Abstract
Penaeid shrimp aquaculture is an important industry in the Americas, and the industry is based almost entirely on the culture of the Pacific White Shrimp, Litopenaeus vannamei. Western Hemisphere shrimp farmers in 14 countries in 2004 produced more than 200,000 metric tons of shrimp, generated more than $2 billion in revenue, and employed more than 500,000 people. Disease has had a major impact on shrimp aquaculture in the Americas since it became a significant commercial entity in the 1970s. Diseases due to viruses, rickettsial-like bacteria, true bacteria, protozoa, and fungi have emerged as major diseases of farmed shrimp in the region. Many of the bacterial, fungal and protozoan caused diseases are managed using improved culture practices, routine sanitation, and the use of chemotherapeutics. However, the virus diseases have been far more problematic to manage and they have been responsible for the most costly epizootics. Examples include the Taura syndrome pandemic that began in 1991–1992 when the disease emerged in Ecuador, and the subsequent White Spot Disease pandemic that followed its introduction to Central America from Asia in 1999. Because of their socioeconomic significance to shrimp farming, seven of the nine crustacean diseases listed by the World Animal Organization (OIE) are virus diseases of shrimp. Of the seven virus diseases of penaeid shrimp, five are native to the Americas or have become enzootic following their introduction. The shrimp virus diseases in the Americas are increasingly being managed by exclusion using a combination of biosecurity and the practice of culturing domesticated specific pathogen-free (SPF) stocks or specific pathogen-resistant (SPR) stocks. Despite the significant challenges posed by disease, the shrimp farming industry of the Americas has responded to the challenges posed by disease and it has developed methods to manage its diseases and mature into a sustainable industry.
Keywords: Penaeid shrimp, Shrimp viruses, Virus diseases of farmed shrimp
1. Introduction
The shrimp farming industry of the Americas produces approximately 20% of the world’s farmed shrimp. The leading producers of farmed raised shrimp in this hemisphere are Ecuador, Brazil, Honduras and Mexico. In terms of significant production from aquaculture, only two penaeid shrimp species have been significant in the Americas. Of these, Litopenaeus vannamei (the Pacific white shrimp; the taxonomy used in this review will follow Perez Farfante and Kensley, 1997) currently accounts for more than 95% of the total production. The second most important species is Litopenaeus stylirostris, the Pacific blue shrimp, and it once accounted for nearly 20% of the hemisphere’s production. However, the high susceptibility of this species to WSSV and to new strains of TSV resulted in the near abandonment of the species in 1999–2000 by most of the industry (Zarain-Herzberg and Ascencio-Valle, 2001, Erickson et al., 2002, OIE, 2006b).
The most important diseases of cultured penaeid shrimp, in terms of economic impact, in the Americas (and in Asia) have infectious agents as their cause (Flegel and Alday-Sanz, 1998, Flegel, 1997, Flegel, 2006, Lightner, 1999, OIE, 2006a, OIE, 2006b). Among the infectious diseases of cultured shrimp, certain virus-caused diseases stand out as the most significant and many of these are listed by the World Animal Health Organization (the OIE – Office International des Epizooties) (Table 1, Table 2 ). Some of the most important diseases (and their etiological agents) were once limited in distribution to either the Western or Eastern Hemisphere (Lightner, 1996a, Lightner, 1996b, Lightner, 2003a, Lightner, 2003b, Flegel and Alday-Sanz, 1998, OIE, 2006b). However, the international movement of live (for aquaculture) and dead (commodity shrimp for reprocessing, direct retail commerce, and for use as bait by sport fishermen) have been implicated or suspected as being responsible for the transfer and establishment of certain pathogens from Asia to the Americas (Lightner, 1996b, Durand et al., 2000, AQUIS, 2000, Hasson et al., 2006). While frozen commodity shrimp have been implicated as the route by which WSSV was moved from Asia to the Americas, TSV was moved in the opposite direction with infected live broodstock from Central America (Nunan et al., 1998b, Tu et al., 1999, Yu and Song, 2000, Durand et al., 2000, Tang and Lightner, 2005).
Table 1.
OIE listed crustacean diseases as of 2006 and those being considered for listing.
| Disease name | Pathogen type | Pathogen name & acronym | Principal host group |
|---|---|---|---|
| Taura syndrome | ssRNA virus | Taura syndrome virus (TSV) | Penaeid shrimp |
| White spot disease | dsDNA virus | White spot syndrome virus (WSSV) | Penaeid shrimp |
| Yellowhead disease | ssRNA virus | Yellow head virus (YHV) & Gill-associated virus (GAV) | Penaeid shrimp |
| Tetrahedral baculovirosis | dsDNA virus | Baculovirus penaei, BP | Penaeid shrimp |
| Spherical baculovirosis | dsDNA virus | Monodon baculovirus, MBV | Penaeid shrimp |
| Infectious hypodermal and hematopoietic necrosis (IHHN) | ssDNA virus | IHHN virus, IHHNV | Penaeid shrimp |
| Infectious myonecrosis (IMN)a | dsRNA virus | IMN virus (IMNV) | Penaeid shrimp |
| Necrotizing hepatopancreatitis (NHP)a | bacteria | NHP-bacterium (NHP-B) | Penaeid shrimp |
| Crayfish plagueb | fungus | Aphanomyces astaci | Freshwater crayfish |
| White tail diseaseb | ssRNA virus | Macrobrachium nodavirus (MrNV) | Macrobrachium rosenbergii |
| Hepatopancreatic parvovirus diseaseb | ssDNA virus | Hepatopancreatic parvovirus (HPV) | Penaeid shrimp |
| Mourilyan virus diseaseb | ssRNA virus | Mourilian virus (MOV) | Penaeid shrimp |
Listing of this disease is under study by the OIE.
Listed as emerging diseases by the OIE.
Table 2.
Estimated losses due to certain OIE listed virus diseases since their emergence and/or.
| Virus – region | Year of emergence | Product loss to industry |
|---|---|---|
| IHHNV – Americasa | 1981 | $0.5–1 billion |
| YHV – Asia | 1991 | $0.5 billion |
| TSV – Americas | 1991/1992 | $1–2 billion |
| TSV – Asia | 1999 | $0.5–1 billion |
| WSSV – Asia | 1992/1993 | $6 billion |
| WSSV– Americas | 1999 | $1–2 billion |
| IMNV – Americas | 2004 | $100–200 million |
| IMNV – Asia | 2006 | ?? |
Includes the Gulf of California fishery for Litopenaeus stylirostris (Lightner, 1996b, Pantoja et al., 1999, Morales-Covarrubias et al., 1999).
As a consequence of the rapid growth and development of the penaeid aquaculture industry, many of the most significant shrimp pathogens were moved from the regions where they initially appeared to new regions even before the “new” pathogen had been recognized, named, proven to cause the “new” disease, and before reliable diagnostic methods were developed. The diseases due to the shrimp viruses IHHNV, TSV and WSSV were all transferred with live shrimp stocks from country to country and from one continent to another well before their etiology was understood and diagnostic methods were available. With some diseases, the introduced pathogen encountered totally naive hosts with little or no innate resistance. The pandemics due to the penaeid viruses WSSV and TSV, and to a lesser extent to IHHNV, IMNV and YHV, have collectively cost the penaeid shrimp industry billions of dollars in lost crops, jobs, and export revenue (Table 2). The social and economic impacts of the pandemics caused by these pathogens have been profound in countries in which shrimp farming constitutes a significant industry. This paper reviews the current status of the virus diseases due to TSV, WSSV, IHHNV, IMNV, and YHV in the Americas in terms of their biology, history, distribution, production impacts, and the methods for their management in shrimp aquaculture in the Americas. Not included in this review will be information on the baculovirus caused diseases caused by Monodon baculovirus (MBV), Baculovirus penaei (BP) and Baculoviral midgut gland necrosis (BMN). Of these only BP is native (and enzootic) in wild American penaeids and is an occasional problem for shrimp farmers (Lightner, 1996a, OIE, 2006b). However, there is little new information on BP-caused disease in the Americas or elsewhere that is not well covered in other reviews (Lightner, 1996a, Flegel, 2006, OIE, 2006b).
2. Infectious hypodermal and hematopoietic necrosis virus (IHHNV)
2.1. Biology of the agent
IHHNV is the smallest of the known penaeid shrimp viruses. The IHHN virion is a 22 nm diameter, nonenveloped icosahedron (Table 1; Fig. 1, Fig. 2 ), with a density of 1.40 g/ml in CsCl. Its genome is linear single-stranded DNA of 4.1 kb in length. Because of its characteristics, IHHNV has been classified as a member of the Parvoviridae and a probable member genus Brevidensovirus (Bonami et al., 1990, Bonami and Lightner, 1991, Mari et al., 1993, Nunan et al., 2000, Shike et al., 2000).
Fig. 1.
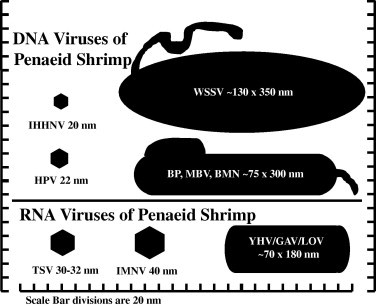
Schematic of the major viruses of penaeid shrimp. The virions are drawn to scale; scale divisions are 20 nm. See text for definition of the acronym shown for each virus.
Fig. 2.
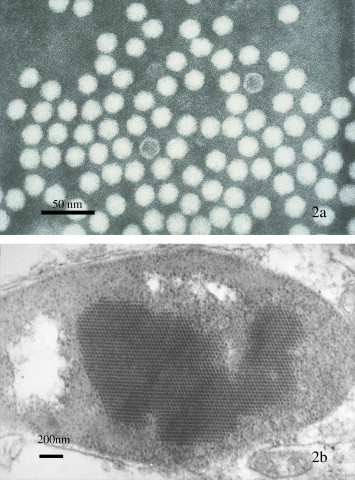
Transmission electron micrographs of IHHN virus from Litopenaeus stylirostris. (a) Purified IHHNV from cesium chloride gradient ultracentrifugation. Most of the hexagonal appearing (in profile) icosahedral particles are full, but two particles lack the viral genome and are shown as only an empty capsid. Stain: 2% PTA and (b) Paracrystalline array of IHHNV in the nucleus of a subcuticular fibrocyte.
2.2. Hosts, history and geographic distribution of IHHN disease
The disease IHHN, and later its causative agent, IHHNV, was first described as the cause of acute epizootics and mass mortalities (>90%) in juvenile and subadult L. stylirostris farmed in super-intensive raceway systems in Hawaii (Brock et al., 1983, Lightner, 1983, Lightner, 1988, Lightner et al., 1983a, Lightner et al., 1983b, Brock and Lightner, 1990). Shortly after its discovery in L. stylirostris, the virus was found in L. vannamei being cultured at the same facility in Hawaii and these L. vannamei were shown to be generally asymptomatic carriers of the virus (Lightner et al., 1983b, Bell and Lightner, 1984). Some members of populations of L. stylirostris and L. vannamei that survive IHHNV infections and/or epizootics, may carry the virus for life and pass the virus onto their progeny and other populations by horizontal and vertical transmission (Bell and Lightner, 1984, Lightner, 1996a, Morales-Covarrubias et al., 1999, Morales-Covarrubias and Chavez-Sanchez, 1999, Motte et al., 2003). IHHNV has been demonstrated by PCR (Motte et al., 2003) and by in-situ hybridization with IHHNV specific probes to be vertically transmitted in the oocytes (Fig. 4 h).
Fig. 4.
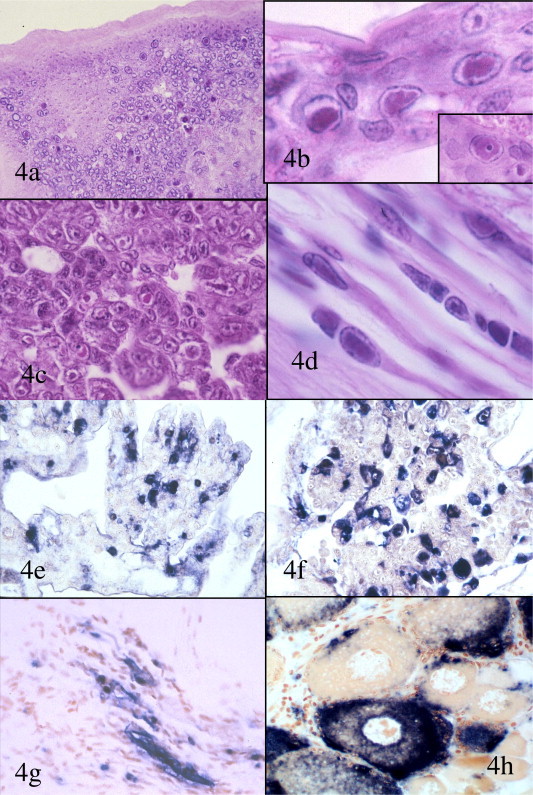
Histopathology of IHHN in Litopenaeus stylirostris and L. vannamei is shown in H&E stained sections (a–d) and by ISH with an IHHNV specific DIG-labeled probe (e–h); (a) low magnification photomicrograph of a section through the cuticular epithelium and subcuticular connective tissues of a small juvenile L. stylirostris with severe acute IHHN (unless otherwise noted, the remaining figures are from L. stylirostris). Intranuclear, haloed, eosinophilic Cowdry type A (CAI) inclusion bodies are evident in some infected cells; (b and c) higher magnification photomicrographs of gills (b and insert), hematopoietic nodule (c) and ventral nerve cord (d) showing examples of IHHNV CAIs; and (e–g). IHHNV-infected cell nuclei, some with distinct CIAs, are stained dark blue–black with the IHHNV DIG-labeled probe in the gills (e), hematopoietic nodule (f), and ventral nerve cord of a L. vannamei (g). cytoplasmic accumulations of IHHNV are apparent in the ventral nerve cord (g) and in developing oocytes in the ovary of a L. vannamei (h) where IHHNV appears to be being deposited as yolk. This may be a mechanism by which IHHNV is transmitted vertically. Mayer–Bennett H&E and DIG-labeled probe with Bismarck Brown counter stain.
A few years after it was reported that L. vannamei could be infected with IHHNV and not cause significant mortalities (Lightner et al., 1983b, Bell and Lightner, 1984), IHHNV was shown to be the cause of ‘runt deformity syndrome’ (RDS) in L. vannamei (Kalagayan et al., 1991). With RDS, affected shrimp present irregular, reduced growth and cuticular deformities and generally no remarkable elevation in mortality (Fig. 3c–f) (Kalagayan et al., 1991, Browdy et al., 1993, Bray et al., 1994, Brock and Main, 1994, Lightner, 1996a). Hence, the economic and production impacts of IHHNV infection in L. vannamei are due to reduced and irregular growth and small count size shrimp at harvest and not to elevated mortality (OIE, 2006b). To mitigate this effect, several strategies have been used. With one strategy, selected lines of L. stylirostris were developed that were not only resistant to IHHN disease but also refractory to infection (Tang et al., 2000, Dhar et al., 2001). IHHNV-free lines of L. vannamei were also developed as SPF (specific pathogen-free) lines and these stocks were the first developed in the SPF stock development program (Wyban et al., 1992, Pruder et al., 1995, Moss et al., 2002).
Fig. 3.

IHHN gross signs. (a) A moribund small juvenile Litopenaeus stylirostris with severe acute IHHN disease. Small buff-colored spots are apparent under the cuticle of the carapace and abdominal tergal plates; (b) large moribund juvenile L. stylirostris with acute IHHN. Mottling in the cuticle is readily apparent in the center shrimp. The spots fade as the disease progresses and shrimp of this species in the final stages of the disease often bluish in color and may present necrotic, melanized edges of the cuticle; (c and d) subadult Litopenaeus vannamei with bent (to the left) rostrums, a classic sign of ‘runt deformity syndrome’ (RDS); (e) a juvenile L. vannamei with RDS. In this specimen the rostrum is bent to the right and the antennal flagella are wrinkled, brittle and mostly broken-off; and (f) juvenile L. vannamei with RDS from a nursery population at ∼day 60 post stocking. The principal effect of RDS shown is the marked disparity in sized of shrimp of the same age.
After its initial discovery in cultured shrimp in Hawaii in 1981, IHHNV was subsequently found to be widely distributed in cultured shrimp in the Americas and in wild shrimp collected along the Pacific coast from Peru to Mexico. As of 2006, the only country in the Americas, which can claim to have IHHNV-free zones or compartments (OIE, 2006a), is the United States. This was achieved with the development and use of SPF shrimp stocks (Pruder et al., 1995). The introduction of IHHNV into shrimp farms in northwestern Mexico and wild shrimp stocks in Mexico’s Gulf of California during the late 1980s and early 1990s resulted not only in significant losses in farmed L. stylirostris, but also in a collapse in 1990 of the wild fishery for L. stylirostris in the northern Gulf of California (Lightner et al., 1992, Martinez-Cordova, 1992, Lightner, 1996b, Pantoja et al., 1999, Morales-Covarrubias and Chavez-Sanchez, 1999, Morales-Covarrubias et al., 1999). A decade later, the L. stylirostris fishery of the northern Gulf of California had recovered sufficiently to support commercial fishing, but the prevalence of IHHNV infection in adult L. stylirostris collected from the northern Gulf fishery remained high (80–100% females and 60% in males) (Morales-Covarrubias et al., 1999, Morales-Covarrubias and Chavez-Sanchez, 1999).
IHHNV has been found to be widely distributed in wild and cultured Penaeus monodon in east and SE Asia where it does not seem to cause production losses (Flegel, 1997, Flegel, 2006, Primavera and Quinitio, 2000, Tang et al., 2003, Chayanburakul et al., 2005, Withyachymnarnkul et al., 2006). Molecular studies show considerable variation among Asian isolates of the virus (Tang et al., 2003, Krabsetsve et al., 2004, Tang and Lightner, 2006), while little variation was found in isolates from the Americas (Tang and Lightner, 2002). All isolates of IHHNV from the Americas are nearly identical with IHHNV from the Philippines. This finding, along with other aspects of its history and epidemiology of IHHN in the Americas, suggests that IHHNV was introduced from the Philippines, perhaps with live P. monodon that were imported in the early 1970s as a candidate aquaculture species during the very early development of shrimp farming in the Americas (Lightner, 1996b, Tang and Lightner, 2002, Tang et al., 2003).
In addition to the Americas/Philippines genotype of IHHNV, three other genetic variants of the virus have been documented. For the purposes of this review, the IHHNV genotype from the Americas/Philippines genotype will be designated as IHHNV-I; the variant from south-east Asia will be designated IHHNV-II; and the IHHNV variant from East Africa, Madagascar and Mauritius and from Australia will be referred to as IHHNV-III. The first two genotypes (IHHNV-I and IHHNV-II) are infectious to the representative penaeids, L. vannamei and P. monodon, while the latter genetic variant has been demonstrated to be not infectious to these species (Tang et al., 2003, Krabsetsve et al., 2004, Tang and Lightner, 2006). The apparent reason for the lack of infectivity of the IHHNV-III genotype was recently explained by the discovery that the DNA fragment represented by IHHNV-III is incorporated into the genome of some genetically distinct populations of P. monodon in the Indo-Pacific region (Duda and Palumbi, 1999, Tang and Lightner, 2006, Tang et al., 2007).
2.3. IHHN gross signs in L. stylirostris
IHHNV often causes an acute disease with very high mortalities in juveniles of this species. Vertically infected larvae and early PL do not become diseased, but in approximately 35-day-old or older juveniles, gross signs of the disease may be observed, followed by mass mortalities (Fig. 3a and b). In horizontally infected juveniles, the incubation period and severity of the disease is somewhat size and/or age dependent, with young juveniles always being the most severely affected. Infected adults seldom show signs of the disease or mortalities (Bell and Lightner, 1984, Bell and Lightner, 1987, Brock and Lightner, 1990, Lightner, 1993, Lightner, 1996a). Gross signs are not IHHN specific, but juvenile L. stylirostris with acute IHHN show a marked reduction in food consumption, followed by changes in behavior and appearance. Shrimp of this species with acute IHHN have been observed to rise slowly in culture tanks to the water surface, there to become motionless and then to roll-over and slowly sink (ventral side up) to the tank bottom. Shrimp exhibiting this behavior may repeat the process for several hours until they become too weak to continue, or until they are attacked and cannibalized by their healthier siblings. L. stylirostris at this stage of infection often have white or buff-colored spots (which differ in appearance and location from the white spots that sometimes occur in shrimp with WSSV infections) in the cuticular epidermis, especially at the junction of the tergal plates of the abdomen, giving such shrimp a mottled appearance (Fig. 3a–b). This mottling later fades in moribund L. stylirostris as such individuals become more bluish. In L. stylirostris and in P. monodon with terminal phase IHHNV infections, moribund shrimp are often distinctly bluish in color, with opaque abdominal musculature (Lightner et al., 1983a, Lightner et al., 1983b, Brock and Lightner, 1990, Lightner, 1996a).
2.4. IHHN gross signs in L. vannamei
The chronic disease, RDS, occurs in this species as a result of IHHNV infection. The severity and prevalence of RDS in infected populations of juvenile or older L. vannamei may be related to infection during the larval or early PL stages (Kalagayan et al., 1991, Motte et al., 2003). RDS has also been reported in cultured stocks of L. stylirostris and P. monodon (Lightner, 1996a, Primavera and Quinitio, 2000). Juvenile shrimp with RDS may display a bent (45–90° bend to left or right) or otherwise deformed rostrum, a deformed 6th abdominal segment, wrinkled antennal flagella, cuticular roughness, ‘bubble-heads’, and other cuticular deformities. Populations of juvenile shrimp with RDS display disparate growth with a wide distribution of sizes and many smaller than expected (‘runted’) shrimp. The coefficient of variation (CV = the standard deviation divided by the mean of different size groups within a population) for populations with RDS is typically greater than 30% and may approach 90%, while IHHNV-free (and thus RDS-free) populations of juvenile L. vannamei and L. stylirostris usually show CVs of 10–30% (Kalagayan et al., 1991, Browdy et al., 1993, Brock and Main, 1994, Carr et al., 1996, Lightner, 1996a, Motte et al., 2003).
2.5. Histopathology of IHHNV infections
Histological demonstration of prominent intranuclear, Cowdry type A inclusion bodies provides a provisional diagnosis of IHHNV infection (Fig. 4a–d). These characteristic IHHN inclusion bodies are eosinophilic and often appear as haloed (with hematoxylin and eosin stains of tissues preserved with fixatives that contain acetic acid, such as Davidson’s AFA and Bouin’s solution), intranuclear inclusion bodies within chromatin-marginated, hypertrophied nuclei of cells in tissues of ectodermal (epidermis, hypodermal epithelium of fore- and hindgut, nerve cord and nerve ganglia) and mesodermal origin (hematopoietic organs, antennal gland, gonads, lymphoid organ, and connective tissue) (Bell and Lightner, 1988, Lightner, 1996a, OIE, 2006b). Intranuclear inclusion bodies due to IHHNV may be easily confused with developing intranuclear inclusion bodies due to WSSV infection (compare Fig. 4a–d to Fig. 10a–d). In-situ hybridization assay of such sections with a specific DNA probe to IHHNV provides a definitive diagnosis of IHHNV infection (Fig. 4e–h) (Lightner, 1996a, Carr et al., 1996, OIE, 2006b).
Fig. 10.
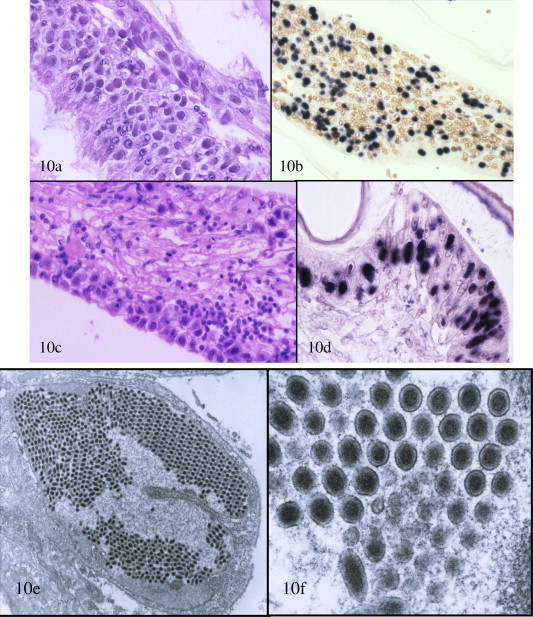
Histopathology of white spot disease (WSD) shown in H&E stained sections (a and c) and by ISH with a WSSV-specific DIG-labeled probe (b and d). Mayer–Bennett H&E and DIG-labeled probe with Bismarck Brown counter stain; (a–d) photomicrographs of sections through the cuticular epithelium and subcuticular connective tissues of a small juvenile Litopenaeus vannamei with severe acute WSD. With H&E, haloed and eosinophilic (early or Cowdry type A) to darkly basophilic (mature) intranuclear inclusion bodies are abundant, and with ISH the inclusion bodies are generally dark blue–black due to reaction of WSSV with the DIG-labeled probe; and (e and f) TEM of a WSSV infected cell in the cuticular epithelium of a juvenile L. vannamei. The reason for the intense ISH-positive reaction in (b) and (d) for WSSV is evident due to the large number of WSSV virions present in the hypertrophied nucleus. Most of the virions are sectioned across their long axis and only a very few show the elliptical morphology of the intact virion. In (f), the dense central nucleocapsid and outer envelope of the virions is readily apparent.
2.6. Antibody-based and ISH diagnostic methods for IHHNV
Although monoclonal antibodies to IHHNV have been reported, no antibody-based antigen detection methods for IHHNV have been successfully developed (Poulos et al., 1994, Lightner and Redman, 1998a, OIE, 2006b). However, molecular techniques using dot-blot and in-situ hybridization (ISH) tests for IHHNV have been developed and a number of methods and commercial products using these methods are available (OIE, 2006b). Non-radioactive digoxigenin-11-dUTP (DIG-11-dUTP)-labeled gene probes for IHHNV detection in dot-blot and ISH formats were the first developed for use in shrimp disease diagnostic applications (Mari et al., 1993). These DIG-labeled probes made the method safe and applicable to most laboratory and field situations (Carr et al., 1996, Lightner, 1996a, OIE, 2006b). DNA probes for dot-blot and ISH applications and PCR methods provide greater diagnostic sensitivity than do more traditional techniques for IHHN diagnosis that employ classic histological approaches. Furthermore, these methods have the added advantage of being applicable to nonlethal testing of valuable broodstock shrimp. A hemolymph sample may be taken with a tuberculin syringe, or an appendage (a pleopod for example) may be biopsied, and used as the sample for a direct dot-blot test (Bell et al., 1990, Carr et al., 1996, OIE, 2006b).
2.7. PCR diagnostic methods for IHHNV
Several single step, nested and real-time PCR methods are available for IHHNV detection (Lightner and Redman, 1998a, Nunan et al., 2000, Tang and Lightner, 2001, Tang and Lightner, 2002, Flegel, 2006, OIE, 2006b). These methods are applicable to routine diagnostic applications in disease situations and for use in surveillance and screening of shrimp stocks or populations to verify their health/disease status. PCR methods have been used to develop, maintain and document the SPF (specific pathogen-free) status of L. vannamei lines developed in the United States for use by the domestic and international shrimp farming industries (Wyban et al., 1992, Pruder et al., 1995, OIE, 2006b). A number of commercial PCR kits are available for IHHNV detection. There are multiple geographical variants of IHHNV, some of which are not distinguishable by all of the available methods for IHHNV (Tang and Lightner, 2002, Tang and Lightner, 2006, Tang et al., 2003). Several primers sets have shown utility in routine use. Among these are 70012F/77353R, 389F/R and 392F/R, and these appear to be the suitable for detecting all the known genetic variants of IHHNV. However, these primer sets do not distinguish infectious IHHNV from a non-infectious variant of IHHNV that is incorporated in the genome of some stocks of P. monodon from the Indian Ocean. Two additional primer sets, MG831F/R and IHHNV 309F/R, respectively, were developed to distinguish the shrimp genome associated variant of IHHNV from the infections free-virus variants (Table 3 ) (Tang and Lightner, 2006, Tang et al., 2007). The lessons learned from applying PCR detection methods to IHHNV and the resulting false positive tests for the virus in some stocks of P. monodon underscore the importance of confirming of unexpected positive and/or negative PCR results for IHHNV with a second primer set, or use of another diagnostic method (i.e. real-time PCR, bioassay, ISH).
Table 3.
Primer sequences used for PCR detection of IHHNV.
| Primer designation | Product | Sequence | Application | Reference |
|---|---|---|---|---|
| 389F | 389 bp | 5′-CGG-AAC-ACA-ACC-CGA-CTT-TA-3′ | All genetic variants | GenBank AF218266 |
| 389R | 5′-GGC-CAA-GAC-CAA-AAT-ACG-AA-3′ | |||
| 77012F | 356 bp | 5′-ATC-GGT-GCA-CTA-CTC-GGA-3′ | All genetic variants | GenBank AF218266 |
| 77353R | 5′-TCG-TAC-TGG-CTG-TTC-ATC-3′ | |||
| 392F | 392 bp | 5′-GGG-CGA-ACC-AGA-ATC-ACT-TA-3′ | All genetic variants | Tang et al. (2000) |
| 392R | 5′-ATC-CGG-AGG-AAT-CTG-ATG-TG-3′ | |||
| 309F | 309 bp | 5′-TCCAACACTTAGTCAAAACCAA -3′ | Infectious virus only | Tang and Lightner (2006) |
| 309R | 5′-TGTCTGCTACGATGATTATCCA-3′ | |||
| 831F | 831 bp | 5′-TTGGGGATGCAGCAATATCT-3′ | IHHNV-realted sequence in P. monodon genome | Tang and Lightner (2006) |
| 831R | 5′-GTCCATCCACTGATCGGACT-3′ | |||
3. Taura syndrome virus (TSV)
3.1. Biology of the agent
TSV is a small, simple RNA virus. The virion is a 32 nm diameter, nonenveloped icosahedron with a buoyant density of 1.338 g/ml (Table 1; Fig. 2, Fig. 5 ). The genome of TSV consists of a linear, positive sense single-stranded RNA of 10,205 nucleotides, excluding the 3′ poly-A tail, and it contains two large open reading frames (ORFs). ORF 1 contains the sequence motifs for nonstructural proteins, such as helicase, protease and RNA-dependent RNA polymerase. ORF 2 contains the sequences for TSV structural proteins, including the three major capsid proteins VP1, VP2 and VP3 (55, 40, and 24 kDa, respectively) (Bonami et al., 1997, Mari et al., 1998, Mari et al., 2002, Robles-Sikisaka et al., 2001). The virus replicates in the cytoplasm of host cells. Based on its characteristics, TSV has been assigned by the International Committee on Taxonomy of Viruses (ICTV) to the newly created genus Cripavirus in new family Dicistroviridae (in the “superfamily” of picornaviruses) (Mayo, 2002a, Mayo, 2002b).
Fig. 5.
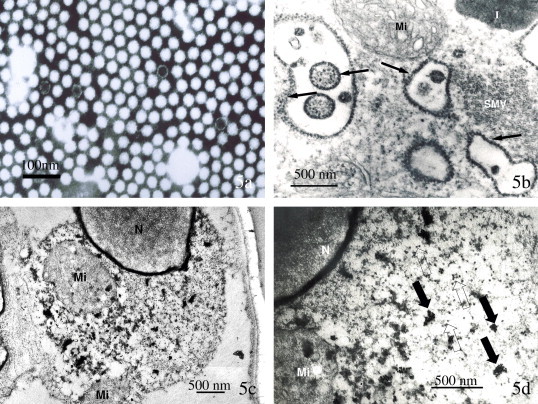
Transmission electron micrographs of Taura syndrome virus (TSV) from Litopenaeus vannamei. (a) Purified TSV from cesium chloride gradient ultracentrifugation. Most of the hexagonal appearing (in profile) icosahedral particles are full, but several particles lack the viral genome and show only empty capsids. Stain: 2% PTA; (b–d) Ultrastructural changes in TSV-infected cells; (b) The cytosol of an infected cell showing accumulations of putative TSV particles (arrows point to examples) associated with membranes of the rough endoplasmic reticulum (RER), masses of small membranous vesicles (SMV) and a distended mitochondria (Mi); (c and d) Detection of TSV by TEM ISH in water soluble resin-embedded tissues. Accumulations of immuno-gold particles are shown reacting to TSV particles in (c) and (d). Solid arrows in (d) point to clumps of immuno-gold particles marking TSV particles, while hollow arrows point to unmarked virions. Of interest is the dense accumulation of immuno-gold particles within the nuclear membrane. Higher magnifications of this area in this and other cells do not show discrete TSV. Hence, the probe may be reacting to free or un-encapsulated ssRNA of TSV.
Physicochemical and more recent molecular studies of TSV suggest that a single strain of the virus was present in the initial TSV pandemic in the Americas, but that new strains are emerging which differ in host range and virulence (Yu and Song, 2000, Zarain-Herzberg and Ascencio-Valle, 2001, Erickson et al., 2002, Chang et al., 2004, Tang and Lightner, 2005, Nielsen et al., 2005). Curiously, the strain of TSV in the early diagnostic cases from the Americas reacts with the monoclonal antibody MAb1A1, but all of the more recently emerged genetic variants of TSV from the Americas do not react with the antibody in Western blot transfers or in IHC (immuno-histochemistry) preparations with paraffin embedded TSV-infected tissues (Erickson et al., 2002, Erickson et al., 2005). Comparison of the cDNA sequence of TSV CP2 from approximately 50 TSV isolates in the author’s collection shows that there are currently four distinct strains (or genetic variants) of the virus (Fig. 8 ) (Tang and Lightner, 2005). While MAb 1A1 reacts with most of the isolates in the clade from the Americas, it does not react with some Mexican isolates in the Americas clade, nor with any of the members of the Belize or Venezuelan clades.
Fig. 8.
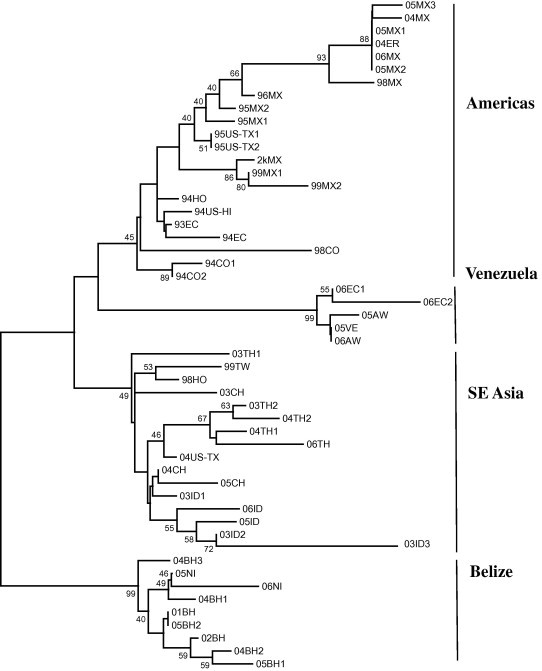
Phylogenetic neighbor-joining (NJ) tree of capsid protein 2 (CP2, 383 amino acids) from 52 TSV isolates. Numbers on branches represent bootstrap values (%) after 1000 replicates. Note that the isolates cluster into four distinct lineages or putative strains or genotypes of the virus.
3.2. History, hosts, and geographic distribution of Taura syndrome (TS)
TSV emerged from an unknown source in Ecuador in 1991. The disease was recognized as a major new disease of farmed L. vannamei by early 1992 and it was named ‘Taura syndrome’ (Jimenez, 1992, Lightner, 1996a, Lightner, 2003b, Brock, 1997, Lightner et al., 1995). The viral etiology of TS was confirmed in 1994 and the virus was named Taura syndrome virus (TSV) (Hasson et al., 1995). In the interest of supporting litigation brought by a group of Ecuadorian shrimp farmers against several international pesticide companies, whose products had been implicated as the cause of a toxicity syndrome they called ‘Taura syndrome,’ Intriago et al. (1997) and Jimenez et al. (2000) reported on the epizootiology of the disease in Ecuador, and they suggested that TSV be assigned the synonym ‘infectious cuticular epithelial necrosis virus (ICENV)’ to distinguish it from the ‘Taura syndrome’ with a putative toxic etiology (Jimenez et al., 2000).
The principal host for TSV is the Pacific white shrimp, L. vannamei, although other species can be infected and present disease (Aguirre Guzman and Valle, 2000, Hasson et al., 1995, Hasson et al., 1999b, Lightner, 1999, Overstreet et al., 1997, Robles-Sikisaka et al., 2001, Srisuvan et al., 2005). Cumulative mortalities due to TSV epizootics have ranged from 40 to >90% in cultured populations of postlarval (PL), juvenile, and subadult L. vannamei. TS is best known as a disease of nursery- or grow-out-phase L. vannamei that occurs within ∼14 to 40 days after stocking postlarvae into grow-out ponds or tanks, hence, shrimp with TS are typically small juveniles of from ∼0.05 g to <5 g. Larger shrimp may also be affected, especially if they are not exposed to the virus until they are larger juveniles or subadults. Survivors of TSV infections may carry the virus for life (Brock et al., 1995, Brock et al., 1997, Hasson et al., 1999b, Lightner, 1996a, Lightner, 1996b, Lotz, 1997b). In regions where the virus is enzootic in farmed stocks, the prevalence of chronic phase TSV infections has been found in various surveys to range from 0% to 100% (Brock, 1997, Jimenez et al., 2000, Laramore, 1997). TSV can also infect other Western Hemisphere penaeid species (i.e. L. stylirostris, L. setiferus, and L. schmitti), sometimes resulting in disease and mortalities in PL or early juvenile stages, but also in asymptomatic persistent infections (Brock et al., 1997, Overstreet et al., 1997). Other Western Hemisphere penaeids (Farfantepenaeus aztecus and Fa. duorarum) and Eastern Hemisphere penaeids (Fenneropenaeus chinensis, P. monodon, and Marsupenaeus japonicus) have been experimentally infected with TSV, but appear not to develop clinical disease (Brock et al., 1997, Overstreet et al., 1997, Flegel, 2006).
Transmission of TSV may be by horizontal or vertical routes. Horizontal transmission by cannibalism or by contaminated water has been demonstrated (Brock, 1997, Hasson et al., 1995, Lightner, 1996a, Lightner, 1996b, Lotz, 1997b, Overstreet et al., 1997, White et al., 2002). Vertical transmission from infected adult broodstock to their offspring is strongly suspected but has not been experimentally confirmed (Lightner, 1996a, Lightner, 2003a, Lightner, 2003b).
TSV was reported to infect human and monkey cell lines, suggesting a zoonotic potential for this virus and that penaeid shrimp could serve as reservoirs for TSV and other members of the ‘picornavirus superfamily’ that infect humans (Audelo del Valle et al., 2003). Because of the experimental design and the improbable results reported by these authors, two other laboratories repeated the study and both found that TSV does not infect or replicate in primate or human cell lines with known susceptibility to human picornaviruses (Luo et al., 2004, Pantoja et al., 2004), effectively refuting the contention that TSV has zoonotic potential.
By 1994, when the viral etiology of TS had been established, the virus had been moved with live shrimp transfers to many of the shrimp growing countries of the Americas (Brock et al., 1995, Hasson et al., 1995, Hasson et al., 1999a, Bonami et al., 1997, Lightner, 1996a, Lightner, 1996b, Lightner, 2003a). While wild postlarvae with TSV infections were reported as being found near shrimp farms with ongoing TSV epizootics (Lightner et al., 1995), TSV infections in wild shrimp have not been further documented, suggesting that TSV does not have a discernable impact on wild populations of shrimp (Brock, 1997). In 1998, TSV was documented in Taiwan in infected stocks of L. vannamei, introduced for aquaculture purposes (Tu et al., 1999, Yu and Song, 2000). In addition to being moved from country to country with live shrimp, there is also evidence that TSV has the potential of being transferred in frozen TSV-infected shrimp products. TSV has been found in frozen commodity shrimp (L. vannamei) products in samples from markets in the USA that originated in Latin America and south-east Asia. Improper disposal of wastes (liquid and solid, i.e. peeled shells, heads, intestinal tracts, etc.) from value-added reprocessing of TSV-infected shrimp at coastal locations may provide a source of TSV that may contaminate wild or farmed stocks near the point of the waste stream discharge (Lightner, 1996b, Nunan et al., 2004, OIE, 2006b).
Shrimp-eating birds and insects may be important factors in the transmission of TSV within shrimp farms and among shrimp farms within a geographical zone or region (OIE, 2006b). TSV has been demonstrated to remain infectious in the feces of sea gulls that have ingested infected shrimp carcasses (Garza et al., 1997, Lightner, 1999). The virus was demonstrated to remain infectious for up to 48 h (after ingestion of TSV-infected shrimp carcasses) in the feces passed by wild or captive sea gulls (Larus atricilla) and chickens (Gallus domesticus, used as a laboratory surrogate for all shrimp-eating birds). These findings implicate birds as being an important mechanical vector for the transmission of the virus within affected farms or farming regions (Vanpatten et al., 2004). There is some evidence that flying aquatic insects such as the water boatman (Trichocorixa reticulata [Corixidae]) that feed on shrimp carcasses can also serve as mechanical vectors of TSV (Brock, 1997, Lightner, 1995, Lightner, 1996b).
3.3. Taura syndrome gross signs in susceptible host species
Gross signs of Taura syndrome have been documented in all life stages (i.e. postlarvae, juveniles and adults) of L. vannamei except in eggs, zygotes and larvae (Brock and Main, 1994, Lightner, 1996a). Following experimental or natural infection, Taura syndrome has three distinct phases in the disease: acute, transition and chronic (Brock, 1997, Hasson et al., 1999b) (Fig. 6). In disease outbreaks at farms, the onset of mortality is often sudden and massive with moribund shrimp coming to the pond surface or edges where large numbers of shrimp-eating birds may be attracted to the dead and dying shrimp. Moribund shrimp in the acute phase of the disease typically present a pink to reddish coloration due to expansion of red cuticular chromatophores (especially in the tail fan), are off feed and with empty guts, and they are lethargic (Fig. 6a). The acute phase is rapid in individual shrimp, probably lasting less than 24 h, but the acute phase in a shrimp farm pond may last for several days in an affected population (Brock, 1997, Garza et al., 1997, Hasson et al., 1999b). As implied by its name, the transition (or recovery in some publications) phase of Taura syndrome is that phase of the disease when affected shrimp may resolve the lesions due to TSV infection that developed in the acute phase. Shrimp in the transition phase typically present randomly distributed variably sized melanized lesions in or under the cuticle (exoskeleton) (Fig. 6b). Those shrimp that successfully resolve the acute-phase TSV lesions and survive the next molting process, typically appear normal. Death due to TSV infection during the three phases most often occurs in the acute phase, probably due to osmotic failure. In the transition phase of the disease, death may also occur due to osmotic failure as a consequence of widespread destruction of the cuticular epithelium or by systemic infections by opportunistic bacteria (Lightner, 1996a, Brock, 1997). Shrimp in the chronic phase of Taura syndrome may carry the virus for life as a persistent infection (Lotz, 1997b, Hasson et al., 1999b). While persistently infected L. vannamei may appear and behave normally, they show slightly less tolerance to low salinity stress than uninfected shrimp (Lotz et al., 2005). Some members of populations of L. vannamei or L. stylirostris that survive TSV infections and/or epizootics may carry the virus for life and, although not documented, pass the virus onto their progeny by vertical transmission (Hasson et al., 1999a, Hasson et al., 1999b, OIE, 2006b).
Fig. 6.
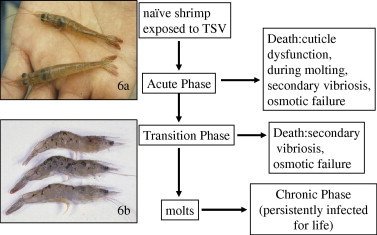
Taura syndrome (TS) infection cycle in Litopenaeus vannamei. (a) Two moribund juvenile shrimp in the acute phase of TS disease. Both shrimp present a soft cuticle, empty guts, extreme lethargy, and expansion of reddish chromatophores, especially in the tail fan and (b) three juvenile shrimp in the transition phase of TS. Random melanized foci, which represent hemocytic inflammation of areas of necrosis of the cuticular epithelium from the acute phase of the disease.
3.4. Histopathology of TSV infections
During the acute phase of TSV infections, the virus infects and replicates primarily in the cuticular epithelium (or hypodermis) of the general exoskeleton, foregut, hindgut, gills and appendages (Fig. 7a–d), and often in the connective tissues, the hematopoietic tissues, and the antennal gland. The enteric organs (endoderm-derived hepatopancreas, midgut and midgut caeca mucosal epithelia) and smooth, cardiac, striated muscle, and the ventral nerve cord, its branches and its ganglia typically show no histological signs of infection by TSV and are usually negative for TSV by ISH (Lightner et al., 1995, Lightner, 1996a, Lightner, 1996b, Lightner, 2003a, Brock, 1997, OIE, 2006b). In H&E stained preparations, acute-phase TSV lesions (Fig. 7a–d) are presented as multifocal areas of necrosis in the cuticular epithelium of the general body surface, appendages, gills, hindgut, and foregut (the esophagus, anterior and posterior chambers of the stomach). Prominent in the multifocal cuticular lesions are conspicuous foci of affected cells that display an increased eosinophilia of the cytoplasm and pyknotic or karyorrhectic nuclei. Cytoplasmic remnants of necrotic cells are often abundant in these TS acute-phase lesions and these are generally presented as spherical bodies (1–20 μm in diameter) that range in staining from eosinophilic to pale basophilic. These structures, along with pyknotic and karyorrhectic nuclei, give acute-phase TS lesions a characteristic ‘peppered’ or ‘buckshot-riddled’ appearance, which is considered to be pathognomonic for TS disease when there is no concurrent necrosis of the parenchymal cells of the lymphoid organ tubules (Fig. 7a–b). The absence of necrosis of the lymphoid organ in acute-phase TSV infections distinguishes TS disease from acute phase yellow head disease in which similar patterns of necrosis to those induced by TSV may occur in the cuticular epithelium and gills (Lightner, 1996a, Bondad-Reantaso et al., 2001, OIE, 2006b). In TSV-infected tissues, pyknotic or karyorrhectic nuclei give a positive (for DNA) Feulgen reaction, which distinguishes them from the less basophilic to eosinophilic cytoplasmic inclusions that do not contain DNA. The absence of hemocytic infiltration or other signs of a significant host-inflammatory response distinguishes the acute phase of TS from the transitional phase of the disease (Lightner, 1996a, Brock, 1997, Hasson et al., 1999a, Hasson et al., 1999b, OIE, 2006b).
Fig. 7.
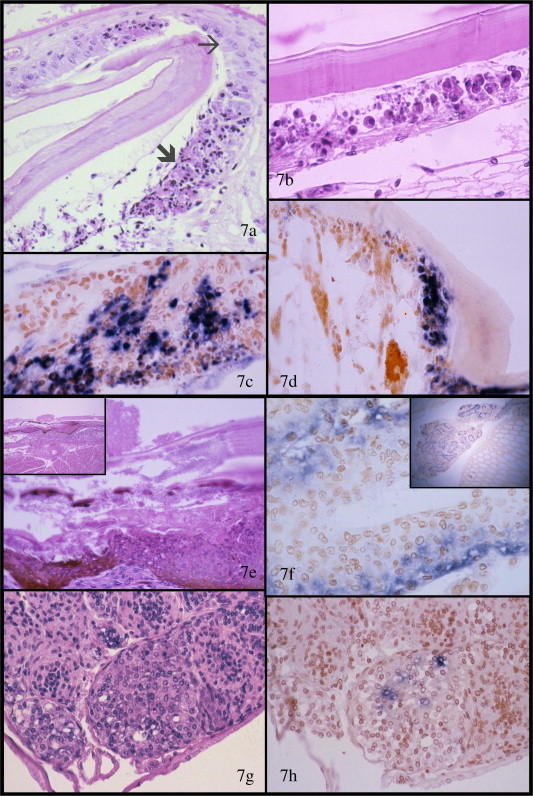
Histopathology of Taura syndrome (TS) is shown in H&E stained sections (a, b, e, and g) and by ISH with a TSV-specific DIG-labeled probe (c, d, f and h). Mayer–Bennett H&E and DIG-labeled probe with Bismarck Brown counterstain; (a–d) photomicrographs of sections through the cuticular epithelium of small juvenile Litopenaeus vannamei with severe acute TS. Prominent foci of necrosis of the cuticular epithelium (thick arrow in a) are shown between normal appearing patches of cuticular epithelium (thin arrow in a); (b–d) higher magnification views of TSV lesions (by H&E and ISH) in the cuticular epithelium showing characteristic necrotic cuticular epithelial with pyknotic and karyorrhectic nuclei, a generally increased cytoplasmic eosinophilia in intact cells, and very numerous, variably staining, spherical remnants of cytoplasm from disrupted cells. The cytoplasmic remnants, pyknotic and karyorrhectic nuclei give the lesion a pathodiagnostic “peppered” or “buckshot-riddled” appearance. The acute nature of the lesion is suggested by the absence of hemocytes in or near the lesion. Demonstration of TSV by ISH with a DIG-labeled probe is shown in (c), (d), (f), and (h). In (c) and (d), TSV is shown in the cytoplasm of still intact cuticular epithelial cells in areas presenting the “buckshot-riddled” appearance that is pathognonomic for TSV acute-phase lesions; (e and insert) low and higher magnification views of a resolving TSV lesion in the cuticular epithelium. The former TS lesion is now in the “wound-healing” process and is marked by multiple layers of melanized and un-melanized hemocytes which have closed the lesion and are providing a foundation for ingrowth of fibrocytes and normal cuticular epithelium from unaffected adjacent areas. A surface plaque of bacteria is shown colonizing the apical surface of the resolving lesion; (f and insert) ISH preparation of the lymphoid organ in which the distal parenchymal cells of the lymphoid organ are accumulating large amounts TSV from the circulating hemolymph during the late acute or transition phase of TS; (g and h) parallel sections (H&E and ISH) of the lymphoid organ (LO) of an experimentally infected L. vannamei in the chronic phase of TS several months after infection. With H&E LO spheroids (LOS) are the only lesion apparent in shrimp in the chronic phase of TS, but the parallel section reacted in ISH with a TSV-specific probe shows the presence of the TSV-infected cells in these LOS. Mayer–Bennett H&E and DIG-labeled probe with Bismarck Brown counter-stain.
In the transition (recovery) phase of TS, which can last several days in individual shrimp and a few days to a week or more in infected populations in ponds, typical acute-phase cuticular lesions decline in abundance and severity and are replaced by conspicuous infiltration and accumulation of hemocytes at the sites of necrosis (Fig. 7e). The masses of hemocytes may become melanized, giving rise to the irregular black spots that characterize the transition phase of the disease (Fig. 6b). In H&E sections, such lesions may show erosion of the cuticle, surface colonization and invasion of the affected cuticle and exposed surface hemocytes by Vibrio spp. (Fig. 7e) (Lightner, 1996a, Hasson et al., 1999b, OIE, 2006b). Sections of the lymphoid organ (LO) during the transition phase of TS may appear normal with H&E staining. However, when sections of the LO are assayed for TSV by ISH with a specific cDNA probe (or by IHC with MAb 1A1 for TSV type A, genotype 1), large quantities of TSV are shown being cleared and concentrated from the hemolymph in the more peripheral parenchymal cells of the LO tubules (Fig. 7f) (Hasson et al., 1999b, Srisuvan et al., 2005).
Shrimp in the chronic phase of TS display no gross signs of infection, and with routine H&E histology the only sign of infection is the presence of numerous prominent lymphoid organ spheroids (LOS), which may remain associated with the main body of the paired LO, or which may detach and become ectopic LOS bodies that lodge in constricted areas of the hemocoel (i.e. the heart, gills, in the subcuticular connectives tissues, etc.). Such LOS are accumulations of LO cells and hemocytes and may be distinguished from normal LO tissues by their spherical nature and the lack of the central vessel that is typical for normal lymphoid organ tubules (Fig. 7g–h) (Anggraeni and Owens, 1998, Hasson et al., 1999b, Srisuvan et al., 2006). When assayed by ISH with a cDNA probe for TSV (or with MAB 1A1 using IHC) some cells in the LOS give positive reactions for the virus, with most of the ISH-positive cells occurring in the spheroids, while no other target tissues react (Fig. 7h) (Hasson et al., 1999b, Srisuvan et al., 2006).
3.5. Molecular diagnostic methods
ISH and RT-PCR tests for TSV have been developed and varieties of these methods are commercially available. A dot-blot method for TSV detection has not been developed. Non-radioactive, DIG-labeled cDNA probes for TSV may be produced in the laboratory. The ISH method provides greater diagnostic sensitivity than does more traditional methods for TSV detection and diagnosis that employ classic histological methods (Mari et al., 1998, Hasson et al., 1999a, Hasson et al., 1999b, Lightner, 1996a, Lightner, 1999, Lightner and Redman, 1998a). The ISH assay of routine histological sections of acute- and transition-phase lesions in the cuticular epithelium, other tissues, and of LOS in transition and chronic phase with a specific DIG-labeled cDNA probe to TSV provides a definitive diagnosis of TSV infection (OIE, 2006b). Pathognomonic TSV-positive lesions display prominent blue to blue-black areas in the cytoplasm of affected cells when reacted with the cDNA probes (Fig. 7c, d, f and h). Not reacting to the probe are the prominent karyorrhectic nuclear fragments and pyknotic nuclei that contribute to the pathognomonic ‘buckshot-riddled’ appearance of TS lesions (compare Fig. 7a and b with Fig. 7c and d). False-negative ISH results may occur with Davidson’s fixed tissues if tissues are left in fixative for more than 24–48 h (Hasson et al., 1997, OIE, 2006b). The low pH of Davidson’s fixative causes acid hydrolysis of the TSV single-stranded RNA genome, resulting in false-negative probe results. This artifact can be avoided through the use of neutral fixatives, including a’RNA-friendly’ fixative developed for shrimp, or by the proper use (avoiding fixation times over 24 h) of Davidson’s fixative (Bell and Lightner, 1988, Hasson et al., 1997, Lightner, 1996b, Lightner and Redman, 1998a).
DNA amplification methods have become the standard for surveillance and SPF status documentation. Numerous one step and nested reverse transcriptase (RT-PCR) methods for detection of TSV are available, and some are commercially available (Mari et al., 1998, Nunan et al., 1998a, Nunan et al., 2004, Chang et al., 2004, Nielsen et al., 2005, Phalitakul et al., 2006, OIE, 2006b). Tissue samples (hemolymph, pleopods, whole small shrimp, etc.) may be assayed for TSV using RT-PCR. Primers designated as 9195 and 9992, amplify a 231 base pair (bp) sequence of the TSV genome (Nunan et al., 1998a. While not as sensitive as nested RT-PCR or real-time RT-PCR methods, the single step method developed by Nunan et al. (1998a) detects all of the known genetic variants of the virus (Table 4, Table 5 ) and is the method currently recommended for surveillance applications by the OIE (OIE, 2006b). The Nunan method detects the known TSV genomic variants because the cDNA fragment amplified is from a conserved sequence that is located in the intergenic region and which extends into ORF 2 of TSV.
Table 4.
Year of collection and origin of the TSV isolates collected from penaeid species.
| TSV isolate | Year | Origin | TSV isolate | Year | Origin |
|---|---|---|---|---|---|
| 93EC | 1993 | Ecuador | 04TH1 | 2004 | Thailand |
| 94CO1 | 1994 | Colombia | 04TH2b,8 | 2004 | Thailand |
| 94CO2 | 1994 | Colombia | 04US-TX | 2004 | US Texas |
| 94USHI1 | 1994 | US-Hawaii | 04MX | 2004 | Mexico |
| 94EC | 1994 | Ecuador | 04BH1 | 2004 | Belize |
| 94HO | 1994 | Honduras | 04BH2 | 2004 | Belize |
| 95MX1 | 1995 | Mexico | 04BH3 | 2004 | Belize |
| 95MX2 | 1995 | Mexico | 04ERb,9 | 2004 | Eritrea |
| 95US-TX1 | 1995 | US-Texas | 04CH | 2004 | China |
| 95US-TX2 | 1995 | US-Texas | 05MX1 | 2005 | Mexico |
| 96MX | 1996 | Mexico | 05MX2 | 2005 | Mexico |
| 98CO | 1998 | Colombia | 05MX3 | 2005 | Mexico |
| 98MX2 | 1998 | Mexico | 05CH | 2005 | China |
| 98HO | 1998 | Honduras | 05VE | 2005 | Venezuela |
| 99MX1a,3 | 1999 | Mexico | 05AW | 2005 | Aruba |
| 99MX2a,4 | 1999 | Mexico | 05ID | 2005 | Indonesia |
| 99TW5 | 1999 | Taiwan | 05BH1 | 2005 | Belize |
| 2KMXa,6 | 2000 | Mexico | 05BH2 | 2005 | Belize |
| 01BH7 | 2001 | Belize | 05NI | 2005 | Nicaragua |
| 02BH | 2002 | Belize | 06ID | 2006 | Indonesia |
| 03TH1 | 2003 | Thailand | 06AW | 2006 | Aruba |
| 03TH2 | 2003 | Thailand | 06EC1 | 2006 | Ecuador |
| 03ID1 | 2003 | Indonesia | 06EC2 | 2006 | Ecuador |
| 03ID2 | 2003 | Indonesia | 06MX | 2006 | Mexico |
| 03ID3 | 2003 | Indonesia | 06TH | 2006 | Thailand |
| 03CH | 2003 | China | 06NI | 2006 | Nicaragua |
Table 5.
Primer set for a single step RT-PCR method for detection of all known genomic variants (strains) of TSV (from Nunan et al. 1998).
| Primer | Product | Sequence | G:C ratio | Annealing Temperature |
|---|---|---|---|---|
| 9992F | 231 bp | 5′-AAGTGAACAGCCGCGCTT-3′ | 55 | 69EC |
| 9195F | 5′-TCAATGAGAGCTTGGTCC-3′ | 50 | 63EC |
4. White spot syndrome virus (WSSV)
4.1. Biology of the agent
The causative agent of WSD is white spot syndrome virus (WSSV) or white spot virus (WSV). WSSV is a very large, enveloped, double-stranded DNA (dsDNA) virus with a density of approximately 1.2 g/ml. WSSV was recently assigned by the ICTV to its own new genus, Whispovirus, and family, Nimaviridae (Table 1) (Mayo, 2002a, Mayo, 2002b). Virions are large (80–120 × 250–380 nm), rod-shaped to elliptical, and with a trilaminar envelope (Wang et al., 1995, Durand et al., 1997, Inouye et al., 1994, Inouye et al., 1996, Kanchanaphum et al., 1998, van Hulten et al., 2001, Vlak et al., 2005, Greenwood et al., 2005). Negatively stained virions purified from shrimp hemolymph show unique, tail-like appendages (Fig. 1, Fig. 9 ) (Wang et al., 1995, Fauquet et al., 2005). The virions are generated in hypertrophied nuclei of infected cells without the production of occlusion bodies (Fig. 10). In initial reports, WSSV was described as a non-occluded baculovirus, but WSSV DNA sequence analysis has shown that it is not related to the baculoviruses (van Hulten et al., 2001, Yang et al., 2001). The size of the WSSV genome has been differently reported for different isolates: 305,107 bp (GenBank Accession No. AF332093), 292,967 bp (GenBank Accession No. AF369029) and 307,287 bp (GenBank Accession No. AF440570) for viruses isolated from the People’s Republic of China, Thailand and Taipei China, respectively. The sequences of these three isolates are almost identical, with the size differences being due mostly to several small insertions and one large (∼12 kbp) deletion. In accordance with a genome size of ∼300 kb, a total of 531 putative open reading frames (ORFs) were identified by sequence analysis, among which 181 ORFs are likely to encode functional proteins. Thirty-six of these 181 ORFs have been identified by screening and sequencing a WSSV cDNA library or else have already been reported to encode functional proteins, many of which show little homology to proteins from other viruses (OIE, 2003).
Fig. 9.
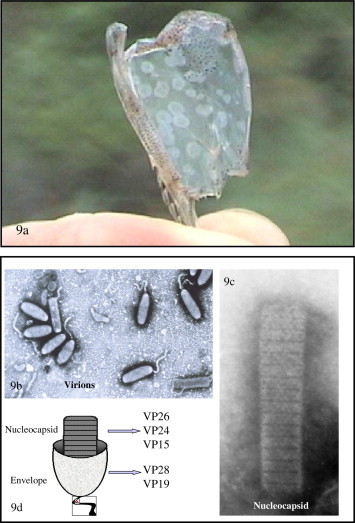
White spot syndrome. (a) Carapace from a juvenile Litopenaeus vannamei that is presenting typical white spots on its inner side due to infection by WSSV. (Photo courtesy of Mr. Diego Buenaventura, Guayaquil, Ecuador, 1999); (b) Transmission electron micrograph (TEM) of semi-purified WSSV virions concentrated from the hemolymph of an experimentally infected juvenile L. vannamei. Intact virions have a tail-like appendage that extends from the envelope; (c) TEM of a nucleocapsid showing the 90° arrangement of the capsid subunits on its surface; and (d) schematic showing in a cut-away view the outer envelope, the inner nucleocapsid, and the principal proteins associated with each. (d was modified from Vlak et al., 2005).
Temperature was found to have a profound effect on the expression of disease in WSSV infected L. vannamei (Vidal et al., 2001, Granja et al., 2003). These authors found that at temperatures above 32 °C WSD did not develop in WSSV infected L. vannamei, but when the same shrimp were cooled to 25 °C the disease would quickly develop with 100% mortality. Subsequent studies demonstrated that the hyperthemic phenomenon also occurred in other penaeids (Guan et al., 2003). Recent work has shown that replication of WSSV is significantly reduced or stopped under hyperthermic conditions (Du et al., 2006). These findings have helped to explain why WSD epizootics occur most often in the cooler seasons in most shrimp farming regions. In the Americas, that information has helped shrimp farmers manage around WSD by avoiding stocking in the cool season, and in some countries like Ecuador and Peru, growing shrimp year-around in temperature controlled greenhouses.
4.2. History and geographic distribution of white spot disease
WSSV has a wide host range among decapod crustaceans (Lo et al., 1996, Flegel, 1997, Flegel and Alday-Sanz, 1998, Nadala and Loh, 1998), and is potentially lethal to most of the commercially cultivated penaeid shrimp species (OIE, 2006b). White spot disease (WSD) caused by WSSV emerged in east Asia in 1992–1993 and it was quickly dispersed with infected seed and broodstock across the Asian continent to SE Asia and India where it caused a major pandemic, and continues to cause significant losses in some regions. WSD outbreaks were first reported from farmed Marsupenaeus japonicus in Japan in 1993 (Inouye et al., 1994, Inouye et al., 1996, Nakano et al., 1994) and the causative agent was named penaeid rod-shaped DNA virus (PRDV) or rod-shaped nuclear virus of Ma. japonicus (RV-PJ). Later, outbreaks of viral disease with similar gross signs and caused by similar rod-shaped viruses were reported from elsewhere in Asia and other names were applied: hypodermal and hematopoietic necrosis baculovirus (HHNBV) in the People’s Republic of China (Huang et al., 1995a, Huang et al., 1995b); white spot baculovirus (WSBV) and PmNOBIII in Taipei China (Chou et al., 1995, Lo et al., 1996); and systemic ectodermal and mesodermal baculovirus (SEMBV) or PmNOBII in Thailand (Wongteerasupaya et al., 1995). The virus from the People’s Republic of China has also been called Chinese baculovirus (CBV) (Lu et al., 1997, Nadala and Loh, 1998, Nadala et al., 1998). Shrimp exhibiting the gross signs and histopathology of WSD have also been reported from Korea (Kim et al., 1998), India (Karunasagar et al., 1998), the Philippines, and the USA (Lightner, 1996b, Durand et al., 2000). WSSV has even reached shrimp farms in southeastern Europe (1997) and the Middle East (1999) via live shrimp movements, and Australia and Spain with introductions of frozen infected shrimp, which were used as fresh food for broodstock (OIE, 2006b).
Beginning in 1999, WSD has also had a severe impact on the shrimp industries of both Central and South America (GAA, 1999a, GAA, 1999b, Durand et al., 2000, Vidal et al., 2001, Lightner, 2003a, Lightner, 2003b, OIE, 2006b). Despite the absence of evidence of live shrimp introductions from Asia to the Americas, WSSV was diagnosed at several sites in 1995–1997 in captive wild shrimp or crayfish and in cultured domesticated shrimp stocks in the eastern and southeastern US (Nunan et al., 1998b, Durand et al., 2000, Lightner et al., 2001). Early in 1999, WSSV was diagnosed as the cause of serious epizootics in Central American shrimp farms. By mid to late 1999, WSSV was causing major losses in Ecuador (then among the world’s top producers of farmed shrimp), and by 2000–2001, export of shrimp from Ecuador was down nearly 70% from pre-WSSV levels (Rosenberry, 2001, Rosenberry, 2003, Lightner, 2003a). Although the documentation is sketchy, WSSV has been found in wild shrimp stocks in the Americas (Nunan et al., 2001, Chapman et al., 2004). In the US, the virus was successfully eradicated from shrimp farms and, except for an outbreak at an isolated shrimp farm on the Island of Kauai, Hawaii in 2004 (CEI, 2004, Ostrowski, 2004), it has not been reported from farmed shrimp stocks in the USA since 1997. However, its sporadic detection in wild shrimp stocks (Pacific Coast of Panama, Gulf of Mexico and SE Atlantic states) (Nunan et al., 2001, Chapman et al., 2004, Hasson et al., 2006) suggests that it has become established in wild penaeid shrimp stocks in coastal waters of the eastern Pacific and southeastern US and the Gulf of Mexico, or that it continues to be introduced perhaps with wastes (peeled shells, etc.) from value-added reprocessing of imported shrimp in coastal packing plants or from infected shrimp used as bait by sport fishermen. It has been proposed that the introductions of WSSV to the Americas were the result of importation of frozen shrimp products from WSSV-affected areas of Asia and the value-added reprocessing of those frozen shrimp for the US market in coastal processing plants (Nunan et al., 1998b, Durand et al., 2000, Lightner et al., 2001, Lightner, 2003a) or possibly due to the use of imported frozen WSSV-infected shrimp as bait by sport fishermen (Hasson et al., 2006). WSSV also reached Spain and Australia in 2000–2001. In both cases successful containment and eradication was reported, and for both events the importation and use of infected frozen shrimp as a fresh feed for broodstock was implicated as the route of introduction (OIE, 2003, Lightner, 2003b). Regardless of where they were obtained, isolates of WSSV have shown little genetic or biological variation, suggesting that the virus emerged and was spread from a single source (OIE, 2003, OIE, 2006b).
4.3. WSD gross signs and histopathology in Litopenaeus vannamei
The gross signs, histopathology and diagnostic procedures (antibody-based and molecular) for WSSV infections have been thoroughly reviewed since the disease emerged in Asia and subsequently in the Americas (Lightner, 1996a, Lightner, 1999, Flegel, 1997, Flegel, 2006, Bell and Lightner, 1988, Loh et al., 1997, Lo and Kou, 1998, Greenwood et al., 2005, OIE, 2006b). The reader is referred to these reviews for additional details on the disease in Asia and the Americas that are not included in the present review.
In L. vannamei acutely affected shrimp with WSD are reported to show a rapid reduction in food consumption, become lethargic, have a loose cuticle with some showing characteristic white spots of 0.5–2.0 mm in diameter, which are most apparent on the inside surface of the carapace (Fig. 9a), but may be present anywhere on the inner surface of the exoskeleton. The white spots represent abnormal deposits of calcium salts by the WSSV-infected cuticular epithelium. In many cases, moribund shrimp with WSD display a pink to reddish-brown coloration, due to expansion of the cuticular chromatophores and few if any white spots. Populations of shrimp showing these signs display high mortality rates with cumulative mortalities reaching 100% within 3–10 days of the onset of clinical signs (Lightner, 1996a, OIE, 2006b).
The principal lesion apparent in routine histological preparations of L. vannamei in the acute phase of WSD is the presence of prominent eosinophilic to pale basophilic (with H&E stains), Feulgen-positive intranuclear inclusion bodies in hypertrophied nuclei of, most commonly, the cuticular epithelial cells and connective tissue cells, and, less frequently, in the antennal gland epithelium, lymphoid organ sheath cells, hematopoietic tissues, and in fixed phagocytes of the heart (Fig. 10). The early stages of inclusion body development in WSD are eosinophilic, centronuclear, and with a halo (an artifact with Davidson’s fixation). The early developing WSSV intranuclear inclusion bodies resemble the appearance of inclusion bodies due to infection by IHHNV, and consequently, at this stage the two diseases can be easily confused. However, the presence of larger, more fully developed, without a halo, pale basophilic inclusion bodies in infected target tissues during the advanced stages of infection clearly distinguishes the two diseases (Fig. 10a–d). Usually WSSV infected nuclei contain a single inclusion body. To reach a differential diagnosis of infection by IHHNV and/or infection by WSSV it is advisable to use a second diagnostic method for one or both disease agents. The available and preferred options are ISH with specific DIG-labeled probes or PCR of parallel samples preserved for that purpose (Lightner, 1996b, OIE, 2006b).
5. Yellow head disease virus (YHV)
5.1. Biology of the agent
The causative agent of YHD is YHV, gill-associated virus (GAV) and other closely related strains of the same virus (Table 1) (OIE, 2003, OIE, 2006b). YHV is an enveloped, rod-shaped, single-stranded RNA (ssRNA) virus in the family Roniviridae in the order Nidovirales. The density of virions is approximately 1.20 g/ml (OIE, 2006b). Transmission electron microscopy (TEM) of YHV-infected tissues shows enveloped bacilliform virions. They range from approximately 150 nm to 200 nm in length and from 40 nm to 50 nm in diameter and are located within vesicles in the cytoplasm of infected cells and in intercellular spaces. The virions arise from longer, filamentous nucleocapsids (approximately 15 nm × 130–800 nm), which accumulate in the cytoplasm and obtain an envelope by budding through the endoplasmic reticulum into intracellular vesicles. Negatively stained YHV virions show regular arrays of short spikes on the viral envelope (Fig. 1, Fig. 11 ) (Boonyaratpalin et al., 1993, Chantanachookin et al., 1993, Lightner, 1996a).
Fig. 11.
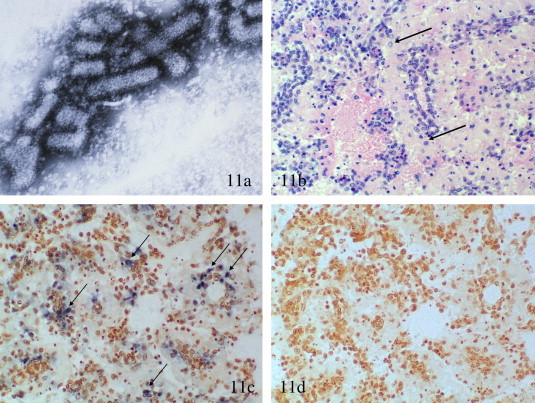
Yellowhead disease (YHD) caused by YHV. (a) TEM of a semi-purified preparation of YHV from the hemolymph of an experimentally infected juvenile Litopenaeus vannamei. Rod-shaped enveloped YHV particles are shown with prominent spikes projecting from the envelope and (b–d) A series of histological sections from L. vannamei that are infected with WSSV and which severe generalized necrosis of the lymphoid organ. Severe necrosis of the LO with generalized nuclear pyknosis as shown here was once considered pathognomonic for YHD. The arrows in (b) (H&E stain) point out two cells with WSSV intranuclear inclusion bodies. (c) Shows reaction of a DIG-labeled WSSV probe to WSSV infected cells (examples are stained blue–black and are indicated by the arrows) in the LO, while the parallel section in (d) shows no reaction to YHV positive cells after being tested by ISH with a DIG-labeled YHV probe. These sections and other information presented in the text illustrate the reason for most of the false positive reports of YHD in the Americas.
YHV was originally described mistakenly as a granulosis-like virus (Boonyaratpalin et al., 1993, Chantanachookin et al., 1993), but it was later found to be a single-stranded, positive sense RNA (ssRNA) virus (Tang and Lightner, 1999) related to nidoviruses in the Coronaviridae and Arteriviridae (Sittidilokratna et al., 2002). GAV, the Australian strain of YHV has been recognized as the type species for the new virus genus Okavirus in the new family Roniviridae (Mayo, 2002a, Mayo, 2002b, OIE, 2003, OIE, 2006b).
5.2. History and geographic distribution of yellow head disease
Yellow head disease (YHD) was first described in 1991 as an epizootic in Thai shrimp farms (Limuswan, 1991), and subsequent outbreaks have been reported from other shrimp farming countries in Asia (OIE, 2003, OIE, 2006b). A closely related strain of YHV, named gill-associated virus (GAV), has been reported from Australian shrimp farms (Walker et al., 2001), and at least six genetic variant of the virus are now recognized (OIE, 2006b). Laboratory trials have shown that YHV can cause high mortality in representative cultured and wild penaeid species from the Americas (Lu et al., 1994, Lu et al., 1997, Lightner, 1999, Pantoja and Lightner, 2003). When it occurs in farms rearing P. monodon, YHD is characterized by high and rapid mortality, that is sometimes accompanied by the gross signs of yellowing of the cephalothorax (from which the disease got its name) and general bleaching of body color. In laboratory studies, American penaeids challenged with YHV did not develop yellow heads or signs of marked discoloration (Lightner and Redman, 1998a). YHV is potentially lethal to most of the commercially cultivated penaeid shrimp species (OIE, 2006a, OIE, 2006b).
5.3. YHD in the Americas?
While there are no confirmed reports of actual YHV caused disease outbreaks in the Americas, there were errant reports several years ago which indicated that YHV infections were co-occurring with white spot disease outbreaks in the USA and in Central America (Pantoja and Lightner, 2003). However, there are several, well documented recent reports that an apparently avirulent genotype of YHV is present in farmed and wild penaeid shrimp in northwest Mexico (de la Rosa-Vélez et al., 2006, Castro-Longoria et al., 2008, Cedano-Thomas et al., 2009, Sánchez-Barajas et al., 2009).
Suspicion that YHV infections were co-occurring with WSSV infections in WSD outbreaks was supported by the discovery that YHV and WSSV were present as co-infecting agents in frozen imported commodity shrimp in the USA (Nunan et al., 1998b, Durand et al., 2000). Subsequent to finding YHV in imported frozen commodity shrimp, the occurrence of YHD, co-occurring with WSD, was incorrectly reported in farmed shrimp from the Americas. Some early unpublished reports from ∼1999 to 2000, were based on the presentation of severe necrosis of the lymphoid organ, a lesion once thought to be pathognomonic for YHD (Fig. 11b–c) (Limuswan, 1991, Lightner, 1996a, Lightner et al., 1998, Lightner and Redman, 1998a, Pantoja and Lightner, 2003). However, the diagnosis of YHV infection in these cases was not confirmed with a second diagnostic method until after the errant diagnostic reports to diagnostic case submitters and to the OIE were released (Lightner, unpublished data). More recent work has shown that the presumptive histological diagnoses were due to severe infections with white spot virus, which can cause histopathology in the lymphoid organ, which mimics that occurring in severe acute YHD (Fig. 11b–d) (Pantoja and Lightner, 2003).
An additional series of recently published papers have documented the occurrence of an apparently avirulent YHV genotype in wild and farmed shrimp in Sonora, Mexico (de la Rosa-Vélez et al., 2006, Castro-Longoria et al., 2008, Cedano-Thomas et al., 2009, Sánchez-Barajas et al., 2009). In the first of these reports, de la Rosa-Vélez et al. (2006) reported the discovery of suspect cases of YHV infections from shrimp farms along the Pacific coast of Mexico. From 39 samples from 26 randomly chosen shrimp farms, 11 YHV positive samples of L. vannamei were found by RT-PCR using primers specific for the virus (Tang and Lightner (1999). Further analysis of selected isolates using primers that amplify other regions of the YHV genome also gave positive results (de la Rosa-Vélez et al., 2006). Of considerable interest in the de la Rosa-Vélez et al. (2006) report was the general absence of notable mortalities in farms with the YHV positive shrimp. However, a laboratory challenge study with the putative YHV agent resulted in 50% mortality in 14 days in the indicator L. vannamei used to assay for the presence of YHV (de la Rosa-Vélez et al., 2006).
In subsequent studies on YHV in Mexico, Bell and Lightner, 1987, Castro-Longoria et al., 2008 surveyed wild blue shrimp, Litopenaeus stylirostris, and L. vannamei collected from the Gulf of California near areas with significant shrimp farms. The samples of wild L. vannamei were negative for YHV, but a few YHV positive specimens we found in the wild L. stylirostris. YHV was confirmed to be present by bioassay with healthy L. vannamei. The presence of YHV in the challenged shrimp was confirmed by RT-PCR, by sequencing the amplicons and by sequence alignment with YHV and GAV sequences in GenBank.
Another recent study demonstrated the presence of YHV in inland freshwater aquaculture systems growing L. vannamei in Colomia in west-central Mexico (Sánchez-Barajas et al., 2009). In this study the prevalence of YHV in the affected farms was 13%, but as was reported in earlier reports of YHV in Mexico, significant mortalities were not observed in the YHV infected shrimp stocks.
In another study run to further confirm that the agent found in affected farms in Mexico was YHV, Cedano-Thomas et al. (2009), amplified replicative and structural protein encoding regions of the several Mexican YHV isolates and compared the sequences obtained with homologous virus sequences from YHV, GAV and other coronaviruses. The authors found that the Mexican YHV isolates differed slightly from YHV and GAV, but nonetheless were closely related to Asian YHV and GAV (Cedano-Thomas et al., 2009).
The finding of a strain of YHV in Mexico (de la Rosa-Vélez et al., 2006, Castro-Longoria et al., 2008, Cedano-Thomas et al., 2009, Sánchez-Barajas et al., 2009), reflects the ongoing risk of additional introductions of YHV into the Western Hemisphere with imported frozen commodity shrimp from Asia (Nunan et al., 1998a, Durand et al., 2000). In addition, because of the possibility that concurrent WSSV/YHV infections may occur Hence, in severe WSSV cases in which YHV infection may also be suspected, all YHV suspect samples should be further analyzed by another method (i.e. RT-PCR or ISH with a YHV specific probe) to confirm or rule out the presence of YHV.
Of interest is the observation that YHD has not emerged as a major disease in cultured stocks of L. vannamei in east and south-east Asia where YHV is enzootic and highly prevalent in wild and farmed stocks of Penaeus monodon (OIE, 2006b, Flegel, 2006). According to FAO statistics the shrimp farming industry in Asia began to switch from culturing P. monodon to L. vannamei in 1999 and by 2005, more than half of the ∼2 million metric tons of production from the region was L. vannamei (FAO, 2006). Despite the predominance of monocultures of L. vannamei, presumably highly susceptible species to YHV, Flegel (2006) did not report the occurrence of any significant outbreaks of yellow head disease in this species in the SE Asian region.
6. Infectious myonecrosis virus (IMNV)
6.1. Biology of the agent
Infectious myonecrosis virus (IMNV) particles are icosahedral in shape and 40 nm in diameter, with a buoyant density of 1.366 g/ml in cesium chloride (Fig. 1, Fig. 12 ). The genome consists of a single, double-stranded RNA (dsRNA) molecule of 7560 bp. Sequencing of the viral genome reveals two non-overlapping open reading frames (ORFs). ORF 1 encodes a RNA-binding protein and a capsid protein. The coding region of the RNA-binding protein is located in the first half of ORF 1 and contains a dsRNA-binding motif. The second half of ORF 1 encodes a capsid protein with a molecular mass of 106 kDa. ORF 2 encodes a RNA-dependent RNA polymerase (RdRp). Based on these characteristics, IMNV is most similar to members of the Totiviridae (Poulos et al., 2006).
Fig. 12.
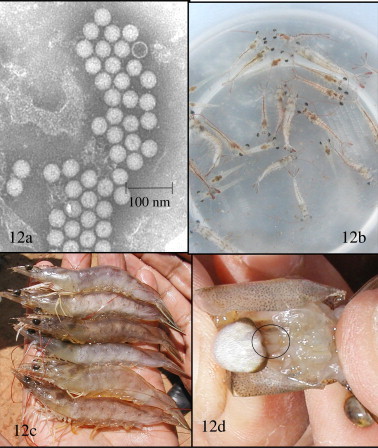
Infectious myonecrosis (IMN) caused by IMNV. (a) TEM of a purified preparation of IMNV from naturally infected Litopenaeus vannamei from Brazil. Mostly full (and one empty) IMNV particles are shown. The 40 nm diameter virions are hexagonal in profile indicating their icosahedral shape. The most prominent gross signs presented by shrimp with IMN is opaque skeletal muscle shown in very young juvenile L. vannamei in (b) and in subadult L. vannamei in (c). The paired lymphoid organs in shrimp with IMN are typically hypertrophied to 2–4 times their normal size as in shown in the circle in (d).
6.2. History and geographic distribution of IMN disease
Infectious myonecrosis (IMN) has been proposed for listing by the OIE (Table 1) (OIE, 2006a). The disease was first described in cultured L. vannamei in northeast Brazil (Lightner, 2003a). IMN causes significant disease and mortalities in juvenile and subadult pond-reared stocks of L. vannamei. In 2003, IMN was reported to have been responsible for millions of dollars in losses in northeast Brazil and by 2004 losses due to IMN in the affected regions of Brazil were estimated at $20 million (Nunes et al., 2004). More recent estimates for IMN losses from 2002 to 2006 in Brazil exceed $100 million (Brazilian Shrimp Farmers Association, unpublished) (Table 2). In Brazil, outbreaks of the disease seemed to be associated with certain types of environment and physical stresses (i.e. extremes in salinity and temperature, collection by cast-net, etc.), and possibly with the use of low quality feeds (Lightner, 2003a). Although IMN seemed to be confined to the NE of Brazil, the disease spread to SE Asia and was reported from Indonesia in May 2006 (Wilkinson, 2006). Because of the ever increasing importance of L. vannamei in the Asia–Pacific and the large scale trans-boundary movement and culture of the species, IMNV was considered important for the region and it was added in January 2006 to the NACA/FAO/OIE (Asian Region) Quarterly Aquatic Animal Disease Report list for the purpose of surveillance and reporting.
6.3. Gross signs and histopathology of IMNV
IMN presents as a disease in L. vannamei with an acute onset of gross signs and elevated mortalities, but it progresses with a more chronic course accompanied by persistent moderate mortalities. To date, IMN appears to be limited to northeast Brazil, but shrimp with similar gross signs have been also reported from other countries of the Caribbean region where L. vannamei are cultured (Lightner, 1993). Affected shrimp present focal to extensive white necrotic areas in the striated muscle, especially of the distal abdominal segments and tail fan (Fig. 12b and c). These may become necrotic and reddened in some individual shrimp. These signs may have a sudden onset following stresses (e.g. capture by cast-net, feeding, sudden changes in temperature or salinity, etc.). Severely affected moribund shrimp may have been feeding just before the onset of stress and will have a full gut (Fig. 12b). Such severely affected shrimp become moribund and mortalities can be instantaneously high and continue for several days. Exposure of the paired lymphoid organs (LO) by simple dissection will show that the paired LO are hypertrophic to twice or more their normal size (Fig. 12d).
By histopathology using routine H&E stained paraffin sections (Bell and Lightner, 1988), shrimp with acute phase IMN present myonecrosis with characteristic coagulative necrosis of striated (skeletal) muscle fibers, often with marked edema among affected muscle fibers (Lightner, 2003a, Poulos et al., 2006). Some shrimp may present with a mix of acute and older lesions (Fig. 13 a and b). In such shrimp, the affected muscle fibers appear to progress from presenting coagulative necrosis to presenting liquefactive necrosis, which is accompanied by moderate infiltration and accumulation of hemocytes. In the most advanced lesions, hemocyte inflamed muscle fibers are replaced by a loose matrix of fibrocytes and connective tissue fibers that are interspersed with hemocytes and foci of (presumed) regenerating muscle fibers (Fig. 13b).
Fig. 13.
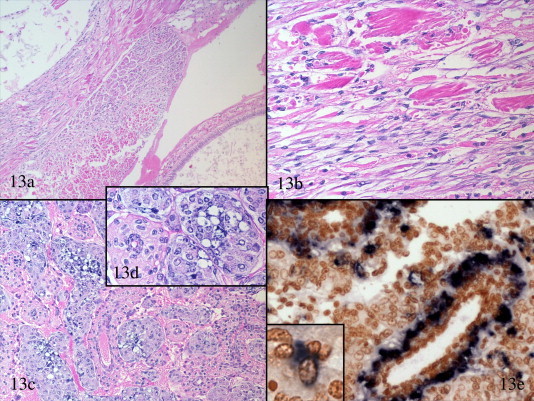
Histopathology of infectious myonecrosis (IMN) shown in H&E stained sections (a–d) and by ISH with a IMNV-specific DIG-labeled probe (e). Mayer–Bennett H&E and DIG-labeled probe with Bismarck Brown counterstain; (a and b) Photomicrographs of sections through skeletal muscles in the abdomen of juvenile Litopenaeus vannamei with severe IMN. Hyaline necrosis of some muscle fibers in (a) is shown with marked edema among the affected fibers, as well as older lesions in (a) and (b) in which necrotic muscle has been infiltrated by hemocytes and replaced by fibrous tissue; (c and d) show large numbers of lymphoid organ (LO) spheroids (LOS) amongst normal LO tubules; (e and inset) photomicrographs of a LO with LOS that has been reacted in ISH with a IMNV-specific DIG-labeled probe. Parenchymal cells in the LO tubule walls show strong positive reactions for IMNV, and the high magnification inset shows that the virus is located in the cytoplasm of the infected cells.
Significant hypertrophy of the lymphoid organ (LO) due to accumulations of lymphoid organ spheroids (LOS) is a highly consistent lesion in shrimp with acute or chronic phase IMN lesions. Often many ectopic LOS are found in other tissues not near the main body of the LO. Common locations for ectopic LOS include the hemocoelom in the gills, heart, near the antennal gland tubules, and ventral nerve cord. In some histological preparations, perinuclear pale basophilic to darkly basophilic inclusion bodies are evident in muscle cells, connective tissue cells, hemocytes, and in cells that comprise lymphoid organ spheroids. When tested by ISH with a specific DIG-labeled cDNA probe to IMNV, these inclusions react with the probe reflecting their presumed high content of IMNV (Lightner et al., 2004, Tang et al., 2005, Poulos et al., 2006). In addition to gross signs and histopathology, diagnostic methods using DIG-labeled cDNA probes to the virus, one step, nested and real-time RT-PCR have been developed for the detection of IMNV (Tang et al., 2005, Poulos et al., 2006, Poulos and Lightner, 2006, Andrade et al., 2007). A nested RT-PCR method for the detection of IMNV is commercially available.
7. Disease management
Until the WSSV pandemic, the penaeid shrimp farming industry in Asia and the Americas remained largely dependent on wild shrimp for stocking its farms, and biosecurity was not part of the shrimp farming industry’s vocabulary. The farming of essentially wild shrimp stocks was accomplished by the practice of collection and use of wild seed (postlarvae) for stocking of its farms directly or by the use of captive wild broodstock for the production of seed stock in hatcheries. This dependence fostered the intensification and spread of the viral diseases in shrimp aquaculture and in wild populations. The shrimp farming industry, as a whole, recognized this fact and has begun to change its farming practices in order to continue to develop, if not survive. While many of the shrimp stocks currently used to stock farms are produced from captive wild broodstock, only those that test negative for WSSV in Asia and WSSV and TSV in the Americas are used to stock biosecure farms. Biosecure production systems (that are designed to exclude potentially infected wild shrimp seed) stocked with shrimp stocks known to be free of the major shrimp pathogens have become a common practice in many shrimp growing regions. A further sign of a maturing industry is its movement towards the expanded development and use of specific pathogen-free (SPF) domesticated shrimp stocks of the most important shrimp species (Pruder et al., 1995, Lotz, 1997a, Lotz, 1997b, Lightner and Redman, 1998b, Moss et al., 2002, Lightner, 2003b, Lightner, 2005, Lee and O’Bryen, 2003). Domesticated lines L. vannamei are now the dominant shrimp farmed in Asia. The rapid change over of the industry to L. vannamei from P. monodon was due in large part to disease (FOA, 2006). Domesticated stocks made it possible to better manage the health status of the farmed stocks, and with L. vannamei SPF domesticated lines were readily available (Lightner, 2005, FAO, 2006). These progresses have advanced the technologies used to farm shrimp and made the industry far more sustainable than it was before the emergence of the virus-caused diseases discussed in the present paper.
Acknowledgments
Grant support for the author of this review was provided by the United States Marine Shrimp Farming Consortium under Grant Nos. 2004-38808-02142 and 2008-38808-19163 from the Cooperative State Research, Education and Extension Service, US Department of Agriculture.
References
- Aguirre Guzman G., Valle F. Infectious disease in shrimp species with aquaculture potential. Recent Res. Develop. Microbiol. 2000;4:333–348. [Google Scholar]
- Andrade T.P.D., Srisuvan T., Tang K.F.J., Lightner D.V. Real-time reverse transcription polymerase chain reaction assay using TaqMan probe for detection and quantification of infectious myonecrosis virus (IMNV) Aquaculture. 2007;264:9–15. [Google Scholar]
- Anggraeni M., Owens L. Evidence for the haemocytic orgin of lymphoidal spheroids in Penaeus monodon. In: Flegel T.W., editor. Advances in Shrimp Biotechnology. National Center for Genetic Engineering and Biotechnology; Bangkok, Thailand: 1998. p. 137. [Google Scholar]
- AQUIS (Australian Quarantine and Inspection Service), 2000. Import Risk Analysis: Prawns and Prawn Products. Draft Import Risk Analysis Paper. Animal Quarantine Policy Memorandum 2000/41, 185 p.
- Audelo del Valle J., Clement-Mellado O., Magana-Hernandez A., Flisser A., Montiel-Aguirre F., Briseno-Garcia B. Infection of cultured human and monkey cell lines with extract of penaeid shrimp infected with Taura syndrome virus. Emerg. Infect. Dis. (CDC) 2003;9:265–266. doi: 10.3201/eid0902.020181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell T.A., Lightner D.V. IHHN virus: Infectivity and pathogenicity studies in Penaeus stylirostris and Penaeus vannamei. Aquaculture. 1984;38:185–194. [Google Scholar]
- Bell T.A., Lightner D.V. IHHN disease of Penaeus stylirostris: effects of shrimp size on disease expression. J. Fish Dis. 1987;10:165–170. [Google Scholar]
- Bell, T.A., Lightner, D.V., 1988. A Handbook of Normal Shrimp Histology. Special Publication No. 1, World Aquaculture Society, Baton Rouge, LA., 114 p.
- Bell T.A., Lightner D.V., Brock J.A. A biopsy procedure for the nondestructive determination of infectious hypodermal and hematopoietic necrosis virus (IHHNV) infection in Penaeus vannamei. J. Aquat. Anim. Health. 1990;2:151–153. [Google Scholar]
- Bondad-Reantaso M.G., McGladdery, S.E., East, I., Subasinghe, R.P., (Eds.), 2001. Asia Diagnostic Guide to Aquatic Animal Diseases. FAO Fisheries Technical Paper 402, Supplement 2. Rome, FAO, 240 pp.
- Bonami J.R., Brehelin M., Mari J., Trumper B., Lightner D.V. Purification and characterization of IHHN virus of penaeid shrimps. J. Gen. Virol. 1990;71:2657–2664. doi: 10.1099/0022-1317-71-11-2657. [DOI] [PubMed] [Google Scholar]
- Bonami J.R., Lightner D.V. Unclassified viruses of Crustacea. In: Adams J.R., Bonami J.R., editors. Atlas of Invertebrate Viruses. CRC Press; Boca Raton, Florida, USA: 1991. pp. 597–622. (Chapter 24) [Google Scholar]
- Bonami J.R., Hasson K.W., Mari J., Poulos B.T., Lightner D.V. Taura syndrome of marine penaeid shrimp: characterization of the viral agent. J. Gen. Virol. 1997;78:313–319. doi: 10.1099/0022-1317-78-2-313. [DOI] [PubMed] [Google Scholar]
- Boonyaratpalin S., Supamattaya K., Kasornchandra J., Direkbusaracom S., Ekpanithanpong U., Chantanachooklin C. Non-occluded baculo-like virus, the causative agent of yellow head disease in the black tiger shrimp (Penaeus monodon) Fish Pathol. 1993;28:103–109. [Google Scholar]
- Bray W.A., Lawrence A.L., Leung-Trujillo J.R. The effect of salinity on growth and survival of Penaeus vannamei, with observations on the interaction of IHHN virus and salinity. Aquaculture. 1994;122:133–146. [Google Scholar]
- Brock J.A. Special topic review: Taura syndrome, a disease important to shrimp farms in the Americas. World Journal of Microbiology & Biotechnology. 1997;13:415–418. [Google Scholar]
- Brock, J.A., Lightner, D.V., Bell, T.A., 1983. A review of four virus (BP, MBV, BMN, and IHHNV) diseases of penaeid shrimp with particular reference to clinical significance, diagnosis and control in shrimp aquaculture. In: Proceedings of the 71st International. Council for the Exploration of the Sea, C.M. 1983/Gen:10/1-18.
- Brock J.A., Lightner D.V. Diseases of Crustacea. Diseases caused by microorganisms. In: Kinne O., editor. vol. 3. Biologische Anstalt Helgoland; Hamburg, Germany: 1990. pp. 245–349. (Diseases of Marine Animals). [Google Scholar]
- Brock, J.A., Main, K., 1994. A Guide to the Common Problems and Diseases of Cultured Penaeus vannamei. Oceanic Institute, Makapuu Point, P.O. Box 25280, Honolulu, Hawaii, USA, 241 pp.
- Brock J.A., Gose R., Lightner D.V., Hasson K.W. An overview on Taura syndrome, an important disease of farmed Penaeus vannamei. In: Browdy C.L., Hopkins J.S., editors. Swimming through Troubled Water, Proceedings of the Special Session on Shrimp Farming, Aquaculture ‘95. 1–4 February 1995. San Diego. World Aquaculture Society; Baton Rouge, LA, USA: 1995. pp. 84–94. [Google Scholar]
- Brock J.A., Gose R.B., Lightner D.V., Hasson K.W. Recent developments and an overview of Taura syndrome of farmed shrimp in the Americas. In: Flegel T.W., MacRae I.H., editors. Diseases in Asian Aquaculture III, Proceedings from the Third Symposium on Diseases in Asian Aquaculture, 29 January–2 February. Bangkok. Fish Health Section, Asian Fisheries Society; Manila: 1997. pp. 275–283. [Google Scholar]
- Browdy C.L., Holloway J.D., King C.O., Stokes A.D., Hopkins J.S., Sandifer P.A. IHHN virus and intensive culture of Penaeus vannamei: effects of stocking density and water exchange rates. J. Crust. Biol. 1993;13:87–94. [Google Scholar]
- Carr W.H., Sweeney J.N., Nunan L.M., Lightner D.V., Hirsch H.H., Reddington J.J. The use of an infectious hypodermal and hematopoietic necrosis virus gene probe serodiagnostic field kit for the screening of candidate specific pathogen-free Penaeus vannamei broodstock. Aquaculture. 1996;147:1–8. [Google Scholar]
- CEI, 2004. White spot disease, United States, April 2, 2004, Impact worksheet. Animal Plant Health Inspection Service, US Department of Agriculture Veterinary Services, Fort Collins, CO. <http://www.aphis.usda.gov/vs/ceah/cei/IW_2004_files/wsd_us_0404_files/wsd_us_0404.htm>.
- Castro-Longoria R., Quintero-Arredondo N., Grijalva-Chon J.M., Ramos-Paredes J. Detection of the yellow-head virus (YHV) in wild blue shrimp, Penaeus stylirostris, from the Gulf of California and its experimental transmission to the Pacific white shrimp, Penaeus vannamei. J. Fish Dis. 2008;31:953–956. doi: 10.1111/j.1365-2761.2008.00978.x. [DOI] [PubMed] [Google Scholar]
- Cedano-Thomas Y., de la Rosa-Vélez J., Bonami J-R., Vargas-Albores F. Gene expression kinetics of the yellow head virus in experimentally infected Litopenaeus vannamei. Aquacult. Res. 2009:1–12. doi: 10.1111/j.1365-2109.2009.02434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y.S., Peng S.E., Yu H.T., Liu F.C., Wang C.H., Lo C.F., Kou G.H. Genetic and phenotypic variations of isolates of shrimp Taura syndrome virus found in Penaeus monodon and Metapeneus ensis in Taiwan. J. Gen. Virol. 2004;85:2963–2968. doi: 10.1099/vir.0.80132-0. [DOI] [PubMed] [Google Scholar]
- Chantanachookin C., Boonyaratpalin S., Kasornchandra J., Direkbusarakom S., Aekpanithanpong U., Supamattaya K., Sriuraitana S., Flegel T.W. Histology and ultrastructure reveal a new granulosis-like virus in Penaeus monodon affected by yellow-head disease. Dis. Aquat. Org. 1993;17:145–157. [Google Scholar]
- Chapman R.W., Browdy C.L., Savin S., Prior S., Wenner E. Sampling and evaluation of white spot syndrome virus in commercially important Atlantic penaeid shrimp stocks. Dis. Aquat. Org. 2004;59:179–185. doi: 10.3354/dao059179. [DOI] [PubMed] [Google Scholar]
- Chayanburakul K., Lightner D.V., Flegel T.W., Sriurairattana S., Withyachumnarnkul B. Different responses to an infection by infectious hypodermal and hematopoietic necrosis virus (IHHNV) in Penaeus mondon and P. vannamei. Dis. Aquat. Org. 2005;67:191–200. doi: 10.3354/dao067191. [DOI] [PubMed] [Google Scholar]
- Chou H.Y., Huang C.Y., Wang C.H., Chiang H.C., Lo C.F. Pathogenicity of a baculovirus infection causing white spot syndrome in cultured penaeid shrimp in Taiwan. Dis. Aquat. Org. 1995;23:165–173. [Google Scholar]
- de la Rosa-Vélez J., Cedano-Thomas Y., Cid-Becerra J., Méndez-Payán J.C., Vega-Pérez C., Zambrano-García J., Bonami J.-R. Presumptive detection of yellow head virus by reverse transcriptase–polymerase chain reaction and dot-blot hybridization in Litopenaeus vannamei and L. stylirostris cultured on the Northwest coast of Mexico. J. Fish Dis. 2006;29:717–728. doi: 10.1111/j.1365-2761.2006.00767.x. [DOI] [PubMed] [Google Scholar]
- Dhar A.K., Roux M.M., Klimpel K.R. Detection and quantification of infectious hypodermal and hematopoeitic necrosis virus and white spot virus in shrimp using real-time quantitative PCR and SYBR green chemistry. J. Clin. Microbiol. 2001;39:2835–2845. doi: 10.1128/JCM.39.8.2835-2845.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H.H., Li W.F., Xu Z.R., Kil Z.S. Effect of hyperthermia on the replication of white spot syndrome virus (WSSV) in Procambarus clarkii. Dis. Aquat. Org. 2006;71:175–178. doi: 10.3354/dao071175. [DOI] [PubMed] [Google Scholar]
- Duda T.F.Jr., Palumbi S.R. Population structure of the black tiger prawn, Penaeus monodon, among western Indian Ocean and western Pacific populations. Mar. Biol. 1999;134:705–710. [Google Scholar]
- Durand S., Lightner D.V., Redman R.M., Bonami J.R. Ultrastructure and morphogenesis of white spot syndrome baculovirus (WSSV) Dis. Aquat. Org. 1997;29:205–211. [Google Scholar]
- Durand S., Tang K.F.J., Lightner D.V. Frozen commodity shrimp: potential avenue for introduction of white spot syndrome virus and yellow head virus. J. Aquat. Anim. Health. 2000;12:128–135. [Google Scholar]
- Erickson H.S., Zarin-Herzberg M., Lightner D.V. Detection of Taura syndrome virus (TSV) strain differences using selected diagnostic methods: diagnostic implications in penaeid shrimp. Dis. Aquat. Org. 2002;52:1–10. doi: 10.3354/dao052001. [DOI] [PubMed] [Google Scholar]
- Erickson H.S., Poulos B.T., Tang K.F.J., Bradley-Dunlop D., Lightner D.V. Taura Syndrome Virus from Belize represents a unique variant. Dis. Aquat. Org. 2005;64:91–98. doi: 10.3354/dao064091. [DOI] [PubMed] [Google Scholar]
- FAO, 2006. State of world aquaculture. FAO Fisheries Technical Paper 500. Food and Agriculture Organization of the United Nations, Rome. 134 p.
- Fauquet, C.M., Mayo, M.A., Maniloff, J., Desselberger, U., Ball, L.A., 2005. Virus taxonomy. Classification and nomenclature of viruses. In: Eighth Report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, 1259 pp.
- Flegel T.W. Major viral diseases of the black tiger prawn (Penaeus monodon) in Thailand. World J. Microbiol. Biotechnol. 1997;13:433–442. [Google Scholar]
- Flegel T.W. Detection of major penaeid shrimp viruses in Asia, a historical perspective with emphasis on Thailand. Aquaculture. 2006;258:1–33. [Google Scholar]
- Flegel T.W., Alday-Sanz V. The crisis in Asian shrimp aquaculture: current status and future needs. J. Appl. Ichthyol. 1998;14:269–273. [Google Scholar]
- GAA (Global Aquaculture Alliance) Shrimp white spot virus confirmed in Central America. GAA Newslett. 1999;2(2) [Google Scholar]
- GAA (Global Aquaculture Alliance) Shrimp white spot disease in Latin America an update. GAA Newslett. 1999;2(3) [Google Scholar]
- Garza J.R., Hasson K.W., Poulos B.T., Redman R.M., White B.L., Lightner D.V. Demonstration of infectious Taura syndrome virus in the feces of sea gulls collected during an epizootic in Texas. J. Aquat. Anim. Health. 1997;9:156–159. [Google Scholar]
- Granja C.B., Aranguren L.F., Vidal O.M., Aragon L., Salazar M. Does hyperthermia increase apoptosis in white spot syndrome virus (WSSV)-infected Litopenaeus vannamei. Dis. Aquat. Org. 2003;54:73–78. doi: 10.3354/dao054073. [DOI] [PubMed] [Google Scholar]
- Greenwood, S., Battison, A., Lavallee, J., 2005. White spot syndrome virus: diagnostic, surveillance and transmission – a literature review. AVC Project Report 05-001, AVC Lobster Science Centre, Charlottetown, Prince Edward Island, Canada, 28p.
- Guan Y., Yu Z., Li C. The effects of temperature on white spot syndrome infections in Marsupenaeus japonicus. J. Invertebr. Pathol. 2003;83:257–260. doi: 10.1016/s0022-2011(03)00068-5. [DOI] [PubMed] [Google Scholar]
- Hasson K.W., Lightner D.V., Poulos B.T., Redman R.M., White B.L., Brock J.A., Bonami J.R. Taura syndrome in Penaeus vannamei: Demonstration of a viral etiology. Dis. Aquat. Org. 1995;23:115–126. [Google Scholar]
- Hasson K.W., Hasson J., Aubert H., Redman R.M., Lightner D.V. A new RNA-friendly fixative for the preservation of penaeid shrimp samples for virological detection using cDNA genomic probes. J. Virol. Methods. 1997;66:227–236. doi: 10.1016/s0166-0934(97)00066-9. [DOI] [PubMed] [Google Scholar]
- Hasson K.W., Lightner D.V., Mohney L.L., Redman R.M., Poulos B.T., Mari J., Bonami J.R. The geographic distribution of Taura syndrome virus (TSV) in the Americas: determination by histology and in situ hybridization using TSV-specific cDNA probes. Aquaculture. 1999;171:13–26. [Google Scholar]
- Hasson K.W., Lightner D.V., Mohney L.L., Redman R.M., Poulos B.T., White B.L. Taura syndrome virus (TSV) lesion development and the disease cycle in the Pacific white shrimp Penaeus vannamei. Dis. Aquat. Org. 1999;36:81–93. [Google Scholar]
- Hasson K.W., Fan Y., Reisinger T., Venuti J., Varner P.W. White-spot syndrome virus (WSSV) introduction into the Gulf of Mexico and Texas freshwater systems through imported frozen bait-shrimp. Dis. Aquat. Org. 2006;71:91–100. doi: 10.3354/dao071091. [DOI] [PubMed] [Google Scholar]
- Huang J., Song X.L., Yu J., Yang C.H. Baculoviral hypodermal and hematopoietic necrosis – study on the pathogen and pathology of the explosive epidemic disease of shrimp. Mar. Fish. Res. 1995;16:1–10. [Google Scholar]
- Huang J., Yu J., Song X.L., Kong J., Yang C.H. Studies on fine structure, nucleic acid, polypeptide and serology of hypodermal and hematopoietic necrosis baculovirus of penaeid shrimp. Mar. Fish. Res. 1995;16:11–23. [Google Scholar]
- Inouye K., Miwa S., Oseko N., Nakano H., Kimura T., Momoyama K., Hiraoka M. Mass mortality of cultured kuruma shrimp Penaeus japonicus in Japan in 1993: electron microscopic evidence of the causative virus. Fish Pathol. 1994;29:149–158. [Google Scholar]
- Inouye K., Yamano K., Ikeda N., Kimura T., Nakano H., Momoyama K., Kobayashi J., Miyajima S. The Penaeid rod-shaped DNA virus (PRDV) which causes penaeid acute viremia (PAV) Fish Pathol. 1996;31:39–45. [Google Scholar]
- Intriago P., Jimenez R., Machuca M., Barniol R., Krauss E., Salvador X. Experiments on toxicosis as the cause of Taura syndrome in Penaeus vannamei (Crustacea: Decapoda) in Ecuador. In: Flegel T.W., MacRae I.H., editors. Diseases in Asian Aquaculture III. Fish Health Section, Asian Fisheries Society; Manila: 1997. pp. 365–379. [Google Scholar]
- Jimenez R. Acuacultura del Ecuador. Camara Nacional de Acuacultura; Guayaquil, Ecuador: 1992. Sindrome de Taura (Resumen) pp. 1–16. [Google Scholar]
- Jimenez R., Barniol R., de Barniol L., Machuca M. Periodic occurrence of epithelial viral necrosis outbreaks in Penaeus vannamei in Ecuador. Dis. Aquat. Org. 2000;42:91–99. doi: 10.3354/dao042091. [DOI] [PubMed] [Google Scholar]
- Kalagayan G., Godin D., Kanna R., Hagino G., Sweeney J., Wyban J., Brock J. IHHN virus as an etiological factor in runt-deformity syndrome of juvenile Penaeus vannamei cultured in Hawaii. J. World Aquacult. Soc. 1991;22:235–243. [Google Scholar]
- Kanchanaphum P., Wongteerasupaya C., Sitidilokratana N., Boonsaeng V., Panyim S., Tassanakajon A., Withyachumnarnkul B., Flegel T.W. Experimental transmission of White-spot syndrome virus (WSSV) from crabs to shrimp Penaeus monodon. Dis. Aquat. Org. 1998;34:1–7. doi: 10.3354/dao034001. [DOI] [PubMed] [Google Scholar]
- Karunasagar I., Otta S.K., Karunasagar I. Disease problems affecting cultured penaeid shrimp in India. Fish Pathol. 1998;33:413–419. [Google Scholar]
- Kim C.K., Kim P.K., Sohn S.G., Sim D.S., Park M.A., Heo M.S., Lee T.H., Lee J.D., Jun H.K., Jang K.L. Development of a polymerase chain reaction (PCR) procedure for the detection of baculovirus associated with white spot syndrome (WSBV) in penaeid shrimp. J. Fish Dis. 1998;21:11–17. doi: 10.1046/j.1365-2761.1998.00324.x. [DOI] [PubMed] [Google Scholar]
- Krabsetsve K., Cullen B.R., Owens L. Rediscovery of the Australian strain of infectious hypodermal and haematopoietic necrosis virus. Dis. Aquat. Org. 2004;61:153–158. doi: 10.3354/dao061153. [DOI] [PubMed] [Google Scholar]
- Laramore, C.R., 1997. Shrimp culture in Honduras following the Taura syndrome virus. In: Proceeding of the 4th Symposium on Aquaculture in Central America: Focusing on Shrimp and Tilapia, Tegucigalpa, Honduras, World Aquaculture Soc., Baton Rouge, Louisiana, USA, pp. 1–7.
- Lee C.S., O’Bryen P.J., editors. Biosecurity in Aquaculture Production Systems: Exclusion of Pathogens and Othr Undesirables. The World Aquaculture Society; Baton Rouge, Louisiana 70803, USA: 2003. p. 93 p. [Google Scholar]
- Lightner D.V. Diseases of cultured penaeid shrimp. In: McVey J.P., editor. vol. 1. CRC Press; Boca Raton, FL, USA: 1983. pp. 289–320. (CRC Handbook of Mariculture. Crustacean Aquaculture). [Google Scholar]
- Lightner D.V. Diseases of cultured penaeid shrimp and prawns. In: Sindermann C.J., Lightner D.V., editors. Disease Diagnosis and Control in North American Marine Aquaculture. Elsevier; Amsterdam, The Netherlands: 1988. pp. 8–127. [Google Scholar]
- Lightner D.V. Diseases of cultured penaeid shrimp. In: McVey J.P., editor. second ed. vol. 1. CRC Press; Boca Raton, FL, USA: 1993. pp. 393–486. (CRC Handbook of Mariculture: Crustacean Aquaculture). [Google Scholar]
- Lightner, D.V., 1995. Taura Syndrome: an economically important viral disease impacting the shrimp farming industries of the Americas including the United States. In: Proceedings Ninety-Ninth Annual Meeting of the US Animal Health Association, Reno, NV, October 28–November 3, 1995, U.S.A.H.A., Richmond, VA, pp. 36–52.
- Lightner D.V., editor. A Handbook of Shrimp Pathology and Diagnostic Procedures for Diseases of Cultured Penaeid Shrimp. World Aquaculture Society; Baton Rouge, Louisiana, USA: 1996. p. 304 pp. [Google Scholar]
- Lightner D.V. Epizootiology, distribution and the impact on international trade of two penaeid shrimp viruses in the Americas. Rev. Sci. Tech. Office Int. Epiz. 1996;15:579–601. doi: 10.20506/rst.15.2.944. [DOI] [PubMed] [Google Scholar]
- Lightner D.V. The penaeid shrimp viruses TSV, IHHNV, WSSV, and YHV: current status in the Americas, available diagnostic methods and management strategies. J. Appl. Aquacult. 1999;9:27–52. [Google Scholar]
- Lightner, D.V., 2003a. The penaeid shrimp viral pandemics due to IHHNV, WSSV, TSV and YHV: history in the Americas and current status. In: Sakai, Y., McVey, J.P., Jang, D., McVey, E., Caesar, M. (Eds.), Proceedings of the Thirty-second US Japan Symposium on Aquaculture. US–Japan Cooperative Program in Natural Resources (UJNR). US Department of Commerce, N.O.A.A., Silver Spring, MD, USA. pp. 6–24. <http://www.lib.noaa.gov/japan/aquaculture/aquaculture_panel.htm>.
- Lightner D.V. Exclusion of specific pathogens for disease prevention in a penaeid shrimp biosecurity program. In: Lee C.-S., O’Bryen P.J., editors. Biosecurity in Aquacvulture Production Systems: Exclusion of Pathogens and Other Undesirables. The World Aquaculture Society; Baton Rouge, LA, USA: 2003. pp. 81–116. [Google Scholar]
- Lightner D.V. Biosecurity in shrimp farming: pathogen exclusion through the use of SPF stock and routine surveillance. J. World Aquacult. Soc. 2005;36:229–248. [Google Scholar]
- Lightner D.V., Redman R.M. Shrimp diseases and current diagnostic methods. Aquaculture. 1998;164:201–220. [Google Scholar]
- Lightner D.V., Redman R.M. Strategies for the control of viral diseases of shrimp in the Americas. Fish Pathol. 1998;33:165–180. [Google Scholar]
- Lightner D.V., Redman R.M., Bell T.A. Infectious hypodermal and hematopoietic necrosis a newly recognized virus disease of penaeid shrimp. J. Invertebr. Pathol. 1983;42:62–70. doi: 10.1016/0022-2011(83)90202-1. [DOI] [PubMed] [Google Scholar]
- Lightner D.V., Redman R.M., Bell T.A., Brock J.A. Detection of IHHN virus in Penaeus stylirostris and P. Vannamei imported into Hawaii. J. World Maricult. Soc. 1983;14:212–225. [Google Scholar]
- Lightner D.V., Bell T.A., Redman R.M., Perez L.A. A collection of case histories documenting the introduction and spread of the virus disease IHHN in penaeid shrimp culture facilities in Northwestern Mexico. ICES Mar. Sci. Symp. 1992;194:97–105. [Google Scholar]
- Lightner D.V., Redman R.M., Hasson K.W., Pantoja C.R. Taura syndrome in Penaeus vannamei (Crustacea: Decapoda): gross signs, histopathology and ultrastructure. Dis. Aquat. Org. 1995;21:53–59. [Google Scholar]
- Lightner D.V., Hasson K.W., Redman R.M., White B.L. Experimental infection of Western Hemisphere penaeid shrimp with Asian white spot syndrome virus and Asian Yellow head virus. J. Aquat. Anim. Health. 1998;10:271–281. [Google Scholar]
- Lightner D.V., Durand S.V., Redman R.M., Mohney L.L., Tang-Nelson K. Qualitative and quantitative studies on the relative virus load of tails and heads of shrimp acutely infected with WSSV: implications for risk assessment. In: Browdy C.L., Jory D.E., editors. The New Wave. Proceedings of the Special Session on Sustainable Shrimp Culture, Aquaculture 2001. The World Aquaculture Society; Baton Rouge, LA, USA: 2001. pp. 285–291. [Google Scholar]
- Lightner D.V., Pantoja C.R., Poulos B.T., Tang K.F.J., Redman R.M., Andrade T.P., Bonami J.R. Infectious myonecrosis: new disease in Pacific white shrimp. Global Aquacult. Advocate. 2004;7:85. [Google Scholar]
- Limuswan C. Tansetakit; Bangkok, Thailand (in Thai): 1991. Handbook for Cultivation of Black Tiger Prawns. [Google Scholar]
- Lo C.F., Kou G.H. Virus-associated white spot syndrome of syndrome in Taiwan: a review. Fish Pathol. 1998;33:365–371. [Google Scholar]
- Lo C.F., Leu J.H., Chen C.H., Peng S.E., Chen Y.T., Chou C.M., Yeh P.Y., Huang C.J., Chou H.Y., Wang C.H., Kou G.H. Detection of baculovirus associated with white spot syndrome (WSBV) in penaeid shrimps using polymerase chain reaction. Dis. Aquat. Org. 1996;25:133–141. [Google Scholar]
- Loh P.C., Tapay L.M., Nadalam E.C. Viral pathogenesis of penaeid shrimp. Adv. Virus Res. 1997;48:263–312. doi: 10.1016/S0065-3527(08)60290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz J.M. Disease control and pathogen status assurance in an SPF-based shrimp aquaculture industry, with particular reference to the United States. In: Flegel T.W., MacRae I.H., editors. Diseases in Asian Aquaculture III. Fish Health Section; Asian Fisheries Society, Manila, The Philippines: 1997. pp. 243–254. [Google Scholar]
- Lotz J.M. Effect of host size on virulence of Taura virus to the marine shrimp Penaeus vannamei (Crustacea: Penaeidae) Dis. Aquat. Org. 1997;30:45–51. [Google Scholar]
- Lotz J.M., Anton L.S., Soto M.A. Effect of chronic Taura syndrome virus infection on salinity tolerance of Litopenaeus vannamei. Dis. Aquat. Org. 2005;65:75–78. doi: 10.3354/dao065075. [DOI] [PubMed] [Google Scholar]
- Lu Y., Tapay L.M., Brock J.A., Loh P.C. Infection of the yellow head baculo-like virus (YBV) in two species of penaeid shrimp Penaeus stylirostris (Stimpson) and Penaeus vannamei (Boone) J. Fish Dis. 1994;17:649–656. [Google Scholar]
- Lu Y., Tapay L.M., Gose R.B., Brock J.A., Loh P.C. Infectivity of yellow head virus (YHV) and the Chinese baculo-like virus (CBV) in two species of penaeid shrimp Penaeus stylirostris (Stimpson) and Penaeus vannamei (Boone) In: Flegel T.W., MacRae I.H., editors. Diseases in Asian Aquaculture III. Asian Fisheries Society; Manila, the Philippines: 1997. pp. 297–304. [Google Scholar]
- Luo P., Hu C.Q., Ren C.H., Sun Z.F. Taura syndrome virus and mammalian cell lines. Emerg. Infect. Dis. (CDC) 2004;10:2260–2261. doi: 10.3201/eid1012.040537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mari J., Bonami J.R., Lightner D.V. Partial cloning of the genome of infectious hypodermal and hematopoietic necrosis virus, an unusual parvovirus pathogenic for penaeid shrimps; diagnosis of the disease using a specific probe. J. Gen. Virol. 1993;74:2637–2643. doi: 10.1099/0022-1317-74-12-2637. [DOI] [PubMed] [Google Scholar]
- Mari J., Bonami J.R., Lightner D.V. Taura syndrome of penaeid shrimp: cloning of viral genome fragments and development of specific gene probes. Dis. Aquat. Org. 1998;33:11–17. doi: 10.3354/dao033011. [DOI] [PubMed] [Google Scholar]
- Mari J., Poulos B.T., Lightner D.V., Bonami J.R. Shrimp Taura syndrome virus: genomic characterization and similarity with members of the genus Cricket paralysis-like viruses. J. Gen. Virol. 2002;83:915–926. doi: 10.1099/0022-1317-83-4-915. [DOI] [PubMed] [Google Scholar]
- Martinez-Cordova L.R. Cultured blue shrimp (Penaeus stylirostris) infected with infectious hypodermal and hematopoietic necrosis virus in northwestern Mexico. Prog. Fish Cultur. 1992;54:265–266. [Google Scholar]
- Mayo M.A. Virus taxonomy – Houston 2002. Arch. Virol. 2002;147(5):1071–1076. doi: 10.1007/s007050200036. [DOI] [PubMed] [Google Scholar]
- Mayo M.A. A summary of taxonomic changes recently approved by ICTV. Arch. Virol. 2002;14(8):1655–1656. doi: 10.1007/s007050200039. [DOI] [PubMed] [Google Scholar]
- Morales-Covarrubias M.S., Chavez-Sanchez M.C. Histopathological studies on wild broodstock of white shrimp Penaeus vannamei in the Platanitos area, adjacent to San Blas, Nayarit, Mexico. J. World Aquacult. Soc. 1999;30:192–200. [Google Scholar]
- Morales-Covarrubias M.S., Nunan L.M., Lightner D.V., Mota-Urbina J.C., Garza-Aguirre M.C., Chavez-Sanchez M.C. Prevalence of IHHNV in wild broodstock of Penaeus stylirostris from the upper Gulf of California, Mexico. J. Aquat. Anim. Health. 1999;11:296 301. [Google Scholar]
- Moss S.M., Arce A.M., Argue B.J., Otoshi C.A., Calderon F.R.O., Tacon A.G.J. Greening of the blue revolution: efforts toward environmentally responsible shrimp culture. In: Browdy C.L., Jory D.E., editors. The New Wave, Proceedings of the Special Session on Sustainable Shrimp Culture, Aquaculture 2001. The World Aquaculture Society; Baton Rouge, LA, USA: 2002. pp. 1–19. [Google Scholar]
- Motte E., Yugcha E., Luzardo J., Castro F., Leclercq G., Rodríguez J., Miranda P., Borja O., Serrano J., Terreros M., Montalvo K., Narváez A., Tenorio N., Cedeño V., Mialhe E., Boulo V. Prevention of IHHNV vertical transmission in the white shrimp Litopenaeus vannamei. Aquaculture. 2003;219:57–70. [Google Scholar]
- Nadala E.C.B., Loh P.C. A comparative study of three different isolates of white spot virus. Dis. Aquat. Org. 1998;33(3):231–234. doi: 10.3354/dao033231. [DOI] [PubMed] [Google Scholar]
- Nadala E.C.B., Tapay L.M., Loh P.C. Characterization of a non-occluded baculovirus-like agent pathogenic to penaeid shrimp. Dis. Aquat. Org. 1998;33(3):221–229. doi: 10.3354/dao033221. [DOI] [PubMed] [Google Scholar]
- Nakano H., Koube H., Umezawa S., Momoyama K., Hiraoka M., Inouye K., Oseko N. Mass mortalities of cultured Kuruma shrimp, Penaeus japonicus, in Japan in 1993: Epizootiological survey and infection trails. Fish Pathol. 1994;29:135–139. [Google Scholar]
- Nielsen L., Sang-oum W., Cheevadhanarak S., Flegel T.W. Taura syndrome virus (TSV) in Thailand and its relationship to TSV in China and the Americas. Dis. Aquat. Org. 2005;63:101–106. doi: 10.3354/dao063101. [DOI] [PubMed] [Google Scholar]
- Nunan L.M., Poulos B.T., Lightner D.V. Reverse transcription polymerase chain reaction (RT-PCR) used for the detection of Taura Syndrome virus (TSV) in experimentally infected shrimp. Dis. Aquat. Org. 1998;34:87–91. doi: 10.3354/dao034087. [DOI] [PubMed] [Google Scholar]
- Nunan L.M., Poulos B.T., Lightner D.V. The detection of white spot syndrome virus (WSSV) and yellow head virus (YHV) in imported commodity shrimp. Aquaculture. 1998;160:19–30. [Google Scholar]
- Nunan L.M., Arce S.M., Staha R.J., Lightner D.V. Prevalence of infectious hypodermal and hematopoietic necrosis virus (IHHNV) and white spot syndrome virus (WSSV) in Litopenaeus vannamei in the Pacific Ocean off the coast of Panama. J. World Aquacult. Soc. 2001;32:330–334. [Google Scholar]
- Nunan L.M., Poulos B.T., Lightner D.V. Use of polymerase chain reaction (PCR) for the detection of infectious hypodermal and hematopoietic necrosis virus (IHHNV) in penaeid shrimp. Mar. Biotechnol. 2000;2:319–328. doi: 10.1007/s101260000003. [DOI] [PubMed] [Google Scholar]
- Nunan L.M., Tang-Nelson K., Lightner D.V. Real-time RT-PCR determination of viral copy number in Penaeus vannamei experimentally infected with Taura syndrome virus (TSV) Aquaculture. 2004;229:1–10. [Google Scholar]
- Nunes A.J.P., Cunha-Martins P., Vasconselos-Gesteira T.-C. Carcinicultura ameacada. Rev. Panoram. Aquic. 2004;83:37–51. (in Portuguese) [Google Scholar]
- OIE (Office International des Epizooties) forth ed. Office International des Epizooties (OIE); Paris, France: 2003. Diagnostic Manual for Aquatic Animal Diseases. 358p. [Google Scholar]
- OIE (Office International des Epizooties) ninth ed. Office International des Epizooties; Paris, France: 2006. Aquatic Animal Health Code. 202p. [Google Scholar]
- OIE (Office International des Epizooties) fifth ed. Office International des Epizooties; Paris, France: 2006. Manual of diagnostic tests for Aquatic Animal diseases. 467p. [Google Scholar]
- Ostrowski, A.C., 2004. Consortium research update FY2004. The Latest Report from the USMSFP Consortium, December 2004. Waimanolo, HI, pp. 1–6. <http://www.usmsfp.org/research.htm>.
- Overstreet R.M., Lightner D.V., Hasson K.W., McIlwain S., Lotz J. Susceptibility to TSV of some penaeid shrimp native to the Gulf of Mexico and southeast Atlantic Ocean. J. Invertebr. Pathol. 1997;69:165–176. doi: 10.1006/jipa.1996.4654. [DOI] [PubMed] [Google Scholar]
- Pantoja C.R., Lightner D.V., Holtschmit H.K. Prevalence and geographic distribution of IHHN parvovirus in wild penaeid shrimp (Crustacea: Decapoda) from the Gulf of California, Mexico. J. Aquat. Anim. Health. 1999;11:23–34. [Google Scholar]
- Pantoja C.R., Lightner D.V. Similarity between the histopathology of White spot syndrome virus and Yellow head syndrome virus and its relevance to diagnosis of YHV disease in the Americas. Aquaculture. 2003;218:47–54. [Google Scholar]
- Pantoja C.R., Navarro S.A., Naranjo J., Lightner D.V., Yerba C.P. Non-susceptibility of three primate cell lines to infection by Taura syndrome virus of penaeid shrimp. Emerg. Infect. Dis. (CDC) 2004;10(12):2106–2112. doi: 10.3201/eid1012.040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Farfante I., Kensley B.F. The penaeoid and sergestoid shrimps and prawns of the world: keys and diagnosis for the families and genera. Mem. Mus. Ď’Hist. Natur. 1997;175:1–233. [Google Scholar]
- Phalitakul S., Wongtawatchai J., Sarikaputi M., Viseshakul N. The molecular detection of Taura syndrome virus emerging with White spot syndrome virus in penaeid shrimp of Thailand. Aquaculture. 2006;260:77–85. [Google Scholar]
- Poulos B.T., Lightner D.V., Trumper B., Bonami J.R. Monoclonal antibodies to a penaeid shrimp parvovirus, infectious hypodermal and hematopoietic necrosis virus (IHHNV) J. Aquat. Anim. Health. 1994;6:149–154. [Google Scholar]
- Poulos B.T., Tang K.F.J., Pantoja C.R., Bonami J.R., Lightner D.V. Purification and characterization of infectious myonecrosis virus of penaeid shrimp. J. Gen. Virol. 2006;87:987–996. doi: 10.1099/vir.0.81127-0. [DOI] [PubMed] [Google Scholar]
- Poulos B.T., Lightner D.V. Detection of infectious myonecrosis virus (IMNV) of penaeid shrimp by reverse-transcriptase polymerase chain reaction (RT-PCR) Dis. Aquat. Org. 2006;73:69–72. doi: 10.3354/dao073069. [DOI] [PubMed] [Google Scholar]
- Primavera J.H., Quinitio E.T. Runt-deformity syndrome in cultured giant tiger prawn Penaeus monodon. J. Crust. Biol. 2000;20:796–802. [Google Scholar]
- Pruder G.D., Brown C.L., Sweeney J.N., Carr W.H. High health shrimp systems: seed supply – theory and practice. In: Browdy C.L., Hopkins J.S., editors. Swimming through Troubled Water, Proceedings of the Special Session on Shrimp Farming, Aquaculture ‘95. 1–4 February 1995. San Diego. World Aquaculture Society; Baton Rouge, LA, USA: 1995. pp. 40–52. [Google Scholar]
- Robles-Sikisaka R., Garcia D.K., Klimpel K.R., Dhar A.K. Nucleotide sequence of 3′-end of the genome of Taura syndrome virus of shrimp suggests that it is related to insect picornaviruses. Arch. Virol. 2001;146:941–952. doi: 10.1007/s007050170126. [DOI] [PubMed] [Google Scholar]
- Rosenberry B., editor. vol. 13. Shrimp News International; San Diego, CA: 2001. p. 324 pp. (World Shrimp Farming 2000). [Google Scholar]
- Rosenberry B., editor. World Shrimp Farming 2003. Shrimp News International; San Diego, CA: 2003. p. 76 p. [Google Scholar]
- Sánchez-Barajas M., Liñán-Cabello M.A., Mena-Herrera A. Detection of yellow-head disease in intensive freshwater production systems of Litopenaeus vannamei. Aquacult. Int. 2009;17:101–112. [Google Scholar]
- Shike H., Dhar A.K., Burns J.C., Shimizu Ch., Jousset F.X., Klimpel K.R., Bergoin M. Infectious hypodermal and hematopoietic necrosis virus of shrimp is related to mosquito Brevidensoviruses. Virology. 2000;277:167–177. doi: 10.1006/viro.2000.0589. [DOI] [PubMed] [Google Scholar]
- Sittidilokratna S., Hodgson R.A.J., Panyim S., Cowley J.A., Boonsaeng V., Walker P.J. Complete ORF-1b-gene sequence indicates yellow head virus is an invertebrate nidovirus. Dis. Aquat. Org. 2002;50:87–93. doi: 10.3354/dao050087. [DOI] [PubMed] [Google Scholar]
- Srisuvan T., Tang K.F.J., Lightner D.V. Experimental infection pf Penaeus monodon with Taura syndrome virus (TSV) Dis. Aquat. Org. 2005;67:1–8. doi: 10.3354/dao067001. [DOI] [PubMed] [Google Scholar]
- Srisuvan T., Noble B.L., Schofield P.J., Lightner D.V. Comparison of four Taura syndrome virus (TSV) isolates in oral challenge studies with Litopenaeus vannamei unselected or selected for resistance to TSV. Dis. Aquat. Org. 2006;71:1–10. doi: 10.3354/dao071001. [DOI] [PubMed] [Google Scholar]
- Tang K.F.J., Lightner D.V. A yellow-head virus gene probe: application to in situ hybridization and determination of its nucleotide sequence. Dis. Aquat. Org. 1999;35:165–173. doi: 10.3354/dao035165. [DOI] [PubMed] [Google Scholar]
- Tang K.F.J., Lightner D.V. Detection and quantification of infectious hypodermal and hematopoietic necrosis virus in penaeid shrimp by real-time PCR. Dis. Aquat. Org. 2001;44:79–85. doi: 10.3354/dao044079. [DOI] [PubMed] [Google Scholar]
- Tang K.F.J., Lightner D.V. Low sequence variation among isolates of infectious hypodermal and hematopoietic necrosis virus (IHHNV) originating from Hawaii and the Americas. Dis. Aquat. Org. 2002;49:93–97. doi: 10.3354/dao049093. [DOI] [PubMed] [Google Scholar]
- Tang K.F.J., Lightner D.V. Phylogenetic analysis of Taura syndrome virus isolates collected between 1993 and 2004 and virulence comparison between two isolates representing different genetic variants. Virus Res. 2005;112:69–76. doi: 10.1016/j.virusres.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Tang K.F.J., Lightner D.V. Infectious hypodermal and hematopoietic necrosis virus (IHHNV) in the genome of the black tiger prawn Penaeus monodon from Africa and Australia. Virus Res. 2006;118:185–191. doi: 10.1016/j.virusres.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Tang K.F.J., Durand S.V., White B.L., Redman R.M., Pantoja C.R., Lightner D.V. Postlarvae and juveniles of a selected line of Penaeus stylirostris are resistant to infectious hypodermal and hematopoietic necrosis virus infection. Aquaculture. 2000;190:203–210. [Google Scholar]
- Tang K.F.J., Poulos B.T., Wang J., Redman R.M., Shih H.-H., Lightner D.V. Geographic variations among infectious hypodermal and hematopoietic necrosis virus (IHHNV) isolates and characteristics of their infection. Dis. Aquat. Org. 2003;53:91–99. doi: 10.3354/dao053091. [DOI] [PubMed] [Google Scholar]
- Tang K.F.J., Pantoja C.R., Poulos B.T., Redman R.M., Lightner D.V. In situ hybridization demonstrates that Litopenaeus vannamei, L. stylirostris and Penaeus monodon are susceptible to experimental infection with infectious myonecrosis virus (IMNV) Dis. Aquat. Org. 2005;63:261–265. doi: 10.3354/dao063261. [DOI] [PubMed] [Google Scholar]
- Tang K.F.J., Navarro S.A., Lightner D.V. PCR assay for discriminating between infectious hypodermal and hematopooietic necrosis virus (IHHNV) and the virus-related sequences in the genome of Penaeus monodon. Dis. Aquat. Org. 2007;74:165–170. doi: 10.3354/dao074165. [DOI] [PubMed] [Google Scholar]
- Tu C., Huang H.T., Chuang S.H., Hsu J.P., Kuo S.T., Li N.J., Hus T.L., Li M.C., Lin S.Y. Taura syndrome in Pacific white shrimp Penaeus vannamei cultured in Taiwan. Dis. Aquat. Org. 1999;38:159–161. [Google Scholar]
- van Hulten M.C.W., Witteveldt J., Peters S., Kloosterboer N., Tarchini R., Fiers M., Sandbrink H., Lankhorst R.K., Vlak J.M. The white spot syndrome virus DNA genome sequence. Virology. 2001;286:7–22. doi: 10.1006/viro.2001.1002. [DOI] [PubMed] [Google Scholar]
- Vanpatten K.A., Nunan L.M., Lightner D.V. Seabirds as potential vectors of penaeid shrimp viruses and the development of a surrogate laboratory model utilizing domestic chickens. Aquaculture. 2004;241:31–46. [Google Scholar]
- Vlak, J.M., Bonami, J.R., Flegel, T.W., Kou, G.H., Lightner, D.V. Lo, C.F. Loh, P.C., Walker, P.W., 2005. Family Nimaviridae, Genus Whispovirus. 8th Report of the ICTV: Virus Taxonomy. In: Fauquet, C.M., Mayo, M.A., Maniloff, J., Desselberger, U., Ball, L.A. (Eds.), Virus Taxonomy. Classification and Nomenclature of Viruses, pp. 187–192. Eighth Report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, 1259 pp.
- Vidal O.M., Granja C.B., Aranguren F., Brock J.A., Salazar M. A profound effect of hyperthermia on survival of Litopenaeus vannamei juveniles infected with White spot syndrome virus. J. World Aquacult. Soc. 2001;32:364–372. [Google Scholar]
- Walker P.J., Cowley J.A., Spann K.M., Hodgson R.A.J., Hall M.R., Withyachumnarnkul B. Yellow head complex viruses: transmission cycles and topogtraphical distbibution in the Asia–Pacific region. In: Browdy C.L., Jory D.E., editors. The New Wave, Proceedings of the Special Session on Sustainable Shrimp Culture, Aquaculture 2001. The World Aquaculture Society; Baton Rouge, LA, USA: 2001. pp. 292–302. [Google Scholar]
- Wang C.H., Lo C.F., Leu J.H., Chou C.M., Yeh P.Y., Chou H.Y., Tung M.C., Chang C.F., Su M.S., Kou G.H. Purification and genomic analysis of baculovirus associated with white spot syndrome (WSBV) of Penaeus monodon. Dis. Aquat. Org. 1995;23:239–242. [Google Scholar]
- White B.L., Schofield P.J., Poulos B.T., Lightner D.V. A laboratory challenge method for estimating Taura syndrome virus resistance in selected lines of Pacific white shrimp Penaeus vannamei. J. World Aquacult. Soc. 2002;33:341–348. [Google Scholar]
- Wilkinson, S., 2006. IMNV Found in Asia–Pacific. NACA (Network of Aquaculture Centers in Asia). <http://www.enaca.org/modules/news/article.php?storyid=825> (posted 21.08.06).
- Withyachymnarnkul B., Chayaburakul K., Lao-Aroon S., Plodpal P., Sritunyalucksana K., Nash G. Low impact of infectious hypodermal and hematopoietic necrosis virus (IHHNV) on growth and reproduction performance of Penaeusmonodon. Dis. Aquat. Org. 2006;69:129–136. doi: 10.3354/dao069129. [DOI] [PubMed] [Google Scholar]
- Wongteerasupaya C., Vickers J.E., Sriurairatana S., Nash G.L., Akarajamorn A., Boonsaeng V., Panyim S., Tassanakajon A., Withyachumnarnkul B., Flegel T.W. A non-occluded, systemic baculovirus that occurs in cells of ectodermal and mesodermal origin and causes high mortality in the black tiger prawn, Penaeus monodon. Dis. Aquat. Org. 1995;21:69–77. [Google Scholar]
- Wyban, J.A., Swingle, J.S., Sweeney, J.N., Pruder, G.D., 1992. Development and commercial performance of high health shrimp using specific pathogen free (SPF) broodstock Penaeus vannamei. In: Wyban, J., (Ed.), Proceedings of the Special Session on Shrimp Farming. World Aquaculture Society, Baton Rouge, LA, pp. 254–259.
- Yang F., He J., Lin X., Li Q., Pan D., Zhang X., Xu X. Complete genome sequence of the shrimp white spot bacilliform virus. J. Virol. 2001;75:11811–11820. doi: 10.1128/JVI.75.23.11811-11820.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C.I., Song Y.L. Outbreaks of Taura syndrome in Pacific white shrimp Penaeus vannamei cultured in Taiwan. Fish Pathol. 2000;32:21–24. [Google Scholar]
- Zarain-Herzberg M., Ascencio-Valle F. Taura syndrome in Mexico: follow-up study in shrimp farms of Sinaloa. Aquaculture. 2001;193:1–9. [Google Scholar]


