Abstract
Aminopeptidase Ey (EC 3.4.11.20) from chicken (Gallus gallusdomesticus) egg yolk is a homodimeric exopeptidase with a broad specificity for N-terminal amino acid residues at P1 position of the substrate. Aminopeptidase Ey is a 300-k metalloexopeptidase, containing 1.0 g atom of zinc per mole of a subunit with a relative molecular mass of 150 k. A full-length cDNA was cloned from chicken (female) liver cDNA library. Analysis of the 3196-base pairs (bp) nucleotide sequence of the cDNA revealed a single open reading frame coding for 967 amino acid residues. The coding region of aminopeptidase Ey gene, apdE, occupies 2901 bp of the cDNA. The predicted amino acid sequence of the enzyme is 66, 65, 64 and 63% identical with those of aminopeptidases N (EC 3.4.11.2) from human, pig, rabbit and rat, respectively. Aminopeptidase Ey contains the metallo-binding sequence motif, His–Glu–Xaa–Xaa–His, found in zinc metallopeptidases. Zinc binding sites, His-386, His-390 and Glu-409, and catalytic site, Glu-387, were conserved in the homologous aminopeptidases N.
Keywords: Aminopeptidase, Aminopeptidase Ey, Aminopeptidase N, Aminopeptidase gene, Aminopeptidase Ey gene, apdE, Egg yolk, Chicken (Gallus gallus domesticus)
1. Introduction
Aminopeptidases (α-aminoacyl-peptide hydrolase, EC 3.4.11.-) catalyze the hydrolysis of amino acid residues from the amino terminus of peptide substrates [33]. These enzymes have broad specificity and occur in several forms. They are widely distributed in many tissues or cells, on cell surfaces, and in soluble or secreted forms in plants and animals [17]. Although some of them are well characterized, their biological roles have not yet been elucidated. Some aminopeptidases have been reported to act on bioactive peptide such as enkephalin [9]and tuftsin [19]. A scheme based on the zinc binding site has been extended to classify zinc metalloproteases into five distinct families by Hooper [8]. Recently Vazeux et al. [34]provided evidence that Glu-408 was the third zinc-coordinating residue of aminopeptidase A (glutamyl-aminopeptidase, EC 3.4.11.7), confirmed the presumed involvement of Glu-386 in the catalytic process of the enzyme, and identified the enzyme as a zinc metallopeptidase functionally similar to thermolysin (EC 3.4.24.27).
There are two distinct aminopeptidases in the chicken's (Gallus gallus domesticus) egg. One is aminopeptidase Ey (EC 3.4.11.20) from yolk 10, 29, 30, 31, 32, and the other is glutamyl aminopeptidase (EC 3.4.11.7) from egg white [24]. In a previous study [10], we purified and characterized a new aminopeptidase Ey from egg yolk. This is a homodimeric enzyme composed of two identical subunits with a relative molecular mass of 150 k 29, 32; we called it aminopeptidase Ey. Aminopeptidase Ey has unique specificities: broad selectivity for amino acid residues at the P1 position of substrates [29]; similar specificity toward the proline residue at the P1′ position 2 of aminopeptidase P (X-prolylaminopeptidase, EC 3.4.11.9); and preference specificity toward small peptides with four or five amino acid residues [30]. In a further study of substrate specificity, we found that aminopeptidase Ey was able to degrade a chemotactic peptide, N-formyl-methionyl-leucyl-phenylalanine (fMet-Leu-Phe) and released N-formyl-methionine, leucine and phenylalanine [31]. The peptide lost its chemotactic activity toward human neutrophile after incubation with aminopeptidase Ey [31].
We describe here the isolation and structural analysis of the entire cDNA of apdE gene encoding aminopeptidase Ey from chicken egg yolk and compare the deduced amino acid sequence in aminopeptidase Ey with those of aminopeptidases N from human [22], pig [23], rabbit [38]and rat [36].
2. Materials and methods
All chemicals used were of analytical grade and readily available from commercial sources. Restriction endonucleases, alkaline phosphatase, T4 polynucleotide kinase and Taq DNA polymerase were purchased from Takara Shuzo (Kyoto, Japan) and T4 DNA ligase was from Gibco (Gaithersberg, MD). Plasmids pUC119 and pBluescript II SK- were purchased from Takara Shuzo (Kyoto, Japan) and Toyobo (Tokyo, Japan), respectively. Chicken (female, adult) liver cDNA library packaged in λgt10 was purchased from Toyobo (Tokyo, Japan). Chicken (female, 40 days) cDNA library packaged in λZAP II was a gift from Professor S. Mizuno (Faculty of Agriculture, Tohoku University).
2.1. Partial amino acid sequences of aminopeptidase Ey
Aminopeptidase Ey was purified as described before [10]; 1 mg of purified aminopeptidase Ey was dissolved in 300 μl of 0.5 M Tris–HCl, 8 M urea, and 10 mM EDTA (pH 8.5). Then 100 μl of 1 M dithiothreitol was added to the enzyme solution and it was incubated at room temperature under N2. It was incubated overnight after adding 2 μl of 4-vinyl pyridine to the mixture. The reaction mixture was dialyzed against 0.5 M Tris–HCl buffer (pH 8.5) containing 2 mM EDTA and then against H2O. After dialysis, the sample was lyophilized. The S-pyridylethylated aminopeptidase Ey was dissolved with 60 μl of 0.1 M Tris–HCl (pH 9.0), containing 8 M urea and 2 mM EDTA, and it was left for 2 h at room temperature before adding 60 μl of 8.33 μg/ml lysylendopeptidase (Wako, Osaka, Japan). Then the mixture was incubated at 30°C for 24 h. The reaction mixture was applied to a reverse-phase column Shodex RSpak RP18-415 (φ 4.6×150 mm) equipped with a Hitachi model L-6200 delivery system. The amino acid sequence of the purified fragment was carried out with Applied Biosystem 473A protein sequencer. Preparation of a C-terminus containing peptide was carried out as described by Kumazaki et al. [11].
2.2. Screening of aminopeptidase Ey cDNA, apdE
A partial clone of aminopeptidase Ey cDNA was obtained from chicken (female, adult) liver cDNA library packaged in λgt10 (Toyobo CLCL 1018a) with polymerase chain reaction (PCR). Two oligonucleotide primers were designed, the sense primer based on the amino acid sequence, 306Glu–Gly–Gln–Gly–Glu–Tyr–312Ala, of lysylendopeptidic peptide of aminopeptidase Ey (rabbit aminopeptidase N numbering), [5′-GA(A/G)GG(T/C/A/G)CA(A/G)GG(T/C/A/G)GA(A/G)TA(T/C)GC-3′] and antisense primer based on the sequence of 344Asp–Phe–Asn–Ala–Gly–Ala–Met–351Glu (rabbit aminopeptidase N numbering) [5′-TCCAT(T/C/A/G)GC(T/C/A/G)CC(T/C/A/G)GC(A/G)TT(A/G)AA(A/G)TC-3′]. Using a DNA thermalcycler (Perkin–Elmer Cetue), 35 cycles of amplification were carried out with a step program consisting of 95°C for 30 s, 48°C for 60 s, and 72°C for 90 s. In the final cycle, 72°C extension time was increased to 7 min. A PCR fragment of 135 bp was obtained. Further screening of 2×105 plaques of the cDNA library with the obtained PCR fragment as the probe led to the isolation of one positive plaque. A cDNA fragment of 1260 bp was obtained. Subsequently, screening of 3.6×105 plaques of chicken (female, 40 days) liver cDNA library packaged in λZAP II led to the isolation of six positive clones. Plaque hybridization was carried out [3]. The positive clones were subcloned into pBluescript SK−. The inserts were 3.5 kb. Both strands were sequenced using Taq dye primer sequencing kit and Applied Biosystem DNA sequencer 373A (Perkin–Elmer, Urayasu, Chiba, Japan).
3. Results
3.1. Partial amino acid sequences of aminopeptidase Ey
Seven lysylendopeptidase-digested peptides were obtained and sequenced. We obtained a total of approximately 150 residues of amino acid sequences, including a short zinc binding consensus sequence motif, His–Glu–Xaa–Xaa–His. Analysis of C-terminal peptide showed that Glu–Val–Val–His–Ala–Trp–Phe–Arg–Ala–Glu–Thr–Ala–Ser was obtained. These results indicate the seven peptides underlined in Fig. 1 .
Fig. 1.

Nucleotide sequence of the cDNA of aminopeptidase Ey gene, apdE, and deduced amino acid sequence of aminopeptidase Ey from chicken's egg yolk. Identical amino acid sequences obtained by lysylendopeptidase digestion were underlined. Open circles (○) identify the His and Glu residues corresponding to the zinc ligands motif in aminopeptidase N from rabbit [38]. Regular box (□) enclose Glu residue identical to the catalytic site corresponding to aminopeptidase N from rabbit [38].
3.2. Cloning and sequencing of aminopeptidase Ey cDNA, apdE
Analysis of the 3196-bp nucleotide sequence of the cDNA revealed a single open reading frame of 2901 nucleotides, starting at the first ATG codon located at 25 nucleotides from 5′-terminal end of the clone and ending with a TAG stop codon. The 3′ untranslated region is 268 nucleotides long and contains a canonical polyadenylation signal (AATAAA) 12 nucleotides upstream of the poly A tail. We designated the gene as apdE. The predicted amino acid sequence of aminopeptidase Ey cDNA, apdE, contains 967 amino acids (Fig. 1). The calculated relative molecular mass of aminopeptidase Ey encoded by the cDNA sequence was 108 669. A survey of the EMBL protein database revealed that the highest degree of identity of aminopeptidase Ey amino acid sequence from chicken egg yolk occurred with aminopeptidases N from human intestine [22], porcine renal brush-border membrane [23], rabbit kidney [38], and rat kidney 16, 36. A detailed comparison of the predicted amino acid sequence of aminopeptidase Ey with human aminopeptidase N and the other aminopeptidases N is shown in Fig. 2 . The amino acid sequence of aminopeptidase Ey exhibits an identity of 66% with that of human intestinal aminopeptidase N [22], 65% with pig kidney aminopeptidase N [23], 64% with rabbit kidney aminopeptidase N [38], and 63% with rat aminopeptidase N [36], respectively. Glutamyl aminopeptidases (aminopeptidases A; EC 3.4.11.7) 13, 37showed only 34% of identity with aminopeptidase Ey.
Fig. 2.
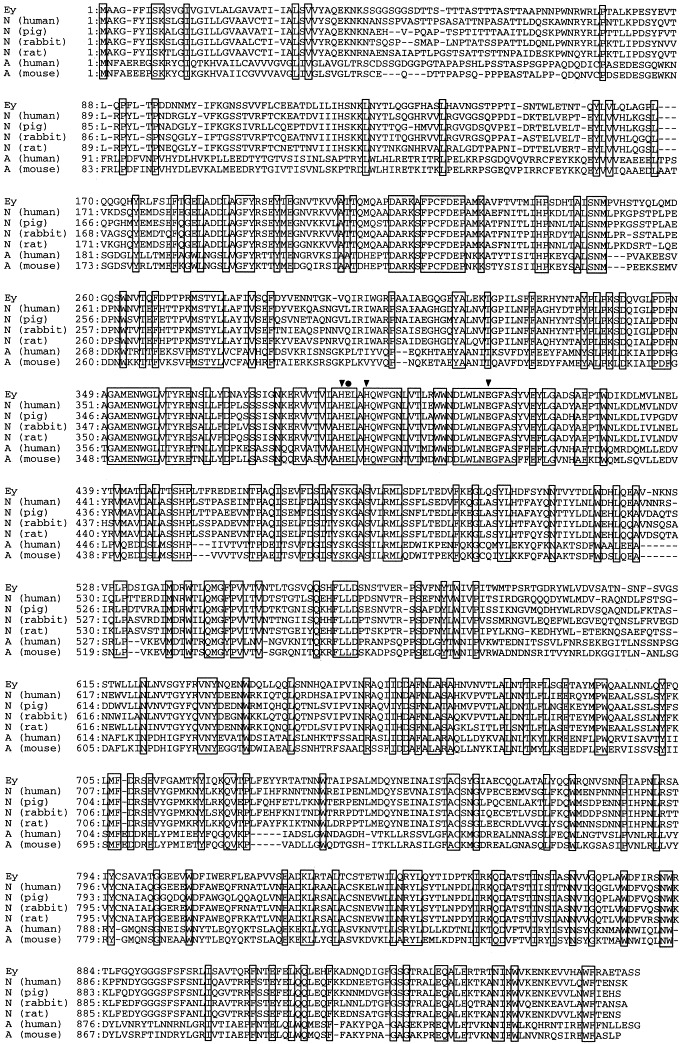
Comparison of amino acid sequences of aminopeptidase Ey, aminopeptidases N from human [22], pig [23], rabbit [38]and rat [36], and aminopeptidase A from human [13]and mouse [37]. Ey, N (human), N (pig), N (rabbit), N (rat), A (human) and A (mouse) indicate aminopeptidase Ey, human intestinal aminopeptidase N, pig kidney aminopeptidase N, rabbit kidney aminopeptidase N, rat kidney aminopeptidase N, human aminopeptidase A and rat aminopeptidase A, respectively. Boxes enclose residues identical to the corresponding aminopeptidase Ey. Triangles (▾) identify the His and Glu residues corresponding to zinc ligands motif in aminopeptidase N from rabbit [38]. Circle (•) shows Glu residues identical to the catalytic site corresponding to aminopeptidase N from rabbit [38].
4. Discussion
Although aminopeptidase Ey was purified from chicken egg yolk, it was observed by Western blot analysis that the large amount of aminopeptidase Ey was located in chicken liver (data not shown). The cDNA of aminopeptidase Ey, apdE, was obtained from chicken liver cDNA library. As the determined amino acid sequences of the lysylendopeptidic peptides and C-terminal peptide of aminopeptidase Ey purified from the chicken egg yolk coincided with the deduced amino acid sequence from apdE and cDNA of aminopeptidase Ey from chicken liver. It affirmed that apdE encoded aminopeptidase Ey. It indicated that the aminopeptidase Ey was expressed in both egg yolk and chicken liver. Human aminopeptidase N is also expressed in a variety of viscera 14, 18, 21, 22, 26, 39.
The deduced amino acid sequence of aminopeptidase Ey was similar to that of aminopeptidases N from mammals. The amino acid sequence of aminopeptidase Ey showed identities of about 63–66% with those of other aminopeptidases N from mammals, but the aminopeptidases N from human, pig, rabbit and rat showed higher identities (77–79%) to each other. We conclude that aminopeptidase Ey represents chicken aminopeptidase N. Aminopeptidase N type had not been previously described in birds. This is the first report of the cloning and sequencing of aminopeptidase N from chicken liver. Recently, cDNA of chicken aminopeptidase H from liver was cloned and sequenced [1], but aminopeptidase H and aminopeptidase Ey do not resemble each other.
Aminopeptidase Ey sequence, as well as mammalian aminopeptidase N, contains the consensus amino acid sequence, His–Glu–Xaa–Xbb–His--//--Glu, characteristic of metallopeptidase. Two histidine residues at 386 and 390, and Glu409 are considered to comprise the zinc binding site, and Glu387 corresponds to active center amino acid [34]. According to Hooper's classification [8], the aminopeptidase Ey, defined by the arrangement of the His–Glu–Xaa–Xbb–His motif and a glutamate as the third ligand, can be classified as a gluzincin. The gluzincins include thermolysin (EC 3.4.24.27), endopeptidase 24.11 (EC 3.4.24.11), aminopeptidase N (EC 3.4.11.2), angiotensin I converting enzyme (EC 3.4.15.1), endopeptidase 24.15, and tetanus and botulinum neurotoxin families.
The first 39 amino acid residues from amino terminus of aminopeptidase Ey and aminopeptidases N are conserved. Using the method of Kyte and Doolittle [12], the hydrophobicity plots of aminopeptidase Ey and aminopeptidase N from rabbit are shown in Fig. 3 . A hydrophobic region at amino acid position 9–32 is found in each aminopeptidase. It has been reported that the hydrophobic region of aminopeptidase N from mammals forms the anchor to the membrane, the region of amino acid position 2–8 is in cytoplasmic space 36, 38, and carboxy terminal regions of the mammalian enzyme containing the aminopeptidase active site signifies the ectodomain. From the results of similarity of hydropathy plot and sequence homology, aminopeptidase Ey can also be assumed to be a membrane protein, but about 95% of the total aminopeptidase Ey activity was found in egg yolk plasma fraction in a soluble form [10]. Many aminopeptidases N are located on the membrane surface, but some of the enzymes occur as the soluble form. Human bile, for instance, contains a soluble form of aminopeptidase N. It is thought that the enzyme is probably released from the biliary canalicular membrane by the detergent activity of bile salts and may be one factor promoting cholesterol crystallization in the gallbladder [21]. Intestinal aminopeptidases N are found as various isoforms. They are fragmented by limited proteolysis with trypsin [4]. The tryptic cleavage site of the enzyme is N-terminal hydrophobic–hydrophilic junction between 39Lys and 40Asn [6]. Aminopeptidase Ey is also thought to be cleaved at the same position as the 39Lys–40Ser bond by an unknown trypsin-like enzyme in egg yolk, and the enzyme might be released from membrane. The following region at amino acid position 40–69 of aminopeptidase Ey is not homologous to that of aminopeptidases N, but these region are Ser- and Thr-rich in both enzymes. Ser- and Thr-rich domains tend to be O-glycosylated [7]. In a present work, amino terminal amino acid sequence of aminopeptidase Ey could not be determined, probably due to limited digestion caused by continuous O-glycosylated Ser/Thr.
Fig. 3.
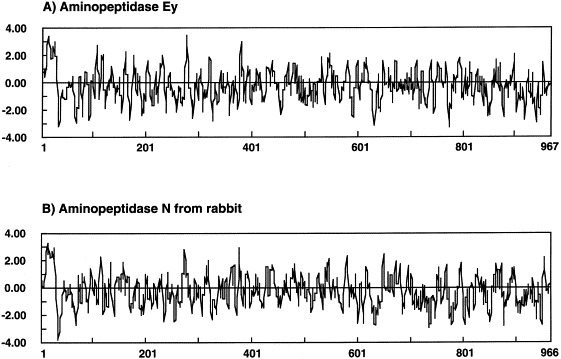
Hydrophobicity plots of aminopeptidase Ey and rabbit aminopeptidase N. A, aminopeptidase Ey; B, aminopeptidase N from rabbit kidney.
There are 16 N-link glycosylation sites in the deduced amino acid sequence of aminopeptidase Ey. Tanaka et al. reported the relative molecular mass of the purified subunit of the enzyme as 150 k and the enzyme contained 14% of carbohydrate including GlcNAc, GalNAc, hexose and sialic acid, and one-third of it (4.8% of the relative molecular mass) was occupied with sialic acid [29]. Purified aminopeptidase Ey has an isoelectric point of 2.8 [29]. After treatment with sialidase, the enzyme shows a sharp band at pI 4.4. As the calculated relative molecular mass of aminopeptidase Ey is 108 669, about 40 kDa of carbohydrate must be linked to the enzyme. This is reminiscent of the human aminopeptidase N which has a deduced molecular mass of aminopeptidase N of 109 542 Da, but the molecular mass of the purified subunit of the enzyme is 150 kDa. The isoelectric point of human aminopeptidase N is 4.7 [28]. Obviously, the human enzyme is also modified with carbohydrates, but the human enzyme may not contain sialic acid.
Mammalian aminopeptidase N is a widespread membrane-binding protein. It is also known as CD13 membrane-surface glycoprotein 14, 35. It plays various roles in vivo. Aminopeptidase N in the human placenta may be to desensitize the action of immunomodulating peptides as well as vasoactive and neuropeptide hormones, and to control both immunology and endocrinology of pregnancy [18]. Rat brain aminopeptidase N is involved in the degradation of regulatory peptides including enkephalins [15]. Cell-surface aminopeptidase N of murine and human metastatic tumor cells may be partly involved in the activation mechanism for type-IV collagenolysis to achieve tumor-cell invasion [26]. Aminopeptidase N of human and porcine is a receptor for coronaviruses, during viral infection. 5, 39. Mammalian intestinal brush-border membrane aminopeptidase N hydrolyzes oligomeric peptides for digestion and transports the resultant free amino acids 2, 20, 25. As there are few reports about avian aminopeptidase N, the role of aminopeptidase Ey in egg yolk is uncertain. Aminopeptidase Ey may participate in the maturation and/or degradation of peptidic hormones concerned with ontogenesis, as mammalian placental aminopeptidase N and brain aminopeptidase N are concerned with the control of the level of peptide hormones. Possibly, aminopeptidase Ey may degrade the oligomeric peptides derived from the storage proteins for supplying amino acids to embryo, as the role of intestinal aminopeptidase N is the digestion of oligopeptides and the absorption of resultant amino acids.
Acknowledgements
We thank Professor S. Mizuno, Department of Applied Biological Chemistry, Tohoku University, for the permission to use of chicken liver cDNA library packaged in λZAPII.
Footnotes
The nucleotide sequence data reported in this paper will appear in the DDBJ, EMBL and GenBank nucleotide sequence database with the accession number D87992.
S1, S2, S3, etc. and S1′, S2′, S3′, etc., corresponding subsites of the peptidase; P1, P2, P3, etc. and P1′, P2′, P3′, etc., amino acid residues of substrate on the amino-terminal and carboxyl-terminal sides of scissile peptide bond, respectively [27].
References
- 1.Adachi H., Tsujimoto M., Fukasawa M., Sato Y., Arai H., Inoue K., Nishimura T. cDNA cloning and expression of chicken aminopeptidase H, possessing endopeptidase as well as aminopeptidase activity. Eur J Biochem. 1997;245:283–288. doi: 10.1111/j.1432-1033.1997.t01-1-00283.x. [DOI] [PubMed] [Google Scholar]
- 2.Antonov V.K., Vorotyntseva T.I., Bessmertnaya L., Mikhailova A.G., Zilberman M.I. Role of intestinal brush border membrane aminopeptidase N in dipeptide transport. FEBS Lett. 1984;171:227–232. doi: 10.1016/0014-5793(84)80493-7. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel F.M., Brent R., Kingston R.E., Moore D.D., Seidman J.G., Smith J.A., Struhl K. Current Protocols in Molecular Biology. Greene Associates and Wiley-Interscience; New York, Chichester, Brisbane, Toronto, Singapore: 1987. pp. 6.0.1–6.4.10. [Google Scholar]
- 4.Benajiba A., Maroux S. Subunit structured of pig small-intestinal brush-border aminopeptidase N. Biochem J. 1981;197:573–580. doi: 10.1042/bj1970573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delmas B., Gelfi J., L'Haridon R., Vogel L.K., Sjostrom H., Noren O., Laude H. Aminopeptidase N is a major receptor for the entero-pathogenic coronavirus TGEV. Nature. 1992;357:417–420. doi: 10.1038/357417a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feracci H., Maroux S., Bonicel J., Desnuelle P. The amino acid sequence of the hydrophobic anchor of rabbit intestinal brush border aminopeptidase N. Biochim Biophys Acta. 1982;684:133–136. doi: 10.1016/0005-2736(82)90057-8. [DOI] [PubMed] [Google Scholar]
- 7.Hansen JE, Lund O, Engelbrecht J, Bohr H, Nielsen JO, Hansen JE. Prediction of O-glycosylation of mammalian proteins: specificity patterns of UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase. Biochem J. 1995;308:801–813. doi: 10.1042/bj3080801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hooper N.M. Families of zinc metalloproteases. FEBS Lett. 1994;354:1–6. doi: 10.1016/0014-5793(94)01079-x. [DOI] [PubMed] [Google Scholar]
- 9.Hui K.S., Wang Y.J., Lajtha A. Purification and characterization of an enkephalin aminopeptidase from rat brain membranes. Biochemistry. 1983;22:1062–1067. doi: 10.1021/bi00274a010. [DOI] [PubMed] [Google Scholar]
- 10.Ichishima E., Yamagata Y., Chiba H., Sawaguchi K., Tanaka T. Soluble and bound forms of aminopeptidase from hen's egg yolk. Agric Biol Chem. 1989;53:1867–1872. [Google Scholar]
- 11.Kumazaki T., Terasawa K., Ishii S. Affinity chromatography on immobilized anhydrotrypsin: general utility for selective isolation of C-terminal peptides from protease digests of proteins. J Biochem (Tokyo) 1987;102:1539–1546. doi: 10.1093/oxfordjournals.jbchem.a122202. [DOI] [PubMed] [Google Scholar]
- 12.Kyte J., Doolittle R.F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 13.Li L., Wang J., Cooper M.D. cDNA cloning and expression of human glutamyl aminopeptidase (aminopeptidase A) Genomics. 1993;17:657–664. doi: 10.1006/geno.1993.1386. [DOI] [PubMed] [Google Scholar]
- 14.Look A.T., Ashmun R.A., Shapiro L.H., Peiper S.C. Human myeloid plasma membrane glycoprotein CD13 (gp150) is identical to aminopeptidase N. J Clin Invest. 1989;83:1299–1307. doi: 10.1172/JCI114015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucius R., Sievers J., Mentlein R. Enkephalin metabolism by microglial aminopeptidase N (CD13) J Neurochem. 1995;64:1841–1847. doi: 10.1046/j.1471-4159.1995.64041841.x. [DOI] [PubMed] [Google Scholar]
- 16.Malfroy B., Kado F.H., Gros C., Giros B., Schwartz J.C., Hellmiss R. Molecular cloning and amino acid sequence of rat kidney aminopeptidase M: a member of a super family of zinc-metallohydrolases. Biochem Biophys Res Commun. 1989;161:236–241. doi: 10.1016/0006-291x(89)91586-6. [DOI] [PubMed] [Google Scholar]
- 17.McDonald J.K., Barrett A.J. Exopeptidases. In: McDonald J.K., Barrett A.J., editors. Mammalian Proteases: A Glossary and Bibliography. Academic Press; London: 1986. pp. 23–154. [Google Scholar]
- 18.Mizutani S., Goto K., Nomura S., Ino K., Goto S., Kikkawa F., Kurauchi O., Goldstein G., Tomoda Y. Possible action of human placental aminopeptidase N in feto-placental unit. Res Commun Chem Pathol Pharmacol. 1993;82:65–80. [PubMed] [Google Scholar]
- 19.Nagaoka I., Yamashita T. Inactivation of phagocytosis-stimulating activity of tuftsin by polymorphonuclear neutrophils. A possible role of leucine aminopeptidase as an ecto-enzyme. Biochim Biophys Acta. 1981;675:85–93. doi: 10.1016/0304-4165(81)90072-6. [DOI] [PubMed] [Google Scholar]
- 20.Noack R., Friedrich M., Proll J., Uhlig J. Digestion and resorption of proteins. Nahrung. 1975;19:891–901. doi: 10.1002/food.19750190922. [DOI] [PubMed] [Google Scholar]
- 21.Offner G.D., Gong D., Afdhal N.H. Identification of a 130-kilodalton human biliary concanavalin A binding protein as aminopeptidase N. Gastroenterology. 1994;106:755–762. doi: 10.1016/0016-5085(94)90712-9. [DOI] [PubMed] [Google Scholar]
- 22.Olsen J., Cowell G.M., Konigshofer E., Danielsen E.M., Moller J., Laustsen L., Hansen O.C., Welinder K.G., Engberg J., Hunziker W. Complete amino acid sequence of human intestinal aminopeptidase N as deduced from cloned cDNA. FEBS Lett. 1988;238:307–314. doi: 10.1016/0014-5793(88)80502-7. [DOI] [PubMed] [Google Scholar]
- 23.Olsen J., Sjostrom H., Noren O. Cloning of the pig aminopeptidase N gene. Identification of possible regulatory elements and the exon distribution in relation to the membrane-spanning region. FEBS Lett. 1989;251:275–281. doi: 10.1016/0014-5793(89)81470-x. [DOI] [PubMed] [Google Scholar]
- 24.Petrovic S., Vitale L. Purification and properties of glutamyl aminopeptidase from chicken egg-white. Comp Biochem Physiol. 1990;95B:589–595. doi: 10.1016/0305-0491(90)90026-p. [DOI] [PubMed] [Google Scholar]
- 25.Plakidou DS, Tanner MJ, McGivan JD. A role for aminopeptidase N in Na(+)-dependent amino acid transport in bovine renal brush-border membranes. Biochem J. 1993;290:59–65. doi: 10.1042/bj2900059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saiki I., Fujii H., Yoneda J., Abe F., Nakajima M., Tsuruo T., Azuma I. Role of aminopeptidase N (CD13) in tumor-cell invasion and extracellular matrix degradation. Int J Cancer. 1993;54:137–143. doi: 10.1002/ijc.2910540122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967;27:157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- 28.Scherberich J.E., Wiemer J., Herzig C., Fischer P., Schoeppe W. Isolation and partial characterization of angiotensinase A and aminopeptidase M from urine and human kidney by lectin affinity chromatography and high-performance liquid chromatography. J Chromatogr. 1990;521:279–289. doi: 10.1016/0021-9673(90)85052-w. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka T., Ichishima E. Molecular properties of aminopeptidase Ey as a zinc-metalloenzyme. Int J Biochem. 1993;25:1681–1688. doi: 10.1016/0020-711x(93)90528-m. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka T., Ichishima E. Substrate specificity of aminopeptidase Ey from hen's (Gallusdomesticus) egg yolk. Comp Biochem Physiol. 1993;105B:105–110. doi: 10.1016/0305-0491(93)90175-5. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka T., Ichishima E. Inactivation of chemotactic peptides by aminopeptidase Ey from hen's (Gallusgallusdomesticus) egg yolk. Comp Biochem Physiol. 1994;107B:533–538. doi: 10.1016/0305-0491(94)90181-3. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka T., Oshida K., Ichishima E. Electron microscopic analysis of dimeric form of aminopeptidase Ey. Agric Biol Chem. 1991;55:2179–2181. [Google Scholar]
- 33.Taylor A. Aminopeptidases: towards a mechanism of action. Trends Biochem Sci. 1993;18:167–172. [PubMed] [Google Scholar]
- 34.Vazeux G., Wang J., Corvol P., Llorens C.C. Identification of glutamate residues essential for catalytic activity and zinc coordination in aminopeptidase A. J Biol Chem. 1996;271:9069–9074. doi: 10.1074/jbc.271.15.9069. [DOI] [PubMed] [Google Scholar]
- 35.Verlinden J, van Leuven F, Cassiman JJ, van den Berghe H. Identification of the human fibroblast surface glycoprotein (FSH) as aminopeptidase M. FEBS Lett. 1981;123:287–290. doi: 10.1016/0014-5793(81)80310-9. [DOI] [PubMed] [Google Scholar]
- 36.Watt V.M., Yip C.C. Amino acid sequence deduced from a rat kidney cDNA suggests it encodes the Zn-peptidase aminopeptidase N. J Biol Chem. 1989;264:5480–5487. [PubMed] [Google Scholar]
- 37.Wu Q., Lahti J.M., Air G.M., Burrows P.D., Cooper M.D. Molecular cloning of the murine BP-1/6C3 antigen: a member of the zinc-dependent metallopeptidase family. Proc Natl Acad Sci USA. 1990;87:993–997. doi: 10.1073/pnas.87.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang X.F., Milhiet P.E., Gaudoux F., Crine P., Boileau G. Complete sequence of rabbit kidney aminopeptidase N and mRNA localization in rabbit kidney by in situ hybridization. Biochem Cell Biol. 1993;71:278–287. doi: 10.1139/o93-042. [DOI] [PubMed] [Google Scholar]
- 39.Yeager C.L., Ashmun R.A., Williams R.K., Cardellichio C.B., Shapiro L.H., Look A.T., Holmes K.V. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature. 1992;357:420–422. doi: 10.1038/357420a0. [DOI] [PMC free article] [PubMed] [Google Scholar]


