Abstract
Introduction
Autoimmune retinopathy (AR) is a sight-threating retinal disorder that is mediated by autoantibodies (AAbs) against retinal proteins. The visual paraneoplastic syndromes, including cancer-associated retinopathy (CAR) and melanoma-associated retinopathy (MAR) are mediated by anti-retinal AAbs. A number of immunochemical techniques have been used to detect serum anti-retinal autoantibodies in patients to help with autoimmune diagnosis.
Area covered
We review techniques used for serum autoantibody evaluation in patients with suspected autoimmune retinopathy.
Expert opinion
Detection of serum AAbs have served as the standard diagnostic tool for autoimmune retinopathies and for management of retinal disorders. An identification of anti-retinal autoantibody or multiple autoantibodies can be useful for not only for diagnosis of autoimmune retinopathies but also for management of retinal disorders. We propose that the line-blotting technique used in conjunction with immunohistochemistry are the best and most reliable assays for detection of serum anti-retinal AAb in the context of clinical history and findings. Clinician should recognize that the majority of antigenic targets identified to date in retinal autoimmunity are ubiquitously expressed proteins (e.g. enolase), which may be difficult to reconcile with the specific patterns of retinal damage observed in CAR, MAR, or AR.
Keywords: autoimmunity, autoantibodies, anti-retinal antibody testing, Western blotting, immunohistochemistry, line blotting, protein arrays, CAR
1. Importance of Autoantibody Testing
A number of techniques have been developed to detect novel autoantibodies (AAbs) in patient’s sera. In retinal disorders, AAbs against retinal antigens have been associated with inflammatory and non-inflammatory conditions. An identification of anti-retinal autoantibody or multiple autoantibodies can be useful for not only for diagnosis but also for management of retinal disorders. Retinal degeneration disorders associated with AAbs against antigens present in the retina and also expressed by cancer cells were reported in the 1980s and were defined as visual paraneoplastic syndromes [1]. Paraneoplastic retinopathies are indirect, remote effects of cancer on the eye associated with specific serum AAbs, and are not caused by neoplastic invasion, metastases, or cancer treatment, or by infections, ischemic processes, metabolic and nutritional deficits [1, 2]. The visual paraneoplastic syndromes include cancer-associated retinopathy (CAR) and melanoma-associated retinopathy (MAR) [3, 4]. In later years, autoimmune retinopathy (AR) was identified as a condition that presents similar symptoms and findings as CAR or MAR, but without cancer [5, 6]. It is a rare disorder of the retina characterized by loss of visual acuity and visual fields, and photoreceptor dysfunction in the presence of anti-retinal AAbs [7]. Similar to AR is a disorder called acute zonal occult outer retinopathy (AZOOR) that can present with symptoms such as visual field loss and ERG findings similar to AR [8, 9]. It is typically bilateral but asymmetric and the majority of patients either stabilize or show partial recovery without treatment. Because autoimmunity is suspected to contribute to the pathogenicity of these retinopathies, measuring of serum AAbs has become an important factor in defining the role of autoimmunity in the syndrome [10–13].
Autoimmune retinopathies are uncommon and difficult to diagnose by clinical examination alone because many patients may have initially negligible retinal changes, often having overlapping symptoms between conditions. Therefore, the presence of specific AAbs against retinal proteins can be used to confirm the diagnosis of different autoimmune disorders and are also becoming increasingly important biomarkers to monitor the progression of the retinal disease [7, 13]. Autoantibodies are likely to appear at a very early stage in the autoimmune disease, which consist of a pre-clinical period, during which the symptoms are almost absent or very mild and vague [14, 15]. Later, abnormal clinical examination may include retinal pigmentary defects, vascular attenuation, reduced rod/cone function examined by electroretinogram, abnormal retinal structure examined by auto fluorescence and spectral domain optical coherence tomography and optic nerve pallor.
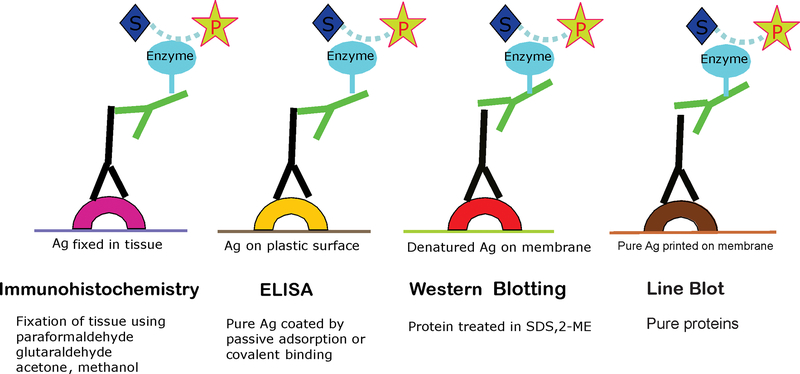
Here, we describe the various immunochemical techniques that have been used to detect specific AAbs. The most common procedures used for detection of anti-retinal AAbs are immunohistochemistry (IHC), western blotting (WB), and less frequently, an enzyme linked immunosorbent assay (ELISA). Each technique has its advantages and disadvantages. The detection of autoantibody-antigen recognition depends on the specific interaction between the antibody and its epitope, thus the appropriate choice of laboratory assay depends on their application (Figure 1).
Figure 1.
Diagram illustrating that different immune techniques are based on the antigen-antibody interaction regardless of antigen source (matrix).
2. Challenges in Autoantibodies Testing
AAbs have been found in the blood of all human sera, regardless of age, gender, or the presence or absence of disease [16]. There are hundreds of AAbs published to be associated with autoimmune and inflammatory disorders of the retina found in blood samples as well as vitreous humor using different approaches [17–22]. Blood samples (plasma or serum) are easy to collect and are most commonly used as a source of AAbs. To determine AAbs related to retinal autoimmune conditions human retinal protein extracts, purified retinal proteins, or antigenic proteins fixed in retinal tissue can be utilized. Particular antigens may have different properties, thus AAb accessibility to proteins may be challenging in different settings, and thus it is important to recognize limitations of available techniques. AAbs that target antigens that are located on the surface of cells are often being considered to be the main pathogenic AAbs. However, the majority of AAbs are directed against intracellular proteins but, in some cases, these proteins can change their localization, migrate to surface of the cell and become more accessible for AAbs, e.g enolase or recoverin [13, 23]. Those antibodies against intracellular antigens can penetrate cells and cause pathologic damage [24].
Over the years, a number of different techniques/approaches have been applied for identification of AAbs using protein extracts, native proteins, recombinant proteins, or protein peptides. Here, we discuss their application for anti-retinal AAb studies.
3. Detection of Autoantigens in Tissue (Immunochemistry)
Localization of a target cell of AAbs in retinal tissue can be examined by immunohistochemistry using frozen sections of primate or human donor retina at different serum dilutions (1:10, 1:50, 1:100) followed by secondary anti-human IgG conjugated to an enzyme horseradish peroxidase (HRP) for detection. Then the blot is immersed in the enzymatic substrate solution until dark brown color develops as a reaction product in the tissue. A commercially available HRP substrate, 3,3′-diaminobenzidine (DAB), is commonly used in the detection of antibody binding to different cells in the retina. Retinal labeling is examined in a light microscope. The use of different dilutions of patient’s serum or purified AAbs is applied to achieve the best sensitivity and specificity of serum examined in order to define positive and negative results.
Antibody binding to retinal cells can be also examined by more sensitive method such as immunofluorescent IHC on frozen retinal slides [23]. Detection of binding is localized by commercially available secondary antibodies labeled with a fluorescent dye and examined in a fluorescent or confocal microscope [22, 25]. In both the colorimetric and fluorescent methods, labeling to retinal layers, such as rods and cones in photoreceptor cell layer, outer nuclear layer, outer plexiform layer, inner nuclear layer, inner plexiform layer, and ganglion cell layer can be evaluated by intensity of the color or of the fluorescence, respectively. Patterns of immunostaining of human retina by different serum AAbs is usually dependent on antibody fine specificity [23]. The major disadvantage of detection of AAbs by IHC technique is that this methodology alone is not sufficient for AAb identification, and has to be followed up by the confirmation of specificity by western blotting or other, more sensitive methods. Another limitation of this method is that some AAbs cannot access an antigen in the tissue, producing a negative result and subsequently, special manipulations are required e.g. antigen retrieval. Preservation of tissue with formaldehyde can change protein chemistry and antibodies do not bind to antigenic epitopes. Antigen retrieval refers to any technique that reverse masked epitope and antibody binding is restored. Two methods are frequently used, protease-induced epitope retrieval and heat-induced epitope retrieval.
4. Identification of Autoantigens by Western Blotting (Immunoblotting)
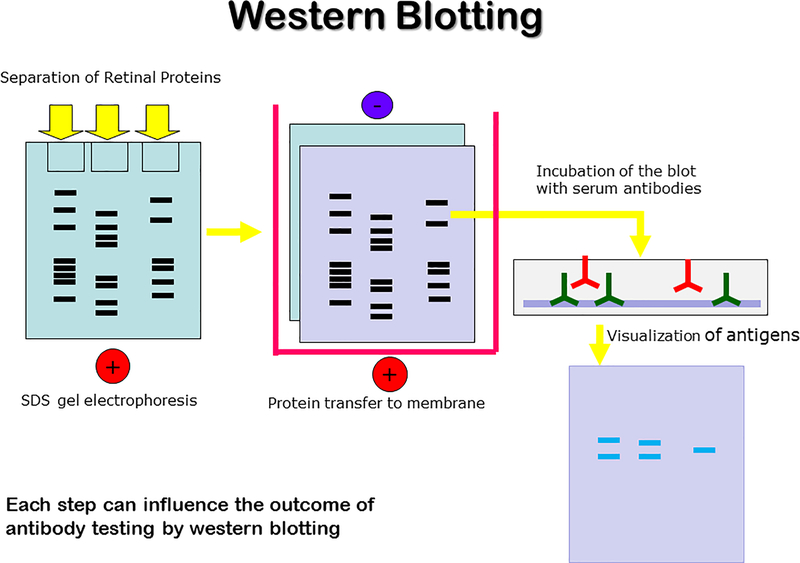
The western blotting method enables the detection of AAbs directed against retinal antigen extracts separated according to their molecular masses using discontinuous polyacrylamide gel electrophoresis (SDS-PAGE), following by transfer to a nitrocellulose or PVDF (polyvinylidene fluoride) membrane. Usually, 10% gels achieve good separation of proteins from 10,000 to 200,000 Da proteins, allowing minor antigenic proteins to be detected. Many companies offer pre-cast gels, which eliminate a reproducibility error. In recent years, novel buffers and materials have been developed that can be used with higher electric field strengths and lower the time of transfer.
Nitrocellulose membrane is easy to handle and wets readily in buffers, reducing time to complete testing. PVDF membrane is naturally hydrophobic and is ideal for western blotting because it ensures high signal with low background and it does not easily break when compared with nitrocellulose. Immunological detection of serum antibody binding to retinal proteins is used to identify specific retinal proteins by the use of the secondary anti-human antibody conjugated to enzymes, such as alkaline phosphatase or HRP (detection sensitivity 10 −100 pg), or to a fluorophore (1 pg – 1 ng), or chemoluminescence detection (1–10 pg), in which there is an enzymatic conversion of a substrate to a fluorescent product [12]. Because of its simplicity and sensitivity, immunoblotting has been widely used in retinal protein research and clinical testing.
Immunoblotting techniques consist of several steps, starting with blocking the membrane containing the transferred proteins and incubation with patient’s serum. The antibody specificity is identified by the presence of precipitation bands with the same molecular mass as those present on a strip, containing standard molecular weight markers. The basic steps of western blotting are illustrated in Fig 2. This method allows for detection of unfolded linear determinants in the protein. A verification of specificity of anti-retinal AAbs requires an additional immunoblotting with purified native or recombinant proteins of the appropriate molecular mass. For example, in our recent studies we have validated the antigen identity of 12 frequently recognized retinal proteins for immunoreactivity with pure native proteins, including recoverin, Rab6, retinal arrestin, heat shock proteins HSP27 and HSP60, carbonic anhydrase II, α-tubulin, aldolase C, α-enolase, GAPDH, PKM2, and CRALBP[17, 23].
Figure 2.
Western blotting workflow.
The major problem in comparing results from different laboratories is that different investigators use various animal tissues extracts (e.g. human, rodent) and protein extraction conditions, which influence the protein composition. Additionally, different electrophoresis systems produce distinct staining patterns after incubation with serum, and in effect, produce different results. Since the protein structure, content, and post-translational modifications are different in human and animal proteins, it is not surprising that different antigens can be identified [5]. In addition, different detection techniques may also contribute to the problem. This lack of standardized methods makes a comparison of results from different laboratories challenging.
Western blotting can be applied to retinal proteins separated by two-dimensional gel electrophoresis (2D). In this technique, proteins are first separated by their isoelectric points and then by molecular mass in SDS-PAGE. In our studies, application of 2D-western blotting allowed us to identify new antigenic proteins for AR patients with and without cancer (HSP60 and collapsin response-mediator protein-2, CRMP-2) [17].
5. Titration of Autoantibodies by Enzyme-linked Immunosorbent Assay (ELISA)
A traditional ELISA detects antibody responses against a single antigen and is one of the most sensitive immunoassays available, yet it is not often used in detection of anti-retinal AAbs. The reason is that this assay requires the use of a purified antigen, which is not always available. Small amounts of native and recombinant autoantigens can be used in ELISA, allowing the analysis of multiple sera in the same assay plate. Flat-bottomed plates, made from polystyrene or polyvinyl chloride, are used in the vast the majority of ELISA assays. There are several forms of ELISAs but the most popular are direct and indirect assays that are simple and rapid methods to identify immune responses against a single antigen. This method can quantify AAb titers in the serum or can measure binding to multiple distinct epitopes of a single antigen fragment/peptide. Non-specific binding is reduced by blocking buffer followed by secondary antibodies conjugated to an enzyme (e.g. HRP) and the application of enzyme substrate produces a colorimetric reaction that is read in an ELISA reader. The color intensity correlates to the amount of AAbs present in the serum sample. Typically, ELISA detects from 0.01 ng to 0.1 ng, but the sensitivity depends on the particular characteristics of the antibody-antigen interaction. In our laboratory, we have used ELISA for antibody titers as well as epitope mapping for different antigens, including rhodopsin, recoverin, enolase, carbonic anhydrase II, and bestrophin 1 [26–29].
6. Detection of Autoantibodies in Antigen Arrays
6.1. Line-blot
Antigen arrays represent another powerful approach for autoantibody detection and can detect many specific AAbs in a single test. Because most patients with retinal autoimmune disorders present heterogeneous phenotypes and multiple AAb responses, assays targeting multiple potential autoantigens generate better disease detection sensitivity.
We designed antigen panels, considering information from other assays and the frequency of antibody positivity in relation to clinical diseases. The most important factor in the preparation of a panel of autoantigens is the source of antigen (native protein vs. recombinant protein) and the purity of proteins used. In most cases, recombinant autoantigens are a convenient and reliable source of antigens that can be used in detection systems, but the purity of each protein needs to be examined by gel electrophoresis and tested with positive and negative controls of a given antibody before they are used in AAb testing. In particular, bacterial protein contaminations in recombinant protein preparations can yield a fault-positive result. Also, recombinant proteins may lack posttranslational modifications or may be improperly protein folded, and as such do not express epitopes in the same way as native antigens and may not fully correlate with that of native antigens. In such cases, the use of native proteins purified from mammalian tissues, or recombinant proteins produced in eukaryotic cells, may be necessary. The isolation of native antigenic proteins from natural sources also has limitations, such as purity, yield, and reproducibility.
Based on findings in our laboratory we have generated panels of antigens for retinal autoimmune disorders. Retinal proteins are printed directly on a membrane and validated with serum that previously tested positive. The panels of reactive autoantigens were selected based on the AAb frequency as determined by Western blotting for AR, CAR, or MAR. Autoantigens selected for use in our panels include photoreceptor proteins (recoverin, retinal arrestin, Rab6, TULP1), heat shock proteins (HSP27 and HSP60), carbonic anhydrase II, α-tubulin, and glycolytic enzymatic proteins that are found in photoreceptor cells (α-enolase, aldolase C, glyceraldehyde 3-phosphate dehydrogenase, pyruvate kinase M2).
The line-blot method enables the identification of IgG-class AAbs found in patients’ sera. The strips containing parallel lines of highly purified and recombinant human proteins are incubated in diluted serum (1:100 with buffer). After washing, anti-human IgG conjugated to alkaline phosphatase is used and then a color reaction is produced with phosphatase substrate. The availability of line-blot methods and the use of recombinant antigens make application, interpretation, and standardization easier. Considering that the line-blot method showed higher sensitivity and less background staining compared to the other methods, we recommend that this method should be the method of choice in searching for autoimmune and paraneoplastic AAbs. The benefits of this methodology include the exceptional stability of the antigens on nitrocellulose and the simplicity of their storage. The format of the line blot assay may also be particularly useful for screening the many antigens that might be considered in cases with nonspecific or atypical clinical presentations.
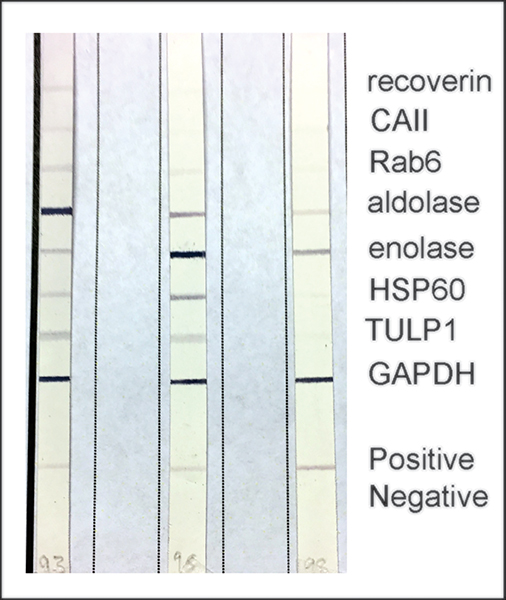
Fig. 3 represent a panel of autoantigens frequently associated with CAR reacting with serum AAbs of 3 randomly selected patients suspected of CAR. Because the disease is highly heterogeneous, several anti-retinal AAbs are frequently found in a single patient as is illustrated in Fig. 3, and patients may differ in their antibody profiles [17, 23, 30]. Antibody profile can also change with the progression of disease, with each stage having its own unique signature.
Figure. 3.
Examples of the CAR line-blot testing for 3 different sera from patient suspected of cancer-associated retinopathy, showing patterns individual for those antibodies. The serum samples were tested at the Ocular Immunology Laboratory in Portland, OR.
6.2. Protein Microarrays for Multiplexed Detection of Autoantibodies
The most advanced techniques for identification of antibody targets are microarrays [31–34]. Antigen arrays for testing of various human autoimmune diseases have been introduced some years ago [35]. In this procedure, proteins are spotted on a solid support (membrane, glass slide) and then incubated with serum, washed to remove unbound antibodies, and subsequently imaged using a secondary anti-IgG antibody conjugated to an enzyme or fluorescent molecule. A robotic microarrayer can be used to spot antigenic proteins onto poly-L lysine-coated microscope slides in duplicate or quadruplicate. This high density antigen microarray offers a major advantage in screening of multiple AAbs, but it is expensive and requires large amounts of purified antigens. Although this technique allow the analysis of many proteins simultaneously, the subsequent identification of reactive proteins is difficult. This technique has been applied in ocular research for an identification of AAb reactivities in serum and corresponding aqueous humor samples of primary open-angle glaucoma patients and non-glaucomatous controls [36]. Increased reactivities against some proteins, such as HSP27, MBP, and α−1-antitrypsin, as well as decreased reactivities against GFAP and β-l-crystalline were discovered in those studies.
A multiplex assay based on a Luminex technology requires microsphere-bound antigens and is more sensitive than Western blotting [37]. In this assay, an antigen suspension bead array using 188 different antigens representing 97 ocular proteins was applied to detect anti-retinal antibodies in sera of patients with presumed AR (n = 24), uveitis (n = 151), and cataract (n = 21)[21]. The results from bead arrays have to be validated with purified antigens by immunoblotting or ELISA. High titers of AAbs against photoreceptor-specific nuclear receptor (PNR) and retinol-binding protein 3 (RBP3) were shown in patients with presumed AR compared to uveitis.
SEREX (serological analysis of recombinantly expressed clones) is a method that combines a molecular cloning technique using a cDNA expression library prepared from retina and serological analysis of human antigens. cDNA library is cloned into phage expression vectors and the phages used to transfect E. coli. Then recombinant proteins are examine by immunoblotting Antibody detection by phage display has led to out-of-frame and 3′ UTR epitopes, reflecting the cDNA libraries used, or defective ribosomal products present in cells [38]. By screening retinal and melanoma cDNA phage libraries by SEREX, using sera of melanoma patients presenting with clinical symptoms of MAR, 20 new antigens were identified, including 6 that had previously been associated with retinal degeneration such as rhodopsin, retinal arrestin, MEK1, SRPX, BBS1, and galectin-3 [39]. Applying the SEREX approach to a retinal cDNA library, mitofilin and titin were also identified as potential targets for AAbs in the pathology of MAR [40].
One antigen that was identified from a retinal cDNA library with serum from a patient with atypical retinal degeneration was lens epithelium-derived growth factor (LEDGF). Western blot analysis revealed that sera from 3 patients reacted with p75/LEDGF, a survival factor that protects cells from oxidative, thermal, and UV damage [41]. Human homologue of polypyrimidine tract binding protein (PTB), a possible autoantigen for cancer-associated retinopathy (CAR), was also isolated from a human retinal cDNA library [42]. Two cDNA clones, recoverin, and tubby-like protein 1 (TULP1), were isolated from a human retinal cDNA library by using serum from a CAR patient as the probe [43].
Phage immunoprecipitation sequencing (PhIP-Seq) is a new technique for autoantigen discovery that is based on a synthetic representation of a complete human peptidome [44, 45]. This method has been used to identify novel autoantigens in neurologic paraneoplastic disorders, multiple sclerosis, and rheumatoid arthritis. PhIP-Seq was also used for an identification of novel autoantigens expressed in the retina in AR patients (RTN3, PRPF6, TRPC6, and B3GNT8), but not in healthy controls [46]. A human proteome (HuProt) microarray, representing >80% of all protein coding genes, was screened with serum from a patient with suspected CAR [25]. This method identified aryl hydrocarbon receptor-interacting protein-like (AIPL)-1, a protein specifically restricted to the retina and the pineal gland. The array also identified lipocalin 8, membrane-associated guanylate kinase WW, PDZ-containing domain 1, and doublecortin domain-containing 2 [25]. Those procedures are limited to the analysis of linear epitopes and the expression of some epitopes in bacteria is difficult.
Accessibility of antigenic epitopes by AAbs present in sera is essential for their detection. Since the protein structure, content, and post-translational modifications are different for different proteomic techniques, it is not surprising that different antigens are often identified. Nevertheless, microarray techniques are useful for screening of large numbers of AAbs and eventually may define the complete set of AAbs in healthy and diseased individuals. However, the effectiveness of microarray techniques in diagnostic testing for individual patients is unrealistic because of high cost and complexity of testing.
7. Conclusions
Serum AAbs have served as standard diagnostic biomarkers for many autoimmune diseases, and now for autoimmune retinopathies. They can be also used to monitor disease activity and treatment response. Immunoblotting techniques have proven reliable for detecting AAbs with different specificity and sensitivity. We propose that the gold standard of detection is the line-blotting technique in combination with immunohistochemical staining of retinal cross-sections. Line-blots of frequent biomarkers are the best and most reliable assays for anti-retinal AAb testing. Clinicians should be aware that the majority of antigen targets identified to date in retinal autoimmunity are ubiquitously expressed proteins (e.g. enolase), which may be difficult to reconcile with the specific patterns of retinal damage observed in CAR, MAR, or AR. Increased titers of such AAbs may be important in diagnostic process.
8. Expert Opinion
In this review, we discussed immunochemical techniques that have been used in search for AAbs against retinal proteins in serum and ocular fluid. There are several methods based on the antigen-antibody interaction in different matrices and all of them have advantages and disadvantages in an accurate determination of antibody specificity related to retinal disease. In the past several years, due to the great interest of ophthalmology specialists and advances in detection techniques, the list of autoantigens involved in humoral responses against retinal antigens has been expanding. Yet, a consensus on which antibody (or antibodies) is the most beneficial in diagnosis of autoimmune retinopathies has been an ongoing debate. The majority of antigen targets identified to date in retinal autoimmunity are ubiquitously expressed proteins (e.g. enolase or carbonic anhydrase II). This suggests that at least some autoimmune process might result in a pathogenic shift from the existing natural autoimmunity to an autoimmune disease. The shift might not need a new autoimmunization, but a loss of tolerance to retinal self-antigens in genetically susceptible individuals to induce autoimmunity. Similarly to other autoimmune diseases, AAbs against those common proteins may also be detected in lower titers in sera of healthy individuals. It is important to consider that the antibody titers against intracellular autoantigens are much higher in disease.
Autoantibodies against common antigens in organ-specific autoimmunity, as in autoimmune retinopathy, target the affected organ and not the entire body, in view of the fact that genetic, environmental, and hormonal factors may be responsible for the onset of autoimmunity. It is conceivable that autoantibodies against intracellular components could cause the clinical manifestations if the antigenic target migrates to the cell surface (e.g. recoverin, glycolytic enzymes, etc.), or if antibodies penetrate into the cell. Regarding antibody pathogenicity, not only recoverin, the first antigen described in patients with CAR, has pathogenic potential, but there is evidence that AAbs against photoreceptor specific proteins as well as more common antigens cause tissue damage in vivo and in vitro. The anatomical localization of autoantigens and metabolic consequences of the AAb causative action are important in antibody evaluation and, in consequence, the significance in their immunodetection. These are important considerations in designing antibody detecting procedures and in evaluating their usefulness in clinical practice.
Advances in techniques for AAb testing, such as antigen microarrays, enable a determination of large AAb repertoire, but they require a verification of autoantigen by a different method (e.g. ELISA), thus the effectiveness of microarrays in diagnostic testing is unrealistic at present because of their technical complexity, specificity, and high cost. We believe that the line-blots, consisting of an array of antigens frequently recognized by patients’ AAbs determined by prior methods, in conjunction with immunohistochemistry of retina are the best, most reliable, and sensitive assays for detection of serum anti-retinal AAbs. Because detection of AAbs by western blotting is not a standardized method used in laboratories, the line-blots could be considered as a gold standard technology in diagnosis of ARs and monitoring the progression of disease.
In clinical practice, diagnosis of autoimmune retinopathies are based on clinical presentation and ophthalmological findings, often ignoring testing for humoral (autoantibodies) or cellular immune responses. We believe that determination of serum anti-retinal AAbs is necessary for autoimmune retinopathies (CAR, MAR, AR) to be diagnosed, and only then, the condition can corroborate autoimmune processes.
Article Highlights.
Detection of anti-retinal autoantibodies can be useful for diagnosis and management of autoimmune retinopathies.
Immunochemical techniques have been used to determine serum anti-retinal autoantibodies in patients with presume autoimmune retinopathy.
The most common procedures for detection of anti-retinal AAbs are immunohistochemistry (IHC), western blotting (WB), and less frequently, an enzyme linked immunosorbent assay (ELISA).
Immunochemical techniques help to identified retina antigens associated with cancer associated retinopathy (CAR) that become important biomarkers to monitor the progression of the retinal disease
Line-blotting technique combined with IHC are the best and most reliable assays for detection of serum anti-retinal autoantibodies
Acknowledgments
Funding
This work was supported by grant P30 EY010572 from the National Institutes of Health (Bethesda, MD) and by unrestricted departmental funding from Research to Prevent Blindness (New York, NY).
Footnotes
Declaration of Interests
The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- [1].Grunwald GB, Simmonds MA, Klein R, Kornguth SE. Autoimmune basis for visual paraneoplastic syndrome in patients with small-cell lung carcinoma. Lancet, 1985;23:658–61. [DOI] [PubMed] [Google Scholar]
- [2].Klingele TG, Burde RM, Rappazzo JA, Isserman MJ, Burgess D, Kantor O. Paraneoplastic retinopathy. J Clin Neuro-ophthalmol, 1984;4:239–45. [PubMed] [Google Scholar]
- [3].Thirkill CE, FitzGerald P, Sergott RC, Roth AM, Tyler NK, Keltner JL. Cancer-Associated Retinopathy (CAR Syndrome) with Antibodies Reacting with Retinal, Optic-Nerve, and Cancer Cells. New Eng J Med, 1989;321:1589–94. [DOI] [PubMed] [Google Scholar]
- [4].Keltner JL, Thirkill CE, Yip PT. Clinical and immunologic characteristics of melanoma-associated retinopathy syndrome: eleven new cases and a review of 51 previously published cases. J Neuroophthalmol, 2001;21:173–87. [DOI] [PubMed] [Google Scholar]
- [5].Heckenlively J, Ferreyra H. Autoimmune retinopathy: A review and summary. Sem Immunopathol, 2008;30:127–34. [DOI] [PubMed] [Google Scholar]
- [6].Khanna S, Martins A, Oakey Z, Mititelu M. Non-paraneoplastic autoimmune retinopathy: multimodal testing characteristics of 13 cases. J Ophthal Inflamm Infec, 2019;9:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fox AR, Gordon LK, Heckenlively JR, Davis JL, Goldstein DA, Lowder CY et al. Consensus on the Diagnosis and Management of Nonparaneoplastic Autoimmune Retinopathy Using a Modified Delphi Approach. Am J Ophthalmol, 2016;168:183–90.** This paper summarized diagnostic criteria for nonparaneoplastic autoimmune retinopathy through expert panel consensus.
- [8].Tagami M, Matsumiya W, Imai H, Kusuhara S, Honda S, Azumi A. Autologous antibodies to outer retina in acute zonal occult outer retinopathy. Jap J Ophthalmol, 2014;58:462–72. [DOI] [PubMed] [Google Scholar]
- [9].Qian CX, Wang A, DeMill DL, Jayasundera T, Branham K, Abalem MF et al. Prevalence of Antiretinal Antibodies in Acute Zonal Occult Outer Retinopathy: A Comprehensive Review of 25 Cases. Am J Ophthalmol, 2017;176:210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Keltner JL, Roth AM, Chang S. Photoreceptor degeneration. Possible autoimmune disorder. Arch Ophthalmol, 1983;101:564–9.* The authors proposed an autoimmune mechanism as being responsible for the retinal degeneration in patients with retinitis pigmentosa and other retinal degenerative diseases
- [11].Keltner JL, Thirkill CE, Tyler NK, Roth AM. Management and monitoring of cancer-associated retinopathy. Arch Ophthalmol, 1992;110:48–53. [DOI] [PubMed] [Google Scholar]
- [12].Adamus G, Ren G, Weleber RG. Autoantibodies against retinal proteins in paraneoplastic and autoimmune retinopathy. BMC Ophthalmol, 2004;4:5.* A high prevalence of anti-retinal autoantibodies in retinopathy patients was discovered, showing a higher incidence of antibodies in CAR than in retinopathy patients without cancer.
- [13].Adamus G Autoantibody Targets and their Cancer Relationship in the Pathogenicity of Paraneoplastic Retinopathy. Autoimmun Rev, 2009;8:410–4.** The study showed that in some patients without diagnosed malignant tumors, the onset of ocular symptoms and the presence of autoantibodies preceded the diagnosis of cancer by months to years.
- [14].Bizzaro N Autoantibodies as predictors of disease: The clinical and experimental evidence. Autoimmun Rev, 2007;6:325–33. [DOI] [PubMed] [Google Scholar]
- [15].Hu ZD, Deng AM. Autoantibodies in pre-clinical autoimmune disease. Clin Chim Acta, 2014;437:14–8. [DOI] [PubMed] [Google Scholar]
- [16].Nagele EP, Han M, Acharya NK, DeMarshall C, Kosciuk MC, Nagele RG. Natural IgG Autoantibodies Are Abundant and Ubiquitous in Human Sera, and Their Number Is Influenced By Age, Gender, and Disease. PLoS One, 2013;8:e60726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Adamus G, Bonnah R, Brown L, David L. Detection of autoantibodies against heat shock proteins and collapsin response mediator proteins in autoimmune retinopathy. BMC Ophthalmol, 2013;13:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Adamus G, Brown L, Schiffman J, Iannaccone A. Diversity in autoimmunity against retinal, neuronal, and axonal antigens in acquired neuro-retinopathy. J Ophthal Inflamm Infec, 2011;1:111–21.* This study showed for the first time the repertoire of anti-optic nerve autoantibodies in retinopathes associated with optic neuropatheis that often differed from anti-retinal antibodies. The major antigenic targets were classical glycolytic enzymes (α and γ enolases, glyceraldehyde 3-phosphate dehydrogenase), neuronal-specific myelin proteins (MBP, MOG), aquaporin 4, and collapsing response mediator protein 5.
- [19].Adamus G, Brown L, Weleber RG. Molecular biomarkers for autoimmune retinopathies: Significance of anti-transducin-alpha autoantibodies. Exp Mol Pathol, 2009;87:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Morohoshi K, Ohbayashi M, Patel N, Chong V, Bird AC, Ono SJ. Identification of anti-retinal antibodies in patients with age-related macular degeneration. Exp Mol Pathol, 2012;93:193–9. [DOI] [PubMed] [Google Scholar]
- [21].ten Berge JC, van Rosmalen J, Vermeer J, Hellström C, Lindskog C, Nilsson P et al. Serum Autoantibody Profiling of Patients with Paraneoplastic and Non-Paraneoplastic Autoimmune Retinopathy. PLoS One, 2016;11:e0167909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yang S, Dizhoor A, Wilson DJ, Adamus G. GCAP1, Rab6, and HSP27: Novel Autoantibody Targets in Cancer-Associated Retinopathy and Autoimmune Retinopathy. Trans Vis SciTech, 2016;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Adamus G, Champaigne R, Yang S. Occurrence of major anti-retinal autoantibodies associated with paraneoplastic autoimmune retinopathy. Clin Immunol, 2020;210:108317.* The study showed that CAR patients with different malignancies have a specific AAb or repertoire of AAbs that could serve as biomarkers for retinal disease.
- [24].Adamus G, Machnicki M, Elerding H, Sugden B, Blocker YS, Fox DA. Antibodies to recoverin induce apoptosis of photoreceptor and bipolar cells in vivo. J Autoimmun, 1998;11:523–33. [DOI] [PubMed] [Google Scholar]
- [25].Dalin F, Adamus G, Yang S, Landgren E, Palle J, Hallgren Å et al. Aryl Hydrocarbon Receptor-Interacting Protein-Like 1 in Cancer-Associated Retinopathy. Ophthalmol, 2016;123:1401–4. [DOI] [PubMed] [Google Scholar]
- [26].Adamus G, Amundson D. Epitope recognition of recoverin in cancer associated retinopathy: Evidence for calcium-dependent conformational epitopes. J Neurosci Res, 1996;45:863–72. [DOI] [PubMed] [Google Scholar]
- [27].Adamus G, Amundson D, Seigel GM, Machnicki M. Anti-enolase alpha autoantibodies in cancer-associated retinopathy: epitope mapping and cytotoxicity on retinal cells. J Autoimmun, 1998;11:671–7. [DOI] [PubMed] [Google Scholar]
- [28].Adamus G, Yang S, Weleber RG. Unique epitopes for carbonic anhydrase II autoantibodies related to autoimmune retinopathy and cancer-associated retinopathy. Exp Eye Res, 2016;147:161–8. [DOI] [PubMed] [Google Scholar]
- [29].Forooghian F, Adamus G, Sproule M, Westall C, O’Connor P. Enolase autoantibodies and retinal function in multiple sclerosis patients. Graefes Arch Clin Exp Ophthalmol, 2007;245:1077–84. [DOI] [PubMed] [Google Scholar]
- [30].Adamus G, Choi D, Raghunath A, Schiffman J. Significance of Anti-retinal Autoantibodies in Cancer-associated Retinopathy with Gynecological Cancers. J Clin Exp Ophthalmol, 2013;4:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Robinson WH. Antigen arrays for antibody profiling. Curr Opin Chem Biol, 2006;10:67–72. [DOI] [PubMed] [Google Scholar]
- [32].Tozzoli R Recent advances in diagnostic technologies and their impact in autoimmune diseases. Autoimmun Rev, 2007;6:334–40. [DOI] [PubMed] [Google Scholar]
- [33].Yuan Y, Wang H, Lin Z-T, Hong X, Heon M, Wu T. Protein Arrays II: Antigen Arrays In: Kaufmann M, Klinger C, Savelsbergh A, editors. Functional Genomics: Methods and Protocols, New York, NY: Springer New York; 2017, p. 271–7. [Google Scholar]
- [34].Ayoglu B, Schwenk JM, Nilsson P. Antigen arrays for profiling autoantibody repertoires. Bioanalysis, 2016;8:1105–26. [DOI] [PubMed] [Google Scholar]
- [35].Robinson WH, DiGennaro C, Hueber W, Haab BB, Kamachi M, Dean EJ et al. Autoantigen microarrays for multiplex characterization of autoantibody responses. Nat Med, 2002;8:295–301. [DOI] [PubMed] [Google Scholar]
- [36].Boehm N, Wolters D, Thiel U, Lossbrand U, Wiegel N, Pfeiffer N et al. New insights into autoantibody profiles from immune privileged sites in the eye: A glaucoma study. Brain Behev Immun, 2012;26:96–102. [DOI] [PubMed] [Google Scholar]
- [37].Maat P, Brouwer E, Hulsenboom E, VanDuijn M, Schreurs MWJ, Hooijkaas H et al. Multiplex serology of paraneoplastic antineuronal antibodies. J Immunol Methods, 2013;391:125–32. [DOI] [PubMed] [Google Scholar]
- [38].Tan HT, Low J, Lim SG, Chung MCM. Serum autoantibodies as biomarkers for early cancer detection. FEBS J, 2009;276:6880–904. [DOI] [PubMed] [Google Scholar]
- [39].Hartmann TB, Bazhin AV, Schadendorf D, Eichmuller SB. SEREX identification of new tumor antigens linked to melanoma-associated retinopathy. Int J Cancer, 2005;114:88–93. [DOI] [PubMed] [Google Scholar]
- [40].Pföhler C, Preuss K-D, Tilgen W, Stark A, Regitz E, Fadle N et al. Mitofilin and titin as target antigens in melanoma-associated retinopathy. Inter J Cancer, 2007;120:788–95. [DOI] [PubMed] [Google Scholar]
- [41].Chin MS, Caruso RC, Detrick B, Hooks JJ. Autoantibodies to p75/LEDGF, a cell survival factor, found in patients with atypical retinal degeneration. J Autoimmun, 2006;27:17–27. [DOI] [PubMed] [Google Scholar]
- [42].Tateiwa H, Gotoh N, Ichikawa M, Kikuchi T, Yoshimura N. Molecular cloning and characterization of human PTB-like protein: a possible retinal autoantigen of cancer-associated retinopathy. J Neuroimmunol, 2001;120:161–9. [DOI] [PubMed] [Google Scholar]
- [43].Kikuchi T, Arai J, Shibuki H, Kawashima H, Yoshimura N. Tubby-like protein 1 as an autoantigen in cancer-associated retinopathy. J Neuroimmunol, 2000;103:26–33. [DOI] [PubMed] [Google Scholar]
- [44].Larman HB, Zhao Z, Laserson U, Li MZ, Ciccia A, Gakidis MAM et al. Autoantigen discovery with a synthetic human peptidome. Nature Biotechnol, 2011;29:535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mohan D, Wansley DL, Sie BM, Noon MS, Baer AN, Laserson U et al. PhIP-Seq characterization of serum antibodies using oligonucleotide-encoded peptidomes. Nat Protoc, 2018;13:1958–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Davoudi S, Ahmadi T, Papavasilieou E, Leskov I, Sobrin L. Phage Immunoprecipitation Sequencing of Autoantigens in Autoimmune Retinopathy. Ocular Immunol Inflamm, 2016:1–8.* This study used PhIP-Seq for first time to identify autoantigens in patients with autoimmune retinopathy.