Abstract
Background
Recently, Allander and co-workers reported the discovery of a new human polyomavirus, KI virus, in respiratory secretions from patients with acute respiratory tract infection (ARTI).
Objective
We examined 951 respiratory samples collected in Queensland, Australia, between November 2002 and August 2003 from patients with respiratory infection, for the presence of the KI virus.
Results
Twenty-four (2.5%) samples were positive for KI virus with 20 (83%) of these from children younger than 5 years. In six (25%) patients KI was co-detected with another virus. Full genome sequencing of three isolates shows a high degree of conservation between the Queensland isolates and the original isolates reported from Swedish patients.
Conclusions
The newly described KI polyomavirus may commonly be found in the respiratory tract of patients with ARTI, particularly children, and results indicate that the virus has global presence.
Keywords: Human polyomavirus, Acute respiratory tract infection, Co-detection, PCR
1. Introduction
Viral infections of the respiratory tract are responsible for significant mortality and morbidity worldwide, particularly in children (Mulholland, 2003). The viral respiratory pathogens that are commonly recognised include rhinoviruses (HRV), coronaviruses (HCoV), influenza viruses (IFV), parainfluenza viruses (PIV), respiratory syncytial virus (RSV) and adenoviruses (ADV). However, despite exhaustive investigation, a significant proportion of respiratory infections cannot be accounted for by these viruses, suggesting that additional respiratory pathogens are still likely to exist (Heikkinen and Jarvinen, 2003). Over the last 6 years, the search for these agents has led to the detection of human metapneumovirus (HMPV) (van den Hoogen et al., 2001), human coronaviruses, SARS (Ksiazek et al., 2003), NL63 (van der Hoek et al., 2004) and HKU1 (Woo et al., 2005), human bocavirus (HBoV) (Allander et al., 2005) and very recently, human KI polyomavirus (KIV) (Allander et al., 2007).
Allander et al. (2007) showed that KIV was phylogenetically related to other primate polyomaviruses in the early region of the genome, but has very little homology in the late region. They found KIV by PCR in 6 (1%) of 637 nasopharyngeal aspirates (NPAs), and in one (0.5%) of 192 faecal samples, but did not detect it in sets of urine and blood samples. So far, the pathogenicity and prevalence of this virus has not yet been established.
The genomes of viruses in the family Polyomaviridae are single molecules of covalently closed, superhelical double-stranded DNA that are replicated in the nucleus. Different family members infect several species of mammals, including humans, other primates, rodents, rabbits as well as birds. Before KI virus, two human polyomaviruses, BK virus (BKV) and JC virus (JCV) were described which are ubiquitous worldwide (Stolt et al., 2003). Although it is postulated that these polyomaviruses utilise a respiratory route for transmission, detection of their nucleic acids in the respiratory tract is rare (Goudsmit et al., 1982, Sundsfjord et al., 1994). Normally infection with JCV and BKV in immunocompetent hosts results in asymptomatic infection, but in the immunocompromised these viruses may cause significant disease. JCV may be the cause of progressive multifocal leukoencephalopathy (PML) while BK virus has been associated with tubular nephritis in renal transplant recipients and haemorrhagic cystitis in bone marrow transplant recipients (Arthur et al., 1986). Following primary infection, these viruses may persist in the kidney and can periodically re-emerge in the host (Behzad-Behbahani et al., 2004, Markowitz et al., 1993). Following the report describing KIV, we sought to determine the presence of this virus in a well-characterised population of respiratory samples collected from Queensland subjects with acute respiratory tract infection (ARTI).
2. Methods
Nine hundred and fifty one respiratory samples, including 887 NPAs and 64 bronchoalveolar lavages, collected in Queensland from November 2002 to August 2003 from patients presenting to hospital with ARTI were examined for the presence of KIV by PCR. These patients ranged in age from 1 month to 95 years with a mean age of 7.5 years and a median age of 1.5 years. Fifty-seven percent of samples were from males, 43% from females, and 78% were collected from children 5 years of age or younger. All samples were previously tested by PCR for the commonly recognized respiratory viruses, HRV, HCoV, IFV, PIV, RSV, ADV, NL63, HKU1, HMPV and HBoV (Arden et al., 2006).
Nucleic acids were extracted from 0.2 ml of each specimen using the High Pure Viral Nucleic Acid kit (Roche Diagnostics, Sydney, Australia). Extracts were stored at −80 °C until analysed by PCR using the two primer sets originally described by Allander et al. (2007) for the detection of KIV (POLVP1-39F & POLVP1-363R) and confirmation of positive results (POLVP1-118F & POLVP1-324R). A 25 μl reaction volume was used in the PCR, containing Qiagen HotStar DNA polymerase (QIAGEN, Melbourne, Australia), and was cycled using reaction parameters as originally described, but extending the number of reaction cycles to 40.
The complete genomes of KIV detected in three patients were sequenced directly from clinical samples using nine primer pairs to amplify overlapping fragments of the circular genome. Amplification products were purified using the High Pure Viral Nucleic Acid kit (Roche Diagnostics, Sydney, Australia) and nucleotide sequencing reactions were performed using ABI BigDye version 3.1 on an ABI 3130xl Genetic Analyser (Applied Biosystems, Scoresby, Victoria, Australia). Raw KIV sequence data were aligned with Swedish KIV sequences using BioEdit version 7.0.5.3. Fractional nucleotide sequence identity matrices were used to examine the variability among Queensland and Swedish KIV sequences.
3. Results
We found 24 of the 951 respiratory specimens were positive for KIV, yielding a prevalence of 2.5% in this sample population. Although positive specimens were obtained from subjects ranging in age from 5 months to 68 years, the majority (83.3%) were detected in children 5 years of age or younger, and from specimens that were collected during May to July inclusive (Table 1 ).
Table 1.
Distribution and rate of KIV-positive results in respiratory samples collected from a Queensland population
| Age group (yrs) | Number tested | KIV positive | % positive |
|---|---|---|---|
| 0–2 | 624 | 12 | 1.9 |
| 3–5 | 117 | 8 | 6.8 |
| 6–10 | 61 | 2 | 3.4 |
| 11–20 | 57 | 0 | 0 |
| 21–30 | 8 | 1 | 11.1 |
| 31–40 | 13 | 0 | 0 |
| 41–50 | 16 | 1 | 5.9 |
| 51–60 | 23 | 0 | 0 |
| >60 | 32 | 0 | 0 |
| All samples | 951 | 24 | 2.5 |
In six cases (25.0%) KIV was detected in patients who showed evidence of co-infection with another respiratory virus. Four were co-detected with RSV, one with influenza A and one with HMPV. All these patients were younger than 3 years of age (Table 2 ).
Table 2.
Clinical presentation and demographic information of 24 patients who tested positive for KIV
| ID | Age | Sex | Month detected | Other virus detected | Clinical presentation |
|---|---|---|---|---|---|
| 1 | 5 months | M | January | Nil | None recorded |
| 2 | 6 months | M | May | Nil | LRTI, febrile |
| 3 | 6 months | M | May | RSV | Trachiitis |
| 4 | 6 months | F | June | Nil | URTI |
| 5 | 7 months | F | July | Nil | LRTI |
| 6 | 9 months | M | June | Nil | Cough, febrile |
| 7 | 10 months | M | June | NIL | Pneumonia |
| 8 | 10 months | M | July | NIL | URTI |
| 9 | 11 months | M | June | RSV | Bronchiolitis |
| 10 | 11 months | M | June | INF A | None recorded |
| 11 | 1 year 2 months | M | June | Nil | Febrile |
| 12 | 1 year 3 months | M | June | RSV | None recorded |
| 13 | 1 year 6 months | M | May | Nil | Bronchiolitis |
| 14 | 1 year 10 months | F | July | Nil | Fever, diarrhoea |
| 15 | 1 year 11 months | M | July | HMPV | Bronchiolitis |
| 16 | 2 years 2 months | M | July | Nil | None recorded |
| 17 | 2 years 9 months | M | April | RSV | None recorded |
| 18 | 3 years 2 months | F | December | Nil | URTI, febrile |
| 19 | 3 years 3 months | M | May | Nil | None recorded |
| 20 | 3 years 7 months | F | July | Nil | None recorded |
| 21 | 5 year 6m | M | June | NIL | Bronchiolitis |
| 22 | 6 years 10 months | M | July | Nil | Cough, respiratory distress |
| 23 | 23 years 6 months | F | January | Nil | Persistent cough |
| 24 | 43 years 11 months | M | July | Nil | Malaise, febrile |
In this study, the 24 patients who yielded positive specimens suffered from a wide range of respiratory symptoms consistent with upper and lower respiratory tract infection including cough, bronchiolitis and pneumonia (Table 2). In all cases there was clear clinical evidence of ARTI and in 18 cases KIV was the only virus detected. However, a significant number of patients still remained that showed similar symptoms in the absence of a recognised respiratory pathogen. Clearly, linking the presence of KIV with respiratory disease remains highly speculative in the absence of studies including “clinically well” control subjects.
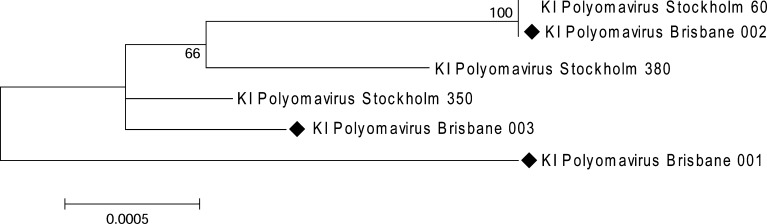
Results of sequencing showed a high level of conservation amongst the Queensland KIV sequences as well as between the Queensland and Swedish sequences with 99.4–100% similarity at a nucleotide level (Fig. 1 ). However these observations are limited by the small number of specimens examined in each study. One genome sequence derived from an NPA collected from a 6-month-old Queensland boy showed a 10 bp insertion at position 60–69 of the KIV genome (Table 3 ), suggesting that variable regions of the KIV genome may occur.
Fig. 1.
Phylogenetic relationships observed in a neighbour-joining analysis in which whole genome sequences from KI polyomavirus strains detected in Queensland children were aligned with Swedish sequences reported by Allander et al. (2007) (GenBank accession numbers EF127906-127908). Data were prepared using BioEdit (version 7.0.5.3), presented on a topology tree that was created using MEGA (version 3.1). Nodal confidence values indicate the results of bootstrap re-sampling (n = 1000). The nucleotide sequences of KI polyomavirus Brisbane 001, 002 and 003 can be accessed in GenBank under EF520287-520289.
Table 3.
Sequences of the first 100 nucleotides of KI virus detected in Queensland children compared to Swedish sequences. Sequence for KI polyomavirus Brisbane 001 shows a 10 base pair insertion at position 60–69 (sequences are numbered as originally described by Allander et al. (2007))
4. Discussion
This study follows the original report from Sweden in showing that KIV may be found in respiratory secretions from patients with ARTI, and is the first to demonstrate likely global presence. The incidence of KIV in our sample population was significantly greater than that reported by Allander et al. (2007) and may reflect genuine differences between these populations, or be due to differences in sample processing and analysis. Our results showed that the majority of positive detections were made in the Australian winter months (May–July). However, considering that the sample population tested in this study was not representative of a complete calendar year, these results should be viewed with caution, and a larger sample population representative of all seasons must be examined before seasonality of infection may be established for this virus. Clearly further, more comprehensive, studies must be performed to establish the true incidence of this virus in different population groups, the genetic diversity of circulating viral strains, and most importantly, its causal role in respiratory and other disease.
This study provides preliminary data examining the role of KIV as a potential respiratory pathogen involved in ARTI. It remains possible that the virus is not involved in respiratory disease and its presence in the respiratory tract simply reflects its mode of transmission similar to JCV and BKV. In 75% of the KIV-positive specimens in our study, no other viral respiratory pathogen was detected hinting at a causal association between KIV and respiratory disease. Establishing the association of KIV with a particular disease will be challenging and will require extensive further investigation. However, considering the oncogenic potential of polyomaviruses in mammals, the results of such studies may have important medical implications.
Acknowledgements
This study was supported by the Royal Children's Hospital Foundation Grant I 922-034, sponsored by the “Woolworths Fresh Futures” Appeal. We thank the staff of the Molecular Diagnostic Unit, Microbiology, Queensland Health Pathology Service Central for their help in the collection and processing of clinical samples.
References
- Allander T., Tammi M.T., Eriksson M., Bjerkner A., Tiveljung-Lindell A., Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci USA. 2005;102:12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allander T., Andreasson K., Gupta S., Bjerkner A., Bogdanovic G., Persson M.A. Identification of a third human polyomavirus. J Virol. 2007;81:4130–4136. doi: 10.1128/JVI.00028-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arden K.E., McErlean P., Nissen M.D., Sloots T.P., Mackay I.M. Frequent detection of human rhinoviruses, paramyxoviruses, coronaviruses, and bocavirus during acute respiratory tract infections. J Med Virol. 2006;78:1232–1240. doi: 10.1002/jmv.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur R.R., Shah K.V., Baust S.J., Santos G.W., Saral R. Association of BK viruria with hemorrhagic cystitis in recipients of bone marrow transplants. N Engl J Med. 1986;315:230–234. doi: 10.1056/NEJM198607243150405. [DOI] [PubMed] [Google Scholar]
- Behzad-Behbahani A., Klapper P.E., Vallely P.J., Cleator G.M., Khoo S.H. Detection of BK virus and JC virus DNA in urine samples from immunocompromised (HIV-infected) and immunocompetent (HIV-non-infected) patients using polymerase chain reaction and microplate hybridisation. J Clin Virol. 2004;29:224–229. doi: 10.1016/S1386-6532(03)00155-0. [DOI] [PubMed] [Google Scholar]
- Goudsmit J., Wertheim-van Dillen P., van Strien A., van der Noordaa J. The role of BK virus in acute respiratory tract disease and the presence of BKV DNA in tonsils. J Med Virol. 1982;10:91–99. doi: 10.1002/jmv.1890100203. [DOI] [PubMed] [Google Scholar]
- Heikkinen T., Jarvinen A. The common cold. Lancet. 2003;361:51–59. doi: 10.1016/S0140-6736(03)12162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Markowitz R.B., Thompson H.C., Mueller J.F., Cohen J.A., Dynan W.S. Incidence of BK virus and JC virus viruria in human immunodeficiency virus-infected and -uninfected subjects. J Infect Dis. 1993;167:13–20. doi: 10.1093/infdis/167.1.13. [DOI] [PubMed] [Google Scholar]
- Mulholland K. Global burden of acute respiratory infections in children: implications for interventions. Pediatr Pulmonol. 2003;36:469–474. doi: 10.1002/ppul.10344. [DOI] [PubMed] [Google Scholar]
- Stolt A., Sasnauskas K., Koskela P., Lehtinen M., Dillner J. Seroepidemiology of the human polyomaviruses. J Gen Virol. 2003;84:1499–1504. doi: 10.1099/vir.0.18842-0. [DOI] [PubMed] [Google Scholar]
- Sundsfjord A., Spein A.R., Lucht E., Flaegstad T., Seternes O.M., Traavik T. Detection of BK virus DNA in nasopharyngeal aspirates from children with respiratory infections but not in saliva from immunodeficient and immunocompetent adult patients. J Clin Microbiol. 1994;32:1390–1394. doi: 10.1128/jcm.32.5.1390-1394.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hoogen B.G., de Jong J.C., Groen J., Kuiken T., de Groot R., Fouchier R.A. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoek L., Pyrc K., Jebbink M.F., Vermeulen-Oost W., Berkhout R.J., Wolthers K.C. Identification of a new human coronavirus. Nat Med. 2004;10:368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P.C.Y., Lau S.K., Chu C.M., Chan K.H., Tsoi H.W., Huang Y. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005;79:884–895. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]