Abstract
A recombinant fowlpox virus (rFPV-IFNγS1) that co-expressed the infectious bronchitis virus (IBV) S1 gene and the chicken interferon-γ gene has been constructed. To evaluate the efficacy of the recombinant fowlpox virus vaccine against heterotypic IBV strains, 60 4-week-old Specific-Pathogen-Free (SPF) chickens were inoculated with this vaccine and 3 weeks post inoculation challenged with the homotypic IBV strain LX4 and the heterotypic IBV strains LHB, LHLJ04XI, LTJ95I and LSC99I. Antibodies against IBV were detected in vaccinated chickens 1-week post inoculation. The number of CD4+ and CD8+ T-lymphocytes in the peripheral blood increased rapidly in the vaccinated groups challenged with strains LX4, LHB and LHLJ04XI. There were significant differences in the number of CD4+ and CD8+ T-lymphocytes between the vaccinated groups challenged with strains LTJ95I and LSC99I and all the control groups. The morbidity was below 30% in vaccinated groups challenge with strains LX4, LHB and LHLJ04XI, but was 40% greater than that in the other groups. In addition, the lesions and the amount of virus shedding were less severe in the vaccinated groups challenged by strains LX4, LHB and LHLJ04XI when compared with the other groups, but there was no significant difference in the average body weight of the chickens in all groups (all p > 0.05). These results indicate that the rFPV-IFNγS1 protected chickens against challenge with homotypic IBV strain LX4 and heterotypic strains LHLJ04XI and LHB.
Keywords: Infectious bronchitis virus, Recombinant fowl poxvirus, IFN-γ, S1 protein
1. Introduction
Infectious bronchitis (IB) is a highly contagious disease of chickens that is caused by infectious bronchitis virus (IBV) [1]. It can lead to serious economic losses in poultry enterprises. The use of live attenuated vaccines against IB has produced very good results. However, the attenuated vaccine can cause latent infection, and may become a source of mutation and restructuring of the virus because of the high degree of variability of IBV, the frequency of deletion and insertion mutations of the RNA genome, as well as homologous recombination between different strains (including the vaccine strains). Another potential problem with attenuated vaccines is reversion to virulence in the course of use.
The use of inactivated vaccines may help to solve the above problems, but deleterious effects have also been reported with inactivated vaccines, such as a longer duration of the local response after immunization. The effects of immunization with inactivated vaccines against coronaviruses are uncertain [2], [3]. Application of live vaccines using viral vectors can resolve these issues because they induce effective humoral and cellular immune responses. They can also stimulate the immune response to live attenuated vaccines [4]. We have used a fowlpox virus as a vector to express IBV S1 gene and the preliminary results showed that the recombinant vaccine has a good immunostimulating effect [5].
Interferons are important cytokines, which have anti-viral and anti-tumor activity, as well as actions in immune regulation. They are widely used in the construction of vaccines using genetic engineering [6]. For example, chicken interferon type II (also known as interferon γ, ChIFNγ) is synthesized and secreted by T cells that have been activated by antigen or mitogen. It is an important immune regulator and has strong adjuvant effects, such as the promotion of expression of MHC II antigen, stimulation of reciprocity of T cells and antigen-presenting cells, and enhancement of the role of T cells in the auxiliary production of antibody. It also has the ability to regulate immune and other activity of cytotoxic T cells. Interferons have been described as an adjuvant [7]. In addition, interferons are of great significance for alleviation of the residual toxicity and side effects of the fowlpox virus vector, so that weight gain is not affected after vaccination with recombinant fowlpox virus [8]. We inserted the gene for ChIFNγ into a fowlpox virus vector and constructed a recombinant fowlpox virus that expressed IBV S1 gene and chicken interferon-γ gene.
The phylogenetic tree of IBV strains isolated in China has been investigated using molecular epidemiology. It has been shown that the S1 gene of different IBV strains is distinct and that strains form independent groups on the molecular basis. These strains may have different origins or have arisen by genetic mutation. These data provide a reference for the prevention and control of IBV in China [9], [10]. The S1 gene of rFPV-IFNγS1 is derived from IBV strain LX4, so rFPV-IFNγS1 can provide protection against IBV strain LX4. The IBV strains used in this study belong to gene cluster I (strains LX4 and LHLJ04XI), gene cluster II (strain LHB), gene cluster III (strain LSC99I) and gene cluster IV (strain LTJ95I). Although all of these strains cause renal disease, their source varies and there are differences in the S1 gene. The results of this study would have theoretical and practical significance for the application of the recombinant vaccine rFPV-IFNγS1 in protection against heterogeneous strains of IBV.
2. Materials and methods
2.1. Virus and vaccine
The IBV strains were a virulent IBV reference strain LX4 (GenBank Accession Nos.: AY189157 and AY326960) and the other four virulent IBV strains (Table 1 ) [10]. The recombinant fowlpox virus rFPV-IFNγS1 showed expression of the S1 gene of IBV strain LX4 and the chicken interferon-γ gene [11]. It was added to 12.5% gelatin–sucrose (1:5) agent, and made into a freeze-dried vaccine before testing (Batch No.: 050310; Harbin Weike Biotechnology Company, Harbin, China).
Table 1.
Four infectious bronchitis virus strains used for challenging chickens.
| IBV strain | Year | Provincea | Production type | Organsb for used virus isolated | GenBank Accession No. | Pathogenicity for chicken embryoc |
|---|---|---|---|---|---|---|
| LHLJ04XI | 2004 | Heilongjiang | Layer hen | Kidney | – | 5.7EID50 |
| LHB96I | 1996 | Hebei | Broiler | Kidney | DQ287912 | 6.5EID50 |
| LSC99I | 1999 | Sichuan | Layer hen | Preventriculus | DQ287915 | 6.4EID50 |
| LTJ95I | 1995 | Tianjin | Layer hen | Kidney | DQ287916 | 7.0EID50 |
Province where the viruses were isolated.
Swollen kidney or swollen preventriculus.
Virus titer was detected by EID50.
2.2. Animal experiments
One hundred and twenty 4-week-old SPF white Leghorn chickens were assigned randomly into two groups. Chickens in Group A (n = 60) were inoculated by scarification of the wing-web with 103 plaque forming units (PFU) of rFPV-IFNγS1. Chickens in Group B (n = 60) served as the unvaccinated controls. At week 4 post-immunization (pi), animals in Groups A and B were divided randomly into five groups, designated sub-groups A1, A2, A3, A4 and A5, and B1, B2, B3, B4, and B5, respectively. One sub-group from Group A and another from Group B were challenged with one of the four IBV strains LX4, LHLJ04XI, LHB, LSC99I and LTJ95I by the intranasal route, respectively. All the chickens were infected with 0.2 mL of allantoic fluid containing 10,000 EID50 [12]. Any clinical signs and the morbidity and mortality percentages were recorded from day 2 post-challenge. Blood samples were collected once a week until day 14 post-challenge and tested for specific antibodies to IBV by enzyme-linked immunosorbent assay (ELISA), and the dynamic variation of CD4+ and CD8+ T lymphocytes in peripheral blood was recorded. On days 6 and 10 post-challenge, one chicken selected randomly from each group was euthanized for harvesting its trachea, liver, spleen, kidney, lung, pancreas and proventriculus for pathological examination.Animal experiments were approved by the Harbin Veterinary Research Institute of the Chinese Academy of Agricultural Sciences and were performed in accordance with animal ethics guidelines and approved protocols.
2.3. Antibody immune response against IBV
Commercial ELISA kits (FlockChek Infectious Bronchitis Virus Antibody Test Kit, IDEXX, Westbrook, ME, USA) were used to test chicken sera for antibodies against IBV according to the manufacturer's instructions. The chicken serum samples were diluted 1:100 in PBS and incubated with 96-well microtiter plates coated with the IBV antigen. The relative level of antibody was determined by calculating the ratio of the sample to a reference positive serum (S/P ratio). Serum samples with S/P ratios of less than or equal to 0.2 were considered to be negative, and serum samples with an S/P ratio greater than 0.2 were considered to be positive.
2.4. Detection of CD4+ and CD8+ T lymphocytes in peripheral blood
Peripheral blood samples (2.0 mL) were collected in heparinized syringes, and the lymphocytes were obtained using lymphocyte separation medium (Sigma–Aldrich, St. Louis, USA), followed by washing twice with PBS. Thirty microliters of mouse anti-chicken CD4-FITC or mouse anti-chicken CD8-FITC (Southern Biotech, Birmingham, AL, USA) diluted 1:100 was added to the cell precipitation. The mixture was incubated in the dark at 4 °C for 30 min and then washed twice, followed by addition of 1.0 mL of PBS. The stained cells were detected by flow cytometry to count the T lymphocytes [13], [14].
2.5. Virus isolation
Throat swab samples were frozen–thawed three times and centrifuged at 1000 × g for 15 min. The resulting supernatant was used to inoculate the allantoic cavity of 9- to 11-day-old SPF chicken embryos. After 96 h, the allantoic fluid was collected and pathological changes in the embryos were recorded. If the embryos appeared to be congested, edematous or hemorrhagic, or if they developed calcium deposition, the samples were considered positive for IBV. Otherwise, the allantoic fluid of the inoculated chicken embryos was collected for three further blind passages and analyzed using RT-PCR. The IBV forward and reverse primers were 5′-ACATTGTTATAGTAGTGGATCAGG-3′ and 5′-AAAAGCACAAATAGGGTGGTAAGA-3′, respectively [15].
2.6. Analysis of body weights
Each chicken was weighed on the day before vaccination, on day 10 post-vaccination, on the day before challenge and on day 10 post-challenge. Body weights were analyzed by ANOVA using the STATISTICA software (StatSoft Inc., Tulsa, OK) [16].
3. Results
3.1. Lesions in chickens challenged with IBV
There were different pathological changes in the kidney between vaccinated groups and control groups after challenge with strains LSC99I and LTJ95I. The kidneys were swollen, pale in color, and showed a white sludge of urate deposition; other organs also showed visible pathological changes. The lesions observed in the liver, spleen, kidneys, lungs and trachea were mild and recovered much sooner in the vaccinated groups that were challenged with strains LHLJ04XI, LHB and LX4 when compared with the control groups. The results showed that lesions of chickens in the vaccinated groups that were challenged with strains LHLJ04XI, LHB and LX4 were less severe than those in the vaccinated groups that were challenged with strains LSC99I and LTJ95I and the control groups (Table 2 ).
Table 2.
Histopathological changes of chickens after challenge with IBV strains LX4, LHLJ04XI, LHB, LSC99I and LTJ95I.a
| Strain | Group | Days after challenge | Kidneyb | Liverc | Spleend | Proventriculuse | Tracheaf | Lungg |
|---|---|---|---|---|---|---|---|---|
| LX4 | Vaccineh | 6 | +i | + | ++ | + | + | + |
| 10 | − | + | + | − | − | − | ||
| Controlj | 6 | ++ | ++ | ++ | ++ | + | ++ | |
| 10 | + | + | − | + | + | ++ | ||
| LHLJ04XI | Vaccine | 6 | + | + | ++ | + | − | + |
| 10 | − | + | − | − | − | − | ||
| Control | 6 | ++ | ++ | + | + | + | ++ | |
| 10 | ++ | + | − | − | + | + | ||
| LHB | Vaccine | 6 | + | + | − | + | ++ | + |
| 10 | − | + | − | − | + | − | ||
| Control | 6 | ++ | ++ | + | ++ | ++ | ++ | |
| 10 | + | + | + | + | ++ | ++ | ||
| LSC99I | Vaccine | 6 | + | ++ | − | + | ++ | + |
| 10 | + | + | − | − | + | + | ||
| Control | 6 | ++ | ++ | + | ++ | ++ | ++ | |
| 10 | + | + | + | + | + | + | ||
| LTJ95I | Vaccine | 6 | ++ | + | + | ++ | + | ++ |
| 10 | + | + | − | + | − | + | ||
| Control | 6 | + | ++ | − | + | ++ | ++ | |
| 10 | + | + | − | + | + | + |
Each chicken was challenged with 0.2 mL of allantoic fluid containing 10,000 EID50 of IBV strain on day 21 post-immunization.
Kidney: decreased focal stromal lymphocytes, renal necrosis, glomerular expansion of glomerulus, necrosis of epithelial cells, glomerular necrosis, interstitial nephritis disease and ureteral epithelial vacuolation and degeneration.
Liver: fatty degeneration and stromal cell-like lesions.
Spleen: proliferation of the red pulp, disorder of the white pulp structure and follicular hyperplasia.
Proventriculus: mucoderm lobular necrosis and decreased lymphocyte.
Trachea: decreased lymphocytes in lamina propria, dropsy of mucous layer, vacuole denature and necrosis and defluvium of epithelium.
Lung: congestion, serosity exudation, decreased focal lymphocytes (more than three in the bronchial mucous membrane), vacuolation and deformation of bronchioloalveolar (II) and edema in the laminae propria.
Chickens were immunized with recombinant fowlpox virus (rFPV-IFNγS1) co-expressing the infectious bronchitis virus (IBV) S1 gene and the chicken interferon-γ gene.
Histopathological changes are expressed as “++++, +++, ++, +” and no changes as “−”.
Chickens were immunized with PBS.
3.2. Antibody responses to IBV in chickens following immunization and challenge
Table 3 shows the changes in levels of antibody against IBV following inoculation of SPF chickens with rFPV-IFNγS1 or PBS and following challenge with IBV. As expected, antibody to IBV was not detected prior to IBV challenge in the control groups. Chickens in the vaccinated groups had the highest level of antibody, and IBV antibody was detected in all chickens on day 7 post-vaccination, which was significantly different from the control group (p < 0.05). One week after challenge, the level of antibodies in the vaccinated and control groups increased significantly, but the levels of antibody differed between the vaccinated groups that were challenged with LHLJ04XI, LHB and LX4 and the other groups.
Table 3.
Antibody titers in sera of chickens following immunization with recombinant fowlpox virus co-expressing the infectious bronchitis virus (IBV) S1 gene and the chicken interferon-γ gene.
| Group | Time (weeks) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 0a | 1 | 2 | 3b | 4 | 5 | 6 | ||
| LX4 | Vaccine | 0 | 1145c | 1731 | 1608 | 2418 | 6393 | 5948 |
| Controld | 0 | 0 | 0 | 135 | 2290 | 4984 | 4039 | |
| LHLJ04XI | Vaccine | 0 | 1145 | 1731 | 1608 | 2250 | 5805 | 5425 |
| Control | 0 | 0 | 0 | 135 | 2462 | 4014 | 4584 | |
| LHB | Vaccine | 0 | 1145 | 1731 | 1608 | 2044 | 9799 | 4685 |
| Control | 0 | 0 | 0 | 135 | 2348 | 3849 | 6088 | |
| LSC99I | Vaccine | 0 | 0 | 0 | 135 | 2602 | 6835 | 4396 |
| Control | 0 | 1145 | 1731 | 1608 | 1433 | 3748 | 3796 | |
| LTJ95I | Vaccine | 0 | 1145 | 1731 | 1608 | 1823 | 3883 | 3198 |
| Control | 0 | 0 | 0 | 135 | 1847 | 4614 | 3519 | |
Values are antibody mean ± S.E.M.
Chickens (n = 12 per group) were immunized with recombinant fowlpox virus co-expressing the infectious bronchitis virus (IBV) S1 gene and the chicken interferon-γ gene at week 0.
Chickens (n = 12 per group) were challenged with 0.2 mL of allantoic fluid containing 10,000 EID50 of IBV strains LX4, LHLJ04XI, LHB, LSC99I and LTJ95I at week 3 post-vaccination.
Chicken sera were collected at weeks 0, 1, 2, 3, 4, 5 and 6.
Control chickens received PBS (n = 12 per group).
3.3. Dynamic changes in peripheral blood CD4+ and CD8+ sub-types of T lymphocytes
Counting of CD4+ and CD8+ T lymphocytes was carried out by flow cytometry. The results showed that the counts of CD4+ (Table 4 ) and CD8+ (Table 5 ) T lymphocytes increased in all the vaccinated groups, but there was a small drop in CD4+ T lymphocytes before challenge; after challenge, the number of CD4+ and CD8+ T lymphocytes continued to rise. Two weeks after the challenge, the percentages of CD4+ and CD8+ T lymphocytes in the vaccinated groups achieved the highest values. The percentages of CD4+ and CD8+ T lymphocytes in the vaccinated groups that were challenged with strains LX4, LHLJ04XI and LHB were significantly higher than those in the control groups and those in the vaccinated groups challenged with strains LSC99I or LTJ95I (p < 0.05).
Table 4.
Dynamics of CD4+ T lymphocytes in peripheral blood of chickens immunized with recombinant fowlpox virus co-expressing the infectious bronchitis virus S1 gene and the chicken interferon-γ gene.
| Group | Time (weeks) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 0a | 1 | 2 | 3b | 4 | 5 | 6 | ||
| LX4 | Vaccine | 7.01 ± 0.21c | 9.36 ± 0.36 | 11.50 ± 0.35* | 10.10 ± 0.33* | 10.90 ± 1.25* | 13.51 ± 0.44* | 14.52 ± 0.62* |
| Controld | 7.01 ± 0.21 | 8.41 ± 0.82 | 10.40 ± 0.34 | 9.47 ± 0.30 | 10.10 ± 0.66 | 11.91 ± 0.65 | 13.10 ± 0.20 | |
| LHLJ04XI | Vaccine | 7.01 ± 0.21 | 9.36 ± 0.36 | 11.50 ± 0.35* | 10.01 ± 0.33* | 10.00 ± 0.79* | 12.21 ± 0.66* | 13.97 ± 0.61* |
| Control | 7.01 ± 0.21 | 8.41 ± 0.82 | 10.40 ± 0.34 | 9.47 ± 0.30 | 10.80 ± 1.42 | 11.61 ± 0.74 | 12.92 ± 0.55 | |
| LHB | Vaccine | 7.01 ± 0.21 | 9.36 ± 0.36 | 11.50 ± 0.35* | 10.10 ± 0.33* | 10.60 ± 0.83* | 12.51 ± 0.61* | 13.44 ± 0.49* |
| Control | 7.01 ± 0.21 | 8.41 ± 0.82 | 10.40 ± 0.34 | 9.47 ± 0.30 | 7.96 ± 2.17 | 10.59 ± 0.94 | 12.68 ± 0.33 | |
| LSC99I | Vaccine | 7.01 ± 0.21 | 9.36 ± 0.36 | 11.50 ± 0.35* | 10.10 ± 0.33* | 10.50 ± 0.82* | 12.23 ± 0.40* | 11.97 ± 0.34 |
| Control | 7.01 ± 0.21 | 8.41 ± 0.82 | 10.40 ± 0.34 | 9.47 ± 0.30 | 9.83 ± 1.53 | 10.71 ± 0.35 | 12.67 ± 0.57 | |
| LTJ95I | Vaccine | 7.01 ± 0.21 | 9.36 ± 0.36 | 11.50 ± 0.35* | 10.10 ± 0.33* | 8.15 ± 1.80 | 10.51 ± 1.07 | 10.77 ± 0.25 |
| Control | 7.01 ± 0.21 | 8.41 ± 0.82 | 10.40 ± 0.34 | 9.47 ± 0.30 | 9.09 ± 1.36 | 14.20 ± 1.68 | 13.31 ± 0.26 | |
Values are mean ± S.E.M.
Value is significantly different from corresponding control value (p < 0.05).
Chickens (n = 12 per group) were immunized with recombinant fowlpox virus co-expressing the infectious bronchitis virus (IBV) S1 gene and the chicken interferon-γ gene at week 0.
Chickens (n = 12 per group) were challenged with 0.2 mL of allantoic fluid containing 10,000 EID50 of IBV strains LX4, LHLJ04XI, LHB, LSC99I and LTJ95I at week 3 post-vaccination.
Blood lymphocytes were harvested at weeks 0, 1, 2, 3, 4, 5 and 6.
Control chickens received PBS (n = 12 per group).
Table 5.
Dynamics of CD8+ T lymphocytes in peripheral blood of chickens immunized with recombinant fowlpox virus co-expressing the infectious bronchitis virus S1 gene and the chicken interferon-γ gene.
| Group | Time (weeks) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 0a | 1 | 2 | 3b | 4 | 5 | 6 | ||
| LX4 | Vaccine | 4.94 ± 0.76c | 7.56 ± 0.49* | 10.21 ± 0.22* | 11.01 ± 0.22* | 14.43 ± 0.68* | 20.75 ± 1.97* | 18.44 ± 4.39* |
| Controld | 4.94 ± 0.76 | 5.14 ± 0.43 | 9.05 ± 0.51 | 10.15 ± 0.36 | 12.26 ± 0.36 | 12.97 ± 1.89 | 12.01 ± 0.48 | |
| LHLJ04XI | Vaccine | 4.94 ± 0.76 | 7.56 ± 0.49* | 10.21 ± 0.22* | 11.01 ± 0.22* | 13.36 ± 0.20* | 17.43 ± 0.68* | 15.42 ± 0.19* |
| Control | 4.94 ± 0.76 | 5.14 ± 0.43 | 9.05 ± 0.51 | 10.15 ± 0.36 | 11.49 ± 0.67 | 12.62 ± 0.69 | 11.36 ± 0.42 | |
| LHB | Vaccine | 4.94 ± 0.76 | 7.56 ± 0.49* | 10.21 ± 0.22* | 11.01 ± 0.22* | 14.91 ± 0.32* | 16.23 ± 0.92* | 14.41 ± 0.61* |
| Control | 4.94 ± 0.76 | 5.14 ± 0.43 | 9.05 ± 0.51 | 10.15 ± 0.36 | 12.43 ± 0.95 | 13.63 ± 0.67 | 11.18 ± 0.21 | |
| LSC99I | Vaccine | 4.94 ± 0.76 | 7.56 ± 0.49* | 10.21 ± 0.22* | 11.01 ± 0.22* | 13.25 ± 0.70 | 12.42 ± 0.67 | 11.09 ± 0.53 |
| Control | 4.94 ± 0.76 | 5.14 ± 0.43 | 9.05 ± 0.51 | 10.15 ± 0.36 | 13.07 ± 0.51 | 13.88 ± 0.40 | 14.37 ± 0.53 | |
| LTJ95I | Vaccine | 4.94 ± 0.76 | 7.56 ± 0.49* | 10.21 ± 0.22* | 11.01 ± 0.22* | 11.59 ± 0.54 | 12.10 ± 0.61 | 11.43 ± 1.80 |
| Control | 4.94 ± 0.76 | 5.14 ± 0.43 | 9.05 ± 0.51 | 10.15 ± 0.36 | 11.03 ± 0.56 | 11.39 ± 0.71 | 12.11 ± 2.28 | |
Values are mean ± S.E.M.
Value is significantly different from corresponding control value (p < 0.05).
Chickens (n = 12 per group) were immunized with recombinant fowlpox virus co-expressing the infectious bronchitis virus (IBV) S1 gene and the chicken interferon-γ gene at week 0.
Chickens (n = 12 per group) were challenged with 0.2 mL of allantoic fluid containing 10,000 EID50 of IBV strains LX4, LHLJ04XI, LHB, LSC99I and LTJ95I at week 3 post-vaccination.
Blood lymphocytes were harvested at weeks 0, 1, 2, 3, 4, 5 and 6.
Control chickens received PBS (n = 12 per group).
3.4. Protection of chickens against IBV challenge
Following challenge with five IBV strains LX4, LHLJ04XI, LHB, LSC99I and LTJ95I, 9/12, 8/11, 7/10, 5/10 and 6/11 chickens in these five vaccination groups, respectively, were protected from clinical signs of IB, and 12/12, 10/11, 9/10, 8/10 and 8/11 of the chickens in these five groups survived. In contrast, chickens (10/12, 11/11, 10/10, 10/10 and 8/11) in the control groups developed clinical signs after challenge with these five IBV strains (Fig. 1 ). All deaths occurred between days 3 and 5 post-challenge. Necropsies revealed typical pathology of acute IB, which was restricted to the upper respiratory tract and kidneys, such as the presence of mucus in the upper respiratory tract, swelling of the throat, bleeding in the trachea and swollen, gray and mottled kidneys.
Fig. 1.
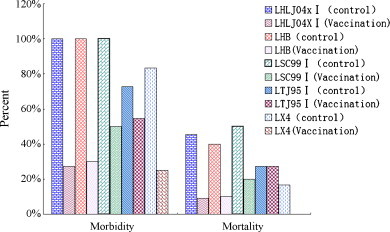
Protection percentages of chickens following immunization with recombinant fowlpox virus co-expressing the infectious bronchitis virus (IBV) S1 gene and the chicken interferon-γ gene at week 0 and challenged with 0.2 mL of allantoic fluid containing 10,000 EID50 of IBV strains LX4, LHLJ04XI, LHB, LSC99I and LTJ95I at week 3 post-vaccination. The chickens were observed daily for clinical symptoms. The clinical symptoms of morbid chicken included apathy, cough, mouth breathing and extension of the neck.
3.5. Virus isolation
From days 3 to 13 after challenge, swabs from five chickens randomly selected from each group were sampled for virus isolation. Table 6 shows that challenge virus was recovered from laryngeal swabs of the vaccinated groups that were challenged with strains LHLJ04XI, LHB and LX4 from days 3 to 6, but from days 3 to 10 in the other groups. The duration of virus shedding in the vaccinated groups that were challenged with strains LHLJ04XI, LHB and LX4 was shorter than those of the other groups.
Table 6.
Isolation of virus from chickens challenged with IBV strains LX4, LHLJ04XI, LHB, LSC99I and LTJ95I.a
| Strain | Group | 3 Days after challenge | 6 Days after challenge | 10 Days after challenge | 13 Days after challenge |
|---|---|---|---|---|---|
| LHLJ04XI | Vaccineb | 2/5c | 3/5 | 0/5 | 0/5 |
| Controld | 3/5 | 5/5 | 2/5 | 0/5 | |
| LHB | Vaccine | 3/5 | 2/5 | 0/5 | 0/5 |
| Control | 4/5 | 5/5 | 1/5 | 0/5 | |
| LSC99I | Vaccine | 4/5 | 3/5 | 1/5 | 0/5 |
| Control | 4/5 | 5/5 | 2/5 | 0/5 | |
| LTJ95I | Vaccine | 3/5 | 4/5 | 1/5 | 0/5 |
| Control | 3/5 | 5/5 | 2/5 | 0/5 | |
| LX4 | Vaccine | 3/5 | 3/5 | 0/5 | 0/5 |
| Control | 3/5 | 4/5 | 1/5 | 0/5 |
Each chicken was challenged with 0.2 mL of allantoic fluid containing 10,000 EID50 of IBV strain on day 21 post-immunization. On days 3, 6, 10, and 13 after challenge, throat swabs were taken from each IBV-challenged chicken for IBV isolation.
Chickens were immunized with recombinant fowlpox virus (rFPV-IFNγS1) co-expressing the infectious bronchitis virus (IBV) S1 gene and the chicken interferon-γ gene.
The number of chickens shedding IBV/the total number of chickens in this group.
Chickens were immunized with PBS.
3.6. Influence of IFNγ on body weights
There were no significant differences in the body weights between the vaccinated and the control groups (Table 7 ; All p > 0.05) when chicken body weights were checked on the day before vaccination, on day 10 post-vaccination, on the day before challenge and on day 10 post-challenge.
Table 7.
| Strain | Group | Before immunization | 10 Days after immunization | Before challenged | 10 Days after challenged |
|---|---|---|---|---|---|
| LHLJ04XI | Vaccinec | 147.80 ± 12.66d | 250.60 ± 21.15 | 404.90 ± 55.06 | 672.40 ± 42.27 |
| Controle | 141.70 ± 15.82 | 241.10 ± 24.19 | 413.50 ± 40.66 | 590.80 ± 66.26 | |
| LHB | Vaccine | 143.10 ± 18.36 | 229.10 ± 25.41 | 420.00 ± 38.59 | 620.20 ± 46.07 |
| Control | 120.70 ± 21.18 | 248.20 ± 28.47 | 406.00 ± 53.39 | 523.33 ± 48.27 | |
| LSC99I | Vaccine | 113.33 ± 23.76 | 235.90 ± 18.79 | 425.30 ± 40.47 | 645.28 ± 81.84 |
| Control | 152.22 ± 14.59 | 228.10 ± 36.98 | 403.10 ± 51.69 | 595.57 ± 78.84 | |
| LTJ95I | Vaccine | 153.90 ± 18.40 | 242.80 ± 28.02 | 444.40 ± 48.92 | 574.50 ± 72.83 |
| Control | 143.40 ± 17.72 | 227.70 ± 33.26 | 398.70 ± 67.95 | 540.00 ± 115.97 | |
| LX4 | Vaccine | 145.40 ± 16.45 | 247.15 ± 26.14 | 436.85 ± 46.24 | 648.24 ± 65.25 |
| Control | 137.82 ± 15.24 | 238.40 ± 22.40 | 419.74 ± 41.25 | 602.45 ± 50.14 |
Each chicken was challenged with 0.2 mL of allantoic fluid containing 10,000 EID50 of virulent IBV strain on day 21 post-immunization.
Data were analyzed by ANOVA using the STATISTICA software. There was no significant difference between mean values of body weights of immunized chickens and the control chickens measured on the same day in the same group in this study (all p > 0.05).
Chickens were immunized with recombinant fowlpox virus (rFPV-IFNγS1) co-expressing the infectious bronchitis virus (IBV) S1 gene and the chicken interferon-γ gene.
Mean value of body weights ± standard deviation (g).
Chickens were immunized with PBS.
4. Discussion
The IBV S1 protein is a critical immunogenic protein for induction of neutralizing antibodies against IBV [17], [18], [19]. It also plays an important role in the serologic classification of the virus. In recent years, S1 gene has become established as the major gene for recombinant vaccine research against IBV infection. In this study, a recombinant fowlpox virus (rFPV-IFNγS1) that co-expressed the IBV S1 gene and the chicken interferon-γ gene was used to vaccinate SPF chickens. The results showed that the recombinant virus protected immunized chickens against challenge with IBV strains LHLJ04XI and LHB, which are closely related to IBV strain LX4. The rates of protection were 72.72% (8/11) and 70% (7/10), which were not significantly different from 75% of IBV strain LX4 (9/12) [5]. However, the rates of protection conferred by the vaccine against IBV strains LSC99I and LTJ95I were only 50% (5/10) and 54.55% (6/11), respectively, and were significantly different (p < 0.05) from that of IBV strain LX4. Therefore, the recombinant vaccine protected chickens challenged with strains that were closely related genetically to the vaccine strain. These results above demonstrated that the recombinant fowlpox virus co-expressing the chicken IFN-γ gene and IBV S1 gene induced strong and reliable immunoprotection, and reduced the growth inhibitory effect of the avian pox virus vector.
Fowlpox virus is known to replicate only in avian cells; it undergoes abortive replication in non-avian cells and cannot be adapted to produce an infective progeny virus in mammalian tissue [20], [21]. Therefore, FPV has been developed as a non-replicating vector for vaccination of mammals [22], [23]. More sophisticated vaccination strategies have included the expression of multiple distinct antigens. Previous studies have successfully constructed a recombinant FPV virus that co-expressed three foreign genes (from two different avian pathogens) [24] and a foreign reporter gene at a single non-essential gene site. Although this recombinant gave rise to few side effects, it still showed slight inhibition of weight gain. Researchers have explored different means to reduce the side effects of recombinant fowlpox virus, one of which is to introduce immune regulatory cytokines to the recombinant vaccine. Interferon is an important cytokine, and chicken IFN-γ is an immune regulator that is secreted by antigen or mitogen-activated T lymphocytes. It not only has immunomodulatory activity but also acts to promote increased body weight and to reduce the effects of stress [25], [26]. There were no significant differences in the body weights between the vaccinated and the control groups in this study, indicating that co-expression of the chicken IFN-γ gene with IBV S1 gene played a role in immune regulation, and reduced the side effects associated with the fowlpox virus vector.
Subgroups of T lymphocytes play a major role in the induction of cellular immunity. When they are stimulated by antigen, the numbers and immune activity of various types of functional T lymphocytes change dramatically. CD4+ T cells can induce and enhance the immune response, secrete a variety of cytokines with immune activity, and stimulate the activation, proliferation, and production of specific antibodies by B lymphocytes. In this study, the number of CD4+ T lymphocytes increased in the vaccinated groups, and the level was higher than that in the control groups; the number of CD4+ T lymphocytes continued to rise after challenge. However, in the third week after challenge, the CD4+ T lymphocyte percentages of the vaccinated groups that were challenged with IBV strains LHLJ04XI, LHB and LX4 were significantly higher than those in the vaccinated groups challenged with IBV strains LSC99I and LTJ95I, indicating that vaccination or challenge with closely related strains promoted B lymphocyte activation and antibody production, as also shown by antibody production against IBV.
Cytotoxicity mediated by CD8+ T cells can inhibit the injury caused by virus infection. Song et al. discussed the cellular immune response to IBV infection [27]. In order to study the mechanism of immune protection, they transplanted T cells into chickens after removal of the CD4+ or CD8+ cells. The T cells were stimulated by IBV, and the chickens were challenged with IBV strain. The results showed that after the removal of CD4+ cells chickens could be protected against challenge with IBV strain G; virus loads in the lung and kidney were reduced. However, chickens in the group with depletion of CD8+ cells and also the control groups were not provided with the same protection; virus could be re-isolated from lung and kidney tissue. These findings confirmed the protective role of CD8+ cells in acute IBV infection. In this study, the number of CD8+ T lymphocytes in the groups vaccinated with the recombinant vaccine was significantly higher than that in the control groups, indicating that cellular immunity was induced. After challenge, the level of CD8+ T lymphocytes in the vaccinated groups challenged with IBV strains LHLJ04XI and LHB was significantly higher than those in the groups challenged with IBV strains LSC99I and LTJ95I. This may be because the recombinant vaccine could not protect against infection with distantly related viruses, leading to serious damage to immune cells, which is consistent with the previous findings [27]. Pathological damage in the immunized groups challenged with IBV strains LX4, LHLJ04XI and LHB was significantly less severe than that in the control group and the groups challenged with IBV strains LSC99I and LTJ95I, again indicating that the recombinant vaccine in this study provided good protection against closely related challenge viruses.
IBV has a number of different serotypes. The analysis of the amino acid sequence of the S1 protein in the different serotypes and strains, and the cross-protection results produced by vaccines of different strains show that there is no correspondence between IBV serotypes and a variety of clinical phenotypes [28]. Ignjatovic and Galli reported that the conventional attenuated vaccines were able to provide effective protection against the different serotypes of IBV [19]. There is cross-protection between some kidney-type strains of the virus and certain respiratory virus strains. However, some respiratory viruses may not exert a cross-protective effect, or may show a poor cross-protective effect. This may be caused by the different origins of the strains or differences in the genes encoding major protective antigens. Of the five IBV strains selected for the experimental challenge in this study, strain LX4 was a reference strain and the other four strains were isolated in different regions in China. They were all the kidney-selective pathogenic type, but from the perspective of genetics, the four strains had very different relationships with the reference strain LX4, which led to the different cross-protective effects. The results in this study show that the recombinant vaccine protected immunized chickens from challenge with IBV strains LHLJ04XI and LHB and there were no significant differences in morbidity and mortality when immunized chickens were challenged either with these IBVs or with the reference strain LX4 whereas the protection conferred by rFPV-IFNγS1 was incomplete in chickens challenged with IBV strains LSC99I and LTJ95I, indicating that the recombinant vaccine incorporating the IBV S1 gene and the chicken interferon-γ gene provided effective immune protection against challenge with heterogeneous IBV strains that have closer phylogenetic relationship in S1 gene.
Acknowledgement
This research was supported by the National High-Tech Research and Development Program of China (863 Plan) (No. 2003AA213021).
References
- 1.Cavanagh D., Naqi S.A. Infectious bronchitis. In: Saif Y.M., Barnes H.J., Fadly A.M., Glisson J.R., McDougald L.R., Swayne D.E., editors. Diseases of poultry. 11th ed. Iowa State University Press; Ames, USA: 2003. pp. 101–119. [Google Scholar]
- 2.Stohlman S.A., Kyuwa S., Cohen M., Bergmann C., Polo J.M., Yeh J. Mouse hepatitis virus nucleocapsid protein-specific cytotoxic T lymphocytes are Ld restricted and specific for the carboxy terminus. Virology. 1992;189:217–224. doi: 10.1016/0042-6822(92)90697-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Z.Y., Kong W.P., Huang Y., Roberts A., Murphy B.R., Subbarao K. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428:561–564. doi: 10.1038/nature02463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webster D.P., Dunachie S., Vuola J.M., Berthoud T., Keating S., Laidlaw S.M. Enhanced T cell-mediated protection against malaria in human challenges by using the recombinant poxviruses FP9 and modified vaccinia virus Ankara. PNAS. 2005;102:4836–4842. doi: 10.1073/pnas.0406381102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian Z.C., Sun Y.K., Wang Y.F., Zhi H.D., Liu S.W., Sun H.L. The immunological efficacies of recombinant fowlpox virus expressing the S1 gene of LX4 strain of infectious bronchitis virus in Specific Pathogen Free (SPF) chickens. Acta Vet Zootech Sin. 2006;37:580–586. [Google Scholar]
- 6.Karaca K., Sharma J.M., Winslow B.J., Junker D.E., Reddy S., Cochran M. Recombinant fowlpox viruses coexpressing chicken type I IFN and Newcastle disease virus HN and F genes: influence of IFN on protective efficacy and humoral responses of chickens following in ovo or post-hatch administration of recombinant viruses. Vaccine. 1998;16:1496–1503. doi: 10.1016/s0264-410x(97)00295-8. [DOI] [PubMed] [Google Scholar]
- 7.Lambrecht B., Gonze M., Morales D., Meulemans G., Vanden B. Comparison of biological activities of natural and recombinant chicken interferon-gamma. Vet Immunol Immunopathol. 1999;70:257–267. doi: 10.1016/s0165-2427(99)00080-x. [DOI] [PubMed] [Google Scholar]
- 8.Rautenschlein S., Sharma J.M., Winslow B.J., McMillen J., Junker D., Cochran M. Embryo vaccination of turkeys against Newcastle disease infection with recombinant fowlpox virus constructs containing interferons as adjuvants. Vaccine. 1999;18:426–433. doi: 10.1016/s0264-410x(99)00254-6. [DOI] [PubMed] [Google Scholar]
- 9.Guo X.B., Liu X., Pu J., Liu Q.F., Wu Q.M., Liu J.H. Different genotypes of nephropathogenic infectious bronchitis viruses co-circulating in chicken population in China. Virus Genes. 2007:333–337. doi: 10.1007/s11262-007-0100-5. [DOI] [PubMed] [Google Scholar]
- 10.Liu S.W., Zhang Q.X., Chen J.D., Han Z.X., Liu X., Feng L. Genetic diversity of avian infectious bronchitis corona virus strains isolated in China between 1995 and 2004. Arch Virol. 2006;151:1133–1148. doi: 10.1007/s00705-005-0695-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun Y.K., Tian Z.C., Wang Y.F., Tong G.Z., Zhi H.D., Liu S.W. Co-expression of S1 gene of infectious bronchitis virus and chicken type II interferon gene in recombinant fowlpox virus. Acta Vet Zootech Sin. 2005;36:800–806. [Google Scholar]
- 12.Toro H., Espnoza C., Ponce V., Rojas V., Morales M.A., Kaleta E.F. Infectious bronchitis: effect of vial doses and routes on specific lacrimal and serum antibody responses in chicken. Avian Dis. 1997;41:379–387. [PubMed] [Google Scholar]
- 13.Collisson E.W., Pei J., Dzielawa J., Seo S.H. Cytotoxic T lymphocytes are critical in the control of infectious bronchitis virus in poultry. Dev Comp Immunol. 2000;24:187–200. doi: 10.1016/s0145-305x(99)00072-5. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L., Jin N., Song Y., Wang H., Ma H., Li Z. Construction and characterization of a recombinant fowlpox virus containing HIV-1 multi-epitope-p24 chimeric gene in mice. Sci China Ser C: Life Sci. 2007;50:212–220. doi: 10.1007/s11427-007-0017-1. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y.F., Shi X.M., Wang M., Liu S.W., Tong G.Z., Cui H.Y. Partial biological character detection of fowlpox virus recombinant co-expressing the IBV-S1gene and chicken IFN-γ gene. Acta Vet Zootech Sin. 2007;38:1077–1082. [Google Scholar]
- 16.StatSoft Inc. STATISTICA (data analysis software system), Version 6.1. Tulsa, OK: StatSoft Inc.; 2003.
- 17.Cavanagh D., Darbyshire J.H., Davis P.J., Peters R.W. Induction of humoral neutralizing and haemagglutination-inhibiting antibody by the spike protein of avian infectious bronchitis virus. Avian Pathol. 1984;13:573–583. doi: 10.1080/03079458408418556. [DOI] [PubMed] [Google Scholar]
- 18.Cavanagh D., Davis P.J., Darbyshire J.H., Peters R.W. Coronavirus IBV: virus retaining spike glycopolypeptide S2 but not S1 is unable to induce virus-neutralizing or haemagglutination-inhibiting antibody, or induce chicken tracheal protection. J Gen Virol. 1986;67:1435–1442. doi: 10.1099/0022-1317-67-7-1435. [DOI] [PubMed] [Google Scholar]
- 19.Ignjatovic J., Galli L. The S glycoprotein but not the N or M protein of avian infectious bronchitis virus induces protection in vaccinated chickens. Arch Virol. 1994;138:117–134. doi: 10.1007/BF01310043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor J., Paoletti E. Fowlpox virus as a vector in non-avian species. Vaccine. 1988;6:466–468. doi: 10.1016/0264-410x(88)90091-6. [DOI] [PubMed] [Google Scholar]
- 21.Taylor J., Weinberg R., Languet B., Desmettre P., Paoletti E. Recombinant fowlpox virus inducing protective immunity in non-avian species. Vaccine. 1988;6:497–503. doi: 10.1016/0264-410x(88)90100-4. [DOI] [PubMed] [Google Scholar]
- 22.Jin N.Y., Zhang H.Y., Yin G.F., Zheng M., Liu T., Jiang W.Z. Immunogenicity of recombinant fowl-pox virus co-expressing structural protein precursor P1-2A and proteinase 3C of FMDV. Chin Sci Bull. 2004;49:823–827. [Google Scholar]
- 23.Li X., Jin N.Y., Lian H., Guan G.F., Sun L.L., Li X.M. Construction and anti-tumor effects of recombinant fowlpox virus expressing Newcastle disease virus hemagglutinin-neuramidinase gene. Chin Sci Bull. 2006;51:2724–2730. [Google Scholar]
- 24.Sun H.L., Wang Y.F., Tong G.Z., Zhang P.J., Miao D.Y., Zhi H.D. Protection of chickens from Newcastle disease and infectious laryngotracheitis with a recombinant fowlpox virus co-expressing the F, HN genes of Newcastle disease virus and gB gene of infectious laryngotracheitis virus. Avian Dis. 2008;52:111–117. doi: 10.1637/7998-041807-Reg. [DOI] [PubMed] [Google Scholar]
- 25.Leong K.H., Ramsay A.J., Boyle D.B., Ramshaw I.A. Selective induction of immune responses by cytokines coexpressed in recombinant fowlpox virus. J Virol. 1994;68:8125–8130. doi: 10.1128/jvi.68.12.8125-8130.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lillehoj H.S., Choi K.D. Recombinant chicken interferon-gamma-mediated inhibition of Eimeria tenella development in vitro and reduction of oocyst production and body weight loss following Eimeria acervulina challenge infection. Avian Dis. 1998;42:307–314. [PubMed] [Google Scholar]
- 27.Song C.S., Lee Y.J., Lee C.W., Sung H.W., Kim J.H., Mo I.P. Induction of protective immunity in chickens vaccinated with infectious bronchitis virus S1 glycoprotein expressed by a recombinant baculovirus. J Gen virol. 1998;79(Pt 4):719–723. doi: 10.1099/0022-1317-79-4-719. [DOI] [PubMed] [Google Scholar]
- 28.Wang C.H., Huang Y.C. Relationship between serotypes and genotypes based on the hypervariable region of the S1 gene of infectious bronchitis virus. Arch Virol. 2000;145:291–300. doi: 10.1007/s007050050024. [DOI] [PMC free article] [PubMed] [Google Scholar]


