Summary
Yellow head virus (YHV) is an invertebrate nidovirus that has caused mass mortality in penaeid shrimp since 1990. Several YHV types are known, but only the original type (YHV-type 1 or YHV-1) is highly virulent. Most studies have focused on acute YHV-1 infections and there is limited work on YHV-1 survivors. We compared moribund and surviving (14%) whiteleg shrimp Penaeus (Litopenaeus) vannamei from an experimental challenge with YHV-1. Although grossly normal, all survivors were positive for YHV-1 by specific, reverse transcriptase polymerase chain reaction (RT-PCR) assays, histological analysis or transmission electron microscopy (TEM), indicating that they were not resistant but tolerant to YHV-1. On the other hand, real-time PCR analysis revealed that mean YHV-1 copies/ng total RNA for survivors (2.8×104±6.9×104) were approximately 40 times lower (P<0.05) than those in moribund shrimp (1.2×106±6.7×105 copies/ng total RNA). This was confirmed by strong positive immunohistochemical and in situ hybridization (ISH) reactions for YHV-1 in lymphoid organ tubules (LOT) of moribund shrimp and weak positive reaction only in lymphoid organ spheroids (LOS) of survivors. TEM revealed morphologically complete YHV virions in both groups. Furthermore, immuno-TEM and Western blot analysis revealed that YHV-1 structural proteins gp116 and p20 were present at comparable reactive levels in each group. Thus, YHV-1 tolerance was not associated with absence of gp116 as previously reported for palaemonid shrimp. Instead, it was associated with the presence of YHV-positive LOS and a relatively low viral load.
Keywords: Yellow head virus, YHV, Penaeus (Litopenaeus) vannamei, Viral tolerance, Shrimp, Survivors, Lymphoid organ, Spheroids, Viral load
1. Introduction
Yellow head disease (YHD) was first described with the occurrence of mass mortalities in farmed black tiger shrimp Penaeus monodon in Thailand [1]. After that, it was reported from cultivation ponds elsewhere in Asia and possibly in the Americas, where it also caused high shrimp mortality [2], [3]. The causative agent of YHD is yellow head virus (YHV). Gross signs often associated with YHV infection include cessation of feeding and the development of yellow coloration of the cephalothorax and gills [4]. Subsequent mass mortality of shrimp occurs within 2–3 days of the first appearance of gross signs of disease. Lymphoid organs (LOs) and gills of moribund shrimp show large numbers of pyknotic and karyorrhectic nuclei and densely basophilic cytoplasmic inclusions. Transmission electron microscopy (TEM) of YHV-infected tissues reveals threadlike nucleocapsids (approximately 15 nm×130–800 nm) together with mature, enveloped, bacilliform virions of approximately 150–200 nm×40–50 nm. Mature virions accumulate within vesicles in the cytoplasm of infected cells and are deposited in intercellular spaces when vesicles fuse with the plasma membrane [4], [5].
YHV is a single-stranded RNA virus [6] with a positive-sense RNA genome [7], [8] of approximately 26 kb and has been classified in the genus Okavirus, family Roniviridae and order Nidovirales [9], [10], [11], [12], [13]. Several genetic variants of YHV have been reported since the original description of yellow head virus type 1 (YHV-1) [11]. These include a closely related form from Australia first called lymphoid organ virus (LOV) in its non-disease state [14] and later gill-associated virus (GAV) in the disease state [15] associated with aquaculture production losses in Australian shrimp farms [8], [11]. Here, the LOV/GAV variant will be referred to as YHV-2. Comparison of nucleic acid and amino acid sequences indicates that YHV-1 and YHV-2 are distinct but closely related viruses [9], [16]. However, YHV-2 is even more closely related to a non-virulent form of YHV (here called YHV-3) that is known to occur in Vietnam [11] and Thailand [17]. A fourth, non-pathogenic type (here called YHV-4) also occurs in Thailand but has not yet been described (T. Flegel, unpublished).
Non-structural proteins encoded by the YHV genome include a large replicase gene in open reading frame (ORF) 1b that appears to be expressed as a polyprotein by ribosomal frame-shift between ORF1a and 1b [12]. Nadala et al. [18] originally reported that YHV particles contained 4 structural proteins of approximately 170, 135, 67 and 22 kDa. Subsequently, only 3 major structural proteins were identified and characterized, including a nucleocapsid protein p20 encoded by ORF2 and envelope glycoproteins gp116 and gp64 encoded by ORF3 [19], [20], [21], [22], [23]. It is generally believed that coronaviruses use envelope proteins as recognition molecules for viral–host binding and cell entry [24]. An in-vitro study has supported this by showing that a polyclonal antibody to gp116 inhibited YHV entry into LO cells, whereas an antibody to gp64 did not [25]. Although antisera against YHV have been produced since 1997 [12], [22], development of monoclonal antibodies (MAb) of IgG type specific for each structural protein (Y19 against p20, Y18 against gp64 and V3–2B against gp116) has allowed improvement in immunodiagnostic methods such as immunohistochemistry, dot-blot analysis and Western blot analysis [20], [21].
Most cultivated penaeid shrimp species are susceptible to YHV-1 infection [2], [3], [26]. In addition, some wild shrimp and crab species may carry persistent infections of YHV-1 without gross signs of disease but remain capable of transmitting lethal infections to cultivated penaeid shrimp species [27], [28]. Thus, they constitute a potential risk in causing YHD outbreaks in farms [2].
Since its original description in Thailand in 1993, the incidence of YHD outbreaks has declined and anecdotal information suggests that the percentage of survivors when outbreaks do occur has increased in successive years [29], [30]. At the same time, it has recently been found that grossly normal shrimp may sometimes be infected with one or more viruses [31], [32]. An explanation for this phenomenon has been proposed in the viral accommodation concept [30], [33] and some support for it has been obtained in studies of model mosquito cells [34] and whole mosquitoes serially challenged with a densovirus (AThDNV) for 5 successive generations [35]. There was some indication that improved survival of persistently infected cells and mosquitoes might be associated with the relatively high prevalence of defective viral particles sometimes called defective interfering particles (DIP) that were generated in host cells. Improvement in shrimp survival upon viral challenge has also been reported to result from feeding or injecting viral envelope proteins [36], [37], [38] or inactivated viral particles [39] as vaccine-like reagents to elicit what has been called a “pseudo-immune” response [40].
As with WSSV outbreaks [40], shrimp sometimes survive YHV-1 outbreaks and remain infected for long periods without signs of disease. The purpose of this study was to carry out a descriptive analysis of survivors of a YHV-1 challenge test to determine whether or not they were infected with YHV-1 and if so to determine some characteristics of the infection in terms of histopathology, viral load and immunochemical characteristics of the virus present. To imitate a natural mode of YHV infection, the test protocol utilized water-borne transmission of YHV-1 via infected shrimp held in cages to physically separate them from uninfected shrimp.
2. Materials and methods
2.1. Shrimp
Experimental Penaeus vannamei juveniles (approximately 100 of 7–10 g BW) were obtained from a commercial farm in Chachoengsao province, Thailand. They originated from specific pathogen-free (SPF) stock and were reared in an outdoor pond that had been treated prior to stocking with insecticide to kill crustacean carrier species and that used controlled water exchanges to prevent carrier entry. Prior to experiments, they were screened for presence of YHV types 1–4 using a commercial RT-PCR kit, IQ2000™ YHV/GAV detection and typing system according to the kit instructions (Farming IntelliGene Technology, Taiwan; www.iq2000kit.com). This kit was designed for detection of YHV-1 and YHV-2, but can also be used to detect YHV-3 since it gives an amplicon of 407 bp identical to that of YHV-2. It can also detect YHV-4 that gives an amplicon at 777 bp only (T.W. Flegel, pers. comm.). This amplicon is equivalent to the outer primer amplicon obtained with heavy infections of YHV-1, -2 and -3. For these tests, pleopods from 30 shrimp at day 0 (d0) were sampled and pooled (5 animals per sample) for RNA extraction and RT-PCR testing. Negative test results confirmed freedom from YHV at 9% prevalence with 95% confidence [41]. The shrimp were acclimatized for 3–5 days in double 1-ton fiberglass tanks containing prepared artificial seawater at 10–15 ppt of salinity. Water depth was 0.80 m and 20% of the water was exchanged daily. Adequate aeration and a simple filter unit were employed. The shrimp were maintained under natural photoperiod (12 h:12 h of dark/light cycle) at 27–30 °C. Commercial pelleted feed was provided twice daily at a rate of 3% BW. The shrimp were grossly normal prior to viral challenge.
2.2. YHV stock solution
YHV-hemolymph stock was collected from moribund P. monodon during a YHD outbreak in a farm, and kept at −80 °C until used. Prior to experiments, a solution containing virulent YHV (YHV-inoculum) was prepared from this stock by filtration with a 0.22 μm membrane filter and dilution in modified Alsever's solution [42] at 1:100. Virulence of the YHV inoculum was confirmed in a separate injection challenge of 20 P. vannamei with 0.1 ml and demonstration of total mortality within 3 days. In addition, sequencing of 2 clones of an 831 bp amplicon obtained by RT-PCR (see below) with RNA extracted from the stock revealed 100% identity to ORF1b gene of YHV-type1 (AY052786).
2.3. YHV challenge protocol and sampling
The overall objective of this test was to compare moribund and surviving shrimp from a group of 50 shrimp challenged with YHV by the water-borne (natural) route. To prepare shrimp as the viral source for the co-habitation challenge, 20 shrimp were removed from the stock of 100 P. vannamei at d0 and injected into the muscle of the third or fourth abdominal segment with 0.1 ml of YHV-inoculum solution. After injection, they were placed in a 1-ton container within 4 floating baskets that did not allow close contact with shrimp outside the baskets. These shrimp served as the source of YHV for immersion challenge of the test shrimp. They also served as a positive YHV-injected, reference control for the shrimp challenged by the water-borne route. The challenge test shrimp (Group 1) comprised 50 shrimp placed in the same 1-ton container outside the baskets so that they would all be exposed to YHV at the same level by the water-borne route. The untreated control shrimp (Group 2) comprised 30 shrimp; 10 of these were sampled at the start of the experiment as 0-h controls and 20 were maintained for 14 days in a separate 300-l-tank. The latter comprised incubation controls to monitor the integrity of the culture system and substantiate freedom from YHV. Tanks were continually monitored so that dead and moribund shrimp could be removed immediately. Dead shrimp were not used for analysis. Moribund shrimp were analyzed and were counted as dead shrimp for purposes of survival values. This challenge protocol was repeated in 4 separate experiments to determine repeatability of the survival profile. However, detailed analysis of shrimp samples was confined to Experiment 4, except for the addition of 4 surviving shrimp (2 each from Experiments 2 and 3) for histopathological analysis by H&E staining, immunohistochemistry and in-situ hybridization (ISH) (see below).
Every shrimp specimen could not be subjected to every test due to the limitation in materials. For example, 1 shrimp specimen has only 2 lobes of the LO and these are very, very small. If one lobe is used for TEM and another for immunohistochemistry, there would be no material left for another test. In our protocol, the 2 lobes from each shrimp were separated as parallel samples. One lobe was immersed in Trizol Reagent (Invitrogen, CA) for RNA extraction, while the other was used variously for histopathology, immuno-TEM and Western blot analysis. In total, 20/50 shrimp were used as representatives for the moribund group and 7 (+4) as representatives for the survivor group (Table 1 ). Shrimp were anesthetized in ice water before tissue samples were aseptically removed.
Table 1.
Key to samples analyzed from the test and control groups from Experiment 4.
| Group | Test |
||||||
|---|---|---|---|---|---|---|---|
| Histology | Immunohistochemistry | In-situ hybridization | RT-PCR | Real-time PCR | TEM of LO | Western blot of LO | |
| Challenge (50) | |||||||
| Moribund (20) | |||||||
| 5 | √ | √ | √ | ||||
| 3 | √ | √ | √ | ||||
| 3 | √ | √ | |||||
| 9 | √ | ||||||
| Survivors (7) | |||||||
| 1 (+4)* | √ | √ | √ | ||||
| 3 | √ | √ | √ | ||||
| 3 | √ | √ | √ | ||||
| Positive control (20) | |||||||
| 5 | √ | √ | √ | ||||
| 3 | √ | √ | |||||
| 3 | √ | √ | |||||
| Control (30) | |||||||
| Day 0 (10) | |||||||
| 3 | √ | √ | √ | √ | |||
| 7 | √ | ||||||
| Day 14 (16) | |||||||
| 3 | √ | √ | √ | ||||
| 3 | √ | ||||||
| Moribund (4) | |||||||
| 4 | √ | ||||||
From Experiments 2 and 3, additional samples of 2 survivors each were processed for histology, immunohistochemistry and in-situ hybridization. These are shown as (+4)* under the heading of survivors in row 8 of the table.
2.4. Histopathology and immunogold labeling for transmission electron microscopy
Parallel LO samples (3–5 pieces per group) were fixed in a cold mixture of 4% paraformaldehyde and 1% glutaraldehyde dissolved in 0.15 M Millonig's buffer at pH 7.4 for 6 h, rinsed in buffer before storage at 4 °C. Subsequently, LO tissues were dehydrated through a graded series of ethanol and embedded in LR White resin (EMS, England). Embedded tissues were polymerized in an oven at 70 °C for 2 days and semi-thin sections were cut, stained with toluidine blue and examined for histopathology under a light microscope (LM). For immuno-TEM, ultrathin sections were cut and immunolabeling was carried out according to methods previously described [43] with MAb V3–2B against YHV envelope protein gp116 and MAb Y19 against nucleocapsid protein p20 [20], [21]. Antibodies that were diluted to optimal dilution were applied to ultrathin sections for 1 h. Anti-mouse immunogold complexes were purchased from Sigma Chemicals (Missouri). Secondary antibody was diluted 1:40 before treatment. The ultrathin sections were contrasted with uranyl acetate and lead citrate for 15 min each and examined using an FEI (Tecnai-20) TEM at high magnification.
2.5. RNA extraction and semi-quantitative RT-PCR
Parallel LO samples were homogenized in Trizol reagent and total RNA was extracted using the phenol–chloroform procedure under sterile conditions. Extracted RNA was re-suspended in DEPC-treated water and quantified by spectrophotometry (OD260) before storage at −80 °C. Primer pairs, P831f and P831r, specific to the ORF1b fragment of YHV and GAV (5′-CAG TCA TTC GCA TTA CAA GC-3′ and 5′-GAA GTC CAT GTG TGT GAG AC-3′, respectively) were designed from AY052786 and AF227196 and synthesized by Proligo (Sigma, Singapore). These were used to monitor YHV type in the injected and challenged shrimp and to generate RT-PCR products for YHV-sequencing analysis. Total RNA extract (100 ng) was used as the template for RT-PCR amplification performed according to the manual supplied with the SuperScript III One-Step RT-PCR kit (Invitrogen, CA). For semi-quantitative estimation of YHV, the number of PCR cycles was varied as 25, 30, 35 and 40 cycles followed by analysis of amplicons by 1.2% agarose gel electrophoresis. Samples that gave visible bands at a lower cycle number were considered to have heavier viral loads than those that gave bands only at a higher cycle number.
2.6. Immunohistochemistry and in-situ hybridization
Shrimp dissection, fixation and paraffin-embedding procedures were based on those of Bell and Lightner [44]. The tissues were sectioned at 5–7 μm thickness and placed on positively charged microscope slides (EMS, CA). Immunolabeling and immunohistochemistry were carried out according to methods previously described [20], [21] with MAb V3–2B against gp116 and Y19 against p20. For immunofluorescence, Alexa Flour 546 (Zymed, San Francisco, CA), 1:2000 dilution was used as the marker for YHV structural proteins and To-Pro-3© fluorescent stain 1:2000 dilution was used as the marker for nuclei (Invitrogen, Singapore). Sections were washed in PBS, mounted using ProLong© Gold anti-fade reagent (Invitrogen, Eugene, OR) and examined and photographed using an Olympus FV-1000 confocal laser scanning microscope (CLSM).
For ISH of parallel tissue sections, digoxigenin-labeled DNA probe for YHV was prepared using a biotin-dUTP-labeling kit (Invitrogen, Singapore) for PCR labeling of a fragment of YHV ORF1b using primers P831f and P831r. The ISH procedure was carried out according to the method for YHV-gene detection described by Tang and Lightner [8]. The slides were counter-stained with 0.5% Bismarck Brown Y for 1 min, washed with tap water and mounted using 1:10 of glycerol/PBS solution before examination by light microscopy.
2.7. YHV quantification by real-time PCR
Real-time PCR was used to determine YHV copy number in LO using cDNAs that were previously reverse transcribed from individual 100 ng total RNA extracts using a Superscript III-RT HRT kit (Invitrogen, CA), according to the manufacturer's recommendations. The real-time primers and TaqMan probe for detection of YHV were designed and selected within the 831-bp amplicon of ORF1b using Primer Express software (Applied Biosystems, CA). The primers YHV70f (5′-CGACATCACTCCAGACAACATCT-3′ and YHV70r (5′-ACAATTGCCGGGACGATATGT-3′) generated a 70-bp DNA fragment after amplification. A TaqMan probe YHV-P (5′-AAGGCGTCTATGACTTCG-3′) that corresponded to the region nucleotides 68–85 of the 831 product was also synthesized and labeled with fluorescent dyes, 6-carboxyfluorescein (FAM) at the 5′-end and N,N,N′,N′-tetramethyl-6-carboxyrhodamine (TAMRA) at the 3′-end. The primers P-EF63f (5′-CTCCTCTCGGACGTTTTGCT-3′) and EF63r (5′-CCTTGATCACACCCACAGCTA-3′), as well as TaqMan probe, EF-P (5′-CCGTCTGCTTCATGTCAC-3′), specific for the EF-1α sequence were selected for quantification as an internal control and cDNA was analyzed from 6 survivors, 6 moribund shrimp and 3 control shrimp. The reaction mixture contained cDNA equivalent to 10 ng of total RNA, each primer at a concentration of 0.3 μM and the TaqMan probe at a concentration of 0.15 μM in a final volume of 25 μl. Each sample was assayed in duplicate with the real-time PCR format. The assays were run on an ABI GeneAmp 7500 sequence detection system. The cycling protocol consisted of 50 °C for 2 min and 95 °C for 10 min to obtain the optimal enzyme activity needed for the TaqMan Universal PCR Master Mix (Applied Biosystems, CA), followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The data acquisition and analysis were carried out using the GeneAmp 7500 sequence detector software. Amplification readings of unknown samples were compared with 8 independent amplification plots from the known target region (831 bp) with 102–109 copies/μg of a YHV-plasmid standard prepared from the cloning strategy described above. Combinations between the readable data and these 8 standard runs were automatically calculated by the software and a threshold cycle (C T) value was set to be above the baseline that began to detect an increase in signal associated with an exponential increase in PCR product. T-test statistical analyses were performed with SigmaStat software Version 2 (Systat Software, Inc., San Jose, CA). Group ranges and means were compared with analysis of variance for the level of YHV copies between moribund and surviving shrimp.
2.8. Quantification of RNA in shrimp tissue
Because our real-time RT-PCR analysis of viral copy numbers was calculated per nanogram of RNA, we desired to translate this into copy numbers per 100 mg shrimp tissue, since it would be a value more easily conceptualized and since 100 mg is a common amount of tissue used to for RNA extraction in shrimp viral detection and quantification methods. To do this, we made 3 preparations of 100 mg fresh shrimp tissue, 2 containing a mixture of cephalothorax tissue and abdominal muscle tissue and 1 containing abdominal muscle tissue. This was done using pre-weighed Eppendorf tubes to weigh the shrimp tissue and using the same RNA extraction, purification and quantification methods described above for RT-PCR analysis.
2.9. Western blot analysis
For Western blot analysis, 3 parallel LO samples were pooled from 3 each of YHV-injected shrimp, moribund shrimp from the co-habitation challenge, surviving shrimp from the co-habitation challenge and control shrimp. These were extracted in tissue lysis buffer (10 mM Tris–HCl, 150 mM NaCl, 1 mM EDTA, 1 mM phenylmethyl–sulfonyl fluoride (PMSF). The total amount of protein in each pooled sample was quantified by Bradford's method using Bio-Rad protein assay kit I (Bio-Rad, CA) with spectometric comparison to a BSA standard at A 595 nm. YHV proteins were detected by 12.5% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) using equal amounts of total proteins (25 μg) and staining with Coomasie brilliant blue R-250. Bands from a duplicate SDS-PAGE were transferred to a nitrocellulose membrane (Amersham, England). It was blocked with 5% skim milk in PBS containing 0.2% Tween-20 for 1 h at room temperature before probing with a MAb raised against gp116 of YHV [21] at a dilution of 1:500 for 1 h. This was followed by treatment with alkaline phosphatase-conjugated goat anti-mouse polyclonal antibodies (Zymed, CA) (dilution 1:2000) and color development using a BCIP/NBT substrate kit (Zymed, CA) for 5–10 min.
3. Results and discussion
3.1. Survival of YHV-challenged P. vannamei
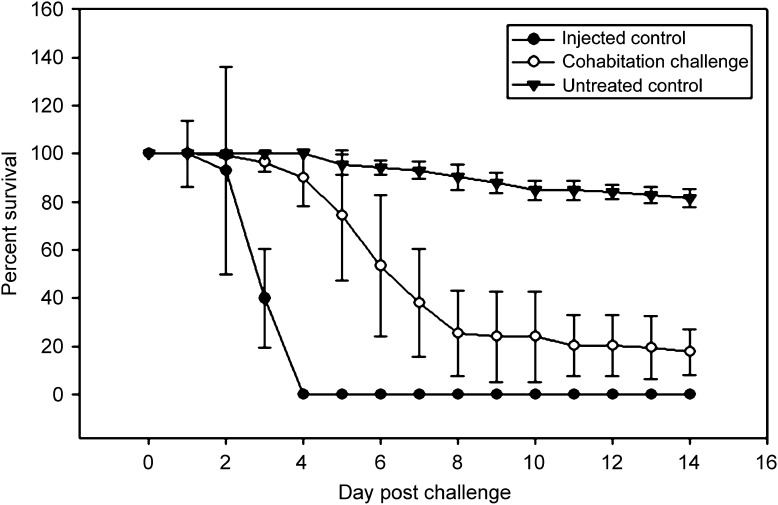
Survival results for control shrimp and for shrimp from co-habitation challenge for 4 separate trials are summarized in Figure 1 . In general, mortality for the co-habitation challenge group began to level off from day 8 (d8) onwards. For specific analysis of Experiment 4, shrimp in Group 1 challenged by the water route showed rapid morbidity or mortality from d3 to d9, although they did not show a yellowish cephalothorax. Survival was 16% (8/50) by d9 and reached 14% (7/50) by d10. Shrimp from d11 onward were defined as survivors. All were normally active and were grossly healthy. One shrimp was removed on d11 for histopathological analysis and the remaining 6 were kept an additional 3 days to d14 when the experiment was terminated. All of the moribund shrimp in Group 1 (20) were positive for YHV by RT-PCR assay or histological plus immunohistochemical and ISH analysis. Survival for the untreated control shrimp in Group 2 was 80% (16/20), indicating that the culture system gave good survival. Like the d0 control shrimp, all incubation control shrimp were negative for YHV by nested RT-PCR assay, including the 4 that died during molting. The absence of YHV in untreated control Group 1 throughout the study confirmed the initial shrimp stock test for freedom from YHV. The caged P. vannamei (20) used as the source of YHV for water-borne challenge by co-habitation started to die 24 h after challenge and showed 100% mortality (i.e., 0% survival) by d5. All were confirmed positive for YHV by RT-PCR and some moribund specimens served as injection-positive YHV controls for the Group 1 shrimp infected by water transmission.
Figure 1.
Graph showing percent survival in the YHV immersion-challenged shrimp and untreated control shrimp from 4 replicated experiments.
Our results showed 14% survival of grossly normal P. vannamei from d11 post-challenge by co-habitation with YHV-1-injected shrimp. This corresponded to field reports of juvenile and adult shrimp that test positive for YHV but show no gross signs of disease [29], [30].
3.2. Confirmation of YHV infection by RT-PCR
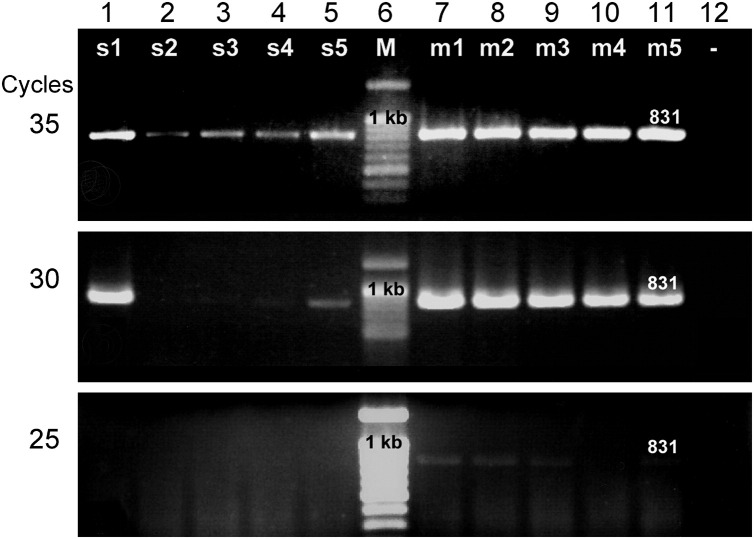
RT-PCR analysis using an IQ2000 commercial kit for YHV or histological analysis confirmed YHV-1 infections in all of the moribund YHV-source shrimp that served as injection positive controls and all of the moribund immersion-challenged Group 1 shrimp. None of the shrimp in control Group 2 gave positive results for any YHV type at any time. Similarly, the P831f and P831r primers specific to the ORF1b region of the YHV complex (Figure 2 ) gave positive results at d14 for both surviving (lanes 1–5) and moribund shrimp (lanes 7–11) from Group 1, showing the expected amplicon of 831 bp. In addition, semi-quantitative RT-PCR tests at 25, 30 and 35 cycles with survivors and moribund shrimp revealed lower band intensities in electrophoresis gels for most of the survivors when compared with those for the moribund shrimp. Band intensity could be used for relative infection level since the template RNA from every sample was dominated by shrimp RNA and adjusted to 100 ng. Using the same ORF1b primers, all of the Group 2 control shrimp gave negative results at d14.
Figure 2.
Ethidium bromide-stained electrophoresis gels showing semi-quantitative RT-PCR results for 35 cycles, 30 cycles and 25 cycles using RNA extracts from unchallenged control shrimp and shrimp from the co-cultivation challenge. Lanes 1–5, surviving shrimp (s1–5). Lanes 7–11, moribund shrimp (m1–5). Lane 6, 100 bp DNA standard marker (M). Lane 12, example of control shrimp result at d14. Only 5 shrimp each from the control and challenge groups are shown in this figure for a 12-comb gel. The remaining 2 shrimp gave similar, group-consistent results.
The positive RT-PCR results for the moribund and surviving shrimp in Group 1 supported the contention that they had been successfully infected with YHV-1 by the water-borne route. The semi-quantitative RT-PCR indicated that the surviving shrimp mostly had lower viral loads than the moribund shrimp. To confirm this, viral loads were measured by real-time PCR.
3.3. Real-time PCR quantitative assay of YHV
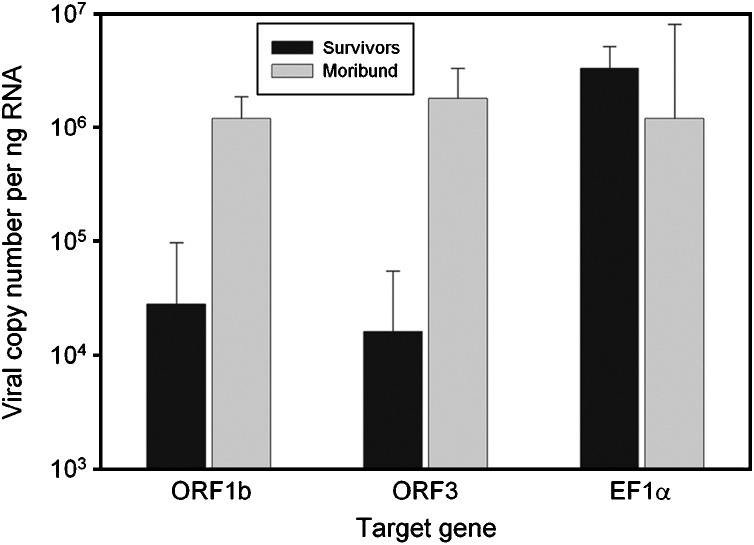
LO samples from 6 surviving shrimp (d14) from immersion-challenged Group 1 and 6 moribund shrimp (3 from immersion-challenged Group 1 and 3 from the YHV injection-positive control) were individually subjected to real-time PCR analysis for the RdRp gene or ORF 1b and for the gp116 envelope protein gene of ORF3. To determine the reproducibility of each reaction, we compared 8 amplification curves from 102 to 109 copies of the plasmid cDNA standard. For the combined data from these 8 independent runs, there was a significant linear relation (R 2=0.992, slope=−2.73, intercept=37.2) between C V and C T values. This is in agreement with similar reports on real-time PCR quantification of shrimp viral loads [45], [46], [47]. Using standard amplification curves for calculations, it was found that YHV copy numbers based on either ORF1b or ORF3 were not significantly different (P>0.05) in the surviving shrimp or in the moribund shrimp (Figure 3 ). Nor was there any significant difference in the mean loads (P>0.05) for the 3 moribund shrimp from the injection group and the 3 from the water challenge group. For the surviving shrimp, the mean of means for ORF1b and ORF3 was 2.2×104±SE 6.0×103 copies/ng RNA and this was 68 times lower (P<0.05) than that for the moribund shrimp (mean=1.5×106±SD 3.0×105 copies/ng RNA). Thus, the real-time RT-PCR results confirmed the preliminary results by semi-quantitative RT-PCR, revealing generally lower viral loads in the surviving shrimp than in the moribund shrimp.
Figure 3.
Compared quantification of 2 ORF from YHV-1 for moribund shrimp and surviving shrimp together with reference EF-1α.
The viral load for our moribund shrimp was similar to that reported by de la Vega et al. [46] for moribund P. monodon challenged with GAV (also called YHV-type 2) in Australia. They found that the highest load of 106 copies/ng of total RNA was in the LO. Because the figure for copy numbers per ng of RNA is difficult to conceptualize, we prepared 3 fresh shrimp tissue samples and measured the mean quantity of total RNA yield per 100 mg shrimp tissue (a common quantity of tissue used for RNA extraction). The mean value obtained was 91.7±11.1 μg/100 mg tissue. Using the mean value, we calculated rough estimates of our viral loads per 100 mg LO tissue to be 2.0×109/100 mg for the surviving shrimp and 1.4×1011/100 mg for the moribund shrimp.
Histological and ultrastructural tests were next performed to prove that the surviving shrimp were actually infected with YHV-1 and not simply RT-PCR positive due to the presence of non-replicating YHV-1 viral particles that were a residual from the challenge.
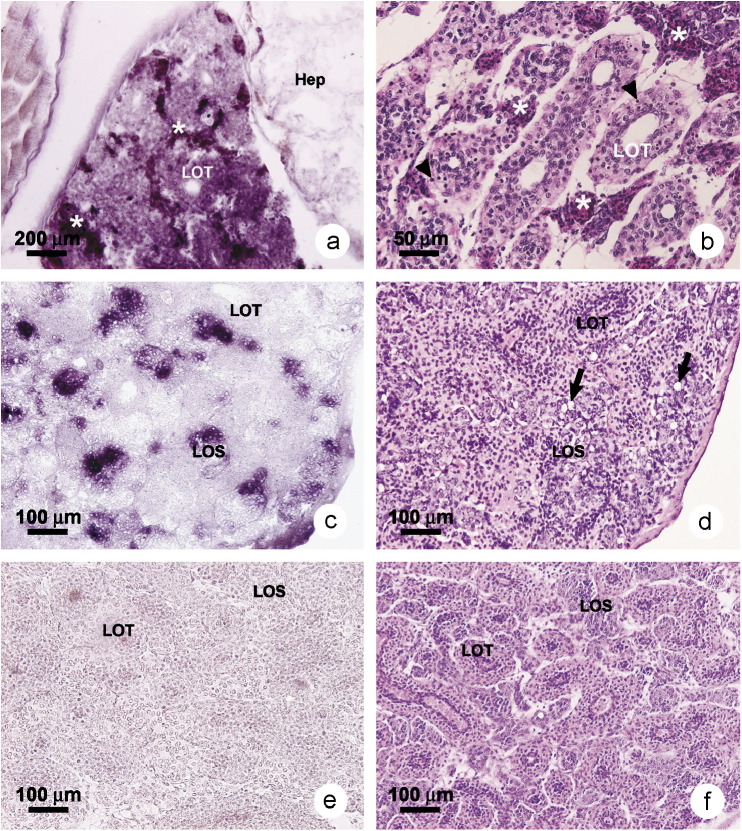
3.4. Histology of the lymphoid organ
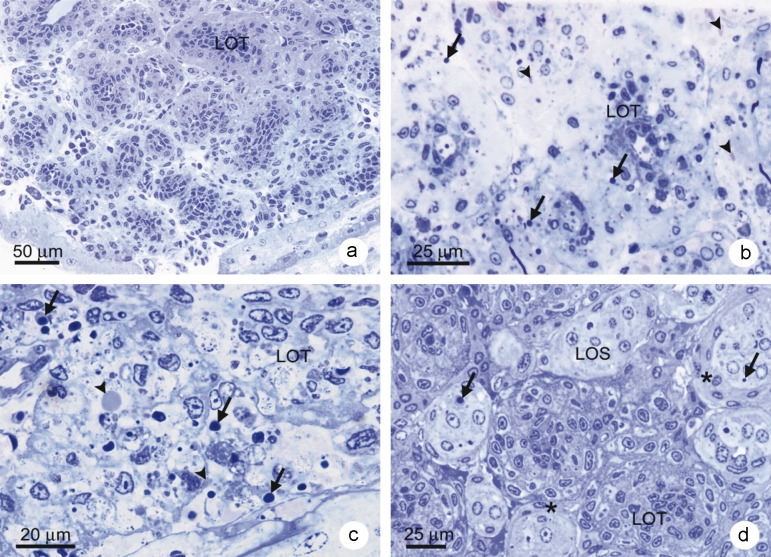
Since the LO is a primary target for YHV-1 [4], semi-thin sections of the LO of shrimp in untreated control Group 2 at d0 (Figure 4a ) were examined to confirm normal histology, including the absence of sinusoidal spheroids or lymphoid organ spheroids (LOS) often associated with viral infections in shrimp [48], [49], [50]. LO of the P. vannamei injected with YHV to serve as viral source and reference positive control showed typical lesions of acute YHD in lymphoid organ tubules (LOT) at d2 after injection (Figure 4b). These signs included pyknotic (Figure 4, arrows) and karyorrhectic nuclei (Figure 4, arrow heads) [4]. Similar lesions were seen in the immersion-challenged Group 1, shrimp at d5 (Figure 4c). In addition, interstitial spaces between the LOT showed aggregates of hemocytes. In contrast, surviving shrimp from Group 1 at d11 and d14 post-challenge exhibited abundant LOS only (Figure 4d). The LOS were formed between the LOT and contained cells exhibiting signs of apoptosis, including nuclear pyknosis and cellular vacuolization. Some LOS were surrounded by a thick capsular sheet (Figure 4, *) that contained vacuolar spaces.
Figure 4.
Photomicrographs of semi-thin sections of lymphoid organ (LO) tissue stained with toluidine blue. (a) Control P. vannamei at d0 showing normal LO tubules (LOT) and absence of LO spheroids (LOS). (b) LO tissue of a moribund shrimp from the YHV source shrimp (also YHV-positive control shrimp) at d2 after YHV injection showing numerous pyknotic nuclei (arrows), karyorrhectic nuclei and cytoplasmic inclusions (arrow heads). (c) LO tissue of moribund shrimp from test Group 1 (immersion challenge) at d5 after YHV challenge showing features similar to those of the YHV-injected shrimp in (b). (d) Surviving shrimp at d14 showing the presence of LOS, some with clearly visible capsular sheaths (*), pyknotic nuclei (arrows) and vacuolated cells.
It is generally believed that LOS indicate the presence of sub-acute to persistent (chronic) viral infections when compared with non-infected shrimp [48], [49], [50]. Semi-thin sections of LO from the incubation control shrimp (Group 2 at d14) showed histological profiles identical to those shown in Figure 4a for the control shrimp at d0 (Group 2 at d0). The Group 2 results confirmed preliminary RT-PCR test results indicating that the shrimp stock used was free of YHV-persistent infections and probably also other viral infections (i.e., because of the absence of spheroids) throughout the experimental period.
In order to prove that the LOS found in the surviving shrimp from immersion-challenged Group 1 were due to YHV-1 infection and not some other pathogen, immunohistochemical and ISH tests were carried out.
3.5. Immunohistochemistry and in-situ hybridization
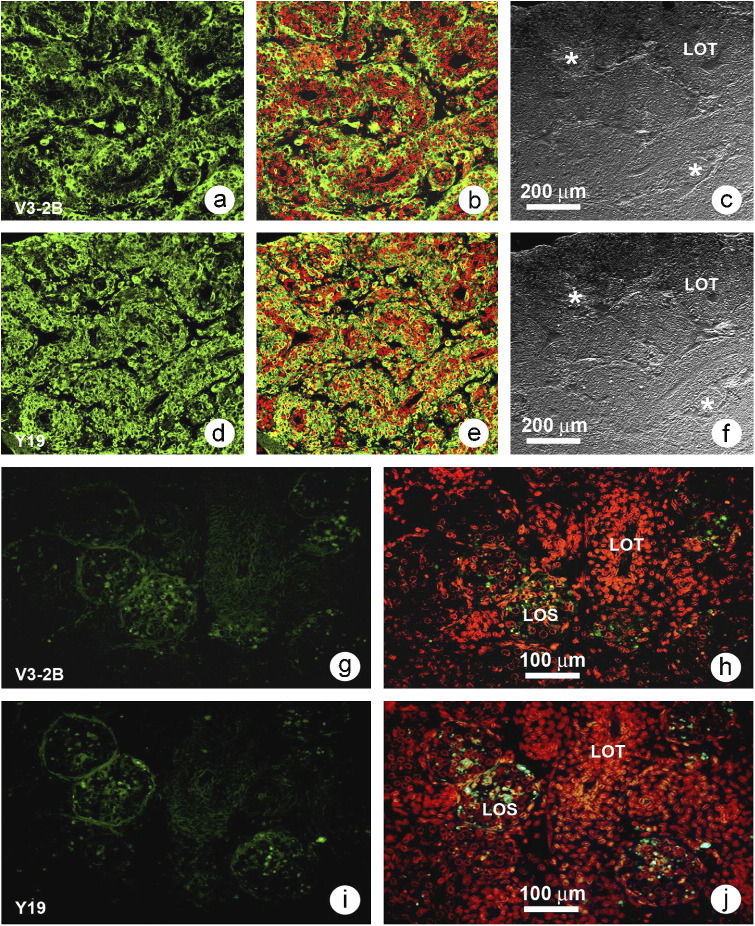
Immunofluorescent labeling for YHV structural proteins in LO sections of moribund P. vannamei collected at d5 from Group 1 (immersion-challenged shrimp) revealed strong immunofluorescent signals for gp116 and p20 at the LOT matrix as well as clusters of aggregated hemocytes (i.e., nascent spheroids) in the inter-tubular spaces (Figures 5a and d ). Micrographs showing combined staining with To-Pro®-3 nuclear stain (red in Figure 5b and e) revealed clusters of newly forming LOS (*) located between LOT. Pyknotic nuclei were observed in the LOT and hemocytes. By contrast, immunoreactions in surviving shrimp from Group 1 collected at d14 (Figures 5g and i) gave mild positive reactions only with cells in LOS, where immunofluorescence was localized as dense spots or diffuse fluorescence in the cytoplasm near the nucleus. No fluorescent signals were seen in shrimp from control Group 2 at d0 or at d14.
Figure 5.
Confocal laser scanning micrographs (CLSM) showing immunofluorescent labeling for gp116 and p20 of YHV in LO sections of P. vannamei challenged with YHV by the water-borne route (a and d). Tissue from moribund shrimp collected at d5 showing an intense, positive yellow-green immunofluorescent signal for gp116 (a) and p20 (d) in LOT matrices and in clusters of aggregated hemocytes (asterisks *) in the matching phase contrast images (c and f). Photomicrographs (b) and (d) show combined images that include nuclear staining (orange). (g–j) Photomicrograps of tissue from surviving shrimp collected at d14 exhibiting weak immunopositive reactions for gp116 (g) and p20 (i) in LOS and no reactions in LOT. Photomicrographs (g) and (i) show respective combined images that include nuclear staining.
ISH in LO using a specific cDNA probe for YHV in moribund shrimp from immersion-challenged Group 1 (Figure 6 ) revealed strong ISH-positive cells in LOT matrix cells and in aggregated hemocytes (*) in inter-tubular spaces (Figure 6a). Parallel sections stained with H&E (Figure 6b) showed typical YHV histopathology previously described (see Figure 4). By contrast, LO sections of surviving shrimp from Group 1 collected at d14 (Figures 6c and d) showed comparatively weak ISH-positive reactions in LOS only (i.e., LOT were negative). No positive ISH reaction was seen in LO from control Group 2 at d0 or d14. Western blot analysis of protein extracts from moribund shrimp and surviving shrimp from immersion-challenged Group 1 confirmed that gp116 was present at a high immunoreactive level in moribund shrimp and a low immunoreactive level in surviving shrimp (Figure 7 ).
Figure 6.
Photomicrographs of serial tissue sections from ISH tests. (a) Representative moribund shrimp specimen from immersion-challenged Group 1 showing an intense, positive ISH reaction in LOT and clusters of aggregated hemocytes (*) at inter-tubular spaces. (b) H&E-stained tissue from another moribund specimen from Group 1 showing typical YHV histopathology (see Figure 2). (c) Representative surviving shrimp specimen from Group 1 showing a positive ISH reaction only in LOS and not in LOT. (d) Adjacent section to that in (c) stained with H&E and showing vacuolated cells (arrows) in LOS. (e) Representative incubation control shrimp from Group 2 at d14 showing a negative ISH reaction in the LO. (f) Serial section to that in (e) stained with H&E.
Figure 7.
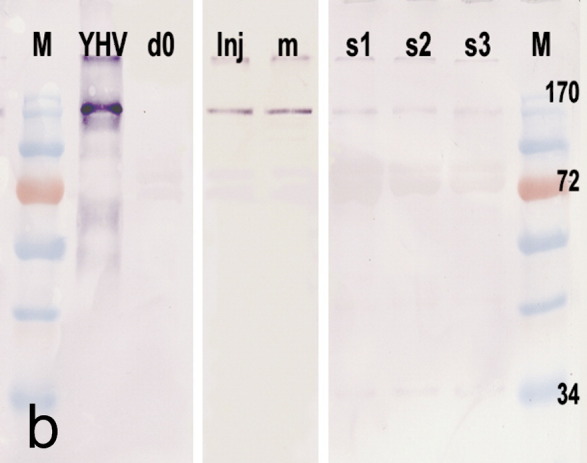
Western blot of SDS-PAGE gel of protein extracted from representative d0 control shrimp and YHV-1-challenged shrimp probed with an MAb against YHV envelope protein gp116. The strongest reaction is at approximately 135 kDa for the semi-purified YHV extract (YHV). The next most intense reactions are for YHV-positive control shrimp (Inj) and for moribund shrimp (m) from the immersion-challenged Group 1. The least intense reactions are from surviving shrimp (s1–s3) from Group 1. There is no reaction for d0 control shrimp (d0). M=molecular weight markers.
Results from immunohistochemistry and ISH tests for YHV infections in the moribund shrimp from the positive control group and from challenge Group 1 were identical to those previously reported for acute YHV infections in P. monodon [21], [51]. The positive results for spheroids in the surviving shrimp from Group 1 resembled those previously reported for shrimp infected with YHV-type viruses in the non-diseased or persistently infected carrier state [17], [52].
Since our challenge system was designed to result in equal viral exposure for all 50 shrimp in Group 1, the simplest explanation for the low viral load in the surviving shrimp is that they responded to the challenge dose by rapidly passing from the early stage of YHV infection (i.e., with pyknotic and karyorrhectic nuclei in the LOT) to the persistently infected carrier state showing LOS (i.e., without experiencing the acute or patent stages of YHD). The mechanisms behind rapid transition to the carrier state are currently unknown but could include factors ranging from shrimp capacity to prevent viral entry (into the body or cells) to factors associated with cellular and humoral defenses. It is unlikely that the surviving shrimp had experienced delayed exposure to YHV, leading to early stages of YHV infection that would have progressed to the acute and patent stages of disease with longer incubation. If so, they would have shown at least some YHV-1 pathology including immunohistochemically positive LOT and absence of immunohistochemically positive LOS. Instead, they showed normal LOT together with spheroids similar to those described shrimp with persistent (chronic) viral infections [48], [49]. Our immunohistochemistry results showed that these spheroids were positive for YHV while the LOT were not. This contrasted with moribund shrimp where spheroids were absent and LOT were positive.
A previous study comparing YHV challenge results in the black tiger shrimp (P. monodon) with those for various palaemonid shrimp indicated that tolerant palaemonid species carried YHV as persistent infections characterized by what appeared to be specific suppression of one of the YHV envelope proteins gp116 when compared with the capsid protein p20 [27]. A similar phenomenon has been reported for a plaemonid shrimp species that is tolerant to white spot syndrome virus (WSSV) infections [53]. In that case, production of the envelope protein vp28 appeared to be suppressed with time after challenge while the level of viral production remained unchanged. The indications were that the ability of these shrimp to tolerate viral infection might be related to control over viral envelope protein production by an unknown mechanism. Presumably, the lack of one or more envelope proteins would reduce the ability of the virus to spread within the host. The P. vannamei survivors in our test system did not show differential suppression of gp116 relative to p20. Instead, both proteins were present at relatively equal but low immunoreactive levels in the survivors when compared with high levels in the moribund shrimp.
Although our immunohistochemistry and ISH results were positive for YHV in LOS of the surviving shrimp, we could not exclude the possibility that the positive reactions resulting from inactivated viral particles or portions of them that remained in the LOS as a residual from a defense reaction. Thus, we carried out additional ultrastructural tests to compare LO from moribund and surviving shrimp from immersion-challenged Group 1.
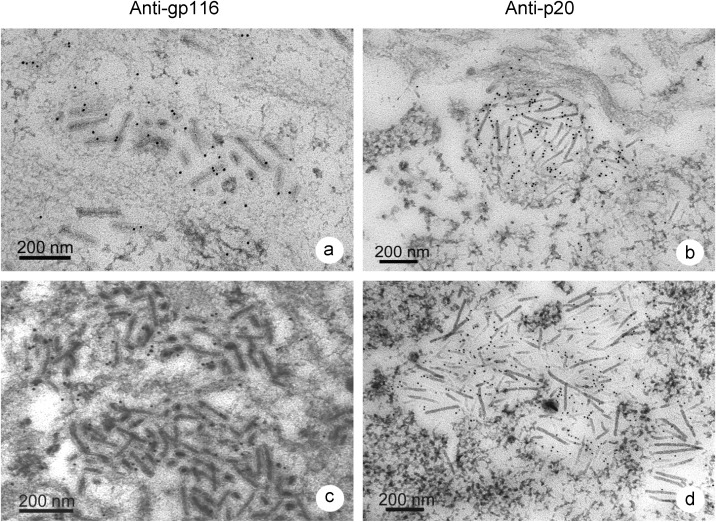
3.6. TEM of LO with immunogold labeling for YHV
TEM of LO tissue from P. vannamei in Group 1 challenged with YHV by immersion revealed that cytoplasm of cells from both surviving shrimp (Figure 8a and b ) and moribund shrimp (Figure 8c and d) contained mature, enveloped, bacilliform virions (approximately 50 nm×150–200 nm) and unenveloped, filamentous nucleocapsids (15 nm×130–800 nm). These were identical to YHV virions in previously published electron micrographs from shrimp with acute YHV infections [4]. However, the number of infected cells was higher in the moribund shrimp. Immunogold labeling for the envelope protein gp116 gave positive results for both surviving (Figure 8a) and moribund (Figure 8c) samples. These resembled previous results using immunogold labeling of mature YHV particles [17]. For the nucleoprotein p20 [21], [22] positive reactions were seen in the area of YHV nucleocapsid assembly, also in both surviving (Figure 8b) and moribund (Figure 8d) shrimp. Acquisition of capsids and envelopes often occurred while the viral material was still in the form of long filaments. Once enveloped, the filaments underwent fragmentation to produce the shorter, rod-shaped, mature virions as previously described for YHV [4]. The fact that both mature and immature developmental stages of YHV were seen in both the moribund and surviving shrimp from Group 1, proved that both had active YHV infections.
Figure 8.
Transmission electron micrographs of LO tissue from Group 1 shrimp specimens (immersion-challenged) showing immunogold labeling for gp116 and p20 in surviving shrimp (a and b) and in moribund shrimp (c and d). Size of the gold particles is 10 nm.
Altogether, our results confirmed that water-borne transmission of YHV-1 was 100% successful for both the moribund and surviving shrimp in our co-habitation challenge. Thus, the reason for survival was not lack of success in viral transmission. Although we did not use the surviving shrimp as a YHV-1 source for ongoing challenge tests with a new batch of naïve SPF shrimp, we expected from previous reports of similar tests that they would be infectious for naïve shrimp (see [35] for a review).
One possible explanation for YHV-1 tolerance in the survivors is that they represented a small and distinct sub-population of the test shrimp that had genetically based, innate tolerance to YHV-1. With respect to innate genetic tolerance to viral pathogens, it has been reported for Taura syndrome virus (TSV) and infectious hypodermal and hematopoetic necrosis virus (IHHNV) that tolerant stocks can be genetically selected in controlled breeding programs by using results from off-site tests with the offspring of breeding stocks [3], [54], [55]. Such commercial stocks are now used widely in the shrimp industry. As a result, we cannot exclude the possibility that the challenge survivors in our experiments had common genetic factors that were not present in the dead/moribund shrimp and that this was the underlying reason for their survival. On the other hand, the exact genetic basis and biochemical mechanisms underlying genetic disease tolerance in commercial stocks is currently unknown and no specific genetic markers linked to it have yet been published. As stated above, they could include factors ranging from cellular and humoral immunity to physical/physiological barriers to viral entry. One way forward would be to carry out detailed genetic profiling of moribund and surviving shrimp from challenge tests such as ours.
Another possible explanation for the survivors is the development of tolerance during the course of YHV-1 challenge. There is some indication that this may be possible in crustaceans [30]. The first report of the phenomenon was for WSSV infections in the Kuruma shrimp Penaeus japonicus where survivors from a WSSV outbreak showed high survival upon re-challenge with a dose of WSSV that was lethal to naïve control shrimp [56]. Subsequent laboratory tests revealed that the phenomenon could be repeated in the laboratory and it was called a quasi-immune response. In all cases, at least some of the quasi-immune shrimp tested positive for WSSV, suggesting that they remained infected after challenge and might better be called tolerant than immune or resistant to WSSV. The situation is similar to that reported for TSV, where survivors remain infected and tolerant possibly for life [48], [49]. For example, it has been shown that YHV-2 (also called GAV) is commonly found in captured broodstock that can transmit the virus to their offspring [57]. There are similar reports for WSSV [58]. Thus, like the question regarding innate genetic tolerance, the question of adaptive tolerance must be left open at this time. Nor is it clear whether these 2 possibilities are mutually exclusive.
In conclusion, we have shown that P. vannamei survivors from a YHV-1 challenge are infected with YHV-1 but that they show approximately 40 times lower levels of virus than moribund shrimp from the same challenge and that they show the presence of LOS positive for YHV-1. Despite the low level of YHV-1, they do produce some complete virions and, by reference to other reports, would probably be infectious for naïve P. vannamei. Thus, they appear to be tolerant rather than resistant to YHV-1 infection. The basis for this tolerance is still unknown but our results suggest that it is not likely to be related to challenge dose. Questions as to whether the tolerance is based on some type of adaptive processes in the survivors, on inherent genetics of the survivors or on a combination of these factors remain unresolved. The association of YHV-1 positive LOS with the tolerant shrimp suggests that closer study of LO function may help to answer some of these questions.
Acknowledgments
This study was supported by grants from the Royal Golden Jubilee (RGJ)—Ph.D. program (No. 4.A.MU/46/I.1), Thailand Research Fund (TRF) and the Centex Shrimp, Mahidol University, Bangkok, Thailand. The authors would like to thank Professor Peter J Walker from CSIRO, Australia, for information regarding his YHV and GAV research. We are also indebted to Dr. Paisarn Sithigorngul from Srinakarinwirot University, Bangkok, for providing monoclonal antibodies against YHV proteins and Dr. Kanokpan Wongprasert at Mahidol University for kind suggestions and instruction on Western blot analysis.
References
- 1.Limsuwan C. Tansetakit Co. Ltd.; Ladyaw, Chatujak, Bangkok: 1991. Handbook for cultivation of black tiger prawns. [Google Scholar]
- 2.Flegel T.W. Special topic review: major viral diseases of the black tiger prawn (Penaeus monodon) in Thailand. World J Microbiol Biotechnol. 1997;13:433–442. [Google Scholar]
- 3.Lightner D.V. The penaeid shrimp viruses TSV, IHHNV, WSSV and YHV: current status in the Americas, available diagnostic methods, and management strategies. J Appl Aquacult. 1999;9:27–52. [Google Scholar]
- 4.Chantanachookin C., Boonyaratanapalin S., Kasornchandra J., Direkbusarakom S., Ekpanithanpong U., Supamataya K. Histology and ultrastructure reveal a new granulosis-like virus in Penaeus monodon affected by “yellow-head” disease. Dis Aquat Org. 1993;17:145–157. [Google Scholar]
- 5.Boonyaratpalin S., Supamattaya K., Kasornchandra J., Direkbusaracom S., Ekpanithanpong U., Chantanachooklin C. Non-occluded baculo-like virus, the causative agent of yellow head disease in the black tiger shrimp (Penaeus monodon) Fish Pathol. 1993;28:103–109. [Google Scholar]
- 6.Wongteerasupaya C., Sriurairatana S., Vickers J.E., Anutara A., Boonsaeng V., Panyim S. Yellow-head virus of Penaeus monodon is an RNA virus. Dis Aquat Org. 1995;22:45–50. [Google Scholar]
- 7.Lightner D.V., Redman R.M. Shrimp diseases and current diagnostic methods. Aquaculture. 1998;164:201–220. [Google Scholar]
- 8.Tang K.F., Lightner D.V. A yellow head virus gene probe: nucleotide sequence and application for in situ hybridization. Dis Aquat Org. 1999;35:165–173. doi: 10.3354/dao035165. [DOI] [PubMed] [Google Scholar]
- 9.Cowley J.A., Dimmock C.M., Wongteerasupaya C., Boonsaeng V., Panyim S., Walker P.J. Yellow head virus from Thailand and gill-associated virus from Australia are closely related but distinct prawn viruses. Dis Aquat Org. 1999;36:153–157. doi: 10.3354/dao036153. [DOI] [PubMed] [Google Scholar]
- 10.Cowley J.A., Dimmock C.M., Spann K.M., Walker P.J. Gill-associated virus of Penaeus monodon prawns: an invertebrate virus with ORF1a and ORF1b genes related to arteri- and coronaviruses. J Gen Virol. 2000;81(Part 6):1473–1484. doi: 10.1099/0022-1317-81-6-1473. [DOI] [PubMed] [Google Scholar]
- 11.Walker P.J., Cowley J.A., Spann K.M., Hodgson R.A.J., Hall M.R., Withyachumanarnkul B. Yellow head complex viruses: transmission cycles and topographical distribution in the Asia-Pacific region. In: Browdy C.L., Jory D.E., editors. The new wave, Proceedings of the special session on sustainable shrimp culture, Aquaculture 2001. World Aquaculture Society; Baton Rouge, LA: 2001. pp. 292–302. [Google Scholar]
- 12.Sittidilokratna N., Hodgson R.A., Cowley J.A., Jitrapakdee S., Boonsaeng V., Panyim S. Complete ORF1b-gene sequence indicates yellow head virus is an invertebrate nidovirus. Dis Aquat Org. 2002;50:87–93. doi: 10.3354/dao050087. [DOI] [PubMed] [Google Scholar]
- 13.Fauquet C.M., Mayo M.A., Maniloff J., Desselberger U., Ball L.A. Elsevier; Amsterdam: 2004. Virus taxonomy. VIIIth Report of the International Committee on Taxonomy of Viruses. [Google Scholar]
- 14.Spann K.M., Vickers J.E., Lester R.J.G. Lymphoid organ virus of Penaeus monodon from Australia. Dis Aquat Org. 1995;23:127–134. [Google Scholar]
- 15.Spann K.M., Cowley J.A., Walker P.J., Lester R.J.G. A yellow-head-like virus from Penaeus monodon cultured in Australia. Dis Aquat Org. 1998;31:169–179. [Google Scholar]
- 16.Walker P.J., Cowley J.A. Viral genetic variation: implications for disease diagnosis and detection of shrimp pathogens. In: Walker P.J., Subasinghe R., editors. FAO fisheries. Technical paper no. 395. FAO, Rome; Bangkok, Thailand: 2000. pp. 54–59. [Google Scholar]
- 17.Soowannayan C., Flegel T.W., Sithigorngul P., Slater J., Hyatt A., Cramerri S. Detection and differentiation of yellow head complex viruses using monoclonal antibodies. Dis Aquat Org. 2003;57:193–200. doi: 10.3354/dao057193. [DOI] [PubMed] [Google Scholar]
- 18.Nadala E.C., Tapay L.M., Cao S., Loh P.C. Detection of yellowhead virus and Chinese baculovirus in penaeid shrimp by the Western blot technique. J Virol Methods. 1997;69:39–44. doi: 10.1016/s0166-0934(97)00136-5. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y.C., Chang P.S. Yellow head virus infection in the giant tiger prawn Penaeus monodon cultured in Taiwan. Fish Pathol. 2000;35:1–10. [Google Scholar]
- 20.Sithigorngul P., Chauychuwong P., Sithigorngul W., Longyant S., Chaivisuthangkura P., Menasveta P. Development of a monoclonal antibody specific to yellow head virus (YHV) from Penaeus monodon. Dis Aquat Org. 2000;42:27–34. doi: 10.3354/dao042027. [DOI] [PubMed] [Google Scholar]
- 21.Sithigorngul P., Rukpratanporn S., Longyant S., Chaivisuthangkura P., Sithigorngul W., Menasveta P. Monoclonal antibodies specific to yellow-head virus (YHV) of Penaeus monodon. Dis Aquat Org. 2002;49:71–76. doi: 10.3354/dao049071. [DOI] [PubMed] [Google Scholar]
- 22.Jitrapakdee S., Unajak S., Sittidilokratna N., Hodgson R.A.J., Cowley J.A., Walker P.J. Identification and analysis of gp116 and gp64 structural glycoproteins of yellow head nidovirus of Penaeus monodon shrimp. J Gen Virol. 2003;84:863–873. doi: 10.1099/vir.0.18811-0. [DOI] [PubMed] [Google Scholar]
- 23.Sittidilokratna N., Phetchampai N., Boonsaeng V., Walker P.J. Structural and antigenic analysis of the yellow head virus nucleocapsid protein p20. Virus Res. 2006;116:21–29. doi: 10.1016/j.virusres.2005.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spaan W., Cavanagh D., Horzinek M.C. Coronavirus structure and genome expression. J Gen Virol. 1988;69:2939–2952. doi: 10.1099/0022-1317-69-12-2939. [DOI] [PubMed] [Google Scholar]
- 25.Assavalapsakul W., Tirasophon W., Panyim S. Antiserum to the gp116 glycoprotein of yellow head virus neutralizes infectivity in primary lymphoid organ cells of Penaeus monodon. Dis Aquat Org. 2005;63:85–88. doi: 10.3354/dao063085. [DOI] [PubMed] [Google Scholar]
- 26.Pantoja C.R., Lightner D.V. Similarity between the histopathology of white spot syndrome virus and yellow head syndrome virus and its relevance to diagnosis of YHV disease in the Americas. Aquaculture. 2003;218:47–54. [Google Scholar]
- 27.Longyant S., Sithigorngul P., Chaivisuthangkura P., Rukpratanporn S., Sithigorngul W., Menasveta P. Differences in susceptibility of palaemonid shrimp species to yellow head virus (YHV) infection. Dis Aquat Org. 2005;64:5–12. doi: 10.3354/dao064005. [DOI] [PubMed] [Google Scholar]
- 28.Longyant S., Sattaman S., Chaivisuthangkura P., Rukpratanporn S., Sithigorngul W., Sithigorngul P. Experimental infection of some penaeid shrimps and crabs by yellow head virus (YHV) Aquaculture. 2006;257:83–91. [Google Scholar]
- 29.Pasharawipas T., Flegel T.W., Sriurairatana S., Morrison D.J. Latent yellow-head infections in Penaeus monodon and implications regarding disease tolerance in crustaceans. In: Menasveta P., Paisarnrat S., Flegel T.W., editors. Shrimp biotechnology in Thailand. National Centre for Genetic Engineering and Biotechnology; Bangkok: 1997. pp. 45–53. [Google Scholar]
- 30.Flegel T.W. Update on viral accommodation, a model for host–viral interaction in shrimp and other arthropods. Dev Comp Immunol. 2007;31:217–231. doi: 10.1016/j.dci.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Flegel T.W., Nielsen L., Thamavit V., Kongtim S., Pasharawipas T. Presence of multiple viruses in non-diseased, cultivated shrimp at harvest. Aquaculture. 2004;240:55–68. [Google Scholar]
- 32.Chayaburakul K., Nash G., Pratanpipat P., Sriurairatana S., Withyachumnarnkul B. Multiple pathogens found in growth-retarded black tiger shrimp Penaeus monodon cultivated in Thailand. Dis Aquat Org. 2004;60:89–96. doi: 10.3354/dao060089. [DOI] [PubMed] [Google Scholar]
- 33.Flegel T.W., Pasharawipas T. Active viral accommodation: a new concept for crustacean response to viral pathogens. In: Flegel T.W., editor. Advances in shrimp biotechnology. National Center for Genetic Engineering and Biotechnology; Bangkok: 1998. pp. 245–250. [Google Scholar]
- 34.Burivong P., Pattanakitsakul S.-N., Thongrungkiat S., Malasit P., Flegel T.W. Markedly reduced severity of Dengue virus infection in mosquito cell cultures persistently infected with Aedes albopictus densovirus (AalDNV) Virology. 2004;329:261–269. doi: 10.1016/j.virol.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 35.Roekring S., Flegel T.W., Malasit P., Kittayapong P. Challenging successive mosquito generations with a densonucleosis virus yields progressive survival improvement but persistent, innocuous infections. Dev Comp Immunol. 2006;30:878–892. doi: 10.1016/j.dci.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Namikoshi A., Wu J.L., Yamashita T., Nishizawa T., Nishioka T., Arimoto M. Vaccination trials with Penaeus japonicus to induce resistance to white spot syndrome virus. Aquaculture. 2004;229:25–36. [Google Scholar]
- 37.Witteveldt J., Vlak J.M., van Hulten M.C.W. Protection of Penaeus monodon against white spot syndrome virus using a WSSV subunit vaccine. Fish Shellfish Immunol. 2004;16:571–579. doi: 10.1016/j.fsi.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Witteveldt J., Cifuentes C.C., Vlak J.M., Van Hulten M.C.W. Protection of Penaeus monodon against white spot syndrome virus by oral vaccination. J Virol. 2004;78:2057–2061. doi: 10.1128/JVI.78.4.2057-2061.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bright Singh I.S., Manjusha M., Somnath Pai S., Philip R. Fenneropenaeus indicus is protected from white spot disease by oral administration of inactivated white spot syndrome virus. Dis Aquat Org. 2005;66:265–270. doi: 10.3354/dao066265. [DOI] [PubMed] [Google Scholar]
- 40.Venegas C.A., Nonaka L., Mushiake K., Shimizu K., Nishizawa T., Muroga K. Pathology of penaeid rod-shaped DNA virus (PRDV) to Kuruma prawn in different developmental stages. Fish Pathol. 1999;34:19–23. [Google Scholar]
- 41.Cameron A. Australian Centre for International Agricultural Research; Canberra: 2002. Survey toolbox for aquatic animal diseases; a practical manual and software package. [Google Scholar]
- 42.Rodriguez J., Boulo V., Mialhe E., Bachere E. Characterisation of shrimp haemocytes and plasma components by monoclonal antibodies. J Cell Sci. 1995;108:1043–1050. doi: 10.1242/jcs.108.3.1043. [DOI] [PubMed] [Google Scholar]
- 43.Hyatt A. Immunogold labelling techniques. In: Harris R., editor. Electron microscopy in biology: a practical approach. Oxford University Press; Oxford: 1991. pp. 59–80. [Google Scholar]
- 44.Bell T.A., Lightner D.V. World Aquaculture Society; Baton Rouge, LA: 1988. A handbook of normal shrimp histology. [Google Scholar]
- 45.Tang K.F.J., Wang J., Lightner D.V. Quantitation of Taura syndrome virus by real-time RT-PCR with a TaqMan assay. J Virol Methods. 2004;115(1):109–114. doi: 10.1016/j.jviromet.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 46.de La Vega E., Degnan B.M., Hall M.R., Cowley J.A., Wilson K.J. Quantitative real-time RT-PCR demonstrates that handling stress can lead to rapid increases of gill-associated virus (GAV) infection levels in Penaeus monodon. Dis Aquat Org. 2004;59(3):195–203. doi: 10.3354/dao059195. [DOI] [PubMed] [Google Scholar]
- 47.de la Vega E., Hall M.R., Degnan B.M., Wilson K.J. Short-term hyperthermic treatment of Penaeus monodon increases expression of heat shock protein 70 (HSP70) and reduces replication of gill associated virus (GAV) Aquaculture. 2006;253(1–4):82–90. [Google Scholar]
- 48.Hasson K.W., Lightner D.V., Mohney L.L., Redman R.M., White B.M. Role of lymphoid organ spheroids in chronic Taura syndrome virus (TSV) infections in Penaeus vannamei. Dis Aquat Org. 1999;38:93–105. [Google Scholar]
- 49.Hasson K.W., Lightner D.V., Mohney L.L., Redman R.M., Poulos B.T., White B.M. Taura syndrome virus (TSV) lesion development and the disease cycle in the Pacific white shrimp Penaeus vannamei. Dis Aquat Org. 1999;36:81–93. [Google Scholar]
- 50.Anggraeni M.S., Owens L. The haemocytic origin of lymphoid organ spheroid cells in the penaeid prawn Penaeus monodon. Dis Aquat Org. 2000;40(2):85–92. doi: 10.3354/dao040085. [DOI] [PubMed] [Google Scholar]
- 51.Soowannayan C., Sithigorngul P., Flegel T.W. Use of a specific monoclonal antibody to determine tissue tropism of yellow head virus (YHV) of Penaeus monodon by in situ immunocytochemistry. Fish Sci. 2002;68(Suppl 1):805–809. [Google Scholar]
- 52.Spann K.M., McCulloch R.J., Cowley J.A., East I.J., Walker P.J. Detection of gill-associated virus (GAV) by in situ hybridization during acute and chronic infections of Penaeus monodon and P. esculentus shrimp. Dis Aquat Org. 2003;56:1–10. doi: 10.3354/dao056001. [DOI] [PubMed] [Google Scholar]
- 53.Yoganandhan K., Sahul Hameed A.S. Tolerance to white spot syndrome virus (WSSV) in the freshwater prawn Macrobrachium rosenbergii is associated with low VP28 envelope protein expression. Dis Aquat Org. 2007;73(3):193–199. doi: 10.3354/dao073193. [DOI] [PubMed] [Google Scholar]
- 54.Argue B.J., Arce S.M., Lotz J.M., Moss S.M. Selective breeding of Pacific white shrimp (Litopenaeus vannamei) for growth and resistance to Taura syndrome virus. Aquaculture. 2002;204(3-4):447–460. [Google Scholar]
- 55.Pruder G.D. Biosecurity: application in aquaculture. Aquacult Eng. 2004;32(1):3–10. [Google Scholar]
- 56.Venegas C.A., Nonaka L., Mushiake K., Nishizawa T., Muroga K. Quasi-immune response of Penaeus japonicus to penaeid rod-shaped DNA virus (PRDV) Dis Aquat Org. 2000;42:83–89. doi: 10.3354/dao042083. [DOI] [PubMed] [Google Scholar]
- 57.Cowley J.A., Hall M.R., Cadogan L.C., Spann K.M., Walker P.J. Vertical transmission of gill-associated virus (GAV) in the black tiger prawn Penaeus monodon. Dis Aquat Org. 2002;50:95–104. doi: 10.3354/dao050095. [DOI] [PubMed] [Google Scholar]
- 58.Hsu H.C., Lo C.F., Lin S.C., Liu K.F., Peng S.E., Chang Y.S. Studies on effective PCR screening strategies for white spot syndrome virus (WSSV) detection in Penaeus monodon brooders. Dis Aquat Org. 1999;39:13–19. doi: 10.3354/dao039013. [DOI] [PubMed] [Google Scholar]