Highlights
► RNA molecules play a major role in all aspects of metabolism and their conformational state is controlled by RNA remodeling motor proteins. ► RNA remodeling proteins can be described in three major structural categories: the hexameric ring proteins, the processive monomeric RNA translocase/helicases, and the functionally diverse DEAD-box remodeling proteins. ► Biophysical studies have revealed new molecular mechanisms and functions for RNA remodeling proteins.
Abstract
It is becoming increasingly clear that RNA molecules play a major role in all aspects of metabolism. The conformational state and stability of RNA are controlled by RNA remodeling proteins, which are ubiquitous motor proteins in the cell. Here, we review advances in our understanding of the structure and function of three major structural families of RNA remodeling proteins, the hexameric ring proteins, the processive monomeric RNA translocase/helicases, and the functionally diverse DEAD-box remodeling proteins. New studies have revealed molecular mechanisms for coupling between ATP hydrolysis and unwinding, the physical basis for regulatory control by cofactors, and novel functions for RNA remodeling proteins.
While only a small portion of our genome is translated into protein, almost the entire genome is transcribed into RNA molecules [1]. Large and small, structured and flexible, coding and noncoding, RNAs are strikingly diverse and involved in every aspect of our metabolism. Many of these RNAs must change shape and structure during the course of their functional lifetimes, sometimes cycling through several conformations in order to regulate linear series of metabolic events, such as the stages of RNA splicing. One of the most dramatic examples of this behavior is ‘RNA thermosensors’, which are regions of RNA that change conformation as a function of temperature, thereby regulating the translation of adjacent genes [2, 3]. However, the structural gymnastics of RNA folding and unfolding often require additional help from proteins [4]. These ‘RNA remodeling enzymes’ fall into several families with diverse structural features and functional behaviors [5, 6, 7]. In most reviews on this topic, these enzymes are grouped and functionally classified through phylogenetic analysis. However, it is becoming increasingly clear that the conserved ‘motor domains’ within these enzymes can be combined and utilized in a variety of ways. As a result, remodeling proteins with the same ‘core’ can perform different tasks, and proteins with divergent ‘cores’ can behave quite similarly. For purposes of this review, the enzymes are grouped by structural and mechanical features, as these can provide different insights into physical behavior.
Ring around the RNA
We typically think of helicases as fuel-driven motors, which couple ATP hydrolysis to protein conformational changes that stimulate RNA unwinding. However, there is growing evidence that some proteins can couple binding energy to RNA duplex destabilization, formally behaving as helicases without ATP hydrolysis. For example, the eukaryotic exosome threads single-stranded RNA through its central ring, unwinding RNA without ATP hydrolysis [8]. One can imagine that the ring-like Ro autoantigen might have similar properties [9].
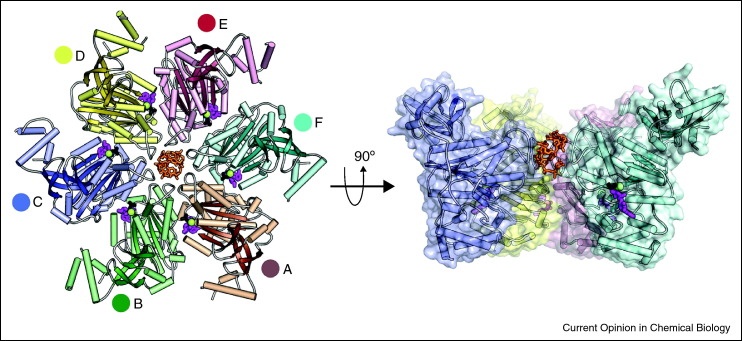
However, hexameric rings are one of the most common types of nucleic acid unwinding motors that encircle single-stranded RNA and strip away complementary strands in an ATP-dependent fashion [10, 11]. The best-studied example is the Rho transcription termination factor, which has been visualized crystallographically in complex with ATP analogs and an RNA translocation substrate [12••] (Figure 1 ). These structures show that the protein subunits wrap around the RNA strand like ‘spiral staircase’. Each subunit projects a loop into the center of the ring, which captures and engages functional groups on the ribose moiety. The relative RNA engagement of each protein subunit is directly coupled to its ATP hydrolysis state, suggesting that each subunit takes its turn at the leading edge of travel, pulling RNA through the ring and moving the protein in a 5′ → 3′ direction. Remarkably, the papillomavirus E1 ring helicase pulls DNA through its central ring in the opposite direction [13], appearing to tug on the backbone with loops in the opposite orientation.
Figure 1.
The hexameric Rho helicase bound to an RNA translocation substrate and ATP analogs. On the left is a top-down view of single-stranded RNA threading through the hexameric ring. The ATP analog ADP-BeF3 (pink) is bound at the interface of each RecA-fold subunit. There is an increasing level of engagement with ADP-BeF3 as one progresses from subunits F to B, signifying their respective place in each hydrolysis/translocation cycle.
Reprinted with permission from Thomsen and Berger [12••].
Biochemical studies on Rho translocation suggest, however, that the overall mechanism is more complicated [11]. Chemogenetic and kinetic methods have shown that some positions along the sugar-phosphate backbone are more important than others. While one might expect that each 2′-hydroxyl group is recognized in the same way by Rho, the functional analysis indicates that every seventh 2′-hydroxyl group plays a particularly important role in the mechanism [14•]. This suggests that future research on Rho may reveal additional events along the translocation pathway or different types of engagement between protein and RNA than has been visualized to date.
Processive, monomeric RNA helicase enzymes
It is often thought that nonhexameric RNA helicases lack the processivity of their phylogenetically related counterparts, the SF1 and SF2 DNA helicases. However, a subset of RNA helicases unwinds RNA in a highly processive manner [6]. Phylogenetically, these are often SF2 helicases that contain the DExH signature in conserved Motif II and which lack the ‘Q’ motif that typifies their DEAD-box cousins [15]. Structurally, all these enzymes contain a fundamental motor unit (Domains 1 and 2) composed of two RecA-like domains that hydrolyze ATP and bind RNA using conserved amino acids at the domain interface (Motifs I–VI) [15]. But they also contain additional domains [16••], such as Domain 3 in Flaviviral helicases. Accessory domains can rigidify the relative position of Domains 1 and 2, perching atop the RNA-binding cleft and providing an extensive RNA interface [6]. Biologically, these proteins have a range of function, including roles in RNA surveillance and decay, as illustrated by new structural work on the Mtr4 helicase [16••]. Some of the most well-studied examples derive from viral systems for replication or transcription. For example, the Dengue [17••, 18] and Hepatitis C NS3 proteins [19••, 20••, 21] and the vaccinia NPH-II proteins [22] have been the subject of intensive structural and mechanistic investigation [6, 7]. Processive RNA helicases from other systems, such as coronaviruses [23], provide rich areas for future investigation.
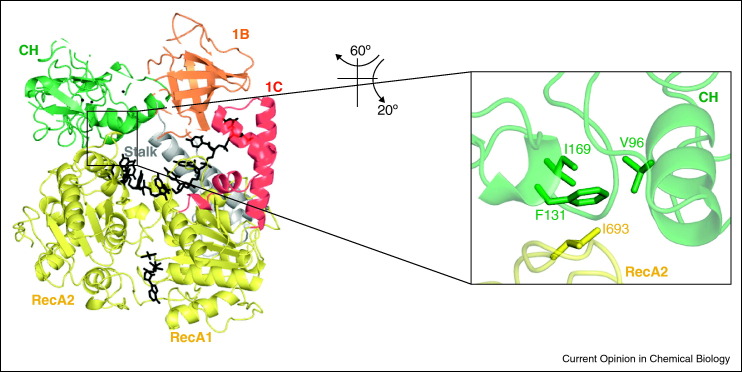
An important exception on many levels is the Upf1 helicase [24], which plays a key role in the quality control of eukaryotic messenger RNA. Upf1 is phylogenetically classified as an SF1 helicase (like UvrD or Rep), but it unwinds RNA in a cofactor-dependent fashion [25]. Its additional allosteric ‘RNA clasping’ domains (1B and CH, in this case) can open and shut, engaging and disengaging from the RNA-binding cleft and thereby controlling helicase behavior [26••] (Figure 2 ). The ‘on–off switch’ [24], that controls dynamics of the cleft is a regulatory protein called Upf2, which binds Upf1 and modulates its function [26••, 27]. Thus, the accessory domains of Upf1 have multiple conformations that permit control of translocation and helicase activity by cofactor proteins.
Figure 2.
Structure of Upf1 containing the CH and 1B domains. These domains (green and orange, respectively) help the RecA folds (yellow) to clamp down on the RNA molecule (black). Note the interface between the CH domain and the RecA2 domain (inset).
Reprinted with permission from Chakrabarti et al. [26••].
The physical basis for processive translocation on RNA was recently revealed through structural studies on the HCV NS3 protein [19••]. By crystallizing full-length NS3 in the presence of bound RNA and an ATP analog, these studies show that Domains 1 and 2 open and close in response to ATP binding, and that RNA translocates by one nucleotide in the process. Consistent with its role in the formation of the RNA-binding cleft, Domain 3 clamps down in response to RNA binding. Similarly, a series of structures capturing a shortened NS3 helicase construct in the presence of ATP and DNA suggest a single nucleotide step for ATP-dependent translocation [20••]. Despite these similarities, there are important differences in NS3 conformation when RNA (the natural substrate) is bound [19••]. For example, conserved residue Thr416, which has long been implicated in translocation of SF2 proteins, is only engaged with the phosphate backbone in the structure containing RNA [19••]. Both these studies are consistent with single molecule investigations of ATP-dependent translocation by NS3 [28].
While translocation of RNA helicases appears to be largely explained from structural and biochemical experimentation, the actual mechanism of unwinding remains an area of intense investigation. Single molecule experiments on HCV NS3 show that strand separation does not take place in one base-pair steps, but in larger units of at least three base pairs [28, 29]. Kinetic studies indicate that the rate constant for unwinding of each three base-pair step corresponds to the rate constant for phosphate release after ATP hydrolysis, suggesting that phosphate release is the power stroke for duplex unwinding [30••]. The release of top-strand RNA occurs in even larger steps of at least 10 base pairs [29, 31, 32]. There is not yet a clear physical understanding of these behaviors, which may be linked to the function of other domains appended to the helicase.
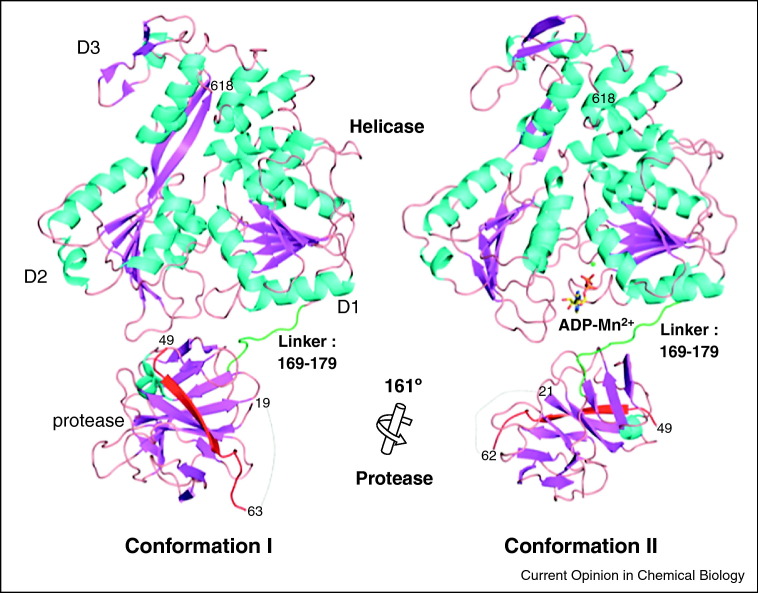
The NS3 proteins from Flaviviral and Hepaciviral replication complexes contain a fourth domain that plays a role in helicase function of these enzymes [6]. This domain is a serine protease, and in the case of HCV NS3, it plays a major role in RNA binding and stepping behavior [33••, 34]. A structural basis for this remained unclear until the crystal structures of the Dengue and West Nile NS3 proteins revealed that the protease domain swings beneath the D1 and D2 ATPase domains [17••, 18, 35], in a location that can readily influence ATPase and unwinding functions (Figure 3 ). Biophysical studies on the full-length HCV NS3 protein suggest that a similar conformation also occurs in HCV NS3, and that it is required for unwinding of RNA [36••]. Intriguingly, crystallographic investigations of full-length HCV NS3 depict the protease domain in a different position, packed against the back of D1–3 [19••, 37]. This enclosed structural state is also biologically relevant and it represents the conformation required for autoproteolysis [36••]. Thus, HCV NS3 has at least two distinct conformational forms, which may allow it to toggle between its dual roles in proteolysis and helicase activity, and potentially regulate viral function.
Figure 3.
Two conformations of the NS3 protein from Dengue virus. In both conformations, the protease domain is located beneath Domains 1 and 2, but it has been visualized in two possible rotational conformations, which differ in their ability to interact with nucleotide.
Reprinted from Luo et al. [17••].
Rearranging and letting go: the multifunctional DEAD-box proteins
The most ubiquitous RNA remodeling machines are the DEAD-box proteins, which are named for the conserved sequence within ATPase Motif II [38]. These proteins contain a minimal ATPase motor consisting only of Domains 1 and 2, which is mechanically coupled to a diversity of other protein substructures. Domains 1 and 2 are loosely connected until the binding of ligands (RNA, small molecules or other proteins) brings them together and forms an ATP-binding cleft at their interface. Structural studies show that the ATP-binding cleft is deeply buried within DEAD-box proteins [39], suggesting particularly tight coupling of ATP hydrolysis with mechanical work. This effect may be accentuated by the presence of the ‘Q-motif’, which is an ATP-binding site unique to DEAD-box proteins that allows for total specificity for ATP rather than other nucleotide triphosphates [38].
Some DEAD-box proteins can function as helicases, and RNA unwinding by these proteins contributes to many biological processes [7, 38]. However, the basic mechanical core of DEAD-box proteins is coupled to diverse protein scaffolds, leading to different mechanical functions by family members containing the same set of conserved ATPase motifs [40•]. DEAD-box proteins serve as anchors for protein complexes [41], RNP remodeling enzymes [38], catalysts for RNA folding [40•, 42••, 43], agents for RNA transport [44••], and many other activities, often regulated by cofactor proteins [44••, 45•]. Thus, it is risky to assume from sequence alone that a DEAD-box protein is a helicase.
For DEAD-box proteins where the mechanochemical cycle has been studied, ATP hydrolysis (specifically, at the stage of Pi release) stimulates the dissociation of bound RNA molecules [46••, 47], allowing recycling of these ‘single-use’ proteins [7]. In some cases, small molecules or bound proteins also stimulate RNA release [44••]. Studies of the DbpA protein have provided insights into the coupling between ATP hydrolysis and RNA unwinding [48••]. DbpA maintains high affinity for RNA in the ATP-bound state and RNA duplex destabilization occurs upon ATP hydrolysis, as the protein enters the ADP.Pi state. Release of Pi causes displacement of the protein from the RNA strand, allowing one round of unwinding a short duplex. It is important to note, however, that single-strand translocation by DEAD-box proteins has not yet been directly investigated. It is entirely possible that there are functions of these proteins (such as translocation) where the protein is not completely released from the lattice during a cycle of ATP hydrolysis and where a cycle of weak and strong binding may instead propel unidirectional motion, as in cytoskeletal motor proteins.
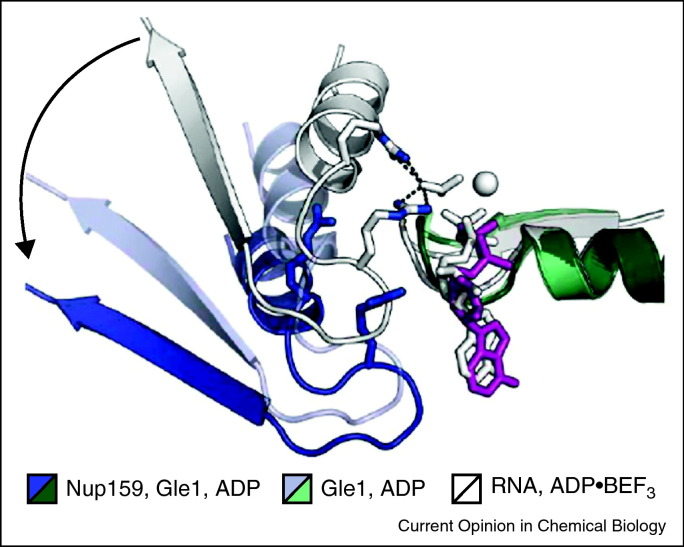
Dbp5 is an RNP remodeling protein that sits on the edge of the nuclear pore complex, where it plays an essential role in RNA export [49, 50]. Dbp5 is activated by the small molecule cofactor inositol 6-phosphate (IsP6) and it releases RNA upon every cycle of ATP hydrolysis, suggesting a model for release of exported RNA [44••]. Recent structural analyses reveal a mechanochemical cycle in which RNA and nucleotide release are stimulated through the binding of protein cofactors Gle1 and Nup159, which crank open the cleft between Domains 1 and 2, releasing ADP and bound RNA [44••] (Figure 4 ).
Figure 4.
Cofactors modulate the active site of Dbp5: the C-terminal domain of Dbp5 moves from an active state that is engaged with nucleotide (white), to a progressively inactive state in the presence of Nup159 (purple), where a catalytically essential arginine is completely displaced.
Reprinted with permission from Montpetit et al. [44••].
During the process of RNA folding in vivo [42••], proteins assist in the destabilization of misfolded intermediates and in the stabilization of weak folding intermediates. Many of these proteins are DEAD-box proteins [43], and Mss116 is a well-studied example [42••]. Mss116 is known to stimulate group II intron self-splicing in vivo [51], and it can enable certain group II introns to self-splice in vitro under physiological ionic conditions [52]. Like many DEAD-box proteins, Mss116 is capable of unwinding short RNA duplexes [46••, 52, 53], however, studies of Mss116 mutants in vivo and in vitro show that its role in splicing is not necessarily dependent on helicase activity [52, 54•]. Ensemble and single molecule experiments [55••, 56], along with folding studies conducted in vivo [42••], show that Mss116 stabilizes an RNA folding intermediate that is required along the pathway to group II intron assembly. ATP hydrolysis is required only during the rapid final stage of intron folding, suggesting that protein release is required for consolidation of the catalytic domains and for turnover of the protein [55••, 56]. Thus, certain DEAD-box proteins may serve as stabilizing RNA-binding proteins, rather than destabilizing helicases. Unlike many other types of RNA-binding proteins, DEAD-box proteins are readily ‘removable’ in a controlled fashion, as ATP hydrolysis stimulates their release from RNA.
Conclusions
In many ways, RNA helicases function similarly to DNA helicases, and they have close phylogenetic relationships. However, the basic motor unit that is shared among all these proteins is utilized in diverse ways in RNA metabolism, resulting in a functional repertoire that goes beyond translocation and unwinding. Thus, the functional demands of RNA metabolism have resulted in molecular motors with new mechanical behaviors and properties.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
References
- 1.Birney E., Stamatoyannopoulos J.A., Dutta A., Guigo R., Gingeras T.R., Margulies E.H., Weng Z., Snyder M., Dermitzakis E.T., Thurman R.E. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447:799–816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giuliodori A.M., Di Pietro F., Marzi S., Masquida B., Wagner R., Romby P., Gualerzi C.O., Pon C.L. The cspA mRNA is a thermosensor that modulates translation of the cold-shock protein CspA. Mol Cell. 2010;37:21–33. doi: 10.1016/j.molcel.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 3.Kortmann J., Sczodrok S., Rinnenthal J., Schwalbe H., Narberhaus F. Translation on demand by a simple RNA-based thermosensor. Nucleic Acids Res. 2011;39:2855–2868. doi: 10.1093/nar/gkq1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owttrim G.W. RNA helicases and abiotic stress. Nucleic Acids Res. 2006;34:3220–3230. doi: 10.1093/nar/gkl408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linder P., Owttrim G.W. Plant RNA helicases: linking aberrant and silencing RNA. Trends Plant Sci. 2009;14:344–352. doi: 10.1016/j.tplants.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Pyle A.M. Translocation and unwinding mechanisms of RNA and DNA helicases. Annu Rev Biophys. 2008;37:317–336. doi: 10.1146/annurev.biophys.37.032807.125908. [DOI] [PubMed] [Google Scholar]
- 7.Jankowsky E. RNA helicases at work: binding and rearranging. Trends Biochem Sci. 2011;36:19–29. doi: 10.1016/j.tibs.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonneau F., Basquin J., Ebert J., Lorentzen E., Conti E. The yeast exosome functions as a macromolecular cage to channel RNA substrates for degradation. Cell. 2009;139:547–559. doi: 10.1016/j.cell.2009.08.042. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs G., Stein A.J., Fu C., Reinisch K.M., Wolin S.L. Structural and biochemical basis for misfolded RNA recognition by the Ro autoantigen. Nat Struct Mol Biol. 2006;13:1002–1009. doi: 10.1038/nsmb1156. [DOI] [PubMed] [Google Scholar]
- 10.Lyubimov A.Y., Strycharska M., Berger J.M. The nuts and bolts of ring-translocase structure and mechanism. Curr Opin Struct Biol. 2011;21:240–248. doi: 10.1016/j.sbi.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabhi M., Tuma R., Boudvillain M. RNA remodeling by hexameric RNA helicases. RNA Biol. 2010;7:655–666. doi: 10.4161/rna.7.6.13570. [DOI] [PubMed] [Google Scholar]
- 12••.Thomsen N.D., Berger J.M. Running in reverse: the structural basis for translocation polarity in hexameric helicases. Cell. 2009;139:523–534. doi: 10.1016/j.cell.2009.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]; A landmark paper that not only reveals mechanistic strategies for the Rho helicase but also provides fresh insights into the molecular basis for unidirectional motion by motor proteins.
- 13.Enemark E.J., Joshua-Tor L. Mechanism of DNA translocation in a replicative hexameric helicase. Nature. 2006;442:270–275. doi: 10.1038/nature04943. [DOI] [PubMed] [Google Scholar]
- 14•.Schwartz A., Rabhi M., Jacquinot F., Margeat E., Rahmouni A.R., Boudvillain M. A stepwise 2′-hydroxyl activation mechanism for the bacterial transcription termination factor Rho helicase. Nat Struct Mol Biol. 2009;16:1309–1316. doi: 10.1038/nsmb.1711. [DOI] [PubMed] [Google Scholar]; This work is complementary to crystallographic investigations of Rho because it utilizes chemical biology methods to examine the functional role of individual RNA atoms in the mechanism.
- 15.Fairman-Williams M.E., Guenther U.P., Jankowsky E. SF1 and SF2 helicases: family matters. Curr Opin Struct Biol. 2010;20:313–324. doi: 10.1016/j.sbi.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16••.Weir J.R., Bonneau F., Hentschel J., Conti E. Structural analysis reveals the characteristic features of Mtr4, a DExH helicase involved in nuclear RNA processing and surveillance. Proc Natl Acad Sci U S A. 2010;107:12139–12144. doi: 10.1073/pnas.1004953107. [DOI] [PMC free article] [PubMed] [Google Scholar]; A paradigmatic example of the diverse ways that auxiliary domains can supplement the functions and activities of the SF2 motor core.
- 17••.Luo D., Wei N., Doan D.N., Paradkar P.N., Chong Y., Davidson A.D., Kotaka M., Lescar J., Vasudevan S.G. Flexibility between the protease and helicase domains of the dengue virus NS3 protein conferred by the linker region and its functional implications. J Biol Chem. 2010;285:18817–18827. doi: 10.1074/jbc.M109.090936. [DOI] [PMC free article] [PubMed] [Google Scholar]; This elegant combination of crystallography and biochemistry underscores the importance auxiliary helicase domains, and shows that the linker regions among domains play specific roles in the mechanism.
- 18.Luo D., Xu T., Hunke C., Gruber G., Vasudevan S.G., Lescar J. Crystal structure of the NS3 protease-helicase from dengue virus. J Virol. 2008;82:173–183. doi: 10.1128/JVI.01788-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19••.Appleby T.C., Anderson R., Fedorova O., Pyle A.M., Wang R., Liu X., Brendza K.M., Somoza J.R. Visualizing ATP-dependent RNA translocation by the NS3 helicase from HCV. J Mol Biol. 2011;405:1139–1153. doi: 10.1016/j.jmb.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first structure of NS3 on its natural substrate, RNA, made particularly significant because NS3 is captured in its full-length form containing all auxiliary domains. The complex is captured through multiple states of ATP hydrolysis, which are coupled with active translocation of an oligonucleotide in the crystal.
- 20••.Gu M., Rice C.M. Three conformational snapshots of the hepatitis C virus NS3 helicase reveal a ratchet translocation mechanism. Proc Natl Acad Sci U S A. 2010;107:521–528. doi: 10.1073/pnas.0913380107. [DOI] [PMC free article] [PubMed] [Google Scholar]; The helicase domain of NS3 is captured bound to DNA, in various states of ATP binding and hydrolysis.
- 21.Matlock D.L., Yeruva L., Byrd A.K., Mackintosh S.G., Langston C., Brown C., Cameron C.E., Fischer C.J., Raney K.D. Investigation of translocation, DNA unwinding, and protein displacement by NS3h, the helicase domain from the hepatitis C virus helicase. Biochemistry. 2010;49:2097–2109. doi: 10.1021/bi901977k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor S.D., Solem A., Kawaoka J., Pyle A.M. The NPH-II helicase displays efficient DNA × RNA helicase activity and a pronounced purine sequence bias. J Biol Chem. 2010;285:11692–11703. doi: 10.1074/jbc.M109.088559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ivanov K.A., Ziebuhr J. Human coronavirus 229E nonstructural protein 13: characterization of duplex-unwinding, nucleoside triphosphatase, and RNA 5′-triphosphatase activities. J Virol. 2004;78:7833–7838. doi: 10.1128/JVI.78.14.7833-7838.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gleghorn M.L., Maquat L.E. UPF1 learns to relax and unwind. Mol Cell. 2011;41:621–623. doi: 10.1016/j.molcel.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Chamieh H., Ballut L., Bonneau F., Le Hir H. NMD factors UPF2 and UPF3 bridge UPF1 to the exon junction complex and stimulate its RNA helicase activity. Nat Struct Mol Biol. 2008;15:85–93. doi: 10.1038/nsmb1330. [DOI] [PubMed] [Google Scholar]
- 26••.Chakrabarti S., Jayachandran U., Bonneau F., Fiorini F., Basquin C., Domcke S., Le Hir H., Conti E. Molecular mechanisms for the RNA-dependent ATPase activity of Upf1 and its regulation by Upf2. Mol Cell. 2011;41:693–703. doi: 10.1016/j.molcel.2011.02.010. [DOI] [PubMed] [Google Scholar]; This paper shows how binding of exogenous factors can serve as ‘on–off’ switches for regulating helicase function, providing a paradigm for mechanical control.
- 27.Clerici M., Mourao A., Gutsche I., Gehring N.H., Hentze M.W., Kulozik A., Kadlec J., Sattler M., Cusack S. Unusual bipartite mode of interaction between the nonsense-mediated decay factors, UPF1 and UPF2. EMBO J. 2009;28:2293–2306. doi: 10.1038/emboj.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Myong S., Bruno M.M., Pyle A.M., Ha T. Spring-loaded mechanism of DNA unwinding by hepatitis C virus NS3 helicase. Science. 2007;317:513–516. doi: 10.1126/science.1144130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dumont S., Cheng W., Serebrov V., Beran R.K., Tinoco I., Jr., Pyle A.M., Bustamante C. RNA translocation and unwinding mechanism of HCV NS3 helicase and its coordination by ATP. Nature. 2006;439:105–108. doi: 10.1038/nature04331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30••.Wang Q., Arnold J.J., Uchida A., Raney K.D., Cameron C.E. Phosphate release contributes to the rate-limiting step for unwinding by an RNA helicase. Nucleic Acids Res. 2010;38:1312–1324. doi: 10.1093/nar/gkp1118. [DOI] [PMC free article] [PubMed] [Google Scholar]; A much-needed investigation into the actual mechanical events that result from ATP binding and hydrolysis by NS3, revealing the basic power stroke during NS3 unwinding.
- 31.Serebrov V., Beran R.K., Pyle A.M. Establishing a mechanistic basis for the large kinetic steps of the NS3 helicase. J Biol Chem. 2009;284:2512–2521. doi: 10.1074/jbc.M805460200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serebrov V., Pyle A.M. Periodic cycles of RNA unwinding and pausing by hepatitis C virus NS3 helicase. Nature. 2004;430:476–480. doi: 10.1038/nature02704. [DOI] [PubMed] [Google Scholar]
- 33••.Rajagopal V., Gurjar M., Levin M.K., Patel S.S. The protease domain increases the translocation stepping efficiency of the hepatitis C virus NS3-4A helicase. J Biol Chem. 2010;285:17821–17832. doi: 10.1074/jbc.M110.114785. [DOI] [PMC free article] [PubMed] [Google Scholar]; A valuable investigation of the mechanical role played by the auxiliary serine protease domain during helicase function.
- 34.Beran R.K., Serebrov V., Pyle A.M. The serine protease domain of hepatitis C viral NS3 activates RNA helicase activity by promoting the binding of RNA substrate. J Biol Chem. 2007;282:34913–34920. doi: 10.1074/jbc.M707165200. [DOI] [PubMed] [Google Scholar]
- 35.Assenberg R., Mastrangelo E., Walter T.S., Verma A., Milani M., Owens R.J., Stuart D.I., Grimes J.M., Mancini E.J. Crystal structure of a novel conformational state of the flavivirus NS3 protein: implications for polyprotein processing and viral replication. J Virol. 2009;83:12895–12906. doi: 10.1128/JVI.00942-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36••.Ding S.C., Kohlway A.S., Pyle A.M. Unmasking the active helicase conformation of nonstructural protein 3 from hepatitis C virus. J Virol. 2011;85:4343–4353. doi: 10.1128/JVI.02130-10. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work shows that, like the NS3 protein from Dengue virus, the serine protease domain exists in two conformations that toggle back and forth, potentially helping to coordinate protease activity with helicase activity.
- 37.Yao N., Reichert P., Taremi S.S., Prosise W.W., Weber P.C. Molecular views of viral polyprotein processing revealed by the crystal structure of the hepatitis C virus bifunctional protease–helicase. Structure. 1999;7:1353–1363. doi: 10.1016/s0969-2126(00)80025-8. [DOI] [PubMed] [Google Scholar]
- 38.Cordin O., Banroques J., Tanner N.K., Linder P. The DEAD-box protein family of RNA helicases. Gene. 2006;367:17–37. doi: 10.1016/j.gene.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 39.Sengoku T., Nureki O., Nakamura A., Kobayashi S., Yokoyama S. Structural basis for RNA unwinding by the DEAD-box protein Drosophila Vasa. Cell. 2006;125:287–300. doi: 10.1016/j.cell.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 40•.Zingler N., Solem A., Pyle A.M. Dual roles for the Mss116 cofactor during splicing of the ai5gamma group II intron. Nucleic Acids Res. 2010;38:6602–6609. doi: 10.1093/nar/gkq530. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work shows that, in a single system, a DEAD-box protein can be playing more than one mechanistic role. Here, the yeast Mss116 protein is shown to act as an annealing enzyme and as a helicase on different parts of an RNA.
- 41.Bono F., Ebert J., Lorentzen E., Conti E. The crystal structure of the exon junction complex reveals how it maintains a stable grip on mRNA. Cell. 2006;126:713–725. doi: 10.1016/j.cell.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 42••.Liebeg A., Mayer O., Waldsich C. DEAD-box protein facilitated RNA folding in vivo. RNA Biol. 2010;7:803–811. doi: 10.4161/rna.7.6.13484. [DOI] [PMC free article] [PubMed] [Google Scholar]; A particularly important analysis of Mss16-assisted RNA folding because it evaluates the functional and structural role of the protein in vivo.
- 43.Pan C., Russell R. Roles of DEAD-box proteins in RNA and RNP folding. RNA Biol. 2010;7:667–676. doi: 10.4161/rna.7.6.13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44••.Montpetit B., Thomsen N.D., Helmke K.J., Seeliger M.A., Berger J.M., Weis K. A conserved mechanism of DEAD-box ATPase activation by nucleoporins and InsP6 in mRNA export. Nature. 2011;472:238–242. doi: 10.1038/nature09862. [DOI] [PMC free article] [PubMed] [Google Scholar]; This structure reveals the Dbp5 export motor imbedded within the complex containing other proteins involved in its function, revealing roles for small molecule activators and a conserved mechanism for ATP hydrolysis in RNA release.
- 45•.Marintchev A., Edmonds K.A., Marintcheva B., Hendrickson E., Oberer M., Suzuki C., Herdy B., Sonenberg N., Wagner G. Topology and regulation of the human eIF4A/4G/4H helicase complex in translation initiation. Cell. 2009;136:447–460. doi: 10.1016/j.cell.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]; A major advance, as it reveals the structural context and mechanistic features of the paradigmatic eIF4A motor.
- 46••.Cao W., Coman M.M., Ding S., Henn A., Middleton E.R., Bradley M.J., Rhoades E., Hackney D.D., Pyle A.M., De La Cruz E.M. Mechanism of Mss116 ATPase reveals functional diversity of DEAD-box proteins. J Mol Biol. 2011;409:399–414. doi: 10.1016/j.jmb.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; The complete kinetic cycle for Mss116 binding and hydrolysis of ATP as a function of RNA coupling. It reveals basic mechanical features of DEAD-box proteins. The work is comparable with Henn et al. [47], on DEAD-box protein DbpA.
- 47.Henn A., Cao W., Hackney D.D., De La Cruz E.M. The ATPase cycle mechanism of the DEAD-box rRNA helicase, DbpA. J Mol Biol. 2008;377:193–205. doi: 10.1016/j.jmb.2007.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48••.Henn A., Cao W., Licciardello N., Heitkamp S.E., Hackney D.D., De La Cruz E.M. Pathway of ATP utilization and duplex rRNA unwinding by the DEAD-box helicase, DbpA. Proc Natl Acad Sci U S A. 2010;107:4046–4050. doi: 10.1073/pnas.0913081107. [DOI] [PMC free article] [PubMed] [Google Scholar]; Detailed kinetic investigation of the role of RNA binding and unwinding in the ATP cycle of a DEAD-box protein. Among the most complete studies to date on cycling.
- 49.Tran E.J., Zhou Y., Corbett A.H., Wente S.R. The DEAD-box protein Dbp5 controls mRNA export by triggering specific RNA:protein remodeling events. Mol Cell. 2007;28:850–859. doi: 10.1016/j.molcel.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 50.Weirich C.S., Erzberger J.P., Flick J.S., Berger J.M., Thorner J., Weis K. Activation of the DExD/H-box protein Dbp5 by the nuclear-pore protein Gle1 and its coactivator InsP6 is required for mRNA export. Nat Cell Biol. 2006;8:668–676. doi: 10.1038/ncb1424. [DOI] [PubMed] [Google Scholar]
- 51.Huang H.R., Rowe C.E., Mohr S., Jiang Y., Lambowitz A.M., Perlman P.S. The splicing of yeast mitochondrial group I and group II introns requires a DEAD-box protein with RNA chaperone function. Proc Natl Acad Sci U S A. 2005;102:163–168. doi: 10.1073/pnas.0407896101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Solem A., Zingler N., Pyle A.M. A DEAD protein that activates intron self-splicing without unwinding RNA. Mol Cell. 2006;24:611–617. doi: 10.1016/j.molcel.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 53.Yang Q., Del Campo M., Lambowitz A.M., Jankowsky E. DEAD-box proteins unwind duplexes by local strand separation. Mol Cell. 2007;28:253–263. doi: 10.1016/j.molcel.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 54•.Bifano A.L., Turk E.M., Caprara M.G. Structure-guided mutational analysis of a yeast DEAD-box protein involved in mitochondrial RNA splicing. J Mol Biol. 2010;398:429–443. doi: 10.1016/j.jmb.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]; A valuable mutational/mechanistic investigation of Mss116 function in vivo.
- 55••.Karunatilaka K.S., Solem A., Pyle A.M., Rueda D. Single-molecule analysis of Mss116-mediated group II intron folding. Nature. 2010;467:935–939. doi: 10.1038/nature09422. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of the first single-molecule investigations that examines the role of proteins in an RNA folding pathway. In this case, it reveals different roles for the Mss116 protein at various stages of a single pathway.
- 56.Fedorova O., Solem A., Pyle A.M. Protein-facilitated folding of group II intron ribozymes. J Mol Biol. 2010;397:799–813. doi: 10.1016/j.jmb.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]