Abstract
A 5′–3′ end interaction leading to stimulation of translation has been described for many cellular and viral mRNAs. Enhancement of viral translational efficiency mediated by 5′ and 3′ untranslated regions (UTRs) has been shown to occur via RNA–RNA interactions or novel RNA–protein interactions. Mammalian RNA viruses make use of end-to-end communication in conjunction with both viral and cellular factors to regulate multiple processes including translation initiation and the switch between translation and RNA synthesis during the viral lifecycle.
Keywords: 5′–3′ Interaction, Mammalian RNA virus, Translational control, Flavivirus, End-to-end communication
1. Mechanisms of translation of positive strand viruses
Due to the complexity of protein synthesis, viruses cannot encode all the components necessary for translation; therefore, they are dependent upon the availability and activity of cellular translation factors. During eukaryotic cap-dependent translation, initiation factors (eIF4F, the cap-binding complex), mediated by the cap-binding protein, eIF4E, recognize an m7GpppN-cap structure at the 5′ end of mRNAs (Gingras et al., 1999). The eIF4F cap-binding complex consists of eIF4E, an adaptor protein (eIF4G), and a helicase (eIF4A) that functions in complex with the co-factor eIF4B (Fig. 1A). In the cell, the 40S ribosome associates with the initiator methionyl-tRNA/eIF2-GTP ternary complex, eIF3, and eIF1A to form the 43S pre-initiation complex (Pestova et al., 2001). Only when bound to the RNA cap structure can the eIF4F complex, mediated by eIF3, recruit the 43S ribosomal complex to the mRNA (Gingras et al., 1999). This forms the 48S complex, which scans the RNA until the AUG initiation codon is encountered, at which point GTP is hydrolyzed, the initiation factors are released, the 60S ribosomal subunit binds the pre-initiation complex to form the 80S ribosome, and translation elongation begins (Pestova et al., 2001).
Fig. 1.
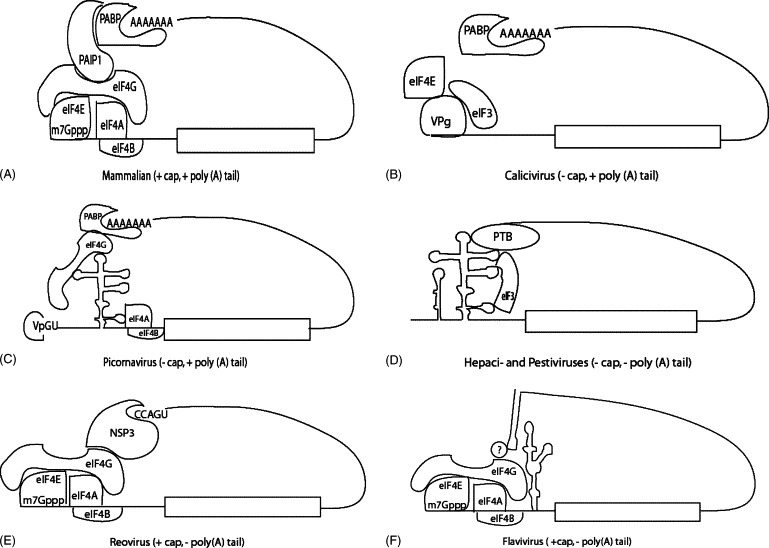
Mechanisms of translation initiation. (A) Mammalian mRNA, (B) calicivirus RNA, (C) picornavirus RNA. Shown is the VPg moiety that is covalently bound to the 5′ end of the genomic RNA, (D) hepaci- and pestivirus RNA, (E) reovirus RNA, and (F) flavivirus RNA.
Two general mechanisms exist by which viruses initiate translation: cap-dependent and cap-independent. For the purpose of this review, the focus will be on mammalian positive-sense RNA viruses, with a particular emphasis on flaviviruses. Genomes of members of the viral families Coronaviridae, Flaviviridae, Reoviridae, and Togaviridae contain an m7GpppN-cap structure at the 5′ end of mRNA and are presumed to initiate translation in a cap-dependent manner. In contrast, RNAs from a diverse group of viruses, which include members of the Caliciviridae, Flaviviridae, and Picornaviridae families, are able to bypass dependency upon an m7G cap structure for translation initiation via various mechanisms. Initiation of protein synthesis through the use of an internal ribosome entry site (IRES) is one such mechanism. The IRES element directs translation in the absence or with a reduced number of cellular translation factors, thus avoiding competition for scarce initiation factors, especially eIF4E, whose availability is among the most highly regulated within the cell (Gingras et al., 1999). Once cellular translation factors have bound to IRES RNA secondary and tertiary structure, they are used to direct ribosomal subunits to the translational start site in the absence of scanning.
While there appears to be no universal mechanism of viral internal ribosome entry, the best characterized viral IRES elements are those of the Picornaviridae family and the hepacivirus (Hepatitis C virus, HCV) and pestivirus genera of the Flaviviridae family. These functionally disparate viral IRES elements associate with different groups of translation factors. Most of the picornavirus IRES elements require the same translation initiation factors as capped mRNAs except for eIF4E, poly(A)-binding protein (PABP), and the N-terminal fragment of eIF4G (Lomakin et al., 2000, Ohlmann et al., 2002, Pestova et al., 1996). The HCV and pestivirus IRESes, on the other hand, can bind and position the 40S subunit specifically and stably in the absence of any eIF, such that the ribosomal P site is placed immediately upstream of the initiator AUG (Pestova et al., 1998). They do, however, require eIF3 for efficient translation initiation (Kieft et al., 2001, Sizova et al., 1998).
Finally, members of the family Caliciviridae undergo cap-independent translation initiation through an entirely different mechanism. The naturally uncapped genomes of caliciviruses are instead covalently linked at their 5′ ends to the viral protein VPg (Fig. 1B) (Herbert et al., 1997). VPg has been found to interact directly with the translation initiation factors eIF4E and eIF3, promoting translation initiation from VPg-linked viral RNA while inhibiting the translation of capped mRNAs (Daughenbaugh et al., 2003, Goodfellow et al., 2005).
2. Communication between the 5′ and 3′ ends of mRNA
2.1. Enhancement of translation
In addition to the 5′ m7GpppN cap structure, nearly all cellular mRNAs contain a region of polyadenylation (poly(A) tail) at their 3′ ends. Translation of mRNAs is most efficient when there is an interaction between the 5′ cap structure and the 3′ poly(A) tail (Sachs et al., 1997, Tarun and Sachs, 1995). It has been shown that both cap-dependent and cap-independent translation are stimulated by factors that bind the poly(A) tail, presumably circularizing the mRNA (Bergamini et al., 2000, Michel et al., 2001). For many mRNAs, this 5′–3′ linkage is mediated by the interaction of the 5′-bound eIF4G and the 3′-bound poly(A)-binding protein (PABP), either directly (Otero et al., 1999, Tarun and Sachs, 1996) or via the bridging protein, PABP-interacting protein (PAIP-1) (Fig. 1A) (Craig et al., 1998). PABP may also interact specifically with ribosomal subunits through the use of RNA recognition motifs (RRM) within PABP, which allow it to bind directly to ribosomal RNA (Imataka et al., 1998).
Many mammalian RNA viruses (e.g. togaviruses, picornaviruses, and coronaviruses) contain genomes that terminate in poly(A) tails, which are similar in length to cellular poly(A) tails and are thought to function in translation like their cellular counterparts. Despite the absence of a cap structure at the 5′-end of the picornavirus genome, the presence of PABP has been shown to enhance initiation of translation from picornavirus IRES elements (Fig. 1C) (De Benedetti et al., 1991, Michel et al., 2001, Paulous et al., 2003). However, poly(A)-mediated stimulation of picornaviral IRES activity depends on the integrity of eIF4G and the interaction of intact eIF4G with PABP (Michel et al., 2001). Thus, since eIF4G is cleaved over the course of picornaviral infection, it is likely that this interaction is important only early in infection. Despite recent advances in understanding the role of the viral 3′UTR in the regulation of translation, the function of mRNA circularization for IRES-dependent translation is not yet fully characterized.
The 3′UTRs of IRES-containing viral genomes are also involved in the regulation of viral protein expression through the binding of cell-specific proteins required for IRES activity. For example, sequences upstream of the poly(A) tail in the picornavirus 3′UTR seem to be involved in the replication of the virus specifically in neuronal cells (Brown et al., 2004, Dobrikova et al., 2003). In addition to stimulating translation via a poly(A)/PABP interaction, sequences in the picornavirus 3′UTR upstream of the poly(A) tail are able to enhance IRES-driven translation in the absence of the poly(A) (Lopez de Quinto et al., 2002). Various cellular proteins may play a role in this process. For example, the cellular proteins eukaryotic elongation factor 1A (eEFIA), La autoantigen (La), murine proliferation-associated protein-1 (Mppl), poly-r(C)-binding protein (PCBP), and polypyrimidine tract binding protein (PTB) have been shown to bind the 5′UTR of picornaviruses and to enhance translation from picornavirus IRES elements (Blyn et al., 1996, Blyn et al., 1997, Borman et al., 1993, Florez et al., 2005, Hellen et al., 1993, Kolupaeva et al., 1996, Meerovitch et al., 1993, Pilipenko et al., 2000, Svitkin et al., 1994), and some of these cellular proteins have also been observed to bind to the 3′UTR of the Norwalk calicivirus (La, PTB, and PABP) (Gutierrez-Escolano et al., 2003) and the HCV genomic RNA (PTB) (Fig. 1D) (Ito and Lai, 1999). Taken together, these observations suggest a potential means of communication between the viral UTRs wherein these cellular proteins interact with the viral RNA to functionally replace certain canonical translation factors.
Several viral genomes lack the poly(A) tail as a translational enhancer, including rotavirus (Reoviridae) and members of the Flaviviridae family. However, enhancement of translational efficiency via interaction of the 5′ and 3′ ends has been demonstrated for these viruses by a range of mechanisms (Chiu et al., 2005, Holden and Harris, 2004, Piron et al., 1998). For example, circularization and translational enhancement of the m7G-capped rotavirus genome are mediated by the virally encoded protein NSP3, which binds to a conserved sequence in the viral 3′UTR (UGUGACC) that functionally substitutes for the poly(A) tail. The 5′ and 3′ ends of each segment of the rotavirus genome (six RNAs in total) are brought together through the direct interaction of the NSP3 protein and eIF4G (Fig. 1E) (Piron et al., 1998, Vende et al., 2000). An additional translation enhancer located only in the 3′UTR of the rotavirus gene 6 mRNA has been shown to augment expression of the major capsid protein VP6 (Yang et al., 2004).
The HCV genome is also not polyadenylated at its 3′ end. Although the HCV RNA does not require eIF4G for translation (Pestova et al., 1998), the HCV IRES has been shown to interact in vitro with recombinant cellular factors such as PTB (Ali and Siddiqui, 1995) and La (Ali and Siddiqui, 1997). PTB can bind to both the IRES (Ali and Siddiqui, 1995) and the X region at the 3′ end of the RNA (Tsuchihara et al., 1997). The multimerized PTB (Perez et al., 1997) may then circularize the HCV genome by binding to both ends of the RNA (Fig. 1D). While this interaction may not be critical for IRES-driven translation in transfected cell lines (Kong and Sarnow, 2002), its importance in the viral lifecycle in vivo remains to be tested.
Finally, although flaviviruses, such as the dengue (DENV) and West Nile (WNV) viruses, are not polyadenylated, the viral 3′UTR contains conserved regions including: the terminal 100 nucleotides (nt), which form a conserved stem loop termed the 3′SL (Brinton et al., 1986); structures that are predicted to form pseudoknots (Shi et al., 1996); and a 3′ cyclization sequence (CS) that is complementary to a CS at the 5′ end of the genome (Hahn et al., 1987). The DENV 3′UTR has been shown to stimulate translation of reporter constructs (Edgil et al., 2003, Holden and Harris, 2004), and half of this stimulation can been attributed to the action of the 3′SL (Fig. 1F) (Holden and Harris, 2004). This has been confirmed by other investigators who have found that the 3′SL contributes significantly to translational enhancement by the 3′UTR in DENV reporter constructs (Chiu et al., 2005). Consistent with these results, recent studies using phosphorodiamidate morpholino oligomers (PMOs) directed to the top of the DENV 3′SL demonstrated an approximately 50% reduction in translation using DENV reporter constructs and DENV replicons containing the nonstructural protein genes in addition to a luciferase reporter (Holden et al., in press). DENV replicons containing mutations in the loops at the top of the 3′SL also reduced translation by approximately 50% compared to the wildtype replicon (K. Clyde, K.L. Holden, E. Harris, unpublished results). Furthermore, similar PMOs targeting the 3′SL of WNV have recently been shown to reduce replication of WNV replicons (Deas et al., 2005), and mutations in the 3′SL of infectious viral RNA negatively impact flavivirus RNA synthesis, host-range and viability (Markoff et al., 2002, Zeng et al., 1998). Conversely, an earlier study using WNV reporter constructs suggested that the WNV 3′SL in the absence of the rest of the viral 3′UTR may inhibit translation of reporter RNAs (Li and Brinton, 2001). While it is clear that the flavivirus 3′UTR plays a role in the modulation of translation efficiency, the mechanism of action is likely regulated by many factors, including the genomic context of the 3′SL.
One of the main functions of the 3′SL seems to be the recruitment of viral and cellular proteins. The flavivirus 3′SL has been shown to bind to the viral proteins NS5, NS3, and NS2A, which presumably form part of the replication complex (Chen et al., 1997, Khromykh et al., 2000), as well as to the cellular proteins eEFIA, La, and PTB (Blackwell and Brinton, 1995, Blackwell and Brinton, 1997, De Nova-Ocampo et al., 2002, Garcia-Montalvo et al., 2004). While the functional consequence of the interaction of these proteins with the 3′UTR has not yet been demonstrated, it may serve to more effectively recruit and/or stabilize critical translation factors at the 5′UTR. These ribonucleoprotein (RNP) complexes may mediate the interaction of the viral UTRs as well as play a role in the switch between translation and replication of the viral genome (see below).
Conserved domains other than the 3′SL in the 3′UTR of flaviviruses have been shown to regulate flavivirus translation. For instance, when both pseudoknot domains are deleted from DENV reporter replicons or reporter constructs, translation is reduced 40–75%, respectively (Alvarez et al., 2005a, Chiu et al., 2005) (K.L. Holden, E. Harris, unpublished results). In addition, these domains appear to play a more dramatic role in regulation of viral RNA synthesis (Alvarez et al., 2005a, Lo et al., 2003, Tilgner et al., 2005). Interestingly, when the entire 3′UTR is deleted from DENV or WNV reporter replicons, little effect on translation is observed (Alvarez et al., 2005a, Tilgner et al., 2005), implying that both positive and negative regulators of translation exist within the flavivirus 3′UTR.
Although it is clear that the circularization of cellular and viral mRNAs stimulates translation, the exact mechanism has not been defined. In the ‘closed-loop’ model of mRNA translation, it is thought that cyclization may serve to stabilize the mRNA and the translation complex. Furthermore, the interaction of the UTRs may ensure translation only of full-length RNAs that contain both the 5′ and 3′ ends. However, under certain conditions, stimulation of translation of cellular mRNAs can be mediated by the poly(A) in trans in a cell-free system, suggesting that it is not circularization of mRNAs, but the interaction of the ends with the translational machinery that prompts maximal translation efficiency (Borman et al., 2002). Alternatively, circularization may promote efficient recycling of ribosomes and rapid re-initiation of translation on the same strand of RNA (Sachs, 2000). This strategy would be particularly advantageous for viral RNAs that need to compete effectively with cellular messages for limited translation factors and ribosomes.
2.2. Alternative strategies of viral translational control
While DENV and other flaviviruses containing capped RNA genomes undergo cap-dependent translation, DENV has been shown to translate efficiently under circumstances in which cap-dependent cellular translation is suppressed through the depletion of eIF4E (Edgil et al., submitted for publication). To investigate this phenomenon, several approaches were used to inhibit cap-dependent translation. Initially, the compounds rapamycin, wortmannin and LY294002 were used to suppress cellular translation initiation through the sequestration of eIF4E. The downstream effect of the interaction of rapamycin with the mammalian target of rapamycin (mTOR) is the hypo-phosphorylation of the eIF4E-binding protein 1 (4E-BP). Similarly, wortmannin and LY294002 inhibit the phosphoinositol-3-kinase (PI3-kinase) pathway, which also results in the hypo-phosphorylation of 4E-BP (Vanhaesebroeck et al., 2001). In its hypo-phosphorylated form, 4E-BP binds to and sequesters eIF4E from the eIF4F translation initiation complex, thus inhibiting cap-dependent translation. The impact of treatment with these drugs on translation of cellular cap-dependent mRNAs was compared to their effect on DENV reporter constructs and on replication of infectious viral RNA. When cells were incubated with DENV in the presence of rapamycin, LY294002 or wortmannin, viral titers remained unchanged at 24 h post-infection (Fig. 2A). These results are consistent with recent studies that have shown both DENV and JEV infection to be resistant to treatment with LY294002 (Lee et al., 2005). Furthermore, whereas metabolic labeling of total cellular protein in inhibitor-treated cells revealed an inhibition of cellular protein synthesis 12 h post-infection, translation of DENV proteins, represented by the DENV RNA-dependent RNA polymerase (NS5), was relatively unaffected (Fig. 2B).
Fig. 2.
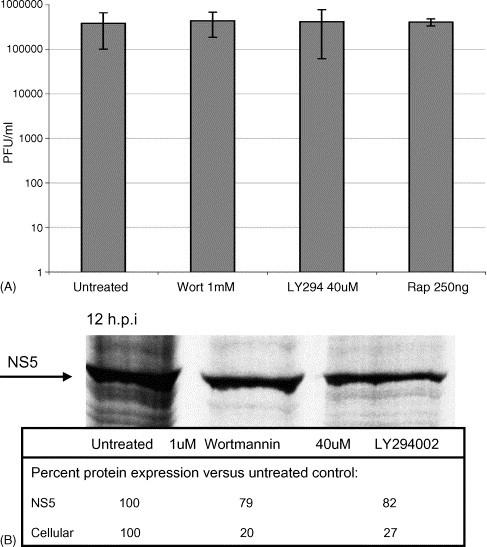
DENV replication and translation are not affected by inhibitors of cap-dependent translation, (A) DENV is replicated in the presence of rapamycin, LY294002 or wortmannin. Cells were exposed to DENV for 1 h in the presence of inhibitors, washed, and incubated with rapamycin (250 ng/ml), LY294002 (40 μM) or wortmannin (1 μM) for 24 h. Supernatants were collected and titered by plaque assay (PFU/ml), (B) DENV RNA is translated in the presence of LY294002 or wortmannin. Cells were prepared as described above. At 12 h post-infection, cells were metabolically labeled and analyzed by SDS-PAGE. ImageQuant software (Molecular Dynamics) was used to quantify the DENV protein NS5 (arrow) and representative cellular proteins.
To measure the impact of these inhibitors on viral translation specifically, a series of reporter constructs containing either the DENV 5′UTR or the control human beta-globin (BG) 5′UTR fused to the firefly luciferase gene (Luc) followed by either the DENV-2 3′UTR, a 60-mer poly(A) tail, or 268 nt of vector sequence was generated (Fig. 3A). Luc activity was measured from RNA transcripts that were generated from the constructs above and transfected into cells in the presence of rapamycin, wortmannin, or LY294002. It was observed that translation of only the reporter construct containing both the DENV 5′ and 3′UTRs was unaffected by the presence of drugs that inhibit translation of cap-dependent cellular messages, whereas that of the other mRNAs was reduced (Fig. 3B). Similar results were observed in cells in which cap-dependent translation was inhibited via small interfering RNA (siRNA)-mediated gene silencing of eIF4E or expression of constitutively hypo-phosphorylated 4E-BP (data not shown). Collectively, these results indicate that, despite the presence of a 5′ cap structure on the DENV genome, DENV replication and DENV translation are resistant to the effects of inhibition of cap-dependent translation.
Fig. 3.
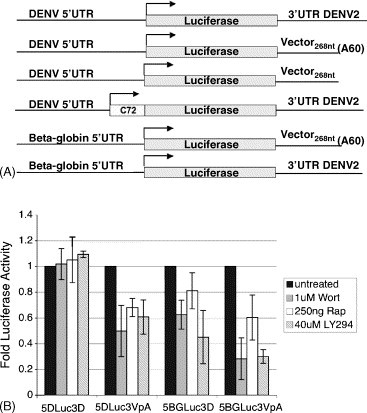
Translation of DENV reporter RNA is not affected by inhibitors of cap-dependent translation (A) DENV RNA reporter constructs. RNA reporter constructs were generated containing either the DENV-2 5′UTR plus or minus the first 72 nt of the DENV coding region or the human β-globin (βg) 5′UTR fused to the firefly luciferase (Luc) gene, followed by either vector sequence, the DENV-2 3′UTR or vector sequence plus a 60-mer poly(A) tail. RNA transcribed in vitro from these constructs using T7 polymerase was transfected into cells, (B) m7G-capped DENV RNA reporter constructs are translated in BHK cells treated with rapamycin, LY294002 or wortmannin. Equal amounts of in vitro transcripts of the constructs described above were capped with m7GpppN and transfected into BHK cells. One hour post-transfection, cells were washed and 250 ng/ml rapamycin, 40 μM LY294002 or 1 μM wortmannin were added. Luc activity was assayed after 12 h by luminometry.
To further examine the ability of the DENV genome to replicate in the absence of a functional cap structure under conditions in which eIF4E is limiting, RNA was transcribed from the DENV-2 infectious clone to contain either a functional 5′ m7GpppA cap structure or a nonfunctional 5′ ApppA cap. Equal amounts of these RNAs were then transfected into cells treated with inhibitors of cap-dependent translation. When supernatants were analyzed by plaque assay 48 h post-transfection, cells containing the ApppA-capped viral RNA released no infectious progeny, compared with titers of ∼103 pfu/ml released by cells that had been transfected with the m7G-capped viral RNA (Table 1 , column 1). These results suggest that, in cells competent for cap-dependent translation, DENV translates exclusively via its 5′ m7G-cap structure. Replication of the m7G-capped viral RNAs was resistant to the presence of the inhibitors of cap-dependent translation (Table 1, row 1), similar to results obtained with the virus itself (Fig. 2A). Moreover, replication of the nonfunctionally capped viral genomes was increased to ∼102 pfu/ml (Table 1, row 2), indicating that the DENV viral genome can be induced to replicate independently of the cap structure under conditions of reduced eIF4E. This data is also consistent with increased levels of translation observed with ApppA-capped DENV reporter RNAs in inhibitor-treated cells relative to ApppN-capped reporter RNAs containing the beta-globin 3′UTR, the poly(A) tail, or both (data not shown).
Table 1.
DENV can replicate independently of a functional 5′ cap under conditions of reduced eIF4E
| Cell treatment |
|||
|---|---|---|---|
| Untreated | 40 μM LY294002 | 1 μM Wortmannin | |
| m7GpppA-capped DENV RNA (pfu/ml) | 822 ± 269 | 1022 ± 758 | 2455 ± 1296 |
| ApppA-capped DENV RNA (pfu/ml) | 0 | 183 ± 42 | 378 ± 270 |
Equal amounts of functionally (m7GpppA) or nonfunctionally (ApppA) capped in vitro transcripts generated from the DENV-2 infectious clone were transfected into cells for 4 h. Cells were washed and incubated with LY294002 or wortmannin for 24 h. Supernatants were then collected and titered on BHK cells.
Together, these data support a model in which DENV is able to alternate from a canonical cap-dependent form of translation initiation to a noncanonical mechanism under conditions of reduced eIF4E (Fig. 4 ). It is clear that the interaction of the DENV 5′ and 3′UTRs stimulates translation; when eIF4E is present, translation initiation presumably occurs through a classical cap-dependent scanning mechanism. However, under conditions in which eIF4E or other cellular translation factors are limiting, such as in differentiated cell types with low levels of eIF4E (Grolleau et al., 1999, Krichevsky et al., 1999) or as a result of the IFN antiviral response (Gil et al., 1999), efficient viral translation requires both the DENV 5′ and 3′UTRs. This data suggests a model in which viral RNA that is translated in cellular conditions where eIF4E is limiting undergoes a reorganization of viral RNP complexes such that RNA structures or sequences in the 3′UTR interact with higher affinity with protein complexes containing eIF4G and eIF4A. This would result in effective delivery by the DENV 3′UTR of key translation initiation factors to the DENV 5′UTR, similar to the mechanism proposed for cap-independent translation of Luteovirus RNAs, such as the barley yellow dwarf virus genome (Guo et al., 2001).
Fig. 4.
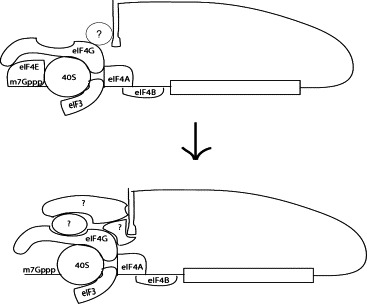
Model of the reorganization of RNP complexes at the 5′ and 3′UTRs of the flavivirus genome under conditions of limiting eIF4E.
2.3. Switch between translation and RNA synthesis
Adding to the complexity of translational control, the 3′UTRs of cellular and viral messages often contain regulatory elements that negatively modulate expression. Whereas the poly(A) tail is thought to stimulate translation, regulatory elements upstream of the poly(A) tail in the 3′UTR may maintain viral RNA in an inactive state via binding of repressor proteins (Fu et al., 1999, Tadauchi et al., 2001). One reason for this level of control may involve the switch from translation to RNA synthesis that must occur in positive-stranded RNA viruses, which are unable to replicate RNA templates undergoing translation due to the collision of translation and replication complexes proceeding in opposite directions. For picornaviruses, the alternate binding of the cellular factor PCBP or the viral protein 3CD to certain viral RNA structures has been shown to control the function of the genomic RNA in translation versus RNA synthesis (Barton et al., 1999, Gamarnik and Andino, 1997, Gamarnik and Andino, 1998). Additionally, both the 5′ and the 3′ termini of the pestivirus bovine viral diarrhea virus (BVDV) RNA genome have been shown to associate specifically with the ‘NFAR’ protein group (Isken et al., 2003). This interaction appears to be important for efficient translation termination and RNA replication and may function to regulate the switch between the two viral states (Isken et al., 2004).
Although the flavivirus DENV is not polyadenylated, the DENV 3′UTR contains conserved regions, including the cyclization sequences (CS) and tandem repeats (Hahn et al., 1987). The 5′CS, which comprises an 11-nt region ∼35 nt downstream of the 5′ end of the capsid gene, and its complementary 3′CS sequence located ∼100 nt from the end of the 3′UTR are the most conserved linear sequences in the flavivirus genome. Recently, additional regions of complementarity between the DENV 5′ and 3′UTRs have been identified and named UAR (for upstream AUG region); the 5′ sequence is 16 nt long and is located immediately upstream of the initiation codon while the 17 nt 3′UAR is found in the stem of the 3′SL (Alvarez et al., 2005b). Similar to the model for alphaviruses and bunyaviruses (Hsu et al., 1974, Talmon et al., 1987), it is hypothesized that base-pairing between the 5′ and 3′ ends, which circularizes the genome, ensures the synthesis of full-length genomic RNAs (Hahn et al., 1987). In fact, visualization of individual DENV viral RNA molecules by atomic force microscopy confirmed that cyclization of the viral genome is a direct result of the physical interaction of the CS and UARs (Alvarez et al., 2005b). Moreover, this circularization appears to be necessary for flavivirus replication; mutation of the CS or UAR sequences in infectious DENV RNA resulted in a lethal phenotype in mammalian cells (Alvarez et al., 2005b, Men et al., 1996). Similarly, PMOs targeted to the 3′CS of a DENV or WNV replicon suppressed RNA replication specifically (Deas et al., 2005, Holden et al., in press). The flavivirus RNA-dependent RNA polymerase (RdRp) also appears to require CS base-pairing for RNA synthesis both in vitro and in vivo (Khromykh et al., 2001, You and Padmanabhan, 1999), whereas the precise role of the UAR sequences in RNA synthesis remains to be determined. Together, these data underscore the importance of the interaction between the 5′ and 3′UTRs in the viral lifecycle.
On the other hand, the flavivirus CS do not appear to be necessary for viral protein synthesis. To measure the influence of the CS on DENV translation, reporter constructs containing the DENV 5′ and 3′UTRs in the presence or absence of the first 72 nt of the capsid protein, which includes the 5′CS, were generated (Fig. 2A). Luc activity measured from cells transfected with either of these RNA constructs demonstrated similar translation efficiencies (Fig. 5 ), indicating that DENV was translated independently of the interaction of the CS. These results are consistent with recent reports demonstrating that hybridization of the 5′ and 3′CS in the context of either DENV reporter constructs or DENV replicons is not necessary for translation, and may even inhibit translation (Alvarez et al., 2005a, Chiu et al., 2005). However, the role of 5′–3′ end interaction in enhancing viral translation combined with the requirement for CS base-pairing for RNA synthesis suggests the existence of alternative conformations of the flavivirus CS and UTRs for distinct viral functions. Like the picornavirus genome, the flavivirus genomic RNA is the template for multiple stages of the viral lifecycle that cannot occur simultaneously on the same RNA. Regulation of these stages depends on the communication of the viral UTRs. During replication, base-pairing of the CS appears to be necessary for viral RNA synthesis. However, this same interaction may inhibit translation of the RNA. We speculate that the switch between translation and replication of the flavivirus genome is mediated by the architectural remodeling of portions of the genome from that of an RNA–protein bridge between the 5′ and 3′UTRs to RNA–RNA interactions between the CS and possibly the UARs at the extreme ends of the viral genome (Fig. 6 ). In this model, the switch between translation and replication is presumably mediated by viral and/or cellular factors that facilitate communication between the viral UTRs.
Fig. 5.
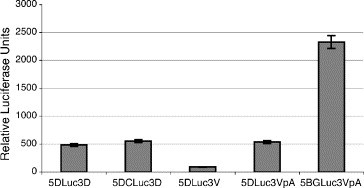
DENV translation is independent of the CS. Equal amounts of m7GpppA-capped in vitro transcripts of the reporter constructs containing the DENV 5′ and 3′UTRs plus or minus the CS were transfected into cells, along with control reporter RNAs (see Fig. 2A). Cells were washed 1 h post-transfection, and Luc activity was assayed after 8 h by luminometry.
Fig. 6.
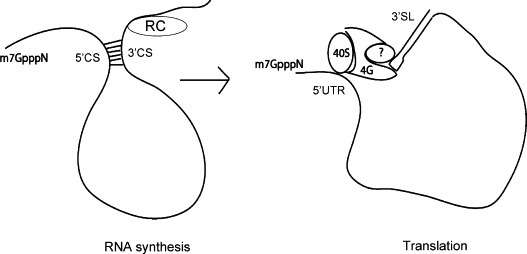
Model depicting the switch between translation and replication of the flavivirus genome mediated through the architectural remodeling of viral RNA.
The genome of RNA viruses is, of necessity, extremely versatile. The compact size of an RNA genome demands ingenuity, in the form of overlapping open reading frames, frame-shifting, and multiple uses of the viral 5′ and 3′UTRs. To maximize the efficacy of the genome, RNA viruses regulate translation, RNA synthesis, and the switch between these two stages through the communication of the UTRs via both RNA–RNA and RNA–protein interactions.
Acknowledgments
We would like to thank members of the Harris laboratory for helpful discussions; Karen Clyde, Anna-Marija Helt, Katherine Holden, and Suman Paranjape for editorial comments; and the Pew Charitable Trusts (#2617SC) and NIH (AI052324) for financial support.
References
- Ali N., Siddiqui A. Interaction of polypyrimidine tract-binding protein with the 5′ noncoding region of the hepatitis C virus RNA genome and its functional requirement in internal initiation of translation. J. Virol. 1995;69:6367–6375. doi: 10.1128/jvi.69.10.6367-6375.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali N., Siddiqui A. The La antigen binds 5′ noncoding region of the hepatitis C virus RNA in the context of the initiator AUG codon and stimulates internal ribosome entry site-mediated translation. Proc. Natl. Acad. Sci. U.S.A. 1997;94:2249–5422. doi: 10.1073/pnas.94.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez D.E., De Lella Ezcurra A.L., Fucito S., Gamarnik A.V. Role of RNA structures present at the 3′UTR of dengue virus on translation, RNA synthesis, and viral replication. Virology. 2005;339:200–212. doi: 10.1016/j.virol.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Alvarez D.E., Lodeiro M.F., Ludueña S.J., Pietrasanta L.I., Gamarnik A.G. Long-range RNA–RNA interactions circularize the dengue virus genome. J. Virol. 2005;79:6631–6643. doi: 10.1128/JVI.79.11.6631-6643.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton D.J., Morasco B.J., Flanegan J.B. Translating ribosomes inhibit poliovirus negative-strand RNA synthesis. J. Virol. 1999;73:10104–10112. doi: 10.1128/jvi.73.12.10104-10112.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamini G., Preiss T., Hentze M.W. Picornavirus IRESes and the poly(A) tail jointly promote cap-independent translation in a mammalian cell-free system. RNA. 2000;6:1781–1790. doi: 10.1017/s1355838200001679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell J.L., Brinton M.A. BHK cell proteins that bind to the 3′ stem-loop structure of the West Nile Virus genome RNA. J. Virol. 1995;69:5650–5658. doi: 10.1128/jvi.69.9.5650-5658.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell J.L., Brinton M.A. Translation elongation factor-1 alpha interacts with the 3′ stem-loop region of West Nile Virus genomic RNA. J. Virol. 1997;71:6433–6444. doi: 10.1128/jvi.71.9.6433-6444.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyn L.B., Swiderek K.M., Richards O., Stahl D.C., Semler B.L., Ehrenfeld E. Poly(rC) binding protein 2 binds to stem-loop IV of the poliovirus RNA 5′ noncoding region: identification by automated liquid chromatography–tandem mass spectrometry. Proc. Natl. Acad. Sci. U.S.A. 1996;93:11115–11120. doi: 10.1073/pnas.93.20.11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyn L.B., Towner J.S., Semler B.L., Ehrenfeld E. Requirement of poly(rC) binding protein 2 for translation of poliovirus RNA. J. Virol. 1997;71:6243–6246. doi: 10.1128/jvi.71.8.6243-6246.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borman A., Howell M.T., Patton J.G., Jackson R.J. The involvement of a spliceosome component in internal initiation of human rhinovirus RNA translation. J. Gen. Virol. 1993;74:1775–1788. doi: 10.1099/0022-1317-74-9-1775. [DOI] [PubMed] [Google Scholar]
- Borman A.M., Michel Y.M., Malnou C.E., Kean K.M. Free poly(A) stimulates capped mRNA translation in vitro through the eIF4G-PABP interaction. J. Biol. Chem. 2002;277:36818–36824. doi: 10.1074/jbc.M205065200. [DOI] [PubMed] [Google Scholar]
- Brinton M.A., Fernandez A.V., Dispoto J.H. The 3′-nucleotides of flavivirus genomic RNA form a conserved secondary structure. Virology. 1986;153:113–121. doi: 10.1016/0042-6822(86)90012-7. [DOI] [PubMed] [Google Scholar]
- Brown D.M., Kauder S.E., Cornell C.T., Jang G.M., Racaniello V.R., Semler B.L. Cell-dependent role for the poliovirus 3′ noncoding region in positive-strand RNA synthesis. J. Virol. 2004;78:1344–1351. doi: 10.1128/JVI.78.3.1344-1351.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.J., Ku M.D., Chien L.J., Hsu S.L., Wang Y.M., Lin J.H. RNA–protein interactions: involvement of NS3, NS5, and 3′ noncoding regions of Japanese encephalitis virus genomic RNA. J. Virol. 1997;71:3466–3473. doi: 10.1128/jvi.71.5.3466-3473.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu W.W., Kinney R.M., Dreher T.W. Control of translation by the 5′- and 3′-terminal regions of the dengue virus genome. J. Virol. 2005;79:8303–8315. doi: 10.1128/JVI.79.13.8303-8315.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A.W., Haghighat A., Yu A.T., Sonenberg N. Interaction of polyadenylate-binding protein with the eIF4G homologue PAIP enhances translation. Nature. 1998;392:520–523. doi: 10.1038/33198. [DOI] [PubMed] [Google Scholar]
- Daughenbaugh K., Fraser C., Hershey J., ME H. The genome-linked protein VPg of the Norwalk virus binds eIF3, suggesting its role in translation initiation complex recruitment. EMBO J. 2003;22:2852–2859. doi: 10.1093/emboj/cdg251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedetti A., Joshi-Barve S., Rinker-Schaeffer C., Rhoads R.E. Expression of antisense RNA against initiation factor eIF-4E mRNA in HeLa cells results in lengthened cell division times, diminished translation rates, and reduced levels of both eIF-4E and the p220 component of eIF-4F. Mol. Cell. Biol. 1991;11:5435–5445. doi: 10.1128/mcb.11.11.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nova-Ocampo M., Villegas-Sepulveda N., del Angel R.M. Translation elongation factor-1 alpha, La, and PTB interact with the 3′ untranslated region of dengue 4 virus RNA. Virology. 2002;295:337–347. doi: 10.1006/viro.2002.1407. [DOI] [PubMed] [Google Scholar]
- Deas T.S., Binduga-Gajewska I., Tilgner M., Ren P., Stein D.A., Moulton H.M., Iversen P.L., Kauffman E.B., Kramer L.D., Shi P.Y. Inhibition of flavivirus infections by antisense oligomers specifically suppressing viral translation and RNA replication. J. Virol. 2005;79:4599–4609. doi: 10.1128/JVI.79.8.4599-4609.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrikova E., Florez P., Bradrick S., Gromeier M. Activity of a type 1 picornavirus internal ribosomal entry site is determined by sequences within the 3′ nontranslated region. Proc. Natl. Acad. Sci. U.S.A. 2003;100:15125–15130. doi: 10.1073/pnas.2436464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgil D., Diamond M.S., Holden K.L., Paranjape S.M., Harris E. Translation efficiency determines differences in cellular infection among dengue virus type 2 strains. Virology. 2003;317:275–290. doi: 10.1016/j.virol.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Edgil, D., Polacek, C., Harris, E. A novel mechanism for dengue virus translation, submitted for publication.
- Florez P.M., Sessions O.M., Wagner E.J., Gromeier M., Garcia-Blanco M.A. The polypyrimidine tract binding protein is required for efficient picornavirus gene expression and propagation. J. Virol. 2005;79:6172–6179. doi: 10.1128/JVI.79.10.6172-6179.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L., Ma W., Benchimol S. A translation repressor element resides in the 3′ untranslated region of human p53 mRNA. Oncogene. 1999;18:6419–6424. doi: 10.1038/sj.onc.1203064. [DOI] [PubMed] [Google Scholar]
- Gamarnik A.V., Andino R. Two functional complexes formed by KH domain containing proteins with the 5′ noncoding region of poliovirus RNA. RNA. 1997;3:882–892. [PMC free article] [PubMed] [Google Scholar]
- Gamarnik A.V., Andino R. Switch from translation to RNA replication in a positive-stranded RNA virus. Genes Dev. 1998;12:2293–2304. doi: 10.1101/gad.12.15.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Montalvo B.M., Medina F., Del Angel R.M. La protein binds to NS5 and NS3 and to the 5′ and 3′ ends of Dengue 4 virus RNA. Virus Res. 2004;102:141–150. doi: 10.1016/j.virusres.2004.01.024. [DOI] [PubMed] [Google Scholar]
- Gil J., Alcami J., Esteban M. Induction of apoptosis by double-stranded-RNA-dependent protein kinase (PKR) involves the alpha subunit of eukaryotic translation initiation factor 2 and NF-kappaB. Mol. Cell. Biol. 1999;19:4653–4663. doi: 10.1128/mcb.19.7.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras A.C., Raught B., Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 1999;68:913–963. doi: 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- Goodfellow I., Chaudhry Y., Gioldasi I., Gerondopoulos A., Natoni A., Labrie L., Laliberte J.-F., Roberts L. Calicivirus translation initiation requires an interaction between VPg and eIF4E. EMBO Rep. 2005;6:968–972. doi: 10.1038/sj.embor.7400510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grolleau A., Sonenberg N., Wietzerbin J., Beretta L. Differential regulation of 4E-BP1 and 4E-BP2, two repressors of translation initiation, during human myeloid cell differentiation. J. Immunol. 1999;162:3491–3497. [PubMed] [Google Scholar]
- Guo L., Allen A.M., Miller W.A. Base-pairing between untranslated regions facilitates translation of uncapped, nonpolyadenylated viral RNA. Mol. Cell. 2001;7:1103–1109. doi: 10.1016/s1097-2765(01)00252-0. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Escolano A.L., Vazquez-Ochoa M., Escobar-Herrera J., Hernandez-Acosta J. La, PTB, and PAB proteins bind to the 3(′) untranslated region of Norwalk virus genomic RNA. Biochem. Biophys. Res. Commun. 2003;311:759–766. doi: 10.1016/j.bbrc.2003.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn C.S., Hahn Y.S., Rice C.M., Lee E., Dalgarno L., Strauss E.G., Strauss J.H. Conserved elements in the 3′ untranslated region of flavivirus RNAs and potential cyclization sequences. J. Mol. Biol. 1987;198:33–41. doi: 10.1016/0022-2836(87)90455-4. [DOI] [PubMed] [Google Scholar]
- Hellen C.U.T., Witherell G.W., Schmid M., Shin S.H., Pestova T.V., Gil A., Wimmer E. The cellular polypeptide p57 that is required for translation of picornavirus RNAs by internal ribosome entry is identical to the nuclear pyrimidine-tract binding protein. Proc. Natl. Acad. Sci. U.S.A. 1993;90:7642–7646. doi: 10.1073/pnas.90.16.7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert T.P., Brierley I., Brown T.D. Identification of a protein linked to the genomic and subgenomic mRNAs of feline calicivirus and its role in translation. J. Gen. Virol. 1997;78(Pt 5):1033–1040. doi: 10.1099/0022-1317-78-5-1033. [DOI] [PubMed] [Google Scholar]
- Holden K.L., Harris E. Enhancement of dengue virus translation: role of the 3′untranslated region and the terminal 3′ stem-loop domain. Virology. 2004;329:119–133. doi: 10.1016/j.virol.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Holden, K.L., Stein, D., Pierson, T.C., Ahmed, A., Clyde, K., Iverson, P., Harris, E. Inhibition of dengue virus translation and RNA synthesis by a morpholino oligomer to the top of the 3′ stem-loop structure. Virology, in press. [DOI] [PubMed]
- Hsu M.T., Kung H.J., Davidson N. An electron microscope study of Sindbis virus RNA. Cold Spring Harb. Symp. Quant. Biol. 1974;38:943–950. doi: 10.1101/sqb.1974.038.01.096. [DOI] [PubMed] [Google Scholar]
- Imataka H., Gradi A., Sonenberg N. A newly identified N-terminal amino acid sequence of human eIF4G binds poly(A)-binding protein and functions in poly(A)-dependent translation. EMBO J. 1998;17:7480–7489. doi: 10.1093/emboj/17.24.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isken O., Grassmann C.W., Sarisky R.T., Kann M., Zhang S., Grosse F., Kao P.N., Behrens S.E. Members of the NF90/NFAR protein group are involved in the life cycle of a positive-strand RNA virus. EMBO J. 2003;22:5655–5665. doi: 10.1093/emboj/cdg562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isken O., Grassmann C.W., Yu H., Behrens S.E. Complex signals in the genomic 3′ nontranslated region of bovine viral diarrhea virus coordinate translation and replication of the viral RNA. RNA. 2004;10:1637–1652. doi: 10.1261/rna.7290904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T., Lai M.M. An internal polypyrimidine-tract-binding protein-binding site in the hepatitis C virus RNA attenuates translation, which is relieved by the 3′-untranslated sequence. Virology. 1999;254:288–296. doi: 10.1006/viro.1998.9541. [DOI] [PubMed] [Google Scholar]
- Khromykh A.A., Meka H., Guyatt K.J., Westway E.G. Essential role of cyclization domains in flavivirus RNA replication. J. Virol. 2001;75:6719–6728. doi: 10.1128/JVI.75.14.6719-6728.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khromykh A.A., Sedlak P.L., Westaway E.G. cis- and trans-acting elements in flavivirus RNA replication. J. Virol. 2000;74:3253–3263. doi: 10.1128/jvi.74.7.3253-3263.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieft J.S., Zhou K., Jubin R., Doudna J.A. Mechanism of ribosome recruitment by hepatitis C IRES RNA. RNA. 2001;7:194–206. doi: 10.1017/s1355838201001790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolupaeva V.G., Hellen C.U., Shatsky I.N. Structural analysis of the interaction of the pyrimidine tract-binding protein with the internal ribosomal entry site of encephalomyocarditis virus and foot-and-mouth disease virus RNAs. RNA. 1996;2:1199–1212. [PMC free article] [PubMed] [Google Scholar]
- Kong L.K., Sarnow P. Cytoplasmic expression of mRNAs containing the internal ribosome entry site and 3′ noncoding region of hepatitis C virus: effects of the 3′ leader on mRNA translation and mRNA stability. J. Virol. 2002;76:12457–12462. doi: 10.1128/JVI.76.24.12457-12462.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krichevsky A.M., Metzer E., Rosen H. Translational control of specific genes during differentiation of HL-60 cells. J. Biol. Chem. 1999;274:14295–14305. doi: 10.1074/jbc.274.20.14295. [DOI] [PubMed] [Google Scholar]
- Lee C.-J., Liao C.-L., Lin Y.-L. Flavivirus activates phosphatidylinositol 3-kinase signaling to block caspase-dependent apoptotic cell death at the early stage of virus infection. J. Virol. 2005;79:8388–8399. doi: 10.1128/JVI.79.13.8388-8399.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Brinton M.A. The 3′ stem loop of the West Nile virus genomic RNA can suppress translation of chimeric mRNAs. Virology. 2001;287:49–61. doi: 10.1006/viro.2001.1015. [DOI] [PubMed] [Google Scholar]
- Lo M.K., Tilgner M., Bernard K.A., Shi P.Y. Functional analysis of mosquito-borne flavivirus conserved sequence elements within 3′ untranslated region of West Nile virus by use of a reporting replicon that differentiates between viral translation and RNA replication. J. Virol. 2003;77:10004–10014. doi: 10.1128/JVI.77.18.10004-10014.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomakin I.B., Hellen C.U., Pestova T.V. Physical association of eukaryotic initiation factor 4G (eIF4G) with eIF4A strongly enhances binding of eIF4G to the internal ribosomal entry site of encephalomyocarditis virus and is required for internal initiation of translation. Mol. Cell. Biol. 2000;20:6019–6029. doi: 10.1128/mcb.20.16.6019-6029.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez de Quinto S., Saiz M., de la Morena D., Sobrino F., Martinez-Salas E. IRES-driven translation is stimulated separately by the FMDV 3′-NCR and poly(A) sequences. Nucleic Acids Res. 2002;30:4398–4405. doi: 10.1093/nar/gkf569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markoff L., Pang X., Houng Hs H.S., Falgout B., Olsen R., Jones E., Polo S. Derivation and characterization of a dengue type 1 host range-restricted mutant virus that is attenuated and highly immunogenic in monkeys. J. Virol. 2002;76:3318–3328. doi: 10.1128/JVI.76.7.3318-3328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerovitch K., Svitkin Y.V., Lee H.S., Lejbkowicz F., Kenan D.J., Chan E.K.L., Agol V.I., Keene J.D., Sonenberg N. La autoantigen enhances and corrects aberrant translation of poliovirus RNA in reticulocyte lysate. J. Virol. 1993;67:3798–3807. doi: 10.1128/jvi.67.7.3798-3807.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Men R., Bray M., Clark D., Chanock R.M., Lai C.-J. Dengue type 4 virus mutants containing deletions in the 3′ noncoding region of the RNA genome: analysis of growth restriction in cell culture and altered viremia pattern and immunogenicity in Rhesus monkeys. J. Virol. 1996;70:3930–3937. doi: 10.1128/jvi.70.6.3930-3937.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel Y.M., Borman A.M., Paulous S., Kean K.M. Eukaryotic initiation factor 4G-poly(A) binding protein interaction is required for poly(A) tail-mediated stimulation of picornavirus internal ribosome entry segment-driven translation but not for X-mediated stimulation of hepatitis C virus translation. Mol. Cell. Biol. 2001;21:4097–4109. doi: 10.1128/MCB.21.13.4097-4109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlmann T., Prevot D., Decimo D., Roux F., Garin J., Morley S.J., Darlix J.L. In vitro cleavage of eIF4GI but not eIF4GII by HIV-1 protease and its effects on translation in the rabbit reticulocyte lysate system. J. Mol. Biol. 2002;318:9–20. doi: 10.1016/S0022-2836(02)00070-0. [DOI] [PubMed] [Google Scholar]
- Otero L.J., Ashe M.P., Sachs A.B. The yeast poly(A)-binding protein Pablp stimulates in vitro poly(A)-dependent and cap-dependent translation by distinct mechanisms. EMBO J. 1999;18:3153–3163. doi: 10.1093/emboj/18.11.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulous S., Malnou C.E., Michel Y.M., Kean K.M., Borman A.M. Comparison of the capacity of different viral internal ribosome entry segments to direct translation initiation in poly(A)-dependent reticulocyte lysates. Nucleic Acids Res. 2003;31:722–733. doi: 10.1093/nar/gkf695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez I., McAfee J.G., Patton J.G. Multiple RRMs contribute to RNA binding specificity and affinity for polypyrimidine tract binding protein. Biochemistry. 1997;36:11881–11890. doi: 10.1021/bi9711745. [DOI] [PubMed] [Google Scholar]
- Pestova T.V., Hellen C.U., Shatsky I.N. Canonical eukaryotic initiation factors determine initiation of translation by internal ribosomal entry. Mol. Cell. Biol. 1996;16:6859–6869. doi: 10.1128/mcb.16.12.6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestova T.V., Kolupaeva V.G., Lomakin I.B., Pilipenko E.V., Shatsky I.N., Agol V.I., Hellen C.U. Molecular mechanisms of translation initiation in eukaryotes. Proc. Natl. Acad. Sci. U.S.A. 2001;98:7029–7036. doi: 10.1073/pnas.111145798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestova T.V., Shatsky I.N., Fletcher S.P., Jackson R.J., Hellen C.U. A prokaryotic-like mode of cytoplasmic eukaryotic ribosome binding to the initiation codon during internal translation initiation of hepatitis C and classical swine fever virus RNAs. Genes Dev. 1998;12:67–83. doi: 10.1101/gad.12.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilipenko E.V., Pestova T.V., Kolupaeva V.G., Khitrina E.V., Poperechnaya A.N., Agol V.I., Hellen C.U. A cell cycle-dependent protein serves as a template-specific translation initiation factor. Genes Dev. 2000;14:2028–2045. [PMC free article] [PubMed] [Google Scholar]
- Piron M., Vende P., Cohen J., Poncet D. Rotavirus RNA-binding protein NSP3 interacts with eIF4GI and evicts the poly(A) binding protein from eIF4F. EMBO J. 1998;17:5811–5821. doi: 10.1093/emboj/17.19.5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs A. Physical and functional interactions between the mRNA cap structure and the poly(A) tail. In: Sonenberg N., Hershey J.W.B., Matthews M.B., editors. Translational Control of Gene Expression. second ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2000. pp. 447–465. [Google Scholar]
- Sachs A.B., Sarnow P., Hentze M.W. Starting at the beginning, middle, and end: translation initiation in eukaryotes. Cell. 1997;89:831–838. doi: 10.1016/s0092-8674(00)80268-8. [DOI] [PubMed] [Google Scholar]
- Shi P.-Y., Brinton M.A., Veal J.M., Zhong Y.Y., Wilson W.D. Evidence for the existence of a pseudoknot structure at the 3′ terminus of the flavivirus genomic RNA. Biochemistry. 1996;35:4222–4230. doi: 10.1021/bi952398v. [DOI] [PubMed] [Google Scholar]
- Sizova D.V., Kolupaeva V.G., Pestova T.V., Shatsky I.N., Hellen C.U. Specific interaction of eukaryotic translation initiation factor 3 with the 5′ nontranslated regions of hepatitis C virus and classical swine fever virus RNAs. J. Virol. 1998;72:4775–4782. doi: 10.1128/jvi.72.6.4775-4782.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkin Y.V., Meerovitch K., Lee H.S., Dholakia J.N., Kenan D.J., Agol V.I., Sonenberg N. Internal translation initiation on poliovirus RNA: further characterization of La function in poliovirus translation in vitro. J. Virol. 1994;68:1544–1550. doi: 10.1128/jvi.68.3.1544-1550.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadauchi T., Matsumoto K., Herskowitz I., Irie K. Post-transcriptional regulation through the HO 3′-UTR by Mpt5, a yeast homolog of Pumilio and FBF. EMBO J. 2001;20:552–561. doi: 10.1093/emboj/20.3.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmon Y., Prasad B.V., Clerx J.P., Wang G.J., Chiu W., Hewlett M.J. Electron microscopy of vitrified-hydrated La Crosse virus. J. Virol. 1987;61:2319–2321. doi: 10.1128/jvi.61.7.2319-2321.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarun S., Sachs A.B. A common function for mRNA 5′ and 3′ ends in translation initiation in yeast. Genes Dev. 1995;9:2997–3007. doi: 10.1101/gad.9.23.2997. [DOI] [PubMed] [Google Scholar]
- Tarun S.Z., Sachs A.B. Association of the yeast poly(A) tail binding protein with translation initiation factor eIF4G. EMBO J. 1996;15:7168–7177. [PMC free article] [PubMed] [Google Scholar]
- Tilgner M., Deas T.S., Shi P.Y. The flavivirus-conserved penta-nucleotide in the 3′ stem-loop of the West Nile virus genome requires a specific sequence and structure for RNA synthesis but not for viral translation. Virology. 2005;331:375–386. doi: 10.1016/j.virol.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Tsuchihara K., Tanaka T., Hijikata M., Kuge S., Toyoda H., Nomoto A., Yamamoto N., Shimotohno K. Specific interaction of polypyrimidine tract-binding protein with the extreme 3′-terminal structure of the hepatitis C virus genome, the 3′X. J. Virol. 1997;71:6720–6726. doi: 10.1128/jvi.71.9.6720-6726.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaesebroeck B., Leevers S.J., Ahmadi K., Timms J., Katso R., Driscoll P.C., Woscholski R., Parker P.J., Waterfield M.D. Synthesis and function of 3-phosphorylated inositol lipids. Annu. Rev. Biochem. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- Vende P., Piron M., Castagne N., Poncet D. Efficient translation of rotavirus mRNA requires simultaneous interaction of NSP3 with the eukaryotic translation initiation factor 3IF4G and the mRNA 3′ end. J. Virol. 2000;74:7064–7071. doi: 10.1128/jvi.74.15.7064-7071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A.D., Barro M., Gorziglia M.I., Patton J.T. Translation enhancer in the 3′-untranslated region of rotavirus gene 6 mRNA promotes expression of the major capsid protein VP6. Arch. Virol. 2004;149:303–321. doi: 10.1007/s00705-003-0211-9. [DOI] [PubMed] [Google Scholar]
- You S., Padmanabhan R. A novel in vitro replication system for Dengue virus. Initiation of RNA synthesis at the 3′-end of exogenous viral RNA templates requires 5′-and 3′-terminal complementary sequence motifs of the viral RNA. J. Biol. Chem. 1999;274:33714–33722. doi: 10.1074/jbc.274.47.33714. [DOI] [PubMed] [Google Scholar]
- Zeng L., Falgout B., Markoff L. Identification of specific nucleotide sequences within conserved 3′-SL in the dengue type 2 virus genome required for replication. J. Virol. 1998;72:7510–7522. doi: 10.1128/jvi.72.9.7510-7522.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


