In the absence of standardized diagnosis and the presence of unique clinical syndromes, it is not surprising that considerable differences exist in the number of reported incidences of disease and the outcomes of various infections in cardiothoracic transplant (CTTX) recipients. Publications to date have employed variable and heterogeneous definitions of CTTX-related infections, thereby limiting the comparison between the types and incidence of infections and the generalizability of these data across transplant centers. Currently, there are no standard international definitions for infections uniquely related to CTTX, with the exception of Chagas disease and toxoplasmosis.1 The purpose of the present working formulation is to provide consensus-derived expert opinion of definitions for infections in CTTX for epidemiologic, research and registry data use.
Scope
Standard definitions of infections specifically related to CTTX will allow for meaningful comparison of the type and incidence of these infections between different types of CTTXs, different regimens of immunosuppression and between different transplant centers, thereby improving the reporting of infection-related morbidity and mortality after cardiothoracic transplantation. The definitions proposed herein are suitable for epidemiologic investigations and are intended to facilitate clinical decision-making.
The definitions described in what follows have been reviewed and approved by a multidisciplinary working group of The International Society for Heart and Lung Transplantation (ISHLT).
Source
These definitions were adapted from surveillance definitions of healthcare-associated sinusitis, tracheobronchitis and pneumonia, used in reporting to the National Healthcare Safety Network (NHSN) and the Centers for Disease Control and Prevention's (CDC) surveillance system for patient and healthcare personnel safety.2 Definitions of invasive fungal infection (IFI) were based on those proposed by the European Organization for Research and Treatment of Cancer and the Mycoses Study Group of the National Institutes of Health (EORTC/MSG),3 whereas definitions from the American Society of Transplantation and other source documents represented the foundation for defining viral infections.1, 4
Bacterial infection overview
All bacterial infections
Bacterial infections are a major contributor of complications in the early post-transplant period in heart- and lung-transplanted patients.5, 6 Some bacterial infections (e.g., pre-transplant colonization or donor-derived infections) have unique issues and implications in CTTX recipients5, 7, 8, 9, 10, 11, 12; therefore, the definitions for these infections for epidemiologic, research or ISHLT registry purposes are specifically addressed herein. Many other bacterial infections (e.g., methicillin-resistant Staphylococcus aureus or vancomysin-resistant enterococcus) are present similarly across hospitalized patients and solid-organ transplant (SOT) recipients and are therefore not directly addressed in what follows.
The existing literature in CTTX has largely classified bacterial infections as “early” (e.g., post-operative) or “late” onset after transplantation, allowing transplant clinicians to determine the source of these infections and focus prevention strategy and early empirical antibiotic treatment regimens on the temporal onset of these infections. A further timeline is used to classify all infections diagnosed in the hospital setting as nosocomial, with onset 48 hours after the patient is admitted to the hospital, and community-acquired infection, with onset at the time of admission or within 48 hours of admission. The latter definitions of infection may be artificial in the setting of CTTX as some infections, although related to healthcare and immunosuppression, may not occur within the established time-line of nosocomial infections. To fully appreciate the impact of the potential source of infection, we propose using the categories of nosocomial (after 48 hours of hospitalization) and community-acquired (prior to 48 hours of hospitalization) with the added category of community-acquired “transplant-related” infections. This category would include infections by pathogens acquired by the CTTX patient prior to time of transplantation and that are clearly related to the immunocompromised state of the CTTX patient after transplantation that may increase the risk for specific bacterial pathogens that are not common in the community. These pathogens may be related to the donor or the recipient via pre-transplant colonization of the respiratory or gastrointestinal (GI) tract and can be therefore regarded as “transplant-related” in this setting.12, 13 It is also to be noted that community-acquired pneumonia may be transplant-related if caused by organisms that are typically associated with transplants (e.g., fungal, multidrug-resistant or atypical bacteria).
Respiratory bacterial infections
Respiratory bacterial infections occur frequently in lung transplant recipients. In one study, 72 episodes per 100 lung transplants per year were reported.14 The source of bacteria-causing pneumonia in lung transplant recipients may be the donor, the recipient or the hospital environment. Nosocomial transmission from other patients or healthcare workers can occur when hand hygiene or appropriate respiratory isolation measures for other hospitalized infected patients are not routinely practiced.11, 15, 16, 17, 18, 19
The definitions of bacterial pneumonia present significant challenges in CTTX. Frequent use of empirical anti-bacterial agents prior to specimen collection and the possibility of concurrent allograft rejection make the use of standard guidelines, as presented by the Centers for Disease Control and Prevention (CDC) for healthcare-associated infections (HCAIs), difficult to apply.2 In addition, some microbiologic diagnostic procedures may not be routinely practiced at many transplant centers and this may limit the employment of diagnostic criteria for infections that require quantification of bacterial colony-forming units per milliliter in the bronchoalveolar lavage (BAL) samples. This methodology has not been validated in the immunocompromised host and is not standardized across institutions. Further, the thresholds proposed may underestimate the episodes of bacterial pneumonia in the CTTX population,20 where early empirical intervention with anti-microbial agents prior to obtaining the samples with suspected pneumonia is common practice. Presence of endobronchial stents in lung transplant recipients further complicates the picture in defining various clinical syndromes.
For these reasons, a specific classification of bacterial pneumoniae in CTTX recipients is proposed based on radiographic findings, clinical symptoms, microbiology and histopathology (including consideration of acute rejection in lung transplant patients).
In lung transplant recipients, the use of differential cytology in BAL may be helpful.21, 22, 23 The predominance of neutrophils with intracellular bacteria (hematoxylin–eosin and gram stain) is more suggestive of the presence of a bacterial pneumonia than a high proportion of lymphocytes or eosinophils in BAL. On the other hand, a lymphocytic or eosinophilic BAL could indicate an acute graft reaction, although cytomegalovirus (CMV), other viruses and atypical pathogens would need to be ruled out.
Acute rejection (AR) of the graft in lung transplant recipients presents a significant consideration in the diagnosis of all pneumonias, including those due to bacteria. There are frequent clinical scenarios where distinction between rejection and infection is critically dependent upon histopathologic findings. In many cases, evidence for infection and rejection coexist. Therefore, in the setting of lung transplantation, the diagnosis of bacterial (or any) pneumonia is more precisely defined by the confirmation or exclusion of AR when microbiologic criteria are not met.24
The determination of AR requires histologic examination. If an AR is documented and clinical and laboratory criteria for bacterial pneumonia are also fulfilled, the diagnosis of AR and concomitant pneumonia is possible.24 Accordingly, pneumonia should be indicated as pneumonia combined with an AR.
Respiratory bacterial infection diagnostic tools
-
•
Direct examination by light microscopy [gram stain, modified acid-fast bacilli (AFB) stain for Nocardia spp, AFB stains for Mycobacteria].
-
•
Culture (including rapid culture methods for Legionella spp, Mycobacteria spp and prolonged culture periods for detection of bacteria-causing infective endocarditis).
-
•
BAL cell analysis: rule-out contamination with <1% epithelial cells,20 then quantitative, semi-quantitative or qualitative culture of BAL material or lung biopsy, if available.
-
•
Quantitative, semi-quantitative or qualitative cytology of BAL.
-
•
Histopathology: special staining of lung tissue (if available) for bacteria (i.e., Brown and Brenn stain), Mycobacteria (AFB stain/auramine) and atypical bacteria (Fite stain for Nocardia, etc.).
-
•
Nucleic acid amplification [including polymerase chain reaction (PCR) methods and real-time PCR] methods for atypical respiratory bacteria (Legionella, Chlamydia and Mycoplasma spp).
-
•
Enzyme immunoassays (EIA) antigen tests for pneumococcal and legionella antigens from urine samples).
-
•
Serology (may be useful for research purposes only in select study designs).
Definitions of bacterial pneumonia and colonization in CTTX are given in Table 1a, whereas the definitions of tracheobronchitis are given in Table 1b.
Table 1a.
Bacterial Pneumonia and Colonization in CTTX
| Infection | Signs/symptoms | Radiology | Microbiology/pathology | Histopathologic evidence of AR |
|---|---|---|---|---|
| Proven pneumonia, acute rejection (AR)- associated OR not AR associated |
|
New/worsening radiographic changes on chest X-ray or CT scan |
|
AR may be present or absent or not investigated |
| Probable pneumonia | As for proven | As for proven | Negative microbiology PLUS absence of AR by histopathology | AR must be excluded |
| Possible pneumonia | As for proven | As for proven | Microbiology negative or not performed PLUS concomitant clinical diagnosis of AR (without histopathology) | No histopathology performed |
| No pneumonia, proven AR | As for proven | As for proven | Negative microbiology PLUS AR proven by histopathology | Histopathologic evidence of AR |
| Colonization |
|
Absent or unchanged | Recovery of pathogen in absence of clinical or radiographic changes | AR present or absent |
Table 1b.
Bacterial Tracheobronchitis and Bronchial Anastomotic Infections in Lung Transplant Recipients
| Infection | Signs/symptoms | Radiology | Microbiology | Histopathologic evidence |
|---|---|---|---|---|
| Proven tracheobronchitis |
|
|
|
Histology showing inflammation with organisms or positive culture from the sterile tissue ALONE |
| Probable tracheobronchitis | As for proven | As for proven | As for proven | Negative histology |
| Proven bronchial anastomotic infection |
|
As for proven tracheobronchitis; may be positive if concurrent pneumonia is present | As for proven tracheobronchitis | As for proven tracheobronchitis |
| Probable bronchial anastomotic infection | As for proven | As for proven | As for proven tracheobronchitis | Negative histopathology |
General comments regarding bacterial pneumonia/tracheobronchitis
-
1
Ventilator-associated pneumonia should be designated when reporting data. A distinction should be made between non-invasive and invasive ventilation.
-
2
Aspiration pneumonia should be considered if the criteria are fulfilled for pneumonia (Table 1a). The cause of this type of pneumonia should be noted.
-
3
Multiple or concurrent episodes of post-transplant pneumonia may occur. To determine a new episode in a single patient, resolution of the initial infection must be determined by clinical, laboratory or histologic evidence. The isolation of a new pathogen alone is not indicative of a new episode of pneumonia. In contrast, a second pneumonia may develop in a patient after single lung transplantation. Here, the contralateral lung may develop an “independent” pneumonia by another organism.
-
4
Sputum samples are frequently contaminated with airway colonizers (e.g., coagulase-negative Staphylococcus and Enterococcus spp), and therefore must be interpreted cautiously.
-
5
The interferon-gamma release assay (IGRA) serum test is not recommended for diagnosis of acute tracheobronchitis disease, although a positive result is an indication of latent disease or recent infection and a useful screening test if baseline IGRA testing is performed prior to transplantation.25
-
6
Episodes of airway colonization are not recorded as infections.
-
7
Histologic representation of chronic graft rejection may not impact the diagnosis of bacterial pneumonia. Therefore, it is not included as criteria for pneumonia definition.26
-
8
The definition of “possible pneumonia” category allows recording of pneumonia after lung transplantation even if required diagnostic procedures were missed, which may occur with prior anti-microbial treatment or delay in diagnostic testing, etc.
-
9
In lung transplant recipients, it is desirable to always give additional information if evidence of acute graft rejection exists either by clinical or by histopathologic diagnosis.
-
10
It is possible to have concurrent infections—pneumonia with sinusitis or anastomotic tracheobronchitis.
-
11
Quantification of organisms in BAL is not considered essential for the diagnosis of ventilator-associated pneumonia (VAP).27 However, invasive diagnostics may help withdraw anti-bacterial therapy, which may prevent further emergence of multi-resistant organisms in future.28, 29
-
12
The category of bacterial tracheobronchitis is classified into probable and proven categories. They can only be diagnosed in the presence of bronchoscopic findings. We have refrained from using the term microbiogically negative tracheobrochitis as it requires more evidence.
-
13
Endobronchial stent–associated tracheobronchitis or bronchial anastomotic infections, both fungal and bacterial, are categorized as probable (Table 2).
-
14
No attempt is made to redefine atypical mycobacterial infections or pulmonary tracheobronchitis in lung transplant recipients and the use of existing definitions from European and North American societies are encouraged until further data emerge.30, 31, 32, 33
-
15
It is preferable to document the use of antibiotics in patients with pneumonia at the time of culture data collection.
Table 2.
Infections Associated With Ventilation or Endobronchial Stents
| Infection | Signs/symptoms | Radiology | Microbiology | Histopathologic evidence |
|---|---|---|---|---|
| Ventilator-associated pneumonia (non-invasive or invasive ventilation); patient on ventilator for at least 48 hours continuously |
|
|
|
|
|
|
|
|
Not applicable |
Viral infection overview
All viral infections
Cardiothoracic transplant recipients are at an increased risk for viral infections with severe clinical sequelae. Some viral infections have unique considerations and implications in CTTX recipients. The definitions for these viral infections are specifically addressed herein and may be used for epidemiologic, research or registry purposes in CTTX recipients.
Many other non-respiratory viral infections present similarly across SOT recipients. Diagnosis and management of these viral infections have been addressed adequately in other guidelines1 and therefore will not be addressed herein.
Respiratory viral infections
Respiratory viral infections, including newly emerging viruses, occur frequently in lung transplant recipients.34 Some epidemiologic studies have suggested an association between respiratory viral infection and the development of bronchiolitis obliterans syndrome (BOS).35, 36, 37, 38, 39, 40 These studies yielded mixed results and the association between respiratory virus infection and BOS remains unclear.41, 42 The recent availability of molecular diagnostics, including PCR and multiplex gene techniques for the recovery of many viruses simultaneously from a single specimen, increased the recovery of pathogens in respiratory infections that previously were considered to be of undetermined etiology.42 Other viruses have been identified and are of uncertain pathogenicity. Further, with the use of molecular diagnostic and deep gene sequencing techniques, novel respiratory viral pathogens, such as human metapneumovirus, human coronaviruses and bocavirus, have been identified.43, 44 These discoveries have led to the expansion of the repertoire of respiratory viral pathogens and infections to be considered in any research of the impact of respiratory viral infections after CTTX. Characteristic histopathologic changes on lung biopsy specimens when these viruses are present have not been identified by these methods, such as with CMV and herpes simplex virus (HSV), but efforts continue, demonstrating the multidisciplinary approach to the accurate diagnosis of infection in this population. The standardization of diagnosis and categorization of respiratory viral infection is essential for the comprehensive evaluation and generalizability of this growing area of study.
Lower respiratory viral infections (LVRIs) may occur with or without acute rejection. Suspected acute rejection should be looked for if all criteria of LRVIs are fulfilled.
CMV in the lung
Definitions for CMV infection and disease, especially for use in research, have been reported in the literature and used in other studies.1, 4 The methodology for CMV recovery has shifted at many centers over the past decade from conventional viral culture methods and antigenemia toward quantitative molecular diagnostics, including PCR and hybrid capture assays.45, 46, 47 However, the issues related to the recovery of CMV in BAL fluid in the absence of histopathologic evidence of CMV remain unresolved,48 and investigations are ongoing to resolve this issue. Asymptomatic viral shedding in the upper oropharynx by CMV is distinguished from active CMV disease in these definitions and will assist in further assessing the role of CMV in CTTX. In early studies, the recovery of CMV by viral culture in the absence of tissue diagnosis was considered diagnostic of CMV pneumonitis.49, 50 However, further studies did not suggest that CMV recovery from BAL was predictive of subsequent CMV pneumonitis.51, 52, 53, 54, 55 With the advent of sophisticated molecular diagnostics, the recovery of CMV from BAL became more specific and reproducible compared with conventional or shell-vial culture,53 and additional studies suggested that CMV viral load in BAL fluid may be correlated with invasive CMV pneumonia.2, 56, 57 However, the utility of CMV viral load in BAL in predicting CMV pneumonitis remains uncertain in studies to date.
CMV diagnostic tools
-
•
Molecular diagnostics (from whole blood, plasma, BAL or tissue).
— Quantitative DNA PCR or hybrid capture assays.
— Qualitative PCR.
— Genotypic resistance testing.
-
•
Antigen pp65.
-
•
Viral culture (conventional or shell-vial centrifugation).
-
•
In situ immunohistochemistry.
-
•
Serology: not recommended for diagnosis.
Definitions for respiratory viral infections are given in Table 3a and 3b.
Table 3a.
Respiratory Viral Infection in CTTX
| Infection | Signs/symptoms | Radiology | Virology |
|---|---|---|---|
| Respiratory viral infections (RVIs) | |||
| Asymptomatic RVI | None | No changes | Recovery of virus from nasopharynx or bronchoalveolar lavagea |
| Clinical RVI |
|
Chest radiograph or CT scan not performed | Lack of confirmatory testing for respiratory viral pathogen (not performed or negative assay) |
| Upper respiratory tract infection | As for clinical RVI | No evidence of lower respiratory tract Infection | Confirmation of a respiratory viral pathogen |
| Lower respiratory tract infection |
|
New/worsening radiographic changes on chest X-ray or CT scan | Confirmation of a respiratory viral pathogen OR histopathologic evidence AND exclusion of AR |
Respiratory viral infection diagnostic tools: nucleic acid amplification (including PCR methods); tissue (cell) culture, both conventional and rapid; culture (shell-vial/R-mix); indirect and direct immunofluorescence antibody (IFA/DFA) tests; and enzyme immunoassays (EIAs).
Table 3b.
Cytomegalovirus (CMV) in CTTX
| CMV infection | Without clinical symptoms | CMV detection in blood by viral culture, antigenemia or molecular diagnostics (DNA-based assay) | |
|---|---|---|---|
| CMV pneumonitis (proven) |
|
New/worsening radiographic changes on chest X-ray or CT scan | Detection of CMV in lung tissue by culture, immunohistochemistry or in situ molecular diagnostics, with OR without CMV detection in blood or BAL by viral culture, antigenemia or molecular diagnostics (DNA-based assay) |
| CMV pneumonitis (probable)8 | As in proven CMV pneumonitis | New/worsening radiographic changes on chest X-ray or CT scan | CMV detection in blood or BAL by viral culture, antigenemia or molecular diagnostics (DNA-based assay) |
| CMV replication without clinical pneumonitis | Without clinical symptoms | No changes to chest X-ray or CT | CMV detection in BAL by viral culture, antigenemia or molecular diagnostics (DNA-based assay) |
Fungal infection overview
All fungal infections
CTTX recipients in general and lung transplant recipients in particular have the highest risk of mold infection.58 Recently published data suggest the cumulative incidence rate at 1 year to be 8%.58, 59 Among mold infections, the overwhelming majority of infections are due to Aspergillus spp, followed by Scedopsorium spp and zygomycetes.60 Despite the advancement in anti-fungal therapy, mortality remains at approximately 29% in Aspergillus infections.60 Candida species was noted to be a major pathogen during the early CTTX period, although it is rarely seen in lung transplant recipients in the current era.61 Although the incidence of invasive candidiasis has remained low in lung transplant recipients, this was the most common fungal infection noted in heart transplant recipients.60
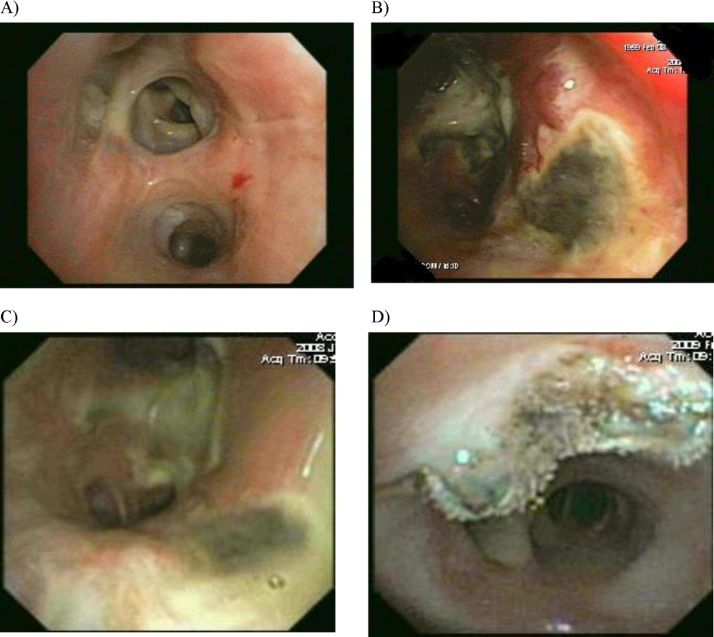
Respiratory fungal infections
Fungal infections in lung transplant recipients have certain characteristics that make them unique compared with other SOT recipients as well as other immunocompromised hosts. This includes the presence of certain risk factors, such as airway ischemia and native lung or unique clinical syndromes, including tracheobronchitis, bronchial anastomotic infection and colonization—syndromes observed only in lung transplant recipients (Figure 2 ).62 Rejection syndromes in lung transplant recipients further complicate the clinical presentation. Diagnosis of fungal infection based on histology alone may not be as accurate due to the concomitant presence of acute or chronic rejection in these individuals.24 Similarly, it is not only the unique clinical syndromes of fungal infection that set them apart from the other immunocompromised hosts, but the diagnostic utility of non-invasive testing also is different. Serum galactomannan has markedly lower sensitivity (30%) in lung transplant recipients as compared with other immunocompromised hosts.63, 64 Similarly, the sensitivity of other serologic markers such as serum cryptococcal or coccidiodal antigen, histoplasma urine antigen may be variable.65, 66, 67, 68 The use of BAL for GM has resulted in sensitivities of >66% when a 0.58 or 0.66 optical density (OD) index was used as a cutoff.69, 70 A higher cutoff (1.5 OD) yielded better results in one study.71 We suggest the use of BAL GM in the diagnosis of invasive aspergillosis (IA) in lung transplant recipients. Similarly, fungal PCRs, especially from BAL specimens, are more likely to be less sensitive for the diagnosis of disease than BAL specimens from other immunocompromised hosts owing to colonization of the airways. Cell wall components of fungi have also been used in the diagnosis of fungal infections. Currently available β-glucan is non-specific and is negative in cases of cryptococcosis and zygomycosis.72 In a recent study of lung transplant recipients, serum β-D-glucan sensitivity was reported to be 93%, whereas specificity was merely 71%.73
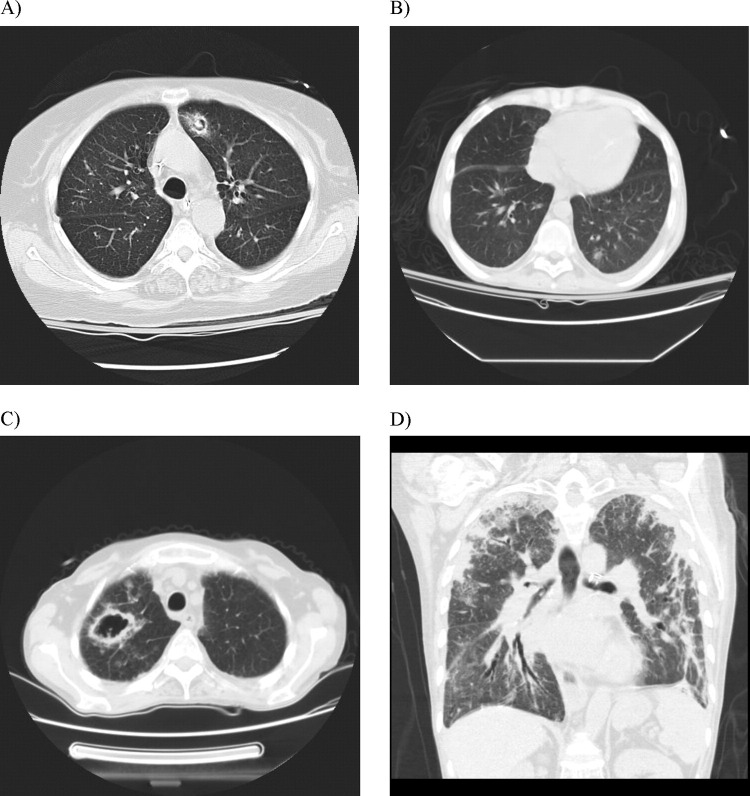
Figure 2.
Common radiologic manifestations of proven invasive aspergillosis in lung transplant recipients. (A) Fungal ball. (B) Solitary pulmonary nodule. (C) Cavitary lesion. (D) Multiple consolidation.
The Mycoses Study Group (MSG) and the European Organization for Research and Treatment (EORTC) recently updated the definitions of fungal infections in immunocompromised hosts.3 These definitions represent an excellent attempt to standardize the reporting of fungal infections in studies. However, they fail to address the unique nature of clinical syndromes in lung transplant recipients, particularly colonization, tracheobronchitis and bronchial anastomotic infections.8, 74, 75 Also, the radiologic presentation of invasive mycoses in cardiothoracic organ transplant recipients may not conform to the classical “halo sign” presentation in neutropenic or stem cell transplant recipients (Figure 2).76 Moreover, the definitions do not account for the differences in the sensitivity of serologic tests, particularly galactomannan in lung transplant recipients.69, 70, 77, 78 In addition, the category of possible fungal infection might not be applicable in lung transplant recipients owing to a multitude of possible diagnoses in these patients. The American Society of Transplantation (AST) also put forward a set of definitions to be used in the study of these infections in SOT recipients.1 The AST definitions do take into account some unique clinical syndromes in lung transplant recipients but lack the detailed description of clinical syndromes. Reported studies of fungal infections in lung transplant recipients used diverse definitions.14, 79, 80, 81, 82, 83 The following sets of definitions are proposed to standardize the reporting of fungal infections, particularly mold and yeast (endemic mycoses, Candida spp and Cryptococcus spp) infections in CTTX recipients, especially among general and lung transplant recipients. The isolation of non-pathogenic molds or other non-pathogenic fungi in BAL or sputum is not believed to satisfy the microbiologic criteria for the diagnosis of probable invasive fungal infections in these patients without histologic confirmation (Tables 4a and 4b). However, these definitions of fungal infections do not address Pneumocystis jiroveci infection, which has previously been adequately defined for use in CTTX.1
Table 4a.
Fungal Pneumonia in CTTX
| Syndromea | Signs/symptoms | Radiology | Laboratory |
|---|---|---|---|
|
|
|
Single positive culture for mold BAL/bloodcOR single positive PCR for mold BAL/blood OR positive galactomannan in the BAL; OR at least TWO positive sputum cultures/PCRs of fungal organisms (excluding Candida species) |
In the absence of biopsy categorize as probable: In the presence of histologic findings of both acute rejection and fungal invasion it should be classified as acute rejection with proven fungal infection.
The presence of mosaic appearance and ground-glass opacity may represent development of bronchiolitis obliterans syndrome or obliterative bronchiolitis.
Isolation of non-pathogenic molds in culture (e.g., Cladosporium spp, Phialemonium, Chaetomium, Cunninghamella, Syncephalastrum, Curvularia, Dactylaria, Graphium or Phialophora) or other non-pathogenic fungi [e.g., Penicillium (non-Marnefii), Paecilomyces or basidiomyctes] do not qualify for the “probable” category. They should only be considered in the “proven” category.
Table 4b.
Fungal Tracheobronchitis in CTTX
| Syndromea | Signs/symptoms | Radiology | Laboratory |
|---|---|---|---|
|
|
|
Single positive culture for mold BALbOR single positive PCR for mold BAL OR positive galactomannan in the BAL OR at least TWO positive sputum cultures/PCRs of fungal organisms (excluding Candida species) |
The presence of mosaic appearance and ground-glass opacity may represent development of bronchiolitis obliterans syndrome or obliterative bronchiolitis.
In the absence of biopsy categorized as probable: In the presence of histologic findings of both acute rejection and fungal invasion it should be classified as acute rejection with proven fungal infection.
Isolation of non-pathogenic molds in culture (e.g., Cladosporium spp, Phialemonium, Chaetomium, Cunninghamella, Syncephalastrum, Curvularia, Dactylaria, Graphium or Phialophora) or other non-pathogenic fungi [e.g., Penicillium (non-Marnefii), Paecilomyces or basidiomyctes] do not qualify for the “probable” category. They should only be considered in the “proven” category.
Fungal infection diagnostic tools
-
•
Direct examination by light microscopy (gram, Giemsa and calcofluor stains).
-
•
Culture.
-
•
Histopathology: routine stains (hemotoxylin–eosin), special (Gomori methenamine silver, mucicarmine, periodic acid–Schiff), direct immunofluorescence and in situ hybridization).
Histopathologic diagnosis is useful in establishing the diagnosis of endemic fungi because of their distinctive morphology.3 However, confusion may occur when attempting to differentiate the hyaline molds that commonly cause invasive disease.84 Fusarium spp and Scedosporium spp cannot be distinguished from Aspergillus spp in tissue sections and even the zygomycetes, which are morphologically quite distinct from Fusarium spp, Scedosporium spp and Aspergillus spp, have been confused with those organisms. The two most commonly encountered yeasts in tissue section from cardiothoracic transplant recipients, Cryptococcus spp and Candida spp, should be easily distinguished in tissue because of the characteristic round shape of the former. Mucicarmine stain of the capsular material of Cryptococus spp may further aid in its histopathologic identification.85
-
•
Nucleic acid amplification, including PCR methods and real-time-PCR (e.g., Myc assay available for clinical specimens).
-
•
Enzyme immunoassay (EIA; cryptococcal antigen test, histoplasma antigen test and galactomannan).
-
•
Cell wall component (β-glucan test).
Definitions of fungal pneumonia, tracheobronchitis, bronchial anastomotic infection and colonization in CTTX are given in Table 4a, Table 4b, Table 4c, Table 4d, respectively.
Table 4c.
Fungal Bronchial Anastomotic Infection in Lung Transplant Recipients
| Syndromea | Signs/symptoms | Radiology | Laboratory |
|---|---|---|---|
|
|
|
Single positive culture for mold in BAL OR single positive PCR for mold in BAL OR positive galactomannan in the BAL OR at least TWO positive sputum cultures/PCRs of fungal organisms (excluding Candida species) |
In the absence of biopsy categorize as probable: In the presence of histologic findings of both acute rejection and fungal invasion it should be classified as acute rejection with proven fungal infection.
Table 4d.
Fungal Colonization in CTTX
| Syndrome | Signs/symptoms | Radiology | Laboratory |
|---|---|---|---|
| Colonization |
|
|
|
Other infectious syndromes in cardiothoracic organ transplant recipients
Non–CTTX-specific infections, such as urinary tract infection (UTI), surgical site infection (SSI), bloodstream infection (BSI), infective endocarditis (IE), Clostridium difficile infection (CDI) and skin and soft tissue infections (SSTIs), are not included herein.2, 86, 87, 88, 89, 90, 91, 92, 93, 94 The consensus opinion of the ISHLT ID council encourages the use of previously published international definitions for these infections, which have been well established outside of the CTTX population. The use of these standard definitions will allow for intercenter comparisons of rates and types of infections that should not be significantly impacted by the transplant.
Disclosure statement
Shahid Husain received grant from Pfizer, Astellas, Merck; Nina Singh received grant from Pfizer; Michael Ison received grants, member of speaking bureau and advisory board member for ADMA, Adamas, BioCryst, Chimerix, GlaxoSmithKlein, Roche, ViraCor, Abbott, Abbott Molecular, Astellas, Biogen Idec; Atul Humar received grant, advisory board member and consultant for Roche, Astellas; Andy Fisher received grant, advisory board member and consultant for Medimmune, GSK, Chiesi, Boehringer Ingelheim; Kate Gould member speaking bureau for Pfizer; Lianne G Singer received grants from Roche, Pfizer, APT; Martha Mooney,Lara Danziger-Isakov, Frauke Mattner, Robin Avery, Robert F. Padera, Leo Lawler, Richard Drew, Amparo Sole, Sean Studer, Patricia Munoz and Margaret Hannan, nothing to disclose.
References
- 1.Humar A., Michaels M. American Society of Transplantation recommendations for screening, monitoring and reporting of infectious complications in immunosuppression trials in recipients of organ transplantation. Am J Transplant. 2006;6:262–274. doi: 10.1111/j.1600-6143.2005.01207.x. [DOI] [PubMed] [Google Scholar]
- 2.Horan T.C., Andrus M., Dudeck M.A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. http://www.cdc.gov/nhsn/PDFs/pscManual/17pscNosInfDef_current.pdf Accessed December 3, 2010. [DOI] [PubMed]
- 3.De Pauw B., Walsh T.J., Donnelly J.P. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ljungman P., Griffiths P., Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34:1094–1097. doi: 10.1086/339329. [DOI] [PubMed] [Google Scholar]
- 5.Mattner F., Fischer S., Weissbrodt H. Post-operative nosocomial infections after lung and heart transplantation. J Heart Lung Transplant. 2007;26:241–249. doi: 10.1016/j.healun.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Dauber J.H., Paradis I.L., Dummer J.S. Infectious complications in pulmonary allograft recipients. Clin Chest Med. 1990;11:291–308. [PubMed] [Google Scholar]
- 7.Horvath J., Dummer S., Loyd J. Infection in the transplanted and native lung after single lung transplantation. Chest. 1993;104:681–685. doi: 10.1378/chest.104.3.681. [DOI] [PubMed] [Google Scholar]
- 8.Kramer M.R., Denning D.W., Marshall S.E. Ulcerative tracheobronchitis after lung transplantation: A new form of invasive aspergillosis. Am Rev Respir Dis. 1991;144:552–556. doi: 10.1164/ajrccm/144.3_Pt_1.552. [DOI] [PubMed] [Google Scholar]
- 9.Flume P.A., Egan T.M., Paradowski L.J. Infectious complications of lung transplantation: Impact of cystic fibrosis. Am J Respir Crit Care Med. 1994;149:1601–1607. doi: 10.1164/ajrccm.149.6.7516251. [DOI] [PubMed] [Google Scholar]
- 10.Frist W.H., Loyd J.E., Merrill W.H. Single lung transplantation: a temporal look at rejection, infection, and survival. Am Surg. 1994;60:94–102. [PubMed] [Google Scholar]
- 11.Gottlieb J., Mattner F., Weissbrodt H. Impact of graft colonization with gram-negative bacteria after lung transplantation on the development of bronchiolitis obliterans syndrome in recipients with cystic fibrosis. Respir Med. 2009;103:743–749. doi: 10.1016/j.rmed.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Mattner F., Kola A., Fischer S. Impact of bacterial and fungal donor organ contamination in lung, heart–lung, heart and liver transplantation. Infection. 2008;36:207–212. doi: 10.1007/s15010-007-7157-x. [DOI] [PubMed] [Google Scholar]
- 13.Pittet D., Hugonnet S., Harbarth S. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene: Infection Control Programme. Lancet. 2000;356:1307–1312. doi: 10.1016/s0140-6736(00)02814-2. [DOI] [PubMed] [Google Scholar]
- 14.Aguilar-Guisado M., Givalda J., Ussetti P. Pneumonia after lung transplantation in the RESITRA Cohort: a multicenter prospective study. Am J Transplant. 2007;7:1989–1996. doi: 10.1111/j.1600-6143.2007.01882.x. [DOI] [PubMed] [Google Scholar]
- 15.Cantrell D., Shamriz O., Cohen M.J. Hand hygiene compliance by physicians: marked heterogeneity due to local culture? Am J Infect Control. 2009;37:301–305. doi: 10.1016/j.ajic.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Quiros D., Lin S., Larson E.L. Attitudes toward practice guidelines among intensive care unit personnel: a cross-sectional anonymous survey. Heart Lung. 2007;36:287–297. doi: 10.1016/j.hrtlng.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitby M., McLaws M.L., Ross M.W. Why healthcare workers don't wash their hands: a behavioral explanation. Infect Control Hosp Epidemiol. 2006;27:484–492. doi: 10.1086/503335. [DOI] [PubMed] [Google Scholar]
- 18.Pittet D., Simon A., Hugonnet S. Hand hygiene among physicians: performance, beliefs, and perceptions. Ann Intern Med. 2004;141:1–8. doi: 10.7326/0003-4819-141-1-200407060-00008. [DOI] [PubMed] [Google Scholar]
- 19.Mattner F., Ruden A.S., Mattner L. Thoracic organ transplantation may not increase the risk of bacterial transmission in intensive care units. Int J Hyg Environ Health. 2007;210:139–145. doi: 10.1016/j.ijheh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Ramirez P., Valencia M., Torres A. Bronchoalveolar lavage to diagnose respiratory infections. Semin Respir Crit Care Med. 2007;28:525–533. doi: 10.1055/s-2007-991524. [DOI] [PubMed] [Google Scholar]
- 21.Mamessier E., Milhe F., Badier M. Comparison of induced sputum and bronchoalveolar lavage in lung transplant recipients. J Heart Lung Transplant. 2006;25:523–532. doi: 10.1016/j.healun.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Riise G.C., Kjellstrom C., Ryd W. Inflammatory cells and activation markers in BAL during acute rejection and infection in lung transplant recipients: a prospective, longitudinal study. Eur Respir J. 1997;10:1742–1746. doi: 10.1183/09031936.97.10081742. [DOI] [PubMed] [Google Scholar]
- 23.Clelland C., Higenbottam T., Stewart S. Bronchoalveolar lavage and transbronchial lung biopsy during acute rejection and infection in heart–lung transplant patients: Studies of cell counts, lymphocyte phenotypes, and expression of HLA-DR and interleukin-2 receptor. Am Rev Respir Dis. 1993;147:1386–1392. doi: 10.1164/ajrccm/147.6_Pt_1.1386. [DOI] [PubMed] [Google Scholar]
- 24.Stewart S., Fishbein M.C., Snell G.I. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–1242. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Manuel O., Humar A., Preiksaitis J. Comparison of quantiferon-TB gold with tuberculin skin test for detecting latent tuberculosis infection prior to liver transplantation. Am J Transplant. 2007;7:2797–2801. doi: 10.1111/j.1600-6143.2007.02011.x. [DOI] [PubMed] [Google Scholar]
- 26.Yousem S.A., Berry G.J., Cagle P.T. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant. 1996;15:1–15. [PubMed] [Google Scholar]
- 27.A randomized trial of diagnostic techniques for ventilator-associated pneumonia. N Engl J Med 2006;355:2619–30. [DOI] [PubMed]
- 28.Kwon Y., Milbrandt E.B., Yende S. Diagnostic techniques for ventilator-associated pneumonia: conflicting results from two trials. Crit Care. 2009;13:303. doi: 10.1186/cc7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fagon J.Y., Chastre J., Wolff M. Invasive and noninvasive strategies for management of suspected ventilator-associated pneumonia: A randomized trial. Ann Intern Med. 2000;132:621–630. doi: 10.7326/0003-4819-132-8-200004180-00004. [DOI] [PubMed] [Google Scholar]
- 30.Chalermskulrat W., Sood N., Neuringer I.P. Non-tuberculous mycobacteria in end stage cystic fibrosis: implications for lung transplantation. Thorax. 2006;61:507–513. doi: 10.1136/thx.2005.049247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griffith D.E., Aksamit T., Brown-Elliott B.A. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 32.Torre-Cisneros J., Doblas A., Aguado J.M. Tuberculosis after solid-organ transplant: incidence, risk factors, and clinical characteristics in the RESITRA (Spanish Network of Infection in Transplantation) cohort. Clin Infect Dis. 2009;48:1657–1665. doi: 10.1086/599035. [DOI] [PubMed] [Google Scholar]
- 33.Aguado J.M., Torre-Cisneros J., Fortun J. Tuberculosis in solid-organ transplant recipients: consensus statement of the group for the study of infection in transplant recipients (GESITRA) of the Spanish Society of Infectious Diseases and Clinical Microbiology. Clin Infect Dis. 2009;48:1276–1284. doi: 10.1086/597590. [DOI] [PubMed] [Google Scholar]
- 34.Hopkins P., McNeil K., Kermeen F. Human metapneumovirus in lung transplant recipients and comparison to respiratory syncytial virus. Am J Respir Crit Care Med. 2008;178:876–881. doi: 10.1164/rccm.200711-1657OC. [DOI] [PubMed] [Google Scholar]
- 35.Weinberg A., Zamora M.R., Li S. The value of polymerase chain reaction for the diagnosis of viral respiratory tract infections in lung transplant recipients. J Clin Virol. 2002;25:171–175. doi: 10.1016/s1386-6532(02)00006-9. [DOI] [PubMed] [Google Scholar]
- 36.Milstone A.P., Brumble L.M., Barnes J. A single-season prospective study of respiratory viral infections in lung transplant recipients. Eur Respir J. 2006;28:131–137. doi: 10.1183/09031936.06.00105505. [DOI] [PubMed] [Google Scholar]
- 37.Gottlieb J., Schulz T.F., Welte T. Community-acquired respiratory viral infections in lung transplant recipients: a single season cohort study. Transplantation. 2009;87:1530–1537. doi: 10.1097/TP.0b013e3181a4857d. [DOI] [PubMed] [Google Scholar]
- 38.Kumar D., Erdman D., Keshavjee S. Clinical impact of community-acquired respiratory viruses on bronchiolitis obliterans after lung transplant. Am J Transplant. 2005;5:2031–2036. doi: 10.1111/j.1600-6143.2005.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khalifah A.P., Hachem R.R., Chakinala M.M. Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. Am J Respir Crit Care Med. 2004;170:181–187. doi: 10.1164/rccm.200310-1359OC. [DOI] [PubMed] [Google Scholar]
- 40.Engelmann I., Welte T., Fuhner T. Detection of Epstein–Barr virus DNA in peripheral blood is associated with the development of bronchiolitis obliterans syndrome after lung transplantation. J Clin Virol. 2009;45:47–53. doi: 10.1016/j.jcv.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Weinberg A., Hodges T.N., Li S. Comparison of PCR, antigenemia assay, and rapid blood culture for detection and prevention of cytomegalovirus disease after lung transplantation. J Clin Microbiol. 2000;38:768–772. doi: 10.1128/jcm.38.2.768-772.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar D., Husain S., Chen M.H. A prospective molecular surveillance study evaluating the clinical impact of community-acquired respiratory viruses in lung transplant recipients. Transplantation. 2010;89:1028–1033. doi: 10.1097/TP.0b013e3181d05a71. [DOI] [PubMed] [Google Scholar]
- 43.Jartti T., van den H.B., Garofalo R.P. Metapneumovirus and acute wheezing in children. Lancet. 2002;360:1393–1394. doi: 10.1016/S0140-6736(02)11391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kesebir D., Vazquez M., Weibel C. Human bocavirus infection in young children in the United States: molecular epidemiological profile and clinical characteristics of a newly emerging respiratory virus. J Infect Dis. 2006;194:1276–1282. doi: 10.1086/508213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maurer J.R., Tullis D.E., Scavuzzo M. Cytomegalovirus infection in isolated lung transplantations. J Heart Lung Transplant. 1991;10:647–649. [PubMed] [Google Scholar]
- 46.Bailey T.C., Buller R.S., Ettinger N.A. Quantitative analysis of cytomegalovirus viremia in lung transplant recipients. J Infect Dis. 1995;171:1006–1010. doi: 10.1093/infdis/171.4.1006. [DOI] [PubMed] [Google Scholar]
- 47.Ettinger N.A., Bailey T.C., Trulock E.P. Cytomegalovirus infection and pneumonitis: Impact after isolated lung transplantation. Am Rev Respir Dis. 1993;147:1017–1023. doi: 10.1164/ajrccm/147.4.1017. [DOI] [PubMed] [Google Scholar]
- 48.Kotton C.N., Kumar D., Caliendo A.M. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795. doi: 10.1097/TP.0b013e3181cee42f. [DOI] [PubMed] [Google Scholar]
- 49.Gutierrez C.A., Chaparro C., Krajden M. Cytomegalovirus viremia in lung transplant recipients receiving ganciclovir and immune globulin. Chest. 1998;113:924–932. doi: 10.1378/chest.113.4.924. [DOI] [PubMed] [Google Scholar]
- 50.Solans E.P., Yong S., Husain A.N. Bronchioloalveolar lavage in the diagnosis of CMV pneumonitis in lung transplant recipients: an immunocytochemical study. Diagn Cytopathol. 1997;16:350–352. doi: 10.1002/(sici)1097-0339(199704)16:4<350::aid-dc9>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 51.Riise G.C., Andersson R., Bergstrom T. Quantification of cytomegalovirus DNA in BAL fluid: a longitudinal study in lung transplant recipients. Chest. 2000;118:1653–1660. doi: 10.1378/chest.118.6.1653. [DOI] [PubMed] [Google Scholar]
- 52.Stephan F., Fajac A., Grenet D. Predictive value of cytomegalovirus DNA detection by polymerase chain reaction in blood and bronchoalveolar lavage in lung transplant patients. Transplantation. 1997;63:1430–1435. doi: 10.1097/00007890-199705270-00011. [DOI] [PubMed] [Google Scholar]
- 53.Chemaly R.F., Yen-Lieberman B., Chapman J. Clinical utility of cytomegalovirus viral load in bronchoalveolar lavage in lung transplant recipients. Am J Transplant. 2005;5:544–548. doi: 10.1111/j.1600-6143.2005.00747.x. [DOI] [PubMed] [Google Scholar]
- 54.Chemaly R.F., Yen-Lieberman B., Castilla E.A. Correlation between viral loads of cytomegalovirus in blood and bronchoalveolar lavage specimens from lung transplant recipients determined by histology and immunohistochemistry. J Clin Microbiol. 2004;42:2168–2172. doi: 10.1128/JCM.42.5.2168-2172.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zedtwitz-Liebenstein K., Jaksch P., Burgmann H. Evaluation of interleukin-6 and interleukin-10 in lung transplant patients with human cytomegalovirus infection. Clin Transplant. 2009;23:687–691. doi: 10.1111/j.1399-0012.2009.01041.x. [DOI] [PubMed] [Google Scholar]
- 56.Westall G.P., Michaelides A., Williams T.J. Human cytomegalovirus load in plasma and bronchoalveolar lavage fluid: a longitudinal study of lung transplant recipients. J Infect Dis. 2004;190:1076–1083. doi: 10.1086/422327. [DOI] [PubMed] [Google Scholar]
- 57.Bewig B., Haacke T.C., Tiroke A. Detection of CMV pneumonitis after lung transplantation using PCR of DNA from bronchoalveolar lavage cells. Respiration. 2000;67:166–172. doi: 10.1159/000029481. [DOI] [PubMed] [Google Scholar]
- 58.Kubak B.M. Fungal infection in lung transplantation. Transpl Infect Dis. 2002;4(suppl 3):24–31. doi: 10.1034/j.1399-3062.4.s3.4.x. [DOI] [PubMed] [Google Scholar]
- 59.Pappas P.G., Alexander B.D., Andes D.R. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET) Clin Infect Dis. 2010;50:1101–1111. doi: 10.1086/651262. [DOI] [PubMed] [Google Scholar]
- 60.Neofytos D., Fishman J.A., Horn D. Epidemiology and outcome of invasive fungal infections in solid organ transplant recipients. Transpl Infect Dis. 2010;12:220–229. doi: 10.1111/j.1399-3062.2010.00492.x. [DOI] [PubMed] [Google Scholar]
- 61.Hadjiliadis D., Howell D.N., Davis R.D. Anastomotic infections in lung transplant recipients. Ann Transplant. 2000;5:13–19. [PubMed] [Google Scholar]
- 62.Husain S. Unique characteristics of fungal infections in lung transplant recipients. Clin Chest Med. 2009;30:307–313. doi: 10.1016/j.ccm.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 63.Husain S., Kwak E.J., Obman A. Prospective assessment of Platelia Aspergillus galactomannan antigen for the diagnosis of invasive aspergillosis in lung transplant recipients. Am J Transplant. 2004;4:796–802. doi: 10.1111/j.1600-6143.2004.00415.x. [DOI] [PubMed] [Google Scholar]
- 64.Pfeiffer C.D., Fine J.P., Safdar N. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin Infect Dis. 2006;42:1417–1427. doi: 10.1086/503427. [DOI] [PubMed] [Google Scholar]
- 65.Singh N., Alexander B.D., Lortholary O. Pulmonary cryptococcosis in solid organ transplant recipients: clinical relevance of serum cryptococcal antigen. Clin Infect Dis. 2008;46:e12–e18. doi: 10.1086/524738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hage C., Kleiman M.B., Wheat L.J. Histoplasmosis in solid organ transplant recipients. Clin Infect Dis. 2010;50:122–123. doi: 10.1086/649056. [DOI] [PubMed] [Google Scholar]
- 67.Cuellar-Rodriguez J., Avery R.K., Lard M. Histoplasmosis in solid organ transplant recipients: 10 years of experience at a large transplant center in an endemic area. Clin Infect Dis. 2009;49:710–716. doi: 10.1086/604712. [DOI] [PubMed] [Google Scholar]
- 68.Assi M.A., Binnicker M.J., Wengenack N.L. Disseminated coccidioidomycosis in a liver transplant recipient with negative serology: use of polymerase chain reaction. Liver Transpl. 2006;12:1290–1292. doi: 10.1002/lt.20820. [DOI] [PubMed] [Google Scholar]
- 69.Husain S., Clancy C.J., Nguyen M.H. Performance characteristics of the platelia Aspergillus enzyme immunoassay for detection of Aspergillus galactomannan antigen in bronchoalveolar lavage fluid. Clin Vaccine Immunol. 2008;15:1760–1763. doi: 10.1128/CVI.00226-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Husain S., Paterson D.L., Studer S.M. Aspergillus galactomannan antigen in the bronchoalveolar lavage fluid for the diagnosis of invasive aspergillosis in lung transplant recipients. Transplantation. 2007;83:1330–1336. doi: 10.1097/01.tp.0000263992.41003.33. [DOI] [PubMed] [Google Scholar]
- 71.Pasqualotto A.C., Xavier M.O., Sanchez L.B. Diagnosis of invasive aspergillosis in lung transplant recipients by detection of galactomannan in the bronchoalveolar lavage fluid. Transplantation. 2010;90:306–311. doi: 10.1097/TP.0b013e3181e49bc1. [DOI] [PubMed] [Google Scholar]
- 72.Pickering J.W., Sant H.W., Bowles C.A. Evaluation of a (1→3)-beta-D-glucan assay for diagnosis of invasive fungal infections. J Clin Microbiol. 2005;43:5957–5962. doi: 10.1128/JCM.43.12.5957-5962.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alexander B.D., Smith P.B., Davis R.D. The (1,3)β-D-glucan test as an aid to early diagnosis of invasive fungal infections following lung transplantation. J Clin Microbiol. 2010;48:4083–4088. doi: 10.1128/JCM.01183-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nunley D.R., Ohori P., Grgurich W.F. Pulmonary aspergillosis in cystic fibrosis lung transplant recipients. Chest. 1998;114:1321–1329. doi: 10.1378/chest.114.5.1321. [DOI] [PubMed] [Google Scholar]
- 75.Nunley D.R., Gal A.A., Vega J.D. Saprophytic fungal infections and complications involving the bronchial anastomosis following human lung transplantation. Chest. 2002;122:1185–1191. doi: 10.1378/chest.122.4.1185. [DOI] [PubMed] [Google Scholar]
- 76.Singh N., Husain S. Aspergillus infections after lung transplantation: clinical differences in type of transplant and implications for management. J Heart Lung Transplant. 2003;22:258–266. doi: 10.1016/s1053-2498(02)00477-1. [DOI] [PubMed] [Google Scholar]
- 77.Clancy C.J., Jaber R.A., Leather H.L. Bronchoalveolar lavage galactomannan in diagnosis of invasive pulmonary aspergillosis among solid-organ transplant recipients. J Clin Microbiol. 2007;45:1759–1765. doi: 10.1128/JCM.00077-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Husain S., Kwak E.J., Obman A. Prospective assessment of Platelia Aspergillus galactomannan antigen for the diagnosis of invasive aspergillosis in lung transplant recipients. Am J Transplant. 2004;4:796–802. doi: 10.1111/j.1600-6143.2004.00415.x. [DOI] [PubMed] [Google Scholar]
- 79.Husain S., Paterson D.L., Studer S. Voriconazole prophylaxis in lung transplant recipients. Am J Transplant. 2006;6:3008–3016. doi: 10.1111/j.1600-6143.2006.01548.x. [DOI] [PubMed] [Google Scholar]
- 80.Weigt S.S., Elashoff R.M., Huang C. Aspergillus colonization of the lung allograft is a risk factor for bronchiolitis obliterans syndrome. Am J Transplant. 2009;9:1903–1911. doi: 10.1111/j.1600-6143.2009.02635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cadena J., Levine D.J., Angel L.F. Antifungal prophylaxis with voriconazole or itraconazole in lung transplant recipients: hepatotoxicity and effectiveness. Am J Transplant. 2009;9:2085–2091. doi: 10.1111/j.1600-6143.2009.02734.x. [DOI] [PubMed] [Google Scholar]
- 82.Sole A., Morant P., Salavert M. Aspergillus infections in lung transplant recipients: risk factors and outcome. Clin Microbiol Infect. 2005;11:359–365. doi: 10.1111/j.1469-0691.2005.01128.x. [DOI] [PubMed] [Google Scholar]
- 83.Sole A., Salavert M. Fungal infections after lung transplantation. Curr Opin Pulm Med. 2009;15:243–253. doi: 10.1097/MCP.0b013e328326f410. [DOI] [PubMed] [Google Scholar]
- 84.Arthurs S.K., Eid A.J., Deziel P.J. The impact of invasive fungal diseases on survival after lung transplantation. Clin Transplant. 2010;24:341–348. doi: 10.1111/j.1399-0012.2009.01076.x. [DOI] [PubMed] [Google Scholar]
- 85.Vance A.M. The use of the mucicarmine stain for a rapid presumptive identification of Cryptococcus from culture. Am J Med Technol. 1961;27:125–128. [PubMed] [Google Scholar]
- 86.Lopez J., Revilla A., Vilacosta I. Definition, clinical profile, microbiological spectrum, and prognostic factors of early-onset prosthetic valve endocarditis. Eur Heart J. 2007;28:760–765. doi: 10.1093/eurheartj/ehl486. [DOI] [PubMed] [Google Scholar]
- 87.Calandra T., Cohen J. The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit Care Med. 2005;33:1538–1548. doi: 10.1097/01.ccm.0000168253.91200.83. [DOI] [PubMed] [Google Scholar]
- 88.Cicalini S., Puro V., Angeletti C. Broadened definition for hospital-acquired infective endocarditis. Clin Infect Dis. 2004;39:1084–1085. doi: 10.1086/423843. [DOI] [PubMed] [Google Scholar]
- 89.Cecchi E., Parrini I., Chinaglia A. New diagnostic criteria for infective endocarditis: A study of sensitivity and specificity. Eur Heart J. 1997;18:1149–1156. doi: 10.1093/oxfordjournals.eurheartj.a015411. [DOI] [PubMed] [Google Scholar]
- 90.Durack D.T., Lukes A.S., Bright D.K. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Am J Med. 1994;96:200–209. doi: 10.1016/0002-9343(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 91.McDonald L.C., Coignard B., Dubberke E. Recommendations for surveillance of Clostridium difficile-associated disease. Infect Control Hosp Epidemiol. 2007;28:140–145. doi: 10.1086/511798. [DOI] [PubMed] [Google Scholar]
- 92.Mermel L.A., Allon M., Bouza E. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Solomkin J.S., Mazuski J.E., Bradley J.S. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:133–164. doi: 10.1086/649554. [DOI] [PubMed] [Google Scholar]
- 94.Stevens D.L., Bisno A.L., Chambers H.F. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373–1406. doi: 10.1086/497143. [DOI] [PubMed] [Google Scholar]