Abstract
Aerosolization procedures during the COVID-19 pandemic place all operating room personnel at risk for exposure. We offer detailed perioperative management strategies and present a specific protocol designed to improve safety during pediatric laryngoscopy and bronchoscopy. Several methods of using disposable drapes for various procedures are described, with the goal of constructing a tent around the patient to decrease widespread contamination of dispersed droplets and generated aerosol. The concepts presented herein are translatable to future situations where aerosol generating procedures increase risk for any pathogenic exposure. This protocol is a collaborative effort based on knowledge gleaned from clinical and simulation experience from Children's Hospital Colorado, Children's Hospital of Philadelphia, The Hospital for Sick Children in Toronto, and Boston Children's Hospital.
Keywords: COVID-19, SARS CoV2, Coronavirus, Pediatric airway, Laryngoscopy, Bronchoscopy, Suspension, Droplet, Precaution, Aerosol generating procedures
1. Introduction
The worldwide COVID-19 pandemic, instigated by the SARS-CoV-2 virus, was first identified in December 2019 in Wuhan, Hubei province, China. Despite the fatality rate appearing to be lower for COVID-19 than other coronavirus outbreaks such as Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS), the elevated transmission rate of SARS-CoV-2, long incubation period and asymptomatic spread have resulted in a large number of deaths, quickly surpassing the combined totals of the SARS and MERS outbreaks [1]. Transmission to healthcare workers caring for patients with COVID-19 was described as early as January 2020, which soon translated to healthcare provider deaths [2,3]. The first reported physician death related to COVID-19 was an otolaryngologist in Wuhan, China [4].
Asymptomatic or mildly symptomatic patients are thought to be responsible for the rapid spread of COVID-19, accounting for up to 79% of documented cases [5]. Transmission of the SARS-CoV-2 virus from these patients is common because viral shedding begins before the onset of symptoms [[6], [7], [8]]. The airway of infected patients has been shown to have a high viral load, especially in the nose, throat and trachea [9]. Otolaryngologists, oral surgeons, dentists, ophthalmologists, and anesthesiologists are among those at highest risk for infection, given the procedures they perform in or near the upper aerodigestive tract. Additionally, airway procedures often result in aerosolization of viral product, placing all healthcare workers present during these procedures at high risk for infection [10].
Various societies have recommended that only essential procedures be performed and that appropriate personal protective equipment (PPE) is required during the COVID-19 pandemic (https://www.entuk.org/covid-19) [11]. Appropriate PPE for all airway procedures should include, at minimum, an N95 respirator, face shield, gown, cap, and gloves that cover the gown [4]. There have been reports of practitioners donning PPE, including an N95 respirator, who have still been infected with COVID-19 during aerosolization procedures such as sinus surgery [10]. The Center for Disease Control and Prevention (CDC) currently recommends prioritizing negative pressure rooms and powered, air-purifying respirators (PAPRs) for aerosol-generating procedures (https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html).
To protect health care workers and hospital staff during and after surgery, it is important to prevent the widespread contamination of dispersed droplets and generated aerosol that are produced by certain interventions. Protocols have been proposed to help protect healthcare workers and their patients in the setting of necessary aerosol-generating procedures including a reusable acrylic barrier enclosure during standard intubation [12]. While having a reusable barrier saves on resources, it may be difficult to fully disinfect and could potentially spread the virus across patients. Other groups have provided extremely helpful broad insights on how to manage various necessary high-risk otolaryngologic procedures during the current pandemic, including rigid microlaryngoscopy and bronchoscopy (MLB) [13,14]. In this article, we offer further detailed perioperative management strategies and present a novel specific protocol designed to improve safety by decreasing intraoperative aerosolized viral product during pediatric MLB by utilizing disposable drapes to create a contained tent immediately around the patient. While this protocol was developed to be used during the COVID-19 pandemic, the concepts are likely translatable to any future pandemic where aerosol generating procedures increase risk of pathogenic exposure. This protocol is the result of a collaborative effort between pediatric otolaryngologists, anesthesiologists, occupational medicine specialists, nursing staff and medical engineers from Children's Hospital Colorado, Children's Hospital of Philadelphia, The Hospital for Sick Children in Toronto, and Boston Children's Hospital.
2. Perioperative management strategies prior to surgery
There is currently a need to preserve PPE for critical situations throughout healthcare. However, donning PPE is essential for MLBs because they are high-risk procedures for aerosolization from an open airway unsecured by an endotracheal tube (ETT) that can place all operating room staff at risk for infection. Therefore, preoperative planning is imperative to avoid unnecessary procedures. Surgeries should be evaluated and ensured to fulfill the following criteria: The surgery is 1) potentially life-saving, 2) may prevent rapid patient deterioration, 3) may prevent permanent disability or dysfunction, or 4) failure to perform the surgery may lead to metastatic or infectious progression. We recommend open discourse between physicians for each case so that various opinions may be solicited, and a consensus agreement achieved regarding the need to proceed. Airway foreign bodies and critical airway obstruction are two presentations that fulfill these criteria.
Once surgery is considered appropriate, preoperative testing for COVID-19 should be performed as soon as possible and results obtained prior to proceeding, provided the patient is clinically stable. A separate pathway for preoperative testing should be established for expedited preoperative results. Currently, there is variability across institutions with respect to access to testing, duration of testing, and reliability of results including false negative rates that at some institutions may be as high as 30–40% [15]. Protocols from northern Italy include obtaining a chest x-ray and oximetry for preoperative patients who screen negative for COVID-19 to capture false negative cases (https://www.youtube.com/watch?v=HQ_IYo44Ixs). While a negative test is insufficient to negate the need for intraoperative airborne precautions given the risk of false negatives and a high-risk procedure, it may decrease the need for pre- and post-operative airborne isolation, thereby preserving limited PPE.
Additional imaging should be considered if the patient is clinically stable. While many centers perform MLB to evaluate for and remove an airway foreign body, there are frequently “negative” bronchoscopies where no foreign body is found [16]. To minimize negative bronchoscopies during the current COVID-19 pandemic, we recommend considering low-dose, non-contrast computerized tomography (CT) to confirm the presence of a foreign body if the patient is an appropriate candidate and does not require general anesthesia to obtain the imaging. CT imaging as a first investigation in suspected airway foreign body has routinely been used prior to the COVID-19 pandemic [17]. It has been shown to be highly sensitive and specific to identify foreign bodies and to decrease negative bronchoscopy rates [18]. If a foreign body is absent and clinical suspicion remains low, the surgery can be cancelled, and the patient can be followed closely as an outpatient. CT imaging may also be used to answer questions normally answered by bronchoscopy, such as evaluating the level and extent of external tracheal compression. With CT imaging, less personnel are at risk and less PPE is consumed compared with rigid bronchoscopy. Conversely, clinically unstable patients should proceed to the operating room with full airborne precautions.
3. Intraoperative protocol
3.1. Prior to procedure
This protocol was developed to protect healthcare workers and to decrease widespread environmental contamination by containing aerosolized viral product in a surgical tent created from clear surgical drapes immediately surrounding the patient (Fig. 1 ). Even with this surgical barrier in place, procedures should be performed in a negative pressure room if possible. The surgeon and all operating room staff should be in full PPE for airborne precautions, preferably with PAPRs where available or fit-tested N95 masks with face shields if PAPRs are not available. Fluid-resistant long-sleeved gowns compliant with at least a level 3 grade by the Association of Advancement of Medical Instruments, gloves that cover the gown sleeves, and shoe covers should also be used by all. Some have suggested wearing two gowns and two gloves for these procedures. A neck covering that can be doffed without contamination should be considered. Again, this approach is not dependent on COVID-19 test results given that this is a high-risk aerosolization procedure.
Fig. 1.
Surgical tent for containing droplet and aerosolized particles during unsecured airway procedure. Three disposable drapes are used to prevent contamination, including 1) drape covering bed, 2) drape covering patient's body, 3) drape suspended over patient's head and chest to create contained working space or tent.
Discussion and planning with all involved personnel before the procedure is essential to ensure the surgery runs smoothly and exposure risks are limited. All potentially necessary instruments are prepared at the beginning of the case including optical forceps, urology stone wire baskets for foreign body removal, biopsy forceps, balloons for dilation, etc. This preparation will minimize delays and reduce equipment related surgical errors [19].
Only essential personnel should be in the operating room to decrease unnecessary healthcare worker exposure and PPE use. Unfortunately, this will mean limiting trainee participation in cases involving an unsecured airway if the procedure can be safely done without the trainee's presence. We have been able to complete these procedures with only an attending anesthesiologist, attending surgeon, circulating nurse, and scrub nurse/technician, all of whom remain in the room for the duration of the procedure. This does not mean that limiting resource expenditure should outweigh patient safety. All necessary resources including personnel and PPE may be utilized to safely complete each case, but care must be given to limit unnecessary waste. The procedures may also be filmed where appropriate and the videos may be used for trainee education. Operating room doors must remain closed to prevent viral spread. Internal and external communication from the OR is essential to decrease the need for repeated door opening. This can be facilitated by using mobile communication devices such as phones or tablet computers.
The OR table should be prepared prior to patient entry. Avoid forced-air warming blankets as they may aerosolize contaminants including viral product [20]. If necessary, place the blanket on the bed and cover it with a liquid-impermeable drape. Prepare the smoke evacuator to clear aerosolized particles from underneath the soon to be constructed surgical tent. Ready any bars or stands required to suspend the drapes above the patient.
3.2. Anesthesia
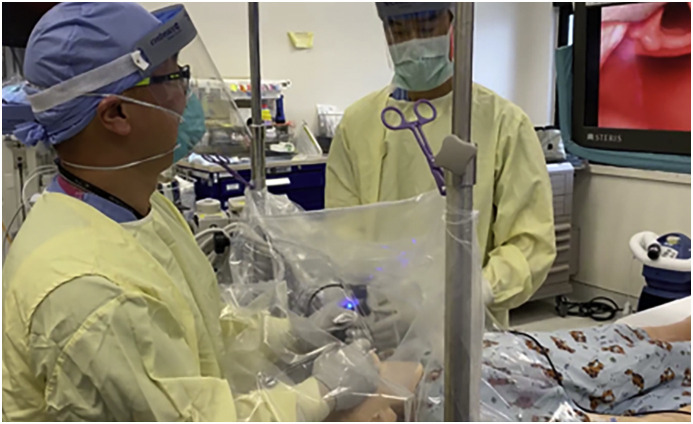
Before anesthetic induction, the anesthesia and surgical team will need to discuss the anticipated procedure and surgical conditions required, particularly spontaneous ventilation versus apnea, and the requirement for laryngeal suspension versus laryngoscopy. If deemed safe, preoperative sedation may calm the patient to facilitate intravenous line placement or mask induction. The anesthesiologist may wish to use an aerosol/droplet barrier between him or her and the patient. If positive pressure ventilation using a facemask is required, it should be performed with low fresh gas flows, small tidal volumes and low ventilatory pressures, using a two-handed technique. After induction, airway management depends on the nature of the procedure being performed. We recommend the placement of a supraglottic airway device or a cuffed endotracheal tube prior to draping the patient to minimize air leak during the draping process. A poorly seated supraglottic airway device may not seal the airway and result in aerosolized particles leaking around it, so a cuffed ETT may be used if not contraindicated. A standard blade videolaryngoscope is favored over a standard laryngoscope for airway exposure as it increases the mouth-to-mouth distance between the patient and anesthesiologist or surgeon (Fig. 2 ) [21].
Fig. 2.
Simulation of videolaryngoscopy under surgical tent. The mouth-to-mouth distance between patient and surgeon is increased compared to standard direct laryngoscopy while maintaining a clear field of view.
3.3. Creation of a surgical tent using disposable drapes
The bed is then rotated towards the surgeon and placed at the desired height. Water-tight adhesive coverings (Tegaderm, 3 M, MN, USA) are used to protect the patient's eyes so the face and body can be washed with soap and water at the end of the case. A drape or surgical towel is placed under the patient's head and a clear 1010 drape (3 M, MN, USA) is placed over the patient's chin, neck and chest. As an alternative, an aperture drape (1020, 1030; 3 M, MN, USA) can be used with the aperture positioned to expose the mouth while the drape covers the rest of the patient.
An ultrafiltration smoke evacuator is secured to the clear drape on the patient's chest with the open end facing towards the patient's head. This device filters viral particles as small as 52–55 nm (HPV) and, therefore, should be effective at filtering the SARS-CoV-2 virus which has a diameter of approximately 120 nm. Placing a surgical towel between the evacuator opening and the drape prevents the latter from being suctioned into the evacuator. The smoke evacuator must be used in addition to the normal surgical suction due to the sheer volume of air that it can clear. This is far superior to the normal surgical suction alone as demonstrated in the following video (https://youtu.be/J6U1iamfZ0A).
An ether screen is then placed over the patient at the level of the chest and secured to the operating room table. As alternatives, a mayo stand with tray removed or IV poles may be used if an ether screen is unavailable. A second ether screen or mayo stand may be positioned 8–12 inches above the patient's abdomen. A clear impermeable surgical drape is then placed over the patient's head and body, and suspended by the ether screen(s). A C-arm drape, O-arm drape, Lap Ped-Neonatal Clear Drape (Medline DYNJCDO284, IL, USA), ECMO cover drape cut along two sides (Uline S12308 60 x 60 2 mm polybag, WI, USA), or two separate clear impermeable slush drapes may be used. The bronchoscopy table should be under the same drape if possible, although certain drapes will not permit this (Fig. 3 ). Regardless of the type of drape and suspensory equipment selected, the principle aim is to create a surgical tent that acts as a barrier to decrease widespread dissemination of aerosolized viral product once the airway is unsecured during bronchoscopy. The smoke evacuator continually removes aerosolized viral product underneath the surgical tent and decreases oxygen accumulation, thereby reducing the risk of a surgical fire. If suspension laryngoscopy will be required for a supraglottoplasty or intratracheal procedure, a microscope drape can be attached to the microscope lens and then inverted over the patient and surgical table instead of draping the microscope. The microscope is then available for use as needed during the procedure (Fig. 4 ). All drapes should cover the patient and operating room table with the open end directed towards the patient's feet. The airway and suction circuits are all passed through the open end. We do not recommend fully enveloping the patient in the drape because it needs to be removed easily at the end of the case to prevent spread of settled viral particles.
Fig. 3.
Bronchoscopy tent with disposable drapes. A) Preoperative preparation involves impermeable blue sheet covering bed, bars ready at side of bed, large drape under which patient is placed. B) Simulation demonstrating bars in place to suspend drape over patient to create working space. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 4.
Simulation of surgical tent for suspension microlaryngoscopy and bronchoscopy created by attaching the microscope drape to the microscope lens and inverting the drape over the patient. The surgeon works through two slits for his or her hands.
3.4. Procedure
As the operating surgeon's arms will be the most directly exposed to contamination since they will be inside the surgical tent, we recommend covering the surgeon's arms with disposable sleeves that can be removed separately and disposed of with the drapes at the end of the procedure. These can be gown sleeves, video camera drapes (Microtek AEF 9604, LOT190904F, Delhi, India) or ultrasound probe drapes. There are two methods for the surgeon to place his or her arms into the field: 1) placing arms under the free edge of the sheet, or 2) placing arms through the sheet. We recommend placing arms through the sheet so the free edge of the sheet does not luff up and direct particles towards the surgeon's face. To do so, either cut a 12-inch working hole in the clear drape near the top of the patient's head, or cut two small slits approximately 1.5 feet apart and gently slide draped arms through the opening(s) (www.ORLPED.com/covid-19-or-draping). The scrub nurse can also don sleeves and insert their arms into the side of the tent to pass instruments safely to and from the surgeon.
Prior to initiating rigid bronchoscopy which inherently increases droplet spread, diagnostic and potentially therapeutic flexible bronchoscopy may be performed through an LMA or ETT (Fig. 5 ). Flexible bronchoscopy may help determine the location of any potential foreign body, critical stenosis, external airway compression, or lesion that needs to be biopsied. If the procedure is for foreign body removal but no foreign body is identified, the rigid bronchoscopy portion can be aborted and aerosolization minimalized. To insert the flexible bronchoscope, a swivel adapter with tape or a Tegaderm (Tegaderm, 3 M, MN, USA) covering the diaphragm opening is attached to the LMA or ETT. A hole is punctured in the tape or Tegaderm and flexible bronchoscopy is performed through the closed airway circuit to minimize leak and aerosolization. Lidocaine may be administered to the vocal cords and carina through the flexible bronchoscope.
Fig. 5.
Confirmatory flexible bronchoscopy through a laryngeal mask airway with swivel connector and Tegaderm for suspected airway foreign body. The surgeon's arms are covered with video camera drapes and are placed through a Lap Ped-Neonatal Clear Drape that is suspended by a Mayo stand with tray removed and empty screw holes covered by Tegaderm adhesives. Equipment for rigid bronchoscopy is at the ready.
Should flexible bronchoscopy be insufficient to accomplish the procedural goals, the flexible scope may be withdrawn, the hole on the swivel adaptor is closed and the tent is prepared for MLB. All surgical instruments including the laryngoscope, suction, rigid bronchoscope, anti-fog, and optical foreign body retrieval instruments, should be placed under the drapes before the MLB. Some practitioners strongly recommend utilizing indirect videolaryngoscopy to maximize the distance between the surgeon's face and the patient's airway (Fig. 2). The ETT or LMA is carefully removed, the MLB is performed, the necessary intervention is completed, and retrieved foreign bodies are discarded (Fig. 6 ).
Fig. 6.
Rigid bronchoscopy for foreign body removal. Surgical tent is constructed from an ether screen (cross bar) and an O-arm drape. The surgeon works through a small slit in the drape. There is a 1010 drape over the patient's chest and a smoke evacuator overtop to filter aerosolized product from under the tent.
4. Post procedure
Following conclusion of the MLB, the aerosolized product under the drape must be contained. If a 12-inch working hole was used in the drape, it is then taped shut. If sleeves through smaller slits were used, the surgeon withdraws their arms while an assistant bunches the sleeve tightly around the arm, bunches the sleeve up with some of the large drape, and seals it with an elastic band (www.ORLPED.com/covid-19-or-draping). Both must ensure the arm drape does not come out of the hole or else viral exposure will occur.
At the conclusion of the MLB, an induction mask, LMA, or cuffed ETT is used to manage the airway under the drape as the patient is kept sedated and the smoke evacuator remains on for 10 min. While this step is not performed under standard MLB conditions, we feel it necessary during the COVID-19 pandemic to clear the aerosolized product from under the drape. If the decision is made to use a cuffed ETT at this point, the benefit of having a completely secured airway needs to be weighed against the increased risk of coughing when the patient awakens. This decision is provider dependent and we recommend at least extubating deeply to minimize coughing.
The large drape is then carefully removed by slowly rolling it under itself, trapping any droplets that are on its undersurface. The adhesive 1010 or aperture drape is rolled over itself, trapping droplets on its surface. The smoke evacuator and suction are disconnected and are rolled into these drapes as they are considered contaminated. The surgical towel or second drape under the patient's head is rolled onto itself and discarded. The patient is then washed with soap and water including the ETT or LMA if present. All equipment that was under the drape is wiped down with appropriate antiviral sanitizing wipes including the anesthesia circuit. Some favor exchanging the anesthesia circuit for a new circuit that has an uncontaminated exterior surface.
The bed is rotated back towards the anesthesiologist. All non-essential personnel are removed from the operating room prior to awakening. Deep emergence from anesthesia and deep extubation, if not contraindicated, can mitigate coughing. Once the LMA or ETT is removed, a mask is promptly placed over the patient's mouth and nose. An additional layer of protection for the anesthesiologist may be used by incorporating a plastic sheet with the mask to decrease aerosolization. We recommend waiting for droplets to settle and for one full circulation of room air to take place (10–25 min) before transferring the patient out of the room.
It is imperative once the procedure is over that all operating room personnel appropriately doff their PPE as this is a high-risk time for contracting infection. Up to 90% of people may inappropriately doff their equipment with errors in sequence or technique while acting on their own [22]. Assisted doffing is generally associated with improved proficiency and therefore safety [23]. Many centers have designated PPE monitors to help assure the doffing process is performed correctly. If no PPE monitor is available, having a written doffing protocol to follow or implementing a buddy system whereby each person watches and coaches the other while doffing can be helpful. The Centers for Disease Control and Prevention (CDC) also has protocols available for suggestions (https://www.cdc.gov/coronavirus/2019-ncov/hcp/using-ppe.html).
5. Discussion
The COVID-19 pandemic has created a greatly increased risk to healthcare personnel, especially those who perform aerosol generating procedures. This pandemic has also highlighted the inexperience of our specialty in dealing with a disease such as this. We present a simple method to increase the safety of MLB for all present during these procedures that can be utilized throughout this pandemic and in future epidemics that may require similar protocols. The additional equipment required can be easily found in most operating rooms or institutions and modifications can be made where required. The principles are sound to decrease aerosolization and droplet contamination in the operating room. This protocol imparts little to no added risk to the patient, as the drapes may be rapidly removed if the patient decompensates or other concerns arise. There is, however, a greatly perceived benefit to the safety of the anesthesiologist, surgeon, and operating room staff involved.
With the rapid onset of the COVID-19 pandemic, there has not yet been time to study the effectiveness of this protocol. Empirically, the described steps make sense and have been developed using multi-institutional and multispecialty expert opinion. The logistics were first tested using simulation. We then tested the ability of the drapes to contain aerosolized products by spraying Bitrex (as used in mask-fit testing) under the drapes which was unable to be detected by operating room staff as long as the drapes were in place and the smoke evacuator was appropriately on. We have also demonstrated the ability of the smoke evacuator to remove aerosolized product from under the drapes (https://youtu.be/J6U1iamfZ0A). Additional testing will be performed to further validate these procedures.
With very little downside, we fully support taking these extra precautions. Each of our institutions have incorporated this protocol into our practices and have successfully performed MLBs on several patients. To date, there have been no known adverse patient or personnel outcomes. We have also successfully used similar methods for transoral drainage of retropharyngeal and parapharyngeal abscesses, esophageal foreign body removal, tracheostomy tube change and drainage of frontal sinus abscess with intracranial spread. The versatility of this protocol lends itself to widespread use.
Advances are occurring daily with respect to improved preoperative viral and antibody COVID-19 testing, treatment, and vaccine development. Though we are confident that the current pandemic will eventually pass, we believe that in the interim this system can help flatten the curve further by decreasing exposure to operating room personnel to hopefully save lives. Although it does not replace the need for individual PPE, it can easily be employed in situations where PPE is scarce and where preoperative testing is unavailable. It is also translatable to future situations where different airborne or droplet infectious vectors are of concern.
6. Conclusion
Creation of a surgical tent using disposable drapes is a relatively simple technique to protect operating room personnel during MLB and other aerosolizing procedures during the COVID-19 pandemic. The few additional resources that are required are generally accessible in any operating room or institution. This technique may prevent aerosolization of other pathogens in the future.
Financial disclosures
The authors have no financial relationships relevant to this manuscript.
Other disclosures not relevant to study
None.
Funding source
No funding was secured for this study.
Declaration of competing interest
The authors have no conflicts of interest.
Acknowledgement
The authors would like to acknowledge the following for their contributions to this work: Alice Oloya, RN; Marie-Eve Mercier, RN; Eloise Salonga, RN; James Drake, BSE, MB, BCh, MSc, FRCSC; Ashley Nagle, CET.
References
- 1.Mahase E. Coronavirus covid-19 has killed more people than SARS and MERS combined, despite lower case fatality rate. BMJ. 2020;368:m641. doi: 10.1136/bmj.m641. [DOI] [PubMed] [Google Scholar]
- 2.Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J. Pediatr. 2020;87:281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease Control and prevention. JAMA, J. Am. Med. Assoc. 2020;323:17–20. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Chan J.Y.K., Wong E.W.Y., Lam W. Practical aspects of otolaryngologic clinical services during the 2019 novel coronavirus epidemic: an experience in Hong Kong. JAMA Otolaryngol. Neck Surg. 2020 doi: 10.1001/jamaoto.2020.0488. [DOI] [PubMed] [Google Scholar]
- 5.Li R., Pei S., Chen B., Song Y., Zhang T., Yang W., Shaman J. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2) Science. 2020;80:eabb3221. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He X., Lau E.H., Wu P., Deng X., Wang J., Hao X., Lau Y.C., Wong J.Y., Guan Y., Tan X., Mo X., Chen Y., Liao B., Chen W., Hu F., Zhang Q., Zhong M., Wu Y., Zhao L., Zhang F., Cowling B.J., Li F., Leung G.M. Temporal dynamics in viral shedding and transmissibility of COVID-19. MedRxiv. 2020 doi: 10.1101/2020.03.15.20036707. 2020.03.15.20036707. [DOI] [PubMed] [Google Scholar]
- 7.Tindale L., Coombe M., Stockdale J.E., Garlock E., Lau W.Y.V., Saraswat M., Lee Y.-H.B., Zhang L., Chen D., Wallinga J., Colijn C. Transmission interval estimates suggest pre-symptomatic spread of COVID-19. MedRxiv. 2020 doi: 10.1101/2020.03.03.20029983. 2020.03.03.20029983. [DOI] [Google Scholar]
- 8.Xia W., Liao J., Li C., Li Y., Qian X., Sun X., Xu H., Mahai G., Zhao X., Shi L., Liu J., Yu L., Wang M., Wang Q., Namat A., Li Y., Qu J., Liu Q., Lin X., Cao S., Huan S., Xiao J., Ruan F., Wang H., Xu Q., Ding X., Fang X., Qiu F., Ma J., Zhang Y., Wang A., Xing Y., Xu S. Transmission of corona virus disease 2019 during the incubation period may lead to a quarantine loophole. MedRxiv. 2020 doi: 10.1101/2020.03.06.20031955. 2020.03.06.20031955. [DOI] [Google Scholar]
- 9.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., Yu J., Kang M., Song Y., Xia J., Guo Q., Song T., He J., Yen H.-L., Peiris M., Wu J. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel Z.M., Fernandez-Miranda J., Hwang P.H., Nayak J.V., Dodd R., Sajjadi H., Jackler R.K. 2020. PRECAUTIONS for ENDOSCOPIC TRANSNASAL SKULL BASE SURGERY during the COVID-19 PANDEMIC, Neurosurgery.https://www.entnet.org/sites/default/files/uploads/covid-19_endosb_lettertoeditor_neurosurgery_update3.23.20.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wahidi M.M., Lamb C., Murgu S., Musani A., Shojaee S., Sachdeva A., Maldonado F., Mahmood K., Kinsey M., Sethi S., Mahajan A., Majid A., Keyes C., Alraiyes A.H., Sung A., Hsia D., Eapen G. American association for bronchology and interventional pulmonology (AABIP) statement on the use of bronchoscopy and respiratory specimen collection in patients with suspected or confirmed COVID-19 infection. J. Bronchology Interv. Pulmonol. 2020:1. doi: 10.1097/lbr.0000000000000681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canelli R., Connor C.W., Gonzalez M., Nozari A., Ortega R. Barrier enclosure during endotracheal intubation. N. Engl. J. Med. 2020;1–2 doi: 10.1056/nejmc2007589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frauenfelder C., Butler C., Hartley B., Cochrane L., Jephson C., Nash R., Hewitt R., Albert D., Wyatt M., Hall A. Practical insights for paediatric otolaryngology surgical cases and performing microlaryngobronchoscopy during the COVID-19 pandemic. Int. J. Pediatr. Otorhinolaryngol. 2020;134:110030. doi: 10.1016/j.ijporl.2020.110030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vukkadala N., Qian Z.J., Holsinger F.C., Patel Z.M., Rosenthal E. Laryngoscope; 2020. COVID-19 and the Otolaryngologist - Preliminary Evidence-Based Review. [DOI] [PubMed] [Google Scholar]
- 15.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA, J. Am. Med. Assoc. 2020:2–3. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cavel O., Bergeron M., Garel L., Arcand P., Froehlich P. Questioning the legitimacy of rigid bronchoscopy as a tool for establishing the diagnosis of a bronchial foreign body. Int. J. Pediatr. Otorhinolaryngol. 2012;76:194–201. doi: 10.1016/j.ijporl.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Pitiot V., Grall M., Ploin D., Truy E., Ayari Khalfallah S. The use of CT-scan in foreign body aspiration in children: a 6 years' experience. Int. J. Pediatr. Otorhinolaryngol. 2017;102:169–173. doi: 10.1016/j.ijporl.2017.08.036. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed O.G., Guillerman R.P., Giannoni C.M. Protocol incorporating airway CT decreases negative bronchoscopy rates for suspected foreign bodies in pediatric patients. Int. J. Pediatr. Otorhinolaryngol. 2018;109:133–137. doi: 10.1016/j.ijporl.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 19.Shah R.K., Kentala E., Healy G.B., Roberson D.W. Classification and consequences of errors in otolaryngology. Laryngoscope. 2004;114:1322–1335. doi: 10.1097/00005537-200408000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Leaper D., Albrecht M., Gauthier R. Forced-air warming: a source of airborne contamination in the operating room? Orthop. Rev. 2009;1:28. doi: 10.4081/or.2009.e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall D., Steel A., Heij R., Eley A., Young P. Videolaryngoscopy increases ‘mouth-to-mouth’ distance compared with direct laryngoscopy. Anaesthesia. 2020:1–2. doi: 10.1111/anae.15047. [DOI] [PubMed] [Google Scholar]
- 22.Phan L.T., Maita D., Mortiz D.C., Weber R., Fritzen-Pedicini C., Bleasdale S.C., Jones R.M. Personal protective equipment doffing practices of healthcare workers. J. Occup. Environ. Hyg. 2019;16:575–581. doi: 10.1080/15459624.2019.1628350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chughtai A.A., Chen X., Macintyre C.R. Risk of self-contamination during doffing of personal protective equipment. Am. J. Infect. Contr. 2018;46:1329–1334. doi: 10.1016/j.ajic.2018.06.003. [DOI] [PubMed] [Google Scholar]