Highlights
-
•
Viral infections are present in large proportion of ICU-admitted patients with CAP.
-
•
Influenza and rhinovirus accounted for a significant number of viral CAP episodes.
-
•
The use of LRT instead of URT samples might be useful in the diagnosis of viral CAP
Keywords: respiratory viruses, CAP, lower respiratory tract infection, molecular diagnostic, respiratory infection
Abstract
Background
The role of respiratory viruses in the etiology of community-acquired pneumonia (CAP) is still debated. The advent of molecular assays has improved the identification of viruses in patients with CAP and according to published studies, viruses account for 11-55% of adult CAP cases.
Objectives and study design
In the present study, the frequency of respiratory viruses was evaluated in respiratory samples collected from 414 patients with CAP admitted to 26 ICUs in the Lombardy Region (10 million inhabitants) during seven winter-spring seasons (2009–2016).
Results
In 226 (54.6%) patients one or more respiratory viruses were identified, while 188 (45.4%) patients were negative. A single virus infection was observed in 214/226 (94.7%) patients; while, in 12/226 (5.3%) at least two respiratory viruses were detected. Influenza A was the most common virus in 140/226 patients (61.9%) followed by rhinoviruses (33/226, 14.6%), respiratory syncytial virus (13/226, 5.8%), influenza B virus (9/226, 4.0%), human coronaviruses (9/226, 4.0%), cytomegalovirus (9/226, 4.0%) and human metapneumovirus (1/226, 0.4%).
Conclusions
Viral infections are present in a consistent proportion of patients admitted to the ICU for CAP. Influenza A and rhinovirus accounted for three-quarters of all CAP in ICU patients. The use of lower respiratory instead of upper respiratory samples might be useful in the diagnosis of viral CAP.
1. Background
In the majority of patients with a poor outcome, who are admitted to intensive care units (ICUs), there is an association with severe respiratory distress syndromes [1]. Severe community-acquired pneumonia (CAP) is usually attributed to bacterial agents, but the introduction of broader diagnostic panels with increased sensitivity have improved the diagnosis of respiratory viruses with a relevant impact on outcomes for ICU-patients [2]. Indeed, based on recent reports, viruses account for 11–55% of the total infections in patients admitted to the ICU with severe respiratory distress [1], [3], [4], [5]. However, many studies have only focused on the role of influenza A virus (e.g. H1N1pdm09) as an etiologic agent of acute respiratory failure [6], [7]. Respiratory viruses other than influenza such as human rhinovirus (HRV), human parainfluenza viruses 1-4 (hPIV1-4), human respiratory syncytial virus (hRSV) and human coronaviruses (hCoVs) are not routinely investigated in respiratory samples of ICU patients, and their role as the cause of ICU admission might be under estimated. Understanding the etiologic agents of CAP may improve treatment decisions and patient outcome. However, despite technological advances, establishing the etiologic causes of pneumonia remains a challenge.
2. Objectives
The aim of this study was to investigate the prevalence of respiratory viruses in patients admitted to 26 ICUs in the Lombardy Region (nearly 10 million inhabitants) during seven consecutive winter seasons (2009–2016).
3. Study design
3.1. Study design and patients
Respiratory samples collected from patients admitted to 26 ICUs in the Lombardy Region with acute respiratory failure, severe pneumonia or acute respiratory distress syndrome assisted by non-invasive or mechanical ventilation or by extra-corporeal membrane oxygenation were analyzed during seven consecutive winter seasons, between November 1st and April 31st (2009–2016). Respiratory samples were collected at admission to the ICU following standard protocols and according to the physician’s orders. Severe respiratory syndrome was defined as acute onset (≤1 week) respiratory failure, with hypoxemia (pO2/FiO2 ratio <300 mmHg while on positive end-expiratory pressure or noninvasive CPAP ≥5 cmH2O) and bilateral opacities at chest imaging [8]. Respiratory samples were sent to the Molecular Virology Unit at the Fondazione IRCCS Policlinico San Matteo, Pavia according to the Influenza Regional Surveillance and Preparedness Plan (DGR IX/1046, 22 Dec. 2010 and DGR 5988, 30 Jun 2011). Local Ethics Committee approval was not required as the study was conducted under the auspices of the Influenza Regional Surveillance and Preparedness Plan (DGR IX/1046, 22 Dec. 2010 and DGR 5988, 30 Jun 2011).
3.2. Identification of respiratory viruses
Respiratory samples were tested with a panel of laboratory-developed real-time RT-PCR or real-time PCR able to detect and quantify the following viruses [9], [10]: influenza virus A and B, including subtype determination, hPIV-3, hRSV types A and B, hCoV types −OC43, −229E, −NL63, and −HKU1, hMPV, HRV and CMV (in a subset of samples).
4. Results
During the study period, a total of 414 (266 male and 148 female) patients were hospitalized in 26 ICUs with severe respiratory distress. Of these, 242/414 (58.2%) patients were adults (18–65 years), 134/414 (32.4%) patients aged >65 years, while 39/414 (9.4%) were children (Table 1 ). Overall, the median age was 56 years (range: 1 mos–86 yrs). In two seasons (2011–2012 and 2014–2015) the overall median age was higher than 60 years, while in one season (2013–2014) it was lower than 50 years. The majority of ICU patients for each year were adults (18–65 yrs), ranging from 45.5% to 66.7% of total patients, over the seven seasons. Of note, in one season (2011–12), patients aged >65 years were the most frequently observed reaching 47.7% of the total patients analyzed (Table 1).
Table 1.
Age distribution of ICU-patients included in the study according to season.
| All seasons (n = 414) |
2009–10 (n = 56) | 2010–11 (n = 84) | 2011–12 (n = 44) | 2012–13 (n = 55) | 2013–14 (n = 36) | 2014–15 (n = 88) | 2015–16 (n = 51) | |
|---|---|---|---|---|---|---|---|---|
| Median age (range) | 56 (1 mos-86) |
52 (3–86) |
52 (1 mo-84) |
63 (1–83) |
57 (9 mos-84) |
47 (1–84) |
62 (3 mos-84) |
59 (1 mos-86) |
| Age group (%) | ||||||||
| <1 year | 8 (1.9) | 0 (0.0) | 3 (3.6) | 0 (0.0) | 1 (1.8) | 0 (0.0) | 2 (2.3) | 2 (3.9) |
| 1–5 years | 14 (3.4) | 1 (1.8) | 3 (3.6) | 1 (2.3) | 5 (9.1) | 3 (8.3) | 1 (1.1) | 0 (0.0) |
| 6–18 years | 17 (4.1) | 4 (7.1) | 2 (2.4) | 2 (4.5) | 4 (7.3) | 2 (5.6) | 1 (1.1) | 2 (3.9) |
| 19–65 years | 242 (58.2) | 37 (66.1) | 56 (66.7) | 20 (45.5) | 29 (52.7) | 23 (63.9) | 46 (52.3) | 30 (58.8) |
| >65 years | 134 (32.4) | 14 (25.0) | 20 (23.8) | 21 (47.7) | 16 (29.1) | 8 (22.2) | 38 (43.2) | 17 (33.3) |
Mo, month.
Respiratory viruses were detected in clinical samples from the lower respiratory tract (LRT) (Broncho alveolar lavage or Broncho aspirate) during 179/414 (43.2%) CAP episodes; while respiratory viruses were detected in samples from the upper respiratory tract (URT) (nasal swab or nasopharyngeal aspirate) during 225/414 (56.8%) episodes.
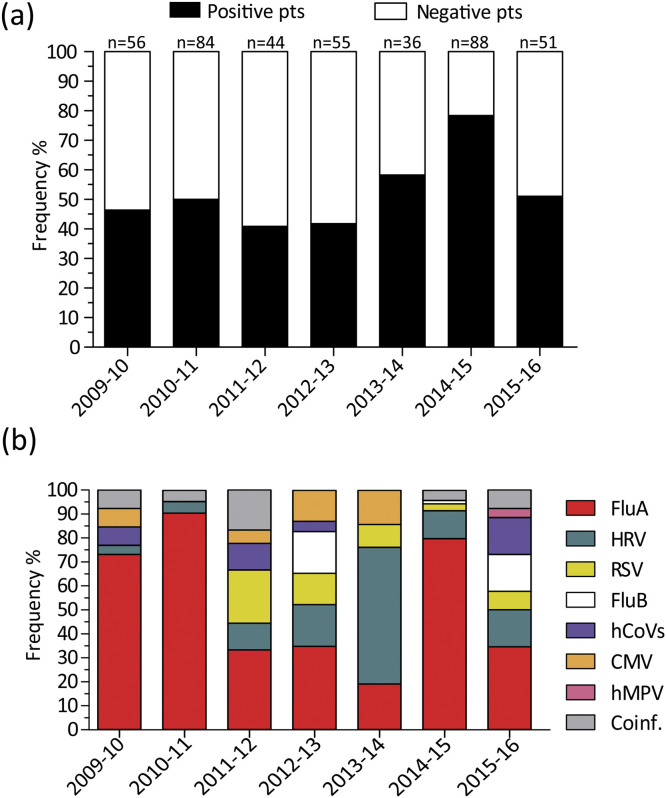
A mean of 59 CAP episodes were analyzed yearly with a minimum of 36 episodes in the 2013–14 season and a maximum of 88 episodes in the 2014–2015 season. In 226/414 (54.6%) patients one or more respiratory viruses were detected, while 188/414 (45.4%) patients resulted negative. The mean positive frequency was 52.4% with a broad difference between seasons (Fig. 1 a). Indeed, the highest positive frequency (78.4%) was observed in the 2014-15 season, while the lowest (40.9%) was observed in the 2011–12 season (Fig. 1a).
Fig. 1.
(a) Seasonal distribution of virus-positive and virus-negative episodes of CAP in ICU-admitted patients. The total number of CAP episodes analyzed yearly are reported above each column. (b) Seasonal distribution of respiratory virus detection.
A single virus infection was observed in 214/226 (94.7%) patients, while in 12/226 (5.3%) at least two respiratory viruses were identified. Overall, influenza A was the most common virus identified (140/226 patients, 61.9%) followed by HRV (33/226, 14.6%), hRSV (13/226, 5.8%), influenza B virus (9/226, 4.0%), hCoVs (9/226, 4.0%), CMV (9/226, 4.0%) and human metapneumovirus (hMPV) (1/226, 0.4%). No hPIVs were detected during the study period. In mixed infections (n = 12), HRV was the most frequently detected virus (7/12, 58.3%) followed by hCoV (6/12, 50.0%), influenza A virus (5/12, 41.7%), hRSV (3/12, 25.0%), CMV (3/12, 25.0%) and influenza B virus (2/12, 16.7%) (data not shown). Influenza A virus accounted for at least 70.0% of virus-positive infections in three seasons (2009–10, 2010–11 and 2014–15) and in another three (2011–12, 2012–13 and 2015–16) seasons it was the most frequently detected virus (33.3–34.8%) (Fig. 1b). In one season (2013–14), HRV was the most frequently detected virus (57.1% Fig. 1b).
In pediatric patients (n = 39; 9.4% of total), 19/39 (48.7) had a viral infection, while 20/39 (51.3%) were virus-negative. In detail, in 11/19 (57.9%) positive patients HRV was detected, in 5/19 (26.3) influenza A was detected, in 2/19 (10.5%) hRSV and in 1/19 (5.3%) influenza B. Among virus-positive CAP episodes (n = 19), 14/19 (73.7%) positive samples were detected in URT samples, while 5/19 (26.3%) were detected in LRT samples. A similar distribution of samples was observed in the virus-negative pediatric group, where 14/20 (70.0%) were URT samples, and 6/20 (30.0) were LRT samples.
The proportion of infections detected in the URT as compared with the LRT samples, for respiratory viruses with at least 10 CAP episodes (influenza A, HRV and hRSV) is reported in Table 2 . hRSV was detected in LRT samples from 8/13 (61.5%) patients compared with 62/140 (44.3%) positive for influenza A and 14/33 (42.4%) positive for HRV. However, due to the low number of hRSV episodes no significant differences were observed between the observed frequencies (p > 0.05).
Table 2.
Distribution of influenza A, HRV and hRSV infections according to sample positivity (URT vs LRT).
| Virus | All seasons | 2009–10 | 2010–11 | 2011–12 | 2012–13 | 2013–14 | 2014–15 | 2015–16 | |
|---|---|---|---|---|---|---|---|---|---|
| Influenza A (n = 140) |
URT | 78 (55.7) | 12 (63.2) | 18 (47.4) | 3 (50.0) | 2 (25.0) | 2 (50.0) | 37 (67.3) | 4 (40.0) |
| LRT | 62 (44.3) | 7 (36.8) | 20 (52.6) | 3 (50.0) | 6 (75.0) | 2 (50.0) | 18 (32.7) | 6 (60.0) | |
| HRV (n = 33) |
URT | 19 (57.6) | 1 | 1(50.0) | 2 (100.0) | 2 (50.0) | 10 (83.3) | 2 (25.0) | 1 (25.0) |
| LRT | 14 (42.4) | 0 (0.0) | 1(50.0) | 0 (0.0) | 2 (50.0) | 2 (16.7) | 6 (75.0) | 3 (75.0) | |
| hRSV (n = 13) |
URT | 5 (38.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (66.7) | 2 (100.0) | 1 (50.0) | 0 (0.0) |
| LRT | 8 (61.5) | 0 (0.0) | 0(0.0) | 4(100.0) | 1 (33.3) | 0 (0.0) | 1 (50.0) | 2 (100.0) | |
URT, upper respiratory tract; LRT, lower respiratory tract; HRV, rhinovirus; hRSV, human respiratory syncytial virus.
A total of 37/414 (8.9%) patients were treated with extracorporeal membrane oxygenation (ECMO). Of these, 22/37 (64.7%) were positive for influenza A, 1/37 (2.7%) for hCoV, 1/37 (2.7%) for HRV, 1/37 (2.7%) for CMV, while 12/37 (32.4%) were virus-negative.
Regarding the nine reported CMV infection episodes, all except one patient had pre-existing co-morbidities or risk factors. In three cases, CMV was detected with at least one respiratory virus (1 hRSV, 1 HRV, and 1hCoV), while in another six cases CMV was observed as a single infection.
5. Discussion
The results of this study suggest that nearly half of patients admitted to the ICU (54.3%) with a severe respiratory syndrome are positive for at least one respiratory virus. Our data are in agreement with other reports focusing on viral etiologies of pneumonia in ICU patients [1], [3], [11], [12]. In these studies, the proportion of viral infections ranged from 23.0 to 49.0%. As recently summarized by Burk and colleagues, a high proportion of viral infections are observed in patients with CAP [13]. In our series, the frequency of different respiratory viruses was assessed in patients hospitalized in 26 ICUs distributed in the Lombardy Region (10 million inhabitants). These results underline the role of viruses in severe respiratory distress for all age groups. In fact, since adequate therapy for influenza is available, early diagnosis and early initiation of anti-viral therapy may improve outcomes in adult and pediatric patients [14], [15].
The use of multiplex molecular assays for the diagnosis of respiratory viruses in ICU patients has increased the proportion of viral infections detected [2], [16]. However, a major weakness of recent studies concerning CAP is that assessments are often performed using only URT samples. [13]. In fact, the use of URT samples for the diagnosis of CAP is controversial [3], [17]. Indeed, in studies that have analyzed paired URT and LRT samples, URT samples often resulted negative while positive results were observed in LRT samples [3], [9], [18], [19]. In addition, studies with >50% LRT samples, showed a higher (44.2%) proportion of viral infections as compared with studies analyzing the etiology of CAP using URT samples only (23.5%) [13]. The high proportion of viral infections described here could indeed be related to the greater frequency (45%) of LRT samples available for the diagnosis as compared to other studies (10–20%) [13]. The importance of LRT testing is also highlighted by a recent study involving ICU patients in whom URT testing failed to detect over 40% of cases of LRT-confirmed influenza [20].
As expected, influenza A was the most common virus detected in ICU-patients [11], [17]. On the other hand, it was somewhat surprising that the second most frequently detected virus was rhinovirus (14.6%). A growing body of evidence has associated rhinovirus with lower respiratory tract disease both in adults [1], [3], [5], [12], [16] and children [21]. Indeed, in the present study, HRV was detected in LRT samples with a frequency (57.6%) similar to that observed for influenza (55.7%) and hRSV (61.5%). This finding provides new insights into the burden and severity of HRV infections. In addition, our data highlight the need for improved assays to identify respiratory viruses other than influenza especially in high-risk patients. More in general, this study underlines the importance of using molecular assays that cover a wide range of viruses for the diagnosis of respiratory infections.
In a few patients, CMV was the only virus detected, however, the importance of CMV detection in ICU-admitted patients remains questionable, especially in the absence of histologic evidence of infection [22].
The results of this study should be interpreted within the context of its limitations. The frequency of different respiratory viruses could be biased by the role of our center as a reference laboratory for the diagnosis and confirmation of severe influenza-like illness and the retrospective nature of this study limited our ability to fully collect clinical information.
In conclusion, viral infections were present in a large proportion of ICU-admitted patients with CAP. In addition to influenza A, rhinovirus was shown to account for a significant number of viral CAP episodes. Finally, the use of LRT samples appears more informative than URT samples to correctly estimate the burden of viral infections in patients with CAP.
Competing interests
None declared.
Funding
This work was supported by the Ministero della Salute, Fondazione IRCCS Policlinico San Matteo Ricerca Corrente grant 80622.
Ethical approval
Local Ethics Committee approval was not required as the study was conducted under the auspices of the Influenza Regional Surveillance and Preparedness Plan (DGR IX/1046, 22 Dec. 2010 and DGR 5988, 30 Jun 2011). All investigations were carried out following the rules of the Declaration of Helsinki (http://www.wma.net/en/30publications/10policies/b3/) revised in 2013.
Acknowledgment
We thank Daniela Sartori for careful preparation of the manuscript and Laurene Kelly for revision of the English.
References
- 1.Wiemken T., Peyrani P., Bryant K., Kelley R.R., Summersgill J., Arnold F. Incidence of respiratory viruses in patients with community-acquired pneumonia admitted to the intensive care unit: results from the Severe Influenza Pneumonia Surveillance (SIPS) project. Eur. J. Clin. Microbiol. Infect. Dis. 2013;32:705–710. doi: 10.1007/s10096-012-1802-8. [DOI] [PubMed] [Google Scholar]
- 2.Templeton K.E., Scheltinga S.A., van den Eeden W.C., Graffelman A.W., van den Broek P.J., Claas E.C. Improved diagnosis of the etiology of community-acquired pneumonia with real-time polymerase chain reaction. Clin. Infect. Dis. 2005;41:345–351. doi: 10.1086/431588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi S.H., Hong S.B., Ko G.B., Lee Y., Park H.J., Park S.Y. Viral infection in patients with severe pneumonia requiring intensive care unit admission. Am. J. Respir. Crit. Care Med. 2012;186:325–332. doi: 10.1164/rccm.201112-2240OC. [DOI] [PubMed] [Google Scholar]
- 4.Wansaula Z., Olsen S.J., Casal M., Golenko C., Erhart L.M., Kammerer P. Surveillance for severe acute respiratory infections (SARI) in Southern Arizona: 2010–2014. Influenza Other Respir. Viruses. 2015;10:161–169. doi: 10.1111/irv.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain S., Self W.H., Wunderink R.G., CDC EPIC Study Team Community-acquired pneumonia requiring hospitalization. N. Engl. J. Med. 2015;373:415–427. doi: 10.1056/NEJMc1511751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez-Padilla R., de la Rosa-Zamboni D., Ponce de Leon S., Hernandez M., Quiñones-Falconi F., Bautista E. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N. Engl. J. Med. 2009;361:680–689. doi: 10.1056/NEJMoa0904252. [DOI] [PubMed] [Google Scholar]
- 7.ANZIC Influenza Investigators, Webb S.A., Pettilä V., Seppelt I., Bellomo R., Bailey M. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N. Engl. J. Med. 2009;361:1925–1934. doi: 10.1056/NEJMoa0908481. [DOI] [PubMed] [Google Scholar]
- 8.ARDS Definition Task Force, Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 9.Piralla A., Baldanti F., Gerna G. Phylogenetic patterns of human respiratory picornavirus species, including the newly identified group C rhinoviruses, during a 1-year surveillance of a hospitalized patient population in Italy. J. Clin. Microbiol. 2011;49:373–376. doi: 10.1128/JCM.01814-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piralla A., Lunghi G., Percivalle E., Viganò C., Nasta T., Pugni L. FilmArray® respiratory panel performance in respiratory samples from neonatal care units. Diagn. Microbiol. Infect. Dis. 2014;79:183–186. doi: 10.1016/j.diagmicrobio.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cillóniz C., Ewig S., Ferrer M., Polverino E., Gabarrús A., Puig de la Bellacasa J. Community-acquired polymicrobial pneumonia in the intensive care unit: aetiology and prognosis. Crit. Care. 2011;15 doi: 10.1186/cc10444. R209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karhu J., Ala-Kokko T.I., Vuorinen T., Ohtonen P., Syrjälä H. Lower respiratory tract virus findings in mechanically ventilated patients with severe community-acquired pneumonia. Clin. Infect. Dis. 2014;59:62–70. doi: 10.1093/cid/ciu237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burk M., El-Kersh K., Saad M., Wiemken T., Ramirez J., Cavallazzi R. Viral infection in community-acquired pneumonia: a systematic review and meta-analysis. Eur. Respir. Rev. 2016;25:178–188. doi: 10.1183/16000617.0076-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith J.R., Ariano R.E., Toovey S. The use of antiviral agents for the management of severe influenza. Crit. Care Med. 2010;38:e43–e51. doi: 10.1097/CCM.0b013e3181c85229. sul. [DOI] [PubMed] [Google Scholar]
- 15.Fiore A.E., Fry A., Shay D., Gubareva L., Bresee J.S., Uyeki T.M. Centers for Disease Control and Prevention (CDC): antiviral agents for the treatment and chemoprophylaxis of influenza- recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm. Rep. 2011;60:1–24. [PubMed] [Google Scholar]
- 16.Minosse C., Selleri M., Zaniratti M.S., Cappiello G., Longo R., Schifano E. Frequency of detection of respiratory viruses in the lower respiratory tract of hospitalized adults. J. Clin. Virol. 2008;42:215–220. doi: 10.1016/j.jcv.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansson N., Kalin M., Tiveljung-Lindell A., Giske C.G., Hedlund J. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin. Infect. Dis. 2010;50:202–209. doi: 10.1086/648678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piralla A., Pariani E., Rovida F., Campanini G., Muzzi A., Emmi V. Segregation of virulent influenza A(H1N1) variants in the lower respiratory tract of critically ill patients during the 2010–2011 seasonal epidemic. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028332. e28332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baldanti F., Campanini G., Piralla A., Rovida F., Braschi A., Mojoli F. Severe outcome of influenza A/H1N1/09 v infection associated with 222G/N polymorphisms in the haemagglutinin: a multicentre study. Clin. Microbiol. Infect. 2011;17:1166–1169. doi: 10.1111/j.1469-0691.2010.03403.x. [DOI] [PubMed] [Google Scholar]
- 20.Reddy K.P., Bajwa E.K., Parker R.A., Onderdonk A.B., Walensky R.P. Relationship between upper respiratory tract influenza test result and clinical outcomes among critically ill influenza patients. Open Forum Infect. Dis. 2016;3 doi: 10.1093/ofid/ofw023. ofw023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louie J.K., Roy-Burman A., Guardia-Labar L., Boston E.J., Kiang D., Padilla T. Rhinovirus associated with severe lower respiratory tract infections in children. Pediatr. Infect. Dis. J. 2009;28:337–339. doi: 10.1097/INF.0b013e31818ffc1b. [DOI] [PubMed] [Google Scholar]
- 22.Al-Omari A., Aljamaan F., Alhazzani W., Salih S., Arabi Y. Cytomegalovirus infection in immunocompetent critically ill adults: literature review. Ann. Intensive Care. 2016;6:110. doi: 10.1186/s13613-016-0207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]