Summary
Objective
To assess the etiological role and the clinical characteristics of HRV and HEV infections in pediatric patients hospitalized for acute respiratory tract infections (ARTIs).
Methods
RT-qPCR assays and molecular sequencing methods were used to identify HRV and HEV strains in nasopharyngeal aspirates of 309 hospitalized pediatric patients with microbiologically unexplained ARTIs and in 210 hospitalized pediatric patients without respiratory symptoms from September 2009 to June 2010 in France.
Results
Among the 309 ARTI cases, 15 HEV and 172 HRV strains were identified whereas only 1 HEV and 37 HRV strains were observed in control patients (187 vs. 38: P < 10−3). HRV strains were identified in 150 of the 164 lower ARTIs whereas HEV strains were identified in only 14 of these cases. Among bronchiolitis and asthma exacerbation cases (n = 133), HEV infected cases were older (Median age (months) 36 vs. 11, P = 0.003) and were more frequently associated with a respiratory distress (P = 0.01) and a need for oxygen supply at the time of admission (P = 0.01) than cases infected by HRV strains.
Conclusion
HRV and HEV strains were identified as potential etiological causes of 60.5% of microbiologically unexplained ARTIs diagnosed in hospitalized pediatric cases. A higher clinical severity was observed in HEV infected bronchiolitis or asthma exacerbation cases in comparison to HRV infected cases.
Keywords: Pediatric patients, Bronchiolitis, Wheezing, Rhinovirus, Enterovirus genus, RT-qPCR assay, Epidemiology, Clinical severity
Introduction
The Enterovirus (EV) genus (Picornaviridae family) consists of small non-enveloped positive RNA viruses classified in 12 species of which 7 are pathogenic for humans: four species (A–D) of human enterovirus (HEV) and three species (A–C) of human rhinovirus (HRV).1 Among the EV genus, HEV and HRV are recognized as leading causes of Acute Respiratory Tract Infections (ARTIs) in humans.2, 3, 4, 5
Human rhinovirus (HRV) of both species A and B are generally considered to be responsible for upper ARTIs, but they are increasingly reported to be associated with lower ARTIs such as bronchiolitis or pneumonia in infants or young children.4, 6 In 2006, a third species was identified with highly prevalence, HRV species C (HRV-C) that could be more specifically responsible for lower ARTIs in pediatric patients.6, 7, 8
Human enterovirus (HEV) can induce non-specific upper and lower ARTIs including severe or fatal bronchiolitis or pneumonia in infants and young children.2, 5, 9 Recently, the use of new molecular tools have demonstrated that new HEV respiratory genotypes have emerged or re-emerged, (e.g. enteroviruses 68, 104, 109, 117 and coxsackievirus A-21) and that these circulating strains could be also responsible for acute bronchitis, bronchiolitis, asthma exacerbation and severe or fatal pneumonia in pediatric patients.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 Recent HEV respiratory outbreaks in Japan, the Philippines and the Netherlands as well as several clusters in the United Kingdom have identified HEV-D68 confirming the rapid circulation of this new emerging respiratory pathogen.10, 12, 13, 14, 15, 16, 18, 19 The clinical presentation of HEV-D68 infections in these outbreaks ranged from mild illness to rare severe pneumonia cases requiring admission in intensive care unit and leading potentially to death.12, 13, 14
The HEV and HRV genotypes detection in the respiratory tract samples remains difficult even using molecular tools because of the large EV genetic diversity, leading to an underestimation of the prevalence and role of HRV and HEV in pediatric ARTIs.4 The detection, identification and monitoring of EV genotypes in ARTIs would be helpful to understand the (re)-emergence of strains responsible for epidemics characterized by severe respiratory symptoms.4 In the present study, we first screened respiratory samples taken from a large cohort of pediatric patients hospitalized in our institution during a 10-month period22, 23, 24 for the presence of EV using reliable specific “Pan-entero-rhino” and HEV molecular detection tools. We secondly performed a VP4-VP2 capsid gene genotyping identification of the EV strains detected. Finally, we assessed the etiological role and the clinical characteristics of HRV and HEV infections in pediatric patients hospitalized for ARTIs.
Patients and methods
Patients
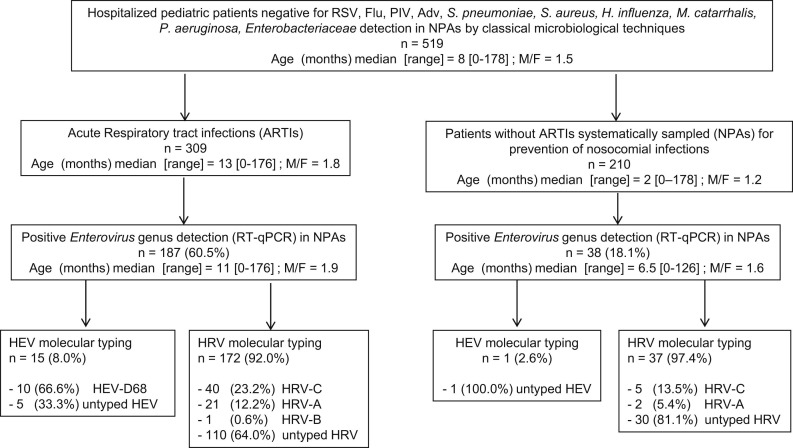
Of the 1195 patients hospitalized in the pediatric department of the University Medical Centre of Reims (Champagne-Ardennes, France) from September 2009 to June 2010 who underwent nasopharyngeal aspirations (NPAs), 519 were retrospectively selected because they were negative by use of classical virological tests consisting of Direct Fluorescent Antibody targeting common respiratory pathogen (respiratory syncytial viruses A and B (RSV), Influenza viruses A and B (Flu A&B), Parainfluenza viruses (PIV) and adenoviruses (Adv)) antigens and negative by use of classical assays for direct detection or isolation of potential bacterial respiratory pathogens (Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, Pseudomonas aeruginosa, Staphylococcus aureus, Enterobacteriaceae). 5, 25 Demographical (age; sex; prematurity), epidemiological (passive smoking at home; familial antecedent of atopia); virological, bacteriological and clinical data (personal antecedent of asthma or infants asthma; symptoms and severity respiratory symptoms at the time of admission: fever, cough, rhinorrhea, wheezing, respiratory distress symptoms and need for oxygen therapy; need for admission to intensive care unit; prescription of antibiotics; length of hospitalization; death and final diagnosis at the end of hospitalization) were extracted from patient records. Of these 519 hospitalized patients negative for the detection of common viral and bacterial respiratory pathogens, 309 were admitted for ARTIs (see definition below) and 210 without any clinical signs consistent with ARTIs were considered as controls patients hospitalized for various non-respiratory diseases and systematically sampled for prevention and control of nosocomial viral infections in pediatric wards (Fig. 1 ). Each control subject was selected during the same period month in regards of selected patients (1–2 patients for one control subject per month; data not shown). Informed consent was obtained from the infants' parents and the hospital's ethics committee (Institutional Review Board of the Reims University hospital) approved the present study.
Figure 1.
Study design and frequency of detection of human enterovirus and human rhinovirus strains by real-time RT-qPCR in nasopharyngeal aspiration samples (NPAs) of pediatric patients hospitalized for acute respiratory tract infections from September 2009 to June 2010 in Northern east of France. HEV (Human enterovirus, specie), HRV (Human rhinovirus, specie), RSV (Respiratory Syncytial Virus), Flu (Influenza viruses), PIV (Parainfluenza Virus), Adv (Adenovirus).
Definitions
The patients were classified in the ARTIs group when they had symptoms consistent with the final diagnosis of rhinitis, bronchitis, bronchiolitis, exacerbated asthma or pneumopathy (e.g. fever, rhinorrhea, cough and wheezing dyspnea). When these symptoms were absent, the patients were classified in the control group. Rhinitis was defined as upper ARTIs whereas bronchitis, bronchiolitis, exacerbated asthma or pneumopathy were defined as lower ARTIs.
Exacerbated asthma corresponded to the majoration of a wheezing dyspnea in a child with personal antecedent of asthma or infant asthma. Asthma was defined as the presence of one episode of wheezing dyspnea in a child of more than 2 years, whereas infant asthma was defined as the presence of more than two events of wheezing dyspnea in a child of less than 2 years. Bronchiolitis was defined according to French consensus conference.26 Briefly, the diagnosis of bronchiolitis was established in case of wheezing dyspnea requiring conventional chest physical therapy in a child of less than 2 years without personal antecedent of infant asthma. Rhinitis, bronchitis and pneumopathy have been defined elsewhere.27, 28
Prematurity was defined as a birth before 37 weeks of amenorrhea. Fever was defined as the presence of an external body temperature > or = to 38 °C. Respiratory distress was defined as the presence of a dyspnea associated with one of the following clinical symptoms: chest in drawing, accessory respiratory muscle use, paradoxical breathing or nasal flaring.
Clinical specimens
Nasopharyngeal aspiration (NPA) was collected for each patient using sterile physiological saline fluid with a disposable mucus extractor at time of hospital admission according to the recent European Respiratory Society guidelines.26, 27, 28 Only one sample per patient was considered, even in case of multiple admissions. Specimens were then rapidly transported to the laboratory where they were divided between two sterile tubes. The first tube was prospectively used to perform routine microbiology diagnostic tests whereas the second tube was immediately stored at −80 °C until doing retrospective molecular analyses.
DNA and RNA extraction from respiratory samples
Total nucleic acid extraction (DNA and RNA) was retrospectively performed from each respiratory specimen using the NucliSens EasyMAG instrument (BioMerieux, Lyon, France) according to the manufacturer's instructions. Briefly, 200 μL (μL) of the nasopharyngeal secretions were subjected to proteinase K (250 μg/ml) (Ambion, Life Technologies, Saint-Aubin, France) digestion in buffer containing 20 mM Tris–HCl pH 8.3 (Sigma–Aldrich, Saint-Louis, USA) and 0.5% SDS (Sigma–Aldrich, Saint-Louis, USA) for 20 min in a water bath at 56 °C. Afterward, it was added to 2 mL of lysis buffer in a plastic vessel and incubated for 10 min at room temperature. Fifty μL of silica were then added to the mixture. This was followed by an automatic magnetic separation step. Nucleic acids were recovered in 50 μL elution buffer and stored at −80 °C until use.
“Pan-entero-rhino” one-step quantitative real-time RT-PCR assay
One-step quantitative real-time RT-PCR (RT-qPCR) assay targeting the highly conserved 5′ nontranslated region of Enterovirus genus (EV) ((human enterovirus (HEV) and human rhinovirus (HRV)) genome was carried out to analyze the clinical samples collected in the course of the study and quantify the viral load in each EV positive specimen. Briefly, 2 μL of total nucleic acid extract were amplified in a 10 μL RT-PCR mixture containing 5 μL 2× Reaction Mix, 2U SuperScript III RT/Platinum Taq Mix (Invitrogen, Life Technologies, Saint-Aubin, France), 0.4 μM of each primer and probe. The primer sequences used were 5′-AGCCTGCGTGGCKGCC-3′ and 5′- GAAACACGGACACCCAAAG-3′ and the probe was 5′-[FAM]-CTCCGGCCCCTGAATGYGGCTAA-3′.22 Reverse transcription (RT) was performed at 55 °C for 30 min, the RT heat inactivated at 94 °C for 2 min, then the cDNA amplified in 40 cycles as follows: denaturation at 94 °C for 15 s and annealing/extension step at 63 °C for 1 min. At the end of the extension step, the Step-One-Plus Real-time PCR Detection system (Applied Biosystem, Saint Aubin, France) monitored real-time PCR amplification by quantitative analysis of the fluorescence emissions. The copy number of viral RNA was determined based on a standard curve of C (t) level versus the copy number of positive-strand transcripts of the CVB3 genome transcribed from pCR21C10A cDNA clone kindly provided by N.M. Chapman (University of Nebraska Medical Center, Omaha NE USA). Transcripts were prepared with MEGAscript High Yield Transcription Kit and purified with MEGAclear (Ambion, Life technologies, Saint-Aubin, France) according to the manufacturer's instructions. A GAPDH real-time RT-PCR assay allows viral load normalization among the clinical samples analyzed using the following calculation: EV RNA load quantified in the NPA divided by GAPDH mRNA copy number assessed in the same specimen.29
HEV and HRV identification
To discriminate HEV from HRV strains in EV positive clinical samples, we performed a specific HEV one-step real-time RT-PCR assay using the primers NC1M-F and E2-R and the probe OL27LC targeting the 5′untranslated region as described previously.30 HEV- and HRV-positive specimens were genotyped in a part of the VP4/VP2 capsid proteins coding region: amplification was performed using the primers VP4/VP2-Pikorna-444-F and VP4/VP2-9565-1193-R according to a previously described protocol23; VP1 amplification of HEV-D68 was performed using the primers VP1-2547-F and VP1-2772-R.16 After amplification, the PCR products were subjected to agarose gel electrophoresis and bands were excised from gel and purified using the QIAquick PCR purification Kit (Qiagen, Courtaboeuf, France). Both strands were sequenced directly with the same PCR primers using the Big Dye terminator v3.1 sequencing kit (Applied Biosystems, Saint Aubin, France). The sequencing products were then analyzed on an ABI 3130 genetic analyzer (Applied Biosystems, Saint Aubin, France). These sequences have been deposited in the GenBank database under accession numbers: HEV-D68: JX402051.1 to JX402060.1; HRV-A: JX876800.1 to JX876814.1; HRV-B: JX876815.1; HRV-C: JX876763.1 to JX876799.1.
Phylogenetic analysis
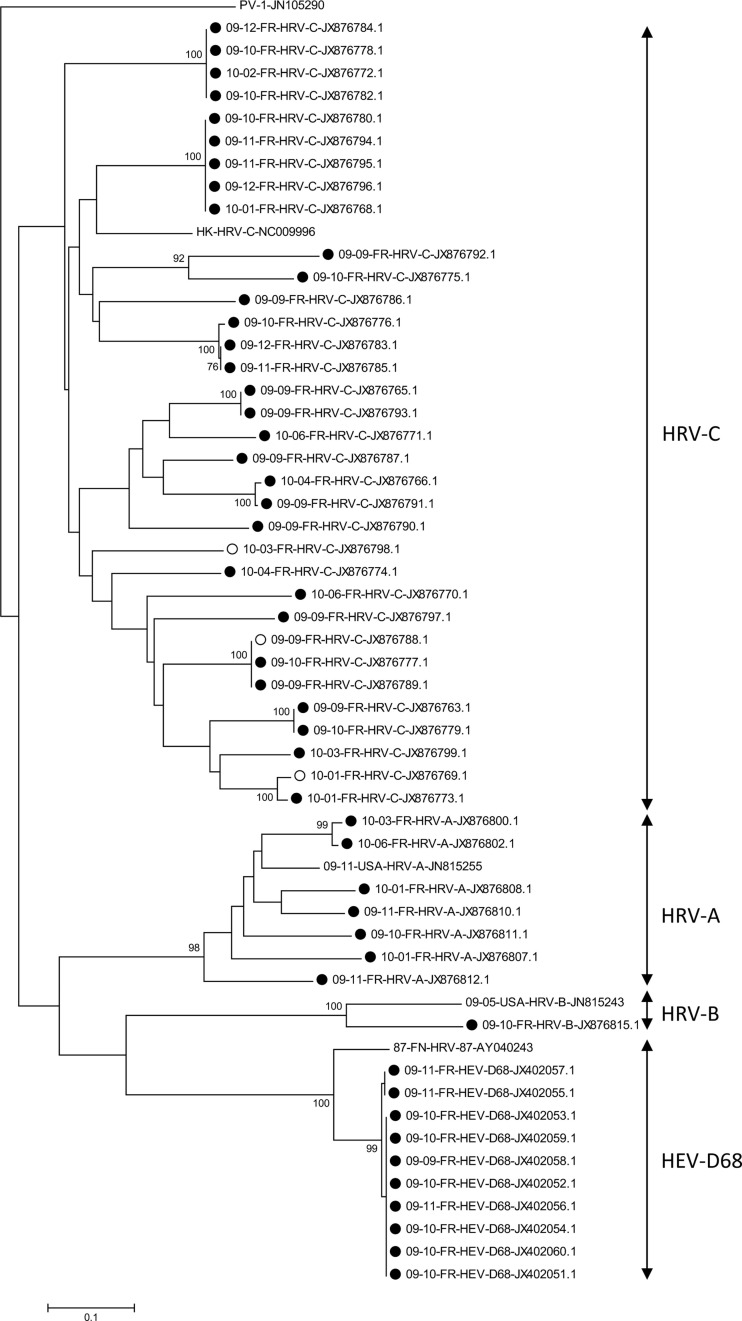
Molecular phylogeny of Enterovirus strains detected in clinical samples was based on the partial VP4/VP2 region nucleotide sequence. Sequences were aligned using Clustal W version 1.81 (www.clustal.org/). Genetic distances between sequences were calculated using the Kimura 2-parameter method. Trees were constructed using the neighbor-joining method as implemented in MEGA 5 software (www.megasoftware.net). Bootstrap values from 1000 replicates are shown at the nodes. Scale bar indicates number of nucleotide substitutions per site.
Statistical analysis
Quantitative variables were described as median ± extreme values and qualitative data as number and percentage. Pearson's chi-square test or the Fisher exact test was used as appropriate to compare qualitative variables and the Mann Whitney U test was used to compare quantitative variables. Results were considered as statistically significant for two-sided P values <0.05. All statistical analyses were performed using the SAS system release 9.0 (SAS Institute Inc, Cary, NC, USA).
Results
Virological findings
Viral RNA belonging to the EV genus (HEV and HRV species) was detected by RT-qPCR assay in 187 (60.5%) of the 309 hospitalized children with ARTIs (Fig. 1), whereas positive EV detection was observed in only 38 of the 210 control pediatric patients (18.1%) (187 vs. 38; P < 10−3). The viral loads assessed in respiratory specimens were not significantly different between patients suffering from ARTIs (median = 6.3 × 107 copies/ml [2.1 × 104–6.5 × 1010]) and control pediatric cases without respiratory symptoms and who were sampled at the same hospital during the same study period (median = 1.88 × 107 copies/ml [6 × 105–5.5 × 1010]; P = 0.16).
Of the 187 EV genus strains detected in hospitalized children with ARTIs, the specific HEV real-time RT-PCR assay evidenced 15 (8%) HEV and 172 (92%) HRV strains. Among the 15 identified HEV strains, HEV-D68 genotype was identified in 10 (67%) cases by phylogenetic analysis on a part of both VP1 and VP4/VP2 coding genes, corresponding to 10/15 (66.6%) and 10/187 (5.3%) of the HEV and EV strains detected respectively (Figs. 1 and 2 ). Among the 172 HRV infected patients, partial VP4/VP2 sequencing and phylogenetic analysis evidenced 40 HRV-C (23.2%), 21 HRV-A (12.2%) and 1 HRV-B (0.6%) while no molecular sequencing subtype identification was possible for 110 strains (64%) (Figs. 1 and 2).
Figure 2.
Molecular phylogeny of enterovirus based on the partial VP4/VP2 region nucleotide sequence. Sequences were aligned using clustal W version 1.81 (www.clustal.org/). Genetic distances between sequences were calculated using the Kimura 2-parameter method. Trees were constructed using the neighbor-joining method as implemented in MEGA 5 software (www.megasoftware.net). Bootstrap values from 1000 replicates are shown at the nodes. Scale bar indicates number of nucleotide substitutions per site. Strains belonging to the control group and strains belonging to the acute airways respiratory diseases are indicated respectively by open and full circles. A reference strain is shown for each virus: Poliovirus Sabin 1 (JN-105290), HRV-A (JN-815255), HRV-B (JN-815243), HRV-C (NC-009996) and HEV-D68/HRV-68 (AY-040243).
Epidemiological findings
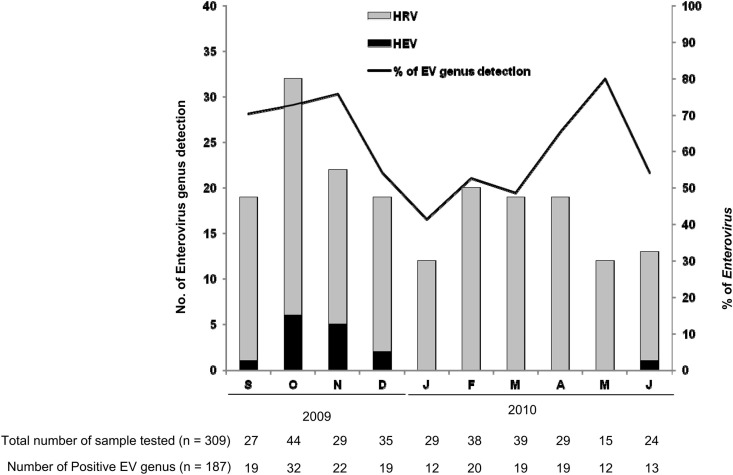
The seasonal pattern of EV genus infections showed an endemic HRV (subtypes A, B and C) co-circulation during the 10-month study period with 2 epidemic peaks in the fall 2009 (October to November) and in the spring 2010 (April to May during which HRV were detected up to 80% of our study children with ARTIs (Fig. 3 ). Interestingly, HEV respiratory infections demonstrated autumnal predominance with 14/15 (93.3%) of HEV and 10/10 (100%) of HEV-D68 infections occurring from September to November 2009 (Fig. 3).
Figure 3.
Seasonal distribution of human enterovirus (HEV) and human rhinovirus (HRV) detection in nasopharyngeal aspiration samples (NPAs) of pediatric patients hospitalized for acute respiratory tract infections (ARTIs) from September 2009 to June 2010.
Clinical findings
Of the 187 EV positive hospitalized pediatric patients, 164 were suffering from lower ARTIs (bronchiolitis or asthma exacerbation (n = 133), bronchitis (n = 10), pneumonia (n = 21)) and 23 from upper ARTIs (rhinitis). HRV strains were identified in 150 (91%) of the 164 lower ARTIs whereas HEV was identified in only 14 (9%) of these cases. Of these 164 HEV or HRV positive ARTI cases, 86 were antibiotics treated during the time of hospitalization whereas in only 7 of them a potential respiratory bacterial pathogen (S. pneumoniae (n = 2), H. influenzae (n = 4) M. catarrhalis (n = 1)) had been evidenced during the hospitalization stage. These bacterial infections had been isolated after the time of hospitalization; they were all associated with the presence of HEV or HRV strains and they were considered as superinfections.
Because bronchiolitis and asthma exacerbation were the most frequently lower ARTIs reported in the present investigation (133/164), we compared the clinical parameters between HEV and HRV infected patients suffering from these two illnesses (Table 1 ). Among the bronchiolitis and asthma exacerbation cases (n = 133), HEV infected cases were older (Median age (months) 36 vs. 11, P = 0.003) and were more frequently associated with a respiratory distress (11 vs. 62, P = 0.01) and a need for oxygen supply at the time of admission (8 vs. 45, P = 0.01) than cases infected by HRV strains (Table 1). Moreover, respiratory viral load levels appeared to be lower in HEV positive patients than those infected by HRV (median values 7.4 × 106 vs. 4.9 × 107 copies/ml, P = 0.005). Taken together our findings suggested that respiratory HEV strains might be more pathogenic than HRV strains and could induce more severe clinical symptoms in hospitalized lower ARTI cases. Among HRV positive lower ARTIs cases with bronchiolitis or exacerbated asthma (n = 121), there was no statistical difference between HRV-C and other HRV subtypes for respiratory distress (P = 0.72), the need for oxygen supply (P = 0.32) and for admission in intensive care unit (P = 0.99). Only 4 patients infected by HRV (1 HRV-C and 3 untyped HRV) were admitted in intensive care unit and all of them presented a good clinical outcome (Table 1).
Table 1.
Clinical characteristics of pediatric patients hospitalized for microbiologically unexplained bronchiolitis or exacerbated asthma and positive for human enterovirus or human rhinovirus detection of by real-time RT-qPCR in nasopharyngeal aspiration samples (NPAs).
| HEV | HRV | md | P | |
|---|---|---|---|---|
| Total number of strains n= (%) | 12 (100.0%) | 121 (100.0%) | 0 | |
| Age (months) median [range] | 36 [6–83] | 11 [0–155] | 0 | 0.003 |
| Sex ratio (M/F) | 2 | 2.3 | 0 | 0.99 |
| Length of hospitalization (days) median [range] | 3 [1–5] | 3 [0–22] | 2 | 0.6 |
| Admission in intensive care unit n= (%) | 0 | 4 (3.3%) | 0 | 0.99 |
| Clinical outcome: number of death n = Medical history: | 0 | 0 | 0 | |
| Asthma or infantile asthmaan= (%) | 6 (50.0%) | 44 (36.3%) | 0 | 0.36 |
| Prematuritybn= (%) | 3 (25.0%) | 21 (17.3%) | 37 | 0.70 |
| Passive smoking n= (%) | 2 (16.6%) | 31 (25.6%) | 70 | 0.24 |
| Family atopic history n= (%) | 6 (50.0%) | 58 (47.9%) | 49 | 0.23 |
| Symptoms at the time of admission: | ||||
| Fevercn = (%) | 8 (75.0%) | 43 (35.5%) | 12 | 0.12 |
| Respiratory distressdn = (%) | 11 (91.6%) | 62 (51.2%) | 2 | 0.01 |
| Need for Oxygen therapy n= (%) | 8 (75.0%) | 49 (40.4%) | 17 | 0.01 |
| Viral load per ml of NPAs samples at the time of admission: Median values copies/ml [range] | 7.4 × 106 [2 × 105–5.4 × 107] | 4.9 × 107 [2 × 105–7.2 × 1010] | 0 | 0.005 |
md: missing data.
Statistical significant values (P < 0.05) are shown in bold.
Asthma is defined as one episode of wheezing dyspnea in a child of more than 2 years. Infantile asthma is defined as more than two episodes of wheezing dyspnea in a child of less than 2 years.
Prematurity is defined as a birth occuring before 37 weeks of amenorrhea.
Fever was defined as the presence of an external body temperature > or = to 38 °C.
Respiratory distress was defined as the presence of dyspnea associated with one of the following clinical symptoms: chest indrawing, accessory respiratory muscle use (e.g. scalene muscles), paradoxical breathing or nasal flaring.
Discussion
Viruses belonging to the Enterovirus genus (HEV and HRV) are well recognized as etiological causes for upper and lower ARTIs in human beings.2, 3, 4, 5 Their prevalence ranged from 1 to 25% of children hospitalized for bronchiolitis indicating that EV might be the second etiological cause of viral lower ARTIs after RSV.31, 32 However, despite their recognized etiological role in pediatric respiratory infections, EV detection in respiratory samples remains an important challenge for the routine virological diagnosis in pediatric patients hospitalized for ARTIs. Because of a large EV genetic diversity combined with the continuous discovery of new species and new serotypes, the EV molecular detection by in-house or commercially available RT-PCR assays could fail and lead to an underestimation of the etiological and clinical role of HRV and HEV strains in pediatric ARTIs.4 Moreover, genotyping by capsid proteins coding region sequencing and phylogenetic analyses are presently required to distinguish between HEV and HRV respiratory infections.4 In the present time, it remains to assess the respective pathogenicity of respiratory HEV and HRV strains in pediatric patients, to identify genotypes or species specifically involved in upper and lower human respiratory diseases and to understand the epidemiology and the molecular mechanisms responsible for emergence of EV epidemic strains.4
In the present study, we retrospectively detected HEV and HRV strains in the respiratory tract of pediatric patients hospitalized for microbiologically unexplained ARTIs by use of a generic “Pan-entero-rhino” one-step RT-qPCR assay that was previously designed to overcome the genetic diversity of both HRV and HEV circulating in humans.22 Viruses belonging to the EV genus were detected in 60.5% of the NPAs and were identified as the potential etiological cause of ARTI in these hospitalized patients. These pediatric patients had been identified as negative for the classical detection of common bacterial and virological respiratory pathogens known as major etiological causes of bronchiolitis or asthma exacerbation in the Northern east of France.5, 33 In the present study, we did not test our samples for the presence of Human Metapneumoviruses and Human Coronaviruses because these viral agents had been previously identified as potential etiological agents in less than 10% of bronchiolitis and asthma exacerbation cases hospitalized in Northern of France.33
Interestingly, EVs were only detected in 18.1% of the children hospitalized during the same period without clinical respiratory symptoms consistent with ARTIs. The differential of EV detection levels between our patient and control groups strongly supported the importance of HRV and HEV strains as major etiological agents in the development of ARTIs in hospitalized pediatric patients.2, 5, 9 Moreover, we observed high EV load levels in cases of ARTIs supporting the hypothesis of an EV ongoing replication instead of an EV viral shedding in the respiratory tract of study patients with respiratory symptoms (Table 1). However, viral load levels appeared to be similar between symptomatic and asymptomatic patients suggesting that the clinical symptoms observed were more likely due to inter-individual susceptibility factors to the EV infection rather than to EV determinants of virulence. The inter-individual factors leading to the development of symptomatic EV infections in humans remained to be determined and could be related to the induction of a local efficient antiviral immune response in the human respiratory tract.34
Distinction between HEV and HRV in pediatric ARTIs revealed the predominance of HRV infections.4, 35 Indeed, only 15 HEV infections have been evidenced in comparison with the 172 HRV infections detected in our 309 pediatric ARTI cases. In our study, all successfully typed HEV strains (10/15) were HEV-D68. This recently emerging virus was isolated from respiratory specimens for the first time in 1962 in the U.S. in children with pneumonia and bronchiolitis.36 However, until recently, reports of respiratory infections due to HEV-D68 were rare.37 Over the past three years, outbreaks in Japan, the Philippines and the Netherlands as well as epidemic clusters in the United Kingdom have implicated HEV-D68 as an emerging respiratory pathogen.12, 13, 14, 18 In the present study, HEV-D68 strains were responsible for a low proportion of pediatric cases hospitalized for ARTIs. Our findings provide further insights into the potential specific lower respiratory tract tropism of HEV-D68 and increase our awareness of the clinical role of these strains. Among our genotyped HRV strains, the HRV-C species appeared to be the leading cause of infection far ahead of HRV-A and B species. However in the present work, the high number of untypable HRV strains highlighted the difficulty to amplify and sequence viral capsid gene RNA sequences extracted from clinical respiratory samples.
Clinical severity between HEV and HRV infections was compared among bronchiolitis and asthma exacerbation cases that were the most frequent lower ARTIs reported in our study. HEV infections were more frequently associated with severity criteria such as respiratory distress and the need for oxygen supply in comparison to HRV infections, despite the fact that HEV strains infected older children than HRV strains (Table 1). Moreover, HEV positive patients were infected with lower viral loads than those with HRV infections (Table 1) and HEV asymptomatic shedding cases were less frequent in control patients (1 out of 210) than in HRV asymptomatic shedding cases (37 out of 210) (Fig. 1). Taking into account all these results, HEV respiratory strains might be more pathogenic and virulent than HRV strains. Moreover, we did not evidence a higher pathogenicity of HRV-C in comparison to HRV-A/B in lower respiratory tract illnesses as reported in recent publications6, 7, 8: this could be linked to the high proportion of untyped HRV strains in the present study (Fig. 1).
In the present retrospective study, we observed that 86 of the 187 HRV or HEV positive pediatric patients with ARTIs received antibiotics whereas only 7 of them had developed a bacterial superinfection that had been evidenced (S. pneumoniae (n = 2), H. influenzae (n = 4) and Branhamella catarrhalis (n = 1)) during the course of hospitalization. This suggested that the severity and duration of HRV or HEV respiratory symptoms could have been responsible for an inadequate and probabilistic prescription of antibiotics by the pediatricians. These findings highlighted the potential interest to routinely perform an RT-PCR assay allowing a rapid EV detection in NPAs samples taken from pediatric patients hospitalized for ARTIs. Moreover it could be interesting to implement the most popular commercially available multiplex respiratory virus assays like the RVP and FilmArray by adding a specific HEV molecular identification using specific probes.38 Indeed, such implemented multiplex RT-PCR assays might allow to reinforce clinical monitoring of HEV positive children and to discontinue antibiotic treatments in case of a positive EV etiological identification. The benefits would include not only economic savings but also the prevention of emergence of antibiotic resistance.
In conclusion, HRV and HEV strains were identified as potential etiological causes of 60.5% of microbiologically unexplained ARTIs diagnosed in hospitalized pediatric cases. A higher clinical severity was observed in HEV-induced bronchiolitis or asthma exacerbation cases in comparison to HRV-related similar cases during the study period. The present work highlights the need to perform a broad HEV and HRV molecular detection in the respiratory samples of pediatric patients hospitalized for lower ARTIs, which results could improve the clinical and therapeutic management of children hospitalized in pediatric wards.
Transparency declaration
The authors have non conflict of interest to declare.
Acknowledgments
This work was supported by clinical research grant from the Reims University Medical Centre (EA4684-CardioVir). Fanny Renois is supported by an official grant from the French Army department (Bourse DGA: Délégation Générale de l’Armement, Ministère de la Défense, Topic: Microbiology, infectious diseases).
References
- 1.Lauber C., Gorbalenya A.E. Toward genetics-based virus taxonomy: comparative analysis of a genetics-based classification and the taxonomy of picornaviruses. J Virol. 2012;86:3905–3915. doi: 10.1128/JVI.07174-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jartti T., Lehtinen P., Vuorinen T., Osterback R., Van den Hoogen B., Osterhaus A.D.M.E. Respiratory picornaviruses and respiratory syncytial virus as causative agents of acute expiratory wheezing in children. Emerg Infect Dis. 2004;10:1095–1101. doi: 10.3201/eid1006.030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antunes H., Rodrigues H., Silva N., Ferreira C., Carvalho F., Ramalho H. Etiology of bronchiolitis in a hospitalized pediatric population: prospective multicenter study. J Clin Virol. 2010;48:134–136. doi: 10.1016/j.jcv.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jartti T., Jartti L., Ruuskanen O., Söderlund-Venermo M. New respiratory viral infections. Curr Opin Pulm Med. 2012;18:271–278. doi: 10.1097/MCP.0b013e328351f8d4. [DOI] [PubMed] [Google Scholar]
- 5.Jacques J., Bouscambert-Duchamp M., Moret H., Carquin J., Brodard V., Lina B. Association of respiratory picornaviruses with acute bronchiolitis in French infants. J Clin Virol. 2006;35:463–466. doi: 10.1016/j.jcv.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Lau S.K.P., Yip C.C.Y., Tsoi H.-w., Lee R.A., So L.-y., Lau Y.-l. Clinical features and complete genome characterization of a distinct human rhinovirus (HRV) genetic cluster, probably representing a previously undetected HRV species, HRV-C, associated with acute respiratory illness in children. J Clin Microbiol. 2007;45:3655–3664. doi: 10.1128/JCM.01254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller E.K., Edwards K.M., Weinberg G.A., Iwane M.K., Griffin M.R., Hall C.B. A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immunol. 2009;123:98–104. doi: 10.1016/j.jaci.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tapparel C., Cordey S., Junier T., Farinelli L., Van Belle S., Soccal P.M. Rhinovirus genome variation during chronic upper and lower respiratory tract infections. PLoS One. 2011;6:e21163. doi: 10.1371/journal.pone.0021163. Vartanian J–P, éditeur. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacques J., Moret H., Minette D., Leveque N., Jovenin N., Deslee G. Epidemiological, molecular, and clinical features of enterovirus respiratory infections in French children between 1999 and 2005. J Clin Microbiol. 2008;46:206–213. doi: 10.1128/JCM.01414-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piralla A., Lilleri D., Sarasini A., Marchi A., Zecca M., Stronati M. Human rhinovirus and human respiratory enterovirus (EV68 and EV104) infections in hospitalized patients in Italy, 2008-2009. Diagn Microbiol Infect Dis. 2012;73:162–167. doi: 10.1016/j.diagmicrobio.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 11.Yozwiak N.L., Skewes-Cox P., Gordon A., Saborio S., Kuan G., Balmaseda A. Human enterovirus 109: a novel interspecies recombinant enterovirus isolated from a case of acute pediatric respiratory illness in nicaragua. J Virol. 2010;84:9047–9058. doi: 10.1128/JVI.00698-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imamura T. Enterovirus 68 among children with severe acute respiratory infection, the Philippines. Emerg Infect Dis. 2011;17:1430–1434. doi: 10.3201/eid1708.101328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaida A. Enterovirus 68 in children with acute respiratory tract infections, Osaka, Japan. Emerg Infect Dis. 2011;17:1494–1497. doi: 10.3201/eid1708.110028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikeda T., Mizuta K., Abiko C., Aoki Y., Itagaki T., Katsushima F. Acute respiratory infections due to enterovirus 68 in Yamagata, Japan between 2005 and 2010. Microbiol Immunol. 2012;56:139–143. doi: 10.1111/j.1348-0421.2012.00411.x. [DOI] [PubMed] [Google Scholar]
- 15.Xiang Z., Gonzalez R., Wang Z., Ren L., Xiao Y., Li J. Coxsackievirus A21, enterovirus 68, and acute respiratory tract infection, China. Emerg Infect Dis. 2012;18:821–824. doi: 10.3201/eid1805.111376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobson L.M., Redd J.T., Schneider E., Lu X., Chern S.-W.W., Oberste M.S. Outbreak of lower respiratory tract illness associated with human enterovirus 68 among American Indian children. Pediat Infects Dis J. 2012;31:309–312. doi: 10.1097/INF.0b013e3182443eaf. [DOI] [PubMed] [Google Scholar]
- 17.Kaida A., Kubo H., Sekiguchi J., Hase A., Iritani N. Enterovirus 104 infection in adult, Japan, 2011. Emerg Infect Dis. 2012;18:882–883. doi: 10.3201/eid1805.111890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauinger I.L., Bible J.M., Halligan E.P., Aarons E.J., MacMahon E., Tong C.Y.W. Lineages, sub-lineages and variants of enterovirus 68 in recent outbreaks. PLoS ONE. 2012;7:e36005. doi: 10.1371/journal.pone.0036005. Schulz TF, éditeur. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meijer A., Van der Sanden S., Snijders B.E.P., Jaramillo-Gutierrez G., Bont L., Van der Ent C.K. Emergence and epidemic occurrence of enterovirus 68 respiratory infections in the Netherlands in 2010. Virology. 2012;423:49–57. doi: 10.1016/j.virol.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 20.Pankovics P., Boros A., Szabó H., Székely G., Gyurkovits K., Reuter G. Human enterovirus 109 (EV109) in acute paediatric respiratory disease in Hungary. Acta Microbiol Immunol Hung. 2012;59:285–290. doi: 10.1556/AMicr.59.2012.2.13. [DOI] [PubMed] [Google Scholar]
- 21.Daleno C., Piralla A., Scala A., Baldanti F., Usonis V., Principi N. Complete genome sequence of a novel human enterovirus C (HEV-C117) identified in a child with community-acquired pneumonia. J Virol. 2012;86:10888–10889. doi: 10.1128/JVI.01721-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tapparel C., Cordey S., Van Belle S., Turin L., Lee W.-M., Regamey N. New molecular detection tools adapted to emerging rhinoviruses and enteroviruses. J Clin Microbiol. 2009;47:1742–1749. doi: 10.1128/JCM.02339-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Savolainen-Kopra C., Blomqvist S., Kaijalainen S., Jounio U., Juvonen R., Peitso A. All known human rhinovirus species are present in sputum specimens of military recruits during respiratory infection. Viruses. 2009;1:1178–1189. doi: 10.3390/v1031178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiang D., Kalra I., Yagi S., Louie J.K., Boushey H., Boothby J. Assay for 5’ noncoding region analysis of all human rhinovirus prototype strains. J Clin Microbiol. 2008;46:3736–3745. doi: 10.1128/JCM.00674-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouscambert-Duchamp M., Lina B., Trompette A., Moret H., Motte J., Andréoletti L. Detection of human metapneumovirus RNA sequences in nasopharyngeal aspirates of young French children with acute bronchiolitis by real-time reverse transcriptase PCR and phylogenetic analysis. J Clin Microbiol. 2005;43:1411–1414. doi: 10.1128/JCM.43.3.1411-1414.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Refabert L. 2000. French consensus reference: bronchiolitis.http://www.has-sante.fr/portail/upload/docs/application/pdf/bronchio.pdf [Google Scholar]
- 27.AFSSAPS . 2005. Antibiothérapie par voie générale en pratique courante au cours des infections respiratoires basses de l'adulte et de l'enfant.http://www.infectiologie.com/site/medias/_documents/consensus/2005-infVRB-recos-afssaps.pdf [Google Scholar]
- 28.AFSSAPS . 2011. Antibiothérapie par voie générale en pratique courante dans les infections respiratoires hautes de l'adulte et de l'enfant.http://www.infectiologie.com/site/medias/Recos/2011-infections-respir-hautes-recommandations.pdf [Google Scholar]
- 29.Wong S.C.C., Chan J.K.C., Lee K.C., Lo E.S.F., Tsang D.N.C. Development of a quantitative assay for SARS coronavirus and correlation of GAPDH mRNA with SARS coronavirus in clinical specimens. J Clin Pathol. 2005;58:276–280. doi: 10.1136/jcp.2004.016592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petitjean J., Vabret A., Dina J., Gouarin S., Freymuth F. Development and evaluation of a real-time RT-PCR assay on the light cycler for the rapid detection of enterovirus in cerebrospinal fluid specimens. J Clin Virol. 2006;35:278–284. doi: 10.1016/j.jcv.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Khetsuriani N., Lamonte-Fowlkes A., Oberst S., Pallansch M.A. Enterovirus surveillance-United States, 1970-2005. MMWR Surveill Summ. 2006;55:1–20. [PubMed] [Google Scholar]
- 32.Antona D., Lévêque N., Chomel J.J., Dubrou S., Lévy-Bruhl D., Lina B. Surveillance of enteroviruses in France, 2000-2004. Eur J Clin Microbiol Infect Dis. 2007;26:403–412. doi: 10.1007/s10096-007-0306-4. [DOI] [PubMed] [Google Scholar]
- 33.Huguenin A., Moutte L., Renois F., Leveque N., Talmud D., Abely M. Broad respiratory virus detection in infants hospitalized for bronchiolitis by use of a multiplex RT-PCR DNA microarray system. J Med Virol. 2012;84:979–985. doi: 10.1002/jmv.23272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Renois F., Jacques J., Talmud D., Deslée G., Lévêque N., Andréoletti L. Respiratory echovirus 30 and coxsackievirus B5 can induce production of RANTES, MCP-1 and IL-8 by human bronchial epithelial cells. Virus Res. 2010;152:41–49. doi: 10.1016/j.virusres.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Wisdom A., Leitch E.C.M., Gaunt E., Harvala H., Simmonds P. Screening respiratory samples for detection of human rhinoviruses (HRVs) and enteroviruses: comprehensive VP4-VP2 typing reveals high incidence and genetic diversity of HRV species C. J Clin Microbiol. 2009;47:3958–3967. doi: 10.1128/JCM.00993-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schieble J.H., Fox V.L., Lennette E.H. A probable new human picornavirus associated with respiratory diseases. Am J Epidemiol. 1967;85:297–310. doi: 10.1093/oxfordjournals.aje.a120693. [DOI] [PubMed] [Google Scholar]
- 37.Oberste M.S., Maher K., Schnurr D., Flemister M.R., Lovchik J.C., Peters H. Enterovirus 68 is associated with respiratory illness and shares biological features with both the enteroviruses and the rhinoviruses. J Gen Virol. 2004;85:2577–2584. doi: 10.1099/vir.0.79925-0. [DOI] [PubMed] [Google Scholar]
- 38.Rand K.H., Rampersaud H., Houck H.J. Comparison of two multiplex methods for detection of respiratory viruses: FilmArray RP and xTAG RVP. J Clin Microbiol. 2011;49:2449–2453. doi: 10.1128/JCM.02582-10. [DOI] [PMC free article] [PubMed] [Google Scholar]