Highlights
-
•
ETEC toxin MEFAs (multiepitope fusion antigen) were constructed.
-
•
Toxin MEFAs induce neutralizing antibodies to all four porcine ETEC toxins.
-
•
Passive maternal antibodies derived from the toxoid MEFA protect suckling piglets against ETEC diarrhea.
-
•
Toxin MEFA can be used for vaccines against porcine ETEC diarrhea.
Keywords: Enterotoxigenic Escherichia coli (ETEC), Pig diarrhea, Toxoid multiepitope fusion antigen (MEFA), Antitoxin vaccine
Abstract
Enterotoxigenic Escherichia coli (ETEC) strains are the main cause of diarrhea in pigs. Pig diarrhea especially post-weaning diarrhea remains one of the most important swine diseases. ETEC bacterial fimbriae including K88, F18, 987P, K99 and F41 promote bacterial attachment to intestinal epithelial cells and facilitate ETEC colonization in pig small intestine. ETEC enterotoxins including heat-labile toxin (LT) and heat-stable toxins type Ia (porcine-type STa) and type II (STb) stimulate fluid hyper-secretion, leading to watery diarrhea. Blocking bacteria colonization and/or neutralizing enterotoxicity of ETEC toxins are considered effective prevention against ETEC diarrhea. In this study, we applied the MEFA (multiepitope fusion antigen) strategy to create toxoid MEFAs that carried antigenic elements of ETEC toxins, and examined for broad antitoxin immunogenicity in a murine model. By embedding STa toxoid STaP12F (NTFYCCELCCNFACAGCY), a STb epitope (KKDLCEHY), and an epitope of Stx2e A subunit (QSYVSSLN) into the A1 peptide of a monomeric LT toxoid (LTR192G), two toxoid MEFAs, ‘LTR192G-STb-Stx2e-STaP12F’ and ‘LTR192G-STb-Stx2e-3xSTaP12F’ which carried three copies of STaP12F, were constructed. Mice intraperitoneally immunized with each toxoid MEFA developed IgG antibodies to all four toxins. Induced antibodies showed in vitro neutralizing activities against LT, STa, STb and Stx2e toxins. Moreover, suckling piglets born by a gilt immunized with ‘LTR192G-STb-Stx2e-3xSTaP12F’ were protected when challenged with ETEC strains, whereas piglets born by a control gilt developed diarrhea. Results from this study showed that the toxoid MEFA induced broadly antitoxin antibodies, and suggested potential application of the toxoid MEFA for developing a broad-spectrum vaccine against ETEC diarrhea in pigs.
1. Introduction
Young piglets commonly develop diarrhea, clinical conditions known as neonatal diarrhea and post-weaning diarrhea. Pig neonatal diarrhea and post-weaning diarrhea are caused by pathogenic bacteria and viruses including diarrheal Escherichia coli, coronaviruses (transmissible gastroenteritis virus – TGE, and porcine epidemic diarrhea virus – PEDV) and rotaviruses, and continue to be the most important swine diseases (Fairbrother et al., 2005, Harvey et al., 2005, USDA, 2002). Diarrhea results in weight loss, slow growth and acute death, causing substantial economic losses to swine producers in the U.S. and other countries (Haesebrouck et al., 2004, Nagy and Fekete, 1999, Verdonck et al., 2002, Vu-Khac et al., 2007). While neonatal diarrhea can be effectively prevented by passive protection of maternal antibodies, through immunization of pregnant sows; there are no effective prevention strategies against post-weaning diarrhea in weaned pigs. Vaccination is considered the most practical and likely the most effective approach to prevent pig diarrhea. Unfortunately, there are no broadly effective vaccines available to protect young pigs against diarrhea particularly post-weaning diarrhea (Melkebeek et al., 2013, Ruan et al., 2011).
E. coli strains have a central role in the etiology of pig diarrhea (Hampson, 1994). Among the types of E. coli causing diarrhea in pigs (Nataro and Kaper, 1998), enterotoxigenic E. coli (ETEC, viz. a group of E. coli strains producing enterotoxins) is by far the most common and important. These ETEC strains produce fimbrial adhesins and enterotoxins. Fimbrial adhesins mediate ETEC bacterial attachment to specific receptors at pig intestinal epithelial cells and facilitate colonization in the pig small intestine (Smith and Linggood, 1971), playing an essential role in initiating the disease. But it is the enterotoxins that disrupt fluid homeostasis in pig small intestinal epithelial cells to cause fluid and electrolyte hyper-secretion, leading to diarrhea (Nataro and Kaper, 1998). Fluid hyper-secretion results in the loss of some of the mucin layer and disruption of tight junctions of microvilli that further enhance bacterial colonization and worsen diarrheal disease (Berberov et al., 2004, Glenn et al., 2009, Zhang et al., 2006). Therefore, fimbrial adhesins and enterotoxins are the key virulence determinants of ETEC diarrhea, and have been long targeted for prevention strategy development.
Fimbrial adhesins expressed by ETEC strains causing diarrhea in pigs are K88 (F4), F18, K99 (F5), 987P (F6), and F41 (F7) (Awad-Masalmeh et al., 1982, Casey et al., 1992, Frydendahl, 2002, Moon, 1990, Moseley et al., 1986, Nagy et al., 1977, Zhang et al., 2007). Toxins produced by porcine ETEC strains are porcine-type heat-labile toxin (pLT; which is homologous to but differs from the LT of ETEC causing human diarrhea—hLT), heat-stable toxin type Ia (pSTa or porcine-type STa), heat-stable toxin type II (STb), Shiga toxin type 2e (Stx2e), and enteroaggregative heat-stable toxin type 1 (EAST1) (Frydendahl, 2002, Lee et al., 1983, Moon et al., 1980, Nakazawa et al., 1987, Osek, 1999, Zhang et al., 2007). Clinical observations and epidemiological studies revealed that a typical ETEC strain expresses one and occasionally two fimbrial adhesins and one, two or more toxins (Francis, 2002, Frydendahl, 2002, Zhang et al., 2007). Laboratory experimental studies demonstrated that an ETEC strain expressing one fimbrial adhesin and LT, STb, or STa enterotoxin causes diarrhea in young pigs (Berberov et al., 2004, Erume et al., 2008, Zhang et al., 2006, Zhang et al., 2008). Unlike LT and STa or STb, Stx2e is a member of the Shiga toxin family, and itself is thought to be primarily associated with Edema disease in young pigs (Bertschiner and Gyle, 1994). But as Stx2e is frequently found in ETEC strains expressing LT and/or ST toxins (Zajacova et al., 2012, Zhang et al., 2007), it was also targeted for ETEC diarrhea prevention. In contrast, E. coli strains expressing fimbriae and EAST1 are found not associated with diarrhea in young pigs (Ruan et al., 2012, Zajacova et al., 2013). It is thought that a vaccine blocking attachment from all ETEC fimbrial adhesins to host receptors and/or eliminating enterotoxicity of ETEC toxins to host epithelial cells would be able to effectively protect against ETEC diarrhea in pigs and humans (Boedeker, 2005, Walker, 2005, Zhang and Sack, 2012).
However, developing effective vaccines against ETEC continues to be difficult because ETEC strains are divergent. Different ETEC bacteria express immunologically heterogeneous fimbrial adhesins and distinctive enterotoxins. To overcome this challenge, innovative antigen preparation approaches are needed. The recently developed MEFA (multiepitope fusion antigen) strategy allowed us to include antigenic elements (epitopes) from multiple human ETEC virulence factors into a single recombinant protein to induce broadly protective antibody responses (Ruan et al., 2015, Ruan et al., 2014a). We also found recently that peptides (or toxoids) derived from mutated LT and STa toxins are safe antigens and LT-STa genetic fusions induce protective antibodies against both LT and ST toxins (Liu et al., 2011, Ruan et al., 2014b, Zhang et al., 2010). By applying the MEFA, toxoid, and genetic fusion approaches, we should be able to include antigenic elements from all porcine ETEC toxins into a single antigen and to develop a safe and broadly effective antitoxin vaccine.
In this study, we applied the toxoid antigens and the MEFA strategy to create LTR192G-STb-Stx2e-STaP12F toxoid MEFAs, examined the toxoid MEFA for broad antitoxin antigenicity, and explored potential application of the toxoid MEFA in development of a broadly effective antitoxin vaccine against ETEC-associated diarrhea in pigs.
2. Methods
2.1. Bacteria strains and plasmids
The E. coli strains and plasmids used in this study are listed in Table 1 . Genomic DNA of porcine E. coli field strains 3030-2 (K88/LT/STb/STa) (Zhang et al., 2006) and 9168 (F18/Stx2e) (Zhang et al., 2007), and STb recombinant strain 8020 (Zhang et al., 2006) were used for PCR amplification of the eltAB genes (LT), the estA gene (STa), the Stx2e A subunit gene and the estB gene (STb), respectively. LT mutant strain 8221 (Zhang et al., 2010) and STa mutant strain 8415 (Zhang et al., 2010) were used for LTR192G and STaP12F gene amplification. Strains 9168 and 8020 were also used in Vero cell test to detect antibody neutralizing activity against Stx2e toxin and STb toxin. Recombinant ETEC strains, 8819 (987P/LT), 8816 (987P/STb) and 8823 (987P/STa) were used in piglet challenge studies. Vector pET28α (Novagen, Madison, WI) was used to clone and to express the toxoid MEFA genes, and vector pMAL-p5X (New England Biolabs, Ipswich, MA) was used to clone the estB gene and the Stx2e A subunit gene for expression of MBP (maltose binding protein)-STb and MBP-Stx2e fusion proteins. E. coli strains BL21 (GE Healthcare, Piscataway, NJ) and DH5α (Promega, Madison, WI) were used to express toxoid MEFA and MBP fusion proteins. Recombinant E. coli strains were cultured in Lysogeny broth (LB) supplemented with kanamycin (30 μg/ml) or ampicillin (100 μg/ml).
Table 1.
Escherichia coli strains and plasmid used in the study.
| Strains | Relevant properties | Sources |
|---|---|---|
| BL21 | B F−, ompT, hsdS (rB−, mB−), gal, dcm. | GE Healthcare |
| DH5α | fhuA2, Δ(argF-lacZ), U169, phoA, glnV44, φ80, Δ(lacZ)M15, gyrA96, recA1, relA1, endA1, thi-1,hsdR17 | Promega |
| 3030–2 | Field isolate, K88/LT/STb/STa | Zhang et al., 2006 |
| 8020 | K88/STb (pRAS1 in 1836–2) | Zhang et al., 2007 |
| 8221 | LTR912G mutant strain | Zhang et al., 2010 |
| 8415 | STaP12F mutant strain | Zhang et al., 2010 |
| 8221 | LTR912G mutant strain | Zhang et al., 2010 |
| 8415 | STaP12F mutant strain | Zhang et al., 2010 |
| 8221 | LTR912G mutant strain | Zhang et al., 2010 |
| 8415 | STaP12F mutant strain | Zhang et al., 2010 |
| 8221 | LTR912G mutant strain | Zhang et al., 2010 |
| 8415 | STaP12F mutant strain | Zhang et al., 2010 |
| 8221 | LTR912G mutant strain | Zhang et al., 2010 |
| 8415 | STaP12F mutant strain | Zhang et al., 2010 |
| 9168 | 04-13812 field isolate, F18/Stx2e | Zhang et al., 2007 |
| 8823 | 987P/STa challenge strain | Zhang et al., 2010 |
| Plasmids | ||
| pET28α | Novagen | |
| pMAL-p5X | New England Biolabs | |
| pET/LTR192G-STaP12F | LTR192G-STaP12F fusion in pET28α at NheI/BamH1 | this study |
| pET/LTR192G-STb-STaP12F | LTR192G-STb-STaP12F fusion in pET28α at NheI/BamH1 | this study |
| pET/LTR192G-STb-Stx2e-STaP12F | LTR192G-STb-stx2e-STaP12F fusion in pET28α at NheI/BamH1 | this study |
| pET/LTR192G-STb-Stx2e-3xSTaP12F | LTR192G-STb-stx2e-3xSTaP12F fusion in pET28α at NheI/BamH1 | this study |
| pMAL-STb | MBP-STb fusion in pMAL-p5X | this study |
| pMAL-Stx2eB | MBP-Stx2eA fusion in pMAL-p5X | this study |
2.2. ETEC toxoid MEFA gene construction
B-cell epitopes from the LT A1 peptide, STb toxin, and the Stx2e A subunit were predicted with web-based software (Odorico and Pellequer, 2003, Saha and Raghava, 2007). Full-length STa toxoid STaP12F gene was first embedded into LTA1 of a modified LT gene coding a monomeric LTR192G (one A subunit and one B subunit into a single peptide); nucleotides coding the STb epitope and the Stx2e A subunit epitope were sequentially embedded into the LTR192G-STaP12F chimeric gene by using SOE (splice overlapping extension) PCR as we described previously (Liu et al., 2011, Ruan et al., 2014b, Zhang et al., 2010).
To embed nucleotides coding the STaP12F mature peptide into the LTA1 of LTR192G, two PCR products were generated and overlapped. The first PCR product was amplified with primers LT192NheI-F3 (5-GTTTGCTAGCAATGGCGACAAATTATAC-′3; NheI site underlined) and LT192-STaP12F-R (5′-GGCAAAATTACAACAAAGTTCACAGCAGTAAAATGTGTTGTT TTCATCAATCACACCAAAATTAACACGATACCA-′3; nucleotides of STaP12F in italic, with mutated nucleotides in shade; nucleotides of LTA1 underlined). The second PCR product was amplified with primers LT192-STaP12F-F (5′-CTTTGTTGTAATTTTGCCTGTGCTGGATGTTAT ATAGCTCCGGCAGAGGATGGTTACAGA-′3; nucleotides of STaP12F in italic, with mutated nucleotides in shade; nucleotides of LTA1 underlined) and LT192BamHI-R1 (5′-GCGTGGATCCCTACTAGTTTTCCATACT-′3; BamH1 site underlined). LTR192G plasmid p8221 was used as DNA templates for both PCRs. Overlapping two PCR products (through the STa complementary nucleotides of primers) generated a LTR192G-STaP12F chimeric gene. Digested with NheI and BamH1 (Biolabs), this LTR192G-STaP12F toxoid chimeric gene was cloned into pET28α. Resultant plasmids were introduced into DH5α competent cells for a LTR192G-STaP12F recombinant strain.
Similar to the insertion of STaP12F into the LTA1 of LTR192G, nucleotides coding the STb epitope and the Stx2e A subunit epitope were sequentially inserted into the A1 fragment of the LTR192G-STaP12F chimeric gene. To insert the STb epitope to create chimeric gene LT192-STb-STaP12F, we overlapped two PCR products: one was amplified with primers T7-F (5′-TAATACGACTCACTATAGGG-′3) and STb-LTAChim-R (5′-ATGTTCACACAGATCTTTTTT GCCGGTTTGTGTTCCTCTCGCGTG-′3; nucleotides of STb epitope in italic, and nucleotides of LTA1 underlined), and the other was amplified with primers LTA-STbChim-F (5′-GATCTGTGTGAACATTAT GTTTCCACTTCTCTTAGTTTGAGAAGT-′3; nucleotides of STb epitope in italic, and nucleotides of LTA1 underlined) and T7-R (5′-TGCTAGTTATTGGTCAGGGGT-′3), with plasmid pLTR192G-STaP12F as the DNA template.
Insertion of nucleotides coding the Stx2e A subunit epitope into chimeric gene LT192-STb-STaP12F was completed by overlapping another two PCR products. One was amplified with T7-F and Stx2e-LTA-R (5′-CGAAGATACATAACTTTGTTG TATATACTGTCCTGCTAAGTGAGC-′3; nucleotides of Stx2e A subunit epitope in italic, and nucleotides of LTA1underlined), and the other was amplified with primers LTA-Stx2e-F (5′-AGTTATGTATCTTCGTTAAAT TATATCGTTATAGCAAATATGTTT-′3; nucleotides of Stx2e A subunit epitope in italic, and nucleotides of LTA1underlined) and T7-R, with plasmid pLTR192G-STb-STaP12F as the DNA template. The overlapped PCR product was amplified with primers T7-F and T7-R, digested with NheI and BamH1, and cloned into pET28α.
To enhance anti-STa antigenicity, two additional copies of STaP12F gene were fused at the 5′ end and the 3′ end of the LTR192G-STb-Stx2e-STaP12F gene for a toxoid MEFA which carries three copies of STa toxoid STaP12F (LTR192G-STb-Stx2e-3xSTaP12F).
2.3. Expression and detection of ETEC toxoid MEFA protein
Expression of toxoid MEFA proteins was examined in a standard sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Toxoid MEFA recombinant strains were cultured at 37 °C in 200 ml Lysogeny broth (LB) supplemented with kanamycin (30 μg/ml). Bacteria in the overnight grown culture, after OD600 reached 0.6, were induced with 0.1 mM isopropyl-1-thio-β-d-galactoside (IPTG) and incubated for 4 more hours. Induced bacteria were collected and used for total protein extraction in B-PER (bacterial protein extraction reagent in phosphate buffer; Pierce, Rockford, IL). B-PER extracted proteins (with a majority of proteins as inclusion bodies; in denaturing buffer) were used for further extraction of 6 × His-tagged toxoid MEFA proteins to a purity of greater than 90% with Ni-nitrolotriacetic acid agarose by following the manufacturer’s protocol (QIAGEN, Valencia, CA). The 6 × His tagged toxoid MEFA proteins were refolded with a Novagen protein refolding kit (Novagen).
The 6xHis-tagged protein (10 μg) of either toxoid MEFA recombinant strain was examined using 12% SDS-PAGE gels with rabbit anti-CT (1:3,000; Sigma, St. Louis, MO), anti-STa (1:5,000; protein A-purified, gift from Dr. DC Robertson, Kansas State Univ.), anti-STb (1:1,000; National Animal Disease Center, Ames, Iowa), and anti-Stx2e serum (1:3,000; National Animal Disease Center). IRDye-labeled goat anti-rabbit IgG (1:5,000; LI-COR, Lincoln, NE) and LI-COR Odyssey premium infrared gel imaging system (LI-COR) were used to detect the toxoid MEFA proteins. Protein purity was assessed using standard Coomassie blue staining as previously described (Ruan et al., 2014a).
2.4. Mouse immunization
Ten 8-week-old female BALB/c mice (Charles River Laboratories International, Inc., Wilmington, MA) in a group were each immunized intraperitoneally with 200 μg of 6 × His-tagged toxoid MEFA protein ‘LTR192G-STb-Stx2e-STaP12F’ or ‘LTR192G-STb-Stx2e-3xSTaP12F’ (in 200 μl 0.02 M Tris–HCl protein buffer), in an equal volume of Freund complete adjuvant (Sigma). Immunized mice received two booster injections in a two-week interval, at the same dose of the primary but with Freund incomplete adjuvant. Ten mice immunized with 200 μl 0.02 M Tris–HCl, with 200 μl Freund complete adjuvant (primary) or incomplete adjuvant (boosters) served as the control. Serum samples collected before the primary and 10 days after the final booster were stored at −80 °C until use. On day 38, all mice were anesthetized with CO2 and exsanguinated. The mouse study complied with the Animal Welfare Act according to National Research Council guidelines (National Research Council, 1996), and was approved by the South Dakota State University Institutional Animal Care and Use Committee and was supervised by the South Dakota state veterinarian.
2.5. Mouse serum antitoxin IgG antibody titration
Serum of each immunized or control mouse was examined for anti-LT, anti-STa, anti-STb and anti-Stx2e IgG antibodies in ELISAs. To titrate anti-LT and anti-STa IgG antibodies, we coated 96-well microtiter plates with cholera toxin (CT, an LT homologue which has been commonly used as the coating antigen to titrate anti-LT antibodies, Sigma; 100 ng per well of Immulon 2HB plates) or STa-ovalbumin conjugates (10 ng per well of Costar plates) as we described previously (Liu et al., 2011, Ruan et al., 2014b, Zhang et al., 2010).
To titrate anti-STb and anti-Stx2e IgG antibodies, we prepared MBP (maltose binding protein)-STb and MBP-Stx2eA fusion proteins and used them as coating antigens. The STb gene was PCR amplified with primers STbNcolI-F (5′-GACCTTTCATCCATGGGCTCTACACAATCAAATAAAAAAGAT-′3; Nco1 site underlined) and STbBamH1-R (5′-GACTTTAGAGGATCCTTAGCATCCTTTTGCTGCAACCAT-′3; BamH1 site underlined), with STb plasmid p8020 (pRAS1) as templates. The Stx2e A subunit gene was amplified with primers Stx2eNco1-F (5′-GACCTTTCATCCATGGGCTTACTGGGTTTTTCTTCGGTATCC-′3; Nco1 site underlined) and Stx2eBamH1-R (5′-GACTTTAGAGGATCCCACGTCTTCCGGCGTCATCGTATA-′3; BamH1 site underlined) with total DNA of field strain 9168 (F18/Stx2e) as templates. PCR amplified STb gene and Stx2e A subunit gene were digested with Nco1 and BamH1, and ligated into pMAL-p5x (BioLabs) for expression of MBP-STb and MBP-Stx2eA fusion proteins. Recombinant fusion proteins MBP-STb and MBP-Stx2eA expressed in E. coli DH5α were extracted with the pMAL™ protein fusion and purification system by following the manufacturer’s protocol (BioLabs). Extracted MBP-STb and MBP-Stx2eA fusion proteins were refolded with a Novagen protein refolding kit (Novagen), verified in Western blot with anti-STb and anti-Stx2e antiserum respectively, and were used to coat Immulon 2HB plates (100 ng per well) to titrate anti-STb and anti-Stx2e IgG antibodies in ELISAs.
2.6. Antitoxin antibody neutralization
Pooled serum samples from the mice immunized with MEFA LTR192G-STb-Stx2e-3xSTaP12F and the control mice were examined for in vitro antibody neutralization activities against LT, STa, STb, and Stx2e. EIA cAMP and cGMP kits (Assay Design, Ann Arbor, MI) and T-84 cells were used to measure neutralizing activity of mouse serum antibodies against LT and STa toxins, as we described previously (Liu et al., 2011, Ruan et al., 2014b, Zhang et al., 2010). Briefly, the serum sample (30 μl) pooled from the immunized group or the control group was incubated with 2 ng STa toxin (gift from Dr. DC Robertson, Kansas State Univ.; diluted in 150 μl Dulbecco modified Eagle medium [DMEM]/F-12 medium) or 10 ng CT (Sigma; in 150 μl DMEM/F-12) for 1 hour at room temperature. Incubated serum/toxin mixture was brought to 300 μl with DMEM/F-12 and added to a confluent monolayer of T-84 cells (105 cells per well, in 700 μl DMEM/F-12) pre-treated with 1 mM IBMX (3-isobutyl-1-methylaxanthine; Sigma). After incubation of 1 h (STa for cGMP) or 3 h (CT for cAMP) at 37 °C in a CO2 incubator, T-84 cells were gently washed and then lysed. Cell lysates were collected and were measured for intracellular cAMP or cGMP levels (pmole/ml) using a cAMP or cGMP ELISA kit by following manufacturer’s protocol (Assay Design).
For antibody neutralization activity against STb toxicity, bacterial culture filtrates of strain 8020 (STb + ) and Vero cells (ATCC; CCL-81) were examined for cytotoxic activity. Vero cells were seeded in a 24-well plate and cultured to reach full confluence. The volume of 8020 filtrates causing over CD50 of Vero cells was pre-determined and used for the in vitro neutralization assay. Mouse serum sample, at 150 μl, 100 μl, 50 μl or 25 μl, pooled from the immunized mice or the control mice was used to be pre-mixed with 300 μl 8020 culture filtrates. Each serum/filtrates mixture was added to Vero cells (in 700 μl Eagle’s Minimum Essential Medium). After incubation in a 37 °C CO2 incubator for 1 h, cells were examined microscopically. The highest dilution that prevented bacteria filtrates from causing CD50 of Vero cells was considered antibody neutralization titer.
To examine antibody neutralization activity against Stx2e toxin, Vero cell test was carried out based on published protocols (Matsuura et al., 2009, Smith and Scotland, 1993), with modification. A volume of filtrates of Stx2e strain 9168 that caused CD50 of Vero cells was pre-determined. That volume (100 μl) of filtrates of 9168 overnight grown culture was mixed with binary diluted mouse serum sample, 50 μl, 25 μl, 12.5 μl, 6.3 μl, 3 μl, or 1.5 μl, pooled from the immunized or the control group. Each mixture was added to Vero cells (confluent monolayer) in a well of a 24-well tissue culture plate (in a final volume of 1 ml with culture medium) and incubated in a CO2 at 37 °C for 3 days. Cells were examined microscopically daily for cell death (round up) and detachment from wells of the tissue culture plates. Vero cells cultured in cell medium alone or with 100 μl bacterial filtrates were used as references.
2.7. Pig immunization and challenge studies
Gilts that had no ETEC diarrhea record and had not been vaccinated were used for this study. Gilt pre-screened without pre-existing antibodies to ETEC toxins were raised in the university swine unit. A pregnant gilt was intramuscularly immunized with 500 μg recombinant ‘LTR192G-STb-Stx2e-3xSTaP12F’ (in 500 μl PBS) and 5 μg double mutant LT adjuvant (dmLT, in 50 μl PBS; provided by Walter Reed Army Institute of Research, Silver Spring, MD) 8 weeks before farrowing, and received a booster 4 weeks later. Another gilt IM injected with 500 μl PBS was used as the control. After 24 h suckling, piglets born by the immunized mother and the control mother were orally inoculated with 8819 (2.5 × 109 CFUs), 8816 (2.5 × 109 CFUs) and 8823 (2.5 × 109 CFUs). Challenged piglets were examined every 3–4 h during 24 h post-inoculation. No piglets were challenged with a Stx2e strain. Pig immunization and challenge studies were approved and supervised by Kansas State University IACUC.
2.8. Statistical analysis
Data generated from this study were analyzed using SAS for Windows, version 8 (SAS Institute, Cary, NC). Results were expressed as means and standard deviations. Nonparametric Mood’s Median Test or Kruskal–Wallis Median Test was carried out using SigmaXL (Kitchener, ON, Canada) at 2-sided and 95% confidence to assess differences at results of antibody titration (OD readings or the serum dilution that gave an OD >0.3 above the background) and antibody neutralization (cAMP or cGMP levels, pmole/ml) studies between the immunized group and the control group. Calculated p values of <0.05 indicated that differences were significant, when treatments were compared with a two-tailed distribution and two-sample equal or unequal variance.
3. Results
3.1. The constructed toxoid MEFA carried epitopes of STb and Stx2e A subunit, and STa toxoid STaP12F
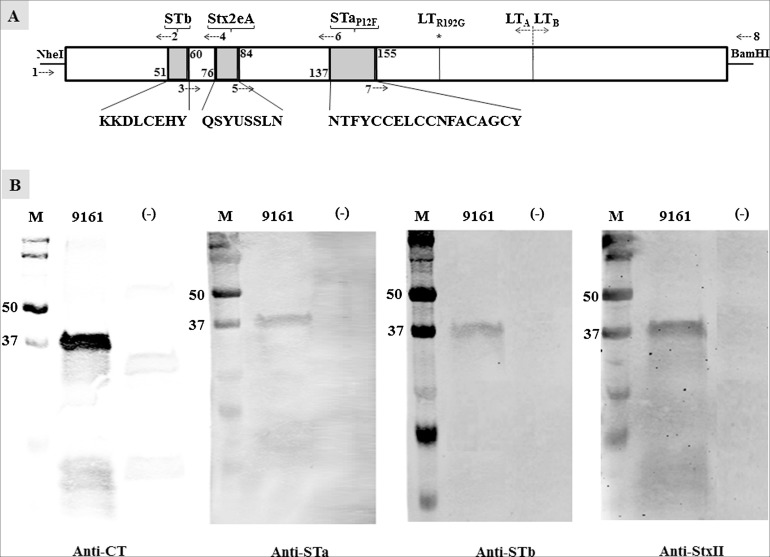
By substituting an in silico predicted surface-exposed but less antigenic epitope of the monomeric LTR192G (138–154 amino acids of the LT A1 peptide) with the full-length mature peptide of STaP12F toxoid, a ‘LTR192G-STaP12F’ toxoid chimeric gene was generated. Toxoid STaP12F was reported to induce neutralizing anti-STa antibodies in rabbits after being genetically fused at the C-terminus of monomeric LTR192G peptide (Zhang et al., 2010). This ‘LTR192G-STaP12F’ was served as the template for subsequent insertions of a STb epitope and a Stx2e epitope. Peptide ‘KKDLCEHY’ was in silico predicted as the only epitope from the poorly immunogenic STb toxin, and ‘QSYVSSLN’ was among the most antigenic epitopes from the Stx2e A subunit. Replacing nucleotides coding the 52–59 amino acids of the LTA1 in the ‘LTR192G-STaP12F’ chimeric gene with nucleotides coding the STb ‘KKDLCEHY’ epitope resulted in ‘LTR192G-STb-STaP12F’ gene. Substituting nucleotides coding the 77–83 amino acids of the LTA1 of ‘LTR192G-STb-STaP12F’ with nucleotides coding the Stx2e A subunit epitope ‘QSYVSSLN’ yielded the ‘LTR192G-STb-Stx2e-STaP12F’ gene (Fig. 1 A). Accordingly, recombinant strains, 8778 (LTR192G-STaP12F), 9137 (LTR192G-STb-STaP12F), and 9161 (LTR192G-STb-Stx2e-STaP12F) were generated (Table 1).
Fig. 1.
Construction and detection of LTR192G-STb-Stx2e-STaP12F toxoid MEFA. (A) Schematic illustration of the toxoid MEFA gene. Nucleotides coding STb epitope (KKDLCEHY), Stx2e A subunit epitope (QSYVSSLN), and full-length STa toxoid STaP12F substituted nucleotides coding the 52–59, 77–83, and 138–154 amino acids of the A1 peptide of the monomeric LT toxoid LTR192G to generate a toxoid MEFA gene using splicing overlap extension (SOE) PCRs. PCR primers: 1, T7-F; 2, STb-LTAChim-R; 3, LTA-STbChim-F; 4, Stx2e-LTA-R; 5, LTA-Stx2e-F; 6, LT192-STaP12F-R; 7, LT192-STaP12F-F; 8, T7-R. Two PCRs using primers LT192NheI-F3 and 6, primers 7 and LT192BamH1-R1 constructed the LTR192G-STaP12F chimeric gene initially. The second set of two PCRs using primers 1 and 2, primers 3 and 8 to insert the STb epitope into the LTR192G-STaP12F toxoid chimeric gene, and the third set of two PCRs with primers 1 and 4, primers 5 and 8 to insert the Ste2e A subunit epitope into the LTR192G-STb-STaP12F chimeric gene. (B) Western blots to show detection of the toxoid MEFA protein with rabbit anti-CT (1:3000; Sigma), anti-STa (1:5000), anti-STb (1:1000), and anti-Stx2e (1:3000) antiserum, with IRDye-labeled goat-anti-rabbit IgG (1:5000; LI-COR) as the secondary antibody. Lane M is the protein marker (in kilo Daltons; Precision Plus Protein Pre-stained standard; Bio-Rad), 9161 represented proteins extracted from the toxoid MEFA recombinant strain, and (−) indicated proteins extracted from E. coli BL21 host strain.
Fusing two additional STaP12F toxoids at the N-terminus and the C-terminus of the ‘LTR192G-STb-Stx2e-STaP12F’ created the second toxoid MEFA, recombinant strain 9403 (LTR192G-STb-Stx2e-3xSTaP12F) (Table 1). DNA sequencing revealed that nucleotide fragments coding the STaP12F toxoid, and epitopes of STb and Stx2e A subunit stayed in a correct reading frame.
Expression of the ‘LTR192G-STb-Stx2e-STaP12F’ or ‘LTR192G-STb-Stx2e-3xSTaP12F’ toxoid MEFA protein was verified in Western blot using anti-CT, anti-STa, anti-STb, and anti-Stx2e antiserum. A protein of approximately 37–40 kDa, an expected molecule weight of the denatured his-tagged toxoid MEFA ‘LTR192G-STb-Stx2e-STaP12F’, was detected from strain 9161 by anti-CT, anti-STa, anti-STb, and anti-StxII antiserum, respectively (Fig. 1B). A protein of approximately 40 kDa was detected from strain 9403 (LTR192G-STb-Stx2e-3xSTaP12F) using the anti-STa and anti-LT antibodies. No proteins of the similar sizes were detected by the same anti-serum from the host E. coli BL21 strain.
3.2. Toxoid MEFA proteins were immunogenic
Both toxoid MEFAs were unable to stimulate cAMP or cGMP in T-84 cells, indicating a lack of LT or STa enterotoxicity (data not shown). Mice immunized with toxoid MEFA ‘LTR192G-STb-Stx2e-STaP12F’ developed strong anti-LT, anti-STb and anti-Stx2e IgG antibody responses (Fig. 2 ). Anti-LT, anti-STb and anti-Stx2e IgG titers (in log10) were 3.75 ± 0.34, 3.37 ± 0.42 and 3.6 ± 0.53, respectively in the immunized mice. These titers were significantly greater than those of the control mice (anti-LT, 0.1 ± 0.1, anti-STb, 0.42 ± 0.61, and anti-Stx2e, 1.26 ± 0.08; p = 0.000 from Mood’s Median test).
Fig. 2.
Mouse serum anti-LT, anti-Stx2e, anti-STb and anti-STa IgG antibody titers (in log10). Anti-LT, anti-Stx2e, anti-STb and anti-STa IgG antibodies in serum samples of the mice immunized with ‘LTR192G-STb-Stx2e-STaP12F’ MEFA and the control mice (10 mice per group) were titrated in ELISAs using CT (100 ng per well of 2HB plates; coating antigens), MBP-Stx2eA fusion (100 ng per well of 2HB plates), MBP-STb fusion (100 ng per well of 2HB plates), and STa-ovalbumin conjugates (10 ng per well of Costar plates), respectively. HRP-conjugated goat anti-mouse IgG (1:5000 for anti-LT, anti-Stx2e and anti-STb; 1:3000 for anti-STa) was used the secondary antibodies. Antibody titers were calculated from the highest dilution of a serum sample that produced an ELISA OD of >0.3 (above the background), and expressed in a scale of log10. Each dot represented an IgG titer from a mouse, and the bar indicated the mean titer of the group. The p value indicated difference of the IgG titers between the immunization group and the control group.
Anti-STa IgG antibody response were detected at a very low titer (0.45 ± 0.21) in the serum of the mice immunized with ‘LTR192G-STb-Stx2e-STaP12F’ (Fig. 2), but still significantly differed from the response in the control mice (p = 0.003 of Mood’s Median test). Mice immunized with toxoid MEFA ‘LTR192G-STb-Stx2e-3xSTaP12F’, which included three copies of the STaP12F toxoid, had anti-STa IgG antibody titers (in log10) detected at 0.87 ± 0.66 from serum samples (Fig. 3 ).
Fig. 3.

Anti-STa IgG titers (in log10) in serum samples of mice immunized with toxoid MEFA ‘LTR192G-STb-Stx2e-3xSTaP12F’. Anti-STa IgG antibodies in the serum samples of mice before the immunization and after the immunization (10 mice in the group) were titrated in ELISA using STa-ovalbumin conjugates (10 ng per well of Costar plates; coating antigen) and HRP-conjugated goat anti-mouse IgG (1:3000; the secondary antibodies). Each dot represented a mouse IgG titer, and the bar indicated the mean titer of the treatment.
3.3. Mouse serum anti-LT and anti-STa antibodies exhibited in vitro neutralizing activities against CT and STa toxins
The pooled serum sample of the immunized mice showed neutralizing activities against CT (Fig. 4 ) and STa (Fig. 5 ). The cAMP level in T-84 cells incubated with 10 ng CT and 30 μl pooled serum of the immunized mice was 15.84 ± 3.40 pmole/ml; the cAMP level in the T-84 cells incubated with the same amount of CT but the pooled serum of the control mice was significantly greater, 41.2 ± 0 pmole/ml (p < 0.05 in Mood’s Median test).
Fig. 4.
Mouse serum in vitro antibody neutralizing activity against CT. Intracellular cyclic AMP levels in T-84 cells measured with an EIA cAMP ELISA kit (Assay Design) showed anti-LT antibody neutralization activity. Neutralizing antibodies prevent CT from stimulating intracellular cAMP in T-84 cells, resulting in a low cAMP level (pmole/ml). The pooled serum sample of the mice immunized with ‘LTR19G-STb-Stx2e-3xSTaP12F’ or the control mice (30 μl in total) was incubated with 10 ng CT toxin (in 150 μl) for 1 h at room temperature, the serum/toxin mixture was brought to 300 μl and added to T-84 cells (in 700 μl cell culture medium). Intracellular cAMP levels in cells were measured after 3 h incubation. Ten ng CT without serum was used as a positive control, and medium (cell culture medium; without toxin or serum) was used as the background reference. Columns and bars indicated mean cAMP levels and standard deviations. The p value of Mood’s Median test indicated difference of antibody neutralizing activity against CT between the immunization group and the control group.
Fig. 5.
Mouse serum in vitro antibody neutralizing activity against STa toxin. Intracellular cyclic GMP levels in T-84 cells measured with an EIA cGMP ELISA kit (Assay Design) showed anti-STa antibody neutralization activity. Neutralizing antibodies prevent STa toxin from stimulating intracellular cGMP in T-84 cells, resulting in a low cGMP level (pmole/ml). The pooled serum sample of the mice immunized with ‘LTR19G-STb-Stx2e-3xSTaP12F’ or the control mice (30 μl in total) was incubated with 2 ng STa toxin (in 150 μl) for 1 h at room temperature, the serum/toxin mixture was brought to 300 μl and added to T-84 cells (in 700 μl cell culture medium). Intracellular cGMP levels in cells were measured after 1 h incubation. Two ng STa without serum was used as a positive control of enterotoxicity and medium (cell culture medium; without toxin or serum) was used as the background reference. Columns and bars indicated mean cGMP levels and standard deviations. The p value of Mood’s Median test indicated difference of antibody neutralizing activity against STa between the immunization group and the control group.
The cGMP level in the T-84 cells incubated with 2 ng STa and 30 μl pooled serum of the immunized mice was 3.45 ± 0.16 pmole/ml. The cGMP level in the T-84 cells incubated with 2 ng STa but the 30 μl pooled serum sample of the control mice was significantly greater (8.32 ± 0.96 pmole/ml; p < 0.01 in Mood’s Median test).
3.4. Serum antibodies showed in vitro neutralizing activity against STb and Stx2e cytotoxicity
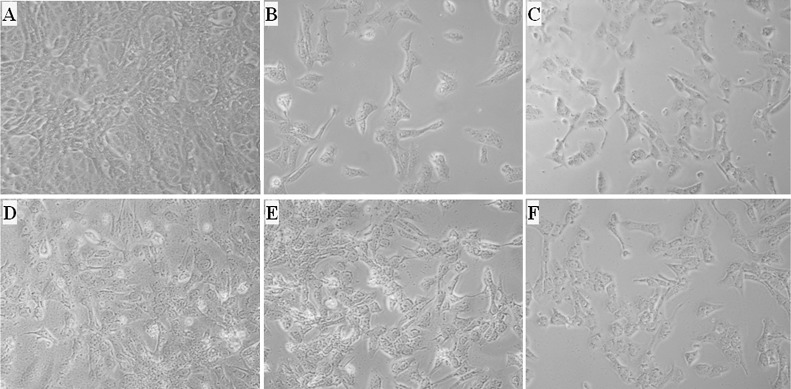
The pooled serum sample of the immunized mice reduced STb cytotoxicity to Vero cells (Fig. 6 ). Vero cells incubated with 300 μl 8020 filtrates alone (Fig. 6B), or 300 μl filtrates and 150 μl pooled serum of the control mice (Fig. 6C), were shown over 50% cell death or detachment. Cells incubated with 300 μl 8020 filtrates and 150 μl (1:7.7 dilution) pooled serum of the immunized mice remained normal (Fig. 6D). When serum of the immunized mice was reduced to 25 μl (1:41 dilution), cells started showing 50% detachment (Fig. 6F).
Fig. 6.
Mouse serum antibody neutralization activity against STb toxin. A: Normal Vero cells grown in cell culture medium. B: Vero cells (in 700 μl culture medium) incubated with 300 μl 8020 (STb) overnight grown culture filtrates, showing over 50% cell death and detachment. C: Vero cells incubated 300 μl 8020 (STb) filtrates pre-mixed with 150 μl pooled serum of the control mice. D: Vero cells incubated with 300 μl 8020 (STb) filtrates pre-mixed with 150 μl pooled serum of the mice immunized with ‘LTR192G-STb-Stx2e-3xSTaP12F’ MEFA. E: Vero cells incubated with 300 μl 8020 (STb) filtrates premixed with 50 μl pooled serum of the immunized mice. F: Vero cells incubated with 300 μl 8020 (STb) filtrates and 25 μl pooled serum of the immunized mice.
Stx2e toxicity Vero cell test showed 50% of the Vero cells became rounded and detached after incubation with 100 μl filtrates of Stx2e strain 9168 overnight grown culture (Fig. 7 B) or in the addition of 50 μl pooled serum sample of the control mice (Fig. 7C). But cells remained normal when incubated with 100 μl 9168 filtrates and 50 μl pooled serum sample (1:20 final dilution, in 1 ml cell culture medium) of the immunized mice (Fig. 7D). Only when less than 12.5 μl pooled serum of the immunized mice was used (a final dilution of 1:80), over 50% of the cells become dead and detached (Fig. 7F).
Fig. 7.
Mouse serum antibody neutralization activity against Stx2e in Vero cell test. A: Vero cells grow in normal cell culture medium, showing normal cell growth. B: Vero cells incubated with 100 μl of 9168 (F18/Stx2e) strain overnight grown culture filtrates, showing over 50% cell death. C: Vero cells incubated with 100 μl 9168 strain filtrates pre-mixed with 50 μl serum pooled from the control mice. D: Vero cells incubated with 100 μl 9168 strain filtrates pre-mixed with 50 μl serum pooled from the immunized mice. E: Vero cells incubated with 100 μl 9168 strain filtrates and 25 μl serum pooled from the immunized mice. F: Vero cells incubated with 100 μl 9168 strain filtrates and 12.5 μl serum pooled from the immunized mice.
3.5. Piglets born by the immunized mother were protected against ETEC challenge
Immunized gilt and piglets born by the immunized gilt had anti-LT, anti-STb, anti-STa and antiStx2e antibodies detected (Table 2 ). After challenged with 8819 (LT), 8816 (STb) and 8823 (STa) recombinant ETEC strains together, all 6 piglets born by the control mother developed severe diarrhea and showed sign of dehydration, whereas 6 out 7 piglets born by the immunized mother remained healthy. Only one piglet born by the immunized mother showed mild diarrhea. No antibodies specific to LT, STa, STb or Stx2e were detected in the control gilt, piglets born by the control gilt, or serum samples of gilts prior to primary immunization.
Table 2.
Anti-LT, –STb, –Stx2e and anti-STa IgG and IgA antibody titers (log10) detected in the immunized or control sows and the piglets born by the immunized or control mothers.
| Anti-LT antibodies | |||||||
|---|---|---|---|---|---|---|---|
| Sows |
Piglets born by |
||||||
| Serum IgG |
Colostrum IgA |
Colostrum IgG |
Serum IgG |
||||
| Immunized | Control | Immunized | Control | Immunized | Control | Immunized | Control |
| 3.40 ± 0.00 | 0 ± 0 | 2.6 ± 0.04 | 0 ± 0 | 3.32 ± 0.01 | 0 ± 0 | 4.15 ± 0.14 | 0 ± 0 |
| Anti-STb antibodies | |||||||
|---|---|---|---|---|---|---|---|
| Sows |
Piglets born by |
||||||
| Serum IgG |
Colostrum IgA |
Colostrum IgG |
Serum IgG |
||||
| Immunized | Control | Immunized | Control | Immunized | Control | Immunized | Control |
| 3.96 ± 0.03 | 0 ± 0 | 2.4 ± 0.02 | 0 ± 0 | 3.32 ± 0.04 | 0 ± 0 | 3.5 ± 0.12 | 0 ± 0 |
| Anti-Stx2e antibodies | |||||||
|---|---|---|---|---|---|---|---|
| Sows |
Piglets born by |
||||||
| Serum IgG |
Colostrum IgA |
Colostrum IgG |
Serum IgG |
||||
| Immunized | Control | Immunized | Control | Immunized | Control | Immunized | Control |
| 3.90 ± 0.02 | 0 ± 0 | 2.2 ± 0.01 | 0 ± 0 | 3.33 ± 0.05 | 0 ± 0 | 3.5 ± 0.12 | 0 ± 0 |
| anti-STa antibodies | |||||||
|---|---|---|---|---|---|---|---|
| Sows |
Piglets born by |
||||||
| Serum IgG |
Colostrum IgA |
Colostrum IgG |
Serum IgG |
||||
| Immunized | Control | Immunized | Control | Immunized | Control | Immunized | Control |
| 1.86 ± 0.01 | 0 ± 0 | 0.78 ± 0.01 | 0 ± 0 | 1.8 ± 0.01 | 0 ± 0 | 1.98 ± 0.10 | 0 ± 0 |
Note: The standard deviations of IgG and IgA antibody titers of the immunized sow and the control sow were from triplicate sampling.
4. Discussion
Since ETEC strains expressing any one, two, or more than two toxins cause diarrhea in neonatal and post-weaning pigs, an effective ETEC antitoxin vaccine need to induce antibodies protecting against LT, STa and STb. Results from the present study indicated that a toxoid MEFA that carried antigenic elements or epitopes of four ETEC toxins induced anti-LT, anti-STa, anti-STb and anti-Stx2e antibodies. Moreover, antibodies derived from the immunized mice showed in vitro neutralization activities against toxicity of all four toxins, and antibodies derived from the immunized gilt showed protection against LT/STa/STb ETEC infection. These results suggest toxoid MEFA LTR192G-STb-Stx2e-3xSTaP12F can potentially be an antigen for developing a protective antitoxin vaccine against ETEC associated diarrhea in young pigs.
STa toxoid STaP12F when was fused to the C-terminus of the LT toxoid LTR192G induced strongly protective anti-STa IgG and IgA antibody responses in IM immunized rabbits (Zhang et al., 2010). Data from the current study, however, showed that MEFA LTR192G-STb-Stx2e-STaP12F, which had the same STa toxoid but embedded inside the A1 peptide of the LTR192G, induced only mild IgG antibody response in the IP immunized mice. That suggested the STa toxoid may not be placed at an optimal position in this toxoid MEFA, negatively affecting its antigen presentation and thus anti-STa antigenicity. Knowing that additional copies of STa toxoid enhanced toxoid fusion anti-STa antigenicity (Zhang et al., 2013), we constructed toxoid MEFA ‘LTR192G-STb-Stx2e-3xSTaP12F’ to carry three copies of the STaP12F. Mice immunized with LTR192G-STb-Stx2e-3xSTaP12F developed similar levels of anti-LT, anti-STb and anti-Stx2e IgG antibody responses compared to the mice immunized with toxin MEFA ‘LTR192G-STb-Stx2e-STaP12F’ (data not shown), but a greater anti-STa IgG antibody titer (Fig. 3). Whether STaP12F is the optimal STa toxoid for toxoid MEFAs to induce protective anti-STa antibodies will need to be further examined. Human-type STa toxoid STaN12S is suggested an optimal STa toxoid for LT-STa toxoid fusions in inducing neutralizing anti-STa antibodies (Ruan et al., 2014b). But the human-type STa and the porcine-type STa show antigenicity differences, whether the analogue porcine-type STaN11S exhibits better antigenic property needs to be further examined. Nevertheless, antibodies induced by the LTR192G-STb-Stx2e-3xSTaP12F showed neutralizing activity against STa toxin, and protected suckling piglets against LT/STb/STa ETEC infection.
LTR192G-STb-Stx2e-STaP12F and LTR192G-STb-Stx2e-3xSTaP12F generated in this study may be used to develop antitoxin subunit vaccines, as a step for further development of practical and economical vaccines to prevent diarrhea in pigs. Unlike live attenuated vaccines, subunit vaccines are not optimal for preventing post-weaning diarrhea in pigs because of a higher production cost and the demand for professional training and labor in parenteral immunization to large numbers of individual pigs. Helpfully, the monomeric ‘LTR192G-STb-Stx2e-STaP12F’ MEFA had the STa toxoid, the STb epitope, and the Stx2e epitope embedded at the A1 peptide of LTR192G. This chimeric gene can be modified to express a LT-like holotoxin-structured toxoid MEFA protein. As we demonstrated previously (Ruan and Zhang, 2013), by simply inserting back the nucleotides coding the native leading signal peptides of the LTA subunit gene (eltA) and the LTB subunit gene (eltB), and also the cistron gene structure between the two LT subunit genes, a monomeric LT gene can be reversed to a LT-like gene coding a LT-like holotoxin-structured protein. Since the native LTB gene expresses the LTB subunit and forms LTB pentamer independently, a modified ‘LTR192G-STb-Stx2e-STaP12F’ fusion protein can form LTB pentamer and binds to GM1 receptors in pig small intestine. If expressed by a nonpathogenic E. coli strain, the modified LT-like-structured ‘LTR192G-STb-Stx2e-STaP12F’ toxoid MEFA could be used to develop a live oral vaccine against porcine ETEC diarrhea. Even for the ‘LTR192G-STb-Stx2e-3xSTaP12F’ toxoid MEFA, if all three copies of STaP12F or a different STa toxoid are embedded at the LT A1 peptide, for an example, at the N- and C-terminus of the A1 peptide, the monomeric ‘LTR192G-STb-Stx2e-3xSTaP12F’ can also be converted to a holotoxin-structured protein and expressed by an E. coli or a Salmonella strain for a live ETEC vaccine candidate.
Double mutant LT, dmLT (LTR192G/L211A) was used as adjuvant for pig immunization. This dmLT has been explored as a safe and effective mucosal adjuvant in human vaccine development. We recently showed that dmLT can be an effective adjuvant in parenteral immunization (data not shown). Adjuvant dmLT included in current pig immunization also served as an antigen to supplement the toxoid MEFA in inducing protective anti-LT antibodies. Future studies with a different adjuvant can conclusively assess protection from anti-LT antibodies derived from this toxoid MEFA. Additionally, studies will be needed to optimize the dose of dmLT adjuvant and also the dose of toxoid MEFA immunogen.
Future studies to assess better the potency of this toxoid MEFA in ETEC vaccine development will be needed. The present study examined only antigen immunogenicity, in vitro antitoxin antibody neutralization and maternal antibody passive protection against ETEC infection. Unlike commercially available kits that were used to measure antibody neutralizing activities against LT or STa, we currently do not have standard assays to measure anti-STb and anti-Stx2e antibody neutralizing activities. In this study, we adopted the Vero cell cytotoxicity assay to assess antibody neutralization activities against STb and Stx2e. Future studies to use purified STb or Stx2e toxin instead of bacterial culture filtrates or to adopt a Shiga toxin quantitative microtiter assay (Gentry and Dalrymple, 1980) may measure better anti-Stx2e or anti-STb antibody neutralization activity. In addition, immunizing neonatal piglets and challenging weaned piglets with the LT+, STb+, and STa+ ETEC strains separately will be needed to assess protective efficacy against each toxin, post-weaning diarrhea, and also perhaps Stx2e associated edema disease as described by Oanh et al. (2012). Moreover, large scaled studies are required to evaluate efficacy of candidate vaccines derived from this toxoid MEFA. Results from this study, nevertheless, indicate the toxoid MEFAs can potentially serve as antigens for multivalent vaccines against ETEC associated diarrhea, and may suggest the MEFA strategy may be a useful platform for developing broadly protective vaccines against other heterogeneous pathogenic strains or isolates.
5. Conclusion
STa toxoid peptide, STb and Stx2e-A subunit epitopes of porcine enterotoxigenic E. coli (ETEC), the main cause of porcine neonatal diarrhea and post-weaning diarrhea, can be embedded into a LT toxoid monomer. Mouse serum antibodies derived against the toxoid MEFA showed neutralizing activities against all four toxins. Pigs IM immunized with LT-STb-Stx2e-3xSTaP12F MEFA developed antibodies specific to each toxin, and maternal passive antibodies protected born piglets against infection of a mixture of a LT+, a STa+ and a STb+ strains. These results suggested that a toxoid MEFA may potentially serve as an antigen for vaccine development against porcine ETEC diarrhea.
Conflict of interest
The authors declaim no conflict of interests from this study.
Acknowledgements
We thank Dr. DC Robertson for providing the anti-STa antiserum and the purified STa toxin. Financial support for this study was provided by the Agricultural Experiment Station of South Dakota State University and Kansas State University.
References
- Awad-Masalmeh M., Moon H.W., Runnels P.L., Schneider R.A. Pilus production, hemagglutination, and adhesion by porcine strains of enterotoxigenic Escherichia coli lacking K88, K99, and 987P antigens. Infect. Immun. 1982;35:305–313. doi: 10.1128/iai.35.1.305-313.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berberov E.M., Zhou Y., Francis D.H., Scott M.A., Kachman S.D., Moxley R.A. Relative importance of heat-labile enterotoxin in the causation of severe diarrheal disease in the gnotobiotic piglet model by a strain of enterotoxigenic Escherichia coli that produces multiple enterotoxins. Infect. Immun. 2004;72:3914–3924. doi: 10.1128/IAI.72.7.3914-3924.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertschiner H.U., Gyle C.L. Oedema disease of pigs. In: Gyles C., editor. Escherichia coli in Domestic Animals and Humans. CAB International; Oxon, UK: 1994. pp. 193–220. [Google Scholar]
- Boedeker E.C. Vaccines for enterotoxigenic Escherichia coli: current status. Curr. Opin. Gastroenterol. 2005;21:15–19. [PubMed] [Google Scholar]
- Casey T.A., Nagy B., Moon H.W. Pathogenicity of porcine enterotoxigenic Escherichia coli that do not express K88, K99, F41, or 987P adhesins. Am. J. Vet. Res. 1992;53:1488–1492. [PubMed] [Google Scholar]
- Erume J., Berberov E.M., Kachman S.D., Scott M.A., Zhou Y., Francis D.H., Moxley R.A. Comparison of the contributions of heat-labile enterotoxin and heat-stable enterotoxin b to the virulence of enterotoxigenic Escherichia coli in F4ac receptor-positive young pigs. Infect. Immun. 2008;76:3141–3149. doi: 10.1128/IAI.01743-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbrother J.M., Nadeau E., Gyles C.L. Escherichia coli in postweaning diarrhea in pigs: an update on bacterial types pathogenesis, and prevention strategies. Anim. Health Res. Rev. 2005;6:17–39. doi: 10.1079/ahr2005105. [DOI] [PubMed] [Google Scholar]
- Francis D.H. Enterotoxigenic Escherichia coli infection in pigs and its diagnosis. J. Swine Health Prod. 2002;10:171–175. [Google Scholar]
- Frydendahl K. Prevalence of serogroups and virulence genes in Escherichia coli associated with postweaning diarrhoea and edema disease in pigs and a comparison of diagnostic approaches. Vet. Microbiol. 2002;85:169–182. doi: 10.1016/s0378-1135(01)00504-1. [DOI] [PubMed] [Google Scholar]
- Gentry M.K., Dalrymple J.M. Quantitative microtiter cytotoxicity assay for Shigella toxin. J. Clin. Microbiol. 1980;12:361–366. doi: 10.1128/jcm.12.3.361-366.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn G.M., Francis D.H., Danielsen E.M. Toxin-mediated effects on the innate mucosal defenses: implications for enteric vaccines. Infect. Immun. 2009;77:5206–5215. doi: 10.1128/IAI.00712-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haesebrouck F., Pasmans F., Chiers K., Maes D., Ducatelle R., Decostere A. Efficacy of vaccines against bacterial diseases in swine: what can we expect? Vet. Microbiol. 2004;100:255–268. doi: 10.1016/j.vetmic.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Hampson D.J. Postweaning Escherichia coli diarrhoea in pigs. In: Gyles C.L., editor. Escherichia coli in Domestic Animals and Humans. CAB International; Oxon, UK: 1994. pp. 171–192. [Google Scholar]
- Harvey R.B., Anderson R.C., Genovese K.J., Callaway T.R., Nisbet D.J. Use of competitive exclusion to control enterotoxigneic strains of Escherichia coli in weaned pigs. J. Anim. Sci. 2005;2005:44–47. [Google Scholar]
- Lee C.H., Moseley S.L., Moon H.W., Whipp S.C., Gyles C.L., So M. Characterization of the gene encoding heat-stable toxin II and preliminary molecular epidemiological studies of enterotoxigenic Escherichia coli heat-stable toxin II producers. Infect. Immun. 1983;42:264–268. doi: 10.1128/iai.42.1.264-268.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Ruan X., Zhang C., Lawson S.R., Knudsen D.E., Nataro J.P., Robertson D.C., Zhang W. Heat-labile- and heat-stable-toxoid fusions (LTR192G-STaP13F of human enterotoxigenic Escherichia coli elicit neutralizing antitoxin antibodies. Infect. Immun. 2011;79:4002–4009. doi: 10.1128/IAI.00165-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura G., Morinaga N., Yahiro K., Komine R., Moss J., Yoshida H., Noda M. Novel subtilase cytotoxin produced by Shiga-toxigenic Escherichia coli induces apoptosis in vero cells via mitochondrial membrane damage. Infect. Immun. 2009;77:2919–2924. doi: 10.1128/IAI.01510-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkebeek V., Goddeeris B.M., Cox E. ETEC vaccination in pigs. Vet. Immunol. Immunopathol. 2013;152:37–42. doi: 10.1016/j.vetimm.2012.09.024. [DOI] [PubMed] [Google Scholar]
- Moon H.W. Colonization factor antigens of enterotoxigenic Escherichia coli in animals. Curr. Top. Microbiol. Immunol. 1990;151:147–165. doi: 10.1007/978-3-642-74703-8_8. [DOI] [PubMed] [Google Scholar]
- Moon H.W., Kohler E.M., Schneider R.A., Whipp S.C. Prevalence of pilus antigens enterotoxin types, and enteropathogenicity among K88-negative enterotoxigenic Escherichia coli from neonatal pigs. Infect. Immun. 1980;27:222–230. doi: 10.1128/iai.27.1.222-230.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley S.L., Dougan G., Schneider R.A., Moon H.W. Cloning of chromosomal DNA encoding the F41 adhesin of enterotoxigenic Escherichia coli and genetic homology between adhesins F41 and K88. J. Bacteriol. 1986;167:799–804. doi: 10.1128/jb.167.3.799-804.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy B., Fekete P.Z. Enterotoxigenic Escherichia coli (ETEC) in farm animals. Vet. Res. 1999;30:259–284. [PubMed] [Google Scholar]
- Nagy B., Moon H.W., Isaacson R.E. Colonization of porcine intestine by enterotoxigenic Escherichia coli: selection of piliated forms in vivo adhesion of piliated forms to epithelial cells in vitro, and incidence of a pilus antigen among porcine enteropathogenic E. coli. Infect. Immun. 1977;16:344–352. doi: 10.1128/iai.16.1.344-352.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa M., Sugimoto C., Isayama Y., Kashiwazaki M. Virulence factors in Escherichia coli isolated from piglets with neonatal and post-weaning diarrhea in Japan. Vet. Microbiol. 1987;13:291–300. doi: 10.1016/0378-1135(87)90060-5. [DOI] [PubMed] [Google Scholar]
- Nataro J.P., Kaper J.B. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . National Academy Press; Washington, DC: 1996. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- Odorico M., Pellequer J.L. BEPITOPE: predicting the location of continuous epitopes and patterns in proteins. J. Mol. Recognit. 2003;16:20–22. doi: 10.1002/jmr.602. [DOI] [PubMed] [Google Scholar]
- Oanh T.K., Nguyen V.K., De Greve H., Goddeeris B.M. Protection of piglets against Edema disease by maternal immunization with Stx2e toxoid. Infect. Immun. 2012;80:469–473. doi: 10.1128/IAI.05539-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osek J. Prevalence of virulence factors of Escherichia coli strains isolated from diarrheic and healthy piglets after weaning. Vet. Microbiol. 1999;68:209–217. doi: 10.1016/s0378-1135(99)00109-1. [DOI] [PubMed] [Google Scholar]
- Ruan X., Zhang W. Oral immunization of a live attenuated Escherichia coli strain expressing a holotoxin-structured adhesin-toxoid fusion (1FaeG-FedF-LTA2:5LTB) protected young pigs against enterotoxigenic E. coli (ETEC) infection. Vaccine. 2013;31:1458–1463. doi: 10.1016/j.vaccine.2013.01.030. [DOI] [PubMed] [Google Scholar]
- Ruan X., Liu M., Casey T.A., Zhang W. A tripartite fusion, FaeG-FedF-LT(192)A2:B, of enterotoxigenic Escherichia coli (ETEC) elicits antibodies that neutralize cholera toxin, inhibit adherence of K88 (F4) and F18 fimbriae, and protect pigs against K88ac/heat-labile toxin infection. Clin. Vaccine Immunol. 2011;18:1593–1599. doi: 10.1128/CVI.05120-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan X., Crupper S.S., Schultz B.D., Robertson D.C., Zhang W. Escherichia coli expressing EAST1 toxin did not cause an increase of cAMP or cGMP levles in cells, and no diarrhea in 5-day old gnotobiotic pigs. PLoS One. 2012;7:e43203. doi: 10.1371/journal.pone.0043203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan X., Knudsen D.E., Wollenberg K.M., Sack D.A., Zhang W. Multiepitope fusion antigen induces broadly protective antibodies that prevent adherence of Escherichia coli strains expressing colonization factor antigen I (CFA/I) CFA/II, and CFA/IV. Clin. Vaccine Immunol. 2014;21:243–249. doi: 10.1128/CVI.00652-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan X., Robertson D.C., Nataro J.P., Clements J.D., Zhang W., the STa Toxoid Vaccine Consortium Group Characterization of heat-stable (STa) toxoids of enterotoxigenic Escherichia coli fused to a double mutant heat-labile toxin (dmLT) peptide in inducing neutralizing anti-STa antibodies. Infect. Immun. 2014;82:1823–1832. doi: 10.1128/IAI.01394-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan X., Sack D.A., Zhang W. Genetic fusions of a CFA/I/II/IV MEFA (multiepitope fusion antigen) and a toxoid fusion of heat-stable toxin (STa) and heat-labile toxin (LT) of enterotoxigneic Escherichia coli retain broad anti-CFA and antitoxin antigenicity. PLoS One. 2015;10:e0121623. doi: 10.1371/journal.pone.0121623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S., Raghava G.P.S. Prediction methods for B-cell epitopes. Methods Mol. Biol. 2007;409:387–394. doi: 10.1007/978-1-60327-118-9_29. [DOI] [PubMed] [Google Scholar]
- Smith H.W., Linggood M.A. Observations on the pathogenic properties of the K88, Hly and Ent plasmids of Escherichia coli with particular reference to porcine diarrhoea. J. Med. Microbiol. 1971;4:467–485. doi: 10.1099/00222615-4-4-467. [DOI] [PubMed] [Google Scholar]
- Smith H.R., Scotland S.M. ACP Broadsheet 135: January 1993: isolation and identification methods for Escherichia coli O157 and other Vero cytotoxin producing strains. J. Clin. Pathol. 1993;46:10–17. doi: 10.1136/jcp.46.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA . USDA:APHIS:VS, CEAH, Nat. Anim. Health Monitoring Syst.; Ft. Collins, CO: 2002. Part II: Reference for swine health and health management in the United States, 2000. [Google Scholar]
- Verdonck F., Cox E., van Gog K., Van der Stede Y., Duchateau L., Deprez P., Goddeeris B.M. Different kinetic of antibody responses following infection of newly weaned pigs with an F4 enterotoxigenic Escherichia coli strain or an F18 verotoxigenic Escherichia coli strain. Vaccine. 2002;20:2995–3004. doi: 10.1016/s0264-410x(02)00220-7. [DOI] [PubMed] [Google Scholar]
- Vu-Khac H., Holoda E., Pilipcinec E., Blanco M., Blanco J.E., Dahbi G., Mora A., Lopez C., Gonzalez E.A., Blanco J. Serotypes virulence genes, intimin types and PFGE profiles of Escherichia coli isolated from piglets with diarrhoea in Slovakia. Vet. J. 2007;174:176–187. doi: 10.1016/j.tvjl.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Walker R.I. Considerations for development of whole cell bacterial vaccines to prevent diarrheal diseases in children in developing countries. Vaccine. 2005;23:3369–3385. doi: 10.1016/j.vaccine.2004.12.029. [DOI] [PubMed] [Google Scholar]
- Zajacova Z.S., Konstantinova L., Alexa P. Detection of virulence factors of Escherichia coli focused on prevalence of EAST1 toxin in stool of diarrheic and non-diarrheic piglets and presence of adhesion involving virulence factors in astA positive strains. Vet. Microbiol. 2012;154:369–375. doi: 10.1016/j.vetmic.2011.07.029. [DOI] [PubMed] [Google Scholar]
- Zajacova Z.S., Faldyna M., Kulich P., Kummer V., Maskova J., Alexa P. Experimental infection of gnotobiotic piglets with Escherichia coli strains positive for EAST1 and AIDA. Vet. Immunol. Immunopathol. 2013;152:176–182. doi: 10.1016/j.vetimm.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Zhang W., Sack D.A. Progress and hurdles in the development of vaccines against enterotoxigenic Escherichia coli in humans. Expert Rev. Vaccines. 2012;11:677–694. doi: 10.1586/erv.12.37. [DOI] [PubMed] [Google Scholar]
- Zhang W., Berberov E.M., Freeling J., He D., Moxley R.A., Francis D.H. Significance of heat-stable and heat-labile enterotoxins in porcine colibacillosis in an additive model for pathogenicity studies. Infect. Immun. 2006;74:3107–3114. doi: 10.1128/IAI.01338-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Zhao M., Ruesch L., Omot A., Francis D. Prevalence of virulence genes in Escherichia coli strains recently isolated from young pigs with diarrhea in the US. Vet. Microbiol. 2007;123:145–152. doi: 10.1016/j.vetmic.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Zhang W., Robertson D.C., Zhang C., Bai W., Zhao M., Francis D.H. Escherichia coli constructs expressing human or porcine enterotoxins induce identical diarrheal diseases in a piglet infection model. Appl. Environ. Microbiol. 2008;74:5832–5837. doi: 10.1128/AEM.00893-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Zhang C., Francis D.H., Fang Y., Knudsen D., Nataro J.P., Robertson D.C. Genetic fusions of heat-labile (LT) and heat-stable (ST) toxoids of porcine enterotoxigenic Escherichia coli elicit neutralizing anti-LT and anti-STa antibodies. Infect. Immun. 2010;78:316–325. doi: 10.1128/IAI.00497-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Knudsen D.E., Liu M., Robertson D.C., Zhang W., the STa Toxoid Vaccine Consortium Group Toxicity and immunogenicity of enterotoxigenic Escherichia coli heat-labile and heat-stable toxoid fusion 3xSTaA14Q-LTS63K/R192G/L211A in a murine model. PLoS One. 2013;8:e77386. doi: 10.1371/journal.pone.0077386. [DOI] [PMC free article] [PubMed] [Google Scholar]