Abstract
Human endogenous retroviruses (HERVs) constitute 5–8% of human genomic DNA and are replication incompetent despite expression of individual HERV genes from different chromosomal loci depending on the specific tissue. Several HERV genes have been detected as transcripts and proteins in the central nervous system, frequently in the context of neuroinflammation. The HERV-W family has received substantial attention in large part because of associations with diverse syndromes including multiple sclerosis (MS) and several psychiatric disorders. A HERV-W-related retroelement, multiple sclerosis retrovirus (MSRV), has been reported in MS patients to be both a biomarker as well as an effector of aberrant immune responses. HERV-H and HERV-K have also been implicated in MS and other neurological diseases but await delineation of their contributions to disease. The HERV-W envelope-encoded glycosylated protein, syncytin-1, is encoded by chromosome 7q21 and exhibits increased glial expression within MS lesions. Overexpression of syncytin-1 in glia induces endoplasmic reticulum stress leading to neuroinflammation and the induction of free radicals, which damage proximate cells. Syncytin-1's receptor, ASCT1 is a neutral amino acid transporter expressed on glia and is suppressed in white matter of MS patients. Of interest, antioxidants ameliorate syncytin-1's neuropathogenic effects raising the possibility of using these agents as therapeutics for neuroinflammatory diseases. Given the multiple insertion sites of HERV genes as complete and incomplete open reading frames, together with their differing capacity to be expressed and the complexities of individual HERVs as both disease markers and bioactive effectors, HERV biology is a compelling area for understanding neuropathogenic mechanisms and developing new therapeutic strategies.
Keywords: Human endogenous retrovirus, Multiple sclerosis, Neuroinflammation, Neurodegeneration, MSRV, Syncytin-1, Endoplasmic reticulum stress
Research Highlights
►HERVs express proteins in the brain. ►HERVs represent 8% of the human genome. ►The HERV-W envelope protein protein, Syncytin-1, is found in MS lesions. ►Syncytin-1 induces endoplasmic reticulum (ER) stress in astrocytes.
1. Introduction
Multiple sclerosis (MS) is the prototypic neuroimmune disease, defined by neuroinflammation within the central nervous system (CNS) accompanied by demyelination and axonal disruption [1]. Viral proteins, in particular glycosylated retrovirus envelope proteins, are potent inducers of inflammation with ensuing cell damage and death [2]. Approximately 8% of the human genome is comprised of retrovirus-like sequences (retroelements) [3], [4], represented chiefly by human endogenous retroviruses (HERVs), which originated through germ-line infection by their exogenous ancestors during primate evolution [5]. Abnormal virus-encoded protein expression (usually glycosylated) has been associated with the unfolded protein response (UPR), causing endoplasmic reticulum (ER) stress with potential adverse outcomes depending on the cell type including inflammation and apoptosis [6]. Herein we review MS pathogenesis together with the different HERVs implicated in MS and their associated disease mechanisms (Table 1 ).
Table 1.
Evidence for role of HERVs in MS.
| HERV | Disease associations | Specific action |
|---|---|---|
| HERV-H/F | Multiple sclerosis in addition to certain cancers | Expressed in MS patients particularly in lymphocytes |
| HERV-W | Multiple sclerosis and schizophrenia | Expressed in white matter lesions in MS patients, schizophrenia also reported |
| HERV- K | Multiple sclerosis | Disease marker |
1.1. Clinical and neuropathological features of MS
Multiple sclerosis (MS) is a common and progressive neurological disease, defined by several phenotypes (relapsing-remitting, primary progressive and secondary progressive together with less common variants such as Marberg's, Balo's and Devic's syndromes), which affects over 1 million people world-wide, usually beginning in the prime of life, ages 20–40 years, and is associated with marked physical and cognitive disabilities and shortened life span [7]. The recent application of rigorous criteria for the diagnosis of MS has refined the inclusion criteria for clinical trials, which may improve the trial outcomes [8]. The neuropathological changes accompanying MS are diverse and have been characterized into four different subtypes [9]. Demyelination and inflammation are the cardinal features of MS and involve the CNS at all levels. Accompanying inflammation and demyelination in MS, is the rediscovery of axonal and neuronal perikaryal injury [10]. Lesions in relapsing-remitting MS patients are usually found in white matter and are characterized by disruption of the blood-brain barrier (BBB), local edema and demyelination, typical of inflammatory processes [11]. Conversely, in primary-progressive MS, inflammatory processes are less predominant but progression to disability and brain atrophy may evolve faster [12]. Interest in the mechanisms by which demyelination and inflammation-related damage to axons and neurons occur [13] has been complemented by neuroimaging studies that suggest that axonal injury accompanied by cerebral atrophy (loss of tissue) constitute a major mechanism by which physical disability progresses during MS [1], [14].
1.2. MS pathogenesis
The role of immunopathogenesis as an etiologic determinant of MS is supported by the large-scale genetic studies showing linkages to multiple immune genes, especially to the MHC Class II loci on chromosome 6 [15], [16] and MS neuropathology, which implicates multiple cell types within the CNS together with immune activation [17]. The current dogma defining MS pathogenesis is that it is a T-cell-driven autoimmune disorder, albeit with diverse phenotypes [1]. Nonetheless, MS clinical and neuropathological phenotypes are highly variable depending on the population and geographic domain. Much of the understanding of MS immunopathogenesis has been influenced by the use of different animal models, especially experimental autoimmune encephalomyelitis (EAE) [18]. Both innate and adaptive immune mechanisms have been shown to participate in the immunopathogenesis of MS [19] although adaptive immunity may predominate early in disease, reflected by selective T and B cell activation accompanying clinical relapses. Multiple groups have demonstrated that enhanced MHC class II expression on myeloid cells and accompanying innate immune activation are associated with damaged myelin and failure of re-myelination [20]. Th1-associated innate immunity is also activated throughout the different phases of disease, as indicated by persistently increased cytokine and chemokine production by macrophages, microglia and astrocytes [21], even into the later or secondary progressive stages of disease [22]. Indeed, modulation/polarization of macrophages by disease-modifying therapies may be an important mechanism by which these compounds exert their therapeutic effects [23]. The mechanisms underlying demyelination and axonal damage remain uncertain although inflammatory molecules including cytokines, chemokines, prostaglandins, reactive oxygen species and matrix metalloproteinases have been demonstrated to contribute to demyelination and axonal/neuronal injury associated with MS [14], [24], [25]. The molecules mentioned above represent a complex cascade of signaling events that originate with activation of lymphocytes, macrophages and astrocytes [17]. Activation of these latter cell types results in the release of soluble intercellular signaling molecules that subsequently either feedback on the activated cells or alternatively, induces cellular changes, usually through receptor-mediated mechanisms to cause damage to myelin, oligodendrocytes, axons and perhaps neuronal cell bodies [13], [26], [27]. Given the substantial role of innate immune mechanisms in MS and the burgeoning recognition of its involvement in prototypic neurodegeneration (e.g., Alzheimer's disease, amyotrophic lateral sclerosis), the contribution of primary neurodegenerative mechanisms (oxidative stress, excitotoxicity, endoplasmic reticulum stress, apoptosis and other programmed cell death pathways) to the development of MS-associated neurological disability has gained greater importance in recent studies of MS pathogenesis [28]. Indeed, the modest clinical benefits of immunosuppressive and immunomodulatory therapies have prompted investigators to re-examine potential pathogenic mechanisms in MS with an increased focus on regulation on neurodegenerative disease mechanisms [29].
Environmental factors including infectious agents, lifestyle (tobacco smoking) and nutritional (vitamin D depletion, dairy products) factors might contribute to MS development and progression [30]. There is remarkable diversity in the evidence that indicates MS is caused by conventional exogenous infectious agents (herpes viruses, coronaviruses, Chlamydial infections, etc.) and in fact, the data are highly controversial in this area (reviewed in [30], [31]). There is also abundant evidence to support the hypothesis that genetics has a pivotal role in an individual's susceptibility to MS, perhaps in conjunction with triggering factors such as viruses. Although MS is not inherited in a Mendelian fashion, concordance rates of 26% in homozygotic twins (3% in dizygotic twins) are typically seen, suggesting substantial discordance (~ 70%) [32]. Nonetheless, family members of MS patients inherit a higher risk of developing MS, arguing for a genetic predisposition to this disease [33]. There is a strong genetic association between MS in Caucasians with the HLA class II DRB1*1501 allele, which is in linkage disequilibrium with DRB5*0101. The latter allele might confer MS risk by selecting for autoreactive CD4+ T cells, while the former is suppressed in EAE by deleting autoreactive T cells in mice transgenic for both the alleles [34]. To date no specific genomic locus has been linked definitively to MS onset or progression although several chromosomal loci and multiple polymorphisms have been associated with MS [35], particularly among genes associated with immune mechanisms.
Two principal hypotheses link MS and infection: the “prevalence hypothesis” states that the causative agent is more common in high risk areas, whereas the “polio hypothesis” and by extension, the “hygiene hypothesis” suggests that early infection induces protective autoimmunity and delayed infection increases the risk of disease [36]. Thus, several infections in early life might protect against the development of MS, which could explain why MS is rare in developing countries or lower socioeconomic groups [37]. Although various infectious agents are linked to MS, their presence may simply provide an appropriate environmental trigger for development of an autoimmune response directed against CNS antigens [38]. Application of sophisticated biochemical and molecular tools has led to identification of several viruses that exhibit an association with MS, albeit no pathogen has been accepted as the causal agent of MS. Studies related to virus infections in MS are largely serological and demonstrated by antibody titers against a particular virus, although it has not been elucidated whether these antibodies are elevated in response to the etiological agent, or through molecular mimicry [39]. Infections contributing to increased MS clinical activity of supposedly viral origin occur in the upper-respiratory tract, are self-limiting and mild, but might include pathogens such as Chlamydia pneumoniae and Mycoplasma pneumoniae [40]. Among viruses, epidemiological studies favor Epstein–Barr virus (EBV) while neuropathological studies support HHV-6, HERVs and perhaps coronaviruses [37]. EBV serology correlates with the distribution and onset of MS. While most adults have been infected with EBV, a proportion of children are EBV seronegative [37]. Meta-analysis studies of EBV infections in MS suggest that there is a role for EBV, particularly with a higher number of patients showing antibodies to EBV nuclear antigen than EBV seropositive controls but whether the oligoclonal IgG from MS brain and cerebrospinal fluid (CSF) is specific to EBV remains unknown [41]. A large Danish study reported that MS risk increases soon after infectious mononucleosis and persists for at least 30 years and there is a confirmed association between EBV infection in children and MS [37]. Although HHV6 and EBV are ubiquitous viruses, seroconversion happens usually before or during puberty into adult life, matching epidemiological evidence for time of exposure to the infectious agent causing MS [41]. Oligoclonal antibodies in CSF of some MS patients were determined to target the EBV-encoded proteins BRRF2 and EBNA-1 [42]. Further, EBV was found to infect B cells, and plasma cells and in brains of MS where viral latent proteins were expressed [43]. A T-cell-mediated immune response to EBV-infected cells leads to damage of neurons and oligodendrocytes as a result of bystander activation [37].
Due to the complex immunophenotype of MS, the immune response to human herpes virus (HHV)-6 has been difficult to predict; concentrations of serum IgG to HHV-6 were higher in RR-MS than CP-MS while HHV-6 DNA was often detected during relapse in 15% of MS patients showing presence of viral DNA. Several cells, including oligodendrocytes in MS brains showed the presence of HHV-6 antigen or genome, but not exclusively associated with MS. Of interest, several retroviral infections exhibit clinical and neuropathological phenotypes that resemble MS including human T cell lymphotropic virus (HTLV)-1-associated myelopathy and human immunodeficiency virus (HIV)-associated leukoencephalopathy [44]. Unlike most conventional exogenous viruses, retroviruses are integrated into the host genome and continue to exert (auto)-immune effects for years after infection, often in the absence of substantial viral replication [45].
1.3. Human endogenous retroviruses (HERVs)
Retroviruses are RNA viruses defined by their expression of reverse transcriptase and best exemplified by the exogenous retroviruses, HIV-1/2, HTLV-I/II, murine leukemia virus (MuLV) and Visna–Maedi virus, an early animal (sheep) model for MS (Fig. 2) [46]. Human DNA contains ~ 3,100,000,000 bp comprising ~ 20,000–25,000 genes, with many RNAs that are non-protein encoding and of no defined function to date [47]. HERVs were first discovered in normal brain tissue as type C-related retroviruses in 1981 [48]. All humans carry human endogenous retrovirus (HERV) sequences as an integral part of their genomes, comprising almost 8.29% of the human genome [49] and are likely vestiges of retroviral infections during evolution. At some point during evolution, exogenous (infectious) progenitors of HERVs inserted themselves into the cells of the germ line, where they have been replicated along with the host's cellular genes following a Mendelian pattern [4], [5]. Integration of endogenous retroviruses into the human germ line is thought to have occurred 2 to about 70 million years ago depending on the individual retrovirus and were introduced by mechanisms involving reverse transcription [3].
Fig. 2.
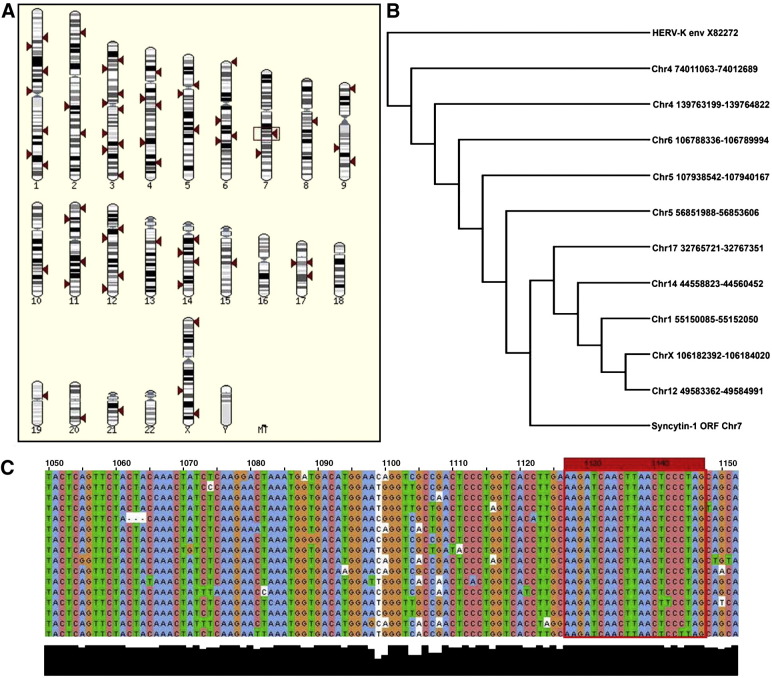
Diversity within syncytin-1. (A) Multiple sites of syncytin-1 integration (indicated by arrowheads) located throughout the human genome were identified by performing a BLAT search with the syncytin-1 ORF from GenBank accession no. NM_014590 using the Ensembl human genome browser (www.ensembl.org). The genomic location of the original syncytin-1 locus on chromosome 7 is indicated by a box. (B) Phylogenetic rooted tree showing sequence diversity within syncytin-1 depending the chromosomal locus (chr# positions). Original syncytin-1 sequence (ERVWE1) is designated as syncytin-ORF chr7 NM 014590. The 10 homologous genomic regions with the highest BLAT scores over the entire length of the syncytin-1 ORF were extracted and aligned using Clustal W, including sequences from syncytin-1 and the HERV-K envelope ORF, as an outgroup (GenBank accession no. X82272). (C) Sequence heterogeneity within syncytin-1 in the vicinity of position 1099; a representative portion of an alignment of the 15 homologous genomic regions with the highest BLAT scores, including sequences truncated relative to the syncytin-1 ORF. A high level of sequence similarity in several regions allows the design of cDNA synthesis primers (red box) that will hybridize with syncytin-1-related mRNA sequences. Sequence divergence nearby will allow unambiguous identification of individual transcripts in order to determine the extent to which other syncytin-1-like transcripts are expressed.
2. HERV classification
The classification of HERVs is based on the tRNA specificity of the primer binding site by adding the one-letter code for the corresponding amino acid to HERV, HERV-H, -T, -W, -K, etc. [50]. Other systems of classification based on sequence identity to known exogenous retroviruses and also to copy numbers [51], [52] have also been used. To date, 31 HERV families have been identified and are named according to the transfer RNA used to prime reverse transcription [53], [54] (Fig. 1 ). However, based on sequence homology, HERVs can be grouped into 80 distinct families [55]. Several members of the HERV-W and -H families have been shown to encode intact envelope proteins and currently 18 full-length HERV env sequences have been defined [54], [56], [57], [58], of which 9 were detected in the brain [54].
Fig. 1.
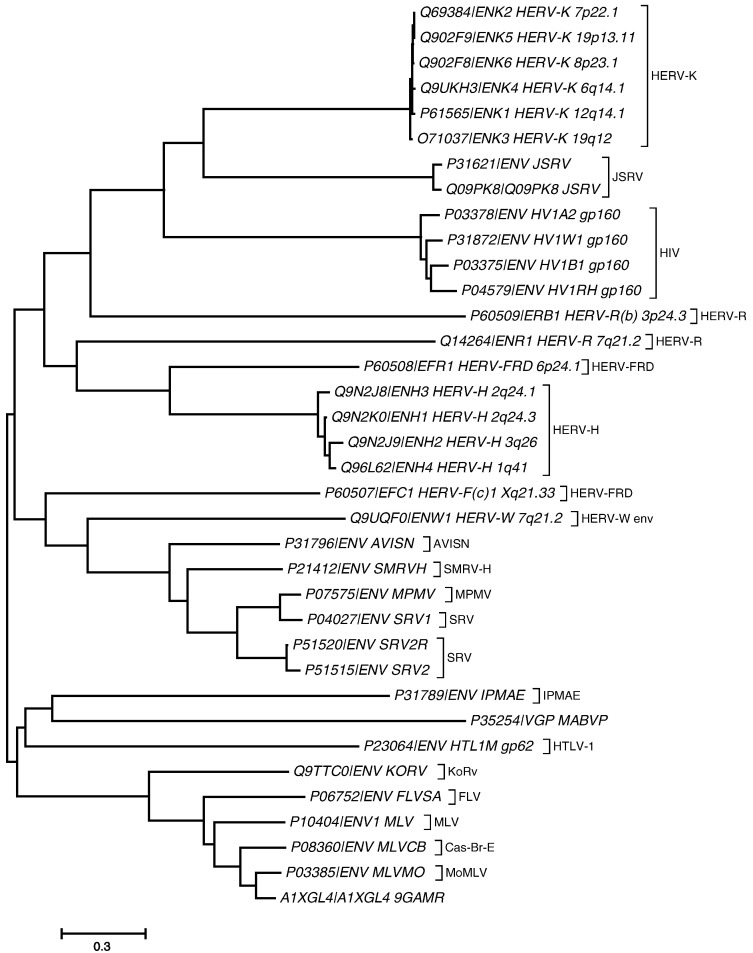
Retrovirus envelope phylogeny. Phylogenetic analysis of endogenous and exogenous retrovirus env sequences from 36 different clones. The sequences were aligned in Clustal X and evolutionary distances were computed using the Poisson correction method. All positions containing gaps and missing data were eliminated from the data set. The evolutionary history was inferred using neighbor-joining method. Sequences are represented by accession numbers and the group to which they belong.
Endogenous retroviruses are divided into two groups based upon the presence or absence of LTRs and are classified as gamma (I)-, beta (II)- and spuma (III)-retrovirus-like retroelements [53]. HERVs are divided into two groups based upon the presence or absence of LTRs. Those with LTRs can be further divided based on infectivity. Infectious endogenized retroelements found in lower species especially rodents and felids with LTRs are retroviruses (e.g. FeLV, some MuLVs), while noninfectious elements with LTRs are retrotransposons and those lacking LTRs are called retroposons [59]. HERVs were discovered using low-stringency screening of human genomic libraries [60], PCR by oligonucleotide homology to viral primer binding sites [61] and during analyses of human gene loci [62]. They are identified as retroviruses because of their provirus-like structure containing LTRs flanked by short direct repeats and primer binding sites flanking internal coding regions. Other retroelements that make up the genome include long interspersed repeat sequences (LINES) that transpose due to inherent reverse transcription activity and processed pseudogenes whose motion is dictated by reverse transcription not encoded by the pseudogenes [63]. Of the retroelements present in the human genome, HERVs are significant in terms of both health and disease.
While HERVs represent a substantial proportion of the human genome little is known about their biological actions, in large part due to the paucity of experimental reagents (complete sequence data and antibodies) and the widely held (incorrect) assumption that HERVs were ‘junk’ DNA. HERVs correspond to approximately 1500–2000 proviruses together with at least 20,000–40,000 copies of solitary LTRs per genome. HERVs have been amplified during evolution by repeated reintegration of reverse-transcribed mRNA into the DNA of germ-line cells [64], [65], [66]. However, it is now clear that HERVs express proteins [67], are capable of being immunogenic [68] and respond to immune stimuli [69] but are probably not replication competent [70], unlike their exogenous counterparts (e.g. HIV-1/2, HTLV-1/2). Of note, some HERVs can infect human cells but do not replicate, likely due to mutations in the pol and gag genes although these mutations do not preclude reverse transcription, protein expression, and the release of viral particles. Thus, HERVs are best regarded as complex host retroelement genes with the capacity influence cellular functions and perhaps survival.
HERV gene expression is assumed to be regulated by their individual LTRs or proximate gene promoters. Varied levels of expression and cell-type specificity of isolated HERV-LTRs in human cell lines suggest that HERV-LTRs may be a valuable source of transcriptional regulatory elements for the construction of targeted retroviral expression vectors [71]. HERVs have been associated with a range of disease processes including neoplasia, auto-immunity and fetal malformations [72], [73]. However, HERVs have also been found to be expressed in healthy normal tissues, such as placenta where they are assumed to exert beneficial effects [57], [74]. We showed that induction of individual HERV expression is neural cell-type- and stimulus-specific but more importantly, was associated with neuroinflammation [69]. Other groups have shown that specific HERVs are associated with select adaptive immune responses depending on the individual HERV and disease context [75], [76]. HERVs might play several roles in neuropathogenesis: cytokine/host gene modulation with neurotoxin production/release, molecular mimicry and insertional mutagenesis [77], [78], [79].
Mobile genetic elements in various species are functionally involved in development of placenta [80] and brain [81], etc. Induction of several HERVs is reported in different cell types derived from patients with various diseases; anti-HERV antibodies and retroviral nucleic acid sequences are detected frequently in autoimmune diseases such as systemic lupus, MS and Type 1 diabetes mellitus, depending on the individual study [75]. The HERV-K Gag protein and transcript have been reported to be highly expressed in teratocarcinoma and breast cancer cell lines [82], [83], HERV-E in prostate carcinoma [84], HERV-H in leukemia cell lines [85] and HERV-W in brain tissue and CSF from MS [57] and schizophrenic patients [86]. The identity of individual HERVs has been controversial in some instances, in large part because of the variation in encoding loci using different detection techniques [87], [88].
2.1. Functions of HERVs
HERV gene expression is principally regulated by their individual LTRs. Cytokines such as TNF-α are known to regulate HERV expression in a temporal and tissue-specific fashion [89], [90]. Although most HERV integration events are ancient, there are human-specific integrations for HERV-K (HML-2, 113 and 115) that are relatively new (400,000–250,000 years ago) and the full-length provirus and pre-integration site alleles are present in the human population [32]. There is also evidence for the ability of HERVs to mediate genomic rearrangements during primate evolution [3]. Comparison of sequences, particularly in the LTRs of certain HERV families, provides insight into the length of time a particular genetic sequence may have been present in the genome. Examples of the nonfunctional viral sequences that may have arisen through several incomplete duplications, recombination events and mutations acquired during primate evolution are HERV-H, HERV-F and HERV-K [3]. The most abundant expression of different HERVs is observed in placenta and embryonic tissues and also in reproductive tissues or cells such as testes [91] and oocytes [92]. The broad expression of HERVs in embryonic tissues may be sufficient for induction of immunological tolerance towards HERV-encoded proteins. HERV proteins could influence feto-maternal immunosuppression during pregnancy when expressed in the placenta [93], [94], particularly in the trophoblast cells [67], [95]. Interestingly, other immunogenic pregnancy-associated glycoproteins are expressed by the placenta but their role(s) in autoimmune diseases remains uncertain [96].
2.2. HERVs and MS pathogenesis
While the cause of MS remains unknown, there is widespread consensus that an infectious agent operating on a background of genetic susceptibility might play a role in the progression of the disease, albeit not the exclusive cause of MS [32]. HERVs could play several roles in MS pathogenesis but the most plausible (and contemporary) explanation for the effects in MS is that they act predominantly as a host component of the pathogenic cascade of events underlying MS (Table 2 ). A retroviral etiology for MS was postulated in the past when 70% of MS patients had cross-reactive antibodies to Human T lymphotropic virus (HTLV-1/2) and human immunodeficiency virus (HIV) antigens [97] although subsequently disproved, underlining the danger of relying on serology for viral detection. Jocher and coworkers found no HTLV-1 sequences in peripheral blood mononuclear cells (PBMCs) or MS brain tissues [41]. As it is now generally accepted that the genetic background of the host plays a crucial role in the disease susceptibility and phenotype but specific haplotypes of HERVs might also predispose an individual to MS. Evidence exists for polymorphisms in HERV-H env alleles at 2q24.3, which interestingly is also the locus for substitutions in 50% of Swedish blood donors [98]. HERV-R (ERV-3), whose allele is homozygous in about 1% of the Caucasian population, exhibits polymorphisms including deletions in the envelope protein [99]. In addition, the HERV-W envelope sequence also shows molecular diversity in blood that is dependent of the specific chromosomal locus [100].
Table 2.
Mechanism of action of HERVs in MS.
| HERV | Disease pathogenesis | Mechanisms |
|---|---|---|
| HERV-W/Syncytin/MSRV | Up regulated in MS lesions | Induction of free radicals, ER stress and subsequent oligodendrocyte death; triggers toll-like receptor (TLR)-4 affecting innate and autoimmunity |
| HERV-H | Up regulated in lymphocytes of MS patients | Induction of antibodies against HERV; induces cell-mediated immune response to gag and env peptides; retrovirus-like particles (RVLPs) observed in cultured patient cells |
A full-length copy of the HERV-W genome is located on chromosome 7q21 [101] and the HERV-W7q envelope-encoded protein, termed syncytin-1, was shown to be expressed in astrocytes, perivascular macrophages and activated parenchymal microglia, in acute and chronic demyelinated lesions [102]. Indeed, syncytin-1 overexpression in astrocytes resulted in cytokine and reactive oxygen species production with ensuing in vitro and in vivo oligodendrocyte injury. Importantly, given the abundance of syncytin-1 encoding RNA and protein in controls and MS patients and its inability to replicate despite the release of HERV-W genome and proteins from cells, it is unlikely to behave like a conventional exogenous retrovirus but rather serves as a modulator of disease. What factors govern syncytin-1 expression in neural cells remain uncertain although recent studies suggest that epigenomic determinants such as DNA methylation are unlikely to contribute to its induction [103].
The MS-associated retrovirus (MSRV) is also a member of the HERV-W family [104] and indeed, the two retroelements share approximately 88% identity within their envelope sequences. Previous studies have shown, using degenerate PCR primers, that the MSRV pol is also upregulated in neuroinflammatory conditions [69] but its DNA copy number does not differ between MS and age-/sex-matched controls [105]. Indeed, the MSRV pol is also upregulated in other neurological disorders [72], [106]. It has been reported that MSRV is replication competent and behaves like an exogenous retrovirus, which has prompted substantial interest about MSRV [107], especially with recent data generated using MSRV envelope-specific PCR primers that did not demonstrate up-regulation of MSRV in MS brains [108], [109]. It is now clear that there are multiple HERV-W members, which vary in their sequence and chromosomal loci. Recent work also indicates that among 7 individual HERV envelopes examined, only syncytin-1 is upregulated in MS brain [102], [108], underscoring the specificity of perturbed syncytin-1 expression in MS but the specific syncytin-1 sequence (or encoding locus) predominating in the CNS remains unknown. Indeed, these findings were confirmed with quantitative PCR analyses wherein syncytin-1 RNA and proviral copy numbers were increased in brains of MS patients although we did not find differences in PBMCs, plasma or CSF from MS and non-MS patients [108]. Other human retroelements have also been posited to participate in MS pathogenesis but a full understanding of their pathogenic role(s) and underlying mechanisms remains to be defined [107]. HERVs potentially might act also as either genetic markers for polymorphisms related to MS, or as markers of environmental/endogenous stress [110], [111]. Induction of HERV-H/RGH, HERV-W and ERV-9 expression was reported when specific cell types (chiefly B cells) from MS patients were cultivated in vitro [112]. Several studies suggest that specific HERVs that occur in few copies in the genome (e.g. ERV-3, HERV-R and HRES-1) may show polymorphic patterns in MS and might act as auto-, super- or neoantigens with the potential to enhance inflammatory responses or induce autoimmune reactions [110], [113]. RNA encoded by these HERVs has been detected by reverse transcriptase polymerase chain reaction (RT-PCR) with degenerate primers in sera/plasma and brain tissues from MS patients, although not exclusively from this patient group [107]. Other methods employed for detecting evidence HERVs in MS and other diseases include electron microscopic identification of ‘virus-like’ particles, reverse transcriptase activity and autoantibodies in blood and CSF, albeit with limited specificity or sensitivity [114].
3. MSRV and its role in MS
Among HERVs associated with MS, MSRV has been the most intensely studied. Leptomeningeal cells (LM7) isolated from the CSF of MS patients, as well as monocytes and EBV-transformed B cells, yielded a novel retroelement that was subsequently identified and characterized as MSRV [104]. Along with a French group who discovered MSRV in MS patients, particles containing reverse transcriptase activity were also reported by a Danish group [58]. MSRV and HERV-H particles and RNA were found to be increased in MS patients relative to controls [32], [115], [116]. However, it is possible that several HERV loci contribute to and are activated in MS since patients exhibit heterogeneous sequences. A pan-retro RT-PCR that amplified a conserved pol sequence among all retroviruses was used [104]. Using MSRV probes obtained from virion-associated RNA, a novel HERV family was identified that is different from ERV9 and genetically related to MSRV sequences and was named HERV-W [57], [117]. There is substantial controversy as to whether MSRV is an endogenous or exogenous virus [118]. MSRV belongs to the ERV9 family of endogenous retroviruses (ERVs), and partial molecular characterization revealed that MSRV has 75% homology to ERV9 [104]. Recent evidence points to MSRV being transcribed from HERV-W loci on chromosomes 3q23, Xq22.3, 15q21.3 and recombinations between loci on chromosomes 3p12.3 and 18q21.32, Xq22.3 and 5p12 [100]. Thus, MSRV is most likely transcribed from an endogenous element, perhaps from truncated or HERV-W pseudogenes, driven by unidentified promoters located upstream of these pseudogenes [100].
MS patients whose serum and CSF samples show detectable levels of MSRV range from 50% in the French population [118] to 100% in Sardinia [119]. It was also detected in blood from control groups without MS (6%) [118], some bipolar disorder patients, 40% of other neurological diseases [120] and in synovial fluid from rheumatoid arthritis patients (22%) [121]. Using primers specific to ERV9/MSRV, although less sensitive than MSRV-specific primers [104], a cohort of South African MS patients did not show the presence of MSRV, except for one pregnant individual [122]. This could be attributed to the degenerate primers used that might have amplified HERVs normally expressed highly in the placenta.
Enhanced expression of MSRV in MS brains [120] and increased MSRV copy number in MS blood DNA [123] are associated with a poorer MS prognosis [124]. MSRV is also induced by inflammatory agents [125], [126] and might also induce a potent inflammatory response [127]. This is particularly evident when none of the MS patients treated with anti-inflammatory drugs showed detectable levels of MSRV [118]. Of particular interest was the finding that IFN-β treatment reduced MSRV load in plasma and MS progression index among patients examined for a year [128]. It is also plausible that MSRV expression is activated in response to certain inflammatory products or infectious agents, particularly viruses (HSV-1, influenza virus). In fact the expression of MSRV was enhanced in the presence of HHV-6 in MS patients [90]. The pol and env genes of MSRV and their expressed proteins have also been shown to be involved in inflammatory responses [129]. Gliotoxins secreted from macrophages expressing MSRV also caused the death of oligodendrocytes, key cells involved in the myelination process [130]. This finding might suggest a direct inflammatory role for MSRV gene products. The pathogenicity of MSRV retroviral particles was evaluated in severe combined immunodeficiency (SCID) mice grafted with human lymphocytes and injected intraperitoneally with MSRV virion. MSRV-injected mice displayed cerebral hemorrhage and died within 5 to 10 days post injection. RT-PCR analyses showed circulating MSRV RNA in serum for mice, with overexpression of TNF-α and IFN-γ in spleen [131]. This in vivo study implies that MSRV retroviral particles from MS cultures might have potent immunopathogenic properties mediated by T cells corroborating previously reported superantigen activity of HERVs in vitro, which appear to be mediated by overexpression of pro-inflammatory cytokines [132]. The pathogenesis of MSRV could also involve an innate immune response to the virus through toll-like receptors (TLRs), which was demonstrated by the stimulation of PBMCs with purified Env protein that resulted in the release of cytokines [127]. However, a subsequent study using a different approach could not demonstrate an immune response to MSRV [114]. In the latter study, a cohort of HLA-B7+-matched MS (n = 24) and healthy controls (n = 29) patients did not exhibit any cellular immune response to MSRV/HERV-W. Further, serum and CSF from MS (n = 50) and healthy controls (n = 29) did not exhibit antibodies against MSRV/HERV-W [114]. Several possible reasons might explain this phenomenon, including a lack of antigens in the cohort studied, insufficiently sensitive methodology or lack of an immune response to the antigen [114].
3.1. ERVWE1/syncytin-1
The HERV-W envelope-encoded glycoprotein, syncytin-1, is readily detected in healthy placenta and is principally expressed by the chromosomal locus, 7q21.2 although there are other loci that encode partial and complete sequences (Fig. 2 ). Syncytin-1 is also detected as both transcript and protein in brain tissues from MS patients [102], especially in astrocytes and microglia within active and chronic MS demyelinating lesions [102]. Syncytin-1 appears to be distinct from MSRV env, but with sequence similarities [100]. Syncytin-1 is overexpressed in brains of individuals with MS [102], [133] and examination of its copy numbers by PCR techniques revealed a significant increase in RNA and DNA copy numbers in the brain tissue but not in peripheral blood mononuclear cells, CSF or plasma of MS patients relative to controls [108], [134]. A major difference between syncytin-1 and MSRV env is the localization of the protein. While the former is found intracellularly and on the plasma membrane, MSRV has been reported to be found as an extracellular virus, visualized by electron microscopy, sedimenting at retrovirus buoyant density, with reverse transcriptase activity and a poly A(+) RNA containing terminal repeats, gag, pol and env sequences. However, aside from point mutations that distinguish MSRV env from syncytin-1 [108], the MSRV env can also be distinguished from syncytin-1 by the presence of a 12 nucleotide insertion in the cytoplasmic tail of the transmembrane region of MSRV. Using specific probes, quantification of DNA, RNA of MSRV and syncytin-1 in PBMCs revealed that MSRV was significantly increased in PBMCs of MS patients compared to controls in contrast to syncytin-1 [109]. While the expression of syncytin-1 in PBMCs in this study agrees with an earlier study using a less sensitive PCR approach [108], the expression pattern of MSRV env is at odds with other studies [100], [108]. Interestingly, syncytin-1-negative/ MSRV env-positive PBMCs from MS patients showed MSRV Env glycoprotein expression, albeit with an antibody that does not distinguish between MSRV env and syncytin-1 [100]. It will be essential to raise antibodies against the 12-nucleotide insertion to distinguish the two at the protein level. Whether the expression of MSRV Env protein in PBMCs corroborates with its expression in the brain is yet to be demonstrated. Nevertheless, these studies imply that an assay to quantitatively determine HERV-W viral load in plasma could be developed as a prognostic tool for MS.
MS patients demonstrate enhanced syncytin-1 protein levels particularly in astrocytes where its expression leads to induction of several ER stress chaperones [135]. One of these chaperones is the old astrocyte-specifically induced substance (OASIS), which enhances the expression of inducible nitric oxide synthase (iNOS). Expression of iNOS has been demonstrated in MS lesions, especially within astrocytes and is a key pathogenic feature of MS [136]. Increased syncytin-1 expression in astrocytes leads to induction of free radicals [102]. The myelin producing cells of the CNS, oligodendrocytes, are particularly vulnerable to free radical mediated damage because the level of antioxidants might be lower in this cell type [137]. Loss of oligodendrocytes and myelin formation was demonstrated in mouse models of demyelination [102]. Implanting TNF-α in the corpus callosum of syncytin-1 transgenic mice not only resulted in ER stress but also revealed loss of the myelin protein CNPase and oligodendrocytes [135].
Several viruses such as herpes simplex virus [138], influenza virus [139] and cytokines such as TNF-α [133] are capable of inducing syncytin-1 expression. This HERV envelope glycoprotein might be misfolded in the endoplasmic reticulum (ER) since it is capable of inducing the chaperones whose main function is to correct misfolded proteins and achieve cellular homeostasis. Retroviruses have been shown to induce an ER stress response; the envelope protein of MuLV is known to upregulate GRP78/BiP as a means to protect cells from apoptosis [140]. However, when ER stress is prolonged, some of the chaperones persist and bind to promoter elements resulting in transcription of genes that alter cellular function. Astrocytes that undergo ER stress as a consequence of syncytin-1 expression release free radicals through the induction of iNOS mediated by OASIS. Free radicals are responsible for much of the pathology in MS as evidenced by axonal damage as well as death and damage of oligodendrocytes. Blocking iNOS using inhibitors or scavenging free radicals using ingredients found in many natural compounds (e.g., ferulic acid) suppressed syncytin-1-mediated damage in cultured neural cells as well as in a mouse model [102]. It could be argued that inhibiting iNOS is not necessarily going to diminish lesions from developing in MS as iNOS as free radicals also have trophic effects in addition to their pathogenic properties [141]. Importantly, syncytin-1 overexpression is restricted to the brain parenchyma in MS patients, which precludes its usefulness as a clinical biomarker [108], [134], [142].
3.2. HERV-W and its receptors
While exogenous retroviruses enter somatic cells by binding to their cognate receptor (s), HERVs reside within the genome and are inherited in a Mendelian fashion. However, HERV proteins, particularly syncytin-1, are known to bind cell surface proteins such as ASCT1 and -2, which are neutral amino acid transporters [143]. The syncytin-1 protein has also retained the ability to interact with the receptor for D-type retroviruses [67], [143] and can confer infectivity to pseudotyped retroviral particles [144]. A widely dispersed interference group of retroviruses that includes the feline endogenous virus (RD114), baboon endogenous virus, HERV-W and type D primate retroviruses uses the human Na-dependent neutral amino acid transporters type 1 and 2 (hASCT1; gene name, SLC1A4 and hASCT2; gene name, SLC1A5) as cell surface receptors. ASCT1 and -2 have been determined to be putative receptors for syncytin-1 [143]; in fact, ASCT1 is predominantly expressed on brain astrocytes [145] and syncytin-1 engagement of ASCT1 results in suppression of ASCT1 [146]. Viruses pseudotyped with syncytin-1 were able to infect astrocytes and macrophages but not neurons [135]. It remains plausible that some HERVs still possess functions of infectious retroviruses that may have been diverted by the host to its benefit. In accordance with a symbiotic role for HERVs, it has been shown that syncytin-1 is a highly fusogenic glycoprotein that is specifically expressed in the placenta and can mediate cell–cell fusion ex vivo [147].
Retrovirus receptor interference or down-regulation proceeds from direct interactions between the virus and the receptor or by indirect (intracellular) mechanisms such as through redox-mediated regulation of the receptor [148]. The receptors for syncytin-1, ASCT1 and 2, are widely expressed on most cell types [143]. ASCT2 abundance is low in the adult brain [149] and recent observations of ASCT2 immunostaining in human brains represent the first report of its expression predominantly in microglial cells [135]. In the mouse brain, ASCT1 was chiefly expressed in GFAP-positive astrocytes in the cerebral cortex and corpus callosum, but not in neurons, oligodendrocytes or activated/resting microglia [150], similar to observations in the white matter of human brains. Interestingly, weak ASCT1 labeling was observed around ER cisterns, revealing its intracellular trafficking pathway or ASCT1 may have a functional role in exchange of ions between cytoplasm and ER lumen [150]. ASCT1 was also found to be significantly down-regulated in glial cells treated with 7-ketocholestrol, a by-product of myelin, damaged by oxidative stress, a key feature of MS [151]. Reduced expression of ASCT1 in astrocytes from MS patients appears to have adverse consequences for oligodendrocytes and perhaps other proximate cells' health and function. ASCT1 is the principal transport system involved in the secretion of L-serine [152], a potent astrocyte-derived neurotrophic factor [150], which is essential for myelination [153] and neuronal survival [154]. Conversely, ASCT1 is also responsible for mediating intracellular transport of the excitotoxic amino acid, cysteine [154], preventing its extracellular accumulation. A reciprocal interaction between syncytin-1 and its putative receptor, ASCT1, along with diminished amino acid influx has also been observed in the placenta [155]. Down-regulation of ASCT1 expression in mouse brain capillaries has been observed during the second postnatal week and is speculated to be due to lowered demand for small neutral amino acids from circulation or their increased synthesis in local glial cells [150]. These results suggest a highly specific role for ASCT1 down-regulation, since another astrocytic, amino acid transporter, EAAT1 [156], was unaffected by syncytin-1 exposure [135]. Indeed, this finding supports earlier studies showing that syncytin-1-mediated effects on oligodendrocytes were not dependent on the glutamate receptors, NMDA-R or AMPA-R [102].
To investigate the mechanism underlying ASCT1 suppression in astrocytes, it was hypothesized that nitric acid (NO) might play a role as NO donors are known to modulate ASCT2 expression [157]. Further, the rationale for using NO donors was that syncytin-1 mediates NO production and formation of peroxynitrites [102], both of which are known to induce ER stress [158]. Sodium nitroprusside (SNP), an NO donor, diminished ASCT1 expression in astrocytes, but also induced Egr1, a repressor protein of ASCT1 in neural cells [159], [160]. Of note, Egr1 suppresses TNF-α [161], which may have pathogenic consequences in MS due to the protective nature of this pro-inflammatory cytokine [162]. Bcl-2-induced Egr1 DNA binding activity has been correlated to oligodendrocyte death [163]. As iNOS and Egr1 are significantly enhanced in brain lesions of MS patients [164] with concurrent down-regulation of ASCT1, there might be a potential role for Egr1 in MS neuropathogenesis. A transgenic mouse model of MS was developed that expressed syncytin-1 in astrocytes under the GFAP promoter. Indeed, syncytin-1 transgenic mice exhibited neurobehavioral features resembling MS. T cell (CD3 immunopositive) infiltration into the corpus callosum were observed in transgenic mice upon TNF-α implantation, suggesting that the adaptive immune component of neuroinflammation is activated in this model. When syncytin-1 was over expressed in human astrocytes, syncytia develop [135], which recapitulates an occasional finding of multinucleated (syncytia) astrocytes in plaques from MS patients [165]. Engagement of ASCT1 by syncytin-1 or treatment with IL-1β results in suppression of its expression although the mechanism by which this reduction might occur remains uncertain [146]. Of interest, ASCT1 was also suppressed in the syncytin-1 transgenic mouse [135]. The down-regulation of ASCT1 in white matter of MS brains has important implications for oligodendrocyte and neuronal survival, given that ASCT1 on astrocytes is responsible for the export of L-serine, which can serve as a neurotrophic factor [154]. Astrocytes are the chief source of L-serine in the CNS [154] and the principal cells type expressing ASCT1 [150]. L-serine biosynthesis plays an important role in multiple cellular reactions, particularly in the brain, as L-serine is a precursor of important metabolites such as nucleotides, phospholipids and the neurotransmitters, L-glycine and D-serine. Disturbances of serine-glycine metabolism in relation to N-methyl-d-aspartate-receptor activation might also play a role in MS pathogenesis [166]. Interestingly, disruption of L-serine biosynthesis, through deficiency in the enzyme, 3-phosphoglycerate dehydrogenase, results in dysmyelination, seizures and psychomotor retardation [167]. Earlier data indicated that treatment of astrocytes with the ASCT1 blocker, benzylserine, mediated oligodendrocyte injury and death [135]. Indeed, the underlying mechanisms by which syncytin-1 mediated its cytotoxic effects appeared to involve activation of the unfolded protein response (UPR) in the CNS, resulting in endoplasmic reticulum (ER) stress, which can lead to inflammation and altered cell survival [168] (Fig. 3 ).
Fig. 3.
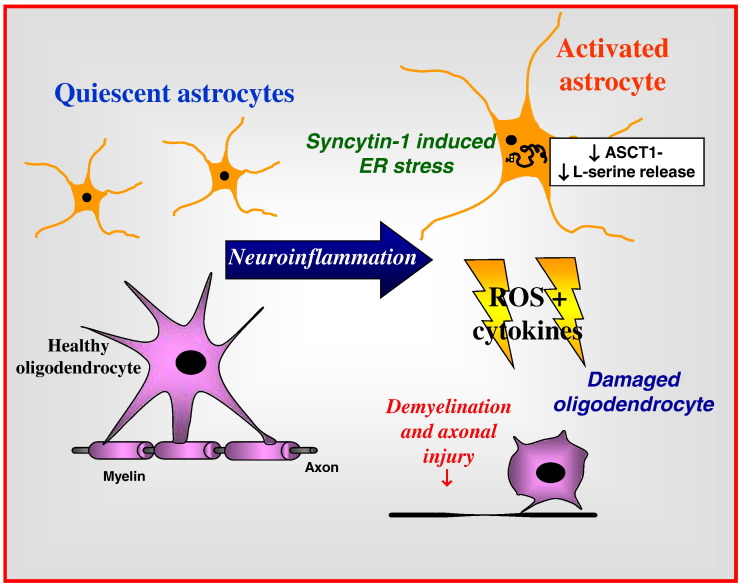
Syncytin-1-mediated neuropathogenesis in multiple sclerosis. Inflammation induces expression of syncytin-1 in astrocytes (and microglia), resulting in the release of cytokines and free radicals together with altered transport of amino acids implicated in neuropathogenesis including L- and D-serine, cysteine due to altered ASCT1 expression on astrocytes, which damage oligodendrocytes leading to demyelination and axonal injury.
3.3. The unfolded protein response (UPR) and endoplasmic reticulum (ER) stress
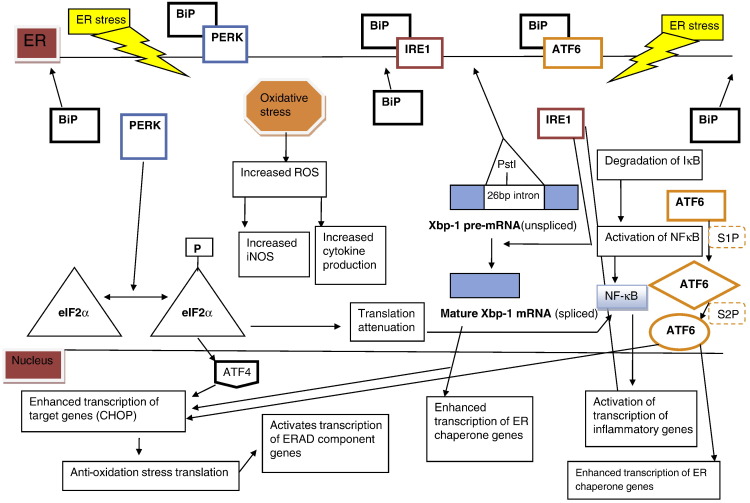
The endoplasmic reticulum has 3 basic functions: (1) protein synthesis, (2) protein folding and (3) calcium storage [169], [170]. When proteins are correctly folded, they are sent to the Golgi apparatus for protein trafficking [170]. However, when the levels of unfolded or misfolded proteins in the ER are too high for effective ER function, four mechanisms of cellular response to this condition can be activated, which are collectively termed the unfolded protein response (UPR) [170]. The first mechanism of the UPR is to increase the expression of ER chaperones to increase the rate of correct protein folding in the ER [170]. If this mechanism fails, the second UPR mechanism is translation attenuation, which decreases the rate at which proteins are synthesized in the ER and hence decreases the rate at which proteins accumulate in the ER [170]. If these two mechanisms are not successful, the third UPR mechanism is ER-associated degradation (ERAD) [170], [171]. During ERAD, proteins are tagged and retrotranslocated out of the ER into the cytoplasm where they are degraded via the ubiquitin–proteosome pathway [171]. However, if ERAD fails, unfolded proteins aggregate and lead to the fourth and final UPR mechanism: ER stress [170]. ER stress can lead to apoptosis, oxidative stress and inflammation [170], [172]. There are 3 principal ER stress sensors: PERK (PRKR-like endoplasmic reticulum kinase), IRE-1 (inositol requirement enzyme-1) and ATF-6 (activating transcription factor 6) [173], [174] (Table 3 ). PERK is a protein kinase that is activated when there is ER stress; its role in ER stress is to phosphorylate eIF-2α and stop translation [169], [175]. IRE-1 has kinase and endoribonuclease activity; however, its cardinal function in ER stress is to cleave a 26-bp intron in pre-mature transcript of XBP-1 (X-box binding protein 1) [172], [175]. The third ER stress sensor, ATF6, is a transcription factor that has a basic region and a leucine zipper motif and its principal role in ER stress is to enhance transcription of ER chaperones [172], [173]. In the absence of ER stress these 3 ER stress sensors are bound to binding immunogenic protein (BIP) and expressed at a very low level [174]. The main control for these three sensors, BiP, is an ER glucose regulated chaperone that plays a major role in ER stress [171], [172], [173]. Upon ER stress (caused by accumulation protein and failure of the other 3 UPR mechanisms), BIP dissociates from the three ER stress sensors and each of which activates its own unique set of pathways [174] (Fig. 4 ).
Table 3.
ER stress proteins and their essential functions (common names underlined).
| ER stress protein | Full name | Other Alias | Function |
|---|---|---|---|
| BiP | Binding Immunoglobulin protein | GRP78 | Folding of glycoprotein, separates from ATF-6, IRE-1 and PERK and binds to unfolded/misfolded proteins when ER stress is present. |
| ATF-6 | Activating Transcription Factor 6 | – | Binds to ER stress response elements and activates the transcription of ER chaperones such as BiP. Activation of CHOP promoter. |
| PERK | PRKR-like Endoplasmic Reticulum Kinase | WRS, HRI | Phosphorylates eIF2a to attenuate translation, and to upregulate expression of ATF4, leading to enhanced transcription of target genes such as CHOP. |
| IRE-1 | Inositol Requirement Enzyme 1 | DIRE-1 | IRE1-alpha and IRE1-beta undergo dimerization and transphosphorylation under ER stress and converts XBP-1 pre-mRNA into XBP-1 mature mRNA |
| OASIS | Old Astrocyte Specifically Induced Substance | CREB3L1 | Activates the transcription of target genes that are mediated by ER stress-responsive and cyclic AMP-responsive elements. |
| CHOP | C ⁄ EBP homologous protein | GADD153 | Apoptosis, growth arrest, oxidative stress, DNA damage. |
| GRP58 | Glucose Regulated Protein | PDIA3 | Folding of nascent proteins. |
| CALR | Calreticulin | – | Folding of glycoproteins. Not directly involved in ER stress. |
| CANX | Calnexin | MHC class 1 antigen binding protein p88 | Folding of glycoproteins. |
| XBP-1 | X box binding protein 1 | TREB5 | Transcription of ERAD components and ER chaperones. Activation of CHOP promoter |
Fig. 4.
Mammalian ER stress mechanisms. In the absence of ER stress, BiP is bound to all 3 ER stress cascade initiators (PERK, IRE1 and ATF6). When ER stress occurs, BiP dissociates from the ER stress cascade initiators and binds to the unfolded and misfolded proteins. PERK is a kinase that phosphorylates EIF2α which leads to the translocation of ATF4 and ultimately activation of CHOP promoter. Endonuclease, IRE1, splices XBP1 pre-mRNA into its mature transcript variant and also leads to the induction of CHOP. ATF6 is a transcription factor that gets cleaved twice before it translocates into the nucleus to enhance the transcription of CHOP and ER chaperone genes. Also ER stress can indirectly lead to increased iNOS and production of pro-inflammatory cytokines through oxidative stress.
Upon dissociation from BiP, PERK will phosphorylate eIF-2α to attenuate translation, thus phosphorylating eIF-2α thereby inactivating it [172]. The alpha subunit of the eukaryotic translation initiation factor (eIF-2α) is involved in the initiation of translation of proteins in the ER [172]. Phosphorylated eIF-2α will enhance translation of specific mRNA for transcription factors like ATF4 or chaperones such as CHOP which will in turn activate the transcription of anti-oxidant genes and stimulate the expression of ERAD component genes [170], [176]. NF-κB is a key transcriptional regulator and once in the nucleus, it will activate transcription of several inflammatory genes [172]. IκB, an inhibitor of NF-κB, has a shorter half life than NF-κB, and therefore, global attenuation of translation by eIF-2α phosphorylation leads to NF-κB dissociation from Iκ-B and its translocation into the nucleus where it is a key transcriptional regulator of inflammatory genes [172]. The resulting increase in expression of ROS-producing enzymes such as iNOS and an increase in cytokine production in the CNS may contribute to neuropathology of neuroinflammation [172], [177].
When IRE-1, the second major ER stress sensor, dissociates from BiP, it will cleave an intron in the pre-mature XBP-1 transcript and yield a mature XBP-1 transcript [174]. XBP-1 is a transcriptional regulator in ER stress and also plays a role in MHC class II regulation [172], [173], [178]. XBP-1 will then translocate into the nucleus and activate the transcription of ER chaperones [170]. IRE-1 can also activate NF-κB when it forms a complex with TRAF2, which will be discussed in the next section (24). The third main ER stress sensor, ATF-6, is a transcription factor that gets cleaved by the proteases SP1 and SP2 and then translocates into the nucleus to activate transcription of ER chaperone genes [174].
Recent studies indicate that ER stress is linked to inflammation, specifically the systemic inflammatory component of innate immunity, called the acute phase response [172]. Members of the membrane-bound transcription factor family with homology to ATF6 include CREBH, Luman and OASIS [179]. CREBH is a hepatocyte-specific bZip transcription factor belonging to the cyclic AMP response element binding protein transcription factor (CREB/ATF) family, which requires proteolytic cleavage for its activation. Interestingly, pro-inflammatory cytokines induce and cleave CREBH, which regulates C-reactive protein (CRP) and serum amyloid P-component (SAP), which are implicated in several pathologies [172] including MS, where levels of these proteins are augmented in the serum [180]. CRP can bind to and activate monocytes/macrophages [181], whereas SAP plays important roles in leukocyte adhesion [182].
3.4. ER stress and viral diseases
Viral infections of mammalian cells elicit cellular responses, such as ER stress and interferon responses [183]. Viruses have evolved mechanisms to challenge these responses that limit/inhibit viral replication. The ER is an essential organelle for viral replication and maturation and in the course of a productive infection, a large amount of viral proteins are synthesized in infected cells, where unfolded or misfolded proteins activate the ER stress response [183]. Several viral proteins trigger Grp78/BiP expression during infection, which in turn associates transiently with folding intermediates of viral glycoproteins. This binding facilitates folding or assembly of viral proteins along the maturation process [183]. Hepatitis C replication stimulates the ATF6 pathway, but suppresses the IRE1-XBP1 pathway [184]. This effect favors translation of viral proteins. There is an increased level of Grp78/BiP, which may be stimulated by viral replication. Infection by cytomegalovirus transiently induces Grp78/BiP at early stages but returns to basal levels at the later stage. This coincides with expression of other markers of UPR. Increased Grp78/BiP at the early stages inhibits the stress response by interacting with PERK, ATF6 and IRE1. Thus, the virus seems to induce Grp78/BiP in order to control ER stress [183].
Caspase-12 is an initiator caspase required for transduction of a death signal from the ER in infected cells and is activated by several viruses and the onset occurs before activation of caspase-8 and -3 [185]. Multiple viruses induce apoptosis mediated by ER stress through the activation of GADD153/CHOP. Virus infection activates the p38 MAPK pathway, which then acts on GADD153/CHOP to initiate apoptosis in infected cells. Through the regulation of as yet unidentified proteins, several viruses including African swine fever virus, block the expression of GADD153/CHOP [183]. It is unclear, however, why some viruses promote ER-mediated apoptosis. Several studies of neurotropic murine retroviruses have reported the induction of inflammation and ER stress-related genes [186]. Moreover, ER stress was related specifically to an individual envelope protein expressed by the retrovirus. A temperature sensitive neurovirulent mutant (ts1) of Moloney murine leukemia virus (MoMuLV) [187] has a single point mutation in the env gene that confers the ability to kill T cells and motor neurons, causing a progressive spongiform encephalopathy [187]. Neurodegeneration is probably due to loss of glial support and release of TNF-α, IL-1β and NO from adjacent ts1-infected glial cells [187]. Neurons display apoptosis, vacuolization and inclusion bodies. Elucidation of the pathogenic mechanisms has revealed a role for ER stress in neuronal death. TNF-α or NO induces the ER to release Ca2+ leading to activation of ER stress signaling pathways and cell death through apoptosis.
Infection by the neurovirulent murine retrovirus, MoMuLV ts1, is associated with phosphorylation of PERK and eIF2α ([186]. During late stages, levels of Grp78/Bip may be insufficient to protect neurons against ER stress associated with neurovirulent murine retroviruses, FrCasBrE and MoMuLV ts1 infection. Neurons, but not astrocytes, show increased levels of ER stress associated genes in infected mice, which result in activation of ER-associated caspase-12 and subsequent cleavage of caspase-3. Stressed neurons also exhibit increases in [Ca2+]i-mediated phosphorylation of calmodulin (CaM) kinase IIα. Since ts1 infects glial cells, but not neurons, an indirect mechanism of neuronal death, perhaps glutamate excitotoxicity associated with NMDA receptor activation due to neurotoxins from infected glia, might lead to neurodegeneration [187]. The envelope protein of an avirulent retroviral strain, F43, binds to Grp78/BiP and is processed through the normal secretory pathway. In contrast, the envelope protein of FrCasBrE bound to Grp78/BiP for a prolonged period is retained in the ER and diverted to the proteasome for degradation [186]. MoMuLV ts1-infected astrocytes induce GADD153/CHOP in neurons but not astrocytes [187], [188]. GADD153/CHOP and Grp78/BiP as well as iNOS and apoptosis are induced in p53 deficient microglial cells when treated with lipopolysaccharide [189] or IFN-γ, suggesting p53-independent NO-induced apoptotic mechanism in microglia [190]. Both FrCasBrE and ts1 strains induce a protein misfolding disease since Grp78/BiP binds to hydrophobic residues and attempts to prevent protein aggregation of misfolded proteins and thus activating an UPR. We have shown that replication-competent feline immunodeficiency virus (FIV) is essential for inducing an ER stress response and a vigorous neurotoxic and immune response in feline macrophages and in vivo. However, mere exposure and/or expression of the envelope protein of FIV were insufficient to induce an ER stress response [191]. ER stress can activate inflammation through activation of NF-κB, as previously described. Similarly TRAF2 ((TNF-α)-receptor-associated factor 2) can form a complex with IRE-1α and also activate inflammatory genes through a phosphorylation cascade [172]. TNF-α is a cytokine that breaches the BBB and causes neuroinflammation [172], [192]. IRE-1α-TRAF2 complex can phosphorylate JNK (JUN N-terminal kinase), which phosphorylates AP1 (transcription factor activator protein 1) [172]. Similar to NF-κB, phosphorylated AP1 can translocate into the nucleus and activate the transcription of inflammatory genes [172].
A recent study by Lin et al. [193] examined ER stress involvement in demyelinating disorders. Interferon (IFN)-γ, a cytokine induced during T cell activation, is potentially involved in demyelinating disorders by causing oligodendrocytes (ODCs) to undergo apoptosis [193]. Lin et al. [193] treated rat ODCs for 48 hours and examined their viability with caspase activation and ER stress transcript levels. By double labeling rat ODCs with CNPase and TUNEL, apoptosis can be visualized specifically in ODCs. ODCs treated with IFN-γ for 48 hours showed significantly higher in double labeling (CNPase and TUNEL) than untreated ODCs [193]. Caspase-3 activity assay in ODC lysates was significantly higher in ODCs treated with IFN-γ for 48 hours compared to untreated ODCs [193]. Western blotting of the phosphorylated form of eIF-2α derived from ODCs treated with IFN-γ for 48 hours shows a more intense immunoreactive band than untreated ODCs when compared to the western blot of unphosphorylated form of eIF-2α [193]. Also in the same study, rat ODCs treated with IFN-γ for 48 hours had increased BiP, CHOP (GADD153) and caspase-12 relative transcript level compared to untreated ODCs [193]. These findings suggest ER stress may have a role in IFN-γ induced ODC apoptosis. IFN-γ- induced ODC apoptosis is likely relevant in the pathogenesis of neuroinflammation [192].
3.5. Other HERVs of relevance to MS pathogenesis
3.5.1. HERV-H
HERV-H has also been studied in MS patients, particularly in Scandinavian populations [107]. The in vitro production of retrovirus-like particles (RVLPs) in cell cultures from MS patients but not healthy controls may be enhanced or activated by infectious triggers such as Herpes viruses (e.g. herpes simplex virus (HSV), EBV). Independent molecular analysis of retroviral RNA associated with RVP revealed two different genetic families of endogenous retroviral elements: MSRV/HERV-W and RGH/HERV-H. Retroviral particles of the latter were reported to be transmitted to mitogen-stimulated lymphocytes from healthy donors [115], [116]. Although this study was not confirmed, it suggests that one or more HERVs may be associated with MS. In a recent study from Spain, of 48 MS patients studied within a patient population of 92, CSF samples showed no HERV-H RNA [194]. In fact, HERV-W was also not detected. In a Danish study, using a well-controlled study group, seroreactivity to select HERV-H peptides was determined by a sensitive time resolved immunofluorometric assay and generally MS patients with an active disease status had antibodies to several HERV-H peptides compared to non-active disease as well as unaffected relatives. At least 50% of the MS patient sera exhibited this response and there was a positive correlation with lower levels of an innate immune molecule, MASP3 [195]. In agreement with other infectious agents associated with MS, HERV-H was found to show a profound increase in cell-mediated immune response when peptides corresponding to the gag and env regions elicited a significant proliferative effect when combined with HSV and HHV-6A [32]. Indeed, splice variants of HERV-H env were also detected in about 40% of MS patients and attributed to the high transcription and translation of HERV-H env-encoded protein, which also elicited a strong serological activity. Interestingly, the splice variants associated with MS did not have any known homology with a host gene and thus ascribing loci was not achieved [116]. Most studies with HERV-H have involved determining whether there is a serological response to HERV-H antigenic peptides and invariably MS patient-derived PBMCs (which are in an activated state) always respond to these peptides more vigorously than control-derived PBMCs [196]. T cells and PBMCs from 25% of MS patients when cultured express retrovirus-like particles, which vary in size from 70 to 100 nm and appear morphologically similar to type C retroviruses in being irregularly rounded, with surface projections on the envelope and a relatively electron dense nucleocapsid/core [107]. Retrovirus-like particles (RVLPs) isolated by both the Danish and French groups from MS patient lymphocyte cell lines were examined by electron microscopy; large numbers of RVLPs were noted in the cytoplasm in case of MSRV, and these were not observed with HERV-H, in addition to differences in core density, particle size and morphology [107].
Due to the apparent ubiquitous presence of several exogenous viruses in MS patient tissues, synergism between some of these viruses and HERVs were examined in various studies. Using the sensitive PERT assay, Brudek et al. [197] examined reverse transcriptase in MS patient lymphocytes and determined that UV-inactivated HSV-1, HHV-6A and VZV could induce HERV reverse transcriptase activity in PBMCs, suggesting that viral proteins are sufficient to induce reverse transcriptase activity and a productive infection is not necessary [197]. In a follow up study, the same group examined the synergism between herpesvirus antigens and HERVs in the release of pro-inflammatory cytokines in PBMCs [198]. Assuming that HERV-H was the retrovirus used for the assay due to their long standing interest in the same, RVLPs were isolated from a B lymphoblastoid cell culture. While HERV-H alone did not induce cytokine production or cell proliferation but in combination with herpesvirus (HSV, VZV), there was a significant increase in IFN-γ, a pro-inflammatory cytokine which is known to exacerbate disease. HHV-6A also induced reverse transcriptase activity as well as a proliferative response [198]; it did not induce cytokines in the latter study [198]. This could be attributed to a different cohort of patients or other aspects of the study. Since nearly 100% of the Danish population is seropositive for HHV-6A and VZV, the role of HERV-H might be stronger than one might have anticipated, due to polymorphisms in the env gene of HERV-H.
3.5.2. HERV-K
An allelic variant member of the HERV-K family, K18, was recently found to be a risk factor for MS [111]. Although the small sample size was a limitation, there was a significant association between HERV-K18 env genotype and risk of MS [111]. HERV-K18 env has previously been described as a superantigen transactivated by EBV in the pathogenesis of type 1 diabetes [75]. Another member of the HERV-K family, K113, with ORFs for all its genes, has a widespread geographic as well as ethnic variation in 0–28% of humans, increasing the possibility of its association with MS since the disease is perhaps geographically and ethnically restricted. However, using a large population of MS (n = 951) and unaffected parents (n = 1902), there was no significant increase in the HERV-K113 provirus allele by PCR analysis [199]. Thus, while K18 shows association in a small subset of MS patients, a larger study with another HERV-K member, K113, reveals no significant differences; disparities in both the number of patients examined and the HERV-K genotype make this association inconclusive.
HERV-K is a relatively new HERV and was present in allelic form in 15% (115 HML-2) to 30% (113 HML-2) in DNA of known and unknown ethnic origins. Although no clear association of a HERV haplotype with host genotype has been made to date in MS patients, there is some evidence that depending on the population, the LTR sequences of HERVs such as HRES-1 show polymorphisms within MS patient haplotypes while those from non-MS patients were identical without geographic restrictions [110].
3.5.3. Conclusions and future directions
Given the diversity of findings related to HERV expression with several conflicting reports, there are issues requiring further attention in this field including standardization of procedures for detecting and defining different human retroelements. Moreover, these types of studies need to be extended to different populations in which MS is common together with analyses of HERV molecular diversity. It is conceivable that HERV allelic variants exert specific effects on the MS phenotype, progression and response to therapy depending on the host genetic background. Indeed, detection of sequences will require confirmatory sequencing to establish the encoding locus before definitive associations between disease and specific HERVs can be made. For example, it is imperative to understand the molecular nature of HERV recombination events that might trigger the emergence of a novel retroelement from individuals with MS, its geographic restriction and epidemiological features. In addition, allelic variation within specific HERVs, already associated with disease, warrants investigation. Since detection of MSRV, syncytin-1 and HERV-H in serum, plasma and brain tissue have depended on the use of RT-PCR, the use and efficacy of this technique for quantification of multicopy genes such as HERVs has recently raised questions about its utility. Quantitative RT-PCR analysis has not been performed in many studies, largely because of the large number of truncated HERV genes in the genome, pseudogenes and the homology between members of the same family. In addition to probes which distinguish HERVs, stringent PCR analyses that conform to standard conditions should be implemented [142].
Production of pathogenic (inflammatory or death signaling) molecules has been associated with retroviral expression [130], especially the envelope protein [2]. It has been postulated that there may exist a pathogenic cascade in MS involving several step-specific pathogens interacting with particular genetic elements leading to enhanced retroviral expression [200]. There is no conclusive proof that any HERV plays a major role in human disease although investigators have postulated several possible pathogenic mechanisms. HERVs, for example, could enhance the transcription of cellular genes downstream of HERV-LTRs that contain promoter elements. HERVs have also been posited to encode superantigens that result in enhanced inflammatory responses or mimic self-antigens leading to autoimmune pathologies such as lupus or MS [201]. Viral proteins interact directly or indirectly with promoters of various inflammatory cytokines and regulate their expression in infected cells. MSRV can be transactivated in vitro by two herpes simplex virus type 1 immediate early proteins [202] and thus act as triggering cofactors of MSRV in the pathogenesis of MS. In a similar fashion, MS long-term lymphocyte cell lines, which are EBV-transformed B cells with a T cell sub-population, with high reverse transcriptase activity attributed to HERV-H RVLP, also produced significant levels of IL-2, IFN-γ, GM-CSF and TNF-α [107]. However, MSRV showed a different cytokine profile with patient lymphocytes and with no correlation to RT activity [130]. In fact, cytokines such as IL-4, IL-6, TNF-α and IFN-γ might induce the release of MSRV particles thereby contributing to the pathogenic feedback loop [125]. These differences in cytokine profiles are to be expected as MS is a heterogeneous disease and compounded by allelic variations among patients, multiple cellular constituents (lymphocyte, PBMC, brains, CSF), stage of the disease, treatment regimen, etc. However, in a recent new study, MSRV env, MSRV gag and syncytin-1 did not provoke immune responses among MS patients who were HLA-B7 matched [114]. This observation suggests that either some specific antigenic epitope among the RVLPs is necessary or a specific protein folding pattern which might not be achieved in insect cells when recombinantly produced or that the pathogenesis is localized to the CNS as argued for syncytin-1 [102], [134], [135].
Syncytin-1 expression is enhanced by various viruses including HSV-1 infection [138]. Yet induced expression of several HERV genes is observed in neuroinflammatory diseases including MS [69], suggesting that HERVs found in MS are a by-product of the inflammatory component of MS, diluting the contention of some researchers that HERVs are a causative pathogen of MS [201]. Although HERVs are not the a priori cause of MS, they might participate in the pathogenesis such as in ER stress, and hence blocking HERV function in the CNS through specific therapies might diminish the progression of MS. While HERVs might also act as auto-, super- or neoantigens (upon expression of oncogenic viruses), HERV variants could participate in antigenic mimicry, all of which have the potential to enhance inflammatory responses or induce autoimmune reactions [110]. However, HERV-W expression in schizophrenia in which there is a lack of inflammation does not support this hypothesis. HERVs are present at conception in humans and if the genes are involved in pathogenesis through polymorphisms or reintegration, there should be a predominant hereditary component of the disease and a minor environmental role. This is not the case with MS, which exhibits significant hereditary and environmental susceptibility factors. Our understanding of HERVs is limited due to variability in the composition of human DNA in terms of copy number variations, deletions, expression patterns associated with methylation and tissue-specific effects. Given the abundance of HERV sequences in the human genome (complete or incomplete ORFs), it is also conceivable that retroelements express non-coding RNAs that act as microRNAs or siRNAs [203]. Because HERVs are conserved evolutionarily, differentially expressed in specific tissues and might participate in disease inheritance, susceptibility and progression, it is imperative to delineate HERV genetics and biology. Indeed, elucidation of individual HERV contributions to pathogenesis might enhance treatments for MS (Table 4 ) and other diseases that involve HERV expression or activation in a patient- or population-specific manner.
Table 4.
MS drugs potentially effecting HERV expression.
| Drug | Example | Drug's principal mode of action | Action against HERVs |
|---|---|---|---|
| Immunomodulatory agents | Interferon-β | Decreases leukocyte transmigration; antiviral; | Reduces HERV-W env RNA load, anti-HERV-W and HERV-H antibodies in sera; regulates syncytin promoter |
| Anti-psychotics | Reduction in intensity, density and compound value of HERV-W gag interneurons in alveus of patients with schizophrenia (Weiss et al., 2007) | ||
| Antiviral | Foscarnet | Inhibits reverse transcriptase | Blocks endogenous retrovirus (Sundquist and Oberg, 1979) |
Acknowledgements
The authors thank Krista Nelles for assistance with manuscript preparation and Dr. Farshid Noorbakhsh for helpful discussions. JMA holds fellowships from the McLaughlin Center for Molecular Medicine and Ontario Ministry of Research and Innovation. AD holds Queen Elizabeth II Studentship. CP is an AHFMR Senior Scholar and holds a Canada Research Chair in Neurological Infection and Immunity.
References
- 1.Sospedra M., Martin R. Immunology of multiple sclerosis. Annu. Rev. Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 2.Power C. Retroviral diseases of the nervous system: pathogenic host response or viral gene-mediated neurovirulence? Trends Neurosci. 2001;24(3):162–169. doi: 10.1016/s0166-2236(00)01737-9. [DOI] [PubMed] [Google Scholar]
- 3.Hughes J.F., Coffin J.M. Evidence for genomic rearrangements mediated by human endogenous retroviruses during primate evolution. Nat. Genet. 2001;29(4):487–489. doi: 10.1038/ng775. [DOI] [PubMed] [Google Scholar]
- 4.Costas J. Characterization of the intragenomic spread of the human endogenous retrovirus family HERV-W. Mol. Biol. Evol. 2002;19(4):526–533. doi: 10.1093/oxfordjournals.molbev.a004108. [DOI] [PubMed] [Google Scholar]
- 5.Urnovitz H.B., Murphy W.H. Human endogenous retroviruses: nature, occurrence, and clinical implications in human disease. Clin. Microbiol. Rev. 1996;9(1):72–99. doi: 10.1128/cmr.9.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang H. Mechanism of arsenic trioxide induced apoptosis in cultured human lens epithelium cells. Zhonghua Yan Ke Za Zhi. 2008;44(10):916–920. [PubMed] [Google Scholar]
- 7.Paty D.W. MRI as a method to reveal in-vivo pathology in MS. J. Neural Transm. Suppl. 1997;49:211–217. doi: 10.1007/978-3-7091-6844-8_22. [DOI] [PubMed] [Google Scholar]
- 8.Polman C.H. Ethics of placebo-controlled clinical trials in multiple sclerosis: a reassessment. Neurology. 2008;70(13 Pt 2):1134–1140. doi: 10.1212/01.wnl.0000306410.84794.4d. [DOI] [PubMed] [Google Scholar]
- 9.Lucchinetti C.F. Distinct patterns of multiple sclerosis pathology indicates heterogeneity on pathogenesis. Brain Pathol. 1996;6(3):259–274. doi: 10.1111/j.1750-3639.1996.tb00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trapp B.D. Axonal transection in the lesions of multiple sclerosis. N Engl J. Med. 1998;338(5):278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 11.Markovic-Plese S., McFarland H.F. Immunopathogenesis of the multiple sclerosis lesion. Curr. Neurol. Neurosci. Rep. 2001;1(3):257–262. doi: 10.1007/s11910-001-0028-4. [DOI] [PubMed] [Google Scholar]
- 12.Prat A., Antel J. Pathogenesis of multiple sclerosis. Curr. Opin. Neurol. 2005;18(3):225–230. doi: 10.1097/01.wco.0000169737.99040.31. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson B. Axonal damage in acute multiple sclerosis lesions. Brain. 1997;120(Pt 3):393–399. doi: 10.1093/brain/120.3.393. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez M. Effectors of demyelination and remyelination in the CNS: implications for multiple sclerosis. Brain Pathol. 2007;17(2):219–229. doi: 10.1111/j.1750-3639.2007.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noseworthy J.H. Progress in determining the causes and treatment of multiple sclerosis. Nature. 1999;399(6738 Suppl):A40–A47. doi: 10.1038/399a040. [DOI] [PubMed] [Google Scholar]
- 16.Robinson J. IMGT/HLA and IMGT/MHC: sequence databases for the study of the major histocompatibility complex. Nucleic Acids Res. 2003;31(1):311–314. doi: 10.1093/nar/gkg070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nair A., Frederick T.J., Miller S.D. Astrocytes in multiple sclerosis: a product of their environment. Cell. Mol. Life Sci. 2008;65(17):2702–2720. doi: 10.1007/s00018-008-8059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Owens G.P. The immunoglobulin G heavy chain repertoire in multiple sclerosis plaques is distinct from the heavy chain repertoire in peripheral blood lymphocytes. Clin. Immunol. 2001;98(2):258–263. doi: 10.1006/clim.2000.4967. [DOI] [PubMed] [Google Scholar]
- 19.Antel J. Multiple sclerosis—emerging concepts of disease pathogenesis. J. Neuroimmunol. 1999;98(1):45–48. doi: 10.1016/s0165-5728(99)00080-6. [DOI] [PubMed] [Google Scholar]
- 20.Ffrench-Constant C. Developmental studies of oligodendrocyte precursor cell migration and their implications for transplantation as therapy for multiple sclerosis. Eye. 1994;8(Pt 2):221–223. doi: 10.1038/eye.1994.50. [DOI] [PubMed] [Google Scholar]
- 21.Barnett M.H., Prineas J.W. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann. Neurol. 2004;55(4):458–468. doi: 10.1002/ana.20016. [DOI] [PubMed] [Google Scholar]
- 22.Bruck W., Lucchinetti C., Lassmann H. The pathology of primary progressive multiple sclerosis. Mult. Scler. 2002;8(2):93–97. doi: 10.1191/1352458502ms785rr. [DOI] [PubMed] [Google Scholar]
- 23.Kim T.G., Befus N., Langridge W.H. Co-immunization with an HIV-1 Tat transduction peptide-rotavirus enterotoxin fusion protein stimulates a Th1 mucosal immune response in mice. Vaccine. 2004;22(3–4):431–438. doi: 10.1016/j.vaccine.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Ransohoff R.M. Mechanisms of inflammation in MS tissue: adhesion molecules and chemokines. J. Neuroimmunol. 1999;98(1):57–68. doi: 10.1016/s0165-5728(99)00082-x. [DOI] [PubMed] [Google Scholar]
- 25.Yong V.W. Metalloproteinases in biology and pathology of the nervous system. Nat. Rev. Neurosci. 2001;2(7):502–511. doi: 10.1038/35081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonetti B., Raine C.S. Multiple sclerosis: oligodendrocytes display cell death-related molecules in situ but do not undergo apoptosis. Ann. Neurol. 1997;42(1):74–84. doi: 10.1002/ana.410420113. [DOI] [PubMed] [Google Scholar]
- 27.Peterson K.E. Differences in cytokine and chemokine responses during neurological disease induced by polytropic murine retroviruses map to separate regions of the viral envelope gene. J. Virol. 2001;75(6):2848–2856. doi: 10.1128/JVI.75.6.2848-2856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mhaille A.N. Increased expression of endoplasmic reticulum stress-related signaling pathway molecules in multiple sclerosis lesions. J. Neuropathol. Exp. Neurol. 2008;67(3):200–211. doi: 10.1097/NEN.0b013e318165b239. [DOI] [PubMed] [Google Scholar]
- 29.Hohlfeld R. The prospects for neuroprotection in MS. Int. MS J. 2003;10(4):103–105. [PubMed] [Google Scholar]
- 30.Pugliatti M. Environmental risk factors in multiple sclerosis. Acta Neurol. Scand. Suppl. 2008;188:34–40. doi: 10.1111/j.1600-0404.2008.01029.x. [DOI] [PubMed] [Google Scholar]
- 31.Mayne M., J.J. Latent and activated brain flore: human herpes virus, endogenous retroviruses,coronaviruses and Chlamydia and their role in neurological disease. In: Johnson C.P.a.R.T., editor. Emerging Neurological Infections. Taylor and Francis; 2005. pp. 363–396. [Google Scholar]
- 32.Brudek T. Simultaneous presence of endogenous retrovirus and herpes virus antigens has profound effect on cell-mediated immune responses: implications for multiple sclerosis. AIDS Res. Hum. Retroviruses. 2004;20(4):415–423. doi: 10.1089/088922204323048168. [DOI] [PubMed] [Google Scholar]
- 33.Sadovnick A.D. Age of onset in concordant twins and other relative pairs with multiple sclerosis. Am. J. Epidemiol. 2009;170(3):289–296. doi: 10.1093/aje/kwp143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gregersen J.W. Functional epistasis on a common MHC haplotype associated with multiple sclerosis. Nature. 2006;443(7111):574–577. doi: 10.1038/nature05133. [DOI] [PubMed] [Google Scholar]
- 35.Oksenberg J.R., Barcellos L.F. Multiple sclerosis genetics: leaving no stone unturned. Genes Immun. 2005;6(5):375–387. doi: 10.1038/sj.gene.6364237. [DOI] [PubMed] [Google Scholar]
- 36.Sotgiu S. Hygiene hypothesis: innate immunity, malaria and multiple sclerosis. Med. Hypotheses. 2008;70(4):819–825. doi: 10.1016/j.mehy.2006.10.069. [DOI] [PubMed] [Google Scholar]
- 37.Holmoy T., Hestvik A.L. Multiple sclerosis: immunopathogenesis and controversies in defining the cause. Curr. Opin. Infect. Dis. 2008;21(3):271–278. doi: 10.1097/QCO.0b013e3282f88b48. [DOI] [PubMed] [Google Scholar]
- 38.Frohman E.M., Racke M.K., Raine C.S. Multiple sclerosis—the plaque and its pathogenesis. N Engl J. Med. 2006;354(9):942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 39.Libbey J.E., McCoy L.L., Fujinami R.S. Molecular mimicry in multiple sclerosis. Int. Rev. Neurobiol. 2007;79:127–147. doi: 10.1016/S0074-7742(07)79006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buljevac D. Prospective study on the relationship between infections and multiple sclerosis exacerbations. Brain. 2002;125(Pt 5):952–960. doi: 10.1093/brain/awf098. [DOI] [PubMed] [Google Scholar]
- 41.Gilden D.H. Infectious causes of multiple sclerosis. Lancet Neurol. 2005;4(3):195–202. doi: 10.1016/S1474-4422(05)01017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cepok S. Identification of Epstein–Barr virus proteins as putative targets of the immune response in multiple sclerosis. J. Clin. Invest. 2005;115(5):1352–1360. doi: 10.1172/JCI23661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lynch D.T., Zimmerman J.S., Rowe D.T. Epstein–Barr virus latent membrane protein 2B (LMP2B) co-localizes with LMP2A in perinuclear regions in transiently transfected cells. J. Gen. Virol. 2002;83(Pt 5):1025–1035. doi: 10.1099/0022-1317-83-5-1025. [DOI] [PubMed] [Google Scholar]
- 44.Boisse L., Gill M.J., Power C. HIV infection of the central nervous system: clinical features and neuropathogenesis. Neurol. Clin. 2008;26(3):799–819. doi: 10.1016/j.ncl.2008.04.002. x. [DOI] [PubMed] [Google Scholar]
- 45.Power C., Gill M.J., Johnson R.T. Progress in clinical neurosciences: the neuropathogenesis of HIV infection: host–virus interaction and the impact of therapy. Can. J. Neurol. Sci. 2002;29(1):19–32. doi: 10.1017/s0317167100001682. [DOI] [PubMed] [Google Scholar]
- 46.Patrick M.K., Johnston J.B., Power C. Lentiviral neuropathogenesis: comparative neuroinvasion, neurotropism, neurovirulence, and host neurosusceptibility. J. Virol. 2002;76(16):7923–7931. doi: 10.1128/JVI.76.16.7923-7931.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Little P.F. Structure and function of the human genome. Genome Res. 2005;15(12):1759–1766. doi: 10.1101/gr.4560905. [DOI] [PubMed] [Google Scholar]
- 48.Martin M.A. Identification and cloning of endogenous retroviral sequences present in human DNA. Proc. Natl Acad. Sci. USA. 1981;78(8):4892–4896. doi: 10.1073/pnas.78.8.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lander E.S. Initial sequencing and analysis of the human genome. Nature. 2001;409(6822):860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 50.Larsson E., Kato N., Cohen M. Human endogenous proviruses. Curr. Top. Microbiol. Immunol. 1989;148:115–132. doi: 10.1007/978-3-642-74700-7_4. [DOI] [PubMed] [Google Scholar]
- 51.Andersson M.L. Diversity of human endogenous retrovirus class II-like sequences. J. Gen. Virol. 1999;80(Pt 1):255–260. doi: 10.1099/0022-1317-80-1-255. [DOI] [PubMed] [Google Scholar]
- 52.Medstrand P., Blomberg J. Characterization of novel reverse transcriptase encoding human endogenous retroviral sequences similar to type A and type B retroviruses: differential transcription in normal human tissues. J. Virol. 1993;67(11):6778–6787. doi: 10.1128/jvi.67.11.6778-6787.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Katzourakis A., Rambaut A., Pybus O.G. The evolutionary dynamics of endogenous retroviruses. Trends Microbiol. 2005;13(10):463–468. doi: 10.1016/j.tim.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 54.Blaise S. Genomewide screening for fusogenic human endogenous retrovirus envelopes identifies syncytin 2, a gene conserved on primate evolution. Proc. Natl Acad. Sci. USA. 2003;100(22):13013–13018. doi: 10.1073/pnas.2132646100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Parseval N., Heidmann T. Human endogenous retroviruses: from infectious elements to human genes. Cytogenet. Genome Res. 2005;110(1–4):318–332. doi: 10.1159/000084964. [DOI] [PubMed] [Google Scholar]
- 56.Dewannieux M., Blaise S., Heidmann T. Identification of a functional envelope protein from the HERV-K family of human endogenous retroviruses. J. Virol. 2005;79(24):15573–15577. doi: 10.1128/JVI.79.24.15573-15577.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blond J.L. Molecular characterization and placental expression of HERV-W, a new human endogenous retrovirus family. J. Virol. 1999;73(2):1175–1185. doi: 10.1128/jvi.73.2.1175-1185.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lindeskog M., Mager D.L., Blomberg J. Isolation of a human endogenous retroviral HERV-H element with an open env reading frame. Virology. 1999;258(2):441–450. doi: 10.1006/viro.1999.9750. [DOI] [PubMed] [Google Scholar]
- 59.Lower R., Lower J., Kurth R. The viruses in all of us: characteristics and biological significance of human endogenous retrovirus sequences. Proc. Natl Acad. Sci. USA. 1996;93(11):5177–5184. doi: 10.1073/pnas.93.11.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ono M., Kawakami M., Takezawa T. A novel human nonviral retroposon derived from an endogenous retrovirus. Nucleic Acids Res. 1987;15(21):8725–8737. doi: 10.1093/nar/15.21.8725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harada F., Tsukada N., Kato N. Isolation of three kinds of human endogenous retrovirus-like sequences using tRNA(Pro) as a probe. Nucleic Acids Res. 1987;15(22):9153–9162. doi: 10.1093/nar/15.22.9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.La Mantia G. Identification of regulatory elements within the minimal promoter region of the human endogenous ERV9 proviruses: accurate transcription initiation is controlled by an Inr-like element. Nucleic Acids Res. 1992;20(16):4129–4136. doi: 10.1093/nar/20.16.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pavlicek A. Processed pseudogenes of human endogenous retroviruses generated by LINEs: their integration, stability, and distribution. Genome Res. 2002;12(3):391–399. doi: 10.1101/gr.216902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jurka J. Repeats in genomic DNA: mining and meaning. Curr. Opin. Struct. Biol. 1998;8(3):333–337. doi: 10.1016/s0959-440x(98)80067-5. [DOI] [PubMed] [Google Scholar]
- 65.Leib-Mosch C., Seifarth W. Evolution and biological significance of human retroelements. Virus Genes. 1995;11(2–3):133–145. doi: 10.1007/BF01728654. [DOI] [PubMed] [Google Scholar]
- 66.Patience C., Wilkinson D.A., Weiss R.A. Our retroviral heritage. Trends Genet. 1997;13(3):116–120. doi: 10.1016/s0168-9525(97)01057-3. [DOI] [PubMed] [Google Scholar]
- 67.Blond J.L. An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J. Virol. 2000;74(7):3321–3329. doi: 10.1128/jvi.74.7.3321-3329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patience C. Packaging of endogenous retroviral sequences in retroviral vectors produced by murine and human packaging cells. J. Virol. 1998;72(4):2671–2676. doi: 10.1128/jvi.72.4.2671-2676.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnston J.B. Monocyte activation and differentiation augment human endogenous retrovirus expression: implications for inflammatory brain diseases. Ann. Neurol. 2001;50(4):434–442. doi: 10.1002/ana.1131. [DOI] [PubMed] [Google Scholar]
- 70.Lower R. The pathogenic potential of endogenous retroviruses: facts and fantasies. Trends Microbiol. 1999;7(9):350–356. doi: 10.1016/s0966-842x(99)01565-6. [DOI] [PubMed] [Google Scholar]
- 71.Schon U. Cell type-specific expression and promoter activity of human endogenous retroviral long terminal repeats. Virology. 2001;279(1):280–291. doi: 10.1006/viro.2000.0712. [DOI] [PubMed] [Google Scholar]
- 72.O'Reilly R.L., Singh S.M. Retroviruses and schizophrenia revisited. Am. J. Med. Genet. 1996;67(1):19–24. doi: 10.1002/(SICI)1096-8628(19960216)67:1<19::AID-AJMG3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 73.York D.F. Nucleotide sequence of the jaagsiekte retrovirus, an exogenous and endogenous type D and B retrovirus of sheep and goats. J. Virol. 1992;66(8):4930–4939. doi: 10.1128/jvi.66.8.4930-4939.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Armbruester V. A novel gene from the human endogenous retrovirus K expressed in transformed cells. Clin. Cancer Res. 2002;8(6):1800–1807. [PubMed] [Google Scholar]
- 75.Conrad B. A human endogenous retroviral superantigen as candidate autoimmune gene in type I diabetes. Cell. 1997;90(2):303–313. doi: 10.1016/s0092-8674(00)80338-4. [DOI] [PubMed] [Google Scholar]
- 76.Portis J.L. Perspectives on the role of endogenous human retroviruses in autoimmune diseases. Virology. 2002;296(1):1–5. doi: 10.1006/viro.2002.1388. [DOI] [PubMed] [Google Scholar]
- 77.Wilkinson David A., D.L.M.a.J.-A.C.L. Endogenous Human Retroviruses. In: Levy J.A., editor. The Retroviridae. Plenum Press; New York: 1994. pp. 465–535. [Google Scholar]
- 78.Power C., Johnson R.T. Neuroimmune and neurovirological aspects of human immunodeficiency virus infection. Adv. Virus Res. 2001;56:389–433. doi: 10.1016/s0065-3527(01)56034-0. [DOI] [PubMed] [Google Scholar]
- 79.Mourier T. Reverse transcription in genome evolution. Cytogenet. Genome Res. 2005;110(1–4):56–62. doi: 10.1159/000084938. [DOI] [PubMed] [Google Scholar]
- 80.Rawn S.M., Cross J.C. The evolution, regulation, and function of placenta-specific genes. Annu. Rev. Cell Dev. Biol. 2008;24:159–181. doi: 10.1146/annurev.cellbio.24.110707.175418. [DOI] [PubMed] [Google Scholar]
- 81.Kwon D.N. Identification of putative endogenous retroviruses actively transcribed in the brain. Virus Genes. 2008;36(3):439–447. doi: 10.1007/s11262-008-0216-2. [DOI] [PubMed] [Google Scholar]
- 82.Boller K. Evidence that HERV-K is the endogenous retrovirus sequence that codes for the human teratocarcinoma-derived retrovirus HTDV. Virology. 1993;196(1):349–353. doi: 10.1006/viro.1993.1487. [DOI] [PubMed] [Google Scholar]
- 83.Wang-Johanning F. Expression of human endogenous retrovirus k envelope transcripts in human breast cancer. Clin. Cancer Res. 2001;7(6):1553–1560. [PubMed] [Google Scholar]
- 84.Wang-Johanning F. Detecting the expression of human endogenous retrovirus E envelope transcripts in human prostate adenocarcinoma. Cancer. 2003;98(1):187–197. doi: 10.1002/cncr.11451. [DOI] [PubMed] [Google Scholar]
- 85.Lindeskog M., Blomberg J. Spliced human endogenous retroviral HERV-H env transcripts in T-cell leukaemia cell lines and normal leukocytes: alternative splicing pattern of HERV-H transcripts. J. Gen. Virol. 1997;78:2575–2585. doi: 10.1099/0022-1317-78-10-2575. [DOI] [PubMed] [Google Scholar]
- 86.Karlsson H. Retroviral RNA identified in the cerebrospinal fluids and brains of individuals with schizophrenia. Proc. Natl Acad. Sci. USA. 2001;98(8):4634–4639. doi: 10.1073/pnas.061021998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Frank O. Variable transcriptional activity of endogenous retroviruses in human breast cancer. J. Virol. 2008;82(4):1808–1818. doi: 10.1128/JVI.02115-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Villesen P. Identification of endogenous retroviral reading frames in the human genome. Retrovirology. 2004;1:32. doi: 10.1186/1742-4690-1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Katsumata K. Cytokine regulation of env gene expression of human endogenous retrovirus-R in human vascular endothelial cells. Clin. Immunol. 1999;93(1):75–80. doi: 10.1006/clim.1999.4762. [DOI] [PubMed] [Google Scholar]
- 90.Mameli G. Brains and peripheral blood mononuclear cells of multiple sclerosis (MS) patients hyperexpress MS-associated retrovirus/HERV-W endogenous retrovirus, but not human herpesvirus 6. J. Gen. Virol. 2007;88(Pt 1):264–274. doi: 10.1099/vir.0.81890-0. [DOI] [PubMed] [Google Scholar]
- 91.Crowell R.C., Kiessling A.A. Endogenous retrovirus expression in testis and epididymis. Biochem. Soc. Trans. 2007;35(Pt 3):629–633. doi: 10.1042/BST0350629. [DOI] [PubMed] [Google Scholar]
- 92.Georgiou I. Retrotransposon RNA expression and evidence for retrotransposition events in human oocytes. Hum. Mol. Genet. 2009;18(7):1221–1228. doi: 10.1093/hmg/ddp022. [DOI] [PubMed] [Google Scholar]
- 93.Larsson E., Andersson A.C., Nilsson B.O. Expression of an endogenous retrovirus (ERV3 HERV-R) in human reproductive and embryonic tissues—evidence for a function for envelope gene products. Ups. J. Med. Sci. 1994;99(2):113–120. doi: 10.3109/03009739409179354. [DOI] [PubMed] [Google Scholar]
- 94.Harris J.R. Placental endogenous retrovirus (ERV): structural, functional, and evolutionary significance. Bioessays. 1998;20(4):307–316. doi: 10.1002/(SICI)1521-1878(199804)20:4<307::AID-BIES7>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 95.Mi S. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature. 2000;403(6771):785–789. doi: 10.1038/35001608. [DOI] [PubMed] [Google Scholar]
- 96.Colmegna I., Garry R.F. Role of endogenous retroviruses in autoimmune diseases. Infect. Dis. Clin. North Am. 2006;20(4):913–929. doi: 10.1016/j.idc.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 97.Azoulay-Cayla A. Is multiple sclerosis a disease of viral origin? Pathol. Biol. (Paris) 2000;48(1):4–14. [PubMed] [Google Scholar]
- 98.Jern P., Sperber G.O., Blomberg J. Definition and variation of human endogenous retrovirus H. Virology. 2004;327(1):93–110. doi: 10.1016/j.virol.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 99.de Parseval N., Heidmann T. Physiological knockout of the envelope gene of the single-copy ERV-3 human endogenous retrovirus in a fraction of the Caucasian population. J. Virol. 1998;72(4):3442–3445. doi: 10.1128/jvi.72.4.3442-3445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Laufer G. Analysis of transcribed human endogenous retrovirus W env loci clarifies the origin of multiple sclerosis-associated retrovirus env sequences. Retrovirology. 2009;6:37. doi: 10.1186/1742-4690-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Voisset C. Chromosomal distribution and coding capacity of the human endogenous retrovirus HERV-W family. AIDS Res. Hum. Retroviruses. 2000;16(8):731–740. doi: 10.1089/088922200308738. [DOI] [PubMed] [Google Scholar]
- 102.Antony J.M. Human endogenous retrovirus glycoprotein-mediated induction of redox reactants causes oligodendrocyte death and demyelination. Nat. Neurosci. 2004;7(10):1088–1095. doi: 10.1038/nn1319. [DOI] [PubMed] [Google Scholar]
- 103.Reiss D., P.C., Moore W., Mager D.L. EndMS, Multiple Sclerosis Society of Canada Meeting 2007: Banff AB. 2007. Role of DNA methylation in the ectopic expression of Syncytin-1 in MS lesions. [Google Scholar]
- 104.Perron H. Molecular identification of a novel retrovirus repeatedly isolated from patients with multiple sclerosis. The Collaborative Research Group on Multiple Sclerosis. Proc Natl Acad Sci U S A. 1997;94(14):7583–7588. doi: 10.1073/pnas.94.14.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mirsattari S.M. Aboriginals with multiple sclerosis: HLA types and predominance of neuromyelitis optica. Neurology. 2001;56(3):317–323. doi: 10.1212/wnl.56.3.317. [DOI] [PubMed] [Google Scholar]
- 106.Frank O. Human endogenous retrovirus expression profiles in samples from brains of patients with schizophrenia and bipolar disorders. J. Virol. 2005;79(17):10890–10901. doi: 10.1128/JVI.79.17.10890-10901.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Christensen T. Association of human endogenous retroviruses with multiple sclerosis and possible interactions with herpes viruses. Rev. Med. Virol. 2005;15(3):179–211. doi: 10.1002/rmv.465. [DOI] [PubMed] [Google Scholar]
- 108.Antony J.M. Comparative expression of human endogenous retrovirus-W genes in multiple sclerosis. AIDS Res. Hum. Retroviruses. 2007;23(10):1251–1256. doi: 10.1089/aid.2006.0274. [DOI] [PubMed] [Google Scholar]
- 109.Mameli G. Novel reliable real-time PCR for differential detection of MSRVenv and syncytin-1 in RNA and DNA from patients with multiple sclerosis. J. Virol. Methods. 2009;161(1):98–106. doi: 10.1016/j.jviromet.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 110.Clausen J. Endogenous retroviruses and MS: using ERVs as disease markers. Int. MS J. 2003;10(1):22–28. [PubMed] [Google Scholar]
- 111.Tai A.K. Human endogenous retrovirus-K18 Env as a risk factor in multiple sclerosis. Mult. Scler. 2008;14(9):1175–1180. doi: 10.1177/1352458508094641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Christensen T. Reverse transcriptase activity and particle production in B lymphoblastoid cell lines established from lymphocytes of patients with multiple sclerosis. AIDS Res. Hum. Retroviruses. 1999;15(3):285–291. doi: 10.1089/088922299311466. [DOI] [PubMed] [Google Scholar]
- 113.Gifford R., Tristem M. The evolution, distribution and diversity of endogenous retroviruses. Virus Genes. 2003;26(3):291–315. doi: 10.1023/a:1024455415443. [DOI] [PubMed] [Google Scholar]
- 114.Ruprecht K. Lack of immune responses against multiple sclerosis-associated retrovirus/human endogenous retrovirus W in patients with multiple sclerosis. J. Neurovirol. 2008;14(2):143–151. doi: 10.1080/13550280801958922. [DOI] [PubMed] [Google Scholar]
- 115.Christensen T. Molecular characterization of HERV-H variants associated with multiple sclerosis. Acta Neurol. Scand. 2000;101(4):229–238. doi: 10.1034/j.1600-0404.2000.101004229.x. [DOI] [PubMed] [Google Scholar]
- 116.Christensen T. A transmissible human endogenous retrovirus. AIDS Res. Hum. Retroviruses. 2002;18(12):861–866. doi: 10.1089/08892220260190344. [DOI] [PubMed] [Google Scholar]
- 117.Komurian-Pradel F. Molecular cloning and characterization of MSRV-related sequences associated with retrovirus-like particles. Virology. 1999;260(1):1–9. doi: 10.1006/viro.1999.9792. [DOI] [PubMed] [Google Scholar]
- 118.Garson J.A. Detection of virion-associated MSRV-RNA in serum of patients with multiple sclerosis. Lancet. 1998;351(9095):33. doi: 10.1016/s0140-6736(98)24001-3. [DOI] [PubMed] [Google Scholar]
- 119.Serra C. Multiple sclerosis and multiple sclerosis-associated retrovirus in Sardinia. Neurol. Sci. 2001;22(2):171–173. doi: 10.1007/s100720170019. [DOI] [PubMed] [Google Scholar]
- 120.Dolei A. Multiple sclerosis-associated retrovirus (MSRV) in Sardinian MS patients. Neurology. 2002;58(3):471–473. doi: 10.1212/wnl.58.3.471. [DOI] [PubMed] [Google Scholar]
- 121.Gaudin P. Infrequency of detection of particle-associated MSRV/HERV-W RNA in the synovial fluid of patients with rheumatoid arthritis. Rheumatology (Oxford) 2000;39(9):950–954. doi: 10.1093/rheumatology/39.9.950. [DOI] [PubMed] [Google Scholar]
- 122.de Villiers J.N. Analysis of viral and genetic factors in South African patients with multiple sclerosis. Metab. Brain Dis. 2006 doi: 10.1007/s11011-006-9016-3. [DOI] [PubMed] [Google Scholar]
- 123.Zawada M. MSRV pol sequence copy number as a potential marker of multiple sclerosis. Pol. J. Pharmacol. 2003;55(5):869–875. [PubMed] [Google Scholar]
- 124.Sotgiu S. Multiple sclerosis-associated retrovirus and MS prognosis: an observational study. Neurology. 2002;59(7):1071–1073. doi: 10.1212/wnl.59.7.1071. [DOI] [PubMed] [Google Scholar]
- 125.Serra C. In vitro modulation of the multiple sclerosis (MS)-associated retrovirus by cytokines: implications for MS pathogenesis. J. Neurovirol. 2003;9(6):637–643. doi: 10.1080/13550280390246462. [DOI] [PubMed] [Google Scholar]
- 126.Rolland A. Correlation between disease severity and in vitro cytokine production mediated by MSRV (multiple sclerosis associated retroviral element) envelope protein in patients with multiple sclerosis. J. Neuroimmunol. 2005;160(1–2):195–203. doi: 10.1016/j.jneuroim.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 127.Rolland A. The envelope protein of a human endogenous retrovirus-w family activates innate immunity through CD14/TLR4 and promotes Th1-Like responses. J. Immunol. 2006;176(12):7636–7644. doi: 10.4049/jimmunol.176.12.7636. [DOI] [PubMed] [Google Scholar]
- 128.Mameli G. Inhibition of multiple-sclerosis-associated retrovirus as biomarker of interferon therapy. J. Neurovirol. 2008;14(1):73–77. doi: 10.1080/13550280701801107. [DOI] [PubMed] [Google Scholar]
- 129.Saresella M. Multiple sclerosis-associated retroviral agent (MSRV)-stimulated cytokine production in patients with relapsing-remitting multiple sclerosis. Mult. Scler. 2009;15(4):443–447. doi: 10.1177/1352458508100840. [DOI] [PubMed] [Google Scholar]
- 130.Menard A. Detection of a gliotoxic activity in the cerebrospinal fluid from multiple sclerosis patients. Neurosci. Lett. 1998;245(1):49–52. doi: 10.1016/s0304-3940(98)00171-2. [DOI] [PubMed] [Google Scholar]
- 131.Firouzi R. Multiple sclerosis-associated retrovirus particles cause T lymphocyte-dependent death with brain hemorrhage in humanized SCID mice model. J. Neurovirol. 2003;9(1):79–93. doi: 10.1080/13550280390173328. [DOI] [PubMed] [Google Scholar]
- 132.Perron H. Multiple sclerosis retrovirus particles and recombinant envelope trigger an abnormal immune response in vitro, by inducing polyclonal Vbeta16 T-lymphocyte activation. Virology. 2001;287(2):321–332. doi: 10.1006/viro.2001.1045. [DOI] [PubMed] [Google Scholar]
- 133.Mameli G. Regulation of the syncytin-1 promoter in human astrocytes by multiple sclerosis-related cytokines. Virology. 2007;362(1):120–130. doi: 10.1016/j.virol.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 134.Antony J.M., Izad M., Bar-Or A., Vodjgani M., Warren K.G., Power C. Quantitative analysis of human endogenous retrovirus-W env in neuroinflammatory diseases. AIDS Res. Hum. Retrovir. 2006;22(12):1253–1259. doi: 10.1089/aid.2006.22.1253. [DOI] [PubMed] [Google Scholar]
- 135.Antony J.M. The human endogenous retrovirus envelope glycoprotein, syncytin-1, regulates neuroinflammation and its receptor expression in multiple sclerosis: a role for endoplasmic reticulum chaperones in astrocytes. J. Immunol. 2007;179(2):1210–1224. doi: 10.4049/jimmunol.179.2.1210. [DOI] [PubMed] [Google Scholar]
- 136.Bo L. Induction of nitric oxide synthase in demyelinating regions of multiple sclerosis brains. Ann. Neurol. 1994;36(5):778–786. doi: 10.1002/ana.410360515. [DOI] [PubMed] [Google Scholar]
- 137.Mattson M.P., Taub D.D. Ancient viral protein enrages astrocytes in multiple sclerosis. Nat. Neurosci. 2004;7(10):1021–1023. doi: 10.1038/nn1004-1021. [DOI] [PubMed] [Google Scholar]
- 138.Ruprecht K. Regulation of human endogenous retrovirus W protein expression by herpes simplex virus type 1: implications for multiple sclerosis. J. Neurovirol. 2006;12(1):65–71. doi: 10.1080/13550280600614973. [DOI] [PubMed] [Google Scholar]
- 139.Nellaker C. Transactivation of elements in the human endogenous retrovirus W family by viral infection. Retrovirology. 2006;3:44. doi: 10.1186/1742-4690-3-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yoshimura F.K. Up-regulation of a cellular protein at the translational level by a retrovirus. Proc. Natl Acad. Sci. USA. 2008;105(14):5543–5548. doi: 10.1073/pnas.0710526105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dalton D.K., Wittmer S. Nitric-oxide-dependent and independent mechanisms of protection from CNS inflammation during Th1-mediated autoimmunity: evidence from EAE in iNOS KO mice. J. Neuroimmunol. 2005;160(1–2):110–121. doi: 10.1016/j.jneuroim.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 142.Garson J.A. Unreliable real-time PCR analysis of human endogenous retrovirus-W (HERV-W) RNA expression and DNA copy number in multiple sclerosis. AIDS Res. Hum. Retrovir. 2009;25(3):377–378. doi: 10.1089/aid.2008.0270. author reply 379-81. [DOI] [PubMed] [Google Scholar]
- 143.Lavillette D. The envelope glycoprotein of human endogenous retrovirus type W uses a divergent family of amino acid transporters/cell surface receptors. J. Virol. 2002;76(13):6442–6452. doi: 10.1128/JVI.76.13.6442-6452.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.An D.S., Xie Y., Chen I.S. Envelope gene of the human endogenous retrovirus HERV-W encodes a functional retrovirus envelope. J. Virol. 2001;75(7):3488–3489. doi: 10.1128/JVI.75.7.3488-3489.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Weiss M.D. Ontogeny and localization of the neutral amino acid transporter ASCT1 in rat brain. Brain Res. Dev. Brain Res. 2001;130(2):183–190. doi: 10.1016/s0165-3806(01)00250-4. [DOI] [PubMed] [Google Scholar]
- 146.Marin M. N-linked glycosylation and sequence changes in a critical negative control region of the ASCT1 and ASCT2 neutral amino acid transporters determine their retroviral receptor functions. J. Virol. 2003;77(5):2936–2945. doi: 10.1128/JVI.77.5.2936-2945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Luabeya M.K. Blood-brain barrier disruption in simian immunodeficiency virus encephalitis. Neuropathol. Appl. Neurobiol. 2000;26(5):454–462. doi: 10.1046/j.1365-2990.2000.00275.x. [DOI] [PubMed] [Google Scholar]
- 148.Saccani A. Redox regulation of chemokine receptor expression. Proc. Natl Acad. Sci. USA. 2000;97(6):2761–2766. doi: 10.1073/pnas.97.6.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kekuda R. Cloning of the sodium-dependent, broad-scope, neutral amino acid transporter Bo from a human placental choriocarcinoma cell line. J. Biol. Chem. 1996;271(31):18657–18661. doi: 10.1074/jbc.271.31.18657. [DOI] [PubMed] [Google Scholar]
- 150.Sakai K. Neutral amino acid transporter ASCT1 is preferentially expressed in L-Ser-synthetic/storing glial cells in the mouse brain with transient expression in developing capillaries. J. Neurosci. 2003;23(2):550–560. doi: 10.1523/JNEUROSCI.23-02-00550.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hackel, D., PhD thesis: Activation of microglia by injured neurons-signal pathways of oxidatively modified lipids. 2005, Magdeburg: Magdeburg. p. 169.
- 152.Yamamoto T. Characterization of rapid and high-affinity uptake of L-serine in neurons and astrocytes in primary culture. FEBS Lett. 2003;548(1–3):69–73. doi: 10.1016/s0014-5793(03)00742-7. [DOI] [PubMed] [Google Scholar]
- 153.Sundaram K.S., Lev M. Inhibition of sphingolipid synthesis by cycloserine in vitro and in vivo. J. Neurochem. 1984;42(2):577–581. doi: 10.1111/j.1471-4159.1984.tb02716.x. [DOI] [PubMed] [Google Scholar]
- 154.Furuya S., Watanabe M. Novel neuroglial and glioglial relationships mediated by L-serine metabolism. Arch. Histol. Cytol. 2003;66(2):109–121. doi: 10.1679/aohc.66.109. [DOI] [PubMed] [Google Scholar]
- 155.Kudo Y., Boyd C.A. Changes in expression and function of syncytin and its receptor, amino acid transport system B(0) (ASCT2), in human placental choriocarcinoma BeWo cells during syncytialization. Placenta. 2002;23(7):536–541. doi: 10.1053/plac.2002.0839. [DOI] [PubMed] [Google Scholar]
- 156.Storck T. Structure, expression, and functional analysis of a Na(+)-dependent glutamate/aspartate transporter from rat brain. Proc. Natl Acad. Sci. USA. 1992;89(22):10955–10959. doi: 10.1073/pnas.89.22.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Uchiyama T. Functional regulation of Na + -dependent neutral amino acid transporter ASCT2 by S-nitrosothiols and nitric oxide in Caco-2 cells. FEBS Lett. 2005;579(11):2499–2506. doi: 10.1016/j.febslet.2005.03.065. [DOI] [PubMed] [Google Scholar]
- 158.Dickhout J.G. Peroxynitrite causes endoplasmic reticulum stress and apoptosis in human vascular endothelium: implications in atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2005;25(12):2623–2629. doi: 10.1161/01.ATV.0000189159.96900.d9. [DOI] [PubMed] [Google Scholar]
- 159.Kanai Y., Hediger M.A. The glutamate/neutral amino acid transporter family SLC1: molecular, physiological and pharmacological aspects. Pflugers Arch. 2004;447(5):469–479. doi: 10.1007/s00424-003-1146-4. [DOI] [PubMed] [Google Scholar]
- 160.Tatarowicz W.A. Repression of the HSV-1 latency-associated transcript (LAT) promoter by the early growth response (EGR) proteins: involvement of a binding site immediately downstream of the TATA box. J. Neurovirol. 1997;3(3):212–224. doi: 10.3109/13550289709018296. [DOI] [PubMed] [Google Scholar]
- 161.Di Battista J.A., Martel-Pelletier J., Pelletier J. Suppression of tumor necrosis factor (TNF-alpha) gene expression by prostaglandin E(2). Role of early growth response protein-1 (Egr-1) Osteoarthritis Cartilage. 1999;7(4):395–398. doi: 10.1053/joca.1998.0222. [DOI] [PubMed] [Google Scholar]
- 162.Korner H. Critical points of tumor necrosis factor action in central nervous system autoimmune inflammation defined by gene targeting. J. Exp. Med. 1997;186(9):1585–1590. doi: 10.1084/jem.186.9.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.FitzGerald U.F. Transcription factor expression and cellular redox in immature oligodendrocyte cell death: effect of Bcl-2. Mol. Cell. Neurosci. 2003;22(4):516–529. doi: 10.1016/s1044-7431(02)00040-4. [DOI] [PubMed] [Google Scholar]
- 164.Mycko M.P. Microarray gene expression profiling of chronic active and inactive lesions in multiple sclerosis. Clin. Neurol. Neurosurg. 2004;106(3):223–229. doi: 10.1016/j.clineuro.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 165.Nishie M. Multinucleated astrocytes in old demyelinated plaques in a patient with multiple sclerosis. Neuropathology. 2004;24(3):248–253. doi: 10.1111/j.1440-1789.2004.00548.x. [DOI] [PubMed] [Google Scholar]
- 166.Stys P.K., Lipton S.A. White matter NMDA receptors: an unexpected new therapeutic target? Trends Pharmacol. Sci. 2007;28(11):561–566. doi: 10.1016/j.tips.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 167.de Koning T.J. Neurotransmitters in 3-phosphoglycerate dehydrogenase deficiency. Eur. J. Pediatr. 2000;159(12):939–940. doi: 10.1007/pl00008379. [DOI] [PubMed] [Google Scholar]
- 168.Zhang K., Kaufman R.J. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454(7203):455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Iwawaki T. A transgenic mouse model for monitoring endoplasmic reticulum stress. Nat. Med. 2004;10(1):98–102. doi: 10.1038/nm970. [DOI] [PubMed] [Google Scholar]
- 170.Yoshida H. ER stress and diseases. FEBS J. 2007;274(3):630–658. doi: 10.1111/j.1742-4658.2007.05639.x. [DOI] [PubMed] [Google Scholar]
- 171.Nishikawa S., Brodsky J.L., Nakatsukasa K. Roles of molecular chaperones in endoplasmic reticulum (ER) quality control and ER-associated degradation (ERAD) J. Biochem. 2005;137(5):551–555. doi: 10.1093/jb/mvi068. [DOI] [PubMed] [Google Scholar]
- 172.Zhang K. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124(3):587–599. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 173.Kimata Y. A role for BiP as an adjustor for the endoplasmic reticulum stress-sensing protein Ire1. J. Cell Biol. 2004;167(3):445–456. doi: 10.1083/jcb.200405153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kondo S. OASIS, a CREB/ATF-family member, modulates UPR signalling in astrocytes. Nat. Cell Biol. 2005;7(2):186–194. doi: 10.1038/ncb1213. [DOI] [PubMed] [Google Scholar]
- 175.Wolfson J.J. Subtilase cytotoxin activates PERK, IRE1 and ATF6 endoplasmic reticulum stress-signalling pathways. Cell. Microbiol. 2008;10(9):1775–1786. doi: 10.1111/j.1462-5822.2008.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Marciniak S.J. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18(24):3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Malhotra J.D. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc. Natl Acad. Sci. USA. 2008;105(47):18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Feldman D.E., Chauhan V., Koong A.C. The unfolded protein response: a novel component of the hypoxic stress response in tumors. Mol. Cancer Res. 2005;3(11):597–605. doi: 10.1158/1541-7786.MCR-05-0221. [DOI] [PubMed] [Google Scholar]
- 179.Omori Y. CREB-H: a novel mammalian transcription factor belonging to the CREB/ATF family and functioning via the box-B element with a liver-specific expression. Nucleic Acids Res. 2001;29(10):2154–2162. doi: 10.1093/nar/29.10.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Boylan M.T. Interferon-beta1a administration results in a transient increase of serum amyloid A protein and C-reactive protein: comparison with other markers of inflammation. Immunol. Lett. 2001;75(3):191–197. doi: 10.1016/s0165-2478(00)00310-2. [DOI] [PubMed] [Google Scholar]
- 181.Crowell R.E. C-reactive protein receptors on the human monocytic cell line U-937. Evidence for additional binding to Fc gamma RI. J. Immunol. 1991;147(10):3445–3451. [PubMed] [Google Scholar]
- 182.Kim J.K., Scott E.A., Elbert D.L. Proteomic analysis of protein adsorption: serum amyloid P adsorbs to materials and promotes leukocyte adhesion. J. Biomed. Mater. Res. A. 2005;75(1):199–209. doi: 10.1002/jbm.a.30424. [DOI] [PubMed] [Google Scholar]
- 183.He B. Viruses, endoplasmic reticulum stress, and interferon responses. Cell Death Differ. 2006 doi: 10.1038/sj.cdd.4401833. [DOI] [PubMed] [Google Scholar]
- 184.Tardif K.D. Hepatitis C virus suppresses the IRE1-XBP1 pathway of the unfolded protein response. J. Biol. Chem. 2004;279(17):17158–17164. doi: 10.1074/jbc.M312144200. [DOI] [PubMed] [Google Scholar]
- 185.Liu N. ATM deficiency induces oxidative stress and endoplasmic reticulum stress in astrocytes. Lab. Invest. 2005;85(12):1471–1480. doi: 10.1038/labinvest.3700354. [DOI] [PubMed] [Google Scholar]
- 186.Dimcheff D.E. Endoplasmic reticulum (ER) stress induced by a neurovirulent mouse retrovirus is associated with prolonged BiP binding and retention of a viral protein in the ER. J. Biol. Chem. 2004;279(32):33782–33790. doi: 10.1074/jbc.M403304200. [DOI] [PubMed] [Google Scholar]
- 187.Kim H.T. Activation of endoplasmic reticulum stress signaling pathway is associated with neuronal degeneration in MoMuLV-ts1-induced spongiform encephalomyelopathy. Lab. Invest. 2004;84(7):816–827. doi: 10.1038/labinvest.3700104. [DOI] [PubMed] [Google Scholar]
- 188.Liu N. Interaction between endoplasmic reticulum stress and caspase 8 activation in retrovirus MoMuLV-ts1-infected astrocytes. Virology. 2006;348(2):398–405. doi: 10.1016/j.virol.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 189.Caney S.M. Expression of chemokine receptors in the feline reproductive tract and large intestine. J. Comp. Pathol. 2002;126(4):289–302. doi: 10.1053/jcpa.2002.0554. [DOI] [PubMed] [Google Scholar]
- 190.Kawahara K. Induction of CHOP and apoptosis by nitric oxide in p53-deficient microglial cells. FEBS Lett. 2001;506(2):135–139. doi: 10.1016/s0014-5793(01)02898-8. [DOI] [PubMed] [Google Scholar]
- 191.Noorbakhsh F. Lentivirus envelope protein exerts differential neuropathogenic effects depending on the site of expression and target cell. Virology. 2006;348(2):260–276. doi: 10.1016/j.virol.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 192.Miller D.H. Biomarkers and surrogate outcomes in neurodegenerative disease: lessons from multiple sclerosis. NeuroRx. 2004;1(2):284–294. doi: 10.1602/neurorx.1.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lin W. Interferon-{gamma} inhibits central nervous system remyelination through a process modulated by endoplasmic reticulum stress. Brain. 2006 doi: 10.1093/brain/awl044. [DOI] [PubMed] [Google Scholar]
- 194.Alvarez-Lafuente R. Herpesviruses and human endogenous retroviral sequences in the cerebrospinal fluid of multiple sclerosis patients. Mult. Scler. 2008;14(5):595–601. doi: 10.1177/1352458507086425. [DOI] [PubMed] [Google Scholar]
- 195.Christensen T. Gene-environment interactions in multiple sclerosis: innate and adaptive immune responses to human endogenous retrovirus and herpesvirus antigens and the lectin complement activation pathway. J. Neuroimmunol. 2007;183(1–2):175–188. doi: 10.1016/j.jneuroim.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 196.Christensen T. Antibodies against a human endogenous retrovirus and the preponderance of env splice variants in multiple sclerosis patients. Mult. Scler. 2003;9(1):6–15. doi: 10.1191/1352458503ms867oa. [DOI] [PubMed] [Google Scholar]
- 197.Brudek T. Activation of endogenous retrovirus reverse transcriptase in multiple sclerosis patient lymphocytes by inactivated HSV-1, HHV-6 and VZV. J. Neuroimmunol. 2007;187(1–2):147–155. doi: 10.1016/j.jneuroim.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 198.Brudek T. Synergistic immune responses induced by endogenous retrovirus and herpesvirus antigens result in increased production of inflammatory cytokines in multiple sclerosis patients. Scand. J. Immunol. 2008;67(3):295–303. doi: 10.1111/j.1365-3083.2007.02067.x. [DOI] [PubMed] [Google Scholar]
- 199.Moyes D.L. HERV-K113 is not associated with multiple sclerosis in a large family-based study. AIDS Res. Hum. Retroviruses. 2008;24(3):363–365. doi: 10.1089/aid.2007.0196. [DOI] [PubMed] [Google Scholar]
- 200.Perron H. Particle-associated retroviral RNA and tandem RGH/HERV-W copies on human chromosome 7q: possible components of a ‘chain-reaction’ triggered by infectious agents in multiple sclerosis? J. Neurovirol. 2000;6(Suppl 2):S67–S75. [PubMed] [Google Scholar]
- 201.Kolson D.L., Gonzalez-Scarano F. Endogenous retroviruses and multiple sclerosis. Ann. Neurol. 2001;50(4):429–430. doi: 10.1002/ana.1235. [DOI] [PubMed] [Google Scholar]
- 202.Perron H. Herpes simplex virus ICP0 and ICP4 immediate early proteins strongly enhance expression of a retrovirus harboured by a leptomeningeal cell line from a patient with multiple sclerosis. J. Gen. Virol. 1993;74(Pt 1):65–72. doi: 10.1099/0022-1317-74-1-65. [DOI] [PubMed] [Google Scholar]
- 203.Levy A., Sela N., Ast G. TranspoGene and microTranspoGene: transposed elements influence on the transcriptome of seven vertebrates and invertebrates. Nucleic Acids Res. 2008;36:D47–D52. doi: 10.1093/nar/gkm949. (Database issue) [DOI] [PMC free article] [PubMed] [Google Scholar]