Abstract
WHO recommendations for early antimicrobial treatment of childhood pneumonia have been effective in reducing childhood mortality, but the last major revision was over 10 years ago. The emergence of antimicrobial resistance, new pneumonia pathogens, and new drugs have prompted WHO to assemble an international panel to review the literature on childhood pneumonia and to develop evidence-based recommendations for the empirical treatment of non-severe pneumonia among children managed by first-level health providers. Treatment should target the bacterial causes most likely to lead to severe disease, including Streptoccocus pneumoniae and Haemophilus influenzae. The best first-line agent is amoxicillin, given twice daily for 3–5 days, although co-trimoxazole may be an alternative in some settings. Treatment failure should be defined in a child who develops signs warranting immediate referral or who does not have a decrease in respiratory rate after 48–72 h of therapy. If failure occurs, and no indication for immediate referral exists, possible explanations for failure should be systematically determined, including non-adherence to therapy and alternative diagnoses. If failure of the first-line agent remains a possible explanation, suitable second-line agents include high-dose amoxicillin–clavulanic acid with or without an affordable macrolide for children over 3 years of age.
Introduction
Each year, clinical pneumonia occurs in an estimated 156 million children aged under 5 years,1 and causes approximately a fifth of all deaths among such children, most of whom are in low-income nations.2 To reduce the morbidity and mortality of pneumonia in children, guidelines currently known as the Integrated Management of Childhood Illness (IMCI) have been developed by WHO and partners for first-level health systems. IMCI has been implemented by many organisations and reduces mortality effectively,3, 4 although implementation can be improved.5, 6 These guidelines include recommendations for the case management of acute respiratory illness. They indicate when referral is needed and specify appropriate antimicrobial agents when referral is not needed.
WHO recommendations for the treatment of pneumonia have provided critical guidance to first-level health-care workers worldwide, but the last major revision is more than 10 years old. The first WHO recommendations for respiratory disease case management were published in a 1981 WHO memorandum.7 The memorandum was aimed at encouraging a systematic approach to the management of children with possible pneumonia in resource-poor settings, based on simple algorithms and empirical treatment. Recommended agents included drugs available at the time of publication, with both intramuscular and oral drugs listed, such as procaine penicillin, ampicillin, erythromycin, co-trimoxazole, and sulfamethoxypyridazine, and noted that there was as yet little experience with co-trimoxazole. Second-line agents for use in children still ill after 48 h included chloramphenicol or oxacillin.
WHO published a revised document in 1991, after much experience had been gained with the case-management approach and empirical treatment.8 For treatment of non-severe pneumonia at the first-level health facility, this document recommended oral co-trimoxazole as the preferred agent, with injectable procaine penicillin and oral amoxicillin as alternatives. A 2005 technical update of the WHO IMCI guidelines recommended oral amoxicillin (50 mg/kg per dose, in two divided doses) or co-trimoxazole (8 mg/kg trimethoprim per dose, in two divided doses) for the treatment of non-severe pneumonia;9 if antimicrobial resistance to co-trimoxazole was high, oral amoxicillin was preferred.
During the past decade, various organisations have issued recommendations for paediatric pneumonia treatment.10, 11, 12, 13, 14 These recommendations are primarily intended for high-income to middle-income nations. New information on antimicrobial resistance, the changing epidemiology of pneumonia, and the availability of a broader range of first-line and second-line antimicrobial agents provides the impetus for updated recommendations for antimicrobial treatment of non-severe pneumonia among children assessed by first-level health providers, often with basic health training.
The WHO Department of Child and Adolescent Health and Development selected and assembled this panel to review the current literature on childhood pneumonia and to define further the most appropriate antimicrobial agents for treatment of non-severe pneumonia. Specifically, we were asked to define the appropriate first-line antimicrobial agent, treatment failure, when to change therapy, and appropriate second-line antimicrobial agents.
Methods
The international panel consisted of nine clinicians and researchers with experience in defining and treating pneumonia among children in various settings. We communicated by regular conference calls and by email. After reviewing the available evidence, we discussed issues such as interpretation of aetiological study results and antimicrobial resistance data. The initial focus was to identify appropriate first-line and second-line antimicrobial agents for the empirical treatment of non-severe pneumonia in children by first-level health-care workers. However, we determined that to address second-line therapy, a definition of treatment failure and determination of when to change therapy were required (panel ).
Panel. Issues addressed by WHO panel.
Aim 1
Identify the most appropriate first-line antimicrobial agent
Aim 2
Define treatment failure
Aim 3
Clarify when it is appropriate to change therapy in children who do not need referral
Aim 4
Prescribe the most appropriate second-line antimicrobial agent for those children who fail the first-line therapy
Search strategy
A literature search was done to generate a foundation for recommendations. The results for appropriate first-line and second-line antimicrobial agents (webtable) were similar those obtained by a Cochrane review.15 Evidence was collected with a focus on publications from January, 1991 (the date of the last recommendations) through September, 2008, by searches of PubMed, EMBASE, and the Cochrane Central Registrar of Controlled Trials, in addition to bibliographies of relevant articles and the author's records. Search terms were “pneumonia”, “child”, “childhood”, “pediatric”, “paediatric”, “antibiotic”, “trial”, and “cohort”, and was limited to available articles in English or an English abstract that included children with pneumonia under age 5 years in the study. For the selection of antimicrobial agents, 36 articles that assessed more than one antimicrobial agent and the seven articles that assessed different durations or doses of antimicrobial agents by use of a randomised controlled trial were included in the review. Articles were excluded if no treatment failure data were available as an outcome or if they exclusively enrolled children with very severe pneumonia. If an article presented results of children with a spectrum of diagnosis (eg, sinusitis, otitis media, and pneumonia) only results for children with pneumonia were included, if possible. These articles provide the basis, along with the panel's experience, for the recommendations.
Development of recommendations
Initial recommendations were drafted and then circulated to a broader group of reviewers for comment. The broader group included experts with experience in the treatment and epidemiology of pneumonia, as well as representatives from the WHO regional offices. Table 1 outlines the recommendations developed, with strength of each major recommendation and the quality of supporting evidence based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system.16, 17 The GRADE quality of evidence categories include high, intermediate, low, and very low, and was determined by the panel's review of the literature. The studies available for this review, with few exceptions, were observational in nature rather than blinded randomised trials, and therefore, the GRADE quality of evidence was rarely higher than intermediate. After the review by the broader group, the strength of each recommendation was determined by the panel members.
Table 1.
Outline of recommendations
| Strength of recommendation | Quality of evidence | |
|---|---|---|
| Recommendation 1 | ||
| Amoxicillin as initial antimicrobial agent for the treatment of non-severe pneumonia. | Strong | High |
| Co-trimoxazole may be an acceptable alternative | Weak | Intermediate |
| Recommendation 2 | ||
| Definitions of treatment failure | Strong | Low |
| Recommendation 3 | ||
| Systematic assessment for treatment or referral | Strong | Very low |
| Recommendation 4 | ||
| Second-line antimicrobial agents | Strong | Very low |
See main text for full details of recommendations.
Recommendation 1
Amoxicillin is the preferred initial antimicrobial agent for the treatment of non-severe pneumonia. The dose of amoxicillin is 50 mg/kg per day in two divided doses for a 3-day treatment course in areas with low HIV prevalence, and 5 days in areas of high HIV prevalence. In some situations, such as where local evidence clearly indicates infrequent resistance, co-trimoxazole (8 mg/kg trimethoprim in two divided doses) may be an acceptable alternative.
Rationale and evidence summary
Pneumonia is, strictly speaking, a pathological diagnosis, determined by clinical means. Radiological consolidation is commonly used as a surrogate, but is often not feasible at first-level health facilities.18 Pneumonia definitions used in the IMCI guidelines were developed and validated to identify children with specific treatment needs,7 such as those needing antimicrobial therapy or referral to higher levels of care. Non-severe pneumonia is diagnosed in a child with cough or difficulty breathing accompanied by tachypnoea, defined as a respiratory rate of at least 40 breaths per min in a child aged 12–59 months, or at least 50 breaths per min in an infant aged 2–11 months. Use of these criteria identifies 80% of children with pneumonia who need antimicrobial therapy.7 Children aged 2–59 months require immediate referral if they have signs of severe or very severe pneumonia (lower chest indrawing or central cyanosis), stridor when calm, or IMCI-defined danger signs (inability to drink or breastfeed, convulsions, persistent vomiting, lethargy, or unconsciousness). Children aged less than 2 months with pneumonia have, by definition, severe pneumonia due to their higher risk for mortality, and thus require referral.
Identification or prediction of the likely organisms that cause pneumonia is the most important step in determining appropriate antimicrobial therapy. Despite the importance of understanding the aetiology of pneumonia, few recent studies have been done in resource-poor settings,19, 20 or in areas with high HIV prevalence.21, 22 The most useful studies that have been done generally use one of two approaches: vaccine probe methods or comprehensive diagnostic testing protocols.
Vaccine probe studies may provide the best possible estimates of pathogen-specific pneumonia burden because they are not limited by insensitive diagnostic testing practices.23 However, these studies are extremely costly and generally estimate only the role of vaccine-type strains of the pathogen and often focus on severe or hospitalised pneumonia cases. By contrast, studies that incorporate a wide range of diagnostic tests offer the possibility of detecting many pathogens. The diagnostic tests used, however, are often insensitive and sometimes not specific, which limits data quality.24
Directly obtaining specimens from the site of infection by lung aspiration before antimicrobial administration, potentially a gold-standard approach in children, produces positive results in 62% of appropriately selected cases, according to a review of 13 studies.25 Although this approach produces a relatively high yield, it is limited to peripheral lobar pneumonias that may selectively decrease the yield of several pathogens and therefore may not be representative of all severe pneumonia.
The results of aetiological studies require cautious interpretation because estimates of the proportion of pneumonia caused by the different pathogens do vary. The sensitivity of culture-based techniques for identifying bacteria is compromised by previous antimicrobial administration, which is often not assessed. If antimicrobial exposure before culture is common, the measured results in culture-based studies may significantly underestimate those organisms that are sensitive to commonly prescribed antimicrobial agents, such as Streptococcus pneumoniae and Haemophilus influenzae, and overestimate organisms such as Staphylococcus aureus.
Although the limitations listed above are numerous, successful treatment ultimately depends on targeting the causative agents. Therefore, despite these limitations, estimates of the common pathogens that cause pneumonia in children are required to determine appropriate empirical antimicrobial therapy. Whereas these recommendations are for non-severe pneumonia, it is important to remember that aetiological studies usually enrol hospital inpatients with severe pneumonia. The common bacterial and viral causes of pneumonia pathogens are shown in table 2 . Estimates include rather broad ranges of the plausible aetiological fractions. Most studies lack comprehensive investigation of viral causes of pneumonia, but in the studies that do provide such estimates, approximately 25% (range 9–64%) of pneumonia acquired in the community has a positive viral diagnostic test.50, 51, 52, 53 Identification of a virus does not necessarily imply that the pneumonia is exclusively caused by that virus, because viral and bacterial agents may coexist, making the role of each pathogen uncertain.58, 59, 60, 61
Table 2.
Common pathogens that cause pneumonia in otherwise healthy children aged 2–59 months
| Estimated percentage* | Comments | |
|---|---|---|
| Bacterial (20–50%) | ||
| Streptococcus pneumoniae | 17–37% | Estimates based on proportion of radiographically confirmed pneumonia prevented by vaccination with 7-valent and 9-valent vaccine (vaccine probe studies),21, 26, 27 and supported by lung aspiration studies25 |
| Haemophilus influenzae | 0–31% | Increasing use of highly efficacious vaccine against disease by H influenzae type b may decrease its role as a pathogen |
| Non-type b may play a greater role in non-severe pneumonia than type b28 | ||
| Found to be a significant cause of pneumonia in all vaccine probe studies,29, 30, 31 except one,32 and in lung aspiration studies25 | ||
| Staphylococcus aureus | 1–33% | Presents clinically as a severe, necrotising pneumonia with rapid progression25 |
| Non-typhoidal salmonellae | 0–28% | Bacteraemia may present with features consistent with a clinical diagnosis of pneumonia33, 34, 35 |
| Estimates are based on studies from tropical Africa33, 36 | ||
| Associated with non-severe pneumonia in some malaria-endemic regions of Africa34 | ||
| Mycoplasma pneumoniae | 5% | Limited diagnostic capacities in low-income countries37, 38, 39, 40 |
| Proportion of pneumonia associated with infection increases with age, the greatest burden is in children aged >3 years41 | ||
| Assumption that infections do not cause significant morbidity or mortality lacks evidence to be either validated or invalidated42, 43 | ||
| Chlamydophila pneumoniae | 3–10% | Limited diagnostic capacities in low-income countries37, 38, 39 |
| Proportion of pneumonia associated with infection increases with age, the greatest burden is in children aged >3 years41 | ||
| Poor quality serological data for very young children44 | ||
| Moraxella catarrhalis | 0–9% | Often not the focus of pneumonia microbiological studies45 |
| Klebsiella pneumoniae | 0–4% | One study noted a higher proportion of 14% in children with previous antimicrobial use45 |
| Rare exception in malnourished children46 | ||
| Viral (9–64%) | ||
| Respiratory syncytial virus | 1–39% | Particularly important in young infants47 |
| Influenza viruses | 0–22% | Important cause throughout age range47 |
| Increasingly documented in the tropics48 | ||
| Adenoviruses | 0–54% | Limited diagnostic testing and use of poor or insensitive tests47 |
| Parainfluenza viruses | 0–46% | Occurrence in alternating years means that single-year studies have limited value49 |
| Human metapneumovirus | 2–8% | Recent but well-documented cause of pneumonia50, 51, 52, 53 |
| Others (including bocavirus, coronaviruses, and rhinoviruses) | 4–30% | Recent PCR-based studies more consistently identify new viruses, but their significance remains to be defined54, 55, 56 |
These estimates of aetiological burden have wide ranges. Variation may be real, due to increased proportions of aetiologies due to high HIV prevalence, as well as seasonal (eg, influenza) and geographical (eg, Salmonella) variability. However, the primary source of variability may be due to measurement, either enrolment criteria (hospitalised versus outpatient enrolment), inadequate diagnostic testing of blood cultures with low yield (eg, blood culture), or misclassification (eg, urine antigen testing). Previous antimicrobial administration also may result in underestimation of some agents, and poor laboratory quality can also play an important part.57 Zero percentages (except in the case of Klebsiella) are often due to lack of diagnostic testing and the use of poor or insensitive tests, which are the important reasons for failure to consistently identify these pathogens, although in some cases true seasonal or geographical variations may contribute.
These recommendations for non-severe pneumonia are limited by the lack of data on the causes of pneumonia among children treated outside hospital, as described above. However, an important goal of treatment for non-severe pneumonia is to prevent the progression to severe pneumonia. Therefore, directing antimicrobial therapy for non-severe pneumonia towards the pathogens known to cause severe pneumonia is believed to be appropriate.
Pathogens such as S pneumoniae and H influenzae are prominent treatable causes of severe pneumonia, and first-line antimicrobial therapy has historically been directed primarily at these pathogens. An approach that targets these two aetiological agents has been repeatedly shown to be effective at reducing pneumonia morbidity and overall mortality through studies and clinical experience during the past 15 years.3 Altering empirical first-line treatment to better cover atypical agents, S aureus, non-typhoidal salmonellae, or others, might be considered on theoretical grounds based on the above-referenced studies, but such an approach lacks evidence that it would reduce morbidity or mortality.
Many studies have compared the efficacy of different antimicrobial agents for treating pneumonia in children (webtable). However, despite the large number of studies, most are limited by small sample sizes, and lack the power to conclusively show non-inferiority. These studies are also hampered by the inability to accurately and consistently define treatment failure, largely due to the limited resources available in the field.
Interpretation is also limited by the so-called “Pollyanna phenomenon”. In settings where an antimicrobial agent is compared with a standard agent, the potentially dangerous subset of bacterial cases that are the intended target of study are diluted by the inclusion of milder, self-limited, viral disease, which results in poor antimicrobial agents seeming to be more efficacious, and superior antimicrobial agents seeming less efficacious against the standard agent, as noted by Marchant and colleagues62 in the setting of otitis media.
The lack of bacterial endpoints complicate our understanding of the clinical relevance of antimicrobial resistant for the management of pneumonia in the community. Our perceptions of the clinical relevance of amoxicillin, macrolide, and co-trimoxazole resistance for oral outpatient management of pneumonia are all based on the failure of these oral agents to eradicate resistant S pneumoniae from the middle ear.62
Although Straus and colleagues63 suggest that co-trimoxazole has a higher failure rate in severe pneumonia treatment than amoxicillin, the role of resistance was unclear, even though resistance has been the most frequently proposed explanation. A sub-analysis did not show a significant increase in failure among co-trimoxazole-resistant S pneumoniae or H influenzae treated with co-trimoxazole.63 Few pneumonia studies have described the clinical impact of co-trimoxazole resistance on the treatment of pneumonia.64
In-vitro resistance to co-trimoxazole correlates with poor clinical outcome in the treatment of these pathogens in acute otitis media. Trials of treatment for acute otitis media have measured bacteriological failures by use of tympanocentesis, comparing middle-ear fluid cultures before and after therapy to show bacterial eradication with treatment. In one tympanocentesis study,65 co-trimoxazole resistance resulted in higher rates of bacteriological failures for H influenzae and S pneumoniae. A caveat is that middle-ear fluid concentrations of many drugs are substantially lower than comparable lung concentrations, and therefore antimicrobial agents used in therapy for acute otitis media may perform more poorly against strains with decreased susceptibility than antimicrobials used in pneumonia therapy. With this caveat, the available data suggest that in vitro co-trimoxazole resistance is meaningful in some clinical circumstances. This conclusion, if relevant for pneumonia, has important implications for countries where co-trimoxazole resistance is common.
First-line antimicrobial agents should be effective, reliable, widely available, and affordable in resource-poor settings. The 2005 technical update of the WHO IMCI guidelines recommended oral amoxicillin or co-trimoxazole as first-line treatment for non-severe pneumonia because of their low cost and wide spectrum of coverage.9
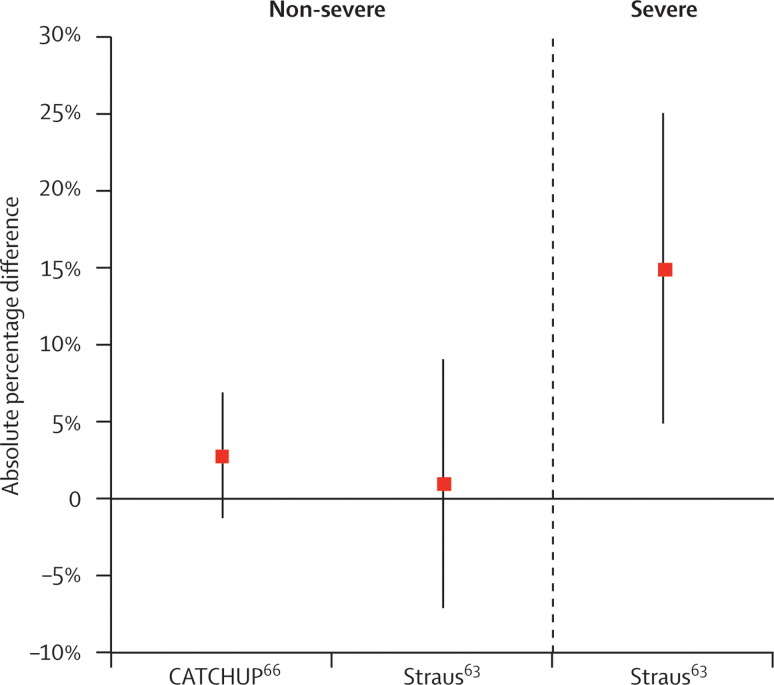
Historically, co-trimoxazole and amoxicillin have been evaluated for the treatment of non-severe pneumonia. Two studies, both done in Pakistan, have compared amoxicillin to co-trimoxazole in children aged under 5 years.63, 66 The absolute difference in treatment failure between children with treatment failure after taking co-trimoxazole relative to amoxicillin is illustrated in figure 1 . The study by Straus and colleagues63 included children with both severe and non-severe pneumonia, whereas the study by the CATCHUP (Co-trimoxazole Amoxicillin Trial in CHildren Under 5 years for Pneumonia) study group66 included only children with non-severe pneumonia.
Figure 1.
Absolute percentage difference in treatment failure among children with pneumonia treated with co-trimoxazole versus amoxicillin
Analysis of two studies done in Pakistan and their pooled results given with 95% CIs. Straus et al63 showed a significant difference in the proportion of children with severe pneumonia who were treatment failures from 33% failing co-trimoxazole to 18% failing amoxicillin. The CATCHUP group66 showed 19% of children failing co-trimoxazole and 16% failing amoxicillin.
The duration and dosage of amoxicillin therapy has been assessed and summarised in a WHO 2003 consultative meeting report.67 Two clinical trials in India and Pakistan examined 3-day (short) versus 5-day (standard) amoxicillin treatment courses for pneumonia and showed equal effectiveness of the two durations.68, 69 Shortened courses have not been as well studied in areas of high HIV prevalence, where a substantially greater burden of severe bacterial pneumonia among HIV-infected persons exists. Prudence indicates that shortening treatment to a 3-day course should be studied before being implemented in such settings. Variations of amoxicillin dose have not been well studied for the treatment of childhood pneumonia. One study of 836 children compared 3 days of amoxicillin in 45 mg/kg versus 90 mg/kg doses and did not show a significant difference in treatment failure 5 days after initiation of therapy.70
Recommendation 2
Treatment failure is defined as the development of lower chest-wall indrawing, central cyanosis, stridor while calm, or IMCI-defined danger signs at any time during a child's illness or a persistently raised respiratory rate at 72 h (48 h in an area of high HIV prevalence).
Rationale and evidence summary
Treatment failure was previously vaguely defined as “the same” (ie, a respiratory rate persistently above the age-appropriate IMCI cut-off) or deterioration after 2 or 3 days of therapy.8 Deterioration is development of chest indrawing, central cyanosis, stridor when calm or IMCI-defined danger signs. If the child does not deteriorate, these signs and symptoms are traditionally assessed at a scheduled follow-up visit 48 h after the initiation of therapy. However, on the basis of results of a study in Pakistan, 72 h may be acceptable in countries with low HIV prevalence.70 These results should be replicated in other low-income countries, particularly those with high HIV prevalence. We believe that children who have a persistently raised respiratory rate and no indication for immediate referral should undergo a brief but systematic assessment to determine whether second-line therapy would be beneficial.
Recommendation 3
A brief but systematic assessment should be used for children who have failed therapy for non-severe pneumonia as determined on follow-up by the health worker. Referral for in-patient treatment should occur, if appropriate. If immediate referral is not warranted, the assessment should determine possible causes of the failure of therapy.
Rationale and evidence summary
The literature does not describe when treatment failure should prompt a change of therapy. Treatment failure may occur for many reasons, only one of which is use of a drug to which the infecting agent is not susceptible. Many of these reasons are described in table 3 , and most require assessment in hospital and specific therapies. If a child develops signs indicative of the need for immediate referral, before or after treatment is initiated, they should be referred immediately.7, 9 We believe that children who fail to improve but do not require immediate referral should be systematically assessed before changing antimicrobial agents. This assessment can begin with the first-level health worker determining whether failure is due to inability or unwillingness to take the medicine appropriately. If the health worker believes that the child has taken the antimicrobial agent correctly, other conditions resulting in treatment failure should be considered. If an initial assessment by the health worker indicates that these considerations are unlikely and referral is not warranted, a second-line agent should expand coverage.
Table 3.
Potential reasons for treatment failure for WHO-defined pneumonia at 72 h and possible solutions
| Frequency* | Possible solution† | |
|---|---|---|
| Wrong diagnosis | ||
| Reactive airways/asthma | Common | Physician referral |
| Malaria | Geographically focused | Hospital for blood smear |
| Foreign body | Rare | Hospital assessment |
| Anaemia | Rare | Hospital assessment |
| Cardiac disease | Rare | Hospital assessment |
| Others | Rare | Hospital assessment |
| Host failure | ||
| HIV/AIDS | Geographically focused | Hospital for HIV test |
| Malnutrition | Geographically focused | Hospital for intensive treatment |
| Pulmonary maldevelopment | Rare | Hospital assessment |
| Others | Rare | Hospital assessment |
| Complication | ||
| Empyema | Uncommon | Hospital for drainage |
| Abscess | Rare | Hospital for radiography |
| Others | Rare | Hospital assessment |
| Non-susceptible pathogen | ||
| Viral infection (respiratory syncytial virus, influenza, others) | Common | Observation or hospital |
| Tuberculosis | Geographically focused | Four drugs and hospital assessment |
| Mycoplasma, Chlamydophila | Uncommon | Appropriate antibiotics (eg, macrolide, doxycycline, or fluroquinolone) |
| Non-susceptible S pneumoniae | Uncommon | Appropriate antibiotics (eg, high-dose amoxicillin, ceftriaxone) |
| Beta-lactamase-producing Haemophlus influenzae | Uncommon | Appropriate antibiotics (eg, amoxicillin–clavulanic acid, ceftriaxone) |
| Non-typhoidal salmonellae | Geographically focused | Appropriate antibiotics in hospital |
| Staphylococcus aureus | Rare | Appropriate antibiotics in hospital |
| Strongyloides, other parasites | Rare | Ivermectin etc, in or out of hospital |
| Endemic fungi | Rare | Hospital assessment and anti-fungal therapy |
| Others | Rare | Hospital assessment |
Common agents may be responsible for at least a third of outpatient pneumonia treatment failures; uncommon agents may be responsible for a minor fraction; rare agents are probably responsible for only occasional treatment failures; globally uncommon agents may be common in certain geographic areas, although they are uncommon as causes for pneumonia treatment failure globally. Based on data from Heffelfinger et al71 and discussions of the panel based on their clinical experience.
Note that referral to the next level facility instead of a hospital may occasionally be appropriate, depending on the resources at the facility and the suspected condition.
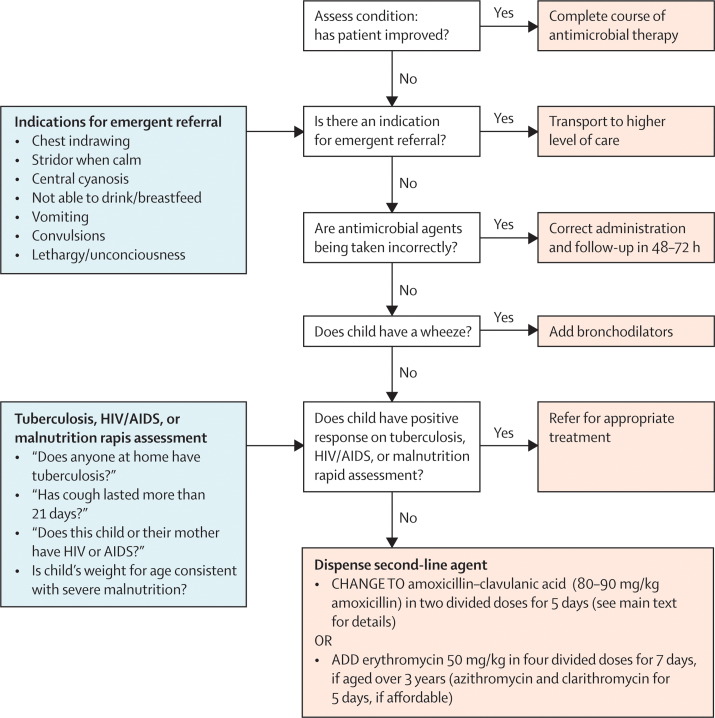
This assessment by the first-level health worker may be guided by an algorithm. The efficacy of algorithms has been shown in the assessments of initial case management by acute respiratory illness guidelines.3, 4, 7 However, we are not aware of any studies that show the efficacy of an algorithm that systematically assesses a child who does not improve after therapy. Figure 2 shows an example of an algorithm developed by us, which aims to be simple for health workers to use accurately and consistently. It addresses the major causes of treatment failure, but is not a validated tool, and is offered here purely as an example.
Figure 2.
Example algorithm of how to systematically assess children aged >2 months and <5 years, initially diagnosed and treated with non-severe pneumonia and who returned for follow-up, in low HIV prevalence settings, based on the experience and recommendations of the panel
This assessment is intended to supplement and not to replace the clinical judgment of the first-level health worker. If Integrated Management of Childhood Illness guidelines have been followed, the child should have been assessed for malnutrition and HIV in settings with high prevalence. This figure is offered only as an example of such an algorithm that can be developed.
Recommendation 4
In the setting of first-level health providers, children initially treated with amoxicillin who have a persistently raised respiratory rate and no indication for referral should receive high-dose amoxicillin with clavulanic acid (80–90 mg/kg per day amoxicillin) for second-line therapy to provide coverage for the major pathogens likely to cause severe disease. A 5-day treatment course should be prescribed for the second-line antimicrobial agents. For children over 3 years of age, an affordable macrolide or azalide (eg, 50 mg/kg erythromycin in four divided doses for 7 days) may be added to the existing regimen for a 5-day or 7-day treatment course. For children failing first-line treatment with co-trimoxazole, the recommendation is to switch to a 5-day course of amoxicillin (50 mg/kg).
Rationale and evidence summary
We define second-line antimicrobial agents as those that are used in the setting of treatment failure when no indication for immediate referral is present (ie, IMCI danger signs, lower chest indrawing, stridor, or central cyanosis) and other reasons for treatment failure have been excluded. No studies were identified that assessed second-line antimicrobial agents; therefore, second-line antimicrobial agents should logically be selected to treat organisms that fail first-line therapy. Whereas a substantial proportion of failures of first-line agents may be due to mild and self-limited viral infections, the second-line agent should ensure coverage of resistant organisms and cover a broader range of organisms that would not be treated with typical first-line agents; in some settings, coverage may be extended to include S aureus or non-typhoidal salmonellae.
A second-line antimicrobial agent should broaden or enhance coverage in the setting of treatment failure. Our recommendations on the best second-line antimicrobial agents are based primarily on efficacy data from clinical trials that assessed antimicrobial activity against the common causes of pneumonia, pharmacokinetic–pharmacodynamic properties, toxicity, tolerability, and ease of administration. Of those antimicrobial agents that were efficacious, cost was thought to be the next most important factor. Only antibacterial agents with activity against the two most important aetiological agents, S pneumoniae and H influenzae, were considered. These attributes for possible antimicrobial agents for the treatment of pneumonia are shown in table 4 from several classes of agents. The listed beta-lactam antimicrobial agents would be the most effective against resistant S pneumoniae and H influenzae, but are not active against Mycoplasma pneumoniae, Chlamydophila pneumoniae, and non-typhoidal salmonellae. Preference is given to antimicrobial agents that are thought to be well-tolerated, and with once daily or, if necessary, twice daily dosing to increase compliance.
Table 4.
Antimicrobial agents used for treatment of community-acquired pneumonia
| Activity according to paediatric pneumonia trials* |
Efficacy in vitro†72, 73, 74 |
Toxic effects73 | Ease of administration(daily doses)73 | Range of antimicrobial agent costs‡for treatment course (US$)75, 76 | Dosage | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| S pneumoniae | H influenzae | S aureus | Non-typhoidal salmonellae | Atypical pneumonia | ||||||
| Aminopenicillins | ||||||||||
| Amoxicillin | yyy | >90% | 70–90% | <40% | 40–70% | <40% | .. | 2 or 3 | 0·11–0·23 | One 250 mg tablet twice daily |
| High-dose amoxicllin | .. | .. | .. | <40% | .. | .. | .. | 2 or 3 | 0·21–0·45 | Two 250 mg tablets twice daily |
| Ampicillin | yy | 70–90% | 40–70% | <40% | <40% | <40% | Diarrhoea | 4 | 0·21–0·65 | One 250 mg tablet four times daily |
| Amoxicillin–clavulanic acid | yy | >90% | >90% | 40–70% | <40% | <40% | Diarrhoea | 2 or 3 | 0·82–3·10 | Half 500 mg tablet twice daily |
| High-dose amoxicillin–clavulanic acid | .. | >90% | >90% | 40–70% | .. | <40% | Diarrhoea | 2 or 3 | 1·63–6·21 | One 500 mg tablet twice daily |
| Penicillins | ||||||||||
| Oral penicillin | y | 40–70% | <40% | <40% | <40% | <40% | .. | 3 or 4 | 0·05–0·58 | .. |
| Intramuscular penicillin§ | yy | 70–90% | <40% | <40% | <40% | <40% | .. | 4 to 6 | 0·08–0·47 | 1 M units |
| Cephalosporins | ||||||||||
| Cephalexin | .. | 40–70% | <40% | 70–90% | <40% | <40% | .. | 4 | 0·22–0·22 | Half 250 mg dose orally every 6 h |
| Cefaclor | .. | 40–70% | 70–90% | 70–90% | .. | <40% | .. | 3 | .. | Syrup |
| Cefuroxime | yy | 70–90% | 70–90% | 70–90% | .. | <40% | .. | 2 | 1·25–3·44 | Half 250 mg dose orally twice daily |
| Cefprozil | .. | 70–90% | 40–70% | 70–90% | .. | <40% | .. | 2 | .. | Syrup |
| Cefpodoxime | .. | 70–90% | 70–90% | 70–90% | .. | <40% | .. | 1 | .. | Syrup |
| Cefixime | .. | 40–70% | >90% | 40–70% | .. | <40% | .. | 1 | 0·20–0·20 | Half 200 mg tablet daily |
| Ceftibuten | .. | 40–70% | >90% | 40–70% | .. | <40% | .. | 2 | … | Syrup |
| Intramuscular ceftriaxone§ | y | >90% | >90% | 70–90% | 70–90% | <40% | .. | 1 | 1·34–14·44 | 500 mg vial |
| Macrolides | ||||||||||
| Erythromycin | yy | 40–70% | 40–70% | 40–70% | <40% | 70–90% | .. | 4 | 0·10–0·22 | Half 250 mg tablet per dose |
| Clarithromycin | y | 40–70% | 40–70% | 70–90% | <40% | 70–90% | .. | 2 | 0·90–0·90 | Syrup |
| Azithromycin | yy | 40–70% | 70–90% | 70–90% | 40–70% | 70–90% | .. | 1 | 0·15–0·57 | Syrup |
| Fluoroquinolones | ||||||||||
| Ciprofloxacin | .. | <40% | 70–90% | 70–90% | 70–90% | 40–70% | Possible cartilage growth | 2 | 0·07–0·15 | 250 mg tablet twice daily |
| Ofloxacin | .. | <40% | 70–90% | 70–90% | 70–90% | 70–90% | Possible cartilage growth | 2 | 0·09–0·22 | Half 200 mg tablet twice daily |
| Levofloxacin | .. | >90% | >90% | 70–90% | 70–90% | 70–90% | Possible cartilage growth | 1 | .. | Half 250 mg tablet orally every 24 h |
| Moxifloxacin | .. | >90% | >90% | 70–90% | 70–90% | 70–90% | Serious cardiac | .. | .. | No paediatric dosing available |
| Tetracyclines | ||||||||||
| Doxycycline | .. | 70–90% | 70–90% | 40–70% | 40–70% | 70–90% | Tooth discolouration (children <7 years) | 2 | .. | 5 mg/kg daily |
| Other agents | ||||||||||
| Co-trimoxazole | yyy | 40–70% | 40–70% | 40–70% | 40–70% | <40% | .. | 3 | 0·03–0·09 | Half 80 mg tablet orally twice daily (based on trimethoprim) |
| Chloramphenicol | yy | 70–90% | >90% | 40–70% | 70–90% | 40–70% | Bone marrow | 4 | 0·11–0·23 | Syrup |
yyy=multiple trials with strong trial evidence, yy=some trials with good evidence, y=minimal trials and evidence.
Pharmacokinetic/pharmacodynamic (PK/PD) properties are used to help determine the susceptibility breakpoints of antimicrobial agents and therefore the agent's likely efficacy. PK/PD properties for drug classes are as follows: aminopenicillins, penicillins, and cephalosporins=time above minimum inhibitory concentration (MIC) 40%; macrolides and fluoroquinolones=area under the curve/MIC above 30.
Costs for non-oral medications do not include administration, syringe, or needle costs.
Treatment course was 5 days and dose was based on a 10 kg child, except intramuscular penicillin and ceftriaxone, for which cost includes only the drug cost and does not include needles and administration.
Although drug cost may be a critically important consideration, it may be acceptable for a second-line antimicrobial agent to be more expensive than first-line agents. Treatment course prices of US$0·50, $1, and $2 were used as cut-offs to assess agent's acceptability (table 4). Appropriate second-line agents must enhance coverage not already provided by the first-line agent. Parenteral antimicrobial agents are often used in hospital settings, but are rarely used in outpatient clinics in low-income countries. The perception by some patients and providers that parenteral agents are always more effective than oral agents can be difficult to correct. Therefore, introduction of a parenteral antimicrobial agent such as ceftriaxone to peripheral settings may be impractical for these and other reasons in most situations.
If a child was initially taking co-trimoxazole at the correct pneumonia treatment dose, the preferred second-line agent would be oral amoxicillin at 50 mg/kg in two divided doses for 5 days. If the first-line agent was amoxicillin, the second antimicrobial agent choice should expand the spectrum or enhance coverage. Use of high-dose amoxicillin–clavulanic acid (80–90 mg/kg daily of divided doses of amoxicillin with a maximum of 6·4 mg/kg clavulanic acid daily) is another possibility to enhance activity against beta-lactamase-producing H influenzae and resistant S pneumoniae, but would not cover atypical bacteria.
Most oral second-generation and third-generation cephalosporin antimicrobial agents are more expensive, but have improved coverage against beta-lactamase-producing H influenzae. Cefuroxime and cefixime are reasonably priced, although they are not as active as high-dose amoxicillin–clavulanic acid against S pneumoniae.72 In addition, these agents do not provide coverage for M pneumoniae or C pneumoniae.
Oral chloramphenicol palmitate is a less desirable second-line agent for non-severe pneumonia. This antimicrobial agent is bacteriostatic against a wide range of potential pathogens and bactericidal against most S pneumoniae and H influenzae, although there is some resistance to chloramphenicol. Bone-marrow toxicity includes reversible, dose-dependent suppression, as well as aplastic anaemia, the latter occurring in approximately one in 24 500 to 40 000 courses.77, 78 Although chloramphenicol is active against a wide range of causal agents and is inexpensive, the potential for bone-marrow toxicity limits its use as a first-line agent and as a universal second-line agent. Some panel members expressed reservations about this agent because of safety concerns with its use as an oral agent for mild disease in the outpatient setting. Also important are concerns about driving resistance in settings where parenteral chloramphenicol may be the sole agent to treat meningococcal meningitis. Oral chloramphenicol may be useful in children if intramuscular antimicrobial agents are not available and immediate transportation to a higher level of care, such as a hospital, is not possible.
Although tetracycline is the appropriate drug for the treatment of some paediatric infections, the small risk of associated side-effects outweigh the potential advantages of widespread use of the drug for non-severe pneumonia treatment. Similarly, the respiratory fluoroquinolones are not optimum second-line drugs for outpatient pneumonia because of the theoretical risk of toxic effects in young children, as well as the measurable risk of promoting resistance to this valuable class of antimicrobial agents.
Azithromycin, clarithromycin, and erythromycin are reasonably priced, but the role of these agents is limited to extending the antimicrobial spectrum to atypical organisms, because these agents are relatively inactive against H influenzae and there is increasing resistance among S pneumoniae.72 Therefore, an affordable macrolide or azalide may be considered for a child who is not better but not worsening at time of re-assessment and is over 3 years of age, when these atypical infections are more likely. Erythromycin may be used in three or four divided doses for a 5-day treatment course at a daily dose of 40 mg/kg.37 Advantages of erythromycin over azithromycin or clarithromycin include low-cost and wide availability and disadvantages include gastrointestinal disturbance and frequent dosing. Macrolides or azalides are also indicated for those who have a documented allergy to penicillin or other beta-lactam agents. Co-trimoxazole was not considered as a second-line agent because co-trimoxazole resistance in S pneumoniae often co-exists with penicillin resistance,79 and, if the first-line agent used was amoxicillin, the addition of co-trimoxazole would probably not be of benefit.
These general recommendations may need to be modified for children living in areas where HIV or malaria are common, or if referral is not possible. Countries introducing algorithms for empirical use by first-level health workers should identify such areas and consider modification of the recommendations.
Areas with a high prevalence of HIV
Special attention is required in regions where HIV prevalence has consistently exceeded 5% in at least one defined subpopulation, as is the case in much of eastern, central, and southern Africa, and certain regions of Asia and Latin America. In these high prevalence areas, HIV counselling and testing is recommended for all children aged under 10 years seen in paediatric health services.80 The burden of HIV-associated pneumonia in such regions is substantial. One study in an area with an HIV-positive prevalence of 5% among children aged under 5 years reported that 45% of hospitalised pneumonia and 85% of pneumonia deaths occurred among HIV-positive children.81 Prophylactic co-trimoxazole has been shown to improve survival and to reduce pneumonia-related deaths in HIV-infected children and should therefore be administered.82
Children presenting with pneumonia should be assessed for symptomatic HIV infection in areas where HIV is a public-health problem and should be tested if indicated by use of methods such as that described by Horwood and colleagues.83 For children living in areas of high HIV prevalence, or who have clinical suspicion or a diagnosis of HIV infection, and who present with non-severe pneumonia, the recommended treatment is amoxicillin, irrespective of co-trimoxazole prophylaxis status. Pneumocystis jirovecii is known to be a cause of severe, progressive pneumonia, especially among children aged 2–6 months,84 but its role in non-severe pneumonia in HIV-infected children was not evaluated as part of this review. If such a child fails first-line therapy for non-severe pneumonia, the child should be referred to hospital for management by WHO guidelines,85, 86 including HIV testing and broad-spectrum parenteral antimicrobial agents. Finally, children in high prevalence areas without clinical suspicion or a diagnosis of HIV infection who fail to improve on first-line therapy should be treated according to the standard recommendations outlined in this review.
Malaria-endemic areas
The clinical overlap between malaria and pneumonia in children is well recognised.87 Symptomatic malaria may have clinical features consistent with a diagnosis of pneumonia, whereas children with pneumonia may have co-incidental malaria parasitaemia.88, 89
Co-trimoxazole therapy for non-severe pneumonia was recommended in some countries where malaria diagnosis was unavailable at first-level facilities, in part because co-trimoxazole has some activity against both malaria and common bacterial causes of pneumonia in children.90, 91 However, co-trimoxazole is not a first-line anti-malaria drug, and amoxicillin, which does not have anti-malarial activity, is now preferred to co-trimoxazole as first-line therapy for non-severe pneumonia. Therefore, if a child has clinical features of non-severe pneumonia but malaria cannot be excluded, the recommended first-line therapies for malaria and pneumonia should both be prescribed. Caution should be exercised in the use of erythromycin as a second-line agent if mefloquine or halofantrine are prescribed for malaria, due to the increased the risk of arrhythmia.
A complication of malaria is severe anaemia that, like pneumonia, can cause rapid breathing. In malaria-endemic regions, children presenting with rapid breathing should be assessed for severe anaemia. If laboratory assessment is not available, marked pallor may be assessed by examining the palms of the hands, nail beds, and conjunctivae. Any child with a clinical diagnosis of pneumonia who also has severe anaemia should be referred to hospital for assessment.
Areas where referral is not possible
A child who fails treatment and meets criteria for referral should be transported to and assessed at a centre that can provide more intensive therapy. However, referral is impossible in some areas. In these situations, the child should receive treatment with agents that provide broader coverage and high activity against pathogens that cause severe pneumonia. These antimicrobial agents include injectable antimicrobial agents such as ceftriaxone, penicillin/gentamicin, or chloramphenicol.
Conclusions
The WHO-recommended systematic case-management approach has greatly reduced the mortality of children with pneumonia. These updated recommendations, now identifying amoxicillin as the preferred first-line agent and specifying the approach to changing to an appropriate second-line agent in the event of treatment failure (table 1), aim to improve treatment protocols for first-level health providers for children with non-severe pneumonia. These recommendations were developed by use of the available evidence for the treatment of children with pneumonia in low-income countries. The evidence basis for some of the recommendations was limited, and more research is needed in several areas. These areas include an improved understanding of the aetiology of pneumonia in children, both severe and non-severe, using optimum diagnostic testing, a better understanding of the reasons for treatment failure, and the determination of the best systematic method for the assessment of treatment failure by first-level health workers. In the meantime, adaptation of these general recommendations to fit country-specific treatment algorithms should help to resolve some of the uncertainty surrounding the appropriate management of young children with non-severe pneumonia.
Search strategy and selection criteria
These are described in detail in the Methods section.
Acknowledgments
Acknowledgments
We would like to thank Richard Adegbola, Trevor Duke, Mike English, Tabish Hazir, Kalle Hoppu, Mary Lou Lindergren, Juan M Lozano, Shabbir Madhi, Trudy Murphy, Stephen Obaro, Emilia Rivadeneira, H P S Sachdev, Frank Shann, and Cynthia Whitney for their reviews. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position or policies of the Centers for Disease Control and Prevention or WHO. © World Health Organization 2009. Published by Elsevier Ltd. All rights reserved.
Conflicts of interest
We declare that we have no conflicts of interest.
Web Extra Material
Summary of studies comparing antimicrobial agents for the treatment of childhood pneumonia
References
- 1.Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ. 2008;86:408–416. doi: 10.2471/BLT.07.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bryce J, Boschi-Pinto C, Shibuya K, Black RE. WHO estimates of the causes of death in children. Lancet. 2005;365:1147–1152. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- 3.Sazawal S, Black RE. Effect of pneumonia case management on mortality in neonates, infants, and preschool children: a meta-analysis of community-based trials. Lancet Infect Dis. 2003;3:547–556. doi: 10.1016/s1473-3099(03)00737-0. [DOI] [PubMed] [Google Scholar]
- 4.Sazawal S, Black RE. Meta-analysis of intervention trials on case-management of pneumonia in community settings. Lancet. 1992;340:528–533. doi: 10.1016/0140-6736(92)91720-s. [DOI] [PubMed] [Google Scholar]
- 5.Marsh DR, Gilroy KE, Van de Weerdt R, Wansi E, Qazi S. Community case management of pneumonia: at a tipping point? Bull World Health Organ. 2008;86:381–389. doi: 10.2471/BLT.07.048462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham SM, English M, Hazir T, Enarson P, Duke T. Challenges to improving case management of childhood pneumonia at health facilities in resource-limited settings. Bull World Health Organ. 2008;86:349–355. doi: 10.2471/BLT.07.048512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO Clinical management of acute respiratory infections in children: a WHO memorandum. Bull World Health Organ. 1981;59:707–716. [PMC free article] [PubMed] [Google Scholar]
- 8.WHO Technical bases for the WHO recommendations on the management of pneumonia in children at first level health facilities [WHO/ARI/91.20] http://www.who.int/child_adolescent_health/documents/ari_91_20/en/index.html (accessed Jan 7, 2009).
- 9.WHO . Technical updates of the guidelines on the Integrated Management of Childhood Illness (IMCI): evidence and recommendations for further adaptations. Department of Child and Adolescent Health and Development, WHO; Geneva: 2005. [Google Scholar]
- 10.Jadavji T, Law B, Lebel MH, Kennedy WA, Gold R, Wang EEL. A practical guide for the diagnosis and treatment of pediatric pneumonia. CMAJ. 1997;156:S703–S711. [PMC free article] [PubMed] [Google Scholar]
- 11.British Thoracic Society of Standards of Care Committee British Thoracic Society guidelines for the management of community acquired pneumonia in childhood. Thorax. 2002;57(suppl 1):i1–i24. doi: 10.1136/thorax.57.90001.i1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Łuczak G, Kozielska E, Tyl J. Antibiotics in lower respiratory tract infection [in Polish] Med Wieku Rozwoj. 2004;8(pt 2):403–410. [PubMed] [Google Scholar]
- 13.Nascimento-Carvalho CM, Souza-Marques HH. Recommendation of the Brazilian Society of Pediatrics for antibiotic therapy in children and adolescents with community-acquired pneumonia [in Portuguese] Rev Panam Salud Publica. 2004;15:380–387. doi: 10.1590/s1020-49892004000600003. [DOI] [PubMed] [Google Scholar]
- 14.Lee PI, Chiu CH, Chen PY, Lee CY, Lin TY. Guidelines for the management of community-acquired pneumonia in children. Acta Paediatr Taiwan. 2007;48:167–180. [PubMed] [Google Scholar]
- 15.Kabra SK, Lodha R, Pandey RM. Antibiotics for community acquired pneumonia in children. Cochrane Database Syst Rev. 2006;3 doi: 10.1002/14651858.CD004874.pub2. CD004874. [DOI] [PubMed] [Google Scholar]
- 16.Atkins D, Best D, Briss PA. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Global Programme on Evidence for Health Policy . Guidelines for WHO Guidelines. World Health Organization; Geneva: 2003. (EIP/GPE/EQC/2003.1). [Google Scholar]
- 18.Hazir T, Nisar YB, Qazi SA. Chest radiography in children aged 2–59 months diagnosed with non-severe pneumonia as defined by World Health Organization: descriptive multicentre study in Pakistan. BMJ. 2006;333:629. doi: 10.1136/bmj.38915.673322.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinberg GA, Spitzer ED, Murray PR. Antimicrobial susceptibility patterns of Haemophilus isolates from children in eleven developing nations. BOSTID Haemophilus Susceptibility Study Group. Bull World Health Organ. 1990;68:179–184. [PMC free article] [PubMed] [Google Scholar]
- 20.Selwyn BJ. The epidemiology of acute respiratory tract infection in young children: comparison of findings from several developing countries. Coordinated Data Group of BOSTID Researchers. Rev Infect Dis. 1990;12(suppl 8):S870–S888. doi: 10.1093/clinids/12.supplement_s870. [DOI] [PubMed] [Google Scholar]
- 21.Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003;349:1341–1348. doi: 10.1056/NEJMoa035060. [DOI] [PubMed] [Google Scholar]
- 22.Jeena P, Thea DM, MacLeod WB. Failure of standard antimicrobial therapy in children aged 3–59 months with mild or asymptomatic HIV infection and severe pneumonia. Bull World Health Organ. 2006;84:269–275. doi: 10.2471/blt.04.015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obaro SK, Madhi SA. Bacterial pneumonia vaccines and childhood pneumonia: are we winning, refining, or redefining? Lancet Infect Dis. 2006;6:150–161. doi: 10.1016/S1473-3099(06)70411-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott JA, Brooks WA, Peiris JS, Holtzman D, Mulhollan EK. Pneumonia research to reduce childhood mortality in the developing world. J Clin Invest. 2008;118:1291–1300. doi: 10.1172/JCI33947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shann F. Etiology of severe pneumonia in children in developing countries. Pediatr Infect Dis. 1986;5:247–252. doi: 10.1097/00006454-198603000-00017. [DOI] [PubMed] [Google Scholar]
- 26.Cutts FT, Zaman SM, Enwere G. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet. 2005;365:1139–1146. doi: 10.1016/S0140-6736(05)71876-6. [DOI] [PubMed] [Google Scholar]
- 27.Madhi SA, Kuwanda L, Cutland C, Klugman KP. The impact of a 9-valent pneumococcal conjugate vaccine on the public health burden of pneumonia in HIV-infected and -uninfected children. Clin Infect Dis. 2005;40:1511–1518. doi: 10.1086/429828. [DOI] [PubMed] [Google Scholar]
- 28.Shann F. Haemophilus influenzae pneumonia: type b or non-type b? Lancet. 1999;354:1488–1490. doi: 10.1016/S0140-6736(99)00232-9. [DOI] [PubMed] [Google Scholar]
- 29.de Andrade AL, de Andrade JG, Martelli CM. Effectiveness of Haemophilus influenzae b conjugate vaccine on childhood pneumonia: a case-control study in Brazil. Int J Epidemiol. 2004;33:173–181. doi: 10.1093/ije/dyh025. [DOI] [PubMed] [Google Scholar]
- 30.Levine OS, Lagos R, Munoz A. Defining the burden of pneumonia in children preventable by vaccination against Haemophilus influenzae type b. Pediatr Infect Dis J. 1999;18:1060–1064. doi: 10.1097/00006454-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Mulholland K, Hilton S, Adegbola R. Randomised trial of Haemophilus influenzae type-b tetanus protein conjugate vaccine [corrected] for prevention of pneumonia and meningitis in Gambian infants [published erratum in Lancet 1997; 350: 524] Lancet. 1997;349:1191–1197. doi: 10.1016/s0140-6736(96)09267-7. [DOI] [PubMed] [Google Scholar]
- 32.Gessner BD, Sutanto A, Linehan M. Incidences of vaccine-preventable Haemophilus influenzae type b pneumonia and meningitis in Indonesian children: hamlet-randomised vaccine-probe trial. Lancet. 2005;365:43–52. doi: 10.1016/s0140-6736(04)17664-2. [DOI] [PubMed] [Google Scholar]
- 33.Berkley JA, Maitland K, Mwangi I. Use of clinical syndromes to target antibiotic prescribing in seriously ill children in malaria endemic area: observational study. BMJ. 2005;330:995. doi: 10.1136/bmj.38408.471991.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graham SM, Molyneux EM, Walsh AL, Cheesbrough JS, Molyneux ME, Hart CA. Nontyphoidal Salmonella infections of children in tropical Africa. Pediatr Infect Dis J. 2000;19:1189–1196. doi: 10.1097/00006454-200012000-00016. [DOI] [PubMed] [Google Scholar]
- 35.O'Dempsey TJ, McArdle TF, Lloyd-Evans N. Importance of enteric bacteria as a cause of pneumonia, meningitis and septicemia among children in a rural community in The Gambia, West Africa. Pediatr Infect Dis J. 1994;13:122–128. doi: 10.1097/00006454-199402000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Bahwere P, De Mol P, Donnen P. Improvements in nutritional management as a determinant of reduced mortality from community-acquired lower respiratory tract infection in hospitalized children from rural central Africa. Pediatr Infect Dis J. 2004;23:739–747. doi: 10.1097/01.inf.0000135663.17018.51. [DOI] [PubMed] [Google Scholar]
- 37.Wubbel L, Muniz L, Ahmed A. Etiology and treatment of community-acquired pneumonia in ambulatory children. Pediatr Infect Dis J. 1999;18:98–104. doi: 10.1097/00006454-199902000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Heiskanen-Kosma T, Korppi M, Jokinen C. Etiology of childhood pneumonia: serologic results of a prospective, population-based study. Pediatr Infect Dis J. 1998;17:986–991. doi: 10.1097/00006454-199811000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Weber MW, Gopalakrishna G, Awomoyi A. The role of Chlamydia pneumoniae in acute respiratory tract infections in young children in The Gambia, West Africa. Ann Trop Paediatr. 2006;26:87–94. doi: 10.1179/146532806X107412. [DOI] [PubMed] [Google Scholar]
- 40.Somer A, Salman N, Yalcin I, Agacfidan A. Role of Mycoplasma pneumoniae and Chlamydia pneumoniae in children with community-acquired pneumonia in Istanbul, Turkey. J Trop Pediatr. 2006;52:173–178. doi: 10.1093/tropej/fml017. [DOI] [PubMed] [Google Scholar]
- 41.Phares CR, Wangroongsarb P, Chantra S. Epidemiology of severe pneumonia caused by Legionella longbeachae, Mycoplasma pneumoniae, and Chlamydia pneumoniae: 1-year, population-based surveillance for severe pneumonia in Thailand. Clin Infect Dis. 2007;45:e147–e155. doi: 10.1086/523003. [DOI] [PubMed] [Google Scholar]
- 42.Shann F, Walters S, Pifer LL. Pneumonia associated with infection with pneumocystis, respiratory syncytial virus, chlamydia, mycoplasma, and cytomegalovirus in children in Papua New Guinea. Br Med J (Clin Res Ed) 1986;292:314–317. doi: 10.1136/bmj.292.6516.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsai MH, Huang YC, Chen CJ. Chlamydial pneumonia in children requiring hospitalization: effect of mixed infection on clinical outcome. J Microbiol Immunol Infect. 2005;38:117–122. [PubMed] [Google Scholar]
- 44.Kutlin A, Roblin PM, Hammerschlag MR. Antibody response to Chlamydia pneumoniae infection in children with respiratory illness. J Infect Dis. 1998;177:720–724. doi: 10.1086/514223. [DOI] [PubMed] [Google Scholar]
- 45.Vuori-Holopainen E, Peltola H. Reappraisal of lung tap: review of an old method for better etiologic diagnosis of childhood pneumonia. Clin Infect Dis. 2001;32:715–726. doi: 10.1086/319213. [DOI] [PubMed] [Google Scholar]
- 46.Fagbule DO. Bacterial pathogens in malnourished children with pneumonia. Trop Geogr Med. 1993;45:294–296. [PubMed] [Google Scholar]
- 47.Weber MW, Mulholland EK, Greenwood BM. Respiratory syncytial virus infection in tropical and developing countries. Trop Med Int Health. 1998;3:268–280. doi: 10.1046/j.1365-3156.1998.00213.x. [DOI] [PubMed] [Google Scholar]
- 48.Finkelman BS, Viboud C, Koelle K, Ferrari MJ, Bharti N, Grenfell BT. Global patterns in seasonal activity of influenza A/H3N2, A/H1N1, and B from 1997 to 2005: viral coexistence and latitudinal gradients. PLoS ONE. 2007;2:e1296. doi: 10.1371/journal.pone.0001296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fry AM, Curns AT, Harbour K, Hutwagner L, Holman RC, Anderson LJ. Seasonal trends of human parainfluenza viral infections: United States, 1990–2004. Clin Infect Dis. 2006;43:1016–1022. doi: 10.1086/507638. [DOI] [PubMed] [Google Scholar]
- 50.Stockton J, Stephenson I, Fleming D, Zambon M. Human metapneumovirus as a cause of community-acquired respiratory illness. Emerg Infect Dis. 2002;8:897–901. doi: 10.3201/eid0809.020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams JV, Harris PA, Tollefson SJ. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350:443–450. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Madhi SA, Ludewick H, Abed Y, Klugman KP, Boivin G. Human metapneumovirus-associated lower respiratory tract infections among hospitalized human immunodeficiency virus type 1 (HIV-1)-infected and HIV-1-uninfected African infants. Clin Infect Dis. 2003;37:1705–1710. doi: 10.1086/379771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Esper F, Boucher D, Weibel C, Martinello RA, Kahn JS. Human metapneumovirus infection in the United States: clinical manifestations associated with a newly emerging respiratory infection in children. Pediatrics. 2003;111(pt 1):1407–1410. doi: 10.1542/peds.111.6.1407. [DOI] [PubMed] [Google Scholar]
- 54.Pierangeli A, Gentile M, Di Marco P. Detection and typing by molecular techniques of respiratory viruses in children hospitalized for acute respiratory infection in Rome, Italy. J Med Virol. 2007;79:463–468. doi: 10.1002/jmv.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dare RK, Fry AM, Chittaganpitch M, Sawanpanyalert P, Olsen SJ, Erdman DD. Human coronavirus infections in rural Thailand: a comprehensive study using real-time reverse-transcription polymerase chain reaction assays. J Infect Dis. 2007;196:1321–1328. doi: 10.1086/521308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fry AM, Lu X, Chittaganpitch M. Human bocavirus: a novel parvovirus epidemiologically associated with pneumonia requiring hospitalization in Thailand. J Infect Dis. 2007;195:1038–1045. doi: 10.1086/512163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fischer J, Peruski LF, Wongjindanon W, et al. Microbial surveillance for Streptococcus pneumoniae in rural Thailand: lessons learned, laboratory capacity, and the case for quality control. Proceedings of the 5th International Symposium on Pneumococci and Pneumococcal Diseases, Alice Springs, Australia; April 2–6, 2006. Abstract P02.19.
- 58.Forgie IM, O'Neill KP, Lloyd-Evans N. Etiology of acute lower respiratory tract infections in Gambian children: I. Acute lower respiratory tract infections in infants presenting at the hospital. Pediatr Infect Dis J. 1991;10:33–41. doi: 10.1097/00006454-199101000-00008. [DOI] [PubMed] [Google Scholar]
- 59.Ghafoor A, Nomani NK, Ishaq Z. Diagnoses of acute lower respiratory tract infections in children in Rawalpindi and Islamabad, Pakistan. Rev Infect Dis. 1990;12(suppl 8):S907–S914. doi: 10.1093/clinids/12.supplement_8.s907. [DOI] [PubMed] [Google Scholar]
- 60.Tupasi TE, Lucero MG, Magdangal DM. Etiology of acute lower respiratory tract infection in children from Alabang, Metro Manila. Rev Infect Dis. 1990;12(suppl 8):S929–S939. doi: 10.1093/clinids/12.supplement_8.s929. [DOI] [PubMed] [Google Scholar]
- 61.Madhi SA, Klugman KP. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med. 2004;10:811–813. doi: 10.1038/nm1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marchant CD, Carlin SA, Johnson CE, Shurin PA. Measuring the comparative efficacy of antibacterial agents for acute otitis media: the “Pollyanna phenomenon”. J Pediatr. 1992;120:72–77. doi: 10.1016/s0022-3476(05)80601-8. [DOI] [PubMed] [Google Scholar]
- 63.Straus WL, Qazi SA, Kundi Z, Nomani NK, Schwartz B. Antimicrobial resistance and clinical effectiveness of co-trimoxazole versus amoxycillin for pneumonia among children in Pakistan: randomised controlled trial. Lancet. 1998;352:270–274. doi: 10.1016/s0140-6736(97)10294-x. [DOI] [PubMed] [Google Scholar]
- 64.Noorani QA, Qazi SA, Rasmussen ZA. Response to cotrimoxazole in the management of childhood pneumonia in first-level health care facilities. Int J Tuberc Lung Dis. 2006;10:932–938. [PubMed] [Google Scholar]
- 65.Leiberman A, Leibovitz E, Piglansky L. Bacteriologic and clinical efficacy of trimethoprim–sulfamethoxazole for treatment of acute otitis media. Pediatr Infect Dis J. 2001;20:260–264. doi: 10.1097/00006454-200103000-00009. [DOI] [PubMed] [Google Scholar]
- 66.CATCHUP Study Group Clinical efficacy of co-trimoxazole versus amoxicillin twice daily for treatment of pneumonia: a randomised controlled clinical trial in Pakistan. Arch Dis Child. 2002;86:113–118. doi: 10.1136/adc.86.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.WHO Consultative meeting to review evidence and research priorities in the management of acute respiratory infections (ARI). Geneva, September 29–October 1, 2003. Meeting report [WHO/FCH/CAH/04.2] http://whqlibdoc.who.int/hq/2004/WHO_FCH_CAH_04.2.pdf [accessed Jan 12, 2009].
- 68.Pakistan Multicentre Amoxycillin Short Course Therapy (MASCOT) pneumonia study group Clinical efficacy of 3 days versus 5 days of oral amoxicillin for treatment of childhood pneumonia: a multicentre double-blind trial [published erratum in Lancet 2003; 361: 788] Lancet. 2002;360:835–841. doi: 10.1016/S0140-6736(02)09994-4. [DOI] [PubMed] [Google Scholar]
- 69.Agarwal G, Awasthi S, Kabra SK. Three day versus five day treatment with amoxicillin for non-severe pneumonia in young children: a multicentre randomised controlled trial. BMJ. 2004;328:791–797. doi: 10.1136/bmj.38049.490255.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hazir T, Qazi SA, Nisar YB. Can WHO therapy failure criteria for non-severe pneumonia be improved in children aged 2–59 months? Int J Tuberc Lung Dis. 2006;10:924–931. [PubMed] [Google Scholar]
- 71.Heffelfinger JD, Davis TE, Gebrian B, Bordeau R, Schwartz B, Dowell SF. Evaluation of children with recurrent pneumonia diagnosed by World Health Organization criteria. Pediatr Infect Dis J. 2002;21:108–112. doi: 10.1097/00006454-200202000-00005. [DOI] [PubMed] [Google Scholar]
- 72.Jacobs MR, Dagan R. Antimicrobial resistance among pediatric respiratory tract infections: clinical challenges. Semin Pediatr Infect Dis. 2004;15:5–20. doi: 10.1053/j.spid.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 73.Gilbert DN, Moellering RC, Eliopoulous GM, Sande MA. The Sanford guide to antimicrobial therapy, 2005. 35th edn. Antimicrobial Therapy Inc; Hyde Park, VT: 2005. [Google Scholar]
- 74.Leibovitz E, Piglansky L, Raiz S. Bacteriologic and clinical efficacy of oral gatifloxacin for the treatment of recurrent/nonresponsive acute otitis media: an open label, noncomparative, double tympanocentesis study. Pediatr Infect Dis J. 2003;22:943–949. doi: 10.1097/01.inf.0000095468.89866.14. [DOI] [PubMed] [Google Scholar]
- 75.McFadyen JE. International drug price indicator guide. Management Sciences for Health; Boston: 2006. [Google Scholar]
- 76.WHO. UNICEF. UNAIDS. Médecins Sans Frontières . Sources and prices of selected medicines and diagnostics for people living with HIV/AIDS. WHO; Geneva: 2006. [Google Scholar]
- 77.Feder HM, Jr, Osier C, Maderazo EG. Chloramphenicol: a review of its use in clinical practice. Rev Infect Dis. 1981;3:479–491. doi: 10.1093/clinids/3.3.479. [DOI] [PubMed] [Google Scholar]
- 78.Paluck E, Katzenstein D, Frankish CJ. Prescribing practices and attitudes toward giving children antibiotics. Can Fam Phys. 2001;47:521–527. [PMC free article] [PubMed] [Google Scholar]
- 79.Hoban DJ, Doern GV, Fluit AC, Roussel-Delvallez M, Jones RN. Worldwide prevalence of antimicrobial resistance in Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin Infect Dis. 2001;32(suppl 2):S81–S93. doi: 10.1086/320181. [DOI] [PubMed] [Google Scholar]
- 80.UNAIDS. WHO Guidance on provider-initiated HIV testing and counseling in health facilities. May, 2007. http://whqlibdoc.who.int/publications/2007/9789241595568_eng.pdf [accessed Jan 12, 2009].
- 81.Madhi SA, Schoub B, Simmank K, Blackburn N, Klugman KP. Increased burden of respiratory viral associated severe lower respiratory tract infections in children infected with human immunodeficiency virus type-1. J Pediatr. 2000;137:78–84. doi: 10.1067/mpd.2000.105350. [DOI] [PubMed] [Google Scholar]
- 82.Chintu C, Bhat GJ, Walker AS. Co-trimoxazole as prophylaxis against opportunistic infections in HIV-infected Zambian children (CHAP): a double-blind randomised placebo-controlled trial. Lancet. 2004;364:1865–1871. doi: 10.1016/S0140-6736(04)17442-4. [DOI] [PubMed] [Google Scholar]
- 83.Horwood C, Liebeschuetz S, Blaauw D, Cassol S, Qazi S. Diagnosis of paediatric HIV infection in a primary health care setting with a clinical algorithm. Bull World Health Organ. 2003;81:858–866. [PMC free article] [PubMed] [Google Scholar]
- 84.Morris A, Lundgren JD, Masur H. Current epidemiology of Pneumocystis pneumonia. Emerg Infect Dis. 2004;10:1713–1720. doi: 10.3201/eid1010.030985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.WHO . Pocket book of hospital care for children: guidelines for the management of common illnesses with limited resources. WHO; Geneva: 2005. [PubMed] [Google Scholar]
- 86.WHO Report of a consultative meeting of children with pneumonia and HIV infection. 30–31 January 2003, Harare, Zimbabwe. http://whqlibdoc.who.int/publications/2004/9241591285.pdf [accessed Jan 12, 2009].
- 87.Redd SC, Bloland PB, Kazembe PN, Patrick E, Tembenu R, Campbell CC. Usefulness of clinical case-definitions in guiding therapy for African children with malaria or pneumonia. Lancet. 1992;340:1140–1143. doi: 10.1016/0140-6736(92)93160-o. [DOI] [PubMed] [Google Scholar]
- 88.O'Dempsey TJ, McArdle TF, Laurence BE, Lamont AC, Todd JE, Greenwood BM. Overlap in the clinical features of pneumonia and malaria in African children. Trans R Soc Trop Med Hyg. 1993;87:662–665. doi: 10.1016/0035-9203(93)90279-y. [DOI] [PubMed] [Google Scholar]
- 89.WHO The overlap in the clinical presentation and treatment of malaria and pneumonia in children: report of a meeting (Geneva, 8 April 1991) [WHO/ARI/92.23 & WHO/MAL/92.1065] http://whqlibdoc.who.int/HQ/1992/WHO_ARI_92.23.pdf [accessed Jan 12, 2009].
- 90.Bloland PB, Redd SC, Kazembe P, Tembenu R, Wirima JJ, Campbell CC. Co-trimoxazole for childhood febrile illness in malaria-endemic regions. Lancet. 1991;337:518–520. doi: 10.1016/0140-6736(91)91299-a. [DOI] [PubMed] [Google Scholar]
- 91.Hamel MJ, Holtz T, Mkandala C. Efficacy of trimethoprim–sulfamethoxazole compared with sulfadoxine–pyrimethamine plus erythromycin for the treatment of uncomplicated malaria in children with integrated management of childhood illness dual classifications of malaria and pneumonia. Am J Trop Med Hyg. 2005;73:609–615. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of studies comparing antimicrobial agents for the treatment of childhood pneumonia