Diarrheal diseases are among the leading causes of death among children in developing countries and claimed an estimated 1.4 to 2.5 million lives in the year 2000.1, 2, 3, 4 In industrialized countries, although deaths from diarrhea are uncommon occurrences, this disease remains an important cause of morbidity and incurs substantial health care costs.5 In addition to its direct health burden, consequences of diarrhea include malnutrition,6 diminished growth,7 and impaired cognitive development.8 In recent years, advances in diagnostics have led to the identification of new causative agents and have improved our understanding of the etiologic role of previously recognized pathogens. Guidelines for the management of diarrhea have been refined, and new strategies for prevention and control have been identified. In this article, we review the current knowledge of the epidemiologic features, causative agents, pathogenic mechanisms, clinical manifestations, management, and strategies for the prevention and control of acute, infectious diarrhea in young children.
Definitions
On the basis of clinical and epidemiologic parameters, episodes of diarrhea are classified into three categories: acute diarrhea, dysentery, and persistent diarrhea. Acute diarrhea is defined as the presence of three or more loose, watery stools within a 24-hour period.9 Dysentery, often termed bloody diarrhea, indicates the presence of visible blood and mucous in diarrheal stools. Episodes of diarrhea lasting more than 14 days are referred to as persistent diarrhea.
Trends in disease burden
In developing countries, deaths caused by diarrhea have declined in incidence consistently during the past 3 decades (Table 1),1, 2, 3, 4, 10, 11, 12, 13, 14, 15, 16, 17 from an estimated 5 million deaths in 197610 to approximately 1.4 to 2.5 million deaths in the year 2000.1, 2, 3, 4 This decline in mortality rate has been attributed to several factors, such as widespread distribution and use of oral rehydrating solutions (ORS),18 improved rates of breastfeeding, improved nutrition, better sanitation and hygiene, and increased coverage of measles immunization.19 Although ascertaining the individual impact of these factors is difficult, the striking inverse association between coverage rates of ORS and rates of mortality from diarrhea in various countries provides a strong argument for the significant contribution of this intervention (Fig 1).19, 20 Although the rate of mortality from diarrhea has declined substantially, morbidity from diarrhea has remained relatively constant during the past two decades, with each child under 5 years of age experiencing an average of three episodes (range: 2.8 to 6.3) annually.4 Whereas most interventions against diarrheal diseases, such as breastfeeding and improved sanitation, would be expected to affect mortality and morbidity simultaneously, increased use of ORS and improvements in nutritional status are likely to have a greater impact on rates of mortality from diarrhea and may explain the relative lack of decline in the incidence of diarrhea. Both the incidence and risk of mortality from diarrheal diseases are greatest among children younger than 1 year of age, and thereafter rates decline incrementally.4
Table 1.
Estimates of Mortality from Diarrheal Diseases Among Children of Developing Countries
| Source | Year of Estimate | Year of Publication | Deaths per Year (Millions) |
|---|---|---|---|
| Rohde10 | 1976 | 1984 | 5 |
| Snyder and Merson11 | 1982 | 1982 | 4.6 |
| Institute of Medicine12 | 1986 | 1986 | 3.5 |
| Martines and Phillips13 | 1990 | 1990 | 3.2 |
| Bern et al.14 | 1992 | 1992 | 3.3 |
| World Development Report15 | 1993 | 1993 | 2.5 |
| Murray and Lopez16 | 1997 | 1997 | 2.4–2.9 |
| Kosek et al.4 | 2000 | 2003 | 2.1–4.7 |
| Parashar et al.3 | 2000 | 2003 | 1.7–3.0 |
| World Health Organization1, 2 | 2001 | 2002 | 1.4 |
| World Health Report17 | 2002 | 2003 | 1.6 |
Figure 1.
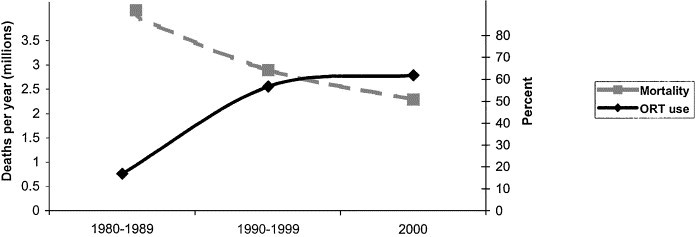
Temporal relationship between diarrheal mortality and oral rehydration therapy (ORT) use, worldwide 1980 to 2000. The point estimates represented for mortality are an average of published estimates for the corresponding time period (Table 1). Percent use of oral rehydration solutions represented by estimates provided by UNICEF for 1986, 1994, and 2000.19
Causative agents
Diarrhea is caused by a mélange of bacterial, viral, and parasitic pathogens. In Europe, North America, and other industrialized countries, the vast majority of episodes of diarrhea are caused by viral pathogens that exhibit distinct winter seasonality.21, 22, 23, 24, 25 In developing countries with poor hygiene and sanitation, enteric bacteria and parasites are more prevalent, and these agents typically peak during the summer months.25, 26, 27, 28, 29 Evidence suggests that as rates of diarrheal mortality have declined in developing countries, viral agents have assumed an increasingly important etiologic role. For example, an assessment of deaths from diarrhea in Mexican children showed that in the 1980s death rates peaked during the summer months when bacterial agents were more prevalent.30 However, as mortality rates declined during the 1990s, the peak in mortality rates shifted toward the winter months, when enteric viruses, particularly rotavirus, were more prevalent.
Differences in the epidemiologic patterns of bacterial and viral agents of diarrhea provide clues to their modes of transmission and have implications for prevention of disease. Because bacterial pathogens are more prevalent in developing countries, fecal-oral spread, often facilitated by conditions of poor hygiene and sanitation, is thought to be the most important mode of transmission. Viral pathogens also are transmitted by this route. However, the fact that certain agents, such as rotavirus, infect nearly all children in both developing and industrialized countries by the time they reach the age of 3 years suggests that transmission may occur through other mechanisms such as fomites and respiratory secretions.31 It also implies that as rates of diarrheal mortality decline and viral agents become more prominent, traditional measures used to improve hygiene and sanitation may not reduce the incidence of disease significantly. Strategies such as vaccines to prevent these illnesses are likely to be more effective. In the next section, we discuss briefly the epidemiologic features of the most common bacterial, viral, and parasitic causes of diarrhea in children in developing countries.
Bacterial agents
Children in developing countries are exposed to a wide range of bacterial enteric pathogens at a very early age and suffer numerous episodes of diarrheal illness as a result. The predominant bacterial pathogens vary with the age of the child and also in time, showing both seasonal and secular trends, as well as geographical variation. The experience of any individual child is unique, but the diarrheagenic Escherichia coli, Shigella spp., Campylobacter spp., Vibrio spp., and Salmonella are among the more commonly recognized bacterial causes of diarrhea among young children. In a published summary of 73 studies of children who sought care for diarrhea in 33 countries, rotavirus was the enteropathogen most frequently identified, with a median of 20 percent.32 However, bacterial pathogens predominated overall, with enterotoxigenic E. coli (median 11%), Campylobacter (median 7%), and Shigella organisms (median 5%) being the agents most commonly identified. Other less frequently identified but clinically important bacterial pathogens include Vibrio spp. (including Vibrio cholerae O1, the cause of cholera) and other diarrheagenic E. coli (including the enterohemorraghic E. coli, such as E. coli O157:H7). Worth noting is that certain enteric pathogens cause mild or asymptomatic infections and that the duration of asymptomatic carriage, particularly in young children, may be prolonged. Therefore, the identification of a pathogenic bacteria, virus, or parasite in a stool specimen from a child with diarrhea does not indicate in all cases that it is the cause of illness. Nonetheless, these studies give us some understanding of the variety and relative importance of the many different enteric pathogens that afflict countless children born and reared in environmental conditions unbefitting the 21st century.
Diarrheagenic E. coli
E. coli is the predominant facultative anaerobe of human colonic flora and colonizes the infant gastrointestinal tract within hours of birth.33 Although most E. coli strains are harmless or even beneficial to their human hosts, several highly adapted clones are capable of causing diarrheal disease. Five classes of diarrheagenic E. coli have been defined by the presence of specific virulent characteristics: enterotoxigenic E. coli (ETEC), enteropathogenic E. coli (EPEC), enteroinvasive E. coli (EIEC), enterohemorrhagic E. coli (EHEC), and enteroaggregative E. coli (EAggEC).34 All five classes of diarrheagenic E. coli cause disease in children in the developing world, but EHEC, including E. coli O157:H7, are the agents of diarrheal disease more commonly identified in developed countries. Only specialized diagnostic tests can distinguish the diarrheagenic E. coli from normal flora present in human stools; thus, they are largely underrecognized outside special studies.
ETEC strains are a frequent cause of acute secretory diarrhea among children in developing countries and also are a major cause of traveler’s diarrhea. EPEC strains cause acute secretory diarrhea, predominantly in children 2 years of age and younger and especially in those who are 6 months or younger. EPEC strains are associated with chronic diarrhea in children but rarely cause disease in adults. EIEC strains cause bloody mucoid diarrhea and share many biochemical and pathogenic properties with Shigella organisms. Watery diarrhea also is seen, and fever is a common finding. EHEC strains also cause bloody diarrhea, and severe hemorrhagic colitis and the hemolytic uremic syndrome are seen in as many as 6 to 8 percent of infections. EHEC infections are more common occurrences in developed countries, although a large outbreak has been described in Swaziland.35 In the United States and in other countries, cattle are the predominant reservoir for EHEC. EAggEC are being recognized increasingly as important diarrheal pathogens, although the mechanism by which they act remains poorly understood. They are associated with watery diarrhea in young children and have been reported as a cause of persistent diarrhea in children and in adults with human immunodeficiency virus (HIV).
Campylobacter
Campylobacter is one of the most frequently isolated bacteria from the stools of infants and children in developing countries.36 It is associated with watery diarrhea and on occasion dysentery, with peak isolation rates found in children 2 years of age and younger. Guillain-Barré syndrome, an autoimmune disorder of the peripheral nervous system that can lead to complete paralysis, is a rare complication of infection with Campylobacter. Poultry is an important source of Campylobacter infections in developed countries, and the presence of an animal in the cooking area is a risk factor for acquiring campylobacteriosis in developing countries.
Shigella species
Shigella is estimated to cause more than 160 million infections annually in developing countries, primarily in children.37 Shigella infections are relatively more common findings in toddlers and older children than in infants. The four recognized subgroups of Shigella are Shigella sonnei, Shigella flexneri, Shigella boydii and Shigella dysenteriae, and multiple serotypes within each subgroup, except S. sonnei. S. sonnei is the subgroup that causes the mildest illness and is seen most commonly in developed countries. S. flexneri, which is more likely to cause dysenteric symptoms and more persistent illness, is the most common subgroup in developing countries, with S. sonnei being the next most common. The other two subgroups comprise less than 5 percent of isolates in most studies but are noteworthy for their association with more severe disease. In particular, S. dysenteriae type 1 (Sd1), which produces the same Shiga-toxin as does EHEC, has caused devastating epidemics of bloody diarrhea, with reported case-fatality rates approaching 10 percent in Asia, Africa, and Central America.
Vibrio cholerae
Many species of Vibrio cause diarrhea in developing countries, but the causes of epidemic cholera, V. cholerae serogroups O1 and O139, are of special importance. No illness produces depletion of volume as rapidly and as severely as does cholera; in the absence of prompt and adequate rehydration, hypovolemic shock and death can occur within 12 to 18 hours after onset of the first symptom.38 Stools are watery, colorless, and flecked with mucus. Vomiting is a common finding, but fever is a rare event. Life-threatening electrolyte imbalances must be avoided by using appropriate solutions for rehydration. Children with cholera are especially prone to the development of hypoglycemia, which can lead to convulsions and death. Because of the potential for epidemic spread, confirmed or suspected cases of cholera (including severe dehydrating diarrheal illness in patients >5 years) should be reported promptly to public health authorities.
Salmonella
More than 2000 serotypes of Salmonella exist, all of which are pathogenic for humans. Infants and the elderly appear to be at greatest risk, but all age groups are susceptible to salmonellosis. Animals are the major reservoir for Salmonellae, and different serotypes predominate among reptiles, fowl, and mammals. The acute onset of nausea, vomiting, and diarrhea that may be watery or, less frequently, dysenteric characterize most cases of Salmonella gastroenteritis. Fever occurs in approximately 70 percent of affected children. Bacteremia occurs in 1 to 5 percent of patients, most frequently in infants. Enteric fever, which classically is associated with Salmonella serotype Typhi (typhoid fever) or serotype Paratyphi A, B, or C, also is seen rarely with non-Typhi serotypes. Typhoid fever is identified most frequently among school-age children but also presents in a milder form in children 2 years of age and younger. In children, the disease not uncommonly presents with diarrhea, with or without blood, but it is characterized by fever lasting 3 or more weeks. Complications of typhoid fever include intestinal hemorrhage and perforation, myocarditis, meningitis, pneumonia, and shock.39
Viral agents
Rotavirus
Rotavirus is the leading cause of severe, dehydrating gastroenteritis among children, accounting for one-third of all hospitalizations for diarrhea and an estimated 500,000 deaths worldwide each year.40 Nearly all children in both industrialized and developing countries are infected with rotavirus by the time they are 3 to 5 years of age. Neonatal infections are common occurrence but often are asymptomatic.21 The incidence of clinical illness peaks in children between 4 and 23 months of age. Rotavirus is associated with gastroenteritis of above-average severity. Thus, the proportion of episodes of diarrhea attributable to rotavirus increases with increasing severity of illness, from 6 to 8 percent of all diarrhea episodes in the community to 15 to 20 percent of episodes requiring outpatient therapy to 25 to 40 percent of episodes requiring hospitalization. Children often are infected with rotavirus multiple times, and each infection confers progressively greater immunity.41 Therefore, severe disease occurs most commonly in young children with first or second infections.
Rotaviruses are classified into different strains (G and P types) on the basis of two outer capsid proteins.21 Theoretically, 80 different strains of rotavirus could result from various combinations of the known 10 G and 8 P types of human rotaviruses. However, four common strains of rotavirus (P[8]G1, P[8]G3, P[8]G4, and P[4]G2) are most prevalent globally. Compared with their counterparts in industrialized countries, children in developing countries experience their first severe infection with rotavirus at a younger age, exhibit year-round rather than seasonal disease, and are more likely to be infected with unusual or multiple strains of rotavirus.42
Human caliciviruses (HuCVs)
HuCVs are a group of nonenveloped, small, round viruses that belong to two genera in the family Caliciviridae, the noroviruses and sapoviruses (previously called “Norwalk-like viruses” and “Sapporo-like viruses,” respectively).43 Infections with the prototype strain, Norwalk virus, were recognized nearly two decades ago to be common occurrences in children, but the etiologic role of HuCV could not be assessed accurately because of the lack of simple and sensitive diagnostic assays. Cloning and sequencing of the genome of Norwalk and several other HuCvs have allowed for the development of polymerase chain reaction-based assays for detection of virus in stool and enzyme immunoassays for detection of serologic responses.44 These assays show that noroviruses are the most common cause of outbreaks of gastroenteritis and that they affect all age groups, whereas sapoviruses affect primarily children.45, 46 The etiologic role of noroviruses in moderate-to-severe childhood gastroenteritis in developing countries is being studied, but preliminary data from industrialized countries indicate that they may be the second most common viral agent after rotavirus, accounting for 4 to 19 percent of episodes of severe gastroenteritis in young children.23
Other viral agents
Enteric adenoviruses of serotypes 40 and 41 and astroviruses are associated with approximately 2 to 12 percent of episodes of gastroenteritis in young children.47, 48 Several other enteric viruses, including coronaviruses, toroviruses, and enteroviruses, have been detected in children with diarrhea, but their etiologic role is poorly defined.
Parasitic agents
Bacterial and viral agents are responsible for the majority of infectious diarrheal illnesses among children in developing countries, with parasitic agents accounting for a relatively small proportion of cases.40 Giardia lamblia, Cryptosporidium parvum, Entamoeba histolytica, and Cyclospora cayetanenesis are the parasites that most commonly cause acute diarrheal illness in children. The prevalence of G. lamblia is low (approximately 2 to 5%) among children in developed countries but can be as high as 20 to 30 percent in developing regions.28 Although often asymptomatic, infection with Giardia organisms can lead to significant anorexia and growth impairment in a small proportion of cases. Infections with both Cryptosporidium and Cyclospora organisms are common occurrences among children in developing countries, but they frequently are asymptomatic. For example, a recent study from Peru showed that children younger than 12 years of age had an average of 0.20 episodes of cyclosporiasis annually and 0.22 episodes of cryptosporidiosis annually but more than two-thirds of infections caused by both agents were asymptomatic; the proportion of cases that resulted in clinical illness declined with increasing age.49 In the developed world, diarrheal infections of parasitic origin are uncommon occurrences and usually restricted to persons who recently traveled to regions where such pathogens are endemic. However, vigilant surveillance for waterborne diseases50, 51 and the advent of several recent outbreaks of cryptosporiodosis and cyclosporiasis in the United States52, 53, 54 and other developed regions55, 56 brings attention to the increasing importance of parasites as human enteropathogens worldwide.
Pathogenic mechanisms
The inoculum required to cause disease varies substantially for different enteric pathogens. For some pathogens, such as Salmonella, approximately 106 to 108 organisms must be ingested to cause illness. On the other hand, for Shigella, Entamoeba, Giardia spp., and Norwalk virus, ingestion of as few as 10 to 100 organisms can cause illness. The expression of certain blood group antigens in the host influences the susceptibility to infection by some organisms, such as V. cholerae and noroviruses. Children with decreased gastric acidity, which may result from chronic asymptomatic infection with Helicobacter pylori or other causes, are at higher risk for the development of infection with bacterial pathogens.
Enteric bacteria produce clinical disease by two primary mechanisms: the production of toxins and invasion of the enteric mucosa. A variety of toxins are produced by enteric pathogens. Some agents, such as V. cholerae and ETEC, produce enterotoxins that cause diarrhea by activation of secretory mechanisms in the intestinal mucosa. ETEC produces both heat-stable (STa and STb) and heat-labile (LT I and LT II) toxins; the heat-labile toxins of ETEC closely resemble cholera toxin. Others, such as S. dysenteriae and Vibrio parahemolyticus, produce cytotoxins that lead to destruction of the intestinal epithelium, resulting in inflammatory diarrhea. Finally, some agents, such as Staphylococcus aureus and Bacillus cereus, induce neurotoxins that induce diarrhea by acting directly on the nervous system. Dysentery results from either the production of cytotoxins or from direct invasion of the intestinal mucosa by pathogens such as Shigella organisms and EIEC. Some enteric pathogens, such as Salmonella Typhi and Yersinia enterocolitica, can penetrate the intestinal mucosa and disseminate through the regional lymphatics to enter the bloodstream, resulting in systemic manifestations.
Enteric viruses cause diarrhea through multiple mechanisms, including interfering with gastrointestinal motility or destroying the intestinal epithelium and reducing brush border enzymes.57 The lack of these enzymes results in the failure to metabolize dietary carbohydrates and, consequently, produces osmotic diarrhea. Studies in mice indicate that a nonstructural protein of rotavirus, NSP4, acts as an enterotoxin and causes secretory diarrhea by altering epithelial cell function and permeability.58, 59 In addition, rotavirus may evoke fluid secretion through activation of the enteric nervous system in the intestinal wall. Recent data indicate that rotavirus antigen and RNA are present in serum of children with acute rotavirus infection, suggesting that rotavirus possibly escapes the intestinal tract in children, resulting in antigeniemia and possible viremia.60 Further investigations are needed to establish the importance of these findings in understanding the pathogenesis of rotavirus and the relation between extraintestinal spread and the severity of disease.
Clinical manifestations
Acute diarrhea usually manifests as an increase in the frequency or volume of stool. Fever is a common occurrence and usually is associated with invasive pathogens. Bloody stools are present in diarrhea caused by invasive pathogens and those that release cytotoxins; the presence of bloody stools in the absence of fecal leukocytes should raise the consideration of EHEC infection. Most viral agents and bacteria that release enterotoxins usually do not cause bloody diarrhea. Vomiting is observed more frequently in viral diarrhea and illness caused by ingestion of preformed bacterial toxins (eg, S. aureus). Despite these clues, determining the causative agent of diarrhea in an individual patient based on clinical grounds alone usually is difficult.
Prognostic factors
The synergistic relationship between malnutrition and diarrhea is well recognized. Approximately 10 percent of children in developing countries are severely underweight, and 8 and 32 percent suffer from wasting and stunting, respectively.19 Diarrhea has prolonged duration and is more severe in children with macronutrient or micronutrient deficiencies, and persistent diarrhea often results in malabsorption and significant weight loss, further promoting this cycle. Children with poor nutritional status, measured by anthropometric growth, also have an elevated risk for diarrheal death.61 Zinc deficiency, in particular, suppresses immune system function and is associated with an increased prevalence of persistent diarrhea.62 Similarly, children with immunosuppression secondary to infection with HIV or other chronic conditions may have an increased risk for the development of clinical illness, prolonged resolution of symptoms, or frequent recurrence of diarrheal episodes.63
Management
Effective prevention of mortality and management of morbidity associated with diarrheal illnesses among children is dependent on making a timely and accurate assessment of the status of hydration, followed by promptly instituting appropriate treatment.
Assessment of the patient
All children presenting with acute diarrhea should be evaluated promptly for features of dehydration to ascertain information about the severity of illness and the need for rapid initiation of therapy. The current World Health Organization (WHO) Integrated Management of Childhood Illness64 guidelines for assessment of dehydration are presented in Table 2. Additional practice guidelines for the treatment of acute gastrointestinal illness in children also are available (American Academy of Pediatrics).65 Because most diarrhea-associated dehydration is isonatremic, measurement of serum electrolytes usually is required in only those children who have either moderate dehydration with atypical clinical history or findings or in children with severe dehydration. Features of hypernatremic dehydration, such as irritability and doughy feel to the skin, should be sought specifically because this condition requires specific rehydration methods. Stool cultures usually are indicated only in cases of dysentery or to determine the etiology of an outbreak or to monitor antimicrobial resistance of bacterial pathogens.
Table 2.
Guidelines for Assessment of Dehydration Status64
| Indicator | Normal/No Dehydration | Some Dehydration (≥2 of these signs) | Severe Dehydration (≥2 of these signs) |
|---|---|---|---|
| Sensorium | Normal | Restless or irritable | Abnormally sleepy or lethargic |
| Sunken eyes* | No | Yes | Yes |
| Drinking | Normal | Drinks eagerly | Drinking poorly or not at all |
| Skin pinch† | Normal (immediate) | Slowly (<2 seconds) | Very Slowly (>2 seconds) |
Assessment of sunken eyes should be done both objectively and by asking the mother/ caregiver, as she is more familiar with the child.
Skin should be pinched longitudinally (eg, thoracoinguinal direction) between the thumb and forefinger, held for 1 to 2 seconds, then released by opening the finger and thumb.
Rehydration therapy
Severe dehydration is a medical emergency, and intravenous rehydration therapy should be instituted immediately. Lactated ringer solution, normal saline, or a similar solution should be administered at a rate of 30 mL/kg of body weight until pulse, perfusion, and mental status return to normal; in some instances, large doses may need to be administered more rapidly, which might require using multiple intravenous lines or, in a few cases, alternative routes of administration, such as intraosseous infusion. Electrolyte levels should be assessed, although therapy can be begun safely in the absence of these data. As soon as the child’s condition is stable and the mental status is normal, therapy can be changed to the oral route, as described below.
For children with mild to moderate dehydration, oral rehydration therapy (ORT) should be initiated with ORS, administered at a rate of 75 mL/kg of body weight per hour over a period of 4 hours (Fig 2).9 ORS contains a mixture of glucose, sodium, potassium, and chloride in a bicarbonate base. The optimal osmolarity of ORS, determined by the concentration of sodium and glucose, was re-evaluated recently.66, 67 WHO and the United Nations Children’s Fund (UNICEF) recently revised their guidelines to suggest use of ORSs that are hyposmotic to solutions previously promoted after several clinical trials documented clear advantages.67, 68 Results of a meta-analysis concluded that children treated with an ORS of lower osmolarity had measurable reductions in stool output and vomiting and less need for intravenous rehydration compared with children treated with the standard ORS.66, 67 The new WHO ORS contains 13.5 g/L glucose, 75 mmol/L sodium, 20 mmol/L potassium, 65 mmol/L chloride, and 30 mmol/L base bicarbonate solution, for a total osmolarity of 245 mOsm/L.68 In addition to WHO ORS, several commercial solutions are available and appropriate for rehydration after losses caused by diarrheal illness.69
Figure 2.
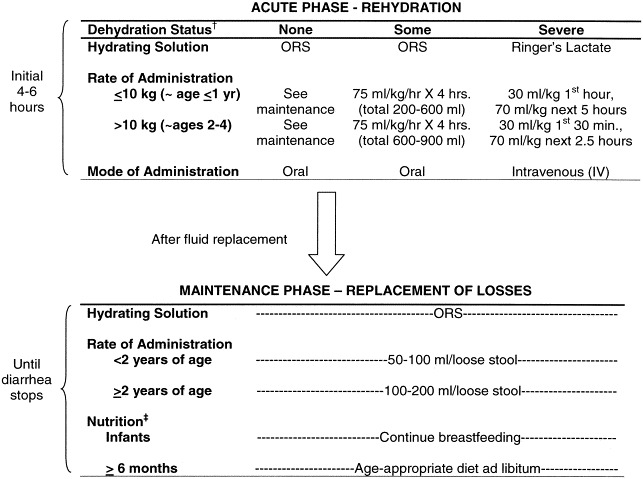
Levels listed are minimums; if child desires additional ORS, give more. †See Table 2 for assessment of dehydration. ‡Breastmilk can be given to infants even during acute rehydration; otherwise, food should not be given during the acute rehydration phase but should be initiated as soon as possible after fluid levels are restored. Children who are still nursing but are 6 months or older should be given supplemental food in addition to breastmilk. Energy and micronutrient-rich foods such as grains, meats, fruits, and vegetables are recommended. Children should try to eat frequent, small meals throughout the day (∼6 meals/day). Energy intake should continue to be increased as tolerated following the diarrheal episode to allow for “catch-up growth” and continued development. Source: World Health Organization. The treatment of diarrhoea. A manual for physicians and other senior health workers. Geneva: World Health Organization; 1995. WHO/CDR/95.3.
For children who are unable to tolerate ORS through the oral route, nasogastric (NG) feeding can be used to administer ORS. NG feeding is particularly beneficial in patients who have persistent vomiting with oral administration. After the first 4 to 6 hours of rehydration therapy has been given, fluid balance should be maintained through continuing administration of ORS.
Dietary therapy
Age-appropriate intake of nutrients, including breastfeeding for infants and nutrient-dense solid food intake for children, should be maintained.9 Concerns about patients having lactose intolerance immediately after having a diarrheal episode appear to be largely unfounded,70 and special formulas or dilutions generally are unnecessary.71 The importance of sufficient caloric intake should be emphasized to offset weight loss from illness and as a fundamental facilitator for proper growth and development. Efforts should be made to educate the mother or caregiver on early recognition of dehydration, appropriate ORS and therapy, and energy intake. Exclusive breastfeeding should be recommended for infants younger than 6 months old,72, 73 and identification of locally available foods that will support nutrient and caloric needs of older children may be useful.74
Pharmacologic therapy
Antimicrobial agents usually are not recommended for the routine treatment of acute diarrheal illness.75 Most episodes caused by bacteria and parasites are self-limiting and can be managed effectively with ORS and adequate food intake, and inappropriate use of antimicrobials can promote antimicrobial resistance. Treatment with antimicrobials is recommended for Campylobacter infections in immunocompromised persons and in those with extraintestinal infections. Erythromycin and azithromycin dihydrate are the agents most commonly used for treatment of Campylobacter infections, but increasing resistance to macrolides and fluoroquinolone agents has been noted in many countries.76 Antimicrobial treatment also decreases the duration of illness of cholera, fluid requirements, and duration of excretion of V. cholerae and may be used in severe cases as an adjunct to rehydration therapy. Trimthoprim-sulfamethoxazole may be used to treat younger children, whereas doxycycline or tetracycline are used more often for older children and adults. Treatment with antimicrobial agents limits the duration and severity of shigellosis and is recommended when the illness is characterized by bloody stools or high fevers.77 However, Shigella organisms rapidly acquire resistance to a variety of antimicrobial agents and strains of Sd1 resistant to all commonly used antimicrobial agents, including fluoroquinolones, recently have been reported from Bangladesh and India, raising the specter of potentially untreatable infections.78 Appropriate treatments for Shigella spp. include, but are not limited to, nalidixic acid, ciprofloxacin, and pivmecillinam; more extensive lists have been published elsewhere.79 The specific antimicrobial for empiric treatment should be based on the regional endemicity, resistance patterns, and availability of Shigella spp. Antimicrobial treatment of non-Typhi salmonellosis should be reserved for extraintestinal infections, but specific antimicrobial therapy with ampicillin, chloramphenicol, or trimethoprim-sulfamethoxazole is indicated to shorten the duration of symptoms and lower the risk of the development of complications for children with illness caused by S. typhi.76 Oral nitazoxanide is recommended for the treatment of diarrheal illness associated with G. lamblia or C. parvum, and trimethoprim-sulfamethoxazole is effective for treating cyclosporiasis.76
A few randomized trials have shown that probiotics such as Lactobacillus GG, a native component of human intestinal flora, hastens recovery from diarrheal episodes and reduces the risk of having recurrent infection.80 These benefits generally are thought to be associated with the ability of probiotics to stimulate host humoral immune defenses, and adjunctive treatment with Lactobacillus casei GG enhances the localized rotavirus-specific IgA response.81 However, adequate data are not available to recommend the routine use of probiotics for treatment of diarrhea in children.
A variety of nonspecific antidiarrhea agents (eg, kaolin), antimotility agents (eg, loperamide), antisecretory agents (eg, bismuth salicylate), and toxin adsorbents (eg, cholestyramine) have been used for treatment of diarrhea in older children and adults. The safety and efficacy of these agents have not been established in children, and they currently are not recommended for treatment.
Micronutrient supplementation
Supplementation with zinc has been shown to be efficacious as an adjunct therapy for acute and persistent diarrheal episodes in children in developing countries.82 Results of a recent meta-analysis that reviewed seven trials for acute diarrhea concluded that treatment with zinc shortens the duration and lessens the severity of illness.82 Administration of zinc supplements to children suffering from persistent diarrhea is recommended by the WHO,64 and randomized trials have demonstrated its utility in reducing mortality rates up to 75 percent (range: 19 to 63 percent) among children with persistent diarrheal illness.82 More research is needed to determine the mechanism of action of zinc and to identify methods for its optimal delivery. Research on vitamin A supplementation is less consistent and generally does not support the use of vitamin A supplementation for reducing morbidity associated with acute diarrhea.83
Prevention
Water, sanitation, and hygiene
A recent estimate attributes 2.2 million deaths to diseases related to water, sanitation, and hygiene, with infectious diarrhea being the primary cause. Moreover, 90 percent of the disease burden occurs among children younger than 5 years of age.84 This disease burden is almost entirely preventable through measures that have been well understood and widely implemented in industrialized countries for more than a century, yet one-fifth of the world’s population lacks access to potable water, and nearly one-half lacks access to adequate sanitation.85 The Millenium Development Goals seek to reduce these proportions by 50 percent; however, earlier global efforts, including the Water and Sanitation Decade (1981 to 1990), fell far short of achieving even this level of success.
Safe water
Traditional approaches to the provision of safe drinking water and disposal of infectious human waste rely on large infrastructure projects (eg, piped treatment systems) that are costly, time-consuming, and labor-intensive to build and maintain. While these remain an essential long-term objective, approaches based on a combination of “distributed” technologies and behavior change communications, such as point-of-use water treatment, offer an immediate means to accelerate the health gains associated with improved water.86, 87 One model for implementation is the Safe Water System program promoted in more than a dozen countries by the U.S. Centers for Disease Control and Prevention (CDC).88
Sanitation
The median reduction in the incidence of diarrheal diseases resulting from improvements in sanitation has been estimated to be 22 percent and as high as 36 percent in selected studies that met more rigorous scientific criteria.89 Convincing people to pay for and regularly use improved sanitation facilities can be challenging, but some community-based and school-based approaches have achieved notable success, with impressive concurrent reductions in the evidence of diarrheal disease.90, 91 Ecological sanitation, which relies on composting latrines or dehydrating latrines with urine separation to enable the recovery of nutrients in waste matter for agricultural use and the minimization of water pollution, is being studied actively as a more economic and environmentally friendly alternative to traditional latrines or sewerage.92
Houseflies can transfer bacterial pathogens, such as Shigella organisms, from the feces of an infected individual to the hands or the food of a susceptible one, and they thereby serve as vectors for transmission of diarrheal disease. Improvements in sanitation will diminish the opportunity that flies have for contact with human excreta, but this objective also can be attained by diminishing the fly density through insecticide spraying or fly traps. Three well-designed studies have demonstrated reductions in the incidence of diarrhea ranging from 23 to 42 percent (and 85% for shigellosis) when interventions for the control of flies were implemented.93, 94, 95
Hygiene
Where sanitation is poor, the potential for proper hygiene to prevent the transmission of diarrheal disease is high because of abundant environmental contamination with human and animal wastes. A recent meta-analysis of 17 studies concluded that promotion of handwashing interventions reduced the risk of diarrhea by 47 percent and of severe intestinal infections by 59 percent.96 Washing with soap enhances the removal of pathogens from hands and may provide pleasant sensory associations that lead to more frequent compliance with handwashing recommendations.97, 98 Waterless hand sanitizing gels, such as the alcohol-based hand sanitizers now widely used in hospitals, eventually may be useful in developing countries where water is scarce, if they can be made available at very low cost. Handwashing at critical times—after defecating, after cleaning an infant or young child who defecated, before preparing food, before eating, and before feeding infants—is important for maximal reductions in diarrheal disease transmission risk. Handwashing by key persons, including those who care for and prepare or serve foods to infants, and those who prepare and serve food as their vocation (eg, street vendors) should be targeted in promotional campaigns.99
Safe food
The increased incidence of diarrheal disease concurrent with the introduction of weaning foods has been recognized for some time, and the protective effects of exclusive breastfeeding have been demonstrated repeatedly.32, 100 The addition of water, infusions, and other types of milk or solid foods to an infant’s diet and the cessation of breastfeeding each is associated independently with a greatly increased risk of developing diarrhea. Exclusive breastfeeding can and should be promoted through existing primary healthcare services.101
Many lines of evidence suggest that weaning foods are vehicles of enteric infection. Numerous studies have demonstrated the presence of bacterial enteropathogens from weaning foods in doses sufficient to cause illness. Moreover, because food reduces gastric acidity, it predisposes one to the development of infection at even lower bacterial inocula than those required through waterborne or “person-to-person” transmission. Proper food preparation and handling are essential for reducing diarrheal disease risks. Infectious human or animal wastes and contaminated water should not be used to fertilize or irrigate fruits and vegetables, especially those grown close to the soil and eaten raw (eg, lettuce). Shellfish should not be harvested from contaminated waters; seafood and other products of animal origin never should be eaten raw; and milk should be boiled or pasteurized. Thorough cooking eliminates most pathogens from foods; however, poor hygiene and improper storage can lead to recontamination multiplication of bacteria. As a result, thoroughly reheating cooked foods before serving them is important.
Micronutrient supplementation
The limited data available suggest that rates of diarrheal illness among children in developing countries who receive supplementation with zinc are 12 to 44 percent lower than that in those who received no supplementation.82 A few randomized trials have demonstrated that vitamin A supplementation reduces the incidence and duration of diarrheal illnesses,102 particularly among children with measles103 or with vitamin A deficiencies.104 In contrast, other trials have failed to identify any significant change in incidence of diarrheal disease associated with vitamin A prophylaxis,105, 106 and some findings suggest that vitamin A megadoses actually may increase the risk of developing diarrheal illness, particularly in younger children (<30 months).106 In addition, a recent review of nine trials found no consistent evidence backing the use of vitamin A supplementation for the primary prevention of diarrhea.83 However, a randomized trial conducted among Bangladeshi children found the combined effect of zinc and vitamin A supplementation on diarrheal incidence and prevalence to be superior to that associated with either macronutrient alone.107 Moreover, findings from the literature highlight that the effectiveness of micronutrient supplementation for reducing the incidence of diarrheal disease depends largely on the overall immunologic and nutritional states of the child. Further research is needed to assess the overall utility of supplementation with micronutrients, identify groups that should be targeted for these interventions, and determine optimal strategies for delivery.
Vaccines
S. Typhi
Two typhoid vaccines currently are approved for clinical use. The Vi capsular polysaccharide vaccine is approved for children older than 2 years of age. A single parenteral dose provides a high level of protection108, 109 and may provide protection for as long as 10 years. The live attenuated vaccine, Ty21a, is similarly efficacious for a period of at least 5 years.110, 111The vaccine is administered in four total doses given every other day. After 5 years, the complete series should be repeated. Ty21a is approved only for children 6 years and older. Typhoid is a major problem among children in developing countries. Unfortunately, no available vaccine provides the convenience that is required for distribution to children in the areas of greatest need.
Shigella organisms
Development of a vaccine for Shigella continues to be a high public health priority because of its low infectious dose and high incidence of postinfectious sequelae, which include protein-losing enteropathy and malnutrition. Antibodies to the Shigella O antigens are known to be protective, and Robbins and coworkers112 have produced parenteral conjugate vaccines consisting of purified Shigella lipopolysaccharide (LPS) conjugated to a protein carrier. Despite the existence of 47 Shigella serotypes, certain types are represented disproportionately. Three conjugate vaccine candidates that have been evaluated in humans are S. dysenteriae 1 LPS conjugated to tetanus toxoid, S. flexneri 2a LPS conjugated to recombinant Pseudomonas exoprotein A, and S. sonnei LPS conjugated to exoprotein A. All three vaccines proved immunogenic and protective against shigellosis in field trials among Israeli soldiers.113, 114, 115 Parenteral Shigella vaccines may be useful for travelers and the military, but they are impractical for use in developing countries.
A more promising vaccine formulation is a single-dose live-attenuated Shigella vaccine that would induce a mild, immunizing form of the disease. Such vaccines are under development in several laboratories. Sansonetti and coworkers116 have constructed a series of Shigella vaccine candidates with mutations in the icsA (virG) gene, which is involved in spread of Shigella through the intestinal epithelium. They also have constructed a S. flexneri 2a vaccine mutated at virG and the iron-scavenging locus iuc. In clinical trials, ingestion of 106 or 108 CFU of this strain (SC602) caused symptoms of mild shigellosis in most volunteers, whereas a dose of 104 caused only mild symptoms in a few volunteers.117 In subsequent challenge studies, volunteers who ingested a single dose of 104 CFU were protected against challenge with wild type S. flexneri 2a. Noriega and coworkers (Kotloff K, personal communication 2004)have had similar success with a S. flexneri 2a strain harboring mutations in the metabolic locus guaBA and the putative enterotoxin loci set and sen. This strain, designated CVD 1208, is well tolerated at doses up to 109 CFU and provides strong serum and intestinal antibody responses.
Whereas a virG mutation alone is not sufficiently attenuating in S. flexneri, a virG mutant of S. sonnei, designated WRSS1, is only mildly reactogenic. In Phase I clinical trials, a single dose of 103, 104, 105, or 106 CFU caused low-grade fever or mild diarrhea in 22 percent of recipients118 and provided strong serum and intestinal antibody responses
V. cholerae
Two oral cholera vaccines, one live-attenuated and one inactivated, have been licensed in industrialized countries. CVD 103HgR (ctxA -, hly -, Classical Inaba strain 569B) was the first recombinant live-attenuated cholera vaccine that proved to be well-tolerated, immunogenic, and protective and is the only such vaccine currently licensed for human use. CVD 103-HgR is derived from V. cholerae O1 Classical Inaba strain 569B, which shows a mild defect in its ability to colonize the mouse and human intestine.119 In addition to deletions in the catalytic A subunit of cholera toxin, CVD 103-HgR also harbors mutations in the putative virulence factor Hly (which is not expressed by classical biotype strains).
CVD 103-HgR has been administered to more than 6000 individuals in a series of placebo-controlled Phase 1 and Phase 2 trials.110, 120, 121, 122, 123, 124, 125, 126, 127 In a randomized, double-blind, placebo-controlled multicenter trial, a single dose of this strain conferred 91 percent protective efficacy against moderate or severe diarrhea after challenge with toxigenic El Tor V. cholerae.128 This vaccine has shown efficacy in field studies in developing countries and is likely to be beneficial for travelers to endemic regions.
Several nonmotile derivatives of V. cholerae have been evaluated as potential vaccines. The most promising of these candidates is Peru-15, derived by deleting the cholera toxin genetic element, introducing the gene encoding cholera toxin B subunit into the recA locus, and screening for nonmotility in a V. cholerae O1 El Tor Inaba strain.129, 130 Peru-15 showed 100 percent efficacy against homologous challenge in a cohort of adult volunteers.129
An oral cholera vaccine consisting of the nontoxic, highly immunogenic cholera toxin B subunit protein in combination with heat- and formalin-killed V. cholerae O1 classical and El Tor vibrios has been developed in Sweden. This B subunit-whole cell (B-WC) vaccine has proved to be safe and protective against cholera and, to a lesser extent, against diarrhea caused by ETEC, producing cholera toxin-like, heat-labile enterotoxin.131, 132, 133 Phase 1 and phase 2 clinical studies established that the B-WC vaccine does not cause any detectable side effects and that it stimulates a gut mucosal IgA antitoxic and antibacterial immune response after multiple doses.132, 134, 135 The duration of protection for this vaccine, however, is likely to be short-lived. In a large field trial, efficacy was estimated to be approximately 60 to 70 percent after 2 years.136 Still higher (ca 70%) longer-term protective efficacy was seen in those older than age 5 years when vaccinated. Killed vaccines offer some promise in developing countries, particularly because they can be produced locally.
ETEC vaccines
ETEC is an extremely common cause of infant diarrhea in the developing world. A vaccine against this infection must be safe and effective in young infants. Antibodies to the colonization factor antigens are thought to be protective against ETEC infection, yet their tremendous antigenic diversity presents a daunting obstacle. The most advanced ETEC vaccine candidate comprises a killed whole cell formulation plus recombinant cholera toxin B subunit. The vaccine contains five strains of formalin-killed ETEC, which collectively express the most prevalent fimbrial antigens: colonization factor antigen/I and CS1 through CS6.137 This vaccine was safe and immunogenic in adult volunteers as well as in children between 2 and 12 years of age.138, 139, 140 Immune responses, as measured by intestinal lavage and antibody-secreting cells, were elicited against cholera toxin B subunit as well as each fimbrial type.139 In addition, this vaccine was immunogenic when tested in endemic areas in adults and children in Bangladesh and Egypt.140, 141, 142
No vaccines currently are available for protection against Shiga toxin-producing E. coli infection. Vaccine candidates under development include Shiga toxoids,143, 144 polysaccharide-based vaccines,145 and live attenuated constructs.146 Candidate vaccines for livestock vaccination also are undergoing development.147
Rotavirus
Efforts to develop rotavirus vaccines were pursued when improvements in hygiene and sanitation did not appear to reduce disease incidence because rates were similar in less developed and industrialized countries. The initial development of rotavirus vaccines was based on the Jennerian approach, which involved the use of a live-attenuated, antigenically related virus derived from a nonhuman host such as a bovine or rhesus strain. Later, after observations in vaccine challenge cross-protection studies in animals suggested that serotype-specific immunity may be important in protection, vaccines were developed by using a modified Jennerian approach, in which animal-human reassortants expressing VP7 proteins of different serotypes were used as the immunogens. More recently, live-attenutated vaccines based on rotavirus strains derived from young children and neonates have been developed.
In 1998, a rotavirus vaccine was licensed in the United States and was recommended for routine immunization of infants. This vaccine, a tetravalent rhesus-human reassortment rotavirus tetravalent vaccine (RRV-TV), was developed to target the four most common G serotypes of rotavirus, G1-G4. However, RRV-TV was withdrawn in 1999 when it was linked causally with intussusception at an estimated rate of 1 case per 11,000 vaccinated infants, and the manufacturer stopped its production. Other rotavirus vaccines are being developed. Two vaccine candidates, a pentavalent bovine rotavirus-human rotavirus reassortant vaccine (Merck & Co. Research Laboratories, West Point, PA) and a monovalent human rotavirus vaccine (Glaxo SmithKline, Philadelphia, PA), are currently in large-scale clinical efficacy trials. Preliminary trial results have shown that these vaccines are efficacious, and evaluation of the safety of their use in children is ongoing.148 A key question that remains unanswered to date is whether Merck’s multivalent rotavirus vaccine, which theoretically should provide better protection against multiple rotavirus strains, offers significant clinical benefits compared with Glaxo SmithKline’s monovalent vaccine. Trial data that address this question are awaited keenly, as theoretically a monovalent vaccine could be produced through a simpler technology and at a lower price and, therefore, be less expensive and more widely available for use in global immunization programs.
Comment
During the past several decades, the rate of diarrheal mortality among children in developing countries has declined significantly. However, the challenge of preventing the estimated 1.4 to 2.5 million deaths caused by diarrhea annually persists. Further reduction of the rate of mortality from diarrhea will require adoption of the best practice guidelines for management of children with diarrhea and appropriate use of prevention strategies. The efficacy of ORT in reducing severe diarrhea has been established unequivocally, but global ORS coverage rates remain less than 50 percent, and efforts must be made to improve coverage.19 Although encouraging progress has been made in reducing the rates of mortality from diarrhea and the potential for future reductions clearly is evident, efforts to protect children from acquiring diarrheal diseases have been less successful. A wide range of preventive measures related to environmental and host factors have been developed and are being refined and implemented, yet significant impact on the global incidence of diarrheal diseases has been elusive. This matter calls for those working in the field, whether on development of vaccines, promotion of micronutrient supplementation and breastfeeding, or novel approaches to water, sanitation, and hygiene, to redouble their efforts, and for the governments and financial interests who support these endeavors to redouble their commitment.
References
- 1.World Health Organization . Global Burden of Disease Estimates 2001. WHO; Geneva: 2002. [Google Scholar]
- 2.Murray C., Lopez A., Mathers C. The Global Burden of Disease 2000 Project: Aims, Methods, and Data Sources. World Health Organization; Geneva: 2001. [Google Scholar]
- 3.Parashar U., Hummelman E., Bresee J. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis. 2003;9:565–572. doi: 10.3201/eid0905.020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kosek M., Bern C., Guerrant R.L. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull World Health Organ. 2003;81:197–204. [PMC free article] [PubMed] [Google Scholar]
- 5.Tucker A., Haddix A., Bresee J. Cost-effectiveness analysis of a rotavirus immunization program for the United States. JAMA. 1998;279:1371–1376. doi: 10.1001/jama.279.17.1371. [DOI] [PubMed] [Google Scholar]
- 6.Black R. Would control of childhood infectious diseases reduce malnutrition? Acta Paediatr Scand Suppl. 1991;374:133–140. doi: 10.1111/j.1651-2227.1991.tb12016.x. [DOI] [PubMed] [Google Scholar]
- 7.Checkley W., Epstein L.D., Gilman R. Effects of acute diarrhea on linear growth in Peruvian children. Am J Epidemiol. 2003;157:166–175. doi: 10.1093/aje/kwf179. [DOI] [PubMed] [Google Scholar]
- 8.Niehaus M., Moore S., Patrick P. Early childhood diarrhea is associated with diminished cognitive function 4 to 7 years later in children in a northeast Brazilian shantytown. Am J Trop Med Hyg. 2002;66:590–593. doi: 10.4269/ajtmh.2002.66.590. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization . The Treatment of Diarrhoea. A Manual for Physicians and Other Senior Health Workers. World Health Organization; Geneva: 1995. WHO/CDR/95.3. [Google Scholar]
- 10.Rohde J. Selective primary health care: strategies for control of disease in the developing world. XV. Acute diarrhea. Rev Infect Dis. 1984;6:840–854. doi: 10.1093/clinids/6.6.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snyder J., Merson M. The magnitude of the global problem of acute diarrhoeal disease: A review of active surveillance data. Bull World Health Organ. 1982;60:604–613. [PMC free article] [PubMed] [Google Scholar]
- 12.Institute of Medicine . Vol 2. National Academy Press; Washington, DC: 1986. p. D13-11-12. (The prospects of immunizing against rotavirus. New Vaccine Development: Diseases of Importance in Developing Countries). [Google Scholar]
- 13.Martines J., Phillips M. Diarrheal diseases. In: Jamison D., Mosley W., Measham A., Bobadilla J., editors. Disease Control Priorities in Developing Countries. Oxford University Press; New York: 1993. pp. 91–116. [Google Scholar]
- 14.Bern C., Martines J., Glass R.I. The magnitude of the global problem of diarrhoeal disease: A ten-year update. Bull World Health Organ. 1992;70:705–714. [PMC free article] [PubMed] [Google Scholar]
- 15.World Bank . World Development Report, Investing in Health. World Bank; New York: 1993. [Google Scholar]
- 16.Murray C., Lopez A. Global mortality, disability, and the contribution of risk factors: global burden of disease study. Lancet. 1997;349:1436–1442. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization . World Health Report 2003: Shaping the Future. WHO; Geneva: 2003. [Google Scholar]
- 18.Victora C.G., Bryce J., Fontain O. Reducing deaths from diarrhoea through oral rehydration therapy. Bull World Health Organ. 2000;78:1246–1255. [PMC free article] [PubMed] [Google Scholar]
- 19.United Nations Children’s Fund . State of the World’s Children 2003. United Nations Children’s Fund; New York: 2003. [Google Scholar]
- 20.United Nations Children’s Fund . Oral Rehydration Therapy. fact sheet on achievements and challenges. 2002. [Google Scholar]
- 21.Bass D., Greenberg H. Group A rotaviruses. In: Blaser M., Smith P., Ravdin J., editors. Vol 1. Raven Press; New York: 1995. pp. 967–982. (Infections of the Gastroinestinal Tract). [Google Scholar]
- 22.Cunliffe N., Kilgore P., Bresee J. Epidemiology of rotavirus diarrhoea in Africa: A review to assess the need for rotavirus immunization. Bull World Health Organ. 1998;76:525–537. [PMC free article] [PubMed] [Google Scholar]
- 23.Pang X.L., Joensuu J., Vesikari T. Human calicivirus-associated sporadic gastroenteritis in Finnish children less than two years of age followed prospectively during a rotavirus vaccine trial. Pediatr Infect Dis J. 1999;18:420–426. doi: 10.1097/00006454-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Mustafa H., Palombo E.A., Bishop R.F. Epidemiology of astrovirus infection in young children hospitalized with acute gastroenteritis in Melbourne, Australia, over a period of four consecutive years, 1995 to 1998. J Clin Microbiol. 2000;38:1058–1062. doi: 10.1128/jcm.38.3.1058-1062.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ono K., Rai S.K., Chikahira M. Seasonal distribution of enteropathogens detected from diarrheal stool and water samples collected in Kathmandu, Nepal. Southeast Asian J Trop Med Public Health. 2001;32:520–526. [PubMed] [Google Scholar]
- 26.Alam M., Akhtar Y.N., Ali S.S. Seasonal variation in bacterial pathogens isolated from stool samples in Karachi, Pakistan. J Pak Med Assoc. 2003;53:125–129. [PubMed] [Google Scholar]
- 27.Adal K., Sterling C., Guerrant R.L. Cryptosporidium and related species. In: Blaser M., Smith P., Ravdin J., editors. Infections of the Gastrointestinal Tract. Raven Press Ltd; New York: 1995. [Google Scholar]
- 28.Farthing M.J. Giardia lamblia. In: Blaser M., Smith P., Ravdin J., editors. Infections of the Gastrointestinal Tract. Raven Press Ltd; New York: 1995. pp. 1081–1105. [Google Scholar]
- 29.Amin O. Seasonal prevalence of intestinal parasites in the United States during 2000. Am J Trop Med Hyg. 2002;66:799–803. doi: 10.4269/ajtmh.2002.66.799. [DOI] [PubMed] [Google Scholar]
- 30.Villa S., Guiscafre H., Martinez H. Seasonal diarrhoeal mortality among Mexican children. Bull World Health Organ. 1999;77:375–380. [PMC free article] [PubMed] [Google Scholar]
- 31.De Wit M.A. Risk Factors for Norovirus, Sapporo-like Virus, and Group A Rotavirus Gastroenteritis. Emerg Infect Dis. 2003;9:1563–1570. doi: 10.3201/eid0912.020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Black R., Lanata C. Epidemiology of diarrheal disease in developing countries. In: Blaser M., Smith P., Ravdin J., editors. Infections of the Gastrointestinal Tract. 2nd ed. Lippincott Williams and Wilkins; Philadelphia: 2002. pp. 11–29. [Google Scholar]
- 33.Nataro J., Kaper J. Diarrheagenic Eschericia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noguera-Obenza M., Cleary T. Diarrheagenic Escherichia coli. Curr Probl Pediatr. 1999:208–216. doi: 10.1016/s0045-9380(99)80027-9. [DOI] [PubMed] [Google Scholar]
- 35.Effler P., Isaacson M., Arntzen L. Factors contributing to the emergence of Escherichia coli O157 in Africa. Emerg Infect Dis. 2001;7:812–819. doi: 10.3201/eid0705.017507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coker A., Isokpehi R., Thomas B. Human campylobacteriosis in developing countries. Emerg Infect Dis. 2002;8:237–243. doi: 10.3201/eid0803.010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kotloff K., Winickoff J., Ivanoff B. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ. 1999;77:651–666. [PMC free article] [PubMed] [Google Scholar]
- 38.Reller M., Tauxe R. Vibrio cholerae (Cholera) In: Long S., Pickering L., Prober C., editors. Principles and Practice of Pediatric Infectious Diseases. Churchill Livingstone; New York: 2003. pp. 864–869. [Google Scholar]
- 39.Cleary T. Salmonella species. In: Long S., Pickering L., Prober C., editors. Principles and Practice of Pediatric Infectious Diseases. Churchill Livingstone; New York: 2003. pp. 830–835. [Google Scholar]
- 40.Glass R.I., Bresee J., Parashar U. The global burden of dehydrating disease. In: Farthing M.J., Mahalanabis D., editors. Vol 51. Vevey Nestec Ltd. and Lippincott Williams & Wilkins; Philadelphia: 2003. pp. 17–30. (The Control of Food and Fluid Intake in Health and Disease). [Google Scholar]
- 41.Velasquez F., Matson D., Calva J. Rotavirus infection in infants as protection against subsequent infections. N Engl J Med. 1996;335:1022–1028. doi: 10.1056/NEJM199610033351404. [DOI] [PubMed] [Google Scholar]
- 42.Bresee J.S., Glass R.I., Ivanoff B. Current status and future priorities for rotavirus vaccine development, evaluation and implementation in developing countries. Vaccine. 1999;17:2207–2222. doi: 10.1016/s0264-410x(98)00376-4. [DOI] [PubMed] [Google Scholar]
- 43.Estes M.K., Hardy M.E. Norwalk virus and other enteric caliciviruses. In: Blaser M., Smith P., Radvin J., editors. Vol 1. Raven Press; New York: 1995. pp. 1009–1034. (Infections of the Gastrointestinal Tract). [Google Scholar]
- 44.Xi J.N., Graham D.Y., Wang K.N. Norwalk virus genome cloning and characterization. Science. 1990;250:1580–1583. doi: 10.1126/science.2177224. [DOI] [PubMed] [Google Scholar]
- 45.Fankhauser R.L., Monroe S.S., Noel J.S. Epidemiologic and molecular trends of “Norwalk-like viruses” associated with outbreaks of gastroenteritis in the United States. J Infect Dis. 2002;186:1–7. doi: 10.1086/341085. [DOI] [PubMed] [Google Scholar]
- 46.Rockx B., De Wit M., Vennema H. Natural history of human calicivirus infection: a prospective cohort study. Clin Infect Dis. 2002;35:246–253. doi: 10.1086/341408. [DOI] [PubMed] [Google Scholar]
- 47.Herrmann J., Blacklow N. Enteric adenoviruses. In: Blaser M., Smith P., Ravdin J., editors. Vol 1. Raven Press; New York: 1995. pp. 1047–1053. (Infections of the Gastrointestinal Tract). [Google Scholar]
- 48.Herrmann J., Taylor D., Echeverria P. Astroviruses as a cause of gastroenteritis in children. N Engl J Med. 1991;324:1757–1760. doi: 10.1056/NEJM199106203242501. [DOI] [PubMed] [Google Scholar]
- 49.Bern C., Ortega Y., Checkley W. Epidemiologic differences between cyclosporiasis and cryptosporidiosis in Peruvian children. Emerg Infect Dis. 2002;8:581–585. doi: 10.3201/eid0806.01-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Levine W.C., Stephenson W.T., Craun G.F. Waterborne disease outbreaks, 1986–1988. MMWR CDC Surveill Summ. 1990;39:1–13. [PubMed] [Google Scholar]
- 51.Dietz V.J., Roberts J.M. National surveillance for infection with Cryptosporidium parvum, 1995–1998: What have we learned? Public Health Rep. 2000;115:358–363. doi: 10.1093/phr/115.4.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee S.H., Levy D.A., Craun G.F. Surveillance for waterborne-disease outbreaks—United States, 1999–2000. MMWR Surveill Summ. 2002;51:1–47. [PubMed] [Google Scholar]
- 53.Corso P.S., Kramer M.H., Blair K.A. Cost of illness in the 1993 waterborne Cryptosporidium outbreak, Milwaukee, Wisconsin. Emerg Infect Dis. 2003;9:426–431. doi: 10.3201/eid0904.020417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herwaldt B. Cyclospora cayetanensis: A review, focusing on outbreaks of cyclosporiasis in the 1990s. Clin Infect Dis. 2000;31:1040–1057. doi: 10.1086/314051. [DOI] [PubMed] [Google Scholar]
- 55.Howe A.D., Forster S., Morton S. Cryptosporidium oocysts in a water supply associated with a cryptosporidiosis outbreak. Emerg Infect Dis. 2002;8:619–624. doi: 10.3201/eid0806.010271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamamoto N., Urabe K., Takaoka M. Outbreak of cryptosporidiosis after contamination of the public water supply in Saitama Prefecture, Japan, in 1996. Kansenshogaku Zasshi. 2000;74:518–526. doi: 10.11150/kansenshogakuzasshi1970.74.518. [DOI] [PubMed] [Google Scholar]
- 57.Moon H.W. Comparative histopathology of intestinal infections. Adv Exp Med Biol. 1997;412:1–19. doi: 10.1007/978-1-4899-1828-4_1. [DOI] [PubMed] [Google Scholar]
- 58.Morris A.P., Scott J.K., Ball J.M. NSP4 elicits age-dependent diarrhea and Ca(2+)mediated I(−) influx into intestinal crypts of CF mice. Am J Physiol. 1999;277:G431–G444. doi: 10.1152/ajpgi.1999.277.2.G431. [DOI] [PubMed] [Google Scholar]
- 59.Estes M.K., Kang G., Zeng C.Q. Pathogenesis of rotavirus gastroenteritis. Novartis Found Symp. 2001;238:82–96. doi: 10.1002/0470846534.ch6. discussion 96–100. discussion 96–100. [DOI] [PubMed] [Google Scholar]
- 60.Blutt S.E., Kirkwood C.D., Parreno V. Rotavirus antigenaemia and viraemia: A common event? Lancet. 2003;362:1445–1449. doi: 10.1016/S0140-6736(03)14687-9. [DOI] [PubMed] [Google Scholar]
- 61.Rice A.L., Sacco L., Hyder A. Malnutrition as an underlying cause of childhood deaths associated with infectious diseases in developing countries. Bull World Health Organ. 2000;78:1207–1221. [PMC free article] [PubMed] [Google Scholar]
- 62.Bitarakwate E., Mworozi E., Kekitiinwa A. Serum zinc status of children with persistent diarrhoea admitted to the diarrhoea management unit of Mulago Hospital, Uganda. Afr Health Sci. 2003;3:54–60. [PMC free article] [PubMed] [Google Scholar]
- 63.Mitra A.K., Hernandez C.D., Hernandez C.A. Management of diarrhoea in HIV-infected patients. Int J STD & AIDS. 2001;12:630–639. doi: 10.1258/0956462011923840. [DOI] [PubMed] [Google Scholar]
- 64.World Health Organization . Improving child health: IMCI, the integrated approach. World Health Organization; Geneva: 1996. WHO/CHD/97.12 Rev. 1. [Google Scholar]
- 65.American Academy of Pediatrics The management of acute gastroenteritis in young children. Pediatrics. 1996;97 Available at: www.aap.org/policy/gastro.htm; accessed June 21, 2004. [Google Scholar]
- 66.Hahn S., Kim Y., Garner P. Reduced osmolarity oral rehydration solution for treating dehydration due to diarrhoea in children: systematic review. BMJ. 2001;323:81–85. doi: 10.1136/bmj.323.7304.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hahn S., Kim S., Garner P. Reduced osmolarity oral rehydration solution for treating dehydration caused by acute diarrhoea in children. 2003. (Cochrane Review); No. Issue 4. Located at: The Cochrane Library, Chichester, UK. [DOI] [PubMed] [Google Scholar]
- 68.World Health Organization . World Health Organization; Geneva: 2002. Oral rehydration salts (ORS): a new reduced osmolarity formulation. [Google Scholar]
- 69.Centers for Disease Control and Prevention Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR. 2003;52:1–16. [PubMed] [Google Scholar]
- 70.Walker-Smith J., Sandhu B., Isolauri E. Guidelines prepared by the ESPGHAN working group on acute diarrhea. J Pediatr Gastroenterol Nutr. 1997;24:619–620. doi: 10.1097/00005176-199705000-00024. [DOI] [PubMed] [Google Scholar]
- 71.Brown K., Peerson J., Fontaine O. Use of nonhuman milks in the dietary management of young children with acute diarrhea: a meta-analysis of clinical trials. Pediatrics. 1994;93:17–27. [PubMed] [Google Scholar]
- 72.World Health Organization . Recent Advances in Research on Feeding During and After Diarrhoea. World Health Organization; Geneva: 1985. WHO/CDD/DDM/85.2. [Google Scholar]
- 73.Jones G., Steketee R.W., Black R.E. How many deaths can we prevent this year? Lancet. 2003;362:65–71. doi: 10.1016/S0140-6736(03)13811-1. [DOI] [PubMed] [Google Scholar]
- 74.Jelliffe D.B., Jelliffe E.P. Dietary Management of Young Children With Acute Diarrhoea: A Manual for Managers of Health Programs. World Health Organization; Geneva: 1991. [Google Scholar]
- 75.World Health Organization . The Rational Use of Drugs in the Management of Acute Diarrhoea in Children. World Health Organization; Geneva: 1990. [Google Scholar]
- 76.American Academy of Pediatrics . Red Book: 2003 Report of the Committee on Infectious Diseases, Vol 26th. American Academy of Pediatrics; Elk Grove Village, IL: 2003. [Google Scholar]
- 77.Bennish M., Wojtnyiak B. Mortality due to shigellosis: community and hospital data. Rev Infect Dis. 1991;13:S245–S251. doi: 10.1093/clinids/13.supplement_4.s245. [DOI] [PubMed] [Google Scholar]
- 78.Bhattacharya S., Sarkar K., Nair G. A regional alert of multi-resistant Shigella dysenteriae type 1 in South Asia. Lancet. 2003;3:751. doi: 10.1016/s1473-3099(03)00829-6. [DOI] [PubMed] [Google Scholar]
- 79.World Health Organization . The management of bloody diarrhoea in young children. World Health Organization Programme for the Control of Diarrhoeal Diseases; Geneva: 1994. WHO/CDD/94.49. [Google Scholar]
- 80.Szajewska H., Mrukowicz J.Z. Probiotics in the treatment and prevention of acute infectious diarrhea in infants and children: a systematic review of published randomized, double-blind, placebo-controlled trials. J Pediatr Gastroenterol Nutr. 2001;33:S17–S25. doi: 10.1097/00005176-200110002-00004. [DOI] [PubMed] [Google Scholar]
- 81.Solis B., Samartin S., Gomez S. Probiotics as a help in children suffering from malnutrition and diarrhoea. Eur J Clin Nutr. 2002;56(suppl 3):57–59. doi: 10.1038/sj.ejcn.1601488. [DOI] [PubMed] [Google Scholar]
- 82.Black R. Zinc deficiency, infectious disease and mortality in the developing world. J Nutr. 2003;133:1485S–1489S. doi: 10.1093/jn/133.5.1485S. [DOI] [PubMed] [Google Scholar]
- 83.Grotto I., Mimouni M., Gdalevich M. Vitamin A supplementation and childhood morbidity from diarrhea and respiratory infections: a meta-analysis. J Pediatr. 2003;142:297–304. doi: 10.1067/mpd.2003.116. [DOI] [PubMed] [Google Scholar]
- 84.Pruss A., Kay D., Fewtrell L. Estimating the burden of disease from water, sanitation, and hygiene at the global level. Environ Health Perspect. 2002;110:537–542. doi: 10.1289/ehp.110-1240845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.United Nations Children’s Fund (UNICEF) Water Supply and Sanitation Council . UNICEF; New York, NY: 2000. Global Water Supply and Sanitation Assessment 2000 Report. [Google Scholar]
- 86.Mintz E., Bartram J., Lochery P. Not just a drop in the bucket: expanding access of point-of-use water treatment systems. Am J Public Health. 2001;91:1565–1570. doi: 10.2105/ajph.91.10.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dooley E. Safe water for all. Environ Health Perspect. 2004;112:A47. doi: 10.1289/ehp.112-1277128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Centers for Disease Control and Prevention . Safe water system. 2004. Available at: www.cdc.gov/safewater; accessed June 21. [Google Scholar]
- 89.Esrey S., Potash J., Roberts L. Effects of improved water supply and sanitation on ascariasis, diarrhoea, dracunculiasis, hookworm infection, schistosomiasis, and trachoma. Bull World Health Organ. 1991;69:609–621. [PMC free article] [PubMed] [Google Scholar]
- 90.Cairncross S. Sanitation in the developing world: Current status and future solutions. Int J Environ Health Res. 2003;(suppl 1):123–131. doi: 10.1080/0960312031000102886. [DOI] [PubMed] [Google Scholar]
- 91.Daniles D., Cousens S., Makoae L. A case-control study of the impact of improved sanitation on diarrhoea morbidity in Lesotho. Bull World Health Organ. 1990;68:455–463. [PMC free article] [PubMed] [Google Scholar]
- 92.Esrey S., Gough J., Rapaport D. Sida; Stockholm: 1998. Ecological Sanitation. [Google Scholar]
- 93.Cohen D., Green M., Block C. Reduction of transmission of shigellosis by control of houseflies (Musca domestica) Lancet. 1991;337:993–997. doi: 10.1016/0140-6736(91)92657-n. [DOI] [PubMed] [Google Scholar]
- 94.Chavasse D., Shier R., Murphy O. Impact of fly control on childhood diarrhoea in Pakistan: community-randomised trial. Lancet. 1999;353:22–25. doi: 10.1016/s0140-6736(98)03366-2. [DOI] [PubMed] [Google Scholar]
- 95.Emerson P., Lindsay S., Walraven G. Effect of fly control on trachoma and diarrhoea. Lancet. 1999;353:1401–1403. doi: 10.1016/S0140-6736(98)09158-2. [DOI] [PubMed] [Google Scholar]
- 96.Curtis V., Cairncross S. Effect of washing hands with soap on diarrhoea risk in the community: a systematic review. Lancet Infect Dis. 2003;3:275–281. doi: 10.1016/s1473-3099(03)00606-6. [DOI] [PubMed] [Google Scholar]
- 97.Hoque B., Briend A. A comparison of local handwashing agents in Bangladesh. J Trop Med Hyg. 1991;94:61–64. [PubMed] [Google Scholar]
- 98.Han A., New O., Aye T. Personal toilet after defecation and the degree of hand contamination according to different methods used. J Trop Med Hyg. 1986;89:237–241. [PubMed] [Google Scholar]
- 99.Sobel J., Mahon B., Mendoza C. A simple system for water purification and storage, handwashing, and beverage storage reduces fecal contamination of street-vended beverages in Guatemala. Am J Trop Med Hyg. 1998;59:380–387. doi: 10.4269/ajtmh.1998.59.380. [DOI] [PubMed] [Google Scholar]
- 100.Gordon J., Chitkara I., Wyon J. Weanling diarrhea. Am J Med Sci. 1963;130:345–377. [PubMed] [Google Scholar]
- 101.Bhandari N., Bahl R., Mazmudar S. Effect of community-based promotion of exclusive breastfeeding on diarrhoeal illness and growth: a cluster randomized controlled trial. Lancet. 2003;361:1421–1423. doi: 10.1016/S0140-6736(03)13134-0. [DOI] [PubMed] [Google Scholar]
- 102.Huttly S., Morris S., Pisani V. Prevention of diarrhoea in young children in developing countries. Bull World Health Organ. 1997;75:163–174. [PMC free article] [PubMed] [Google Scholar]
- 103.D’Souza R., D’Souza R. Vitamin A for preventing secondary infections in children with measles—a systematic review. J Trop Pediatr. 2002;48:72–77. doi: 10.1093/tropej/48.2.72. [DOI] [PubMed] [Google Scholar]
- 104.Chowdhury S., Kumar R., Ganguly N. Effect of vitamin A supplementation on childhood morbidity and mortality. Indian J Med Sci. 2002;56:259–264. [PubMed] [Google Scholar]
- 105.Abdeljaber M.H., Monto A.S., Tilden R.L. The impact of vitamin A supplementation on morbidity: a randomized community intervention trial. Am J Public Health. 1991;81:1654–1656. doi: 10.2105/ajph.81.12.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dibley M.J., Sadjimin T., Kjolhede C.L. Vitamin A supplementation fails to reduce incidence of acute respiratory illness and diarrhea in preschool-age Indonesian children. J Nutr. 1996;126:434–442. doi: 10.1093/jn/126.2.434. [DOI] [PubMed] [Google Scholar]
- 107.Rahman M.M., Vermund S.H., Wahed M.A. Simultaneous zinc and vitamin A supplementation in Bangladeshi children: randomised double blind controlled trial. BMJ. 2001;323:314–318. doi: 10.1136/bmj.323.7308.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Acharya I., Lowe C., Thapa R. Prevention of typhoid fever in Nepal with the Vi capsular polysaccharide of Salmonella typhi. A preliminary report. N Engl J Med. 1987;317:1101–1104. doi: 10.1056/NEJM198710293171801. [DOI] [PubMed] [Google Scholar]
- 109.Dizer U., Gorenek L., Guner O. Assessment of the antibody response in 110 healthy individuals who have been subject to Vi capsular polysaccharide vaccine. Vaccine. 2002;20:3052–3054. doi: 10.1016/s0264-410x(02)00257-8. [DOI] [PubMed] [Google Scholar]
- 110.Cryz S.J., Jr, Que J.U., Levine M.M. Safety and immunogenicity of a live oral bivalent typhoid fever (Salmonella typhi Ty21a)-cholera (Vibrio cholerae CVD 103-HgR) vaccine in healthy adults. Infect Immun. 1995;63:1336–1339. doi: 10.1128/iai.63.4.1336-1339.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Levine M.M., Ferreccio C., Cryz S. Comparison of enteric-coated capsules and liquid formulation of Ty21a typhoid vaccine in randomised controlled field trial. Lancet. 1990;336(8720):891–894. doi: 10.1016/0140-6736(90)92266-k. [DOI] [PubMed] [Google Scholar]
- 112.Robbins J., Chu C., Schneerson R. Hypothesis for vaccine development: Protective immunity to enteric diseases caused by nontyphoidal Salmonellae and Shigellae may be conferred by serum IgG antibodies o the O-specific polysaccharides of their lipopolysaccharides. Clin Infect Dis. 1992;15:346–351. doi: 10.1093/clinids/15.2.346. [DOI] [PubMed] [Google Scholar]
- 113.Cohen D., Ashkenazi S., Green M. Safety and immunogenicity of investigational Shigella conjugate vaccines in Israeli volunteers. Infect Immun. 1996;64:4074–4077. doi: 10.1128/iai.64.10.4074-4077.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chu C.Y., Liu B.K., Watson D. Preparation, characterization, and immunogenicity of conjugates composed of the O-specific polysaccharide of Shigella dysenteriae type 1 (Shiga’s bacillus) bound to tetanus toxoid. Infect Immun. 1991;59:4450–4458. doi: 10.1128/iai.59.12.4450-4458.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Taylor D.N., Trofa A.C., Sadoff J. Synthesis, characterization, and clinical evaluation of conjugate vaccines composed of the O-specific polysaccharides of Shigella dysenteriae type 1, Shigella flexneri type 2a, and Shigella sonnei (Plesiomonas shigelloides) bound to bacterial toxoids. Infect Immun. 1993;61:3678–3687. doi: 10.1128/iai.61.9.3678-3687.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bernardini M., Mounier J., d’Hauteville H. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci USA. 1989;86:3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Coster T.S., Hoge C.W., VanDeVerg L.L. Vaccination against shigellosis with attenuated Shigella flexneri 2a strain SC602. Infect Immun. 1999;67:3437–3443. doi: 10.1128/iai.67.7.3437-3443.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kotloff K.L., Taylor D.N., Sztein M.B. Phase I evaluation of delta virG Shigella sonnei live, attenuated, oral vaccine strain WRSS1 in healthy adults. Infect Immun. 2002;70:2016–2021. doi: 10.1128/IAI.70.4.2016-2021.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Levine M.M., Kaper J.B., Herrington D. Safety, immunogenicity, and efficacy of recombinant live oral cholera vaccines, CVD 103 and CVD 103-HgR. Lancet. 1988;2:467–470. doi: 10.1016/s0140-6736(88)90120-1. [DOI] [PubMed] [Google Scholar]
- 120.Cookson S.T., Stamboulian D., Demonte J. A cost-benefit analysis of programmatic use of CVD 103-HgR live oral cholera vaccine in a high-risk population. Int J Epidemiol. 1997;26:212–219. doi: 10.1093/ije/26.1.212. [DOI] [PubMed] [Google Scholar]
- 121.Cryz S.J., Jr, Levine M.M., Kaper J.B. Randomized double-blind placebo controlled trial to evaluate the safety and immunogenicity of the live oral cholera vaccine strain CVD 103-HgR in Swiss adults. Vaccine. 1990;8:577–580. doi: 10.1016/0264-410x(90)90012-b. [DOI] [PubMed] [Google Scholar]
- 122.Cryz S.J., Jr, Levine M.M., Losonsky G. Safety and immunogenicity of a booster dose of Vibrio cholerae CVD 103-HgR live oral cholera vaccine in Swiss adults. Infect Immun. 1992;60:3916–3917. doi: 10.1128/iai.60.9.3916-3917.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kaper J.B., Michalski J., Ketley J.M. Potential for reacquisition of cholera enterotoxin genes by attenuated Vibrio cholerae vaccine strain CVD 103-HgR. Infect Immun. 1994;62:1480–1483. doi: 10.1128/iai.62.4.1480-1483.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kotloff K.L., Wasserman S.S., O’Donnell S. Safety and immunogenicity in North Americans of a single dose of live oral cholera vaccine CVD 103-HgR: Results of a randomized, placebo-controlled, double-blind crossover trial. Infect Immun. 1992;60:4430–4432. doi: 10.1128/iai.60.10.4430-4432.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lagos R., San Martin O., Wasserman S.S. Palatability, reactogenicity and immunogenicity of engineered live oral cholera vaccine CVD 103-HgR in Chilean infants and toddlers. Pediatr Infect Dis J. 1999;18:624–630. doi: 10.1097/00006454-199907000-00011. [DOI] [PubMed] [Google Scholar]
- 126.Richie E.E., Punjabi N.H., Sidharta Y.Y. Efficacy trial of single-dose live oral cholera vaccine CVD 103-HgR in North Jakarta, Indonesia, a cholera-endemic area. Vaccine. 2000;18:2399–2410. doi: 10.1016/s0264-410x(00)00006-2. [DOI] [PubMed] [Google Scholar]
- 127.Simanjuntak C.H., O’Hanley P., Punjabi N.H. Safety, immunogenicity, and transmissibility of single-dose live oral cholera vaccine strain CVD 103-HgR in 24- to 59-month-old Indonesian children. J Infect Dis. 1993;168:1169–1176. doi: 10.1093/infdis/168.5.1169. [DOI] [PubMed] [Google Scholar]
- 128.Tacket C.O., Cohen M.B., Wasserman S.S. Randomized, double-blind, placebo-controlled, multicentered trial of the efficacy of a single dose of live oral cholera vaccine CVD 103-HgR in preventing cholera following challenge with Vibrio cholerae O1 El tor inaba three months after vaccination. Infect Immun. 1999;67:6341–6345. doi: 10.1128/iai.67.12.6341-6345.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cohen M.B., Giannella R.A., Bean J. Randomized, controlled human challenge study of the safety, immunogenicity, and protective efficacy of a single dose of Peru-15, a live attenuated oral cholera vaccine. Infect Immun. 2002;70:1965–1970. doi: 10.1128/IAI.70.4.1965-1970.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sack D.A., Sack R.B., Shimko J. Evaluation of Peru-15, a new live oral vaccine for cholera, in volunteers. J Infect Dis. 1997;176:201–205. doi: 10.1086/514025. [DOI] [PubMed] [Google Scholar]
- 131.van Loon F.P., Clemens J.D., Chakraborty J. Field trial of inactivated oral cholera vaccines in Bangladesh: Results from 5 years of follow-up. Vaccine. 1996;14:162–166. doi: 10.1016/0264-410x(95)00122-h. [DOI] [PubMed] [Google Scholar]
- 132.Jertborn M., Svennerholm A.M., Holmgren J. Immunological memory after immunization with oral cholera B subunit-whole-cell vaccine in Swedish volunteers. Vaccine. 1994;12:1078–1082. doi: 10.1016/0264-410x(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 133.Holmgren J., Czerkinsky C., Lycke N. Strategies for the induction of immune responses at mucosal surfaces making use of cholera toxin B subunit as immunogen, carrier, and adjuvant. Am J Trop Med Hyg. 1994;50(5 suppl):42–54. [PubMed] [Google Scholar]
- 134.Svennerholm A.M., Jertborn M., Gothefors L. Mucosal antitoxic and antibacterial immunity after cholera disease and after immunization with a combined B subunit-whole cell vaccine. J Infect Dis. 1984;149:884–893. doi: 10.1093/infdis/149.6.884. [DOI] [PubMed] [Google Scholar]
- 135.Jertborn M., Svennerholm A.M., Holmgren J. Safety and immunogenicity of an oral recombinant cholera B subunit-whole cell vaccine in Swedish volunteers. Vaccine. 1992;10:130–132. doi: 10.1016/0264-410x(92)90030-n. [DOI] [PubMed] [Google Scholar]
- 136.Clemens J.D., Sack D.A., Harris J.R. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet. 1990;335:270–273. doi: 10.1016/0140-6736(90)90080-o. [DOI] [PubMed] [Google Scholar]
- 137.Holmgren J., Svennerholm A. Vaccines against diarrheal diseases. In: Wigzell H., editor. Vol 133. Springer-Verlag; Berlin-Heidelberg-New York: 1998. pp. 291–328. (Handbook of Experimental Pharmacology). [Google Scholar]
- 138.Ahren C., Jertborn M., Svennerholm A.M. Intestinal immune responses to an inactivated oral enterotoxigenic Escherichia coli vaccine and associated immunoglobulin A responses in blood. Infect Immun. 1998;66:3311–3316. doi: 10.1128/iai.66.7.3311-3316.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jertborn M., Ahren C., Holmgren J. Safety and immunogenicity of an oral inactivated enterotoxigenic Escherichia coli vaccine. Vaccine. 1998;16:255–260. doi: 10.1016/s0264-410x(97)00169-2. [DOI] [PubMed] [Google Scholar]
- 140.Savarino S.J., Brown F.M., Hall E. Safety and immunogenicity of an oral, killed enterotoxigenic Escherichia coli-cholera toxin B subunit vaccine in Egyptian adults. J Infect Dis. 1998;177:796–799. doi: 10.1086/517812. [DOI] [PubMed] [Google Scholar]
- 141.Qadri F., Wenneras C., Ahmed F. Safety and immunogenicity of an oral, inactivated enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine in Bangladeshi adults and children. Vaccine. 2000;18:2704–2712. doi: 10.1016/s0264-410x(00)00056-6. [DOI] [PubMed] [Google Scholar]
- 142.Savarino S.J., Hall E.R., Bassily S., PRIDE Study Group Oral, inactivated, whole cell enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine: results of the initial evaluation in children. J Infect Dis. 1999;179:107–114. doi: 10.1086/314543. [DOI] [PubMed] [Google Scholar]
- 143.Ishikawa S., Kawahara K., Kagami Y. Protection against Shiga toxin 1 challenge by immunization of mice with purified mutant Shiga toxin 1. Infect Immun. 2003;71:3235–3239. doi: 10.1128/IAI.71.6.3235-3239.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Keusch G., Acheson D., Marchant C. Toxoid-based active and passive immunization to prevent and/or modulate hemolytic-uremic syndrome due to Shiga toxin-producing Escherichia coli. In: O’Brien A., editor. Escherichia coli O157:H7 and Other Shiga Toxin-Producing E. coli Strains. American Society for Microbiology; Washington, DC: 1998. pp. 409–418. [Google Scholar]
- 145.Conlan J.W., Cox A.D., KuoLee R. Parenteral immunization with a glycoconjugate vaccine containing the O157 antigen of Escherichia coli O157: H7 elicits a systemic humoral immune response in mice, but fails to prevent colonization by the pathogen. Can J Microbiol. 1999;45:279–286. [PubMed] [Google Scholar]
- 146.Tzschaschel B.D., Guzman C.A., Timmis K.N. An Escherichia coli hemolysin transport system-based vector for the export of polypeptides: export of Shiga-like toxin IIeB subunit by Salmonella typhimurium aroA. Nat Biotechnol. 1996;14:765–769. doi: 10.1038/nbt0696-765. [DOI] [PubMed] [Google Scholar]
- 147.Dean-Nystrom E.A., Gansheroff L.J., Mills M. Vaccination of pregnant dams with intimin (O157) protects suckling piglets from Escherichia coli O157: H7 infection. Infect Immun. 2002;70:2414–2418. doi: 10.1128/IAI.70.5.2414-2418.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lynch M., Bresee J.S., Gentsch J. Rotavirus vaccines. Curr Opin Infect Dis. 2000;13:495–502. doi: 10.1097/00001432-200010000-00011. [DOI] [PubMed] [Google Scholar]


