Abstract
Purpose of Review
Perivascular adipose tissue (PVAT) has a complex, bidirectional relationship with the vascular wall. In disease states, PVAT secretes pro-inflammatory adipocytokines which may contribute to atherosclerosis. Recent evidence demonstrates that pericoronary adipose tissue (PCAT) may also function as a sensor of coronary inflammation. This review details PVAT biology and its clinical translation to current imaging phenotyping.
Recent Findings
PCAT attenuation derived from routine coronary computed tomography (CT) angiography is a novel noninvasive imaging biomarker of coronary inflammation. Pro-inflammatory cytokines released from the arterial wall diffuse directly into the surrounding PCAT and inhibit adipocyte lipid accumulation in a paracrine manner. This can be detected as an increased PCAT CT attenuation, a metric which associates with high-risk plaque features and independently predicts cardiac mortality. There is also evidence that PCAT attenuation relates to coronary plaque progression and is modified by systemic anti-inflammatory therapies.
Summary
Due to its proximity to the coronary arteries, PCAT has emerged as an important fat depot in cardiovascular research. PCAT CT attenuation has the potential to improve cardiovascular risk stratification, and future clinical studies should examine its role in guiding targeted medical therapy.
Keywords: Perivascular adipose tissue, Atherosclerosis, Inflammation, Cardiac computed tomography angiography
Introduction
Coronary artery disease (CAD) remains the leading single cause of death worldwide [1]. Despite progress in primary and secondary prevention, a substantial risk of recurrent cardiovascular (CV) events persists [2]. Vascular inflammation is considered a key driver of atherogenesis and atherosclerotic plaque rupture resulting in acute coronary syndrome (ACS) [3]. Randomized studies demonstrate a residual inflammatory risk even after aggressive lowering of low-density lipoprotein cholesterol [4]. The recent CANTOS trial [5••] showed that targeting of interluekin-1β with the monoclonal antibody canakinumab reduced recurrent CV event rates, hence validating the inflammatory hypothesis of atherosclerosis.
There is burgeoning research interest into the detection of coronary inflammation, which has important implications for CV risk stratification and targeted medical therapy. Perivascular adipose tissue (PVAT) surrounds blood vessels and has important metabolic and vasoprotective functions. Dysfunctional PVAT secretes pro-inflammatory adipocytokines which may induce atherosclerosis—the “out-side-to-inside” theory of vascular inflammation [6]. Human epicardial coronary arteries are encased in pericoronary adipose tissue (PCAT), recently shown to undergo morphological changes in response to coronary inflammation via “inside-to-outside” signaling pathways [7••]. These changes can be characterized by noninvasive imaging with coronary computed tomography coronary angiography (CCTA).
In this review, we first discuss the anatomy and biological role of PVAT in health. We then turn to dysfunctional PVAT and its contribution to atherosclerosis, with a special focus on cardiovascular disease. Next, we summarize recent evidence for the imaging phenotyping of PCAT as a promising biomarker of coronary inflammation (see Appendix Table 2).
Adipose Tissue Structure and Function
Excess adiposity arising from the accumulation of adipose tissue (AT) is an independent risk factor for CV disease and the metabolic syndrome [8, 9]. Body mass index (BMI), the traditional measure of obesity, fails to account for regional differences in AT quality and distribution, which are key drivers of its cardiometabolic effects [10]. In humans, AT serves as the main site for energy storage and is located throughout the body in distinct subcutaneous and visceral depots, with the latter being more strongly associated with an adverse metabolic risk profile [11]. AT is comprised of adipocytes, macrophages, fibroblasts, nerve tissue, stromal vascular cells, and pre-adipocytes at various stages of development [12]. Adipocyte differentiation occurs in distinct stages under the regulation of adipogenic proteins, during which pre-adipocytes accumulate intracellular lipid droplets and enlarge to become mature adipocytes [13].
Role of Perivascular Adipose Tissue in Health
PVAT is defined as the AT surrounding blood vessels, including large arteries and veins, organ-specific vasculature, and skeletal muscle microvessels [6]. In large vessels, PVAT is contiguous with the adventitial layer of the vascular wall without a dividing fascial plane, whereas in small vessels, perivascular adipocytes are integrated into the vascular wall itself [14]. Historically, PVAT was perceived as scaffolding for blood vessels; however, ample research has shown it to be a metabolically active endocrine organ which modulates vascular function. Adipocytes express and secrete a wide range of bioactive molecules, known as adipokines, which can act in a paracrine or vasocrine manner [15]. Given its anatomical proximity to the vessel wall, PVAT may have more immediate and direct effects on the underlying vasculature compared with distant AT depots which can only act via a circulating pool of messengers.
In the healthy state, PVAT secretes vasoprotective adipokines (e.g., adiponectin and omentin-1) which promote vasodilatation and exert anti-inflammatory, anti-fibrotic, and anti-oxidant effects. Macrophages and T-lymphocytes residing in PVAT can also release classical implicated cytokines, including interleukin (IL)-6, tumor necrosis factor (TNF)-α, monocyte chemoattractant protein-1 (MCP-1), and plasminogen activator inhibitor-1 (PAI-1) [16, 17]. The immune cells actively partner with adipocytes to maintain the balance of cytokines and regulate inflammatory responses to external stimuli.
Dysfunctional PVAT in Obesity
When exposed to chronic caloric excess, PVAT undergoes expansion and pathological remodeling. There is hypertrophy of existing adipocytes and hyperplasia of pre-adipocytes [18], with outstripping of the vascular supply leading to hypoxia, adipocyte dysfunction, and apoptosis [19]. This is accompanied by a shift in the secretory profile and cellular composition of PVAT to an inflammatory phenotype. Adipocytes downregulate release of vasoprotective adipokines and upregulate release of pro-inflammatory adipokines (e.g., leptin, resistin, and visfatin) [10, 14, 20, 21]. Adipocyte hypertrophy also stimulates secretion of MCP-1 which promotes macrophage recruitment [22, 23]; in obesity, these M1 or “classically activated” macrophages have enhanced secretion of the pro-inflammatory cytokines TNF-α, IL-6, and IL-8 [24]. The subsequent infiltration of PVAT by immune cells, activation of inflammatory signaling pathways, and release of reactive oxygen species contribute to the chronic low-grade systemic inflammation associated with obesity [25].
Response of PVAT to Vascular Disease
Vascular inflammation has long been believed to follow an “inside-to-outside” model, in which intimal injury leads to expression of vascular adhesion molecules, release of inflammatory signals, and migration of immune cells to the endothelium [3]. This intimal inflammation then spreads into the media and adventitia [26]. In a murine model of PVAT, balloon-induced or wire-induced vessel injury triggered rapid upregulation of MCP-1, IL-6, and TNF-α, and downregulation of adiponectin [27]. Furthermore, a porcine experiment of drug-eluting stent-induced coronary vasoconstriction showed PVAT inflammation, as assessed by [18]F-fluorodeoxyglucose positron emission tomography (18F-FDG PET), to be greater at stent edges compared with control sites [28]. Arterial hypertension may also influence the phenotype of PVAT, with morphological changes in adipocytes, increased complement secretion, and adventitial thickening demonstrated in deoxycorticosterone acetate-salt hypertensive rats [29]. Similarly, leptin is downregulated in the PVAT of spontaneously hypertensive rats, leading to angiotensin II-mediated vasoconstriction [30].
PVAT may also release adiponectin as a protective mechanism in advanced CV disease states. A recent study of patients undergoing coronary artery bypass grafting (CABG) showed that myocardial oxidative stress releases lipid peroxidation products which diffuse into the surrounding EAT and upregulate peroxisome proliferator-activator receptor gamma (PPARγ)-mediated adiponectin expression [31]. Serum adiponectin levels are significantly elevated in patients with heart failure [32], thought to be driven by circulating brain natriuretic peptide which can override the suppressive effect of inflammation on adiponectin expression and release [33]. Hence, PVAT undergoes dynamic, phenotypic changes as a result of its interactions with the CV system.
Role of PVAT in Atherosclerosis
The recognition that PVAT inflammation may contribute to atherosclerosis has led to the “outside-to-inside” theory of vascular inflammation, whereby this process begins in AT then propagates inward to the vasculature [6]. Dysfunctional PVAT secretes pro-inflammatory adipokines and cytokines (“adipocytokines”), which can diffuse directly into the vessel wall due the lack of a dividing fascial plane. Leptin, resistin, MCP-1, and IL-8 all promote monocyte migration and activation into macrophages [34–36]. Once in the vascular space, macrophages release additional pro-inflammatory cytokines including IL-1β, IL-6, and TNF-α [37]. Adventitial vasa vasorum neovascularization also occurs during vascular injury and inflammation [38], providing a direct route to transmit adipocytokines from PVAT to the inner vasculature. Paracrine and vasocrine effects of PVAT on the vessel wall may result in (i) endothelial dysfunction from decreased nitric oxide production; (ii) monocyte chemotaxis and adhesion to the endothelium via increased expression of adhesion molecules; (iii) hypercoagulability through upregulated PAI-1; and (iv) vascular smooth muscle cell (VSMC) proliferation [39]. These mechanisms initiate and propagate plaque formation and plaque-specific inflammation.
The concept of PVAT inflammation inducing atherosclerosis in the underlying vessel is supported by early studies of human abdominal aortic PVAT and epicardial AT (EAT). PVAT from atherosclerotic abdominal aortas was found to secrete MCP-1 and IL-8, resulting in the accumulation of macrophages and T-lymphocytes at the PVAT-adventitia interface [34]. In an ex vivo study, EAT from patients undergoing CABG had higher levels of IL-1 β, IL-6, TNF-α, and MCP-1 than paired subcutaneous fat samples [40]; this was associated with a dense macrophage and T-lymphocytic infiltrate in EAT. Many subsequent reports have confirmed the increased expression and secretion of pro-inflammatory cytokines in the EAT of patients with CAD [41–44]. Similarly, autopsy studies have demonstrated active inflammation in the pericoronary EAT of subjects with CAD [45] and the extent of inflammatory infiltrate to correlate with histological plaque size and composition [46].
The atherogenic effect of PVAT was clearly demonstrated in a study involving apolipoprotein-E-deficient mice [47], in which visceral AT was transplanted immediately adjacent to the right common carotid artery—a site that typically does not develop spontaneous atherosclerosis. This resulted in larger, more complex atherosclerotic lesions, and higher serum MCP-1 compared with mice who received subcutaneous fat transplants. In another murine model, transplantation of thoracic aortic PVAT to wire-injured carotid arteries accelerated neointimal hyperplasia in an MCP-1-dependent manner [48]. Experimental evidence has also shown PVAT inflammation to result in increased expression of transforming growth factor (TGF)-β [49] and leptin [50], which promote VSMC proliferation and neointimal formation.
Hence, communication between PVAT and the vascular wall is bidirectional, and PVAT may have a direct local role in atherogenesis via adipocytokines and their paracrine and vasocrine effects (Fig. 1a).
Fig. 1.
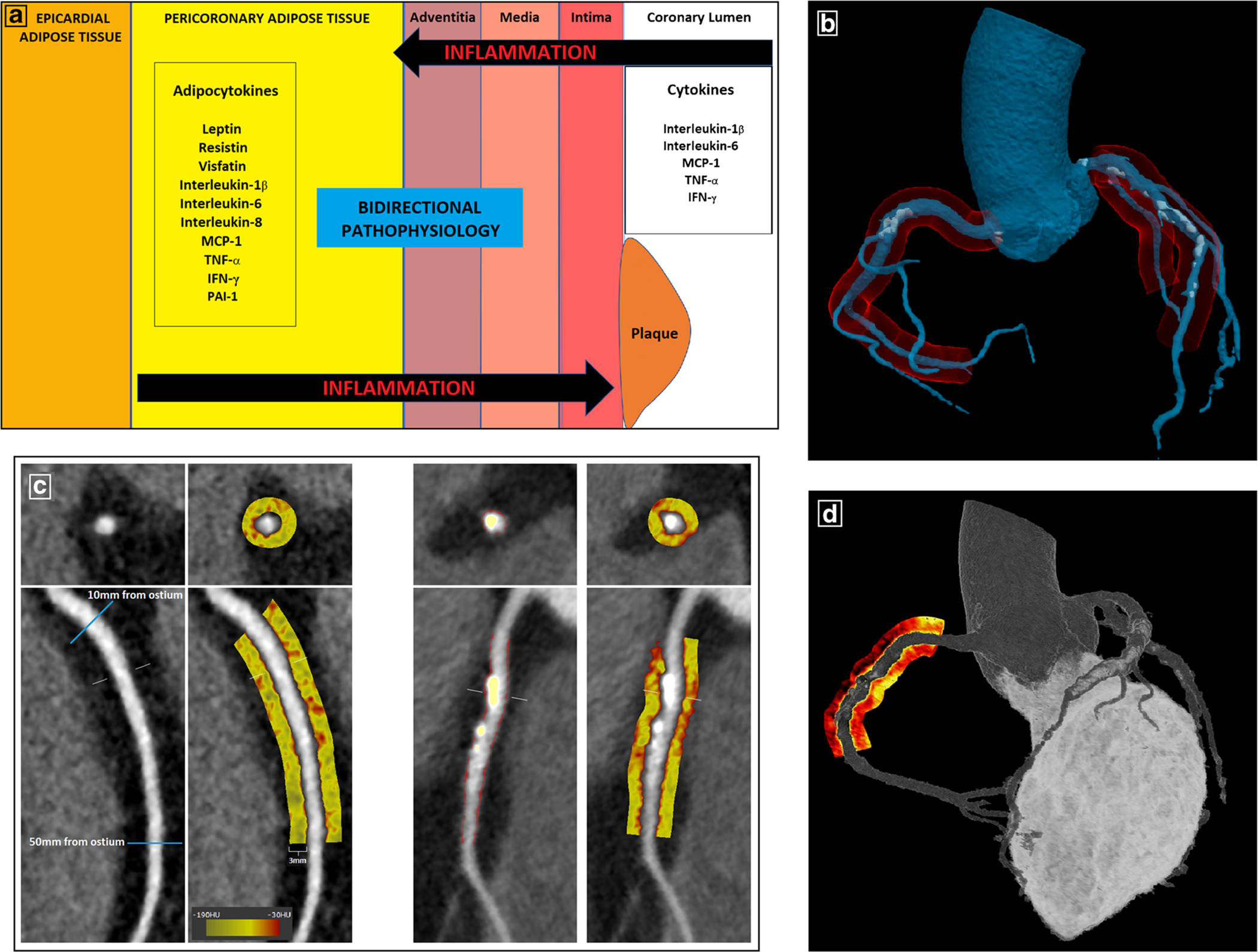
Pericoronary adipose tissue—from biology to imaging phenotyping. a Bidirectional communication between PCAT and the coronary arterial wall. Dysfunctional PCAT secretes pro-inflammatory adipocytokines which diffuse directly into the vessel wall and contribute to atherosclerosis via paracrine and vasocrine mechanisms. The recent discovery of “inside-to-outside” signaling pathways demonstrates that PCAT can also function as a sensor of coronary inflammation. b Schematic representation of PCAT (red) surrounding the 3 major coronary arteries in a 3D anatomical model generated from CCTA. c PCAT quantification on CCTA using semi-automated software (Autoplaque v2.5). Left-sided panels show the proximal segment of the RCA (10–50 mm from RCA ostium) in curved and cross-sectional views, with PCAT visualized within a 3-mm radius around the vessel on a color map (Hounsfield unit scale inset). Right-sided panels show plaque quantification in the proximal RCA (non-calcified plaque in red overlay and calcified in yellow overlay) and the corresponding PCAT color map. d 3D rendering of a PCAT “heat map” around the proximal RCA
Clinical Studies Associating PVAT with CV Risk
Various AT depots surround the heart, which can be classified according to their anatomical location in relation to the pericardium and coronary arteries. EAT, the true cardiac visceral fat depot, is located between the myocardium and visceral pericardium and supplied by branches of the coronary arteries [51]. Pericoronary AT, a component of EAT, refers to the fat directly surrounding the coronary arteries and contiguous with the adventitia [52] (Fig. 1b). Paracardial AT is situated on the external surface of the parietal pericardium and also referred to as thoracic [53] fat, while pericardial AT refers to the sum of both epicardial and paracardial AT [54].
There is growing evidence to support the imaging and quantification of PVAT for CV risk stratification. CT is considered the reference standard for EAT assessment due to its spatial resolution and three-dimensional aquisition [55, 56]. In recent years, the automation of EAT volume quantification [55] and development of deep-learning algorithms [57•] has revolutionized our assessment of this fat depot. EAT volume measured from routine non-contrast CT is highly correlated with visceral adiposity [54, 58] and associates with multiple independent risk factors including high triglycerides, low HDL-cholesterol, fasting glucose, systolic blood pressure, and C-reactive protein [58–60].
The role of EAT in coronary atherosclerosis has been extensively examined in large-scale epidemiological studies, with CT-derived EAT volume shown to associate with coronary calcification [58, 61], calcium progression [62], prevalent ischemic heart disease [63], and incident myocardial infarction [64]. Hospital registries of patients with low to intermediate CV risk have demonstrated higher EAT volumes to relate to the extent of CAD [65, 66], stenosis severity [67], high-risk plaque (HRP) [67, 68], and myocardial ischemia [69, 70].
Association of PCAT Volume and Quality with Coronary Atherosclerosis
Due to the proximity of PCAT to the coronary arteries, several imaging studies have specifically examined its influence on coronary atherosclerosis (Table 1). While recognized as being phenotypically distinct from the remaining EAT, PCAT has had various definitions in the literature [52, 72–74, 79, 80] due to the lack of clear anatomical borders. Coronary CT angiography is a reliable modality for the detection and characterization of coronary atherosclerotic plaque [81], and its high-image quality and delineation of the vessel wall enables the simultaneous quantification of PCAT. In patients with known or suspected CAD undergoing CCTA, PCAT volume is associated with plaque presence and stenosis severity in the underlying coronary segment, independently of CV risk factors [71, 72, 79]. Higher PCAT volumes have been observed around culprit lesions in myocardial infarction (MI) [74], and in coronary segments with mixed plaque compared with segments with non-calcified or calcified plaque [72, 79].
Table 1.
Studies examining the association of CT-derived PCAT volume and attenuation with coronary atherosclerosis
| Authors | Study population | n | Male (%) | Age (years) | CAD measure | Main findings | Variables in multiple models |
|---|---|---|---|---|---|---|---|
| A. Cross-sectional studies | |||||||
| Mahabadi et al. [71] (2010) | Patients with known or suspected CAD | 78 | 68 | 61 ± 12 | Plaque presence |
|
Age, gender, BMI, DM, hypertension, dyslipidemia, smoking, family history of CAD |
| Maurovich et al. [72] (2011) | ROMICAT trial | 51 | 65 | 50 ± 5 | Plaque presence and morphology |
|
BMI, hypertension, dyslipidemia, hs-CRP |
| Marwan et al. [73] (2017) | Stable patients undergoing invasive angiography | 29 | 75 | 59 ± 10 | Plaque presence and morphology (IVUS) |
|
No multivariable model |
| Konishi et al. [45] (2010) | ACS patients (n = 39) Stable patients with suspected CAD (n = 69) | 108 | 51 | 65 ± 11 | Culprit lesion Presence of CAD |
|
Age, gender, waist circumference, hypertension, dyslipidemia, HbA1c |
| Balcer et al. [74] (2018) | Acute MI patients | 46 | 71 | 64 ± 16 | Culprit lesion |
|
Age, gender, BMI, SBP, hypertension medication, LDL-C, HDL-C, lipid medication, DM, active smoking, family history of CAD, EAT volume |
| Antonopoulos et al. [7••] (2017) | Stable patients who underwent clinical CCTA | 273 | 27 | 62 ± 1 | Presence of CAD |
|
Age, gender, hypertension, dyslipidemia, DM, smoking, thoracic AT volume, CCS |
| 21 | 80 | 58 ± 3 | Plaque burden | ||||
| CCS | |||||||
| MI treated with PCI (n = 10) | Culprit lesion (stented) | ||||||
| Stable CAD with PCI (n = 11) | |||||||
| Goeller et al. [75•] (2018) | ACS patients (n = 19) | 35 | 86 | 60 ± 11 | Culprit lesion Plaque attenuation |
|
Age, gender, number of risk factors + low-attenuation, intermediate-attenuation, and high-attenuation plaque burden |
| Stable CAD patients (n = 16) | |||||||
| Kwiecinski et al. [76•] (2019) | Stable patients with HRP | 41 | 68 | 65 ± 6 | Active coronary microcalcification (18F-NaF uptake on PET) |
|
Total plaque volume, NCP volume, lesion quantitative % stenosis |
| B. Longitudinal studies | |||||||
| Oikonomou et al. [77••] (2018) | CRISP-CT study (Patients undergoing clinically indicated CCTA, with derivation (n = 1872) and validation (n = 2040) cohorts) | 3912 | 59 | 17–89 | Cardiac and all-cause mortality |
|
Age, gender, hypertension, dyslipidemia, DM, smoking status, EAT volume, Duke CAD index, number of HRP features, tube voltage + CCS |
| MI | |||||||
| Goeller et al. [78•] (2019) | Patients with stable CAD who underwent serial CCTA | 111 | 77 | 59 ± 10 | NCP progression |
|
Gender, LDL change, statin use, number of CV risk factors |
ACS, acute coronary syndrome; BMI, body mass index; CAD, coronary artery disease; CCS, coronary artery calcium score; CCTA, coronary computed tomography coronary angiography; CRISP-CT, Cardiovascular RISk Prediction using Computed Tomography; CV, cardiovascular; DM, diabetes mellitus; EAT, epicardial adipose tissue; 18F-NaF, 18F-sodium fluoride; HDL-C, high-density lipoprotein cholesterol; HRP, high-risk plaque; hs-CRP, high-sensitivity C-reactive protein; HU, Hounsfield units; IVUS, intravascular ultrasound; LAD, left anterior descending; LCx, left circumflex; LDL-C, low-density lipoprotein cholesterol; NCP, non-calcified plaque; PCAT, pericoronary adipose tissue; PCI, percutaneous coronary intervention; PET, positron emission tomography; RCA, right coronary artery; ROMICAT, Rule Out Myocardial Infarction using Computer Assisted Tomography; SBP, systolic blood pressure; SUV, standard uptake value; TBR, target-to-background ratio
Experimental studies have demonstrated changes in the quality of AT in obese subjects, focusing on inflammatory characteristics such as adipocyte size [82], macrophage infiltration [83], arteriolar dysfunction [84], and angiogenesis [85]. However, obtaining all these measures requires invasive tissue biopsy. Noninvasive imaging provides a practical means for the qualitative characterization of AT in the clinical setting. Mazurek et al. [86] used 18F-FDG PET-CT to detect PCAT inflammation in a cohort of patients with ACS and found the maximum standardized uptake value (SUV) in PCAT surrounding the proximal segments of all three major coronary arteries to be higher than in the adjacent EAT [87]. The authors reported the total PCAT SUV to be positively correlated with plaque burden and necrotic core rate determined on IVUS. In a subsequent study, the same investigators showed per vessel PCAT SUV to be greater in patients with stable CAD than in non-CAD controls, and to independently associate with coronary stenosis severity. Nevertheless, PET imaging is limited by its cost, clinical availability, low spatial resolution, and complex imaging protocols.
Routine CT employs a Hounsfield units (HU) scale of attenuation (reduction in signal), which may be used as a noninvasive measure of AT quality. AT is detected within the window of – 190 to – 30 HU [55, 88], and experimental animal studies have shown lower HU to be associated with more lipid dense AT [89]. EAT attenuation is known to correlate with CV risk factors [59, 90] and associate with measures of coronary atherosclerosis, including coronary calcium score (CCS) [91], HRP [68], and incident CV events [59]. Similarly, Konishi et al. [45] demonstrated PCAT CT attenuation in regions of interest (> 10 mm2) placed 5 mm from the vessel wall to be higher around culprit lesions compared with non-culprit lesions in patients with ACS. Marwan et al. [73] showed the average CT attenuation of PCAT—measured within a manually contoured 3-mm radius from the coronary artery—to be higher around segments with plaque than segments without plaque on intravascular ultrasound (IVUS).
Detecting PCAT Inflammation on CCTA
The link between biopsy-proven PVAT inflammation and CT attenuation was recently established in a landmark study by Antonopoulos et al. [7••] In patients undergoing CABG, PCAT, and non-PCAT EAT, samples were harvested from around the proximal right coronary artery (RCA) for histology, gene expression studies, and CT imaging. The authors showed that exposure of PCAT to pro-inflammatory cytokines suppressed the differentiation of pre-adipocytes while triggering their proliferation, resulting in numerous smaller adipocytes with fewer intracellular lipid droplets. This was paralleled by reduced gene expression of the adipocyte differentiation markers PPARγ, CCAAT/enhancer binding protein α (CEBPA), and fatty acid binding protein-4 (FABP4). On ex vivo CT scans of AT explants and in vivo CCTA, Antonopoulos et al. demonstrated an inverse association of PCAT attenuation with histological adipocyte size and degree of adipocyte differentiation, with higher PCAT attenuation (less negative HU) reflecting smaller adipocytes with a lower lipid content.
The authors quantified PCAT on multiplanar reconstructed CCTA images using bespoke research software, with the RCA chosen for analysis due to the absence of major side branches and abundance of surrounding AT. PCAT was semi-automatically segmented into concentric cylindrical 1-mm-thick layers around a 40-mm-long segment of the RCA (10th to 50th mm from its ostium). AT was identified as all voxels with CT attenuation between – 190 and – 30 HU, and PCAT attenuation was defined as the mean attenuation of AT within a radial distance from the outer coronary artery wall equal to the average diameter of the vessel. Antonopoulos et al. showed that PCAT CT attenuation was higher in patients with CAD than those without CAD and was associated with the presence of > 50% stenosis in any artery. In a subset of 40 patients, PCAT attenuation was analyzed around a proximal 40-mm segment of all three major coronary arteries and found to correlate with the underlying fibrous plaque volume. PCAT attenuation showed a weak association with CCS in the RCA and no association with total CCS. This bench-to-bedside study demonstrated the “inside-to-outside” effect of vascular inflammation on PCAT, and CCTA-derived PCAT emerged as a novel surrogate measure of coronary inflammation.
The same investigators proceeded to a prognostic validation of this imaging biomarker, in a post hoc analysis of 2 prospectively recruited cohorts with a total of 3912 patients who underwent clinically indicated CCTA [77••]. PCAT attenuation around the proximal RCA was used as a representative biomarker of global coronary inflammation, given its strong correlation with equivalent measurements around the proximal left anterior descending and left circumflex arteries. The study population was stratified by “high” and “low” PCAT attenuation based on an optimum threshold of – 70.1 HU. In the validation cohort (n = 2040), high-PCAT attenuation (≥ 70.1 HU vs < 70.1 HU) was associated with increased risk of cardiac mortality (hazard ratio (HR) 5.62, p < 0.0001) and all-cause mortality (HR 3.69, p < 0.0001) at a median follow-up of 54 months, adjusted for age, sex, risk factors, modified Duke CAD index, and number of HRP features. The addition of high-PCAT attenuation to a risk prediction model incorporating the same variables improved the discriminatory value for both cardiac and all-cause mortality.
PCAT Attenuation and High-Risk Plaque
Goeller et al. [75•] sought to determine the per lesion association of PCAT attenuation with HRP. In a retrospective case-control study, patients with ACS (n = 19) who underwent CCTA prior to invasive angiography were matched to controls with stable CAD (n = 16). Plaque quantification was performed in coronary lesions using validated semi-automated software [92], with automated contouring of the vessel wall and manual adjustments made by an expert reader if necessary. Following this, PCAT was automatically sampled in three-dimensional layers moving away radially in 1-mm increments from the outer coronary wall. As the average lesion diameter was 3 mm, PCAT attenuation was defined as the mean CT attenuation of AT (− 190 HU to − 30 HU) within a tubular volume between the vessel wall and an outer radial distance of 3 mm from the vessel wall. The authors reported a higher PCAT attenuation around culprit lesions compared with non-culprit lesions in ACS patients (− 69.1 HU vs − 74.8 HU, p = 0.01) and the highest-grade stenosis lesions of controls (− 69.1 HU vs − 76.4 HU, p = 0.01). In ACS patients, PCAT attenuation correlated only with the burden of intermediate-attenuation (30 to 130 HU) non-calcified plaque (NCP; r = 0.393, p = 0.001). This association of PCAT CT attenuation with HRP characteristics may reflect vascular inflammation causing plaque instability and phenotypic changes in PCAT.
The same research group [76•] studied the relationship between PCAT CT attenuation and coronary arterial uptake of [18]F-sodium fluoride (18F-NaF) onPET—a marker of active plaque microcalcification in response to coronary inflammation. In 41 patients with HRP identified on CCTA, 23 had coronary 18F-NaF uptake. Lesions with 18F-NaF uptake exhibited higher surrounding PCAT CT attenuation than those without 18F-NaF uptake (− 73 HU vs − 86 HU, p < 0.001). There was a moderate correlation between PCAT attenuation and PET tracer uptake determined by target-to-background ratio (TBR; r = 0.68, p < 0.00), and on multivariable analysis PCAT attenuation was an independent predictor of 18F-NaF TBR. These findings support the link between vascular inflammation and coronary microcalcification at a noninvasive imaging level and suggest that PCAT attenuation provides important information regarding plaque metabolic activity. Furthermore, the lack of association between PCAT attenuation and CT-derived coronary calcium measures observed in the previous studies [7••, 75•] is consistent with coronary macrocalficiation being a stabilizer of atherosclerotic plaque and barrier to the spread of inflammation [93].
Tracking Changes in Coronary Inflammation
Goeller et al. [78•] evaluated PCAT attenuation around the proximal RCA, the most standardized method for PCAT analysis, in relation to plaque changes on serial CCTA (median interval 3.4 ± 1.6 years) in a stable CAD cohort. Their automated method of PCAT quantification is shown in Fig. 1 c and d. The authors demonstrated progression of NCP burden to be associated with an increase in PCAT attenuation, and regression of NCP burden to be associated with a decrease in PCAT attenuation (4.4 vs. − 2.78 HU, p < 0.0001). Changes in PCAT attenuation correlated with changes in the burden of NCP and low-density NCP—the inflammatory plaque components—however not with the burden of calcified plaque. A high-baseline PCAT attenuation (≥ − 75 HU) independently predicted NCP progression. These findings suggest that PCAT attenuation can detect changes in plaque-specific inflammation quantified by NCP and low-density NCP burden, and may help to identify patients at increased risk of future NCP progression.
Recently, Elnabawi et al. [94] used PCAT attenuation around the proximal RCA to track changes in the coronary inflammatory status in response to systemic anti-inflammatory treatments. In a prospective study of 134 patients with psoriasis who underwent serial CCTA, biologic therapy was associated with a significant decrease in PCAT attenuation at 1-year follow-up (− 71.2 vs − 76.1 HU, p < 0.001). No change in PCAT attenuation was observed in those not receiving biologic therapy. These findings were independent of the presence of CAD and consistent among patients receiving different biologic agents.
Future Perspectives
PCAT CT attenuation is a promising imaging biomarker with several potential clinical applications; however, gaps currently exist in the evidence. Standardization of PCAT attenuation measurement across different CT vendors and scan parameters will need to be achieved before its integration into clinical workstations. PCAT quantification around culprit lesions in ACS and around HRP in stable CAD patients requires validation in larger, prospective cohorts. Furthermore, the natural history of PCAT attenuation and its response to conventional treatment following ACS remains unknown.
The ability to reliably detect inflamed coronary arteries has important treatment implications. Individuals without CAD but a high-PCAT attenuation and hence increased CV risk may benefit from early primary preventative measures. PCAT attenuation may also identify patients with inflamed, unstable plaques who require intensification of medical therapy. The recent CANTOS trial demonstrated that specific targeting of interleukin-1β with canakinumab reduced CV events, and trials of other anti-inflammatory agents are underway. Current evidence shows that PCAT attenuation may be able to track the response of the coronaries to systemic anti-inflammatory therapies, paving the way for future randomized studies assessing whether this biomarker can be modified by targeted anti-inflammatory interventions. PCAT attenuation contributes to the burgeoning research in the complex interplay between inflammation and atherosclerosis but at this time, there remains a dearth of information to allow modification of clinical practice.
Conclusion
A complex “cross-talk” exists between PVAT and the vasculature. PVAT modulates local vascular biology via adipocytokines and their paracrine effects on the vessel wall, and dysfunctional PVAT may incite atherosclerosis. PCAT surrounding the coronary arteries undergoes distinct phenotypic changes in response to coronary inflammation, and this can be detected using PCAT CT attenuation, a novel metric derived from routine CCTA. This imaging biomarker has been shown to associate with plaque vulnerability, track changes in plaque-specific inflammation, and independently predict cardiac mortality. Measurement of PCAT attenuation complements current CCTA-based plaque analysis and has the potential to enhance CV risk stratification and guide individualized primary and secondary prevention.
Funding information
Dr Nerlekar is supported by a post-doctoral scholarship from the National Heart Foundation and a Robertson Family Research Fellowship. Dr. Dey is supported in part by a National Heart, Lung, and Blood Institute grant 1R01HL133616.
Appendix
Table 2.
Search strategy
| Number | Query | Results |
|---|---|---|
| 1 | Perivascular adipose tissue | 559 |
| 2 | Pericoronary adipose tissue | 29 |
| 3 | Epicardial adipose tissue | 1019 |
| 4 | Coronary atherosclerosis | 12827 |
| 5 | Computed tomography | 555610 |
| 6 | Inflammation | 514604 |
| 7 | Obesity | 313732 |
| 8 | 1 or 2 or 3 | 1563 |
| 9 | 8 and 4 | 139 |
| 10 | 8 and 5 | 292 |
| 11 | 8 and 6 | 391 |
| 12 | 8 and 7 | 449 |
Example search strategy using Medline as performed on 15 August 2019
Footnotes
Conflict of Interest Andrew Lin, Damini Dey, Dennis T.L. Wong, and Nitesh Nerlekar declare they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.WHO. Disease burden and mortality estimates. 2016.
- 2.Cholesterol Treatment Trialists’ C, Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libby P, Ridker Paul M, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–43. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM. How common is residual inflammatory risk? Circ Res. 2017;120(4):617–9. [DOI] [PubMed] [Google Scholar]
- 5.••.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with Canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–31 Landmark study validating the inflammatory hypothesis of atherosclerosis.
- 6.Britton KA, Fox CS. Perivascular adipose tissue and vascular disease. Clini Lipidol. 2011;6(1):79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.••.Antonopoulos AS, Sanna F, Sabharwal N, Thomas S, Oikonomou EK, Herdman L, et al. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med. 2017;9(398) Seminal study linking biopsy-proven coronary inflamamtion to a PCAT CT attenuation, a novel imaging biomarker.
- 8.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67(5):968–77. [DOI] [PubMed] [Google Scholar]
- 9.Hughes-Austin J, Larsen B, Allison M. Visceral adipose tissue and cardiovascular disease risk 2013.
- 10.Fuster JJ, Ouchi N, Gokce N, Walsh K. Obesity-induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ Res. 2016;118(11): 1786–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116(1):39–48. [DOI] [PubMed] [Google Scholar]
- 12.Armani A, Mammi C, Marzolla V, Calanchini M, Antelmi A, Rosano G, et al. Cellular models for understanding adipogenesis, adipose dysfunction, and obesity. 2010. 564–72 p. [DOI] [PubMed] [Google Scholar]
- 13.Ntambi JM, Young-Cheul K. Adipocyte differentiation and gene expression. JNutr. 2000;130(12):3122s–6s. [DOI] [PubMed] [Google Scholar]
- 14.Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, et al. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res. 2009;104(4): 541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szasz T, Webb RC. Perivascular adipose tissue: more than just structural support. Clin Sci (London, England : 1979). 2012;122(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajsheker S, Manka D, Blomkalns AL, Chatteijee TK, Stoll LL, Weintraub NL. Crosstalk between perivascular adipose tissue and blood vessels. Curr Opin Pharmacol. 2010;10(2):191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thalmann S, Meier CA. Local adipose tissue depots as cardiovascular risk factors. Cardiovasc Res. 2007;75(4):690–701. [DOI] [PubMed] [Google Scholar]
- 18.Hyvonen MT, Spalding KL. Maintenance of white adipose tissue in man. Int J Biochem Cell Biol. 2014;56:123–32. [DOI] [PubMed] [Google Scholar]
- 19.Shimizu I, Walsh K. The whitening of brown fat and its implications for weight management in obesity. Curr Obes Rep. 2015;4(2): 224–9. [DOI] [PubMed] [Google Scholar]
- 20.de Souza Batista CM, Yang R-Z, Lee M-J, Glynn NM, Yu D-Z, Pray J, et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes. 2007;56(6):1655. [DOI] [PubMed] [Google Scholar]
- 21.Park SY, Kim KH, Seo KW, Bae JU, Kim YH, Lee SJ, et al. Resistin derived from diabetic perivascular adipose tissue up-regulates vascular expression of osteopontin via the AP-1 signalling pathway. J Pathol. 2014;232(1):87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karastergiou K, Mohamed-Ali V. The autocrine and paracrine roles of adipokines. Mol Cell Endocrinol. 2010;318(1–2):69–78. [DOI] [PubMed] [Google Scholar]
- 23.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci USA. 2003;100(12):7265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wensveen FM, Valentic S, Sestan M, Turk Wensveen T, Polic B. The “Big Bang” in obese fat: events initiating obesity-induced adipose tissue inflammation. Eur J Immunol. 2015;45(9):2446–56. [DOI] [PubMed] [Google Scholar]
- 26.Moreno PR, Purushothaman KR, Fuster V, O’Connor WN. Intimomedial interface damage and adventitial inflammation is increased beneath disrupted atherosclerosis in the aorta: implications for plaque vulnerability. Circulation. 2002;105(21):2504–11. [DOI] [PubMed] [Google Scholar]
- 27.Takaoka M, Suzuki H, Shioda S, Sekikawa K, Saito Y, Nagai R, et al. Endovascular injury induces rapid phenotypic changes in perivascular adipose tissue. Arterioscler Thromb Vasc Biol. 2010;30(8):1576–82. [DOI] [PubMed] [Google Scholar]
- 28.Ohyama K, Matsumoto Y, Amamizu H, Uzuka H, Nishimiya K, Morosawa S, et al. Association of coronary perivascular adipose tissue inflammation and drug-eluting stent-induced coronary hyperconstricting responses in pigs. Arterioscler Thromb Vasc Biol. 2017;37(9):1757–64. [DOI] [PubMed] [Google Scholar]
- 29.Ruan CC, Zhu DL, Chen QZ, Chen J, Guo SJ, Li XD, et al. Perivascular adipose tissue-derived complement 3 is required for adventitial fibroblast functions and adventitial remodeling in deoxycorticosterone acetate-salt hypertensive rats. Arterioscler Thromb Vasc Biol. 2010;30(12):2568–74. [DOI] [PubMed] [Google Scholar]
- 30.Galvez-Prieto B, Bolbrinker J, Stucchi P, de Las Heras AI, Merino B, Arribas S, et al. Comparative expression analysis of the renin-angiotensin system components between white and brown perivascular adipose tissue. J Endocrinol. 2008;197(1):55–64. [DOI] [PubMed] [Google Scholar]
- 31.Antonopoulos AS, Margaritis M, Verheule S, Recalde A, Sanna F, Herdman L, et al. Mutual regulation of epicardial adipose tissue and myocardial redox state by PPAR-gamma/adiponectin signalling. Circ Res. 2016;118(5):842–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.George J, Patal S, Wexler D, Sharabi Y, Peleg E, Kamari Y, et al. Circulating adiponectin concentrations in patients with congestive heart failure. Heart. 2006;92(10):1420–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antonopoulos Alexios S, Margaritis M, Coutinho P, Digby J, Patel R, Psarros C, et al. Reciprocal effects of systemic inflammation and brain natriuretic peptide on adiponectin biosynthesis in adipose tissue of patients with ischemic heart disease. Arterioscler Thromb Vasc Biol. 2014;34(9):2151–9. [DOI] [PubMed] [Google Scholar]
- 34.Henrichot E, Juge-Aubry CE, Pernin A, Pache JC, Velebit V, Dayer JM, et al. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler Thromb Vasc Biol. 2005;25(12):2594–9. [DOI] [PubMed] [Google Scholar]
- 35.Knudson JD, Dick GM, Tune JD. Adipokines and coronary vasomotor dysfunction. Exp Biol Med (Maywood, NJ). 2007;232(6): 727–36. [PubMed] [Google Scholar]
- 36.Weber C, Schober A, Zernecke A. Chemokines: key regulators of mononuclear cell recruitment in atherosclerotic vascular disease. Arterioscler Thromb Vasc Biol. 2004;24(11):1997–2008. [DOI] [PubMed] [Google Scholar]
- 37.Thorlacius H, Lindbom L, Raud J. Cytokine-induced leukocyte rolling in mouse cremaster muscle arterioles in P-selectin dependent. Am J Phys. 1997;272(4 Pt 2):H1725–9. [DOI] [PubMed] [Google Scholar]
- 38.Kwon HM, Sangiorgi G, Ritman EL, Lerman A, McKenna C, Virmani R, et al. Adventitial vasa vasorum in balloon-injured coronary arteries: visualization and quantitation by a microscopic three-dimensional computed tomography technique. J Am Coll Cardiol. 1998;32(7):2072–9. [DOI] [PubMed] [Google Scholar]
- 39.Verhagen SN, Visseren FL. Perivascular adipose tissue as a cause of atherosclerosis. Atherosclerosis. 2011;214(1):3–10. [DOI] [PubMed] [Google Scholar]
- 40.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108(20):2460–6. [DOI] [PubMed] [Google Scholar]
- 41.Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kremen J, Dolinkova M, Krajickova J, Blaha J, Anderlova K, Lacinova Z, et al. Increased subcutaneous and epicardial adipose tissue production of proinflammatory cytokines in cardiac surgery patients: possible role in postoperative insulin resistance. J Clin Endocrinol Metab. 2006;91(11):4620–7. [DOI] [PubMed] [Google Scholar]
- 43.Baker AR, Harte AL, Howell N, Pritlove DC, Ranasinghe AM, da Silva NF, et al. Epicardial adipose tissue as a source of nuclear factor-kappaB and c-Jun N-terminal kinase mediated inflammation in patients with coronary artery disease. J Clin Endocrinol Metab. 2009;94(1):261–7. [DOI] [PubMed] [Google Scholar]
- 44.Hirata Y, Kurobe H, Akaike M, Chikugo F, Hori T, Bando Y, et al. Enhanced inflammation in epicardial fat in patients with coronary artery disease. Int Heart J. 2011;52(3):139–42. [DOI] [PubMed] [Google Scholar]
- 45.Konishi M, Sugiyama S, Sato Y, Oshima S, Sugamura K, Nozaki T, et al. Pericardial fat inflammation correlates with coronary artery disease. Atherosclerosis. 2010;213(2):649–55. [DOI] [PubMed] [Google Scholar]
- 46.Verhagen SN, Vink A, van der Graaf Y, Visseren FL. Coronary perivascular adipose tissue characteristics are related to atherosclerotic plaque size and composition. A post-mortem study. Atherosclerosis. 2012;225(1):99–104. [DOI] [PubMed] [Google Scholar]
- 47.Öhman MK, Luo W, Wang H, Guo C, Abdallah W, Russo HM, et al. Perivascular visceral adipose tissue induces atherosclerosis in apolipoprotein E deficient mice. Atherosclerosis. 2011;219(1):33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manka D, Chatterjee TK, Stoll LL, Basford JE, Konaniah ES, Srinivasan R, et al. Transplanted perivascular adipose tissue accelerates injury-induced neointimal hyperplasia: role of monocyte chemoattractant protein-1. Arterioscler Thromb Vasc Biol. 2014;34(8):1723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moe KT, Naylynn TM, Yin NO, Khairunnisa K, Allen JC, Wong MC, et al. Tumor necrosis factor-alpha induces aortic intima-media thickening via perivascular adipose tissue inflammation. J Vasc Res. 2013;50(3):228–37. [DOI] [PubMed] [Google Scholar]
- 50.Schroeter Marco R, Eschholz N, Herzberg S, Jerchel I, Leifheit-Nestler M, Czepluch Frauke S, et al. Leptin-dependent and leptin- independent paracrine effects of perivascular adipose tissue on neointima formation. Arterioscler Thromb Vasc Biol. 2013;33(5):980–7. [DOI] [PubMed] [Google Scholar]
- 51.Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2(10):536–43. [DOI] [PubMed] [Google Scholar]
- 52.Demircelik MB, Yilmaz OC, Gurel OM, Selcoki Y, Atar IA, Bozkurt A, et al. Epicardial adipose tissue and pericoronary fat thickness measured with 64-multidetector computed tomography: potential predictors of the severity of coronary artery disease. Clinics (Sao Paulo, Brazil). 2014;69(6):388–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dey D, Nakazato R, Li D, Berman DS. Epicardial and thoracic fat—noninvasive measurement and clinical implications. Cardiovasc Diagn Ther. 2012;2(2):85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wheeler GL, Shi R, Beck SR, Langefeld CD, Lenchik L, Wagenknecht LE, et al. Pericardial and visceral adipose tissues measured volumetrically with computed tomography are highly associated in type 2 diabetic families. Investig Radiol. 2005;40(2): 97–101. [DOI] [PubMed] [Google Scholar]
- 55.Ding X, Terzopoulos D, Diaz-Zamudio M, Berman DS, Slomka PJ, Dey D. Automated pericardium delineation and epicardial fat volume quantification from noncontrast CT. Med Phys. 2015;42(9): 5015–26. [DOI] [PubMed] [Google Scholar]
- 56.Nerlekar N, Baey Y-W, Brown AJ, Muthalaly RG, Dey D, Tamarappoo B, et al. Poor correlation, reproducibility, and agreement between volumetric versus linear epicardial adipose tissue measurement. JACC Cardiovasc Imaging. 2018;11(7): 1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.•.Commandeur F, Goeller M, Betancur J, Cadet S, Doris M, Chen X, et al. Deep learning for quantification of epicardial and thoracic adipose tissue from non-contrast CT. IEEE Trans Med Imaging. 2018;37(8):1835–46 A novel deep-learning approach for quantification of EAT volume.
- 58.Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117(5):605–13. [DOI] [PubMed] [Google Scholar]
- 59.Goeller M, Achenbach S, Marwan M, Doris MK, Cadet S, Commandeur F, et al. Epicardial adipose tissue density and volume are related to subclinical atherosclerosis, inflammation and major adverse cardiac events in asymptomatic subjects. J Cardiovasc Comput Tomogr. 2018;12(1):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uygur B, Celik O, Ozturk D, Erturk M, Otcu H, Ustabasioglu FE, et al. The relationship between location-specific epicardial adipose tissue volume and coronary atherosclerotic plaque burden in type 2 diabetic patients. Kardiol Pol. 2017;75(3):204–12. [DOI] [PubMed] [Google Scholar]
- 61.McClain J, Hsu F, Brown E, Burke G, Carr J, Harris T, et al. Pericardial adipose tissue and coronary artery calcification in the Multi-ethnic Study of Atherosclerosis (MESA). Obesity (Silver Spring). 2013;21(5):1056–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mahabadi AA, Lehmann N, Kalsch H, Robens T, Bauer M, Dykun I, et al. Association of epicardial adipose tissue with progression of coronary artery calcification is more pronounced in the early phase of atherosclerosis: results from the Heinz Nixdorf recall study. JACC Cardiovasc Imaging. 2014;7(9):909–16. [DOI] [PubMed] [Google Scholar]
- 63.Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009;30(7):850–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mahabadi AA, Berg MH, Lehmann N, Kalsch H, Bauer M, Kara K, et al. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall Study. J Am Coll Cardiol. 2013;61(13): 1388–95. [DOI] [PubMed] [Google Scholar]
- 65.Bettencourt N, Toschke AM, Leite D, Rocha J, Carvalho M, Sampaio F, et al. Epicardial adipose tissue is an independent predictor of coronary atherosclerotic burden. Int J Cardiol. 2012;158(1):26–32. [DOI] [PubMed] [Google Scholar]
- 66.Versteylen MO, Takx RA, Joosen IA, Nelemans PJ, Das M, Crijns HJ, et al. Epicardial adipose tissue volume as a predictor for coronary artery disease in diabetic, impaired fasting glucose, and non-diabetic patients presenting with chest pain. Eur Heart J Cardiovasc Imaging. 2012;13(6):517–23. [DOI] [PubMed] [Google Scholar]
- 67.Ito T, Suzuki Y, Ehara M, Matsuo H, Teramoto T, Terashima M, et al. Impact of epicardial fat volume on coronary artery disease in symptomatic patients with a zero calcium score. Int J Cardiol. 2013;167(6):2852–8. [DOI] [PubMed] [Google Scholar]
- 68.Lu MT, Park J, Ghemigian K, Mayrhofer T, Puchner SB, Liu T, et al. Epicardial and paracardial adipose tissue volume and attenuation—association with high-risk coronary plaque on computed tomographic angiography in the ROMICAT II trial. Atherosclerosis. 2016;251:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hell MM, Ding X, Rubeaux M, Slomka P, Gransar H, Terzopoulos D, et al. Epicardial adipose tissue volume but not density is an independent predictor for myocardial ischemia. J Cardiovasc Comput Tomogr. 2016; 10(2): 141–9. [DOI] [PubMed] [Google Scholar]
- 70.Tamarappoo B, Dey D, Shmilovich H, Nakazato R, Gransar H, Cheng VY, et al. Increased pericardial fat volume measured from noncontrast CT predicts myocardial ischemia by SPECT. JACC Cardiovasc Imaging. 2010;3(11):1104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mahabadi AA, Reinsch N, Lehmann N, Altenbernd J, Kalsch H, Seibel RM, et al. Association of pericoronary fat volume with atherosclerotic plaque burden in the underlying coronary artery: a segment analysis. Atherosclerosis. 2010;211(1): 195–9. [DOI] [PubMed] [Google Scholar]
- 72.Maurovich-Horvat P, Kallianos K, Engel LC, Szymonifka J, Fox CS, Hoffmann U, et al. Influence of pericoronary adipose tissue on local coronary atherosclerosis as assessed by a novel MDCT volumetric method. Atherosclerosis. 2011;219(1):151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marwan M, Hell M, Schuhback A, Gauss S, Bittner D, Pflederer T, et al. CT attenuation of pericoronary adipose tissue in normal versus atherosclerotic coronary segments as defined by intravascular ultrasound. J Comput Assist Tomogr. 2017;41(5):762–7. [DOI] [PubMed] [Google Scholar]
- 74.Balcer B, Dykun I, Schlosser T, Forsting M, Rassaf T, Mahabadi AA. Pericoronary fat volume but not attenuation differentiates culprit lesions in patients with myocardial infarction. Atherosclerosis. 2018;276:182–8. [DOI] [PubMed] [Google Scholar]
- 75.•.Goeller M, Achenbach S, Cadet S, Kwan AC, Commandeur F, Slomka PJ, et al. Pericoronary adipose tissue computed tomography attenuation and high-risk plaque characteristics in acute coronary syndrome compared with stable coronary artery disease. JAMA Cardiol. 2018;3(9):858–63 Demonstrates association of PCAT attenuation with high-risk plaque.
- 76.•.Kwiecinski J, Dey D, Cadet S, Lee S-E, Otaki Y, Huynh PT, et al. Peri-coronary adipose tissue density is associated with 18F-sodium fluoride coronary uptake in stable patients with high-risk plaques. JACC Cardiovasc Imaging. 2019; Combined assessment of PCAT CT attenuation and 18F-NaF uptake, two novel imaging biomarkers.
- 77.••.Oikonomou EK, Marwan M, Desai MY, Mancio J, Alashi A, Hutt Centeno E, et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet. 2018;392(10151):929–39 Progonstic validation of PCAT CT attenuation.
- 78.•.Goeller M, Tamarappoo BK, Kwan AC, Cadet S, Commandeur F, Razipour A, et al. Relationship between changes in pericoronary adipose tissue attenuation and coronary plaque burden quantified from coronary computed tomography angiography. Eur Heart J Cardiovasc Imaging. 2019;20(6):636–43 Demonstrates the association of PCAT attenuation with coronary plaque progression.
- 79.Hassan M, Said K, Rizk H, ElMogy F, Donya M, Houseni M, et al. Segmental peri-coronary epicardial adipose tissue volume and coronary plaque characteristics. Eur Heart J Cardiovasc Imaging. 2016;17(10):1169–77. [DOI] [PubMed] [Google Scholar]
- 80.Hell MM, Achenbach S, Schuhbaeck A, Klinghammer L, May MS, Marwan M. CT-based analysis of pericoronary adipose tissue density: relation to cardiovascular risk factors and epicardial adipose tissue volume. J Cardiovasc Comput Tomogr. 2016;10(1):52–60. [DOI] [PubMed] [Google Scholar]
- 81.Voros S, Rinehart S, Qian Z, Joshi P, Vazquez G, Fischer C, et al. Coronary atherosclerosis imaging by coronary CT angiography: current status, correlation with intravascular interrogation and meta-analysis. J Am Coll Cardiol Img. 2011;4(5):537–48. [DOI] [PubMed] [Google Scholar]
- 82.Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia. 2000;43(12):1498–506. [DOI] [PubMed] [Google Scholar]
- 83.Apovian CM, Bigornia S, Mott M, Meyers MR, Ulloor J, Gagua M, et al. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol. 2008;28(9):1654–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Farb MG, Ganley-Leal L, Mott M, Liang Y, Ercan B, Widlansky ME, et al. Arteriolar function in visceral adipose tissue is impaired in human obesity. Arterioscler Thromb Vasc Biol. 2012;32(2):467–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gealekman O, Guseva N, Hartigan C, Apotheker S, Gorgoglione M, Gurav K, et al. Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation. 2011;123(2):186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mazurek T, Kochman J, Kobylecka M, Wilimski R, Filipiak KJ, Krolicki L, et al. Inflammatory activity of pericoronary adipose tissue may affect plaque composition in patients with acute coronary syndrome without persistent ST-segment elevation: preliminary results. Kardiol Pol. 2014;72(5):410–6. [DOI] [PubMed] [Google Scholar]
- 87.•.Mazurek T, Kobylecka M, Zielenkiewicz M, Kurek A, Kochman J, Filipiak KJ, et al. PET/CT evaluation of (18)F-FDG uptake in pericoronary adipose tissue in patients with stable coronary artery disease: Independent predictor of atherosclerotic lesions’ formation? J Nucl Cardiol. 2017;24(3):1075–84 First non-invasive imaging study to document PCAT inflammation in patients with CAD.
- 88.Mihl C, Loeffen D, Versteylen MO, Takx RA, Nelemans PJ, Nijssen EC, et al. Automated quantification of epicardial adipose tissue (EAT) in coronary CT angiography; comparison with manual assessment and correlation with coronary artery disease. J Cardiovasc Comput Tomogr. 2014;8(3):215–21. [DOI] [PubMed] [Google Scholar]
- 89.Baba S, Jacene HA, Engles JM, Honda H, Wahl R. CT Hounsfield units of brown adipose tissue increase with activation: preclinical and clinical studies. J Nucl Med. 2010;51:246–50. [DOI] [PubMed] [Google Scholar]
- 90.Franssens BT, Nathoe HM, Leiner T, van der Graaf Y, Visseren FL. Relation between cardiovascular disease risk factors and epicardial adipose tissue density on cardiac computed tomography in patients at high risk of cardiovascular events. Eur J Prev Cardiol. 2017;24(6):660–70. [DOI] [PubMed] [Google Scholar]
- 91.Abazid RM, Smettei OA, Kattea MO, Sayed S, Saqqah H, Widyan AM, et al. Relation between epicardial fat and subclinical atherosclerosis in asymptomatic individuals. J Thorac Imaging. 2017;32(6):378–82. [DOI] [PubMed] [Google Scholar]
- 92.Dey D, Schepis T, Marwan M, Slomka PJ, Berman DS, Achenbach S. Automated three-dimensional quantification of noncalcified coronary plaque from coronary CT angiography: comparison with intravascular US. Radiology. 2010;257(2):516–22. [DOI] [PubMed] [Google Scholar]
- 93.Nakahara T, Dweck MR, Narula N, Pisapia D, Narula J, Strauss HW. Coronary artery calcification: from mechanism to molecular imaging. JACC Cardiovasc Imaging. 2017;10(5):582–93. [DOI] [PubMed] [Google Scholar]
- 94.•.Elnabawi YA, Oikonomou EK, Dey AK, Mancio J, Rodante JA, Aksentijevich M, et al. Association of biologic therapy with coronary inflammation in patients with psoriasis as assessed by perivascular fat attenuation indexassociation of biologic therapy with coronary inflammation in psoriasisassociation of biologic therapy with coronary inflammation in psoriasis. JAMA Cardiol. 2019; Demonstrates that PCAT CT attenuation can be modified by anti-inflammatory treatments.


