |
| C-12 | 1 |
| C-13 | 1 |
| C-14 | 3 |
| C-15 | 1 |
| C-16 | 1 |
| C-19 | 4 |
| C-23 | 1 |
| C-24 | 1 |
| C-25 | 1 |
| C-28 | 1 |
| C-30 | 1 |
| C-34 | 4 |
| C-35 | 4 |
| C-38 | 1 |
| C-39 | 1 |
| C-41 | 1 |
| C-42 | 2 |
| C-43 | 1 |
| C-46 | 3 |
| C-49 | 1 |
| C-50 | 4 |
| C-51 | 4 |
| C-54 | 4 |
All qPCR assays were optimized for primer concentration and annealing/extension temperature. The optimal annealing temperature was determined to be 60 °C with a denaturation time of 20 s and an annealing/extension time of 80 s. The PCR conditions were selected to produce the maximum fluorescent signal generated after 40 amplification cycles.
Serial 2-fold dilutions of 10 concentrations of each RNA transcript was prepared in PCR-grade water and tested to determine the limit of detection of the assays. Using Poisson regression analysis, the limit for a 95% probability of detection was estimated to be 1147 copies/mL for assay-1, and 4765 copies/mL, 1138 copies/mL and 1470 copies/mL respectively for assays 2–4.
Nucleic acid extracts from other respiratory pathogens, including influenza A and B, human respiratory syncytial virus, human metapneumovirus, parainfluenza viruses 1–4, human adenovirus, human bocavirus, human coronaviruses (HCoV-229E, HCoV-NL-63, HCoV-OC43, and HCoV-HKU-1), Mycoplasma pneumoniae and Streptococcus pneumoniae were non-reactive in each of the RV-C real-time assays. In addition, a BLASTn search performed to check the specificity of the primer and probe sets used in the assays showed no genomic cross-reactivity with other virus families, bacteria or cells. However as anticipated there was cross reactivity with other enterovirus species.
Linearity was assessed in triplicate over five independent experiments, and in all assays it spanned more than 7 orders of magnitude (Table A1 ). All assays demonstrated a strong linear relationship (r2 = >0.995) between Cq values and RNA copy number (Table A2 ). All assays demonstrated amplification efficiencies of more than 95% (Table A1). Typical calibration and amplification curves are shown in Fig. 2 .
Table A1.
The analytical performance of the individual PCR assays for the detection of matched RV-C RNA transcript.
| RV-C Assay-1 |
RV-C Assay-2 |
RV-C Assay-3 |
RV-C Assay-4 |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | %CV | Mean ± SD | %CV | Mean ± SD | %CV | Mean ± SD | %CV | |
| Slope | −3.32 ± 0.11 | 3.33 | −3.38 ± 0.11 | 3.23 | −3.44 ± 0.09 | 2.72 | −3.37 ± 0.05 | 1.52 |
| Efficiency | 0.98 ± 0.05 | 5.02 | 0.97 ± 0.05 | 4.68 | 0.95 ± 0.04 | 3.93 | 0.97 ± 0.03 | 3.12 |
| Y-intercept | 34.00 ± 2.46 | 7.23 | 38.00 ± 2.72 | 7.17 | 36.12 ± 1.00 | 7.55 | 33.45 ± 1.59 | 8.42 |
| Goodness of fit (R2) | 0.999 | 0.11 | 0.999 | 0.47 | 0.999 | 0.09 | 0.999 | 0.08 |
| Range of Linearity | 100–108 | 100–108 | 100–108 | 100–108 | ||||
SD—standard deviation, % CV—percentage coefficient of variation.
Table A2.
A comparison of RNA transcript concentration and Cq values for the four RV-C assays.
| RV-C Assay-1 |
RV-C Assay-2 |
RV-C Assay-3 |
RV-C Assay-4 |
|
|---|---|---|---|---|
| RNA transcript concentration (copies/reaction) | Mean Cq ± SD | Mean Cq ± SD | Mean Cq ± SD | Mean Cq ± SD |
| 100 | 32.77 + 0.14 | 33.25 + 1.48 | 32.66 + 0.31 | 31.84 + 1.01 |
| 101 | 29.11 + 0.12 | 28.22 + 0.46 | 29.62 + 0.06 | 27.37 + 0.07 |
| 102 | 25.86 + 0.11 | 25.37 + 0.06 | 25.95 + 0.07 | 24.20 + 0.14 |
| 103 | 22.07 + 0.06 | 22.25 + 0.07 | 22.52 + 0.04 | 20.96 + 0.03 |
| 104 | 18.68 + 0.06 | 18.91 + 0.06 | 19.08 + 0.08 | 17.53 + 0.10 |
| 105 | 15.35 + 0.04 | 15.39 + 0.04 | 15.52 + 0.11 | 14.20 + 0.14 |
| 106 | 11.73 + 0.10 | 13.13 + 0.11 | 12.05 + 0.08 | 10.79 + 0.04 |
| 107 | 8.52 + 0.04 | 8.78 + 0.02 | 8.55 + 0.02 | 7.81 + 0.06 |
| 108 | 5.27 + 0.12 | 5.17 + 0.20 | 5.19 + 0.16 | 4.77 + 0.07 |
Cq—quantification cycle value, SD—standard deviation, Each test was performed using RNA transcript with a primer-probe target sequence completely homologous to the respective RV-C assay.
Fig. 2.
Amplification and standard curves generated using the primer and probe set of assay one. A serial 10-fold dilution of synthetic RNA transcript of a segment in the 5′UTR of RV-C genotype 2 was used to generate the amplification curves.
3.1.2. Repeatability and reproducibility
To evaluate repeatability and reproducibility of each assay, dilutions (101,102,104,106) of RNA transcript were tested in triplicate. Intra-assay %CV of the four RV-C assays ranged from 0.10% to 8.58% and in most cases variability increased proportionally with dilution (Table A3 ). Inter-assay variability was evaluated using results from five independent experiments and demonstrated %CV of less than 15% (Table A3).
Table A3.
Intra and Inter assay variability of the four RV-C qRT-PCR assays.
| Intra-assay variationa |
Inter-assay variationb |
|||
|---|---|---|---|---|
| RNA target and input target copies | Quantity range (Calculated copies/reaction) | %CV range | Quantity Mean (Calculated copies/reaction) | %CV |
| RV-C transcript-1 106 | 3030000–3560000 | 0.16–2.39 | 3480000 | 6.89 |
| RV-C transcript-1 104 | 30000–36700 | 0.27–2.33 | 37500 | 5.67 |
| RV-C transcript-1 102 | 125–409 | 0.16–7.07 | 361 | 8.92 |
| RV-C transcript-1 101 | 21–40 | 2.10–7.33 | 39 | 5.88 |
| RV-C transcript-2 106 | 3560000–4210000 | 0.22–0.61 | 3810000 | 7.50 |
| RV-C transcript-2 104 | 39100–49700 | 1.70–2.42 | 43400 | 10.56 |
| RV-C transcript-2 102 | 379–405 | 1.44–2.75 | 396 | 3.04 |
| RV-C transcript-2 101 | 35–42 | 3.89–5.82 | 39 | 7.22 |
| RV-C transcript-3 106 | 4080000–5790000 | 0.37–2.30 | 4760000 | 14.58 |
| RV-C transcript-3 104 | 35700–46200 | 0.23–1.23 | 42000 | 9.26 |
| RV-C transcript-3 102 | 372–470 | 0.40–8.58 | 440 | 9.21 |
| RV-C transcript-3 101 | 42–59 | 0.93–7.78 | 47 | 14.57 |
| RV-C transcript-4 106 | 4020000–4860000 | 0.10–1.77 | 4360000 | 8.22 |
| RV-C transcript-4 104 | 48500–52800 | 0.28–1.61 | 50000 | 4.89 |
| RV-C transcript-4 102 | 423–608 | 1.07–4.76 | 525 | 11.05 |
| RV-C transcript-4 101 | 42–49 | 1.47–5.16 | 46 | 5.36 |
% CV—percentage coefficient of variation.
Assays were performed in triplicate.
Five independent experiments.
3.2. Probe mismatch
To demonstrate the need for separate assays this study examined the impact of probe-target sequence mismatches on viral load. Each RV-C transcript was prepared at seven different concentrations ranging from 107 to 101 copies/μL with the calculated copy numbers (means of three experiments) for each transcript expressed as percentages of the copy number obtained with the perfectly matched RV-C transcript-1. At concentrations between 107 and 102 copies/μL there was minimal (<15%) difference in copy number yield between transcript-1 and transcript-2. However, at the lowest copy number (101), a single nucleotide mismatch (near the 5’end) in the probe target region (Fig. 1) resulted in an inaccurate viral load determination (Table 2 ). Multiple mismatches between the probe and target (transcript-3 and-4) resulted in substantial inaccuracy in RV-C load measurement across the concentration range (Table 2).
Table 2.
Variation in calculated copy number yield (%) of transcripts 1–4 compared to the number of probe mismatches.
| Calculated copy number of transcripts 2–4a |
Probe mismatches | |||||||
|---|---|---|---|---|---|---|---|---|
| 107 | 106 | 105 | 104 | 103 | 102 | 101 | ||
| Transcripts | ||||||||
| 1 | 100% | 100% | 100% | 100% | 100% | 100% | 100% | |
| 2 | 95% | 90% | 76% | 72% | 90% | 87% | 28% | 1 |
| 3 | <1% | <1% | <1% | <1% | <1% | <1% | <1% | 4 |
| 4 | 7% | 10% | 12% | 5% | 10% | 3% | 3% | 3 |
Each RV-C transcript was tested at 7 different concentrations ranging from 101–107 copies/μL in RV Assay-1. The calculated copy numbers (means of three independent experiments) for each transcript is presented as a percentage of the perfectly matched RV-transcript 1.
3.3. Clinical studies
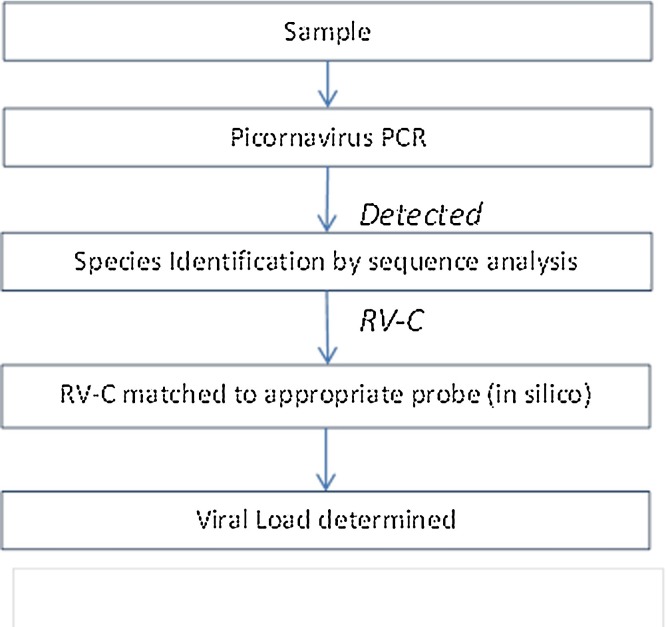
An algorithm was developed to guide viral load determination for RV-C positive samples (Fig. 3 ). Using this algorithm RV-C positive samples (n = 40) from children presenting with acute wheeze were matched to the appropriate assay.
Fig. 3.
The algorithm for the determination of RV-C viral load in clinical samples.
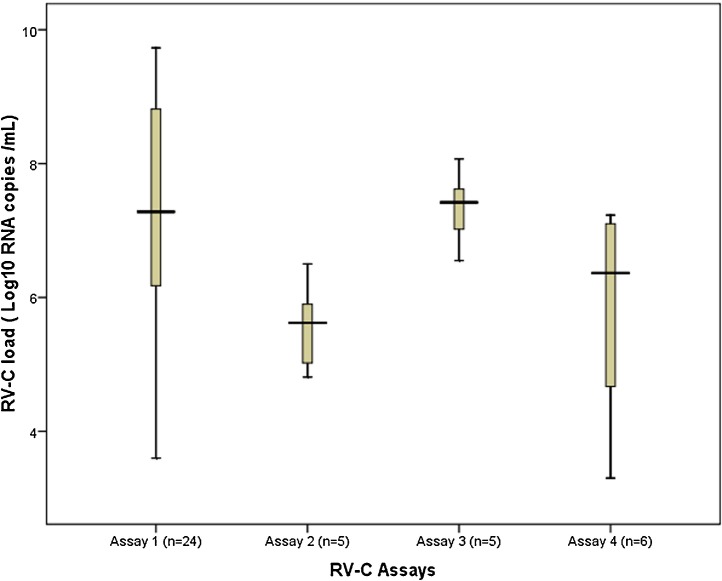
In this group of patients, a total of 23 genotypes were identified with the most commonly detected genotypes being C-16 (n = 5), C-35 (n = 3), C-42 (n = 3), C-14 (n = 3) and C-11 (n = 3) (Table A4 ). In silico analysis demonstrated that assay-1 aligned completely with the target region of 24/40 (16/23 genotypes) samples. Assays 2–4 were suitable for the remaining genotypes (n = 7) (Table A4). As illustrated in Fig. 4 , 67.5% (27/40) patients had RV-C viral loads that ranged between 4 and 8 log10 RNA copies/mL, 11/40 (27.5%) patients had viral load levels higher than 8 log 10 RNA copies/mL and 2/40 patients (5%) had viral loads less than 4 log10 RNA copies/mL. Overall, median RV-C load in this patient group was 6.8 Log10 RNA copies/mL (IQR: 5.7-8.2 Log10 copies/mL). All samples met criteria for adequate sample collection and nucleic acid extraction with GAPDH Cq values within the accepted range (Table A4).
Table A4.
RV-C load determinations for 40 patients enrolled in an asthma exacerbation study.
| RNA copies/mL | SD | %CV | Log10 RNA copies/mL | Genotype | Assay | GAPDH Mean Cq | GAPDH SD |
|---|---|---|---|---|---|---|---|
| 9.66E + 06 | 1.55E + 06 | 0.16 | 6.99 | C-03 | 1 | 26.64 | 0.4 |
| 1.35E + 05 | 4.73E + 04 | 0.35 | 5.13 | C-04 | 1 | 30.51 | 0.8 |
| 2.98E + 08 | 4.47E + 07 | 0.15 | 8.47 | C-06 | 1 | 24.54 | 0.1 |
| 5.33E + 09 | 7.46E + 08 | 0.14 | 9.73 | C-06 | 1 | 25.62 | 0.8 |
| 3.15E + 06 | 8.19E + 05 | 0.26 | 6.50 | C-08 | 2 | 27.71 | 0.6 |
| 6.44E + 04 | 1.67E + 04 | 0.26 | 4.81 | C-08 | 2 | 27.74 | 0.3 |
| 1.06E + 07 | 2.65E + 06 | 0.25 | 7.02 | C-14 | 3 | 23.03 | 0.3 |
| 4.20E + 07 | 7.56E + 06 | 0.18 | 7.62 | C-14 | 3 | 22.81 | 0.3 |
| 3.55E + 06 | 8.17E + 05 | 0.23 | 6.55 | C-14 | 3 | 28.84 | 0.3 |
| 5.33E + 09 | 8.53E + 08 | 0.16 | 9.73 | C-16 | 1 | 27.61 | 0.6 |
| 4.28E + 06 | 1.03E + 06 | 0.24 | 6.63 | C-16 | 1 | 27.72 | 0.2 |
| 2.96E + 07 | 6.51E + 06 | 0.22 | 7.47 | C-16 | 1 | 25.43 | 0.4 |
| 1.91E + 08 | 3.44E + 07 | 0.18 | 8.28 | C-16 | 1 | 27.33 | 0.5 |
| 1.76E + 08 | 2.82E + 07 | 0.16 | 8.25 | C-16 | 1 | 28.23 | 0.1 |
| 3.28E + 07 | 6.56E + 06 | 0.20 | 7.52 | C-23 | 1 | 25.61 | 0.3 |
| 7.53E + 08 | 1.28E + 08 | 0.17 | 8.88 | C-24 | 1 | 21.42 | 0.6 |
| 1.98E + 06 | 6.73E + 05 | 0.34 | 6.30 | C-25 | 1 | 25.81 | 0.8 |
| 5.60E + 08 | 8.40E + 07 | 0.15 | 8.75 | C-25 | 1 | 20.22 | 0.5 |
| 2.28E + 09 | 3.42E + 08 | 0.15 | 9.36 | C-28 | 1 | 21.61 | 0.4 |
| 3.48E + 05 | 1.08E + 05 | 0.31 | 5.54 | C-30 | 1 | 27.02 | 0.4 |
| 1.99E + 03 | 6.17E + 02 | 0.31 | 3.30 | C-35 | 4 | 29.81 | 0.4 |
| 1.69E + 06 | 6.76E + 05 | 0.40 | 6.23 | C-35 | 4 | 27.32 | 0.4 |
| 3.19E + 06 | 8.61E + 05 | 0.27 | 6.50 | C-35 | 4 | 25.61 | 0.3 |
| 1.25E + 07 | 2.13E + 06 | 0.17 | 7.10 | C-35 | 4 | 22.33 | 1.3 |
| 3.26E + 09 | 5.87E + 08 | 0.18 | 9.51 | C-38 | 1 | 23.71 | 0.5 |
| 4.45E + 06 | 1.29E + 06 | 0.29 | 6.65 | C-39 | 1 | 24.20 | 0.1 |
| 1.04E + 05 | 2.08E + 04 | 0.20 | 5.02 | C-42 | 2 | 28.84 | 0.1 |
| 7.88E + 05 | 2.99E + 05 | 0.38 | 5.90 | C-42 | 2 | 30.04 | 0.8 |
| 4.15E + 05 | 1.66E + 04 | 0.04 | 5.62 | C-42 | 2 | 29.93 | 0.2 |
| 1.11E + 06 | 3.66E + 05 | 0.33 | 6.05 | C-43 | 1 | 28.22 | 0.4 |
| 2.65E + 07 | 5.04E + 06 | 0.19 | 7.42 | C-46 | 3 | 25.11 | 0.6 |
| 1.18E + 08 | 1.53E + 07 | 0.13 | 8.07 | C-46 | 3 | 23.53 | 0.2 |
| 4.73E + 04 | 1.32E + 04 | 0.28 | 4.67 | C-51 | 4 | 24.83 | 0.8 |
| 8.51E + 08 | 1.62E + 08 | 0.19 | 8.93 | C-04 | 1 | 27.52 | 0.1 |
| 1.70E + 07 | 3.74E + 06 | 0.22 | 7.23 | C-19 | 4 | 29.81 | 0.1 |
| 2.93E + 05 | 7.62E + 04 | 0.26 | 5.47 | C-11 | 1 | 24.52 | 0.6 |
| 3.29E + 05 | 8.23E + 04 | 0.25 | 5.52 | C-11 | 1 | 28.61 | 0.4 |
| 1.23E + 07 | 2.34E + 06 | 0.19 | 7.09 | C-11 | 1 | 23.24 | 0.1 |
| 2.04E + 06 | 4.90E + 05 | 0.24 | 6.31 | C-24 | 1 | 25.33 | 0.2 |
| 3.95E + 03 | 1.46E + 03 | 0.37 | 3.60 | C-13 | 1 | 24.63 | 0.1 |
Clinical samples were tested in triplicate and mean viral load calculated. SD—standard deviation, %CV—coefficient of variation GAPDH—Glyceraldehyde 3-phosphate dehydrogenase; internal control, Cq—quantification cycle value.
Fig. 4.
Box plots of RV-C load in samples from young children presenting to the Emergency Department with acute wheeze.
4. Discussion
This study presents the development and validation of four qRT-PCR assays that, in combination are able to accurately and reliably measure the viral load of circulating RV-C genotypes.
Reports of an association between RV-C infection and severe respiratory disease have been mixed as some studies have found an association (Bizzintino et al., 2011, Bochkov et al., 2011, Camargo et al., 2012, Piralla et al., 2009) but others have not (Iwane et al., 2011, Linsuwanon et al., 2009). Similar to other acute viral respiratory tract infections where a correlation exists between viral load and disease severity (DeVincenzo et al., 2010, Li et al., 2010, Roussy et al., 2014, To et al., 2010), it is suspected that RV-C load may also drive disease severity. Inaccurate quantitative methods complicate the evaluation of viral factors that may contribute to disease severity (Schibler et al., 2012). Previously published quantitative assays have tried to address the genetic heterogeneity of RV-C by either using an intercalating dye in place of a specific hydrolysis probe or adding degenerate bases in either the primer or probe sequence (Bochkov et al., 2011, Granados et al., 2012). However, accuracy maybe impaired since these techniques may lead to non-specific amplification and reduced amplification efficiencies of the assays (Chemidlin Prevost-Boure et al., 2011, Schibler et al., 2012).
In this study, performance evaluation of each assay was conducted in accordance with the MIQE guidelines (Bustin et al., 2009). A portion in the 5′UTR was chosen as a target for our assays since previous work has demonstrated that 5′UTR sequence can be used for RV-C genotypic assignment at almost identical accuracy to the VP4/VP2 and VP1 and with superior clinical sensitivity (Lee et al., 2012). All assays demonstrated a broad dynamic range, high sensitivity, efficiency and performance in RV-C viral load determination with both clinical samples and in vitro RNA transcripts. Viral load in our respiratory samples were within the 1 × 103 and 1 × 1012 copies/mL range which is in concordance with previous publications (Li et al., 2010, Roussy et al., 2014, Schibler et al., 2012). All assays demonstrated high repeatability and reproducibility with CV of below 8% and 15% respectively. All assays were nonreactive with a range of other potential respiratory pathogens but cross-reactivity with other picornavirus species requires sequencing of the 5′UTR to confirm RV-C identification prior to quantification. This was also needed to determine the appropriate primer-probe combination.
To accurately measure viral load in clinical samples it is vital that primers and probes are designed to match the target sequence. Indeed, previously published studies have shown that the positioning of the mismatch is a crucial determinant of probe binding affinity (Benovoy et al., 2008, Letowski et al., 2004) which may in turn impact upon the accuracy of the calculated viral load. Mismatches throughout or near the middle of the probe target region destabilize hybridization more than those near the ends (Letowski et al., 2004). Another recent study demonstrated that multiple mismatches in the probe target region may have a greater impact on accuracy than a single mismatch (Randhawa et al., 2011). This is consistent with our findings which showed that at most dilutions a single probe mismatch between assay probe and transcript material had minimal impact on accuracy but when multiple mismatches were present there was a substantial effect on viral load measurement across the dilution range. Together, these findings demonstrate the need for multiple qRT-PCR assays to achieve accurate RV-C loads for the different genotypes. However, we were able to show that this could be achieved with a small number of assays, requiring only four different probes to cover the 34 genotypes with known 5′UTR sequences. It is anticipated that these four assays will cover more of the RV-C genotypes, but that awaits further sequence data.
Fortunately other studies have demonstrated that while a large proportion of RV-C genotypes circulate simultaneously in various geographical regions worldwide they are dominated by C-1, C-2, C-6, C-16, C17, C-18 and C43 genotypes (Lu et al., 2014, McIntyre et al., 2013). All of these genotypes were quantified at equal efficiencies in this study, suggesting that our assays can be used to accurately determine RV-C load of various genotypes from different geographical regions as well as to properly investigate differences in pathogenesis between RV-C genotypes. A limitation of the current method is it cannot reveal the presence of a mixed infection and therefore may not be able to accurately quantify the viral load of each genotype present.
In conclusion, this study describes a reliable and accurate PCR based method of quantifying RV-C load in clinical samples containing a wide range of RV-C genotypes. These assays will provide a reliable tool for investigating the role of RV-C in respiratory illness, and for evaluating the effectiveness of future antiviral therapies.
References
- Benovoy D., Kwan T., Majewski J. Effect of polymorphisms within probe–target sequences on olignonucleotide microarray experiments. Nucleic Acids Res. 2008;36:4417–4423. doi: 10.1093/nar/gkn409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzintino J., Lee W.-M., Laing I.A., Vang F., Pappas T., Zhang G., Martin A.C., Khoo S.-K., Cox D.W., Geelhoed G.C., McMinn P.C., Goldblatt J., Gern J.E., Le Souëf P.N. Association between human rhinovirus C and severity of acute asthma in children. Eur. Respir. J. 2011;37:1037–1042. doi: 10.1183/09031936.00092410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochkov Y.A., Palmenberg A.C., Lee W.-M., Rathe J.A., Amineva S.P., Sun X., Pasic T.R., Jarjour N.N., Liggett S.B., Gern J.E. Molecular modeling, organ culture and reverse genetics for a newly identified human rhinovirus C. Nat. Med. 2011;17:627–632. doi: 10.1038/nm.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin S.A., Benes V., Garson J.A., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M.W., Shipley G.L., Vandesompele J., Wittwer C.T. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Camargo C.N., Carraro E., Granato C.F., Bellei N. Human rhinovirus infections in symptomatic and asymptomatic subjects. Braz. J. Microbiol. 2012;43:1641–1645. doi: 10.1590/S1517-838220120004000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemidlin Prevost-Boure N., Christen R., Dequiedt S., Mougel C., Lelievre M., Jolivet C., Shahbazkia H.R., Guillou L., Arrouays D., Ranjard L. Validation and application of a PCR primer set to quantify fungal communities in the soil environment by real-time quantitative PCR. PLoS One. 2011;6:e24166. doi: 10.1371/journal.pone.0024166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S.-H., Hong S.-B., Kim T., Kim S.-H., Huh J.W., Do K.-H., Lee S.-O., Kim M.-N., Lim C.-M., Kim Y.S., Koh Y., Woo J.H., Choi S.-H., Sung H. Clinical and molecular characterization of rhinoviruses A, B, and C in adult patients with pneumonia. J. Clin. Virol. 2015;63:70–75. doi: 10.1016/j.jcv.2014.12.016. [DOI] [PubMed] [Google Scholar]
- Cooney M.K., Wise J.A., Kenny G.E., Fox J.P. Broad antigenic relationships among rhinovirus serotypes revealed by cross-immunization of rabbits with different serotypes. J. Immunol. 1975;114:635–639. [PubMed] [Google Scholar]
- Cooney M.K., Fox J.P., Kenny G.E. Antigenic groupings of 90 rhinovirus serotypes. Infect. Immun. 1982;37:642–647. doi: 10.1128/iai.37.2.642-647.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D.W., Bizzintino J., Ferrari G., Khoo S.K., Zhang G., Whelan S., Lee W.M., Bochkov Y.A., Geelhoed G.C., Goldblatt J., Gern J.E., Laing I.A., Le Souëf P.N. Human rhinovirus species C infection in young children with acute wheeze is associated with increased acute respiratory hospital admissions. Am. J. Respir. Crit. Care Med. 2013;188:1358–1364. doi: 10.1164/rccm.201303-0498OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVincenzo J.P., Wilkinson T., Vaishnaw A., Cehelsky J., Meyers R., Nochur S., Harrison L., Meeking P., Mann A., Moane E., Oxford J., Pareek R., Moore R., Walsh E., Studholme R., Dorsett P., Alvarez R., Lambkin-Williams R. Viral load drives disease in humans experimentally infected with respiratory syncytial virus. Am. J. Respir. Crit. Care Med. 2010;182:1305–1314. doi: 10.1164/rccm.201002-0221OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawkner-Corbett D.W., Khoo S.K., Duarte M.C., Bezerra P., Bochkov Y.A., Gern J.E., Le Souef P.N., McNamara P.S., Rose K., Fonceca A.M., Hopkins M., Britto M., Cuevas L.E., Correia J.B. Rhinovirus-C detection in children presenting with acute respiratory infection to hospital in Brazil. J. Med. Virol. 2015 doi: 10.1002/jmv.24300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz A., Adams O., Willems R., Bonzel L., Neuhausen N., Schweizer-Krantz S., Ruggeberg J.U., Willers R., Henrich B., Schroten H., Tenenbaum T. Correlation of viral load of respiratory pathogens and co-infections with disease severity in children hospitalized for lower respiratory tract infection. J. Clin. Virol. 2010;48:239–245. doi: 10.1016/j.jcv.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gama R.E., Hughes P.J., Bruce C.B., Stanway G. Polymerase chain reaction amplification of rhinovirus nucleic acids from clinical material. Nucleic Acids Res. 1988;16:9346. doi: 10.1093/nar/16.19.9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granados A., Luinstra K., Chong S., Goodall E., Banh L., Mubareka S., Smieja M., Mahony J. Use of an improved quantitative polymerase chain reaction assay to determine differences in human rhinovirus viral loads in different populations. Diagn. Microbiol. Infect. Dis. 2012;74:384–387. doi: 10.1016/j.diagmicrobio.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueudin M., Vabret A., Petitjean J., Gouarin S., Brouard J., Freymuth F. Quantitation of respiratory syncytial virus RNA in nasal aspirates of children by real-time RT-PCR assay. J. Virol. Methods. 2003;109:39–45. doi: 10.1016/S0166-0934(03)00042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, T.A.S.-., 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT, pp. 95–98.
- Ireland D.C., Kent J., Nicholson K.G. Improved detection of rhinoviruses in nasal and throat swabs by seminested RT-PCR. J. Med. Virol. 1993;40:96–101. doi: 10.1002/jmv.1890400204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwane M.K., Prill M.M., Lu X., Miller E.K., Edwards K.M., Hall C.B., Griffin M.R., Staat M.A., Anderson L.J., Williams J.V., Weinberg G.A., Ali A., Szilagyi P.G., Zhu Y., Erdman D.D. Human rhinovirus species associated with hospitalizations for acute respiratory illness in young US children. J. Infect. Dis. 2011;204:1702–1710. doi: 10.1093/infdis/jir634. [DOI] [PubMed] [Google Scholar]
- Jackson D.J., Gangnon R.E., Evans M.D., Roberg K.A., Anderson E.L., Pappas T.E., Printz M.C., Lee W.-M., Shult P.A., Reisdorf E., Carlson-Dakes K.T., Salazar L.P., DaSilva D.F., Tisler C.J., Gern J.E., Lemanske R.F. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am. J. Respir. Crit. Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R.R., Schinkel J., Dek I., Koekkoek S.M., Visser C.E., de Jong M.D., Molenkamp R., Pajkrt D. Quantitation of respiratory viruses in relation to clinical course in children with acute respiratory tract infections. Pediatr. Infect. Dis. J. 2010;29:82–84. doi: 10.1097/INF.0b013e3181b6de8a. [DOI] [PubMed] [Google Scholar]
- Kennedy J.L., Shaker M., McMeen V., Gern J., Carper H., Murphy D., Lee W.M., Bochkov Y.A., Vrtis R.F., Platts-Mills T., Patrie J., Borish L., Steinke J.W., Woods W.A., Heymann P.W. Comparison of viral load in individuals with and without asthma during infections with rhinovirus. Am. J. Respir. Crit. Care Med. 2014;189:532–539. doi: 10.1164/rccm.201310-1767OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W.-M., Lemanske R.F., Evans M.D., Vang F., Pappas T., Gangnon R., Jackson D.J., Gern J.E. Human rhinovirus species and season of infection determine illness severity. Am. J. Respir. Crit. Care Med. 2012;186:886–891. doi: 10.1164/rccm.201202-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letowski J., Brousseau R., Masson L. Designing better probes: effect of probe size, mismatch position and number on hybridization in DNA oligonucleotide microarrays. J. Microbiol. Methods. 2004;57:269–278. doi: 10.1016/j.mimet.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Li C.C., Wang L., Eng H.L., You H.L., Chang L.S., Tang K.S., Lin Y.J., Kuo H.C., Lee I.K., Liu J.W., Huang E.Y., Yang K.D. Correlation of pandemic (H1N1) 2009 viral load with disease severity and prolonged viral shedding in children. Emerg. Infect. Dis. 2010;16:1265–1272. doi: 10.3201/eid1608.091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsuwanon P., Payungporn S., Samransamruajkit R., Posuwan N., Makkoch J., Theanboonlers A., Poovorawan Y. High prevalence of human rhinovirus C infection in Thai children with acute lower respiratory tract disease. J. Infect. 2009;59:115–121. doi: 10.1016/j.jinf.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q.-B., Wo Y., Wang L.-Y., Wang H.-Y., Huang D.-D., Zhang X.-A., Liu W., Cao W.-C. Molecular epidemiology of human rhinovirus in children with acute respiratory diseases in Chongqing, China. Sci. Rep. 2014;4:6686. doi: 10.1038/srep06686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger V., Ampuero S., Palomino M.A., Chnaiderman J., Levican J., Gaggero A., Larranaga C.E. Comparison of virological profiles of respiratory syncytial virus and rhinovirus in acute lower tract respiratory infections in very young Chilean infants, according to their clinical outcome. J. Clin. Virol. 2014;61:138–144. doi: 10.1016/j.jcv.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre C.L., Knowles N.J., Simmonds P. Proposals for the classification of human rhinovirus species A, B and C into genotypically assigned types. J. Gen. Virol. 2013;94:1791–1806. doi: 10.1099/vir.0.053686-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E., Edwards K., Weinberg G., Iwane M., Griffin M., Hall C., Zhu Y., Szilagyi P., Morin L., Heil L. A novel group of rhinoviruses is associated with asthma hospitalizations. J. Allergy Clin. Immunol. 2009;123 doi: 10.1016/j.jaci.2008.10.007. 98–104 e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piralla A., Rovida F., Campanini G., Rognoni V., Marchi A., Locatelli F., Gerna G. Clinical severity and molecular typing of human rhinovirus C strains during a fall outbreak affecting hospitalized patients. J. Clin. Virol. 2009;45:311–317. doi: 10.1016/j.jcv.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Puro V., Minosse C., Cappiello G., Lauria F.N., Capobianchi M.R. Rhinovirus and lower respiratory tract infection in adults. Clin. Infect. Dis. 2005;40:1068–1069. doi: 10.1086/428359. [DOI] [PubMed] [Google Scholar]
- Randhawa P., Kant J., Shapiro R., Tan H., Basu A., Luo C. Impact of genomic sequence variability on quantitative PCR assays for diagnosis of polyomavirus BK infection. J. Clin. Microbiol. 2011;49:4072–4076. doi: 10.1128/JCM.01230-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussy J.-F., Carbonneau J., Ouakki M., Papenburg J., -È. Hamelin M., De Serres G., Boivin G. Human metapneumovirus viral load is an important risk factor for disease severity in young children. J. Clin. Virol. 2014;60(2):133–140. doi: 10.1016/j.jcv.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Schibler M., Yerly S., Vieille G., Docquier M., Turin L., Kaiser L., Tapparel C. Critical analysis of rhinovirus RNA load quantification by real-time reverse transcription-PCR. J. Clin. Microbiol. 2012;50:2868–2872. doi: 10.1128/JCM.06752-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To K.K.W., Chan K.-H., Li I.W.S., Tsang T.-Y., Tse H., Chan J.F.W., Hung I.F.N., Lai S.-T., Leung C.-W., Kwan Y.-W., Lau Y.-L., Ng T.-K., Cheng V.C.C., Peiris J.S.M., Yuen K.-Y. Viral load in patients infected with pandemic H1N1 2009 influenza A virus. J. Med. Virol. 2010;82:1–7. doi: 10.1002/jmv.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]