Highlights
-
•
Avian genomes contain a smaller immune repertoire.
-
•
TLR8, RIG-I and downstream components are missing in chickens.
-
•
Birds are missing IgD, and ducks make a truncated IgY.
-
•
Chicken leukocyte Ig-like receptors are missing in ducks.
-
•
Birds have a minimal MHC that affects function.
Keywords: RIG-I, pathway, TLR8, Lymph node, Duck, Chicken, Major Histocompatibility Complex
Abstract
Birds have a smaller repertoire of immune genes than mammals. In our efforts to study antiviral responses to influenza in avian hosts, we have noted key genes that appear to be missing. As a result, we speculate that birds have impaired detection of viruses and intracellular pathogens. Birds are missing TLR8, a detector for single-stranded RNA. Chickens also lack RIG-I, the intracellular detector for single-stranded viral RNA. Riplet, an activator for RIG-I, is also missing in chickens. IRF3, the nuclear activator of interferon-beta in the RIG-I pathway is missing in birds. Downstream of interferon (IFN) signaling, some of the antiviral effectors are missing, including ISG15, and ISG54 and ISG56 (IFITs). Birds have only three antibody isotypes and IgD is missing. Ducks, but not chickens, make an unusual truncated IgY antibody that is missing the Fc fragment. Chickens have an expanded family of LILR leukocyte receptor genes, called CHIR genes, with hundreds of members, including several that encode IgY Fc receptors. Intriguingly, LILR homologues appear to be missing in ducks, including these IgY Fc receptors. The truncated IgY in ducks, and the duplicated IgY receptor genes in chickens may both have resulted from selective pressure by a pathogen on IgY FcR interactions. Birds have a minimal MHC, and the TAP transport and presentation of peptides on MHC class I is constrained, limiting function. Perhaps removing some constraint, ducks appear to lack tapasin, a chaperone involved in loading peptides on MHC class I. Finally, the absence of lymphotoxin-alpha and beta may account for the observed lack of lymph nodes in birds. As illustrated by these examples, the picture that emerges is some impairment of immune response to viruses in birds, either a cause or consequence of the host-pathogen arms race and long evolutionary relationship of birds and RNA viruses.
1. Birds have a reduced complement of immune genes
A survey of genomic resources demonstrates that the avian immune gene complement is reduced compared to mammals. An initial investigation of the immune genes in the chicken genome, a Red Jungle Fowl, suggested that birds have a reduced immune gene repertoire (Consortium, 2004). As this sequence assembly and annotation has been improved, some of these missing genes have been identified, however others are clearly not present. As genomes are sequenced for other birds, including turkey (Dalloul et al., 2010), zebrafinch (Warren et al., 2010) and duck (http://pre.ensembl.org/Anas_platyrhynchos/Info/Index), synteny along the chromosome allowed identification of genes. Thus, immune genes could be identified even if significantly diverged. A comparison of immune genes between three species of birds, confirmed that immune genes show greater divergence between species than other genes, with higher dN/dS ratio than other parts of the genome and evidence of positive selection on specific codons within genes (Ekblom et al., 2010). The sequencing of cDNA libraries as expressed sequence tags (ESTs) (Carre et al., 2006), and BLAST homology searches helped to identify the genes. Nonetheless, some genes are still unaccounted for. This appears true for all birds, although species differences exist. For some of these genes missing from the avian defense arsenal, the evidence is overwhelming, while others are less certain. In all cases, the completion and quality of the genome sequence and annotation determines whether a gene can be identified or not. Gaps exist in the genome sequences, and immune genes are often present in gene families, which are particularly prone to problems with assembly. EST libraries are incomplete, and immune gene expression may be restricted to certain tissues or cell types, and most importantly, only following immune activation. Thus, until genomes are complete and error-free it may be premature to say that a gene is not there. Nonetheless, claiming that a gene is missing certainly inspires research aimed at confirming or disproving this, or demonstrating that another gene plays an analogous or compensatory role. Thus, it is worth highlighting the genes that appear to be missing.
The contracted immune gene repertoire of birds was discussed in recent review of the progress in avian immunology since the availability of the chicken genome (Kaiser, 2010, Kaiser, 2012). In comparison with mammals, birds have partial repertoires of pattern recognition receptors including TLR receptors (Boyd et al., 2007, Brownlie and Allan, 2011, Cormican et al., 2009) and RIG-like receptors (Barber et al., 2010, Karpala et al., 2012). Others have extensively examined the repertoire of avian cytokines (Kaiser et al., 2005) and chemokines (Hughes et al., 2007, Kaiser et al., 2005) interferons (Schultz et al., 2004) (Schultz and Magor, 2008) and defensins (Lynn et al., 2007) noting the genes missing from these repertoires. The immunoglobulin locus been characterized in ducks (Lundqvist et al., 2001), and encodes just three antibody isotypes (Magor, 2011). Finally, the chicken Major Histocompatibility Complex (Kaufman, 2013) is a minimal MHC, where only the most essential genes have been retained. These reviews of each system, although excellent, do not dwell on the genes not found.
Over the course of our analysis of immune systems of ducks, we have often invested significant effort to identify homologues of the chicken or mammalian immune system. Despite our best efforts, some genes have eluded our search. Here we will focus on components of three parts of the immune system that we are investigating in ducks (pattern recognition, antibodies and MHC) and identify the genes that are not there in the duck or the chicken or both. We will assess the strength of the data suggesting the absence of the gene, and consider the effect of the gene loss on the immune system of the animal. Finally, we will speculate on the selective forces that may have led to the loss of the gene.
2. Toll-like receptors
Innate immunity provides the first line of defense against pathogens. Recognition of the pathogen through the molecular patterns of conserved pathogen components, or pattern recognition activates a signaling cascade to turn on genes for the effectors of the immune response. Toll like receptors (TLRs) detect foreign invaders by sensing pathogen-associated molecular patterns. Binding of agonists to TLRs on the cell surface, or within the endosomal compartment, activate signal transduction pathways to turn on antimicrobial peptides, cytokines, interferons and cellular killing mechanisms. Birds possess genes for ten TLRs. These include two TLR1 genes, two TLR2 genes, TLR3, TLR4, TLR5, TLR7, TLR15 and TLR21. Several excellent reviews have been written recently on avian TLR genes (Brownlie and Allan, 2011, Cormican et al., 2009). Two genes are missing in comparison to fish and mammals, TLR8 and TLR9. TLR9, which detects CpG, has been functionally compensated by TLR21 (Brownlie et al., 2009, Keestra et al., 2010).
2.1. TLR8 is missing in birds
TLR7 and TLR8 are phylogenetically related as the product of an ancient gene duplication and both can recognize single-stranded RNA, oligoribonucleotides, and nucleic acid analogues in the endosomal compartment (reviewed by Cervantes et al., 2012). TLR8, which is present in fish and mammals, is absent in birds. Sequencing downstream of chicken TLR7 showed only fragments of TLR8 (Philbin et al., 2005). Further, PCR evidence suggested that TLR8 was disrupted in all the galliform birds, but not anseriform birds, and there was speculation that this could account for the increased susceptibility of chickens to influenza relative to ducks (Philbin et al., 2005). To follow up on this observation, we cloned duck TLR7 cDNA, and isolated a genomic clone for duck TLR7, sequenced it, and examined the region downstream. As seen for chickens, we could identify only small fragments of TLR8, and a CR1 element disrupted the gene in both ducks and chickens (MacDonald et al., 2007). TLR8 is also absent from the zebra finch (Cormican et al., 2009) and turkey genome (Ramasamy et al., 2012). Given the evolutionary distance of galliform birds and zebra finch, TLR8 is likely missing from the entire avian lineage.
For several years, mouse TLR8 had been presumed non-functional, based on the lack of response to TLR7/8 agonists in the TLR7−/− mouse (Hemmi et al., 2002). However, when peripheral blood monocytes from mice are treated with selective TLR8 agonists, imidazoquinoline 3M002 and poly T oligonucleotides, mouse TLR8 activation is demonstrated while TLR7 is suppressed (Gorden et al., 2006). TLR8 is expressed in monocytes/macrophages and myeloid DCs, while TLR7 is expressed in pDCs and B cells (Hornung et al., 2002). TLR8 also plays a role in detecting bacterial RNA, including RNA from Borrelia burgdorferi, the agent of Lyme disease, inducing production of IFN-beta through IRF7 (Cervantes et al., 2011). TLR8 is upregulated by the phagocytosis of Mycobacterium, including the attenuated BCG vaccine strain, Mycobacterium bovis, and Mycobacterium tuberculosis (Davila et al., 2008). Human TLR8 allelic variants are associated with increased susceptibility to pulmonary tuberculosis (Davila et al., 2008). The protective allele is associated with decreased translation of TLR8, presumably resulting in a decrease in sensing and activation, and less inflammation (Davila et al., 2008). Effectively, loss of TLR8 expression is protective against tuberculosis.
It is not clear why the loss of TLR8 was selected for in birds. The simplest explanation is the similarity of function between TLR7 and TLR8 rendered the second gene non-functional. In this scenario, however, there is no selection for the deletion of the gene. Alternatively, TLR8 became detrimental, perhaps by recognizing self-antigens and initiating autoimmunity. Negative selection would then likely lead to the loss of this receptor. TLR7 has been implicated in induction of autoimmunity (Mills, 2011). Early experiments used chickens to demonstrate thyroid autoimmunity (Sundick et al., 1992, Wick et al., 1974) but it is not known to what extent avian species suffer autoimmunity in nature. Ironically, knockout of TLR8 in mice leads to autoimmunity through overexpression and disregulation of TLR7 (Demaria et al., 2010). Intriguingly, nucleic acid sensing TLRs are implicated in preventing reactivation of host retroviral elements and consequent tumor production (Yu et al., 2012). TLR7 has been directly implicated in this immunosurveillance, as lack of antibodies against endogenous retroviral elements correlates with absence of TLR7 in knockout mice strains. This crucial role of TLR7 in immunosurveillance of endogenous retroviruses would provide the selective pressure to retain TLR7 in the genome, regardless of how TLR8 was lost. Since TLR8 appears to have been inactivated by a CR1 repetitive element, it is tempting to speculate that TLR8 was lost in a hypothetical reactivation of endogenous retroviral elements that disrupted the genome in a distant avian ancestor. Jim Kaufman has alluded to such a catastrophic ‘avian big bang’ in describing the loss of several avian MHC genes (Kaufman and Wallny, 1996). Another theoretical possibility is that TLR8 became the target of a pathogen that subverted it for its own benefit (discussed in Barber, 2011). Viral subversion of TLR3 is such that its absence increases host survival from many pathogens. TLR3-induced host proinflammatory cytokines allow West Nile Virus to cross the blood brain barrier (Wang et al., 2004). TLR3 activity has also been implicated in influenza-induced pneumonia (Le Goffic et al., 2006) and morbidity from vaccinia infection (Hutchens et al., 2008). Along these lines, we can envision a pathogen that subverted avian TLR8 for increased susceptibility. This could include viral targeting of TLR8 receptor for increased inflammation and pathology, or subversion of an endosomal TLR8 for entry of a mycobacterial pathogen into the cell. Mycobacteria are initially engulfed by macrophages, but survive and multiply intracellularly. Thus, bacterial or viral subversion of a PRR may drive selection to disable the gene.
Whether cause or effect, the lack of TLR8 in avian monocytes/macrophages likely does contribute to the susceptibility of birds to RNA viruses (West Nile virus, Newcastle disease virus, influenza virus and others) and intracellular bacterial infections, including mycobacteria. Mycobacterium avium is a significant pathogen of birds, particularly those raised in small flocks, while modern flock hygiene has reduced the incidence in commercial poultry. Susceptibility to mycobacteriosis in birds varies, with chickens, pheasants, partridges being most susceptible, ducks and geese moderately resistant, and pigeons being very resistant (reviewed in Tell et al., 2001).
3. RIG-I signaling pathway
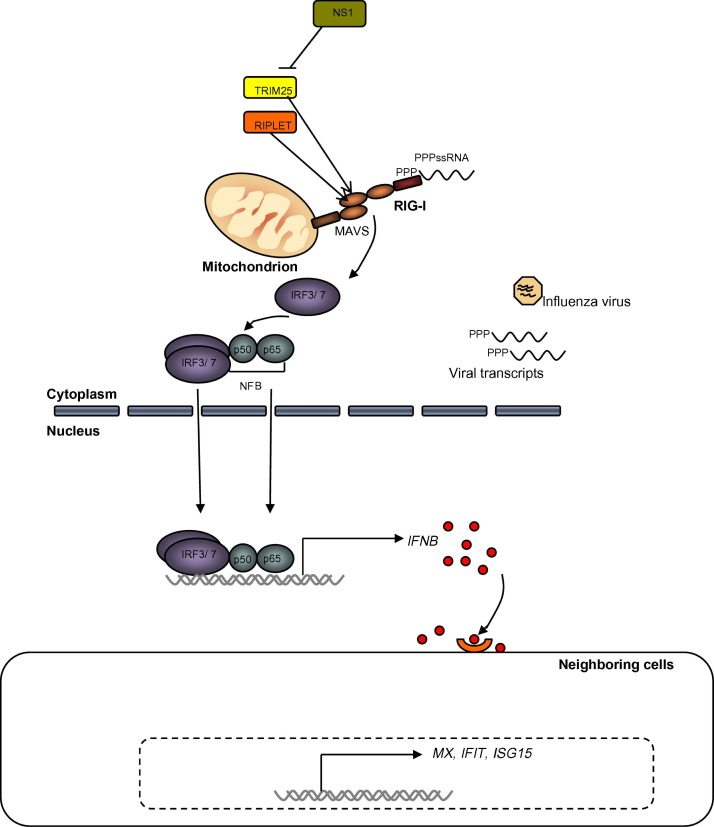
RIG-I is a cytoplasmic pattern recognition receptor for single-stranded 5′-triphosphate RNA with short double-stranded conformation, such as panhandle structures of viral genomes (Hornung et al., 2006, Pichlmair et al., 2006, Schlee et al., 2009, Yoneyama et al., 2004). Both RIG-I, and the related pattern recognition receptor for intracellular RNA, MDA5, share the same pathway signaling through MAVS on the mitochondrion (Fig. 1 ). After detection of viral RNA by RIG-I, a conformational change releases the CARD domains (Kolakofsky et al., 2012, Kowalinski et al., 2011, Luo et al., 2011, Takahasi et al., 2008). TRIM25, an E3 ubiquitin ligase, interacts with the CARD domains of RIG-I to activate it through attached (Gack et al., 2007) or unanchored K63-polyubiquitin chains (Jiang et al., 2012, Zeng et al., 2010). The relative importance of these two mechanisms in the activation of RIG-I is still controversial, but activation leads to oligomerization and RIG-I translocation to the mitochondria. Translocation of RIG-I and TRIM25 to the mitochondrial membrane involves the mitochondrial chaperone 14-3-3ɛ (Liu et al., 2012), allowing interaction with MAVS at the mitochondrion. This interaction induces prion-like aggregates of MAVS (Hou et al., 2011) that initiate signaling leading to IRF3/7 translocation and the production of type I interferons and proinflammatory cytokines.
Fig. 1.
A simplified schematic of the RIG-I signaling pathway. RIG-I detects 5′ triphosphate RNA and undergoes conformational changes that allow it to engage MAVS on the mitochondrion. TRIM25 and Riplet/RNF135 are E3 ubiquitin ligases involved in activation of RIG-I. MAVS multimerization initiates a signaling cascade that ultimately results in dimerization and translocation of IRF3 or IRF7, along with NFkB into the nucleus to activate IFNβ. Type I IFNs acting on neighboring cells turn on downstream interferon stimulated genes including MX, IFIT, ISG15 and many others. This figure is adapted from Bowie and Unterholtzner (2008).
3.1. RIG-I is missing in chickens
The gene encoding RIG-I, DDX58, is not annotated in the chicken genome sequence, and is missing in some fish species, but MDA5 homologues are present in all vertebrate families (Zou et al., 2009). We demonstrated that ducks have a functional RIG-I (Barber et al., 2010). In contrast, the DDX58 gene appears absent in chickens by analysis of the syntenic region of the Z chromosome, although we can identify the flanking gene. We also cannot find RIG-I in a search of the expressed sequence tag database for chickens. Thus RIG-I is missing in the genome of the ancestral chicken represented by the Red Jungle Fowl, and the sequences from modern commercial chicken breeds. Our Southern blots show a duck RIG-I probe cross-hybridizes with pigeon DNA, but not with chicken DNA (Barber et al., 2010). We also cannot detect the gene in DNA of turkey or partridge, suggesting that the gene is missing in galliformes (Barber, 2011). Furthermore, we showed that chicken DF-1 cells cannot detect RIG-I ligand, but if we transfect the cells with duck RIG-I we can reconstitute the pathway (Barber et al., 2010). The loss of RIG-I likely contributes to the susceptibility of chickens to infection compared to ducks to a variety of single-strand RNA viruses, including influenza A virus and Newcastle disease virus, both of which cause more harm in chickens than ducks. The related RNA detector, MDA5, can partially compensate and detect avian influenza in chicken cells to generate an interferon response (Karpala et al., 2011, Liniger et al., 2012).
It is difficult to speculate on the selective forces resulting in loss of RIG-I in some birds. Some have suggested the possible existence of a compensatory yet-to-be identified alternate receptor (Karpala et al., 2011), which could certainly facilitate the loss from the genome. RIG-I was initially identified in a leukemia cell line upregulated by retinoic acid (Liu et al., 2000), and indeed it is upregulated by a variety of stress inducers. RIG-I is implicated in a number of other biological events including cell proliferation, apoptosis, senescence, and acute and chronic inflammatory diseases (Liu and Gu, 2011). It is possible that selection to eliminate RIG-I from aberrant activation in one of these alternate roles had resulted in the loss of RIG-I in galliform birds. Finally, there remains the intriguing possibility that the RIG-I receptor was the prey of one of the many single-strand RNA viruses that infect birds, including influenza virus, Newcastle disease virus, West Nile virus, and coronaviruses, and was usurped for the virus’ advantage. The TLR and RLR signaling pathways are targets for viral subversion (reviewed by Es-Saad et al., 2012, Ramos and Gale, 2011). Influenza virus interferes in the mammalian RIG-I pathway in several places, through the action of NS1 protein (Gack et al., 2009). In a similar manner, paramyxoviruses make the V protein that interferes with signaling by the chicken MDA5 receptor (Childs et al., 2007) involving direct protein–protein interaction and preventing interaction with the RNA ligand (Motz et al., 2013). While this interference renders the receptor non-functional during an infection, it would not necessarily lead to selection to eliminate the receptor. However, we can envision interactions with the RIG-I receptor where regulation is aberrant, or excessive activation leads to death. In this scenario, loss of RIG-I could provide a selective advantage to survive a lethal infection with an unknown pathogen. Suggesting that RIG-I is also involved in development in some capacity, RIG-I knockout mice are embryonic lethal due to liver damage (Kato et al., 2005). Aberrant or loss of expression of RIG-I during development due to pathogen subversion, and associated embryonic lethality, could result in selective loss of this receptor. While this raises the question of how the birds without RIG-I survived, we note that RIG-I is not absolutely essential for development, since RIG-I knockout mice have since been made on a different genetic background which are fertile and viable (Wang et al., 2007). Given that RIG-I expression is impaired in lethal infections (Kobasa et al., 2007), it is possible to envision a scenario by which aberrant expression of RIG-I leads to death, and the gene is selectively lost in a common ancestor of chickens and turkeys.
Human RIG-I is regulated through polyubiquitinylation, ISGylation, sumolyation, and phosphorylation and alternate splicing (Eisenacher and Krug, 2012, Loo and Gale, 2011, Maelfait and Beyaert, 2012, Oshiumi et al., 2012, Wang et al., 2011). A major on–off switch for human RIG-I upon viral infection is polyubiquitination by host E3 ubiquitin ligase, tripartite motif protein 25 (TRIM25) (Gack et al., 2007). Sequences at the T55 residue implicated in interaction with TRIM25 and the site of attachment of polyubiquitin chains, K172, are not conserved in duck or zebra finch RIG-I (Barber et al., 2010) or goose RIG-I (Sun et al., 2013). Thus activation of avian RIG-I involves ubiquitination at alternate residues, or interaction with unanchored polyubiquitin chains, not attached to any protein, can activate RIG-I (Zeng et al., 2010).
Given the lack of RIG-I in chickens, the recent observation that knockdown of chicken TRIM25 impairs the interferon response of chicken cells is intriguing (Rajsbaum et al., 2012). Perhaps TRIM25 is involved in the activation of chMDA5. The binding of unanchored K63 polyubiquitin chains can activate human MDA5 in vitro (Jiang et al., 2012). A role for TRIM25 in generating or attaching ubiquitin chains to MDA5 could explain the importance of chTRIM25 in the interferon response of chicken cells.
3.2. RLR modifier Riplet is missing in chickens
Riplet/RNF135 is a cytoplasmic E3-ligase identified by yeast two-hybrid as one of the proteins binding RIG-I, and is essential for RIG-I activation in human cell lines upon infection with an RNA virus (Oshiumi et al., 2009, Oshiumi et al., 2010). Riplet shares 60.8% identity with TRIM25 in humans (Oshiumi et al., 2009), and also has an N-terminal RING domain and C-terminal PRY/SPRY domain. The RING domain confers ubiquitin E3 ligase activity (Nisole et al., 2005) and also contributes to other protein–protein interactions (Borden, 2000). Riplet also mediates K63-polyubiquitination of RIG-I (Gao et al., 2009, Oshiumi et al., 2010). However, there is debate as to whether Riplet interacts with the CARD domains of RIG-I (Gao et al., 2009) the C-terminal repressor domain of RIG-I or both (Oshiumi et al., 2010). Riplet is crucial for RIG-I activation in cells regardless of expression of TRIM25 (Oshiumi et al., 2010). Knockout of Riplet (Oshiumi et al., 2010) or TRIM25 (Gack et al., 2007) impaired the RIG-I dependent innate immune response, suggesting that both are required. Knockout of Riplet resulted in animals that were deficient in the production of interferon in response to RNA, but not DNA viruses (Oshiumi et al., 2010).
Riplet is present in zebra finch (Taeniopygia guttata), but we were unable to find the ortholog in the chicken (Gallus gallus) genome. In the duck, we have located a putative Riplet coding region, but it lacks exon 1. In repeated 5′ RACE experiments, all clones recovered contain sequences that correspond to an intact open reading frame, but lack the expected RING domain. In mice, deletion of the RING domain prevents RIG-I activation (Oshiumi et al., 2010) therefore we hypothesize that deletion of the RING domain in ducks may render it functionally inactive. Nonetheless, we saw upregulation of Riplet and TRIM25 in duck lung at 1dpi with highly pathogenic avian influenza virus (Fleming-Canepa X. et al., unpublished data). Because Riplet may not be functional in ducks, but still highly upregulated during influenza infection, we speculate that Riplet is acting as a decoy for the viral NS1. Influenza A NS1 protein interacts with TRIM25 (Gack et al., 2009) and Riplet (Rajsbaum et al., 2012) causing inhibition of innate immune signaling. Alternatively, Riplet may dimerize with other E3 ligases to function, as recently shown for TRIM16 (Bell et al., 2012).
Comparison of embryonic fibroblast cells from RIG-I knockout and wild-type mice upon influenza infection, reveal genes that are downstream of RIG-I signaling. These genes have been referred to as the RIG-I bioset, the genes induced by influenza infection in a RIG-I dependent manner. In mouse fibroblast cells, the genes include IFNB, IRF3, IRF7, STAT1, STAT2, PKR, OAS, MX1, IFIT2 (ISG54), IFIT1 (ISG56) and RSAD2 (viperin) (Loo et al., 2008). While the overlap between RIG-I and MDA5 inducible genes downstream of MAVS signaling in chicken cells is unknown, we used a microarray approach to examine the genes turned on by RIG-I in chicken cells. Using chicken DF-1 cells, transfected with duck RIG-I, the expression of the RIG-I gene bioset in avian species is augmented (Barber et al., 2013). We noted that some essential genes of the mouse RIG-I bioset are missing in avian species, including IRF3, ISG15, and IFIT2 (ISG54) and IFIT1 (ISG56).
3.3. IRF3 is missing in birds
Interferon regulatory factor-3 is a critical player in the induction of type I IFNs following virus infection (Au et al., 1995). IRF3 and IRF7 have different and crucial roles in the induction of INF-α/β (Honda et al., 2006, Honda and Taniguchi, 2006). IRF3 is constitutively expressed, and is activated by C-terminal phosphorylation that allows dimerization and nuclear localization (Lin et al., 1998). This led to the suggestion that IRF3 was responsible for the initial upregulation of the IFNB gene, followed by interferon dependent induction of IRF7. However, IRF7−/− knockout mice are severely impaired in interferon production upon infection with ssRNA viruses (Honda et al., 2005), suggesting the contribution of IRF3 is minor. Although a gene has been named IRF3 in chickens (Grant et al., 1995), it is interferon inducible and more similar to IRF7. Others have noted the absence of IRF3 in chickens (Huang et al., 2010) and in avian species (Cormican et al., 2009). It is not known which IRF is translocating to the nucleus to activate interferon in the RIG-I/MDA5 pathway in avian species. We speculate that IRF7 fulfills the nuclear translocation and activation of type I IFNs in both TLR and RLR signaling, but this has not been experimentally examined.
3.4. ISG15 is missing in birds
Interferon stimulated gene 15 (ISG15) is highly up regulated by interferon treatment and was the first ubiquitin-like modifier identified. The amino acid sequence of ISG15 is similar to a linear ubiquitin dimer (reviewed in Zhang and Zhang, 2011). ISG15 is conjugated to proteins like ubiquitin, through a process called ISGylation. Among the identified ISGylated substrates are interferon-induced proteins like PKR, RIG-I, MXA (Zhao et al., 2005). IRF3 ISGylation by HERC5 (the main ISG15 E3 ligase in human) increases stability of IRF3, exerting a positive regulation in the RIG-I pathway (Shi et al., 2010). Negative feedback on RIG-I expression and signaling is mediated by ISG15 conjugation to RIG-I (Kim et al., 2008). ISG15 is also involved in a direct antiviral mechanism where ISGylation of influenza A virus NS1 protein impairs viral replication (Zhao et al., 2010).
No genes homologous to human ISG15 have been annotated in any of the available avian genomes. In the chicken, genes located adjacent to human ISG15 were predicted; including HES1 (homologous to human HES4) and AGRN. Within this syntenic region of the chicken genome no ubiquitin-like gene was present. Similarly, homologs of HES1 and ARGN genes were found in the duck scaffolds (scaffold 1197 and 2665, respectively) but synteny analysis cannot be performed because the scaffolds do not overlap.
Enzymes involved in the ISGylation system (including UbE1L, UbCH8, HERC5 and USP18) are present in the chicken and duck genomes, but there is not yet any functional evidence of ISGylation in these species. USP18, which is responsible for cleavage of ISG15 from ISGylated substrates, correlates with survival of influenza-infected chickens, indirectly suggesting some functionality of the ISGylation system (Uchida et al., 2012).
ISG15 conjugation plays many roles in mammalian antiviral immunity, including ISGylation of MX, PKR, RIG-I, and IRF3, and influenza NS1 protein (reviewed in Skaug and Chen, 2010). However, given the absence of several ISG15 targets, including RIG-I in chickens, IRF3 in birds, and evidence that MX is non-functional in chickens (Schusser et al., 2011) and ducks (Bazzigher et al., 1993), the absence of this ubiquitin modifier in birds would be less significant. It is not known whether NS1 is modified by ISG15 in avian hosts. We cannot rule out the possibility that we have failed to identify the avian ISG15 homolog because of low sequence conservation with human ISG15. It is also possible that another unknown ubiquitin-like modifier in birds plays the role of ISG15 within the ISGylation system. Intriguingly, the most similar sequence to ISG15 in the duck and chicken genome lies within the C-terminal end of 2′,5′-oligoadenylate synthetase-like (OASL) gene. OASL has two tandem ubiquitin-like domains that share 37% amino acid identity to human ISG15. The antiviral activity of the human P59 protein, encoded by human OASL, is dependent on the C-terminal ubiquitin-like domain (Marques et al., 2008). However, the biological function of the OASL ubiquitin-like domains is not yet clear and its role as ubiquitin-like modifier has not been described.
3.5. Birds have a single IFIT gene
The interferon-induced proteins with tetratricopeptide repeats (IFIT) genes are highly upregulated by type I IFNs or by viral infection (Bluyssen et al., 1994, Levy et al., 1986, Wathelet et al., 1986). The human IFIT gene family consists of IFIT1 (ISG56), IFIT2 (ISG54), IFIT3 (ISG60), and IFIT5 (ISG58), while the mouse IFIT family lacks IFIT5 and contains IFIT1, IFIT2, and IFIT3 (Bluyssen et al., 1994). The IFIT family appears to be limited to a single gene in marsupials, birds, frogs and fish (reviewed by Zhou et al., 2013). While these proteins have served as markers of viral infection, only recently have their functions in the innate antiviral response been elucidated (Daffis et al., 2010, Fensterl et al., 2012, McDermott et al., 2012, Pichlmair et al., 2011, Schmeisser et al., 2010). IFIT proteins reside within the cytoplasm of cells, and all contain multiple tetratricopeptide repeats (TPRs) (Lamb et al., 1995). The TPRs within these proteins consist of a helix-turn-helix motif and facilitate protein–protein interactions (Blatch and Lassle, 1999). IFIT1 and IFIT2 mediate their antiviral activity by a disruption of translation via an interaction with eukaryotic initiation factor 3 (eIF3) (Li et al., 2010). IFIT1 and IFIT2 also inhibit the translation of viral mRNAs lacking a 2′-O methylation cap structure (Daffis et al., 2010). IFIT1 and IFIT5 have the ability to bind to, and sequester viral 5′-triphosphate RNA (Pichlmair et al., 2011). The crystal structure of IFIT2 has revealed an RNA binding domain that also may function in an antiviral context (Yang et al., 2012). The multi-functional IFIT1 protein can also restrict the replication of human papilloma virus (HPV) by binding the viral helicase E1, and restricting its function in viral replication (Saikia et al., 2010). Interestingly, IFIT1 has also been associated with negative feedback regulation of genes upregulated during viral infection, further demonstrating the diverse function of these genes (Li et al., 2009). The IFIT gene family represents a significant contributor to the broad-ranged antiviral activity of interferons, and plays an important role in the cellular, innate antiviral response.
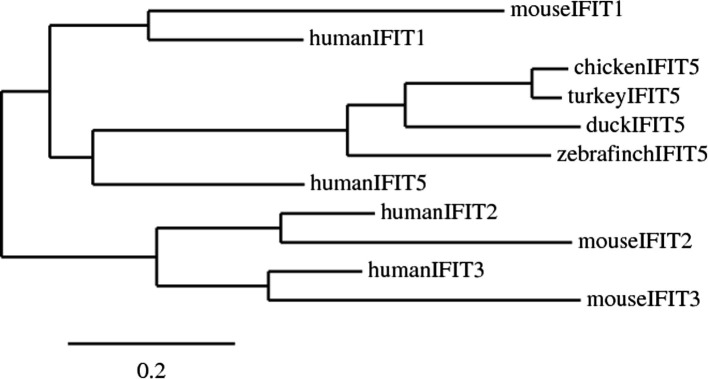
In avian species, the only identifiable IFIT gene encodes a protein that aligns with other IFIT5 proteins in a phylogenetic tree (Fig. 2 ). The upregulation of IFIT5 following viral infection of chicken cells expressing duck RIG-I (Barber et al., 2013) or infection of ducks (Vanderven et al., 2012) suggests IFIT5 is an important antiviral effector in avian species. The apparent absence of an expanded IFIT gene family in avian species suggests that several of the functions attributed to IFIT proteins will be missing. Indeed, the specific role of avian IFIT5 during a viral infection is unknown.
Fig. 2.
A phylogenetic tree showing similarity of avian and mammalian IFIT sequences. Sequences were aligned and phylogenetic tree generated using a maximum likelihood estimation using a program called PhyML using www.phylogeny.fr. (Dereeper et al., 2008). Accession numbers for the IFIT sequences were: chicken IFIT5 (XM_421662.3), turkey IFIT5 (XM_003208028.1), zebra finch IFIT5 (XM_002188552.1), human IFIT1 (NM_001270927.1), mouse IFIT1(NM_008331.3), human IFIT2 (NM_001547.4), mouse IFIT2 (NM_008332.3), human IFIT3 (NM_001031683.2), mouse IFIT3 (NM_010501.2), human IFIT5 (NM_012420.2). Note the duck IFIT sequence is a partial sequence.
4. Birds have a minimal immunoglobulin locus
Birds have only three antibody isotypes, IgM, IgA and IgY. IgY, the avian serum Ig most similar to mammalian IgG, is a precursor to IgG and IgE that has composite function of both isotypes (Warr et al., 1995). Ducks make a truncated version of IgY (Magor et al., 1992). In addition, birds use a single light chain gene of the λ type (Magor et al., 1994a, Reynaud et al., 1983).
4.1. IgD is missing from the duck IgH locus
Ducks have three immunoglobulin heavy chain genes arranged in the gene order IGHM, IGHA and IGHY encoding the mu, alpha and upsilon chains for IgM, IgA and IgY, respectively (Lundqvist et al., 2001, Magor et al., 1999). The IGHA gene, encoding alpha is inverted in the locus, and IGHD (delta) is absent. Despite availability of chicken, zebrafinch and turkey genomes, no other avian immunoglobulin heavy chain locus has yet been assembled. From the limited analysis that has been published for chicken IgH (Zhao et al., 2000), it shares the same organization. The transposition of IGHA from the 3′ most position in the locus, to an inverted position downstream of IGHM, may have also resulted in the loss of IGHD. Lack of IGHD is evident from genomic sequencing for ducks. Early studies reported a δ chain in chickens (Chen et al., 1982), but it is generally accepted that there is no avian homologue of IgD. Since IgD has been identified in teleosts (Bengten et al., 2002, Wilson et al., 1997) frogs (Zhao et al., 2006) and reptiles (Cheng et al., 2013, Wei et al., 2009) the IGHD gene was lost in birds.
IgD is an enigmatic antibody that exists in a wide variety of forms in different species, except birds. The function of IgD is beginning to emerge from observations first made for fish, and subsequently for human IgD. IgD functions at the interface of innate and adaptive immune responses. In fish, IgD specific B cells have been identified, and secreted IgD lacks the variable region suggesting it functions more like a pattern recognition receptor (Edholm et al., 2010). IgD is found on the surface of granulocytes in fish, which do not make the IgD transcript, and involves a specific receptor (Edholm et al., 2010). In humans, circulating IgD binds to basophils and activates antimicrobial and inflammatory factors (Chen et al., 2009). IgD from IgD+ IgM- B cells binds to basophils, and can also bind to certain bacteria in the respiratory tract. The basophil binds IgD through a specific receptor, and cross-linking of IgD leads to the production of B cell activating factors (BAFF) and pro-inflammatory cytokines. Serum IgD is elevated in patients with chronic infections, and specific IgD antibodies could be demonstrated in a number of these infections (reviewed by Chen and Cerutti, 2010). This ancient surveillance system serves to instruct the B cells of the type of pathogens in the respiratory tract. As the specific functions of IgD are elucidated, the consequences of the lack of IgD antibody in birds will become evident. Birds have basophils, but it is unclear whether a different Ig isotype can bind to the basophil IgD receptor to compensate, or whether the receptor exists in birds. Indeed, it remains to be demonstrated that a homologous receptor is involved in the IgD binding by basophils of humans and fish.
4.2. Duck IgY lacks the Fc domain
Duck IgY is made in two secreted forms, a full-length form and a truncated form. The truncated form, called IgYΔFc, lacks the Fc region entirely. It arises from alternate splicing that adds an exon encoding just two amino acids after the CH1 and CH2 domains, and uses an alternate polyadenylation site (Magor et al., 1992) (Magor et al., 1994b). What controls the alternate splicing is unknown, but the truncated form predominates later in the immune response. The IgYΔFc antibodies would be expected to be defective in several processes such as antigen internalization, which is required for appropriate presentation of antigens needed to generate T cell help. The truncated IgY also does not participate in complement fixation, opsonization, precipitation reactions, and reportedly also cannot participate in hemagglutination inhibition (HI) (Higgins et al., 1987). Of benefit to ducks, perhaps the truncated IgY helps prevent viral internalization through receptor-mediated endocytosis and subsequent infection of macrophages and other leukocytes (Magor, 2011).
5. Expansion of the leukocyte immunoglobulin-like receptor family in chickens
The chicken Ig-like receptor (CHIR) genes (Dennis et al., 2000) are counterparts of the leukocyte immunoglobulin-like receptor family (LILR). The CHIR genes constitute a large and diverse family of genes in the chicken, with more than 100 members located in a region syntenic to the mammalian leukocyte receptor complex (LRC) (Laun et al., 2006, Nikolaidis et al., 2005, Viertlboeck and Gobel, 2011, Viertlboeck et al., 2005) and vast diversity within an individual (Viertlboeck et al., 2010). CHIR are expressed in a variety of myeloid and lymphoid cells, with individual receptors expressed in a cell-type restricted manner (Viertlboeck et al., 2005). Receptor diversity includes variation within a hypervariable region, the putative binding region, alternate transcript splicing, and presence or absence of functional activation or inhibitory motif. The extensive expansion and diversification of this family in chickens, and leukocyte expression, suggests their evolution is in response to the pressure of pathogens, as suggested for human LILR and mouse PIR genes (Barclay and Hatherley, 2008). Human LILR receptors are involved in self/non-self recognition and some engage MHC class I targets, as well as pathogen mimics of MHC proteins (Anderson and Allen, 2009, Brown et al., 2004). Staphylococcus aureus targets the mouse inhibitory receptor PIR-B for increased virulence (Nakayama et al., 2012). In turn, activating PIR-A receptors may have evolved in response to the selective pressure from pathogens, as indicated by the relict ITIMs in the PIR-A gene suggesting it is derived from a PIR-B ancestor (Nakayama et al., 2012). Thus, counterbalance through inhibitory and activating CHIR proteins may have evolved in response to pathogen manipulation of immune signaling through these receptors.
5.1. LILR receptors are missing in ducks
We have searched unsuccessfully for immunoglobulin superfamily members homologous to the chicken CHIR receptors in ducks. In high and low stringency Southern blots, genomic DNA from chickens shows an extensive pattern of hybridization, while DNA from ducks shows no significant hybridization (MacDonald et al., 2007). All efforts to amplify these genes by polymerase chain reaction using several sets of degenerate primers are completely unsuccessful. Our searches of the draft assembly of the duck genome, and 70,000 expressed tag sequences generated by 454 sequencing, also find no evidence of CHIR homologues. We cannot rule out the possibility that we have simply missed the CHIR genes due to weak homology, if they have evolved to be quite different in ducks. This in itself is quite intriguing. The rapid species-specific divergence of primate LILR genes, with only some genes showing clear orthologous relationships between species (Canavez et al., 2001), while others have evolved to be unique in each species is thought to reflect their species-specific interactions with pathogens. Alternatively, these genes are truly absent from ducks, despite their presence in chickens. There are several examples where different vertebrates have employed different families of leukocyte receptors (Parham and Moffett, 2013). For example, cattle use KIR as NK cell receptors, which have undergone expansion (McQueen et al., 2002) while horses use Ly49 (Takahashi et al., 2004).
5.2. CHIR-AB1, the chicken Fc receptor for IgY, is missing in ducks
Although there are hundreds of CHIR genes, the only CHIR with a known ligand is CHIR-AB1, which functions as the chicken IgY Fc receptor (Viertlboeck et al., 2007). Chickens have a large number of CHIR-AB1 genes that have varying specificities for IgY (Viertlboeck et al., 2009). Remarkably, we cannot find identifiable homologues of the CHIR-AB1 in ducks. Duck full-length IgY does not bind the chicken Fc receptor (Viertlboeck et al., 2007). As noted above ducks also make a truncated IgYΔFc that would be expected to not to bind Fc receptor. Göbel speculated that the loss of the IgY Fc fragment, and the duplication and divergence of the chicken CHIR-AB1 (Fc receptor) family were both strategies to evade a pathogen interfering with the IgY-Fc receptor interaction in birds (Purzel et al., 2009). Ducks evade this pathogen by production of an antibody lacking the Fc region, retaining the specificity for the antigen, as this truncated form predominates in the later immune response. In chickens, selection favored the duplication of the CHIR receptor family to make a large number of potential ‘decoy receptors’ for IgY. While no known pathogen targets the Fc-IgY interaction in birds, this is a very interesting hypothesis. Chickens may elude this pathogen through the binding of decoy receptors, while ducks may avoid the internalization of an intracellular pathogen through the production of the IgYΔFc.
6. Birds have a ‘minimal MHC’
The vertebrate MHC is the most dynamic part of the genome, showing repeated cycles of ‘birth-and-death’ evolution (Kelley et al., 2005). Polygeny and polymorphism are hallmarks of the region, with varying numbers of genes between species (and sometimes between individuals). Usually it is not possible to identify orthologous genes between species. The MHC class I and class II genes are the most polymorphic genes in the vertebrate genome.
The MHC of the chicken has been referred to as the ‘minimal MHC’ (Kaufman et al., 1995), fulfilling all the requirements of an MHC region, with a limited set of genes. The B locus, or genomic MHC region, contains just 19 genes within 92 kb (Kaufman et al., 1999). MHC class I genes flank either side of the transporters for antigen processing (TAP) genes. They are referred to as the major (BF2) and minor (BF1) MHC class I loci. Similarly, the MHC class II genes (BLB1 and BLB2) are located on either side of tapasin (TAPBP), and in close proximity to the chaperones involved in MHC class II loading (DMA and DMB). Several genes are notably absent, including the proteasome genes LMP2 and LMP7, as well as genes encoding TNF alpha, and lymphotoxin alpha and beta.
Kaufman argues the MHC organization critically affects function because proximity of the genes involved in antigen transport and presentation, allows their encoded proteins to evolve to work together. Indeed, TAP1 and TAP2 genes are also polymorphic, and using a peptide translocation assay, Kaufman recently showed that TAP determines specificity for the linked dominant MHC class I gene (Walker et al., 2011). The limitation to one MHC class I gene in chickens, impairs defense against viral pathogens, as ability to defend against a particular pathogen is completely dependent on whether or not it can load peptides from that pathogen. The best illustration of the consequences of limited MHC class I presentation is the ability of chickens of one genotype to defend against Rous sarcoma virus, while other strains cannot (Wallny et al., 2006).
The duck has a functionally similar MHC class I region, with 5 MHC class I genes encoded adjacent to the TAP genes (Moon et al., 2005). Ducks predominantly express one gene, which is adjacent to the TAP2 gene, which is also polymorphic (Mesa et al., 2004). In ducks, as in chickens, this is expected to have functional consequences for the defense against viruses. Viruses can easily change the one or two epitopes that can be presented by alleles encoded by one MHC class I gene, and thus escape the cytotoxic T cells focused on these epitopes.
6.1. Tapasin is missing in duck MHC
We have been unable to identify the tapasin (TAPBP) gene in the duck MHC. Tapasin bridges the gap between the TAP transporter and empty MHC class I molecules, bringing them into close proximity to the translocation core where peptides are loaded (Sadasivan et al., 1996). In the absence of tapasin, empty MHC class I molecules weakly associate with TAP leading to binding and cell surface expression of less than optimal peptides (Grandea et al., 1995). Tapasin has been identified adjacent to MHC class II in other birds, including chicken (Frangoulis et al., 1999), quail (Shiina et al., 1999), turkey (Chaves et al., 2009), pheasant (Ye et al., 2012) and zebra finch (Balakrishnan et al., 2010) and black grouse (Wang et al., 2012). Also, the tapasin gene is polymorphic in chicken, turkey and pheasant (Sironi et al., 2006). Through analysis of the unannotated duck genome (PreEnsemble) we can identify the location of the duck MHC class II genes, but a search for tapasin within proximity is unsuccessful. Using primers based on the chicken sequence, or conserved regions identified in aligned avian tapasin sequences, our attempts to amplify tapasin from mallards or a domestic duck by RT-PCR or from genomic DNA fail to yield a tapasin product. It is possible that failure to amplify tapasin from ducks is due to sequence divergence of tapasin between avian species. The galliform tapasin proteins are about 90% identical (Wang et al., 2012), but the human and chicken tapasin share only 36% amino acid identity (Frangoulis et al., 1999). In low stringency southern blot analysis, distinctive bands could be detected in chicken, however the probe does not hybridize to duck genomic DNA (Petkau, 2012).
Although tapasin seems to play an important role in antigen presentation, certain human MHC class I alleles can function in a tapasin-independent manner (Park et al., 2003, Lewis et al., 1998). A single amino acid substitution in HLA-B from Asp116 to Tyr116 allows the latter to function in a completely tapasin independent manner (Sieker et al., 2007). It also appears that tapasin influences the peptide repertoire presented to MHC class I, favoring certain peptides over others. In the absence of tapasin antigen presentation is altered, rather than deficient, and is still sufficient to induce in an immune response (Boulanger et al., 2010). Similarly, the absence of immunoproteasomes in mice, results in a change in 50% of the loaded peptide repertoire (Kincaid et al., 2012).
We could speculate that loss of tapasin was advantageous in ducks. We presume that ducks, like chickens, already had constraints on presentation of antigens by MHC class I due to potential co-evolution of the TAP transporters and adjacent MHC class I genes. Evidence for this is that both TAP1 and TAP2 are polymorphic in ducks, and they express one dominant MHC class I gene (Mesa et al., 2004). Perhaps the loss of tapasin permits closer interaction of the TAP transporter and the specific MHC class I molecule intended for loading. Proteins encoded by genes co-evolving along a haplotype reach a best fit. Tapasin as the bridge between TAP and MHC class I molecules could serve to bring the incorrect MHC class I into proximity of the TAP transporters from the other haplotype, unless it was also evolving to keep step.
6.2. Lymphotoxin-alpha and beta are missing from the avian MHC
The genes encoding TNF-alpha (TNF-α), and lymphotoxin-alpha and beta (LTα and LTβ) are missing from the avian MHC. Extensive efforts by PCR, EST mining, and hybridization to identify TNF-α in chickens and ducks have failed. Similarly, the two genes encoding LTα and LTβ are missing in chickens (Kaiser, 2012), and a scan of the genome sequence shows they are also absent in ducks. Lymphotoxin-alpha knockout mice lack lymph nodes (De Togni et al., 1994), and similarly chickens have no lymph nodes. Primitive lymph nodes were previously described in ducks (Berens von Rautenfeld and Burdras, 1983) and immunoglobulin transcripts were analyzed in lymphatic tissues isolated from ducks (Bando and Higgins, 1996, Magor et al., 1994a). However, we question whether these tissues contain recognizable lymph nodes containing secondary lymphoid tissue, as we are unable to identify anything in the lymphatic tissue that resembles a lymph node, even tracking with injected India ink. We examined isolated lymphatic tissues in ducks for mRNA expression of CCL19 and CCL21, the two chemokines which are responsible for recruiting naïve T cells and dendritic cells to lymph nodes. We showed expression of these chemokines was negligible in lymphatic tissues, and abundant in spleen and influenza-infected lung tissues (Fleming-Canepa et al., 2011). Clearly, the lymphatic tissues of ducks are not sites of recruitment of lymphocytes and dendritic cells as expected for secondary lymphoid tissues. Thus, ducks and chickens, like all other non-mammalian vertebrates (Hofmann et al., 2010), lack true lymph nodes.
7. Comparative genomics going forward
Through comparison of the immune arsenal of ducks and chickens we highlight several immune genes that are ‘MIA or missing in action’ from the flight divisions. We refer to these genes as MIA, because we cannot say with certainty that these genes are not present, at least until the sequencing and annotation of avian genomes is more complete. If these genes are truly absent, what emerges is a picture in which chickens, missing TLR8 and RIG-I, have less ability to detect RNA viruses and intracellular bacteria than ducks, which lack only TLR8. In addition, birds are missing components in the RIG-I pathway, and interferon-responsive antiviral effectors. Chickens have a large expanded family of leukocyte receptors, which are apparently missing in ducks, which include the IgY Fc receptors. Notably, ducks make a truncated IgY that is lacking the Fc region as their most abundant serum antibody. By similarity to their human homologues, the LILR receptors, other members of the CHIR family are presumed to be involved in self/non-self recognition, and their expansion may somehow compensate for the deficit due to the minimal avian MHC. In contrast, ducks may have lost tapasin, and with it lost some of the constraint on antigen presentation due to co-evolving linked MHC class I and TAP transporter genes. Whether the host-pathogen arms race is cause or consequence of these gene losses, birds have had a long evolutionary relationship with RNA viruses, including those causing zoonoses.
Acknowledgements
The idea for this review came from a conversation with Martin Flajnik in the beer tent at the Comparative Immunology Workshop, held in Waterloo, Ontario in 2010.
References
- Anderson K.J., Allen R.L. Regulation of T-cell immunity by leucocyte immunoglobulin-like receptors: innate immune receptors for self on antigen-presenting cells. Immunology. 2009;127:8–17. doi: 10.1111/j.1365-2567.2009.03097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au W.C., Moore P.A., Lowther W., Juang Y.T., Pitha P.M. Identification of a member of the interferon regulatory factor family that binds to the interferon-stimulated response element and activates expression of interferon-induced genes. Proc. Natl. Acad. Sci. USA. 1995;92:11657–11661. doi: 10.1073/pnas.92.25.11657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan C.N., Ekblom R., Volker M., Westerdahl H., Godinez R., Kotkiewicz H., Burt D.W., Graves T., Griffin D.K., Warren W.C., Edwards S.V. Gene duplication and fragmentation in the zebra finch major histocompatibility complex. BMC Biol. 2010;8:29. doi: 10.1186/1741-7007-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bando Y., Higgins D. Duck lymphoid organs: their contribution to the ontogeny of IgM and IgY. Immunology. 1996;89:8–12. doi: 10.1046/j.1365-2567.1996.d01-703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber M.R., Aldridge J.R., Jr., Fleming-Canepa X., Wang Y.D., Webster R.G., Magor K.E. Identification of avian RIG-I responsive genes during influenza infection. Mol. Immunol. 2013;54:89–97. doi: 10.1016/j.molimm.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber, M.R.W., 2011. Antiviral pattern recognition receptors in the natural host of influenza, ducks (Anas platyrhynchos). Ph.D. Thesis, University of Alberta.
- Barber M.R.W., Aldridge J.R., Jr., Webster R.G., Magor K.E. Association of RIG-I with innate immunity of ducks to influenza. Proc. Natl. Acad. Sci. USA. 2010;107:5913–5918. doi: 10.1073/pnas.1001755107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay A.N., Hatherley D. The counterbalance theory for evolution and function of paired receptors. Immunity. 2008;29:675–678. doi: 10.1016/j.immuni.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzigher L., Schwarz A., Staeheli P. No enhanced influenza virus resistance of murine and avian cells expressing cloned duck Mx protein. Virology. 1993;195:100–112. doi: 10.1006/viro.1993.1350. [DOI] [PubMed] [Google Scholar]
- Bell J.L., Malyukova A., Holien J.K., Koach J., Parker M.W., Kavallaris M., Marshall G.M., Cheung B.B. TRIM16 acts as an E3 ubiquitin ligase and can heterodimerize with other TRIM family members. PloS One. 2012;7:e37470. doi: 10.1371/journal.pone.0037470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengten E., Quiniou S.M., Stuge T.B., Katagiri T., Miller N.W., Clem L.W., Warr G.W., Wilson M. The IgH locus of the channel catfish, Ictalurus punctatus, contains multiple constant region gene sequences: different genes encode heavy chains of membrane and secreted IgD. J. Immunol. 2002;169:2488–2497. doi: 10.4049/jimmunol.169.5.2488. [DOI] [PubMed] [Google Scholar]
- Berens von Rautenfeld D., Burdras K.-D. Topography, ultrastructure and phagocytic capacity of avian lymph nodes. Cell Tissue Res. 1983;228:389–403. doi: 10.1007/BF00204887. [DOI] [PubMed] [Google Scholar]
- Blatch G.L., Lassle M. The tetratricopeptide repeat: a structural motif mediating protein–protein interactions. Bioessays. 1999;21:932–939. doi: 10.1002/(SICI)1521-1878(199911)21:11<932::AID-BIES5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Bluyssen H.A., Vlietstra R.J., Faber P.W., Smit E.M., Hagemeijer A., Trapman J. Structure, chromosome localization, and regulation of expression of the interferon-regulated mouse Ifi54/Ifi56 gene family. Genomics. 1994;24:137–148. doi: 10.1006/geno.1994.1591. [DOI] [PubMed] [Google Scholar]
- Borden K.L. RING domains: master builders of molecular scaffolds? J. Mol. Biol. 2000;295:1103–1112. doi: 10.1006/jmbi.1999.3429. [DOI] [PubMed] [Google Scholar]
- Boulanger D.S., Oliveira R., Ayers L., Prior S.H., James E., Williams A.P., Elliott T. Absence of tapasin alters immunodominance against a lymphocytic choriomeningitis virus polytope. J. Immunol. 2010;184:73–83. doi: 10.4049/jimmunol.0803489. [DOI] [PubMed] [Google Scholar]
- Bowie A.G., Unterholzner L. Viral evasion and subversion of pattern-recognition receptor signalling. Nat Rev Immunol. 2008;8:911–922. doi: 10.1038/nri2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd A., Philbin V.J., Smith A.L. Conserved and distinct aspects of the avian Toll-like receptor (TLR) system: implications for transmission and control of bird-borne zoonoses. Biochem. Soc. Trans. 2007;35:1504–1507. doi: 10.1042/BST0351504. [DOI] [PubMed] [Google Scholar]
- Brown D., Trowsdale J., Allen R. The LILR family: modulators of innate and adaptive immune pathways in health and disease. Tissue Antigens. 2004;64:215–225. doi: 10.1111/j.0001-2815.2004.00290.x. [DOI] [PubMed] [Google Scholar]
- Brownlie R., Allan B. Avian toll-like receptors. Cell Tissue Res. 2011;343:121–130. doi: 10.1007/s00441-010-1026-0. [DOI] [PubMed] [Google Scholar]
- Brownlie R., Zhu J., Allan B., Mutwiri G.K., Babiuk L.A., Potter A., Griebel P. Chicken TLR21 acts as a functional homologue to mammalian TLR9 in the recognition of CpG oligodeoxynucleotides. Mol. Immunol. 2009;46:3163–3170. doi: 10.1016/j.molimm.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Canavez F., Young N.T., Guethlein L.A., Rajalingam R., Khakoo S.I., Shum B.P., Parham P. Comparison of chimpanzee and human leukocyte Ig-like receptor genes reveals framework and rapidly evolving genes. J. Immunol. 2001;167:5786–5794. doi: 10.4049/jimmunol.167.10.5786. [DOI] [PubMed] [Google Scholar]
- Carre W., Wang X., Porter T.E., Nys Y., Tang J., Bernberg E., Morgan R., Burnside J., Aggrey S.E., Simon J., Cogburn L.A. Chicken genomics resource: sequencing and annotation of 35,407 ESTs from single and multiple tissue cDNA libraries and CAP3 assembly of a chicken gene index. Physiol. Genomics. 2006;25:514–524. doi: 10.1152/physiolgenomics.00207.2005. [DOI] [PubMed] [Google Scholar]
- Cervantes J.L., Dunham-Ems S.M., La Vake C.J., Petzke M.M., Sahay B., Sellati T.J., Radolf J.D., Salazar J.C. Phagosomal signaling by Borrelia burgdorferi in human monocytes involves Toll-like receptor (TLR) 2 and TLR8 cooperativity and TLR8-mediated induction of IFN-beta. Proc. Natl. Acad. Sci. USA. 2011;108:3683–3688. doi: 10.1073/pnas.1013776108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes J.L., Weinerman B., Basole C., Salazar J.C. TLR8: the forgotten relative revindicated. Cell. Mol. Immunol. 2012;9:434–438. doi: 10.1038/cmi.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves L.D., Krueth S.B., Reed K.M. Defining the turkey MHC: sequence and genes of the B locus. J. Immunol. 2009;183:6530–6537. doi: 10.4049/jimmunol.0901310. [DOI] [PubMed] [Google Scholar]
- Chen C.L., Lehmeyer J.E., Cooper M.D. Evidence for an IgD homologue on chicken lymphocytes. J. Immunol. 1982;129:2580–2585. [PubMed] [Google Scholar]
- Chen K., Cerutti A. New insights into the enigma of immunoglobulin D. Immunol. Rev. 2010;237:160–179. doi: 10.1111/j.1600-065X.2010.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Xu W., Wilson M., He B., Miller N.W., Bengten E., Edholm E.S., Santini P.A., Rath P., Chiu A., Cattalini M., Litzman J., Bussel B., Huang B., Meini A., Riesbeck K., Cunningham-Rundles C., Plebani A., Litzman J., Cerutti A. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat. Immunol. 2009;10:889–898. doi: 10.1038/ni.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G., Gao Y., Wang T., Sun Y., Wei Z., Li L., Ren L., Guo Y., Hu X., Lu Y., Wang X., Liu G., Zhang C., Yu J., Pan-Hammarstrom Q., Hammarstrom L., Wu X., Li N., Zhao Y. Extensive diversification of IgH subclass-encoding genes and IgM subclass switching in crocodilians. Nat. Commun. 2013;4:1337. doi: 10.1038/ncomms2317. [DOI] [PubMed] [Google Scholar]
- Childs K., Stock N., Ross C., Andrejeva J., Hilton L., Skinner M., Randall R., Goodbourn S. Mda-5, but not RIG-I, is a common target for paramyxovirus V proteins. Virology. 2007;359:190–200. doi: 10.1016/j.virol.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Consortium I.C.G Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature. 2004;432:695–716. doi: 10.1038/nature03154. [DOI] [PubMed] [Google Scholar]
- Cormican P., Lloyd A.T., Downing T., Connell S.J., Bradley D., O’Farrelly C. The avian Toll-Like receptor pathway-subtle differences amidst general conformity. Dev. Comp. Immunol. 2009;33:967–973. doi: 10.1016/j.dci.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Daffis S., Szretter K.J., Schriewer J., Li J., Youn S., Errett J., Lin T.Y., Schneller S., Zust R., Dong H., Thiel V., Sen G.C., Fensterl V., Klimstra W.B., Pierson T.C., Buller R.M., Gale M., Jr., Shi P.Y., Diamond M.S. 2′-O methylation of the viral mRNA cap evades host restriction by IFIT family members. Nature. 2010;468:452–456. doi: 10.1038/nature09489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalloul, R.A., Long J.A., Zimin, A.V., Aslam L., Beal, K., Ann Blomberg, L., Bouffard P., Burt, D.W., Crasta, O., Crooijmans, R.P., Cooper, K., Coulombe, R.A., De S., Delany M.E., Dodgson, J.B., Dong, J.J., Evans, C., Frederickson, K.M., Flicek, P., Florea L., Folkerts, O., Groenen, M.A., Harkins, T.T., Herrero, J., Hoffmann, S., Megens, H.J., Jiang A., de Jong, P., Kaiser, P., Kim, H., Kim, K.W., Kim, S., Langenberger, D., Lee, M. K., Lee, T., Mane S., Marcais, G., Marz, M., McElroy, A.P., Modise, T., Nefedov, M., Notredame, C., Paton, I.R., Payne, W.S., Pertea, G., Prickett, D., Puiu, D., Qioa, D., Raineri, E., Ruffier, M., Salzberg, S.L., Schatz, M.C., Scheuring, C., Schmidt, C.J., Schroeder, S., Searle, S.M., Smith, E.J., Smith, J., Sonstegard, T.S., Stadler, P.F., Tafer, H., Tu, Z.J., Van Tassell, C.P., Vilella, A.J., Williams, K.P., Yorke, J.A., Zhang, L., Zhang, H.B., Zhang, X., Zhang, Y., Reed, K.M. 2010. Multi-platform next-generation sequencingMulti-platform next-generation sequencing of the domestic turkey (Meleagris gallopavo): genome assembly and analysis. PLoS Biol 8. [DOI] [PMC free article] [PubMed]
- Davila S., Hibberd M.L., Hari Dass R., Wong H.E., Sahiratmadja E., Bonnard C., Alisjahbana B., Szeszko J.S., Balabanova Y., Drobniewski F., van Crevel R., van de Vosse E., Nejentsev S., Ottenhoff T.H., Seielstad M. Genetic association and expression studies indicate a role of toll-like receptor 8 in pulmonary tuberculosis. PLoS Genet. 2008;4:e1000218. doi: 10.1371/journal.pgen.1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Togni P., Goellner J., Ruddle N.H., Streeter P.R., Fick A., Mariathasan S., Smith S.C., Carlson R., Shornick L.P., Strauss-Schoenberger J. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- Demaria O., Pagni P.P., Traub S., de Gassart A., Branzk N., Murphy A.J., Valenzuela D.M., Yancopoulos G.D., Flavell R.A., Alexopoulou L. TLR8 deficiency leads to autoimmunity in mice. J. Clin. Invest. 2010;120:3651–3662. doi: 10.1172/JCI42081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis G., Jr., Kubagawa H., Cooper M.D. Paired Ig-like receptor homologs in birds and mammals share a common ancestor with mammalian Fc receptors. Proc. Natl. Acad. Sci. USA. 2000;97:13245–13250. doi: 10.1073/pnas.230442897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dereeper A., Guignon V., Blanc G., Audic S., Buffet S., Chevenet F., Dufayard J.F., Guindon S., Lefort V., Lescot M., Claverie J.M., Gascuel O. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008;36:W465–469. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edholm E.S., Bengten E., Stafford J.L., Sahoo M., Taylor E.B., Miller N.W., Wilson M. Identification of two IgD+ B cell populations in channel catfish, Ictalurus punctatus. J. Immunol. 2010;185:4082–4094. doi: 10.4049/jimmunol.1000631. [DOI] [PubMed] [Google Scholar]
- Eisenacher K., Krug A. Regulation of RLR-mediated innate immune signaling – it is all about keeping the balance. Eur. J. Cell Biol. 2012;91:36–47. doi: 10.1016/j.ejcb.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Ekblom R., French L., Slate J., Burke T. Evolutionary analysis and expression profiling of zebra finch immune genes. Genome Biol. Evol. 2010;2:781–790. doi: 10.1093/gbe/evq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Es-Saad S., Tremblay N., Baril M., Lamarre D. Regulators of innate immunity as novel targets for panviral therapeutics. Curr. Opin. Virol. 2012;2:622–628. doi: 10.1016/j.coviro.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fensterl V., Wetzel J.L., Ramachandran S., Ogino T., Stohlman S.A., Bergmann C.C., Diamond M.S., Virgin H.W., Sen G.C. Interferon-induced Ifit2/ISG54 protects mice from lethal VSV neuropathogenesis. PLoS Pathog. 2012;8:e1002712. doi: 10.1371/journal.ppat.1002712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming-Canepa X., Brusnyk C., Aldridge J.R., Ross K.L., Moon D., Wang D., Xia J., Barber M.R., Webster R.G., Magor K.E. Expression of duck CCL19 and CCL21 and CCR7 receptor in lymphoid and influenza-infected tissues. Mol. Immunol. 2011;48:1950–1957. doi: 10.1016/j.molimm.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangoulis B., Park I., Guillemot F., Severac V., Auffray C., Zoorob R. Identification of the Tapasin gene in the chicken major histocompatibility complex. Immunogenetics. 1999;49:328–337. doi: 10.1007/s002510050500. [DOI] [PubMed] [Google Scholar]
- Gack M.U., Albrecht R.A., Urano T., Inn K.S., Huang I.C., Carnero E., Farzan M., Inoue S., Jung J.U., Garcia-Sastre A. Influenza A virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I. Cell Host Microbe. 2009;5:439–449. doi: 10.1016/j.chom.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gack M.U., Shin Y.C., Joo C.H., Urano T., Liang C., Sun L., Takeuchi O., Akira S., Chen Z., Inoue S., Jung J.U. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446:916–920. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- Gao D., Yang Y.K., Wang R.P., Zhou X., Diao F.C., Li M.D., Zhai Z.H., Jiang Z.F., Chen D.Y. REUL is a novel E3 ubiquitin ligase and stimulator of retinoic-acid-inducible gene-I. PLoS One. 2009;4:e5760. doi: 10.1371/journal.pone.0005760. (Electronic Resource) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorden K.K., Qiu X.X., Binsfeld C.C., Vasilakos J.P., Alkan S.S. Cutting edge: activation of murine TLR8 by a combination of imidazoquinoline immune response modifiers and polyT oligodeoxynucleotides. J. Immunol. 2006;177:6584–6587. doi: 10.4049/jimmunol.177.10.6584. [DOI] [PubMed] [Google Scholar]
- Grandea A.G., 3rd, Androlewicz M.J., Athwal R.S., Geraghty D.E., Spies T. Dependence of peptide binding by MHC class I molecules on their interaction with TAP. Science. 1995;270:105–108. doi: 10.1126/science.270.5233.105. [DOI] [PubMed] [Google Scholar]
- Grant C.E., Vasa M.Z., Deeley R.G. CIRF-3, a new member of the interferon regulatory factor (IRF) family that is rapidly and transiently induced by dsRNA. Nucleic Acids Res. 1995;23:2137–2146. doi: 10.1093/nar/23.12.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmi H., Kaisho T., Takeuchi O., Sato S., Sanjo H., Hoshino K., Horiuchi T., Tomizawa H., Takeda K., Akira S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- Higgins D.A., Shortridge K.F., Ng P.L. Bile immunoglobulin of the duck (Anas platyrhynchos). II. Antibody response in influenza A virus infections [published erratum appears in Immunology 1988 Mar; 63(3):559] Immunology. 1987;62:499–504. [PMC free article] [PubMed] [Google Scholar]
- Hofmann J., Greter M., Du Pasquier L., Becher B. B-cells need a proper house, whereas T-cells are happy in a cave: the dependence of lymphocytes on secondary lymphoid tissues during evolution. Trends Immunol. 2010;31:144–153. doi: 10.1016/j.it.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Honda K., Takaoka A., Taniguchi T. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25:349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Honda K., Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat. Rev. 2006;6:644–658. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- Honda K., Yanai H., Negishi H., Asagiri M., Sato M., Mizutani T., Shimada N., Ohba Y., Takaoka A., Yoshida N., Taniguchi T. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- Hornung V., Ellegast J., Kim S., Brzozka K., Jung A., Kato H., Poeck H., Akira S., Conzelmann K.K., Schlee M., Endres S., Hartmann G. 5′-Triphosphate RNA is the ligand for RIG-I. [see comment] Science. 2006;314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- Hornung V., Rothenfusser S., Britsch S., Krug A., Jahrsdorfer B., Giese T., Endres S., Hartmann G. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 2002;168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- Hou F., Sun L., Zheng H., Skaug B., Jiang Q.-X., Chen Z.J. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell. 2011;146:448–461. doi: 10.1016/j.cell.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B., Qi Z.T., Xu Z., Nie P. Global characterization of interferon regulatory factor (IRF) genes in vertebrates: glimpse of the diversification in evolution. BMC Immunol. 2010;11:22. doi: 10.1186/1471-2172-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S., Poh T.Y., Bumstead N., Kaiser P. Re-evaluation of the chicken MIP family of chemokines and their receptors suggests that CCL5 is the prototypic MIP family chemokine, and that different species have developed different repertoires of both the CC chemokines and their receptors. Dev. Comp. Immunol. 2007;31:72–86. doi: 10.1016/j.dci.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Hutchens M., Luker K.E., Sottile P., Sonstein J., Lukacs N.W., Nunez G., Curtis J.L., Luker G.D. TLR3 increases disease morbidity and mortality from vaccinia infection. J. Immunol. 2008;180:483–491. doi: 10.4049/jimmunol.180.1.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Kinch L.N., Brautigam C.A., Chen X., Du F., Grishinm N.V., Chen Z.J. Ubiquitin-induced oligomerization of the RNA sensors RIG-I and MDA5 activates antiviral immune response. Immunity. 2012;36:959–973. doi: 10.1016/j.immuni.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser P. Advances in avian immunology–prospects for disease control: a review. Avian Pathol. 2010;39:309–324. doi: 10.1080/03079457.2010.508777. [DOI] [PubMed] [Google Scholar]
- Kaiser P. The long view: a bright past, a brighter future? Forty years of chicken immunology pre- and post-genome. Avian Pathol. 2012;41:511–518. doi: 10.1080/03079457.2012.735359. [DOI] [PubMed] [Google Scholar]
- Kaiser P., Poh T.Y., Rothwell L., Avery S., Balu S., Pathania U.S., Hughes S., Goodchild M., Morrell S., Watson M., Bumstead N., Kaufman J., Young J.R. A genomic analysis of chicken cytokines and chemokines. J. Interferon Cytokine Res. 2005;25:467–484. doi: 10.1089/jir.2005.25.467. [DOI] [PubMed] [Google Scholar]
- Karpala A.J., Lowenthal J.W., Bean A.G. Identifying innate immune pathways of the chicken may lead to new antiviral therapies. Vet. Immunol. Immunopathol. 2012;148:100–109. doi: 10.1016/j.vetimm.2011.05.031. [DOI] [PubMed] [Google Scholar]
- Karpala A.J., Stewart C., McKay J., Lowenthal J.W., Bean A.G. Characterization of chicken Mda5 activity: regulation of IFN-beta in the absence of RIG-I functionality. J. Immunol. 2011;186:5397–5405. doi: 10.4049/jimmunol.1003712. [DOI] [PubMed] [Google Scholar]
- Kato H., Sato S., Yoneyama M., Yamamoto M., Uematsu S., Matsui K., Tsujimura T., Takeda K., Fujita T., Takeuchi O., Akira S. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23:19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Kaufman J. Antigen processing and presentation: evolution from a bird’s eye view. Mol. Immunol. 2013;55:159–161. doi: 10.1016/j.molimm.2012.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J., Milne S., Gobel T.W., Walker B.A., Jacob J.P., Auffray C., Zoorob R., Beck S. The chicken B locus is a minimal essential major histocompatibility complex. Nature. 1999;401:923–925. doi: 10.1038/44856. [DOI] [PubMed] [Google Scholar]
- Kaufman J., Volk H., Wallny H.J. A “minimal essential Mhc” and an “unrecognized Mhc”: two extremes in selection for polymorphism. Immunol. Rev. 1995;143:63–88. doi: 10.1111/j.1600-065x.1995.tb00670.x. [DOI] [PubMed] [Google Scholar]
- Kaufman J., Wallny H.J. Chicken MHC molecules, disease resistance and the evolutionary origin of birds. Curr. Top. Microbiol. Immunol. 1996;212:129–141. doi: 10.1007/978-3-642-80057-3_12. [DOI] [PubMed] [Google Scholar]
- Keestra A.M., de Zoete M.R., Bouwman L.I., van Putten J.P. Chicken TLR21 is an innate CpG DNA receptor distinct from mammalian TLR9. J. Immunol. 2010;185:460–467. doi: 10.4049/jimmunol.0901921. [DOI] [PubMed] [Google Scholar]
- Kelley J., Walter L., Trowsdale J. Comparative genomics of major histocompatibility complexes. Immunogenetics. 2005;56:683–695. doi: 10.1007/s00251-004-0717-7. [DOI] [PubMed] [Google Scholar]
- Kim M.J., Hwang S.Y., Imaizumi T., Yoo J.Y. Negative feedback regulation of RIG-I-mediated antiviral signaling by interferon-induced ISG15 conjugation. J. Virol. 2008;82:1474–1483. doi: 10.1128/JVI.01650-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid E.Z., Che J.W., York I., Escobar H., Reyes-Vargas E., Delgado J.C., Welsh R.M., Karow M.L., Murphy A.J., Valenzuela D.M., Yancopoulos G.D., Rock K.L. Mice completely lacking immunoproteasomes show major changes in antigen presentation. Nat. Immunol. 2012;13:129–135. doi: 10.1038/ni.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobasa D., Jones S.M., Shinya K., Kash J.C., Copps J., Ebihara H., Hatta Y., Kim J.H., Halfmann P., Hatta M., Feldmann F., Alimonti J.B., Fernando L., Li Y., Katze M.G., Feldmann H., Kawaoka Y. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445:319–323. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- Kolakofsky D., Kowalinski E., Cusack S. A structure-based model of RIG-I activation. RNA. 2012;18:2118–2127. doi: 10.1261/rna.035949.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalinski E., Lunardi T., McCarthy A.A., Louber J., Brunel J., Grigorov B., Gerlier D., Cusack S. Structural basis for the activation of innate immune pattern-recognition receptor RIG-I by viral RNA. Cell. 2011;147:423–435. doi: 10.1016/j.cell.2011.09.039. [DOI] [PubMed] [Google Scholar]
- Lamb J.R., Tugendreich S., Hieter P. Tetratrico peptide repeat interactions: to TPR or not to TPR? Trends Biochem. Sci. 1995;20:257–259. doi: 10.1016/s0968-0004(00)89037-4. [DOI] [PubMed] [Google Scholar]
- Laun K., Coggill P., Palmer S., Sims S., Ning Z., Ragoussis J., Volpi E., Wilson N., Beck S., Ziegler A., Volz A. The leukocyte receptor complex in chicken is characterized by massive expansion and diversification of immunoglobulin-like Loci. PLoS Genet. 2006;2:e73. doi: 10.1371/journal.pgen.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goffic R., Balloy V., Lagranderie M., Alexopoulou L., Escriou N., Flavell R., Chignard M., Si-Tahar M. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2006;2:e53. doi: 10.1371/journal.ppat.0020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D., Larner A., Chaudhuri A., Babiss L.E., Darnell J.E., Jr. Interferon-stimulated transcription: isolation of an inducible gene and identification of its regulatory region. Proc. Natl. Acad. Sci. USA. 1986;83:8929–8933. doi: 10.1073/pnas.83.23.8929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J.W., Sewell A., Price D., Elliott T. HLA-A∗0201 presents TAP-dependent peptide epitopes to cytotoxic T lymphocytes in the absence of tapasin. Eur. J. Immunol. 1998;28:3214–3220. doi: 10.1002/(SICI)1521-4141(199810)28:10<3214::AID-IMMU3214>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Li H.T., Su Y.P., Cheng T.M., Xu J.M., Liao J., Chen J.C., Ji C.Y., Ai G.P., Wang J.P. The interaction between interferon-induced protein with tetratricopeptide repeats-1 and eukaryotic elongation factor-1A. Mol. Cell Biochem. 2010;337:101–110. doi: 10.1007/s11010-009-0289-9. [DOI] [PubMed] [Google Scholar]
- Li Y., Li C., Xue P., Zhong B., Mao A.P., Ran Y., Chen H., Wang Y.Y., Yang F., Shu H.B. ISG56 is a negative-feedback regulator of virus-triggered signaling and cellular antiviral response. Proc. Natl. Acad. Sci. USA. 2009;106:7945–7950. doi: 10.1073/pnas.0900818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R., Heylbroeck C., Pitha P.M., Hiscott J. Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol. Cell Biol. 1998;18:2986–2996. doi: 10.1128/mcb.18.5.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liniger M., Summerfield A., Zimmer G., McCullough K.C., Ruggli N. Chicken cells sense influenza A virus infection through MDA5 and CARDIF signaling involving LGP2. J. Virol. 2012;86:705–717. doi: 10.1128/JVI.00742-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Gu J. Retinoic acid inducible gene-I, more than a virus sensor. Protein Cell. 2011;2:351–357. doi: 10.1007/s13238-011-1045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.M., Loo Y.M., Horner S.M., Zornetzer G.A., Katze M.G., Gale M., Jr. The mitochondrial targeting chaperone 14-3-3epsilon regulates a RIG-I translocon that mediates membrane association and innate antiviral immunity. Cell Host Microbe. 2012;11:528–537. doi: 10.1016/j.chom.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T.X., Zhang J.W., Tao J., Zhang R.B., Zhang Q.H., Zhao C.J., Tong J.H., Lanotte M., Waxman S., Chen S.J., Mao M., Hu G.X., Zhu L., Chen Z. Gene expression networks underlying retinoic acid-induced differentiation of acute promyelocytic leukemia cells. Blood. 2000;96:1496–1504. [PubMed] [Google Scholar]
- Loo Y.M., Fornek J., Crochet N., Bajwa G., Perwitasari O., Martinez-Sobrido L., Akira S., Gill M.A., Garcia-Sastre A., Katze M.G., Gale M., Jr. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J. Virol. 2008;82:335–345. doi: 10.1128/JVI.01080-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo Y.M., Gale M., Jr. Immune signaling by RIG-I-like receptors. Immunity. 2011;34:680–692. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist M.L., Middleton D.L., Hazard S., Warr G.W. The immunoglobulin heavy chain locus of the duck. Genomic organization and expression of D, J, and C region genes. J. Biol. Chem. 2001;276:46729–46736. doi: 10.1074/jbc.M106221200. [DOI] [PubMed] [Google Scholar]
- Luo D., Ding S.C., Vela A., Kohlway A., Lindenbach B.D., Pyle A.M. Structural insights into RNA recognition by RIG-I. Cell. 2011;147:409–422. doi: 10.1016/j.cell.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn D.J., Higgs R., Lloyd A.T., O’Farrelly C., Herve-Grepinet V., Nys Y., Brinkman F.S., Yu P.L., Soulier A., Kaiser P., Zhang G., Lehrer R.I. Avian beta-defensin nomenclature: a community proposed update. Immunol. Lett. 2007;110:86–89. doi: 10.1016/j.imlet.2007.03.007. [DOI] [PubMed] [Google Scholar]
- MacDonald M.R., Veniamin S.M., Guo X., Xia J., Moon D.A., Magor K.E. Genomics of antiviral defenses in the duck, a natural host of influenza and hepatitis B viruses. Cytogenet. Genome Res. 2007;117:195–206. doi: 10.1159/000103180. [DOI] [PubMed] [Google Scholar]
- Maelfait J., Beyaert R. Emerging role of ubiquitination in antiviral RIG-I signaling. Microbiol. Mol. Biol. Rev. 2012;76:33–45. doi: 10.1128/MMBR.05012-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magor K.E. Immunoglobulin genetics and antibody responses to influenza in ducks. Dev. Comp. Immunol. 2011 doi: 10.1016/j.dci.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Magor K.E., Higgins D.A., Middleton D.L., Warr G.W. CDNA sequence and organization of the immunoglobulin light chain gene of the duck, Anas platyrhynchos. Dev. Comp. Immunol. 1994;18:523–531. doi: 10.1016/s0145-305x(06)80006-6. [DOI] [PubMed] [Google Scholar]
- Magor K.E., Higgins D.A., Middleton D.L., Warr G.W. One gene encodes the heavy chains for three different forms of IgY in the duck. J. Immunol. 1994;153:5549–5555. [PubMed] [Google Scholar]
- Magor K.E., Higgins D.A., Middleton D.L., Warr G.W. Opposite orientation of the alpha- and upsilon-chain constant region genes in the immunoglobulin heavy chain locus of the duck. Immunogenetics. 1999;49:692–695. doi: 10.1007/s002510050666. [DOI] [PubMed] [Google Scholar]
- Magor K.E., Warr G.W., Middleton D., Wilson M.R., Higgins D.A. Structural relationship between the two IgY of the duck, Anas platyrhynchos: molecular genetic evidence. J. Immunol. 1992;149:2627–2633. [PubMed] [Google Scholar]
- Marques J., Anwar J., Eskildsen-Larsen S., Rebouillat D., Paludan S.R., Sen G., Williams B.R., Hartmann R. The p59 oligoadenylate synthetase-like protein possesses antiviral activity that requires the C-terminal ubiquitin-like domain. J. Gen. Virol. 2008;89:2767–2772. doi: 10.1099/vir.0.2008/003558-0. [DOI] [PubMed] [Google Scholar]
- McDermott J.E., Vartanian K.B., Mitchell H., Stevens S.L., Sanfilippo A., Stenzel-Poore M.P. Identification and validation of Ifit1 as an important innate immune bottleneck. PloS One. 2012;7:e36465. doi: 10.1371/journal.pone.0036465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen K.L., Wilhelm B.T., Harden K.D., Mager D.L. Evolution of NK receptors: a single Ly49 and multiple KIR genes in the cow. Eur. J. Immunol. 2002;32:810–817. doi: 10.1002/1521-4141(200203)32:3<810::AID-IMMU810>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Mesa C.M., Thulien K.T., Moon D.A., Veniamin S.M., Magor K.E. The dominant MHC class I gene is adjacent to the polymorphic TAP2 gene in the duck, Anas platyrhynchos. Immunogenetics. 2004;56:192–203. doi: 10.1007/s00251-004-0672-3. [DOI] [PubMed] [Google Scholar]
- Mills K.H. TLR-dependent T cell activation in autoimmunity. Nat. Rev. 2011;11:807–822. doi: 10.1038/nri3095. [DOI] [PubMed] [Google Scholar]
- Moon D.A., Veniamin S.M., Parks-Dely J.A., Magor K.E. The MHC of the duck (Anas platyrhynchos) contains five differentially expressed class I genes. J. Immunol. 2005;175:6702–6712. doi: 10.4049/jimmunol.175.10.6702. [DOI] [PubMed] [Google Scholar]
- Motz C., Schuhmann K.M., Kirchhofer A., Moldt M., Witte G., Conzelmann K.K., Hopfner K.P. Paramyxovirus V proteins disrupt the fold of the RNA sensor MDA5 to inhibit antiviral signaling. Science. 2013;339:690–693. doi: 10.1126/science.1230949. [DOI] [PubMed] [Google Scholar]
- Nakayama M., Kurokawa K., Nakamura K., Lee B.L., Sekimizu K., Kubagawa H., Hiramatsu K., Yagita H., Okumura K., Takai T., Underhill D.M., Aderem A., Ogasawara K. Inhibitory receptor paired Ig-like receptor B is exploited by Staphylococcus aureus for virulence. J. Immunol. 2012;189:5903–5911. doi: 10.4049/jimmunol.1201940. [DOI] [PubMed] [Google Scholar]
- Nikolaidis N., Makalowska I., Chalkia D., Makalowski W., Klein J., Nei M. Origin and evolution of the chicken leukocyte receptor complex. Proc. Natl. Acad. Sci. USA. 2005;102:4057–4062. doi: 10.1073/pnas.0501040102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisole S., Stoye J.P., Saib A. TRIM family proteins: retroviral restriction and antiviral defence. Nat. Rev. Microbiol. 2005;3:799–808. doi: 10.1038/nrmicro1248. [DOI] [PubMed] [Google Scholar]
- Oshiumi H., Matsumoto M., Hatakeyama S., Seya T. Riplet/RNF135, a RING finger protein, ubiquitinates RIG-I to promote interferon-beta induction during the early phase of viral infection. J. Biol. Chem. 2009;284:807–817. doi: 10.1074/jbc.M804259200. [DOI] [PubMed] [Google Scholar]
- Oshiumi H., Matsumoto M., Seya T. Ubiquitin-mediated modulation of the cytoplasmic viral RNA sensor RIG-I. J. Biochem. 2012;151:5–11. doi: 10.1093/jb/mvr111. [DOI] [PubMed] [Google Scholar]
- Oshiumi H., Miyashita M., Inoue N., Okabe M., Matsumoto M., Seya T. The ubiquitin ligase Riplet is essential for RIG-I-dependent innate immune responses to RNA virus infection. Cell Host Microbe. 2010;8:496–509. doi: 10.1016/j.chom.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Parham P., Moffett A. Variable NK cell receptors and their MHC class I ligands in immunity, reproduction and human evolution. Nat. Rev. 2013;13:133–144. doi: 10.1038/nri3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B., Lee S., Kim E., Ahn K. A Single Polymorphic Residue Within the Peptide- Binding Cleft of MHC Class I Molecules Determines Spectrum of Tapasin Dependence. J. Immunol. 2003;170:961–968. doi: 10.4049/jimmunol.170.2.961. [DOI] [PubMed] [Google Scholar]
- Petkau, K., 2012. Allelic diversity of TAP in wild mallards. M.Sc Thesis, University of Alberta, Edmonton, Canada.
- Philbin V.J., Iqbal M., Boyd Y., Goodchild M.J., Beal R.K., Bumstead N., Young J., Smith A.L. Identification and characterization of a functional, alternatively spliced Toll-like receptor 7 (TLR7) and genomic disruption of TLR8 in chickens. Immunology. 2005;114:507–521. doi: 10.1111/j.1365-2567.2005.02125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichlmair A., Lassnig C., Eberle C.A., Gorna M.W., Baumann C.L., Burkard T.R., Burckstummer T., Stefanovic A., Krieger S., Bennett K.L., Rulicke T., Weber F., Colinge J., Muller M., Superti-Furga G. IFIT1 is an antiviral protein that recognizes 5′-triphosphate RNA. Nat. Immunol. 2011;12:624–630. doi: 10.1038/ni.2048. [DOI] [PubMed] [Google Scholar]
- Pichlmair A., Schulz O., Tan C.P., Naslund T.I., Liljestrom P., Weber F., Reis e Sousa C. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. [see comment] Science. 2006;314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- Purzel J., Schmitt R., Viertlboeck B.C., Gobel T.W. Chicken IgY binds its receptor at the CH3/CH4 interface similarly as the human IgA: Fc alpha RI interaction. J. Immunol. 2009;183:4554–4559. doi: 10.4049/jimmunol.0901699. [DOI] [PubMed] [Google Scholar]
- Rajsbaum R., Albrecht R.A., Wang M.K., Maharaj N.P., Versteeg G.A., Nistal-Villan E., Garcia-Sastre A., Gack M.U. Species-specific inhibition of RIG-I ubiquitination and IFN induction by the influenza A virus NS1 protein. PLoS Pathog. 2012;8:e1003059. doi: 10.1371/journal.ppat.1003059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy K.T., Reddy M.R., Verma P.C., Murugesan S. Expression analysis of turkey (Meleagris gallopavo) toll-like receptors and molecular characterization of avian specific TLR15. Mol. Biol. Rep. 2012;39:8539–8549. doi: 10.1007/s11033-012-1709-6. [DOI] [PubMed] [Google Scholar]
- Ramos H.J., Gale M., Jr. RIG-I like receptors and their signaling crosstalk in the regulation of antiviral immunity. Curr. Opin. Virol. 2011;1:167–176. doi: 10.1016/j.coviro.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud C.A., Dahan A., Weill J.C. Complete sequence of a chicken lambda light chain immunoglobulin derived from the nucleotide sequence of its mRNA. Proc. Natl. Acad. Sci. USA. 1983;80:4099–4103. doi: 10.1073/pnas.80.13.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadasivan B., Lehner P.J., Ortmann B., Spies T., Cresswell P. Roles for calreticulin and a novel glycoprotein, tapasin, in the interaction of MHC class I molecules with TAP. Immunity. 1996;5:103–114. doi: 10.1016/s1074-7613(00)80487-2. [DOI] [PubMed] [Google Scholar]
- Saikia P., Fensterl V., Sen G.C. The inhibitory action of P56 on select functions of E1 mediates interferon’s effect on human papillomavirus DNA replication. J. Virol. 2010;84:13036–13039. doi: 10.1128/JVI.01194-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlee M., Roth A., Hornung V., Hagmann C.A., Wimmenauer V., Barchet W., Coch C., Janke M., Mihailovic A., Wardle G., Juranek S., Kato H., Kawai T., Poeck H., Fitzgerald K.A., Takeuchi O., Akira S., Tuschl T., Latz E., Ludwig J., Hartmann G. Recognition of 5′ triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative-strand virus. Immunity. 2009;31:25–34. doi: 10.1016/j.immuni.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeisser H., Mejido J., Balinsky C.A., Morrow A.N., Clark C.R., Zhao T., Zoon K.C. Identification of alpha interferon-induced genes associated with antiviral activity in Daudi cells and characterization of IFIT3 as a novel antiviral gene. J. Virol. 2010;84:10671–10680. doi: 10.1128/JVI.00818-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz U., Kaspers B., Staeheli P. The interferon system of non-mammalian vertebrates. Dev. Comp. Immunol. 2004;28:499–508. doi: 10.1016/j.dci.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Schultz U., Magor K.E. Avian Immunology; 2008. Comparative Immunology of Agricultural Birds. [Google Scholar]
- Schusser B., Reuter A., von der Malsburg A., Penski N., Weigend S., Kaspers B., Staeheli P., Hartle S. Mx is dispensable for interferon-mediated resistance of chicken cells against influenza A virus. J. Virol. 2011;85:8307–8315. doi: 10.1128/JVI.00535-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H.X., Yang K., Liu X., Liu X.Y., Wei B., Shan Y.F., Zhu L.H., Wang C. Positive regulation of interferon regulatory factor 3 activation by Herc5 via ISG15 modification. Mol. Cell Biol. 2010;30:2424–2436. doi: 10.1128/MCB.01466-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiina T., Shimizu C., Oka A., Teraoka Y., Imanishi T., Gojobori T., Hanzawa K., Watanabe S., Inoko H. Gene organization of the quail major histocompatibility complex (MhcCoja) class I gene region. Immunogenetics. 1999;49:384–394. doi: 10.1007/s002510050511. [DOI] [PubMed] [Google Scholar]
- Sieker F., Springer S., Zacharias M. Comparative molecular dynamics analysis of tapasin-dependent and -independent MHC class I alleles. Protein Sci. 2007;16:299–308. doi: 10.1110/ps.062568407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sironi L., Lazzari B., Ramelli P., Gorni C., Mariani P. Single nucleotide polymorphism discovery in the avian Tapasin gene. Poult. Sci. 2006;85:606–612. doi: 10.1093/ps/85.4.606. [DOI] [PubMed] [Google Scholar]
- Skaug B., Chen Z.J. Emerging role of ISG15 in antiviral immunity. Cell. 2010;143:187–190. doi: 10.1016/j.cell.2010.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Ding N., Ding S.S., Yu S., Meng C., Chen H., Qiu X., Zhang S., Yu Y., Zhan Y., Ding C. Goose RIG-I functions in innate immunity against Newcastle disease virus infections. Mol. Immunol. 2013;53:321–327. doi: 10.1016/j.molimm.2012.08.022. [DOI] [PubMed] [Google Scholar]
- Sundick R.S., Bagchi N., Brown T.R. The role of iodine in thyroid autoimmunity: from chickens to humans: a review. Autoimmunity. 1992;13:61–68. doi: 10.3109/08916939209014636. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Yawata M., Raudsepp T., Lear T.L., Chowdhary B.P., Antczak D.F., Kasahara M. Natural killer cell receptors in the horse: evidence for the existence of multiple transcribed LY49 genes. Eur. J. Immunol. 2004;34:773–784. doi: 10.1002/eji.200324695. [DOI] [PubMed] [Google Scholar]
- Takahasi K., Yoneyama M., Nishihori T., Hirai R., Kumeta H., Narita R., Gale M., Jr., Inagaki F., Fujita T. Nonself RNA-sensing mechanism of RIG-I helicase and activation of antiviral immune responses. Mol. Cell. 2008;29:428–440. doi: 10.1016/j.molcel.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Tell L.A., Woods L., Cromie R.L. Mycobacteriosis in birds. Rev. Sci. Technol. 2001;20:180–203. doi: 10.20506/rst.20.1.1273. [DOI] [PubMed] [Google Scholar]
- Uchida Y., Watanabe C., Takemae N., Hayashi T., Oka T., Ito T., Saito T. Identification of host genes linked with the survivability of chickens infected with recombinant viruses possessing H5N1 surface antigens from a highly pathogenic avian influenza virus. J. Virol. 2012;86:2686–2695. doi: 10.1128/JVI.06374-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderven H., Petkau K., Ryan-Jean K.E., Aldridge J.R., Webster R.G., Magor K.E. Avian influenza rapidly induces antiviral genes in duck lung and intestine. Mol. Immunol. 2012;51:316–324. doi: 10.1016/j.molimm.2012.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viertlboeck B.C., Gick C.M., Schmitt R., Du Pasquier L., Gobel T.W. Complexity of expressed CHIR genes. Dev. Comp. Immunol. 2010;34:866–873. doi: 10.1016/j.dci.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Viertlboeck B.C., Gobel T.W. The chicken leukocyte receptor cluster. Vet. Immunol. Immunopathol. 2011;144:1–10. doi: 10.1016/j.vetimm.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Viertlboeck B.C., Habermann F.A., Schmitt R., Groenen M.A., Du Pasquier L., Gobel T.W. The chicken leukocyte receptor complex: a highly diverse multigene family encoding at least six structurally distinct receptor types. J. Immunol. 2005;175:385–393. doi: 10.4049/jimmunol.175.1.385. [DOI] [PubMed] [Google Scholar]
- Viertlboeck B.C., Schweinsberg S., Hanczaruk M.A., Schmitt R., Du Pasquier L., Herberg F.W., Göbel T.W. The chicken leukocyte receptor complex encodes a primordial, activating, high-affinity IgY Fc receptor. Proc. Natl. Acad. Sci. USA. 2007;104:11718–11723. doi: 10.1073/pnas.0702011104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viertlboeck B.C., Schweinsberg S., Schmitt R., Herberg F.W., Göbel T.W. The chicken leukocyte receptor complex encodes a family of different affinity FcY receptors. J. Immunol. 2009;182:6985–6992. doi: 10.4049/jimmunol.0803060. [DOI] [PubMed] [Google Scholar]
- Walker B.A., Hunt L.G., Sowa A.K., Skjodt K., Gobel T.W., Lehner P.J., Kaufman J. The dominantly expressed class I molecule of the chicken MHC is explained by coevolution with the polymorphic peptide transporter (TAP) genes. Proc. Natl. Acad. Sci. USA. 2011;108:8396–8401. doi: 10.1073/pnas.1019496108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallny H.J., Avila D., Hunt L.G., Powell T.J., Riegert P., Salomonsen J., Skjodt K., Vainio O., Vilbois F., Wiles M.V., Kaufman J. Peptide motifs of the single dominantly expressed class I molecule explain the striking MHC-determined response to Rous sarcoma virus in chickens. Proc. Natl. Acad. Sci. USA. 2006;103:1434–1439. doi: 10.1073/pnas.0507386103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Ekblom R., Strand T.M., Portela-Bens S., Hoglund J. Sequencing of the core MHC region of black grouse (Tetrao tetrix) and comparative genomics of the galliform MHC. BMC Genomics. 2012;13:553. doi: 10.1186/1471-2164-13-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Liu X.J., Wei B. Mitochondrion: an emerging platform for host antiviral signalling. Expert Opin. Ther. Targets. 2011;15:647–665. doi: 10.1517/14728222.2011.561321. [DOI] [PubMed] [Google Scholar]
- Wang T., Town T., Alexopoulou L., Anderson J.F., Fikrig E., Flavell R.A. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat. Med. 2004;10:1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhang H.X., Sun Y.P., Liu Z.X., Liu X.S., Wang L., Lu S.Y., Kong H., Liu Q.L., Li X.H., Lu Z.Y., Chen S.J., Chen Z., Bao S.S., Dai W., Wang Z.G. Rig-I−/− mice develop colitis associated with downregulation of G alpha i2. Cell Res. 2007;17:858–868. doi: 10.1038/cr.2007.81. [DOI] [PubMed] [Google Scholar]
- Warr G.W., Magor K.E., Higgins D.A. IgY: clues to the origins of modern antibodies. Immunol. Today. 1995;16:392–398. doi: 10.1016/0167-5699(95)80008-5. [DOI] [PubMed] [Google Scholar]
- Warren W.C., Clayton D.F., Ellegren H., Arnold A.P., Hillier L.W., Kunstner A., Searle S., White S., Vilella A.J., Fairley S., Heger A., Kong L., Ponting C.P., Jarvis E.D., Mello C.V., Minx P., Lovell P., Velho T.A., Ferris M., Balakrishnan C.N., Sinha S., Blatti C., London S.E., Li Y., Lin Y.C., George J., Sweedler J., Southey B., Gunaratne P., Watson M., Nam K., Backstrom N., Smeds L., Nabholz B., Itoh Y., Whitney O., Pfenning A.R., Howard J., Volker M., Skinner B.M., Griffin D.K., Ye L., McLaren W.M., Flicek P., Quesada V., Velasco G., Lopez-Otin C., Puente X.S., Olender T., Lancet D., Smit A.F., Hubley R., Konkel M.K., Walker J.A., Batzer M.A., Gu W., Pollock D.D., Chen L., Cheng Z., Eichler E.E., Stapley J., Slate J., Ekblom R., Birkhead T., Burke T., Burt D., Scharff C., Adam I., Richard H., Sultan M., Soldatov A., Lehrach H., Edwards S.V., Yang S.P., Li X., Graves T., Fulton L., Nelson J., Chinwalla A., Hou S., Mardis E.R., Wilson R.K. The genome of a songbird. Nature. 2010;464:757–762. doi: 10.1038/nature08819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathelet M., Moutschen S., Defilippi P., Cravador A., Collet M., Huez G., Content J. Molecular cloning, full-length sequence and preliminary characterization of a 56-kDa protein induced by human interferons. Eur. J. Biochem. 1986;155:11–17. doi: 10.1111/j.1432-1033.1986.tb09452.x. [DOI] [PubMed] [Google Scholar]
- Wei Z., Wu Q., Ren L., Hu X., Guo Y., Warr G.W., Hammarstrom L., Li N., Zhao Y. Expression of IgM, IgD, and IgY in a reptile, Anolis carolinensis. J Immunol. 2009;183:3858–3864. doi: 10.4049/jimmunol.0803251. [DOI] [PubMed] [Google Scholar]
- Wick G., Sundick R.S., Albini B. A review: the obese strain (OS) of chickens: an animal model with spontaneous autoimmune thyroiditis. Clin. Immunol. Immunopathol. 1974;3:272–300. doi: 10.1016/0090-1229(74)90015-4. [DOI] [PubMed] [Google Scholar]
- Wilson M., Bengten E., Miller N.W., Clem L.W., Du Pasquier L., Warr G.W. A novel chimeric Ig heavy chain from a teleost fish shares similarities to IgD. Proc. Natl. Acad. Sci. USA. 1997;94:4593–4597. doi: 10.1073/pnas.94.9.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Liang H., Zhou Q., Li Y., Chen H., Ye W., Chen D., Fleming J., Shu H., Liu Y. Crystal structure of ISG54 reveals a novel RNA binding structure and potential functional mechanisms. Cell Res. 2012;22:1328–1338. doi: 10.1038/cr.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., He K., Wu S.Y., Wan Q.H. Isolation of a 97-kb minimal essential MHC B locus from a new reverse-4D BAC library of the golden pheasant. PloS One. 2012;7:e32154. doi: 10.1371/journal.pone.0032154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama M., Kikuchi M., Natsukawa T., Shinobu N., Imaizumi T., Miyagishi M., Taira K., Akira S., Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. [see comment] Nat. Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- Yu P., Lubben W., Slomka H., Gebler J., Konert M., Cai C., Neubrandt L., da Costa O.P., Paul S., Dehnert S., Dohne K., Thanisch M., Storsberg S., Wiegand L., Kaufmann A., Nain M., Quintanilla-Martinez L., Bettio S., Schnierle B., Kolesnikova L., Becker S., Schnare M., Bauer S. Nucleic acid-sensing Toll-like receptors are essential for the control of endogenous retrovirus viremia and ERV-induced tumors. Immunity. 2012;37:867–879. doi: 10.1016/j.immuni.2012.07.018. [DOI] [PubMed] [Google Scholar]
- Zeng W., Sun L., Jiang X., Chen X., Hou F., Adhikari A., Xu M., Chen Z.J. Reconstitution of the RIG-I pathway reveals a signaling role of unanchored polyubiquitin chains in innate immunity. Cell. 2010;141:315–330. doi: 10.1016/j.cell.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Zhang D.E. Interferon-stimulated gene 15 and the protein ISGylation system. J. Interferon Cytokine Res. 2011;31:119–130. doi: 10.1089/jir.2010.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Denison C., Huibregtse J.M., Gygi S., Krug R.M. Human ISG15 conjugation targets both IFN-induced and constitutively expressed proteins functioning in diverse cellular pathways. Proc. Natl. Acad. Sci. USA. 2005;102:10200–10205. doi: 10.1073/pnas.0504754102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Hsiang T.Y., Kuo R.L., Krug R.M. ISG15 conjugation system targets the viral NS1 protein in influenza A virus-infected cells. Proc. Natl. Acad. Sci. USA. 2010;107:2253–2258. doi: 10.1073/pnas.0909144107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Pan-Hammarstrom Q., Yu S., Wertz N., Zhang X., Li N., Butler J.E., Hammarstrom L. Identification of IgF, a hinge-region-containing Ig class, and IgD in Xenopus tropicalis. Proc. Natl. Acad. Sci. USA. 2006;103:12087–12092. doi: 10.1073/pnas.0600291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Rabbani H., Shimizu A., Hammarstrom L. Mapping of the chicken immunoglobulin heavy-chain constant region gene locus reveals an inverted alpha gene upstream of a condensed upsilon gene. Immunology. 2000;101:348–353. doi: 10.1046/j.1365-2567.2000.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Michal J.J., Zhang L., Ding B., Lunney J.K., Liu B., Jiang Z. Interferon induced IFIT family genes in host antiviral defense. Int. J. Biol. Sci. 2013;9:200–208. doi: 10.7150/ijbs.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J., Chang M., Nie P., Secombes C.J. Origin and evolution of the RIG-I like RNA helicase gene family. BMC Evol. Biol. 2009;9:85. doi: 10.1186/1471-2148-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]