Abstract
Ficolins are collagenous lectins that bind N-acetylated glycans and participate in innate immune responses, including phagocytosis and complement activation. Related collagenous lectins such as mannan binding lectin (MBL) and surfactant proteins A and D possess antiviral activity, but this activity has not been demonstrated for ficolins. In these studies, we used purified porcine plasma ficolin α and recombinant ficolin α to assess their ability to bind and neutralize porcine reproductive and respiratory virus (PRRSV) in various assays. Recombinant ficolin α was designed with a C-terminal 6-histidine tag using a pcDNA3.1 expression vector system in CHO K1 cells. Plasma-purified and recombinant ficolin α reduced cytopathic effect of PRRSV-infected Marc-145 cells in neutralization assays and inhibited replication of infectious viral particles in a GlcNAc-dependent manner. In vitro replication determined by plaque assay was inhibited in the presence of plasma-purified ficolin α and recombinant ficolin. Immunoreactive plasma ficolin α and recombinant ficolin α also bound PRRSV-coated wells in a GlcNAc-dependent manner. These studies indicate that porcine ficolin can bind and neutralize a common arterivirus that is a major pathogen of swine.
Abbreviations: ANOVA, analysis of variance; CHO K1 cells, Chinese hamster ovary K1 cell line; CPE, cytopathic effect; ELISA, enzyme-linked immunosorbent assay; GlcNAc, N-acetyl-d-glucosamine; kDa, kilodaltons; MBL, mannan binding lectin; MALDI, matrix-assisted laser desorption/ionization; Marc-145 cells, African monkey kidney cell line; MS/MS, tandem mass spectrometry; PFU, plaque-forming units; pFCN, plasma ficolin α; pI, isoelectric point; PLSD, protected least significant difference; PRRSV, porcine reproductive and respiratory syndrome virus; rFCN, recombinant ficolin; SDS-PAGE, sodium dilauryl sulfate-polyacrylamide gel electrophoresis
Keywords: Ficolins, Mannan binding lectins, Innate immunity, Pigs, N-Acetylglucosamine, PRRSV
1. Introduction
Ficolins are complement-activating proteins (Taira et al., 2000, Matsushita et al., 2000) that bind N-acetyl groups in various saccharides, especially N-acetylglucosamine (GlcNAc) (Ohashi and Erickson, 1997, Matsushita et al., 1996, Brooks et al., 2003b, Frederiksen et al., 2005). N-Acetyl groups are present on various microbial surface glycans including peptidoglycan (van Heijenoort, 2001), chitin (Munro and Gow, 2001), lipopolysaccharide core regions (Rietschel et al., 1994), and some bacterial capsules (Altman et al., 1992). The classification of ficolins as lectins is controversial because ficolins can also bind to N-acetyl groups on various non-carbohydrate compounds (Frederiksen et al., 2005, Krarup et al., 2004, Brooks et al., 2003b) so some ficolin interactions with pathogens might be glycan independent.
Various bacteria-binding functions of human (Endo et al., 2006), murine (Ohashi and Erickson, 1998) and porcine (Brooks et al., 2003a) ficolins have been reported, but there are recent indications that ficolins might have other roles. In humans, three ficolin proteins, H-ficolin, L-ficolin and M-ficolin, have been described (Teh et al., 2000, Le et al., 1998, Matsushita et al., 1996). Human plasma H-ficolin and L-ficolin bind and opsonize apoptotic cells (Honore et al., 2007, Jensen et al., 2007, Kuraya et al., 2005) and necrotic cells (Honore et al., 2007, Jensen et al., 2007) and can interact with DNA (Palaniyar et al., 2004, Jensen et al., 2007). Serum ficolin is reduced, while L- and H-ficolins are increased, in the placentas of women with preeclampsia, suggesting that ficolin might mediate a pro-inflammatory cascade (Wang et al., 2007). Ficolin-α and ficolin-β, which were the first ficolins characterized, were cloned from a porcine uterus cDNA library (Ichijo et al., 1993). Ficolin α is mainly a plasma protein produced in substantial amounts by the porcine liver (Ohashi and Erickson, 1998) but few microbial binding targets have been identified (Brooks et al., 2003b). Porcine ficolin β is expressed in neutrophils and secreted when neutrophils are activated, but the binding functions of ficolin β are unknown (Brooks et al., 2003c).
The ability to recognize aberrant host molecules suggests that ficolins might also bind some viruses that can present host glycans on their surfaces (Vigerust and Shepherd, 2007). Viral glycoproteins are often decorated with complex-type oligosaccharides, which are capped by two terminal GlcNAc residues (Ansari et al., 2006, Balzarini, 2007). Other related collagenous lectins bind surface glycoproteins on influenza A virus and human immunodeficiency virus (Ezekowitz et al., 1989), herpes simplex virus (Gadjeva et al., 2004) and non-enveloped rotavirus (Reading et al., 1998). MBL (Hartshorn et al., 1994, Kawai et al., 2007), SP-D (Hartshorn et al., 1997, Hawgood et al., 2004), SP-A (Hawgood et al., 2004, Benne et al., 1995) and bovine conglutinin (Kawai et al., 2007) inhibit influenza virus hemagglutination and infectivity, and MBL also promotes complement-mediated lysis of infected cells (Reading et al., 1995). In vivo and in vitro studies have shown that strains of influenza A virus vary with respect to their susceptibility to collectin-mediated neutralization and/or destruction; this variation relates to the level of glycosylation of the hemagglutinin molecule (Hartley et al., 1997).
To examine the possible ability of ficolins to interact with viruses, we studied the interactions between porcine ficolin α and porcine reproductive and respiratory syndrome virus (PRRSV), an important swine pathogen responsible for substantial economic losses for swine producers worldwide (Neumann et al., 2005). PRRSV is an enveloped, single-stranded, positive-sense RNA virus of 40–70 nm diameter, and undergoes a high rate of mutation underlying variations in antigen expression, plaque size, pathogenicity, and production of neutralizing antibodies (Rossow, 1998). We used ficolin purified from porcine plasma (predominantly ficolin α) (Brooks et al., 2003a, Brooks et al., 2003b) and also GlcNAc-affinity-purified His-tagged recombinant porcine ficolin α (rFCN) to show that GlcNAc-dependent PRRSV binding activity of porcine plasma ficolin is not explained by ficolin β or other GlcNAc-binding proteins that might co-purify with plasma ficolin α.
2. Materials and methods
2.1. Reagents and media
All chemicals utilized were obtained from Fisher Scientific (Ottawa, Ont., Canada) except as noted. 50:50 D-MEM: F-12 media, 5% fetal bovine serum, 2 mM l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin were purchased from Sigma (Oakville, Ont.) and Opti-MEM medium was purchased from Invitrogen (Burlington, Ont.). Immobiline Drystrip Gels, IPG buffer and high molecular weight markers were obtained from Amersham Biosciences Inc. (Baie D’Urfe PQ). Whole blood was collected from healthy adult Yorkshire cross pigs into 3.8% buffered sodium citrate (pH 7.4, 9:1 blood to citrate ratio by volume). Platelet-poor plasma was isolated by centrifugation (1000 × g) for 30 min at room temperature, and stored at −70 °C until used. Ficolin was purified from pig plasma using affinity chromatography with N-acetylglucosamine (GlcNAc)-conjugated AF Epoxy 650 M Toyopearl beads (Tosoh Bioscience LLC, Montgomeryville, PA) as previously described (Brooks et al., 2003b). Rabbit anti-porcine ficolin antiserum was previously prepared against affinity-purified 38–42 kDa subunit bands of plasma ficolin (DeLay, 1999, Brooks et al., 2003b). Native anti-porcine ficolin antiserum was prepared as previously described (Brooks et al., 2003a). Goat anti-rabbit polyclonal horseradish peroxidase (HRP) was obtained from the Dako Corporation (Santa Barbara, CA).
2.2. Virus assays
The possible interactions between porcine ficolin α and PRRSV were examined using three different assays that examined the ability of ficolin to reduce cytopathic effect (CPE) in virus infected cells (neutralization assay), reduce replication of viral particles (plaque assay) and directly bind virus-coated wells (ELISA). Marc-145 cells (Kim et al., 1993) were grown as monolayers in Dulbecco's modified Eagle's medium supplemented with 5% fetal bovine serum, 2 mM L-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin (Sigma, Oakville, Ont.). The cells were maintained at 37 °C with 5% CO2. Stocks of PRRSV (North American genotype strain PA8) (Wootton et al., 2000) were prepared in Marc-145 cells, and purified on sucrose cushion gradients. Specifically, Marc-145 cells were infected with PA8 stock at 200 TCID50 and incubated at 37 °C with 5% CO2 for 72 h. The infected cell monolayers were then stored at −70 °C, and released from the cells by three freeze–thaw cycles. These lysates were then layered on 10–60% (w/w) sucrose gradients and centrifuged at 90,000 × g at 4 °C for 4 h. The purified virus band was collected. Centrifugation was performed at 90,000 × g for 1 h to pellet the purified virus, and the pellet was resuspended in 1 ml PBS. Neutralization of CPE was assayed in Marc-145 cells in 96-well plates infected with wild-type PRRSV (PA8) stock diluted in D-MEM to make 200 plaque-forming units (PFU) in a 50 μl volume. The diluted virus was pre-mixed with 0–100 μg/ml of purified plasma ficolin in 50 μl volumes. Mixtures were then incubated for 1 h at 37 °C, and approximately 2 × 105 Marc-145 cells in 100 μl were added to each well. At 3 days post-infection, individual wells were scored by CPE (Lee and Yoo, 2006).
The effect of ficolin α on the formation of infectious PRRSV particles via plaque formation was also assessed. In initial experiments, Marc-145 cells were infected with 10-fold serial dilutions of purified PA8 for 1 h at 37 °C. Once the concentration of infectious viral particles was determined, all subsequent plaque assays used a 10−4 dilution of virus stock (∼5 × 105 PFU/ml). Cells were infected with virus alone, or pre-incubated for 20 min at room temperature with various concentrations of pFCN or rFCN (0–10 μg/ml). In competition assays, 100 μl aliquots of pFCN and rFCN were pre-incubated for 20 min with either 100 μl of 100 mM GlcNAc or 100 μl of a 1:5000 dilution of native anti-porcine ficolin antibody, before incubation with the virus, and subsequent infection of the cells. After the 20 min incubation period, the infected cell monolayers were washed with PBS once, and overlaid with D-MEM with 5% FBS containing 0.8% SeaPlaque agarose (FMC Bioproducts, Rockland, ME). After 72 h, the agarose plugs were removed and the cell monolayer was incubated with staining solution (20% formaldehyde, 9.0% ethanol, and 0.1% crystal violet) for 30 min at room temperature. The cells were washed gently with water to remove excess dye and air dried to examine and count the plaques.
To determine if ficolin α could directly bind to PRRS virions, 96-well, at-bottom polystyrene tissue culture plates (Costar, Fisher-Scientific, Mississauga, Ont.) that do not bind ficolin were coated with 100 μl of ∼5 × 105 PFU/ml PA8 PRRSV or 5% BSA diluted in PBS. After overnight incubation at 4 °C, wells were blocked with 5% BSA for 60 min at room temperature, washed with PBS a minimum of 3 times, and then incubated for 2 ± 8 h with 100 μl of various dilutions of pig plasma (PP), ficolin-depleted plasma (FDP), pFCN or rFCN. After three washes with PBS, the presence of immunoreactive bound ficolin was detected by incubating with 100 μl of 1:5000 dilution of native polyclonal anti-ficolin antibody for 1 h, followed by 100 μl of a 1:5000 dilution of peroxidase-conjugated anti-rabbit immunoglobulin (Dako, Santa Barbara, CA). After further washing with PBS, bound anti-ficolin antibody was measured colorimetrically after incubation with SureBlue TMB substrate and Stop solution (Mandel, Guelph, Ont.). In some experiments, the 100 μl aliquots of various test protein preparations were pre-incubated with 100 μl of 100 mM GlcNAc (Sigma, Oakville, Ont.) or 100 μl of a 10−4 dilution of native anti-ficolin antibody, before incubating with the PRRSV-coated plates.
2.3. Recombinant porcine ficolin α
Plasmid Constructs: Blunt-end PCR products of the complete coding region of porcine ficolin α were generated by PCR with Advantage® cDNA PCR kit (Clontech, Mountain View, CA) and corresponding 5′- and 3′-primers (Forward primer: 5′-CACCATGGACACACGCGGAGTG-3′, Reverse primer: 5′-GCCCTGAAAATAAAGATTCTCCGTGGCCCGAAACTTCAT-3′). The PCR-generated DNA fragments containing a CACC sequence in front of the start codon, a Kozak sequence but no stop codon, were subcloned into a mammalian expression vector pcDNA™3.1D/V5-His TOPO using a TA directional cloning kit (Invitrogen, Burlington, Ont.). The expression construct contained cDNA encoding porcine ficolin α with a C-terminal fused segment encoding a V5 epitope of 14 amino acids followed by six histidines (6 × His) and a TEV protease recognition site (ENLYFQG, encoded from the reverse primer sequence: GCCCTGAAAATAAAGATTCTC), in order to cleave the tags if necessary. The tags and spacer regions add approximately 5 kDa to the ficolin α polypeptide. The PCR-amplified product was TOPO-cloned into the pcDNA3.1D/V5-His-TOPO vector (Invitrogen, Burlington, Ont.), yielding the pcDNA3.1D/V5-His-ficolin α expression construct. The construct was transformed and propagated in chemically competent E. coli (One Shot TOP10 E. coli, Invitrogen, Burlington, Ont.). Large-scale purification of plasmids was carried out using a Maxi-Prep kit (Qiagen, Mississauga, Ont.) according to the manufacturer's instructions. The presence of the correct cDNA ficolin sequence was verified by DNA sequencing (Guelph Molecular Supercentre, Guelph, Ont.) using the following primers: T7 extended forward primer 5′-TAA TAC GAC TCA CTA TAG GGA GAC-3′, BGH extended reverse primer 5′-TAG AAG GCA CAG TCG AGG ATG-3′.
Chinese hamster ovary cells (CHOK1) cells were cultured in 50:50 D-MEM: F-12 media (Sigma, Oakville, Ont.), supplemented with 5% fetal bovine serum, 2 mM l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin (Sigma, Oakville, Ont.). The expression plasmid was transfected into CHO K1 cells using a cationic lipid-based transfection reagent (Lipofectamine 2000, Invitrogen, Burlington, Ont.) according to the manufacturer's recommendations. For each transfection reaction, a 3:1 ratio of vector to transfection reagent was utilized, with each being mixed with 2 ml of serum-free Opti-MEM (Invitrogen, Burlington, Ont.). After a 20 min incubation, the DNA–lipofectamine complexes were then added to 75-cm2 flasks (Corning, Fisher-Scientific, Mississauga Ont.) of CHO K1 cells that were 75–85% confluent (approximately 1 × 106 cells/ml). Cells were incubated for up to 48 h at 37 °C, 5% CO2. CHO K1 cells, treated only with empty liposomes, were cultured in parallel and provided negative control supernatant and cells. Transfection efficiency was measured by utilizing the manufacturer-supplied Lac Z expression vector (Invitrogen, Burlington, Ont.). Supernatants were collected and recombinant ficolin α (rFCN) was purified with affinity chromatography on Nickel-charged Probond columns (Invitrogen, Burlington, Ont.), followed by purification on GlcNAc-conjugated Toyopearl beads (Brooks et al., 2003b) and proteins were dialyzed (Novagen, Madison WI) overnight in 10 volumes of PBS at 4 °C. Protein concentrations were determined using the Bradford protein assay (BioRad, Mississauga, Ont.).
2.4. Characterization of rFCN
Purity, higher-order oligomerization and function of rFCN were evaluated by electrophoresis. Eluted proteins were supplemented with a cocktail of protease inhibitors (Complete; Roche Molecular Biochemicals, Mississauga, Ont.) and run on 1-dimensional, 2-dimensional, and non-denaturing 4–12% gradient polyacrylamide gels. Briefly, eluates were first characterized by 12% one-dimensional SDS-PAGE (with a 4% stacking region) in reducing conditions (Laemmli, 1970) using the Bio-Rad modular Mini-Protean II system (BioRad, Mississauga, Ont.). Pre-stained broad range markers (BioRad, Mississauga, Ont.) were used to identify apparent molecular weights of isolated proteins. Electrophoresis was carried out at 4 °C for 1.5 h at 150 V. The resulting protein band at approximately 44 kDa was silver-stained and excised, then stored in 2% acetic acid before protein identification via MALDI-MS/MS peptide mass fingerprinting (Mass Spectrometry Facility, Hospital for Sick Children, Toronto, Ont.). Non-denaturing PAGE (4–12% acrylamide gradient) was performed as described (Van Leuven et al., 1981).
For Western blots, proteins separated by 1D-PAGE were electroblotted onto nitrocellulose membranes. Membranes were blocked in PBS-T (PBS pH 7.4 containing 1% BSA, and 0.1% Tween-20) and exposed to rabbit anti-ficolin polyclonal antibody (DeLay, 1999) or anti-V5 antibody conjugated with HRP (Invitrogen Burlington, Ont.), diluted 1:5000 in PBS-T. Membranes were washed in a minimum of five changes of PBS-T. If anti-ficolin antibody was the primary antiserum, the blot was then exposed to goat anti-rabbit immunoglobulins conjugated to HRP (Dako, Santa Barbara, CA) (1:5000 dilution in PBS-T). Western blots were then developed by chemiluminescence.
For 2D-PAGE, eluates were denatured in rehydration buffer (8 M urea, 2% CHAPS, 5% IPG buffer, 0.2% dithiothreitol and bromophenol blue) at room temperature for 1 h, then applied to Immobiline DryStrip gels (pH 3–11 nonlinear). Strips were rehydrated overnight (∼12 h) before first dimension isoelectric focusing was performed according to manufacturer-recommended protocols on an IPGphor Isoelectric Focusing System (GE Healthcare, Oakville, Ont.). Proteins were resolved on 12% SDS polyacrylamide gels (2nd-dimension) according to manufacturer's recommended protocols for the IPGphor system.
2.5. Bacterial binding assays
To determine if rFCN purified by affinity chromatography was functional, as compared to the previously characterized bacterial binding functions of GlcNAc-affinity-purified porcine plasma ficolin, binding assays were performed using two bacterial species previously shown to bind plasma-purified ficolin α (Actinobacillus pleuropneumoniae 5b and Salmonella enteritidis) (Nahid and Sugii, 2006, Brooks et al., 2003a). Pig plasma (500 μl) or 100 μg of either affinity-purified pFCN or rFCN were incubated with 108 colony-forming units (CFU) of bacteria in 1.5 ml overnight at 4 °C. Bacteria were pelleted and resuspended in PBS 4 times, and bound proteins were eluted from the bacterial surface with 300 mM GlcNAc. All eluants were separated by 1D-SDS-PAGE, and were electrotransferred to a nitrocellulose membrane (Trans-Blot, BioRad, Mississauga, Ont.) in a 25 mM Tris–HCl buffer, containing 100 mM glycine and 20% methanol, at 4 °C for 1 h at 100 V, and incubated overnight in PBS containing 5% BSA, to block non-specific binding. Bound ficolin was detected by immunoblots with polyclonal anti-ficolin antibody (pFCN and rFCN) or anti-V5 antibody (rFCN only). After incubation with primary antiserum for 1 h, immunoblots were washed twice for 15 min in PBS containing 0.01% Tween-20 (PBS/Tween) and incubated for 45 min in a 1:5000 diluted secondary antibody, peroxidase-labelled goat polyclonal anti-rabbit IgG, H + L (Biomeda Corp, Foster City, CA). All antibodies were diluted in PBS, 0.01% Tween-20 and 1% BSA. After washing 4 times for 15 min in PBS/Tween, the signal was revealed using a chemiluminescence substrate (ECL, Amersham Biosciences Inc., Baie d’Urfe, QC) following the manufacturer's recommendations.
2.6. Data analysis
Data are presented as means (±standard deviations) from either duplicate or triplicate experiments with 2–4 replicates per experiment. Parametric data were analyzed by one-way analysis of variance (ANOVA). Multigroup comparisons were analyzed using the Fisher protected least significant difference (PLSD) test. ED50 values were calculated using Probit analysis. Statistical analyses were performed using Statview software (Cary, NC) with an α of 0.05.
3. Results
Purified porcine plasma ficolin α (pFCN) at concentrations of 0.1–100 μg/ml significantly (p < 0.0001) reduced the numbers of cytopathic foci induced by 200 PFU of PRRSV in Marc-145 cells (Fig. 1 ), consistent with methods previously described (Lee and Yoo, 2006). Similarly, pFCN (0.001–10 μg/ml) had a significant (p < 0.0001) dose-dependent inhibitory effect on the numbers of PRRSV lytic plaques induced in Marc-145 cells (Fig. 2 ). The mean viral titer of 10.4 × 105/ml, as assayed by plaque counts in the presence of 0 μg/ml pFCN, was reduced to 8.25 × 105/ml in the presence of only 0.001 μg/ml of pFCN (p < 0.01). The greatest reduction in the formation of infectious viral particles detected in the plaque assay was observed in the presence of 10 μg/ml of pFCN, where the infectious mean viral titer was reduced to 1.45 × 105/ml (p < 0.0001). This inhibitory effect of purified plasma ficolin was substantially diminished when 10 μg/ml pFCN was pre-incubated with 100 mM of GlcNAc (p < 0.01) (Fig. 2b). To determine whether pFCN could bind to PRRSV, the amounts of immunoreactive pFCN bound to PRRSV-coated wells was measured by ELISA (Fig. 3 ). Immunoreactive pFCN bound PRRSV in a dose-dependent manner (p < 0.0001), but binding above background levels was only obtained at higher concentrations (10 and 100 μg/ml of pFCN). When pFCN was pre-incubated with either 100 mM GlcNAc or 10−4 anti-ficolin antibody, the amounts of immunoreactive ficolin bound to PRRSV-coated wells was reduced at ficolin concentrations of 10 μg/ml (p < 0.001) and 100 μg/ml (p < 0.0001) (Fig. 3).
Fig. 1.
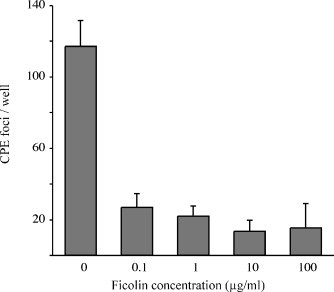
Neutralizing effect of GlcNAc-affinity-purified porcine plasma ficolin (pFCN) on numbers of cytopathic foci observed by light microscopy of monolayers of Marc-145 cells at 72 h after being infected with PRRSV. Values are means (±S.D.) of four replicates per treatment in one experiment, repeated twice. The reduction in CPE was significant at all concentrations of ficolin (p < 0.0001).
Fig. 2.
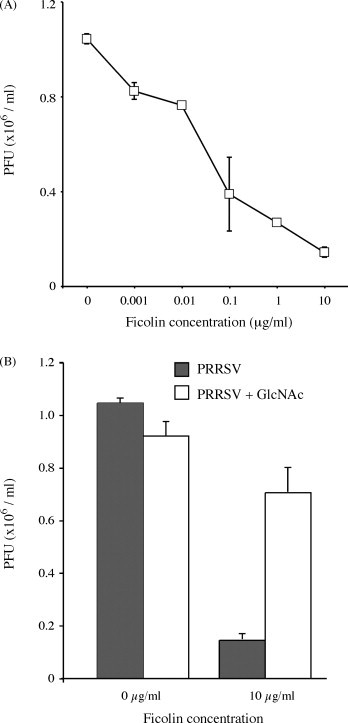
Neutralizing effect of GlcNAc-affinity-purified porcine plasma ficolin (pFCN) on numbers of lytic plaques observed in stained monolayers of Marc-145 cells 72 h after being infected with ∼5 × 105 PFU of PRRSV. Numbers of PRRSV-induced plaques were significantly reduced at all concentrations of pFCN preparations between 0.001 and 10 μg/ml (Panel A), with an ED50 of 0.055 μg/ml (p = 0.0016). The differences between virus alone and the two lowest concentrations of ficolin (0.001, 0.01 μg/ml) was significant with a p < 0.01, while the remaining differences were more highly significant with a p < 0.0001. The effect of pFCN (10 μg/ml) on PRRSV-induced plaques (solid bars) was substantially reduced in the presence of 100 mM GlcNAc (open bars) (p < 0.01) but GlcNAc had no effect on PRRSV alone (Panel B).
Fig. 3.
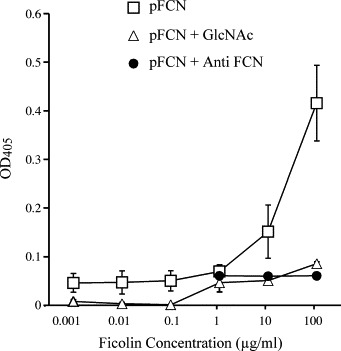
Binding of various concentrations of pFCN to PRRSV-coated wells, detected by ELISA with polyclonal antibody to ficolin. At pFCN concentrations of 10 or 100 μg/ml, binding of pFCN to PRRSV-coated wells was significantly (p < 0.0001) reduced by pre-incubation with 100 mM GlcNAc or anti-ficolin antibody. Points are means (±S.D.) from three separate experiments, each performed in triplicate.
Affinity-purified preparations of porcine plasma ficolins contain additional minor bands at ∼85 and ∼25 kDa consistent with heavy and light chains of IgM as described previously (Brooks et al., 2003b). These do not react in Western blots with anti-ficolin antibodies that were raised against the major ficolin subunit extracted from PAGE lanes (DeLay, 1999). However, we were concerned that some of the PRRSV neutralizing effects of purified pFCN might result from contaminants that co-purified from plasma (e.g. ficolin β, IgM, mannan-binding lectins, etc.), so we prepared recombinant porcine ficolin α (rFCN) and purified it by nickel- and GlcNAc-affinity chromatography. The recombinant porcine ficolin α was cloned into a mammalian expression vector and produced in CHO K1 cells in order to enhance protein glycosylation and folding, similar to that of the native protein. Recombinant ficolin α (rFCN) purified by affinity chromatography from culture supernatants of transfected CHO K1 cells contained an approximately 44 kDa reduced band (Fig. 4A, lane 2) that was consistent with porcine ficolin α by MALDI-TOF trypsin fragment mass fingerprint (5 peptides at 894.3856, 1045.474, 1123.5258, 1618.7651, 3069.4492) with over 95% confidence. The ∼44 kDa immunoreactive band that migrated just above pFCN was detected on immunoblot with anti-ficolin antibody (Fig. 4B, lane 2). As expected, only rFCN was detected by anti-V5 antibody that recognized the C-terminal tag of the recombinant protein (Fig. 4B, lane 4). The addition of the TEV protease recognition site (ENLYFQG), V5 epitope (GKPIPNPLLGLDST) and 6 histidine residues on the recombinant protein likely accounts for the apparent molecular weight difference between pFCN and rFCN. Affinity-purified preparations of porcine plasma ficolins contain additional bands at 29 and 81 kDa (Fig. 4A, lane 1) consistent with heavy and light chains of IgM, as described previously (Brooks et al., 2003b). An additional band of ∼65 kDa is noted in the rFCN preparations and cross-reacts with both anti-ficolin and anti-V5 antibodies (Fig. 4B, lanes 2 and 4). When separated by non-denaturing (native) PAGE, both pFCN and rFCN preparations contained high molecular weight bands consistent with formation of higher-order oligomers (Fig. 5 ) (Brooks et al., 2003b). pFCN preparations (lane 3) contain a band at approximately 800 kDa as well as an additional band at approximately 1000 kDa, while rFCN preparations (lane 5) reveal multiple bands from approximately 750–850 kDa (Fig. 5). Lanes 1 and 2 are molecular weight markers specifically for non-denaturing gels (Amersham Biosciences), while lane 4 contains GlcNAc-affinity-purified recombinant Lac Z CHO K1 supernatant, which was loaded as a negative control, as it was not expected to bind GlcNAc.
Fig. 4.
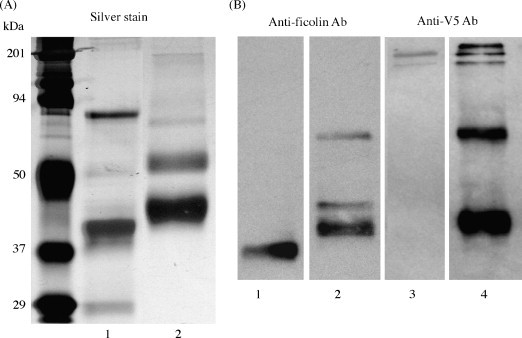
Comparison of immunoreactive ficolin subunits of GlcNAc-affinity-purified pFCN and rFCN under 12% SDS-PAGE (reducing) conditions. Preparations of porcine ficolin α purified from plasma (pFCN) contain a major ∼40 kDa band in a 38–42 kDa triplet (A, lane 1) that is immunoreactive with antibodies to ficolin (B, Lane 1) consistent with previous studies (Brooks et al., 2003a, Brooks et al., 2003b). By comparison, GlcNAc-affinity-purified rFCN migrated mainly around 44 kDa (A, lane 2), and was recognized by polyclonal anti-ficolin antibody (B, lane 2) or anti-V5 antibody which detects the C-terminal tag of rFCN (B, lane 4). Plasma-purified ficolin was not detected with anti-V5 antibody (B, lane 3).
Fig. 5.
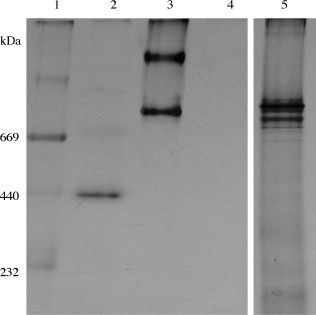
Comparison of high molecular weight forms of bands (∼800 kDa) in lanes of GlcNAc-affinity-purified pFCN (Lane 3) and rFCN (Lane 5) in silver-stained non-denaturing PAGE (4–12%). Lane 1 is MW markers; Lane 2 is Ferritin (440 kDa); Lane 4 is GlcNAc-eluted proteins from recombinant LacZ without rFCN.
Isoelectric points of pFCN and rFCN were similar on silver-stained gels although pFCN had five spots (∼40 kDa, pI 5.2–6.0) compared with only three visible forms of rFCN (∼44 kDa, pI 5.5–5.7) (Fig. 6 ). Vertical streaking, a characteristic elecrophoretic property of plasma ficolins (Brooks et al., 2003b) was observed with pFCN, but was less apparent with rFCN.
Fig. 6.

Comparison of the forms of GlcNAc-affinity-purified pFCN (A) and rFCN (B) in silver-stained two-dimensional SDS-PAGE (15%) gels. For pFCN, five spots with typical vertical streaking were visible at ∼40–42 kDa between pI 5.2 and 6.0. For rFCN, three less abundant spots at ∼44 kDa in the pI range of 5.5–5.7 were detected.
Bacterial binding properties of rFCN were similar to those of pFCN. Ficolins that were affinity column-purified from either pig plasma (pFCN) or CHO K1 supernatants (rFCN) were similarly eluted with 300 mM GlcNAc from intact Actinobacillus pleuropneumoniae serotype 5b (APP 5b) and Salmonella enteritidis (Fig. 7 ). Immunoreactive ficolin was detected at approximately 40–42 kDa for pFCN and 44 kDa for rFCN. Additional higher molecular weight immunoreactive bands were seen in both preparations.
Fig. 7.
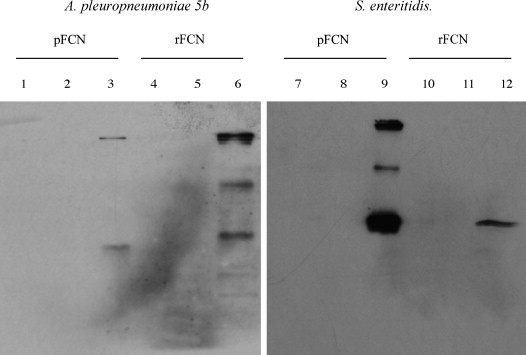
Comparison of the bacterial-binding activity of GlcNAc-affinity-purified pFCN and rFCN in suspension cultures of intact bacteria (Actinobacillus pleuropneumoniae serotype 5b and Salmonella enteritidis) that are known to bind porcine ficolin α. Bound proteins were eluted with GlcNAc (300 mM) after prior washing to remove non-specifically bound proteins, and eluted proteins were separated by12% SDS-PAGE (reducing) and detected by immunoblots with polyclonal anti-ficolin antibody. Lanes 1, 4, 7 and 10 are unbound fractions, lanes 2, 5, 8 and 11 are the final wash fractions, and lanes 3, 6, 9 and 12 are the GlcNAc-eluted proteins.
In plaque assays, pFCN and rFCN both reduced formation of infectious PRRSV in Marc-145 cell monolayers (Fig. 8A). Pre-incubation with 10 μg of pFCN reduced plaques by 93% from 21.2 × 104 to 2.0 × 104 (p = 0.002), whereas the same amount of rFCN reduced plaques by 70% to 5.8 × 104/ml (p = 0.009) (Fig. 8B). The inhibitory effects of both pFCN and rFCN were almost completely negated by co-incubation of ficolin with 100 mM GlcNAc or anti-ficolin antibody (p < 0.01) (Fig. 8A and B). The numbers of plaques formed in the presence of virus with 100 mM GlcNAc or virus with anti-ficolin antibody did not differ significantly (p > 0.05) from those that also included pFCN or rFCN (Fig. 8).
Fig. 8.
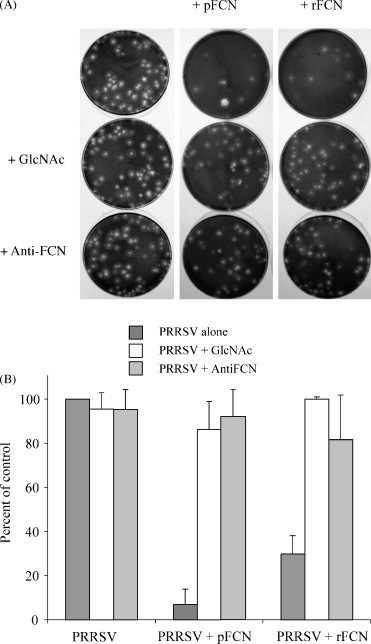
Inhibition of PRRSV replicative efficiency by pFCN or rFCN in plaque assays. Panel A shows plaques in monolayer cultures of Marc-145 cells in 60 mm dishes fixed and stained with crystal violet. Panel B shows numbers of plaques per dish expressed as a percent (±S.D.) of numbers of plaques in cultures with PRRSV alone. Cultures were incubated for 72 h after infection with PRRSV alone, or with PRRSV pre-incubated with either 100 mM GlcNAc or anti-ficolin antibody. The number of visible plaques were significantly reduced in the presence of either 10 μg/ml of pFCN (p = 0. 002) or rFCN (p = 0.009), and their effects were similarly blocked by pre-incubation of PRRSV with either 100 mM GlcNac or anti-ficolin antibody. Each bar is the mean (±S.D.) from three separate experiments, each culture in duplicate.
Pre-incubation with 100 mM GlcNAc reduced the amounts of immunoreactive ficolin detected in PRRSV-coated wells incubated with pig plasma (PP), affinity-purified ficolin (pFCN) and rFCN (Fig. 9 ) (p < 0.0001). By comparison, there was no GlcNAc-competible binding of immunoreactive ficolin in virus-coated wells incubated with ficolin-depleted plasma (FDP), nor in wells which were coated with mock-infected Marc-145 cell lysates (mock virus) or GlcNAc affinity fraction from the recombinant LacZ transfected CHO K1 cell control (mock lysate) (Fig. 9).
Fig. 9.
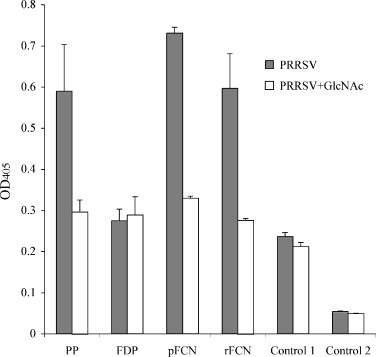
Binding (means ± S.D.) of immunoreactive ficolin to PRRSV-coated wells, detected by ELISA with polyclonal antibody to ficolin. Significant (p < 0.0001) GlcNAc-competible binding above wells containing 100 mM GlcNAc pre-incubation (open bars) was observed for pig plasma (PP), GlcNAc-affinity-purified plasma ficolin (pFCN), and recombinant porcine ficolin α (rFCN). There was insignificant GlcNAc-competible binding of immunoreactive ficolin observed in ficolin-depleted plasma (FDP), in lysate from Marc-145 cells without PRRSV (control 1) and in lysates from Lac Z-transfected CHO K1 cells without FCN (Control 2). Bars are from three separate experiments, each performed in triplicate.
4. Discussion
These studies provide initial evidence that porcine plasma ficolin can bind PRRSV in a GlcNAc-dependent manner, and that this binding can reduce infection of Marc-145 cells. This suggests that ficolin may have the ability to neutralize PRRSV and reduce its replication but the biological significance of such ficolin binding is still unknown. Ficolin binding and neutralization of PRRSV in these in vitro studies occurred in concentration ranges (1–100 μg/ml) that correspond to concentrations of 10–80 μg/ml that have been reported for plasma ficolins in pigs (Ohashi and Erickson, 1998, Seebaransingh, 1999). The cytopathic effect, lytic plaque effect and direct binding to PRRSV were all reduced substantially in the presence of purified plasma ficolin and recombinant ficolin α, and these effects were counteracted by GlcNAc. This indicates that the inhibitory activity of ficolin is dependent on its N-acetyl-binding functions (Krarup et al., 2004, Brooks et al., 2003a).
While the PRRSV inhibitory effect of ficolin purified from pig plasma was inhibited by GlcNAc and by an antibody that binds pig ficolin α and β (Brooks et al., 2003a, Brooks et al., 2003b), we were concerned that the effect could be explained by other plasma proteins such as ficolin β, mannan-binding lectin (MBL) or immunoglobulin that bind GlcNAc and co-purify with ficolin α (Brooks et al., 2003a). However, we produced recombinant porcine ficolin α, and showed that it had similar inhibitory and binding effects as we observed with pig plasma ficolins, and these were similarly inhibited by GlcNAc and ficolin-specific antiserum. The preparation of recombinant ficolin α (rFCN) was functionally similar to plasma-purified ficolin α (pFCN) because it was purified from culture supernatants by GlcNAc affinity chromatography, and it was similarly eluted with GlcNAc from A. pleuropneumoniae serotype 5b (APP 5b) and S. enteritidis.
There were several structural differences between the plasma and recombinant forms but both resolved as higher-order oligomers in native PAGE gels, and had similar pI range on 2D-electrophoresis. However, electrophoretic mobility under reducing conditions differed between pFCN and rFCN, likely due to the C-terminal tag on rFCN that was designed for additional antibody detection, specific for the recombinant form. On 2D-PAGE, rFCN was observed as ∼44 kDa (3 spots in pI 5.5–5.7) compared with ∼40 kDa for pFCN (5 spots in pI 5.2–6.0) (Brooks et al., 2003a, Brooks et al., 2003b). The narrower apparent migration of rFCN on the second dimension is suggestive of a difference in the glycosylation, since treatment of pFCN with N-glycosidase reduces the number and shape of ficolin spots on 2D gels but does not interfere with its GlcNAc-dependent binding (Brooks et al., 2003b). Inadequate glycosylation of recombinant proteins can impair protein folding and function (Bhatia and Mukhopadhyay, 1999), therefore an additional comparison between pFCN and rFCN was conducted to determine if the recombinant form had similar functions to native plasma-purified ficolin. pFCN selectively binds Actinobacillus pleuropneumoniae serotype 5b (APP 5b) and Salmonella enteriditis (S. enteriditis) (Brooks et al., 2003a, Nahid and Sugii, 2006), and is elutable with GlcNAc; rFCN could also bind these bacterial species in a GlcNAc-dependent manner.
The biological relevance of this interaction between ficolin and PRRSV is unknown. While ficolin bound to PRRS virions and inhibited replication in Marc-145 cells using concentrations similar to that found in pig plasma, the mechanism of infection for the permissive Marc-145 cells is not identical to porcine macrophages, which are the main target cell for PRRSV in vivo (Nauwynck et al., 1999, Duan et al., 1997). Binding of ficolin to PRRSV was neutralizing in these in vitro assays but it is possible that ficolins might provide a route of infection of macrophages via the C1q receptor that can bind and internalize ficolins (Kuraya et al., 2005).
The PRRSV genome encodes six structural proteins which include membrane glycoproteins GP2, GP3, GP4, and GP5. The functions of GP 2 through 5 are still largely unresolved, although GP5 has been shown to be necessary for proper viral assembly (Wissink et al., 2004). Both GP2 and 5 contain two predicted N-glycosylation sites that are highly conserved between North American and European strains of PRRSV. GP5 contains high-mannose type sugars and complex-type sugars with terminal GlcNAc residues while the remaining envelope glycoproteins contain complex N-glycans at various asparagine residues (Ansari et al., 2006). GP3 is the most glycosylated protein with seven N-linked glycosylation sites that are highly conserved (Wissink et al., 2004). Ficolin α may bind one or more of the terminal GlcNAc residues that decorate the PRRSV envelope glycoproteins, inhibiting viral entry into alveolar macrophages and thereby reducing infection. Other collagenous lectins have been reported to have antiviral effects related to direct binding and virus neutralization (Hartshorn et al., 1997, Reading et al., 1997, Ezekowitz et al., 1989, Reading et al., 1998, Kawai et al., 2007, Haurum et al., 1993b, Haurum et al., 1993a), including SP-A and SP-D, which can bind to the G-protein of respiratory syncytial virus (RSV) in a carbohydrate-dependent manner (Ghildyal et al., 1999, Hickling et al., 2000, Hickling et al., 1999). In vivo relevance is suggested as SP-A-deficient mice suffered more severe RSV infections with severe pneumonia and impaired superoxide and hydrogen peroxide production by alveolar macrophages (LeVine et al., 1999). If these mice were supplemented with exogenous SP-A together with RSV inoculation, the infection was less severe, comparable to that seen in controls (LeVine et al., 1999). Alternatively, ficolins could bind PRRSV and enhance presentation of the virus to macrophages, thereby enhancing viral infection, and likely exacerbating the disease pathogenesis. MBL binding to HIV was shown to enhance inflammation and HIV replication in peripheral blood mononuclear cells (Heggelund et al., 2005, Ji et al., 2005).
Some lectins derived from various species are currently being considered as anti-viral drugs. Lectins with anti-HIV activity have been of particular interest, and have been isolated from cyanobacteria (Bokesch et al., 2003, Lagenaur and Berger, 2005), sea coral (Kljajic et al., 1987), algae (Mori et al., 2005), and a number of plants (Balzarini et al., 2004, Balzarini et al., 2005). The carbohydrate specificity is predominantly to high-mannose and GlcNAc residues (Keyaerts et al., 2007, Balzarini, 2006). Plant lectins that recognize mannan or GlcNAc terminal residues have been reported to have antiviral effects against coronaviruses such as SARScoV and feline infectious peritonitis virus (FIPV) (Keyaerts et al., 2007). The antiviral effects appeared to target either initial viral attachment to host cells, or viral release following a replicative phase (Keyaerts et al., 2007). Recently, the commensal Lactobacillus jensenii was engineered to express an HIV-binding cyanobacterial lectin (cyanovirin) at high levels, in order to deliver and maintain an active antiviral protein in the vaginal mucosa, preventing HIV transmission (Liu et al., 2006, Lagenaur and Berger, 2005).
The finding in this study is the first evidence of antiviral activity by ficolins in any species. While the mechanism is still unknown, the results in this study suggest that ficolin can bind to N-acetylated glycans on the viral surface and inhibit infection. This suggests that ficolins might have antiviral roles similar to those that have recently been proposed for MBL (Kawai et al., 2007, Ji et al., 2005) which is structurally similar but can bind mannose in viral glycoproteins. A better understanding of the mechanisms by which ficolins influence viral infection and disease might lead to new antiviral interventions.
Acknowledgements
We appreciate the technical assistance of Barbara Jefferson and Dr. Jutta Hammermueller. Funding from the National Sciences and Engineering Research Council of Canada (NSERC), Ontario Pork, Ontario Ministry of Agriculture and Food and the Canadian Institutes of Health Research supported this work.
References
- Altman E., Brisson J.R., Gagne S.M., Perry M.B. Structure of the capsular polysaccharide of Actinobacillus pleuropneumoniae serotype 5b. Eur. J. Biochem. 1992;204:225–230. doi: 10.1111/j.1432-1033.1992.tb16628.x. [DOI] [PubMed] [Google Scholar]
- Ansari I.H., Kwon B., Osorio F.A., Pattnaik A.K. Influence of N-linked glycosylation of porcine reproductive and respiratory syndrome virus GP5 on virus infectivity, antigenicity, and ability to induce neutralizing antibodies. J. Virol. 2006;80:3994–4004. doi: 10.1128/JVI.80.8.3994-4004.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzarini J. Inhibition of HIV entry by carbohydrate-binding proteins. Antiviral. Res. 2006;71:237–247. doi: 10.1016/j.antiviral.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Balzarini J. Carbohydrate-binding agents: a potential future cornerstone for the chemotherapy of enveloped viruses? Antivir. Chem. Chemother. 2007;18:1–11. doi: 10.1177/095632020701800101. [DOI] [PubMed] [Google Scholar]
- Balzarini J., Hatse S., Vermeire K., Princen K., Aquaro S., Perno C.F., De Clercq E., Egberink H., Vanden Mooter G., Peumans W., Van Damme E., Schols D. Mannose-specific plant lectins from the Amaryllidaceae family qualify as efficient microbicides for prevention of human immunodeficiency virus infection. Antimicrob. Agents Chemother. 2004;48:3858–3870. doi: 10.1128/AAC.48.10.3858-3870.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzarini J., Van Laethem K., Hatse S., Froeyen M., Van Damme E., Bolmstedt A., Peumans W., De Clercq E., Schols D. Marked depletion of glycosylation sites in HIV-1 gp120 under selection pressure by the mannose-specific plant lectins of Hippeastrum hybrid and Galanthus nivalis. Mol. Pharmacol. 2005;67:1556–1565. doi: 10.1124/mol.104.005082. [DOI] [PubMed] [Google Scholar]
- Benne C.A., Kraaijeveld C.A., van Strijp J.A., Brouwer E., Harmsen M., Verhoef J., van Golde L.M., van Iwaarden J.F. Interactions of surfactant protein A with influenza A viruses: binding and neutralization. J. Infect. Dis. 1995;171:335–341. doi: 10.1093/infdis/171.2.335. [DOI] [PubMed] [Google Scholar]
- Bhatia P.K., Mukhopadhyay A. Protein glycosylation: implications for in vivo functions and therapeutic applications. Adv. Biochem. Eng. Biotechnol. 1999;64:155–201. doi: 10.1007/3-540-49811-7_5. [DOI] [PubMed] [Google Scholar]
- Bokesch H.R., O’Keefe B.R., McKee T.C., Pannell L.K., Patterson G.M., Gardella R.S., Sowder R.C., Turpin J., Watson K., Buckheit R.W., Boyd M.R. A potent novel anti-HIV protein from the cultured cyanobacterium Scytonema varium. Biochemistry. 2003;42:2578–2584. doi: 10.1021/bi0205698. [DOI] [PubMed] [Google Scholar]
- Brooks A.S., DeLay J.P., Hayes M.A. Characterization of porcine plasma ficolins that bind Actinobacillus pleuropneumoniae serotype 5B. Immunobiology. 2003;207:327–337. doi: 10.1078/0171-2985-00245. [DOI] [PubMed] [Google Scholar]
- Brooks A.S., DeLay J.P., Hayes M.A. Purification and binding properties of porcine plasma ficolin that binds Actinobacillus pleuropneumoniae. Dev. Comp. Immunol. 2003;27:835–844. doi: 10.1016/s0145-305x(03)00057-0. [DOI] [PubMed] [Google Scholar]
- Brooks A.S., Hammermueller J., DeLay J.P., Hayes M.A. Expression and secretion of ficolin beta by porcine neutrophils. Biochim. Biophys. Acta. 2003;1624:36–45. doi: 10.1016/j.bbagen.2003.09.004. [DOI] [PubMed] [Google Scholar]
- DeLay J.P., 1999. Biochemical characterization and bacterial binding functions of porcine ficolins. DVSc., thesis. University of Guelph.
- Duan X., Nauwynck H.J., Pensaert M.B. Effects of origin and state of differentiation and activation of monocytes/macrophages on their susceptibility to porcine reproductive and respiratory syndrome virus (PRRSV) Arch. Virol. 1997;142:2483–2497. doi: 10.1007/s007050050256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo Y., Liu Y., Fujita T. Structure and function of ficolins. Adv. Exp. Med. Biol. 2006;586:265–279. doi: 10.1007/0-387-34134-X_18. [DOI] [PubMed] [Google Scholar]
- Ezekowitz R.A., Kuhlman M., Groopman J.E., Byrn R.A. A human serum mannose-binding protein inhibits in vitro infection by the human immunodeficiency virus. J. Exp. Med. 1989;169:185–196. doi: 10.1084/jem.169.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen P.D., Thiel S., Larsen C.B., Jensenius J.C. M-ficolin, an innate immune defence molecule, binds patterns of acetyl groups and activates complement. Scand. J. Immunol. 2005;62:442–473. doi: 10.1111/j.1365-3083.2005.01685.x. [DOI] [PubMed] [Google Scholar]
- Gadjeva M., Paludan S.R., Thiel S., Slavov V., Ruseva M., Eriksson K., Lowhagen G.B., Shi L., Takahashi K., Ezekowitz A., Jensenius J.C. Mannan-binding lectin modulates the response to HSV-2 infection. Clin. Exp. Immunol. 2004;138:304–311. doi: 10.1111/j.1365-2249.2004.02616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghildyal R., Chapman A., Peroulis I., Mills J., Meanger J. Expression and characterisation of the ovine respiratory syncytial virus (ORSV) G protein for use as a diagnostic reagent. Vet. Res. 1999;30:475–482. [PubMed] [Google Scholar]
- Hartley C.A., Reading P.C., Ward A.C., Anders E.M. Changes in the hemagglutinin molecule of influenza type A (H3N2) virus associated with increased virulence for mice. Arch. Virol. 1997;142:75–88. doi: 10.1007/s007050050060. [DOI] [PubMed] [Google Scholar]
- Hartshorn K.L., Crouch E.C., White M.R., Eggleton P., Tauber A.I., Chang D., Sastry K. Evidence for a protective role of pulmonary surfactant protein D (SP-D) against influenza A viruses. J. Clin. Invest. 1994;94:311–319. doi: 10.1172/JCI117323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorn K.L., White M.R., Shepherd V., Reid K., Jensenius J.C., Crouch E.C. Mechanisms of anti-influenza activity of surfactant proteins A and D: comparison with serum collectins. Am. J. Physiol. 1997;273:L1156–L1166. doi: 10.1152/ajplung.1997.273.6.L1156. [DOI] [PubMed] [Google Scholar]
- Haurum J.S., Thiel S., Haagsman H.P., Laursen S.B., Larsen B., Jensenius J.C. Studies on the carbohydrate-binding characteristics of human pulmonary surfactant-associated protein A and comparison with two other collectins: mannan-binding protein and conglutinin. Biochem. J. 1993;293(Pt 3):873–878. doi: 10.1042/bj2930873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haurum J.S., Thiel S., Jones I.M., Fischer P.B., Laursen S.B., Jensenius J.C. Complement activation upon binding of mannan-binding protein to HIV envelope glycoproteins. AIDS. 1993;7:1307–1313. doi: 10.1097/00002030-199310000-00002. [DOI] [PubMed] [Google Scholar]
- Hawgood S., Brown C., Edmondson J., Stumbaugh A., Allen L., Goerke J., Clark H., Poulain F. Pulmonary collectins modulate strain-specific influenza A virus infection and host responses. J. Virol. 2004;78:8565–8572. doi: 10.1128/JVI.78.16.8565-8572.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggelund L., Mollnes T.E., Espevik T., Muller F., Kristiansen K.I., Aukrust P., Froland S.S. Modulatory effect of mannose-binding lectin on cytokine responses: possible roles in HIV infection. Eur. J. Clin. Invest. 2005;35:765–770. doi: 10.1111/j.1365-2362.2005.01579.x. [DOI] [PubMed] [Google Scholar]
- Hickling T.P., Bright H., Wing K., Gower D., Martin S.L., Sim R.B., Malhotra R. A recombinant trimeric surfactant protein D carbohydrate recognition domain inhibits respiratory syncytial virus infection in vitro and in vivo. Eur. J. Immunol. 1999;29:3478–3484. doi: 10.1002/(SICI)1521-4141(199911)29:11<3478::AID-IMMU3478>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Hickling T.P., Malhotra R., Bright H., McDowell W., Blair E.D., Sim R.B. Lung surfactant protein A provides a route of entry for respiratory syncytial virus into host cells. Viral Immunol. 2000;13:125–135. doi: 10.1089/vim.2000.13.125. [DOI] [PubMed] [Google Scholar]
- Honore C., Hummelshoj T., Hansen B.E., Madsen H.O., Eggleton P., Garred P. The innate immune component ficolin 3 (Hakata antigen) mediates the clearance of late apoptotic cells. Arthritis Rheum. 2007;56:1598–1607. doi: 10.1002/art.22564. [DOI] [PubMed] [Google Scholar]
- Ichijo H., Hellman U., Wernstedt C., Gonez L.J., Claesson-Welsh L., Heldin C.H., Miyazono K. Molecular cloning and characterization of ficolin, a multimeric protein with fibrinogen- and collagen-like domains. J. Biol. Chem. 1993;268:14505–14513. [PubMed] [Google Scholar]
- Jensen M.L., Honore C., Hummelshoj T., Hansen B.E., Madsen H.O., Garred P. Ficolin-2 recognizes DNA and participates in the clearance of dying host cells. Mol. Immunol. 2007;44:856–865. doi: 10.1016/j.molimm.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Ji X., Gewurz H., Spear G.T. Mannose binding lectin (MBL) and HIV. Mol. Immunol. 2005;42:145–152. doi: 10.1016/j.molimm.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Kawai T., Kase T., Suzuki Y., Eda S., Sakamoto T., Ohtani K., Wakamiya N. Anti-influenza A virus activities of mannan-binding lectins and bovine conglutinin. J. Vet. Med. Sci. 2007;69:221–224. doi: 10.1292/jvms.69.221. [DOI] [PubMed] [Google Scholar]
- Keyaerts E., Vijgen L., Pannecouque C., Van Damme E., Peumans W., Egberink H., Balzarini J., Van Ranst M. Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antiviral Res. 2007;75:179–187. doi: 10.1016/j.antiviral.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.S., Kwang J., Yoon I.J., Joo H.S., Frey M.L. Enhanced replication of porcine reproductive and respiratory syndrome (PRRS) virus in a homogeneous subpopulation of MA-104 cell line. Arch. Virol. 1993;133:477–483. doi: 10.1007/BF01313785. [DOI] [PubMed] [Google Scholar]
- Kljajic Z., Schroder H.C., Rottmann M., Cuperlovic M., Movsesian M., Uhlenbruck G., Gasic M., Zahn R.K., Muller W.E. A d-mannose-specific lectin from Gerardia savaglia that inhibits nucleocytoplasmic transport of mRNA. Eur. J. Biochem. 1987;169:97–104. doi: 10.1111/j.1432-1033.1987.tb13585.x. [DOI] [PubMed] [Google Scholar]
- Krarup A., Thiel S., Hansen A., Fujita T., Jensenius J.C. L-ficolin is a pattern recognition molecule specific for acetyl groups. J. Biol. Chem. 2004;279:47513–47519. doi: 10.1074/jbc.M407161200. [DOI] [PubMed] [Google Scholar]
- Kuraya M., Ming Z., Liu X., Matsushita M., Fujita T. Specific binding of L-ficolin and H-ficolin to apoptotic cells leads to complement activation. Immunobiology. 2005;209:689–697. doi: 10.1016/j.imbio.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lagenaur L.A., Berger E.A. An anti-HIV microbicide comes alive. Proc. Natl. Acad. Sci. U.S.A. 2005;102:12294–12295. doi: 10.1073/pnas.0505960102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Y., Lee S.H., Kon O.L., Lu J. Human L-ficolin: plasma levels, sugar specificity, and assignment of its lectin activity to the fibrinogen-like (FBG) domain. FEBS Lett. 1998;425:367–370. doi: 10.1016/s0014-5793(98)00267-1. [DOI] [PubMed] [Google Scholar]
- Lee C., Yoo D. The small envelope protein of porcine reproductive and respiratory syndrome virus possesses ion channel protein-like properties. Virology. 2006;355:30–43. doi: 10.1016/j.virol.2006.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVine A.M., Gwozdz J., Stark J., Bruno M., Whitsett J., Korfhagen T. Surfactant protein-A enhances respiratory syncytial virus clearance in vivo. J. Clin. Invest. 1999;103:1015–1021. doi: 10.1172/JCI5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Lagenaur L.A., Simpson D.A., Essenmacher K.P., Frazier-Parker C.L., Liu Y., Tsai D., Rao S.S., Hamer D.H., Parks T.P., Lee P.P., Xu Q. Engineered vaginal lactobacillus strain for mucosal delivery of the human immunodeficiency virus inhibitor cyanovirin-N. Antimicrob. Agents Chemother. 2006;50:3250–3259. doi: 10.1128/AAC.00493-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita M., Endo Y., Taira S., Sato Y., Fujita T., Ichikawa N., Nakata M., Mizuochi T. A novel human serum lectin with collagen- and fibrinogen-like domains that functions as an opsonin. J. Biol. Chem. 1996;271:2448–2454. doi: 10.1074/jbc.271.5.2448. [DOI] [PubMed] [Google Scholar]
- Matsushita M., Endo Y., Fujita T. Cutting edge: complement-activating complex of ficolin and mannose-binding lectin-associated serine protease. J. Immunol. 2000;164:2281–2284. doi: 10.4049/jimmunol.164.5.2281. [DOI] [PubMed] [Google Scholar]
- Mori T., O’Keefe B.R., Sowder R.C., Bringans S., Gardella R., Berg S., Cochran P., Turpin J.A., Buckheit R.W., McMahon J.B., Boyd M.R. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J. Biol. Chem. 2005;280:9345–9353. doi: 10.1074/jbc.M411122200. [DOI] [PubMed] [Google Scholar]
- Munro C.A., Gow N.A. Chitin synthesis in human pathogenic fungi. Med. Mycol. 2001;39(Suppl 1):41–53. [PubMed] [Google Scholar]
- Nahid A.M., Sugii S. Binding of porcine ficolin-alpha to lipopolysaccharides from Gram-negative bacteria and lipoteichoic acids from Gram-positive bacteria. Dev. Comp. Immunol. 2006;30:335–343. doi: 10.1016/j.dci.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Nauwynck H.J., Duan X., Favoreel H.W., Van Oostveldt P., Pensaert M.B. Entry of porcine reproductive and respiratory syndrome virus into porcine alveolar macrophages via receptor-mediated endocytosis. J. Gen. Virol. 1999;80(Pt 2):297–305. doi: 10.1099/0022-1317-80-2-297. [DOI] [PubMed] [Google Scholar]
- Neumann E.J., Kliebenstein J.B., Johnson C.D., Mabry J.W., Bush E.J., Seitzinger A.H., Green A.L., Zimmerman J.J. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J. Am. Vet. Med. Assoc. 2005;227:385–392. doi: 10.2460/javma.2005.227.385. [DOI] [PubMed] [Google Scholar]
- Ohashi T., Erickson H.P. Two oligomeric forms of plasma ficolin have differential lectin activity. J. Biol. Chem. 1997;272:14220–14226. doi: 10.1074/jbc.272.22.14220. [DOI] [PubMed] [Google Scholar]
- Ohashi T., Erickson H.P. Oligomeric structure and tissue distribution of ficolins from mouse, pig and human. Arch. Biochem. Biophys. 1998;360:223–232. doi: 10.1006/abbi.1998.0957. [DOI] [PubMed] [Google Scholar]
- Palaniyar N., Nadesalingam J., Clark H., Shih M.J., Dodds A.W., Reid K.B. Nucleic acid is a novel ligand for innate, immune pattern recognition collectins surfactant proteins A and D and mannose-binding lectin. J. Biol. Chem. 2004;279:32728–32736. doi: 10.1074/jbc.M403763200. [DOI] [PubMed] [Google Scholar]
- Reading P.C., Hartley C.A., Ezekowitz R.A., Anders E.M. A serum mannose-binding lectin mediates complement-dependent lysis of influenza virus-infected cells. Biochem. Biophys. Res. Commun. 1995;217:1128–1136. doi: 10.1006/bbrc.1995.2886. [DOI] [PubMed] [Google Scholar]
- Reading P.C., Morey L.S., Crouch E.C., Anders E.M. Collectin-mediated antiviral host defense of the lung: evidence from influenza virus infection of mice. J. Virol. 1997;71:8204–8212. doi: 10.1128/jvi.71.11.8204-8212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading P.C., Holmskov U., Anders E.M. Antiviral activity of bovine collectins against rotaviruses. J. Gen. Virol. 1998;79:2255–2263. doi: 10.1099/0022-1317-79-9-2255. [DOI] [PubMed] [Google Scholar]
- Rietschel E.T., Kirikae T., Schade F.U., Mamat U., Schmidt G., Loppnow H., Ulmer A.J., Zahringer U., Seydel U., Di Padova F., Schreier M., Brade H. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 1994;8:217–225. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- Rossow K.D. Porcine reproductive and respiratory syndrome. Vet. Pathol. 1998;35:1–20. doi: 10.1177/030098589803500101. [DOI] [PubMed] [Google Scholar]
- Seebaransingh, R., 1999. Plasma ficolins and acutre phase proteins of young pigs. MSc. thesis., University of Guelph.
- Taira S., Kodama N., Matsushita M., Fujita T. Opsonic function and concentration of human serum ficolin/P35. Fukushima J. Med. Sci. 2000;44:13–23. doi: 10.5387/fms.46.13. [DOI] [PubMed] [Google Scholar]
- Teh C., Le Y., Lee S.H., Lu J. M-ficolin is expressed on monocytes and is a lectin binding to N-acetyl-d-glucosamine and mediates monocyte adhesion and phagocytosis of Escherichia coli. Immunology. 2000;101:225–232. doi: 10.1046/j.1365-2567.2000.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heijenoort J. Recent advances in the formation of the bacterial peptidoglycan monomer unit. Nat. Prod. Rep. 2001;18:503–519. doi: 10.1039/a804532a. [DOI] [PubMed] [Google Scholar]
- Van Leuven F., Cassiman J.J., Van den Berghe H. Functional modifications of alpha 2-macroglobulin by primary amines. I. Characterization of alpha 2 M after derivatization by methylamine and by factor XIII. J. Biol. Chem. 1981;256:9016–9022. [PubMed] [Google Scholar]
- Vigerust D.J., Shepherd V.L. Virus glycosylation: role in virulence and immune interactions. Trends Microbiol. 2007;15:211–218. doi: 10.1016/j.tim.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.C., Yim K.W., Poon T.C., Choy K.W., Chu C.Y., Lui W.T., Lau T.K., Rogers M.S., Leung T.N. Innate immune response by ficolin binding in apoptotic placenta is associated with the clinical syndrome of preeclampsia. Clin. Chem. 2007;53:42–52. doi: 10.1373/clinchem.2007.074401. [DOI] [PubMed] [Google Scholar]
- Wissink E.H., Kroese M.V., Maneschijn-Bonsing J.G., Meulenberg J.J., van Rijn P.A., Rijsewijk F.A., Rottier P.J. Significance of the oligosaccharides of the porcine reproductive and respiratory syndrome virus glycoproteins GP2a and GP5 for infectious virus production. J. Gen. Virol. 2004;85:3715–3723. doi: 10.1099/vir.0.80402-0. [DOI] [PubMed] [Google Scholar]
- Wootton S., Yoo D., Rogan D. Full-length sequence of a Canadian porcine reproductive and respiratory syndrome virus (PRRSV) isolate. Arch. Virol. 2000;145:2297–2323. doi: 10.1007/s007050070022. [DOI] [PMC free article] [PubMed] [Google Scholar]


