Abstract
Background
Recently, two new polyomaviruses (PyV), termed WUPyV and KIPyV, were identified in respiratory tract specimens from children with acute respiratory tract infections (ARTIs). However, their roles in the disease have not been determined.
Objectives
To determine the prevalence of WUPyV and KIPyV in the Chinese population suffering from ARTIs in Beijing, China, and to examine their possible role in causing disease.
Study design
Nasopharyngeal aspirates, nasal swabs and throat swabs were collected from 415 children and 297 immunocompetent adults with lower ARTIs (LARTIs). The specimens were screened by polymerase chain reaction for the presence of WUPyV, KIPyV, and other common respiratory pathogens.
Results
Although none of the adults sampled were positive for either virus, WUPyV in 10 (2.4%) children and KIPyV was detected in 2 (0.5%) of the children sampled, respectively. Eleven of the positive cases were co-detected with either rhinovirus (6/11), respiratory syncytial virus (4/11), parainfluenzavirus virus (3/11) or Mycoplasma pneumoniae (2/11). Phylogenetic analysis of the WUPyV and KIPyV isolates showed that the nucleotide sequences were homologous to those of previously reported strains.
Conclusions
The presence of WUPyV and KIPyV in samples from children but not from immunocompetent adults suffering from LARTIs suggests that these viruses primarily infect the young population. Co-detection of additional respiratory pathogens in most of the specimens containing either WUPyV or KIPyV suggests that these viruses do not cause disease independently.
Abbreviations: PyV, polyomaviruses; WUPyV, WU polyomavirus; KIPyV, KI polyomavirus; BKPyV, BK polyomavirus; JCPyV, JC polyomavirus; PIV, parainfluenzavirus; HRV, human rhinovirus; RSV, respiratory syncytial virus; MP, Myoplasma pneumoniae; hMPV, human metapneumovirus; NPA, nasopharyngeal aspirates; ARTIs, acute respiratory tract infections; ALRTIs, acute lower respiratory tract infections
Keywords: KI polyomavirus, WU polyomavirus, Acute lower respiratory tract infection
1. Introduction
Two new polyomaviruses (PyV), termed WUPyV and KIPyV, have been identified in respiratory tract specimens from children with acute respiratory tract infections (ARTIs),1, 2 suggesting a potential role for polyomaviruses in human ARTIs. Full-length genome sequencing demonstrated that WUPyV and KIPyV are genetically similar and together form a new subfamily within the Polyomaviridae.1, 2 Previous studies have examined the prevalence of these two novel viruses within different regions, such as Australia, Korea, Canada, USA, Germany, and Thailand, and their association with the population suffering from ARTIs.3, 4, 5, 6, 7, 8 However, the clinical significance of WUPyV and KIPyV remains to be established.9 In order to further investigate the association between viral infection and the manifestation of ARTIs, we examined the prevalence, as well as clinical and molecular features, of WuPyV and KIPyV infection among children and adults with lower ARTIs (LARTIs) in Beijing, China.
2. Methods
Nasopharyngeal aspirates (NPAs) were collected, upon admission, from 415 hospitalized children with LARTIs (262 males and 153 females) at the Beijing Children's Hospital between March and December 2007. Children ranged in age from 1 month to 14 years, with a mean age of 2.6 years and a median age of 8.2 months.
Nasal and throat swabs were collected from 297 immunocompetent adult patients (148 males and 149 females) when they were diagnosed with LARTIs at the Outpatient Clinic of the Peking Union Medical College Hospital between May 2005 and December 2007. Of the 297 adults sampled, 74 were identified and sampled during the same period as that for the pediatric study described above. For each adult, both nasal and throat swabs were collected simultaneously and pooled into one tube containing virus transport medium. Adults ranged in age from 15 years to 97 years, with a mean age of 47.2 years and a median age of 43 years.
Viral nucleic acids were extracted from each specimen using the NucliSens easyMAG™ system and the NucliSens Isolation Reagents (bioMérieux). All specimens were tested for the presence of common respiratory viruses including parainfluenza viruses (PIV) 1–4, human rhinovirus (HRV), enterovirus, influenza viruses, respiratory syncytial virus (RSV), human coronaviruses (229E, OC43, NL63 and HKU1), human metapneumovirus, adenovirus, and human bocavirus using several molecular tests, as previously described.10, 11, 12, 13, 14, 15 The presence of Mycoplasma pneumoniae (MP), was established using MP-specific IgM antibody and the gelatin particle agglutination test kit (SERODIA-MYCO II, Fujirebio Inc., Japan).
WUPyV was detected by PCR analysis using primers targeting its VP2 gene, generating a 250-bp amplicon.1 In addition, to exclude the false-negative results, the WUPyV samples negative for VP2 were retested using primers targeting the large T antigen (LTAg) gene.1 KIPyV was detected by nested PCR using primers targeting its VP1 gene,2 amplifying a 207-bp fragment. BKPyV and JCPyV were detected by PCR analysis as described previously before.1 All PCR amplicons were confirmed by DNA sequence analysis and by alignment with sequences available in the GenBank database. The homology of the WUPyV and KIPyV isolates was assessed by phylogenetic analysis using the Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0 and the neighbor-joining method.
3. Results
Of the 415 specimens from pediatric patients, 10 (2.4%) samples tested positive for WUPyV. Among these positive specimens, eight were collected from male patients and two were collected from females. The ages of WUPyV-positive patients ranged from 2 months to 7 years (mean age of 25.4 months; median age of 12.7 months). Eight (2.7%) were from 297 patients <3 years old and 2 (1.7%) from 118 patients >3 years old. WUPyV-positive cases were detected in 7 of the 10 months during which specimens were collected (not in May, June, and August; no collection in January or February), suggesting that WUPyV infection is not seasonally associated. Co-infection with additional respiratory pathogens was detected in all 10 WUPyV-positive specimens, with HRV (6/10) and RSV (4/10) being the most frequently co-detected viral agents. PIV3 (1/10), PIV4 (1/10) and MP (2/10) were also co-detected in some of WUPyV-positive samples. One sample was WUPyV-negative using primers targeting the VP2 gene and WUPyV-positive using primers targeting the LTAg gene. It is unclear why this sample tested negative initially. We subsequently amplified the VP2 gene in a nested PCR reaction using two sets of primers: those targeting the full-length VP2 gene, and the original VP2-specific detecting primers described before.1
KIPyV was detected in two pediatric patients (0.5%) aged 3 months and 3.5 years. Of the two KIPyV-positive cases, one was positive for KIPyV alone, while the other was positive for both KIPyV and PIV2.
None of the 297 samples from adults tested positive for WUPyV or KIPyV, and none of the pediatric or adult patient samples tested positive for the presence of BK or JC PyVs.
The 12 children who tested positive for either WUPyV or KIPyV suffered a wide range of respiratory diseases including bronchopneumonia (7/12), peribronchitis (2/12), and pneumonia (3/12) (Table 1 ). In addition, five had underlying serious medical conditions. Notably, patient BCH-370A, who was hospitalized due to pneumonia and tested positive for KIPyV alone, had severe health problems including prematurity (born at 33 weeks), patent foramen ovale, newborn wet lung, newborn aspiration pneumonitis, and bronchopulmonary dysplasia. Each of the WUPyV- or KIPyV-positive patients eventually recovered.
Table 1.
Clinical manifestation of the WU and KI polyomavirus (PyV) positive patients
| Patient no. | Age/sex | Date of collection | Symptoms/health deficiencies or sign | Clinical diagnosis | Time in hospital (days) | Pathogens detected |
|---|---|---|---|---|---|---|
| BCH-151A | 3.5 years/F | August 14 | Cough, fever/low O2 sat | Peribronchitis | – | KIPyV + PIV2 |
| BCH-370A | 3.6 months/M | December 15 | Cough, gasping/premature (33 w), patent foramen ovale, newborn wet lung, newborn aspiration pneumonitis, bronchopulmonary dysplasia | Pneumonia Congestive heart failure | 11 | KIPyV |
| BCH-004A | 8.5 months/M | March 27 | Cough, vomit, diarrhea, fever | Bronchopneumonia Myocardial damage | 9 | WUPyV + RSV |
| BCH-34A | 2.2 months/F | April 12 | Cough, gasping, fever/patent ductus arteriosus | Bronchopneumonia | 9 | WUPyV + HRV |
| BCH-123A | 2.7 mon/M | July 30 | Cough | Bronchopneumonia | 6 | WUPyV + PIV3 + PIV4 |
| BCH-172A | 4.3 y/M | September 6 | Cough, fever/patent foramen ovale | Bronchopneumonia | 12 | WUPyV + MP |
| BCH-200A | 8 months/M | October 15 | Cough, gasping | Peribronchitis | – | WUPyV + HRV |
| BCH-235A | 2.9 years/M | October 29 | Cough, fever, vomit, diarrhea | Bronchopneumonia Myocardial damage | 13 | WUPyV + HRV |
| BCH-259A | 2.7 years/M | November 8 | Cough, fever | Pneumonia Pleural effusion | 14 | WUPyV + HRV |
| BCH-312A | 1.2 years/M | November 22 | Cough | Bronchopneumonia | 15 | WUPyV + HRV + RSV |
| BCH-313A | 11 months/M | November 25 | Cough, vomit, fever/metabolic acidosis | Bronchopneumonia Cardiac insufficiency | 7 | WUPyV + HRV + RSV |
| BCH-404A | 7.4 years/F | December 24 | Cough, nausea, vomit, fever | Pneumonia | 7 | WUPyV + RSV + MP |
KIPyV, KI polyomavirus; PIV, parainfluenzavirus; WUPyV, WU polyomavirus; RSV, respiratory syncytial virus; HRV, Rhinovirus; MP, Myoplasma pneumoniae.
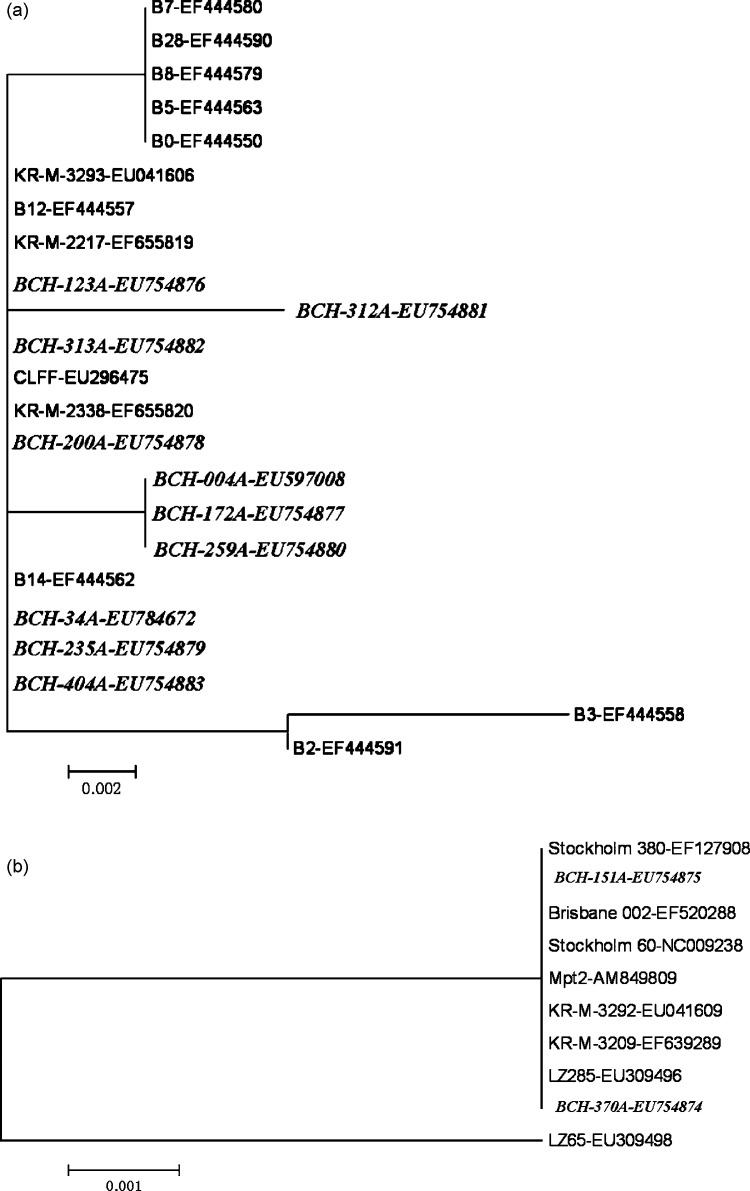
Phylogenetic analysis revealed a very high level of identity between the 10 WUPyV isolates (GenBank accession numbers: EU597008, EU754876–83, EU784672), as well as between the 2 KIPyV isolates (GenBank accession numbers: EU754874–75). The nucleotide sequences of the VP2 gene from the WUPyV strains found showed 98–100% homology with previously described strains. The VP1 gene sequences from the KIPyV isolates showed 99–100% homology with the reference strains (Fig. 1 ). Despite the limited sequences analyzed, the minimal variations suggest the stability of the virus genomes and the global similarity of the epidemic strains.
Fig. 1.
Phylogenetic analysis of nucleotide sequences of VP2 of WUPyV (a) and VP1 of KIPyV (b) isolates (the isolates identified during this study are shown in italics). The tree was built with the MEGA4.0 software by using distance method and the neighbor-joining algorithm with Kimura 2 parameters. Strains isolated in Beijing are indicated by a specific identification code (BCH) followed by the patient number. The analysis included WUPyV reference sequences from GenBank including B0, B12, B3, B14, B5, B8, B7, B28, B2, KR-M-2217, CLFF, KR-M-3293, KR-M-2338 (GenBank accession numbers: EF444550, EF444557, EF444558, EF444562, EF444563, EF444579, EF444580, EF444590, EF444591, EF655819, EU296475, EU041606, EF655820, respectively), and KIPyV reference sequences including LZ65, LZ285, KR-M-3209, KR-M-3292,Stockholm 60, Brisbane 002, Stockholm 380, Mpt2 (GenBank accession numbers: EU309498, EU309496, EF639289, EU041609, NC_009238, EF520288, EF127908, AM849809).
4. Discussion
Our investigation provides valuable insights into the population that is susceptible to infection with these viruses. Consistent with previous reports,8 our finding indicates that the majority (8/10) of WUPyV-positive samples originated from children less than 3 years old. However, because the positive sample number is low, further investigation with larger positive patient pools is required to accurately estimate the age distribution of WUPyV and KIPyV infections.
In an effort to further study the clinical relevance of WUPyV and KIPyV infection in LARTIs, we assayed for the presence of these viruses in immunocompetent adults diagnosed with LARTIs. Significantly, no positive cases were detected among the population sampled. Although prior studies have reported the presence of WUPyV and KIPyV in adults, the populations sampled in these studies corresponded to immunocompromised patients suffering from leukemia, HIV infection or other diseases, or undergoing transplant therapy.1, 9 Thus, in combination with previous reports, our data suggest that WUPyV and KIPyV infection of adults only occurs when they are immunosuppressed or have other significant health deficiencies. However, before making this conclusion, we must consider the limitations associated with particular specimen types on viral detection. In our study, adult specimens were collected using throat and nasal swabs. As the viral load in such specimens is usually lower than in NPAs,16 it is possible that the prevalence of WUPyV and KIPyV has been considerably underestimated. Future studies focusing on the prevalence of WUPyV and KIPyV in adults should therefore assay lower respiratory tract samples, such as bronchoalveolar lavage fluid (BALF).
All but one of the WUPyV- or KIPyV-positive pediatric patients were co-infected with other respiratory pathogens, suggesting that WUPyV and KIPyV, may not directly cause disease, but rather may act by one, or a combination, of the following: (1) as opportunistic pathogens in LARTIs; (2) colonizing the respiratory tract without causing any disease; (3) a part of the endogenous viral flora, which could be reactivated by other viral infections;1 or (4) playing a role in causing severe diseases in the presence of other pathogens. One pediatric patient was positive for KIPyV alone. However, the possibility that this patient was also infected with other pathogens cannot be excluded as we assessed only for the presence of common respiratory viruses and MP during this study.
Acknowledgments
This study was supported by grants from bioMérieux and the Basic Research Fund for Central Nonprofit Institutes (Institute of Pathogen Biology) (2008IPB113).
Contributor Information
Kunling Shen, Email: kunling_shen@hotmail.com.
Jianwei Wang, Email: wangjw28@163.com.
References
- 1.Gaynor A.M., Nissen M.D., Whiley D.M., Mackay I.M., Lambert S.B., Wu G. Identification of a novel polyomavirus from patients with acute respiratory tract infections. PLoS Pathog. 2007;3:e64. doi: 10.1371/journal.ppat.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allander T., Andreasson K., Gupta S., Bjerkner A., Bogdanovic G., Persson M.A. Identification of a third human polyomavirus. J Virol. 2007;81:4130–4136. doi: 10.1128/JVI.00028-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bialasiewicz S., Whiley D.M., Lambert S.B., Jacob K., Bletchly C., Wang D. Presence of the newly discovered human polyomaviruses KI and WU in Australian patients with acute respiratory tract infection. J Clin Virol. 2008;41:63–68. doi: 10.1016/j.jcv.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han T.H., Chung J.Y., Koo J.W., Kim S.W., Hwang E.S. WU polyomavirus in children with acute lower respiratory tract infections, South Korea. Emerg Infect Dis. 2007;13:1766–1768. doi: 10.3201/eid1311.070872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abed Y., Wang D., Boivin G. WU polyomavirus in children, Canada. Emerg Infect Dis. 2007;13:1939–1941. doi: 10.3201/eid1312.070909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le B.M., Demertzis L.M., Wu G., Tibbets R.J., Buller R., Arens M.Q. Clinical and epidemiologic characterization of WU polyomavirus infection, St. Louis, Missouri. Emerg Infect Dis. 2007;13:1936–1938. doi: 10.3201/eid1312.070977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neske F., Blessing K., Ullrich F., Pröttel A., Wolfgang Kreth H., Weissbrich B. WU polyomavirus infection in children, Germany. Emerg Infect Dis. 2008;14:680–681. doi: 10.3201/eid0104.071325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Payungporn S., Chieochansin T., Thongmee C., Samransamruajkit R., Theamboolers A., Poovorawan Y. Prevalence and molecular characterization of WU/KI polyomaviruses isolated from pediatric patients with respiratory disease in Thailand. Virus Res. 2008;135:230–236. doi: 10.1016/j.virusres.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norja P., Ubillos I., Templeton K., Simmonds P. No evidence for an association between infections with WU and KI polyomaviruses and respiratory disease. J Clin Virol. 2007;40:307–311. doi: 10.1016/j.jcv.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coiras M.T., Aguilar J.C., García M.L., Casas I., Pérez-Breña P. Simultaneous detection of fourteen respiratory viruses in clinical specimens by two multiplex reverse transcription nested-PCR assays. J Med Virol. 2004;72:484–495. doi: 10.1002/jmv.20008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coiras M.T., Pérez-Breña P., García M.L., Casas I. Simultaneous detection of influenza A, B, and C viruses, respiratory syncytial virus, and adenoviruses in clinical samples by multiplex reverse transcription nested-PCR assay. J Med Virol. 2003;69:132–144. doi: 10.1002/jmv.10255. [DOI] [PubMed] [Google Scholar]
- 12.Woo P.C., Lau S.K., Chu C.M., Chan K.H., Tsoi H.W., Huang Y. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005;79:884–889. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peiris J.S., Tang W.H., Chan K.H., Khong P.L., Guan Y., Lau Y.L. Children with respiratory disease associated with metapneumovirus in Hong Kong. Emerg Infect Dis. 2003;9:628–633. doi: 10.3201/eid0906.030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allard A.K., Girones R., Juto P., Wadell G. Polymerase chain reaction for detection of adenoviruses in stools. J Clin Microbiol. 1990;28:2659–2667. doi: 10.1128/jcm.28.12.2659-2667.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung J.Y., Han T.H., Kim C.K., Kim S.W. Bocavirus infection in hospitalized children, South Korea. Emerg Infect Dis. 2006;12:1254–1256. doi: 10.3201/eid1208.060261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stensballe L.G., Trautner S., Kofoed P.E., Nante E., Hedegaard K., Jensen I.P. Comparison of nasopharyngeal aspirate and nasal swab specimens for detection of respiratory syncytial virus in different settings in a developing country. Trop Med Int Health. 2002;7:317–321. doi: 10.1046/j.1365-3156.2002.00867.x. [DOI] [PubMed] [Google Scholar]