Abstract
Objectives
To evaluate effectiveness of a nasal resveratrol/carboxymethyl-β-glucan solution compared to nasal saline solution: a) on common cold symptoms by means of a validated measure scale (CARIFS score), b) on Rhinovirus infection and CCL2, CCL5, IL8, IL6, CXCL10 and TLR2 expression in nasal swabs, c) on frequency of relapses after 30 days of follow-up.
Methods
89 infants with respiratory infection symptoms were randomly assigned to receive either a nasal resveratrol/carboxymethyl-β-glucan solution or nasal saline solution.
All patients were evaluated with CARIFS score at enrollment, after 48 h, 7 and 30 days by physicians and parents. Nasal swabs were obtained at enrollment, after 48 h and after one week.
Results
CARIFS score improved in both groups. Episodes of sneezing and cough were fewer in study group after 7 days of follow-up (p < 0.05). No significant differences were found on nasopharyngeal swabs in Rhinovirus detection and cytokines expression after 48 h, nor in 30 days relapses. TLR2 expression was significantly higher in Rhinovirus infected children of the study group. No adverse effects occurred.
Conclusions
These data suggest that a solution containing resveratrol plus carboxymethyl-β-glucan might have a positive impact on both clinical and socio-economic burden due to infant common cold.
Keywords: "Common cold" [Mesh], Resveratrol/carboxymethyl-β-glucan, "Rhinovirus" [Mesh], Biological sciences, Natural product, Antioxidant, Molecular biology, Biomolecules, Microbiology, Natural product chemistry, Toxicology, Pharmaceutical science
"Common cold" [Mesh]; Resveratrol/carboxymethyl-β-glucan; "Rhinovirus" [Mesh]; Biological science; Natural product; Antioxidant; Molecular biology; Biomolecules; Microbiology; Natural product chemistry; Toxicology; Pharmaceutical science.
1. Introduction
The common cold is an acute viral disease affecting the upper airways that causes an inflammation of nasal and pharyngeal mucosa. It is the most frequent human disease with a significant impact on public health and quality of life [1, 2].
The common cold usually causes mild symptoms, but it may be severe in infants.
Human Rhinoviruses (HRVs) are the prevalent cause of common cold in adults and children. In total, more than 200 viruses have been identified to cause common cold and flu-like symptoms [2, 3].
Ninety % of the HRV-A and HRV-B serotypes bind the ICAM-1 glycoproteins on the respiratory epithelial cells, while the remaining viruses bind members of the LDL-R family [4].
The interaction between HRV and the epithelial ICAM-1 receptor induces secretion of cytokines, chemokines and growth factors, such as GM-CSF, RANTES, IL-6, IL-8 and interferon-β [5, 6]. Type I-interferons are inducers of IP-10 (also known as CXCL10) which is responsible for recruitment of lymphocytes and other cell types to the airway mucosa in response to Rhinovirus infection. IP-10 is associated with increase in virus titres and symptomatic colds [7]. Cytokines and chemokines are necessary for the recruitment of inflammatory cells as well as for the host antiviral response.
The most frequent symptoms of the common cold are rhinorrhoea, nasal congestion, sneezing, sore/scratchy throat and chest congestion, followed by cough, headache and fever [8, 9].
Usually the common cold is self-limiting and lasts around 10 days. In children, the most common bacterial complication is acute otitis media [10]. Other complications are sinusitis, bronchiolitis, pneumonia, and exacerbations of asthma [11, 12].
Treatment of the common cold is symptomatic, based on antihistamines, antitussives and decongestants, drugs that are not recommended in children, especially under 2 years of age [13]. However, there are few options to treat even children with over-the-counter cough & cold medication. A recent evaluation by the Pharmacovigilance Risk Assessment Committee (PRAC) concluded that no age restrictions need to be considered for ambroxol and bromhexine [14]. Furthermore, some nasal decongestants have been specifically designed for babies (low concentrations, metered dropper devices).
Resveratrol (trans-3,5,4′-trihydroxystilbene) is a polyphenol that occurs as a phytoalexin in different plant species [15, 16] and shows beneficial effects in inflammatory conditions, in oncology, in cardiovascular diseases, obesity, type 2 diabetes and neurodegenerative diseases [17, 18, 19, 20, 21, 22].
Resveratrol modulates the inflammatory response inhibiting the transcriptional activity of NF-kB and reducing the secretion of IL-6, IL-8 and RANTES in HRV-infected nasal epithelia either alone or in combination with carboxymethyl-β-glucan [23]. The reduction of cytokines production was correlated with the inhibition of HRV replication resveratrol-induced [23]. Resveratrol has low solubility, low bioavailability and becomes unstable due to self-oxidation and photosensitivity. The combination of resveratrol with a modified β-glucan (carboxymethyl-β-glucan; CM-glucan) in aqueous solution improves its stability without modifying its biological properties [24]. β-glucan is a polysaccharide able to stimulate the immune system [25].
A nasal spray containing resveratrol and CM-glucan can reduce nasal symptoms and respiratory complications in children with allergic rhinitis, also reducing the use of antihistamines [26]. This solution is also effective in reducing nasal symptoms in children with upper respiratory tract infections and a preliminary study showed the preventive effect of an aerosol formulation containing a resveratrol/CM-glucan combination in children with recurrent respiratory infections [27, 28]. At this time, there are no data regarding utilization of resveratrol/CM-glucan combination in children under 6 months of age.
The principal aim of this study was to verify the effectiveness of the resveratrol/carboxymethyl-β-glucan solution, compared with saline solution, on common cold symptoms treatment in infants. Secondary outcomes were the evaluation of Rhinovirus infection, the expression in nasal swabs of some cytokines (CCL2, CCL5, IL8, IL6, CXCL10, and TLR2) and the frequency of relapses after 30 days of follow-up in the studied population.
2. Methods
2.1. Study design and patients
This was a double-blind, randomized, placebo-controlled clinical trial (clinicaltrials.gov: NCT03683108), performed to assess the efficacy of a nasal resveratrol/carboxymethyl-β-glucan solution on common cold symptoms.
Consecutive infants from 0 to 6 months, who had been admitted with signs and symptoms suggestive for acute respiratory illness at the Outpatient Services of the Neonatology Unit, Department of Biomedical and Human Oncological Science, University of Bari, Bari, Italy between December 2015 and March 2018, were enrolled.
The presence of one nasal symptom such as (a) rhinorrhoea, (b) nasal congestion and (c) sneezing was considered as inclusion criteria.
Exclusion criteria were: (a) preterm infants with diagnosis of bronchopulmonary dysplasia; (b) other major acute or chronic diseases; (c) respiratory malformations, history of asthma, allergic rhinitis, autoimmune disease, or allergy; (d) participation to other clinical trials; (e) use of other respiratory symptomatic treatments during the study protocol.
Informed consent was obtained at the enrollment, in accordance with the local Ethics Committee, (University of Bari, Policlinico Hospital of Bari, Medical School, Bari, Italy) which approved the study protocol.
All infants were randomized to receive either a nasal resveratrol/carboxymethyl-β-glucan solution or a placebo, 3 drops in each nostril, 4 times a day for seven days.
Randomization was performed using a computer-generated allocation sequence (nQuery Advisor v.7.0 software, Statistical Solutions Ltd., Cork, Ireland). To avoid disproportionate numbers of patients in each group, a randomization scheme was performed in blocks of four participants.
All participants, as well as scientific and medical personnel dedicated to the study and distributing the study agents or assessing the samples and analyses, were blinded to group assignment. Randomization codes were secured until all data were analyzed.
The nasal resveratrol/carboxymethyl-β-glucan solution was produced by Noos, Rome, Italy and currently sold in Italy as Class I EC Medical Device, under the brand Linfovir® plus. It is an isotonic solution in drops containing resveratrol 0.05% (extracted by Polygonum cuspidatum) and carboximethyl-β-glucan 0.01%.
The placebo was composed by isotonic saline solution and was identical in visual and sensory properties in order to maintain a double-blind status.
Infants characteristics were collected at baseline. Each patient underwent a clinical evaluation at enrollment, after 48 h and after 7 and 30 days.
Parents were asked to notify the research team of the relapse of common cold during the 30 days after enrollment.
All data was recorded in a standardized informatic form.
2.2. Canadian Acute Respiratory Illness and Flu Scale
The common cold symptoms were evaluated by parents by means of a validated scale for childhood respiratory infections called Canadian Acute Respiratory Illness and Flu Scale (CARIFS) during all visits.
The CARIFS score consisted of 18 items each answered on a 4-point scale (no problem = 0, minor problem = 1, moderate problem = 2, major problem = 3).
CARIFS items “headache”, “sore-throat”, “muscle aches or pains” were not applicable to infants, hence we used 15 items. The mean of the points of all applicable items was considered as a measure of the child's overall illness level.
2.3. Medical assessment
During all visits, an independent assessment of the child's overall illness level was completed by the same physician (M.E.B.) according to anamnesis and clinical evaluation.
Presence and severity of signs and symptoms associated with a clinical cold were recorded (i.e. mucopurulent rhinorrhoea, nasal congestion, snoring, sneezing, productive or non-productive cough, fever, night waking, infantile colic and lack of appetite).
Each sign or symptom was rated by the clinician and assigned a numeric score from 0 to 4 (0 = no symptom; 1 = mild symptom; 2 = moderate; 3 = severe). Scores for individual signs/symptoms were summed to create a mean total medical symptom score. A subset analysis was also considered to investigate difference in each sign or symptom.
2.4. Virus detection and cytokine/chemokine expression
Nasal samples were obtained from each patient at enrollment and after 48 h and 7 days. A nasal swab (MIDTURBINATE FL/PEDIAT. PF50MM, Copan Italia, Brescia, Italy) was inserted approximately one-half the distance between the nares and bridge of the nose and collected in 1 ml eNAT™ medium (Copan Italia) optimized for molecular applications. Samples were stored at -80 °C for further analyses. Each specimen was sent frozen for virus and cytokine/chemokine analyses to the laboratory of Microbiology (Sapienza University, Rome). Upon receipt, 3 aliquots were prepared and stored at –80 °C.
2.5. Viral RNA extraction and real-time RT-PCR
Viral RNA was extracted from nasal samples (200 μl of clinical sample) using QIAamp® MinElute® Virus Spin kit (Qiagen). Extracted RNA was tested for HRVs, Respiratory Syncitial Virus (RSV) and human metapneumovirus (hMPV) by One-step RT-PCR assays: Rhino&EV/Cc r-gene® and RSV/hMPV r-gene®, respectively (bioMérieux) according to the manufacturer's instructions. Each sample was tested in duplicate. Cellular control was included to assesses the quality of the sample collection by validating the presence of cells and the absence of inhibitors. Real-time PCR reactions were performed on an ABI 7500 thermocycler (Applied Biosystems).
2.6. Immunological markers assays
Total RNA was extracted from clinical samples (Mini Rneasy Plus kit, Qiagen, Hilden, Germany) according to the manufacturer's instructions. Extracted RNA was eluted with 30 μL of RNase-free water and reverse transcribed to cDNA by SuperScript™ IV VILO™ Master Mix (Invitrogen). cDNA was tested for gene expression by customized Taqman array (ThermoFisher Scientific). Plates containing TaqMan probe and specific PCR primer sets for CCL2, CCL5, IL-8, IL-6, CXCL10 and TLR2 were run on an ABI 7500 thermocycler (Applied Biosystems). Quantitative Real-time PCR results were normalized to 18S rRNA expression (housekeeping gene). Fold change in gene expression was expressed as 2−ΔΔCt and non-infected group at T0 was chosen as control.
2.7. Statistical analysis
Comparisons between groups at baseline were made by t-Student and chi-square test for numerical and categorical variables respectively. When necessary Yates correction for chi-square test was used. To evaluate the trend of symptoms and scores among the observation times in relation to groups a two-way analysis of variance for repeated measures was performed. Gene expression data from non infected and HRV infected subjects at baseline were compared by using unpaired t-test or Mann Whitney test depending on the result of normality test (Kolmogov-Smirnov test). Paired t-test or Wilcoxon test was used for testing groups at 48h follow up.
Statistical analyses were performed using SPSS statistics version 23 (IBM Corporation, Armonk, NY, USA) and Graphpad Prism version 5.0 (Graphpad Software, San Diego, CA, USA).
3. Results
Of the 100 infants enrolled, 89 (89%) completed the entire 30 days follow-up and entered in the final analysis. Demographical data of infants are shown in Table 1. At enrollment 38 samples (43%) were positive for HRV, one for hMPV (1%) and none for RSV.
Table 1.
Demographic and baseline characteristics of patients in relation to treatments.
| Characteristics of subjects | Linfovir® plus, n = 42 (47,2%) | Placebo, n = 47 (52,8%) | p |
|---|---|---|---|
| Male, n (%) Female, n (%) |
21 (50) 21 (50) |
21 (55,3) 26 (44,7) |
n.s. |
| Birth weight, mean (SD) | 2432 (908) | 2250 (909) | n.s. |
| Gestational age at birth, weeks, mean (SD) | 34,6 (3,8) | 34 (4,01) | n.s. |
| Day of life at enrollment, mean (SD) | 102 (66) | 98 (106) | n.s. |
| Maternal age at birth, years, mean (SD) | 32,7 (5,6) | 33,3 (7,1) | n.s. |
| Paternal age at birth, years, mean (SD) | 36,7 (6,8) | 36,5 (7,5) | n.s. |
| HRV positive swab at baseline, n (%) | 20 (47,6) | 18 (38) | n.s. |
| hMPV positive swab at baseline, n (%) | 0 | 1 (2) | n.s. |
| RSV positive swab at baseline, n (%) | 0 | 0 | n.s. |
No significative difference between the two groups according to demographical data and positivity to HRV, hMPV and RSV at baseline were reported.
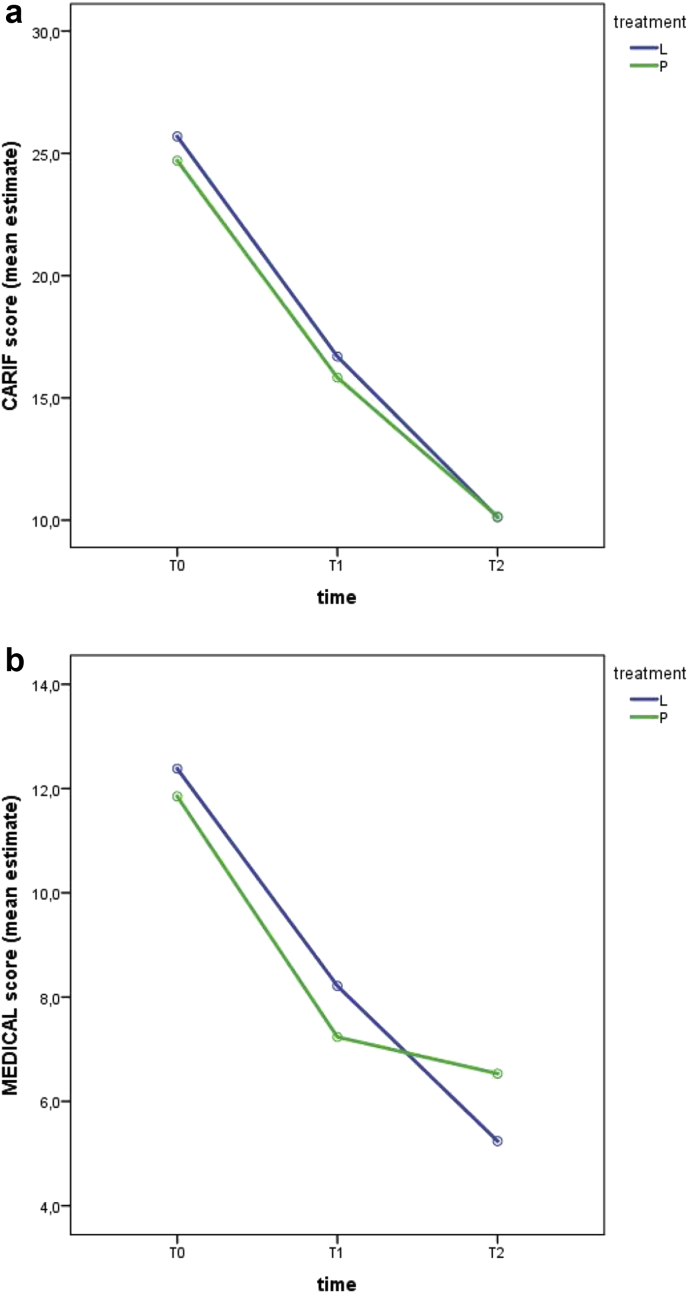
CARIFS and MEDICAL mean scores at enrollment and during follow-up in both groups are shown in Figure 1.
Figure 1.
CARIFS (a) and MEDICAL (b) mean scores in relation to time of observation and treatment.
CARIFS score at baseline showed no significant difference between the two groups (t = 1.23 p = 0.22).
After beginning of treatment, we recorded a significant reduction of CARIFS score in both groups with no significant difference between groups (time effect F = 913,233 p < 0.0001, treatment effect F = 1.453 p = 0.231, interaction effect F = 1.24 p = 0.296). At each time point, a significant reduction was found throughout the study period (T1vs T0 F = 780.968 p < 0.0001; T2 vs T1 F = 363.78 p < 0.0001).
Medical assessment at baseline showed no significant difference between the two groups (t = 0.49 p = 0.62).
After beginning of treatment, we recorded a significant improvement of symptoms in each group without significant differences between groups (time effect F = 64.977 p < 0.0001, treatment effect F = 0.013 p = 0.91, interaction effect F = 2.298 p = 0.103). At each time point, a significant reduction was found throughout the study period (T1vs T0 F = 63.543 p < 0.0001; T2 vs T1 F = 12.731 p = 0.001). Between T1 and T2 the score reduction was less in the placebo group than in Linfovir® plus group (interaction effect F = 4.866 p = 0.03).
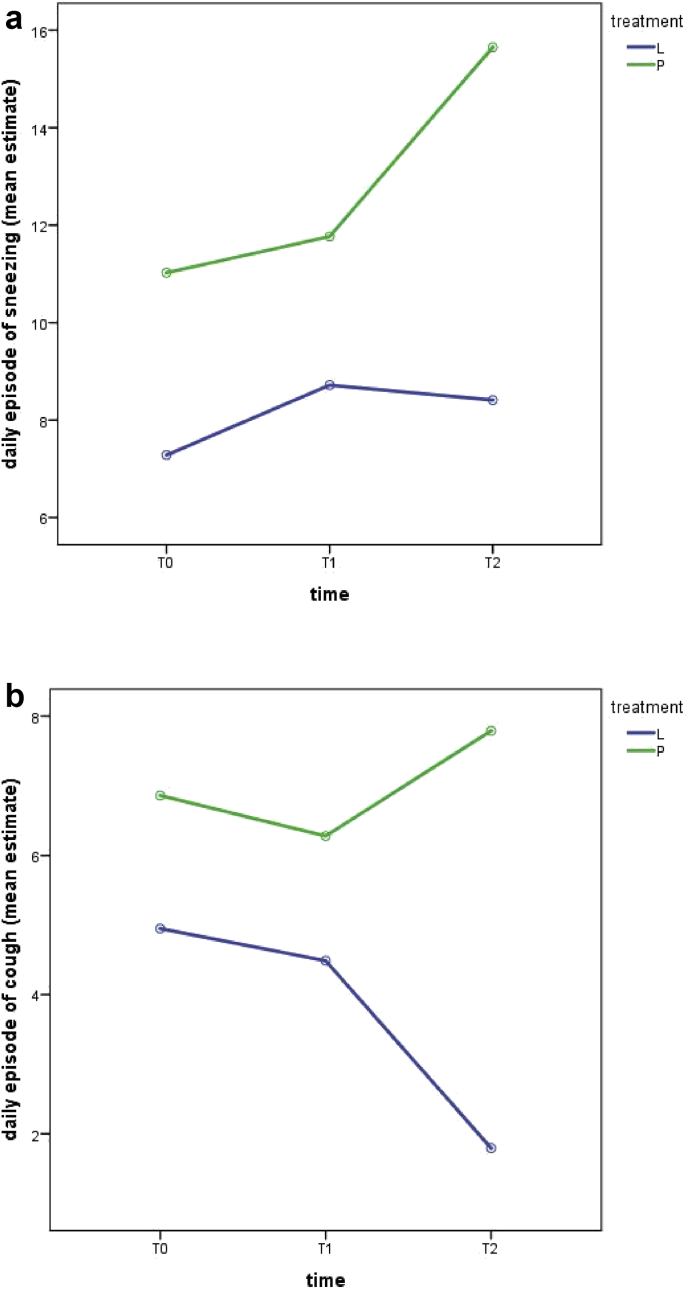
When symptoms were analyzed individually, episodes of sneezing and productive or non-productive cough were different in Linfovir® plus group compared to placebo group (Figure 2).
Figure 2.
Mean estimate of daily episodes of sneezing (a) and cough (b) in relation to time of observation and treatments.
Episodes of sneezing showed a different trend in the two groups (interaction effect F = 3.16 p = 0.045). In Linfovir® plus group there was a significant increase in sneezing during the first 48 h follow up (F = 11.86 p = 0.001), followed by a slight but no significant reduction after one week; in placebo group a slight increase in sneezing episodes, even if not significant, occurred in the first 48 h (F = 0.84 p = 0.37) followed by a significant increase in sneezing after 7 days of follow-up (F = 6.43 p = 0.015). The difference between the two groups was highly significant after 7 days of follow-up (t = 2.47, p = 0.016).
Regarding episodes of productive or non-productive cough within 48h, the symptoms increased significantly in placebo group (F = 5.245 p = 0.027) and decreased significantly in Linfovir® plus group (F = 3.98 p = 0.05) (interaction effect F = 4.06 p = 0.02). The difference between the two groups was highly significant after 7 days of follow-up (t = 2,89, p = 0.006). Notably, in HRV infected infants episodes of cough were significantly lower in Linfovir® plus group in comparison to placebo group after 7 days of treatment (t = 2.48 p = 0.03).
No difference was detected regarding muco-purulent rhinorrhoea, nasal congestion, snoring, fever, night wakings, infantile colic and lack of appetite during the follow-up (data not shown).
After 48 h HRV disappeared in 20% of Linfovir® plus children and in 25% of placebo group children (χ2 = 0, p = 1). The HRV negative samples after 7 days of follow-up were greater in Linfovir® plus group compared to placebo group, without significance (61% vs 44%, χ2 = 1.0, p = 0.32).
Results of immunological markers analysis are shown in Table 2. Highly variable cytokine expression levels were detected in both groups, without statistically significant differences between the study groups, neither at enrollment nor after 48 h of treatment.
Table 2.
Levels of nasal immunological markers gene expression at enrollment and after 48 h of treatment.
| Cytokine median (range) | Linfovir |
Placebo |
||
|---|---|---|---|---|
| Enrollment | 48 h | Enrollment | 48 h | |
| CCL2 | 1.39 (0.03–62.43) | 1.96 (0.05–138.7) | 3.13 (0.01–283.4) | 1.59 (0.05–47.24) |
| CCL5 | 0.88 (0.05–17.43) | 1.56 (0.02–21.87) | 1.28 (0.11–13.66) | 0.78 (0.06–4.66) |
| IL-8 | 2.41 (0.07–287.6) | 4.24 (0.07–70.74) | 3.57 (0.09–56.51) | 4.65 (0.05–124.8) |
| IL-6 | 1.51 (0.06-155-3) | 2.89 (0.01–83.30) | 2.37 (0.06–413.7) | 1.23 (0.03-62-95) |
| CXCL10 | 0.8 (0.02–118.1) | 0.41 (0.01–164.7) | 1.04 (0.01–163.2) | 0.21 (0.0–8.95) |
| TLR2 | 1.61 (0.27–16) | 3.11 (0.21–16.66) | 1.34 (0.35–60.19) | 2.12 (0.25–15.97) |
Fold change for each gene is expressed as 2-ΔΔCt.
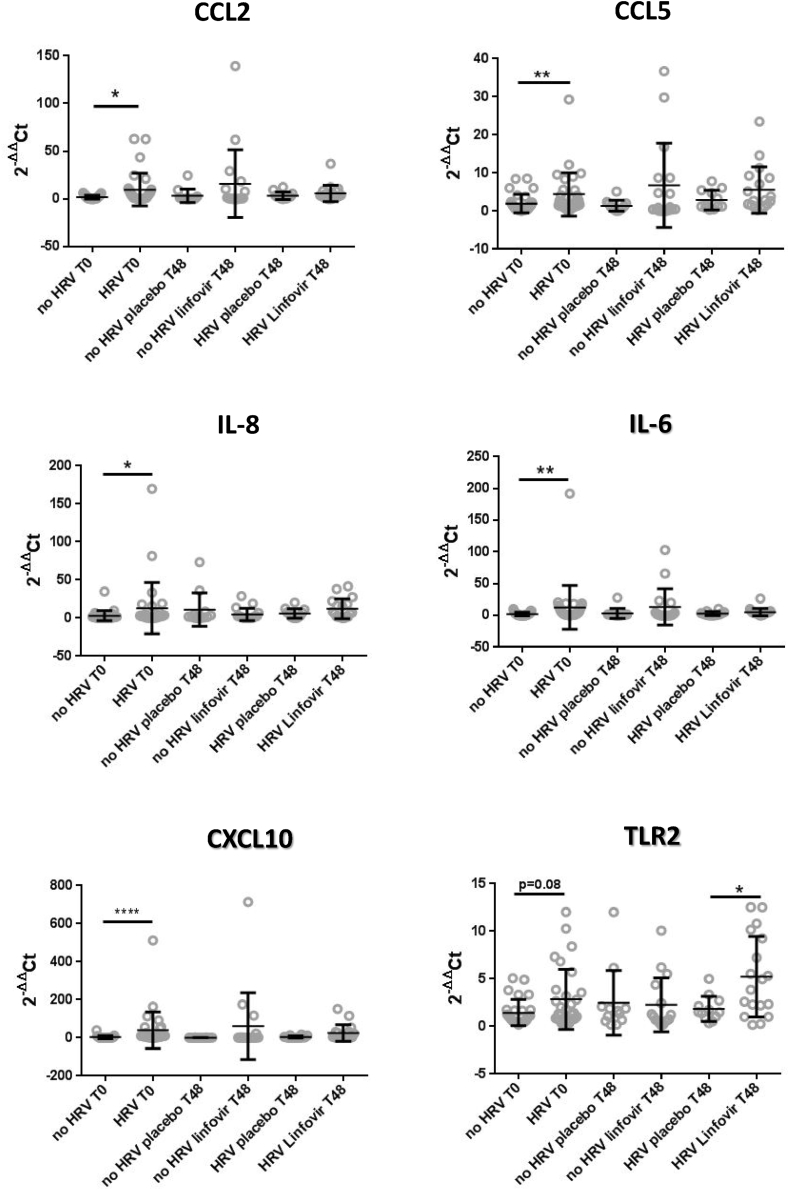
Since infection with HRVs influences the expression of several cytokines, the data obtained were also analyzed in both groups based on the presence/absence of viral infection. Results of mRNA expression analysis (Figure 3) revealed that levels of CCL2, CCL5, IL-8, IL-6 and CXCL10 were significantly up-regulated in HRV infected children at baseline. In particular CXCL10 showed a 10-fold increase in expression in response to HRV infection (p < 0.0001).
Figure 3.
mRNA expression of immunological markers in nasal samples of HRV-infected and non infected children. Expression is displayed as fold change relative to no HRV T0 group according to 2 −ΔΔCt method and normalized to 18S housekeeping gene. Data are represented as mean ± SD; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001.
Although not significant, we also found an increase of TLR-2 mRNA level in HRV infected compared to HRV uninfected children at baseline. After 48 h of follow-up, TLR-2 gene expression in HRV infected children treated with Linfovir® plus was significantly increased compared to HRV infected subjects of placebo group (p < 0.05).
30-day relapses were found to be lower in Linfovir® plus group, although without significant differences between the two groups (15% vs 28.9%, p = 0.15). No adverse effects were recorded.
4. Discussion
Resveratrol has been extensively studied for its ability to inhibit the growth of bacteria, fungi and viruses [29, 30, 31]. In particular, preclinical studies demonstrated that resveratrol was able to inhibit the replication of several respiratory viruses such as rhinovirus, influenza A virus, respiratory syncytial virus, MERS-CoV (Middle East respiratory syndrome - coronavirus), and human metapneumovirus and to reduce virus-induced inflammatory mediators [23, 32, 33, 34, 35, 36].
For the low bioavailability of resveratrol after oral administration, only few studies evaluated the therapeutic application of resveratrol against infectious diseases. Topical application of resveratrol in mice was able to reduce both skin lesion and vaginal replication due to herpes simplex infection [37, 38]. To our knowledge this is the first human trial on the effect of topical application of resveratrol on respiratory infections and related symptoms.
Despite the overall non superiority of this novel approach, the present study showed that intranasal instillation of drops containing resveratrol plus carboxymethyl-β-glucan may provide some beneficial effects in infants with the common cold. The most notable finding in this respect is the significant reduction in sneezing and productive or non-productive cough episodes. This finding is in keeping with previous results showing the capacity of resveratrol and CM-glucan to decrease nasal symptoms and respiratory complications in children with allergic rhinitis and acute rhinopharyngitis [26, 27].
Despite our samples being tested for the major viruses that cause respiratory infections in newborns, only 44% of the samples showed the presence of viruses (i.e. 20 in the treated group and 19 in the placebo group). In all these samples, except one, the presence of HRV was identified. It is noteworthy that in HRV infected infants episodes of cough were significantly lower in Linfovir® plus group in comparison to placebo group after 7 days of treatment. Moreover, TLR-2 mRNA level in HRV infected children treated with Linfovir® plus was significantly increased compared to HRV infected subjects of the placebo group at 48 h follow-up. Since it has been demonstrated that TLR-2 can be involved in the type I IFNs antiviral response upon binding of viral antigens to this pattern recognition receptor [39], our results suggest that Linfovir® plus could promote antiviral defense mainly through TLR-2 up-regulation.
The positive effects observed in the Linfovir® plus group might be related to the anti-inflammatory and anti-viral mechanisms of resveratrol and to the immune-modulation and osmotic activities provided by glucan [23, 24, 25].
According to our results, the solution containing resveratrol plus carboxymethyl-β-glucan may be an interesting option in the treatment of infant common cold to reduce some respiratory symptoms and decrease relapses, since there is a limited availability of symptomatic treatment for viral infections in this age group.
Thus, our results suggest that resveratrol plus carboxymethyl-β-glucan might have a clinical and socio-economic positive impact on the common cold, but its beneficial effects warrant large scale clinical trials to be further assessed.
The limitations of our study are a) the relatively low number of virus infected samples; b) the possible difference in the duration of viral infection at the time of enrollment that can influence the temporal evolution of the infection; c) the absence of a diagnosis of bacterial infection.
5. Conclusions
Despite the promising results obtained in this study, more evidence-based studies on larger cohorts are needed to assess the therapeutic potential of a solution containing resveratrol plus carboxymethyl-β-glucan and its application in clinical settings.
Declarations
Author contribution statement
M.E. Baldassarre: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
P. Mastromarino and E. Schiavi: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data.
G. Labellarte and M.C. Pignatelli: Contributed reagents, materials, analysis tools or data.
M. Fanelli: Analyzed and interpreted the data.
N. Laforgia, A. Di Mauro, M. Capozza and R. Panza: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Maria Passioti, Paraskevi Maggina, Spyridon Megremis, Papadopoulos Nikolaos G. The common cold: potential for future prevention or cure. Curr. Allergy Asthma Rep. 2014;14(2):413. doi: 10.1007/s11882-013-0413-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee Jennifer W., Davis Jonathan M. Future applications of antioxidants in premature infants. Curr. Opin. Pediatr. 2011;23(2):161–166. doi: 10.1097/MOP.0b013e3283423e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winther B. Rhinovirus infections in the upper airway. Proc. Am. Thorac. Soc. 2011;8(1):79–89. doi: 10.1513/pats.201006-039RN. [DOI] [PubMed] [Google Scholar]
- 4.Bochkov Yury A., Gern James E. Rhinoviruses and their receptors: implications for allergic disease. Curr. Allergy Asthma Rep. 2016;16(4):30. doi: 10.1007/s11882-016-0608-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartlett Nathan W., Walton Ross P., Edwards Michael R., Juliya Aniscenko, Gaetano Caramori, Zhu Jie. Mouse models of rhinovirus-induced disease and exacerbation of allergic airway inflammation. Nat. Med. 2008;14(2):199–204. doi: 10.1038/nm1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly John T., Busse William W. Host immune responses to rhinovirus: mechanisms in asthma. J. Allergy Clin. Immunol. 2008;122(4):671–682. doi: 10.1016/j.jaci.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spurrell Jason C.L., Shahina Wiehler, Zaheer Raza S., Sanders Scherer P., David Proud. Human airway epithelial cells produce IP-10 (CXCL10) in vitro and in vivo upon rhinovirus infection. Am. J. Physiol. Lung Cell Mol. Physiol. 2005;289(1):L85–95. doi: 10.1152/ajplung.00397.2004. [DOI] [PubMed] [Google Scholar]
- 8.Pappas Diane E., Hendley J., Owen Hayden Frederick G., Birgit Winther. Symptom profile of common colds in school-aged children. Pediatr. Infect. Dis. J. 2008;27(1):8–11. doi: 10.1097/INF.0b013e31814847d9. [DOI] [PubMed] [Google Scholar]
- 9.Emanuel Troullos, Lisa Baird, Shyamalie Jayawardena. Common cold symptoms in children: results of an Internet-based surveillance program. J. Med. Internet Res. 2014;16(6):e144. doi: 10.2196/jmir.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armengol Carlos Eladio. Owen Hendley J. Winther birgit. Occurrence of acute otitis media during colds in children younger than four years. Pediatr. Infect. Dis. J. 2011;30(6):518–520. doi: 10.1097/INF.0b013e3182044930. [DOI] [PubMed] [Google Scholar]
- 11.Imakita M., Shiraki K., Yutani C., Ishibashi-Ueda H. Pneumonia caused by rhinovirus. Clin. Infect. Dis. 2000;30(3):611–612. doi: 10.1086/313723. [DOI] [PubMed] [Google Scholar]
- 12.Kusel Merci M.H., de Klerk Nicholas H., Holt Patrick G., Tatiana Kebadze, Johnston Sebastian L., Sly Peter D. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: a birth cohort study. Pediatr. Infect. Dis. J. 2006;25(8):680–686. doi: 10.1097/01.inf.0000226912.88900.a3. [DOI] [PubMed] [Google Scholar]
- 13.Stock Ingo. Human rhinovirus diseases--epidemiology, treatment and prevention. Med. Monatsschr. Pharm. 2014;37(2):44–53. [PubMed] [Google Scholar]
- 14.European Medicines Agency . Ambroxol and Bromhexine Expectorants: Safety Information Updated Risk of Allergy and Skin Reactions Included in the Product Information. 2015. [Google Scholar]
- 15.Mehdi Koushki, Nasrin Amiri-Dashatan, Nayebali Ahmadi, Allah Abbaszadeh Hojjat, Mostafa Rezaei-Tavirani. Resveratrol: a miraculous natural compound for diseases treatment. Food Sci. Nutr. 2018:2473–2490. doi: 10.1002/fsn3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Celotti E., Ferrarini R., Zironi R., Conte L.S. Resveratrol content of some wines obtained from dried Valpolicella grapes: recioto and Amarone. J. Chromatogr. A. 1996;730(1–2):47–52. doi: 10.1016/0021-9673(95)00962-0. [DOI] [PubMed] [Google Scholar]
- 17.Aggarwal Bharat B, Bhardwaj Anjana, Aggarwal Rishi S, Seeram Navindra P, Shishodia Shishir, Takada Yasunari. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res n.d.;24(5A):2783–2840. [PubMed]
- 18.Silvia Bradamante, Livia Barenghi, Villa Alessandro. Cardiovascular protective effects of resveratrol. Cardiovasc. Drug Rev. 2004;22(3):169–188. doi: 10.1111/j.1527-3466.2004.tb00139.x. [DOI] [PubMed] [Google Scholar]
- 19.Nady Braidy, Bat-Erdene Jugder, Anne Poljak, Tharusha Jayasena, Hussein Mansour, Mohammad Nabavi Seyed. Resveratrol as a potential therapeutic candidate for the treatment and management of alzheimer’s disease. Curr. Top. Med. Chem. 2016;16(17):1951–1960. doi: 10.2174/1568026616666160204121431. [DOI] [PubMed] [Google Scholar]
- 20.Ester Tellone, Antonio Galtieri, Russo Annamaria, Bruno Giardina, Silvana Ficarra. Resveratrol: a focus on several neurodegenerative diseases. Oxid Med Cell Longev. 2015;2015:392169. doi: 10.1155/2015/392169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rivera Leonor, Rocío Morón, Antonio Zarzuelo, Milagros Galisteo. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem. Pharmacol. 2009;77(6):1053–1063. doi: 10.1016/j.bcp.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 22.Palsamy P., Subramanian S. Ameliorative potential of resveratrol on proinflammatory cytokines, hyperglycemia mediated oxidative stress, and pancreatic beta-cell dysfunction in streptozotocin-nicotinamide-induced diabetic rats. J. Cell. Physiol. 2010;224(2):423–432. doi: 10.1002/jcp.22138. [DOI] [PubMed] [Google Scholar]
- 23.Paola Mastromarino, Daniela Capobianco, Cannata Federica, Chiara Nardis, Elena Mattia, De Leo Alessandra Resveratrol inhibits rhinovirus replication and expression of inflammatory mediators in nasal epithelia. Antivir. Res. 2015;123:15–21. doi: 10.1016/j.antiviral.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Antonio Francioso, Paola Mastromarino, Alessandra Masci, d’Erme Maria, Luciana Mosca. Chemistry, stability and bioavailability of resveratrol. Med. Chem. 2014;10(3):237–245. doi: 10.2174/15734064113096660053. [DOI] [PubMed] [Google Scholar]
- 25.Lehtovaara Benjamin C., Gu Frank X. Pharmacological, structural, and drug delivery properties and applications of 1,3-β-glucans. J. Agric. Food Chem. 2011;59(13):6813–6828. doi: 10.1021/jf200964u. [DOI] [PubMed] [Google Scholar]
- 26.Miraglia Del Giudice M., Maiello N., Decimo F., Capasso M., Campana G., Leonardi S. Resveratrol plus carboxymethyl-β-glucan may affect respiratory infections in children with allergic rhinitis. Pediatr. Allergy Immunol. 2014;25(7):724–728. doi: 10.1111/pai.12279. [DOI] [PubMed] [Google Scholar]
- 27.Varricchio Alfonso Maria, Michele Capasso, Della Volpe Antonio, Luigi Malafronte, Nicola Mansi, Attilio Varricchio. Resveratrol plus carboxymethyl-β-glucan in children with recurrent respiratory infections: a preliminary and real-life experience. Ital. J. Pediatr. 2014;40(1):93. doi: 10.1186/s13052-014-0093-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antonio Francioso, Riccardo Cossi, Fanelli Sergio, Paola Mastromarino, Luciana Mosca. Studies on trans-resveratrol/carboxymethylated (1,3/1,6)-β-d-glucan association for aerosol pharmaceutical applications. Int. J. Mol. Sci. 2017;18(5):967. doi: 10.3390/ijms18050967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin Vestergaard, Hanne Ingmer. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents. 2019:716–723. doi: 10.1016/j.ijantimicag.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Abba Yusuf. Hasliza Hassim, Hazilawati Hamzah, Mohamed Mustapha Noordin. Antiviral activity of resveratrol against human and animal viruses. Adv Virol. 2015;2015:184241. doi: 10.1155/2015/184241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michela Campagna, Carmen Rivas. Antiviral activity of resveratrol. Biochem. Soc. Trans. 2010;38(Pt 1):50–53. doi: 10.1042/BST0380050. [DOI] [PubMed] [Google Scholar]
- 32.Palamara Anna T., Lucia Nencioni, Katia Aquilano, De Chiara Giovanna, Hernandez Leyanis, Federico Cozzolino. Inhibition of influenza A virus replication by resveratrol. J. Infect. Dis. 2005;191(10):1719–1729. doi: 10.1086/429694. [DOI] [PubMed] [Google Scholar]
- 33.Lin Chao-jen, Hui-Ju Lin, Ter-Hsin Chen, Hsu Yu-An, Liu Chin-San, Hwang Guang-Yuh. Polygonum cuspidatum and its active components inhibit replication of the influenza virus through toll-like receptor 9-induced interferon beta expression. PloS One. 2015;10(2) doi: 10.1371/journal.pone.0117602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao-Hong Xie, Zang Na, Si-min Li, Wang Li-jia, Deng Yu, He Yun. Resveratrol Inhibits respiratory syncytial virus-induced IL-6 production, decreases viral replication, and downregulates TRIF expression in airway epithelial cells. Inflammation. 2012;35(4):1392–1401. doi: 10.1007/s10753-012-9452-7. [DOI] [PubMed] [Google Scholar]
- 35.Shih-Chao Lin, Chi-Tang Ho, Wen-Ho Chuo, Li Shiming, Wang Tony T., Chi-Chen Lin. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect. Dis. 2017;17(1):144. doi: 10.1186/s12879-017-2253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narayana Komaravelli, Kelley John P., Garofalo Matteo P., Wu Haotian, Antonella Casola, Deepthi Kolli. Role of dietary antioxidants in human metapneumovirus infection. Virus Res. 2015;200:19–23. doi: 10.1016/j.virusres.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Docherty John J., Fu Ming Ming, Hah Jennifer M., Sweet Thomas J., Faith Seth A., Tristan Booth. Effect of resveratrol on herpes simplex virus vaginal infection in the mouse. Antivir. Res. 2005;67(3):155–162. doi: 10.1016/j.antiviral.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Docherty John J., Smith Jennifer S., Fu Ming, Ming Stoner Terri, Tristan Booth. Effect of topically applied resveratrol on cutaneous herpes simplex virus infections in hairless mice. Antivir. Res. 2004;61(1):19–26. doi: 10.1016/j.antiviral.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 39.Shepardson Kelly M., Benjamin Schwarz, Larson Kyle, Morton Rachelle V., John Avera, Kimberly McCoy. Induction of antiviral immune response through recognition of the repeating subunit pattern of viral capsids is toll-like receptor 2 dependent. mBio. 2017;8(6) doi: 10.1128/mBio.01356-17. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]