Abstract
Infectious bronchitis (IB) is a worldwide disease affecting chickens of all ages and causing important economic losses in poultry industry. Despite being one of the predominant IB virus (IBV) serotype in several European countries, slightly is known about pathogenesis and pathogenicity of Italy 02 serotype. In this study chicks and old hens were infected by oculo-nasal route with Italy 02 serotype. Clinical signs, gross and microscopic findings were evaluated, viral nucleic acid detection was assessed by in situ hybridization (ISH) in several tissues and viral RNA was detected by RT-PCR in trachea, kidney and nasal and cloacal swabs.
Italy 02 serotype was demonstrated to cause severe respiratory and renal damage in one-day old chicks but not in adult hens in which only respiratory disease and drop in egg production was observed. The use of ISH technique demonstrated the presence of viral RNA in nasal turbinates prior to trachea, but more consistent and longer replication periods in enterocytes of lower gastrointestinal tract. The detection of viral nucleic acid in gut by RT-PCR was consistent and more persistent viral shedding was detected in faeces than in nasal exudates.
We describe a complete update of IBV distribution in tissues by the use of molecular techniques and we also provide and in-depth pathological characterization of the new Italy 02 IBV serotype. Furthermore, new data about IBV pathogenesis essential in field control is afforded.
Keywords: Infectious bronchitis, Italy 02, Molecular localization, Pathogenesis
1. Introduction
Avian infectious bronchitis (IB) is an acute, highly contagious disease with severe economic consequences in poultry industry worldwide. Although IBV is mainly considered a respiratory infection, other clinical manifestations including renal, enteric and reproductive signs can also be observed and therefore, economic losses due to low performance and mortality because of secondary infections are experienced (Cavanagh and Naqi, 2003, OIE, 2004). The causative virus agent of IB (IBV) is a member of the Coronaviridae family (Cavanagh and Naqi, 2003). The virus isolates present a high antigenic and immunogenic diversity as was revealed early after its first identification (Hofstad, 1958). Since then, newly emergent isolates that differ not only in antigenic properties but also in tissue tropism and pathogenicity are continuously being reported all over the world (Gelb et al., 1991, OIE, 2004, Shaw et al., 1996, Zanella et al., 2000).
Use of molecular techniques in epidemiological surveys, particularly reverse transcription-polymerase chain reaction (RT-PCR) and gene sequencing, have widely contributed to faster identification of new emergent IBV isolates. However, these new isolates are rarely further characterized and thereby, molecular data cannot be correlated neither with antigenic nor pathogenic features of these new identified isolates, as was the case of the Italy 02 IBV serotype. Even when after its first report, this serotype turned to be one of the predominant genotypes in several European countries, including Spain (Dolz et al., 2006, Jones et al., 2005, Worthington and Jones, 2006, Worthington et al., 2004). Recently in some of these countries the number of isolations of Italy 02 strains has decreased during the last years, but it has not been the case of Spain where it still remains the predominant genotype (Dolz et al., 2009). However, despite the epidemiologic relevance acquired, very few data is available regarding in vivo pathologic traits of Italy 02 serotype strains. Italy 02 viruses have been isolated mostly from broiler flocks that experienced respiratory signs, and also from adult birds, broiler breeders and layers, associated with drop in egg production (Worthington et al., 2004). However, tissue tropism, pathogenicity and exact pathogenesis of this novel serotype remain unknown and even the pathogenic ability of this serotype is questioned.
IBV pathogenesis was deeply investigated in 1970s and 1980s, most of these studies relying on the use of virus isolation, immunofluorescence and immunohistochemical techniques (Albassam et al., 1986, Alexander and Gough, 1978, Cook, 1968, Cook, 1971, Crinion et al., 1971, Chen et al., 1996, Chong and Apostolov, 1982, Hofstad and Yoder, 1966, Jones and Jordan, 1970, Jones and Jordan, 1971, McMartin, 1968, Nakamura et al., 1991, Owen et al., 1991). With the advancement of molecular techniques, antigen detection sensibilities greatly improved. However, these techniques have been rarely implemented in IBV pathogenesis investigations despite the extensive use in epidemiological studies (Kapczynski et al., 2002, Lee et al., 2002). Therefore, the application of molecular techniques in pathogenesis studies could greatly improve the current knowledge in IBV infection and tissue distribution. The aim of the present study was to investigate the pathogenic traits of Italy 02 IBV serotype in young chickens and adult laying hens. Clinical signs, gross and microscopic lesions were evaluated and sequential antigen detection was assessed by RT-PCR and in situ hybridization in several tissues of infected birds.
2. Materials and methods
2.1. Virus
Spanish field isolate Spain/00/337, previously characterized to belong to Italy 02 serotype (Dolz et al., 2006) was used in this study. Spain/00/337 was isolated in 2000 from trachea and kidney of 44-day-old broiler chickens and it was propagated following standard procedures at 37 °C in the allantoic cavities of 11-day-old specific pathogen free (SPF) embryonated chicken eggs. The virus infectious titre was determined by inoculation of 10-fold serial dilutions in SPF embryonated eggs and measured as egg infectious doses 50% (EID50) following the Reed and Muench method (Reed and Muench, 1938, Villegas, 1998). Allantoic fluid was stored at −80 °C until use.
2.2. Birds and experimental infections
Two experimental infections were carried out: one in day-old SPF chickens and the other one in 40-week-old SPF hens. All animal experimental procedures were approved by the Ethical and Animal Welfare Committee of the Universitat Autònoma de Barcelona (UAB).
Fifty-day-old SPF chickens were obtained from SPF eggs (Charles River Spafas, Preston, US). One day-old chickens were oculo-nasally administrated with 0.1 ml of either 106.5 EID50/ml of Spain/00/337 (n = 39) or 0.1 ml of saline solution (n = 11). Both groups were housed separately in negative-pressure boxes units and clinical signs were daily recorded. On days 1, 3, 5, 7, 9, 12, 15, 18, 21, 24 and 27 post infection (PI), three infected and one control animals were randomly selected and euthanized to evaluate macroscopic lesions. Tissue samples from nasal turbinates, conjunctiva, trachea, lung, kidney, proventriculus, caecal tonsil, cloaca, liver, skin, ciatic nerve and heart were collected in 10% neutral-buffered formalin. In addition, nasal and cloacal swabs and tissue samples from proximal trachea and kidney were collected and frozen at −80 °C for RT-PCR detection. To evaluate tracheal ciliary activity, four tracheal rings from each bird were obtained and placed in 1 ml of Hank's balanced salt solution with sodium bicarbonate and antibiotics.
Twenty-five SPF adult hens (40-week-old) were obtained from SPF chicken embryonated eggs (Charles River Spafas, Preston, US) and raised in one negative-pressure box until they reached the laying period. When constant laying was achieved, hens were divided into two groups of 4 (non-infected group) and 21 (infected group) birds housed separately in negative-pressure box units. At 40 weeks of age, hens were oculo-nasally administrated with 0.1 ml of either 106.5 EID50/ml Spain/00/337 or 0.1 ml of saline solution. Clinical signs were daily recorded. On days 2, 4, 6, 8, 11, 15 and 19 PI, three hens from the infected group were euthanized. Control birds were euthanized on days 2, 6, 11 and 19 PI. Prior to sacrifice, nasal and cloacal swabs were collected from birds. At necropsy, the same samples as in experiment of day-old SPF chicks were collected. Additionally, magnum and ovary samples were placed in 10% neutral-buffered formalin.
2.3. Gross pathology and histopathology
All birds which died or were sacrificed were examined for the presence of gross lesions. Sections of the tissues mentioned above were fixed in 10% neutral-buffered formalin, processed by the conventional paraffin embedding procedure for haematoxylin and eosin staining and examined under light microscope. Microscopic lesions in respiratory tissues were classified into three stages, following the classification described by Nakamura and colleagues (Nakamura et al., 1991): degenerative stage, hyperplastic stage and recovery stage.
2.4. Ciliostasis
Ciliary activity was measured in four tracheal rings for each bird. For each ring, ciliary activity was assessed by a semi-quantitative scoring (0–4 indicating ciliostasis, ciliary activity in one quarter of the ring, two quarters of the ring, three quarters of the ring or complete ciliary activity; respectively).
2.5. RNA extraction and RT-PCR
Viral RT-PCR detection was assessed from nasal and cloacal swabs and tracheal and renal tissues, from both, day-old chicks and adult hens. Viral RNA was extracted from 30 mg of tissue or 150 μl of liquid suspensions. Swabs were placed in 1 ml of Hank's balanced salt solution with sodium bicarbonate and antibiotics and 150 μl of liquid suspension was used to extract viral RNA using a modified protocol from a commercially available kit (Nucleospin RNA virus; Macherey-Nagel, Düren, Germany) as previously described (Dolz et al., 2005). The amplification reaction was performed as described by Adzhar et al. (1996) using a universal pair of primers, UTR1− and UTR2+, designed to amplify a fragment of the 3′ untranslated region of IBV. Amplified RT-PCR products were analyzed by electrophoresis on 2% agarose gels and stained with ethidium bromide. Negative controls were included in both, extraction and amplification steps, each 4 samples to detect potential cross contamination. Also, one positive sample and one positive extraction were included in all RNA extractions and RT-PCRs respectively.
2.6. In situ hybridization (ISH)
The same RT-PCR carried out to detect viral RNA from samples, was used to synthesize a digoxigenin (DIG)-labeled riboprobe which hybridized with the 3′ unstranslated region of Spain/00/337 genome by using the PCR DIG Probe Synthesis kit (Roche, Penzberg, Germany) following manufacturer instructions. The commercial kit contains all reagents for the direct DIG-labeling of DNA fragments generated by PCR process. Briefly, once cDNA was synthesized it was used to synthesize the DIG-labeled probe. Amplification was developed in a 50 μl reaction containing 0.24 μM of each mentioned primer (UTR1− and UTR2+), 5 μl of 10× PCR buffer with MgCl2, 2.62 U of enzyme mix, 5 μl of PCR DIG labeling mix, 33.05 μl of diethylpyrocarbonate (DEPC)-treated water and 5 μl of synthesized cDNA. All amplification reaction reagents with the exception of primers and DEPC-treated water RNAse free were supplied with the PCR DIG Probe Synthesis kit. Labeled products were analyzed in 2% agarose gels stained with ethidium bromide and purified by QIAquick PCR Purification Kit (Qiagen Inc., Valencia, CA) following manufacturer's instructions. To determine the optimum concentration of probe to use in ISH, several dilutions from 1:50 to 1:200 were tested for each batch of labeled probe.
In situ hybridization was carried out in nasal turbinates, proximal and distal trachea, lung, kidney, proventriculus, duodenum, caecal tonsil, cloaca and bursal tissues as previously described (Rosell et al., 1999). In laying hens, ISH was also performed in magnum and ovary tissues. All solutions and buffers were prepared in DEPC-treated water and all labware was rinsed with RNase AWAY® (Invitrogen Ltd., Paisley, UK) solution to be free of RNAse activity. Hybridization with the probe was performed overnight at 37 °C. Colour was developed by the addition of a NBT/BCIP chromogen solution commercially available (NBT/BCIP Stock Solution, Roche, Mannheim, Germany) for 20 min at 37 °C. Positive and negative controls were included in each ISH. In the same way, all tissues were tested without probe to discard potential non-probe specific staining.
3. Results
3.1. Clinical signs
In one day-old SPF chicks, clinical signs were first observed at day 3 PI, starting with cough. At day 5 PI most of the chicks were affected showing depression, ruffled feathers, nasal discharge, coughing, gasping and conjunctivitis. These signs were noted in most of the birds between 5 and 15 days PI, but thereafter only some birds still showed mild respiratory signs and ultimately all signs disappeared at day 18 PI. A total of 6 birds died during the infection period, 1 at 5 days PI, 2 at 12 days PI and 3 at day 15 PI. No clinical signs or dead birds were recorded in the non-infected control group.
For 40-week-old SPF hens, some of them showed nasal discharge at days 2 and 4 PI. From 6 to 11 days PI most of the hens showed clinical signs including conjunctivitis, nasal discharge, tracheal rales and coughing. Only one hen still showed respiratory signs at day 15 PI and clinical signs disappeared up to the end of the experiment. Non infected control hens did not show clinical signs. At the beginning of the experimental period hens lay consistent with an average daily production of 80% approximately. In infected-hens group, egg production declined up to 21% at day 11 PI. Fifteen days PI, egg production increased to 40% and at day 19 PI it was recovered up to 75%. Egg production in non infected hens group was maintained consistent around 80%.
3.2. Gross pathology and tracheal ciliostasis
Gross lesions in day-old chicks were mostly observed in upper respiratory tract and kidney. Slight congestion and presence of catarrhal exudate at nasal turbinates and trachea was observed at day 3 PI. From day 5 to 15 PI, gross lesions in the upper respiratory tract progressed and become more severe with mild to moderate mucosal congestion and thickening and intense catarrhal exudate. No gross lesions were observed from day 18 PI up to the end of the experiment. Total ciliostasis was observed in two out of the three infected birds at day 3 PI and in all killed infected birds until 21 days PI. Twenty-four days PI, partial recovery of ciliary activity in infected birds was observed and progressed to total recovery at day 27 PI. First renal gross lesions were observed at day 5 PI and consisted of slight enlargement and paleness in two out of the three birds. From day 5 to 15 PI kidneys from almost all infected birds necropsied were moderately enlarged and swollen. Multifocal pale areas and distended ureters with urates were also frequently observed. Only slight paleness was observed in two out of the three infected birds killed at day 18 PI and no gross lesions were observed up to the end of the experiment. No gross lesions were observed in non-infected chicks and ciliary activity was maximum in this group during all experiment.
In adult hens gross lesions were restricted to the upper respiratory tract. At day 2 PI, all birds showed mild mucosal congestion of nasal turbinates with presence of catarrhal exudate. Two out of three birds also showed a very small amount of catarrhal exudate in the trachea with slight mucosal hyperaemia. From 4 to 8 days PI higher amounts of catarrhal exudate and mucosal hyperemia were observed in nasal turbinates and trachea. Between 11 and 15 days PI nasal cavity was totally normal and slight lesions were observed in trachea with presence of small amounts of serous or catarrhal exudates. No gross lesions in the trachea were observed at day 19 PI. Ciliary activity was completely lost at day 4 PI. Partial recovery of ciliary activity was observed between 8 and 15 days PI and it was totally recovered 19 days PI.
3.3. Histopathological findings
In day-old SPF chicks microscopical lesions were observed in nasal turbinates (Fig. 1A.1) and trachea at day 3 PI. Characteristic lesions of a degenerative stage were observed from 3 to 7 day PI in nasal turbinates and from 3 to 9 days PI in trachea. Hyperplastic stage lesions persisted up to 15 days in nasal turbinates and up to 18 days in trachea. Recovery of respiratory tissues was observed at day 18 PI in nasal turbinates and at day 21 PI in trachea. Although it was not evaluated in all birds, 7 days PI lateral nasal gland showed mild lymphocytic interstitial infiltration and necrosis of glandular epithelial cells (Fig. 1B.1). In kidney, main lesions appeared at day 5 PI, including degenerative necrotic changes in epithelial cells of collecting tubules and multifocal to diffuse interstitial infiltration of lymphocytes and few heterophils (Fig. 1C.1). From day 5 to 9 PI, degenerative and inflammatory changes were more pronounced and frequently cellular debris was observed in dilated tubules. From 12 to 15 days PI, degenerative tubular changes decreased and moderate infiltration with slight to moderate fibrosis in medullary regions were present. From 18 to 27 days PI formation of lymphoid nodules in the tubular interstitium was observed. Occasionally, necrosis of isolated tubular cells and cellular debris in tubular lumina were still present associated with slight lymphocytic infiltrates in the interstitium. However, regenerated tubular cells with basophilic cytoplasm were frequently observed during this period, and most tubules had recovered to the end of the experiment. No significant lesions were observed in any of the other tissues evaluated or in uninfected birds.
Fig. 1.
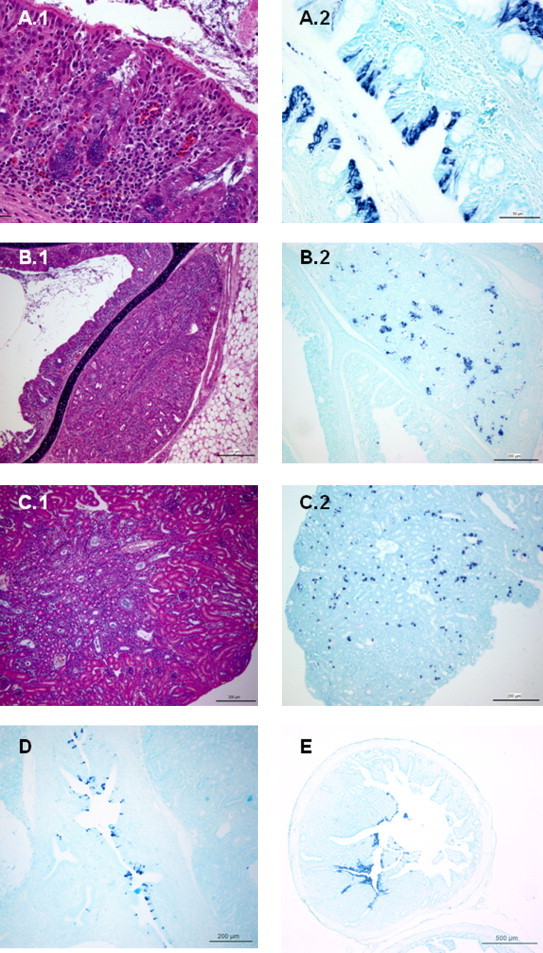
One-day old chickens infected with IBV Italy 02 serotype. (A) Nasal turbinates, day 1 PI. A.1: diffuse moderate lymphocytic infiltration and congestion in lamina propia of nasal turbinates (H/E; 50 μm); A.2: intense and extensive IBV genome specific staining of the cytoplasm of epithelial cells of nasal turbinates (ISH; 50 μm). (B) Lateral nasal gland, day 7 PI. B.1: mild lymphocytic infiltration and glandular cells necrosis (H/E; 200 μm); B.2: numerous IBV genome specific stained-cells are observed within the inflammed gland (ISH; 200 μm). (C) Kidney, day 5 PI. C.1: focal interstitial lymphocytic infiltration is observed associated to tubular renal damage and urate crystals within tubules (H/E; 200 μm); C.2: multifocal staining of tubular epithelial cells (ISH; 200 μm). (D) Ureter, day 5 PI. Numerous epithelial cells with IBV genome specific staining (ISH; 200 μm). (E) Caecal tonsil, day 5 PI. Numerous enterocytes showing strong IBV genome specific staining (ISH; 500 μm).
In case of adult hens, 2 days PI clear degenerative changes were observed in trachea (Fig. 2A.1), whereas only moderate to severe lymphocytic infiltrate in lamina propia was observed in nasal turbinates. Degenerative stage was observed in nasal turbinates and trachea from 4 to 6 days PI. Hyperplastic changes started at day 6 PI in trachea and at 8 days PI in nasal turbinates. Between 11 and 15 days PI signs of recovery were observed in trachea, whereas from 15 days PI started recovery stage in nasal turbinates. Mild renal lesions were observed in 40-week-old SPF hens. From 6 and 8 PI some birds showed mild multifocal interstitial infiltration of lymphocytes and plasma cells surrounding renal tubules. At day 11 PI, interstitial infiltration was more pronounced (Fig. 2B) and one bird showed degeneration and necrosis of epithelial cells of collecting tubules. Occasionally, debris of sloughed cells was observed in the lumina of affected tubules. At days 15 and 19 PI, no tubular alterations were observed, but moderate interstitial lymphocytic infiltration was observed in most of infected birds necropsied. No significant microscopical changes were detected in ovary and oviduct examined up to 6 days PI. At 8, 11 and 15 days PI multifocal moderate infiltration with lymphocytes and plasma cells and slight to moderate edema in the lamina propia of oviduct was observed in some infected hens. At day 19 PI, one bird showed degeneration, necrosis and sloughing of epithelial cells of magnum (Fig. 2D.1). Cellular debris and desquamated cells were present in the lumen of oviduct. Significant lesions were observed neither in any of the other tissues nor in control birds evaluated.
Fig. 2.
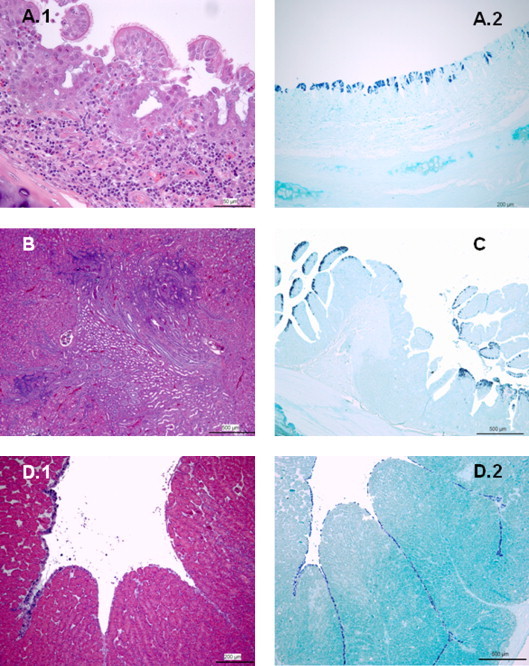
Adult hens infected with IBV Italy 02 serotype. (A) Trachea, day 2 PI. A.1: severe diffuse lymphocytic infiltration and hyperemia of lamina propia; note loss of glandular structures, mild hyperplasia and desquamation of ciliated epithelial cells lining tracheal mucosa (H/E; 50 μm); A.2: numerous ciliated epithelial cells of tracheal mucosa showed strong IBV genome specific staining in the cytoplasm (ISH; 200 μm). (B) Kidney, day 11 PI. Mild multifocal lymphocytic infiltration is observed in renal interstitium (H/E; 500 μm). (C) Rectum, day 2 PI. Numerous strong positive-stained enterocytes were observed in the rectal mucosa (ISH; 500 μm). (D) Oviduct, day 19 PI. D.1: desquamation and degenerative changes are observed in epithelial cells lining glandular tissue (H/E; 200 μm); D.2: extensive IBV genome specific staining of epithelial cells is shown (ISH; 500 μm).
3.4. Viral nucleic acid tissue distribution by in situ hybridization
Results related to IBV detection by ISH in day-old chicks are summarized in Table 1 . In all tissues where viral genome was detected by ISH, positive staining was restricted to the cytoplasm of the infected cells. Viral genome was detected by ISH in epithelial cells of nasal turbinates, trachea, lung, kidney, caecal tonsil, cloaca and bursa of Fabricius of day-old chicks. IBV genome was detected in upper respiratory tract from 1 to 12 days PI. At day 1 PI extensive replication was observed in nasal turbinates of all three infected birds (Fig. 1A.2), whereas it was only detected in tracheal epithelium of one bird. In addition to ciliated epithelial cells, IBV nucleic acid was also detected 7 days PI in glandular cells of lateral nasal glands (Fig. 1B.2). Only two birds showed focal positive staining in epithelial cells of the principal bronchi in the lung, at day 9 PI. IBV specific genome staining was detected by ISH in tubular renal epithelial cells (Fig. 1C.2) and epithelial cells of ureters (Fig. 1D) from day 5 to 15 PI in day-old SPF chicks. When nucleic acid presence was evaluated in gut, this was detected neither in proventriculus nor duodenum at any time. However, massive replication was identified in enterocytes of caecal tonsil (Fig. 1E) and rectum. In these locations, virus-specific staining was demonstrated as early as day 3 PI and up to 24 days PI. Extensive staining of epithelial cells lining the mucosa of caecal tonsil and rectum was observed between 3 and 9 days PI in all infected chicks examined. The number of genome-positive cells and intensity of staining and also the number of chicks positive decreased between 12 and 18 days PI in both tissues. From 18 to 27 days PI, scattered positive enterocytes were still observed in rectum, whereas in caecal tonsil no staining was observed at day 27 PI. Scattered positive cells were observed in the epithelium of bursa of Fabricius from 3 to 7 days PI.
Table 1.
Detection by RT-PCR and ISH of Italy 02 virus in chickens infected at one day of age. Results are expressed as the number of positive birds per total of birds tested. In bold letters is represented the last positive result obtained. ND = Not done. NA = Not available.
| Tissues |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RT-PCR |
ISH |
||||||||||||
| Trachea | Nasal swab | Kidney | Cloacal swab | Nasal turbinate | Trachea | Lung | Kidney | Proventriculus | Duodenum | Caecal tonsil | Rectum | Bursa Fabricius | |
| 1 day PI | 3/3 | NA | 1/3 | 0/3 | 3/3 | 1/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 |
| 3 days PI | 3/3 | NA | 3/3 | 3/3 | 3/3 | 3/3 | 0/3 | 0/3 | 0/3 | 0/3 | 1/3 | 3/3 | 2/3 |
| 5 days PI | 3/3 | 2/3 | 3/3 | 3/3 | 3/3 | 2/3 | 0/3 | 3/3 | 0/3 | 0/3 | 2/2 | 3/3 | 1/3 |
| 7 days PI | 3/3 | 2/3 | 3/3 | 2/3 | 3/3 | 3/3 | 0/3 | 1/3 | 0/3 | 0/3 | 3/3 | 3/3 | 1/3 |
| 9 days PI | 3/3 | 2/3 | 3/3 | 3/3 | 2/3 | 3/3 | 1/3 | 3/3 | 0/3 | 0/3 | 3/3 | 3/3 | 0/3 |
| 12 days PI | 3/3 | 1/3 | 2/3 | 3/3 | 1/3 | 1/3 | 0/3 | 2/3 | 0/3 | 0/3 | 0/3 | 2/3 | 0/1 |
| 15 days PI | 3/3 | 0/3 | 3/3 | 3/3 | 0/3 | 0/3 | 0/3 | 3/3 | 0/3 | 0/3 | 1/3 | 3/3 | 0/3 |
| 18 days PI | 2/3 | 0/3 | 0/3 | 2/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 1/2 | 2/3 | 0/3 |
| 21 days PI | 3/3 | 0/3 | 1/3 | 1/3 | ND | ND | ND | ND | 0/3 | 0/3 | 2/3 | 2/3 | 0/3 |
| 24 days PI | 0/3 | 0/3 | 0/3 | 3/3 | ND | ND | ND | ND | 0/3 | 0/3 | 2/2 | 3/3 | 0/3 |
| 27 days PI | 0/3 | 1/3 | 0/3 | 2/3 | ND | ND | ND | ND | 0/3 | 0/3 | 0/3 | 1/3 | 0/3 |
Data concerning IBV detection by ISH in 40-week-old SPF hens are summarized in Table 2 . In all the tissues where viral nucleic acid was detected by ISH, positive staining was restricted to the cytoplasm of the infected cell. IBV nucleic acid was detected in regions of upper respiratory tract at different times post infection. Whereas in nasal turbinates it was detected from 2 to 8 days PI, shorter detection was shown in trachea, from 2 to 6 days PI (Fig. 2A.2). In addition, no positive staining was observed in lung tissue. Similarly to day-old chicks, enterocytes lining caecal tonsil and rectum (Fig. 2C) of 40-week-old SPF hens showed extensive and persistent specific-positive staining. Intense and massive positive staining was observed in both tissues as early as 2 days PI. Both tissues were positive in the same degree and in all infected hens up to 6 days PI. From 8 to 11 days PI the number of positive cells slightly decreased and no staining was observed in some birds. Both tissues were positive up to the end of the experiment at day 19 PI when only a few number of enterocytes showed positive staining. No positive cells were observed in duodenum, but two birds at day 4 and 6 PI showed positive staining of few epithelial cells of proventricular mucosa. Although ovary and oviduct region were evaluated for viral genome presence at all times post-infection by ISH, only one hen at day 19 PI showed massive positive staining of epithelial cells lining of tubular glands of magnum (Fig. 2D.2). No viral genome was detected following ISH in the kidney.
Table 2.
Detection of Italy 02 virus by RT-PCR and ISH in hens infected at 40 weeks of age. Results are expressed as the number of positive birds per total of birds tested. In bold letters the last positive result obtained. ND = Not done.
| Tissues |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RT-PCR |
ISH |
|||||||||||||
| Trachea | Nasal swab | Kidney | Cloacal swab | Nasal turbinate | Trachea | Lung | Kidney | Proventriculus | Duodenum | Caecal tonsil | Rectum | Ovary | Uterus | |
| 2 days PI | 3/3 | 1/3 | 2/3 | 2/3 | 3/3 | 3/3 | 0/3 | 0/3 | 0/3 | 0/3 | 2/2 | 2/2 | 0/3 | 0/3 |
| 4 days PI | 3/3 | 1/3 | 3/3 | 3/3 | 3/3 | 3/3 | 0/3 | 0/3 | 1/3 | 0/3 | 3/3 | 3/3 | 0/3 | 0/3 |
| 6 days PI | 3/3 | 1/3 | 2/3 | 3/3 | 3/3 | 3/3 | 0/3 | 0/3 | 1/3 | 0/3 | 3/3 | 3/3 | 0/3 | 0/3 |
| 8 days PI | 3/3 | 1/3 | 2/3 | 3/3 | 1/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 1/3 | 2/3 | 0/3 | 0/3 |
| 11 days PI | 2/3 | 0/3 | 2/3 | 3/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 2/3 | 1/3 | 0/3 | 0/3 |
| 15 days PI | 1/3 | 0/3 | 2/3 | 3/3 | ND | ND | ND | ND | 0/3 | 0/3 | 2/2 | 3/3 | 0/3 | 0/3 |
| 19 days PI | 1/2 | 0/2 | 1/2 | 1/2 | ND | ND | ND | ND | 0/2 | 0/3 | 1/2 | 1/2 | 0/2 | 1/2 |
3.5. Viral nucleic acid tissue distribution and shedding by RT-PCR
Results of viral RNA detection by RT-PCR in trachea and kidney tissues and in tracheal and cloacal swabs from day-old chicks and SPF adult hens are summarized in Table 1, Table 2. All control birds were tested and no viral RNA was detected in any of them.
4. Discussion
IBV pathogenesis has been extensively studied by both virus isolation and immunohistochemistry techniques (Alexander and Gough, 1977, Benyeda et al., 2010, Crinion and Hofstad, 1972, Chen et al., 1996, Chong and Apostolov, 1982, Hofstad and Yoder, 1966, Jones and Jordan, 1972, Owen et al., 1991). In this study, an ISH technique based on a digoxigenin-labeled riboprobe synthesized by direct labeling of a PCR product, has been successfully used to detect Italy 02 serotype of IBV in formalin fixed tissues. This method provides a fast system to synthesize own probes regardless of the strain and therefore, it can be rapidly applied to any new emergent IBV strain.
Although pathogenesis of IBV in respiratory tract is well documented, the majority of studies have been focused on trachea and lungs, but few is known about nasal turbinates, which have been demonstrated to be highly affected in other avian viral respiratory infections, such as turkey rhinotracheitis (Nakamura et al., 1991, Purcell and McFerran, 1972). The present study demonstrates widespread virus nucleic acid detection in ciliated epithelial cells of nasal turbinates, associated to severe microscopic lesions. Moreover, Italy 02 serotype of IBV detection in nasal turbinates was not only limited to epithelial cells but was also detected in glandular cells of the lateral nasal glands, inducing severe degenerative and inflammatory changes in these glands. These findings demonstrate that infection with this particular IBV serotype causes, not only tracheitis, but also severe rhinitis and adenitis of nasal glands which, apart from inducing ciliostasis, it is likely to compromise the first defensive barrier of respiratory tract and facilitate secondary infections.
It has already been demonstrated that the ability of some IBV strains to replicate in kidney and cause renal damage varies among strains (Albassam et al., 1986, Ignjatovic et al., 2002, Lee et al., 2004). Our data indicate that Italy 02 virus is able to cause gross and microscopic lesions in kidney of day-old chicks. Furthermore, viral nucleic acid was detected in epithelial tubular renal cells and ureters by ISH, which correlated with the detection of IBV genome by RT-PCR. Thereby, there is no doubt that Italy 02 can induce renal disease in young chickens. However, contradictory results were obtained regarding virus detection in kidney of adult hens: slight microscopic lesions were observed and IBV genome was consistently detected by RT-PCR, but no evidences of viral detection by ISH was observed. Although it is possible that RT-PCR might detect virus nucleic acid that was not actively replicating in renal tissue but was circulating in bloodstream, viraemia periods reported for IBV are mostly short and thereby it would be inconsistent with such a persistent detection (Chong and Apostolov, 1982, Raj and Jones, 1997). On the other hand, some controversial findings, as isolation of IBV from kidney tissue in absence of renal disease, lesions or viral replication evidenced by other techniques, have also been previously reported in adult birds, by other authors (Ignjatovic et al., 2002, Lee et al., 2004). These different findings could be due to IBV strain differences.
Most enveloped viruses do not have the ability to infect the gastrointestinal (GI) tract because viral envelopes are susceptible to dissociation by enzymes, bile salts and pH values present in GI. However, coronaviruses are enveloped viruses which can withstand the harsh conditions in the GI tract (Flint et al., 2000). In the present study we have confirmed this statement, by detecting IBV in enterocytes from caecal tonsil, ileum and rectum. Despite very extensive virus replication was evidenced in caecal tonsil, ileum and rectum, it could not be confirmed by ISH in duodenum or proventriculus. These observations are supported by other studies in which; although IBV was isolated from proventriculus and duodenum, replication was not observed in these regions (Raj and Jones, 1996). Both, changes in cell receptors and adverse luminal content conditions, can alter virus infectivity and replication extent within GI tract regions. Moreover, it is well established that in birds, the lower intestine moves uric acid into proximal regions of the colon and caeca through retrograde peristalsis (Laverty et al., 2006). Our results showed that IBV genome can be evidenced in kidney as early as day 1 PI, and we detected positivity by ISH, not only in epithelial tubular cells, but also in epithelial cells of ureters. Thus, IBV is likely to be shed in uric acid, which on the other hand, could reach upper gut regions by retroperistaltic movement. It is therefore possible that this physiological feature may contribute to the successful and persistent establishment of IBV replication in lower GI tract regions.
Aerosols are considered the main route of horizontal transmission of IBV within an infected flock and respiratory droplets are assumed as the main source of virus. However, virus has been evidenced to be shed in faeces by virus isolation. Indeed, transmission via contaminated faecal material is considered to occur during recovery stages of the disease (Alexander and Gough, 1977, Alexander and Gough, 1978, Jones and Ambali, 1987). In the present study, both routes of viral shedding were investigated by RT-PCR detection in nasal and cloacal swabs in day-old chicks and adult SPF hens and surprisingly, virus shedding in faeces was detected more consistently and for longer periods (up to the end of the experiment) than in nasal discharge. These observations have clear practical consequences in both disease diagnosis and control. On one hand, based in our results, cloacal swab samples would improve virus detection and therefore rapid diagnosis of the disease even in the acute stage of the disease. On the other hand, our results indicate that faeces are likely to be a major source of virus for susceptible birds and would greatly contribute to IBV transmission among animals and probably also among farms. Although IBV is considered to be poor resistant to environmental conditions, our findings highlight the importance of good disinfection and cleaning practices in effective control of the disease, in addition of control people movements among farms even when no clinical signs are observed.
In the present study, infected adult hens showed a decline in egg production soon after experiencing respiratory signs, but no alterations of outer or inner egg quality were macroscopically observed. Interestingly, microscopic changes, mostly consistent on inflammatory infiltration in the oviduct, were observed in not all but some hens from 8 days PI up to the end of the experiment. More surprisingly, one hen at the end of the experiment showed clear degenerative changes of epithelial cells lining glandular tissue on magnum which were associated to widespread viral nucleic acid detection in epithelial cells evidenced by ISH. Thus, it is likely that Italy 02 can barely replicate in the oviduct of adult hens. Our results are in concordance with those reported previously suggesting that the alteration in the oviduct may contribute to diminished egg production (Cavanagh, 2003). Taking into account that viral replication periods reported for IBV in oviduct reach no further than 9 days PI (Jones and Jordan, 1971), it seems unlikely that IBV would have not replicated in epithelial cells of the oviduct prior to day 19 PI in our experiment. It appears more probably that IBV had been replicating, in some birds, at lower rates in the oviduct, which would be supported by inflammatory microscopic changes found in those birds. Severity of reproductive disease has been demonstrated to vary greatly depending on virus strain, period of lay and host individual factors (Raj and Jones, 1997), this latter probably related with the late replication observed in the present study.
Current investigation reports, for the first time, the pathogenesis of the novel IBV serotype Italy 02 in both, young and adult SPF birds. The present data have considerable implications not only for a better understanding of IBV pathogenesis but also for an improvement of diagnostic and particularly control of IB.
Conflict of interest
The authors declare that they have no competing interests.
Acknowledgements
This work was funded by Centre de Recerca en Sanitat Animal (CReSA), UAB-IRTA.
References
- Adzhar A., Shaw K., Britton P., Cavanagh D. Universal oligonucleotides for the detection of infectious bronchitis virus by the polymerase chain reaction. Avian Pathol. 1996;25:817–836. doi: 10.1080/03079459608419184. [DOI] [PubMed] [Google Scholar]
- Albassam M.A., Winterfield R.W., Thacker H.L. Comparison of the nephropathogenicity of four strains of infectious bronchitis virus. Avian Dis. 1986;30:468–476. [PubMed] [Google Scholar]
- Alexander D.J., Gough R.E. Isolation of avian infectious bronchitis virus from experimentally infected chickens. Res. Vet. Sci. 1977;23:344–347. [PubMed] [Google Scholar]
- Alexander D.J., Gough R.E. A long-term study of the pathogenesis of infection of fowls with three strains of avian infectious bronchitis virus. Res. Vet. Sci. 1978;24:228–233. [PubMed] [Google Scholar]
- Benyeda Z., Szeredi L., Mató T., Süveges T., Balka G., Abonyi-Tóth Z., Rusvai M., Palya V. Comparative histopathology and immunohistochemistry of QX-like, Massachusetts and 793/B serotypes of infectious bronchitis virus infections in chickens. J. Comp. Pathol. 2010;143:276–283. doi: 10.1016/j.jcpa.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 2003;32(6):567–582. doi: 10.1080/03079450310001621198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D., Naqi S. Infectious Bronchitis. In: Saif Y.M., Barnes H.J., Glisson J.R., Fadly A.M., McDougald L.R., Swayne D.E., editors. Diseases of Poultry. Iowa State University Press; Ames: 2003. pp. 101–120. [Google Scholar]
- Chen B.Y., Hosi S., Nunoya T., Itakura C. Histopathology and immunohistochemistry of renal lesions due to infectious bronchitis virus in chicks. Avian Pathol. 1996;25:269–283. doi: 10.1080/03079459608419141. [DOI] [PubMed] [Google Scholar]
- Chong K.T., Apostolov K. The pathogenesis of nephritis in chickens induced by infectious bronchitis virus. J. Comp. Pathol. 1982;92:199–211. doi: 10.1016/0021-9975(82)90078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J.K. Duration of experimental infectious bronchitis in chickens. Res. Vet. Sci. 1968;9:506–514. [PubMed] [Google Scholar]
- Cook J.K. Recovery of infectious bronchitis virus from eggs and chicks produced by experimentally inoculated hens. J. Comp. Pathol. 1971;81:203–211. doi: 10.1016/0021-9975(71)90093-4. [DOI] [PubMed] [Google Scholar]
- Crinion R.A., Hofstad M.S. Pathogenicity of four serotypes of avian infectious bronchitis virus for the oviduct of young chickens of various ages. Avian Dis. 1972;16:351–363. [PubMed] [Google Scholar]
- Crinion R.A., Ball R.A., Hofstad M.S. Pathogenesis of oviduct lesions in immature chickens following exposure to infectious bronchitis virus at one day old. Avian Dis. 1971;15:32–41. [PubMed] [Google Scholar]
- Dolz R., Majo N., Ordonez G., Porta R. Viral genotyping of infectious bursal disease viruses isolated from the 2002 acute outbreak in Spain and comparison with previous isolates. Avian Dis. 2005;49:332–339. doi: 10.1637/7299-110204R1.1. [DOI] [PubMed] [Google Scholar]
- Dolz R., Pujols J., Ordonez G., Porta R., Majo N. Antigenic and molecular characterization of isolates of the Italy 02 infectious bronchitis virus genotype. Avian Pathol. 2006;35:77–85. doi: 10.1080/03079450600597295. [DOI] [PubMed] [Google Scholar]
- Dolz R., Bertran K., Majó N. First report of IBV QX-like strains in Spain. VI International symposium on Avian Corona- and Pneumoviruses and complicating pathogens; Rauischholzhausen, Germany; 2009. pp. 27–33. [Google Scholar]
- Flint S.J., Enquist L.W., Krug R.M., Racaniello V.R., Skalka A.M. ASM Press; Washington, D.C.: 2000. Principles of Virology: Molecular Biology, Pathogenesis, and Control. pp. 595–598. [Google Scholar]
- Gelb J., Jr., Wolff J.B., Moran C.A. Variant serotypes of infectious bronchitis virus isolated from commercial layer and broiler chickens. Avian Dis. 1991;35:82–87. [PubMed] [Google Scholar]
- Hofstad M.S. Antigenic differences among isolates of avian infectious bronchitis virus. Am. J. Vet. Res. 1958;19:740–743. [PubMed] [Google Scholar]
- Hofstad M.S., Yoder H.W., Jr. Avian infectious bronchitis – virus distribution in tissues of chicks. Avian Dis. 1966;10:230–239. [PubMed] [Google Scholar]
- Ignjatovic J., Ashton D.F., Reece R., Scott P., Hooper P. Pathogenicity of Australian strains of avian infectious bronchitis virus. J. Comp. Pathol. 2002;126:115–123. doi: 10.1053/jcpa.2001.0528. [DOI] [PubMed] [Google Scholar]
- Jones R.C., Ambali A.G. Re-excretion of an enterotropic infectious bronchitis virus by hens at point of lay after experimental infection at day old. Vet. Rec. 1987;120:617–618. doi: 10.1136/vr.120.26.617. [DOI] [PubMed] [Google Scholar]
- Jones R.C., Jordan F.T. The exposure of day-old chicks to infectious bronchitis and the subsequent development of the oviduct. Vet. Rec. 1970;87:504–505. doi: 10.1136/vr.87.17.504. [DOI] [PubMed] [Google Scholar]
- Jones R.C., Jordan F.T. The site of replication of infectious bronchitis virus in the oviduct of experimentally infected hens. Vet. Rec. 1971;89:317–318. doi: 10.1136/vr.89.11.317. [DOI] [PubMed] [Google Scholar]
- Jones R.C., Jordan F.T. Persistence of virus in the tissues and development of the oviduct in the fowl following infection at day old with infectious bronchitis virus. Res. Vet. Sci. 1972;13:52–60. [PubMed] [Google Scholar]
- Jones R.C., Worthington K.J., Gough R.E. Detection of the Italy 02 strain of infectious bronchitis virus in the UK. Vet. Rec. 2005;156:260. [PubMed] [Google Scholar]
- Kapczynski D.R., Sellers H.S., Rowland G.N., Jackwood M.W. Detection of in ovo-inoculated infectious bronchitis virus by immunohistochemistry and in situ hybridization with a riboprobe in epithelial cells of the lung and cloacal bursa. Avian Dis. 2002;46:679–685. doi: 10.1637/0005-2086(2002)046[0679:DOIOII]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Laverty G., Elbrond V.S., Árnason S.S., Skadhauge E. Epithelial structure and function in the hen lower intestine. In: Perry G.C., editor. Avian Gut Function in Health and Disease. CABI International; Oxon, UK: 2006. pp. 65–84. [Google Scholar]
- Lee C.W., Brown C., Jackwood M.W. Tissue distribution of avian infectious bronchitis virus following in ovo inoculation of chicken embryos examined by in situ hybridization with antisense digoxigenin-labeled universal riboprobe. J. Vet. Diagn. Invest. 2002;14:377–381. doi: 10.1177/104063870201400503. [DOI] [PubMed] [Google Scholar]
- Lee C.W., Brown C., Hilt D.A., Jackwood M.W. Nephropathogenesis of chickens experimentally infected with various strains of infectious bronchitis virus. J. Vet. Med. Sci. 2004;66:835–840. doi: 10.1292/jvms.66.835. [DOI] [PubMed] [Google Scholar]
- McMartin D.A. The pathogenicity of an infectious bronchitis virus for laying hens, with observations on pathogenesis. Br. Vet. J. 1968;124:576–581. doi: 10.1016/s0007-1935(17)39033-4. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Cook J.K., Otsuki K., Huggins M.B., Frazier J.A. Comparative study of respiratory lesions in two chicken lines of different susceptibility infected with infectious bronchitis virus: histology, ultrastructure and immunohistochemistry. Avian Pathol. 1991;20:241–257. doi: 10.1080/03079459108418761. [DOI] [PubMed] [Google Scholar]
- Office edi, 2004, Avian infectious bronchitis, In: OIE (Ed.) Manual of diagnostic tests and vaccines for terrestrial animals. Paris, France.
- Owen R.L., Cowen B.S., Hattel A.L., Naqi S.A., Wilson R.A. Detection of viral antigen following exposure of one-day-old chickens to the Holland 52 strain of infectious bronchitis virus. Avian Pathol. 1991;20:663–673. doi: 10.1080/03079459108418805. [DOI] [PubMed] [Google Scholar]
- Purcell D.A., McFerran J.B. The histopathology of infectious bronchitis in the domestic fowl. Res. Vet. Sci. 1972;13:116–122. [PubMed] [Google Scholar]
- Raj G.D., Jones R.C. Immunopathogenesis of infection in SPF chicks and commercial broiler chickens of a variant infectious bronchitis virus of economic importance. Avian Pathol. 1996;25:481–501. doi: 10.1080/03079459608419157. [DOI] [PubMed] [Google Scholar]
- Raj G.D., Jones R.C. Infectious bronchitis virus: immunopathogenesis of infection in the chicken. Avian Pathol. 1997;26:677–706. doi: 10.1080/03079459708419246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed L.J., Muench H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- Rosell C., Segales J., Plana-Duran J., Balasch M., Rodriguez-Arrioja G.M., Kennedy S., Allan G.M., McNeilly F., Latimer K.S., Domingo M. Pathological, immunohistochemical, and in-situ hybridization studies of natural cases of postweaning multisystemic wasting syndrome (PMWS) in pigs. J. Comp. Pathol. 1999;120:59–78. doi: 10.1053/jcpa.1998.0258. [DOI] [PubMed] [Google Scholar]
- Shaw K., Britton P., Cavanagh D. Sequence of the spike protein of the Belgian B164S isolate of nephropathogenic infectious bronchitis virus. Avian Pathol. 1996;25:607–611. doi: 10.1080/03079459608419165. [DOI] [PubMed] [Google Scholar]
- Villegas P. Titration of biological suspensions. In: Swayne D.E., Glisson J.R., Jackwood M.W., Pearson J.E., Reed W.M., editors. A laboratory Manual for the Isolation and Identification of Avian Pathogens. The American Association of Avian Pathologists; Pennsylvania: 1998. pp. 248–254. [Google Scholar]
- Worthington K.J., Jones R.C. An update of the european RT-PCR IBV survey and recent findings on a novel IBV genotype. V International Symposium on Avian Corona- and Pneumoviruses and Complicating Pathogens; Rauischholzhausen, Germany; 2006. pp. 176–188. [Google Scholar]
- Worthington K.J., Savage C., Naylor C.J., Wijmenga W., Jones R.C. An RT-PCR survey of infectious bronchitis virus genotypes in the UK and selected European countries between 2002 and 2004 and the results from a vaccine trial. Fourth International Symposium on Avian Corona- and Pneumovirus Infections; Rauischholzhausen, Germany; 2004. pp. 125–133. [Google Scholar]
- Zanella A., Coaro R., Fabris G., Marchi R., Lavazza A. Avian infectious bronchitis virus: isolation of an apparently new variant in Italy. Vet. Rec. 2000;146:191–193. doi: 10.1136/vr.146.7.191. [DOI] [PubMed] [Google Scholar]


