Abstract
Objective
To evaluate the effects of influenza vaccination with or without heptavalent pneumococcal conjugate vaccination on respiratory tract infections (RTIs) in children.
Study design
This was a randomized, double-blind, placebo-controlled trial comprising 579 children age 18 to 72 months with a previous history of physician-diagnosed RTI, recruited between 2003 and 2005. The children were assigned to 2 doses of parenteral inactivated trivalent subunit influenza plus heptavalent pneumococcal conjugate vaccination (TIV+PCV7), influenza plus placebo vaccination (TIV+plac), or control hepatitis B virus vaccination plus placebo (HBV+plac). Main outcome measures were febrile RTI and related polymerase chain reaction (PCR)-confirmed influenza, primary care visits, antibiotic prescriptions, and acute otitis media (AOM) episodes.
Results
During influenza seasons, febrile RTI were reduced by 24% (95% confidence interval [CI] = 1% to 42%) in the TIV+PCV7 group and by 13% (95% CI = -12% to 32%) in the TIV+plac group compared with the control group. The occurrence of PCR-confirmed influenza was reduced by 52% (95% CI = 7% to 75%) in the TIV+PCV7 group and by 51% (95% CI = 3% to 75%) in the TIV+plac group. Episodes of AOM were reduced by 57% (95% CI = 6% to 80%) in the TIV+PCV7 group and by 71% (95% CI = 30% to 88%) in the TIV+plac group. Outside of the influenza seasons, no significant effects of vaccinations were demonstrated on the studied outcomes.
Conclusions
During influenza seasons, influenza vaccination with or without pneumococcal conjugate vaccination substantially reduced cases of confirmed influenza and AOM episodes.
Abbreviations: AOM, Acute otitis media; CI, Confidence interval; GP, General practitioner; HBV+plac, Hepatitis B virus and placebo vaccination; PCR, Polymerase chain reaction; RTI, Respiratory tract infection; TIV+PCV7, Influenza and heptavalent pneumococcal conjugate vaccination; TIV+plac, Influenza and placebo vaccination
Respiratory tract infections (RTIs) continue to be the leading cause of acute illness worldwide, particularly in children. Despite their marginal effects, ear, nose, and throat surgery and antibiotic therapy remain common treatments for RTIs.1, 2, 3 Because frequent use of antibiotics has been associated with increasing bacterial resistance,4 preventive strategies, particularly immunizations, are becoming increasingly attractive.
See related article, p 771
In children, influenza viruses and Streptococcus pneumoniae are common pathogens in RTIs, and both are currently among a limited number of respiratory disease pathogens that may be preventable by vaccination. In addition, evidence from animal and human studies suggests that influenza virus and S pneumoniae interact synergistically to cause complicated infections;5 thus, simultaneous immunization against influenza virus and S pneumoniae may be particularly effective.
The US Advisory Committee on Immunization Practices recently extended its recommendations for influenza vaccination to include all children age 6 to 59 months.6 Many European countries are considering extending recommendations for influenza vaccination to young children. However, little data are available from randomized controlled trials on the effects of influenza vaccination in these groups.7, 8, 9 Moreover, studies on the effects of pneumococcal conjugate vaccination on common RTIs in this age group are scarce, and are absent for influenza plus pneumococcal conjugate vaccination.10, 11 The current study was conducted to evaluate the effects of parenteral influenza vaccination plus heptavalent pneumococcal conjugate vaccination and influenza vaccination alone in preventing RTIs in preschool children.12
Methods
Participants and Recruitment
General practitioners (GPs) in the central Netherlands selected children age 18 to 72 months with a previously diagnosed RTI, registered according to the International Classification of Primary Care13, 14: acute otitis media (AOM) (H71); cough (with fever) (R05); acute upper RTI (R74), including common cold and pharyngitis; sinusitis (R75); acute tonsillitis (R76); acute laryngitis/tracheitis (R77); acute bronchitis/bronchiolitis (R78); influenza (R80); pneumonia (R81); pleurisy/pleural effusion (R82); and other respiratory infection (R83). Using this selection criterion increased the probability of the children having an RTI during the study period. Exclusion criteria were verified using questionnaires completed by both the GPs and the parents and included chronic asthma or recurrent wheezing (for longer than 3 months) treated with corticosteroids; other disorders predisposing to recurrent RTIs, such as Down syndrome and cleft palate; and clinically significant hypersensitivity to eggs. Children with previous serious adverse reactions to vaccines also were excluded, as were children who previously received influenza, pneumococcal, or hepatitis B virus (HBV) vaccinations and those with conditions for which these vaccinations are already recommended, such as chronic cardiac and respiratory conditions. The Medical Ethical Review Board of the University Medical Center Utrecht approved the study protocol. Parents were told about the study by their GP, and written informed consent was obtained from both parents or legal guardian of each child before study enrollment.
Procedures
Three cohorts of children were enrolled during September to October 2003, 2004, and 2005 (Table I). The children were randomly assigned in blocks of 3 to trivalent influenza plus heptavalent pneumococcal conjugate vaccination (TIV+PCV7), TIV plus placebo vaccination (TIV+plac), or control HBV plus placebo vaccination (HBV+plac) in a 1:1:1 ratio. The treatment group assignments were not revealed to parents, investigators, research personnel conducting the follow-up, or health care providers, all of whom remained blinded throughout the study. The injections were administered by nonblinded research nurses who were not involved in subsequent follow-up and were instructed to not reveal the intervention allocation. The vaccines were administered intramuscularly in the deltoid or gluteal muscle (in separate extremities). The pneumococcal and placebo vaccines were administered in the right extremity; the influenza and HBV vaccines, in the left extremity.
Table I.
Vaccination schedule
| Cohort | Vaccination group (n) | First vaccination (September-October) | Second vaccination⁎ (November-December) | Third vaccination (October next year) | Total follow-up time |
|---|---|---|---|---|---|
| 2003 and 2004 (n = 363) | Influenza plus pneumococcal vaccination (122) | TIV+PCV7 | TIV+PCV7 | TIV | 18 months |
| Influenza vaccination alone (117) | TIV+plac | TIV+plac | TIV | 18 months | |
| Control vaccination (124) | HBV+plac | HBV+plac | HBV | 18 months | |
| 2005 (n = 216) | Influenza plus pneumococcal vaccination (75) | TIV+PCV7 | TIV+PCV7 | — | 6 months |
| Influenza vaccination alone (70) | TIV+plac | TIV+plac | — | 6 months | |
| Control vaccination (71) | HBV+plac | HBV+plac | — | 6 months |
Maximum of 60 days after the first vaccination.
The vaccination schedule was similar for all 3 study arms (Table I). The children received 2 vaccinations 4 to 8 weeks apart in the year of inclusion, and the first 2 cohorts of children received a subsequent vaccination in the subsequent year. To evaluate blinding, parents of the last 2 cohorts of children were asked which vaccinations that they thought their child had received just after the vaccinations were given and at the end of the study. Just after the vaccination, 87% of the parents either did not know or identified the wrong set of vaccinations; at the end of the study, this percentage was 80%, indicating successful blinding.
Vaccines
Inactivated TIV (Influvac) was supplied by Solvay, Weesp, the Netherlands. The strains in the 2003-2004 formulation were A/New Caledonia/20/99 (H1N1), A/Moscow/10/99 (H3N2), and B/HongKong/330/01. The strains in the 2004-2005 formulation were A/New Caledonia/20/99 (H1N1), A/Fuijan/411/2002 (H3N2), and B/Shanghai/361/2002. The strains in the 2005-2006 formulation included A/New Caledonia/20/99 (H1N1), A/California/7/2004 (H3N2), and B/Shanghai/361/2002. The PCV7 (Prevenar; Wyeth Vaccines Research, Berkshire, United Kingdom) comprised 2 μg each of capsular polysaccharides of pneumococcal serotypes 4, 9V, 14, 19F, and 23 F; 4 μg of serotype 6B polysaccharide; and 2 μg of serotype 18C oligosaccharide, each conjugated individually to the CRM197 protein. The placebo was a standard diluent (0.9% phosphate-buffered NaCl; Solvay, Weesp, the Netherlands). The control vaccine was the recombinant HBV vaccine (Engerix-B Junior; GlaxoSmithKline, Rixenart, Belgium).
Clinical Outcome Measures
Follow-up of clinical outcomes for each participant began 14 days after the second set of vaccinations and continued for 18 or 6 months, depending on the year of inclusion (Table I). Each parent was instructed to keep a daily diary, recording any clinical signs or symptoms associated with RTI, including general weakness/malaise, rhinitis, sore throat, earache, coughing, wheezing/shortness of breath, and shivering/muscle aches, and to characterize their severity on a scale of 1 (mild) to 3 (severe). The parent also was instructed to measure the child's body temperature (preferably at the end of the day) using a validated electronic tympanic thermometer provided by the trial center.15 The parent also was asked to record all GP visits due to their child's RTI-related complaints. For each such visit, the GP was instructed to complete a form including information on the diagnosis and possible antibiotic prescriptions.
The primary study outcome was a febrile RTI, defined as fever (tympanic temperature ≥ 38.0°C) for at least 2 consecutive days accompanied by 1 or more of the aforementioned signs or symptoms of RTI with a moderate (ie, 2) or severe (ie, 3) severity score. An incident RTI episode was recorded as such only when it occurred after a period of at least 5 days without fever and other RTI symptoms. Secondary outcomes were febrile RTI–related polymerase chain reaction (PCR)-confirmed influenza (see below), GP visits, antibiotic prescriptions, or a physician-diagnosed episode of AOM.
During influenza seasons, the parent was instructed to contact the trial center for evaluation for influenza if the child had fever (tympanic temperature ≥ 38.0 °C) for more than 1 day accompanied by at least 1 RTI-associated sign or symptom of severity score ≥ 2. Within 4 days of onset of fever and symptoms, a trained research assistant obtained a nasopharyngeal swab for viral determination. Each sample was analyzed by real-time PCR for the presence of influenza A and B viruses.16 Only 1 nasopharyngeal swab was obtained per influenza season per child, to minimize discomfort to the child. For this study, influenza season was defined as the period with more than 50 GP visits for influenza-like illness per 100 000 inhabitants per week in the Netherlands (a total of 28 weeks over 3 consecutive years), as recorded by the European Influenza Surveillance Scheme (http://www.eiss.org).
Characteristics of Influenza Seasons
In the Netherlands, the 2003-04 influenza season peaked with 149 patients visiting a GP for influenza-like illness per 100 000 inhabitants.17 Almost all of the isolates were influenza A/H3N2 viruses and appeared closely related to influenza virus A/Fujian/411/02, a variant that had circulated in the 2002-03 season and deviated somewhat from the vaccine strain A/Moscow/10/99 and formerly circulating strains.17 The 2004-05 influenza season peaked with 240 GP-attended influenza-like illnesses per 100 000 inhabitants.18 Again, most isolates were influenza A/H3N2 viruses and appeared related to the new variant A/California/7/04, which deviated to some extent from the vaccine strains. Although the influenza A/H1N1 viruses were similar to the vaccine strains, the influenza B virus isolates deviated significantly from the vaccine strains.18 The 2005-06 influenza season included 2 peaks of activity with 138 and 98 GP-attended influenza-like illnesses per 100 000 inhabitants.19 The first peak was due mainly to influenza B viruses, which differed significantly from the vaccine strains. The second peak was attributed mainly to influenza A/H3N2-viruses, which generally matched the vaccine strains.19
Dutch Pneumococcal and Influenza Vaccination Guidelines for Children
In the Netherlands, pneumococcal conjugate vaccination was added to the national infant vaccination program in April 2006, with no catch-up program for children under age 5 years. For influenza, Dutch immunization guidelines recommend vaccination only for children with high-risk medical conditions, such as chronic cardiac or respiratory disease.
Statistical Analyses
We estimated that we would need at least 163 study subjects per study arm (assuming a hazard rate for the primary outcome of 5.0 per 1 000 days of follow-up during an average influenza season duration of 60 days) to achieve 80% power to detect a 45% reduction (hazard rate, 0.55), with a 2-sided α of .05. Taking into account a 15% potential loss to follow-up, we calculated that 187 subjects per arm were required. Alternatively, we also considered a more conservative approach, estimating that we would need 200 study subjects per study arm to detect a similar 45% reduction (risk ratio, 0.55) in the probability of developing at least 1 febrile RTI, assuming a 15% probability of this outcome in the control group, a power of 80%, and a 2-sided α of 0.05. Thus, our goal was to enroll 200 children in each of the 3 study arms.
All analyses were carried out on an intention-to-treat basis. Relative risk (RR)—that is, the proportion of children with at least 1 febrile RTI and febrile RTI with confirmed influenza during influenza season in the vaccination groups versus the control group—along with its 95% confidence interval (CI) was determined using the Episheet spreadsheet for the analysis of epidemiologic data (http://members.aol.com/krothman/episheet.xls). Using the statistical package SAS version 8.02 (SAS Institute, Cary, North Carolina), the incidence rates of each outcome (ie, number of events per day) during influenza seasons and outside of influenza seasons were modeled using Poisson regression, accounting for the potential dependency of observations within individuals. An offset was included in the model to account for different observation times per individual. The incidence rate ratios with 95% CIs were based on maximum likelihood estimates and their corresponding standard errors.
Results
Study Population
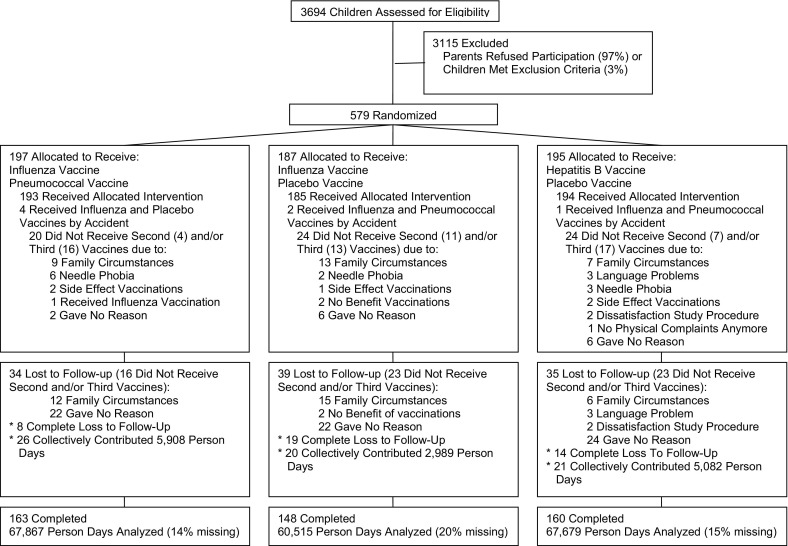
A total of 579 children were enrolled, including 171 in the first cohort, 192 in the second cohort, and 216 in the third cohort (Table I). The baseline characteristics were similar in all 3 study arms (Table II). A total of 471 children (81%) completed the study; 41 (7%) were completely lost to follow-up, and 67 (12%) had only partial follow-up (Figure). In general, the vaccinations were well tolerated, and no immediate or severe adverse events were recorded.
Table II.
Baseline characteristics of patients
| TIV+PCV7 (n = 197) | TIV+plac (n = 187) | HBV+plac (n = 195) | |
|---|---|---|---|
| Male sex, % | 58.9 | 55.6 | 50.3 |
| Mean age, years | 3.0 | 3.1 | 3.1 |
| Number of RTI episodes in preceding year, % | |||
| ≤3 | 46 | 44 | 42 |
| 4 to 6 | 37 | 40 | 41 |
| ≥7 | 17 | 16 | 17 |
| GP-diagnosed RTI in preceding year, n | 3.0 | 3.1 | 3.2 |
| Courses of antibiotics prescribed in preceding year, n | 1.6 | 1.6 | 1.8 |
| History of ENTsurgery, % | 28.6 | 27.6 | 29.5 |
| Asthma/wheezing in preceding year, % | 28.3 | 27.4 | 31.6 |
| Hay fever/allergic rhinitis in preceding year, % | 16.6 | 14.1 | 12.4 |
| Eczema in preceding year, % | 21.6 | 18.3 | 22.9 |
| Day care, % | 69.7 | 62.0 | 66.3 |
| Mean number of siblings, n | 0.8 | 0.9 | 1.0 |
| Breast-feeding < 3 months, % | 58.2 | 62.7 | 55.5 |
| Tobacco smoke exposure indoors, % | 22.6 | 25.9 | 26.6 |
Figure.
Flowchart of the trial.
Febrile RTI
During influenza seasons
During the influenza seasons, 28% of the subjects in the TIV+PCV7 group and 34% of those in the TIV+plac group had at least 1 febrile RTI episode versus 39% in the control HBV+plac group, corresponding to RRs of 0.72 (95% CI = 0.57 to 0.92) and 0.88 (95% CI = 0.70 to 1.10), respectively. In all 3 influenza seasons together, a total of 330 episodes of febrile RTI were reported, for an overall incidence rate of 6.1 per 1 000 child-days of follow-up (5.2 in the TIV+PCV7 group, 6.1 in the TIV+plac group, and 6.9 in the control HBV+plac group). This relates to reductions of 24% (95% CI = 2% to 42%) in the TIV+PCV7 group and 13% (95% CI = −13% to 33%) in the TIV+plac group compared with the control group (Table III). These figures correspond to the need to vaccinate between 9 and 17 children to prevent 1 such RTI episode. Of the 330 febrile RTI episodes occurring during the influenza seasons, 271 (82%) were in separate children, and nose/throat swabs for influenza virus PCR analysis were obtained in 117 of these episodes. Influenza virus type A or B was identified in a total of 48 children, including 9% of the children in the HBV+plac group, 4% of those in the TIV+PCV7 group (RR = 0.48; 95% CI = 0.25 to 0.93), and 5% of those in the TIV+plac group (RR = 0.49; 95% CI = 0.25 to 0.97). In these 48 confirmed cases of influenza, 5 involved AOM for which a physician was consulted (3 cases in the HBV+plac group, 2 cases in the PCV7+plac group, and no cases in the TIV+plac group).
Table III.
Incidence rates of outcomes by follow-up period and treatment group
| Condition | Group | Incidence per 1000 child days | Incidence rate ratio (95% CI) | P value for overall differences among the 3 study groups |
|---|---|---|---|---|
| Influenza season | ||||
| Febrile RTI | TIV+PCV7 | 5.2 (4.2 to 6.5) | 0.76 (0.58 to 0.99) | .13 |
| TIV+plac | 6.0 (5.0 to 7.2) | 0.87 (0.68 to 1.12) | ||
| HBV+plac | 6.9 (5.9 to 8.2) | |||
| GP visit | TIV+PCV7 | 2.2 (1.6 to 3.0) | 0.75 (0.50 to 1.12) | .15 |
| TIV+plac | 2.0 (1.4 to 2.7) | 0.67 (0.45 to 1.02) | ||
| HBV+plac | 2.9 (2.3 to 3.7) | |||
| Antibiotic prescription | TIV+PCV7 | 1.0 (0.6 to 1.6) | 0.73 (0.40 to 1.32) | .57 |
| TIV+plac | 1.2 (0.8 to 1.9) | 0.89 (0.50 to 1.61) | ||
| HBV+plac | 1.4 (0.9 to 2.1) | |||
| AOM | TIV+PCV7 | 0.5 (0.3 to 1.0) | 0.43 (0.20 to 0.94) | .01 |
| TIV+plac | 0.4 (0.2 to 0.8) | 0.29 (0.12 to 0.70) | ||
| HBV+plac | 1.2 (0.8 to 1.9) | |||
| Outside influenza season | ||||
| Febrile RTI | TIV+PCV7 | 4.0 (3.4 to 4.7) | 1.20 (0.95 to 1.52) | .29 |
| TIV+plac | 3.5 (2.9 to 4.2) | 1.05 (0.82 to 1.34) | ||
| HBV+plac | 3.3 (2.8 to 3.9) | |||
| GP visit | TIV+PCV7 | 1.2 (0.9 to 1.6) | 0.82 (0.56 to 1.21) | .54 |
| TIV+plac | 1.2 (0.9 to 1.7) | 0.82 (0.55 to 1.23) | ||
| HBV+plac | 1.5 (1.1 to 1.9) | |||
| Antibiotic prescription | TIV+PCV7 | 0.7 (0.5 to 1.1) | 0.89 (0.54 to 1.44) | .33 |
| TIV+plac | 0.6 (0.4 to 0.9) | 0.67 (0.39 to 1.16) | ||
| HBV+plac | 0.8 (0.6 to 1.2) | |||
| AOM | TIV+PCV7 | 0.5 (0.3 to 0.7) | 2.00 (1.02 to 3.93) | .13 |
| TIV+plac | 0.3 (0.2 to 0.6) | 1.41 (0.65 to 3.03) | ||
| HBV+plac | 0.2 (0.1 to 0.4) | |||
Of the 330 febrile RTI episodes, 128 (39%) resulted in a primary care visit, 66 (20%) resulted in an antibiotic prescription, and 39 (12%) resulted in a physician diagnosis of AOM. No significant differences in the rates of primary care visits or antibiotic prescriptions were seen between the TIV+PCV7 and TIV+plac groups and the control group (Table III). AOM episodes were reduced by 57% (95% CI = 10% to 80%) in the TIV+PCV7 group and by 71% (95% CI = 29% to 88%) in the TIV+plac group compared with the control HBV+plac group (Table III).
Outside of influenza seasons
Outside of the influenza seasons, a total of 504 episodes of febrile RTI and 183 (36%) related GP visits, 101 (20%) antibiotic prescriptions, and 51 (10%) physician-diagnosed episodes of AOM were recorded. No overall differences in the rates of any of these outcomes were found among the 3 study arms (Table III). Similar trends in vaccine effects were observed when data were analyzed by age at randomization (18 to 36 months vs 36 months and older) (Table IV; available at www.jpeds.com).
Table IV.
Incidence rates of outcomes by follow-up period, treatment group, and age at randomization
| Condition | Group | Incidence per 1000 child days |
|
|---|---|---|---|
| <3.0 years at randomization | ≥3.0 years at randomization | ||
| Influenza season | |||
| Febrile RTI | TIV+PCV7 | 5.2 (3.9 to 6.9) | 5.4 (4.0 to 7.4) |
| TIV+plac | 6.7 (5.4 to 8.4) | 4.9 (3.6 to 6.6) | |
| HBV+plac | 7.2 (5.9 to 8.9) | 6.5 (5.0 to 8.4) | |
| GP visit | TIV+PCV7 | 2.2 (1.4 to 3.3) | 2.2 (1.4 to 3.5) |
| TIV+plac | 2.5 (1.8 to 3.6) | 1.0 (0.5 to 2.1) | |
| HBV+plac | 3.2 (2.3 to 4.3) | 2.5 (1.6 to 3.9) | |
| Antibiotic prescription | TIV+PCV7 | 0.9 (0.5 to 1.6) | 1.3 (0.7 to 2.6) |
| TIV+plac | 1.6 (1.9 to 2.7) | 0.6 (0.2 to 1.7) | |
| HBV+plac | 1.5 (1.0 to 2.4) | 1.1 (0.6 to 2.4) | |
| AOM | TIV+PCV7 | 0.6 (0.2 to 1.3) | 0.5 (0.2 to 1.4) |
| TIV+plac | 0.6 (0.3 to 1.3) | 0.0 | |
| HBV+plac | 1.5 (0.9 to 2.5) | 0.8 (0.4 to 1.7) | |
| Outside influenza season | |||
| Febrile RTI | TIV+PCV7 | 4.4 (3.6 to 5.4) | 3.3 (2.4 to 4.4) |
| TIV+plac | 4.1 (3.3 to 5.0) | 2.7 (1.9 to 3.6) | |
| HBV+plac | 3.4 (2.8 to 4.2) | 3.2 (2.4 to 4.2) | |
| GP visit | TIV+PCV7 | 1.3 (0.9 to 1.8) | 1.1 (0.7 to 1.8) |
| TIV+plac | 1.4 (1.0 to 2.0) | 0.9 (0.5 to 1.6) | |
| HBV+plac | 1.5 (1.0 to 2.0) | 1.5 (0.1 to 2.2) | |
| Antibiotic prescription | TIV+PCV7 | 0.8 (0.5 to 1.2) | 0.7 (0.4 to 1.2) |
| TIV+plac | 0.6 (0.4 to 1.1) | 0.5 (0.2 to 1.0) | |
| HBV+plac | 0.9 (0.6 to 1.3) | 0.8 (0.5 to 1.4) | |
| AOM | TIV+PCV7 | 0.5 (0.3 to 0.8) | 0.4 (0.2 to 0.8) |
| TIV+plac | 0.4 (0.2 to 0.7) | 0.3 (0.2 to 0.9) | |
| HBV+plac | 0.2 (0.1 to 0.5) | 0.3 (0.1 to 0.6) | |
Discussion
This randomized, double-blind, placebo-controlled trial comprising 579 children found that influenza vaccination with or without heptavalent pneumococcal conjugate vaccination was effective against confirmed influenza and AOM episodes during the influenza seasons but appeared to be less effective against febrile RTI episodes in general. These vaccinations appeared to have no beneficial effects outside of the influenza seasons.
Recent meta-analyses on the effects of influenza vaccination in healthy children of all ages reported reductions of 62% (95% CI = 45% to 75%),7 65% (95% CI = 45% to 77%),8 and 65% (95% CI = 47% to 76%)9 in confirmed influenza by parenteral inactivated influenza vaccination. Within a subgroup of healthy preschool children, a statistically nonsignificant reduction in confirmed influenza of 58% (95% CI = −20% to 86%) was reported based on 2 randomized controlled trials covering each influenza season with a total size of only 132 children.7 In the current study, comprising 579 children and covering 3 influenza seasons, we had adequate power to observe statistically significant effects of 51% (95% CI = 3% to 75%) and 52% (95% CI = 7% to 75%) on confirmed influenza in the 2 vaccination groups, corresponding to the estimates from meta-analyses.
The effects on influenza-like illness in healthy children of all ages reported by meta-analyses have varied from reductions of 28% (95% CI = 22% to 33%) to 45% (95% CI = 33% to 55%).7, 8, 9 In children under age 6 years, a statistically nonsignificant reduction in influenza-like illness of 48% (95% CI = −98% to 86%) was found.7 A recent randomized study in preschool-age children covering a single influenza season found significant reductions of 33% in upper RTI, 22% in lower RTI, 26% in febrile respiratory illness, and 32% in antibiotic prescriptions from influenza vaccination. Such reductions are difficult to interpret, however, because the control group did not receive placebo or control vaccinations.20, 21 We found reductions in febrile RTI of 24% (95% CI = 2% to 48%) in the TIV+PCV7 group and 13% (95% CI = −13% to 33%) in the TIV+plac group during the influenza seasons.
These vaccine effect estimates appear low compared with those reported in previous studies. But our definition of febrile RTI likely covers more illnesses than those more narrowly defined as influenza-like illnesses. Typically, less-specific definitions of diagnoses will result in smaller relative reductions. Obviously, even during the influenza season, a large proportion of febrile RTIs in children is caused by other pathogens, including rhinoviruses, human coronaviruses, parainfluenza viruses, adenoviruses, and particularly seasonally active respiratory syncytial virus, which often co-circulates with influenza virus.22, 23 Nonetheless, we found reductions in AOM episodes of 57% (95% CI = 6% to 80%) in the TIV+PCV7 group and 71% (95% CI = 30% to 88%) in the TIV+plac group, indicating the pivotal role of influenza viruses in this condition.
Our findings are in agreement with those of previous nonrandomized and/or unblinded studies in preschool children that reported a 40% to 62% reduction in AOM from parenteral inactivated subunit influenza vaccination.24, 25 A recent meta-analysis of 6 (quasi-) randomized controlled trials reported a 32% reduction in AOM (95% CI = −16% to 60%) by parenteral inactivated influenza vaccination among healthy children of all ages.9
Although pneumococcal conjugate vaccinations have been demonstrated to be highly effective against invasive pneumococcal disease and, to a lesser extent, against pneumonia,26 they appear to be less effective in mucosal infections such as upper RTIs, including AOM. Two randomized controlled trials on pneumococcal conjugate vaccinations in infants have reported reductions in AOM of 6% to 8%.27, 28 Studies of pneumococcal conjugate vaccinations in older children are scarce. One previous double-blind, randomized, placebo-controlled study on nonavalent pneumococcal conjugate vaccinations in children age 12 to 35 months attending day care reported reductions of 15% (95% CI = 4% to 24%) in upper RTIs, 16% (95% CI = 2% to 28%) in lower RTIs, and 17% (95% CI = −2% to 33%) in AOM episodes.10 However, another trial of PCV7 followed by pneumococcal polysaccharide vaccination in children with recurrent AOM found no beneficial effect on AOM.11 We found no overall significant difference in the occurrence of GP-diagnosed AOM episodes among our study groups outside the influenza seasons. Our data suggest that preventing the viral infection that precedes AOM is an important variable.
Although the present study was not adequately powered to detect relatively small effects, such as 8% reduction, our data suggest a trend toward increase in the PCV7 group. The lack of an effect on either all-cause RTI or physician-attended AOM episodes by PCV7 may have been caused, at least in part, by changes in nasopharyngeal carriage after vaccination, with a shift from serotypes covered by the vaccine to nonvaccine serotypes and other colonizing bacteria of the nasopharynx.29, 30, 31 Although rates of vaccine serotype RTI may decrease, the replacement pathogens may have pathogenic potential,27 possibly even causing an increase in the rates of RTI by nonvaccine serotype pneumococci and other nasopharyngeal colonizing flora.31, 32 This replacement phenomenon may affect older children in particular.11, 33 Future studies should address this issue in more depth.
The fact that we did not obtain nasopharyngeal swabs for influenza PCR testing in every RTI episode prevented us from determining the total incidence of confirmed influenza in the different study arms and thereby the estimated absolute vaccine effect. But because the episodes during which swabs were obtained were not selected based on patient characteristics (eg, age), disease characteristics (other than the previously defined criterion of at least 2 days of fever accompanied by at least 1 RTI-associated sign or symptom with a severity score of 2), or study arm (randomized, double-blind trial), it is unlikely that the missing data had an impact on the relative vaccine effect. Another potential limitation of this study is the fact that influenza virus activity was mild during the 2003-04 and 2005-06 influenza seasons, and that the circulating influenza virus A subtype H3N2 strain suboptimally matched the vaccine strain in the 2003-04 and 2004-05 influenza seasons, as also occurred for the influenza B viruses in the 2004-05 and 2005-06 seasons.17, 18, 19 This mild influenza virus activity and suboptimal vaccine matching may have led to underestimation of the effect of influenza vaccination that can occur during a severe epidemic.
Acknowledgments
The authors thank the children and parents who participated in this study, as well as Yvonne Schönbeck and Nelly van Eden for their help with the trial protocol, patient recruitment, and data collection.
Footnotes
Financial support information is available at www.jpeds.com.
Appendix
This study was funded by the Netherlands Organization for Health Research and Development ZONMW (grant 2200.0121). The ZONMW played no role in the design and conduct of the study; the collection, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript. Influenza vaccines were provided by Solvay, Weesp, the Netherlands. Pneumococcal vaccines were provided by Wyeth Vaccines Research, Berkshire, UK. Hepatitis B vaccines were provided by GlaxoSmithKline BV, Rixensart, Belgium. Jansen wrote the first draft of the manuscript. No honorarium, grant, or other form of payment was given to produce the manuscript. The authors declare no conflicts of interest.
References
- 1.Arroll B. Antibiotics for upper respiratory tract infections: an overview of Cochrane reviews. Respir Med. 2005;99:255–261. doi: 10.1016/j.rmed.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 2.van Staaij B.K., van den Akker E.H., van der Heijden G.J.M.G., Schilder A.G., Hoes A.W. Adenotonsillectomy for upper respiratory infections: evidence-based? Arch Dis Child. 2005;90:19–25. doi: 10.1136/adc.2003.047530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Staaij B.K., van den Akker E.H., Rovers M.M., Hordijk G.J., Hoes A.W., Schilder A.G.M. Effectiveness of adenotonsillectomy in children with mild symptoms of throat infections or adenotonsillar hypertrophy: an open, randomised, controlled trial. BMJ. 2004;329:651–656. doi: 10.1136/bmj.38210.827917.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goossens H., Ferech M., van der Stichele R., Elseviers M. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365:579–587. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- 5.McCullers J.A. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19:571–582. doi: 10.1128/CMR.00058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith N.M., Bresee J.S., Shay D.K., Uyeki T.M., Cox N.J., Strikas R.A., Advisory Committee on Immunization Practices Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55:1–42. [PubMed] [Google Scholar]
- 7.Jefferson T., Smith S., Demicheli V., Harnden A., Rivetti A., Di Pietrantonj C. Assessment of the efficacy and effectiveness of influenza vaccines in healthy children: a systematic review. Lancet. 2005;365:773–780. doi: 10.1016/S0140-6736(05)17984-7. [DOI] [PubMed] [Google Scholar]
- 8.Negri E., Colombo C., Giordano L., Groth N., Apolone G., La Vecchia C. Influenza vaccine in healthy children: a meta-analysis. Vaccine. 2005;23:2851–2861. doi: 10.1016/j.vaccine.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 9.Manzoli L., Schioppa F., Boccia A., Villari P. The efficacy of influenza vaccine for healthy children: a meta-analysis evaluating potential sources of variation in efficacy estimates including study quality. Pediatr Infect Dis J. 2007;26:97–106. doi: 10.1097/01.inf.0000253053.01151.bd. [DOI] [PubMed] [Google Scholar]
- 10.Dagan R., Sikuler-Cohen M., Zamir O., Janco J., Givon-Lavi N., Fraser D. Effect of a conjugate pneumococcal vaccine on the occurrence of respiratory infections and antibiotic use in day-care center attendees. Pediatr Infect Dis J. 2001;20:951–958. doi: 10.1097/00006454-200110000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Veenhoven R., Bogaert D., Uiterwaal C., Brouwer C., Kiezebrink H., Bruin J. Effect of conjugate pneumococcal vaccine by polysaccharide pneumococcal vaccine on recurrent acute otitis media: a randomised study. Lancet. 2003;361:2189–2195. doi: 10.1016/S0140-6736(03)13772-5. [DOI] [PubMed] [Google Scholar]
- 12.Schönbeck Y., Sanders E.A.M., Hoes A.W., Schilder A.G.M., Verheij T.J.M., Hak E. Rationale and design of the Prevention of Respiratory Infections and Management in Children (PRIMAKid) study: a randomized controlled trial on the effectiveness and costs of combined influenza and pneumococcal vaccination in pre-school children with recurrent respiratory tract infections. Vaccine. 2005;23:4906–4914. doi: 10.1016/j.vaccine.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 13.World Organization of Family Doctors (WONCA) Classification Committee . 3rd edition. Oxford University Press; New York: 1983. ICHPPC-2 Defined. [Google Scholar]
- 14.World Organization of Family Doctors (WONCA) Classification Committee . 2nd edition. Oxford University Press; Oxford, UK: 1998. ICPC-2, International Classification of Primary Care. [Google Scholar]
- 15.van Staaij B.K., Rovers M.M., Schilder A.G., Hoes A.W. Accuracy and feasibility of daily infrared tympanic membrane temperature measurements in the identification of fever in children. Int J Pediatr Otorhinolaryngol. 2003;67:1091–1097. doi: 10.1016/s0165-5876(03)00198-8. [DOI] [PubMed] [Google Scholar]
- 16.van Elden L., Nijhuis M., Schipper P., Schuurman R., van Loon A.M. Simultaneous detection of influenza viruses A and B using real-time quantitative PCR. J Clin Microbiol. 2001;39:196–200. doi: 10.1128/JCM.39.1.196-200.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rimmelzwaan G.F., de Jong J.C., Bartelds A.I.M., Wilbrink B., Fouchier R.A.M., Osterhaus A.D.M.E. The 2003/2004 influenza season in the Netherlands with a limited epidemic of the virus variant A/Fujian, and the vaccine composition for the 2004/2005 season. Ned Tijdschr Geneeskd. 2004;148:1984–1988. [in Dutch] [PubMed] [Google Scholar]
- 18.de Jong J.C., Rimmelzwaan G.F., Bartelds A.I.M., Meijer A., Fouchier R.A.M., Osterhaus A.D.M.E. The influenza season 2004/05 in the Netherlands with the largest epidemic of the last 5 years caused by the virus variant A/California and the composition of the vaccine for the season 2005/06. Ned Tijdschr Geneeskd. 2005;149:2355–2361. [in Dutch] [PubMed] [Google Scholar]
- 19.Rimmelzwaan G.F., de Jong J.C., Donker G.A., Meijer A., Fouchier R.A.M., Osterhaus A.D.M.E. The 2005-2006 influenza season in the Netherlands and the vaccine composition for the 2006-2007 season. Ned Tijdschr Geneeskd. 2006;150:2209–2214. [in Dutch] [PubMed] [Google Scholar]
- 20.Esposito S., Marchisio P., Bosis S., Lambertini L., Claut L., Faelli N. Clinical and economic impact of influenza vaccination on healthy children aged 2-5 years. Vaccine. 2006;24:629–635. doi: 10.1016/j.vaccine.2005.08.054. [DOI] [PubMed] [Google Scholar]
- 21.Bueving H.J., van der Wouden J.C. Influenza vaccination in healthy children. Vaccine. 2006;24:4901. doi: 10.1016/j.vaccine.2006.03.085. [DOI] [PubMed] [Google Scholar]
- 22.van Gageldonk-Lafeber A.B., Heijnen M.L.A., Bartelds A.M., Peters M.F., van der Plas S.M., Wilbrink B. A case-control study of acute respiratory tract infection in general practice patients in the Netherlands. Clin Infect Dis. 2005;41:490–497. doi: 10.1086/431982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zambon M.C., Stockton J.D., Clewley J.P., Fleming D.M. Contribution of influenza and respiratory syncytial virus to community cases of influenza-like illness: an observational study. Lancet. 2001;358:1410–1416. doi: 10.1016/s0140-6736(01)06528-x. [DOI] [PubMed] [Google Scholar]
- 24.Heikkinen T., Ruuskanen O., Waris M., Ziegler T., Arola M., Halonen P. Influenza vaccination in the prevention of acute otitis media in children. Am J Dis Child. 1991;145:445–448. doi: 10.1001/archpedi.1991.02160040103017. [DOI] [PubMed] [Google Scholar]
- 25.Ozgur S.K., Beyazova U., Kemaloglu Y.K., Maral I., Sahin F., Camurdan A.D. Effectiveness of inactivated influenza vaccine for prevention of otitis media in children. Pediatr Infect Dis J. 2006;25:401–404. doi: 10.1097/01.inf.0000217370.83948.51. [DOI] [PubMed] [Google Scholar]
- 26.Lucero M.G., Dulalia V.E., Parreno R.N., Lim-Quianzon D.M., Nohynek H., Makela H. Pneumococcal conjugate vaccines for preventing vaccine-type invasive pneumococcal disease and pneumonia with consolidation on x-ray in children under two years of age. Cochrane Database Syst Rev. 2004 doi: 10.1002/14651858.CD004977. CD004977. [DOI] [PubMed] [Google Scholar]
- 27.Eskola J., Kilpi T., Palmu A., Jokinen J., Haapakoski J., Herva E. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. 2001;344:403–409. doi: 10.1056/NEJM200102083440602. [DOI] [PubMed] [Google Scholar]
- 28.Fireman B., Black S.B., Shinefield H.R., Lee J., Lewis E., Ray P. Impact of the pneumococcal conjugate vaccine on otitis media. Pediatr Infect Dis J. 2003;22:10–16. doi: 10.1097/00006454-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Dagan R., Melamed R., Muallem M., Piglansky L., Greenberg D., Abramson O. Reduction of nasopharyngeal carriage of pneumococci during the second year of life by a heptavalent conjugate pneumococcal vaccine. J Infect Dis. 1996;174:1271–1278. doi: 10.1093/infdis/174.6.1271. [DOI] [PubMed] [Google Scholar]
- 30.Obaro S.K., Adegbola R.A., Banya W.A.S., Greenwood B.M. Carriage of pneumococci after pneumococcal vaccination. Lancet. 1996;348:271–272. doi: 10.1016/s0140-6736(05)65585-7. [DOI] [PubMed] [Google Scholar]
- 31.Block S.L. Searching for the holy grail of acute otitis media. Arch Dis Child. 2006;91:959–961. doi: 10.1136/adc.2006.097063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shouval D.S., Greenberg D., Givon-Lavi N., Porat N., Dagan R. Site-specific disease potential of individual Streptococcus pneumoniae serotypes in pediatric invasive disease, acute otitis media and acute conjunctivitis. Pediatr Infect Dis J. 2006;25:602–607. doi: 10.1097/01.inf.0000220231.79968.f6. [DOI] [PubMed] [Google Scholar]
- 33.Syrjänen R.K., Kilpi T.M., Kaijalainen T.H., Herva E.E., Takala A.K. Nasopharyngeal carriage of Streptococcus pneumoniae in Finnish children younger than 2 years old. J Infect Dis. 2001;184:451–459. doi: 10.1086/322048. [DOI] [PubMed] [Google Scholar]