Summary
Respiratory viruses are responsible for a large proportion of acute respiratory illness in adults as well as children, and are associated with a huge socio-economic burden worldwide. Development of accurate point-of-care tests (POCT) for respiratory viruses has been listed as a priority by the World Health Organisation and replacing the current paradigm of empirical antimicrobial use with directed use is a listed goal of the movement for reduction in antimicrobial resistance. POCTs for respiratory viruses have previously been limited by the poor sensitivity of antigen detection based tests and by a limited range of detectable viruses. Highly accurate molecular platforms are now able to test for a comprehensive range of viruses, can be operated by non-laboratory staff and can generate a result in approximately 1 h, making them potentially deployable as POCTs. The potential clinical benefits of POC testing for respiratory viruses in adults include a reduction in unnecessary antibiotic use, improved antiviral prescribing for influenza and rationalisation of isolation facilities. We review here the burden of disease, the currently available molecular platforms with potential for POCT use and the existing evidence for clinical and economic benefits of testing for respiratory viruses in adults.
Keywords: Point-of-care testing, Respiratory viruses, Influenza, Adults, Acute respiratory illness, Antimicrobial resistance
Highlights
-
•
There is a large burden of respiratory virus infection in hospitalised adults.
-
•
Replacing empirical antimicrobials with pathogen directed use is a global priority.
-
•
Molecular platforms now exist with potential for use as point-of-care tests (POCT).
-
•
Potential benefits of POCT include a reduction in unnecessary antibiotic use.
-
•
High quality trials evaluating clinically relevant outcomes are urgently needed.
Introduction
Acute respiratory tract infections are responsible for an estimated 4.25 million deaths each year and are the third most common cause of death worldwide.1 Although bacteria have previously been considered to be the principal aetiological agents of severe respiratory infection, the global importance of respiratory viruses in all age groups has been increasingly recognised in recent years.2, 3, 4 Diagnostic technology for respiratory virus detection has evolved rapidly over the last two decades from viral culture and immunofluorescence to the current standard of molecular detection by polymerase chain reaction (PCR). This review focuses on the currently available molecular diagnostic platforms for respiratory virus detection with potential for use as point-of-care tests (POCT) and explores the current landscape for POCT in adults.
Respiratory viruses: clinical and economic burden of disease
Improvements in the sensitivity of diagnostic testing for respiratory viruses with the widespread use of nucleic amplification techniques such as PCR have helped to accurately define the burden of viral disease over the past two decades. In children respiratory viruses have been detected by molecular diagnostic techniques in 43–67% of cases of community acquired pneumonia (CAP),5 over 90% of infants with bronchiolitis,6 and approximately 85% of asthma exacerbations.7 In adults approximately 20–40% of CAP cases,8, 9, 10, 11, 12 50–70% of asthma exacerbations13 and 30–50% of chronic obstructive pulmonary disease exacerbations14 are associated with respiratory virus detection. In hospitalised adults with acute respiratory illness, viruses are the most commonly detectable pathogen (being detected in around 50%) with bacterial detection being much less frequent, although antibiotic use is almost universal.4 Furthermore, preceding viral infection is thought to be a key predisposing event to secondary bacterial infections in the lung and other sites in the respiratory tract.15, 16, 17 Respiratory viruses including influenza have also been implicated in precipitating non-respiratory illnesses such as myocardial infarction, venous thromboembolism, stroke and loss of diabetic control.18, 19, 20, 21, 22
Infections with respiratory viruses are frequent events in all age groups and result in an enormous burden on health systems as well as the economic costs in direct medical expenses and indirect productivity losses. Direct medical expenses include outpatient clinic visits, emergency department visits, hospitalisations and treatment costs, including over-the-counter medication and drug prescriptions. Indirect productivity losses include missed workdays for adult patients and caregivers. In Europe direct costs attributed to pneumonia are estimated at approximately €10.1 billion annually and indirect costs of lost work days at €3.6 billion.23
Based on the 2003 population size, seasonal influenza epidemics resulted in an average of 610,660 life-years lost, 3.1 million hospital days and 31.4 million outpatient visits in the USA.24 Direct medical costs averaged US$10.4 billion annually, and projected lost earnings due to illness and loss of life amounted to US$16.3 billion annually. The total economic burden of annual influenza epidemics using projected statistical life values amounted to US$87.1 billion.25 The common cold also causes a significant economic burden with a US-based study estimating that non-influenza, viral respiratory tract illnesses (mostly common colds) cost around US$40 billion in 2001.26
Influenza
The influenza virus causes seasonal epidemics leading to excess hospitalisations and death mainly in the elderly and in patients with co-morbidity.27, 28 It causes severe illness in up to 5 million people and around half a million deaths per year worldwide.29 Annual seasonal influenza vaccine is recommended in at risk groups30 however vaccine uptake is sub-optimal31, 32 and high quality evidence for significant protection in the elderly is lacking.33, 34 The rate of hospitalisation in adults with influenza has been estimated at 5 to 20 per 100,000 overall35, 36 and may be as high as 1200 per 100,000 in those over 85 years old.37 In adults hospitalised with laboratory confirmed influenza, 10–30% are admitted to critical care units and 3–15% die in hospital,38, 39 with outcomes being predicted by co-morbidity.40 As noted above, in addition to acute respiratory presentations, influenza may precipitate decompensated cardiovascular disease, myocardial infarction, collapse or diabetic emergencies20, 21, 22, 41 and so many hospitalised cases of influenza are likely to remain undiagnosed. A recent Canadian study estimated that only around 1 in 14 emergency department visits due to influenza virus infection were correctly attributed to influenza.42 It is likely, therefore, that the burden of influenza and its economic impact have been under-estimated.
Respiratory syncytial virus
RSV is the principle cause of bronchiolitis in infants but is now increasingly recognised as a major cause of severe respiratory illness in adults, with some studies suggesting a disease burden similar to that of influenza.43, 44 RSV affects all age groups and a study of hospitalised children and adults that calculated disability adjusted life years (DALYs) concluded that influenza and RSV were consistently the greatest causes of disease across all age groups.45 Adults at high risk of severe RSV disease include the frail elderly, those with chronic cardio-respiratory disease and the immunocompromised. The mortality rate of RSV infection in adults and the elderly is similar to that of influenza (7–8%) but may reach 30–70% in the heavily immunocompromised46 contrasting with the negligible RSV-related mortality in infected children.
Rhinovirus
Picornaviruses are responsible for the majority of common colds and adults typically suffer two to four symptomatic episodes per year.47 They are also responsible for the majority of exacerbations of asthma in adults and a significant proportion of exacerbations of COPD.13, 14, 48 Common colds cause an estimated 20 million lost workdays per year in the US, with an estimated annual cost of around US$410 million for rhinovirus-related asthma exacerbations.49
Other respiratory viruses
Adenovirus infections are a common cause of mild acute respiratory illness in children, but also cause epidemics among adult military recruits and can cause serious infections in the immunocompromised and occasionally in immunocompetent adults.50, 51 The clinical impact of human coronaviruses 229E and OC43 infection has only recently been explored, with early data suggesting a prevalence in adults hospitalised with acute respiratory illnesses of between 3% and 11%.4, 52 A US multi-centre study showed that the prevalence of human metapneumovirus (hMPV) infection in hospitalised adults with respiratory symptoms was 2.6% and patients had similar clinical characteristics to those infected with RSV infection with increasing age being a risk factor for emergency department visit and hospitalisation.44 Parainfluenza viruses seem to be of less importance in adults compared to paediatric populations, however they can cause influenza-like illness in adults and are detected in adults hospitalised with acute respiratory illness at low frequency.4, 45, 53
Diagnostic tests for respiratory viruses
Laboratory PCR
Nucleic acid amplification techniques such as PCR have now largely superseded cell culture and direct fluorescent antibody testing as the method of choice for routine diagnostic testing for respiratory viruses, due to their superior diagnostic accuracy and faster turnaround time. PCR is highly sensitive and specific but generally has a turnaround time of at least 24 h and requires specialist laboratory facilities and expertise.54, 55
Rapid antigen detection tests for respiratory viruses
There are several commercially available FDA approved and CE marked, rapid diagnostic tests for respiratory viruses including influenza and RSV, which use antigen detection by either immune-chromatographic assay or immunofluorescence. Time to result is generally around fifteen minutes, they are easy to use and do not require laboratory support, and generally involve visual inspection of a test line (in addition to a control line). Unfortunately the clinical utility of rapid antigen based detection for respiratory viruses has been limited by their unacceptably poor sensitivity, especially in adults where it is around 50% for influenza56 and lower for RSV,57 meaning that they cannot be used to rule out infection. The Sofia Fluorescent Immunoassay Analyzer (Quidel, San Diego, CA, USA) is a benchtop analyser that combines lateral flow immunofluorescence-based antigen detection test kits with an optical sensor and provides a result in around 10 min. Test kits are available for influenza and RSV which are both FDA approved and CE marked. Sensitivity for detection of influenza is around 80% compared to PCR and so may be higher than for some other rapid antigen tests.58 Although it has not been evaluated in adults, in children the sensitivity for detection of RSV was around 70% compared to PCR but would be expected to be lower in adults.59
Multiple respiratory virus antigen detection
MariPOC (ArcDia Laboratories, Turku, Finland) is a CE marked, multi-analyte immunofluorescence-based antigen detection platform that can simultaneously detect 8 respiratory viruses (influenza A and B, RSV, adenovirus, hMPV, and parainfluenza types 1, 2, and 3) in addition to Streptococcus pneumonia. It has not been evaluated in adults but when compared to RT-PCT in children with acute respiratory illness diagnostic accuracy was generally moderate although sensitivity was as low as 12.5% for some viral targets.60, 61
Molecular platforms with point-of-care testing potential
Alere i Influenza A&B
The Alere i Influenza A&B (Alere, San Diego, CA, USA) is an FDA approved and CE marked isothermal nucleic acid amplification-based system that uses a fluorescence-based molecular signal to detect influenza A and B. Results are generated within 15 min, with around 2 min of “hands on” time. The testing kits and analyser have been specifically designed to be used by non-laboratory clinical staff in an acute care environment and it is the only molecular platform that is FDA approved specifically as a POCT. In a study examining diagnostic accuracy involving 545 respiratory specimens from symptomatic patients (85% children and 15% adults), the sensitivity and specificity of the Alere i Influenza A&B assay was 99.3% and 98.1% for influenza A, and 97.6% and 100% for influenza B compared to viral culture and PCR.62 However, a Swiss study of 436 participants (broadly two-thirds children and one-third adults) showed a lower pooled influenza A and B sensitivity of 82.3% (mostly influenza A rather than B) compared to PCR.63 Another study using samples predominantly from adults, showed an even lower sensitivity for influenza A at 73.2%.64 The high specificity demonstrated, simplicity of use and fast turnaround time make the Alere i Influenza A&B test an exciting prospect for point-of-care use however there have been no clinical trials evaluating clinical or health economic outcomes and the lower sensitivity in adults for influenza A and the limited range of pathogens detected limit its usefulness.
Biofire FilmArray Respiratory Panel
The FilmArray Respiratory Panel (BioFire Diagnostics, Salt Lake City, UT, USA) is and FDA approved and CE marked platform that uses nested real-time PCR to detect 20 respiratory pathogens (17 viral targets and 3 bacteria). The FilmArray requires 2 min of “hands on” time and produces a test result in one hour.65 Several published studies have evaluated the ease-of-use and turnaround time of the system and comparing the diagnostic accuracy to laboratory PCR. These studies have shown superiority of the FilmArray system in terms of ease-of-use and turnaround times compared to laboratory PCR.66, 67, 68 Sensitivity and specificity compared to laboratory PCR (with confirmatory cell culture and sequencing) are excellent. Initial pooled sensitivity for viral targets was around 90% – principally due to poor sensitivity in adenovirus detection, however following improvements in the adenovirus assay this has risen to over 95%.66, 67, 69, 70 It is notable that these studies were all conducted within a laboratory rather at the point-of-care and a large proportion of these studies were conducted using samples from children rather than adults. A notable limitation of the system is the workflow as only a single specimen can be tested at any one time on the analyser.
A single study has examined clinical outcomes in children hospitalised with acute respiratory illness and tested with the FilmArray respiratory panel compared with standard laboratory PCR.71 This was not a randomised controlled trial but examined outcomes pre and post intervention and the FilmArray respiratory panel was not used as a POCT but was housed within the existing laboratory. This study demonstrated that in patients tested with the FilmArray, the test result was available to clinicians after a mean time of 6 h versus around 24 h with standard laboratory PCR. The duration of antibiotics was shorter in those tested with the FilmArray although this was dependent on receiving the test results within 4 h. The duration of inpatient stay and the time in isolation facilities were shorter in those tested with the FilmArray if the results were positive for viruses. There have been no trials in adults and no randomised controlled trials to date examining the potential clinical benefits of using this system as a POCT.
Cepheid GeneXpert Flu and Flu/RSV
The Xpert Flu (Cepheid, Sunnyvale, CA, USA) real-time PCR test cartridge is an FDA approved and CE marked test for use on the integrated, automated GeneXpert platform and detects influenza A and B with a turnaround time of about 75 min and reported “hands on” time of 2 min.72, 73 The modular multiple port system allows on-demand, random-access testing so that up to 16 tests can be run simultaneously (depending on the number of ports in the testing unit). The system has been evaluated in a prospective trial using samples from 300 adults with acute respiratory illness in emergency departments. In this group the sensitivity for detection of influenza was 95.3% (84.2%–99.4%) with a specificity of 99.2% (95% CI: 97.0%–99.9%) compared to laboratory PCR.74 Although a comparatively easy to use test, there are currently no published trials evaluating its use as a point-of-care test or evaluating the potential clinical or health economic benefits of its use in emergency departments. The combined Xpert Flu/RSV cartridge has been evaluated in a retrospective study using adult and paediatric samples and demonstrated a sensitivity of 97% for influenza A, 100% for influenza B and 98% for RSV, with specificity 100% for all three viruses compared to laboratory PCR.75 Although Xpert Flu and Xpert Flu/RSV have excellent sensitivity and clear point-of-care potential the restricted range of viruses currently detected is a limiting feature of this system.
This review focuses on platforms with a well established peer-reviewed evidence base incorporating clinical specimens however there are numerous other platforms in earlier stages of development and using a variety of sensing technologies, which are not included. Examples include the FDA cleared and CE marked Cobas Liat Influenza A/B (Roche Diagnostics, Indianapolis, IN, USA)76 which uses PCR to detect influenza A and B in less than 20 min, and the GenMark eSensor Respiratory virus panel (GenMark Diagnostics, Inc., Carlsbad, CA) which uses cartridge based multiplexed PCR to detect a panel of respiratory viruses in 60–90 min.77 A comparison of the reviewed molecular platforms for respiratory virus detection with POCT potential is shown in Table 1 .
Table 1.
Comparison of molecular platforms with point-of-care potential for detecting respiratory viruses.
| System and panel | Benefits | Limitations | References |
|---|---|---|---|
| Alere i Influenza A&B | 15 min run-time 2 min “hands on” time Simplicity |
Moderate sensitivity for Influenza A Only influenza viruses detected |
62, 63, 64 |
| Biofire FilmArray Respiratory Panel | 60 min run-time 2 min “hands on” time Wide range of viruses detected |
Unable to process multiple samples simultaneously | 65, 66, 67, 68, 69, 70, 71 |
| Cepheid GeneXpert (Xpert Flu and Flu/RSV) | 75 min run-time 2 min “hands on” time Modular system allows multiple simultaneous tests |
Limited range of viruses detected | 72, 73, 74, 75 |
Respiratory virus point-of-care testing in the wider context
The UK Department of Health commissioned report into UK pathology services in 2006 noted the importance of developing clinically relevant point-of-care diagnostic tests to reduce turnaround times and improve patient pathways.78 Despite this POCT for infectious diseases in the UK and globally have not advanced far beyond dipstick testing for urinary tract infection with in vitro diagnostic tests for infection remaining confined to large centralised laboratories. The associated slow turnaround times mean that results are only available to clinicians many hours to several days after the patient has presented, and long after antimicrobial decisions have been made, perpetuating the current paradigm of empirical antimicrobial use rather than pathogen directed use. The Infectious Diseases Society of America policy paper ‘Better Tests, Better Care: Improved Diagnostics for Infectious Diseases’ acknowledges the ongoing culture of empirical antimicrobial use and the unmet need for rapid accurate tests for infectious diseases to allow appropriate pathogen directed therapy.79
The Wold Economic Forum has stated that antimicrobial resistance is the arguably the greatest threat to global human health and current high profile initiatives on combating resistance have focused attention on antimicrobial stewardship, which seeks to preserve existing antimicrobial agents and slow the development of resistance. One of the strategies to achieve this goal is the development of rapid diagnostic tests and biomarkers to ensure appropriate use of antimicrobials so that antimicrobial use is pathogen directed rather than empirical and only used in those where there is clear evidence of benefit.80, 81 In addition the increasing recognition of the huge global burden of respiratory viruses has led to the creation of the WHO's global Battle against Respiratory Viruses Initiative (BRaVe) initiative which aims to improve research into strategies to prevent, diagnose and manage respiratory virus infection. Priority areas include improved diagnostic tests for respiratory viruses including the creation and use of cheap, accurate and easy to use POCTs.82
Potential clinical benefits of point-of-care testing
Reduction in antibiotic use
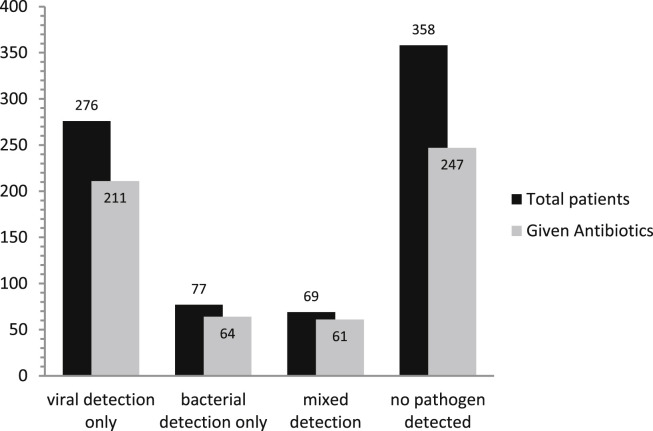
The current culture of empirical antimicrobial use in patients with suspected infection is no longer considered sustainable due the emergence and proliferation of antibiotic resistance. Antibiotic use in hospitalised patients with acute respiratory illness is near universal despite the predominance of viruses and the low frequency of detectable bacteria in much of this patient group. Fig. 1 shows antibiotic use by detected pathogen in a large study of hospitalised adults from the UK.4 Furthermore patients with acute respiratory illness syndromes that are known to be principally virally induced, such as exacerbations of asthma and acute bronchitis, are often treated with antibiotics despite the lack of evidence for benefit, and in the case of asthma, national guidelines discouraging their use.83, 84, 85 In the study from the UK referenced above almost 60% of patients hospitalised with an exacerbation of asthma received at least one dose of antibiotic whilst in hospital.4 The use of respiratory virus POCT could potentially reduce unnecessary antibiotic use in patients with acute respiratory illness especially in those conditions known to be principally viral and where antibiotic use has not shown benefit, by demonstrating to clinicians that the patient's illness and fever are explained by the presence of a virus. Patients with uncomplicated Influenza-like illness caused by respiratory viruses including influenza, are often treated with antibiotics due to diagnostic confusion with bacterial infection. In these patients the early detection of a respiratory virus in the absence of evidence of concomitant bacterial infection may prevent unnecessary antibiotic use.
Figure 1.
Antibiotic use in patients by detected aetiology from a study of hospitalised adults with acute respiratory illness (n = 758). Mixed detection refers to the concurrent detection of viruses and bacteria in the same patient.
[Reproduced from: Clark TW, et al. Adults hospitalised with acute respiratory illness rarely have detectable bacteria in the absence of COPD or pneumonia; viral infection predominates in a large prospective UK sample. J Infect 2014;69(5):507–15].
The evidence base for respiratory virus testing reducing unnecessary antibiotic use is limited and mainly consists of trials using rapid antigen based testing for influenza. A large randomised control trial evaluating the clinical and health economic benefits of rapid antigen testing for influenza in hospitalised adults did not demonstrate any improvement in antibiotic use or other clinical or health economic benefits in those tested with rapid antigen tests compared to laboratory testing.86 In a small non-randomised study of hospitalised adults rapid diagnostic testing for influenza using antigen detection demonstrated small reductions in antibiotic use (74% vs 99%) with no increase in adverse events in those where antibiotics were withheld.87 Studies in children including several small randomised controlled trials have evaluated the impact of routine rapid antigen testing for influenza on antibiotic use, with inconsistent results.88, 89, 90, 91, 92 For molecular tests the evidence base is even more limited with trials using molecular testing platform within the laboratory rather than as POCT, with the associated prolonged turnaround times. The impact of respiratory virus testing in adult outpatients with acute respiratory illness was assessed in a small randomised controlled trial using laboratory-based PCR. Results were available the next day in those randomised to viral PCR testing and even with this delay antibiotic prescribing was significantly reduced compared to those treated with standard care (5% vs 12%).93 As noted above the FilmArray respiratory platform has been clinically evaluated in a single centre paediatric study where the platform was not used as a POCT but was housed within the central laboratory. Although not a randomised controlled trial it demonstrated reductions in antibiotic use in those testing positive for viruses with the FilmArray versus standard laboratory PCR, although only when results were available within 4 h, underscoring the importance of rapid results and suggesting possible further reductions if the platform was used as a POCT.71
A recent Cochrane review evaluating the use of rapid viral diagnostics for acute febrile respiratory illness in children in the emergency department concluded that there is currently insufficient evidence to support rapid viral testing to reduce antibiotic use in this setting. The authors have suggested an adequately powered trial with antibiotic use as the primary outcome measure.94
Directed antiviral agent use
Influenza
The neuraminidase inhibitors (NAI) Oseltamivir and Zanamivir are licensed antivirals for the treatment and prevention of influenza and are recommend by UK Public Health England for the treatment of hospitalised adults with suspected and confined influenza A and B.95 Although there has been controversy regarding the evidence for their efficacy from the original pharma sponsored trials, there is now a large body of evidence from observational studies suggesting a significant reduction in mortality in hospitalised adults with confirmed influenza.96 Although the degree of benefit from NAIs is probably greatest when they are started with 48 h of symptom onset there is evidence in adults to suggest ongoing benefit when started beyond this time and up to 5 days of symptoms.96, 97 This is particularly pertinent as patients infected with influenza often present to hospital after 48 h of symptom duration.4 In current UK practise patients with suspected influenza are generally treated empirically with NAI whilst awaiting the results of laboratory PCR testing.95 This strategy leads to unnecessary NAI exposure with the associated risk of side effects in patients who are subsequently found not to have influenza. Following the recent publication of the independent meta-analysis of oseltamivir trials,98 an editorial letter (published in the Lancet) suggested that, in view of the modest efficacy and moderate risk of nausea and vomiting with oseltamivir, the administration of this drug should ideally be directed with the use of diagnostic tests rather than used empirically.99 The use of a molecular point-of-care test has the potential to allow implementation of directed rather than empirical NAI treatment thus maximising clinical benefit for those with influenza infection and minimising unnecessary antiviral exposure and drug related adverse events in those without.
As noted above several studies in children and a single study in adults have suggested improved use of NAIs using rapid antigen testing for influenza versus routine clinical care87, 88, 89, 90, 91, 92 although there have been no studies evaluating molecular platform POCTs with this outcome measure. In addition to NAIs there are several promising novel and re-purposed anti-influenza agents currently in late stages of clinical development including nitazoxanide, favipiravir (T-705) and anti-m2e monoclonal antibody.100, 101, 102
Respiratory syncytial virus
There are currently no specific antiviral agents licensed for RSV infection. The broad spectrum antiviral agent ribavirin is sometimes used in immunocompromised adults with severe RSV infection but its use is limited by safety concerns and difficulties with administering the nebulised solution103, 104 and there have been no randomised controlled trials to evaluate its efficacy. Several small molecule anti-RSV agents are in the late stages of clinical development including Gilead's GS-5806, a RSV fusion protein inhibitor which has demonstrated a reduction in symptoms and viral load105 in a challenge study of healthy adults and is currently being trialled in hospitalised adults.106
Rhinovirus
There are currently no specific antiviral agents licensed for the prevention or treatment of rhinovirus infection. The rhinovirus capsid binding agent pleconaril107 showed promise in clinical trials of naturally occurring colds but was rejected by the US Food and Drug Administration due to the relatively high frequency of side effects, drug interactions and concerns over resistance.108 Phase 2 trials of the human rhinovirus capsid binder vapendavir (BTA798) are currently underway in adult asthmatics with naturally acquired rhinovirus infection.109 A single centre randomised controlled trial of inhaled beta interferon has demonstrated reduced severity of rhinovirus induced asthma exacerbation in severe asthmatics110 and larger confirmatory trials are ongoing.
Promising candidate antiviral agents in late stages of development are listed in Table 2 .
Table 2.
Examples of promising candidate antiviral agents currently in late stage development.
| Antiviral agent | Target | Mechanism | Developmental stage | Reference |
|---|---|---|---|---|
| Nitazoxanide | Influenza | Multiple | Phase 2/3 | 100 |
| Favipiravir (T-705) | Influenza and other viruses | Inhibition of RNA polymerase | Phase 3 | 101 |
| Anti-M2e monoclonal antibodies | Influenza | Binding to M2e epitope | Phase 2 | 102 |
| GS-5806 | RSV | Fusion inhibitor | Phase 2 | 105 |
| Vapendavir (BTA798) | Rhinovirus | Capsid binder | Phase 2 | 109 |
| Inhaled beta-interferon | Rhinovirus and other viruses | Restored antiviral response in asthmatics | Phase 2/3 | 110 |
Infection control
Respiratory viruses are known to be highly infectious and to cause nosocomial outbreaks and so testing and isolation of suspected cases is a central tenet of infection control practices in hospitals. Currently cases are isolated based on clinical suspicion with laboratory testing providing definitive results in 24–48 h. This leads to patients without infection occupying valuable isolation facilities unnecessarily for several days and reducing patient flow through the hospital. Although intuitively POCTs for respiratory viruses performed in emergency departments should improve isolation facility use and patient flow there is a paucity of quality evidence for the effects of POCTs in this setting. A systematic review of published literature on the subject of POCTs for the diagnosis of infectious diseases concluded that although POCTs may have a role in infection control the lack of good, consistent clinical data surrounding their use outside of the laboratory is a limiting factor in their implementation.111 A single centre non-randomised paediatric study from the UK suggested significant improvement in isolation facility use and patient flow with rapid antigen based POCT for RSV during the winter months.112 The previously mentioned non-randomised pre and post intervention paediatric study using the FilmArray respiratory panel as a POCT demonstrated a reduction in the time spent in isolation facilities in patients tested with POCT versus standards laboratory PCR testing.71
Other benefits
Several studies evaluating the use of antigen detection based POCTs for influenza in children have shown a decrease in the number of investigations performed on influenza positive patients compared with those tested with standard of care.89, 90, 91, 92, 113 One of these also suggested a reduction in the duration of hospitalisation for those testing positive for influenza89 as did the previously mentioned study using the FilmArray in children.71 The potential clinical benefits listed above could translate in to an overall economic benefit for health care organisations. A study using decision analytic modelling to ascertain the most cost effective testing strategy in children presenting to the emergency department with influenza-like illness suggested that rapid PCR using the FilmArray respiratory panel was superior to standard laboratory PCR or rapid antigen testing. However the incremental costs per QALY were high and many questionable assumptions about the effects of diagnosing a respiratory virus infection on investigations, antibiotic and antiviral use were made.114 Another study using health economic modelling evaluated the cost effectiveness of PCR based rapid diagnostics (Cepheid Xpert Flu assay) for the diagnosis of influenza in high risk adults presenting to the emergency department. They concluded that PCR based rapid testing was the most cost effective strategy although this depended on the prevalence of influenza and again was based on strong assumptions of antiviral use and efficacy.115
The potential benefits of POC testing for respiratory viruses in adults are listed in Table 3 .
Table 3.
Summary of potential benefits of POCT for respiratory viruses along with complicating factors.
| Potential benefit of POCT | Complicating factors |
|---|---|
| Reduced unnecessary antibiotic use | Co-infection with bacteria is common |
| Improved antiviral usea | Ongoing controversy over efficacy of neuraminidase inhibitors |
| Improved isolation facility use | Increased detection of viruses may stretch limited resources |
| Reduced economic cost | Health economic modelling assumption are largely speculative |
Currently only neuraminidase inhibitors for influenza.
Conclusion
The current global priority of replacing empirical antimicrobial use with pathogen directed therapy to help combat resistance, coupled with the recent development of rapid, accurate and easy-to-use molecular test platforms for respiratory viruses, sets the scene for rolling out a point-of-care testing strategy in patients presenting with acute respiratory illness. The potential benefits of such a strategy include a reduction in unnecessary antibiotic use, improved use of directed antiviral therapy for influenza, improved use of isolation facilities in secondary care, a reduction in the number of investigations performed and a reduction in the duration of hospitalisation in some situations. These effects could be associated with an overall health economic benefit. There is a substantial evidence gap and high quality randomised controlled trials evaluating molecular POCTs in adults and using clinically relevant outcomes such as antibiotic use, directed antiviral use and infection control facility use are urgently needed. In addition to the existing agents active against influenza there may soon also be available a range of clinically effective antiviral agents active against other respiratory viruses such as RSV and rhinovirus. For these agents to be utilised effectively, especially in hospitalised patients with severe disease, routine early testing for the presence of viruses with molecular POCTs will be necessary to allow targeted treatment.
Author contributions
NJB, HFS and TWC were all involved in the design, literature search and writing the manuscript, including the final version submitted.
Funding source
None.
Conflicts of interest
None declared for all authors.
References
- 1.Schluger N.W. World Lung Foundation; 2010. Acute respiratory infections Atlas. [Google Scholar]
- 2.Jain S., Williams D.J., Arnold S.R., Ampofo K., Bramley A.M., Reed C. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835–845. doi: 10.1056/NEJMoa1405870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'Acremont V., Kilowoko M., Kyungu E., Philipina S., Sangu W., Kahama-Maro J. Beyond malaria – causes of fever in outpatient Tanzanian children. N Engl J Med. 2014;370(9):809–817. doi: 10.1056/NEJMoa1214482. [DOI] [PubMed] [Google Scholar]
- 4.Clark T.W., Medina M.J., Batham S., Curran M.D., Parmar S., Nicholson K.G. Adults hospitalised with acute respiratory illness rarely have detectable bacteria in the absence of COPD or pneumonia; viral infection predominates in a large prospective UK sample. J Infect. 2014;69(5):507–515. doi: 10.1016/j.jinf.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruuskanen O., Lahti E., Jennings L.C., Murdoch D.R. Viral pneumonia. Lancet. 2011;377(9773):1264–1275. doi: 10.1016/S0140-6736(10)61459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mansbach J.M., Piedra P.A., Teach S.J., Sullivan A.F., Forgey T., Clark S. Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Arch Pediatr Adolesc Med. 2012;166(8):700. doi: 10.1001/archpediatrics.2011.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnston S.L., Pattemore P.K., Sanderson G., Smith S., Lampe F., Josephs L. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995;310(6989):1225. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jennings L.C., Anderson T.P., Beynon K.A., Chua A., Laing R.T., Werno A.M. Incidence and characteristics of viral community-acquired pneumonia in adults. Thorax. 2008;63(1):42–48. doi: 10.1136/thx.2006.075077. [DOI] [PubMed] [Google Scholar]
- 9.Johansson N., Kalin M., Tiveljung-Lindell A., Giske C.G., Hedlund J. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis. 2010;50(2):202–209. doi: 10.1086/648678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Templeton K.E., Scheltinga S.A., van den Eeden W.C., Graffelman A.W., van den Broek P.J., Claas E.C. Improved diagnosis of the etiology of community-acquired pneumonia with real-time polymerase chain reaction. Clin Infect Dis. 2005;41(3):345–351. doi: 10.1086/431588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnstone J., Majumdar S.R., Fox J.D., Marrie T.J. Viral infection in adults hospitalized with community-acquired pneumonia: prevalence, pathogens, and presentation. Chest. 2008;134(6):1141–1148. doi: 10.1378/chest.08-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lieberman D., Shimoni A., Shemer-Avni Y., Keren-Naos A., Shtainberg R., Lieberman D. Respiratory viruses in adults with community-acquired pneumonia. Chest. 2010;138(4):811–816. doi: 10.1378/chest.09-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papadopoulos N.G., Christodoulou I., Rohde G., Agache I., Almqvist C., Bruno A. Viruses and bacteria in acute asthma exacerbations – a GA2LEN-DARE* systematic review. Allergy. 2011;66:458–468. doi: 10.1111/j.1398-9995.2010.02505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohan A., Chandra S., Agarwal D., Guleria R., Broor S., Gaur B. Prevalence of viral infection detected by PCR and RT-PCR in patients with acute exacerbation of COPD: a systematic review. Respirology. 2010;15(3):536–542. doi: 10.1111/j.1440-1843.2010.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madhi S.A., Klugman K.P. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med. 2004;10(8):811–813. doi: 10.1038/nm1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.George S.N., Garcha D.S., Mackay A.J., Patel A.R., Singh R., Sapsford R.J. Human rhinovirus infection during naturally occurring COPD exacerbations. Eur Respir J. 2014;44(1):87–96. doi: 10.1183/09031936.00223113. [DOI] [PubMed] [Google Scholar]
- 17.McCullers J.A. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat Rev Microbiol. 2014;12(4):252–262. doi: 10.1038/nrmicro3231. [DOI] [PubMed] [Google Scholar]
- 18.Falsey A.R., Hennessey P.A., Formica M.A., Cox C., Walsh E.E. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352(17):1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 19.Warren-Gash C., Bhaskaran K., Hayward A., Leung G.M., Lo S.V., Wong C.M. Circulating influenza virus, climatic factors, and acute myocardial infarction: a time series study in England and Wales and Hong Kong. J Infect Dis. 2011;203(12):1710–1718. doi: 10.1093/infdis/jir171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu T., Carcaillon L., Martinez I., Cambou J.P., Kyndt X., Guillot Association of influenza vaccination with reduced risk of venous thromboembolism. Thromb Haemost. 2009;102(6):1259–1264. doi: 10.1160/TH09-04-0222. [DOI] [PubMed] [Google Scholar]
- 21.Kwok C.S., Aslam S., Kontopantelis E., Myint P.K., Zaman M.J., Buchan I. Influenza, influenza-like symptoms and their association with cardiovascular risks: a systematic review and meta-analysis of observational studies. Int J Clin Pract. 2015 May 4 doi: 10.1111/ijcp.12646. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madjid M., Curkendall S., Blumentals W.A. The influence of oseltamivir treatment on the risk of stroke after influenza infection. Cardiology. 2009;113(2):98–107. doi: 10.1159/000172796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welte T., Torres A., Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 2012;67(1):71–79. doi: 10.1136/thx.2009.129502. [DOI] [PubMed] [Google Scholar]
- 24.van Asten L., van den Wijngaard C., van Pelt W., van de Kassteele J., Meijer A., van der Hoek W. Mortality attributable to 9 common infections: significant effect of influenza A, respiratory syncytial virus, influenza B, norovirus, and parainfluenza in elderly persons. J Infect Dis. 2012;206(5):628–639. doi: 10.1093/infdis/jis415. [DOI] [PubMed] [Google Scholar]
- 25.Molinari N.A., Ortega-Sanchez I.R., Messonnier M.L., Thompson W.W., Wortley P.M., Weintraub E. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25(27):5086–5096. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 26.Fendrick A.M., Monto A.S., Nightengale B., Sarnes M. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch Intern Med. 2003;163(4):487–494. doi: 10.1001/archinte.163.4.487. [DOI] [PubMed] [Google Scholar]
- 27.Thompson W.W., Shay D.K., Weintraub E., Brammer L., Bridges C.B., Cox N.J. Influenza-associated hospitalizations in the United States. J Am Med Assoc. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 28.Thompson W.W., Shay D.K., Weintraub E., Brammer L., Cox N., Anderson L.J. Mortality associated with influenza and respiratory syncytial virus in the United States. J Am Med Assoc. 2003;289:179–188. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization (WHO) March 2014. WHO seasonal influenza factsheet N°211. [Google Scholar]
- 30.World Health Organization (WHO), Regional Office for Europe . 2014. WHO/Europe recommendations on influenza vaccination during the 2014/2015 winter season. [Google Scholar]
- 31.Begum F., Pebody R. Health Protection Agency, Department of Health; London: 2010. Seasonal influenza vaccine uptake among those 65 years and over and under 65 years at risk in England: winter season 2009–2010. [Google Scholar]
- 32.Blank P.R., Schwenkglenks M., Szucs T.D. Vaccination coverage rates in eleven European countries during two consecutive influenza seasons. J Infect. 2009;58:446–458. doi: 10.1016/j.jinf.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Osterholm M.T., Kelley N.S., Sommer A., Belongia E.A. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(1):36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 34.Simonsen L., Taylor R.J., Viboud C., Miller M.A., Jackson L.A. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy review article. Lancet Infect Dis. 2007;7(10):658–666. doi: 10.1016/S1473-3099(07)70236-0. [DOI] [PubMed] [Google Scholar]
- 35.Dao C.N., Kamimoto L., Nowell M., Reingold A., Gershman K., Meek J. Emerging Infections Program Network: adult hospitalizations for laboratory-positive influenza during the 2005–2006 through 2007–2008 seasons in the United States. J Infect Dis. 2010;202:881–888. doi: 10.1086/655904. [DOI] [PubMed] [Google Scholar]
- 36.Widmer K., Zhu Y., Williams J.V., Griffin M.R., Edwards K.M., Talbot H.K. Rates of hospitalizations for respiratory syncytial virus, human metapneumovirus, and influenza virus in older adults. J Infect Dis. 2012 Jul 1;206(1):56–62. doi: 10.1093/infdis/jis309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irwin D.E., Weatherby L.B., Huang W.Y., Rosenberg D.M., Cook S.F., Walker A.M. Impact of patient characteristics on the risk of influenza/ILI-related complications. BMC Health Serv Res. 2001;1:8. doi: 10.1186/1472-6963-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mauskopf J., Klesse M., Lee S., Herrera-Taracena G. The burden of influenza complications in different high-risk groups: a targeted literature review. J Med Econ. 2013;16(2):264–277. doi: 10.3111/13696998.2012.752376. [DOI] [PubMed] [Google Scholar]
- 39.Li G., Yilmaz M., Kojicic M., Fernández-Pérez E., Wahab R., Huskins W.C. Outcome of critically ill patients with influenza virus infection. J Clin Virol. 2009;46:275–278. doi: 10.1016/j.jcv.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meier C.R., Napalkov P.N., Wegmuller Y., Jefferson T., Jick H. Population-based study of incidence, risk factors, clinical complications and drug utilisation associated with influenza in the United Kingdom. Eur J Clin Microbiol Infect Dis. 2000;19:834–842. doi: 10.1007/s100960000376. [DOI] [PubMed] [Google Scholar]
- 41.Warren-Gash C., Smeeth L., Hayward A.C. Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: a systematic review. Lancet Infect Dis. 2009;9:601–610. doi: 10.1016/S1473-3099(09)70233-6. [DOI] [PubMed] [Google Scholar]
- 42.Schanzer D.L., Schwartz B. Impact of seasonal and pandemic influenza on emergency department visits, 2003–2010, Ontario, Canada. Acad Emerg Med. 2013;20(4):388–397. doi: 10.1111/acem.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Falsey A.R., McElhaney J.E., Beran J., van Essen G.A., Duval X., Esen M. Respiratory syncytial virus and other respiratory viral infections in older adults with moderate to severe influenza-like illness. J Infect Dis. 2014;209(12):1873–1881. doi: 10.1093/infdis/jit839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Widmer K., Griffin M.R., Zhu Y., Williams J.V., Talbot H.K. Respiratory syncytial virus- and human metapneumovirus-associated emergency department and hospital burden in adults. Influenza Other Respir Viruses. 2014;8(3):347–352. doi: 10.1111/irv.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaunt E.R., Harvala H., McIntyre C., Templeton K.E., Simmonds P. Disease burden of the most commonly detected respiratory viruses in hospitalized patients calculated using the disability adjusted life year (DALY) model. J Clin Virol. 2011;52(3):215–221. doi: 10.1016/j.jcv.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walsh E.E., Falsey A.R. Respiratory syncytial virus infection in adult populations. Infect Disord Drug Targets. 2012;12(2):98–102. doi: 10.2174/187152612800100116. [DOI] [PubMed] [Google Scholar]
- 47.Heikkinen T., Järvinen A. The common cold. Lancet. 2003;361(9351):51–59. doi: 10.1016/S0140-6736(03)12162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greenberg S.B. Viral respiratory infections in elderly patients and patients with chronic obstructive pulmonary disease. Am J Med. 2002;112(Suppl. 6A):28S–32S. doi: 10.1016/S0002-9343(01)01061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monto A.S., Fendrick A.M., Sarnes M.W. Respiratory illness caused by picornavirus infection: a review of clinical outcomes. Clin Ther. 2001;23(10):1615–1627. doi: 10.1016/S0149-2918(01)80133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lynch J.P., 3rd, Fishbein M., Echavarria M. Adenovirus. Semin Respir Crit Care Med. 2011;32(4):494–511. doi: 10.1055/s-0031-1283287. [DOI] [PubMed] [Google Scholar]
- 51.Clark T.W., Fleet D.H., Wiselka M.J. Severe community-acquired adenovirus pneumonia in an immunocompetent 44-year-old woman: a case report and review of the literature. J Med Case Rep. 2011;5:259. doi: 10.1186/1752-1947-5-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walsh E.E., Shin J.H., Falsey A.R. Clinical impact of human coronaviruses 229E and OC43 infection in diverse adult populations. J Infect Dis. 2013;208(10):1634–1642. doi: 10.1093/infdis/jit393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu W.K., Liu Q., Chen D.H., Liang H.X., Chen X.K., Huang W.B. Epidemiology and clinical presentation of the four human parainfluenza virus types. BMC Infect Dis. 23 Jan 2013;13:28. doi: 10.1186/1471-2334-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mackay I.M. Real-time PCR in the microbiology laboratory. Clin Microbiol Infect. 2004;10:190–212. doi: 10.1111/j.1198-743x.2004.00722.x. [DOI] [PubMed] [Google Scholar]
- 55.Mahony J.B. Detection of respiratory viruses by molecular methods. Clin Microbiol Rev. 2008;21(4):716–747. doi: 10.1128/CMR.00037-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chartrand C., Leeflang M.M., Minion J., Brewer T., Pai M. Accuracy of rapid influenza diagnostic tests: a meta-analysis. Ann Intern Med. 2012;156(7):500–511. doi: 10.7326/0003-4819-156-7-201204030-00403. [DOI] [PubMed] [Google Scholar]
- 57.Casiano-Colón A.E., Hulbert B.B., Mayer T.K., Walsh E.E., Falsey A.R. Lack of sensitivity of rapid antigen tests for the diagnosis of respiratory syncytial virus infection in adults. J Clin Virol. 2003;28(2):169–174. doi: 10.1016/s1386-6532(03)00002-7. [DOI] [PubMed] [Google Scholar]
- 58.Lee C.K., Cho C.H., Woo M.K., Nyeck A.E., Lim C.S., Kim W.J. Evaluation of Sofia fluorescent immunoassay analyzer for influenza A/B virus. J Clin Virol. 2012;55(3):239–243. doi: 10.1016/j.jcv.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 59.Kanwar N., Hassan F., Nguyen A., Selvarangan R. Head-to-head comparison of the diagnostic accuracies of BD Veritor System RSV and Quidel Sofia RSV FIA systems for respiratory syncytial virus (RSV) diagnosis. J Clin Virol. 2015;65:83–86. doi: 10.1016/j.jcv.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 60.Brotons P., Launes C., Iñigo M., Peris N., Selva L., Muñoz-Almagro C. Performance of a rapid multi-analyte 2-photon excitation assay in children with acute respiratory infection. Diagn Microbiol Infect Dis. 2014;79(2):190–193. doi: 10.1016/j.diagmicrobio.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ivaska L., Niemelä J., Heikkinen T., Vuorinen T., Peltola V. Identification of respiratory viruses with a novel point-of-care multianalyte antigen detection test in children with acute respiratory tract infection. J Clin Virol. 2013;57(2):136–140. doi: 10.1016/j.jcv.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bell J., Bonner A., Cohen D.M., Birkhahn R., Yogev R., Triner W. Multicenter clinical evaluation of the novel Alere i Influenza A&B isothermal nucleic acid amplification test. J Clin Virol. 2014;61(1):81–86. doi: 10.1016/j.jcv.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 63.Beckmann C., Hirsch H.H. Diagnostic performance of near-patient testing for influenza. J Clin Virol. 2015;67:43–46. doi: 10.1016/j.jcv.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nie S., Roth R.B., Stiles J., Mikhlina A., Lu X., Tang Y.W. Evaluation of Alere i Influenza A&B for rapid detection of influenza viruses A and B. J Clin Microbiol. 2014;52(9):3339–3344. doi: 10.1128/JCM.01132-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Poritz M.A., Blaschke A.J., Byington C.L., Meyers L., Nilsson K., Jones D.E. FilmArray, an automated nested multiplex PCR system for multi-pathogen detection: development and application to respiratory tract infection. PLoS One. 2011;6(10):e26047. doi: 10.1371/journal.pone.0026047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Popowitch E.B., O'Neill S.S., Miller M.B. Comparison of the Biofire FilmArray RP, Genmark eSensor RVP, Luminex xTAG RVPv1, and Luminex xTAG RVP fast multiplex assays for detection of respiratory viruses. J Clin Microbiol. 2013;51(5):1528–1533. doi: 10.1128/JCM.03368-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pierce V.M., Elkan M., Leet M., McGowan K.L., Hodinka R.L. Comparison of the Idaho Technology FilmArray system to real-time PCR for detection of respiratory pathogens in children. J Clin Microbiol. 2012;50(2):364–371. doi: 10.1128/JCM.05996-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang J., Simons D.B., Adams J.L., Jerris R.C., Rogers B.B. Multiplex viral polymerase chain reaction testing using the FilmArray device compared with direct fluorescent antibody testing. Lab Med. 2014;45(1):62–64. doi: 10.1309/lmomixq6n4japdx1. [DOI] [PubMed] [Google Scholar]
- 69.Butt S.A., Maceira V.P., McCallen M.E., Stellrecht K.A. Comparison of three commercial RT-PCR systems for the detection of respiratory viruses. J Clin Virol. 2014;61(3):406–410. doi: 10.1016/j.jcv.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Loeffelholz M.J., Pong D.L., Pyles R.B., Xiong Y., Miller A.L., Bufton K.K. Comparison of the FilmArray Respiratory Panel and Prodesse real-time PCR assays for detection of respiratory pathogens. J Clin Microbiol. 2011;49(12):4083–4088. doi: 10.1128/JCM.05010-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rogers B.B., Shankar P., Jerris R.C., Kotzbauer D., Anderson E.J., Watson J.R. Impact of a rapid respiratory panel test on patient outcomes. Arch Pathol Lab Med. 2015;139(5):636–641. doi: 10.5858/arpa.2014-0257-OA. [DOI] [PubMed] [Google Scholar]
- 72.Popowitch E.B., Rogers E., Miller M.B. Retrospective and prospective verification of the Cepheid Xpert influenza virus assay. J Clin Microbiol. 2011;49(9):3368–3369. doi: 10.1128/JCM.01162-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jenny S.L., Hu Y., Overduin P., Meijer A. Evaluation of the Xpert Flu A panel nucleic acid amplification-based point-of-care test for influenza A virus detection and pandemic H1 subtyping. J Clin Virol. 2010;49(2):85–89. doi: 10.1016/j.jcv.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dugas A.F., Valsamakis A., Gaydos C.A., Forman M., Hardick J., Kidambi P. Evaluation of the Xpert Flu rapid PCR assay in high-risk emergency department patients. J Clin Microbiol. 2014;52(12):4353–4355. doi: 10.1128/JCM.02343-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salez N., Nougairede A., Ninove L., Zandotti C., de Lamballerie X., Charrel R.N. Prospective and retrospective evaluation of the Cepheid Xpert Flu/RSV XC assay for rapid detection of influenza A, influenza B, and respiratory syncytial virus. Diagn Microbiol Infect Dis. 2015;81(4):256–258. doi: 10.1016/j.diagmicrobio.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Binnicker M.J., Espy M.J., Irish C.L., Vetter E.A. Direct detection of influenza A and B viruses in less than 20 minutes using a commercially available rapid PCR assay. J Clin Microbiol. 2015;53(7):2353–2354. doi: 10.1128/JCM.00791-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ruggiero P., McMillen T., Tang Y.W., Babady N.E. Evaluation of the BioFire FilmArray respiratory panel and the GenMark eSensor respiratory viral panel on lower respiratory tract specimens. J Clin Microbiol. 2014;52(1):288–290. doi: 10.1128/JCM.02787-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Report of the review of NHS pathology services in England. August 2006. [Google Scholar]
- 79.Caliendo A.M., Gilbert D.N., Ginocchio C.C., Hanson K.E., May L., Quinn T.C., Infectious Diseases Society of America (IDSA) Better tests, better care: improved diagnostics for infectious diseases. Clin Infect Dis. 2013;57(Suppl. 3):S139–S170. doi: 10.1093/cid/cit578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Antimicrobial resistance. Global report on surveillance. World Health Organisation; 2014. [Google Scholar]
- 81.Spellberg B., Bartlett J.G., Gilbert D.N. The future of antibiotics and resistance. N Engl J Med. 2013;368(4):299–302. doi: 10.1056/NEJMp1215093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Addressing the public health burden of respiratory viruses: the Battle against Respiratory Viruses (BRaVe) initiative. Future Virol. 2013;8(10):953–968. [Google Scholar]
- 83.Graham V.A., Milton A.F., Knowles G.K., Davies R.J. Routine antibiotics in hospital management of acute asthma. Lancet. 1982;1(8269):418–420. doi: 10.1016/s0140-6736(82)91619-1. [DOI] [PubMed] [Google Scholar]
- 84.Smith S.M., Fahey T., Smucny J., Becker L.A. Antibiotics for acute bronchitis. Cochrane Database Syst Rev. 2014;3:CD000245. doi: 10.1002/14651858.CD000245.pub3. [DOI] [PubMed] [Google Scholar]
- 85.Little P., Stuart B., Moore M., Coenen S., Butler C.C., Godycki-Cwirko M. Amoxicillin for acute lower-respiratory-tract infection in primary care when pneumonia is not suspected: a 12-country, randomised, placebo-controlled trial. Lancet Infect Dis. 2013;13(2):123–129. doi: 10.1016/S1473-3099(12)70300-6. [DOI] [PubMed] [Google Scholar]
- 86.Nicholson K.G., Abrams K.R., Batham S., Medina M.J., Warren F.C., Barer M. Randomised controlled trial and health economic evaluation of the impact of diagnostic testing for influenza, respiratory syncytial virus and Streptococcus pneumoniae infection on the management of acute admissions in the elderly and high-risk 18- to 64-year-olds. Health Technol Assess. 2014;18(36):1–274. doi: 10.3310/hta18360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Falsey A.R., Murata Y., Walsh E.E. Impact of rapid diagnosis on management of adults hospitalized with influenza. Arch Intern Med. 2007;167:354–360. doi: 10.1001/archinte.167.4.ioi60207. [DOI] [PubMed] [Google Scholar]
- 88.Noyola D.E., Demmler G.J. Effect of rapid diagnosis on management of influenza A infections. Pediatr Infect Dis J. 2000;19(4):303–307. doi: 10.1097/00006454-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 89.Bonner A.B., Monroe K.W., Talley L.I., Klasner A.E., Kimberlin D.W. Impact of the rapid diagnosis of influenza on physician decision-making and patient management in the pediatric emergency department: results of a randomized, prospective, controlled trial. Pediatrics. 2003;112(2):363–367. doi: 10.1542/peds.112.2.363. [DOI] [PubMed] [Google Scholar]
- 90.Poehling K.A., Zhu Y., Tang Y.W., Edwards K. Accuracy and impact of a point-of-care rapid influenza test in young children with respiratory illnesses. Arch Pediatr Adolesc Med. 2006;160(7):713–718. doi: 10.1001/archpedi.160.7.713. [DOI] [PubMed] [Google Scholar]
- 91.Blaschke A.J., Shapiro D.J., Pavia A.T., Byington C.L., Ampofo K., Stockmann C. A national study of the impact of rapid influenza testing on clinical care in the emergency department. J Pediatr Infect Dis Soc. 2014;3(2):112–118. doi: 10.1093/jpids/pit071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Iyer S.B., Gerber M.A., Pomerantz W.J., Mortensen J.E., Ruddy R.M. Effect of point-of-care influenza testing on management of febrile children. Acad Emerg Med. 2006;13(12):1259–1268. doi: 10.1197/j.aem.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 93.Brittain-Long R., Westin J., Olofsson S., Lindh M., Andersson L.M. Access to a polymerase chain reaction assay method targeting 13 respiratory viruses can reduce antibiotics: a randomised, controlled trial. BMC Med. 2011;9:44. doi: 10.1186/1741-7015-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Doan Q., Enarson P., Kissoon N., Klassen T.P., Johnson D.W. Rapid viral diagnosis for acute febrile respiratory illness in children in the Emergency Department. Cochrane Database Syst Rev. 2014;9:CD006452. doi: 10.1002/14651858.CD006452.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Health Protection Services . Public Health England; December 2013. PHE guidance on use of antiviral agents for the treatment and prophylaxis of influenza. Version 4. [Google Scholar]
- 96.Muthuri S.G., Venkatesan S., Myles P.R., Leonardi-Bee J., Al Khuwaitir T.S., Al Mamun A. Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med. 2014;2(5):395–404. doi: 10.1016/S2213-2600(14)70041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fry A.M., Goswami D., Nahar K., Sharmin A.T., Rahman M., Gubareva L. Efficacy of oseltamivir treatment started within 5 days of symptom onset to reduce influenza illness duration and virus shedding in an urban setting in Bangladesh: a randomised placebo-controlled trial. Lancet Infect Dis. 2014;14(2):109–118. doi: 10.1016/S1473-3099(13)70267-6. [DOI] [PubMed] [Google Scholar]
- 98.Dobson J., Whitley R.J., Pocock S., Monto A.S. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet. 2015;385(9979):1729–1737. doi: 10.1016/S0140-6736(14)62449-1. [DOI] [PubMed] [Google Scholar]
- 99.Kelly H., Cowling B.J. Influenza: the rational use of oseltamivir. Lancet. 2015;385(9979):1700–1702. doi: 10.1016/S0140-6736(15)60074-5. [DOI] [PubMed] [Google Scholar]
- 100.Haffizulla J., Hartman A., Hoppers M., Resnick H., Samudrala S., Ginocchio C., US Nitazoxanide Influenza Clinical Study Group Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double-blind, randomised, placebo-controlled, phase 2b/3 trial. Lancet Infect Dis. 2014;14(7):609–618. doi: 10.1016/S1473-3099(14)70717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Furuta Y., Gowen B.B., Takahashi K., Shiraki K., Smee D.F., Barnard D.L. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res. 2013;100(2):446–454. doi: 10.1016/j.antiviral.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ramos E.L., Mitcham J.L., Koller T.D., Bonavia A., Usner D.W., Balaratnam G. Efficacy and safety of treatment with an anti-m2e monoclonal antibody in experimental human influenza. J Infect Dis. 2015;211(7):1038–1044. doi: 10.1093/infdis/jiu539. [DOI] [PubMed] [Google Scholar]
- 103.Turner T.L., Kopp B.T., Paul G., Landgrave L.C., Hayes D., Jr., Thompson R. Respiratory syncytial virus: current and emerging treatment options. Clinicoecon Outcomes Res. 2014;25(6):217–225. doi: 10.2147/CEOR.S60710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Walsh E.E. Respiratory syncytial virus infection in adults. Semin Respir Crit Care Med. 2011;32(4):423–432. doi: 10.1055/s-0031-1283282. [DOI] [PubMed] [Google Scholar]
- 105.DeVincenzo J.P., Whitley R.J., Mackman R.L., Scaglioni-Weinlich C., Harrison L., Farrell E. Oral GS-5806 activity in a respiratory syncytial virus challenge study. N Engl J Med. 2014;371(8):711–722. doi: 10.1056/NEJMoa1401184. [DOI] [PubMed] [Google Scholar]
- 106.Efficacy, pharmacokinetics, and safety of GS-5806 in hospitalized adults with Respiratory Syncytial Virus (RSV) infection. ClinicalTrials.gov.
- 107.Hayden F.G., Herrington D.T., Coats T.L., Kim K., Cooper E.C., Villano S.A. Efficacy and safety of oral pleconaril for treatment of colds due to picornaviruses in adults: results of 2 double-blind, randomized, placebo-controlled trials. Clin Infect Dis. 2003;36(12):1523–1532. doi: 10.1086/375069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schwitzer G. How the media left the evidence out in the cold. BMJ. 2003;326(7403):1403–1404. [Google Scholar]
- 109.A phase 2 study of BTA798 in asthmatic adults with Symptomatic Human Rhinovirus Infection (RHINO). ClinicalTrials.gov.
- 110.Djukanović R., Harrison T., Johnston S.L., Gabbay F., Wark P., Thomson N.C., INTERCIA Study Group The effect of inhaled IFN-β on worsening of asthma symptoms caused by viral infections. A randomized trial. Am J Respir Crit Care Med. 2014;190(2):145–154. doi: 10.1164/rccm.201312-2235OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moore C. Point-of-care tests for infection control: should rapid testing be in the laboratory or at the front line? J Hosp Infect. 2013;85(1):1–7. doi: 10.1016/j.jhin.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 112.Mills J.M., Harper J., Broomfield D., Templeton K.E. Rapid testing for respiratory syncytial virus in a paediatric emergency department: benefits for infection control and bed management. J Hosp Infect. 2011;77(3):248–251. doi: 10.1016/j.jhin.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 113.Hojat K., Duppenthaler A., Aebi C. Impact of the availability of an influenza virus rapid antigen test on diagnostic decision making in a pediatric emergency department. Pediatr Emerg Care. 2013;29(6):696–698. doi: 10.1097/PEC.0b013e3182948f11. [DOI] [PubMed] [Google Scholar]
- 114.Nelson R.E., Stockmann C., Hersh A.L., Pavia A.T., Korgenksi K., Daly J.A. Economic analysis of rapid and sensitive polymerase chain reaction testing in the emergency department for influenza infections in children. Pediatr Infect Dis J. 2015;34(6):577–582. doi: 10.1097/INF.0000000000000703. [DOI] [PubMed] [Google Scholar]
- 115.Dugas A.F., Coleman S., Gaydos C.A., Rothman R.E., Frick K.D. Cost-utility of rapid polymerase chain reaction-based influenza testing for high-risk emergency department patients. Ann Emerg Med. 2013;62(1):80–88. doi: 10.1016/j.annemergmed.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]