Abstract
Background
A previously unidentified species of human rhinovirus, HRV-C, was described in 2006 in association with lower respiratory tract infection (LRTI). Features of infection in immunosuppressed adults are poorly characterised.
Objectives
This study aims to determine the epidemiology of HRV-C in haematopoietic stem cell transplant (HSCT) recipients in a single centre.
Study design
A prospective cohort study of all HSCT recipients admitted to Westmead Hospital, Westmead, Australia from 1 July 2005 to 30 September 2007 was undertaken. Nose/throat samples were collected from all patients at the time of admission and patients developing pre-defined symptoms and/or signs of respiratory infection during the admission. Samples were processed and tested for rhinoviruses and 14 other respiratory viruses using nucleic acid-based methods, immunofluorescence and culture. HRV genotyping was performed by sequencing a region of the rhinovirus 5′ untranslated region (UTR). Clinical data on each episode were collected prospectively.
Results
HRVs were identified in 24 episodes: 8% of 299 episodes of clinically- defined respiratory infections and 39% of 61 episodes in which respiratory viruses were detected. HRV-C was most frequent (HRV-C: nine, HRV-A: eight and HRV-B: two). Seven episodes of HRV-C, five with pneumonia, occurred within 100 days of HSCT. Co-pathogens were frequent.
Conclusions
The newly described HRV-C was the most common rhinovirus group detected in HSCT recipients with respiratory infection, with co-pathogens being frequent. Further research is required to understand the activity and pathogenicity of this virus in HSCT recipients.
Keywords: Rhinovirus, Respiratory virus, Pneumonia, Haematopoietic stem cell transplant, Immunosuppressed
1. Background
A previously unidentified human rhinovirus (HRV) species, HRV-C, was described in 20061, 2 in association with lower respiratory tract infection and wheezing in children.3, 4 Features of infection in adults are poorly described. While HRVs are the most frequent respiratory viruses detected by polymerase chain reaction (PCR) in adult haematopoietic stem cell transplant (HSCT) recipients,5, 6 the epidemiology of HRV-C in this population is unknown.
2. Objectives
This study aims to describe the clinical epidemiology of HRV-C in a cohort of adult HSCT recipients with acute respiratory infection.
3. Study design
A prospective cohort study of HSCT recipients admitted to Westmead Hospital, Westmead, Australia, between 1 July 2005 and 30 September 2007 was undertaken. This centre performs 40–55 allogeneic and 15–25 autologous adult HSCTs annually. Institutional ethics approval was granted.
The nursing staff completed a daily check list comprising six clinical features of possible respiratory virus infection: cough; fever >38 °C in the last 24 h; sneezing or runny nose; dyspnoea; oxygen saturation <95% on air; and clinician-documented crackles on chest examination.7 Paired nose/throat swabs (NTSs) were collected into a viral transport medium (Copan Diagnostics, Murrieta, CA, USA) when ≥2 clinical features were present, and from all patients on admission to the haematology ward regardless of symptoms. Bronchoalveolar lavage (BAL) was performed at the discretion of treating physicians. Repeated samples were collected only if symptoms persisted.
Immunofluorescence was performed for influenzaviruses A and B (IFVs), parainfluenzaviruses (PIV) 1–3, respiratory syncytial virus (RSV), human adenovirus (HAdV; Chemicon Inc., Temecula, CA, USA) and human metapneumovirus (HMPV; D3DFA, Diagnostic Hybrids, Athens, OH, USA) detection (Institute of Clinical Pathology and Medical Research, Westmead Hospital, Westmead, NSW, Australia). NTS negative by IFV, and all BAL samples, were cultured for viruses.
A sample aliquot was stored at −80 °C for PCR for HRVs,2, 8 IFVs, PIV 1–3, RSV, HMPV, HAdV, coronaviruses OC43, 229E, NL63 and HKU1, polyomaviruses WU and KI and human bocavirus3, 4, 7 (Queensland Paediatric Infectious Diseases Laboratory, Herston, QLD, Australia). Samples were batched, with results not available to clinicians. A region of the HRV 5′ untranslated region (UTR) was sequenced, with a type assigned when it shared ≥97% nucleotide identity with the same region of a fully sequenced HRV using methods described previously (Table 1 ).9 Briefly, 2 μl of nucleic acid extract was reverse transcribed in a total 20 μl reaction volume (OneStep RT-PCR kit, QIAGEN, Australia; 50 °C for 60 min), subjected to a hot-start (95 °C for 15 min) then a ∼380 bp complementary DNA (cDNA) was amplified using 45 cycles of 94 °C for 20 s, 55 °C for 20 s and 72 °C for 50 s with a final incubation at 72 °C for 10 min. The product from positive reactions was subjected to nucleotide sequencing using the PRISM BigDye sequencing kit v3.1 (Applied Biosystems, Foster City, CA, USA). Sequence analysis on products ranging from >200 nucleotides (nt) in length was conducted using Geneious Pro.10 This assay has been shown to be at least as clinically sensitive as others widely used.11 If an HRV was repeatedly positive in the screening assay but no useful sequence could be obtained it was called untypeable. Sequences containing two picornavirus templates (a member of an enterovirus (EV) and HRV species) that could not be further interpreted were designated “double” infections. A post hoc analysis of all HRV samples and review of clinical data were undertaken.
Table 1.
Oligonucleotide sequences of conventional HRV screening.
| Oligonucleotide Name | Oligonucleotide sequence (5′–3′) |
|---|---|
| OL26 01.1 | GCACTTCTGTTTCCCCC |
| OL26 02.1 | CGGACACCCAAAGTAG |
4. Definitions
4.1. Upper respiratory tract infection (URTI)
The presence of two or more of rhinorrhoea, sneezing or cough, with a normal chest examination and radiological imaging (chest X-ray or computed tomography).
4.2. Lower respiratory tract infection (LRTI or pneumonia)
The presence of fever and hypoxia or pulmonary infiltrates reported on radiological imaging.
4.3. Nosocomial acquisition
Symptom onset 4 or more days following hospital admission, in keeping with previous definitions.12, 13
4.4. Co-pathogens
Other respiratory viruses (including those detected by PCR), bacteraemia or candidaemia detected from samples collected during the episode of respiratory infection.
5. Results
Respiratory virus testing was performed in 213 of the 299 episodes of respiratory tract information (RTI) from 193 HSCT recipients. In symptomatic patients, the first sample was collected a median of 2 days (range 0–5) following symptom onset. At least one respiratory virus was detected in 61 episodes, with HRV in 24 (39%; 12 URTIs, 12 LRTIs, including four with upper respiratory symptoms). Asymptomatic infection was detected from four of 205 (2.0%) samples (three episodes).
Phylogenetic analysis was performed on the 26 HRVs identified by PCR; the viral species was identified in 19 (73%). One culture-positive, PCR-negative isolate was unavailable for sequencing. Four HRVs were untypeable, one showed double infection, one was not reproducible and another was identified as EV-D68 (Table 2 ).
Table 2.
Clinical details of all rhinovirus episodes (n = 27).
| Patient (episode) | HRV | Gender (age, years) | HSCT type (stem cell source) | Time post-HSCT | Clinical syndrome | Co-pathogen | GVHD | Immunosuppression |
|---|---|---|---|---|---|---|---|---|
| 1 (1) | A | M (58) | Pre-autologous (PBSCT) | −4 days | URTI | Nil | N/A | Nil |
| 2 (1) | A | M (42) | Pre-MUD (PBSCT) | −14 days | URTI | Nil | N/A | MTX, MP |
| 3 (1) | A | F (33) | MR RIC (PBSCT) | 7 months | U&LRTI | Yesa | Yes | Prednisolone, tacrolimus, sirolimus |
| 4 (1) | A | M (62) | Autologous (PBSCT) | 23 months | Asymptomatic | Nil | No | Nil |
| 5 (1) | A | M (28) | MUD (Cord) | 15 months | LRTI | Yesa | Yes | CSA |
| 6 (1) | A | M (36) | MUD (Cord) | 29 months | URTI | Yesa | Yes | Tacrolimus |
| 7 (1) | A | M (65) | MR (BMT) | 152 months | LRTI | Nil | No | Cyclophosphamide, vincristine, doxorubicin, glivec |
| 8 (1) | A | M (48) | MUD (PBSCT) | 22 days | URTI | Nil | Yes | CSA, MTX, MP |
| 9 (1) | B | M (52) | MR RIC (BMT) | 13 days | U&LRTI | Nil | No | CSA |
| 10 (1) | B | F (21) | MR (PBSCT) | 12 months | LRTI | Yesc | No | Prednisolone, tacrolimus, sirolimus |
| 11 (1) | C | M (19) | MUD (Cord) | 59 days | LRTI | Nil | No | CSA |
| 12 (1) | C | M (56) | MUD (PBSCT) | 4 days | LRTI | Yesd | No | CSA, MTX, MP |
| 13 (1) | C | M (24) | MUD (Cord) | 12 days | U&LRTI | Yese | Yes | CSA, MP |
| 14 (1) | C | M (18) | MUD RIC (PBSCT) | 4 days | U&LRTI | Yesg | No | CSA, MP, MMF, alemtuzumab |
| 15 (1) | C | M (51) | Pre-MR RIC (PBSCT) | −23 days | Asymptomatic | Nil | N/A | Nil |
| 16 (1) | C | M (20) | MUD (BMT) | 47 months | URTI | Nil | No | Tacrolimus, prednisolone |
| 16 (2) | C | M (20) | MUD (BMT) | 49 months | LRTI (fatal) | Yesf | No | CSA, prednisolone |
| 17 (1) | C | M (63) | MR (PBSCT) | 76 days | URTI | Nil | No | CSA, MP, dacluzimab, infliximab, MMF |
| 18 (1) | C | F (54) | MUD RIC (PBSCT) | 39 days | URTI | Nil | No | CSA |
| 19 (1) | Untypeable | M (61) | MUD RIC (PBSCT) | 7 months | URTI | Nil | Yes | CSA |
| 20 (1) | Untypeable | M (49) | MR (PBSCT) | 7 days | URTI | Nil | Yes | CSA, MTX, MP |
| 20 (2) | Enterovirus | M (49) | MR (PBSCT) | 78 days | URTI | Nil | No | CSA, prednisolone |
| 21 (1) | Untypeable | M (53) | Autologous (PBSCT) | −7 days | Asymptomatic | Nil | No | Nil |
| 22 (1) | Untypeable | M (48) | MR (PBSCT) | 28 months | LRTI | Nil | No | Nil |
| 23 (1) | Positive not repeated | M (53) | MR (PBSCT) | 7 days | LRTI | Nil | Yes | CSA, MTX |
| 24 (1) | Double infection | M (35) | MR (PBSCT) | 29 days | URTI | Nil | Yes | CSA |
| 25 (1) | N/Ab | M (32) | MUD (PBSCT) | 7 months | URTI | Nil | No | Nil |
BMT: bone marrow transplant; CSA: cyclosporine A; MMF: mycophenolatemofetil; MP: methylprednisolone; MR: matched related donor; MTX: methotrexate; MUD: matched unrelated donor; N/A: not available; PBSCT: peripheral blood stem cell transplant; RIC: reduced intensity conditioning; a second episode of HRV infection requires a seven day period free of symptoms and hypoxia, resolution of imaging changes in those with LRTI; and a respiratory sample negative for the previously identified virus.
Adenovirus.
PCR negative, culture positive.
Probable invasive aspergillosis and H. influenzae (BAL).
E. faecium & C. glabrata in blood cultures.
Polyomavirus KI & A. xyloxidans bacteraemia.
Polyomavirus KI & E. coli bacteraemia.
Polyomavirus KI.
HRV-C was most frequent (nine, 47%), followed by HRV-A (eight, 42%) and HRV-B (two, 11%) (Table 2, Table 3 ). Pneumonia was present in five of nine episodes with HRV-C (56%), both with HRV-B, and three of eight with HRV-A. Infection was acquired nosocomially in eight episodes. Co-pathogens were present in seven episodes, with co-viruses in six (Table 2). Invasive ventilation was required in two HRV-C episodes (patients #10 and #12), both with co-pathogens. Patient #10 died from overwhelming sepsis with Haemophilus influenzae bacteraemia and probable invasive aspergillosis within 24 h of symptom onset.
Table 3.
HRV species with associated clinical features.
| A (no. = 8) | B (no. = 2) | C (no. = 9) | Total (no. = 19) | ||
|---|---|---|---|---|---|
| Demographics | Male:female | 7:1 | 1:1 | 7:2 | 15:4 |
| Median age years (range) | 63 (33–65) | 37 (21–52) | 24 (18–63) | 39 (18–65) | |
| Graft type | Allogeneic recipient | 5 | 2 | 8 | 15 |
| Donor | Related donor | 2 | 2 | 1 | 5 |
| Unrelated donor | 3 | 0 | 7 | 10 | |
| Acquisition | Pre or during HSCT admission | 3 | 1 | 3 | 7 |
| During conditioning or <100 days | 3 | 1 | 7 | 11 | |
| Nosocomial acquisition | 2 | 1 | 5 | 8 | |
| Clinical illness | Asymptomatic | 1 | 0 | 1 | 2 |
| URTI | 4 | 0 | 3 | 7 | |
| LRTI | 2 | 1 | 3 | 6 | |
| U&LRTI | 1 | 1 | 2 | 4 | |
| All LRTI | 3 | 2 | 5 | 10 | |
| No LRTI | 5 | 0 | 4 | 9 | |
| Wheeze | 0 | 1 | 2 | 3 | |
| Co-pathogen | 3 | 1 | 4 | 8 | |
| Co-viruses | 3 | 0 | 3 | 6 | |
| Outcomes | Respiratory support | 0 | 2 | 2 | 4 |
| Fatal outcome | 0 | 0 | 1 | 1 |
Nosocomial acquisition – onset following four or more days of hospitalization.
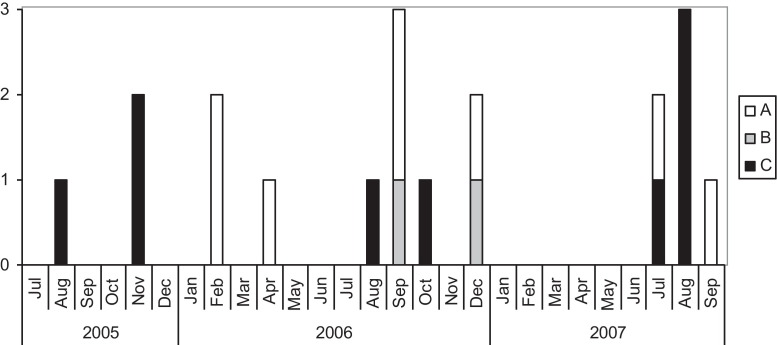
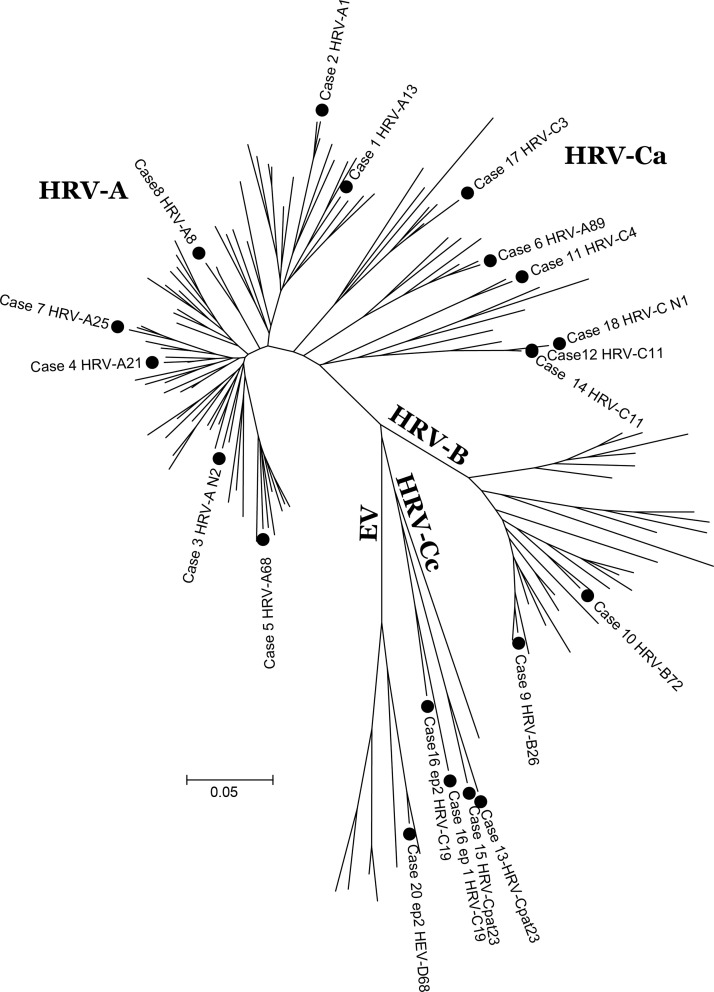
While episodes occurred over 26 months (Fig. 1 ), a cluster of four cases of HRV-C occurred in July–August 2007. The phylogenetic tree (Fig. 2 ) shows the same strain (C11) causing two of these episodes; these were nosocomially acquired and related in time and place (cases #12 and #14).
Fig. 1.
Epidemic curve of HRV-A, -B and -C.
Fig. 2.
Topology tree of detected HRV-C.
6. Discussion
The newly described HRV-C was present in almost half of all episodes of rhinovirus infection in this cohort. This follows a report of HRV-C predominance in a cohort of Thai children hospitalised with LRTI.14 HRV-A has dominated cohorts of adults and children hospitalised with RTI, with HRV-C in 25–35% of episodes.4, 15, 16, 17, 18 This includes renal transplant recipients and others with immunocompromise.15, 16
Although HRV-C and HRV-A caused a similar number of episodes, HRV-C had a higher proportion with pneumonia (56% and 38%, respectively), occurring in the early post-transplant period (78% and 38%) and associated with co-pathogens (44% and 25%). An association with HRV-C, but not HRV-A, and pneumonia in adults with underlying medical conditions or advanced age has been noted,18 but not in all cohorts, including outpatient renal transplant recipients.16 Seven of the nine episodes of HRV-C occurred within 100 days of HSCT when patients have severe immunosuppression and high infection risk, particularly post allogeneic transplant.
While HRV-B and HRV-C were more commonly associated with pneumonia, in only one episode was HRV-C the sole pathogen. The presence of co-pathogens in earlier HRV-C studies is limited to co-virus detections in 8% in adults17 and 26% in children,19 with a similar proportion in this cohort. Polyomavirus KI was the most frequent co-virus; however, its clinical importance remains uncertain.20
This study is limited by the small number of HRV detected from symptomatic samples compared with 30% detection in a prior HSCT cohort6 in which routine weekly testing occurred within the first 100 days post HSCT, and BAL sampling was frequent in pneumonia. In our study, 52 BAL samples were collected from 223 episodes of pneumonia. A diagnostic BAL was obtained in only one of 12 HRV-associated pneumonias, with the remaining episodes inferred from positive upper respiratory samples. It is recognised that upper respiratory samples are less sensitive in pneumonia21 and HRV in this setting may be missed. We acknowledge that without lower respiratory samples for histopathology and further microbiologic testing we cannot state definitively that HRV-C caused pneumonia.
In summary, HRV-C was detected in adult HSCT recipients with viral RTIs including pneumonia, with the poorest outcomes in the presence of co-pathogens. Patients appear to be most at risk during the early post-HSCT period when immunosuppression is greatest. Further characterisation of the epidemiology and pathogenicity of HRV-C in other geographically representative adult HSCT populations is warranted.
Competing interests
All authors report no conflicts of interest relevant to this article.
Funding
This study was supported by grants from the National Health and Medical Research Council, Department of Health and Ageing, Australian Government to The NHMRC Clinical Centre for Research Excellence in Bioethics and Haematological Malignancies (Grant 264625); New South Wales Department of Health Capacity Building Infrastructure Grant 2003/04–2005/06; New South Wales Department of Health Capacity Building Infrastructure Grant 2006/07–2008/09; Queensland Children's Medical Research Institute Grants 10281, Children's Hospital Foundation Queensland, and a National Health and Medical Research Council of Australia post-graduate medical scholarship to PEF. TCS is a Sydney Medical Foundation Fellow; the Sydney Emerging Infections and Biosecurity Institute is also supported by the Sydney Medical School Foundation.
Ethical approval
Ethical approval was given by Western Sydney Area Health Service Ethics Committee, HREC2006/2/4.32(2309).
Acknowledgements
Ken McPhie and Mala Ratnamohan for IF and virus isolation.
References
- 1.Arden K.E., McErlean P., Nissen M.D., Sloots T.P., Mackay I.M. Frequent detection of human rhinoviruses, paramyxoviruses, coronaviruses, and bocavirus during acute respiratory tract infections. J Med Virol. 2006;78(September (9)):1232–1240. doi: 10.1002/jmv.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamson D., Renwick N., Kapoor V., Liu Z., Palacios G., Ju J. MassTag polymerase-chain-reaction detection of respiratory pathogens, including a new rhinovirus genotype, that caused influenza-like illness in New York State during 2004–2005. J Infect Dis. 2006;November (15):1398–1402. doi: 10.1086/508551. 194(10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lau S.K., Yip C.C., Tsoi H.W., Lee R.A., So L.Y., Lau Y.L. Clinical features and complete genome characterization of a distinct human rhinovirus (HRV) genetic cluster, probably representing a previously undetected HRV species, HRV-C, associated with acute respiratory illness in children. J Clin Microbiol. 2007;45(November (11)):3655–3664. doi: 10.1128/JCM.01254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller E.K., Khuri-Bulos N., Williams J.V., Shehabi A.A., Faouri S., Jundi Al I. Human rhinovirus C associated with wheezing in hospitalised children in the middle east. J Clin Virol. 2009;46(September (1)):85–89. doi: 10.1016/j.jcv.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Kraaij M.G.J., van Elden L.J.R., van Loom A.M., al E. Frequent detection of respiratory viruses in adult recipients of stem cell transplants with the use of real-time polymerase chain reaction, compared with viral culture. Clin Infect Dis. 2005;40:662–669. doi: 10.1086/427801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milano F., Campbell A.P., Guthrie K.A., Kuypers J., Englund J.A., Corey L. Human rhinovirus and coronavirus detection among allogeneic hematopoietic stem cell transplantation recipients. Blood. 2010;11(March 115(10)):2088–2094. doi: 10.1182/blood-2009-09-244152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson P.E., Gilroy N.M., Sloots T.P., Nissen M.D., Dwyer D.E., Sorrell T.C. Evaluation of a clinical scoring system and directed laboratory testing for respiratory virus infection in hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2011;13(October (5)):448–455. doi: 10.1111/j.1399-3062.2011.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gama R.E., Horsnell P.R., Hughes P.J., North C., Bruce C.B., al-Nakib W. Amplification of rhinovirus specific nucleic acids from clinical samples using the polymerase chain reaction. J Med Virol. 1989;28(June (2)):73–77. doi: 10.1002/jmv.1890280204. [DOI] [PubMed] [Google Scholar]
- 9.Mackay I.M., Lambert S.B., Faux C.E., Arden K.E., Nissen M.D., Sloots T.P. Community-wide, contemporaneous circulation of a broad spectrum of human rhinoviruses in healthy Australian preschool-aged children during a 12-month period. J Infect Dis. 2012;September doi: 10.1093/infdis/jis476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drummond AJ, Ashton B, Buxton S, Cheung M, Cooper A, Duran C, et al. Geneious v5.4. http://www.geneious.com; 2011.
- 11.Faux C.E., Arden K.E., Lambert S.B., Nissen M.D., Nolan T.M., Chang A.B. Usefulness of published PCR primers in detecting human rhinovirus infection. Emerg Infect Dis. 2011;3(February (2)):296–298. doi: 10.3201/eid1702.101123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martino R., Porras R.P., Rabella N., Williams J.V., Ramila E., Margall N. Prospective study of the incidence, clinical features, and outcome of symptomatic upper and lower respiratory tract infections by respiratory viruses in adult recipients of hematopoietic stem cell transplants for hematologic malignancies. Biol Blood Marrow Transplant. 2005;11(October (10)):781–796. doi: 10.1016/j.bbmt.2005.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zambon M., Bull T., Sadler C.J., Goldman J.M., Ward K.N. Molecular epidemiology of two consecutive outbreaks of parainfluenza 3 in a bone marrow transplant unit. J Clin Microbiol. 1998;36(8):2289–2293. doi: 10.1128/jcm.36.8.2289-2293.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linsuwanon P., Payungporn S., Samransamruajkit R., Posuwan N., Makkoch J., Theanboonlers A. High prevalence of human rhinovirus C infection in Thai children with acute lower respiratory tract disease. J Infect. 2009;59(August (2)):115–121. doi: 10.1016/j.jinf.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piralla A., Rovida F., Campanini G., Rognoni V., Marchi A., Locatelli F. Clinical severity and molecular typing of human rhinovirus C strains during a fall outbreak affecting hospitalized patients. J Clin Virol. 2009;45(August (4)):311–317. doi: 10.1016/j.jcv.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe A., Carraro E., Kamikawa J., Leal E., Granato C., Bellei N. Rhinovirus species and their clinical presentation among different risk groups of non-hospitalized patients. J Med Virol. 2010;82(December (12)):2110–2115. doi: 10.1002/jmv.21914. [DOI] [PubMed] [Google Scholar]
- 17.Xiang Z., Gonzalez R., Xie Z., Xiao Y., Liu J., Chen L. Human rhinovirus C infections mirror those of human rhinovirus A in children with community-acquired pneumonia. J Clin Virol. 2010;49(October (2)):94–99. doi: 10.1016/j.jcv.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau S.K.P., Yip C.C.Y., Lin A.W.C., Lee R.A., So L.-Y., Lau Y.-L. Clinical and molecular epidemiology of human rhinovirus C in children and adults in Hong Kong reveals a possible distinct human rhinovirus C subgroup. J Infect Dis. 2009;200(October (7)):1096–1103. doi: 10.1086/605697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calvo C., Casas I., Garcia-Garcia M.L., Pozo F., Reyes N., Cruz N. Role of rhinovirus C respiratory infections in sick and healthy children in Spain. Pediatr Infect Dis J. 2010;29(August (8)):717–720. doi: 10.1097/INF.0b013e3181d7a708. [DOI] [PubMed] [Google Scholar]
- 20.Jiang M., Abend J.R., Johnson S.F., Imperiale M.J., Jiang M., Abend J.R. The role of polyomaviruses in human disease. Virology. 2009;384(February (2)):266–273. doi: 10.1016/j.virol.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blyth C.C., Iredell J.R., Dwyer D.E. Rapid-test sensitivity for novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;361(December (25)):2493. doi: 10.1056/NEJMc0909049. [DOI] [PubMed] [Google Scholar]