Abstract
Severe pneumonia and ARDS caused by human adenovirus B21 infections (HAdV-B21) is a rare, but a devastating disease with rapid progression to multiorgan failure and death. However, only a few cases were reported so far.
Infections appear associated with increased disease severity and higher mortality in infected critically ill patients. Possible factors contributing to infection are underlying psychiatric disease resulting in institutionalization of respective patients, and polytoxicomania. Controlled data on the therapy of severe adenovirus infections are lacking and remains experimental.
In conclusion, data on HAdV-B21 infections causing severe pneumonia or ARDS are scarce. Controlled clinical trials on the therapy of adenovirus pneumonia are non existent and thus there is no established therapy so far. ICU physicians should be aware of this potentially devastating disease and further studies are needed.
Keywords: Critical illness, Respiratory failure, Viral 21B, HAdV-B21
1. Introduction
Acute Respiratory Distress Syndrome (ARDS) is a life-threatening disease characterized by fluid accumulation in the interstitial and alveolar spaces due to increased vascular permeability resulting from inflammation [1]. Infectious pneumonia is the most common disease leading to ARDS [[1], [2], [3], [4]] with viral pneumonia accounting for approximately 9% of pneumonia cases [1,5]. However, the proportion of viral pneumonia leading to ARDS is unknown [1,5]. Infections with some respiratory viruses are associated with a high incidence of lung injury, ARDS, and increased mortality [1]. They include influenza A H1N1, avian influenza A viruses H5N1 and H7N9, and the SARS and MERS-Coronaviruses [1,6].
Human adenoviruses are a frequent cause of acute upper respiratory tract infection in children and have high respiratory morbidity, in particular in immune-compromised hosts [[7], [8], [9]]. Interestingly, adenoviruses can affect almost all organ systems depending on the adenovirus type and host factors as e.g. immunodeficiency [10,11]. Adenovirus infections are mostly self-limiting in immune-competent patients with treatment mainly relying on supportive measures [10,11]. Life threatening adenovirus infections, e.g. viral pneumonia and ARDS were reported and associated with a few adenovirus types, but appear to be rare [8,11]. These cases are mainly attributed to disseminated infections by adenovirus serotypes 7, 21 and 3, all belonging to subgroup B1 viruses.
Here we report two cases of severe ARDS caused by a highly virulent strain of adenovirus B21, subtype 21a (HAdV-B21) and review the literature on adenovirus-induced ARDS.
1.1. Case 1
A 53 year old male patient was referred to our hospital from an outpatient psychiatric clinic due to upper respiratory tract infection progressing to lower respiratory tract involvement. The patient presented with worsening tachypnoea and increasing respiratory distress. His personal history included paranoid schizophrenia, a history of intravenous drug use with current enrollment in an official drug substitution program, structural epilepsy, and chronic hepatitis C. Amoxicillin with clavulanic acid and clarithromycin was prescribed for suspected pneumonia and he was admitted to the normal ward. Within a short time his respiratory function deteriorated rapidly and he was transferred to the intensive care unit (ICU) for intubation. Antibiotic therapy was escalated to piperacillin/tazobactam. Initially, the patient presented with a clinical picture suggesting septic shock with hyperdynamic cardiocirculatory failure due to marked vasoplegia. Differentiated catecholamine and volume therapy was started. Bilateral pleural effusions were drained. Over the ensuing days, his condition stabilized, a decline in inflammation parameters was observed and catecholamine support was stopped. Cultures of respiratory specimens were without growth.
Five days later, a secondary decline in respiratory function was observed and imaging studies were suggestive of ARDS. Lung protective ventilation was initiated, prone positioning performed, and antibiotic therapy was changed to cefepime and metronidazole. Laboratory parameters documented increased systemic inflammation (C-reactive protein, CRP 319 mg/l) and the patient developed pancytopenia (white blood cell count 2.9G/l, hemoglobin 85 g/l, platelet count 32G/l). By PCR highvirus loads of HAdV-B21 was detected in plasma and broncho-alveolar lavage (BAL)-fluid.
Despite extensive medical therapy, the patient developed rapidly deteriorating multi-organ dysfunction. The installation of extra corporal membrane (ECMO) support was carefully considered, but decided against. The patient died shortly after being put on comfort care. Autopsy confirmed severe ARDS with bilateral diffuse parenchymal defects and interstitial thickening. Additionally, hemorrhagic infarction of the right upper lobe was noted.
1.2. Case 2
A 35 year old female presented to a regional hospital with a two-week history of lower respiratory tract infection not responding to treatment with amoxicillin with clavulanic acid for 10 days. Her personal history included seropositive rheumatoid arthritis treated with infliximab and leflunomide and borderline psychiatric disease. Despite escalation of antibiotic therapy to piperacillin/tazobactam and non-invasive ventilation, respiratory function worsened rapidly. The patient was intubated and transferred to our ICU.
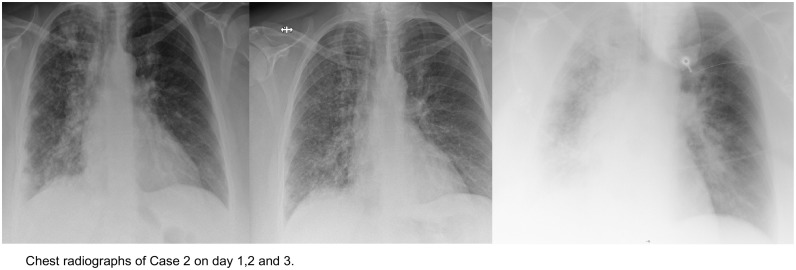
At admission, the patient had severe ARDS (oxygenation index 80) and needed mechanical ventilation with high ventilatory pressures (plateau pressure 35 mmHg). She had diffuse bilateral infiltrates (Fig. 1 ) without evidence for cardiac dysfunction. Her CRP level was elevated to 168 mg/l, the total white blood cell count was 15.1 G/l with 13.2 G/l neutrophils. She had, acute kidney injury (creatinine 259 umol/l), severe non-lactic metabolic acidosis (pH 7.19), increased lactate dehydrogenase (5631 U/l), creatinine kinase (4052 U/l), and alanin-aminotransferase (244 U/l). There was no evidence of reactivation of rheumatoid arthritis. Blood cultures taken before and at ICU admission all returned negative.
Fig. 1.
Radiograph of Case 2.
Antibiotic therapy was changed to meropenem, vancomycin and fluconazole. In addition, the patient underwent lung protective ventilation and prone positioning as well as received inhaled nitric-oxide and methylprednisolone for supportive care. Additionally, continous veno-venous hemodiafiltration for progressive acute kidney injury and metabolic acidosis was initiated.
Gastro-esophagoscopy due to suspected upper gastrointestinal bleeding showed ubiquitous ulcerations with necrotic tissue in the lower esophagus, but not in the stomach. Acyclovir was added to the treatment regime.
In the BAL fluid a high concentration of HAdV-B21 was detected and cidofovir C was given in addition.
Despite maximal medical therapy with high-dose catecholamine support the patient's condition worsened progressively and veno-venous ECMO support was started. She died 2 days later of multi-organ dysfunction. An autopsy was not performed.
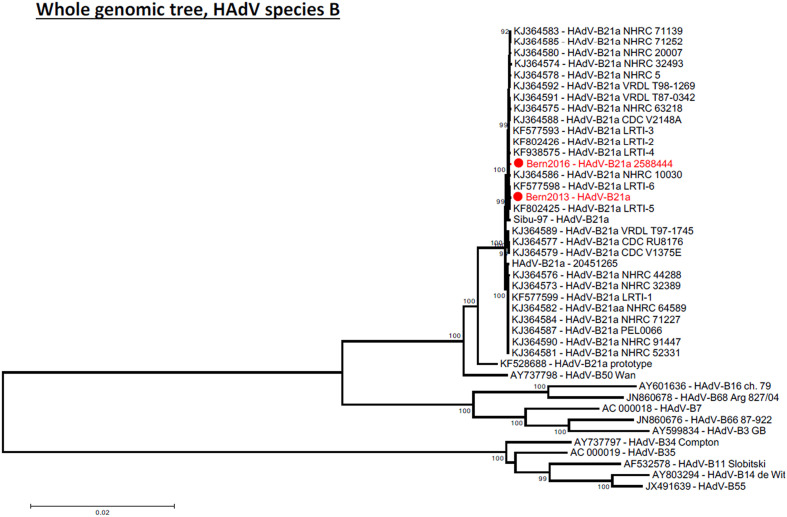
2. Microbiological assessment
Adenovirus was detected and quantified in BAL (1.05 × 109 copies/mL) and in plasma (4.9 × 107 copies/mL) by quantitative real-time PCR (Adenovirus r-gene, Argene bioMérieux) in case 1. No quantitative PCR was performed in case 2 but the cycle threshold (CT) values by qualitative real-time PCR were very low in BAL (15.2) and in plasma (19.7), which corresponds to a high virus load in both specimen types, with the virus load in BAL higher than in plasma, similar to case 1. In both cases adenovirus was isolated by viral culture on A549 cells and initial typing by partial sequencing of the hexon gene [12] resulted in HAdV-B21. Complete genomic sequencing confirmed these results and identified this virus as subtype 21a (see Fig. 2 ). In case 1, adenovirus DNA was also quantified in autoptic tissue specimens. The adenovirus DNA concentration was high in specimens of the left and right lung (74 and 312 copies of adenovirus DNA/cell, respectively) and low (<0.1 copies/cell) in all other organs and tissues (liver, kidney, spleen, stomach, jejunum, colon, pancreas, thyroid, lymph node) tested, with exception of the pancreas, which had a virus load of 4 copies/cell. These results indicate that adenovirus replication in this case was almost completely limited to the lung tissue. Therefore, the high virus loads detected in plasma probably originated from the lungs. By contrast, disseminated adenovirus infections of immunosuppressed patients, which result also in high virus loads in peripheral blood, are caused by virus replication in multiple internal organs [13]. However, in the immunosuppressed patient, these infections are often associated with other types of adenoviruses than in healthy hosts and may therefore have that other replication rates in the different organs than HAdV-B21 infections.
Fig. 2.
Phylogenetic analysis comparing whole genomic HAdV sequences from the two pneumonia cases with other subtype 21a strains, the 1956 prototype of HAdV type 21 and other types of species HAdV-B. The tree were generated with MEGA 5.2 using neighbor-joining method; bootstrap values (%) were generated with 1000 pseudo-replicates and are presented as numbers on tree nodes.
In both cases, no bacteria or additional viruses were found in BAL fluid (Influenza A and B, Respiratory Syncytial Virus, Parainfluenzaviruses, human Metapneumovirus, Coronaviruses, Rhinovirus, Cytomegalovirus and Herpes simplex virus type 1 all tested negative by qualitative real-time PCR). Blood cultures and routine bacterial cultures also remained negative.
3. Literature review
A systematic literature search was conducted on Medline (www.pubmed.org) with the following MeSH terms “adenovirus B21”, “adenovirus B21 pneumonia”, “adenovirus B21 severe pneumonia” and “adenovirus B21 ARDS”.
The following exclusion criteria were applied: articles not featuring HAdV-B21 infections, articles in languages other than English and German, articles on biological or microbiological aspects of adenovirus infections with no relation to clinical practice, and articles older than 20 years.
Older clinical work was excluded as it has been described that non-virulent strains HAdV-B21 have circulated in Europe between 1960 and 1980 [11].
The literature research resulted in 8 articles related to HAdV-B21 infection. Of these, five publications were excluded because they reported microbiological or technical details with no relation to clinical practice [[14], [15], [16], [17]] or were only available in Russian [18], see Table 1 . The remaining papers were used for further evaluation [9,11]. Included papers are summarized in Table 2 .
Table 1.
Studies excluded from this review after full analysis with reason for exclusion.
| N | Article | Reason for exclusion | Reference |
|---|---|---|---|
| 1 | Dehghan S, Seto J, Jones MS, Dyer DW, Chodosh J, Seto D. Simian adenovirus type 35 has a recombinant genome comprising human and simian adenovirus sequences, which predicts its potential emergence as a human respiratory pathogen. Virology. 2013;447(1–2):265–73. | Microbiological aspects | [14] |
| 2 | Barrero PR, Valinotto LE, Tittarelli E, Mistchenko AS. Molecular typing of adenoviruses in pediatric respiratory infections in Buenos Aires, Argentina (1999–2010). Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology. 2012;53(2):145–50 | Technical aspects | [15] |
| 3 | Seto J, Walsh MP, Metzgar D, Seto D. Computational analysis of adenovirus serotype 5 (HAdV-C5) from an HAdV coinfection shows genome stability after 45 years of circulation. Virology. 2010;404(2):180–6. | Microbiological and technical aspects | [16] |
| 4 | Metzgar D, Gibbins C, Hudson NR, Jones MS. Evaluation of multiplex type-specific real-time PCR assays using the LightCycler and joint biological agent identification and diagnostic system platforms for detection and quantitation of adult human respiratory adenoviruses. Journal of clinical microbiology. 2010;48(4):1397–403. | Technical aspects | [17] |
| 5 | Tsybalova LM, Popova TL, Karpukhin GI. [HLA system antigens in persons with differing susceptibility to the causative agents of acute respiratory diseases]. Zhurnal mikrobiologii, epidemiologii, i immunobiologii. 1989 [10]:64–8. | Article in Russian | [18] |
Table 2.
Studies included in this review.
| N | Author (year) | Design | Study population | Total study population | Geographical region | Main findings concerning human adenovirus B21 | R |
|---|---|---|---|---|---|---|---|
| 1 | Scott M et al. (2016) | Prospective observational registry study | General population with human adenovirus infections | 198 (20 adenovirus B21) | United States |
|
[9] |
| 2 | Hage E et al. (2014) | Observational study | Patients with human adenovirus B21 infections and pneumonia | 6 (6 adenovirus B21) | Germany |
|
[11] |
| 3 | Gray et al. (2007) | Observational study | General population with human adenovirus infections | 2237 (32 adenovirus B21) | United States |
|
[19] |
3.1. Clinical aspects
The first paper presents results of a cluster analysis of emergent severe respiratory tract infections caused by adenoviruses in Oregon, USA, from 2013 to 2014 [9]. Ten percent of the patients were infected with HAdV-B21, the third most common type of adenovirus isolated following adenovirus B7 (59%) and C2 (13%). Patients suffering from adenovirus B21 infection were significantly older than those infected with other adenovirus types. HAdV-B21 infections resulted in pneumonia in >21% of cases and required hospitalization in 81%. Thirty patients (30%) were admitted to the ICU, 23% required mechanical ventilation. No fatalities were noted.
The second publication describes a case series of 7 cases of HAdV-B21 infections between 2005 and 2013 in Germany [11]. The patients' age ranged from 2.5 months to 61 years. Four out of seven patients were male [11]. Six patients suffered from severe pneumonia, and one from keratoconjunctivitis. All patients with pneumonia required hospitalization in an ICU for mechanical ventilation, two received ECMO therapy and two others not on ECMO died. Interestingly, four out of seven patients had psychiatric disease (schizophrenia or polytoxicomania), and one patient received immunosuppressants following solid organ and stem cell transplantation.
The third article evaluates data on genotyping of adenovirus infections from 22 US hospitals over 25 month of observation [19]. Infection with HAdV-B21 was associated with an OR of 7.6 for severe disease.
3.2. Microbiological aspects
Adenoviridae are ubiquitous DNA viruses. They are associated with respiratory disease, but also gastrointestinal, ophthalmological, cardiac, neurological and genitourinary infections depending on the tropism of the adenovirus type causing the infection [10]. Adenoviridae comprise 7 species pathogenic for humans (A–G), and >80 different adenovirus types were classified [[9], [10], [11]]. Transmission occurs by droplets, via the fecal-oral route, and via contaminated fomites. Viral shedding from the respiratory or gastrointestinal tracts may continue for weeks, even in asymptomatic patients [9]. The incubation period is 5–10 days [10]. In immunocompetent patients, the disease is typically self-limiting [10,11], but persistent infections can be noted in the immune-incompetent host resulting in reactivation and severe disease in case of secondary immunosuppression [20].
HAdV-B21 was first isolated in 1956 from a patient with conjunctivitis [11]. Although HAdV-B21 may occasionally cause conjunctivitis, earlier evidence [21,22] and case reports in children [23,24] showed an association between HAdV-B21 and respiratory tract infections. HAdV-B21 can cause severe (broncho-) pneumonia and ARDS [9,11,19,23,24] and the latter may be associated with the emergence of the new subtype 21a, which was also found in our two cases. Main genetic features of this subtype were a shorter penton “RGD” (Arginine-Glycine-Aspartic acid) loop, which binds to the secondary cellular receptor, and thus might influence tropism and virulence. Moreover, subtype 21a was found to have a recombinant genome with the E4 gene region sequence derived from adenovirus type 3, a frequently observed respiratory pathogen [11].
4. Discussion
Even though HAdV-B21 infections are known since 1950`ies and have affected major organizations by regular outbreaks such as the US military [25], only few severe infections with HAdV-B21 have been reported [9,11]. After careful evaluation of our cases and previously reported ones, three aspects appear to stand out.
First, HAdV-B21 pneumonia was associated with increased rates of severe lung injury and a fatal outcome [11]. The high virulence of the currently circulating HAdV-B21 may contribute to rapid disease progression. Lack of awareness of treating ICU physicians for the severity of adenoviral lower respiratory tract infection may result in delayed treatment. However, if early treatment of viral ARDS and particularly of HAdV-B21 related disease would make a difference in terms of outcome, remains unclear. The effectiveness of antiviral treatment in patients with viral ARDS, the choice of drug, its dosage and treatment duration, remain an area of debate and research, even in case of the more prevalent influenza virus infections [[26], [27], [28]]. In 2014, a meta-analysis of 29,234 patients by Muthuri and co-authors found a significant reduction of mortality in adult patients with influenza A treated early with neuraminidase inhibitors [29]. The effect on mortality was lower when treatment was delayed and not significant in children [29]. Two recently published studies do not support high-dose treatment with oseltamivir [26,27]. On the other hand, a Cochrane review on prevention and treatment of influenza with neuraminidase inhibitors found no effect on serious influenza complications such as ARDS [30]. There is little evidence to guide treatment of adenovirus-related ARDS. No prospective randomized clinical trials have been performed to date to support the use of any antiviral agent for adenovirus infection [31,32]. Cidofovir and brincidofovir appear to be effective treatments for severe adenovirus infections and are frequently used in immunocompromised patients [[31], [32], [33]], but none of these substances is approved for this indication [34]. Therefore, further research is certainly warranted.
Second, a significant percentage of HAdV-B21 cases had psychiatric diseases (e.g. schizophrenia or drug abuse). It was suggested that a psychiatric disorder may make patients more vulnerable to adenovirus infections because of close contacts with fellow in-patients [35]. Facilitated spread of infection and reduced standards of hygiene may be important with regard to children in day-care facilities [36]. Last but not least patients with psychiatric disorders may be at increased risk for malnourishment [37].
Third, respiratory adenovirus infections are frequently transmitted by droplets and thus start as upper respiratory tract infections. An adequate response of the patient (e.g. bed rest) may limit or delay spread of the infection to the lower respiratory tract. Pneumotropic adenovirus types may effectively disseminate through the respiratory tract because apical infection of ciliated airway cells is more efficient and subsequently more virus is shed into the airways [38]. Tissue injury may be promoted by proinflammatory cytokines such as IP-10 and I-Tac induced by pneumotropic adenovirus types [38]. Host factors impacting on the occurrence of HAdV-B21 are currently unclear and should be studied in future analyses.
5. Conclusions
Severe pneumonia and ARDS caused by HAdV-B21 infections are rare, but may be fatal. Especially infections due to HAdV-B21 appear associated with increased disease severity and mortality. The reason for the apparently increased risk for severe pulmonary disease in patients with underlying psychiatric disease needs to be elucidated. In patients with severe ARDS in which the disease progresses despite maximal therapy, rare viral infections such as HAdV-B21 should be suspected and evaluated by real time PCR in BAL and plasma fluid.
Controlled data on the therapy of severe adenovirus infections is lacking and the use of antiviral drugs like cidofovir remains experimental.
In conclusion, data on HAdV-B21 infections causing severe pneumonia or ARDS are scarce. ICU physicians should be aware of this potentially devastating disease and further studies are needed.
Ethical approval and consent to participate
Not applicable
Consent for publication
All authors consent to publication
Availability of supporting data
Not applicable
Competing interests
The authors declare that they have no competing interest
Authors' contributions
CAP: designed the strategy, performed the literature review, drafted the first manuscript (clinical part), revision for important intellectual content
MTB: drafted the first manuscript (microbiological part), revision for important intellectual content
JCS: designed the strategy, drafted the manuscript, revision for important intellectual content
EH: Performed the microbiological analysis, revision for important intellectual content
AH: Performed the microbiological analysis, revision for important intellectual content
SZ: Designed the strategy, revision for important intellectual content
Acknowledgments
Acknowledgements
“Not applicable.
Conflict of interest
None.
Funding
Self-funding.
Contributor Information
Carmen Andrea Pfortmueller, Email: carmen.pfortmueller@insel.ch.
Maria Teresa Barbani, Email: mariateresa.barbani@ifik.unibe.ch.
Joerg Christian Schefold, Email: joerg.schefold@insel.ch.
Albert Heim, Email: heim.albert@mh-hannover.de.
Stefan Zimmerli, Email: stefan.zimmerli@insel.ch.
References
- 1.Bauer T.T., Ewig S., Rodloff A.C., Muller E.E. Acute respiratory distress syndrome and pneumonia: a comprehensive review of clinical data. Clin Infect Dis. 2006;43(6):748–756. doi: 10.1086/506430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Estenssoro E., Dubin A., Laffaire E., Canales H., Saenz G., Moseinco M. Incidence, clinical course, and outcome in 217 patients with acute respiratory distress syndrome. Crit Care Med. 2002;30(11):2450–2456. doi: 10.1097/00003246-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Valta P., Uusaro A., Nunes S., Ruokonen E., Takala J. Acute respiratory distress syndrome: frequency, clinical course, and costs of care. Crit Care Med. 1999;27(11):2367–2374. doi: 10.1097/00003246-199911000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Luhr O.R., Antonsen K., Karlsson M., Aardal S., Thorsteinsson A., Frostell C.G. Incidence and mortality after acute respiratory failure and acute respiratory distress syndrome in Sweden, Denmark, and Iceland. The ARF study group. Am J Respir Crit Care Med. 1999;159(6):1849–1861. doi: 10.1164/ajrccm.159.6.9808136. [DOI] [PubMed] [Google Scholar]
- 5.de Roux A., Marcos M.A., Garcia E., Mensa J., Ewig S., Lode H. Viral community-acquired pneumonia in nonimmunocompromised adults. Chest. 2004;125(4):1343–1351. doi: 10.1378/chest.125.4.1343. [DOI] [PubMed] [Google Scholar]
- 6.Pariani E., Martinelli M., Canuti M., Jazaeri Farsani S.M., Oude Munnink B.B., Deijs M. Influenza and other respiratory viruses involved in severe acute respiratory disease in northern Italy during the pandemic and postpandemic period (2009-2011) Biomed Res Int. 2014;2014 doi: 10.1155/2014/241298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun B., He H., Wang Z., Qu J., Li X., Ban C. Emergent severe acute respiratory distress syndrome caused by adenovirus type 55 in immunocompetent adults in 2013: a prospective observational study. Crit Care. 2014;18(4):456. doi: 10.1186/s13054-014-0456-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson C.M., Singh G., Henquell C., Walsh M.P., Peigue-Lafeuille H., Seto D. Computational analysis and identification of an emergent human adenovirus pathogen implicated in a respiratory fatality. Virology. 2011;409(2):141–147. doi: 10.1016/j.virol.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott M.K., Chommanard C., Lu X., Appelgate D., Grenz L., Schneider E. Human adenovirus associated with severe respiratory infection, Oregon, USA, 2013-2014. Emerg Infect Dis. 2016;22(6):1044–1051. doi: 10.3201/eid2206.151898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flomenberg P. Adenovirus infections. Medicine. 2014;42(1):42–44. [Google Scholar]
- 11.Hage E., Huzly D., Ganzenmueller T., Beck R., Schulz T.F., Heim A. A human adenovirus species B subtype 21a associated with severe pneumonia. J Infect. 2014;69(5):490–499. doi: 10.1016/j.jinf.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Madisch I., Wolfel R., Harste G., Pommer H., Heim A. Molecular identification of adenovirus sequences: a rapid scheme for early typing of human adenoviruses in diagnostic samples of immunocompetent and immunodeficient patients. J Med Virol. 2006;78(9):1210–1217. doi: 10.1002/jmv.20683. [DOI] [PubMed] [Google Scholar]
- 13.Forstmeyer D., Henke-Gendo C., Brocker V., Wildner O., Heim A. Quantitative temporal and spatial distribution of adenovirus type 2 correlates with disease manifestations and organ failure during disseminated infection. J Med Virol. 2008;80(2):294–297. doi: 10.1002/jmv.21071. [DOI] [PubMed] [Google Scholar]
- 14.Dehghan S., Seto J., Jones M.S., Dyer D.W., Chodosh J., Seto D. Simian adenovirus type 35 has a recombinant genome comprising human and simian adenovirus sequences, which predicts its potential emergence as a human respiratory pathogen. Virology. 2013;447(1–2):265–273. doi: 10.1016/j.virol.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrero P.R., Valinotto L.E., Tittarelli E., Mistchenko A.S. Molecular typing of adenoviruses in pediatric respiratory infections in Buenos Aires, Argentina (1999-2010) J Clin Virol. 2012;53(2):145–150. doi: 10.1016/j.jcv.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Seto J., Walsh M.P., Metzgar D., Seto D. Computational analysis of adenovirus serotype 5 (HAdV-C5) from an HAdV coinfection shows genome stability after 45 years of circulation. Virology. 2010;404(2):180–186. doi: 10.1016/j.virol.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Metzgar D., Gibbins C., Hudson N.R., Jones M.S. Evaluation of multiplex type-specific real-time PCR assays using the LightCycler and joint biological agent identification and diagnostic system platforms for detection and quantitation of adult human respiratory adenoviruses. J Clin Microbiol. 2010;48(4):1397–1403. doi: 10.1128/JCM.01600-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsybalova L.M., Popova T.L., Karpukhin G.I. HLA system antigens in persons with differing susceptibility to the causative agents of acute respiratory diseases. Zh Mikrobiol Epidemiol Immunobiol. 1989;(10):64–68. [PubMed] [Google Scholar]
- 19.Gray G.C., McCarthy T., Lebeck M.G., Schnurr D.P., Russell K.L., Kajon A.E. Genotype prevalence and risk factors for severe clinical adenovirus infection, United States 2004-2006. Clin Infect Dis. 2007;45(9):1120–1131. doi: 10.1086/522188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kosulin K., Geiger E., Vecsei A., Huber W.D., Rauch M., Brenner E. Persistence and reactivation of human adenoviruses in the gastrointestinal tract. Clin Microbiol Infect. 2016;22(4) doi: 10.1016/j.cmi.2015.12.013. (381.e1-.e8) [DOI] [PubMed] [Google Scholar]
- 21.Van Der Veen J., Dijkman J.H. Association of type 21 adenovirus with acute respiratory illness in military recruits. Am J Hyg. 1962;76:149–159. doi: 10.1093/oxfordjournals.aje.a120270. [DOI] [PubMed] [Google Scholar]
- 22.Shult P.A., Polyak F., Dick E.C., Warshauer D.M., King L.A., Mandel A.D. Adenovirus 21 infection in an isolated antarctic station: transmission of the virus and susceptibility of the population. Am J Epidemiol. 1991;133(6):599–607. doi: 10.1093/oxfordjournals.aje.a115932. [DOI] [PubMed] [Google Scholar]
- 23.James A.G., Lang W.R., Liang A.Y., Mackay R.J., Morris M.C., Newman J.N. Adenovirus type 21 bronchopneumonia in infants and young children. J Pediatr. 1979;95(4):530–533. doi: 10.1016/s0022-3476(79)80756-8. [DOI] [PubMed] [Google Scholar]
- 24.Lang W.R., Howden C.W., Laws J., Burton J.F. Bronchopneumonia with serious sequelae in children with evidence of adenovirus type 21 infection. Br Med J. 1969;1(5636):73–79. doi: 10.1136/bmj.1.5636.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kajon A.E., Hang J., Hawksworth A., Metzgar D., Hage E., Hansen C.J. Molecular epidemiology of adenovirus type 21 respiratory strains isolated from US military trainees (1996-2014) J Infect Dis. 2015;212(6):871–880. doi: 10.1093/infdis/jiv141. [DOI] [PubMed] [Google Scholar]
- 26.Noel Z.R., Bastin M.L., Montgomery A.A., Flannery A.H. Comparison of high-dose versus standard dose oseltamivir in critically ill patients with influenza. J Intensive Care Med. 2017 Dec;32(10):574–577. doi: 10.1177/0885066616638649. Epub 2016 Mar 18. [DOI] [PubMed] [Google Scholar]
- 27.Flannery A.H., Thompson Bastin M.L. Oseltamivir dosing in critically ill patients with severe influenza. Ann Pharmacother. 2014;48(8):1011–1018. doi: 10.1177/1060028014535362. [DOI] [PubMed] [Google Scholar]
- 28.Wintermeyer S.M., Nahata M.C. Rimantadine: a clinical perspective. Ann Pharmacother. 1995;29(3):299–310. doi: 10.1177/106002809502900312. [DOI] [PubMed] [Google Scholar]
- 29.Muthuri S.G., Venkatesan S., Myles P.R., Leonardi-Bee J., Al Khuwaitir T.S., Al Mamun A. Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med. 2014;2(5):395–404. doi: 10.1016/S2213-2600(14)70041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jefferson T., Jones M.A., Doshi P., Del Mar C.B., Hama R., Thompson M.J. Vol. 4. 2014. Neuraminidase Inhibitors for Preventing and Treating Influenza in Healthy Adults and Children. The Cochrane Database of Systematic Reviews. (Cd008965) [Google Scholar]
- 31.Florescu M.C., Miles C.D., Florescu D.F. What do we know about adenovirus in renal transplantation? Nephrol Dial Transplant. 2013;28(8):2003–2010. doi: 10.1093/ndt/gft036. [DOI] [PubMed] [Google Scholar]
- 32.Tollefson A.E., Spencer J.F., Ying B., Buller R.M., Wold W.S., Toth K. Cidofovir and brincidofovir reduce the pathology caused by systemic infection with human type 5 adenovirus in immunosuppressed Syrian hamsters, while ribavirin is largely ineffective in this model. Antiviral Res. 2014;112:38–46. doi: 10.1016/j.antiviral.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Grimley M.S., Chemaly R.F., Englund J.A., Kurtzberg J., Chittick G., Brundage T.M. Brincidofovir for asymptomatic adenovirus viremia in pediatric and adult allogeneic hematopoietic cell transplant recipients: a randomized placebo-controlled phase II trial. Biol Blood Marrow Transplant. 2017;23(3):512–521. doi: 10.1016/j.bbmt.2016.12.621. [DOI] [PubMed] [Google Scholar]
- 34.Ison M.G., Hayden R.T. Adenovirus. Microbiol Spectr. 2016;4(4) doi: 10.1128/microbiolspec.DMIH2-0020-2015. [DOI] [PubMed] [Google Scholar]
- 35.Rump B., De Boer M., Reis R., Wassenberg M., Van Steenbergen J. Signs of stigma and poor mental health among carriers of MRSA. J Hosp Infect. 2017 Mar;95(3):268–274. doi: 10.1016/j.jhin.2016.09.010. Epub 2016 Sep 17. [DOI] [PubMed] [Google Scholar]
- 36.Amoah A.O., Witherspoon N.O., Perodin J., Paulson J.A. Findings from a pilot environmental health intervention at early childhood centers in the District of Columbia. J Public Health (Oxf) 2016;38(3) doi: 10.1093/pubmed/fdv135. (e209-e17) [DOI] [PubMed] [Google Scholar]
- 37.Oster G., Berger A., Edelsberg J., Weber D.J. Initial treatment failure in non-ICU community-acquired pneumonia: risk factors and association with length of stay, total hospital charges, and mortality. J Med Econ. 2013;16(6):809–819. doi: 10.3111/13696998.2013.794805. [DOI] [PubMed] [Google Scholar]
- 38.Lam E., Ramke M., Warnecke G., Schrepfer S., Kopfnagel V., Dobner T. Effective apical infection of differentiated human bronchial epithelial cells and induction of proinflammatory chemokines by the highly pneumotropic human adenovirus type 14p1. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0131201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable