Abstract
Chronic obstructive pulmonary disease (COPD) is characterised by progressive airflow obstruction that is only partly reversible, inflammation in the airways, and systemic effects or comorbities. The main cause is smoking tobacco, but other factors have been identified. Several pathobiological processes interact on a complex background of genetic determinants, lung growth, and environmental stimuli. The disease is further aggravated by exacerbations, particularly in patients with severe disease, up to 78% of which are due to bacterial infections, viral infections, or both. Comorbidities include ischaemic heart disease, diabetes, and lung cancer. Bronchodilators constitute the mainstay of treatment: β2 agonists and long-acting anticholinergic agents are frequently used (the former often with inhaled corticosteroids). Besides improving symptoms, these treatments are also thought to lead to some degree of disease modification. Future research should be directed towards the development of agents that notably affect the course of disease.
Introduction
Chronic obstructive pulmonary disease (COPD) is currently defined as “a preventable and treatable disease with some significant extrapulmonary effects that may contribute to the severity in individual patients. Its pulmonary component is characterized by airflow limitation that is not fully reversible. The airflow limitation is usually progressive and associated with an abnormal inflammatory response of the lung to noxious particles or gases”.1 The general view of this disease is that it will become one of the major health challenges of the next few decades. Prevalence surveys suggest that up to almost a quarter of adults aged 40 years and older have mild airflow obstruction.2, 3, 4 COPD is presently the fourth leading cause of death, but WHO predicts that it will become the third leading cause by 2030;5 mortality owing to cardiac diseases and stroke decreased over the period 1970–2002, but that of COPD doubled over the same period.6
In the past two decades important progress has been made in the understanding of the epidemiology, pathophysiology, diagnosis, and treatment of COPD, but important issues remain unresolved. Particular concerns are the causes and mechanisms of disease, mechanisms of inflammation, whether to diagnose early, identification of effective biomarkers, the relation between airway disease and comorbidities, and the development of treatments to increase disease modification. In this Seminar we provide an overview of the insights that have been gained and those that are lacking. We also attempt to define some research questions for the next decade.
Epidemiology and causes
Age-adjusted mortality for COPD in the USA doubled from 1970 to 2002, although in other developed countries reports suggest stabilisation of or even falls in the incidence and prevalence.7, 8 These declines are related to decreases in the prevalence of smoking and reductions in air pollutants. In developing countries prevalence has risen strikingly, owing to increased smoking rates and reductions in other causes of death, particularly from severe infections. Worldwide prevalence of COPD of Global Initiative on Obstructive Lung Disease (GOLD) stage 2 or higher in adults aged 40 years and older is 9–10%.9 The Burden of Obstructive Lung Disease initiative2 used standardised methods to investigate the prevalence of COPD around the world and showed important differences between countries. Prevalence ranged from 9% in Reykjavik, Iceland, to 22% in Cape Town, South Africa, for men, and from 4% in Hannover, Germany, to 17% in Cape Town for women.
Most prevalence studies use the GOLD definition of chronic airflow obstruction, for which the threshold is a postbronchodilator ratio of forced expiratory volume in 1 s (FEV1) to forced vital capacity (also known as the Tiffeneau index) of 0·7.1 The ratio decreases with age and, therefore, is controversial as an epidemiological tool because its use might lead to overdiagnosis of COPD in elderly patients.10 Thus, exposure to risk factors and the presence of respiratory symptoms or FEV1 less than 80% of predicted must also be taken into account. This method of assessment is simple, not tied to reference values derived from complex equations, enables comparison of prevalence estimates across sites, and can be easily understood by patients and the wider population. Four GOLD stages are distinguished on the basis of severity of airflow obstruction: stage 1 (mild, FEV1 ≥80% of predicted) stage 2 (moderate, FEV1 50–80% of predicted), stage 3 (severe, FEV1 30–50% of predicted), and stage 4 (very severe, FEV1 <30% of predicted).
Another important feature in the epidemiology of COPD is the high risk of underdiagnosis. 60–85% of patients, mainly with mild to moderate disease, are thought to remain undiagnosed.11, 12 In a survey in Spain, 10% of adults aged 40–80 years had COPD but in only 27% of them had it previously been diagnosed.11 The use of spirometry at all levels of health care is crucial to improve diagnosis and detect COPD early. Moreover, although COPD has been typically seen in men, prevalence in women has risen because of the increasing number of those older than 50 years who smoke. For the first time in the USA, similar numbers of men and women died from COPD in the year 2000, although the mortality rate remains lower in women.13
COPD results from the interplay between genetic susceptibility and exposure to environmental stimuli. Smoking cigarettes is the main cause, but other causes might increase the risk of and lead to disease in non-smokers. Maternal smoking, childhood asthma, and childhood respiratory infections are significantly associated with reduced FEV1,14 and previous tuberculosis, outdoor air pollution, occupational exposure to dusts and fumes, exposure to second-hand smoke, and biomass smoke inhalation have been particularly associated with the development of airflow obstruction and chronic respiratory symptoms.15 A well established genetic cause of COPD, α1 antitrypsin deficiency, is present in 1–2% of individuals with COPD.16 Genome-wide association studies, however, have identified regions on chromosome 4 near HHIP and in FAM13A and on chromosome 15 in CHRNA and IREB2 that are unequivocally associated with COPD susceptibility.17, 18, 19 A pooled analysis identified a single-nucleotide polymorphism in MMP12 as a protective factor for COPD.20 Moreover, several case-control studies of candidate genes have linked specific loci to phenotypes related to COPD.21
Pathophysiology
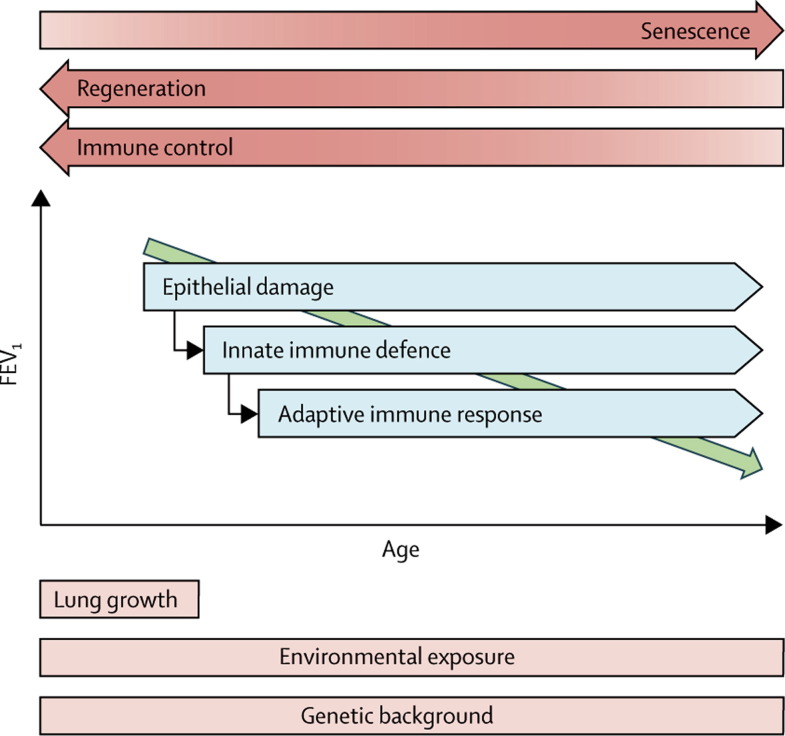
The principal feature of COPD is limitation of airflow that is not fully reversible. Remodelling of the small-airway compartment and loss of elastic recoil by emphysematous destruction of parenchyma result in progressive decline of FEV1, inadequate lung emptying on expiration, and subsequent static and dynamic hyperinflation.22 At the pathological level, exposure to smoke leads to infiltration of the mucosa, submucosa, and glandular tissue by inflammatory cells. Increased mucus content, epithelial-cell hyperplasia, and disturbed tissue repair with wall thickening in the small conducting airways are cardinal features of COPD (figure 1 ).23 This progressive narrowing, obliteration, and even removal of the terminal bronchioles is accompanied by emphysema, which typically starts in the respiratory bronchioles.24 The mechanisms that lead to thickening of the small-airway walls and destruction of lung tissue are far from understood,23 but they are likely to be multifactorial pathobiological processes25 that are interacting on a complex background of genetic determinants,26 lung growth,14 and environmental stimuli (figure 2 ).3, 13 Within this framework we discuss the pathogenesis of COPD as a progressive immunological disorder.27
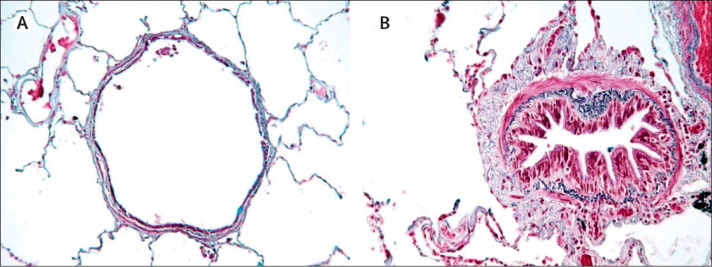
Figure 1.
Comparison of airway features in a healthy individual and in a patient with chronic obstructive pulmonary disease
(A) Normal airway. (B) In chronic obstructive pulmonary disease airways are narrowed by infiltration of inflammatory cells, mucosal hyperplasia, and deposition of connective tissue in the peribronchiolar space.23
Figure 2.
Schematic representation of the mechanisms involved in the pathogenesis of chronic obstructive pulmonary disease
The process is essentially characterised by a decline in FEV1 with increasing age. FEV1=forced expiratory volume in 1 s.
Cigarette smoke causes direct injury of airway epithelial cells, which leads to the release of endogenous intracellular molecules or danger-associated molecular patterns. These signals are identified by pattern-recognition receptors, such as Toll-like receptors 4 and 2 on epithelial cells, and a non-specific inflammatory response is triggered.28 Upon the release of early cytokines (tumour necrosis factor α and interleukins 1 and 8), macrophages, neutrophils, and dendritic cells are recruited to the site of inflammation to orchestrate the innate immune response.29, 30 Proteolytic enzymes and reactive oxygen species are released and, if not sufficiently counterbalanced by antiproteases and antioxidant factors, further damage will occur.31 Immature dendritic cells pick up self-antigens released from damaged tissue and foreign antigens from incoming pathogens and present them to naive T cells in the draining lymph nodes.29 Once activated into T-helper-1 cells, these antigen-specific CD4 and CD8 cells and antibody-producing B cells are drawn to the lungs to neutralise the antigens. As the disease progresses, tertiary lymphoid aggregates, including an oligoclonal selection of the B and T cells involved, develop around the small airways.32, 33 Although the exact nature and function of these aggregates needs to be elucidated, adaptive or autoimmune responses are thought to perpetuate the inflammation years after smoking cessation.27, 34
Apart from these basic immunological processes, several other mechanisms might contribute to the inflammatory cascade. Tapering of the immune response by regulatory T cells protects against uncontrolled inflammation,35 and reduced populations of these cells have been seen in the lungs of patients with COPD.34, 36 By contrast, the numbers of proinflammatory T-helper-17 cells rise,37, 38 which suggests impaired immune regulation in COPD. Pulmonary emphysema and cellular ageing share some features:25, 39 senescence leads to cells becoming non-proliferative but metabolically active, which predisposes individuals to increased inflammation, reduced cell regeneration, and carcinogenesis. Cigarette smoke and oxidative stress promote senescence.40 As such, COPD can be interpreted as accelerated ageing of the lung, and hence age will increase susceptibility to COPD.41 Finally, cellular apoptosis and matrix destruction are continuously compensated for by cellular renewal and matrix repair to maintain lung homoeostasis.42 Resident stem cells within the lung are activated by epithelial damage43 but cigarette smoke limits alveolar repair44 and dysregulates repair processes involving transforming growth factor β, which leads to fibrosis.45 The underlying molecular signals are poorly understood, but in COPD repair mechanisms eventually fail.
The complexity of the pathogenesis of COPD is reflected in the broad variation of clinical phenotypes. Further research is needed to clarify to what extent mechanisms offer potential for new targeted interventions.
Exacerbations
The chronic and progressive course of COPD is frequently aggravated by exacerbations—short periods (at least 48 h) of increased cough, dyspnoea, and production of sputum that can become purulent. Mild exacerbations require increased doses of bronchodilators, moderate exacerbations need treatment with systemic corticosteroids, antibiotics, or both, and severe exacerbations frequently necessitate admission to hospital. Some patients have no or few exacerbations, whereas others have frequent exacerbations. Frequency may increase with increasing severity of COPD. In a systematic review by Hoogendoorn and colleagues,46 37 studies were identified that defined exacerbations according to use of resources. Patients with mild COPD had a mean of 0·82 exacerbations per year, and the rates increased to 1·17, 1·61, and 2·01 in patients with moderate, severe, and very severe disease, respectively. Other characteristics besides severity of COPD are strongly associated with frequent exacerbations, but the best predictor is a history of frequent exacerbations.47
Exacerbations reduce quality of life,48 speed disease progression, and increase the risk of death.49 Because of their impact on the natural history of the disease, a primary goal of treatment is to reduce the number of exacerbations. They are diagnosed on the basis of clinical symptoms. No clear biological markers have been identified.50 The most promising biomarker so far is amyloid A in serum. In proteomic studies raised concentrations distinguished patients in exacerbated states from those with stable disease and those with severe exacerbations from patients with milder exacerbations.51 These data require confirmation in large multicentre studies.
Several causes of exacerbations are suggested for patients with COPD, such as heart failure, pneumonia, pulmonary embolism, non-adherence to inhaled medication, or inhalation of irritants, such as tobacco smoke or particles (panel 1 ). The most frequent cause is viral or bacterial infection. In patients admitted to hospital for COPD exacerbations, viral infections, bacterial infections, or both, were detected in 78% of cases and, more importantly, the exacerbations were more severe than those in patients with non-infectious causes, shown by more marked impairment in lung function and longer times in hospital.52
Panel 1. Causes of acute exacerbations of chronic obstructive pulmonary disease52.
Infectious (60–80% of all exacerbations)
Frequent (70–85% of all infectious exacerbations)
-
•
Haemophilus influenzae
-
•
Streptococcus pneumoniae
-
•
Moraxella catarrhalis
-
•
Viruses (influenza and parainfluenza viruses, rhinoviruses, coronaviruses)
Infrequent (15–30% of all infectious exacerbations)
-
•
Pseudomonas aeruginosa *
-
•
Opportunistic gram-negative species
-
•
Staphylococcus aureus
-
•
Chlamydophila pneumoniae
-
•
Mycoplasma pneumoniae
Non-infectious (20–40% of all exacerbations)
-
•
Heart failure
-
•
Pulmonary embolism
-
•
Non-pulmonary infections
-
•
Pneumothorax
-
•
Pneumonia
Precipitating and environmental factors
-
•
Cold air
-
•
Air pollution
-
•
Allergens
-
•
Tobacco smoking
-
•
Non-adherence to respiratory medication
The accepted gold standard for the diagnosis of bacterial causes is the isolation of a potentially pathogenic micro-organism in sputum culture. This method, however, is neither sensitive nor specific enough to be accurate. Clinical criteria must, therefore, also be taken into account to aid in the decision of whether or not to use antibiotics. The presence of green (purulent) as opposed to white (mucoid) sputum is one of the best and easiest methods of predicting the need for antibiotic therapy.53, 54 Measurement of procalcitonin concentrations in serum has shown promise as a guide of whether to use antibiotics,55 although C-reactive protein has shown better results in the prediction of response to antibiotic therapy.56 Future research will establish the usefulness of both these biomarkers in ambulatory patients treated in the community.
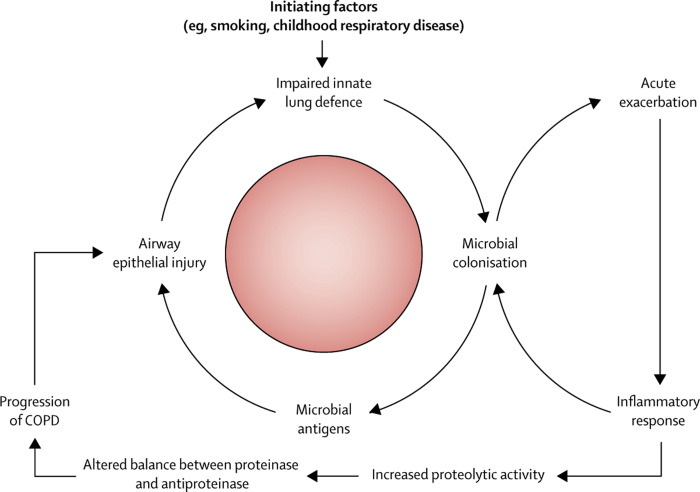
An additional difficulty in the identification of microbial causes for COPD exacerbations is that a substantial proportion of patients with stable disease have bacterial colonisation in the lower airways. Various species colonise the airways, but Haemophilus influenzae is most frequently seen.57 The production of purulent sputum when patients are in a stable state indicates colonisation by potentially pathogenic micro-organisms.58 Colonising bacteria promote bronchial and systemic inflammation and release antigens that result in bronchial epithelial injury and facilitate the acquisition of new microbial strains,59 which is associated with an increased risk of developing an exacerbation.60 Strain changes for H influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis are associated with increased bronchial and systemic inflammation and the development of exacerbations.61 Colonising bacteria can, therefore, accelerate the progression of COPD through an increase in the frequency of exacerbations and also through direct injury to the lung tissue (figure 3 ).59, 62, 63
Figure 3.
Hypothetical cycle of infection and inflammation in COPD
COPD=chronic obstructive pulmonary disease. Reproduced from reference 59 by permission of Massachusetts Medical Society.
The type of infecting species depends partly on the severity of the underlying COPD.64 In mild disease, S pneumoniae is predominant, whereas in patients with low FEV1, H influenzae and M catarrhalis are more frequently seen. Pseudomonas aeruginosa might be seen in patients with severe obstruction, and acquisition of a new strain is associated with the development of an exacerbation and colonisation of bronchial epithelium.65 Disease evolution can be worsened without prompt appropriate antibiotic therapy66 or if a multidrug-resistant strain is present.67 Some of the risk factors associated with P aeruginosa infection are summarised (panel 2 ).64, 68, 69
Panel 2. Risk factors for Pseudomonas aeruginosa infection in chronic obstructive pulmonary disease.
-
•
FEV1 <35%
-
•
Previous treatment with antibiotics
-
•
Oral corticosteroid use
-
•
Poor score on the BODE index
-
•
Previous admission to hospital
-
•
Previous isolation of Pseudomonas aeruginosa
-
•
Absence of influenza vaccination
FEV1=forced expiratory volume in 1 s. BODE=score of disease severity based on body-mass index, FEV1, Medical Research Council dyspnoea score, and 6 min walking distance.
Viruses are thought to account for 15–25% of all infective exacerbations, particularly human rhinovirus, influenza and parainfluenza viruses, and adenoviruses. Concomitant infection with viruses and bacteria are seen in 25% of patients with exacerbations who are admitted to hospital.52 Viral exacerbations are strongly correlated with colds at presentation, high frequency of exacerbations, and severe respiratory symptoms during exacerbations. Mallia and co-workers70 used experimental rhinovirus infection in individuals with COPD and noted the development of lower-airway respiratory symptoms, airflow obstruction, systemic inflammation, and inflammation of the airways. A significant correlation was also seen between viral load and concentrations of inflammatory markers. These findings strongly support a causal relation between rhinovirus infection and COPD exacerbations. No diagnostic test is available for viral exacerbations of COPD. Increased concentrations of interferon-γ-inducible protein 10 in serum was useful in one study to identify rhinovirus infection in patients with COPD.71 The presence of fever has also been associated with virus detection during exacerbations.72 Viral infections might facilitate subsequent bacterial infection or increases in the numbers of bacteria already colonising the lower airways. Although viral infection could be self-limiting, secondary bacterial infection might prolong exacerbations.70
Systemic manifestations and comorbidities
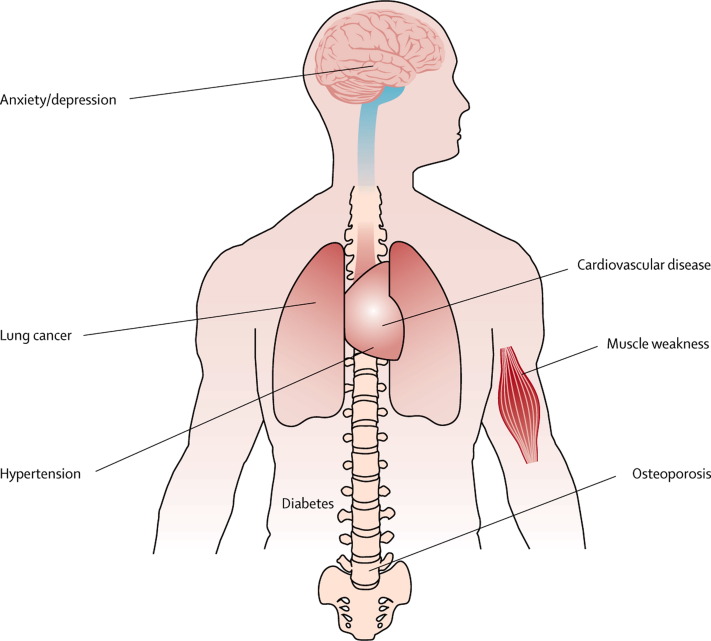
Although COPD is a lung disease, it is associated with systemic manifestations and comorbid conditions.73, 74 The most common comorbidities are ischaemic heart disease, diabetes, skeletal muscle wasting, cachexia, osteoporosis, depression, and lung cancer (figure 4 ).73 These comorbidities affect health outcomes, increase the risks of admission to hospital and death, and account for more than 50% of use of health-care resources for COPD.75, 76 They also explain why clinical features in patients with COPD do not correlate well with FEV1.77 These chronic diseases can develop in patients with or without COPD, but their frequent association with the disorder—certainly with severe disease—suggests common risk factors and mechanistic pathways. For instance, cigarette smoking is a major risk for COPD and cardiovascular disease, osteoporosis, and lung cancer.78 Abundant evidence shows that physical inactivity, which is frequently observed in people who develop COPD,79 is linked to the major comorbidities.80, 81 Finally, ageing is a major risk factor for chronic diseases. Almost half of all people aged 65 years or more have at least three chronic medical disorders,82 and for COPD in particular a cluster analysis indicated that age rather than FEV1 accounted for most of the comorbidities and symptoms.83
Figure 4.
Comorbidities of chronic obstructive pulmonary disease
One common denominator across comorbidities is systemic inflammation. Increased concentrations of circulating cytokines (tumour necrosis factor α and interleukins 6 and 8), adipokines (leptin, ghrelin), and acute-phase proteins (C-reactive protein, fibrinogen) are seen in most of the diseases. Furthermore, all described risk factors have been directly linked to the presence of systemic inflammation.84, 85, 86 In several studies biomarkers of systemic inflammation have been seen in patients with COPD, particularly when disease is severe and during acute exacerbations.87 Whether these systemic markers spill over from the lungs into the systemic circulation or merely reflect the proinflammatory state is unclear.84, 88, 89
Clinical management
Stable COPD
The most widely applied guideline for treatment is the GOLD guideline (table ).1 For all patients, smoking cessation, reduction in exposure to environmental and occupational risk factors, and yearly influenza vaccinations are recommended. The first step must be smoking cessation. This intervention lessens the decline of FEV1 by about 35 mL per year,90, 91 which slows disease progression and lowers mortality by 18%.92 Inhaled bronchodilators are the mainstay treatment for COPD (table). For very severe disease (GOLD stage 4) surgical options include lung transplantation and lung-volume reduction if quality of life is unacceptably low. Respiratory rehabilitation should be considered at all stages (table) in patients with muscle weakness, deconditioning, and poor quality of life. This treatment should be aimed at improving quality of life and exercise capacity.93, 94, 95
Table.
Therapy at each stage of chronic obstructive pulmonary disease, by GOLD stage1
| 1 (mild) | 2 (moderate) | 3 (severe) | 4 (very severe) | |
|---|---|---|---|---|
| FEV1:FVC | <0·70 | <0·70 | <0·70 | <0·70 |
| FEV1 | ≥80% of predicted | 50–80% of predicted | 30–50% of predicted | <30% of predicted or <50% of predicted plus chronic respiratory failure |
| Treatment | Influenza vaccination and short-acting bronchodilator* when needed | Influenza vaccination, short-acting and ≥1 long-acting bronchodilator* when needed; consider respiratory rehabilitation | Influenza vaccination and short-acting and ≥1 long-acting bronchodilator* when needed, inhaled glucocorticosteroid if repeated exacerbations; consider respiratory rehabilitation | Influenza vaccination and short-acting and ≥1 long-acting bronchodilator* when needed, inhaled glucocorticosteroid if repeated exacerbations, long-term oxygen if chronic respiratory failure occurs; consider respiratory rehabilitation and surgery |
GOLD=Global Initiative on Obstructive Lung Disease.
β2 agonists or anticholinergics.
Two large-scale, long-term, landmark studies have confirmed the efficacy of a fixed combination of a long-acting β2 agonist (salmeterol) and inhaled corticosteroid (fluticasone)96 and a long-acting anticholinergic agent (tiotropium).97 The two regimens had similar effects on prebronchodilator and postbronchodilator FEV1 (increases of 87–103 mL and 47–92 mL, respectively), scores on the St George's respiratory questionnaire for health status (reductions of three units), and the frequency of exacerbations (reductions of 14–25%). The effects of tiotropium seemed smaller, but might be explained by the use of other active treatments: up to 74% of the patients took long-acting β2 agonists, inhaled corticosteroids, or both.97 Greater effects on health status and frequency of exacerbations in patients receiving tiotropium versus placebo had been reported previously.98, 99, 100 A direct comparison of a fixed combination and tiotropium confirmed similar effects on the frequency of exacerbations, but the effect on health status was slightly larger with the combined therapy.101
Inhaled medications used for the treatment of COPD have good safety profiles. Typical side-effects of inhaled anticholinergics are dry mouth and prostatism,102 and for inhaled steroids are skin bruising103 ocular effects, and osteoporosis.104 β2 agonists are associated with tremor and cardiac effects.104 Cardiovascular side-effects of tiotropium and ipratropium were reported in a meta-analysis by Singh and colleagues,105 but this finding was not confirmed in a trial of tiotropium106 and a later review of all tiotropium trials.102 The latter was accepted as sufficient proof of the absence of toxic effects by the US Food and Drug Administration.107 A fixed combination of β2 agonists and inhaled steroids has been associated with the risk of pneumonia.96, 108, 109, 110, 111, 112 This risk does not seem to be present with budesonide,113 although the reason for this discrepancy is unclear.
Several issues related to the treatment of COPD are the subjects of debate. The most important is probably whether bronchodilators, inhaled corticosteroids, or both, modify the disease course. No direct evidence clearly shows a disease-modifying role, but circumstantial evidence is accumulating. Two large landmark trials have shown a trend for reduced mortality with fixed combinations96 and significantly reduced mortality was seen with a long-acting anti-cholinergic agent in two analyses, although this effect was not seen in a third analysis.97 A post-hoc analysis showed a significant reduction in the decline of FEV1, by 16 mL per year, with fixed combinations,114 and a prespecified subgroup analysis showed a reduction of 6 mL per year with a long-acting anticholinergic agent in patients with GOLD stage 2 COPD.115 In the latter analysis most of the patients in the control group were taking a combination of a long-acting β2 agonists and an inhaled steroid and, therefore, these effects on annual rate of decline seem to be additive. Thus, with the appropriate combination of agents, tangible effects might be obtained. In a smaller subgroup of patients who took no maintenance medications at the beginning of the trial, tiotropium slowed the rate of decline in postbronchodilator FEV1 by 11 mL per year and of deterioration in health-related quality of life by two-thirds.116 Finally, safety data indicate that reductions in the incidence of respiratory failure and myocardial infarction as a serious adverse events have been seen with tiotropium.97
Another question of great interest is whether early pharmacotherapy—when disease is in GOLD stages 1 and 2—is warranted in COPD.117 Again, no direct evidence presently supports this practice because no prospective, randomised, controlled trials have been done. The studies of fixed combinations and tiotropium mentioned above both showed that the effects of pharmacotherapy were similar in patients with GOLD stage 2 COPD and those with more advanced disease.115, 118 Decline in FEV1, and hence disease progression, is faster in the early stages of COPD than in the later stages.90, 96, 97 Early intervention, therefore, seems as though it would be beneficial.
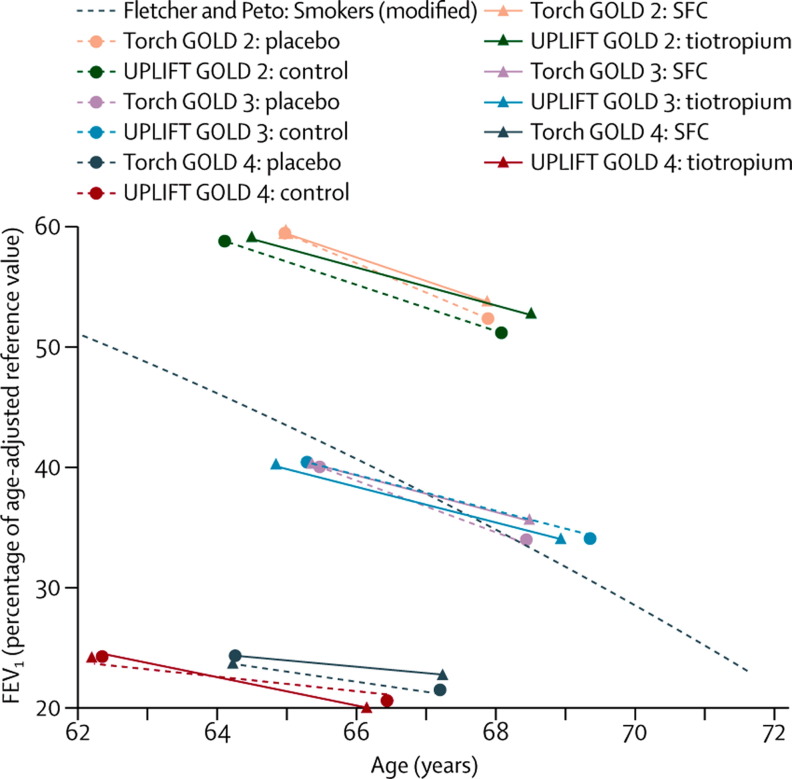
Figure 5 summarises, in a so-called Fletcher-Peto diagram, the data on FEV1 decline in relation to age from two landmark studies.117, 119 Although some evidence of a reduced rate of decline in FEV1 is present in GOLD stage 2 particularly,116, 118 the lines depicting the decline in FEV1 in the different GOLD stages are essentially parallel. Thus, how patients would move from GOLD stage 2 to 3, and further to 4, is hard to conceive. Several potential explanations for this pattern may be offered. First, patients who reach GOLD stages 3–4 have poor pulmonary function at diagnosis (lower intercept). For instance, patients who have low lung function at presentation because of genetic factors or exposure to environmental factors before age 4 years are at increased risk of developing chronic respiratory disease.4, 120 Second, patients who reach GOLD stages 3–4 also have a faster decline earlier in the disease course than patients in whom disease reaches less advanced stages.90, 115, 118 Finally, comorbidities of COPD arise in the early disease stages.73, 74 No randomised controlled trials have yet been done to investigate whether treatment with bronchodilators or inhaled corticosteroids reduces the prevalence or severity of comorbidities. Observational studies suggest that statins, β blockers, and angiotensin-converting-enzyme inhibitors improve outcomes and survival,121, 122, 123 although these studies might be statistically flawed simply because of their observational nature.124
Figure 5.
FEV1 decline in relation to increasing age in patients with COPD in GOLD stages 2–4 treated with a combined long-acting β2 agonist and inhaled corticosteroid or tiotropium
Data from the TORCH96 and UPLIFT97 studies are plotted on a Fletcher-Peto diagram. The black dashed line represents the original Fletcher-Peto curve.119 GOLD=Global Initiative on Obstructive Lung Disease. SFC=salmeterol and fluticasone combined. FEV1=forced expiratory volume in 1 s. Reproduced from reference 117 by permission of BMJ Publishing Group Ltd.
A last relevant question is whether continuous treatment with antibiotics is useful in stable COPD. Macrolides, particularly azithromycin, have been the subject of substantial interest in the past 5 years. In one randomised study of long-term erythromycin compared with placebo in patients with COPD, exacerbations were less frequent (35% reduction) and shorter (13 vs 9 days) in the macrolide group.125 In a randomised study of 1577 patients, treatment with azithromycin for 1 year lowered the risk of an exacerbation by 27% and improved quality of life by 2·8 units on the St George's respiratory questionnaire but caused decrements in hearing more frequently than placebo.126 Potential effects of azithromycin have been related to increased alveolar macrophage phagocytic function.127, 128 One study assessed the effect of pulse therapy with moxifloxacin for 5 days every 8 weeks. A 19% reduction in the frequency of exacerbations was seen,129 but this effect requires confirmation in other studies.
Other agents that might be of benefit in the treatment of COPD include phosphodiesterase inhibitors, such as theophylline, mucolytic agents, and antioxidants.130 The only effect that seems to have been reported is a 21% reduction in the rate of exacerbations with mucolytic agents.131 This effect might be larger in patients only taking short-acting bronchodilators,132 and is not seen in patients taking inhaled corticosteroids.133
Exacerbations
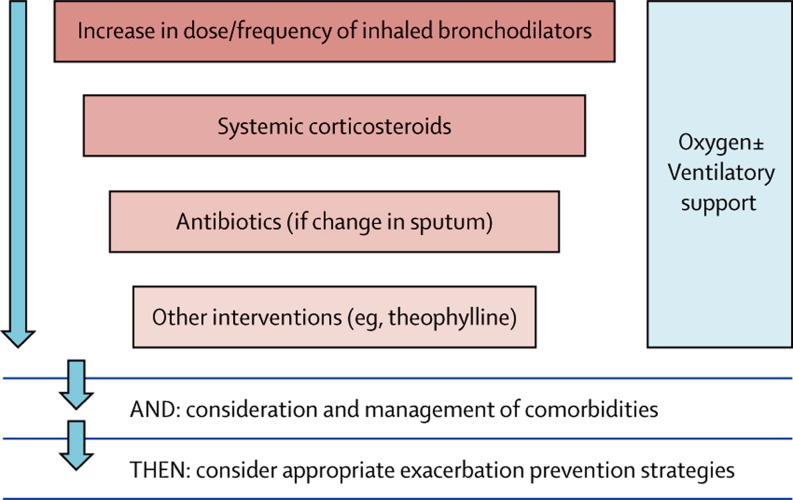
Several national and international guidelines for the treatment of COPD exacerbations are available.134, 135 The first step is to increase the dose and frequency of short-acting β2 agonists, anticholinergic bronchodilators, or both. If no response is noted, oral corticosteroids may be added. If changes are seen in expectorated sputum, antibiotics can be used, and if severity increases, other interventions, such as theophylline, should be considered. Oxygen and ventilatory support is recommended if respiratory failure occurs (figure 6 ).136
Figure 6.
General approach to management of exacerbations in chronic obstructive pulmonary disease136
Non-invasive ventilatory support is the first approach to hypercapnic respiratory failure and is effective in avoiding intubation and reducing the risk of death.137 Cochrane reviews support the use of short-acting bronchodiolators,138 systemic corticosteroids,139 antibiotics,140 and non-invasive ventilation,137 and a meta-analysis supports the use of theophylline.141 Besides these respiratory treatments, the complexity (eg, associated with pneumonia, heart failure, or pneumothorax) need to be taken into account and comorbidities need to be treated appropriately.
Conclusions and future directions
Substantial unmet needs remain in COPD and improved insight is required into the pathophysiology and effective treatments. From a diagnostic point of view, specific disease biomarkers, improved methods for early detection and diagnosis of exacerbations, and enhanced understanding of the relations between COPD and comorbidities would be helpful. Additionally, an important question that so far remains unanswered is whether different phenotypes of the disease exist and, if so, whether they respond differently to treatment.
Research is being done into new compounds to treat COPD.142 Roflumilast, an oral specific phosphodiesterase 4 inhibitor, improved postbronchodilator FEV1 by about 48 mL and was associated with a 17% reduction in the frequency of exacerbations in patients with GOLD stage 3–4 COPD and history of exacerbations, cough, and sputum changes, but had no effect on health-related quality of life or systemic inflammation.143 Similar effects were seen in patients who received roflumilast in addition to salmeterol or tiotropium.144 Indacaterol is a new, once-daily, long-acting β2 agonist that provides sustained bronchodilation with an acceptable safety profile.145, 146 Improvements in FEV1 of 160–170 mL have been reported and discussed.145 Several new, once-daily, long-acting anticholinergic agents and β2 agonists are under development and being tested alone and in combination, with each other and with once-daily inhaled steroids. Additionally, agents that possess simultaneous anticholinergic and β2-agonist activities (muscarinic antagonist β2 agonists) are being developed.147 These latter agents will probably improve symptomatic control, but it seems unlikely that they will modify disease substantially more than available agents. By contrast, enhanced disease modification is expected from a range of novel inflammatory blockers that includes inhibitors of phosphodiesterase 4, agents targeting CD8+ T cells,142 and inhibitors of NF-κB,142 chemokine-receptors 2 and 3,142, 148 T-helper-17 cells,142 and MAP kinase p38.142, 149
Interest has been shown in the role of the vitamin D pathway in chronic diseases in general and in COPD in particular.150, 151 In one study a high prevalence of vitamin D deficiency was found and variants in the vitamin-D-binding gene (GC) correlated with vitamin D levels and the risk of COPD.152 Impaired macrophage function, which improves after vitamin D supplementation, is probably related to the development of COPD.128 Randomised controlled trials are being done to assess the effects of vitamin D supplementation on the disease course of COPD (ClinicalTrials.gov NCT00977873).153 In the first of these studies, vitamin D supplementation did not reduce COPD exacerbations in the whole study population, but it did in a subgroup with low vitamin D levels (<10 ng/mL) at baseline.153
Finally, entirely new care models need to be developed for patients with COPD as well as other chronic diseases. In view of the comorbidities frequently seen in patients with COPD, new models for multidisciplinary care, clinical pathways, self-management,154 teleconsultating,155 telemonitoring, and rehabilitation99 are required. In studies the latter four modalities have been associated with clear effects on outcomes, but access to these services remains poor—for instance, rehabilitation is applied to less than 5% of the eligible patients.99, 153, 154
Search strategy and selection criteria
We searched the Cochrane Library, Medline, and Embase for papers published in 2006–10. We used the terms “COPD-Epidemiology and causes”, “COPD-Pathophysiology”, “COPD-Exacerbations”, “COPD-Systemic manifestations”, “COPD-Co-morbidities”, “COPD-Clinical management”, and “COPD-Specific drug classes”. We also searched the reference lists of identified articles for further relevant papers, and we included older widely referenced publications.
Contributors
MD wrote the first draft of the paper, with input from WJ and MM, and all authors collaborated to develop the final content of the manuscript. MD took final responsibility for the decision to submit.
Conflicts of interest
MD has received speaker fees from Boehringer-Pfizer, GlaxoSmithKline, and AstraZeneca, consulting fees from Boehringer-Pfizer, GlaxoSmithKline, AstraZeneca, Dompé, Novartis, and Nycomed, and grant support from AstraZeneca, GlaxoSmithKline, UCB, and Chiesi. WJ received consulting fees from AstraZeneca, Boehringer-Pfizer, and Novartis. MM has received speaker fees from Boehringer-Pfizer, AstraZeneca, Bayer Schering, Talecris, and Novartis, consulting fees from Boehringer-Pfizer, GlaxoSmithKline, AstraZeneca, Bayer Schering, Dompé, Almirall, Novartis, and Nycomed, and grant support from Talecris.
Footnotes
In patients with severe impairment of forced expiratory volume in 1 s and other known risk factors.52
References
- 1.Global Initiative for Chronic Obstructive Lung Disease Global strategy for the diagnosis, management, and prevention of COPD: updated 2010. http://www.goldcopd.org/uploads/users/files/GOLDReport_April112011.pdf (accessed Oct 20, 2011).
- 2.Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (The BOLD Study): a population-based prevalence study. Lancet. 2007;370:741–750. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 3.Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370:765–773. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 4.Menezes AM, Perez-Padilla R, Jardim JR, et al. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet. 2005;366:1875–1881. doi: 10.1016/S0140-6736(05)67632-5. [DOI] [PubMed] [Google Scholar]
- 5.WHO World health statistics 2008. http://www.who.int/whosis/whostat/EN_WHS08_Full.pdf (accessed Oct 20, 2011).
- 6.Jemal A, Ward E, Hao Y, et al. Trends in the leading causes of death in the United States, 1970–2002. JAMA. 2005;294:1255–1259. doi: 10.1001/jama.294.10.1255. [DOI] [PubMed] [Google Scholar]
- 7.Gershon AS, Wang C, Wilton AS, et al. Trends in chronic obstructive pulmonary disease prevalence, incidence, and mortality in Ontario, Canada, 1996 to 2007: a population-based study. Arch Intern Med. 2010;170:560–565. doi: 10.1001/archinternmed.2010.17. [DOI] [PubMed] [Google Scholar]
- 8.Soriano JB, Ancochea J, Miravitlles M, et al. Recent trends in COPD prevalence in Spain: a repeated cross-sectional survey 1997–2007. Eur Respir J. 2010;36:758–765. doi: 10.1183/09031936.00138409. [DOI] [PubMed] [Google Scholar]
- 9.Halbert RJ, Natoli JL, Gano A, et al. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28:523–532. doi: 10.1183/09031936.06.00124605. [DOI] [PubMed] [Google Scholar]
- 10.Vollmer WM, Gislason T, Burney P, et al. Comparison of spirometry criteria for the diagnosis of COPD: results from the BOLD study. Eur Respir J. 2009;34:588–597. doi: 10.1183/09031936.00164608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miravitlles M, Soriano JB, Garcia-Rio F, et al. Prevalence of COPD in Spain: impact of undiagnosed COPD on quality of life and daily life activities. Thorax. 2009;64:863–868. doi: 10.1136/thx.2009.115725. [DOI] [PubMed] [Google Scholar]
- 12.Hvidsten SC, Storesund L, Wentzel-Larsen T, et al. Prevalence and predictors of undiagnosed chronic obstructive pulmonary disease in a Norwegian adult general population. Clin Respir J. 2010;4:13–21. doi: 10.1111/j.1752-699X.2009.00137.x. [DOI] [PubMed] [Google Scholar]
- 13.Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance-United States, 1971–2000. MMWR Surveill Summ. 2002;51:1–16. [PubMed] [Google Scholar]
- 14.Svanes C, Sunyer J, Plana E, et al. Early life origins of chronic obstructive pulmonary disease. Thorax. 2010;65:14–20. doi: 10.1136/thx.2008.112136. [DOI] [PubMed] [Google Scholar]
- 15.Eisner MD, Anthonisen N, Coultas D, et al. An official American Thoracic Society public policy statement: novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182:693–718. doi: 10.1164/rccm.200811-1757ST. [DOI] [PubMed] [Google Scholar]
- 16.Gooptu B, Ekeowa UI, Lomas DA. Mechanisms of emphysema in α1-antitrypsin deficiency: molecular and cellular insights. Eur Respir J. 2009;34:475–488. doi: 10.1183/09031936.00096508. [DOI] [PubMed] [Google Scholar]
- 17.Hancock DB, Eijgelsheim M, Wilk JB, et al. Meta-analyses of genome-wide association studies identify multiple loci associated with pulmonary function. Nat Genet. 2010;42:45–52. doi: 10.1038/ng.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pillai SG, Ge D, Zhu G, et al. A genome-wide association study in chronic obstructive pulmonary disease (COPD): identification of two major susceptibility loci. PLoS Genet. 2009;5:e1000421. doi: 10.1371/journal.pgen.1000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilk JB, Chen TH, Gottlieb DJ, et al. A genome-wide association study of pulmonary function measures in the Framingham Heart Study. PLoS Genet. 2009;5:e1000429. doi: 10.1371/journal.pgen.1000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunninghake GM, Cho MH, Tesfaigzi Y, et al. MMP12, lung function, and COPD in high-risk populations. N Engl J Med. 2009;361:2599–2608. doi: 10.1056/NEJMoa0904006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lambrechts D, Buysschaert I, Zanen P, et al. The 15q24/25 susceptibility variant for lung cancer and chronic obstructive pulmonary disease is associated with emphysema. Am J Respir Crit Care Med. 2010;181:486–493. doi: 10.1164/rccm.200909-1364OC. [DOI] [PubMed] [Google Scholar]
- 22.O'Donnell DE. Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:180–184. doi: 10.1513/pats.200508-093DO. [DOI] [PubMed] [Google Scholar]
- 23.Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol. 2009;4:435–459. doi: 10.1146/annurev.pathol.4.110807.092145. [DOI] [PubMed] [Google Scholar]
- 24.Hogg JC, McDonough JE, Gosselink JV, et al. What drives the peripheral lung-remodeling process in chronic obstructive pulmonary disease? Proc Am Thorac Soc. 2009;6:668–672. doi: 10.1513/pats.200907-079DP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacNee W, Tuder RM. New paradigms in the pathogenesis of chronic obstructive pulmonary disease I. Proc Am Thorac Soc. 2009;6:527–531. doi: 10.1513/pats.200905-027DS. [DOI] [PubMed] [Google Scholar]
- 26.Silverman EK, Spira A, Pare PD. Genetics and genomics of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6:539–542. doi: 10.1513/pats.200904-021DS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med. 2009;360:2445–2454. doi: 10.1056/NEJMra0804752. [DOI] [PubMed] [Google Scholar]
- 28.Opitz B, van Laak V, Eitel J, Suttorp N. Innate immune recognition in infectious and noninfectious diseases of the lung. Am J Respir Crit Care Med. 2010;181:1294–1309. doi: 10.1164/rccm.200909-1427SO. [DOI] [PubMed] [Google Scholar]
- 29.Demedts IK, Bracke KR, Van Pottelberge G, et al. Accumulation of dendritic cells and increased CCL20 levels in the airways of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:998–1005. doi: 10.1164/rccm.200608-1113OC. [DOI] [PubMed] [Google Scholar]
- 30.Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 2003;22:672–688. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- 31.Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J. 2006;28:219–242. doi: 10.1183/09031936.06.00053805. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan AK, Simonian PL, Falta MT, et al. Oligoclonal CD4+ T cells in the lungs of patients with severe emphysema. Am J Respir Crit Care Med. 2005;172:590–596. doi: 10.1164/rccm.200410-1332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Strate BW, Postma DS, Brandsma CA, et al. Cigarette smoke-induced emphysema: a role for the B cell? Am J Respir Crit Care Med. 2006;173:751–758. doi: 10.1164/rccm.200504-594OC. [DOI] [PubMed] [Google Scholar]
- 34.Lee SH, Goswami S, Grudo A, et al. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med. 2007;13:567–569. doi: 10.1038/nm1583. [DOI] [PubMed] [Google Scholar]
- 35.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barcelo B, Pons J, Ferrer JM, et al. Phenotypic characterisation of T-lymphocytes in COPD: abnormal CD4+CD25+ regulatory T-lymphocyte response to tobacco smoking. Eur Respir J. 2008;31:555–562. doi: 10.1183/09031936.00010407. [DOI] [PubMed] [Google Scholar]
- 37.Alcorn JF, Crowe CR, Kolls JK. TH17 cells in asthma and COPD. Annu Rev Physiol. 2010;72:495–516. doi: 10.1146/annurev-physiol-021909-135926. [DOI] [PubMed] [Google Scholar]
- 38.Di Stefano A, Caramori G, Gnemmi I, et al. T helper type 17-related cytokine expression is increased in the bronchial mucosa of stable chronic obstructive pulmonary disease patients. Clin Exp Immunol. 2009;157:316–324. doi: 10.1111/j.1365-2249.2009.03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence in patients with pulmonary emphysema. Am J Respir Crit Care Med. 2006;174:886–893. doi: 10.1164/rccm.200509-1374OC. [DOI] [PubMed] [Google Scholar]
- 40.Tsuji T, Aoshiba K, Nagai A. Cigarette smoke induces senescence in alveolar epithelial cells. Am J Respir Cell Mol Biol. 2004;31:643–649. doi: 10.1165/rcmb.2003-0290OC. [DOI] [PubMed] [Google Scholar]
- 41.Ito K, Barnes PJ. COPD as a disease of accelerated lung aging. Chest. 2009;135:173–180. doi: 10.1378/chest.08-1419. [DOI] [PubMed] [Google Scholar]
- 42.Tuder RM, Yoshida T, Fijalkowka I, et al. Role of lung maintenance program in the heterogeneity of lung destruction in emphysema. Proc Am Thorac Soc. 2006;3:673–679. doi: 10.1513/pats.200605-124SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giangreco A, Arwert EN, Rosewell IR, et al. Stem cells are dispensable for lung homeostasis but restore airways after injury. Proc Natl Acad Sci USA. 2009;106:9286–9291. doi: 10.1073/pnas.0900668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rennard SI, Togo S, Holz O. Cigarette smoke inhibits alveolar repair: a mechanism for the development of emphysema. Proc Am Thorac Soc. 2006;3:703–708. doi: 10.1513/pats.200605-121SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol. 2010;298:L715–L731. doi: 10.1152/ajplung.00361.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoogendoorn M, Feenstra TL, Hoogenveen RT, et al. Association between lung function and exacerbation frequency in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2010;5:435–444. doi: 10.2147/COPD.S13826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 48.Doll H, Miravitlles M. Health-related QOL in acute exacerbations of chronic bronchitis and chronic obstructive pulmonary disease: a review of the literature. Pharmacoeconomics. 2005;23:345–363. doi: 10.2165/00019053-200523040-00005. [DOI] [PubMed] [Google Scholar]
- 49.Soler-Cataluna JJ, Martinez-Garcia MA, Roman SP, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hurst JR, Donaldson GC, Perera WR, et al. Use of plasma biomarkers at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;174:867–874. doi: 10.1164/rccm.200604-506OC. [DOI] [PubMed] [Google Scholar]
- 51.Bozinovski S, Hutchinson A, Thompson M, et al. Serum amyloid A is a biomarker of acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:269–278. doi: 10.1164/rccm.200705-678OC. [DOI] [PubMed] [Google Scholar]
- 52.Papi A, Bellettato CM, Braccioni F, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173:1114–1121. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- 53.Stockley RA, O'Brien C, Pye A, et al. Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. Chest. 2000;117:1638–1645. doi: 10.1378/chest.117.6.1638. [DOI] [PubMed] [Google Scholar]
- 54.Soler N, Agusti C, Angrill J, et al. Bronchoscopic validation of the significance of sputum purulence in severe exacerbations of chronic obstructive pulmonary disease. Thorax. 2007;62:29–35. doi: 10.1136/thx.2005.056374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131:9–19. doi: 10.1378/chest.06-1500. [DOI] [PubMed] [Google Scholar]
- 56.Daniels JM, Schoorl M, Snijders D, et al. Procalcitonin vs C-reactive protein as predictive markers of response to antibiotic therapy in acute exacerbations of COPD. Chest. 2010;138:1108–1115. doi: 10.1378/chest.09-2927. [DOI] [PubMed] [Google Scholar]
- 57.Monso E, Ruiz J, Rosell A, et al. Bacterial infection in chronic obstructive pulmonary disease. A study of stable and exacerbated outpatients using the protected specimen brush. Am J Respir Crit Care Med. 1995;152:1316–1320. doi: 10.1164/ajrccm.152.4.7551388. [DOI] [PubMed] [Google Scholar]
- 58.Miravitlles M, Marin A, Monso E, et al. Colour of sputum is a marker for bacterial colonisation in chronic obstructive pulmonary disease. Respir Res. 2010;11:58. doi: 10.1186/1465-9921-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355–2365. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 60.Sethi S, Evans N, Grant BJ, et al. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347:465–471. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 61.Sethi S, Wrona C, Eschberger K, et al. Inflammatory profile of new bacterial strain exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:491–497. doi: 10.1164/rccm.200708-1234OC. [DOI] [PubMed] [Google Scholar]
- 62.Wilkinson TM, Patel IS, Wilks M, et al. Airway bacterial load and FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:1090–1095. doi: 10.1164/rccm.200210-1179OC. [DOI] [PubMed] [Google Scholar]
- 63.Marin A, Monso E, Garcia-Nunez M, et al. Variability and effects of bronchial colonisation in patients with moderate COPD. Eur Respir J. 2010;35:295–302. doi: 10.1183/09031936.00126808. [DOI] [PubMed] [Google Scholar]
- 64.Miravitlles M, Espinosa C, Fernandez-Laso E, et al. Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Study Group of Bacterial Infection in COPD. Chest. 1999;116:40–46. doi: 10.1378/chest.116.1.40. [DOI] [PubMed] [Google Scholar]
- 65.Murphy TF, Brauer AL, Eschberger K, et al. Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:853–860. doi: 10.1164/rccm.200709-1413OC. [DOI] [PubMed] [Google Scholar]
- 66.Ewig S, Soler N, Gonzalez J, et al. Evaluation of antimicrobial treatment in mechanically ventilated patients with severe chronic obstructive pulmonary disease exacerbations. Crit Care Med. 2000;28:692–697. doi: 10.1097/00003246-200003000-00015. [DOI] [PubMed] [Google Scholar]
- 67.Montero M, Dominguez M, Orozco-Levi M, et al. Mortality of COPD patients infected with multi-resistant Pseudomonas aeruginosa: a case and control study. Infection. 2009;37:16–19. doi: 10.1007/s15010-008-8125-9. [DOI] [PubMed] [Google Scholar]
- 68.Monso E, Garcia-Aymerich J, Soler N, et al. Bacterial infection in exacerbated COPD with changes in sputum characteristics. Epidemiol Infect. 2003;131:799–804. doi: 10.1017/s0950268803008872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Allegra L, Blasi F, Diano P, et al. Sputum color as a marker of acute bacterial exacerbations of chronic obstructive pulmonary disease. Respir Med. 2005;99:742–747. doi: 10.1016/j.rmed.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 70.Mallia P, Message SD, Gielen V, et al. Experimental Rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med. 2011;183:734–742. doi: 10.1164/rccm.201006-0833OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Quint JK, Donaldson GC, Goldring JJ, et al. Serum IP-10 as a biomarker of human rhinovirus infection at exacerbation of COPD. Chest. 2010;137:812–822. doi: 10.1378/chest.09-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McManus TE, Marley AM, Baxter N, et al. Respiratory viral infection in exacerbations of COPD. Respir Med. 2008;102:1575–1580. doi: 10.1016/j.rmed.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Decramer M, Rennard S, Troosters T, et al. COPD as a lung disease with systemic consequences-clinical impact, mechanisms, and potential for early intervention. COPD. 2008;5:235–256. doi: 10.1080/15412550802237531. [DOI] [PubMed] [Google Scholar]
- 74.Fabbri LM, Luppi F, Beghe B, et al. Complex chronic comorbidities of COPD. Eur Respir J. 2008;31:204–212. doi: 10.1183/09031936.00114307. [DOI] [PubMed] [Google Scholar]
- 75.Mannino DM, Thorn D, Swensen A, et al. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32:962–969. doi: 10.1183/09031936.00012408. [DOI] [PubMed] [Google Scholar]
- 76.Sin DD, Anthonisen NR, Soriano JB, et al. Mortality in COPD: role of comorbidities. Eur Respir J. 2006;28:1245–1257. doi: 10.1183/09031936.00133805. [DOI] [PubMed] [Google Scholar]
- 77.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 78.Edwards R. The problem of tobacco smoking. BMJ. 2004;328:217–219. doi: 10.1136/bmj.328.7433.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pitta F, Troosters T, Spruit MA, et al. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171:972–977. doi: 10.1164/rccm.200407-855OC. [DOI] [PubMed] [Google Scholar]
- 80.Booth FW, Gordon SE, Carlson CJ, et al. Waging war on modern chronic diseases: primary prevention through exercise biology. J Appl Physiol. 2000;88:774–787. doi: 10.1152/jappl.2000.88.2.774. [DOI] [PubMed] [Google Scholar]
- 81.Heath GW. Physical activity transitions and chronic disease. Am J Lifestyle Med. 2009;3:27S–31S. doi: 10.1177/1559827609334504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294:716–724. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 83.Burgel PR, Paillasseur JL, Caillaud D, et al. Clinical COPD phenotypes: a novel approach using principal component and cluster analyses. Eur Respir J. 2010;36:531–539. doi: 10.1183/09031936.00175109. [DOI] [PubMed] [Google Scholar]
- 84.Fabbri LM, Rabe KF. From COPD to chronic systemic inflammatory syndrome? Lancet. 2007;370:797–799. doi: 10.1016/S0140-6736(07)61383-X. [DOI] [PubMed] [Google Scholar]
- 85.Mora S, Cook N, Buring JE, et al. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116:2110–2118. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yanbaeva DG, Dentener MA, Creutzberg EC, et al. Systemic effects of smoking. Chest. 2007;131:1557–1566. doi: 10.1378/chest.06-2179. [DOI] [PubMed] [Google Scholar]
- 87.Broekhuizen R, Wouters EF, Creutzberg EC, et al. Raised CRP levels mark metabolic and functional impairment in advanced COPD. Thorax. 2006;61:17–22. doi: 10.1136/thx.2005.041996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33:1165–1185. doi: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]
- 89.Sinden NJ, Stockley RA. Systemic inflammation and comorbidity in COPD: a result of ‘overspill’ of inflammatory mediators from the lungs? Review of the evidence. Thorax. 2010;65:930–936. doi: 10.1136/thx.2009.130260. [DOI] [PubMed] [Google Scholar]
- 90.Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994;272:1497–1505. [PubMed] [Google Scholar]
- 91.Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med. 2002;166:675–679. doi: 10.1164/rccm.2112096. [DOI] [PubMed] [Google Scholar]
- 92.Anthonisen NR, Skeans MA, Wise RA, et al. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142:233–239. doi: 10.7326/0003-4819-142-4-200502150-00005. [DOI] [PubMed] [Google Scholar]
- 93.Lacasse Y, Goldstein R, Lasserson TJ, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;4 doi: 10.1002/14651858.CD003793.pub2. CD003793. [DOI] [PubMed] [Google Scholar]
- 94.Troosters T, Casaburi R, Gosselink R, et al. Pulmonary rehabilitation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:19–38. doi: 10.1164/rccm.200408-1109SO. [DOI] [PubMed] [Google Scholar]
- 95.Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131:4S–42S. doi: 10.1378/chest.06-2418. [DOI] [PubMed] [Google Scholar]
- 96.Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 97.Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543–1554. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 98.Casaburi R, Mahler DA, Jones PW, et al. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19:217–224. doi: 10.1183/09031936.02.00269802. [DOI] [PubMed] [Google Scholar]
- 99.Niewoehner DE, Rice K, Cote C, et al. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomized trial. Ann Intern Med. 2005;143:317–326. doi: 10.7326/0003-4819-143-5-200509060-00007. [DOI] [PubMed] [Google Scholar]
- 100.Dusser D, Bravo ML, Iacono P. The effect of tiotropium on exacerbations and airflow in patients with COPD. Eur Respir J. 2006;27:547–555. doi: 10.1183/09031936.06.00062705. [DOI] [PubMed] [Google Scholar]
- 101.Wedzicha JA, Calverley P, Seemungal T, et al. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008;177:19–26. doi: 10.1164/rccm.200707-973OC. [DOI] [PubMed] [Google Scholar]
- 102.Kesten S, Celli B, Decramer M, et al. Tiotropium HandiHaler in the treatment of COPD: a safety review. Int J Chron Obstruct Pulmon Dis. 2009;4:397–409. doi: 10.2147/copd.s4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pauwels RA, Lofdahl CG, Laitinen LA, et al. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. N Engl J Med. 1999;340:1948–1953. doi: 10.1056/NEJM199906243402503. [DOI] [PubMed] [Google Scholar]
- 104.Decramer M, Ferguson G. Clinical safety of long-acting β2-agonist and inhaled corticosteroid combination therapy in COPD. COPD. 2006;3:163–171. doi: 10.1080/15412550600830263. [DOI] [PubMed] [Google Scholar]
- 105.Singh S, Loke YK, Furberg CD. Inhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300:1439–1450. doi: 10.1001/jama.300.12.1439. [DOI] [PubMed] [Google Scholar]
- 106.Celli B, Decramer M, Leimer I, et al. Cardiovascular safety of tiotropium in patients with COPD. Chest. 2010;137:20–30. doi: 10.1378/chest.09-0011. [DOI] [PubMed] [Google Scholar]
- 107.Michele TM, Pinheiro S, Iyasu S. The safety of tiotropium—the FDA's conclusions. N Engl J Med. 2010;363:1097–1099. doi: 10.1056/NEJMp1008502. [DOI] [PubMed] [Google Scholar]
- 108.Crim C, Calverley PM, Anderson JA, et al. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur Respir J. 2009;34:641–647. doi: 10.1183/09031936.00193908. [DOI] [PubMed] [Google Scholar]
- 109.Nannini LJ, Cates CJ, Lasserson TJ, et al. Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2007;4 doi: 10.1002/14651858.CD006826. CD006826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Singh S, Amin AV, Loke YK. Long-term use of inhaled corticosteroids and the risk of pneumonia in chronic obstructive pulmonary disease: a meta-analysis. Arch Intern Med. 2009;169:219–229. doi: 10.1001/archinternmed.2008.550. [DOI] [PubMed] [Google Scholar]
- 111.Drummond MB, Dasenbrook EC, Pitz MW, et al. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300:2407–2416. doi: 10.1001/jama.2008.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ernst P, Gonzalez AV, Brassard P, et al. Inhaled corticosteroid use in chronic obstructive pulmonary disease and the risk of hospitalization for pneumonia. Am J Respir Crit Care Med. 2007;176:162–166. doi: 10.1164/rccm.200611-1630OC. [DOI] [PubMed] [Google Scholar]
- 113.Sin DD, Tashkin D, Zhang X, et al. Budesonide and the risk of pneumonia: a meta-analysis of individual patient data. Lancet. 2009;374:712–719. doi: 10.1016/S0140-6736(09)61250-2. [DOI] [PubMed] [Google Scholar]
- 114.Celli BR, Thomas NE, Anderson JA, et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med. 2008;178:332–338. doi: 10.1164/rccm.200712-1869OC. [DOI] [PubMed] [Google Scholar]
- 115.Decramer M, Celli B, Kesten S, et al. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet. 2009;374:1171–1178. doi: 10.1016/S0140-6736(09)61298-8. [DOI] [PubMed] [Google Scholar]
- 116.Troosters T, Celli B, Lystig T, et al. Tiotropium as a first maintenance drug in COPD: secondary analysis of the UPLIFT trial. Eur Respir J. 2010;36:65–73. doi: 10.1183/09031936.00127809. [DOI] [PubMed] [Google Scholar]
- 117.Decramer M, Cooper CB. Treatment of COPD: the sooner the better? Thorax. 2010;65:837–841. doi: 10.1136/thx.2009.133355. [DOI] [PubMed] [Google Scholar]
- 118.Jenkins CR, Jones PW, Calverley PM, et al. Efficacy of salmeterol/fluticasone propionate by GOLD stage of chronic obstructive pulmonary disease: analysis from the randomised, placebo-controlled TORCH study. Respir Res. 2009;10:59. doi: 10.1186/1465-9921-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fletcher C, Peto R. The natural history of chronic airflow obstruction. BMJ. 1977;1:1645–1648. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Weiss ST. Lung function and airway diseases. Nat Genet. 2010;42:14–16. doi: 10.1038/ng0110-14. [DOI] [PubMed] [Google Scholar]
- 121.Mancini GB, Etminan M, Zhang B, et al. Reduction of morbidity and mortality by statins, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers in patients with chronic obstructive pulmonary disease. J Am Coll Cardiol. 2006;47:2554–2560. doi: 10.1016/j.jacc.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 122.Rutten FH, Zuithoff NP, Hak E, et al. β-Blockers may reduce mortality and risk of exacerbations in patients with chronic obstructive pulmonary disease. Arch Intern Med. 2010;170:880–887. doi: 10.1001/archinternmed.2010.112. [DOI] [PubMed] [Google Scholar]
- 123.van Gestel YR, Hoeks SE, Sin DD, et al. Effect of statin therapy on mortality in patients with peripheral arterial disease and comparison of those with versus without associated chronic obstructive pulmonary disease. Am J Cardiol. 2008;102:192–196. doi: 10.1016/j.amjcard.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 124.Suissa S. Co-morbidity in COPD: the effects of cardiovascular drug therapies. Respiration. 2010;80:3–7. doi: 10.1159/000315387. [DOI] [PubMed] [Google Scholar]
- 125.Seemungal TA, Wilkinson TM, Hurst JR, et al. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;178:1139–1147. doi: 10.1164/rccm.200801-145OC. [DOI] [PubMed] [Google Scholar]
- 126.Albert RK, Connett J, Bailey WC, et al. COPD Clinical Research Network Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689–698. doi: 10.1056/NEJMoa1104623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hodge S, Hodge G, Jersmann H, et al. Azithromycin improves macrophage phagocytic function and expression of mannose receptor in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178:139–148. doi: 10.1164/rccm.200711-1666OC. [DOI] [PubMed] [Google Scholar]
- 128.Taylor AE, Finney-Hayward TK, Quint JK, et al. Defective macrophage phagocytosis of bacteria in COPD. Eur Respir J. 2010;35:1039–1047. doi: 10.1183/09031936.00036709. [DOI] [PubMed] [Google Scholar]
- 129.Sethi S, Jones PW, Theron MS, et al. Pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Respir Res. 2010;11:10. doi: 10.1186/1465-9921-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Decramer M, Janssens W. Mucoactive therapy in COPD. Eur Respir Rev. 2010;19:134–140. doi: 10.1183/09059180.00003610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Poole P, Black PN. Mucolytic agents for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2010;2 doi: 10.1002/14651858.CD001287.pub3. CD001287. [DOI] [PubMed] [Google Scholar]
- 132.Zheng JP, Kang J, Huang S-G, et al. Effect of carbocisteine on acute exacerbation of chronic obstructive pulmonary disease (PEACE Study): a randomised placebo-controlled study. Lancet. 2008;371:2013–2018. doi: 10.1016/S0140-6736(08)60869-7. [DOI] [PubMed] [Google Scholar]
- 133.Decramer M, Rutten-van Mölken M, Dekhuijzen PNR, et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): a randomised placebo-controlled trial. Lancet. 2005;365:1552–1560. doi: 10.1016/S0140-6736(05)66456-2. [DOI] [PubMed] [Google Scholar]
- 134.Chronic obstructive pulmonary disease National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax. 2004;59(suppl 1):1–232. [PMC free article] [PubMed] [Google Scholar]
- 135.Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 136.Hurst JR, Wedzicha JA. Management and prevention of chronic obstructive pulmonary disease exacerbations: a state of the art review. BMC Med. 2009;7:40. doi: 10.1186/1741-7015-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ram FS, Picot J, Lightowler J, et al. Non-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2004;3 doi: 10.1002/14651858.CD004104.pub3. CD004104. [DOI] [PubMed] [Google Scholar]
- 138.McCrory DC, Brown CD. Anticholinergic bronchodilators versus beta2-sympathomimetic agents for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2003;4 doi: 10.1002/14651858.CD003900. CD003900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Walters JA, Gibson PG, Wood-Baker R, et al. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2009;1 doi: 10.1002/14651858.CD001288.pub3. CD001288. [DOI] [PubMed] [Google Scholar]
- 140.Ram FS, Rodriguez-Roisin R, Granados-Navarrete A, et al. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;2 doi: 10.1002/14651858.CD004403.pub2. CD004403. [DOI] [PubMed] [Google Scholar]
- 141.Barr RG, Rowe BH, Camargo CA., Jr Methylxanthines for exacerbations of chronic obstructive pulmonary disease: meta-analysis of randomised trials. BMJ. 2003;327:643. doi: 10.1136/bmj.327.7416.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8:183–192. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- 143.Calverley PM, Rabe KF, Goehring UM, et al. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374:685–694. doi: 10.1016/S0140-6736(09)61255-1. [DOI] [PubMed] [Google Scholar]
- 144.Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009;374:695–703. doi: 10.1016/S0140-6736(09)61252-6. [DOI] [PubMed] [Google Scholar]
- 145.Beeh KM, Beier J. Indacaterol, a novel inhaled, once-daily, long-acting beta2-agonist for the treatment of obstructive airways diseases. Adv Ther. 2009;26:691–699. doi: 10.1007/s12325-009-0044-3. [DOI] [PubMed] [Google Scholar]
- 146.Tashkin DP. Indacaterol maleate for the treatment of chronic obstructive pulmonary disease. Expert Opin Pharmacother. 2010;11:2077–2085. doi: 10.1517/14656566.2010.499358. [DOI] [PubMed] [Google Scholar]
- 147.Ray NC, Alcaraz L. Muscarinic antagonist-beta-adrenergic agonist dual pharmacology molecules as bronchodilators: a patent review. Expert Opin Ther Pat. 2009;19:1–12. doi: 10.1517/13543770802630331. [DOI] [PubMed] [Google Scholar]
- 148.Bracke KR, Demedts IK, Joos GF, et al. CC-chemokine receptors in chronic obstructive pulmonary disease. Inflamm Allergy Drug Targets. 2007;6:75–79. doi: 10.2174/187152807780832292. [DOI] [PubMed] [Google Scholar]
- 149.Singh D, Smyth L, Borrill Z, et al. A randomized, placebo-controlled study of the effects of the p38 MAPK inhibitor SB-681323 on blood biomarkers of inflammation in COPD patients. J Clin Pharmacol. 2010;50:94–100. doi: 10.1177/0091270009347873. [DOI] [PubMed] [Google Scholar]
- 150.Janssens W, Lehouck A, Carremans C, et al. Vitamin D beyond bones in chronic obstructive pulmonary disease: time to act. Am J Respir Crit Care Med. 2009;179:630–636. doi: 10.1164/rccm.200810-1576PP. [DOI] [PubMed] [Google Scholar]
- 151.Chishimba L, Thickett DR, Stockley RA, et al. The vitamin D axis in the lung: a key role for vitamin D-binding protein. Thorax. 2010;65:456–462. doi: 10.1136/thx.2009.128793. [DOI] [PubMed] [Google Scholar]
- 152.Janssens W, Bouillon R, Claes B, et al. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax. 2010;65:215–220. doi: 10.1136/thx.2009.120659. [DOI] [PubMed] [Google Scholar]
- 153.Lehouck A, Mathieu C, Carremans C, et al. High doses of vitamin D to reduce exacerbations in COPD: a randomized controlled trial. Ann Intern Med. 2012;156:105–114. doi: 10.7326/0003-4819-156-2-201201170-00004. [DOI] [PubMed] [Google Scholar]
- 154.Bourbeau J, Julien M, Maltais F, et al. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Arch Intern Med. 2003;163:585–591. doi: 10.1001/archinte.163.5.585. [DOI] [PubMed] [Google Scholar]
- 155.Casas A, Troosters T, Garcia-Aymerich J, et al. Integrated care prevents hospitalisations for exacerbations in COPD patients. Eur Respir J. 2006;28:123–130. doi: 10.1183/09031936.06.00063205. [DOI] [PubMed] [Google Scholar]