Abstract
The physicochemical properties of nanomaterials play a key role in tissue-specific targeting by reducing nonspecific background uptake as well as controlling biodistribution and clearance. Due to the strong influence of surface chemistry, chemical modulation of bioinert exosomes with chargeable and traceable small molecule fluorophores has a significant effect on the targeting, stability, and toxicity of the final conjugates. In this study, charge-variable exosomes are designed by conjugating their surface proteins with near-infrared fluorophores to control the in vivo fate of exosomes. Interestingly, zwitterionic fluorophore-labeled exosomes show rapid renal clearance with minimum to none nonspecific tissue uptake, whereas anionic exosomes are excreted through the hepatobiliary route with high uptake in the liver. The biodistribution and pharmacokinetics of exosome conjugates are comparable to their corresponding free fluorophores, demonstrating that the surface characteristics govern the fate of final conjugates in the living organism. Such unique surface properties of chemically modulated exosomes are confirmed in the lymphatic system after intradermal administration, which results in distinctive kinetic profiles in the secondary lymphoid tissues. This finding can subsequently serve as the foundation for developing tissue-specific exosome-based therapeutics.
Keywords: bioengineered exosome, fate control, near-infrared imaging, optical fluorescence imaging, surface protein engineering
1. Introduction
The emergence of exosomes in biology and immunology has become crucial in understanding the biological model of intercellular interactions.[1,2] Exosomes possess inherent molecular signatures of the cells that they originated from, and donor exosomes are involved in the reprogramming of target recipient cells via nonau-tonomous cell regulation.[3,4] Although exosomes primarily affect normal physiological processes, such as aging, nerve regeneration, immune responses, and pathological proceeding,[5,6] they have also been successfully utilized in drug delivery, cancer vaccinations, and cancer immunotherapy due to their bioinert property and functional roles in biological processes.[7,8] However, when used for targeted drug delivery, the inherent characteristic of exosome with high uptake in normal tissues may lead to undesirable side effects and relatively low targeting efficacy. Developing a systematic method to modify the original distribution of exosome and controlling their in vivo fate is, therefore, necessary for efficient targeting and therapy using exosomes.
Previous reports presented that the in vivo behavior of exosomes is subjected to the characteristics of the cells from which they originated.[9] Fluorophore-labeled exosomes derived from bone marrow dendritic cells showed more accumulation in the spleen, whereas melanoma-and muscle-cell-derived exosomes tended to move toward the lung and liver, respectively.[9] Different surface molecular organizations seemed to affect the differential, inherent tissue homing capability of exosomes. Other evidence emphasizing the tumorigenic role of exosomes demonstrated that pancreatic ductal adenocarcinomas-shed exosomes altered the normal liver microenvironment to create tumor-favorable niches by triggering Kupffer cells to release TGF-β, followed by fibronectin production and recruitment of macrophage infiltration. This, in turn, contributed to the high liver metastasis.[10] Moreover, exosomes released from primary tumors create premetastatic niches, where metastatic cancers reside in, and surface protein subtypes in exosomes play essential roles in determining the target organ to which cancer is supposed to metastasize.[11,12] The ability of exosomes to find their target organs appears to depend on the types or quantity of exosome-specific surface proteins. Therefore, chemical manipulation of the surface protein on exosomes may offer a critical method for controlling in vivo behavior of exosomes.
Delicate formulation design of the surface charge or lipophilicity of small molecules or nanoparticles (NPs) is crucial for exploring the controllability of their pharmacokinetics. By optimizing the surface charge, which would affect the cellular internalization, in vivo distribution, and excretion, the targeting efficiency toward the desired organs could be enhanced.[13–17] The importance of surface charges on NPs in different immunological processes was also documented.[18,19] Surface charge modification determines the specific uptake of NPs as an immune cell subtype: cationic NPs preferentially induce lung dendritic cell mediated adaptive immunity while anionic formulations engulfed by alveolar macrophage elicit less adaptive immune responses.[19] Furthermore, the distinct surface charge of systemically administered NPs may affect serum–protein interaction, the duration of their stay in the blood, and the mononuclear phagocyte system’s (MPS) sequestration of them. Positively charged NPs largely accumulate in the liver while neutral and negatively charged NPs seem to evade MPS with their longer circulation time.[20] It has also been reported that systemic administration of zwitterionic NPs resulted in minimum to none nonspecific uptake in major organs while showing active and rapid renal clearance.[14,21]
Herein, we applied a charge-based surface modification of nanomaterials to control the fate of exosomes in the body. The surface proteins of exosomes were modified with variable inherent charges of near-infrared (NIR) fluorophores, which resulted in the altered pharmacokinetics and organ distribution of exosome with renal and hepatobiliary-favorable excretion. The significance of this strategy was validated by modulating the retention time of intradermally injected NIR fluorophore-conjugated exosomes in the draining lymph nodes.
2. Results
2.1. Chemical Modification of Plasma-Isolated Autologous Exosomes
Surface proteins anchored in the phospholipid membrane of exosomes have been found to play a crucial role in determining in vivo fate of exosomes.[11] To investigate the effect of charge-based surface modification on the fate of the exosome, we collected autologous exosomes from freshly isolated mouse plasma. Typical round shape exosomes with a size of ≈100 nm were clearly visualized under a transmission electron microscope (Figure 1a).[1,2] Western blot analysis demonstrated that the canonical exosomal protein markers, including CD9 and CD63, were highly expressed in the isolated exosomes while nonexosomal endoplasmic reticulum calnexin proteins were not detected (Figure 1b). The organotropisms of exosome were modified using a series of NIR fluorophores possessing different physicochemical properties to ultimately control the in vivo fate of exosomes (Figure 1c). Especially, we focused on the effect of the surface charges of fluorophores on the biodistribution and clearance of exosomes. TG42 is a zwitterionic pentamethine fluorophore composed of +3 and −3 charges,[22] which endows the total surface charge of 0 to exosome after its conjugation. The indole nitrogen (light blue) is not a static charge and buried within the hydrophobic core of the pentamethine backbone; therefore, it has minimum to no effect on the surface charge (Figure 1d).[23] TG44 is composed of +3 and −1 charges, resulting in +2 surface charge after conjugation. WuA108 and WuA110 include +1/−3 and +1/−5, respectively, and the final surface charges of −2 and −4, respectively. The log D value (related to hydrophilicity/lipophilicity) of each fluorophore is between −3.2 and 3.0, determined using Marvin and JChem calculator plugins (ChemAxon, Budapest, Hungary).[24]
Figure 1.

Schematic illustration of in vivo fate controllable NIR exosomes. Exosomes were classified through the confirmation of a) the morphology by the electron microscope and b) the expression of canonical exosomal proteins (CD63 and CD9) by the Western blot. c) Physicochemical and optical properties of NIR fluorophores with distinct surface charges, net charges, and hydrophilicity/lipophilicity. d) Chemical and schematic drawings of exosomes labeled with NIR fluorophores: zwitterionic TG42, positively charged TG44, and negatively charged WuA108 and WuA110 were conjugated on the surface of plasma exosomes. Shown in red are the surface charge of each fluorophore. The indole nitrogen (light blue) is not a static charge and buried within the hydrophobic core of pentamethine backbone.
2.2. Bioconjugation of autologous Exosomes with NIR Fluorophores
The bioconjugation of NIR fluorophores on the surface of exosomes was made by converting the carboxylic group into the amine-reactive N-hydroxysuccinimide (NHS) ester.[24] After vigorous stirring for 1 h, the NIR fluorophore-conjugated exosomes (NIR-exo) were purified using a P-6 size exclusion column to remove unbound free fluorophores. The small peptides and debris that are smaller than 5 nm were removed through centrifugal filtrations (50 kDa MWCO). Then, the fluorescent signal of both retentive and flow-through was compared to verify complete removal of free fluorophores after conjugation (Figure S1a, Supporting Information). Prior to in vivo experiment, the stability of NIR-exo was also confirmed by incubating the purified TG42-exo in 100% mouse serum for 24 h at 37 °C, which proved that >95% of bioconjugates were stable at a physiological condition (Figure S1b, Supporting Information). Then, the successful conjugation and labeling ratios were confirmed by the unique absorbance peak of each fluorophore at its absorbance maximum and exosomes at 280 nm (Figure S1c, Supporting Information). The average labeling ratio was found to be about 100 based on our calculation using concentrations of fluorophore exosomes based on the Beer–Lambert law. The optical properties of each fluorophore including extinction coefficient and quantum yields were not changed after conjugation to exosomes (Figure S1d, Supporting Information). The average diameter of exosomes before conjugation was measured to be 91.1 nm while the NIR fluorophore-conjugated exosomes were ranged from 91.2 to 98.4 nm in diameter measured by DLS (Figure S1e, Supporting Information). Additionally, the zeta potential (ζ) values of exosomes were reflected by the change of surface charges after introduction of various charges (Figure S1e, Supporting Information). The intact exosomes showed slightly negative charged value (ζ = −10.72).
2.3. Surface Charges Affect the Biodistribution of Exosome Conjugates
To examine the biodistribution and clearance of NIR fluorophores, 10 nmol of each fluorophore or NIR-exo were injected intravenously into CD-1 mice and their in vivo behaviors were monitored under the intraoperative FLARE imaging system (Figure 2a). Zwitterionic TG42 showed minimum to no nonspecific uptake except in kidneys, which seemed to show renal clearance of the majority of injected dose within 4 h (>55%) while lipophilic WuA108 (log D = 3.0, pH 7.4) showed fast excretion as feces through the hepatobiliary route (Figure S2, Supporting Information). Interestingly, TG44 and WuA110 were cleared through both renal and hepatobiliary routes with high nonspecific uptake in major organs, including the heart, lungs, liver, and pancreas (Figure 2b). We then examined whether the exosomes conjugated with different charged fluorophores follow the fate of the original fluorophores or not. Zwitterionic TG42-coated exosomes (TG42-exo) showed rapid renal clearance, evidenced by high signals in the kidneys and bladder at 4 h post-injection (Figure S2, Supporting Information), whereas +2 charged TG44-exo showed hepatobiliary clearance with relatively high uptake in the lungs due to high positive surface charges. These results correspond with the biodistribution of TG42 and TG44, respectively.[24,25] Interestingly, WuA108 coating led the exosome conjugates to be trapped in the liver due to the relatively high lipophilicity, resulting in hepatobiliary clearance at 24 post-injection (highest signals in the duodenum) as WuA108 did. Finally, high negative charges in WuA110-exo derived them into the kidneys while preventing renal excretion because the glomerular capillary membrane is negatively charged, and penetration of polyanions is simply limited by the Coulomb repulsion.[25,26] The signals in the lungs and liver were found in all mice injected with variously charged exosomes because of their high molecular weights and large hydrodynamic diameters (>90 nm). These exosomes were mostly cleared out of the body within 24 h post-injection. TG42-exo and WuA110-exo were found in the bladder, but minimum to no renal excretion was observed in the mice injected with TG44-exo and WuA108exo. WuA108, in fact, demonstrated the slowest clearance, as evidenced by the high signals in the blood and intestine at 24 h post-injection (Figure S2, Supporting Information). We concluded that the surface charge and lipophilicity contributed to the differential biodistribution of exosome groups because the size of exosomes was equally distributed as determined by DLS (Figure S1d, Supporting Information).
Figure 2.
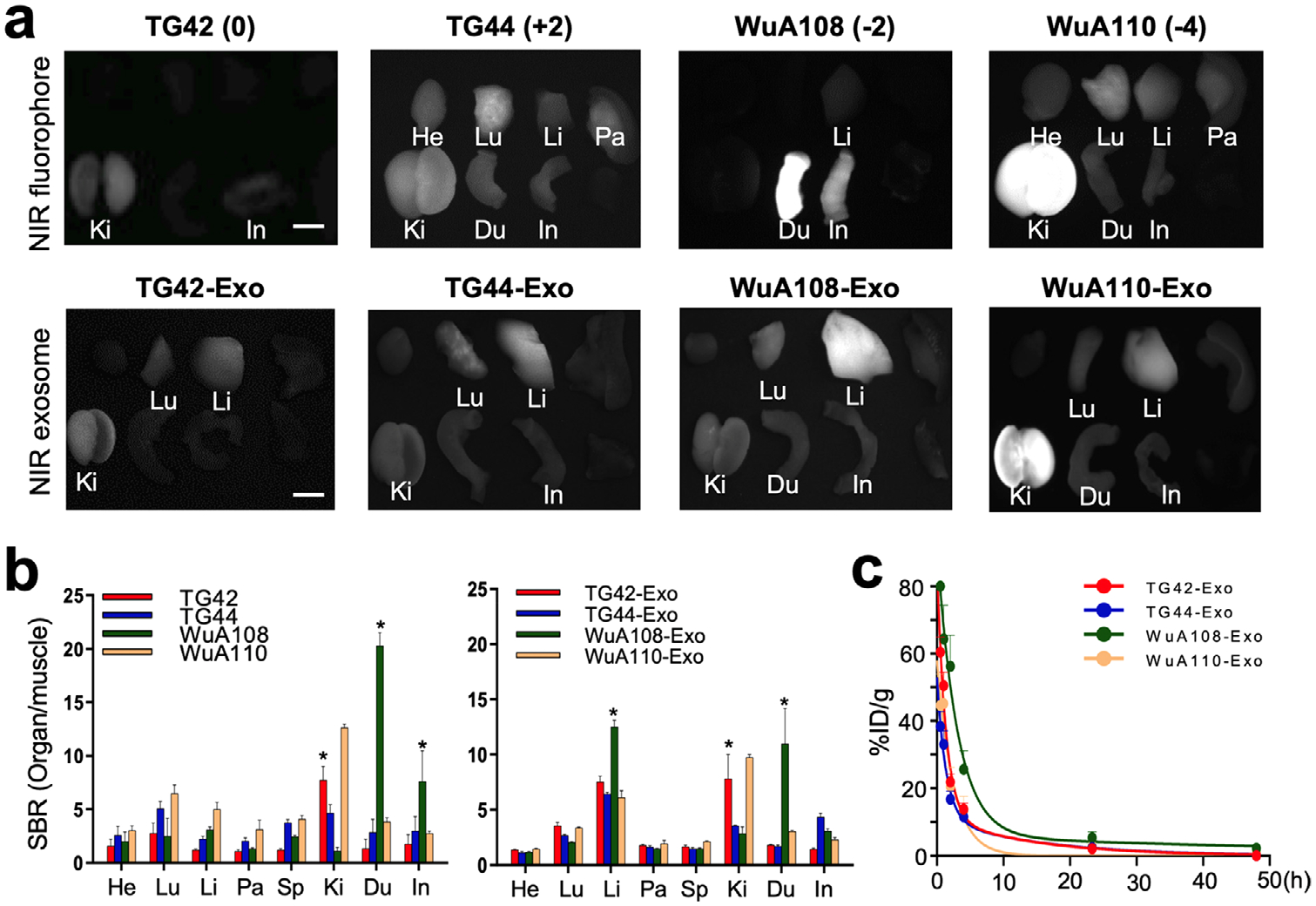
Differential in vivo fate of bioengineered NIR exosomes. a) 10 nmol of each fluorophore (top) and NIR-exo (bottom) were injected intravenously into CD-1 mice 4 h prior to imaging. Biodistribution and clearance were monitored under the m channel of the FLARE imaging system. Representative images of mice 4 h post-intravenous injection are presented. b) SBR of the resected organs was obtained by analyzing NIR images of the free NIR fluorophores (left) and NIR exosomes (right) at 4 h post-intravenous injection (n = 3, mean ± SD). c) Blood half-life was calculated using the NIR signal intensity of capillary tube containing blood serum at each time point up to 48 h post-injection (n = 3, mean ± SD). Du, duodenum; He, heart; In, intestine; Ki, kidneys; Li, liver; Lu, lungs; Pa, pancreas; Sp, spleen. Exposure time = 200 ms; scale bars = 5 mm.
To verify whether the pharmacokinetics of exosomes were influenced by each corresponding NIR fluorophore possessing different chemical compositions, blood samples were collected using capillary tubes over the time course of 48 h and their fluorescence signals were measured using the FLARE imaging system (Figure 2c). All NIR fluorophore-conjugated exosomes distributed quickly in the tissues and organs (t1/2α< 10 min) except for WuA108-exo while the terminal half-life (t1/2β) values ranged from 149 to 792 min, depending on their charges and lipophilicity (Table 1). Interestingly, WuA108-exo showed the slowest blood-to-tissue distribution (t1/2α = 26 min) as well as the slowest elimination from the blood (t1/2β = 792 min), which was about fivefold longer than those of TG42-exo. These data corresponded with the pharmacokinetic data obtained from free NIR fluorophore tested in CD-1 mice.[24] This finding also seemed to support that unique pharmacokinetic properties of exosomes labeled with multiple NIR fluorophores could be dominated by the substituted molecules on the surface.[14,24]
Table 1.
Pharmacokinetic parameters of bioengineered exosomes.
| Pharmacokinetics | TG42-Exo | TG44-Exo | WuA108-Exo | WuA110-Exo |
|---|---|---|---|---|
| Half-life t1/2α [min] | 6.85 | 7.84 | 26.18 | 8.79 |
| Half-life t1/2β [min]) | 149 | 185 | 792 | 390 |
| kel [min−1] | 0.0052 | 0.0041 | 0.0003 | 0.0009 |
| AUC [%ID·g−1 min−1] | 11537 | 9871 | 25759 | 5922 |
| Plasma clearance [mL·min−1] | 0.0118 | 0.0154 | 0.0054 | 0.0126 |
| Volume of distribution [mL] | 2.4321 | 3.0356 | 5.3709 | 8.4773 |
2.4. Differential Fate of Exosomes in the Lymphatic System
Altering the kinetics of antigen in the lymphatic vessel and its retention time in the lymph node plays an important role in inducing optimal immune responses.[27–29] Surface charges of NPs are indeed critical for their specific uptake by distinct immune cell subtypes in the secondary lymphoid tissue and adaptive immune response.[18,19] To examine if the physicochemical properties of exosomes—including surface charges and lipophilicity—affect their translocation rate and retention time in the lymphatic system, NIR-exo conjugated with four different surface charges were inoculated into the footpad of BALB/c mice. The kinetic profiles at each time point were monitored by the real-time NIR fluorescence imaging system (Figure 3a). Upon intradermal administration, TG42-exo translocated rapidly through the lymphatic vessel and appeared in the popliteal lymph node (PN) within 5 min post-injection. Peak signals in both PN and sciatic lymph node (SN) occurred around 2 h. TG44-exo showed a slower translocation compared to TG42-exo, reaching PN at 30 min post-injection. However, both TG42-exo and TG44-exo moved to the second tier (SN) at 2 h. WuA108-exo demonstrated the fastest movement into both PN and SN, which happened within 5 min post-injection, followed by a gradual signal decrease in lymph nodes. WuA110exo, on the other hand, exhibited the slowest initial translocation and accumulated in the PN at 2 h post-intradermal injection. However, they remained in both PN and SN up to 48 h post-injection. Unlike biodistribution and clearance upon intravenous administration, the fate of NIR-exo through the lymphatic system after intradermal injection was quite different from the behavior of free fluorophores. Small molecule fluorophores showed faster translocation in the lymphatic system compared to exosomes, showing rapid diffusion and fast clearance from the lymphatics to the surrounding tissue (Figure S3, Supporting Information). This difference is considered to be due to the small size of the free fluorophores (≈1 nm) compared to that of NIR-exo (≈100 nm) since molecules smaller than 10 nm freely travel from the lymphatics to the blood capillaries.[30]
Figure 3.

Kinetic and retention profile of bioengineered exosomes in lymphatics. a) 10 μL of each bioengineered exosome (4 μg) was administered into the footpad region of BALB/c mice and tracked by the FLARE imaging system in real time. The NIR signal intensity in each lymph node was compared using the image processing feature of the ImageJ. Representative images from each group are presented. b) Quantitative region of interest (ROI) analysis of the popliteal and sciatic LNs at the time points of 3, 5, 10, 30, 60, 120, 180, and 240 min. A standard curve of SBR was in the range of 0.5–20 μm of fluorophore exosomes, and the obtained NIR signal intensity value was converted to the concentration (n = 3, means ± SD). *p < 0.05, **p < 0.01, and ***p < 0.001. c) Representative images of popliteal and sciatic LNs 48 h post-injection are shown in order to compare the signal intensity.
Quantitative analysis of NIR fluorescent signal was conducted at each time point by drawing a region of interest (ROI) around the PN and SN areas, and the result was expressed as a signal-to-background ratio (SBR; Figure 3b). SBR at 24 and 48 h post-injection was not quantified due to the possibility of inaccurate analysis based on weak signals in the lymph nodes. The uptake of TG42-exo and TG44-exo was observed consistently in both lymph nodes up to 180 min, which decreased gradually afterward. WuA108-exo was rapidly found in the PN within 5 min of injection whereas the uptake of WuA110-exo in both lymph nodes continued up to 240 min. To confirm the remaining signals in the lymph nodes, both PN and SN were isolated from mice injected with NIR-exo at 48 h post-injection, and the resected lymph nodes were imaged using the NIR fluorescence imaging system (Figure 3c).
3. Discussion
Although exosomes have been explored for targeted drug delivery, their nonspecific uptake in tissues and organs limits their theranostic applications. In this study, we demonstrated for the first time that the fate of exosomes could be controlled by surface protein engineering using chargeable and traceable small molecule fluorophores. The translocation and retention time of exosomes in the secondary lymphoid tissue was also modulated, which shows an ample potential for application in immunotherapy. The ability to control the behavior of exosomes by manipulating their physicochemical properties, including surface charges and lipophilicity, is essential for the achievement of tissue-specific delivery as well as optimal biodistribution and clearance. The surface membrane proteins of exosomes have been considered as a key determinant of tissue targetability in vivo, and modulating the surface of exosome with well-balanced NIR fluorophores could be crucial for determining the fate of exosomes in the body.
Since the surface area of a globular-shaped exosome (≈100 nm in diameter) is 10 000-fold larger than that of a small molecule fluorophore (≈1 nm in diameter), more than 100 small molecules are able to be conjugated on the surface of an exosome. Therefore, we believe that the behavior of bioconjugated exosomes could be controlled by the physicochemical properties (e.g., surface charges and lipophilicity) of conjugated small molecules. For example, unlike other exosomes, zwitterionic TG42-coated exosomes with unique physiological properties seemed to show rapid renal excretion through the kidneys and low nonspecific uptake in major organs, including heart, lungs, and liver.[31] Such unique biodistribution of TG42-exo corresponds with the behavior of free fluorophore TG42.[22] This result is also consistent with our previous study where the surface charges and size of NPs governed the in vivo fate of globular-shaped quantum dots after a bolus intravenous injection.[13,25] In addition, we proved that zwitterionic fluorophore-conjugated antibodies[14] and polymers[21] show the same physiological properties, including rapid renal clearance and ultralow nonspecific uptake, as their corresponding fluorophores. Nevertheless, due to the large size of exosome, further research is required to find out whether fluorescence signals in the bladder would result from the intact TG42-exo or partially metabolized fragment of TG42-exo after tissue uptake.
Exosomes, compared to other NPs or polymers, have served as a promising vaccine delivery carrier due to their biostable and bioinert properties.[32–34] In contrast to liposomes, synthesized vesicles with no natural membrane components, exosomes naturally possess surface proteins and lipids that provide sufficient bioavailability. Exosomes also have a lower likelihood of undesirable immunogenicity and nonspecific engulfing by circulating monocyte compared to liposomes, which may trigger Toll-like receptor activation.[32–34] However, the therapeutic vaccine efficacy of exosomes alone is not likely to be sufficient to boost enough anti-tumor T lymphocyte immune responses.[35] While the uptake efficacy of antigen by antigen-presenting cells (APC) is a key factor for promoting vaccine-specific immune responses, the retention time and dose of antigen vaccine in the regional lymph nodes also play a critical role in triggering APC maturation and activating T cells.[36] To maximize the therapeutic efficacy of vaccines, modifying antigen vaccine properties, such as their size, shape, charge, hydrophobicity, and surface molecular composition, can be an effective therapeutic strategy. Such surface engineering of vaccines or vaccine-loaded NPs can eventually improve vaccine therapy via alteration of their structures. The exosomes with their size ranging around 40–150 nm provide a large surface area, and the high avidity of their receptors may enhance the uptake rate of exosome vaccines. The modification of surface charge and lipophilicity of exosomes may promote immune responses by controlling the physiological behavior of exosomes in the lymphatic system, including the movement toward the lymphoid organs from peripheral tissue.[36] Interestingly, there were significant differences in the kinetics among four NIR-exo groups with different physicochemical properties. Zwitterionic TG42-exo showed a fast translocation from the footpad to lymph nodes due to the nonsticky property of ZW800–1.[14,21,23] Surprisingly, WuA108-exo (the total surface charges > −200) translocated rapidly toward the draining lymph nodes compared with other exosomes. This is presumably due to the electrostatic repulsion between the negative charges of WuA108-exo and the slightly negative charged interstitial matrix of the lymphatic vessel and injection site.[37,38]
In summary, we conclude that NIR fluorophores on the surface proteins of exosomes likely govern the in vivo fate of exosome bioconjugates. These findings imply that the targeting properties of exosomes can be improved with chemical engineering of exosomes by obtaining optimal biodistribution with minimal nonspecific uptake. Furthermore, such delicate surface modification of exosomes may generate more desirable immune responses due to its effect on the retention time of antigen vaccines in lymph nodes in addition to exosome’s structural ability to carry a large number of antigens. NIR fluorescence-based real-time tracking of exosomes enables monitoring of the direction and translocation rate of exosomes in the lymphatic system, which would lay the foundation for the development of potent vaccines and further surgical interventions.
4. Experimental Section
Materials:
All chemicals and solvents were of American Chemical Society grade or HPLC purity and were used as received. Dipyrrolidino(N-succinimidyloxy)carbenium hexafluorophosphate (HSPyU), N,N-diisopropylethylamine (DIEA), N-hydroxysuccinimide (NHS), and ethyl acetate were purchased from Fisher Scientific (Pittsburgh, PA), Sigma-Aldrich (Saint Louis, MO), or Acros Organics (Morris Plains, NJ). Other solvents were purchased from VWR International (West Chester, PA) and American Bioanalytic (Natick, MA).
Exosome Isolation from Mouse Plasma:
To extract autologous exosomes, mouse blood was rapidly collected with a 28-gauge syringe by cardiac puncture under isoflurane. Approximately 800 μL of whole blood was drawn from a single mouse and added into an EDTA-coated tube. The plasma sample was centrifuged at 2000 × g for 20 min at 4 °C to remove cells and debris. After samples were re-centrifuged to remove debris, exosome precipitation reagent (Invitrogen) with a volume equivalent to the 1/5 of the total sample volume was added to the samples. The samples were thoroughly mixed by vortexing and incubated for 10 min at room temperature, followed by centrifugation at 10 000 × g for 5 min at 4 °C. After removing supernatant, 100 μL PBS was added in order to resuspend the exosome. Exosomes were loaded into 50 kDa cut-off centrifugal filter (Sartorius Vivaspin 500 Centrifugal Concentrators) and centrifuged at 15 000 × g for 15 min to remove small peptides.
Western Blot Analysis for Exosome Marker Expression:
After exosomal protein concentration was determined using the bicinchoninic acid (BCA) assay (Thermo Scientific), 25 μg of protein was fractionated using 12% acrylamide sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and blotted to polyvinylidene fluoride membranes (Millipore). The membranes were blocked using 5% nonfat skimmed milk powder in PBS-T (1% Tween-20) for 2 h at room temperature. The membrane was probed with exosome-specific markers, such as CD9 (1:500, Anti-rabbit antibody, System Biosciences), CD63 (1:500, Anti-rabbit antibody, Santa Cruz Biotechnology), the ER membrane marker, Calnexin (1:1000, Anti-rabbit antibody, Abcam), and β-actin (1:1000, Anti-rabbit antibody, Abcam) overnight at 4 °C. Membranes were incubated with HRP-linked secondary antibody (Cell Signaling) for 2 h at room temperature, and protein bands were visualized using a chemiluminescence detection reagent (Promega).
NIR Fluorophores Labeling on Surface Proteins on Exosomes:
For surface membrane protein labeling of exosomes, NIR fluorophores were converted to the NHS ester form. Briefly, each compound was completely dissolved in anhydrous methyl sulfoxide and HSPyU (10.0 eq.) was added in the presence of DIEA (2.5 eq.). The reaction mixture was stirred at room temperature for 2 h, followed by addition of the NHS and precipitating in ethyl acetate. The precipitated powder was washed with ethyl acetate and dried in vacuo. For bioconjugation on exosomes, 20 nmol of each NHS ester was mixed with 40 μg of exosome in PBS at pH 8.0 for 1 h in a shaker. Unbound fluorophores were removed using a Bio-Spin P-6 size exclusion column (Bio-Rad) by centrifuging at 1000 × g for 2 min twice. The exosomal total protein concentration was measured in BCA protein assay based on the known standard BSA concentration. The labeling ratio of NIR fluorophores on an exosome was calculated using the concentration of exosomes and fluorophore exosome. First, the concentration of fluorophore-exo was calculated using Beer–Lambert law with the measured extinction coefficient values of each fluorophore at 650 nm (TG42: 136000, TG44: 146000, WuA108:148000, WuA110: 104000) and the maximum absorbance value of each fluorophore-exo. Next, the concentration of exosomes was calculated by counting a number of exosomes (4 × 1015 exosomes per liter for TG42 conjugation) using NP tracking analysis (NTA) system (Nanosight NS300; Malvern), which was then converted into concentration by multiplying Avogadro’s number (6.64 nm for TG42 conjugation). Finally, the conjugation ratio was calculated by dividing the concentration of fluorophore exosome by concentration of exosomes. The same calculation method was applied for rest of the groups with exosome concentration (TG42: 6.64 nm, TG44: 7.8 nm, WuA108: 7.5 nm, WuA110: 7.2 nm), which resulted in conjugations ratios of 103 (TG42), 101 (TG44), 105 (WuA108), and 109 (WuA110), respectively.
Serum Stability and Optical Property of Exosomes:
To test the stability of NIR fluorophore-conjugated exosomes, TG42-exo was added to the 100% mouse serum and incubated up to 24 h. The NIR fluorescence intensity was measured at 10 min, 30 min, 60 min, 2 h, 4 h, 24 h time points using a UV/vis/NIR spectrometer (USB2000, Ocean Optics, Dunedin, FL) with a laser excitation wavelength of 655 nm. Absorbance and fluorescence spectra of each fluorophore were measured using fiber optic HR2000 (200–1100 nm) spectrometers by a 5 mW, 655 nm laser diode (Opcom Inc., Xiamen, China) coupled through a 300 mm core diameter; NA 0.22 fiber (Fiberguide Industries, Stirling, NJ). 5 × 10−6 m of each fluorophore solution was prepared in 10% fetal bovine serum (FBS) in PBS. In silico calculations of physiochemical properties such as molecular weight, charge distribution, log D at pH 7.4, pKa, rotatable bonds were calculated using JChem calculator plugins (ChemAxon, Budapest, Hungary). Data was plotted using Prism software version 7.0 (GraphPad, San Diego, CA) and Microsoft Excel (Redmond, WA).
Size Measurement of Exosomes before and after Conjugation:
NIR fluorophore-exosomes size distribution and particle number were analyzed using the NTA system (Nanosight NS300; Malvern). For size measurement, exosomes were diluted 2000-fold in particle-free PBS. A laser beam was adjusted to focus on suspended particles of interest. All measurements were recorded for further size and particle number analysis by NTA software. All samples were analyzed in triplicate.
Animal Models and Biodistribution Study:
Animals were housed in an AAALAC-certified facility, and all procedures were performed under the supervision of MGH IACUC with approved protocol #2016N000136. Six-weeks-old male CD-1 or BALB/c mice were fed alfalfa-free chow at least 4 days prior to imaging to minimize autofluorescence. Mice were anesthetized by injecting a mixed solution of 100 mg per kg ketamine and 10 mg per kg xylazine intraperitoneally (Webster Veterinary, Fort Devens, MA). For pharmacokinetics study, 10 nmol of each NIR fluorescent exosomes were inoculated into the mice, and blood was extracted at individual time points (3, 5, 10, 30, 60, 120, 240 min, 24 h, 48 h) by cutting the tip of the tail vein of mice. Before injection, a few drops of blood were obtained from the tail vein and stored in the heparinized capillary tubes (Fisher Scientific, Pittsburgh, PA) as a reference. All the capillary tubes containing collected blood were placed on ice to prevent blood clotting. To separate the serum from whole blood, each blood sample was centrifuged at 3000 rpm for 10 min, and the supernatant was then loaded into capillary microtubes. All mice were monitored using the FLARE intraoperative NIR fluorescence imaging system (see below) to measure signals for distribution (t1/2α) and elimination (t1/2) blood half-life. Fluorescence intensity results were expressed as a bi-exponential decay curve and pharmacokinetic parameters, including plasma concentration, the volume of distribution, bioavailability, and clearance were measured using Prism 8 software.
Real-Time NIR Fluorescence Imaging and Kinetics Analyses:
All animals were imaged using the intraoperative FLARE imaging system. Animals were imaged using a 660 nm excitation laser source (2 mW cm−2) with white light (400–650 nm; 40000 lux). Color and NIR fluorescence signals and bright-field images were simultaneously obtained using customized software at rates of 15 Hz over a 15 cm field of view. Each image from this experiment was acquired under the same exposure time and condition. Animals were sacrificed after time-course NIR fluorescence imaging, and major organs were isolated for further biodistribution analysis. For ex vivo biodistribution study, all organs were isolated 4, 24, and 48 h after injection and NIR signals from individual organs were measured and displayed as photons per second per cm2. For kinetics analyses in the lymphatic system, BALB/c mice were anesthetized and shaved on the right and left lower half of the body. 0.1 nmol of exosomes (4 μg per 10 μL) were inoculated in the footpad. The 0.5 s flame of real-time video images were captured during initial 5 min period, followed by snapshot images at 5 min, 30 min, 2 h, 4 h, 24 h, and 48 h, using the FLARE imaging system. An exposure time of 500 ms was applied to all lymph node tracing images. The standard linear curve with fluorophore-exosomes’ concentration (0.5–5 μm) was used to convert the NIR signal intensity value of each NIR-exo to concentration value. After each signal at the lymph node and noise signals were converted into molar concentration values based on standard curve data, noise signal obtained at each time point were subtracted from each lymph node signal, which was expressed as SBR value. The graph was drawn at time point intervals of 3, 5, 10, 30, 60, 120, 180, 240, 1440, and 2880 min (n = 3).
Statistical Analyses:
For semi-quantitative analysis, the ROI was drawn at popliteal and sciatic lymph nodes at each time point by ImageJ v1.48 (NIH, Bethesda, MD). At least three animals were analyzed at each time point, and statistical analysis was carried out using the Student’s t-test in order to analyze the significant differences in SBR among the groups. p-values less than 0.05 were considered significant: *p < 0.05, **p < 0.01, and ***p < 0.001. The experiments were not randomized, and the investigators were not blinded to allocation during experiments and outcome assessment. Results were presented as mean ± SD and curve fitting was performed using Microsoft Excel and Prism 8 (GraphPad software Inc., San Diego, CA) software.
Supplementary Material
Acknowledgements
D.W.H. and M.J.J. contributed equally to this work. The thank Ivey Choi for manuscript editing. This study was supported by the US NIH grants NIBIB #R01EB022230, NHLBI #R01HL143020, and NCI #R21CA223270. This work was also supported by the MSIP #2017M3C7A1048079 through the National Research Foundation of Korea, the Joint Research Project for Outstanding Research Institutions funded by the Gimhae Industry Promotion and Biomedical Foundation, and Therabest Co., LTD.
Footnotes
The ORCID identification number(s) for the author(s) of this article can be found under https://doi.org/10.1002/adtp.201900111
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Conflict of Interest
The authors declare no conflict of interest.
Contributor Information
Do Won Hwang, Gordon Center for Medical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA; Department of Molecular Medicine and Biopharmaceutical Sciences, Graduate School of Convergence Science and Technology, College of Medicine and College of Pharmacy, Seoul National University, Seoul 08826, South Korea; 142 Unjungro, Seongnam, Gyeonggi 13466, South Korea.
Min Joo Jo, Gordon Center for Medical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA.
Jeong Heon Lee, Gordon Center for Medical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA.
Homan Kang, Gordon Center for Medical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA.
Kai Bao, Gordon Center for Medical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA.
Shuang Hu, Gordon Center for Medical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA.
Yoonji Baek, Gordon Center for Medical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA.
Hyung Geun Moon, Division of Pulmonary, Critical Care, Sleep and Allergy, Department of Medicine, University of Illinois at Chicago, Chicago, IL 60612, USA.
Dong Soo Lee, Department of Molecular Medicine and Biopharmaceutical Sciences, Graduate School of Convergence Science and Technology, College of Medicine and College of Pharmacy, Seoul National University, Seoul 08826, South Korea.
Satoshi Kashiwagi, Gordon Center for Medical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA.
Maged Henary, Department of Chemistry, Center for Diagnostics and Therapeutics, Georgia State University, Atlanta GA 30303, USA.
Hak Soo Choi, Gordon Center for Medical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA.
References
- [1].Tkach M, Thery C, Cell 2016, 164, 1226. [DOI] [PubMed] [Google Scholar]
- [2].S ELA, Mager I, Breakefield XO, Wood MJ, Nat. Rev. Drug Discovery 2013, 12, 347. [DOI] [PubMed] [Google Scholar]
- [3].Camussi G, Deregibus MC, Bruno S, Grange C, Fonsato V, Tetta C, Am. J. Cancer Res 2011, 1, 98. [PMC free article] [PubMed] [Google Scholar]
- [4].Rak J, Front. Pharmacol 2013, 4, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhang Y, Kim MS, Jia B, Yan J, Zuniga-Hertz JP, Han C, Cai D, Nature 2017, 548, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Buzas EI, Gyorgy B, Nagy G, Falus A, Gay S, Nat. Rev. Rheumatol 2014, 10, 356. [DOI] [PubMed] [Google Scholar]
- [7].Vader P, Mol EA, Pasterkamp G, Schiffelers RM, Adv. Drug Delivery Rev 2016, 106, 148. [DOI] [PubMed] [Google Scholar]
- [8].Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D, Acta Pharmacol. Sin 2017, 38, 754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wiklander OP, Nordin JZ, O’Loughlin A, Gustafsson Y, Corso G, Mager I, Vader P, Lee Y, Sork H, Seow Y, Heldring N, Alvarez-Erviti L, Smith CI, Le Blanc K, Macchiarini P, Jungebluth P, Wood MJ, Andaloussi SE, Extracell J. Vesicles 2015, 4, 26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, Xiang J, Zhang T, Theilen TM, Garcia-Santos G, Williams C, Ararso Y, Huang Y, Rodrigues G, Shen TL, Labori KJ, Lothe IM, Kure EH, Hernandez J, Doussot A, Ebbesen SH, Grandgenett PM, Hollingsworth MA, Jain M, Mallya K, Batra SK, et al. , Nat. Cell Biol 2015, 17, 816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, et al. , Nature 2015, 527, 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, Psaila B, Kaplan RN, Bromberg JF, Kang Y, Bissell MJ, Cox TR, Giaccia AJ, Erler JT, Hiratsuka S, Ghajar CM, Lyden D, Nat. Rev. Cancer 2017, 17, 302. [DOI] [PubMed] [Google Scholar]
- [13].Choi HS, Liu W, Liu F, Nasr K, Misra P, Bawendi MG, Frangioni JV, Nat. Nanotechnol 2010, 5, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Choi HS, Gibbs SL, Lee JH, Kim SH, Ashitate Y, Liu F, Hyun H, Park G, Xie Y, Bae S, Henary M, Frangioni JV, Nat. Biotechnol 2013, 31, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Choi HS, Ipe BI, Misra P, Lee JH, Bawendi MG, Frangioni JV, Nano Lett 2009, 9, 2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Huang J, Li J, Lyu Y, Miao Q, Pu K, Nat. Mater 2019. 10.1038/s41563-019-0378-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [17].Li J, Pu K, Chem. Soc. Rev 2019, 48, 38. [DOI] [PubMed] [Google Scholar]
- [18].Fromen CA, Rahhal TB, Robbins GR, Kai MP, Shen TW, Luft JC, DeSimone JM, Nanomedicine 2016, 12, 677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fromen CA, Robbins GR, Shen TW, Kai MP, Ting JP, DeSimone JM, Proc. Natl. Acad. Sci. U. S. A 2015, 112, 488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Blanco E, Shen H, Ferrari M, Nat. Biotechnol 2015, 33, 941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kang H, Gravier J, Bao K, Wada H, Lee JH, Baek Y, El Fakhri G, Gioux S, Rubin BP, Coll JL, Choi HS, Adv. Mater 2016, 28, 8162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hyun H, Henary M, Gao T, Narayana L, Owens EA, Lee JH, Park G, Wada H, Ashitate Y, Frangioni JV, Choi HS, Mol. Imaging Biol 2016, 18, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Choi HS, Nasr K, Alyabyev S, Feith D, Lee JH, Kim SH, Ashitate Y, Hyun H, Patonay G, Strekowski L, Henary M, Frangioni JV, Angew. Chem., Int. Ed 2011, 50, 6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Koo H, Lee JH, Bao K, Wu Y, El Fakhri G, Henary M, Yun SH, Choi HS, Adv. Healthcare Mater 2016, 5, 2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV, Nat. Biotechnol 2007, 25, 1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chang RL, Deen WM, Robertson CR, Brenner BM, Kidney Int. 1975, 8, 212. [DOI] [PubMed] [Google Scholar]
- [27].Tozuka M, Oka T, Jounai N, Egawa G, Ishii KJ, Kabashima K, Takeshita F, J. Dermatol. Sci 2016, 82, 38. [DOI] [PubMed] [Google Scholar]
- [28].Rappuoli R, Mandl CW, Black S, De Gregorio E, Nat. Rev. Immunol 2011, 11, 865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Moyer TJ, Zmolek AC, Irvine DJ, J. Clin. Invest 2016, 126, 799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bagby TR, Cai S, Duan S, Thati S, Aires DJ, Forrest L, Pharmaceutics 2012, 4, 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].van Balkom BW, Pisitkun T, Verhaar MC, Knepper MA, Kidney Int. 2011, 80, 1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].van den Boorn JG, Schlee M, Coch C, Hartmann G, Nat. Biotechnol 2011, 29, 325. [DOI] [PubMed] [Google Scholar]
- [33].El Andaloussi S, Lakhal S, Mager I, Wood MJ, Adv. Drug Delivery Rev 2013, 65, 391. [DOI] [PubMed] [Google Scholar]
- [34].Escudier B, Dorval T, Chaput N, Andre F, Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S, Boccaccio C, Bonnerot C, Dhellin O, Movassagh M, Piperno S, Robert C, Serra V, Valente N, Le Pecq JB, Spatz A, Lantz O, Tursz T, Angevin E, Zitvogel L, J. Transl. Med 2005, 3, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, Li G, Mol. Ther 2008, 16, 782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bachmann MF, Jennings GT, Nat. Rev. Immunol 2010, 10, 787. [DOI] [PubMed] [Google Scholar]
- [37].Helle M, Rampazzo E, Monchanin M, Marchal F, Guillemin F, Bonacchi S, Salis F, Prodi L, Bezdetnaya L, ACS Nano 2013, 7, 8645. [DOI] [PubMed] [Google Scholar]
- [38].Ali Khan A, Mudassir J, Mohtar N, Darwis Y, Int. J. Nanomed 2013, 8, 2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


