Abstract
We determined the complete nucleotide and predicted amino acid sequence of the genomic RNA of PL97-1, the first Korean strain of porcine reproductive and respiratory syndrome virus (PRRSV), which was isolated from the serum of an infected pig in 1997. We found that the 15411-nucleotide genome of PL97-1 consisted of a 189-nucleotide 5′ noncoding region (NCR), a 15071-nucleotide protein-coding region, and a 151-nucleotide 3′NCR, followed by a poly (A) tail. The 5′-end of PL97-1 began with 1ATG ACG TAT AGG12. Comparison of the PL97-1 genome with the 11 fully sequenced PRRSV genomes currently available revealed sequence divergence ranging from 0.3% (the VR-2332-derived vaccine MLV RespPRRS/Repro strain) to 38% (the Dutch Lelystad strain). To better understand the genetic relationships between these different strains, phylogenetic analyses were performed on the full-length PRRSV genomes. Significantly, the phylogenetic tree based on the ORF1b or ORF7 genes most closely resembled the tree based on the full-length genomes. Thus, these single genes will be the most useful in revealing the genetic relationships between the different strains relative to their geographical distribution. Extensive phylogenetic analyses using the ORF7 sequences of 111 PRRSV isolates available revealed that PL97-1 is most closely related to the North American genotype VR-2332, a VR-2332-derived vaccine strain, and Chinese BJ-4. It is distantly related to the European genotype Lelystad. This study provides the largest full-length genome phylogenetic analysis of PRRSV that has been published to date, and supports an earlier genetic grouping of the many temporally and geographically diverse PRRSV strains currently isolated.
Keywords: PRRSV, PL97-1, Complete genome sequence, Phylogeny
1. Introduction
Porcine reproductive and respiratory syndrome (PRRS) is characterized by the reproductive losses of sows and respiratory disorders in piglets and was first reported in the US in 1987 (Keffaber, 1989) and in Europe in 1990 (Paton et al., 1991, Wensvoort et al., 1991). PRRS is a global disease that has an immense economic impact on the swine industry. The causative agent, the PRRS virus (PRRSV), was first described as the Lelystad virus in Europe (Wensvoort et al., 1991) and VR-2332 in the US (Benfield et al., 1992, Collins et al., 1992). PRRSV is a member of the family Arteriviridae and belongs to the order Nidovirales along with equine arteritis virus (EAV), lactate dehydrogenase-elevating virus (LDV), and simian hemorrhagic fever virus (SHFV) (Cavanagh, 1997, Snijder and Meulenberg, 1998).
PRRSV is a small-enveloped virus containing a positive-sense, single-stranded ≈15 kb RNA genome with a poly (A) tail at its 3′-end. The genome contains nine open reading frames (ORFs) flanked by 5′ and 3′ noncoding regions (NCRs). The replicase proteins are encoded by two overlapping ORFs (ORF1a and 1b). The viral structural proteins are encoded by ORFs 2–7 (Meulenberg et al., 1993). ORF5, 6, and 7 encode the three major structural proteins (the envelope glycoprotein, membrane, and nucleocapsid proteins, respectively), while ORF2a, 2b, 3 and 4 express four minor structural proteins (Meulenberg et al., 1997, Snijder and Meulenberg, 1998, Snijder and Meulenberg, 2001, Dea et al., 2000).
PRRSV emerged almost simultaneously in Europe and North America with very similar disease symptoms. Surprisingly, however, the two PRRSV isolates share only 55–70% nucleotide identity in their genes (Kwang et al., 1994, Mardassi et al., 1994, Meng et al., 1994, Meng et al., 1995a, Morozov et al., 1995, Murtaugh et al., 1995, Gagnon and Dea, 1998, Nelsen et al., 1999). Thus, Lelystad and VR-2332 are considered as the reference strains of the European and the American genotypes, respectively. Additional PRRSV strains have since been isolated from pigs at different times and locations. Most have been partially sequenced. Previous phylogenetic analyses have mainly focused on partial ORF5 sequences (Meng et al., 1995b, Suarez et al., 1996, Andreyev et al., 1997, Madsen et al., 1998, Goldberg et al., 2000, Indik et al., 2000, Key et al., 2001, Forsberg et al., 2002, Stadejek et al., 2002, Mateu et al., 2003), which appears to be the most variable protein when the American and European isolates are compared (Murtaugh et al., 1995, Kapur et al., 1996) and shows the highest degree of genetic diversity within a single genotype (Suarez et al., 1996, Pirzadeh et al., 1998). Other studies have also investigated the PRRSV genetic relationships by comparing the ORF7 nucleotide sequences (Meng et al., 1995a, Suarez et al., 1996, Drew et al., 1997, Chueh et al., 1998, Le Gall et al., 1998, Madsen et al., 1998, Medveczky et al., 2001, Forsberg et al., 2002, Stadejek et al., 2002) or other proteins (Meng et al., 1995b, Drew et al., 1997, Madsen et al., 1998, Nelsen et al., 1999, Oleksiewicz et al., 2000).
Here, we determined the complete nucleotide and amino acid sequences of PL97-1, the first PPRSV Korean isolate that was isolated in 1997. We also investigated the genetic relationships between PL97-1 and the 11 fully-sequenced worldwide-distributed PRRSV strains. We found that the phylogenic trees based on the nonstructural ORF1b and the structural ORF7 genes resembled the tree based on the full-length PRRSV genome better than the other PRRSV genes. We therefore extensively analyzed PRRSV isolates using 111 selected ORF7 gene sequences from a wide range of PRRSV strains isolated from different places or at different times. The epidemiological implications of this study are discussed.
2. Materials and methods
2.1. Virus and cells
PL97-1 was originally isolated from the serum of an infected pig (80 days old) displaying clinical respiratory distress in the Korean province Kyounggi-Do in 1997. The herd, from which PL97-1 was isolated, was not vaccinated. The virus was passaged twice on subconfluent monolayers of MARC-145 cells in minimum essential medium supplemented with 5% fetal bovine serum, nonessential amino acids, sodium pyruvate, and antibiotics in 5% CO2 at 37 °C. The virus was collected when 70% of the cells showed cytopathology. The supernatant was stored at −80 °C as the viral stock.
2.2. Reverse transcription
Viral RNA was extracted from 100 μl virus stock with 300 μl TRIzol LS reagent as recommended by the manufacturer (GIBCO/BRL, Gaitherburg, MD). 5 μg glycogen (Boehringer Mannheim, Indianapolis, IN) was added as a carrier to extracted samples prior to isopropanol precipitation to improve viral RNA recovery. The viral RNA was used as a template for cDNA synthesis using Superscript II RNaseH(−) RT (GIBCO/BRL) and primers based on the consensus sequence of nine fully sequenced PRRSV RNA genomes available from GenBank (Lelystad, NVSL 97-7985, CH-1a, SP, 16244B, PA8, VR-2332, RespPRRS MLV, and BJ-4). Reverse transcription was conducted as described previously (Yun et al., 2003) with an appropriate primer (see below).
2.3. Synthesis of overlapping cDNAs encompassing the PL97-1 genome
Four long overlapping cDNAs (PF1, PF2, PF3, and PF4) encompassing the entire viral RNA genome apart from the 5′- and 3′-termini were obtained by optimized long RT-PCR using the low-error-rate Pfu DNA polymerase and primer pairs, designed according to the consensus sequence of nine fully sequenced PRRSV RNA genomes (Table 1 ). The cDNA synthesis and PCR of PF1 (nt 180–5297) employed the PR1RT and the PR1F/PR1R primers, respectively. PF2 (nt 3708–9108) amplification used the PR2RT and the PR2F/PR2R primers, respectively, while PF3 (nt 7688–13051) employed the PR3RT and the PR3F/PR3R primers, respectively. PF4 amplification (nt 9610–15238) utilized the PR4RT and the PR4F/PR4R primers, respectively. 5 μl of standard RT reactions performed as described above were used for amplification with Pyrobest DNA polymerase (Takara Bio Inc., Shiga, Japan) employing 35 cycles of denaturation (94 °C for 30 s), annealing (60 °C for 30 s), and extension (72 °C for 6 min), with a final extension step (72 °C for 10 min). The four overlapping PCR products were amplified as dominant bands in reactions containing an RNaseH(−) RT, but not in reactions lacking the enzyme (data not shown).
Table 1.
Oligonucleotides used for ligation, cDNA synthesis, and PCR amplification
| Oligonucleotide | Sequencea | Positionb | Polarity |
|---|---|---|---|
| PR1RT | 5′-TAGGATGGTGAGGGGGTG | 5332–5349 | Antisense |
| PR1F | 5′-CCCTTTAACCATGTGT | 180–195 | Sense |
| PR1R | 5′-CAAAGCAACCAGGTAA | 5282–5297 | Antisense |
| PR2RT | 5′-GAGCATGTCCTCAAACTT | 9168–9185 | Antisense |
| PR2F | 5′-CCGGATATGGTCGCGG | 3708–3723 | Sense |
| PR2R | 5′-CCATATGCTGTGCATA | 9093–9108 | Antisense |
| PR3RT | 5′-CACATTCCCTATCCCGAA | 13074–13091 | Antisense |
| PR3F | 5′-GTTTAAACTGCTAGCC | 7688–7703 | Sense |
| PR3R | 5′-GTGTAGCTGAAGGACA | 13036–13051 | Antisense |
| PR4RT | 5′-CTAATTGAATAGGTGACT | 15342–15359 | Antisense |
| PR4F | 5’-ATTATGAGGGGAAGAA | 9610–9625 | Sense |
| PR4R | 5′-ACGCGGATCAGGCGCA | 15223–15238 | Antisense |
| PR41 | 5′-GGAGAAGCCCCATTTTCC | 15038–15055 | Sense |
| PR49 | 5′-CGACCCGTACCATTCTTT | 476–493 | Antisense |
| PR50 | 5′-AAAAGTCTTCAGGCTTGG | 692–709 | Antisense |
| PRX | 5’-CCAGTGTTGTGGGCTGCAGGGCGAATT | ||
| PRXR | 5’-GATGAATTCGCCCTGCAGGCCACAACA |
PRRSV-specific sequences are shown in boldface type.
Nucleotide position refers to the complete genome sequence of the PRRSV PL97-1 strain.
To sequence the 5′-terminus of the PL97-1 RNA genome, we adopted a 5′RACE protocol with a minor modification. First-strand cDNA was first synthesized from the viral RNA by Superscript II RT using the 5′-end-unphosphorylated primer PR50. To remove RNA from the first-strand cDNA–RNA hybrid, it was degraded at 30 °C for 1 h in a 75 μl reaction mixture containing 60 U RNase H, 20 μl first-strand cDNA reaction mixture, and the buffer supplied by the manufacturer (Takara). The resulting first-strand cDNA was phenol-extracted, precipitated with 100% ethanol, and resuspended in 14 μl RNase-free water. To introduce a primer-binding site, the 3′-end of the first-strand cDNA was ligated to synthetic oligonucleotides. A synthetic oligonucleotide X (PRX) was phosphorylated at its 5′-end and modified at its 3′-end by the incorporation of ddATP to prevent intra- and inter-molecular ligation, as described previously (Yun et al., 2003). The first-strand cDNA was ligated with this PRX at 16 °C for 12 h in a 40 μl reaction mixture containing 40 U T4 RNA ligase, 7 μl single-stranded cDNA, 10 pmol PRX, 20% PEG #6000, and the buffer (Takara). The PRX-ligated cDNA was then phenol-extracted, ethanol-precipitated, and resuspended in 20 μl RNase-free water. One-twentieth of this was PCR amplified using the PR49 forward primer and the PRXR reverse primer with 30 cycles of the same program described above except that the extensions at 72 °C took 1–5 min. This was followed by a 10 min extension step at 72 °C. Agarose gel electrophoresis revealed that the products amplified in reactions containing RT migrated predominantly as a band of ≈500 bp (data not shown). No band was observed in reactions lacking PRX during ligation or RT during cDNA synthesis (data not shown). The ≈500 bp cDNA amplicon was purified and the 334 bp Pst I-Sac I PCR fragment was inserted into the Pst I-Sac I-digested pRS2 vector. We sequenced both uncloned cDNA amplicons and 10 randomly picked independent clones containing the insert.
To sequence the 3′-terminus of the genome, we adopted a 3′RACE protocol (Yun et al., 2003) that involves ligating synthetic oligonucleotides to the 3′-end of the viral RNA to provide a specific primer-binding site during RT-PCR. Briefly, 5′-phosphorylated and 3’-blocked PRX was ligated to the 3′-end of the viral RNA at 16 °C for 12 h in a 20 μl reaction mixture containing 10 U T4 RNA ligase (New England Biolabs Inc., Beverly, MA), 40 U RNaseOUT, 10 pmol PRX, extracted viral RNA, and the buffer supplied by the manufacturer. After incubation, the PRX-ligated viral RNA was phenol-extracted, precipitated with 100% ethanol, and resuspended in 20 μl RNase-free water. Half was subsequently used for cDNA synthesis using Superscript II RT and the PRXR primer, as described above. First-strand cDNA was amplified using forward primer PR41 and reverse primer PRXR. For PCR, one quarter of the RT product was amplified by 30 cycles of the same program described above except that the extension at 72 °C took 1 min. This was followed by the usual 10 min extension step. As resolved by agarose gel electrophoresis, minor products were nonspecifically amplified in the absence of PRX during ligation (data not shown). These minor products were not further analyzed since no product was found in a nested PCR with a pair of PRRSV-specific inner primers. The appearance of these minor products might be explained by self-priming of the 3′-end portion of PRRSV RNA to the complementary sequence present in its upstream of the viral genome and subsequent nonspecific PCR amplification. In contrast, a prominent band of ≈450 bp was produced from the PRX-ligated genomic RNA (data not shown). No bands were observed in reactions lacking RT, as expected (data not shown). The ≈450 bp cDNA amplicon was purified and the 384 bp Mfe I-Pst I fragment of the cDNA amplicons was cloned into the EcoR I-Pst I-digested pRS2 vector. Both uncloned cDNA amplicons and 10 randomly picked independent clones containing the insert were sequenced. This full-length PL97-1 nucleotide sequence has been submitted to the GenBank database under accession number AY585241.
2.4. Multiple alignments and phylogenetic analyses
The GenBank accession numbers of the fully sequenced PRRSV strains used in the sequence alignments and phylogenetic analyses are detailed in Table 2 . These strains include the PL97-1 strain and the 11 other PRRSV strains whose full-length nucleotide sequences are presently available in GenBank. MLV RespPRRS/Repro and RespPRRS MLV are the same vaccine strain derived from the parent virus VR-2332. The differences in the genomes reflect different outcomes of sequencing by two independent laboratories. Our initial analysis of the viral ORF7 gene involved 191 strains that are available in GenBank. In the final analysis, one representative sequence was selected from several candidate strains isolated from the same country in the same year that showed very high levels of sequence similarity.
Table 2.
History of the porcine reproductive and respiratory syndrome virus strains used in this study
| Virus straina | Place and year of isolation | GenBank accession no. |
|---|---|---|
| AV30 | Belgium, 1992 | AY035946 |
| PA8 | Canada, 1995 | AF 176348 |
| 93-47324 | Canada, 1993 | AF043969 |
| IAF-exp91 | Canada | L40898 |
| HB-1(sh)/2002 | China, 2002 | AY 150312 |
| BJ-4 | China | AF 331831 |
| CH-1a | China | AY 032626 |
| Ye | China | AF142476 |
| 28639/98 | Denmark, 1998 | AY035957 |
| 20567 A | Denmark, 1997 | AY035952 |
| 21191 | Denmark, 1997 | AY035953 |
| 24554/97 | Denmark, 1997 | AY035955 |
| 12985 | Denmark, 1996 | AY035949 |
| 14474B | Denmark, 1996 | AY035950 |
| 17704A | Denmark, 1996–1997 | AF095479 |
| 17738B | Denmark, 1996–1997 | AF095480 |
| 17875 | Denmark, 1996–1997 | AF095484 |
| 17876 | Denmark, 1996–1997 | AF095485 |
| 18013 | Denmark, 1996–1997 | AF095486 |
| 18027 | Denmark, 1996–1997 | AF095487 |
| 18031 | Denmark, 1996–1997 | AF095488 |
| 18033 | Denmark, 1996–1997 | AF095489 |
| 18253 | Denmark, 1996–1997 | AF095490 |
| 18338 | Denmark, 1996–1997 | AF095491 |
| 19020 | Denmark, 1996–1997 | AF095495 |
| 21192 | Denmark, 1996–1997 | AF095497 |
| 21317 | Denmark, 1996–1997 | AF095498 |
| 5767-6 | Denmark, 1995 | AY035962 |
| 12654 | Denmark, 1995 | AY035947 |
| 12770/95 | Denmark, 1995 | AY035948 |
| Denmark 49 | Denmark, 1995 | AF297103 |
| 340-1 | Denmark, 1994 | AY035959 |
| 228 A | Denmark, 1993 | AY035954 |
| 18794 | Denmark, 1993 | AY035951 |
| 48/92-1 | Denmark, 1992 | AY035961 |
| France 50-18 | France, 1995 | AF297102 |
| SDRPV4A | France, 1993 | AY035965 |
| SDRPIV4A | France, 1992 | AY035964 |
| 2.46 | Germany, 1993 | AY035967 |
| 2.96 | Germany, 1993 | AY035968 |
| ABV 32-13 | Hungary, 1999 | AF297104 |
| 974/98 | Italy, 1998 | AY035978 |
| 1142/97 | Italy, 1997 | AY035941 |
| 2029/97 | Italy, 1997 | AY035973 |
| 2481/97 | Italy, 1997 | AY035975 |
| 2567/96 | Italy, 1996 | AY035976 |
| 3943/96 | Italy, 1996 | AY035977 |
| 7571/96 | Italy, 1996 | AY035943 |
| 1/93 | Italy, 1993 | AY035969 |
| 1999/93 | Italy, 1993 | AY035972 |
| 3391/93 | Italy, 1993 | AY035942 |
| 2156 | Italy, 1992 | AY035974 |
| 1828 | Italy | AY035971 |
| Kitasato 93-1 | Japan | AB023782 |
| EDRD-1 | Japan | D45852 |
| PL97-1 | Korea, 1997 | This study |
| Aus | Lithuania, 2000 | AF438362 |
| Sid | Lithuania, 2000 | AF438363 |
| The Netherlands 60 | The Netherlands, 1994 | AF297100 |
| The Netherlands 3.2 | The Netherlands, 1993 | AF297101 |
| Lelystad | The Netherlands, 1991 | M 96262 |
| Boxmeer 10 | The Netherlands | L04493 |
| Nie | Poland, 1997 | AF438361 |
| Rak | Poland, 1997 | AF438360 |
| L56/2/91 | Spain, 1991 | AY035979 |
| 65/2/91 | Spain, 1991 | AY035980 |
| AF317692 | Spain | AF317692 |
| MD-001 | Taiwan, 1991 | AF121131 |
| NY4 | UK, 1994 | L77926 |
| Be1 | UK, 1993 | L77914 |
| Ha1 | UK, 1992 | L77918 |
| L1-D767 | UK, 1992 | AY035982 |
| L2 | UK, 1992 | L77920 |
| No1 | UK, 1992 | L77924 |
| NY3-D769 | UK, 1992 | AY035983 |
| H3 | UK, 1991 | L77916 |
| Ox1 | UK | L77927 |
| 16244B | USA, 1997 | AF 046869 |
| NVSL 97-7985 | USA, 1997 | AF 325691 |
| P129 | USA, 1995 | AF 494042 |
| 95-13536 | USA, 1995 | AF043959 |
| 95-15299 | USA, 1995 | AF043960 |
| 95-33010 | USA, 1995 | AF043961 |
| 94-18310 | USA, 1994 | AF043958 |
| 94-36893 | USA, 1994 | AF043970 |
| 93-6351 | USA, 1993 | AF043952 |
| 93-14620 | USA, 1993 | AF043951 |
| 93-22326 | USA, 1993 | AF043964 |
| 93-22330 | USA, 1993 | AF043953 |
| 93-27687 | USA, 1993 | AF043954 |
| 93-44927 | USA, 1993 | AF043957 |
| 92-6725 | USA, 1992 | AF043971 |
| 92-01205 | USA, 1992 | AF043956 |
| 92-11824 | USA, 1992 | AF043950 |
| 92-19698 | USA, 1992 | AF043963 |
| 92-22332 | USA, 1992 | AF043965 |
| 91-46907 | USA, 1991 | AF043955 |
| 89-46448 | USA, 1989 | AF043949 |
| 89-46489 | USA, 1989 | AF043966 |
| VR-2332 | USA | U 87392 |
| 28523 | USA | AF043973 |
| ISU-P | USA | AF043974 |
| SU-22 | USA | U18749 |
| ISU-55 | USA | U18751 |
| SU-79 | USA | U18752 |
| ISU-1894 | USA | U18748 |
| SU-3927 | USA | U18750 |
| A-D21 | USA | AF043972 |
| RespPRRS MLV | Vaccine strain | AF 066183 |
| MLV RespPRRS/Repro | Vaccine strain (NOBL Lab) | AF 159149 |
| SP | Vaccine strain (Prime Pac) | AF 184212 |
Thirteen fully sequenced PRRSV strains are indicated in boldface type.
Multiple sequence alignments were performed using ClustalX program (Thompson et al., 1997). Percentage sequence divergences between aligned nucleotide sequences were calculated using ClustalX. The phylogenetic unrooted and rooted trees were reconstructed on aligned nucleotide sequences by using the neighbor-joining method (Saitou and Nei, 1987). The genomes of LDV (U15146) and SHFV (AF180391), two other members of the Arteriviridae family, were also used in the sequence alignments and phylogenetic analyses. The EAV genome (NC002532) was used as an outgroup in all analyses. Constructed neighbor-joining trees were subjected to bootstrap analysis using 1000 replicates (Felsenstein, 1985) to assess confidence values of virus groupings and a distance matrix was obtained from bootstrapped datasets by the Kimura method (Kimura, 1980). All trees were drawn using TreeView software (Page, 1996).
3. Results
3.1. Full-length nucleotide and amino acid sequence analyses
To characterize PL97-1, the first Korean PRRSV strain that was isolated from the serum of an infected pig (80 days old) in 1997, we determined its full-length nucleotide sequence as described in Materials and Methods in detail. Six RT-PCR cDNA amplicons covering the entire RNA genome were directly sequenced to avoid selection bias that might have taken place during cloning. To ensure sequencing accuracy, we not only repeated the RT-PCR with two independently isolated viral RNAs but also sequenced both strands of the amplicons. Thus, the PL97-1 strain RNA genome was sequenced at least once with a minimum redundancy of 4.0. The complete PL97-1 genome was found to be 15411 nucleotides long excluding the poly(A) tail. It consists of a 189-nucleotide 5′NCR, a 15071-nucleotide protein-coding region (9 ORFs), and a 151-nucleotide 3′NCR. Comparison of this genome with that of 11 other fully sequenced PRRSV strains (Table 2) available from GenBank showed sequence divergence ranged from 0.3 to 38.0%. PL97-1 diverged most from the Dutch Lelystad strain (38.0%) (Table 3 , upper right). High divergence from the Chinese HB-1(sh)/2002 (10.2%), the American NVSL 97-7985 (8.9%), the Chinese CH-1a (8.7%), and the American P129 (8.6%) strains was also observed, while lower divergence was observed with the VR-2332-derived vaccine strain designated as MLV RespPRRS/Repro (0.3%) or RespPRRS MLV (0.4%), and the American VR-2332 (0.5%) and the Chinese BJ-4 (0.5%) strains (Table 3, upper right). Similar sequence divergence profiles were observed when only the ORF1b genes of the various strains were compared (Table 3, lower left).
Table 3.
Pairwise comparisons of full-length genome and ORF1b gene sequences of PRRSV isolates
| Virus strain | % Nucleotide sequence divergencea |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CH-1a | HB-1(sh)/2002 | P129 | NVSL 97-7985 | BJ-4 | RespPRRS MLV | MLV RespPRRS/Repro | VR-2332 | PL97-1 | PA8 | 16244B | SP | Lelystad | |
| CH-1a | 4.0 | 5.0 | 5.9 | 8.6 | 8.6 | 8.5 | 8.5 | 8.7 | 8.8 | 9.1 | 9.4 | 38.4 | |
| HB-1(sh)/2002 | 3.6 | 7.1 | 7.9 | 10.2 | 10.2 | 10.1 | 10.1 | 10.2 | 10.4 | 10.5 | 10.9 | 38.6 | |
| P129 | 4.7 | 6.5 | 5.7 | 8.6 | 8.5 | 8.5 | 8.4 | 8.6 | 8.7 | 9.1 | 9.2 | 38.1 | |
| NVSL 97-7985 | 4.6 | 6.2 | 5.1 | 8.9 | 8.8 | 8.8 | 8.8 | 8.9 | 9.1 | 9.3 | 9.6 | 38.3 | |
| BJ-4 | 7.5 | 8.6 | 7.1 | 7.1 | 0.2 | 0.2 | 0.4 | 0.5 | 0.8 | 1.6 | 6.5 | 38.1 | |
| RespPRRS MLV | 7.5 | 8.5 | 7.1 | 7.0 | 0.1 | 0.1 | 0.3 | 0.4 | 0.7 | 1.5 | 6.4 | 38.0 | |
| MLV RespPRRS/Repro | 7.5 | 8.5 | 7.1 | 7.0 | 0.1 | 0.0 | 0.3 | 0.3 | 0.6 | 1.4 | 6.4 | 38.0 | |
| VR-2332 | 7.6 | 8.6 | 7.1 | 7.1 | 0.4 | 0.3 | 0.3 | 0.5 | 0.7 | 1.5 | 6.4 | 38.0 | |
| PL97-1 | 7.7 | 8.7 | 7.3 | 7.3 | 0.4 | 0.3 | 0.3 | 0.6 | 0.8 | 1.6 | 6.5 | 38.0 | |
| PA8 | 7.7 | 8.7 | 7.3 | 7.3 | 0.6 | 0.5 | 0.5 | 0.8 | 0.8 | 1.9 | 6.7 | 38.0 | |
| 16244B | 7.9 | 9.1 | 7.6 | 7.4 | 1.3 | 1.2 | 1.1 | 1.4 | 1.4 | 1.6 | 7.2 | 38.0 | |
| SP | 7.3 | 8.5 | 7.1 | 7.0 | 4.1 | 4.0 | 4.0 | 4.0 | 4.2 | 4.2 | 4.6 | 38.2 | |
| Lelystad | 35.9 | 36.3 | 35.6 | 35.9 | 35.7 | 35.7 | 35.6 | 35.7 | 35.7 | 35.7 | 35.5 | 35.9 | |
The percent nucleotide sequence divergences of the complete genomes are presented at the upper right. The percent nucleotide sequence divergences of the ORF1b genes are shown in the lower left. The percentages of PL97-1 sequence divergence are indicated in boldface type.
The PL97-1 5′NCR nucleotide sequence was aligned with that of the other fully sequenced PRRSV genomes. The 5′-end nucleotides of PL97-1 begin with 1ATG ACG TAT AGG12. These were also observed in the CH-1a, HB-1(sh)/2002, P129, VR-2332, PA8, and SP strains (Fig. 1A ). However, in BJ-4 and RespPRRS MLV, an additional T was reported to be present (Fig. 1A). An additional T has been found previously in the 5′-end of the VR-2332 genome (Oleksiewicz et al., 1999, Shen et al., 2000) but not in the 5′-ends of the two vaccine strains RespPRRS MLV (Oleksiewicz et al., 1999) and SP (Shen et al., 2000). The 5′-end of the 16244B strain was defined as 1CGC CCG GGC AGG12 (Fig. 1A) (Allende et al., 1999). The 23- and 24-nucleotides at the utmost 5′-end of NVSL 97-7985 and MLV RespPRRS/Repro, respectively, were not reported (Fig. 1A, open box). Although Lelystad diverges considerably from other strains, including PL97-1, the 3′-end portion of its 5′NCR is highly conserved (Fig. 1A, dash-line box).
Fig. 1.
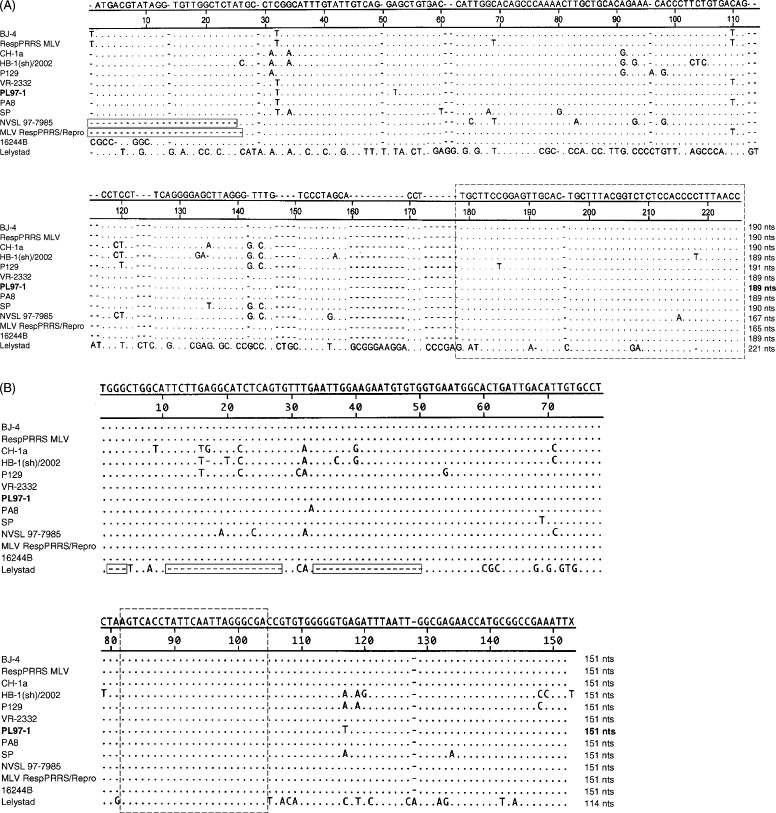
Nucleotide sequence alignment of the 5′NCR (A) and 3′NCR (B) regions of the 12 available fully sequenced PRRSV strains including PL97-1. Except for PL97-1, which was sequenced in this study, all other sequence information was obtained from the GenBank database indicated in Table 2. The consensus sequence of all 12 PRRSV strains is shown on top, and only differences from that sequence in the PRRSV strains are indicated. Deletions are indicated by hyphens. Dash-line boxes indicate a highly conserved region in the 5′NCRs (A) and 3′NCRs (B) present in all 12 PRRSV genomes. (A) The open box indicates a stretch of 23–24 nucleotides at the utmost 5′-end of the NVSL 97-7985 and MLV RespPRRS/Repro PRRSV strains, respectively, which were not reported. (B) The open box represents a string of deletions of 3, 18, and 17 nucleotides in the beginning of the 3′NCR of the Lelystad PRRSV strain.
We found that the sequence 15394AAC CAT GCG GCC GAA ATT15411 terminates the 3′-end of the viral genome, followed by a 54–64 bp poly(A) tail. Comparison of the 3′NCR sequences revealed a high degree of genetic conservation (Fig. 1B). The 3′NCRs of all strains except Lelystad were invariably 151 nucleotides long. The 37-nucleotide shorter 3′NCR of the Lelystad strain is due to a string of 3, 18, and 17 nucleotide deletions at the beginning of its 3′NCR (Fig. 1B, open box) as well as an insertion of A at position 128. The 151-nucleotide 3′NCR of HB-1(sh)/2002 involves a deletion of A at position 17 and an addition of T at the 3′-end of the genome prior to the poly(A) tail (Fig. 1B). Notably, a stretch of 23 bases at positions 82–104 is absolutely conserved between Lelystad and the other PRRSVs, including PL97-1 (Fig. 1B, dash-line box).
We plotted the number of nucleotide (Fig. 2A ) and amino acid (Fig. 2B) alterations at each residue throughout all 11 fully sequenced PRRSV genomes of the North American genotypes relative to their consensus sequence. Higher nucleotide sequence variations were noted in four subregions of the genome, such as the 5′ half of ORF1a, the 3′end portion of ORF1b, ORF4, and ORF5 (indicated as graded bars in Fig. 2A). However, frequent nucleotide alterations were found to be evenly distributed throughout the genomes (Fig. 2A). At the amino acid level, however, more local accumulation of amino acid alterations was found in the N-terminal half of the nonstructural protein ORF1a corresponding to the nsp1b and nsp2 proteins and the structural proteins such as ORF4 and ORF5, whereas less variation was found in the C-terminal half of the nonstructural protein ORF1a and the entire nonstructural protein ORF1b (Fig. 2B). This suggests that there has been strong selection for the nonstructural proteins, which are less tolerant of amino acid alterations.
Fig. 2.
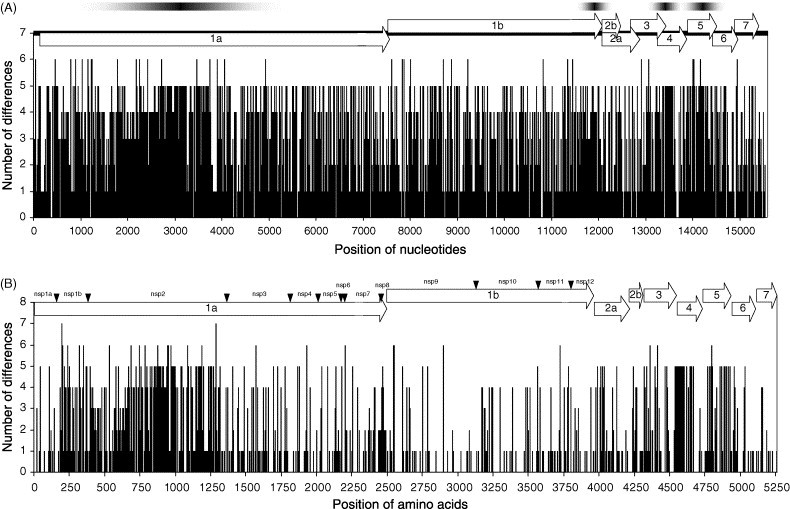
Distribution of nucleotide (A) and amino acid (B) differences throughout the entire PRRSV genome. Nucleotide and amino acid sequences were compared by the multiple sequence alignment method using ClustralX. The number of differences throughout the entire genome was plotted. (A) Schematic diagram of the full-length PRRSV genome is schematically depicted on top. The four subregions containing higher nucleotide sequence variation are indicated as graded bars on the top of the PRRSV genome structure. (B) Each ORF is drawn on two separate lines, and linearly illustrated on top for the purpose of discussion. Arrowheads indicate predicted proteolytic cleavage sites in the ORF1 polyproteins.
3.2. Phylogenetic analyses
To establish the genetic relationships of the fully sequenced PRRSV strains, phylogenetic analyses were performed. A full-length genome-based phylogenetic tree reveals that there are two distinct phylogenetic groups based on their geographical origin (Fig. 3A ). The European genotype consists only of the Dutch Lelystad strain, while the North American genotype contains the other 11 PRRSV isolates, which are from Canada, China, Korea, and USA. Two separate clusters with high bootstrap support were defined in the North American genotype. In the first cluster, the two American isolates P129 and NVSL 97-7985 were most closely related to the two Chinese isolates CH-1a and HB-1(sh)/2002, but each formed a separate minor branch. In the second cluster, three isolates from Canada (PA8), Korea (PL97-1), and USA (VR-2332) were closely related to the VR-2332-derived vaccine strain designated as RespPRRS MLV or MLV RespPRRS/Repro and the Chinese BJ-4 isolate, but each formed a single minor branch. The American 16244B strain was also grouped in the second cluster but formed a single minor branch. While the SP vaccine strain was related to the other PRRSV isolates in the second cluster, it formed a distinct branch. The genomes of LDV and SHFV, two other members of the Arteriviridae family, were included in the phylogenetic analyses to provide information on divergence levels in comparison to other arteriviruses. Both viruses appeared in all phylogenetic trees as a separate distant branch, showing that these viruses are significantly different from all PRRSV strains (Fig. 3).
Fig. 3.
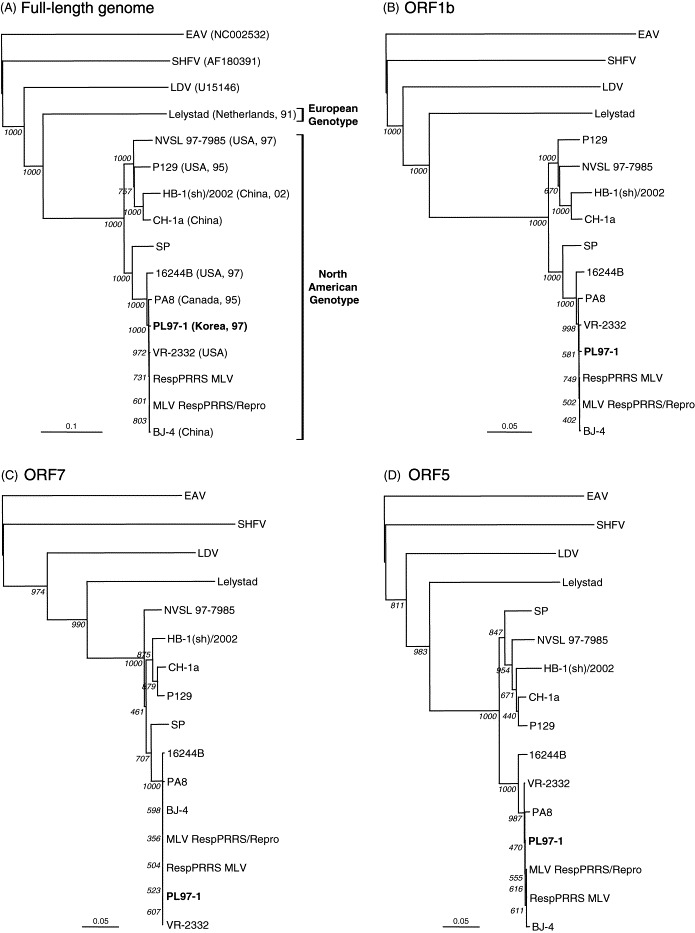
Phylogenetic tree constructed with the nucleotide sequence of the full-length genome (A), or the ORF1b (B), ORF7 (C), or ORF5 (D) gene of all 12 available PRRSV strains. Phylogenetic trees were constructed using the neighbor-joining method in ClustralX (Thompson et al., 1997). The scale bars at the bottom of each tree represent the number of nucleotide substitutions per site. The numbers at each node indicate bootstrap replicate support. The trees were rooted using the nucleotide sequence of EAV, a member of the Arteriviridae family. The genomes of LDV and SHFV were included in the phylogenetic analyses to provide information on divergence levels in comparison to other arteriviruses. The strain name is followed by the country and the year of isolation in two digits.
Over 200 PRRSV isolates have been isolated at different times and places worldwide but only 12, including PL97-1, have been fully sequenced, while six isolates have been characterized and published (Meulenberg et al., 1997, Allende et al., 1999, Allende et al., 2000, Nelsen et al., 1999, Shen et al., 2000, Wootton et al., 2000, Yuan et al., 2001). This partial sequence information makes difficult to fully investigate the genetic relationships between the large and heterogeneous pool of the PRRSV isolates. Consequently, we searched for an optimal subregion that would accurately represent the full-length genome-based phylogenetic topology and performed phylogenetic analyses based on each viral gene, including ORF5 and ORF7, and the 5′NCR or 3′NCR, using the 12 fully sequenced strains. The resulting phylogenetic trees were compared to the full-length genome-based phylogenetic tree. The ORF1b gene-based phylogenetic tree most closely resembled the full-length genome-based phylogenetic tree in that it shows the two distinct phylogenetic groups and clusters of the latter tree (Fig. 3B, compared to Fig. 3A). The phylogenetic trees based on the ORF5 and 7 genes, which are the most frequently sequenced genes in the database to date, revealed overall similar tree topologies to the full-length genome-based phylogenetic tree, but with minor differences. In the ORF7 tree, NVSL 97-7985 was related to the members of the first cluster of the North American genotype but forms a distinct branch (Fig. 3C). The ORF5 gene-based phylogenetic tree showed that the SP strain was more closely related to the members of the first cluster within the North American genotype than those of the second cluster (Fig. 3D).
To investigate how PL97-1 relates genetically to the wide variety of temporally and geographically diverse PRRSV strains available, we performed an extensive phylogenetic analysis with a selection of 111 ORF7 genes. Two distinct phylogenetic groupings that corresponded to the European and North American genotypes were identified with 100% bootstrap support (Fig. 4A ), as previously described (Meulenberg et al., 1993, Murtaugh et al., 1995, Nelsen et al., 1999, Dea et al., 2000, Meng, 2000). The majority of strains in the European genotype group were defined by four clusters (Fig. 4B). The first consisted of two early Spanish and Danish isolates (L56/2/91, 1991, and 340-1, 1994), a relatively recent Hungarian isolate (ABV 32-13, 1999), and two Italian isolates (7571/96, 1996 and 2481/97, 1997). The second cluster contained Italian isolates from the early 1990s and one from 1997 (1142/97), while the third consisted of most Danish isolates from 1992 to 1998. The fourth cluster contained early 1990 isolates from the Netherlands, Denmark, Spain, Germany, UK, Italy, Belgium, and France, including Lelystad. In addition, four Italian strains (2567/96, 2029/97, 3943/96, and 974/98) isolated in the late 1990’s and two Polish strains (Nie and Rak) isolated in 1997 were closely related to the other PRRSV isolates in this genotype, but each formed a single minor branch. While two recent Lithuanian isolates in this genotype were closely related to each other, they were found to be the most divergent from these isolates with a high bootstrap support.
Fig. 4.
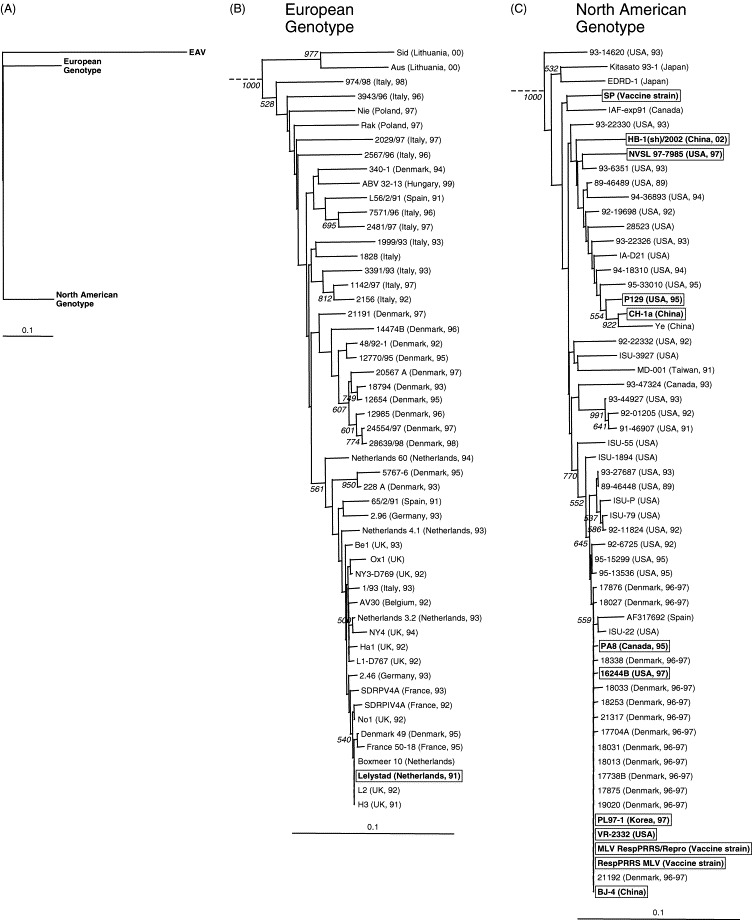
Phylogenetic relationships predicted from the ORF7 nucleotide sequences of 111 selected PRRSV strains isolated from different geographic regions worldwide at different time periods. (A) All 111 PRRSV strains used in this analysis were classified into two distinct phylogenetic groups corresponding to the European and North American genotypes. (B–C) A branch of the European genotype (B) or the North American genotype (C) in (A) was magnified for the purpose of illustration. Detailed information regarding the PRRSV strains used in this analysis is provided in Table 2. The 12 fully sequenced PRRSV strains are boxed. Numbers at each node indicate bootstrap replicate values greater than 500 (1000 replicates). For details of the trees, see legend to Fig. 3.
In the North American genotype group, the majority of strains were also separated into four clusters (Fig. 4C). The first consisted of isolates from China, including HB-1(sh)/2002 and CH-1a, and strains from USA, including NVSL 97-7985 and P129. The second consisted of early isolates from USA (92-22332 and ISU-3927) and Taiwan (MD-001, 1991), while the third contained early isolates from USA and Canada (93-47324, 1993). Interestingly, the fourth cluster was comprised of strains from Denmark (1996–1997) and USA isolated in the early 1990s, including VR-2332 and 16244B (1997), and the fully sequenced Canadian PA8 isolate (1995). This latter cluster also contained a Spanish strain (GenBank accession no. AF317692) and the two fully sequenced Asian strains PL97-1 (Korea, 1997) and BJ-4 (China). The VR-2332-derived vaccine strain designated as MLV RespPRRS/Repro or RespPRRS MLV also belonged to this cluster. However, the SP vaccine strain appeared to be closely related to the Canadian IAF-exp91 isolate, and formed a separate branch. Two closely related Japanese isolates (Kitasato 93-1 and EDRD-1) did not belong to any of these clusters in the North American genotype, which indicates that they are distantly related to other North American strains. The USA 93-14620 isolate also formed a distinguishable branch and was less closely related to other isolates in the North American genotype.
3.3. Detailed comparison of PL97-1 with RespPRRS MLV and VR-2332 strains
Our results showed that the PL97-1 strain was closely related to the VR-2332-derived live vaccine strain RespPRRS MLV and its parent virus VR-2332. More detailed analyses showed that the full-length genome of PL97-1 was found to have 62 and 72 nucleotide changes relative to that of RespPRRS MLV and VR-2332, respectively (data not shown). On the other hand, a total of 41 nucleotide changes have previously been identified between RespPRRS MLV and VR-2332 (Yuan et al., 2001). In addition, phylogenetic analysis also revealed that RespPRRS MLV was more closely related to the parent virus VR-2332 than the PL97-1 analyzed in this study.
Next, we analyzed for the amino acid changes of PL97-1 compared to the two PRRSV strains RespPRRS MLV and VR-2332. As shown in Table 4 , a total of 45 residues were varied and the majority of which were located in the ORF1a and 1b. Of these, the PL97-1 strain contained 29 and 31 unique amino acid substitutions compared to the corresponding amino acid sequences of RespPRRS MLV and VR-2332, respectively (Table 4). Comparison of RespPRRS MLV with VR-2332 revealed 30 unique amino acid changes, as previously described (Yuan et al., 2001). Furthermore, the amino acid residues at position 13 and 151 of ORF5 were previously shown to be different between the vaccine strain and the parent virus (Wesley et al., 1999). The VR-2332 has a positively charged Arg at both positions and Arg151 was found in all field isolates of PRRSV including VR-2332 (Wesley et al., 1999). In comparison, the RespPRRS MLV has a Gln at residue 13 and a Gly at residue 151. Interestingly, the PL97-1 has a Gln at residue 13 and an Arg at residue 151, indicating an intermediate genotype (Table 4).
Table 4.
Amino acid substitutions in the PRRSV PL97-1 strain relative to PRRSV strains RespPRRS MLV and VR-2332
| Protein | Amino acid positiona | PL97-1b | RespPRRS MLV | VR-2332 |
|---|---|---|---|---|
| ORF1a | 199 | I | I | V |
| 321 |  |
L | L | |
| 331 | S | F | S | |
| 349 |  |
S | S | |
| 361 |  |
P | P | |
| 668 | F | F | S | |
| 720 |  |
Y | Y | |
| 741 |  |
K | K | |
| 951 | N | N | D | |
| 1042 |  |
T | T | |
| 1090 | D | N | D | |
| 1248 | 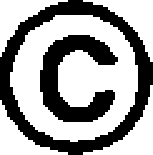 |
Y | Y | |
| 1263 | 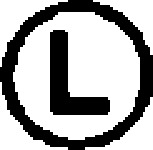 |
F | F | |
| 1487 | N | T | N | |
| 1498 | A | A | S | |
| 1505 | 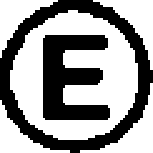 |
K | K | |
| 1506 | A | T | A | |
| 1756 | 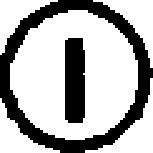 |
L | L | |
| 1876 |  |
I | I | |
| 2162 | L | L | P | |
| 2296 |  |
I | I | |
| ORF1b | 754 | E | E | G |
| 946 | Y | H | Y | |
| 982 |  |
V | V | |
| 1036 | R | R | C | |
| 1097 |  |
Y | Y | |
| 1120 | T | T | S | |
| 1175 | E | E | G | |
| 1178 | L | L | V | |
| 1211 | A | A | G | |
| ORF2a | 10 | L | F | L |
| 122 | S | S | A | |
| 128 | K | R | K | |
| 130 | V | M | V | |
| ORF2b | 9 | D | Y | D |
| ORF3 | 83 | E | E | G |
| 94 |  |
I | I | |
| 106 | S | S | G | |
| 251 | A | T | A | |
| ORF4 | 69 | C | Y | C |
| 125 | V | A | V | |
| ORF5 | 13 | Q | Q | R |
| 151 | R | G | R | |
| ORF6 | 16 | Q | E | Q |
| 121 | G | G | R |
The amino acid residue is numbered based on the PL97-1.
The amino acid residues that are not represented by either RespPRRS MLV or VR-2332 are circled.
4. Discussion
We have determined the complete nucleotide sequence of PL97-1, the first Korean PRRSV strain and characterized its genome at a molecular level. Its genetic relationship with a large selection of PRRSV strains isolated at different time periods from different geographic regions was also assessed. Significantly, the 5′-end of the fully sequenced PRRSV genomes appears to be heterogeneous. The 5′-end of PL97-1 begins with 1ATG ACG TAT AGG12, which is identical to six other fully sequenced PRRSV genomes, including VR-2332 (U87392). However, this sequence is not present in the 5′-ends of NVSL 97-7985 (AF325691) and MLV RespPRRS/Repro (AF159149), whose 5′-ends consist of T and G, respectively. An additional T was identified in BJ-4 (AF331831) and RespPRRS MLV (AF066183). Previous studies also reported an additional T in VR-2332 (Oleksiewicz et al., 1999, Shen et al., 2000). However, this additional T is not found in the 5′-ends of the two vaccine strains RespPRRS MLV (Oleksiewicz et al., 1999) and SP (Shen et al., 2000). Moreover, Yuan et al. (2001) observed an additional T in the 5′-end of the RespPRRS vaccine strain but not in VR-2332. An additional dinucleotide AC was also identified in 111/92 (Oleksiewicz et al., 1999). The 5′-end of 16244B was defined as 1CGC CCG GGC AGG12 (Allende et al., 1999). Thus, it appears that the 5′-end of the PRRSV genome can consist of any of the four nucleotides. This is unusual given that the 5′ and 3′-end nucleotide sequences of positive-sense RNA viruses are highly conserved because they are generally important in RNA replication and transcription. This heterogenicity of the PRRSV 5′-end sequence should be further investigated for its relevance. Interestingly, both ends of viral sequences have been shown to not only play an important role in the replication of coronaviruses, another member of the order Nidovirales (Williams et al., 1999), but they are also related to poliovirus attenuation (Westrop et al., 1989).
We found significant nucleotide sequence differences in the PRRSV 5′NCR, but its last 3′ quarter of about 45 nucleotides was highly conserved, even between the North American and the European genotypes. However, the predicted secondary RNA structures of the 5′NCR has suggested that its 5′ three-quarters forms three conserved stem-loop structures in the North American genotypes and two in the European strains, whereas the 3′ quarter that includes the highly conserved 45 nucleotides was predicted to be a variable domain (Tan et al., 2001). Thus, the nucleotide sequence of the highly conserved 45 nucleotides, rather than its secondary structure, might be critical for viral replication/transcription. In comparison, the PRRSV 3′NCR sequence is less divergent and a 23-nucleotide stretch in the middle of the 3′NCR was absolutely conserved in all 12 fully sequenced PRRSV genomes. Thus, it may play an important role in viral replication. The Lelystad genome also contained three deletions of 3-, 18-, and 17-nucleotides in the beginning of its 3′NCR, which suggests that this region may not be required for viral replication. A recent study of infectious Lelystad cDNA revealed that the deletion of the 7 nucleotides, but not of 32 nucleotides, immediately downstream of ORF7 did not affect infectious virus production (Verheije et al., 2001). Further investigations are needed to elucidate this issue.
Although over 200 PRRSV strains have been isolated from widely different geographical regions, most have been only partially sequenced. In general, only the ORF5 and ORF7 genes have been sequenced. These studies classified PRRSV into two distinct phylogenetic groups, namely, the European and North American genotypes. Our assessment of the genetic relationships between all 12 full-length PRRSV genomes supports this classification, which reflects their geographical origin. We showed that the Lelystad strain was distantly related to the other fully sequenced strains and formed the only fully sequenced European genotype isolate to date, while the other 11 strains, including PL97-1, formed the North American genotype. Notably, the SP vaccine strain was closely related to the other North American genotype strains but formed a distinct branch with high bootstrap support.
Significantly, when the phylogenetic trees based on single genes or the 5′ and 3′NCRs of the 12 fully sequenced PRRSV genomes were compared to the tree based on the entire genome, the ORF1b or 7 gene-based tree corresponded well to the full-length genome-based tree. Extensive ORF7 gene-based phylogenetic analysis using 111 selected strains confirmed the phylogenetic relationships determined with the 12 fully sequenced strains. The ORF7 gene-based analysis also showed high genetic variation exists not only between two genotypes but also within each genotype, as indicated previously (Meng et al., 1995b, Forsberg et al., 2002). Moreover, major nucleotide differences in the ORF1 have also been described previously (Allende et al., 1999). Although the functional significance of this genetic variation remains to be determined, antigenic differences between two genotypes on a serological basis have been reported (Drew et al., 1995, Katz et al., 1995, Sorensen et al., 1998). Thus, our findings and others suggest that PRRSV heterogenicity may be a consideration in vaccine development.
From an evolutionary point of view, it appears that two different PRRSV genotypes rose simultaneously in Europe and North America (Wensvoort et al., 1991, Benfield et al., 1992, Collins et al., 1992). Each genotype contains strains that were isolated from the same geographic location in the same or another year yet belong to different clusters. This may reflect the introduction of a new variant strain into the same geographic location by, for example, animal or semen transport. This is supported by a recent study suggested that the genetic variation of the different strains in the same herd was caused by animal or semen transport rather than by local evolution (Madsen et al., 1998, Goldberg et al., 2000). Alternatively, the preexisting strains may evolve locally into new variants during intra- and inter-animal spread due to a combination of the error-prone viral RNA polymerase and selective pressure. These two scenarios are not mutually exclusive and depend largely on geographic location and time of isolation. With regard to the Japanese encephalitis virus, all known five viral genotypes including the oldest are found in a region of Indonesia–Malaysia. However, newer genotypes appear to have spread from this region into other geographical locations at given time periods (Solomon et al., 2003). If this is the case for PRRVS as well, the origin of PRRSV where both genotypes can be found remains to be discovered. The recent genetic characterization of PRRSV strains isolated from Eastern Europe suggests that the current European isolates might be derived from a common ancestor that is closely related to the North American isolates (Stadejek et al., 2002).
The Korean PL97-1 strain sequenced in this study was found to be highly similar to the American wild-type VR-2332 and the VR-2332-derived vaccine strain designated as RespPRRS MLV or MLV RespPRRS/Repro. There are two possible explanations for this similarity. First, the modified live vaccine used in Korea since 1996 may have persisted and mutated into a less attenuated variant, causing clinical signs upon infection and spread. That PRRSV persists and undergoes limited but consistent mutations has been demonstrated experimentally in infected pigs (Allende et al., 2000). A vaccine-derived field isolate was demonstrated directly to cause reproductive problems by experimental inoculation (Nielsen et al., 1998). Moreover, that vaccine strains can revert into more virulent variants has been suggested to occur in both US (Mengeling et al., 1999) and Denmark (Botner et al., 1997, Madsen et al., 1998, Storgaard et al., 1999). Alternatively, the prevalent field isolates in Korea were already coincidentally similar to VR-2332 prior to the introduction of the modified live vaccine.
Of these two possibilities, the high genetic similarity to the VR-2332-derived vaccine strain and its parent virus is very suggestive that the PL97-1 might be reverted from the vaccine strain. Especially, this idea is supported by analyzing for the amino acid changes of PL97-1 compared to the VR-2332-derived vaccine and several vaccine-derived field isolates. The PL97-1 strain appeared to contain the three reversion mutations (codon positions 331 of ORF1a, 946 of ORF1b, and 151 of ORF5), which invariably found in all field isolates of PRRSV reported by other investigators (Madsen et al., 1998, Storgaard et al., 1999, Wesley et al., 1999, Nielsen et al., 2001). The former two reversion mutations were observed independently in all seven Danish vaccine-derived field isolates (Nielsen et al., 2001) and in the pathogenic American vaccine-like isolate, 16244B (Allende et al., 1999, Allende et al., 2000). This indicates strong parallel selective pressure on these positions in the vaccine virus when used in swine herds, involved in the attenuation of the vaccine strain and the subsequent reversion to virulence. The amino acid residue at position 151 of ORF5 was shown to be different between the vaccine strain (Gly151) and its parent virus (Arg151) and a positively charged Arg151 was found in most of field isolates of PRRSV (Meng et al., 1994, Meng et al., 1995b, Mardassi et al., 1995, Kapur et al., 1996, Andreyev et al., 1997, Madsen et al., 1998, Wesley et al., 1998, Wesley et al., 1999, Storgaard et al., 1999, Allende et al., 2000). Besides, the amino acid residue at position 16 of ORF6 has been identified as a candidate virulence determinant, reverting from a Glu to the Gln found in the VR-2332 strain (Madsen et al., 1998, Storgaard et al., 1999, Allende et al., 2000). We found that the PL97-1 had also undergone exactly the same reversion mutation.
In summary, this study provides the complete nucleotide sequence of the first Korean PRRSV isolate and the largest phylogenetic tree analyzed to date. The latter reinforces the earlier grouping of PRRSV strains that are widely separated by time and geography. Molecular characterization of PL97-1 will help elucidate viral protein functions and viral pathogenetic mechanisms, which may help to control the spread of the disease. Furthermore, a better understanding of the phylogenetic relationships between the PRRSV strains worldwide will aid the development of new, safe and effective vaccines against this pathogen.
Acknowledgements
This work was supported by grant no. R01-2001-000-00249-0 from the Korea Science & Engineering Foundation.
References
- Allende R., Lewis T.L., Lu Z., Rock D.L., Kutish G.F., Ali A., Doster A.R., Osorio F.A. North American and European porcine reproductive and respiratory syndrome viruses differ in non-structural protein coding regions. J. Gen. Virol. 1999;80:307–315. doi: 10.1099/0022-1317-80-2-307. [DOI] [PubMed] [Google Scholar]
- Allende R., Laegreid W.W., Kutish G.F., Galeota J.A., Wills R.W., Osorio F.A. Porcine reproductive and respiratory syndrome virus: description of persistence in individual pigs upon experimental infection. J. Virol. 2000;74:10834–10837. doi: 10.1128/jvi.74.22.10834-10837.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreyev V.G., Wesley R.D., Mengeling W.L., Vorwald A.C., Lager K.M. Genetic variation and phylogenetic relationships of 22 porcine reproductive and respiratory syndrome virus (PRRSV) field strains based on sequence analysis of open reading frame 5. Arch. Virol. 1997;142:993–1001. doi: 10.1007/s007050050134. [DOI] [PubMed] [Google Scholar]
- Benfield D.A., Nelson E., Collins J.E., Harris L., Goyal S.M., Robison D., Christianson W.T., Morrison R.B., Gorcyca D., Chladek D. Characterization of swine infertility and respiratory syndrome (SIRS) virus (isolate ATCC VR-2332) J. Vet. Diagn. Invest. 1992;4:127–133. doi: 10.1177/104063879200400202. [DOI] [PubMed] [Google Scholar]
- Botner A., Strandbygaard B., Sorensen K.J., Have P., Madsen K.G., Madsen E.S., Alexandersen S. Appearance of acute PRRS-like symptoms in sow herds after vaccination with a modified live PRRS vaccine. Vet. Rec. 1997;141:497–499. doi: 10.1136/vr.141.19.497. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch. Virol. 1997;142:629–633. [PubMed] [Google Scholar]
- Chueh L.L., Lee K.H., Wang F.I., Pang V.F., Weng C.N. Sequence analysis of the nucleocapsid protein gene of the porcine reproductive and respiratory syndrome virus Taiwan MD-001 strain. Adv. Exp. Med. Biol. 1998;440:795–799. doi: 10.1007/978-1-4615-5331-1_103. [DOI] [PubMed] [Google Scholar]
- Collins J.E., Benfield D.A., Christianson W.T., Harris L., Hennings J.C., Shaw D.P., Goyal S.M., McCullough S., Morrison R.B., Joo H.S., Gorcyca D.E., Chladek D.W. Isolation of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) in North America and experimental reproduction of the disease in gnotobiotic pigs. J. Vet. Diagn. Invest. 1992;4:117–126. doi: 10.1177/104063879200400201. [DOI] [PubMed] [Google Scholar]
- Dea S., Gagnon C.A., Mardassi H., Pirzadeh B., Rogan D. Current knowledge on the structural proteins of porcine reproductive and respiratory syndrome (PRRS) virus: comparison of the North American and European isolates. Arch. Virol. 2000;145:659–688. doi: 10.1007/s007050050662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew T.W., Lowings J.P., Yapp F. Variation in open reading frames 3, 4, and 7 among porcine reproductive and respiratory syndrome virus isolates in the UK. Vet. Microbiol. 1997;55:209–221. doi: 10.1016/s0378-1135(96)01328-4. [DOI] [PubMed] [Google Scholar]
- Drew T.W., Meulenberg J.J.M., Sands J.J., Paton D.J. Production, characterization and reactivity of monoclonal antibodies to porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 1995;76:1361–1369. doi: 10.1099/0022-1317-76-6-1361. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Forsberg R., Storgaard T., Nielsen H.S., Oleksiewicz M.B., Cordioli P., Sala G., Hein J., Botner A. The genetic diversity of European type PRRSV is similar to that of the North American type but is geographically skewed within Europe. Virology. 2002;299:38–47. doi: 10.1006/viro.2002.1450. [DOI] [PubMed] [Google Scholar]
- Gagnon C.A., Dea S. Differentiation between porcine reproductive and respiratory syndrome virus isolates by restriction fragment length polymorphism of their ORFs 6 and 7 genes. Can. J. Vet. Res. 1998;62:110–116. [PMC free article] [PubMed] [Google Scholar]
- Goldberg T.L., Hahn E.C., Weigel R.M., Scherba G. Genetic, geographical and temporal variation of porcine reproductive and respiratory syndrome virus in Illinois. J. Gen. Virol. 2000;81:171–179. doi: 10.1099/0022-1317-81-1-171. [DOI] [PubMed] [Google Scholar]
- Indik S., Valicek L., Klein D., Klanova J. Variations in the major envelope glycoprotein GP5 of Czech strains of porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 2000;81:2497–2502. doi: 10.1099/0022-1317-81-10-2497. [DOI] [PubMed] [Google Scholar]
- Kapur V., Elam M.R., Pawlovich T.M., Murtaugh M.P. Genetic variation in porcine reproductive and respiratory syndrome virus isolates in the midwestern United States. J. Gen. Virol. 1996;77:1271–1276. doi: 10.1099/0022-1317-77-6-1271. [DOI] [PubMed] [Google Scholar]
- Katz J.B., Shafer A.L., Eernisse K.A., Landgraf J.G., Nelson E.A. Antigenic differences between European and American isolates of porcine reproductive and respiratory syndrome virus (PRRSV) are encoded by the carboxylterminal portion of viral open reading frame 3. Vet. Microbiol. 1995;44:65–76. doi: 10.1016/0378-1135(94)00113-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keffaber K.K. Reproductive failure of unknown etiology. Am. Assoc. Swine Pract. Newslett. 1989;1:1–9. [Google Scholar]
- Key K.F., Haqshenas G., Guenette D.K., Swenson S.L., Toth T.E., Meng X.J. Genetic variation and phylogenetic analyses of the ORF5 gene of acute porcine reproductive and respiratory syndrome virus isolates. Vet. Microbiol. 2001;83:249–263. doi: 10.1016/s0378-1135(01)00427-8. [DOI] [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Kwang J., Kim H.S., Joo H.S. Cloning, expression, and sequence analysis of the ORF4 gene of the porcine reproductive and respiratory syndrome virus MN-1b. J. Vet. Diagn. Invest. 1994;6:293–296. doi: 10.1177/104063879400600302. [DOI] [PubMed] [Google Scholar]
- Le Gall A., Legeay O., Bourhy H., Arnauld C., Albina E., Jestin A. Molecular variation in the nucleoprotein gene (ORF7) of the porcine reproductive and respiratory syndrome virus (PRRSV) Virus Res. 1998;54:9–21. doi: 10.1016/s0168-1702(97)00146-9. [DOI] [PubMed] [Google Scholar]
- Madsen K.G., Hansen C.M., Madsen E.S., Strandbygaard B., Botner A., Sorensen K.J. Sequence analysis of porcine reproductive and respiratory syndrome virus of the American type collected from Danish swine herds. Arch. Virol. 1998;143:1683–1700. doi: 10.1007/s007050050409. [DOI] [PubMed] [Google Scholar]
- Mardassi H., Mounir S., Dea S. Identification of major differences in the nucleocapsid protein genes of a Quebec strain and European strains of porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 1994;75:681–685. doi: 10.1099/0022-1317-75-3-681. [DOI] [PubMed] [Google Scholar]
- Mardassi H., Mounir S., Dea S. Molecular analysis of the ORFs 3–7 of porcine reproductive and respiratory syndrome virus, Quebec reference strain. Arch. Virol. 1995;140:1405–1418. doi: 10.1007/BF01322667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateu E., Martin M., Vidal D. Genetic diversity and phylogenetic analysis of glycoprotein 5 of European-type porcine reproductive and respiratory virus strains in Spain. J. Gen. Virol. 2003;84:529–534. doi: 10.1099/vir.0.18478-0. [DOI] [PubMed] [Google Scholar]
- Medveczky I., Balint A., Makranszky L., Steverink P., Jacobs L. Sequence analysis of the membrane protein gene and nucleocapsid gene of porcine reproductive and respiratory syndrome virus isolated from a swine herd in Hungary. Acta Vet. Hung. 2001;49:237–244. doi: 10.1556/004.49.2001.2.14. [DOI] [PubMed] [Google Scholar]
- Meng X.J. Heterogeneity of porcine reproductive and respiratory syndrome virus: implications for current vaccine efficacy and future vaccine development. Vet. Microbiol. 2000;74:309–329. doi: 10.1016/S0378-1135(00)00196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X.J., Paul P.S., Halbur P.G. Molecular cloning and nucleotide sequencing of the 3′-terminal genomic RNA of the porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 1994;75:1795–1801. doi: 10.1099/0022-1317-75-7-1795. [DOI] [PubMed] [Google Scholar]
- Meng X.J., Paul P.S., Halbur P.G., Lum M.A. Phylogenetic analyses of the putative M (ORF 6) and N (ORF 7) genes of porcine reproductive and respiratory syndrome virus (PRRSV): implication for the existence of two genotypes of PRRSV in the USA and Europe. Arch. Virol. 1995;140:745–755. doi: 10.1007/BF01309962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X.J., Paul P.S., Halbur P.G., Morozov I. Sequence comparison of open reading frames 2–5 of low and high virulence United States isolates of porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 1995;76:3181–3188. doi: 10.1099/0022-1317-76-12-3181. [DOI] [PubMed] [Google Scholar]
- Mengeling W.L., Vorwald A.C., Lager K.M., Clouster D.F., Wesley R.D. Identification and clinical assessment of suspected vaccine-related field strains of porcine reproductive and respiratory syndrome virus. Am. J. Vet. Res. 1999;60:334–340. [PubMed] [Google Scholar]
- Meulenberg J.J.M., den Besten A.P., de Kluyver E.P., van Nieuwstadt A., Wensvoort G., Moormann R.J.M. Molecular characterization of Lelystad virus. Vet. Microbiol. 1997;55:197–202. doi: 10.1016/S0378-1135(96)01335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulenberg J.J.M., Hulst M.M., de Meijer E.J., Moonen P.L.J.M., den Besten A., de Kluyver E.P., Wensvoort G., Moormann R.J.M. Lelystad virus, the causative agent of porcine epidemic abortion and respiratory syndrome (PEARS), is related to LDV and EAV. Virology. 1993;192:62–72. doi: 10.1006/viro.1993.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozov I., Meng X.J., Paul P.S. Sequence analysis of open reading frames (ORFs) 2–4 of a US isolate of porcine reproductive and respiratory syndrome virus. Arch. Virol. 1995;140:1313–1319. doi: 10.1007/BF01322758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtaugh M.P., Elam M.R., Kakach L.T. Comparison of the structural protein coding sequences of the VR-2332 and Lelystad virus strains of the PRRS virus. Arch. Virol. 1995;140:1451–1460. doi: 10.1007/BF01322671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelsen C.J., Murtaugh M.P., Faaberg K.S. Porcine reproductive and respiratory syndrome virus comparison: divergent evolution on two continents. J. Virol. 1999;73:270–280. doi: 10.1128/jvi.73.1.270-280.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, J., Botner, A., Oleksiewicz, M., Storgaard, T., 1998. Experimental inoculation of late-term pregnant sows with a field isolate of PRRS vaccine-like virus. In: Done, S., Thomson, J., Varley, M. (Eds.), Proceedings of the 15th International Pig Veterinary Society Congress, vol. 2, Nottingham University Press, Nottingham, pp. 127.
- Nielsen H.S, Oleksiewicz M.B., Forsberg R., Stadejek T., Botner A., Storgaard T. Reversion of a live porcine reproductive and respiratory syndrome virus vaccine investigated by parallel mutations. J. Gen. Virol. 2001;82:1263–1272. doi: 10.1099/0022-1317-82-6-1263. [DOI] [PubMed] [Google Scholar]
- Oleksiewicz M.B., Botner A., Nielsen J., Storgaard T. Determination of 5′-leader sequences from radically disparate strains of porcine reproductive and respiratory syndrome virus reveals the presence of highly conserved sequence motifs. Arch. Virol. 1999;144:981–987. doi: 10.1007/s007050050560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleksiewicz M.B., Botner A., Toft P., Grubbe T., Nielsen J., Kamstrup S., Storgaard T. Emergence of porcine reproductive and respiratory syndrome virus deletion mutants: correlation with the porcine antibody response to a hypervariable site in the ORF 3 structural glycoprotein. Virology. 2000;267:135–140. doi: 10.1006/viro.1999.0103. [DOI] [PubMed] [Google Scholar]
- Page R.D.M. Treeview: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- Paton D.J., Brown I.H., Edwards S., Wensvoort G. ‘Blue ear’ disease of pigs. Vet. Rec. 1991;128:617. doi: 10.1136/vr.128.26.617. [DOI] [PubMed] [Google Scholar]
- Pirzadeh B., Gagnon C.A., Dea S. Genomic and antigenic variations of porcine reproductive and respiratory syndrome virus major envelope GP5 glycoprotein. Can. J. Vet. Res. 1998;62:170–177. [PMC free article] [PubMed] [Google Scholar]
- Saitou N., Nei M. The Neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Shen S., Kwang J., Liu W., Liu D.X. Determination of the complete nucleotide sequence of a vaccine strain of porcine reproductive and respiratory syndrome virus and identification of the Nsp2 gene with a unique insertion. Arch. Virol. 2000;145:871–883. doi: 10.1007/s007050050680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder E.J., Meulenberg J.J.M. The molecular biology of arteriviruses. J. Gen. Virol. 1998;79:961–979. doi: 10.1099/0022-1317-79-5-961. [DOI] [PubMed] [Google Scholar]
- Snijder, E.J., Meulenberg, J.J.M., 2001. Arteriviruses. In: Knipe, D.M., Howley, P.M. (Eds.), Fields Virology, fourth ed., vol. 1. Lippincott Williams and Wilkins, PA, pp. 1205–1220.
- Solomon T., Ni H., Beasley D.W.C., Ekkelenkamp M., Cardosa M.J., Barrett A.D.T. Origin and evolution of Japanese encephalitis virus in Southeast Asia. J. Virol. 2003;77:3091–3098. doi: 10.1128/JVI.77.5.3091-3098.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen K.J., Strandbygaard B., Botner A., Madsen E.S., Nielsen J., Have P. Blocking ELISA’s for the distinction between antibodies against European and American strains of porcine reproductive and respiratory syndrome virus. Vet. Microbiol. 1998;60:169–177. doi: 10.1016/s0378-1135(98)00159-x. [DOI] [PubMed] [Google Scholar]
- Stadejek T., Stankevicius A., Storgaard T., Oleksiewicz M.B., Belak S., Drew T.W., Pejsak Z. Identification of radically different variants of porcine reproductive and respiratory syndrome virus in Eastern Europe: towards a common ancestor for European and American viruses. J. Gen. Virol. 2002;83:1861–1873. doi: 10.1099/0022-1317-83-8-1861. [DOI] [PubMed] [Google Scholar]
- Storgaard T., Oleksiewicz M., Botner A. Examination of the selective pressures on a live PRRS vaccine virus. Arch. Virol. 1999;144:2389–2401. doi: 10.1007/s007050050652. [DOI] [PubMed] [Google Scholar]
- Suarez P., Zardoya R., Martin M.J., Prieto C., Dopazo J., Solana A., Castro J.M. Phylogenetic relationships of European strains of porcine reproductive and respiratory syndrome virus (PRRSV) inferred from DNA sequences of putative ORF-5 and ORF-7 genes. Virus Res. 1996;42:159–165. doi: 10.1016/0168-1702(95)01305-9. [DOI] [PubMed] [Google Scholar]
- Tan C., Chang L., Shen S., Liu D.X., Kwang J. Comparison of the 5′ leader sequences of North American isolates of reference and field strains of porcine reproductive and respiratory syndrome virus (PRRSV) Virus Genes. 2001;22:209–217. doi: 10.1023/A:1008179726163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J.D., Gibson T.J., Plewniak F., Jeanmougin F., Higgins D.G. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheije M.H., Kroese M.V., Rottier P.J., Meulenberg J.J. Viable porcine arteriviruses with deletions proximal to the 3′-end of the genome. J. Gen. Virol. 2001;82:2607–2614. doi: 10.1099/0022-1317-82-11-2607. [DOI] [PubMed] [Google Scholar]
- Wensvoort G., Terpstra C., Pol J.M., ter Laak E.A., Bloemraad M., de Kluyver E.P., Kragten C., van Buiten L., den Besten A., Wagenaar F., Broekhuijsen J.M., Moonen P.L.J.M., Zetstra T., de Boer E.A., Tibben H.J., de Jong M.F., van’t Veld P., Groenland G.J.R., van Gennep J.A., Voets M.T., Verheijden J.H.M., Braamskamp J. Mystery swine disease in the Netherlands: the isolation of Lelystad virus. Vet. Q. 1991;13:121–130. doi: 10.1080/01652176.1991.9694296. [DOI] [PubMed] [Google Scholar]
- Wesley R.D., Mengeling W.L., Lager K.M., Clouser D.F., Landgraf J.G., Frey M.L. Differentiation of a porcine reproductive and respiratory syndrome virus vaccine strain from North American field strains by restriction fragment length polymorphism analysis of ORF5. J. Vet. Diagn. Invest. 1998;10:140–144. doi: 10.1177/104063879801000204. [DOI] [PubMed] [Google Scholar]
- Wesley R.D., Mengeling W.L., Lager K.M., Vorwald A.C., Roof M.B. Evidence for divergence of restriction fragment length polymorphism patterns following in vivo replication of porcine reproductive and respiratory syndrome virus. Am. J. Vet. Res. 1999;60:463–467. [PubMed] [Google Scholar]
- Westrop G.D., Wareham K.A., Evans D.M., Dunn G., Minor P.D., Magrath D.I., Taffs F., Marsden S., Skinner M.A., Schild G.C., Almond J.W. Genetic basis of attenuation of the Sabin type 3 oral poliovirus vaccine. J. Virol. 1989;63:1338–1344. doi: 10.1128/jvi.63.3.1338-1344.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G.D., Chang R.Y., Brian D.A. A phylogenetically conserved hairpin-type 3′ untranslated region pseudoknot functions in coronavirus RNA replication. J. Virol. 1999;73:8349–8355. doi: 10.1128/jvi.73.10.8349-8355.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootton S., Yoo D., Rogan D. Full-length sequence of a Canadian porcine reproductive and respiratory syndrome virus (PRRSV) isolate. Arch. Virol. 2000;145:2297–2323. doi: 10.1007/s007050070022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S., Mickelson D., Murtaugh M.P., Faarberg K.S. Complete genome comparison of porcine reproductive and respiratory syndrome virus parental and attenuated strains. Virus Res. 2001;79:189–200. doi: 10.1016/S0168-1702(01)00295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun S.-I., Kim S.-Y., Rice C.M., Lee Y.-M. Development and application of a reverse genetics system for Japanese encephalitis virus. J. Virol. 2003;77:6450–6465. doi: 10.1128/JVI.77.11.6450-6465.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


