Abstract
In vitro anti-rotavirus activity of Alpinia katsumadai (AK) extracts were evaluated against bovine G8P[7] and porcine G5P[7] rotaviruses in two different assay strategies, a mixed treatment assay and a post treatment assay. In the mixed treatment assay, six AK extracts [AK-1 (EtOH extract), AK-3 (H2O layer), AK-5 (40% methanol fraction), and AK-9–11 (H2O extract, polysaccharide fraction, supernatant fraction)] exhibited inhibitory activities against G5P[7] rotavirus with the EC50 values ranging from 0.7 ± 0.4 to 33.7 ± 6.5 μg/mL. Extracts AK-1, AK-3, and AK-5 inhibited rotavirus infection against G8P[7] rotavirus, the with EC50 values of 8.4 ± 2.2 μg/mL, 6.5 ± 0.8 μg/mL and 8.4 ± 5.0 μg/mL, respectively. By hemagglutination inhibition (HI) assay, six AK extracts completely inhibited viral adsorption onto human RBCs in both strains of rotaviruses at less than 11 μg/mL. However, in the post treatment assay, there was no anti activity shown against both strains of rotaviruses. As a result, six AK extracts were attributed mainly to having a strong interaction with hemagglutinin protein on the outer surface of rotavirus, resulting to blockage of viral adsorption.
Keywords: Alpinia katsumadai, Anti-rotavirus, Viral adsorption, Hemagglutination inhibition
1. Introduction
Rotaviruses, belonging to the Reoviridae family are large non-enveloped viruses, consisting of triple layered particles which surround the viral genome composed of 11 segments of double-stranded RNA (Estes and Kapikian, 2007). The rotaviruses are major pathogens that cause severe, acute dehydrating gastroenteritis in young children and in a wide variety of domestic animals (Estes and Kapikian, 2007, Gentsch et al., 2005, Glass et al., 1997). Specifically, rotaviruses cause severe diarrheal diseases in neonatal and post-weaning piglets and calves (Estes and Kapikian, 2007).
The anti-rotavirus effects of some commercially available antiviral drugs such as ribavirin, interferon, dipyridamole, cimentidine, and famodine have been examined in vitro and in animal experiments (Gu et al., 2000) and have shown anti-rotavirus activities (Lecce et al., 1990, Smee et al., 1982, Tonew et al., 1977). Dipyridamole has also been reported to possess antiviral activity against representatives of other virus families (e.g., Picornaviridae, Togaviridae, Orthomyxoviridae, Paramyxoviridae, Herpesviridae, and Poxviridae) (Lecce et al., 1990, Smee et al., 1982, Tonew et al., 1977). Cimentidine and famodine do not only have anti-HIV activity, but also exhibit therapeutic effects against herpes simplex virus infection (Bourinbaiar and Fruhstorfer, 1996, Kabuta et al., 1989). Although these drugs act by inhibiting rotavirus replication and adsorption, any side-effects have not been examined in clinical studies. Immunoglobulins have also been used to treat diarrhea caused by rotaviruses; however, these drugs are very costly and the side-effects are unknown (Guarino et al., 1994, Madkour et al., 1993, Yolken et al., 1985). Antiviral agents from natural sources such as black tea, Citrus aurantium, marine sponges, soy, and Stevia rebaudiana were considered as ideal drug candidates because they are less toxic, have fewer side-effects, and cheaper, but may have more effective reaction than those commercially available anti-rotavirus agents (Andres et al., 2009, Bae et al., 2000, Clark et al., 1998, Da Silva et al., 2006, Takahashi et al., 2001). However, these drugs are not currently available for human or animal use. Nevertheless, the diarrhea caused by rotaviruses remains uncontrolled, thus new drugs are urgently needed to control rotavirus infection.
Alpinia katsumadai Hayata (Zingiberaceae) (AK) has been utilized as a traditional Chinese herbal drug for its anti-emetic and stomachic mechanism of action (Tang and Eisenbrand, 1992). It has been reported to contain a variety of diarylheptanoids, monoterpenes, sesquiterpenoid, flavonoids, and chalcones as major constituents (Kuroyanagi et al., 1983, Ngo and Brow, 1998, Yang et al., 1999). The extracts and compounds isolated from this plant have shown anti-emetic activity and a plasma cholesterol-lowering effect by cholesterol esterase inhibitory activity, and anti-oxidant activity (Kim et al., 2000, Lee et al., 2003, Yang et al., 1999). Recently, compounds isolated from this plant showed in vitro neuraminidase inhibitory activities against human influenza virus A/PR/8/34 of subtype H1N1 and four H1N1 swine influenza viruses with antiviral effects in plaque reduction assays (Grienke et al., 2010). However, to date the anti-rotavirus activities of the extracts and compounds isolated from this plant have not been previously evaluated. Therefore, in this study, we have found the in vitro anti-rotaviral activity of AK extracts and its mechanism of anti-rotaviral activity.
2. Materials and methods
2.1. Preparation of A. katumadai extracts and fractions
The dried seeds (4.8 kg) of A. katsumadaii were ground and macerated with ethanol (1.5 L × 20) for one week at room temperature, and then filtered and the clarified solvent was evaporated under reduced pressure to afford the ethanol extract (289 g, AK-1). The combined ethanol extract was dissolved in 2.0 L of a mixture of water and ethanol (1:9) and successively partitioned with EtOAc and water, yielding a layer of EtOAc (192 g, AK-2) and a layer of water (70 g, AK-3). The water soluble fraction AK-3 was subjected to dianion (HP-20) column chromatography, eluted with MeOH in water in a step-gradient manner from 20% to 100% to make five fractions [20% methanol (AK-4): 3.9 g, 40% methanol (AK-5): 11.9 g, 60% methanol (AK-6): 32.7 g, 80% methanol (AK-7): 3.8 g, and 100% methanol (AK-8): 1.1 g]. In order to obtain the polysaccharide fraction, we performed another procedure. The dried and pulverized seeds of A. katsumadaii (600 g) were mixed with 1.5 L of water and shaken at 80 °C for 12 h. The water extract (98 g, AK-9) was filtered through filter paper to remove debris, and the solution was precipitated by the addition of ethanol with a 1:4 ratio (v/v) at room temperature. After overnight precipitation, the precipitate was collected by centrifugation (12,000 rpm, 30 min at 4 °C) and washed with acetone and freeze-dried. This fractionation procedure was repeated three times. The corresponding fraction (56 g, AK-10) was light a brown powder (polysaccharide fraction), and the remaining supernatant was concentrated in a rotary evaporator under reduced pressure, yielding a supernatant fraction (28 g, AK-11).
2.2. Cells
African rhesus monkey kidney (MA-104) cells were obtained from the American Type Culture Collection (ATCC CRL-2373.1; Manassas, VA, USA) and grown in Eagle’s minimum essential medium (EMEM) supplemented with 5% fetal bovine serum (FBS), 100 U/mL penicillin, 100 μg/mL streptomycin, and 100 U/mL amphotericin B (Park et al., 2006).
2.3. Viruses
KJ56-1 (bovine rotavirus, G8P[7]) and KJ25-1 (porcine rotavirus, G5P[7]) viruses isolated from fecal samples of Korean diarrheic calves and piglets were used in this study. These viruses were kindly provided by Dr. Kyoung-Oh Cho (Chonnam National University, South Korea). The rotaviruses were preactivated with 10 μg/mL trypsin (1:250; GIBCO Invitrogen Corporation, California) for 30 min at 37 °C before being inoculated onto confluent MA-104 cells. The infected cells were maintained in the presence of 1 μg/mL trypsin.
2.4. Cytotoxicity assay
MA-104 cells (1 × 105 cells/well) were grown in 96-well plates for 48 h. The media were removed and replaced by new media containing serial dilutions of extracts under test. After incubation for 72 h, the media were discarded, and 5 μL of MTT (3-[4,5-dimethylthiozol-2-yl]-2,5-diphenyltetrazolium bromide; Sigma, St. Louis, MO) solution was added to each well. Plates were then incubated at 37 °C for 4 h. The solution was removed, and 100 μL of 0.04 M HCl-isopropanol was added to each well to dissolve formazan crystals (Fig. 1 ). Using a microplate reader, the absorbance of each well was measured at 540 nm. After subtracting the background absorbance at 655 nm, the 50% cytotoxic concentration (CC50) of each extract was estimated by regression analysis.
Fig. 1.

Anti-rotaviral assay strategies with A. katumadai (AK) extracts. Virus inoculation after virus incubation with AK extracts at 4 °C for 1 h for mixed treatment assay (A), treatment of AK extracts after viral infection for post treatment assay (B), and serial dose treatment of AK extracts for cytotoxicity assay (C).
2.5. Antiviral assay
The antiviral assays used in this study have been previously described (Barnard et al., 1997), and the visualization of these assays was performed by neutral red method as briefly described.
In the mixed treatment assay: Each extract was mixed with a 0.01 multiplicity of infection (MOI) of the rotaviruses at various concentrations (0.1–133.3 μg/mL) and incubated at 4 °C for 1 h. The mixtures were inoculated in triplicates onto near confluent MA-104 cell monolayers (1 × 105 cells/well) for 1 h with occasional rocking. The solution was removed and the cells replaced with EMEM containing 1 μg/mL trypsin. The cells were incubated for 72 h at 37 °C under 5% CO2 atmosphere until the cells in the infected, untreated control showed complete viral CPE by light microscopy (Fig. 1). The 50% effective concentration (EC50) was estimated by regression analysis.
In the post treatment assay: The rotaviruses at a 0.01 MOI were inoculated onto near confluent MA-104 cell monolayers (1 × 105 cells/well) for 1 h with occasional rocking. The solution was removed and replaced by EMEM containing 1 μg/mL trypsin and various concentrations (0.1–133.3 μg/mL) of each extract in triplicates. The cells were cultured for 72 h at 37 °C until cells in the infected, untreated control well showed complete viral CPE by light microscopy. Neutral red solution was added to each well at 0.034% (w/v), and plates were incubated for 2 h at 37 °C in the absence of light. The neutral red solution was removed, cells were washed with PBS (pH 7.4), and destaining solution (1% glacial acetic acid, 49% H2O and 50% ethanol) was added. The plates were incubated in the dark for 15 min at room temperature, and the absorbance of each well at 540 nm was read using a microplate reader (Fig. 1). EC50 of each extracts was estimated by regression analysis.
2.6. Hemagglutination inhibition (HI) assay
Standardized solutions of human red blood cells (hRBC, type O) were prepared according to the WHO manual 2002 (WHO, 2002). A 25 μL aliquot of rotavirus solution containing 4 HAU was mixed with 25 μL of serial dilutions in PBS (pH 7.4) of each extract and incubated for 1 h at 4 °C. Fifty microliters of a 1% (v/v) hRBC suspension was added, and the samples were incubated for 1 h at room temperature.
3. Results
3.1. Cytotoxicity of A. katsumadai extracts in MA-104 cells
The cytotoxicity of AK extracts was evaluated by the MTT assay at 50% cell toxicity (CC50). Confluent MA-104 cells were incubated with EMEM media in the absence or presence of two fold diluted AK extracts (0.1–133.3 μg/mL) for 72 h, and the MTT reagents were treated onto the cells. Cytotoxicity among the six AK extracts showed a different concentration. AK-1 extract had low CC50 at 30.7 μg/mL, while AK-3, AK-5, AK-9, AK-10, and AK-11 with high CC50 at 70.1-over 133.3 μg/mL (Table 1). Hence, experiments to evaluate the antiviral effect were carried out at AK extracts concentration of more than 90% cell viability in this study.
Table 1.
In vitro anti-rotavirus activities of A. katumadai (AK) extracts against KJ56-1 (bovine rotavirus, G8P[7]) and KJ25-1 (porcine rotavirus, G5P[7]) on MA-104 cells using the mixed treatment assay.
| Extract | CC50 (μg/mL)a | KJ56-1 (G8P[7]) |
KJ25-1 (G5P[7]) |
||
|---|---|---|---|---|---|
| EC50 (μg/mL)b | SIc | EC50 (μg/mL)b | SIc | ||
| EtOH extract (AK-1) | 30.7 | 8.4 ± 2.2 | 3.7 | 8.9 ± 4.8 | 3.4 |
| H2O layer (AK-3) | 70.1 | 6.5 ± 0.8 | 10.8 | 0.7 ± 0.4 | 100.1 |
| 40% methanol fraction (AK-5) | >133.3 | 8.4 ± 5.0 | >15.9 | 1.6 ± 0.5 | >83.3 |
| H2O extract (AK-9) | >133.3 | – | – | 33.7 ± 6.5 | >4.0 |
| Polysaccharide fraction (AK-10) | 88.0 | – | – | 14.9 ± 2.6 | 5.9 |
| Supernatant fraction (AK-11) | >133.3 | – | – | 23.5 ± 1.8 | >5.7 |
CC50: mean (50%) value of cytotoxic concentration.
EC50: mean (50%) value of effective concentration.
SI: selective index, CC50/EC50.
3.2. Antiviral activity of A. katsumadai on rotavirus adsorption
In the mixed treatment assay, after various concentrations of AK extracts and bovine rotavirus (G8P[7]) or porcine rotavirus (G5P[7]) were mixed and incubated, the mixtures were then inoculated into the confluent MA-104 cells. Of the 11 AK extracts, six extracts [AK-1 (EtOH extract), AK-3 (H2O layer), AK-5 (40% methanol fraction), and AK-9-11 (H2O extract, polysaccharide fraction, supernatant fraction)] exhibited inhibitory activities against G5P[7] rotavirus with EC50 values ranging from 0.7 ± 0.4 to 33.7 ± 6.5 μg/mL (Table 1 ). Against G8P[7] rotavirus, the AK-1, AK-3, and AK-5 extracts inhibited rotavirus infection with EC50 values of 8.4 ± 2.2 μg/mL, 6.5 ± 0.8 μg/mL, and 8.4 ± 5.0 μg/mL, respectively (Table 1). The AK-1, AK-3, and AK-5 inhibited both G8P[7] and G5P[7] rotavirus infection in MA-104 cells. Whereas AK-9 (H2O extract), AK-10 (polysaccharide fraction), and AK-11 (supernatant fraction) only exhibited inhibitory effect against G5P[7] rotavirus. Extracts AK-3 and AK-5 showed the highest SI values of 10.8 and >15.9 in G8P[7] and 100.1 and >83.3 in G5P[7] rotavirus, respectively. Therefore, among the six AK extracts, AK-3 had the most inhibitory effect in the both G8P[7] and G5P[7] rotaviruses.
3.3. Antiviral activity of A. katsumadai on rotavirus replication
In order to test the ability of the six AK extracts in inhibiting the replication of rotaviruses G8P[7] and G5P[7] in MA-104 cells, the post treatment assay was used. However, in contrast to the mixed treatment assay, no inhibitory effects against both rotaviruses were shown in the post treatment assay. These results indicate that AK extracts exert potent anti-rotaviral activity only before viral adsorption.
3.4. Hemagglutination inhibition activity
To determine whether the AK extracts can block virus adsorption or cell entry, we evaluated whether AK extracts could inhibit rotavirus-induced hemagglutination by binding to O-type hRBCs. The six AK extracts completely inhibited viral adsorption onto hRBCs in both G8P[7] and G5P[7] rotaviruses at less than 11 μg/mL (Fig. 2 ). Among the six extracts, AK-1, AK-3, and AK-5 particularly showed strong inhibition of hemagglutination with 0.7–4.3 μg/mL in both rotaviruses. AK-9 had a stronger inhibitory activity of hemagglutination in G8P[7] rotavirus than G5P[7] rotavirus contrary to the pretreatment assay result. AK-10 (7.0 and 7.4 μg/mL) and AK-11 (6.7 and 5.5 μg/mL) showed similar inhibitory concentrations to hemagglutination of G8P[7] and G5P[7] rotaviruses. As a result, six AK extracts were attributed mainly to having a strong interaction with hemagglutinin protein on the outer surface of rotavirus, resulting in blockage of viral adsorption.
Fig. 2.
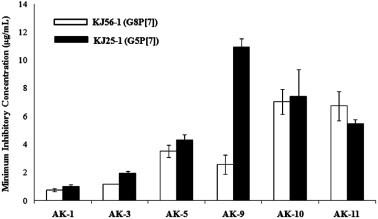
Hemagglutination inhibitory activity of A. katumadai (AK) extracts. Four HAU of bovine (G8P[7]) and porcine (G5P[7]) rotavirus were incubated with two fold dilutions of AK extracts or PBS (negative control), and human RBC (hRBC), for 1 h at room temperature. The minimum concentration of AK extract inhibiting the viral hemagglutination was determined. AK-1: EtOH extract; AK-3: H2O layer; AK-5: 40% methanol fraction; AK-9: H2O extract; AK-10: polysaccharide fraction; and AK-11: supernatant fraction.
4. Discussion and conclusion
Various steps of the viral replication cycle are the targets of anti-rotavirus agents: adsorption, penetration into cells, uncoating, transcription, translation, assembly and viral release from infected cells (Estes and Kapikian, 2007). We hypothesized that antiviral effects of AK extracts would act at the first two steps: (1) blockage of virus adsorption to cells and/or (2) inhibition of viral replication after entry cell. Time-of-addition experiments were performed to determine the stage at which AK extracts would exert inhibitory activities. The six AK extracts were incubated with MA-104 cells at two distinct time points: after incubation for 1 h at 4 °C with virus prior to virus infection (mixed treatment assay), and at 1 h after virus inoculation (post treatment assay) (Fig. 1).
Specifically, rotavirus entry into cells is a multistep process in which several interactions between its outer-layer proteins, VP4 and VP7, and the cell surface receptors including sialic acids (SA), integrins, and heat Shock Protein hsc70 occur (Lopez and Arias, 2006). VP4 is responsible for the hemagglutination (HA) activity and the binding SA which could be different according to rotavirus strains (Isa et al., 2006, Lopez and Arias, 2006). The HA activity of some rotavirus strains lead to the idea that SA is involved in the cell binding and infectivity of some animal rotavirus as in the case of influenza A virus, reovirus type 3, various coronaviruses, and Sendai virus (Isa et al., 2006). Therefore, hemagglutination inhibition assay was employed to assess the inhibitory effects of AK extracts on viral adsorption to host cells. As a result, the six AK extracts completely inhibited viral adsorption onto hRBCs in both G8P[7] and G5P[7] rotaviruses below 11 μg/mL (Fig. 2). Particularly, AK-1, AK-3, and AK-5 showed strong inhibition of hemagglutination with EC50 values of 0.7–4.3 μg/mL in both rotaviruses. The HI assay results are in agreement with the mixed treatment assay results, indicating that the AK extracts could probably exert a potential anti-rotaviral activity via blockage of viral attachment to SA of host cell surfaces.
In conclusion, the present study has shown that AK extracts can inhibit both G8P[7] bovine rotavirus and G5P[7] porcine rotavirus infection probably by blocking viral adsorption leading to no virus entry into the cell. In this study, the AK extracts were classified as active compounds in the prevention of rotavirus infection; however, further studies about its specific mechanism of action in rotavirus infection should be explored.
Acknowledgments
This research was supported by National Research Foundation grant funded by the Korea government (MEST) (No. 2010-0002047) and KRIBB Research Initiative Program, Republic of Korea.
References
- Andres A., Donovan S.M., Kuhlenschmidt M.S. Soy isoflavones and virus infections. Journal of Nutritional Biochemistry. 2009;20:563–569. doi: 10.1016/j.jnutbio.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae E.A., Han M.J., Lee M., Kim D.H. In vitro inhibitory effect of some flavonoids on rotavirus infectivity. Biological & Pharmaceutical Bulletin. 2000;23:1122–1124. doi: 10.1248/bpb.23.1122. [DOI] [PubMed] [Google Scholar]
- Barnard D.L., Hill C.L., Gage T., Matheson J.E., Huffman J.H., Sidwell R.W., Sidwell M.I., Otto M.I., Schinazi R.F. Potent inhibition of respiratory syncytial virus by polyoxometalates of several structural classes. Antiviral Research. 1997;34:27–37. doi: 10.1016/s0166-3542(96)01019-4. [DOI] [PubMed] [Google Scholar]
- Bourinbaiar A.S., Fruhstorfer E.C. The effect of histamine type 2 receptor antagonists on human immunodeficiency virus replication: identification of a new class of antiviral agents. Life Sciences. 1996;59:365–370. doi: 10.1016/s0024-3205(96)00553-x. [DOI] [PubMed] [Google Scholar]
- Clark K.J., Grant P.G., Sarr A.B., Belakere J.R., Swaggerty C.L., Philips T.D., Woode G.N. An in vitro study of theaflavins extracted from black tea to neutralize bovine rotavirus and bovine coronavirus infections. Veterinary Microbiology. 1998;63:147–157. doi: 10.1016/S0378-1135(98)00242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva A.C., Kratz J.M., Farias F.M., Henriques A.T., Santos J., Leonel R.M., Lerner C., Mothes B., Barardi C.R.M., Simoes C.M. In vitro antiviral activity of marine sponges collected off Brazilian coast. Biological & Pharmaceutical Bulletin. 2006;29:135–140. doi: 10.1248/bpb.29.135. [DOI] [PubMed] [Google Scholar]
- Estes M.K., Kapikian A.Z. Rotaviruses. In: Knipe D.M., Griffin D.E., Lamb R.A., Straus S.E., Howley P.M., Martin M.A., Roizman B., editors. Fields Virology. fifth ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2007. pp. 1917–1974. [Google Scholar]
- Gentsch J.R., Laird A.R., Bielfelt B., Griffin D.D., Bányai K., Ramachandran M., Jain V., Cunliffe N.A., Nakagomi O., Kirkwood C.D., Fischer T.K., Parashar U.D., Bresee J.S., Jiang B., Glass R.I. Serotype diversity and reassortment between human and animal rotavirus strains: implications for rotavirus vaccine programs. Journal of Infectious Diseases. 2005;192(Suppl. 1):S146–S159. doi: 10.1086/431499. [DOI] [PubMed] [Google Scholar]
- Glass R.I., Bressee J.S., Parashar U., Miller M., Gentsch J.R. Rotavirus vaccines at the threshold. Nature Medicine. 1997;3:1324–1325. doi: 10.1038/nm1297-1324. [DOI] [PubMed] [Google Scholar]
- Grienke U., Schmidtke M., Kirchmair J., Pfarr K., Wutzler P., Dürrwald R., Wolber G., Liedl K.R., Stuppner H., Rollinger J.M. Antiviral potential and molecular insight into neuraminidase inhibiting diarylheptanoids from Alpiniakatsumadai. Journal of Medicinal Chemistry. 2010;53:778–786. doi: 10.1021/jm901440f. [DOI] [PubMed] [Google Scholar]
- Gu Y., Gu Q., Kodama H., Mueller W.E., Ushijima H. Development of antirotavirus agents in Asia. Pediatrics International: Official Journal of the Japan Pediatric Society. 2000;42:440–447. doi: 10.1046/j.1442-200x.2000.01248.x. [DOI] [PubMed] [Google Scholar]
- Guarino A., Canani R.B., Russo S. Oral immunoglobulins for treatment of acute rotaviral gastroenteritis. Pediatrics. 1994;93:12–16. [PubMed] [Google Scholar]
- Isa P., Arias C.F., López S. Role of sialic acids in rotavirus infection. Glycoconjugate Journal. 2006;23:27–37. doi: 10.1007/s10719-006-5435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabuta H., Yamamoto S., Shingu M. The effect of cimetidine on survival of mice infected with herpes simplex virus type 2, murine encephalomyelitis virus and vesicular stomatitis virus infections. Kurume Medical Journal. 1989;36:95–99. doi: 10.2739/kurumemedj.36.95. [DOI] [PubMed] [Google Scholar]
- Kim H.S., Kim J.Y., Choi J.W., Huh Y.M., Suh P.G., Ryu S.H. Plasma cholesterol-lowering effects of Alpinia katumadai extract as an inhibiter of pancreatic cholesterol esterase activity. Korean J. Food Sci. Technol. 2000;32:200–205. [Google Scholar]
- Kuroyanagi M., Noro T., Fukushima S., Aiyama R., Ikuta A., Itokawa H., Morita M. Studies on the constituents of the seeds of Alpinia katsumadai Hayata. Chemical and Pharmaceutical Bulletin. 1983;31:1544–1550. [Google Scholar]
- Lecce J.G., Cummins J.M., Richards A.B. Treatment of rotavirus infection in neonate and weanling pigs using natural human interferon alpha. Molecular Biotherapy. 1990;2:211–216. [PubMed] [Google Scholar]
- Lee S.E., Shin H.T., Hwang H.J., Kim J.H. Antioxidant activity of extracts from Alpinia katsumadai seed. Phytotherapy Research: PTR. 2003;17:1041–1047. doi: 10.1002/ptr.1291. [DOI] [PubMed] [Google Scholar]
- Lopez S., Arias C.F. Early steps in rotavirus cell entry. Current Topics in Microbiology and Immunology. 2006;309:39–66. doi: 10.1007/3-540-30773-7_2. [DOI] [PubMed] [Google Scholar]
- Madkour A.A., Madina E.M., el-Azzouni O.E. Smectite in acute diarrhea in children: a double-blind placebo-controlled clinical trial. Journal of Pediatric Gastroenterology and Nutrition. 1993;17:176–181. doi: 10.1097/00005176-199308000-00008. [DOI] [PubMed] [Google Scholar]
- Ngo K.S., Brow G.D. Stilbenes, monoterpenes, diarylheptanoids, labdanes and halcons from Alpinia katsumadai. Phytochemistry. 1998;47:1117–1123. [Google Scholar]
- Park S.H., Saif L.J., Jeong C., Lim G.K., Park S.I., Kim H.H., Park S.J., Kim Y.J., Jeong J.H., Kang M.I., Cho K.O. Molecular characterization of novel G5 bovine rotavirus strains. Journal of Clinical Microbiology. 2006;44:4101–4112. doi: 10.1128/JCM.01196-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee D.F., Sidwell R.W., Clark S.M., Barnett B.B., Spendlove R.S. Inhibition of rotavirus by selected antiviral substances: mechanism of viral inhibition and in vivo activity. Antimicrobial Agents and Chemotherapy. 1982;21:66–73. doi: 10.1128/aac.21.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Matsuda M., Ohashi K., Taniguchi K., Nakagomi O., Abe Y., Mori S., Sato N., Okutani K., Shigeta S. Analysis of anti-rotavirus activity of extract from Stevia rebaudiana. Antiviral Research. 2001;49:15–24. doi: 10.1016/s0166-3542(00)00134-0. [DOI] [PubMed] [Google Scholar]
- Tang W., Eisenbrand G. Springer; Berlin: 1992. Chinese Drugs of Plant Origin. p. 87. [Google Scholar]
- Tonew M., Tonew E., Mentel R. The antiviral activity of dipyridamole. Acta Virologica. 1977;21:146–150. [PubMed] [Google Scholar]
- Yang Y., kinoshita K., Koyama K., Takahashi K., Tai T., Nunoura Y., Watanabe K. Anti-emetic principles of Alpinia katsumadai Hayata. Natural Product Sciences. 1999;5:20–24. doi: 10.1021/np990096e. [DOI] [PubMed] [Google Scholar]
- Yolken R.H., Losonsky G.A., Vonderfecht S., Leister F., Wee S.B. Antibody to human rotavirus in cow’s milk. The New England Journal of Medicine. 1985;312:605–610. doi: 10.1056/NEJM198503073121002. [DOI] [PubMed] [Google Scholar]


