Abstract
Midbrain dopamine (DA) neurons have received significant attention in brain research because of their central role in reward processing and their dysfunction in neuropsychiatric disorders such as Parkinson’s disease, drug addiction, depression and schizophrenia. Until recently, it has been thought that DA neurons form a homogeneous population whose primary function is the computation of reward prediction errors. However, through the implementation of viral vector strategies, an unexpected complexity and diversity has been revealed at the anatomical, molecular and functional level. In this review, we discuss recent viral vector approaches that have been leveraged to dissect how different circuits involving distinct DA neuron subpopulations may contribute to the role of DA in reward- and aversion-related behaviors. We focus on studies that have used cell type- and projection-specific optogenetic manipulations, discuss the strengths and limitations of each approach, and critically examine emergent organizational principles that have led to a reclassification of midbrain DA neurons.
Keywords: dopamine, mesocorticolimbic, reward, aversion, ventral tegmental area, optogenetics
1. Introduction
A major goal in neurobiology is to understand which neurons communicate with each other, how this communication is relevant to behavior, and how miscommunication leads to the diseased brain. However, the complexity of the mammalian central nervous system remains a large obstacle towards achieving this goal, due to its enormous diversity of cell types that differ in morphology, connectivity and function. With the development and implementation of viral vector strategies, a completely new perspective on brain structure, function, and the causes of disease at the circuit level has been revealed. Indeed, engineered viral vectors represent the most effective means of gene transfer to modify specific cell types within larger neural networks, and they have become the workhorse for almost all current approaches used to describe the architecture, molecular features and functions of neural circuits. They are currently used to integrate neural circuit information across multiple levels of investigation, including functional manipulations (e.g., optogenetics), neural activity readouts (e.g., calcium imaging, electrophysiology), molecular profiling and trans-synaptic tracing using modified rabies viruses (Boyden, 2015; Callaway, 2008; Deisseroth, 2011; Deisseroth and Schnitzer, 2013; Wickersham et al., 2007).
Midbrain dopamine (DA) neurons have received significant attention in brain research because of their central role in reward processing and their dysfunction in neuropsychiatric disorders such as Parkinson’s disease, drug addiction, depression and schizophrenia (Deisseroth, 2014; Lammel et al., 2014; Lüscher, 2016; Nestler and Carlezon, 2006; Schultz, 2016; Volkow and Morales, 2015; Wise, 2004). The midbrain DA system is mainly comprised of DA neurons in the ventral tegmental area (VTA, A10) and in the neighboring substantia nigra pars compacta (SN, A9). Predominant projection targets of VTA DA neurons are the medial prefrontal cortex (mPFC), the ventral striatum (nucleus accumbens [NAc] core, medial and lateral shell) and the basolateral amygdala (BLA), while DA SN neurons project strongly to the dorsal striatum (Bjorklund and Dunnett, 2007). Until recently, midbrain DA neurons had been thought to form a homogeneous cell population. Classical work has shown that they are phasically excited in response to rewards and reward-predicting stimuli, and are inhibited in response to aversive stimuli (Schultz, 1997; Ungless et al., 2004). However, recent studies that have integrated advanced technologies including cell type-specific electrophysiology, rabies virus-based trans-synaptic tracing, voltammetry, calcium imaging and optogenetics-based approaches have brought to light a much greater diversity of DA cell properties and functions than previously supposed. This work has shown that VTA DA neurons are heterogeneous not only in regard to their anatomical, molecular and electrophysiological properties, but also in their response to salient appetitive and aversive stimuli. For a comprehensive discussion on the heterogeneity of VTA DA neurons, the reader is recommended to refer to recent reviews on this topic (Anderegg et al., 2015; Bariselli et al., 2016; Bromberg-Martin et al., 2010; Fields et al., 2007; Hu, 2016; Juarez and Han, 2016; Lammel et al., 2014; Lerner et al., 2016; Pignatelli and Bonci, 2015; Roeper, 2013). In this review, we discuss recent advances that have implemented viral vector strategies to dissect the midbrain DA system anatomically and functionally, and highlight the strengths and caveats associated with these approaches. We also discuss how this work has given strong emphasis to the possibility that distinct DA subtypes may contribute differentially to the role of DA in reward- and aversion-related behaviors.
2. Viral vector strategies for neuroanatomical dissection of the midbrain dopamine system
Traditionally, investigations into the circuit architecture of the midbrain DA system have mainly relied on anterograde and retrograde tracer substances such as PHA-L and fluorescent retrobeads, respectively. For example, classical anterograde tracing experiments have established that major inputs to the VTA arise from widespread brain regions including the mPFC, lateral habenula (LHb), dorsal raphe nucleus (DRN), NAc, ventral pallidum, preoptic area, lateral hypothalamus (LH) and laterodorsal tegmentum (LDT) (Geisler et al., 2007; Yetnikoff et al., 2014). Conversely, using classical retrograde tracing techniques, several studies have correlated a DA neuron’s projection target with different features at the molecular, anatomical, and electrophysiological level (Beckley et al., 2013; Beier et al., 2015; Ford et al., 2006; Ikemoto, 2007; Lammel et al., 2008; 2011; 2012; Lerner et al., 2015; Margolis et al., 2006, 2008). By combining retrograde tracing, molecular profiling and acute brain slice patch clamp recordings in adult mice, subpopulations of “nonconventional” DA neurons located in the medial posterior VTA were identified and characterized (Lammel et al., 2008). These DA neurons project to mPFC, NAc medial shell, NAc core and BLA, and for the most part do not send axon collaterals to other brain regions (Albanese and Minciacchi, 1983; Fallon, 1981; Fallon and Loughlin, 1982; Lammel et al., 2008). In addition, they have a molecular make-up and firing properties that are clearly different from “conventional” DA neurons, which are located in the lateral VTA and the SN and project to NAc lateral shell and dorsal striatum, respectively (Lammel et al., 2008). The results from this and the other studies (see above) suggest that VTA DA neurons are heterogeneous in regard to their anatomical, molecular and electrophysiological properties, a finding that has strongly highlighted the possibility that individual groups of DA neurons provide unique information to other brain regions about how to respond to specific environmental stimuli.
However, a major caveat of conventional tracer substances is that although they can identify connections between two discrete anatomical areas, they are limited by the inability to distinguish between neuronal cell types. Cell-type specificity is particularly important in heterogeneous brain regions such as the VTA, which in addition to DA neurons also contains GABAergic and glutamatergic neurons (Hnasko et al., 2012; Nair-Roberts et al., 2008; Yamaguchi et al., 2007). Thus, cell type- and projection-specific approaches are needed in order to understand the full complexity of the midbrain DA system. The advent of modified rabies virus (RV)-based tracing approaches has made it possible to identify direct monosynaptic partners of genetically identified subpopulations of neurons within larger neural networks. Three different RV-based approaches have been used for studying anatomical connectivity in the midbrain DA system: cell type-specific, projection-specific and combined cell type- and projection-specific trans-synaptic tracing.
Cell type-specific trans-synaptic tracing using modified rabies viruses
Trans-synaptic tracing leverages the well-understood mechanisms of RV, which infects neural tissue, spreads across synapses and is strictly transported retrogradely (Wickersham et al., 2007). Because a natural rabies virus would theoretically infect an unlimited number of neurons, researchers have developed a rabies virus with two important modifications: the virus is pseudotyped with avian virus envelope protein A (EnvA), and the gene that codes for rabies glycoprotein (RG) has been replaced with a gene for a fluorescent protein (e.g., EnvA-dG-RV-GFP; Osakada and Callaway, 2013; Wickersham et al., 2007). The EnvA modification renders the virus incapable of infecting mammalian cells unless they express the avian tumor virus A (TVA) receptor. Moreover, because RG is necessary for trans-synaptic spread of the virus, when EnvA-dG-RV-GFP does infect a neuron (i.e., a starter cell), it will only infect that neuron’s direct presynaptic partners and is unable to spread beyond these cells because they lack RG. The modified RV can be combined with the Cre/lox recombination system, which involves Cre-dependent viral vectors and transgenic Cre-driver mouse lines, to achieve cell-type specificity (Gelman et al., 2003; Gong et al., 2007; see below). Cre recombinase (Cre) is a protein originally discovered in the P1 bacteriophage which recognizes the specific loxP DNA sequence. When pairs of loxP sites flank an exogene (i.e., it is “floxed”) in an inverted orientation (termed “double inverted open reading frame,” or DIO), Cre mediates an inversion of the exogene between the loxP sites. Transgenic Cre-driver mice that express Cre under a cell type-specific promoter can be injected with viral vectors encoding the Cre-dependent genes for TVA and RG (e.g., AAV-DIO-TVA and AAV-DIO-RG) such that TVA and RG are only expressed in this genetically defined subpopulation of neurons. When EnvA-dG-RV-GFP is subsequently injected into the same brain region, it will only infect this small subset of cells. It trans-complements with the RG being exogenously expressed by these cells and thereby enables trans-synaptic spread of the rabies virus. Because the direct presynaptic partners do not express RG, the modified RV cannot spread any further, and fluorescent protein (e.g., GFP) expression will be limited to these cells and the starter cell population. In this way, modified rabies virus enables the identification of cells that make direct monosynaptic connections onto a genetically defined starter cell population (Wall et al., 2010).
In a foundational study, Uchida and colleagues have used this approach to identify monosynaptic inputs to VTA or SN DA neurons throughout the entire brain (Watabe-Uchida et al., 2012). Even though most of the inputs to VTA and SN DA neurons were consistent with previous studies using conventional tracer substances, the authors found some important differences that were previously unreported. Specifically, they found that DA neurons in the SN receive particularly strong inputs from somatosensory and motor cortices, as well as from the subthalamic nucleus, whereas DA neurons in the VTA receive strong inputs from the LH. The finding that VTA and SN DA neurons receive distinct inputs may contribute to their different firing patterns and diverse behavioral functions. Moreover, cell type-specific trans-synaptic RV tracing has also been leveraged to identify afferents to glutamatergic and GABAergic neurons in the VTA, showing that these cell populations receive inputs from similar brain regions but in varying proportions. Specifically, glutamatergic VTA neurons have been found to receive proportionally more cortical input (e.g., somatosensory, motor, insular, and cingulate cortices) compared to VTA GABA and DA neurons. On the other hand, VTA GABA cells receive significantly more input from the LDT, rostromedial tegmental nucleus (RMTg) and LHb (Faget et al., 2016).
Projection-specific trans-synaptic tracing using TRIO and cTRIO
A second type of monosynaptic RV tracing has been developed to identify inputs to projection-defined subpopulations, accordingly named TRIO (Tracing of the Relationship between Input and Output; Beier et al., 2015; Lerner et al., 2015; Schwarz et al., 2015). TRIO involves injecting a canine adenovirus (CAV) encoding the gene for Cre recombinase into a known projection target for a specific cell population. Consequently, only cells that project to this area express Cre recombinase. Helper viruses carrying Cre-dependent genes for RG and TVA (e.g., AAV-DIO-TVA and AAV-DIO-RG) are then injected into the nucleus of interest, so that only a projection-defined subset of neurons will express TVA and RG (i.e., starter cells). Thus, following injection of a modified RV (EnvA-dG-RV-GFP), only neurons that make direct monosynaptic connections onto the projection-defined neurons and the starter cell population will express a fluorescent marker protein.
This approach can be further refined to yield a third type of monosynaptic tracing that is both projection- and cell type-specific: cTRIO (cell-type specific Tracing of the Relationship between Input and Output; Beier et al., 2015). cTRIO is a technique in which a CAV vector carrying the Cre-dependent gene for Flp-recombinase (Flp) is injected into a projection target of a nucleus from a transgenic Cre-driver mouse. Much like Cre-lox recombination, Flp-FRT recombination allows for site-specific recombination of DNA sequences flanked by flippase recognition target (FRT) sites. In this way, Flp can be expressed in a population of neurons that is defined by both a cell type-specific genetic marker and a projection target. Flp-dependent viruses carrying genes for TVA and RG can then be injected into this nucleus, so that only this projection- and cell type-specific subpopulation will be susceptible to infection with a modified RV.
Experiments employing TRIO and cTRIO have found quantitative, but not qualitative, differences in the inputs to projection-defined subpopulations of VTA DA neurons. For example, VTA DA neurons projecting to the NAc lateral shell receive proportionally more input from the anterior cortex and striatal regions, but less inputs from the DRN, compared to VTA DA neurons projecting to the NAc medial shell (Beier et al., 2015). Uchida and colleagues found that VTA DA cells projecting to the posterior “tail” of the striatum form an anatomically distinct subclass within the VTA as they receive significantly less input from the ventral striatum and proportionally more input from the subthalamic nucleus, zona incerta and ventral pallidum (Menegas et al., 2015).
RV-based trans-synaptic tracing has been used to confirm many of the inputs to midbrain DA neurons previously reported using conventional tracing techniques. At the same time, however, these studies have produced a more nuanced picture of the input-output relationships of this complex system, and many important questions remain after trans-synaptic tracing approaches have revealed unexpected inputs to DA neurons that could not be validated by complimentary approaches. For example, electrophysiological recordings (Lammel et al., 2012) and electron microscopy (Carr and Sesack, 2000) have failed to provide evidence for a direct synaptic connection from the LHb onto NAc-projecting DA neurons, as well as from the mPFC onto NAc-projecting DA neurons, but their presence could be detected using cTRIO (Beier et al., 2015). In addition, RV-based trans-synaptic tracing studies consistently report that the NAc provides strong monosynaptic inputs to VTA DA neurons (Beier et al., 2015; Watabe-Uchida et al., 2012; Faget et al., 2016; Menegas et al., 2015). In contrast, studies that combine synaptic electrophysiology and optogenetics show conflicting results, with one study showing functional connections (Matsui et al., 2014) while other studies observe very little or no connectivity (Bocklisch et al., 2013; Kupchik et al., 2015; Xia et al., 2011). Several methodological differences could contribute to these apparent contradictions, including specificity of the viral injections, viral serotypes or different Cre-driver lines. Further investigations are needed to rule out false positive results and verify the presence of unexpected synaptic connections. Another important caveat of RV-based tracing is that the amount of presynaptic labeling does not convey any information about the strength of a synaptic connection (i.e., sparse labeling does not necessarily indicate a weak synaptic connection). Moreover, many brain regions that innervate VTA DA neurons are highly heterogeneous and contain neurons of variegated neurochemical identity such as the DRN, which sends not only serotonergic, but also glutamatergic projections to the VTA (Qi et al., 2014). Because trans-synaptic tracing approaches cannot detect differences in the cellular identity of direct presynaptic inputs, complementary experimental approaches involving electrophysiology, immunohistochemistry and/or in situ hybridization are necessary to fully characterize afferent inputs to midbrain DA neurons.
3. Viral vector strategies for optogenetic manipulations of midbrain dopamine neurons
Targeting midbrain dopamine neurons
Optogenetics uses photostimulation to control the activity of specific cell populations which have been genetically transduced to express microbial opsins that are activated by light. It can be used to manipulate neurons on a millisecond time scale with high spatial and temporal precision both in vitro and in freely moving animals (Deisseroth, 2011; Rajasethupathy et al., 2016; Tye and Deisseroth, 2012; Yizhar et al., 2011). An important prerequisite for optogenetic manipulations of midbrain DA neurons is cell type-specificity, which is particularly significant in the case of the VTA as it contains heterogeneous subpopulations of DAergic, GABAergic and glutamatergic neurons (Nair-Roberts et al., 2008). The most widely implemented approach for targeting midbrain DA neurons involves the Cre/lox system (Gong et al., 2007; Lindeberg et al., 2004; Pupe and Wallén-Mackenzie, 2015), in which a Cre-dependent viral vector coding for a transgene of interest (e.g., channelrhodopsin-2 [ChR2]) can be infused into the VTA or SN of a transgenic Cre-driver mouse line expressing the gene for Cre under the control of a cell type-specific promoter (see above). Using this approach, only cells expressing Cre will reverse the inverted transgene to induce expression of the functional protein. Ultimately, the integration of viral vectors with transgenic animals combines the anatomical specificity afforded by stereotaxic microinjection with the cell type-specificity that is possible with genetically engineered animals.
An important consideration of all transgenic Cre-driver lines is the actual cell type-specificity of Cre expression, which must be confirmed individually for each line. For example, in bacterial artificial chromosome (BAC) transgenic mouse lines, ectopic gene expression can occur if upstream regulatory elements are incomplete relative to those involved in regulating the endogenous gene (e.g., a distant enhancer sequence may be omitted in the BAC), leading to differential control of the transgene compared to the endogenous gene. Alternatively, depending on where the BAC integrates into the host genome, regulatory sequences and processes at the locus of integration may be different from and/or disrupt those mediated by the promoter included in the BAC to target transgene expression to specific cell types. Ectopic gene expression may also be observed in cells where the endogenous promoter is transiently activated during development, leading to Cre expression which can persist in the cytoplasm even after the promoter has been turned off. This can occur in both BAC and knock-in transgenic mice. Finally, ectopic Cre expression can also be observed in cells where expression of the endogenous protein is regulated post-transcriptionally via mechanisms like RNA interference, which would lead to mRNA transcription of both the endogenous gene and Cre, but translation of Cre only. In all of these cases, ectopic Cre expression can lead to the recruitment of neurons outside of the intended cell type, causing off-target effects and potentially confounding the interpretation of supposedly cell type-specific results (Lammel et al., 2015; Vuong et al., 2015).
Importantly, transgenic Cre-driver mouse lines expressing Cre under the control of the promoter for tyrosine hydroxylase (TH), the rate-limiting enzyme in the synthesis of DA and the gold standard for identifying DA neurons, exhibit substantial ectopic transgene expression patterns in non-DA neurons (Lammel et al., 2015). These mice exhibit Cre expression in TH-immunonegative cells that reside in the midline region of the VTA and in the interpeduncular nucleus (IPN), a brain region located just ventral of the VTA. It is surprising that some IPN neurons express TH mRNA, even though it is unlikely that these cells are bona fide DA neurons (i.e., cells that synthesize and release the neurotransmitter dopamine) as they lack detectable TH protein expression as well as other DA-specific markers (e.g., DAT, VMAT2). Nevertheless, these TH-immunonegative cells are likely to be recruited during optogenetic experiments utilizing TH-Cre mice, suggesting that TH as a molecular marker alone may not be reliable for identifying and targeting DA neurons in the ventral midbrain. On the other hand, Cre-driver mice expressing Cre under the dopamine transporter (DAT) promoter exhibit DA-specific Cre expression patterns (Lammel et al., 2015); however, these DAT-Cre mice may prove to be less amenable to studies seeking to investigate the projection from medial VTA DA neurons to the mPFC, since these neurons have been shown to lack DAT expression (Lammel et al., 2008). Recently, transgenic mice that express Cre under the control of the Pituitary homeobox 3 promoter, a developmental marker for DA neurons (Pitx3-Cre mice), have also become available (Smidt et al., 2012). Although a detailed quantitative description of the cell type-specificity of Cre expression in these mice is still lacking and additional work will be necessary to fully characterize them, initial results point to a cell population in the midline VTA that expresses Cre but lacks detectable TH protein expression (Smidt et al., 2012; Zhao et al., 2004).
In addition to transgenic Cre-driver mice, Cre-driver rats are also highly desirable because rats can carry out more complex behavioral tasks than mice and are the preferred animal model in many classical psychological assays (Zalocusky and Deisseroth, 2013). Development of these transgenic rats has been delayed because rats are less genetically tractable compared to mice, but recently a line expressing Cre under the control of the TH promoter has been developed (Witten et al., 2011). Though Cre expression in these animals has yet to be thoroughly characterized, preliminary work suggests that in rats, unlike in mice, TH mRNA expression correlates with TH protein expression (Yamaguchi et al., 2015), suggesting that non-DA specific Cre expression in the IPN is less likely to occur in these animals.
Optogenetic control of midbrain dopamine neurons
Channelrhodopsin-2, in conjunction with transgenic rodent lines, is the most commonly used opsin for photostimulation of midbrain DA neurons since it produces strong and stable photocurrents that activate cells at low to moderate frequencies with high temporal precision (Yizhar et al., 2011). The manipulations facilitated by this technology have been used to investigate the role of phasic DA activity for reward-related behaviors. According to the reward prediction error (RPE) hypothesis, midbrain DA neurons show characteristic phasic responses to rewards and reward-predicting cues, and are inhibited by aversive events (Schultz, 1997; Ungless et al., 2004). The RPE hypothesis is supported by extensive empirical data, including functional magnetic resonance imaging (fMRI) studies in humans (D’Ardenne et al., 2008; Schultz, 2016). Additional evidence for a causal role of phasic DA activity in promoting reward-related behavior comes from a landmark study by Deisseroth and colleagues, who demonstrated that phasic, but not tonic, optogenetic stimulation of VTA DA neurons is sufficient to drive behavioral conditioning (Tsai et al., 2009). Though this work was carried out using TH-Cre mice, which have since been shown to exhibit ectopic Cre expression in midline VTA and IPN cells that lack detectable levels of TH protein, the results of this study remain likely to be cell-type specific because the authors here stimulated cells in the lateral VTA which does not contain Cre positive cells that lack TH protein (Lammel et al., 2015).
Subsequent work by Uchida and colleagues employed viral vector targeting strategies and in vivo extracellular electrophysiology to identify VTA neurons encoding RPE responses in a classical conditioning task (Cohen et al., 2012). In these experiments, Cre-dependent viral vectors expressing ChR2 were infused into the VTA of DAT-Cre or VGAT-Cre mice to target DA or GABA neurons, respectively. VTA DA or GABA neurons were optogenetically “tagged” by correlating spontaneous spike waveforms and light-evoked voltage responses. As expected, VTA DA neurons showed phasic excitation after reward-predicting stimuli – a response that seems to be remarkably uniform across DA neurons in the lateral VTA (Cohen et al., 2012; Eshel et al., 2016). VTA GABA neurons, on the other hand, show increased activity during the delay of reward prediction and during its delivery (Cohen et al., 2012). Moreover, VTA GABA neurons exert strong inhibitory influence over VTA DA neurons (Matsui et al., 2014), and optogenetic stimulation of these cells disrupts reward consumption and promotes aversion-related behaviors (Tan et al., 2012; van Zessen et al., 2012). Thus, a ‘subtractive model’ has been proposed, which is based on a local inhibitory microcircuit that contributes to the computation of DA RPEs (Eshel et al., 2015). Concordant with these results is the finding that tonic optogenetic stimulation of VTA DA neurons elicits an aversive response, likely by occluding the phasic bursting activity necessary to signal the presence of a reward or a reward-associated cue (Mikhailova et al., 2016).
A second line of research by Janak and colleagues engaged TH-Cre rats in an associative blocking task (Steinberg et al., 2013). Concurrent optogenetic stimulation of VTA DA neurons with reward delivery caused a long-lasting increase in cue-elicited reward-seeking behavior. Thus, upregulation of VTA DA activity at the time of a reward mimics a positive prediction error that informs the animal’s behavior. In agreement with this line of thinking is a recent study in rhesus monkeys demonstrating that photostimulation to reward outcomes promotes learning of reward predicting stimuli (Stauffer et al., 2016). While non-human primates typically do not offer the same possibilities for cell type-specific manipulations as in rodents, this was elegantly circumvented by using a novel viral cocktail technique with DA specific opsin expression levels greater than 95% (Stauffer et al., 2016).
Conversely, photoinhibition of DA neurons can be achieved using inhibitory microbial opsins such as halorhodopsin (eNpHR3.0), archaerhodopsin (Arch), and chloride conducting channelrhodopsins (Berndt et al., 2014; Wietek et al., 2014; Yizhar et al., 2011; Zhang et al., 2007). However, it should be noted that eNpHR3.0 is probably the most suitable existing tool for optogenetic silencing of terminals, since Arch may paradoxically increase the spontaneous release of neurotransmitter in response to long periods of light stimulation, and chloride conducting channelrhodopsins may cause neurotransmitter release at light onset (Mahn et al., 2016). Inhibitory opsins have been leveraged to examine the encoding of RPE during inhibition of VTA DA neurons. Consistent with the RPE hypothesis and the ‘subtractive model’, it was shown that optogenetic inhibition of VTA DA neurons elicits conditioned place aversion (Berndt et al., 2015). Furthermore, inhibiting VTA DA cells during the delivery of a reward is sufficient to mimic a negative reward prediction error response (Chang et al., 2016). Altogether, most studies that have employed optogenetic stimulation or inhibition of VTA DA cells are in line with classical extracellular electrophysiological experiments in behaving non-human primates and with theoretical frameworks that have hypothesized a causal role for temporally precise DA signaling in cue-reward learning and RPE signaling (Schultz, 1997; Schultz, 2016; Stauffer et al., 2016).
Optogenetic control of dopaminergic projections
An important limitation of photoactivating or -inhibiting DA cell bodies is the intrinsic difficulty in determining precisely whether one specific DA subpopulation is preferentially activated over another. VTA DA neurons with distinct projection targets are generally intermingled in the VTA, even though some anatomical separation exists between DA subpopulations in the medial and lateral VTA (Beier et al., 2015; Lammel et al., 2008). For example, VTA DA cells projecting to the NAc lateral shell are predominantly located in the dorsolateral region of the VTA. If light is directed to the lateral VTA, these cells would be preferentially activated while other DA subpopulations in ventral or medial subregions of the VTA remain unaffected, and it is possible, if not likely, that such biased activation of one DA subpopulation over another may drive drastically different behavioral phenotypes. Indeed, Tye and colleagues have observed that selective photostimulation of a discrete subpopulation of DA neurons in the DRN promoted conditioned place aversion (Matthews et al., 2016), which is in stark contrast to the rewarding phenotype that has been observed in response to stimulation of DA neurons in the VTA (Tsai et al., 2009).
Given the pronounced heterogeneity of DA neurons, it is not surprising that many recent studies have begun to employ projection-specific optogenetic manipulations to parse out the different biological functions of DA subsystems. Projection-specific optogenetic manipulations can be performed by directing light stimulation to opsin-expressing axons and terminals in brain regions that are innervated by midbrain DA neurons (Tye and Deisseroth, 2012; Yizhar et al., 2011). Consistent with the idea that projection-defined DA subpopulations subserve different biological functions is the finding that photostimulation of DA projections to the NAc, but not the mPFC, is sufficient to increase social interaction. In contrast, photostimulation of projections to the mPFC promotes conditioned place aversion (Gunaydin et al., 2014). De Lecea and colleagues also carried out a detailed optogenetic dissection for DA projections to the NAc, mPFC, central amygdala (CeA), and dorsolateral striatum (DLS) to reveal a complex circuitry for regulating sleep-wake behaviors (Eban-Rothschild et al., 2016). Stimulation of NAc, CeA, and DLS, but not mPFC, projections promoted a transition from non-REM sleep to wakefulness, whereas stimulation of mPFC and CeA projections promoted a transition from REM sleep to wakefulness. Thus, projection-specific manipulations are particularly important for the elicitation of complex behaviors mediated by distinct DA subsystems. An important limitation of the latter two studies is that TH-Cre mice have been used, which show non-DA specific Cre expression patterns (see above). Additionally, some VTA DA neurons may co-release other neurotransmitters such as glutamate (Stuber et al., 2010), and optogenetic stimulation of VTA glutamate inputs in the NAc is sufficient to promote aversion-related behavior by activating striatal GABAergic interneurons (Qi et al., 2016). Thus, future studies are needed to further parse out the precise contribution of VTA neurons in multiplexed neurochemical signaling.
Optogenetic control of inputs to the VTA
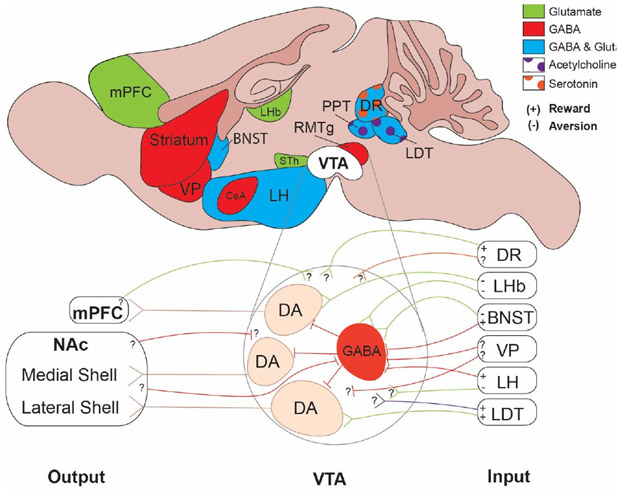
Midbrain DA neurons receive excitatory and inhibitory inputs from widespread brain regions (Yetnikoff et al., 2014). Because DA burst firing activity is highly regulated by glutamatergic afferent inputs (Grace and Bunney, 1984a, 1984b; Grace et al., 2007), an important line of research is to determine the behavioral functions and synaptic connectivity of specific afferent inputs to midbrain DA neurons (Figure 1). A high density of synaptic inputs to the VTA originates from the DRN (Beier et al., 2015; Faget et al., 2016; Watabe-Uchida et al., 2012). Optogenetic studies that stimulated DRN inputs to the VTA in freely behaving animals mainly observed reward-related behaviors, which is consistent with electrical stimulation of the DRN (Rompre and Miliaressis, 1985; Simon et al., 1976). However, some controversy remains regarding the neurochemical identity of this pathway. While one group found that VTA-projecting DRN neurons encode reward-related behaviors through both serotonin and glutamate (Liu et al., 2014), other groups reported that these neurons encode reward behaviors primarily through glutamate but not serotonin (Fonseca et al., 2015; McDevitt et al., 2014; Qi et al., 2014).
Figure 1. Input-Output relationships of dopamine neurons in the ventral tegmental area.
Top: Brain regions that send major inputs to the VTA are color coded according to the cellular identity of input neurons. Inset: detailed view of VTA functional connectivity based on recent studies that have combined synaptic electrophysiology and in vivo optogenetics. Note, that GABA neurons include those that are located in the VTA as well as RMTg. (+) and (−) indicate that optogenetic stimulation of a particular input induced reward (measured as place preference) or aversion (measured as place aversion). Question marks indicate that cell type- and/or projection-specific synaptic connectivity has not yet been established.
The mesopontine laterodorsal tegmental nucleus (LDT) and pedunculopontine nucleus (PPT) are heterogeneous brain regions located in close proximity to the DRN and provide cholinergic, glutamatergic and GABAergic input to the VTA (Wang and Morales, 2009). Classical studies that stimulated LDT neurons electrically demonstrated increased burst firing in VTA DA neurons as well as increased DA levels in the NAc (Forster and Blaha, 2000; Lodge and Grace, 2006). Consistent with this is the finding that VTA DA neurons projecting to the NAc lateral shell subregion receive substantial excitatory input from the LDT (Lammel et al., 2012). More recently, it has been reported that optogenetic stimulation of both glutamatergic and cholinergic LDT projections to the VTA is sufficient to promote reward-related behaviors in freely behaving rodents (Lammel et al., 2012; Steidl et al., 2016; Xiao et al., 2016). PPT neurons, on the other hand, appear to preferentially influence motor control via cholinergic projections to the SN, but not VTA (Xiao et al., 2016), whereas glutamatergic PPT neurons target non-DA VTA neurons, which have been demonstrated to be necessary for the acquisition of stimulus-reward associations (Yau et al., 2016).
Altogether, both DRN and LDT appear to be uniquely positioned to exercise control of burst firing via their direct excitatory projections to VTA DA neurons projecting to NAc. Since increased DA levels in the NAc promote reinforcement (Steinberg and Janak, 2013), these pathways may represent key players in the brain’s reward circuitry.
Conversely, optogenetic stimulation of inhibitory inputs to the VTA can also promote reward-related behaviors. For example, optogenetic stimulation of both BNST and LH GABAergic inputs to the VTA produces a rewarding phenotype. Given that these cells preferentially innervate non-DA VTA neurons (i.e., putative GABAergic neurons), a circuit model has been proposed where di-synaptic disinhibition of VTA DA neurons drives reward-related behaviors (Jennings et al., 2013; Nieh et al., 2016).
While most VTA DA neurons are inhibited by aversive stimuli (Ungless et al., 2004), some DA neurons in the ventromedial VTA appear to be phasically excited by aversive stimuli (Brischoux et al., 2009). This result seems to be at odds with the RPE hypothesis, but supports the notion that DA neurons form a heterogenous population tuned to either rewarding and/or aversive stimuli. In this regard an important finding is that optogenetic stimulation of specific VTA afferent pathways can produce aversion-related behaviors (Jennings et al., 2013; Lammel et al., 2012; Nieh et al., 2016; Stamatakis and Stuber, 2012). Accordingly, optogenetic stimulation of glutamatergic LHb, BNST and LH terminals in the VTA induced robust place aversion. ChR2-assisted circuit mapping revealed that these inputs target GABAergic neurons in the VTA/RMTg, indicating that di-synaptic inhibition of VTA DA neurons may contribute to the aversive behavior. It certainly can be argued that this result is consistent with the RPE hypothesis and ‘subtractive model’ (see above), but BNST, LH and LHb also send direct excitatory inputs to VTA DA neurons (Jennings et al., 2013; Lammel et al., 2012; Nieh et al., 2016; Stamatakis and Stuber, 2012). Obvious questions are whether these inputs target specific DA subpopulations and whether excitatory postsynaptic currents lead to a propagated action potential and subsequent DA release in target regions. While specific DA subpopulations have not yet been determined for LH and BNST inputs, it has been already demonstrated that LHb inputs preferentially target mesoprefrontal DA neurons (Lammel et al., 2012). Altogether, these results suggest that in addition to the anatomical origin and neurochemical identity (e.g., GABA, glutamate) of VTA afferents, synaptic connectivity in the VTA is an important determinant that can influence behavior in profoundly different ways.
Optogenetic functional Magnetic Resonance Imaging
The integration of optogenetics and behavioral paradigms has emerged as a powerful way to investigate whether stimulating or silencing a defined group of cells induces specific behavioral changes. However, this approach offers no insights into the corresponding neural activity that occurs to produce these changes. Fortunately, integration of optogenetics with electrophysiology (Cohen et al., 2012) or recently developed in vivo calcium imaging approaches (Deisseroth and Schnitzer, 2013; see below) provide the ability to observe changes in neural activity while performing optogenetic manipulations in freely moving animals. Unfortunately, however, there is a fundamental bias in the choice of which cells and brain regions are recorded and monitored using these techniques, which makes it difficult to understand whole-brain consequences of an optogenetic manipulation.
Optogenetic functional Magnetic Resonance Imaging (ofMRI) is a way of overcoming this bias. ofMRI is a recently developed technique that provides the ability to measure brain-wide activity changes as a consequence of an optogenetic manipulation (Albaugh et al., 2016; Decot et al., 2016; Ferenczi et al., 2016). In ofMRI experiments, optogenetic manipulations are performed in a custom-built animal fMRI scanner that combines the Blood Oxygen Level Dependent (BOLD) response, a measure of metabolic activity as a proxy for neural activity, with optogenetic stimulation. Like all fMRI methods, ofMRI suffers from the temporal lag that exists between changes in neuron firing and hemodynamic changes, which places the temporal resolution on the order of seconds. However, the main advantage of ofMRI is its ability to collect global, unbiased spatial information about how optogenetic manipulations affect activity throughout the brain. Moreover, because more powerful magnets can be safely used on animals than on people, the spatial resolution of ofMRI is at the submillimeter range. Thus, ofMRI has recently emerged as an integrated approach that combines local, temporally precise neuronal manipulations with global, temporally delayed brain responses. For example, ofMRI has been used to reveal that the mPFC exerts top-down control over midbrain DA interactions with the striatum and that, when elevated, activity in the mPFC can suppress natural reward-related behavior. Specifically, optogenetic manipulation of mPFC neurons using step-function opsins produced a decrease in BOLD responses in the NAc and behavioral correlates of anhedonia (i.e., reduced sucrose preference; Ferenczi et al., 2016). This groundbreaking study exemplifies how functional brain imaging and optogenetic techniques can be integrated to investigate brain-wide circuit dynamics underlying motivated behavior. Finally, fMRI in animal models has recently seen the integration of magnetically active heme proteins that bind to and signal concentrations of DA such that the BOLD signal quantitatively relays information about DA neurotransmitter concentration (Lee et al., 2014). Though this technology is in the early stages and currently requires a supply of L-DOPA and a dopamine reuptake inhibitor to maximize DA concentrations, it shows a lot of promise.
As optogenetic approaches shed light on neural circuit function in animals, it is of crucial importance to validate that analogous circuits exist in humans. For example, it has been reported that the same network comprising the LHb, VTA and mPFC previously identified in rodents (Lammel et al., 2012) is also activated during aversive processing in humans (Hennigan et al., 2015). Such translational approaches will be vital in developing new therapeutic interventions for neuropsychiatric disorders. Future research in this area will likely be directed toward overcoming some of the current limitations of these studies, including immobilization during brain imaging (animals must be sedated or extensively acclimated to the scanner head fixation device), limited temporal resolution associated with BOLD responses, and relatively coarse spatial resolution.
4. Viral vector strategies for neural activity readouts in the midbrain dopamine system
Optogenetic control over neural activity represents the most precise method currently available for functionally investigating mammalian circuits. However, it is also beset by certain limitations. First, optogenetic stimulation of axons projecting to one region may result in backpropagating action potentials that activate undesired brain areas via axon collaterals. Second, optogenetic stimulation shines light in pulses that activate all virally transduced neurons at the same time, thus possibly driving the activity of thousands of neurons in synchrony, which is unlikely to occur naturally. Third, artificially stimulating neurons is unlikely to recapitulate their endogenous, physiological patterns of activity. Because of these limitations, optogenetic studies should be complemented with investigations of the naturally occurring activity patterns of neurons during various behavioral tasks and in response to various environmental stimuli. In vivo calcium imaging allows the visualization of calcium dynamics in genetically-defined neurons with unprecedented cellular and subcellular spatial resolution (Hamel et al., 2015; Resendez and Stuber, 2015). Calcium signaling can be visualized using genetically encoded calcium sensitive fluorescent indicators that bind to intracellular Ca2+ ions (e.g., GCaMP6; Akerboom et al., 2012). To achieve cell-type specificity, Cre-dependent viral vectors encoding these calcium indicators can be injected into the brain of transgenic Cre-driver mice, resulting in transient calcium fluorescence in specific cell populations. This fluorescence signal can serve as a proxy for neural activity since periods of increased neural activity are associated with fluctuations in intracellular calcium levels, which correlate with action potential generation and neurotransmitter exocytosis (Hamel et al., 2015). There are currently three methodological approaches for performing in vivo calcium imaging: (1) in a head-fixed setup with sub-cellular resolution (two-photon calcium imaging), (2) using a miniature epi-fluorescence microscope and implanted microendoscope (one-photon calcium imaging), and (3) using an implanted optical fiber (fiber photometry). A major limitation of two-photon calcium imaging is that it currently must be performed in head-fixed animals. This greatly limits its use for investigating many standardized and previously validated rodent behavioral assays (e.g., conditioned place preference assay). In contrast, calcium imaging using microendoscopes or fiber photometry can be performed in freely moving animals, but do not offer the same sub-cellular resolution as two-photon calcium imaging. Furthermore, fiber photometry has a low spatial resolution, but offers the unique possibility of monitoring both population- and projection-specific activity signals in real time from genetically-defined cell populations.
Notably, several recent studies that have used in vivo fiber photometry to correlate a DA neuron’s projection target with its functional role have provided strong evidence for the diverse biological functions of DA signaling in the mammalian brain. Deisseroth and colleagues measured calcium dynamics in DA axons and terminals in the mPFC or NAc while administering a rewarding or an aversive stimulus. As expected, and consistent with the RPE hypothesis, DA terminals in the NAc exhibited increased activity following a rewarding stimulus and decreased activity in response to electrical foot shock. In contrast, DA terminals in the mPFC showed the opposite pattern – calcium dynamics were increased in response to foot shock but remained unaffected by a rewarding stimulus (Kim et al., 2016). In another fiber photometry study from the same group, two distinct functional classes of DA neurons were discovered. DA neurons in the medial SN projecting to the dorsomedial striatum showed a marked decrease in calcium activity in response to an aversive stimulus, whereas DA neurons in the lateral SN projecting to the dorsolateral striatum showed a significant increase (Lerner et al., 2015). This result appears to be consistent with findings of Matsumoto and Hikosaka, who reported a population of putative DA neurons in the lateral SN that increased firing in response to aversive stimuli (Matsumoto and Hikosaka, 2009). However, the projection target of these cells remains speculative, since the latter study was performed in non-human primates, which have a more widespread DA projection system compared to rodents. For example, primate mesoprefrontal DA neurons originate in both the VTA and SN (Williams and Goldman-Rakic, 1998), while in mice these cells are located selectively in the medial VTA (Lammel et al., 2008).
Using two-photon calcium imaging and fiber photometry, Howe and Dombeck found widespread populations of DA axons projecting to the dorsal striatum that displayed rapid phasic signaling associated with motor control but not unpredicted reward (Howe and Dombeck, 2016). The DA signals in the dorsal striatum were largely distinct from those that were found in the ventral striatum (i.e., NAc), which responded to unexpected reward. These findings are in agreement with another fiber photometry study by Parker et al., which also found functional specialization of DA subpopulations, observing key differences in the encoding of reward and choice in DA terminals in dorsal versus ventral striatum (Parker et al., 2016). A notable difference between the two studies is that Parker et al. observed more reward-related signaling in the dorsal striatum compared to Howe and Dombeck. However, it is possible that responses to reward exist in dorsomedial-projecting SN DA neurons (observed by Lerner et al., 2015), and in that case any difference between the two studies could be due to sampling bias. Nevertheless, these studies suggest that DA neuromodulation can differentially impact motor control and reward learning, thereby providing further evidence for a specialization of DA function that is based on a DA neuron’s projection target.
5. Conclusions and future directions
Understanding how motivational systems are organized in the brain and how they impact neural circuits that direct behavior is a major question in neuroscience. In this review, we have focused on a series of recent studies that have used viral vector strategies for anatomical and functional dissection of the midbrain DA system. Even though genetically-encodable optical tools and whole-brain mapping approaches have provided scientists with the necessary tools to unravel the precise identity and role of the various neuronal subpopulations located within the VTA and SN, understanding the role of DA circuits involved in motivated behaviors has been a challenging pursuit. We propose a projection-based model of DA function which is well suited to explain the seemingly opposing findings of electrophysiological, fiber photometry and voltammetry studies. Accordingly, phasic DA release is not uniformly broadcasted to all brain regions in response to reward, but is selectively evoked in distinct regions with respect to unpredicted reward, aversion or locomotion. A projection-based model also aligns well with the cellular heterogeneity of DA neurons in terms of projections, inputs, gene expression and electrophysiological properties. Future studies will need to investigate how co-release of glutamate and/or GABA from DA synapses contributes to motivated behaviors. Moreover, additional work will be necessary to reliably define and target midbrain DA neuronal subtypes, which will be critical for the dissection of the functions of individual, projection target-defined subgroups of neurons. Although VTA DA neurons are known to have largely non-overlapping projections, novel technologies that employ barcoding (e.g., MAPseq; Kebschull et al., 2016) could also be used to screen for more complex connectivity patterns even within the VTA. Finally, it will be necessary to develop technologies to better integrate readouts of natural neural activity with optogenetic stimulation patterns in order to drive activity in specific circuits at physiologically relevant frequencies. This information can then be leveraged toward identifying and manipulating specific DA subcircuits that are altered in disease. Given that perturbations of midbrain DA neurons are well known to be implicated in several neuropsychiatric disorders (e.g., Parkinson’s disease, depression, substance abuse disorder, schizophrenia), identifying molecular targets in specific DA subpopulations could prove critical to developing novel therapeutic interventions.
Highlights.
Viral vector strategies are discussed that have been used to dissect how different subpopulations of dopamine neurons contribute to motivated behaviors.
Acknowledgements
The authors thank James Peck, Christine Liu, Amanda Tose and Vivian J. Han for critical reading of the manuscript, and Johannes W. de Jong for designing the figure. Work in the authors’ laboratory is supported by grants from the Whitehall Foundation, Brain Research Foundation, Brain & Behavior Research Foundation (NARSAD Young Investigator Grant) and Wayne and Gladys Valley Foundation (John P. Stock Faculty Fellowship).
Abbreviations:
- VTA
Ventral Tegmental Area
- DA
dopamine
- NAc
Nucleus Accumbens
- SN
Substantia Nigra pars compacta
- PHA-L
Phaseolus vulgaris leucoagglutinin
- mPFC
medial prefrontal cortex
- LHb
lateral habenula
- DRN
dorsal raphe nucleus
- VP
ventral pallidum
- LH
lateral hypothalamus
- LDT
laterodorsal tegmental nucleus
- RMTg
rostromedial tegmental nucleus
- GABA
gamma-aminobutyric acid
- RV
rabies virus
- RG
rabies glycoprotein
- EnvA
Avian sarcoma leucosis virus- A envelope
- TVA
Tumor virus receptor A
- Cre
Cre-recombinase
- GFP
green fluorescent protein
- TRIO
tracing of the relationship between input and output
- cTRIO
cell-type specific tracing of the relationship between input and output
- CAV
canine adeno virus
- AAV
adeno-associated virus
- Flp
Flp-recombinase
- DIO
double floxed inverted open reading frame
- BAC
bacterial artificial chromosome
- IPN
interpeduncular nucleus
- TH
tyrosine hydroxylase
- DAT
dopamine transporter
- Pitx3
Pituitary homeobox 3
- ChR2
Channelrhodopsin2
- fMRI
functional magnetic resonance imaging
- RPE
reward prediction error
- eNpHR3.0
halorhodopsin
- Arch
archaerhodopsin
- CeA
central amygdala
- DLS
dorsolateral striatum
- REM
rapid eye movement
- 5HT
five hydroxytryptamine
- vGlut2
vesicular glutamate transporter 2
- PPT
pedunculopontine tegmental nucleus
- ACh
acetylcholine
- BNST
bed nucleus of the stria terminalis
- ofMRI
optogenetic functional magnetic resonance imaging
- BOLD
blood oxygen level dependent
- L-DOPA
Levodopa
- FRT
flippase recognition target
References
- Akerboom J, Chen T-W, Wardill TJ, Tian L, Marvin JS, Mutlu S, Calderón NC, Esposti F, Borghuis BG, Sun XR, et al. (2012). Optimization of a GCaMP calcium indicator for neural activity imaging. J. Neurosci 32, 13819–13840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanese A, and Minciacchi D (1983). Organization of the ascending projections from the ventral tegmental area: a multiple fluorescent retrograde tracer study in the rat. J. Comp. Neurol 216, 406–420. [DOI] [PubMed] [Google Scholar]
- Albaugh DL, Salzwedel A, Van Den Berge N, Gao W, Stuber GD, and Shih Y-YI (2016). Functional Magnetic Resonance Imaging of Electrical and Optogenetic Deep Brain Stimulation at the Rat Nucleus Accumbens. Sci. Rep 6, 31613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderegg A, Poulin J-F, and Awatramani R (2015). Molecular heterogeneity of midbrain dopaminergic neurons - Moving toward single cell resolution. FEBS Lett. 589, 3714–3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bariselli S, Glangetas C, Tzanoulinou S, and Bellone C (2016). Ventral tegmental area subcircuits process rewarding and aversive experiences. J. Neurochem 139, 1071–1080. [DOI] [PubMed] [Google Scholar]
- Beckley JT, Evins CE, Fedarovich H, Gilstrap MJ, and Woodward JJ (2013). Medial prefrontal cortex inversely regulates toluene-induced changes in markers of synaptic plasticity of mesolimbic dopamine neurons. J. Neurosci 33, 804–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, Gao XJ, Kremer EJ, Malenka RC, and Luo L (2015). Circuit Architecture of VTA Dopamine Neurons Revealed by Systematic Input-Output Mapping. Cell 162, 622–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt A, Lee SY, Ramakrishnan C, and Deisseroth K (2014). Structure-guided transformation of channelrhodopsin into a light-activated chloride channel. Science 344, 420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt A, Lee SY, Wietek J, Ramakrishnan C, Steinberg EE, Rashid AJ, Kim H, Park S, Santoro A, Frankland PW, et al. (2015). Structural foundations of optogenetics: Determinants of channelrhodopsin ion selectivity. Proc. Natl. Acad. Sci 113, 822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund A, and Dunnett SB (2007). Dopamine neuron systems in the brain: an update. Trends Neurosci. 30, 194–202. [DOI] [PubMed] [Google Scholar]
- Bocklisch C, Pascoli V, Wong JCY, House DRC, Yvon C, de Roo M, Tan KR, and Luscher C (2013). Cocaine Disinhibits Dopamine Neurons by Potentiation of GABA Transmission in the Ventral Tegmental Area. Science 341, 1521–1525. [DOI] [PubMed] [Google Scholar]
- Boyden ES (2015). Optogenetics and the future of neuroscience. Nat. Neurosci 18, 1200–1201. [DOI] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, and Ungless MA (2009). Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc. Natl. Acad. Sci 106, 4894–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, and Hikosaka O (2010). Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 68, 815–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway EM (2008). Transneuronal circuit tracing with neurotropic viruses. Curr. Opin. Neurobiol 18, 617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, and Sesack SR (2000). Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J. Neurosci 20, 3864–3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CY, Esber GR, Marrero-Garcia Y, Yau H-J, Bonci A, and Schoenbaum G (2016). Brief optogenetic inhibition of dopamine neurons mimics endogenous negative reward prediction errors. Nat. Neurosci 19, 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JY, Haesler S, Vong L, Lowell BB, and Uchida N (2012). Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature 482, 85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ardenne K, McClure SM, Nystrom LE, and Cohen JD (2008). BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science 319, 1264–1267. [DOI] [PubMed] [Google Scholar]
- Decot HK, Namboodiri VMK, Gao W, McHenry JA, Jennings JH, Lee S-H, Kantak PA, Jill Kao Y-C, Das M, Witten IB, et al. (2016). Coordination of Brain-Wide Activity Dynamics by Dopaminergic Neurons. Neuropsychopharmacol. 42, 615–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K (2011). Optogenetics. Nat. Methods 8, 26–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K (2014). Circuit dynamics of adaptive and maladaptive behaviour. Nature 505, 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K, and Schnitzer MJ (2013). Engineering Approaches to Illuminating Brain Structure and Dynamics. Neuron 80, 568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eban-Rothschild A, Rothschild G, Giardino WJ, Jones JR, and de Lecea L (2016). VTA dopaminergic neurons regulate ethologically relevant sleep–wake behaviors. Nat. Neurosci 19, 1356–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshel N, Bukwich M, Rao V, Hemmelder V, Tian J, and Uchida N (2015). Arithmetic and local circuitry underlying dopamine prediction errors. Nature 525, 243–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshel N, Tian J, Bukwich M, and Uchida N (2016). Dopamine neurons share common response function for reward prediction error. Nat. Neurosci 19, 479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faget L, Osakada F, Duan J, Ressler R, Johnson AB, Proudfoot JA, Yoo JH, Callaway EM, and Hnasko TS (2016). Afferent Inputs to Neurotransmitter-Defined Cell Types in the Ventral Tegmental Area. Cell Rep. 15, 2796–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JH (1981). Collateralization of monoamine neurons: mesotelencephalic dopamine projections to caudate, septum, and frontal cortex. J. Neurosci 1, 1361–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JH, and Loughlin SE (1982). Monoamine innervation of the forebrain: collateralization. Brain Res. Bull 9, 295–307. [DOI] [PubMed] [Google Scholar]
- Ferenczi EA, Zalocusky KA, Liston C, Grosenick L, Warden MR, Amatya D, Katovich K, Mehta H, Patenaude B, Ramakrishnan C, et al. (2016). Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science 351, aac9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL, Hjelmstad GO, Margolis EB, and Nicola SM (2007). Ventral Tegmental Area Neurons in Learned Appetitive Behavior and Positive Reinforcement. Annu. Rev. Neurosci 30, 289–316. [DOI] [PubMed] [Google Scholar]
- Fonseca MS, Murakami M, and Mainen ZF (2015). Activation of Dorsal Raphe Serotonergic Neurons Promotes Waiting but Is Not Reinforcing. Curr. Biol 25, 306–315. [DOI] [PubMed] [Google Scholar]
- Ford CP, Mark GP, and Williams JT (2006). Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J. Neurosci 26, 2788–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster GL, and Blaha CD (2000). Laterodorsal tegmental stimulation elicits dopamine efflux in the rat nucleus accumbens by activation of acetylcholine and glutamate receptors in the ventral tegmental area. Eur. J. Neurosci 12, 3596–3604. [DOI] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, and Zahm DS (2007). Glutamatergic Afferents of the Ventral Tegmental Area in the Rat. J. Neurosci 27, 5730–5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman DM, Noan D, Avale ME, Otero V, Low MJ, and Rubinstein M (2003). Transgenic mice engineered to target Cre/loxP-mediated DNA recombination into catecholaminergic neurons. Genesis 36, 196–202. [DOI] [PubMed] [Google Scholar]
- Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, and Gerfen CR (2007). Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J. Neurosci 27, 9817–9823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, and Bunney BS (1984a). The control of firing pattern in nigral dopamine neurons: burst firing. J. Neurosci 4, 2877–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, and Bunney BS (1984b). The control of firing pattern in nigral dopamine neurons: single spike firing. J. Neurosci 4, 2866–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, and Lodge DJ (2007). Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 30, 220–227. [DOI] [PubMed] [Google Scholar]
- Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, et al. (2014). Natural Neural Projection Dynamics Underlying Social Behavior. Cell 157, 1535–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel EJO, Grewe BF, Parker JG, and Schnitzer MJ (2015). Cellular Level Brain Imaging in Behaving Mammals: An Engineering Approach. Neuron 86, 140–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennigan K, D’Ardenne K, and McClure SM (2015). Distinct midbrain and habenula pathways are involved in processing aversive events in humans. J. Neurosci. 35, 198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Hjelmstad GO, Fields HL, and Edwards RH (2012). Ventral Tegmental Area Glutamate Neurons: Electrophysiological Properties and Projections. J. Neurosci. 32, 15076–15085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe MW, and Dombeck DA (2016). Rapid signalling in distinct dopaminergic axons during locomotion and reward. Nature. 535, 505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H (2016). Reward and Aversion. Annu. Rev. Neurosci 39, 297–324. [DOI] [PubMed] [Google Scholar]
- Ikemoto S (2007). Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev 56, 27–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pled KE, Kash TL, and Stuber GD (2013). Distinct extended amygdala circuits for divergent motivational states. Nature 496, 224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez B, and Han M-H (2016). Diversity of Dopaminergic Neural Circuits in Response to Drug Exposure. Neuropsychopharmacol. 41, 2424–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebschull JM, Garcia da Silva P, Reid AP, Peikon ID, Albeanu DF, and Zador AM (2016). High-Throughput Mapping of Single-Neuron Projections by Sequencing of Barcoded RNA. Neuron 91, 975–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CK, Yang SJ, Pichamoorthy N, Young NP, Kauvar I, Jennings JH, Lerner TN, Berndt A, Lee SY, Ramakrishnan C, et al. (2016). Simultaneous fast measurement of circuit dynamics at multiple sites across the mammalian brain. Nat. Methods 13, 325–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupchik YM, Brown RM, Heinsbroek JA, Lobo MK, Schwartz DJ, and Kalivas PW (2015). Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat. Neurosci 18, 1230–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Hackel O, Jones I, Liss B, and Roeper J (2008). Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron 57, 760–773. [DOI] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, and Malenka RC (2012). Input-specific control of reward and aversion in the ventral tegmental area. Nature 491, 212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, and Malenka RC (2014). Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology 76 PtB, 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Steinberg EE, Földy C, Wall NR, Beier K, Luo L, and Malenka RC (2015). Diversity of transgenic mouse models for selective targeting of midbrain dopamine neurons. Neuron 85, 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Cai LX, Lelyveld VS, Hai A, and Jasanoff A (2014). Molecular-level functional magnetic resonance imaging of dopaminergic signaling. Science 344, 533–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner TN, Shilyansky C, Davidson TJ, Evans KE, Beier KT, Zalocusky KA, Crow AK, Malenka RC, Luo L, Tomer R, et al. (2015). Intact-Brain Analyses Reveal Distinct Information Carried by SNc Dopamine Subcircuits. Cell 162, 635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner TN, Ye L, and Deisseroth K (2016). Communication in Neural Circuits: Tools, Opportunities, and Challenges. Cell 164, 1136–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeberg J, Usoskin D, Bengtsson H, Gustafsson A, Kylberg A, Suderstrom S, and Ebendal T (2004). Transgenic expression of Cre recombinase from the tyrosine hydroxylase locus. Genesis 40, 67–73. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhou J, Li Y, Hu F, Lu Y, Ma M, Feng Q, Zhang J, Wang D, Zeng J, et al. (2014). Dorsal Raphe Neurons Signal Reward through 5-HT and Glutamate. Neuron 81, 1360–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, and Grace AA (2006). The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proc. Natl. Acad. Sci 103, 5167–5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C (2016). The Emergence of a Circuit Model for Addiction. Annu. Rev. Neurosci 39, 257–276. [DOI] [PubMed] [Google Scholar]
- Mahn M, Prigge M, Ron S, Levy R, and Yizhar O (2016). Biophysical constraints of optogenetic inhibition at presynaptic terminals. Nat. Neurosci 19, 554–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, and Fields HL (2006). Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc. Natl. Acad. Sci 103, 2938–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Mitchell JM, Ishikawa J, Hjelmstad GO, and Fields HL (2008). Midbrain dopamine neurons: projection target determines action potential duration and dopamine D(2) receptor inhibition. J. Neurosci 28, 8908–8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui A, Jarvie BC, Robinson BG, Hentges ST, and Williams JT (2014). Separate GABA Afferents to Dopamine Neurons Mediate Acute Action of Opioids, Development of Tolerance, and Expression of Withdrawal. Neuron 82, 1346–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, and Hikosaka O (2009). Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature 459, 837–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews GA, Nieh EH, Vander Weele CM, Halbert SA, Pradhan RV, Yosafat AS, Glober GF, Izadmehr EM, Thomas RE, Lacy GD, et al. (2016). Dorsal Raphe Dopamine Neurons Represent the Experience of Social Isolation. Cell 164, 617–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt RA, Tiran-Cappello A, Shen H, Balderas I, Britt JP, Marino RAM, Chung SL, Richie CT, Harvey BK, and Bonci A (2014). Serotonergic versus nonserotonergic dorsal raphe projection neurons: differential participation in reward circuitry. Cell Rep. 8, 1857–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegas W, Bergan JF, Ogawa SK, Isogai Y, Umadevi Venkataraju K, Osten P, Uchida N, and Watabe-Uchida M (2015). Dopamine neurons projecting to the posterior striatum form an anatomically distinct subclass. eLife 4, e10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhailova MA, Bass CE, Grinevich VP, Chappell AM, Deal AL, Bonin KD, Weiner JL, Gainetdinov RR, and Budygin EA (2016). Optogenetically-induced tonic dopamine release from VTA-nucleus accumbens projections inhibits reward consummatory behaviors. Neuroscience 333, 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, and Ungless MA (2008). Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience 152, 1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, and Carlezon WA (2006). The Mesolimbic Dopamine Reward Circuit in Depression. Biol. Psychiatry 59, 1151–1159. [DOI] [PubMed] [Google Scholar]
- Nieh EH, Vander Weele CM, Matthews GA, Presbrey KN, Wichmann R, Leppla CA, Izadmehr EM, and Tye KM (2016). Inhibitory Input from the Lateral Hypothalamus to the Ventral Tegmental Area Disinhibits Dopamine Neurons and Promotes Behavioral Activation. Neuron 90, 1286–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakada F, and Callaway EM (2013). Design and generation of recombinant rabies virus vectors. Nat. Protoc. 8, 1583–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker NF, Cameron CM, Taliaferro JP, Lee J, Choi JY, Davidson TJ, Daw ND, and Witten FB (2016). Reward and choice encoding in terminals of midbrain dopamine neurons depends on striatal target. Nat. Neurosci 19, 845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatelli M, and Bonci A (2015). Role of Dopamine Neurons in Reward and Aversion: A Synaptic Plasticity Perspective. Neuron 86, 1145–1157. [DOI] [PubMed] [Google Scholar]
- Pupe S, and Wallen-Mackenzie Å (2015). Cre-driven optogenetics in the heterogeneous genetic panorama of the VTA. Trends Neurosci. 38, 375–386. [DOI] [PubMed] [Google Scholar]
- Qi J, Zhang S, Wang H-L, Wang H, de Jesus Aceves Buendia J, Hoffman AF, Lupica CR, Seal RP, and Morales M (2014). A glutamatergic reward input from the dorsal raphe to ventral tegmental area dopamine neurons. Nat. Commun 5, 5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Zhang S, Wang H-L, Barker DJ, Miranda-Barrientos J, and Morales M (2016). VTA glutamatergic inputs to nucleus accumbens drive aversion by acting on GABAergic intemeurons. Nat. Neurosci 19, 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasethupathy P, Ferenczi E, and Deisseroth K (2016). Targeting Neural Circuits. Cell 165, 524–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez SL, and Stuber GD (2015). In vivo calcium imaging to illuminate neurocircuit activity dynamics underlying naturalistic behavior. Neuropsychopharmacol. 40, 238–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeper J (2013). Dissecting the diversity of midbrain dopamine neurons. Trends Neurosci. 36, 336–342. [DOI] [PubMed] [Google Scholar]
- Rompre PP, and Miliaressis E (1985). Pontine and mesencephalic substrates of self-stimulation. Brain Res. 359, 246–259. [DOI] [PubMed] [Google Scholar]
- Schultz W (1997). Dopamine neurons and their role in reward mechanisms. Curr. Opin. Neurobiol 7, 191–197. [DOI] [PubMed] [Google Scholar]
- Schultz W (2016). Dopamine reward prediction-error signalling: a two-component response. Nat. Rev. Neurosci 17, 183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz LA, Miyamichi K, Gao XJ, Beier KT, Weissbourd B, DeLoach KE, Ren J, Ibanes S, Malenka RC, Kremer EJ, et al. (2015). Viral-genetic tracing of the input–output organization of a central noradrenaline circuit. Nature 524, 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon H, Le Moal M, and Cardo B (1976). Intracranial self-stimulation from the dorsal raphe nucleus of the rat: effects of the injection of para-chlorophenylalanine and of alpha-methylparatyrosine. Behav. Biol 16, 353–364. [DOI] [PubMed] [Google Scholar]
- Smidt MP, von Oerthel L, Hoekstra EJ, Schellevis RD, and Hoekman MFM (2012). Spatial and Temporal Lineage Analysis of a Pitx3-Driven Cre-Recombinase Knock-In Mouse Model. PLoS ONE 7, e42641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, and Stuber GD (2012). Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat. Neurosci 15, 1105–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer WR, Lak A, Yang A, Borel M, Paulsen O, Boyden ES, and Schultz W (2016). Dopamine Neuron-Specific Optogenetic Stimulation in Rhesus Macaques. Cell 166, 1564–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steidl S, Wang H, Ordonez M, Zhang S, and Morales M (2016). Optogenetic excitation in the ventral tegmental area of glutamatergic or cholinergic inputs from the laterodorsal tegmental area drives reward. Eur. J. Neurosci (in press) doi: 10.1111/ejn.13436 [DOI] [PubMed] [Google Scholar]
- Steinberg EE, and Janak PH (2013). Establishing causality for dopamine in neural function and behavior with optogenetics. Brain Res. 1511, 46–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg EE, Keiflin R, Boivin JR, Witten EB, Deisseroth K, and Janak PH (2013). A causal link between prediction errors, dopamine neurons and learning. Nat. Neurosci 16, 966–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Hnasko TS, Britt JP, Edwards RH, and Bonci A (2010). Dopaminergic Terminals in the Nucleus Accumbens But Not the Dorsal Striatum Corelease Glutamate. J. Neurosci 30, 8229–8233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KR, Yvon C, Turiault M, Mirzabekov JJ, Doehner J, Labouebe G, Deisseroth K, Tye KM, and Liischer C (2012). GABA neurons of the VTA drive conditioned place aversion. Neuron 73, 1173–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H-C, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, and Deisseroth K (2009). Phasic Firing in Dopaminergic Neurons Is Sufficient for Behavioral Conditioning. Science 324, 1080–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, and Deisseroth K (2012). Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat. Rev. Neurosci 13, 251–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, and Bolam JP (2004). Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science 303, 2040–2042. [DOI] [PubMed] [Google Scholar]
- Volkow ND, and Morales M (2015). The Brain on Drugs: From Reward to Addiction. Cell 162, 712–725. [DOI] [PubMed] [Google Scholar]
- Vuong HE, Perez de Sevilla Muller L, Hardi CN, McMahon DG, and Brecha NC (2015). Heterogeneous transgene expression in the retinas of the TH-RFP, TH-Cre, TH-BAC-Cre and DAT-Cre mouse lines. Neuroscience 307, 319–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall NR, Wickersham IR, Cetin A, De La Parra M, and Callaway EM (2010). Monosynaptic circuit tracing in vivo through Cre-dependent targeting and complementation of modified rabies virus. Proc. Natl. Acad. Sci 107, 21848–21853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H-L, and Morales M (2009). Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur. J. Neurosci 29, 340–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, and Uchida N (2012). Whole-Brain Mapping of Direct Inputs to Midbrain Dopamine Neurons. Neuron 74, 858–873. [DOI] [PubMed] [Google Scholar]
- Wickersham IR, Finke S, Conzelmann KK, and Callaway EM (2007). Retrograde neuronal tracing with a deletion-mutant rabies virus. Nat. Methods 4, 47–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wietek J, Wiegert JS, Adeishvili N, Schneider F, Watanabe H, Tsunoda SP, Vogt A, Elstner M, Oertner TG, and Hegemann P (2014). Conversion of channelrhodopsin into a light-gated chloride channel. Science 344, 409–412. [DOI] [PubMed] [Google Scholar]
- Williams SM, and Goldman-Rakic PS (1998). Widespread origin of the primate mesofrontal dopamine system. Cereb. Cortex 8, 321–345. [DOI] [PubMed] [Google Scholar]
- Wise RA (2004). Dopamine, learning and motivation. Nat. Rev. Neurosci 5, 483–494. [DOI] [PubMed] [Google Scholar]
- Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, Yizhar O, Cho SL, Gong S, Ramakrishnan C, et al. (2011). Recombinase-Driver Rat Lines: Tools, Techniques, and Optogenetic Application to Dopamine-Mediated Reinforcement. Neuron 72, 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Driscoll JR, Wilbrecht L, Margolis EB, Fields HL, and Hjelmstad GO (2011). Nucleus Accumbens Medium Spiny Neurons Target Non-Dopaminergic Neurons in the Ventral Tegmental Area. J. Neurosci 31, 7811–7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Cho JR, Zhou C, Treweek JB, Chan K, McKinney SL, Yang B, and Gradinaru V (2016). Cholinergic Mesopontine Signals Govern Locomotion and Reward through Dissociable Midbrain Pathways. Neuron 90, 333–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Sheen W, and Morales M (2007). Glutamatergic neurons are present in the rat ventral tegmental area. Eur. J. Neurosci 25, 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Qi J, Wang H-L, Zhang S, and Morales M (2015). Glutamatergic and dopaminergic neurons in the mouse ventral tegmental area. Eur. J. Neurosci 41, 760–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau H-J, Wang DV, Tsou J-H, Chuang Y-F, Chen BT, Deisseroth K, Ikemoto S, and Bonci A (2016). Pontomesencephalic Tegmental Afferents to VTA Non-dopamine Neurons Are Necessary for Appetitive Pavlovian Learning. Cell Rep. 16, 2699–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetnikoff L, Lavezzi HN, Reichard RA, and Zahm DS (2014). An update on the connections of the ventral mesencephalic dopaminergic complex. Neuroscience 282, 23–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Davidson TJ, Mogri M, and Deisseroth K (2011). Optogenetics in neural systems. Neuron 71, 9–34. [DOI] [PubMed] [Google Scholar]
- Zalocusky K, and Deisseroth K (2013). Optogenetics in the behaving rat: integration of diverse new technologies in a vital animal model. Optogenetics 1, 1–17. [Google Scholar]
- van Zessen R, Phillips JL, Budygin EA, and Stuber GD (2012). Activation of VTA GABA neurons disrupts reward consumption. Neuron 73, 1184–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang L-P, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, et al. (2007). Multimodal fast optical interrogation of neural circuitry. Nature 446, 633–639. [DOI] [PubMed] [Google Scholar]
- Zhao S, Maxwell S, Jimenez-Beristain A, Vives J, Kuehner E, Zhao J, O’Brien C, de Felipe C, Semina E, and Li M (2004). Generation of embryonic stem cells and transgenic mice expressing green fluorescence protein in midbrain dopaminergic neurons. Eur. J. Neurosci 19, 1133–1140. [DOI] [PubMed] [Google Scholar]