Summary
Carbohydrates are chemical compounds that contain only oxygen, hydrogen and carbon. They are classified by their number of sugar units: monosaccharides (such as glucose and fructose), and disaccharides (such as sucrose and lactose) are simple carbohydrates; oligosaccharides and polysaccharides (such as starch, glycogen and cellulose) are complex carbohydrates. Carbohydrates play a crucial role in diverse biological systems [Hricovín M. Structural aspects of carbohydrates and the relation with their biological properties. Curr Med Chem 2004;11:2565–83].
According to Roseman [Sugars of the cell membrane. In: Weissmann G, Clairborn E, editors. Cell membranes. Biochemistry, Cell Biology, Pathology. New York: H. P. Publ. Co; 1975. p. 55–64], two classes of glycoproteins are described. Free glycoproteins are localised in the surface coat of the membranes and form a thick mobile layer, without any association to the membrane itself. Functionally, however, they are located in a close association with the membrane (e.g. in the duodenal mucosa). The other group consists of the membrane glycoproteins, which are integral to the membranes and are located in the outer layer. The oligosaccharide chains are bound to the N-terminal part of proteins, and are situated in the hydrophilic zone.
Glycoproteins have diverse functions. They are important in specific receptor functions, in immunological cell destruction and play a significant role in reactions with lectins, antibodies, as well as in cell association and mutual recognition of the cells.
This paper focuses on aspects of a summary of polarisation optical investigations and biological functions of the following three groups of carbohydrates: oligosaccharides, glycoproteins and glycosaminoglycans.
Keywords: Glycoproteins, Sialic acid, Amyloid, Blood cell membranes, Polarisation microscopy, Glycosaminoglycans, Topo-optical staining reactions
Introduction
The functional significance of carbohydrates in biology is a topic of intense current research. Carbohydrates are permanent components of many biological structures and biomembranes in human and in animal cells, as well as in bacterial and plant cell walls in the form of polysaccharides, glycoproteins and glycolipids. Whether chains of oligosaccharides and polysaccharides have a distinct orientation in biomembranes or in cellular structures could not be solved until Romhányi and Fischer developed the aldehyde bisulfite toluidine blue reaction (ABT reaction) or “anisotropic PAS reaction” (Romhányi et al., 1975) as a modification of the classical periodic acid-Schiff (PAS) reaction (McManus, 1946).
At the light miscroscopic level, the periodate reactive monosaccharides, disaccharides and polysaccharides are demonstrable by conventional PAS reaction giving an intense purple colour (review in Romhányi, 1978). The disadvantage of the PAS reaction is the lack of birefringence (isotropic staining reaction) between crossed polars and so it does not provide any insight into the molecular order of the reactive components in tissues. However, with post-staining precipitation using a mixture of potassium ferricyanide solution and potassium iodide solution it is suitable to produce an anisotropic effect. Upon long-time exposure to polarised light, also the post-precipitation-complex is not stable and disintegrates (Makovitzky, unpublished data). Electron microscopy can ultrastructurally prove the presence of these components. They are localised as a carbohydrate rich cell coat at the cell surface (Rambourg, 1971; Rambourg and Leblond, 1967; Geyer, 1977; Geyer et al., 1973). By autoradiography (fluorography) cell-surface glycoproteins can be examined by means of incorporated radioactive molecules. New glycoprotein fractions were visualised with the help of this method (Gahmberg, 1981; Gahmberg and Hakomori, 1973; Gahmberg and Andersson, 1977).
A new era – that of lectin histochemistry – made it possible, by its employment along with other methods, to represent certain sugar molecules and sialic acids at the membrane surface (Yamada and Shimizu, 1979; Muresan et al., 1982; Scocco et al., 1999). The same applies to the demonstration of periodate reactive carbohydrate components at the ultrastructural level, based on the silver reduction capacity of periodate induced aldehyde groups (Movat, 1961; Rambourg, 1971; Rambourg and Leblond, 1967; Ainsworth et al., 1972) or to the binding of labeled lectins to cell-surface carbohydrates (Nicolson and Singer, 1971, Nicolson and Singer, 1974).
In summary, all these reactions result in formation of an amorphous aggregate on the reductive or binding sites and give information only on the presence and localisation on carbohydrate components at the ultrastructural level, but do not provide information about the molecular order and spatial orientation of the chains of the reacting carbohydrate components. In this regard, polarisation microscopy with the topo-optical reactions offers new possibilities. Using the ABT reaction or “anisotropic PAS reaction” carbohydrates can be detected with great sensitivity and their spatial location at a molecular level can be analysed. The reaction shows that the carbohydrate components are present in a highly linearly oriented fashion (parallel or perpendicularly to the length). Thus, it becomes possible to discover structural differences in biological systems (Romhányi et al., 1975; Fischer, 1976, Fischer, 1978; Fischer and Emödy, 1976, Fischer and Emödy, 1978).
The space of the glycolitic (vicinal) hydroxyl groups within carbohydrate residues is between 2 and 5 Å, this distance is a pre-condition for the creation of the metachromasia, for the anisotropy, and thus for the positive ABT reaction (Fischer, 1978; Sylvén, 1954; Romhányi, 1963, Romhányi, 1989, 1983; Fischer and Romhányi, 1977; Szirmai and Balázs, 1958; Romhányi et al., 1975). Only at this distance an interaction of the dye molecules bound to the OH groups takes place, which leads to the anisotropy and metachromasia. If the space is larger than 5 Å, only basophilia and isotropy, like after the first step in the Feulgen reaction combined with the bisulfite toluidine blue reaction, is observed (Fischer, 1978). Due to the distance of the OH groups taking part in the ABT reaction, the indirect resolution range of the polarisation microscopy is theoretically between 2 and 5 Å (Romhányi, 1978; Fischer, 1978).
Fischer stressed it in his PhD work (Fischer, 1978) that carbohydrates are among the most important elements of biological structures – in botany and microbiology as cell wall-forming-elements, in human biology as structural and connective tissue components. He worked on the polarisation optical and histochemical investigation of carbohydrates and clarified the spatial orientation of these macromolecules at a molecular level.
Using the ABT reaction in the heart musculature at the electron microscopic level Sótonyi and Somogyi (1983) confirmed that there is a similar localisation of the sugars in comparison to the polarisation optical ABT reaction. According to them, the electron microscopic ABT reaction is more sensitive than the periodic acid silver methenamin staining procedure for electron microscopy.
The development of the aldehyde bisulfite toluidine blue reaction (Romhányi et al., 1975)
The classical view of the mechanism of the periodic acid-Schiff procedure is that the oxidation of vicinal diols yields dialdehydes that condense with Schiff's reagent to give a coloured product (review by Reid and Park, 1990). According to Guthrie (1961) the structure of the product results from the oxidation of mono-, di-, oligo- and polysaccharides. The next step is the visualisation and/or blockade of aldehydes, i.e. the addition of sodium bisulfite (Malinin, 1970) or cyanide (Lillie, 1952, Lillie, 1965; Lillie and Pizzolato, 1972). Other possibilities of the blockade and visualisation of this reaction are reviewed by Reid and Park (1990).
The original reaction after periodic acid oxidation following a selective bisulfite addition and toluidine blue staining at pH 1 was first described by Malinin (1970) for the light microscopic demonstration of sugar components (glycogen) and basophilia. He carried out only light microscopic observations without stabilization of the most labile metachromatic staining reaction and did not perform polarisation optical investigations.
Romhányi and Molnár (1974) as well as Romhányi et al. (1975) have adopted this reaction for polarising microscopy. The last named authors called the reaction the “aldehyde bisulfite toluidine blue reaction” (ABT reaction). The ABT reaction is an inversive topo-optical staining reaction and is characterized by a metachromatic basophilia, anisotropy and linear negative dichroism (Fischer, 1978; Makovitzky 1984a; Módis, 1991; Romhányi et al., 1975).
The ABT reaction as a selective topo-optical reaction of vicinal OH groups in polysaccharides
The first step is based on the selective oxidation of the OH groups with 1% periodic acid (30 min at room temperature) – equivalent to the first step in the PAS reaction, which is a common method in carbohydrate histochemistry (McManus, 1946). The oxidation mechanism is specific for the chemical groups shown in Figure 1 . The newly formed CHO groups react selectively with bisulfite, whereby they are converted into sulfonic acid (Fischer, 1978; Romhányi et al., 1975). The sulfonic acid has a strong negative charge and therefore the new aldehyde bisulfite-complex selectively reacts with basic dyes like toluidine blue (Tb) at pH 1 or various thiazine dyes, i.e azure a, azure b, methylene blue and 1,9-dimethyl methylene blue (1,9 dmmb) at pH 1 (Figure 2 ).
Figure 1.
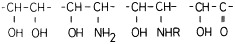
Chemical groups oxidised by periodic acid in the first step of the ABT reaction.
Figure 2.
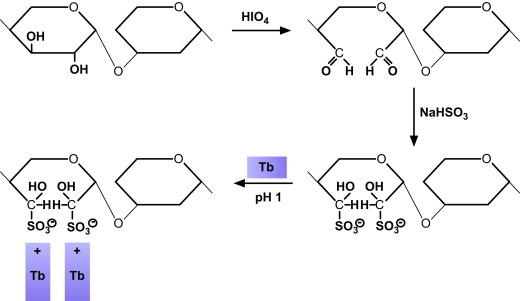
Diagram illustrating the mechanism of the ABT reaction (after Richter, 2005a).
As described by Romhányi et al. (1975) the ABT reaction is a useful method with higher intensity than the PAS reaction. The reaction is specific to the selective demonstration of vicinal OH groups by metachromatic basophilia and the detection of linear order of the vicinal (glycolitic) OH groups in polysaccharide chains.
In our experience, the ABT reaction is a sensitive method to show the exact localisation of periodate reactive carbohydrates in tissue sites with recognizable morphological characteristics. The great advantage of this topo-optical reaction over chemical or biochemical techniques is that it is inexpensive and easy to carry out. The entire mechanism of the ABT reaction is discussed in detail in our previous publications (Richter, 2005a, Richter, 2005b; Richter and Makovitzky, 2006). The polarisation optical analysis makes it possible to determine the spatial situation of the sugar chains on a molecular level, which is not possible with the conventional PAS reaction and with the electron microscopic PAS reaction (Figure 3 ).
Figure 3.
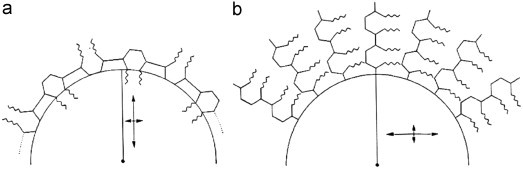
Polarisation optical analysis of the ABT reaction (after Romhányi et al., 1975; Fischer, 1977). (a) The sign is negative with respect to the length of the polysaccharide chains. Radially positive birefringence (optically positive spherulites) of the cell walls is indicative of radially oriented dye molecules and in turn of surface-parallel orientation of the reacting polysaccharide chains. (b) Radially negative birefringence (optically negative spherulites) induced by the reaction is indicative of surface-parallel dye molecule orientation and of mainly radially oriented, linear OH groups which are probably fixed to a circular structure possibly resulting from radial or helical polysaccharide chains.
Culling and Reid (1980), Culling et al. (1974), Reid et al., 1976, Reid et al., 1978, Klessen, 1977, Klessen, 1978 as well as Klessen and Graumann (1978) could selectively react the O-acyl radicals of the sugar molecules with an alkaline effect by potassium hydroxide (KOH) followed by the PAS reaction.
Fischer (1976) selectively showed the O-acyl radicals using the KOH-ABT reaction and a new periodic acid-borohydride ABT reaction. The original periodic acid-borohydride PAS reaction was described first by Reid et al. (1976). Fischer and Emödy (1976) obtained similar results in bacteria and fungi using the periodic acid-borohydride ABT reaction and the KOH-ABT reaction.
The polarisation optical identification of sialic acid residues
Sialic acids are one of the most important molecules of life since they occupy the terminal position on macromolecules and cell membranes and are involved in many biological and pathological phenomena. Over 40 neuraminic acid derivates have been elucidated. The family of sialic acids play a dual role: they are not only indispensable for protection and adaptation, but are also utilised by life-threatening infectious microorganisms (Schauer, 1988, Schauer, 2000, Schauer, 2004).
The sialic acid specific topo-optical reaction based on the mild PAS reaction and the ABT reaction
The sialic acid specific topo-optical staining reaction is based on the mild PAS (mPAS) reaction combined with bisulfite addition (Makovitzky, 1979, Makovitzky, 1980). The selective staining reaction of sialic acid is possible by the use of very dilute periodic acid (selective or mild periodate oxidation) followed by treatment with Schiff's reagent (Klessen, 1977, Klessen, 1978; Roberts, 1977; Klessen and Graumann, 1978; Veh and Kornadt, 1989; Veh et al., 1982). The basis of the sialic acid specific topo-optical reaction is as follows:
-
a.
Selective oxidation of vicinal OH groups on C8–C9 (including C7) carbon atoms of N-acetyl neuraminic acid.
-
b.
Presence of these groups in unsubstituted form.
-
c.
High suitability of the generated aldehyde groups for bisulfite addition.
The first step – mild periodic acid oxidation (0.01% periodic acid for 10 min at 4 °C) – produces a Schiff-positive C7-aldehydo-neuraminic acid residue. Subsequently, bisulfite addition and the 1,9 dmmb staining reaction and/or azure b staining reaction were carried out at pH 1–2 (Figure 4 ).
Figure 4.
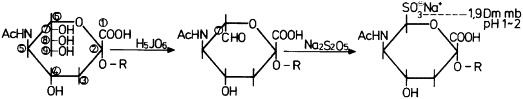
Mechanism of the sialic acid specific (mPAS) topo-optical staining reaction (after Makovitzky, 1980).
The specificity of the reaction has been proved by neuraminidase digestion and sialic acid extraction with 0.1 N H2SO4 (Feuerstein and Geyer, 1978; Makovitzky, 1984a; Richter and Makovitzky, 2006). As shown in our previous publications, methods based upon acid hydrolysis are comparable to digestions with neuraminidase (Makovitzky, 1984a; Richter, 2005a, Richter, 2005b; Makovitzky et al., 2006). In the mPAS reaction, 2.7 moles periodate were consumed per mole oxidised sialic acid (Spiro, 1964). These reaction conditions are specific for sialic acid, since after an oxidation time of 10 min 1.15 mol periodic acid are consumed by N-acetyl neuraminic (sialic) acid, compared with less than 0.07 mol for all other carbohydrates together (Roberts, 1977). According to Schauer and Faillard (1968) and Schauer (1973) the consumption of periodic acid per mol oxidised N-acetyl neuraminic acid is 1.4 mol after 10 min, and 2 mol after 40 min.
This reaction also determines, similarly to the ABT reaction, the sign of birefringence and thus the adjustment of dye molecules attached to linear oriented OH groups, i.e. parallel or perpendicular orientation (Makovitzky, 1984a, Makovitzky, 1987a; Richter, 2005a, Richter, 2005b; Richter and Makovitzky, 2006).
The finding that after mild periodic acid treatment the C8–C9 nonsubstituted OH groups of sialic acid are selectively oxidised, whereas no other tissue carbohydrates are affected, opens new methodological prospects.
By development of the KOH-sialic acid reaction and the periodate borohydride sialic acid reaction from the KOH-ABT reaction and PB-ABT reaction, it became possible to demonstrate the O-acyl sialic acid molecules selectively (Makovitzky, 1979, Makovitzky, 1981a, Makovitzky, 1981b).
The periodate oxidation of the glycosaminoglycans (GAGs)
Romhányi et al. (1978) described the “one-step” anisotropic PAS reaction with 2% periodic acid, which is a specific reaction for hyaluronic acid (proved with hyaluronidase digestion).
The “two-step” PAS reaction gives a positive reaction of acid mucopolysaccharides (proteoglycans and glycosaminoglycans) with vicinal diols located at the C2 and C3 position of the oxidised uronic acid residues (Scott and Dorling, 1969). Módis (1991) reinvestigated the two-step PAS reaction and combined it with the ABT reaction to the so called “chemically intensified basophilic reaction”, abbreviated as CIBR (Richter, 2005a, Richter, 2005b; Richter and Makovitzky, 2006). Oxidation with freshly prepared periodic acid (2% solution) is the first step of this reaction, yielding aldehyde groups at the sites of vicinal diols located on carbohydrate residues (Módis, 1991; Richter, 2005a, Richter, 2005b; Richter and Makovitzky, 2006). The newly produced aldehyde groups in this position are blocked by subsequent treatment with sodium borohydride. A further prolonged oxidation with periodic acid (1% solution, 24 h) results in the oxidation of vicinal OH groups of glucuronic acid or iduronic acid-containing glycosaminoglycans (hyaluronic acid, chondroitin sulfate and dermatan sulfate). After bisulfite addition, the newly produced hydroxysulfonate groups in the position C2 and C3 are selectively stained with toluidine blue or 1,9-dimethyl methylene blue at pH 1 (Figure 5 ). Sulfate groups bound primarily to glycosaminoglycans do also participate in this reaction, however they do not affect the oriented dye binding. It should be observed that the specificity of the staining is dependent on the low pH of the dye bath where the carboxyl groups are suppressed (Richter, 2005a, Richter, 2005b; Richter and Makovitzky, 2006).
Figure 5.
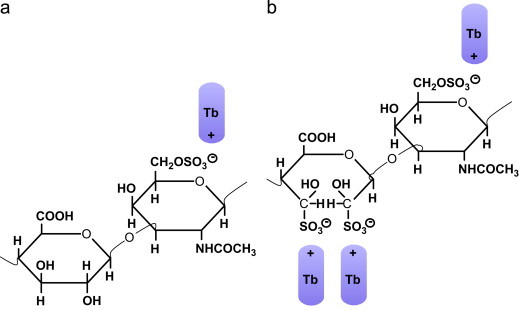
Mechanism of the CIBR demonstrated in a repeating disaccharide unit (d-glucuronic acid and N-acetyl-d-galactosamine) of chondroitin-6-sulfate. The prolonged treatment with periodic acid results in the oxidation of vicinal hydroxyl groups of the uronic acid residues and the newly formed aldehyde groups are transformed to hydroxysulfonate groups by the addition of sodium disulfite which can be stained with Tb at pH 1. The uronic acid residue will bind two dye molecules after CIBR which will be added to the dye molecule bound by the sulfate groups of the hexosamine residue. The outcome ist that three Tb molecules are bound to one disaccharide unit in comparison to the control (toluidine blue topo-optical reaction) where one dye molecule is taken up. (a) Tb pH 1 (b) CIBR with Tb at pH 1 (after Richter, 2005a).
The sugar components and their orientation in the cell walls
According to Frey-Wyssling, 1953, Frey-Wyssling, 1954, Frey-Wyssling, 1959, the sign of unstained cellulose “elementar fibrils” is linear positive to the length. After Congo red staining, cellulose gives an additive topo-optical staining reaction, and the dye molecules are oriented parallel to the surface (Wälchli, 1945). Cellulose is a neutral polysaccharide, a linear ß-glycosidic polymer of d-glucose with chain lengths of over 2000 glucose units and with a regular linear crystalline order of polysaccharide chains and is rich in OH groups. The fibrillar structure of cellulose has been proved by X-ray diffraction and electron microscopic investigations (Frey-Wyssling, 1954; Frey-Wyssling et al., 1948; Hagen, 1969).
Fischer found for the cellulose of the root of Alium cepea (onion) that after pectinase digestion and ABT reaction, an inversive topo-optical reaction, the sign was linear negative to the axis with metachromasia and negative dichroismus: the toluidine blue dye molecules and the linear oriented OH groups are perpendicularly oriented to the surface (Romhányi et al., 1975; Fischer, 1978). In contrast, for amylose it was supposed that three different molecular conformations of the alpha-1-4-glucosan amylose are possible: stretched, folded or helical chains. Frey-Wyssling (1953) studied the polarisation of starch granules by optical and electron microscopy. These are known to consist of 22% amylose and 78% amylopectin (Bates et al., 1943). The starch granules, when unstained, have a high anisotropy and are linear negative (radially positive spherulites); therefore, the hexose molecules in starch granules are oriented opposite to those in cellulose. In starch granules, the hexose molecules are oriented radially and show a high intensity of anisotropy. This indicates a similar orientation of amylose and amylopectin in starch granules (Frey-Wyssling, 1953). The radial orientation confirm the results of the X-ray diffraction and the polarisation optical analysis of Frey-Wyssling, 1954, Frey-Wyssling, 1959, Frey-Wyssling, 1976. The amylose has a crystalline helical structure: a helical unit has 6 hexose molecules. Using the ABT reaction we found a radial orientation of the hexose molecules in complex starch granules, so it is an inversive topo-optical staining reaction (Figure 6 ). The sign is linear positive to the axis. The polysaccharide chains are parallel to the surface, in a helical orientation (Fischer, 1977; Makovitzky, 2003).
Figure 6.
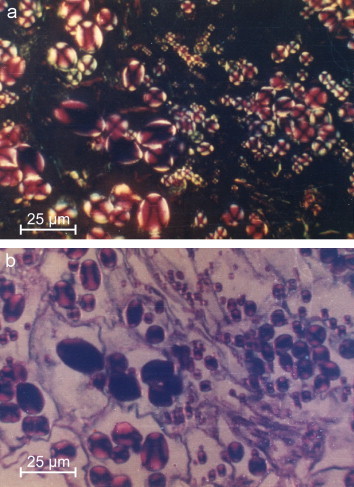
ABT reaction of starch granules in polarised light without (a) and after additive compensation (b) ×300. Scale bars represent 25 μm.
Glycogen is made of glucose units that are connected by alpha-1,4- and alpha-1,6 bonds. The 1,4-connections form long glucose chains, whereas the 1,6 bonds are responsible for the interconnection of the chains. The unstained glycogen is isotropic. In the ABT reaction, (Fischer, 1978) recorded a granular mosaic anisotropy with low intensity and no dichroismus. In opposition to the green polarisation colour of cellulose after ABT reaction, the glycogen shows a red polarisation colour. This is not the optimal polarisation colour and can be explained by the branched structure of glycogen, indicating bundles of carbohydrate chains with various orientations. The more complex polysaccharides dextran and galactan are isotropic with the ABT reaction. This observation is probably caused by the non-specific orientation of the polysaccharide chains.
The ABT reaction of isolated cellulose
Unstained isolated cellulose (long cellulose fibers and microcrystalline cellulose) shows a high anisotropy, the sign of the anisotropy is linear negative to the length. Congo red staining applied for 10 min revealed a light microscopically weak congophilia and a weak anisotropy, 20 min staining enhanced the effects. Small fragments show a yellow colour by light microscopy, whereas large fragments show an orange colour. Both exhibit anisotropy with an orange polarisation colour. The sign of the anisotropy of the larger fragments is linear negative, i.e. the Congo red molecules are perpendicularly oriented to the cellulose. The small fragments exhibit a linear positive anisotropy with, therefore, parallel oriented Congo red molecules.
The ABT reaction gives a similar result: light microscopically we found a weak metachromasia with the exception of some non-reactant and unstained fragments. Both types of fragments show a red-orange polarisation colour. The sign of the birefringence of the larger fragments is linear positive (inversive topo-optical reaction), the smaller fragments show a linear negativity.
We have found with the aldehyde bisulfite 1,9-dimethylmethylene blue (ABD) reaction a strong staining reaction with metachromatic basophilia and an anisotropy with linear positive sign with respect to the length (or axis) by light microscopy.
The molecular orientation of rhodopsin in the rod outer segment of frog retina
Romhányi et al. (1978) removed acid polysaccharides by hyaluronidase and blocked the participation of the free aldehyde groups present in formalin-fixed rods by treatment with borohydride, which reduces aldehyde groups to non-reactive OH groups in the ABT reaction. He found a strong reaction of the rod outer segments for vicinal OH groups. The sign of the anisotropy was negative with respect to the axis of the rods, indicating that the carbohydrate chains were oriented perpendicularly to the disc membranes.
The orientation of the sugar components in basement membranes
Romhányi developed a mild sulfation reaction after acetylation and methylation to demonstrate the polyhydroxyl groups of the collagen fibers. This is an inversive topo-optical reaction of the collagen fibers and collagen fibers stain selectively with toluidine blue at pH 1. Light microscopically, the fibers show an intensive selective basophilia, a deep green polarisation colour and the sign of anisotropy is linear negative. Romhányi blocked oxidation of the glycolitic OH groups of the sugars with periodic acid, sulfated the oriented OH groups of the collagen fiber (of hydroxyprolin and hydroxylysin) with sulfuric acid (“mild sulfation”) and stained them with toluidine blue at pH 1. The sign of anisotropy was linear negative to the length as a selective presentation of the collagen fibers in the basement membranes (Romhányi, 1988; Romhányi et al., 1973, Romhányi et al., 1974).
After ABT reaction and/or ABD reaction, basement membranes show an intensive basophilic staining and a strong birefringence with a deep polarisation colour. The sugars are present as disaccharide units in the basement membranes and are bound with glycosidic bonds to the OH groups of the hydroxylysine molecules of the protocollagen fibers (Romhányi, 1975, Romhányi, 1978, Romhányi, 1988, Romhányi, 1989; Spiro, 1967, Spiro, 1973; Spiro et al., 1971). The ABT reaction has been used by Deák (1976) to study the arrangement of sugar components (disaccharides) in epithelial- and capillary basement membranes.
The ABT reaction of glycolipids
The anisotropy of myelin sheaths was first investigated by Schmidt (1936). He presented them as highly oriented lipid membranes. Frozen sections of peripheral nerves show unstained an intense birefringence (Schmidt, 1936; Romhányi, 1978; Wolman, 1970). The sign of anisotropy is linear negative to the axis. After the ABT reaction, the frozen sections of the myelin sheaths show an intense basophilic staining reaction and a multiplication of the original birefringence. The linear oriented OH groups are parallel to the length axis. It is interesting that the myelin sheath, after formalin fixation (4% phosphate buffered formalin at pH 7.4) and paraffin wax embedding, shows a positive ABT reaction. After lipid extraction, Romhányi et al. (1975) found a positive ABT reaction and interpreted this result as the presence of oriented carbohydrates. However, in addition, one has to consider free aldehyde groups and the aldehyde groups produced from unsaturated lipids by periodic acid. In order to distinguish between the two possibilities, i.e. carbohydrates or lipids, Romhányi et al. (1975) considered several experimental results:
-
a.
The free groups of aldehydes can be stained selectively with a bisulfite toluidine blue reaction.
-
b.
The unsaturated bonds can be shown with a performic acid-Schiff reaction (Lillie, 1952) and/or with the polarisation optical variant – the performic acid bisulfite toluidine blue reaction.
-
c.
The carbohydrate component can be stained selectively with a performic acid-borohydride ABT reaction (Romhányi et al., 1975). In this reaction, the free aldehyde groups and aldehyde groups produced by periodic acid from double bonds are converted into OH groups by the effect of borohydride. These newly formed OH groups are not vicinal and therefore do not participate in the ABT reaction.
Romhányi et al. (1975) described the reactivity of glycol groups in glycolipids of the cerebroside deposits in Gaucher cells. Age pigment granules of the heart and brain were rendered strongly basophilic by ABT reaction, but proved to be isotropic (Romhányi and Deák, 1975).
The complex structural organisation of the carbohydrate components in microbial cell walls
The ABT reaction is also used to selectively stain microbes in daily routine diagnostic practice. Microbes such as fungi and bacteria have highly oriented carbohydrates in their cell walls (Fischer, 1976, Fischer, 1977; Fischer and Emödy, 1976). Between 1976 and 1981 Fischer and Emödy analysed the molecular structure of the cell walls of different fungi and bacteria with the ABT reaction and later with the KOH-ABT reaction (specific for the occurrence of O-acyl groups in the sugar molecules). They also examined the mechanism of bacterial phagocytosis and found that at the surface of leukocytes a de novo O-acyl sialic acid synthesis takes place (Fischer, 1981, Fischer, 1987; Fischer and Emödy, 1978).
The spatial orientation of the sugar chains and sialic acids in human erythrocyte membranes
We determined the spatial position of the carbohydrate chains of erythrocyte membranes and lymphocyte membranes based on analysis of the aldehyde bisulfite 1,9-dimethylmethylene blue reaction (ABD reaction, Makovitzky, 1984a, Makovitzky, 1987b). According to our analysis, oligosaccharide chains containing sialic acids are linked to the peptide skeleton and oriented parallel to the membrane (Stibenz, 1985; Stibenz and Geyer, 1980, Stibenz and Geyer, 1982; Geyer and Makovitzky, 1980; Geyer et al., 1973).
Glycophorins are a group of transmembrane proteins whose extracellular parts are highly glycosylated and that carry the largest amount of the membrane carbohydrates (Steck, 1974, Steck, 1978; Furthmayr, 1978a, Furthmayr, 1978b). Glycophorin has a molecular mass of 31,000 g/mol. It consists of 60% carbohydrates and 40% protein (Furthmayr, 1978b; Marchesi, 1979a, Marchesi, 1979b, Marchesi, 1983). From the amino acid sequences, the maximum length of the extracellular segment of glycophorin A is 168 Å (16.8 nm). Glycophorin A is highly glycosylated and its carbohydrate moiety amounts to 64% of the dry mass (Winzler, 1970; Stibenz, 1985). All saccharide chains are linked by O-glycosidic bonds to the N-terminal segment. Of the 16 saccharide chains of glycophorin A, 15 carry 30 terminal sialic acid residues (Furthmayr, 1978a, Furthmayr, 1978b) and this may affect the three-dimensional structure under physiochemical conditions. Later findings (Stibenz and Geyer, 1980; Geyer and Makovitzky, 1980) suggest that at physiologic pH and ionic strength, sialic acids are uniformly arranged and distributed, due to the spatial orientation of the glycoprotein segment and the high charge density of sialic acids. These reports were fully corroborated by the sialic acid specific topo-optical reaction.
The oriented dye binding of toluidine blue to the erythrocyte membrane between pH 5–7.4 can be explained by sialic acids of the oligosaccharides, whose COOH groups are optimally dissociated at this pH. These sialic acids are oriented terminally and partially overlap (Geyer and Makovitzky, 1980; Halbhuber et al., 1983; Gliesing, 1987). The binding sites of the sugar chains have a distance of 0.4–0.5 nm in glycophorins to each other (Stibenz, personal communication 1980) and by parallel orientation of the carbohydrate chains this can also be assumed for the COOH groups of the sialic acid molecules. Between pH 5–7.4, this structure leads to metachromasia and anisotropy and to an optimal dye binding, characterized by a deep green polarisation colour in contrast to the toluidine blue topo-optical staining reaction (Figure 7 ).
Figure 7.
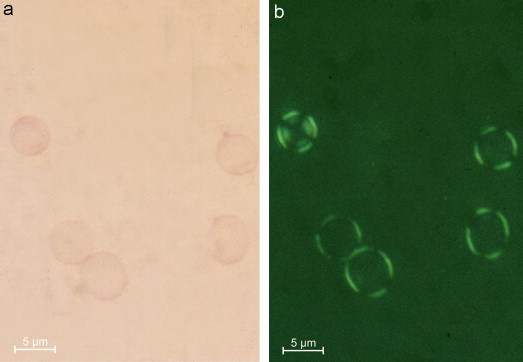
Human erythrocytes stained with the sialic acid specific topo-optical reaction. (a) Light microscopy and (b) polarisation optical × 160. Scale bars represent 5 μm.
After neuraminidase digestion and combined trypsin-neuraminidase digestion, the erythrocyte membrane shows anisotropy with decreased intensity upon toluidine blue topo-optical staining between pH 5–7.4. This underlines the leading role of the COOH groups of the sialic acid molecules in oriented dye binding (Makovitzky and Geyer, 1977; Gliesing, 1987). The neuraminidase- or trypsin-treated human erythrocytes have a reduced concentration of sialic acids (110 μg/g; 210 μg/g) compared to the control cells (290 μg/g) (Stibenz, personal communication; Halbhuber et al., 1984a, Halbhuber et al., 1984b).
The spatial orientation of the sialic acid carrying glycophorin A is important for the oriented dye binding and for the sialic acid specific topo-optical staining reaction. Using electron microscopy, Skutelsky et al. (1977) found that the average distance between sialic acids to the outer lipid lamella is 5–7 nm. Donath (1979) and Donath and Gingell (1983) recorded a gain of the glycocalyx thickness at 30 mM NaCl concentration from 3.5 to 12.1 nm by use of isotonic NaCl solution at pH 7.4. Based on biochemical results under physiological conditions, the external hydrophilic N-terminal segments of glycophorins are parallel oriented to the outer lamella of the lipid bilayer (Geyer, 1980; Geyer and Makovitzky, 1980; Stibenz and Geyer, 1982; Stibenz, 1985; Halbhuber et al., 1990, Halbhuber et al., 1992).
Pretreatments of the cells (hypotonic milieu or procain hydrochloride) as well as various ghost preparations (Makovitzky, 1979; Geyer and Makovitzky, 1980; Gliesing and Halbhuber, 1989) decreased the original membrane anisotropy. These treatments may affect the conformational state and change the original spatial orientation of the N-terminal hydrophilic segments of the glycophorin A. Stibenz and Geyer, 1980, Stibenz and Geyer, 1982 suggest two conformational states of hydrophilic segments of glycophorin A based on calculation of probability according to their amino acid sequence: (a) to run parallel to and (b) to have polar or polar and apolar interactions with the outer lamella of the lipid bilayer. On the basis of molecular theory of the toluidine blue topo-optical staining reaction (Makovitzky and Geyer, 1977; Geyer and Makovitzky, 1980), the topo-optical staining reaction behaviour of the various prepared ghost membranes can be understood by conformational changes, because all ghost preparations were carried out in the presence of a protease inhibitor. The reconstituted ghosts did not give the original intensity of control erythrocyte membranes (Geyer and Makovitzky, unpublished data; Gliesing and Halbhuber, 1989). Interestingly, the membrane anisotropy is restored after neuraminidase or combined enzymatic treatments with the aldehyde bisulfite 1,9-dimethylmethylene blue reaction (ABD). The residues obviously have some linear oriented OH groups and these groups are reactive.
The sugar chains and sialic acids in human lymphocyte membranes
The principle structure of human lymphocyte and erythrocyte membranes is the same. The sialic acid is bound to the terminal part of oligosaccharide chains and is responsible for the negative charge of lymphocytes. The amount of sialic acids on the surface coat is higher in lymphocyte membranes compared to erythrocyte membranes (Ruhenstroth-Bauer, 1961; Ruhenstroth-Bauer and Lücke-Huhle, 1968). Kósa and Módis (1989) found that the sialic acid concentration on the cells of patients with chronic lymphatic leukaemia (CLL) is higher than in control lymphocytes, i.e. the cells of CLL-patients show an intensified anisotropy compared to control lymphocytes. They also found that after neuraminidase digestion, the anisotropy is decreased or abolished. Makovitzky, 1983, Makovitzky, 1984b was able to show that with the sialic and O-acyl sialic acid topo-optical staining reaction NK/Ly leukaemia ascites cells exhibit the most intense anisotropy .
Using the 1,9-dimethylmethylene blue topo-optical staining reaction at pH 1 we have demonstrated ultrastructural differences between the lymphocyte membrane and the erythrocyte membrane, as illustrated in Table 1 . The erythrocyte membrane is not stained with 1,9 dmmb at pH 1. We demonstrated the GAG components of the surface coat on the membrane of human lymphocyte. With the KOH–ABD reaction and/or KOH-sialic acid specific reaction, we demonstrated O-acyl residues in the sugar chains of the surface coat on the membrane of human lymphocytes (Makovitzky, 1979, Makovitzky, 1981a, Makovitzky, 1981b). We obtained similar results with the periodic acid borohydride–KOH–ABD reaction or—periodic acid borohydride KOH-sialic acid topo-optical reaction (light microscopically by Culling and Reid, 1980; Culling et al., 1974; Reid and Park, 1990; Reid et al., 1976, Reid et al., 1978). We demonstrated the membrane bound RNA (mRNA) with the PBT –reaction (permanganate-bisulfite toluidine blue-reaction (Fischer, 1978) on the surface of human lymphocytes (Makovitzky, 1987a, Makovitzky, 1987b).
Table 1.
Differences between erythrocyte and lymphocyte membrane with topooptical staining reactions.
| Sialic acid | 9-O-Acyl sialic acid | mRNA | sGAG | |
|---|---|---|---|---|
| Erythrocyte membrane | + | − | − | − |
| Lymphocyte membrane | + | T−, B+ | + | + |
The B-lymphocytes have highly oriented 9-O-acyl sialic acid and N-acetyl neuraminic acid (sialic acid) on its outer membrane, as demonstrated by quantitative colorimetry, thin-layer chromatography and combined gas–liquid chromatography–mass spectrometry (Kamerling JP et al., 1980, Kamerling et al., 1982).
The metabolism of acetylated sialic acids is under the control of two groups of enzymes: O-acetyl transferases and 9-O-acetyl esterases (Klein and Roussel, 1998). Shen et al., 2004a, Shen et al., 2004b found 9-O-acetyl sialic acids in the colon and the goblet cells and discussed their regulation. Reid et al. (1978) and Reid and Park (1990) performed many histochemical reactions for the selective detection of 9-O-acyl sialic acids. These reactions were adopted for polarisation microscopy (Makovitzky, 1984a, Makovitzky, 1987a, Makovitzky, 1987b) making it possible to determine the orientation of the linear oriented OH groups of sialic acid molecules (Figure 8 ). At the same time, it was possible to demonstrate the direction of the linearly oriented OH groups from sialic acid.
Figure 8.
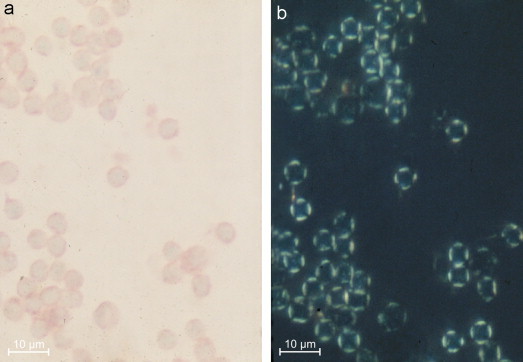
Human lymphocytes stained with the O-acyl sialic acid specific topo-optical reaction. (a) Light microscopy and (b) polarisation optical ×80. Scale bars represent 10 μm.
Detection of 9-O-acetylated sialoglycoproteins is an immunological tool for monitoring childhood acute lymphoblastic leukaemia (Pal et al., 2004a, Pal et al., 2004b). The overexpression of 9-O-acetylated sialoglycoconjugates on lymphoblasts and concomitant anti 9-O-acetylated sialoglycoconjugates have been found to have a diagnostic and prognostic potential (Bandyopadhyay et al., 2005). Dot-blot analysis demonstrated the potential application of immune complexed antigen as a disease specific marker (monitoring marker) and its efficacy as a sensitive and specific method that could serve as an economical yet effective index for monitoring disease status.
Rinniger et al. (2006) have presented the localisation and distribution of O-acetylated N-acetyl neuraminic acids, the endogenous substrates of the hemagglutinin-esterase of murine coronaviruses, in mouse tissue.
The ultrastructure of human platelet membrane glycoproteins
Using the ABT/ABD reaction and the sialic acid specific topo-optical reaction it became possible to demonstrate glycoproteins in the surface location of human platelets. The results are comparable to those obtained with biochemical techniques. The spatial orientation of oligosaccharide chains and the localisation of terminal sialic acids are similarly to the human erythrocyte membrane and lymphocyte membrane (Makovitzky, 1984a, Makovitzky, 1984b; Tsuji and Osawa, 1986; Tandon et al., 1989).
Microvilli and mucus film of the epithelial surface layer of the intestinal mucus membrane
In the duodenum, jejunum and ileum, the sialic acids and 9-O-acyl sialic acids are oriented in opposite directions in the outer and inner layer. The microvillous surface layer is a bilayer: the microvilli are linear negative with respect to the axis of the inner layer and the mucus film in the outer layer is linear positive. Optical analysis indicate that the 1,9-dimethylmethylene blue dye molecules are bound perpendicularly to the inner layer on the membranes of the individual microvilli. In the outer layer, the dye molecules are oriented parallel (Makovitzky, 1987a, Makovitzky, 1990; Romhányi et al., 1975; Romhányi, 1978). Thus, the vicinal OH groups of the sugar chains and the sialic acid molecules are oriented opposite (Figure 9 ).
Figure 9.
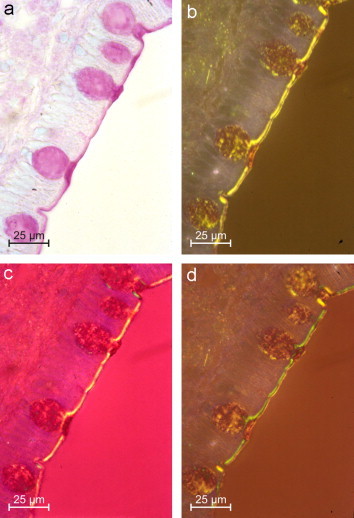
Sialic acid specific topo-optical reaction of the human duodenum in bright-field (a), polarised light (b), after additive (c), and subtractive compensation (d) × 400. Scale bars represent 25 μm.
Oriented sugar chains in the bronchial epithelium
Németh, 1974, Németh, 1976 found that the mucociliar surface and mucus film of the bronchial epithelium show unstained a linear positive anisotropy to the length. After toludine blue topo-optical staining and/or ABT reaction, he found by compensation an opposite phenomenon: the mucus film bound the dye molecules perpendicular to the length in the two reactions, whereas the mucociliar surface bound the dye molecules parallel to the length. Based on his polarisation optical analysis, the sugar chains are parallel oriented in the mucus film, and perpendicular in the mucociliar surface. The mucus film swims on the mucociliar units under physiological hydrated conditions.
Polarisation optical and histochemical investigations of the proteoglycans and glycosaminoglycans (GAGs)
Proteoglycans contain a linear protein core to which one or more glycosaminoglycans chains (chondroitin sulfate, dermatan sulfate, heparan sulfate, heparin and keratan sulfate) are covalently linked. The highly anionic GAGs consist of repeating disaccharide units, containing hexosamine (either d-glucosamine or d-galactosamine) and hexuronic acid (either d-glucuronic acid or l-iduronic acid). The only GAG which is not bound to a protein core is hyaluronic acid. Proteoglycans represent a diverse group of glycoconjugates sharing the common basic feature that they all have at least one GAG side chain. The core protein is generally linear in shape – except some globular domains – and the extended GAGs are perpendicularly oriented to the protein backbone (Romhányi, 1963; Módis, 1974, Módis, 1991; Snow and Wight, 1989). The GAGs have a helical conformation and they – due to their high density of anionic charges (sulfates and carboxyls) – can easily interact with microcations like Na+, K+, Ca2+ and polycations. The polarisation optical classification of proteoglycans has been reviewed by Módis (1991).
Fischer (1978) and Módis (1991) adapted the ABT reaction for the polarisation microscopic analysis of the glycosaminoglycans (“two-step” method and CIBR). The light microscopical reaction was developed originally by Scott and Dorling (1969). GAG components can be analysed using the toluidine blue reaction or 1,9-dimethylmethylene blue topo-optical reaction (Appel and Makovitzky, 2003; Makovitzky, 2001; Módis, 1991; Richter, 2005a, Richter, 2005b; Richter and Makovitzky, 2006). Fischer (1978) was first to demonstrate that GAGs also yield a pronounced ABT reaction after prolonged periodic acid incubation. In the literature, only marginal PAS-positivity of GAGs has been described. This is in contrast to the known linearly oriented OH groups of the uronic acids (Fischer, 1978). Thus, Fischer analysed the synovial fluid and its hyaluronic acid components with a prolonged ABT reaction. The hyaluronic acid components showed linear negative birefringence with respect to the length, i.e. the polysaccharide chains are oriented linear parallel to the length (axis).
Topo-optical reactions of carbohydrate residues in amyloid deposits
Amyloid is a generic term referring to tissue deposits of highly organised insoluble fibrils. They are composed of three major components: the amyloid fibril protein, amyloid P component (AP) and GAGs. The amyloid P component comprises up to 15% of the amyloid deposits and consists of neutral hexoses and their derivates. Sialic acid and fucose are found in terminal positions in the oligosaccharide chains of the glycoprotein (Bladen et al., 1966; Binette et al., 1971; Haupt et al., 1972; Hess et al., 1988). In recent studies of the authors (Richter, 2005a, Richter, 2005b; Richter and Makovitzky, 2006), topo-optical reactions were used to establish the structure, distribution and location of carbohydrate residues that occur within amyloid deposits. Congo red-marked amyloid deposits correlated pathomorphologically with ABT-positive structures, indicating a linkage of amyloid fibrils and periodate reactive carbohydrates (Figure 10 a–d). Using the sialic acid specific topo-optical reaction, it became possible to visualise small amounts of sialic acids bound to the amyloid fibrils (Figure 10e, f). After the toluidine blue topo-optical reaction, the amyloid deposits exhibited metachromasia in bright light and birefringence with a red polarisation colour between crossed polars indicating the presence of sulfated GAGs. The critical electrolyte concentration (CEC) method revealed hyaluronic acid, chondroitin sulfate, keratan sulfate and heparan sulfate in amyloid deposits and tissue-isolated amyloid fibrils (Richter, 2005a, Richter, 2005b; Appel et al., 2005; Makovitzky et al., 2006).
Figure 10.
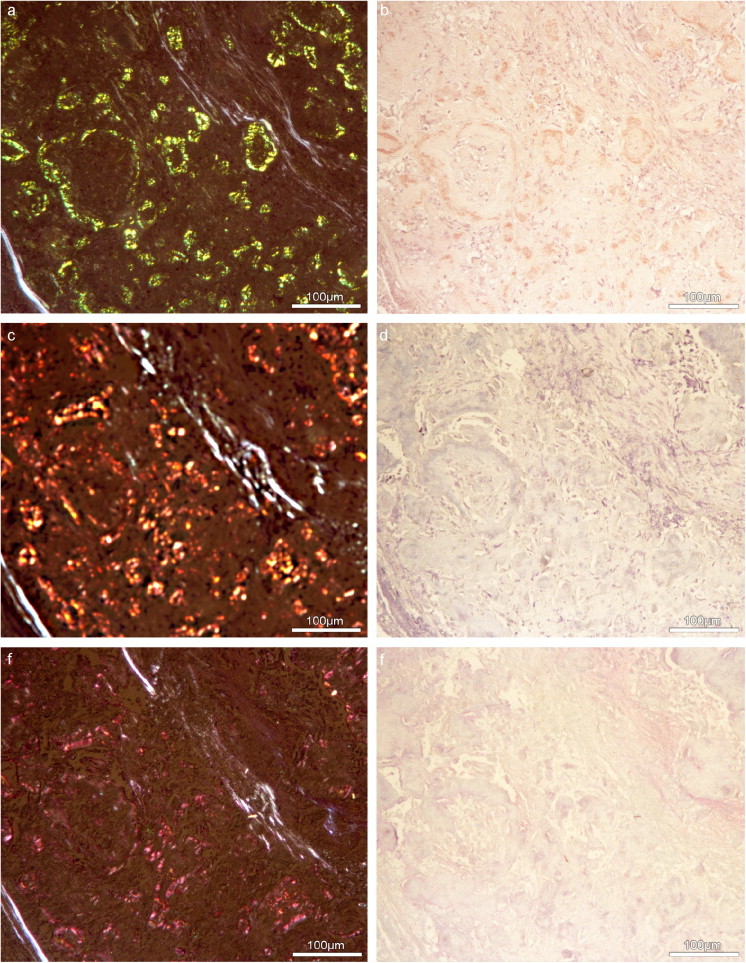
Visualisation of amyloid deposits in pulmonary biopsy specimens from a 49-year-old female patient with nonsecretory multiple myeloma (NSMM). Consecutive serial sections stained with Congo red (a,b), ABT reaction (c,d) and the sialic acid specific topo-optical reaction (e,f). Congo red-marked areas where congruent with areas shown in c–f. Metachromasia appears in bright light (d,f) and birefringence with a red polarisation colour in polarised light (c,e) corresponding to a close pathomorphological relationship between amyloid fibrils and periodate reactive carbohydrates with terminally bound sialic acids. In polarised light (a,c,e) and bright light (b,d,f) ×40. According to Richter, (2005a). Scale bars represent 100 μm.
It has been postulated that GAGs play a role in the pathogenesis of amyloidosis which includes initiating fibrillogenesis, enhancing the stability of preformed fibrils, determining the anatomical distribution of amyloid deposits and decreasing amyloid susceptibility to proteolysis (Snow and Wight, 1989; Fraser et al., 1992; De Lorenzi et al., 2004). We have examined the effect of the “chemically intensified basophilic reaction” (CIBR) for the first time on amyloid deposits and found that it is a sensitive topo-optical visualisation reaction of uronic acid-containing GAGs with potential vicinal diols (Richter, 2005a, Richter, 2005b; Richter and Makovitzky, 2006). In conclusion, we showed that there is a close pathomorphological relationship between amyloid fibrils, periodate reactive carbohydrates – including sialic acids – and GAGs.
Concluding remarks
The findings of Romhányi et al. (1975), Fischer (1978) as well as Fischer and Emödy (1976) indicate that the polarisation optical analysis, by means of the ABT reaction, is a very sensitive and useful method to study the molecular order of the polysaccharide chains of plant cell walls, yeast cell walls and capsules. Our analyses show highly oriented sugar components and sialic acids in the erythrocyte membranes and lymphocyte membranes using the ABT reaction or ABD reaction, as well as sialic acid topo-optical reaction and its variants. A high intensity is often characteristic for the topo-optical staining reactions of erythrocyte and lymphocyte membranes, detecting ultrastructural differences between the membranes of red blood cells and lymphocytes, not amenable to other ultrastructural morphological methods.
The “one-step ABT reaction” yields results on the spatial orientation of hyaluronic acids in tissue sites. Further, the CIBR provides information on the orientation of primarily uronic acid-containing GAGs with potential vicinal diols (hyaluronic acid, chondroitin sulfate and dermatan sulfate) in human and animal amyloid deposits (Appel and Makovitzky, 2003; Appel et al., 2005; Makovitzky, 2001; Richter, 2005a, Richter, 2005b; Richter and Makovitzky, 2006; Kröger et al., 2008). Based on the applied topo-optical reactions, it can be confirmed that the “non-fibrilar” component of amyloid deposits (the amyloid P component with carbohydrate residues – including sialic acids – and GAGs) has a complex oriented structure and establishes an entity with the amyloid fibril proteins as well as lipids. We confirm the helical model for the amyloid fibril structure proposed by Inoue and Kisilevsky (1996).
References
- Ainsworth S.K., Ito S., Karnovsky M.J. Alkaline bismuth reagent for high resolution ultrastructural demonstration of periodate-reactive sites. J Histochem Cytochem. 1972;20:995–1005. doi: 10.1177/20.12.995. [DOI] [PubMed] [Google Scholar]
- Appel T.R., Makovitzky J. Romhányi's staining methods applied to tissue-isolated amyloid fibrils. Acta Histochem. 2003;105:371–372. doi: 10.1078/0065-1281-00725. [DOI] [PubMed] [Google Scholar]
- Appel T.R., Richter S., Linke R.P., Makovitzky J. Histochemical and topo-optical investigations on tissue-isolated and in vitro amyloid fibrils. Amyloid. 2005;12:174–183. doi: 10.1080/13506120500221906. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S., Mukherjee K., Chatterjee M., Bhattacharya D.K., Mandal C. Detection of immune-complexed 9-O-acetylated sialoglycoconjugates in the sera of patients with pediatric acute lymphoblastic leukemia. J Immunol Methods. 2005;297:13–26. doi: 10.1016/j.jim.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Bates F.L., French D., Rundle R.L. Amylose and amylopectin content of starches determined by their iodine complex formation. J Am Chem Soc. 1943;65:142–148. [Google Scholar]
- Binette P., Matsuzaki M., Calkins E., Alper R., Winzler R.J. Carbohydrate composition of amyloid components. Proc Soc Exp Biol Med. 1971;137:165–167. doi: 10.3181/00379727-137-35536. [DOI] [PubMed] [Google Scholar]
- Bladen H.A., Nylen M.U., Glenner G.G. The ultrastructure of human amyloid as revealed by the negative staining technique. J Ultrastruct Res. 1966;14:449–459. doi: 10.1016/s0022-5320(66)80075-8. [DOI] [PubMed] [Google Scholar]
- Culling C.F., Reid P.E. Specific techniques for the identification of O-acylated sialic acids in colonic mucins. J Microsc. 1980;119:415–425. doi: 10.1111/j.1365-2818.1980.tb04113.x. [DOI] [PubMed] [Google Scholar]
- Culling C.F., Reid P.E., Clay M.G., Dunn W.L. The histochemical demonstration of O-acetylated sialic acid in gastrointestinal mucins. Their association with the potassium hydroxyde-periodic acid-Schiff effect. J Histochem Cytochem. 1974;22:826–831. doi: 10.1177/22.8.826. [DOI] [PubMed] [Google Scholar]
- De Lorenzi E., Giorgetti S., Grossi S., Merlini G., Caccialanza G., Bellotti V. Pharmaceutical strategies against amyloidosis: old and new drugs in targeting a “protein misfolding disease”. Curr Med Chem. 2004;11:1065–1084. doi: 10.2174/0929867043455549. [DOI] [PubMed] [Google Scholar]
- Deák G. Nodular thickening of peritubular basement membranes in diabetic kidneys. Acta Morphol Acad Sci Hung. 1976;24:191–202. [PubMed] [Google Scholar]
- Donath E. Zur Charakterisierung der elektrostatischen Eigenschaften von Zelloberflächen mit vorgelagerter Glycocalyx. Thesis. Humboldt Universität, Berlin, 1979.
- Donath E., Gingell D. A sharp cell surface conformational transition at low ionic strength changes the nature of the adhesion of enzyme-treated red blood cells to a hydrocarbon interface. J Cell Sci. 1983;63:113–124. doi: 10.1242/jcs.63.1.113. [DOI] [PubMed] [Google Scholar]
- Feuerstein H., Geyer G. Eine Modifikation der Sialinsäure-Bestimmung am Erythrozyten. Folia Haematol. 1978;105:679–683. [PubMed] [Google Scholar]
- Fischer J. Demonstration on microorganisms in tissues by ABT and KOH-ABT topo-optical reactions. Acta Morphol Acad Sci Hung. 1976;28:203–214. [PubMed] [Google Scholar]
- Fischer J. Optical polarization reveals different ultrastructural molecular arrangement of polysaccharides in the yeast cell walls. Acta Biol. 1977;24:49–58. [PubMed] [Google Scholar]
- Fischer J. Topo-optical analysis of complex biological structures transformable to polyaldehydes. PhD thesis, Pécs, Hungary, 1978.
- Fischer J. Accumulation of glycoproteins in activated neutrophilic granulocytes. Morphol Igazsagugyi Orv Sz. 1981;21:215–223. [PubMed] [Google Scholar]
- Fischer J. Structure and lectin binding of tissue glycoconjugates. DSc Thesis, Pécs, Hungary, 1987.
- Fischer J., Emödy L. Molecular order of carbohydrate components in cell walls of bacteria, fungi and algae according to the topo-optical reaction of the vicinal OH groups. Acta Microbiol Acad Sci Hung. 1976;23:97–108. [PubMed] [Google Scholar]
- Fischer J., Emödy L. Baktériális sejtfal és membránpolysaccharidák ultrastruk- turája és változásai a fagocitózis során. MTA Biol Oszt Közl. 1978;21:465–473. [Google Scholar]
- Fischer J., Romhányi G. Optical studies on the molecular-sterical mechanism of metachromasia. Acta Histochem. 1977;59:29–39. doi: 10.1016/S0065-1281(77)80076-7. [DOI] [PubMed] [Google Scholar]
- Fraser P.E., Nguyen J.T., Chin D.T., Kirschner D.A. Effects of sulfate ions on alzheimer β/A4 peptide assemblies: implications for amyloid fibril-proteoglycan interactions. J Neurochem. 1992;59:1531–1540. doi: 10.1111/j.1471-4159.1992.tb08470.x. [DOI] [PubMed] [Google Scholar]
- Frey-Wyssling A. Elsevier Publishing Company; 1953. Submicroscopic of protoplasm. [Google Scholar]
- Frey-Wyssling A. The fine structure of cellulose microfibrils. Science. 1954;119:80–82. doi: 10.1126/science.119.3081.80. [DOI] [PubMed] [Google Scholar]
- Frey-Wyssling A. Springer; Berlin-Göttingen-Heidelberg: 1959. Die pflanzliche Zellwand. [Google Scholar]
- Frey-Wyssling A. Submikroskopische morphologie mit hilfe indirekter und direkter methoden. Ber Bot Ges. 1976;89:525–529. [Google Scholar]
- Frey-Wyssling A., Mühlethaler K., Wyckoff R.G.W. Mikrofibrillenbau der pflanzlichen Zellwände. Experientia. 1948;4:475–476. [Google Scholar]
- Furthmayr H. Glycophorins A, B, and C: a family of sialoglycoproteins. Isolation and preliminary characterization of trypsin derived peptides. J Supramol Struct. 1978;9:79–95. doi: 10.1002/jss.400090109. [DOI] [PubMed] [Google Scholar]
- Furthmayr H. Structural comparison of glycophorins and immunochemical analysis of genetic variants. Nature. 1978;271:519–524. doi: 10.1038/271519a0. [DOI] [PubMed] [Google Scholar]
- Gahmberg C.G. Membrane glycoproteins and glycolipids: structure, localisation and function of the carbohydrates. In: Finean J.B., Michell R.H., editors. Membrane structure. Elsevier; Amsterdam: 1981. pp. 127–161. [Google Scholar]
- Gahmberg C.G., Andersson L.C. Selective radioactive labeling of cell surface sialoglycoproteins by periodate-tritiated borohydride. J Biol Chem. 1977;252:5888–5894. [PubMed] [Google Scholar]
- Gahmberg C.G., Hakomori S.I. External labeling of cell surface galactose and galactosamine in glycolipid and glycoprotein of human erythrocytes. J Biol Chem. 1973;248:4311–4317. [PubMed] [Google Scholar]
- Geyer G. Elektronenmikroskopische histochemie. In: Graumann W., Neumann K., editors. Handbuch der Histochemie Bd. 1/3. Fischer Verlag; Stuttgart-New York: 1977. [Google Scholar]
- Geyer G. How sensitive may cytochemical methods detect alterations of the glycocalyx? Basic Appl Histochem. 1980;24:3–15. [PubMed] [Google Scholar]
- Geyer G., Makovitzky J. Erythrocyte membrane topo-optical staining reflects glycoprotein conformational changes. J Microsc. 1980;119:407–414. doi: 10.1111/j.1365-2818.1980.tb04112.x. [DOI] [PubMed] [Google Scholar]
- Geyer G., Linss W., Stibenz D. Ultrahistochemical demonstration of positively charged groups at the surface of human red blood cells. Acta Histochem. 1973;46:244–252. [PubMed] [Google Scholar]
- Gliesing M. Topo-optische Untersuchungen an der Membran intakter und alterierter Erythrozyten. MD thesis, Jena, Germany, 1987.
- Gliesing M., Halbhuber K.J. Topo-optical studies of gradually disintegrated erythrocyte membrane derivates: different kinds of ghosts. Acta Histochem. 1989;86:117–121. doi: 10.1016/S0065-1281(89)80077-7. [DOI] [PubMed] [Google Scholar]
- Guthrie R.D. The “dialdehydes” from the periodate oxidation of carbohydrates. Adv Carbohydr Chem. 1961;16:105–158. doi: 10.1016/s0096-5332(08)60261-1. [DOI] [PubMed] [Google Scholar]
- Hagen A. Pflanzliche Zellwand. In: Bielka H., editor. Molekulare Biologie der Zelle. Fischer Verlag; Stuttgart: 1969. pp. 293–303. [Google Scholar]
- Halbhuber K.J., Feuerstein H., Stibenz D., Linss W. Membrane alteration during banking of red blood cells. Biomed Biochim Acta. 1983;42:337–341. [PubMed] [Google Scholar]
- Halbhuber K.J., Linss W., Lemke C., Fröber R., Gliesing M., Makovitzky J. Histochemical methods for the detection of alterations in erythrocyte membrane. Histochem J. 1984;16:351–353. doi: 10.1007/BF01002847. [DOI] [PubMed] [Google Scholar]
- Halbhuber K.J., Gliesing M., Stibenz D., Makovitzky J. Topo-optical investigations of human erythrocyte glycocalyx age-related changes. Histochemistry. 1984;81:187–193. doi: 10.1007/BF00490116. [DOI] [PubMed] [Google Scholar]
- Halbhuber K.J., Oehring H., Gliesing M., Stibenz D. Polarization-optical investigation (topo-optical analysis) of the structure of the human erythrocyte glycocalyx. Influence of pH, ionic strength and diamide-induced spectrin cross linking. Cell Mol Biol. 1990;36:643–657. [PubMed] [Google Scholar]
- Halbhuber K.J., Klinger M., Oehring H., Linss W., Gliesing M. Structural alteration of the membrane skeleton and of the glycocalyx-preconditions for the IgG-receptor expression in the red blood cell (RBC) membrane. In: Wegmann R.J., Wegmann M.A., editors. Recent advances in cellular and molecular biology. Peeters Press; Leuven: 1992. pp. 227–242. [Google Scholar]
- Haupt H., Heimburger N., Kranz T., Baudner S. Humanserumproteine mit hoher Affinität zu Carboxymethylcellulose, III. Physikalisch-chemische und immunologische Charakterisierung eines metallbindenden 9,5S-α1-Glykproteins. Hoppe Seylers Z Physiol Chem. 1972;353:1841–1849. [PubMed] [Google Scholar]
- Hess D., Ohishi H., Skinner M., Cohen A.S., Schmid K. The carbohydrate composition of human serum amyloid P component. Clin Chim Acta. 1988;173:331–336. doi: 10.1016/0009-8981(88)90022-8. [DOI] [PubMed] [Google Scholar]
- Hricovín M. Structural aspects of carbohydrates and the relation with their biological properties. Curr Med Chem. 2004;11:2565–2583. doi: 10.2174/0929867043364414. [DOI] [PubMed] [Google Scholar]
- Inoue S., Kisilevsky R. A high resolution ultrastructural study of experimental murine AA amyloid. Lab Invest. 1996;74:670–683. [PubMed] [Google Scholar]
- Kamerling JP, Makovitzky J, Schauer R, Vliegenthart JF, Wember M. The nature and histochemistry of sialic acids in human lymphocytes. 13. FEBS Meeting, Jerusalem, 1980, Abstract p. 180 C4-P3.
- Kamerling J.P., Makovitzky J., Schauer R., Vliegenthart J.F., Wember M. The nature of sialic acids in human lymphocytes. Biochim Biophys Acta. 1982;714:351–355. doi: 10.1016/0304-4165(82)90344-0. [DOI] [PubMed] [Google Scholar]
- Klein A., Roussel P. O-acetylation of sialic acids. Biochimie. 1998;80:49–57. doi: 10.1016/s0300-9084(98)80056-4. [DOI] [PubMed] [Google Scholar]
- Klessen C. Spezifität und Kontrollreaktionen in der Kohlenhydrathistochemie. Acta Histochem Suppl. 1977;18:45–58. [PubMed] [Google Scholar]
- Klessen C. Demonstration eines Alkali PAS-Effekts bei Verwendung von Perjodsäure in niedriger Konzentration. Histochemistry. 1978;56:299–305. doi: 10.1007/BF00495991. [DOI] [PubMed] [Google Scholar]
- Klessen C., Graumann W. Histochemische Untersuchung zum KOH-PAS-effekt. Acta Histochem Suppl. 1978;20:319–323. [Google Scholar]
- Kósa K., Módis L. A sejtfelszin szénhidrátok vizsgálata krónikus lymphoid leukaemiás betegek perifériás lymphocytáiban. Magy Belorv Arch Suppl. 1989;42:158–162. (Hungarian) [Google Scholar]
- Kröger M, Richter S, Gruys E, Kovács MB, Makovitzky J. Topo-optical investigations of AA-amyloid deposits from various species. Abstract. Acta Histochem 2008. [DOI] [PubMed]
- Lillie R.D. Ethylenic reaction of ceroid with performic acid and Schiff reagent. Stain Technol. 1952;27:37–45. doi: 10.3109/10520295209105058. [DOI] [PubMed] [Google Scholar]
- Lillie R.D. third ed. McGraw-Hill; New York-Toronto-Sydney-London: 1965. Histopathologic technique and practical histochemistry. [Google Scholar]
- Lillie R.D., Pizzolato P. Histochemical use of borohydrides as aldehyde blocking reagents. Stain Technol. 1972;47:13–16. doi: 10.3109/10520297209116528. [DOI] [PubMed] [Google Scholar]
- Makovitzky J. Glycoconjugates. Proceedings of the fifth international symposium Kiel. G Thieme Publishers; Stuttgart: 1979. Topo-optical examination of lymphocyte membrane (a polarization microscopic study) pp. 195–196. [Google Scholar]
- Makovitzky J. Ein topo-optischer Nachweis von C8–C9-unsubstituierten Neuraminsäureresten in der Glykokalyx von Erythrocyten. Acta Histochem. 1980;66:192–196. [PubMed] [Google Scholar]
- Makovitzky J. Glycoconjugates. Proceedings of the sixth international symposium. Japan Scientific Press; Tokyo: 1981. On the composition of the membrane of human lymphocytes; pp. 126–127. [Google Scholar]
- Makovitzky J. Topo-optical reactions of the membrane of human lymphocytes. Haematologia. 1981;14:439. [Google Scholar]
- Makovitzky J. Topo-optic reactions for the selective demonstration of sialic acid on human blood cells. Acta Histochem Suppl. 1983;28:213–217. [PubMed] [Google Scholar]
- Makovitzky J. Polarization optical analysis of blood cell membranes. Prog Histochem Cytochem. 1984;15:1–100. doi: 10.1016/s0079-6336(84)80005-4. [DOI] [PubMed] [Google Scholar]
- Makovitzky J. Components of Ehrlich ascites tumor cell surface. Acta Histochem Suppl. 1984;29:121–127. [PubMed] [Google Scholar]
- Makovitzky J. The variation of Romhányi's aldehyde-bisulfite-toluidineblue (ABT)-reaction: the aldehyde-bisulfite-1,9-dimethyl methylene blue (ABD)- and sialic acid specific topo-optical reaction. Acta Histochem. 1987;81:35–39. doi: 10.1016/s0065-1281(87)80075-2. [DOI] [PubMed] [Google Scholar]
- Makovitzky J. The study of original and induced negative charges of human lymphocyte membrane. Acta Histochem Suppl. 1987;34:185–194. [PubMed] [Google Scholar]
- Makovitzky J. The sterical order of the sialic acid in microvillous layer of the human duodenal mucosa. Acta Histochem Suppl. 1990;38:189–193. [PubMed] [Google Scholar]
- Makovitzky J. The nature of the localized meniscus amyloid. In: Bély M, Apáthy À, editors. Amyloid an Amyloidosis. The Proceedings of the IXth International Symposium on Amyloidosis, July 15–21 2001, Budapest, Ed by M. p. 409–11.
- Makovitzky J. Polarisationsmikroskopie in der submikroskopischen Strukturforschung: Geschichte und Theorie. BIOspektrum (Heidelb.) 2003;9:375–376. [Google Scholar]
- Makovitzky J., Geyer G. Untersuchungen über die Toluidinblaufärbung der Glykokalyx. Histochemistry. 1977;50:261–270. doi: 10.1007/BF00491073. [DOI] [PubMed] [Google Scholar]
- Makovitzky J., Richter S., Appel T.R. Topooptical investigations and enzymatic digestions on tissue-isolated amyloid fibrils. Acta Histochem. 2006;108:193–196. doi: 10.1016/j.acthis.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Malinin G.I. Metachromatic staining of sodium bisulfite addition derivates of glycogen. J Histochem Cytochem. 1970;18:834–841. doi: 10.1177/18.11.834. [DOI] [PubMed] [Google Scholar]
- Marchesi V.T. Spectrin: present status of a putative cyto-skeletal protein of the red cell membrane. J Membr Biol. 1979;51:101–131. doi: 10.1007/BF01869164. [DOI] [PubMed] [Google Scholar]
- Marchesi V.T. Functional proteins of the human red blood cell membrane. Semin Hematol. 1979;16:3–20. [PubMed] [Google Scholar]
- Marchesi V.T. The red cell membrane skeleton: recent progress. Blood. 1983;61:1–11. [PubMed] [Google Scholar]
- McManus J.F. Histological demonstration of mucin after periodic acid. Nature. 1946;158:202. doi: 10.1038/158202a0. [DOI] [PubMed] [Google Scholar]
- Módis L. Topo-optical investigations of mucopolysaccharides (acid glycosaminoglycans) In: Graumann W., editor. Vol. II. Gustav Fischer Verlag; Stuttgart: 1974. (Handbuch der Histochemie). [Google Scholar]
- Módis L. CRC Press; Boca Raton, FL: 1991. Organization of the extracellular matrix: a polarization microscopic approach. [Google Scholar]
- Movat H.Z. Silver impregnation methods for electron microscopy. Am J Clin Pathol. 1961;35:528–537. doi: 10.1093/ajcp/35.6.528. [DOI] [PubMed] [Google Scholar]
- Muresan V., Iwanij V., Smith Z.D., Jamieson J.D. Purification and use of limulin: a sialic acid specific lectin. J Histochem Cytochem. 1982;30:938–946. doi: 10.1177/30.9.6897073. [DOI] [PubMed] [Google Scholar]
- Németh À. A polarization microscopic study of granular pneumocytes and alveolar interface in rat lung. Acta Morphol Acad Sci Hung. 1974;22:115–127. [PubMed] [Google Scholar]
- Németh À. Topo-optical study of the pulmonary ciliary membrane surface. Acta Morphol Acad Sci Hung. 1976;24:247–259. [PubMed] [Google Scholar]
- Nicolson G.L., Singer S.J. Ferritin-conjugated plant agglutinins as specific saccharide stains for electron microscopy: application to saccharides bound to cell membranes. Proc Natl Acad Sci USA. 1971;68:942–945. doi: 10.1073/pnas.68.5.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G.L., Singer S.J. The distribution and asymmetry of mammalian cell surface saccharides utilizing ferritin-conjugated plant agglutinins as specific saccharide stains. J Cell Biol. 1974;60:236–248. doi: 10.1083/jcb.60.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S., Ghosh S., Bandyopadhyay S., Mandal C., Bandyopadhyay S., Kumar Bhattacharya D. Differential expression of 9-O-acetylated sialoglyco-conjugates on leukemic blats: a potential tool for long term monitoring of children with acute lymphoblastic leukemia. Int J Cancer. 2004;111:270–277. doi: 10.1002/ijc.20246. [DOI] [PubMed] [Google Scholar]
- Pal S., Ghosh S., Mandal C., Kohla G., Brossmer R., Isecke R. Purification and characterization of 9-O-acetylated sialoglycoproteins from leucemic cells and their potential as immunological tool for monitoring childhood acute lymphoblastic leucemia. Glycobiology. 2004;14:859–870. doi: 10.1093/glycob/cwh111. [DOI] [PubMed] [Google Scholar]
- Rambourg A. Morphological and histochemical aspects of glycoproteins at the surface of animal cells. Int Rev Cytol. 1971;31:57–114. doi: 10.1016/s0074-7696(08)60057-1. [DOI] [PubMed] [Google Scholar]
- Rambourg A., Leblond C.P. Electron microscope observations on the carbohydrate-rich cell coat present at the surface of cells in the rat. J Cell Biol. 1967;32:27–53. doi: 10.1083/jcb.32.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid P.E., Park C.M. Progess in Histochemistry and Cytochemistry. Gustav Fischer Verlag; Stuttgart, New York: 1990. Carbohydrate histochemistry of epithelial glycoproteins. [DOI] [PubMed] [Google Scholar]
- Reid P.E., Culling C.F., Dunn W.L., Clay M.G. The use of a transesterification technique to distinguish between certain neuraminidase resistant epithelial mucins. Histochemistry. 1976;46:203–207. doi: 10.1007/BF02462784. [DOI] [PubMed] [Google Scholar]
- Reid P.E., Culling C.F., Dunn W.L. A histochemical method for the identification of 9-O-acyl sialic acid. An investigation of bovine submaxillary gland and intestinal mucins. J Histochem Cytochem. 1978;26:187–192. doi: 10.1177/26.3.632555. [DOI] [PubMed] [Google Scholar]
- Richter S. Amyloidose des Respirationstraktes. Eine polarisationsoptisch-histochemische Untersuchung mit klinischem Bezug. MD thesis, Rostock, Germany, 2005a.
- Richter S. Amyloidosis of the respiratory tract. A polarization optical and histochemical investigation with clinical findings. Amyloid. 2005;12:259–261. [Thesis Reports] [Google Scholar]
- Richter S., Makovitzky J. Topo-optical visualization reactions of carbohydrate-containing amyloid deposits in the respiratory tract. Acta Histochem. 2006;108:181–191. doi: 10.1016/j.acthis.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Rinniger A., Richet C., Pons A., Kohla G., Schauer R., Bauer H.C. Localisation and distribution of O-acetylated N-acetylneuraminic acids, the endogenous substrates of the hemagglutinin-esterases of murine coronaviruses, in mouse tissue. Glycoconj J. 2006;23:73–84. doi: 10.1007/s10719-006-5439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts G.P. Histochemical detection of sialic acid residues using periodate oxidation. Histochem J. 1977;9:97–102. doi: 10.1007/BF01007012. [DOI] [PubMed] [Google Scholar]
- Romhányi G. Über die submikroskopische Grundlage der metachromatischen Reaktion. Acta Histochem. 1963;15:201–233. [PubMed] [Google Scholar]
- Romhányi G. A biológiai membránok ultrastrukturájáról optikai analizisek tükrében. MTA Biol OsztKözl. 1975;18:1–19. [Google Scholar]
- Romhányi G. Ultrastructure of biomembranes as shown by topo-optical reactions. Acta Biol Acad Sci Hung. 1978;29:311–365. [PubMed] [Google Scholar]
- Romhányi G. The topo-optical reactions and their roles in biological ultrastructure research. In: Tolnai M., editor. Lecture at the Hungarian Academy of Sciences (25th March 1983) Akadémia Kiadó; Budapest: 1988. [Google Scholar]
- Romhányi G. A membrán glikoproteinek ultrastrukturális és sejtbiológiai vonatkozásai. In: Somogyi J., editor. A biomembránok szerkezete és müködése. Akadémiai Kiadó; Budapest: 1989. [Google Scholar]
- Romhányi G, Deák G. Topo-optical studies on age pigment, corpora amylacea and senile amyloid like substances of brain. In: Környey St, Tariska St, Gosztonyi G. editors, VII. International Congress of Neuropathology 1–7 September 1974 Budapest Proceedings. Vol. II Excerpta Medica Amsterdam, 1975, p. 123–26.
- Romhányi G., Molnár L. Optical polarisation indicates linear arrangement of rhodopsin oligosaccharide chain in rod disk membranes of frog retina. Nature. 1974;249:486–488. doi: 10.1038/249486a0. [DOI] [PubMed] [Google Scholar]
- Romhányi G., Bukovinszky A., Deák G. Sulfation as a collagen-specific reaction. The ultrastructure of sulfate collagen, basement membranes and reticulin fibers as shown by topo-optical staining reactions. Histochemie. 1973;36:125–138. doi: 10.1007/BF00304388. [DOI] [PubMed] [Google Scholar]
- Romhányi G., Bukovinszky A., Deák G. Selective proteolytic sensitivity of sulfate-collagen and basement membranes. Histochemistry. 1974;42:199–209. doi: 10.1007/BF00533271. [DOI] [PubMed] [Google Scholar]
- Romhányi G., Deák G., Fischer J. Aldehyde bisulfite-toluidine blue (ABT) staining as a topo-optical reaction for demonstration of linear order of vicinal OH groups in biological structures. Histochemistry. 1975;43:333–348. doi: 10.1007/BF00490192. [DOI] [PubMed] [Google Scholar]
- Romhányi G., Fischer J., Corless J.M. Orientation of acidic polysaccharides and rhodopsin-oligosaccharides in frog retinal rod outer segments. Histochemistry. 1978;56:65–77. doi: 10.1007/BF00492254. [DOI] [PubMed] [Google Scholar]
- Roseman S. Sugars of the cell membrane. In: Weissmann G., Clairborn E., editors. Cell membranes. Biochemistry, Cell Biology, Pathology. H. P. Publ. Co; New York: 1975. pp. 55–64. [Google Scholar]
- Ruhenstroth-Bauer G. Experiences with electrophoresis of blood cells. Bibl Haematol. 1961;12:5–19. [PubMed] [Google Scholar]
- Ruhenstroth-Bauer G., Lücke-Huhle C. Elektrophoretische Untersuchungen an Zellen der lymphatischen Organe. Z Naturforsch B. 1968;23:115. [PubMed] [Google Scholar]
- Schauer R. Chemistry and biology of acetylneuraminic acids. Angew Chem Int Ed Engl. 1973;12:127–138. doi: 10.1002/anie.197301271. [DOI] [PubMed] [Google Scholar]
- Schauer R. Sialic acids as antigenic determinants of complex carbohydrates. Adv Exp Med Biol. 1988;228:47–72. doi: 10.1007/978-1-4613-1663-3_2. [DOI] [PubMed] [Google Scholar]
- Schauer R. Achievements and challenges of sialic acid research. Glycoconj J. 2000;17:485–499. doi: 10.1023/A:1011062223612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer R. Sialic acids: fascinating sugars in higher animals and man. Zoology. 2004;107:49–64. doi: 10.1016/j.zool.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Schauer R., Faillard H. Action specificity of neuraminidase. Hoppe Seylers Z Physiol Chem. 1968;349:961–968. [PubMed] [Google Scholar]
- Schmidt WJ. Doppelbrechung und Feinbau der Markscheide der Nervenfasern Z Zellforsch 1936;23:657–76.
- Scocco P., Menghi G., Cecccarelli P., Pedini V. Lectin histochemistry and identification of O-acetylated sialoderivates in the horse sublingual gland. Eur J Histochem. 1999;43:47–54. [PubMed] [Google Scholar]
- Scott J.E., Dorling J. Periodate oxidation of acid polysaccharides. A PAS method for chrondroitin sulphates and other glycosamino-glycuronans. Histochemie. 1969;19:295–301. [PubMed] [Google Scholar]
- Shen Y., Tiralongo J., Kohla G., Schauer R. Regulation of sialic acid O-acetylation in human colon mucosa. Biol Chem. 2004;385:145–152. doi: 10.1515/BC.2004.033. [DOI] [PubMed] [Google Scholar]
- Shen Y., Kohla G., Lrhorfi A.L., Sipos B., Kalthoff H., Gerwig G.J. O-acetylation and de-O-acetylation of sialic acids in human colorectal carcinoma. Eur J Biochem. 2004;271:281–290. doi: 10.1046/j.1432-1033.2003.03927.x. [DOI] [PubMed] [Google Scholar]
- Skutelsky E., Danon D., Wilchek M., Bayer E.A. Localization of sialyl residues on cell surfaces by affinity cytochemistry. J Ultrastruct Res. 1977;61:325–335. doi: 10.1016/s0022-5320(77)80057-9. [DOI] [PubMed] [Google Scholar]
- Snow A.D., Wight T.N. Proteoglycans in the pathogenesis of Alzheimer's disease and other amyloidoses. Neurobiol Aging. 1989;10:481–497. doi: 10.1016/0197-4580(89)90108-5. [DOI] [PubMed] [Google Scholar]
- Sótonyi P., Somogyi E. Cytochemical demonstration of the molecular forms of cardiac glycosides in the heart muscle. Acta Histochem. 1983;72:117–122. doi: 10.1016/s0065-1281(83)80018-x. [DOI] [PubMed] [Google Scholar]
- Spiro R.G. Periodate oxidation of the glycoprotein fetuin. J Biol Chem. 1964;239:567–573. [PubMed] [Google Scholar]
- Spiro R.G. The structure of the disaccharide unit of the renal glomerular basement membrane. J Biol Chem. 1967;242:4813–4823. [PubMed] [Google Scholar]
- Spiro R.G. Glycoproteins. Adv Protein Chem. 1973;27:349–467. doi: 10.1016/s0065-3233(08)60451-9. [DOI] [PubMed] [Google Scholar]
- Spiro R.G., Lucas F., Rudall K.M. Glycosylation of hydroxylysine in collagens. Nat New Biol. 1971;231:54–55. doi: 10.1038/newbio231054a0. [DOI] [PubMed] [Google Scholar]
- Steck T.L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974;62:1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck T.L. The band 3 protein of human red cell membranes: a review. J Supramol Struct. 1978;8:311–324. doi: 10.1002/jss.400080309. [DOI] [PubMed] [Google Scholar]
- Stibenz D. Milieu- und konservierungsbedingte Strukturveränderungen der Membran menschlicher Erythrozyten. Habilitationsschrift, Jena, Germany, 1985.
- Stibenz D., Geyer G. Conformational calculations of the N-terminal hydrophilic segment of human erythrocyte glycophorin. Folia Haemat Int Mag Klin Morphol Blutforsch. 1980;107:787–792. [PubMed] [Google Scholar]
- Stibenz D., Geyer G. Remarks on the spatial structure of the external segment of glycophorin A. Folia Haemat Int Mag Klin Morphol Blutforsch. 1982;109:251–254. [PubMed] [Google Scholar]
- Sylvén B. Metachromatic dye-substrate interaction. Q J Microsc Sci. 1954;95:327–358. [Google Scholar]
- Szirmai J.A., Balázs E.A. Metachromasia and the quantitative determination of dyebinding. Acta Histochem Suppl. 1958;(Suppl 1):1:56–179. [PubMed] [Google Scholar]
- Tandon N.N., Lipsky R.H., Burgess W.H., Jamieson G.A. Isolation and characterization of platelet glycoprotein IV (CD 36) J Biol Chem. 1989;264:7570–7575. [PubMed] [Google Scholar]
- Tsuji T., Osawa T. Purification and chemical characterization of human platelet membrane glycoprotein IV. J Biochem (Tokyo) 1986;100:1077–1085. doi: 10.1093/oxfordjournals.jbchem.a121787. [DOI] [PubMed] [Google Scholar]
- Veh R.W., Kornadt T. Histochemical demonstration of 7,9-disubstituted sialic acids: strong periodate oxidation of sialic acid mixtures on the biochemical and histochemical level. Acta Histochem Suppl. 1989;37:185–186. [PubMed] [Google Scholar]
- Veh R.W., Meersen D., Kuntz H.D., May B. Histochemical demonstration of side-chain substituted sialic acids. In: Maic R.A., Williamson R.C.N., editors. Colonic Carcinogenesis (Falk Symposium 31) M T P Press Ltd. Int Med Publ; Lancaster (UK): 1982. [Google Scholar]
- Wälchli O. Die Einlagerung von Kongorot in Zellulose Dr. rer nat. ETH Zürich, Switzerland, 1945.
- Winzler R.J. Carbohydrates in cell surfaces. Int Rev Cytol. 1970;29:77–125. doi: 10.1016/s0074-7696(08)60033-9. [DOI] [PubMed] [Google Scholar]
- Wolman M. On the use of polarized light in pathology. Pathol Annu. 1970;5:381–416. [PubMed] [Google Scholar]
- Yamada K., Shimizu S. The use of peroxidase-labelled Limulus polyphemus agglutinin for the histochemistry of sialic acid-containing glycoproteins in light microscopy. Histochem J. 1979;11:457–471. doi: 10.1007/BF01002773. [DOI] [PubMed] [Google Scholar]


