Highlights
-
•
Recombinant porcine IL-22 has been expressed with activation of STAT3 signal.
-
•
pIL-22 reversed apoptosis induced by DON and protected the integrity of epithelial.
-
•
pIL-22 defended ETEC K88 infection with increased pBD-1 expression.
Keywords: Porcine interleukin 22, Deoxynivalenol, ETEC K88, Intestinal epithelial cells
Abstract
Pigs suffer enteritis induced by pathogenic bacteria infection and toxins in the moldy feed, which cause intestinal epithelial damage and diarrhea through the whole breeding cycle. Interleukin-22 (IL-22) plays a critical role in maintaining intestinal mucosal barrier function through repairing intestinal epithelial damage. However, little was known about the effects of IL-22 against apoptosis caused by toxins and infection of intestinal pathogens in the intestinal epithelium, especially in pigs. In this study, we had successfully used prokaryotic expression system to produce recombinant porcine interleukin-22. Meanwhile, purified rIL-22 could activate STAT3 signal pathway and have been demonstrated to be safe to IPEC-J2 cells by increasing E-cadherin expression, without proinflammatory cytokines changes. Furthermore, rIL-22 reversed apoptosis induced by deoxynivalenol (DON) and played a vital part in repairing the intestinal injury. We also found that rIL-22 stimulated epithelial cells to secrete pBD-1 against enterotoxigenic E. coli (ETEC) K88 infection, as well as alleviating apoptosis ratio. This study provided a theoretical basis for curing intestinal inflammation caused by ETEC infection and epithelial apoptosis induced by DON with rIL-22 in pigs.
1. Introduction
Interleukin-22 (IL-22), a member of the IL-10 cytokine family, mainly produced by the lymphatic system cells of innate and adaptive immunity, including αβ T cells, γδ T cells, NKT cells, and innate lymphoid cells (ILCs) (Dudakov et al., 2015). IL-22 acts biology function via the signal transduction in immune response by heterodimeric receptor complex consisting of IL-22R1 and IL-10R2 (Witte et al., 2010). In combined with receptor complex, IL-22 activates the receptor-associated Janus kinases, Jak1 and Tyk2, and then leads to the phosphorylation of STAT3 and activation of p38 mitogen activated protein kinase pathway (Lejeune et al., 2002). IL-22 has been described as the cytokine of epithelium protection, which has been widely studied for its different effects on cell proliferation, tissue regeneration, cellular defense and inflammation (Lindemans et al., 2015). Especially, IL-22 in the gut is able to stimulate intestinal epithelial cells to produce antimicrobial peptides, enhance the intestinal epithelial barrier of mucus and promote intestinal repair (Hou et al., 2018; Parks et al., 2015). Pathogens such as enterotoxigenic Escherichia coli (ETEC) and mycotoxin in contaminated feed are destructive to intestinal epithelial cells, which cause enteritis and seriously threatens gut health of pigs (Alizadeh et al., 2015; Lee et al., 2016; Zhang et al., 2018).
Deoxynivalenol (DON) is a mycotoxin of the trichothecenes family that is one of the most common mycotoxin in animal feed (Bouhet and Oswald, 2005). Pigs are the most sensitive species to DON, which can cause abdominal distress, diarrhea, vomiting and even shock or death (Pinton and Oswald, 2014). Besides, DON can induce apoptosis and breakdown intestinal barrier integrity in epithelial cells (Akbari et al., 2014; Ma et al., 2012), which increases the susceptibility of animals to intestinal pathogen infection (Antonissen et al., 2014). Besides feeds, enteropathogens, such as enterotoxigenic Escherichia coli (ETEC) K88, commonly induce diarrhea in piglets, which cause mortality and reduce growth rate, resulting in heavy economic losses (Jin and Zhao, 2000). Recent studies demonstrated that IL-22 could effectively suppress infection of several intestinal viruses through activation of STAT3 signaling (Xue et al., 2017).Moreover, IL-22 also activated STAT3 to induce the expressions of transcription factors, such as Pou5f1, Sox2, and Nanog, and promoted tumor progression in human colorectal cancer cells (Koltsova and Grivennikov, 2014; Kryczek et al., 2014). However, IL-22-STAT3 pathway plays a protective role in intestinal epithelium during colitis by promoting expression of genes involved in apoptosis, proliferation, migration, and survival (Akira, 2000; Chung et al., 2006). Studies have shown that STAT3 activation in intestinal epithelial is protective of mucosal wound healing and (Pickert et al., 2009; Sugimoto et al., 2008). Previous researches reported that IL-22 could modulate antimicrobial peptides production to guarantee barrier functions (Mulcahy et al., 2016; Sovran et al., 2015; Treerat et al., 2017). However, it is still unclear whether porcine IL-22 has a protective role against intestinal epithelial damage.
Therefore, we hypothesized that recombinant porcine IL-22 would have the positive effect on intestinal injury, which can effectively rescue the apoptosis induced by DON via STAT3 signal pathway and stimulate epithelial cells to secrete antimicrobial peptides against infection of ETEC K88.
2. Material and methods
2.1. Cells culture and reagents
The porcine intestinal epithelial cells IPEC-J2 (GuangZhou Jennio Biotech Co, China) were cultured in 5% CO2 at 37℃ in high-glucose Dulbecco’s Modified Eagle’s Medium (DMEM, GIBCO) containing 10% fetal bovine serum (FBS, GIBCO). Medium was changed every 3 days and cells were passed once a week. For CCK8 assays, cells were seeded in 96-well plates (Costar, USA). For total protein extraction, cells were seeded in 6-well plates (Costar, USA). ETEC K88 strain comes from pig and stocked in our lab, which contains toxins (heat-labile enterotoxin 1, LT1; heat-stable enterotoxin, ST; enteroaggregative heat-stable toxin1, EAST1), adhesins (F4) and hemolysins (α-hly) (Yu et al., 2012; Zhou et al., 2014).
DON (Sigma, USA) was dissolved in DMSO (Sigma, USA) to a 5 mM stock solution at −20 °C and working dilutions were prepared in cell culture medium. Control samples were treated with DMSO in equivalent concentrations.
2.2. ETEC K88 and culture conditions
ETEC K88 was grown in Luria-Bertani (LB) broth. Bacterial titers (colony forming units, CFU) were determined by serial dilution and plating onto solid medium. Bacteria were harvested during mid log phase by centrifugation at 3000 × g for 10 min and then resuspended in DMEM medium free of antibiotics.
2.3. Clone and prokaryotic expression of rIL-22
The gene encoding porcine IL-22 (GenBank: KX588234.1) was gained from artificial synthesis (Genscript Biotech Corporation, China), contained Hind Ⅲ and Kpn I restriction enzyme sites (TaKaRa, Japan) and homologous arm of pET-32a, which was cloned into a pET-32a vector containing a 6-histidine tag at the N-terminus by Hind Ⅲ and Kpn I through One Step Cloning Kit (Vazyme, China). The recombinant plasmid pET-32a-IL-22 was transformed into BL21 (DE3). A colony of positive cells was selected on LB agar plates containing ampicillin (100 μg/ml) and screened by direct colony PCR with IL-22 primer (Table 1 ). The extracted plasmid designated pET-32a-IL-22 was subjected to DNA sequencing by Genscript, Nanjing, China.
Table 1.
Primers used for RT-PCR.
| Gene | Primer Sequence (5’-3’ Product Size | Reference | |
|---|---|---|---|
| β-actin | F: AGATCAAGATCATCGCGCCT | 170 bp | Xia et al. (2017) |
| R: ATGCAACTAACAGTCCGCCT | |||
| IL-6 | F:CCTCGGCAAAATCTCTGCAA | 189 bp | Xia et al. (2017) |
| R: TGAAACTCCACAAGACCGGT | |||
| IL-8 | F: CCTCATTCCTGTGCTGGTCA | 273 bp | Xia et al. (2017) |
| R: TGCAAGTTGAGGCAAGAAGAC | |||
| TNF-α | F: GCCCTTCCACCAACGTTTTC | 158 bp | Xia et al. (2017) |
| R: TCCCAGGTAGATGGGTTCGT | |||
| pBD-1 | F: CTCCTCCTTGTATTCCTCCT | 141 bp | Wan et al. (2013) |
| R: GGTGCCGATCTGTTTCAT | |||
| pBD-2 | F: GACTGTCTGCCTCCTCTC | 148 bp | Wan et al. (2013) |
| R: GGTCCCTTCAATCTGTTG | |||
| IL-22 | F: CCCAGATCTGGGTACCATGGTCCCG | 477bp | primer premier 5.0 |
| R: GCCTTTAATACGACATTGGGACAGTT | |||
Supplementary Fig. 1 verification of rIL-22. (A) Identification of pET32a-IL-22 and double-enzyme digestion. lane 1 shows the recombinant plasmid of pET32a-IL-22; lane 2 shows the products after pET32a-IL-22 was digested by Kpn I and Hind III, Lane 3 shows the DNA marker; (B) Verification of colony PCR. Lane1 shows the DNA marker; lane 2 shows negative control; lane 3–10 show positive colony. (C) Western blot analysis of expressed recombinant rIL-22. Lane M2: Western blot marker; Lane 3: Supernatant of cell lysate with induction for 16 h at 15 °C; Lane 4: Pellet of cell lysate with induction for 16 h at 15 °C; Lane 5: Supernatant of cell lysate with induction for 4 h at 37 °C; Lane 6: Pellet of cell lysate with induction for 4 h at 37 °C.
pET-32a-IL-22 transformed cells were cultured in LB medium containing ampicillin (100 μg/ml) (GIBICO, USA) at 37℃ with shaking until the optical density of the culture at 600 nm reached 0.5. Isopropyl β-d-Thiogalactoside (IPTG) (Sigma, USA) was then added to a final concentration of 1 mM to induce expression at 37℃ for 4 h or 15℃ for 16 h. The empty pET−32a vector transformed culture was used as control. The bacteria were pelleted at 8000×g, at 4℃ for 5 min, then pellets were resuspended in buffer I (50 mM Tris-HCl, 2 mM EDTA, 300 mM NaCl, 0.5% Triton X-100, 1 mM PMSF, 1 mg/ml lysozyme, pH 8.0) and was sonicated on ice five times, each for 30 s with 30 s intervals. After sonication, the lysate was centrifuged at 5,000 rpm for 10 min, and supernatant was collected and pellet was softly resuspended in buffer II (50 mM Tris-HCl, 2 mM EDTA, 300 mM NaCl, 0.5% Triton X-100, 2 M urea, pH 8.0). The cell lysate, supernatant and pellet of cell lysate were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis.
Due to rIL-22 was mainly in the form of inclusion body verified by SDS-PAGE and western blot analysis, the inclusion body was denatured in the buffer III (50 mM Tris-HCl, 300 mM NaCl, 100 mM GSH, 8 M urea, pH 8.0) and put into a dialysis bag. The purified protein-containing dialysis bag was put into renaturation solution (50 mM Tris-HCl, 300 mM NaCl, 0.5% Triton X-100, 0.5 M arginine, 3 mM GSH, 0.6 mM GSSG, 8 M urea, pH 8.0) at 4 ℃ for 12 h, then treated with renaturation solution by decreasing urea gradient ranged from 8 M, 6 M, 4 M, 2 M to 1 M for 12 h respectively. The protein was pelleted at 8000×g, at 4℃ for 10 min.
2.4. Cell viability assay
The activity of IPEC-J2 cells was assayed using the Cell Counting Kit (CCK-8) (Dojindo, Japan) method. IPEC-J2 cells were plated at a density of 4 × 105 cells/ml in 96-well plates at 37 °C and treated with 25 μM and 50 μM DON for 48 h, followed by treatment with 10 μl/well CCK8 solution (5 mg/ml). The cells were incubated for an additional 2 h. The OD value was read at 570 nm on a microplate reader. The cell viability (%) was calculated as the percent ratio of absorbance of the samples against the non-treated control medium.
2.5. RNA extraction and RT-PCR
IPEC-J2 cells were harvested and the total RNA was extracted using TRIzol (Invitrogen) according to manufacturer’s instructions. The concentration of total RNA was measured by a NanoDrop-ND1000 spectrophotometer (Thermo Fisher Scientific, USA). Then cDNA was synthesized using HiScript II Q RT SuperMix for qPCR (Vazyme, China). Quantitative RT-PCR was performed using ChamQ SYBR qPCR Master Mix (Vazyme, China) and primers (Genscript, China) used for this study were listed in Table 1. Levels of mRNA were calculated using 2−ΔΔCt method and normalized to those of GAPDH mRNA.
2.6. Western blot analysis
Protein lysates were obtained from IPEC-J2 cells using ice-cold lysis RIPA buffer (Solarbio, China) containing 10 mM PMSF (Solarbio, China), followed by a quantitation of protein using a BCA protein assay kit (Thermo Scientific, USA). Cell lysates that contained equal amounts of protein were separated by SDS-PAGE and transferred to a PVDF membrane (Millipore, USA). Membranes were blocked with 5% bovine serum albumin (BSA) for 2 h at room temperature. The blotted membranes were probed with primary antibodies at 4 ℃ overnight: p-STAT3 (Tyr705) (Cell Signaling Technology, 1:2000); STAT3 (Cell Signaling Technology, 1:1000); ZO-1 (Thermo Scientific, 1:2000); E-cadherin (Abcam, 1:1000) GAPDH (Abcam, 1:10,000) and β-Tubulin (Vazyme, 1:1000); Subsequently, the membranes were washed and incubated with goat anti-rabbit or goat anti-mouse IgG-HRP (Vazyme, 1:5000) for 2 h at room temperature. Membranes were then washed four times in TBST buffer (5 min per wash) (Solarbio, China). The target protein was visualized with enhanced chemiluminescence (ECL) system (Bio-Rad, USA), followed by analysis using Quantity One software analysis (Bio-Rad, USA).
2.7. Assessment of apoptosis by flow cytometry
In order to evaluate the protective effect of rIL-22 against DON, IPEC-J2 cells were first treated with DON (25 μM) for 24 h and then cocultured with 100 ng/ml rIL-22 for another 24 h, while cells were treated with rIL-22 for 24 h or DON for 48 h respectively in other groups. For ETEC K88 study, IPEC-J2 cells were first treated with rIL-22 (10 or 100 ng/ml) for 24 h and followed with ETEC K88 (MOI = 100) for another 2.5 h, while cells were treated with rIL-22 for 12 h or ETEC K88 for 2.5 h respectively in other groups.
Treated cells were harvested and labeled with an anti-Annexin V-FITC Apoptosis Detection Kit (Vazyme, China). Floating cells were collected, then attached cells were washed with 0.01 M PBS and trypsinized for 5 min. Finally, trypsinized cells and floating cells were added together to centrifugate at 300×g for 10 min, then stained with AnnexinV-FITC and propidium iodide (PI). The intensity of the markers was examined by flow cytometry (FACSCantoII, BD Biosciences, USA). All flow cytometric data were analyzed by using FlowJo software (BD Biosciences, USA).
2.8. Adhesion of ETEC K88
IPEC-J2 cells were grown to confluency in 12-well tissue-culture plates, washed three times with antibiotic free medium, added to 1 ml/well DMEM/F12 medium (without serum and antibiotics), pretreated with 100 ng/ml rIL-22 for 12 h and PBS as a control for 12 h. After 12 h, incubated with 10 ul DMEM/F12 medium (without serum and antibiotics) containing ETEC K88 (MOI = 100) for 2.5 h at 37℃. The monolayers were washed three times with DMEM/F12 medium and lysed in 0.5% Triton X-100 in PBS at room temperature for 5 min in order to release the bacteria. The suspensions were serially diluted with sterile distilled water and 100 μl of each dilution was plated on solid LB broth medium. The plates were incubated for 12 h at 37℃. The number of adhering bacteria was determined by plating colony-counting methods.
2.9. Statistical analysis
Results are expressed as the means ± standard deviation from three independent experiments. Statistical analyses were performed using GraphPad Prism with Student’s t-test. Differences were considered significant at *P < 0.05, **P < 0.01, *** P < 0.001.
3. Results
3.1. Expression and purification of recombinant IL-22
Firstly, we successfully constructed recombinant plasmid pET32a-pIL-22 (Fig. 1 a), confirmed by restriction enzyme reaction (Supplementary Fig. 1a). The recombinant plasmid was transformed into BL21 (DE3) and verified by colony PCR (Supplementary Fig. 1b). After induction by IPTG, pIL-22 is mainly detected in the form of inclusion body in cell lysis through SDS-PAGE (Fig. 1b) and Western blot (Supplementary Fig. 1c). The protein was approximate 35.6 kDa as expected and the expression of the protein peaked at 15℃ 16 h or 37℃ 4 h post induction. After denaturing in urea, gradient dialysis and purified by a Ni–NTA column, refolded porcine IL-22 is obtained and the protein purity reached 95.7% through Quantity One software analysis (Bio-Rad, USA) (Fig. 1c).
Fig. 1.
Clone and prokaryotic expression of rIL-22. (A) The recombinant plasmid map of pET32a-IL-22. (B) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Lane M1: Protein marker; Lane PC1: BSA (1 μg); Lane PC2: BSA (2 μg) ; Lane NC: Cell lysate without induction; Lane 1: Cell lysate with induction for 16 h at 15 °C; Lane 2: Cell lysate with induction for 4 h at 37 °C; Lane NC1: Supernatant of cell lysate non-induced; Lane NC2: Pellet of cell lysate non-induced; Lane 3: Supernatant of cell lysate with induction for 16 h at 15 °C; Lane 4: Pellet of cell lysate with induction for 16 h at 15 °C; Lane 5: Supernatant of cell lysate with induction for 4 h at 37 °C; Lane 6: Pellet of cell lysate with induction for 4 h at 37 °C. (C) SDS-PAGE analysis of purified rIL-22. Lane 1 shows the total protein extract of rIL-22-His inclusion body pellet following 1 mM IPTG induction at 15 °C for 16 h (protein purity: 72.73%); Lane 2 shows the total protein extract of rIL-22-his through gradient dialysis (protein purity: 77.65%) ; Lane 3 purified rIL-22-His by a Ni–NTA column (protein purity: 95.7%); Lane 4 shows the protein marker. The analysis of protein purity used Quantity One software.
3.2. Biological activity of recombinant IL-22
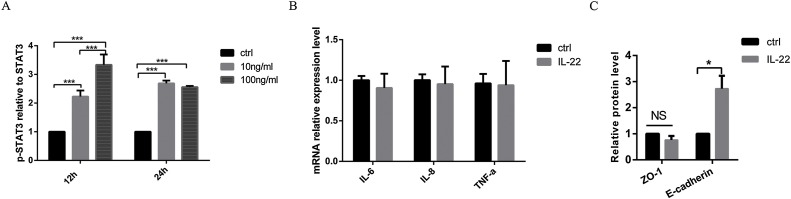
STAT3 signaling in the intestinal epithelium plays an important role in mucosal wound healing and is activated by IL-22 during acute experimental colitis (Nagalakshmi et al., 2004; Pickert et al., 2009). To study the biological activity of recombinant rIL-22, we detected the phosphorylation level of STAT3 induced by rIL-22 on IPEC-J2 cells. Compared with basal levels of phosphorylation of STAT3 in unstimulated controls, phosphorylation STAT3 was clearly increased by rIL-22 after 12 h and 24 h (Fig. 2 a). Moreover, we found that 100 ng/ml rIL-22 that we used is a safe concentration on IPEC-J2 cells, which did not affect the expressions of inflammatory factors, IL-6, IL-8 and TNF-α (Fig. 2b). Apart from that, rIL-22 at 100 ng/ml increased the expression of E-cadherin (Fig. 2c).
Fig. 2.
Biological activity of recombinant rIL-22. (A) Western blot analysis for phosphorylation levels of STAT3 (Tyr705). IPEC-J2 cells were treated by 100 ng/ml rIL-22 for 12 h and 24 h respectively. (B) Effect of rIL-22 on proinflammatory cytokines. Relative mRNA levels were measured for IL-6, IL-8 and TNF-α in untreated (control) and rIL-22 (100 ng/ml, 24 h) treated cells using RT-PCR. (C) Western blot analysis for ZO-1, E-cadherin. Data are expressed as means ± standard errors from three independent experiments. Differences were considered significant at (*) 0.01 < P < 0.05, (***) P < 0.001.
3.3. Effect of recombinant rIL-22 on apoptosis induced by DON
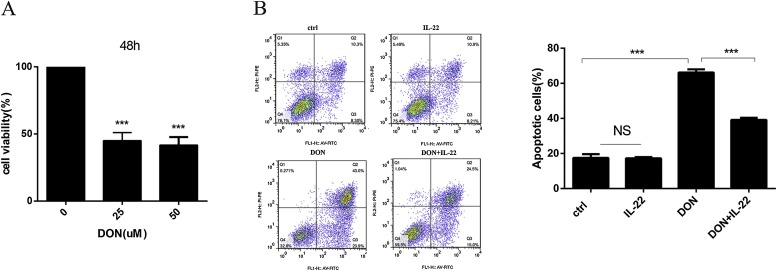
To develop an injury model induced by DON, the influence of DON on viability of IPEC-J2 cells at 25 and 50 μM concentrations for 48 h was evaluated by CCK-8 assay. Cell viability was significantly decreased by approximately 60% after 48 h by DON compared to the control (P < 0.001) (Fig. 3 a). The flow cytometric analysis showed that significant differences (P < 0.05) in apoptosis were observed after treated with 25 μM DON for 48 h with highest percentage of apoptotic IPEC-J2 cells being 66.9%. Compared with exposure to DON, the apoptosis of IPEC-J2 cells were significantly reduced to 39.5% in the presence of DON plus rIL-22 (P < 0.001) (Fig. 3b).
Fig. 3.
Effect of recombinant rIL-22 on apoptosis induced by DON. (A) CCK8 assay for viability of IPEC-J2 cells at 25 μM and 50 μM DON for 48 h. (B) Effects of rIL-22 on DON-induced cell apoptosis in IPEC-J2 cells. Cell distribution analysis of apoptosis of IPEC-J2 cells treated with rIL-22, DON, and DON plus rIL-22. In each diagram, Q1 represents the percentage of non-viable, necrotic cells, Q2 represents the percentage of late apoptotic IPEC-J2 cells, Q3 represents the percentages of early apoptotic IPEC-J2 cells and Q4 represents the percentage of live IPEC-J2 cells. The statistical analysis of cell distribution data among samples, apoptotic cells included Q2 with Q3. Data are expressed as means ± standard errors from three independent experiments. Differences were considered significant at (***) P < 0.001.
3.4. Effect of recombinant rIL-22 on ETEC K88 infection
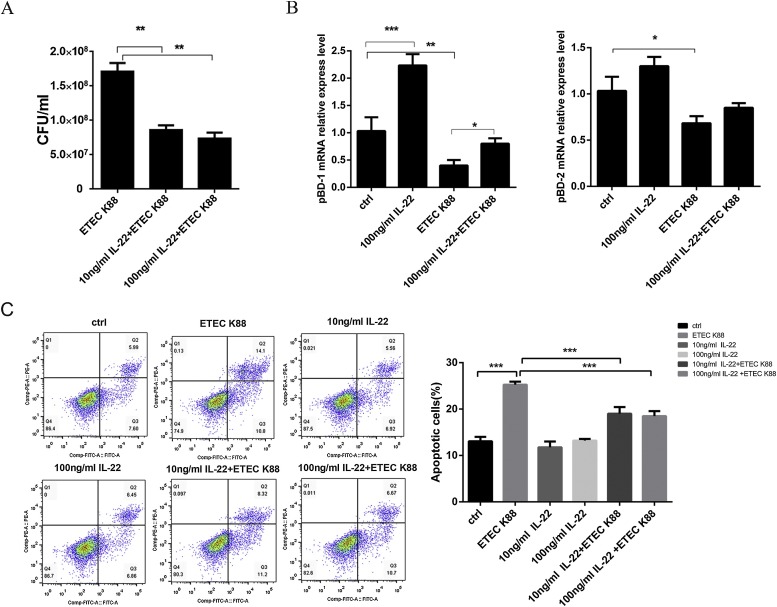
As shown in Fig. 4 a, rIL-22 effectively reduced the adhesion of ETEC K88 at 10 ng/ml and 100 ng/ml (P < 0.01) (Fig. 4a). Moreover, rIL-22 also obviously augmented the express level of pBD-1 (P < 0.01), but not pBD-2 (P > 0.05) (Fig. 4b). Meanwhile, 100 ng/ml rIL-22 alleviated the reduced level of pBD-1 caused by ETEC K88 infection (P < 0.05) (Fig. 4b). These results demonstrated that rIL-22 can induce epithelial cells to produce pBD-1 and reduce the adhesion of ETECK88. Meanwhile, we also found that the viability of cells infected ETEC K88 for 2.5 h decreased significantly, and 10 ng/ml and 100 ng/ml rIL-22 pretreatment decreased apoptosis ratio of intestinal epithelial cells induced by ETEC K88 significantly (P < 0.001) (Fig. 4c).
Fig. 4.
Effect of recombinant rIL-22 on infection of ETEC K88. (A) rIL-22 decreased ETEC K88 adhesion. Mock cells, rIL-22-pretreated cells were incubated with equal numbers of ETEC K88 for 2.5 h, and the number of adherent bacteria (CFU) was determined. (B) Relative mRNA levels were measured for pBD-1 and pBD-2 in untreated (control) and treated cells using RT-PCR. (C) Effect of recombinant rIL-22 on apoptosis after infection with ETEC K88. Cell distribution analysis of apoptosis of IPEC-J2 cells. Data are expressed as means ± standard errors from three independent experiments. Differences were considered significant at (*) 0.01 < P < 0.05, (**) 0.001 < P < 0.01, (***) P < 0.001.
4. Discussion
Intestinal barrier is the basis for nutrients absorption and an important protective barrier against pathogens invasion (Choi et al., 2017; Odenwald and Turner, 2017). However, pig suffers intestinal inflammation since its born, such as PEDV and E. coli infection, weaning stress, feed mildew and so on, which cause huge economic loss in porcine industry (Curry et al., 2017; Yu et al., 2017). Antibiotics are always used to treat porcine diarrhea, which could induce antibiotic resistance, drug residue and antibiotic-associated diarrhea (AAD) (Huang et al., 2018; Kim et al., 2017). IL-22 regulates early host defense against attaching and effacing of Citrobacter rodentium and ameliorates intestinal mucosal wound healing via a receptor complex, which are expressed in epithelial cells of small and large bowel (Parks et al., 2015). However, little is uncovered to the function of porcine IL-22, which may be a good alternative to prevent and cure porcine diarrhea.
Previous research have found that IL-22 play an important role against the infection of enteric coronaviruses (Xue et al., 2017). In this study, prokaryotic expression system was successfully used to produce recombinant protein rIL-22, which was able to lead to phosphorylation of STAT3 and rescue the apoptosis induced by DON. Combined with its safety on cell viability and balance proinflammatory cytokines expressions, rIL-22 is a novel idea to cure the vomit and apoptosis of intestinal epithelial cells induced by toxin.
Enteropathogens always damaged intestinal epithelial and induced intestinal inflammation and diarrhea. ETEC K88, is a vital pathogens, which caused serious intestinal disorder in piglets. IL-22 mainly actives STAT3 signal pathway that related to suppress apoptosis to maintain intestinal epithelial barrier function, ameliorate intestinal inflammation and prevent infection of gastrointestinal bacteria via regulation of antimicrobial peptides expression (Wittkopf et al., 2015). Porcine intestinal epithelial cells could produce β-defensin to maintain the integrity of intestinal mucosal barrier (Mariani et al., 2009). β-defensin attenuated intestinal inflammation and mucosal lesions in dextran sodium sulfate (DSS) induced colitis and displayed broad antimicrobial activity against pathogenic intestinal bacteria (Veldhuizen et al., 2008; Wittkopf et al., 2015). In our experiment, we found that rIL-22 significantly improved the expression of pBD-1 against the infection of ETEC K88, although the increase of pBD-2 was not obvious at statistical level. Researches showed that pBD-1 had a strong inhibitory activity against E. coli, S. typhimurium, L. monocytogenes, C. albicans and B. pertussis (Elahi et al., 2006; Shi et al., 1999). It’s possible that rIL-22 decreased the adhesion of ETEC K88 via antibacterial effect of pBD-1.
In conclusion, our study firstly suggested porcine IL-22 can contribute to anti-apoptosis induced by DON and decrease the intestinal epithelial damage of ETEC K88. Together, these results project a new protective role for rIL-22 in repairing epithelial cell injury caused by DON or E. coli. However, the detailed mechanism of the anti-apoptosis and resist infection activity of IL-22 remains elusive and need further study.
Funding information
The funding information should be: This study was supported by National Key Research and Development Program of China (2018YFD0500600), Fundamental Research Funds for the Central Universities (JCQY201906),National Natural Science Foundation of China (31502024), and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Conflicts of interest
There are no conflicts of interest (financial, professional or personal) related to this manuscript.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.vetmic.2019.02.027.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Akbari P., Braber S., Gremmels H., Koelink P.J., Verheijden K.A., Garssen J., Fink-Gremmels J. Deoxynivalenol: a trigger for intestinal integrity breakdown. FASEB J. 2014;28:2414–2429. doi: 10.1096/fj.13-238717. [DOI] [PubMed] [Google Scholar]
- Akira S. Roles of STAT3 defined by tissue-specific gene targeting. Oncogene. 2000;19:2607–2611. doi: 10.1038/sj.onc.1203478. [DOI] [PubMed] [Google Scholar]
- Alizadeh A., Braber S., Akbari P., Garssen J., Fink-Gremmels J. Deoxynivalenol impairs weight gain and affects markers of gut health after low-dose, short-term exposure of growing pigs. Toxins. 2015;7:2071–2095. doi: 10.3390/toxins7062071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonissen G., Martel A., Pasmans F., Ducatelle R., Verbrugghe E., Vandenbroucke V., Li S., Haesebrouck F., Van Immerseel F., Croubels S. The impact of Fusarium mycotoxins on human and animal host susceptibility to infectious diseases. Toxins (Basel) 2014;6:430–452. doi: 10.3390/toxins6020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhet S., Oswald I.P. The effects of mycotoxins, fungal food contaminants, on the intestinal epithelial cell-derived innate immune response. Vet. Immunol. Immunopathol. 2005;108:199–209. doi: 10.1016/j.vetimm.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Choi W., Yeruva S., Turner J.R. Contributions of intestinal epithelial barriers to health and disease. Exp. Cell Res. 2017;358:71–77. doi: 10.1016/j.yexcr.2017.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y.J., Park B.B., Kang Y.J., Kim T.M., Eaves C.J., Oh I.H. Unique effects of Stat3 on the early phase of hematopoietic stem cell regeneration. Blood. 2006;108:1208–1215. doi: 10.1182/blood-2006-01-010199. [DOI] [PubMed] [Google Scholar]
- Curry S.M., Schwartz K.J., Yoon K.J., Gabler N.K., Burrough E.R. Effects of porcine epidemic diarrhea virus infection on nursery pig intestinal function and barrier integrity. Vet. Microbiol. 2017;211:58–66. doi: 10.1016/j.vetmic.2017.09.021. [DOI] [PubMed] [Google Scholar]
- Dudakov J.A., Hanash A.M., van den Brink M.R.M. Interleukin-22: immunobiology and pathology. Annu. Rev. Immunol. 2015;33:747–785. doi: 10.1146/annurev-immunol-032414-112123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elahi S., Buchanan R.M., Attah-Poku S., Townsend H.G., Babiuk L.A., Gerdts V. The host defense peptide beta-defensin 1 confers protection against Bordetella pertussis in newborn piglets. Infect. Immun. 2006;74:2338–2352. doi: 10.1128/IAI.74.4.2338-2352.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q.H., Ye L.L., Liu H.F., Huang L.L., Yang Q., Turner J.R., Yu Q.H. Lactobacillus accelerates ISCs regeneration to protect the integrity of intestinal mucosa through activation of STAT3 signaling pathway induced by LPLs secretion of IL-22. Cell Death Differ. 2018;25:1657–1670. doi: 10.1038/s41418-018-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.C., Vlasova A.N., Kumar A., Kandasamy S., Fischer D.D., Deblais L., Paim F.C., Langel S.N., Alhamo M.A., Rauf A., Shao L., Saif L.J., Rajashekara G. Effect of antibiotic, probiotic, and human rotavirus infection on colonisation dynamics of defined commensal microbiota in a gnotobiotic pig model. Benef. Microbes. 2018;9:71–86. doi: 10.3920/BM2016.0225. [DOI] [PubMed] [Google Scholar]
- Jin L.Z., Zhao X. Intestinal receptors for adhesive fimbriae of enterotoxigenic Escherichia coli (ETEC) K88 in swine--a review. Appl. Microbiol. Biotechnol. 2000;54:311–318. doi: 10.1007/s002530000404. [DOI] [PubMed] [Google Scholar]
- Kim Y.J., Kim S.H., Ahn J., Cho S., Kim D., Kim K., Lee H., Son H., Lee H.J., Yong D., Choi J.Y., Kim H.R., Shin J.H. Prevalence of clostridium perfringens toxin in patients suspected of having antibiotic-associated diarrhea. Anaerobe. 2017;48:34–36. doi: 10.1016/j.anaerobe.2017.06.015. [DOI] [PubMed] [Google Scholar]
- Koltsova E.K., Grivennikov S.I. IL-22 gets to the stem of colorectal cancer. Immunity. 2014;40:639–641. doi: 10.1016/j.immuni.2014.04.014. [DOI] [PubMed] [Google Scholar]
- Kryczek I., Lin Y., Nagarsheth N., Peng D., Zhao L., Zhao E., Vatan L., Szeliga W., Dou Y., Owens S., Zgodzinski W., Majewski M., Wallner G., Fang J., Huang E., Zou W. IL-22(+)CD4(+) T cells promote colorectal cancer stemness via STAT3 transcription factor activation and induction of the methyltransferase DOT1L. Immunity. 2014;40:772–784. doi: 10.1016/j.immuni.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I.K., Kye Y.C., Kim G., Kim H.W., Gu M.J., Umboh J., Maaruf K., Kim S.W., Yun C.H. Stress, nutrition, and intestinal immune responses in pigs - a review. Asian-Australas. J. Anim. Sci. 2016;29:1075–1082. doi: 10.5713/ajas.16.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejeune D., Dumoutier L., Constantinescu S., Kruijer W., Schuringa J.J., Renauld J.C. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J. Biol. Chem. 2002;277:33676–33682. doi: 10.1074/jbc.M204204200. [DOI] [PubMed] [Google Scholar]
- Lindemans C.A., Calafiore M., Mertelsmann A.M., O’Connor M.H., Dudakov J.A., Jenq R.R., Velardi E., Young L.F., Smith O.M., Lawrence G., Ivanov J.A., Fu Y.Y., Takashima S., Hua G., Martin M.L., O’Rourke K.P., Lo Y.H., Mokry M., Romera-Hernandez M., Cupedo T., Dow L., Nieuwenhuis E.E., Shroyer N.F., Liu C., Kolesnick R., van den Brink M.R.M., Hanash A.M. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015;528:560–564. doi: 10.1038/nature16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Zhang A., Shi Z., He C., Ding J., Wang X., Ma J., Zhang H. A mitochondria-mediated apoptotic pathway induced by deoxynivalenol in human colon cancer cells. Toxicol. In Vitro. 2012;26:414–420. doi: 10.1016/j.tiv.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Mariani V., Palermo S., Fiorentini S., Lanubile A., Giuffra E. Gene expression study of two widely used pig intestinal epithelial cell lines: IPEC-J2 and IPI-2I. Vet. Immunol. Immunopathol. 2009;131:278–284. doi: 10.1016/j.vetimm.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Mulcahy M.E., Leech J.M., Renauld J.C., Mills K.H., McLoughlin R.M. Interleukin-22 regulates antimicrobial peptide expression and keratinocyte differentiation to control Staphylococcus aureus colonization of the nasal mucosa. Mucosal Immunol. 2016;9:1429–1441. doi: 10.1038/mi.2016.24. [DOI] [PubMed] [Google Scholar]
- Nagalakshmi M.L., Rascle A., Zurawski S., Menon S., de Waal Malefyt R. Interleukin-22 activates STAT3 and induces IL-10 by colon epithelial cells. Int. Immunopharmacol. 2004;4:679–691. doi: 10.1016/j.intimp.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Odenwald M.A., Turner J.R. The intestinal epithelial barrier: a therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017;14:9–21. doi: 10.1038/nrgastro.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks O.B., Pociask D.A., Hodzic Z., Kolls J.K., Good M. Interleukin-22 signaling in the regulation of intestinal health and disease. Front. Cell Dev. Biol. 2015;3:85. doi: 10.3389/fcell.2015.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickert G., Neufert C., Leppkes M., Zheng Y., Wittkopf N., Warntjen M., Lehr H.A., Hirth S., Weigmann B., Wirtz S., Ouyang W., Neurath M.F., Becker C. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 2009;206:1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P., Oswald I.P. Effect of deoxynivalenol and other type B trichothecenes on the intestine: a review. Toxins (Basel) 2014;6:1615–1643. doi: 10.3390/toxins6051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Zhang G., Wu H., Ross C., Blecha F., Ganz T. Porcine epithelial beta-defensin 1 is expressed in the dorsal tongue at antimicrobial concentrations. Infect. Immun. 1999;67:3121–3127. doi: 10.1128/iai.67.6.3121-3127.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sovran B., Loonen L.M., Lu P., Hugenholtz F., Belzer C., Stolte E.H., Boekschoten M.V., van Baarlen P., Kleerebezem M., de Vos P., Dekker J., Renes I.B., Wells J.M. IL-22-STAT3 pathway plays a key role in the maintenance of ileal homeostasis in mice lacking secreted mucus barrier. Inflamm. Bowel Dis. 2015;21:531–542. doi: 10.1097/MIB.0000000000000319. [DOI] [PubMed] [Google Scholar]
- Sugimoto K., Ogawa A., Mizoguchi E., Shimomura Y., Andoh A., Bhan A.K., Blumberg R.S., Xavier R.J., Mizoguchi A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Invest. 2008;118:534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treerat P., Prince O., Cruz-Lagunas A., Munoz-Torrico M., Salazar-Lezama M.A., Selman M., Fallert-Junecko B., Reinhardt T.A., Alcorn J.F., Kaushal D., Zuniga J., Rangel-Moreno J., Kolls J.K., Khader S.A. Novel role for IL-22 in protection during chronic Mycobacterium tuberculosis HN878 infection. Mucosal Immunol. 2017;10:1069–1081. doi: 10.1038/mi.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuizen E.J., Rijnders M., Claassen E.A., van Dijk A., Haagsman H.P. Porcine beta-defensin 2 displays broad antimicrobial activity against pathogenic intestinal bacteria. Mol. Immunol. 2008;45:386–394. doi: 10.1016/j.molimm.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Wan M.L., Woo C.S., Allen K.J., Turner P.C., El-Nezami H. Modulation of porcine beta-defensins 1 and 2 upon individual and combined Fusarium toxin exposure in a swine jejunal epithelial cell line. Appl. Environ. Microbiol. 2013;79:2225–2232. doi: 10.1128/AEM.03277-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte E., Witte K., Warszawska K., Sabat R., Wolk K. Interleukin-22: a cytokine produced by T, NK and NKT cell subsets, with importance in the innate immune defense and tissue protection. Cytokine Growth Factor Rev. 2010;21:365–379. doi: 10.1016/j.cytogfr.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Wittkopf N., Pickert G., Billmeier U., Mahapatro M., Wirtz S., Martini E., Leppkes M., Neurath M.F., Becker C. Activation of intestinal epithelial Stat3 orchestrates tissue defense during gastrointestinal infection. PLoS One. 2015;10 doi: 10.1371/journal.pone.0118401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L., Dai L., Yu Q., Yang Q. Persistent transmissible gastroenteritis virus infection enhances enterotoxigenic Escherichia coli K88 adhesion by promoting epithelial-mesenchymal transition in intestinal epithelial cells. J. Virol. 2017;91 doi: 10.1128/JVI.01256-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M., Zhao J., Ying L., Fu F., Li L., Ma Y., Shi H., Zhang J., Feng L., Liu P. IL-22 suppresses the infection of porcine enteric coronaviruses and rotavirus by activating STAT3 signal pathway. Antiviral Res. 2017;142:68–75. doi: 10.1016/j.antiviral.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., Wang Z., Yang Q. Lactobacillus amylophilus D14 protects tight junction from enteropathogenic bacteria damage in Caco-2 cells. J. Dairy Sci. 2012;95:5580–5587. doi: 10.3168/jds.2012-5540. [DOI] [PubMed] [Google Scholar]
- Yu H.T., Ding X.L., Li N., Zhang X.Y., Zeng X.F., Wang S., Liu H.B., Wang Y.M., Jia H.M., Qiao S.Y. Dietary supplemented antimicrobial peptide microcin J25 improves the growth performance, apparent total tract digestibility, fecal microbiota, and intestinal barrier function of weaned pigs. J. Anim. Sci. 2017;95:5064–5076. doi: 10.2527/jas2017.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Xu Y., Zhang Z., You J., Yang Y., Li X. Protective immunity of a multivalent vaccine candidate against piglet diarrhea caused by enterotoxigenic Escherichia coli (ETEC) in a pig model. Vaccine. 2018;36:723–728. doi: 10.1016/j.vaccine.2017.12.026. [DOI] [PubMed] [Google Scholar]
- Zhou M.X., Guo Z.Y., Yang Y., Duan Q.D., Zhang Q., Yao F.H., Zhu J., Zhang X.J., Hardwidge P.R., Zhu G.Q. Flagellin and F4 fimbriae have opposite effects on biofilm formation and quorum sensing in F4ac + enterotoxigenic Escherichia coli. Vet. Microbiol. 2014;168:148–153. doi: 10.1016/j.vetmic.2013.10.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.