Abstract
Objectives
We aimed to identify patients' clinical characteristics associated with respiratory viruses identified among patients presenting with influenza-like illness (ILI).
Methods
A sample of patients of all ages presenting with ILI was included by physicians of the French Sentinelles network during two seasons (2015/16 and 2016/17). Nasopharyngeal samples were tested for the presence of influenza virus (IV), respiratory syncytial virus (RSV), human rhinovirus (HRV) and human metapneumovirus (HMPV). Patients' characteristics associated with each of the four virus classes were studied using multivariate logistic regressions.
Results
A total of 5859 individuals were included in the study: 48.0% tested positive for IV, 7.9% for HRV, 7.5% for RSV and 4.1% for HMPV. Cough was associated with IV (OR 2.14, 95% CI 1.81–2.52) RSV (OR 2.52, 95% CI 1.75–3.74) and HMPV detection (OR 2.15, 95% CI 1.40–3.45). Rhinorrhoea was associated mainly with HRV detection (OR 1.75, 95% CI 1.34–2.32). Headache was associated with IV detection (OR 1.75, 95% CI 1.34–2.32), whereas absence of headache was associated with RSV and HMPV detection. Dyspnoea was associated with RSV detection (OR 2.33, 95% CI 1.73–3.12) and absence of dyspnoea with IV detection. Conjunctivitis was associated with IV detection (OR 1.27, 95% CI 1.08–1.50). Some associations were observed only in children: dyspnoea and cough with RSV detection (age <5 years), conjunctivitis with IV detection (age <15 years). Period of onset of symptoms differed among aetiological diagnoses. Seasonal influenza vaccination decreased the risk of IV detection (OR, 0.67, 95% CI 0.51–0.86).
Conclusions
This study allowed the identification of symptoms associated with several viral aetiologies in patients with ILI. A proper knowledge and understanding of these clinical signs may improve the medical management of patients.
Keywords: Influenza, Influenza-like illness, Primary care, Respiratory infections, Respiratory viruses, Surveillance
Introduction
Precise aetiological diagnoses of respiratory infections require virological analyses as none of the common respiratory viruses provide a pathognomonic clinical picture [1], [2]. Clinical symptoms and impact according to patient characteristics differ depending on the virus responsible for the infection, and seasonal dynamics vary according to the virus as well as across seasons [1], [3], [4]. Knowledge of factors associated with the virological status might improve treatment decisions and medical care proposed by the physician at the time of diagnosis, especially for influenza, for which antivirals are recommended for the at-risk population [1], [5], [6].
In France, in primary care, since 1984, the Sentinelles network has been conducting surveillance of patients with influenza-like illness, with a specific definition for the purpose of influenza surveillance (called thereafter Sentinelles influenza-like illness (S-ILI)) [7], [8]. Since 2014, this surveillance has been linked to virological analyses to detect and characterize circulating respiratory viruses [9]. This surveillance is based on general practitioners and paediatricians who collect nasopharyngeal swabs from patients with S-ILI. These are routinely tested for four common respiratory virus classes: influenza virus (IV), respiratory syncytial virus (RSV), human metapneumovirus (HMPV) and human rhinovirus (HRV).
As improved characterization of community-acquired respiratory infections could help health-care professionals in the management of these infections, the aim of this study was to identify the clinical characteristics collected by the physician in patients with S-ILI that were associated with each of the four tested viruses (IV, RSV, HRV and HMPV). Primary analyses were conducted by comparing patients positive for one virus to the rest of the cohort. Secondary analyses were performed considering only patients who tested positive for any of the virus classes, and considering different age groups.
Methods
Study design and data collection
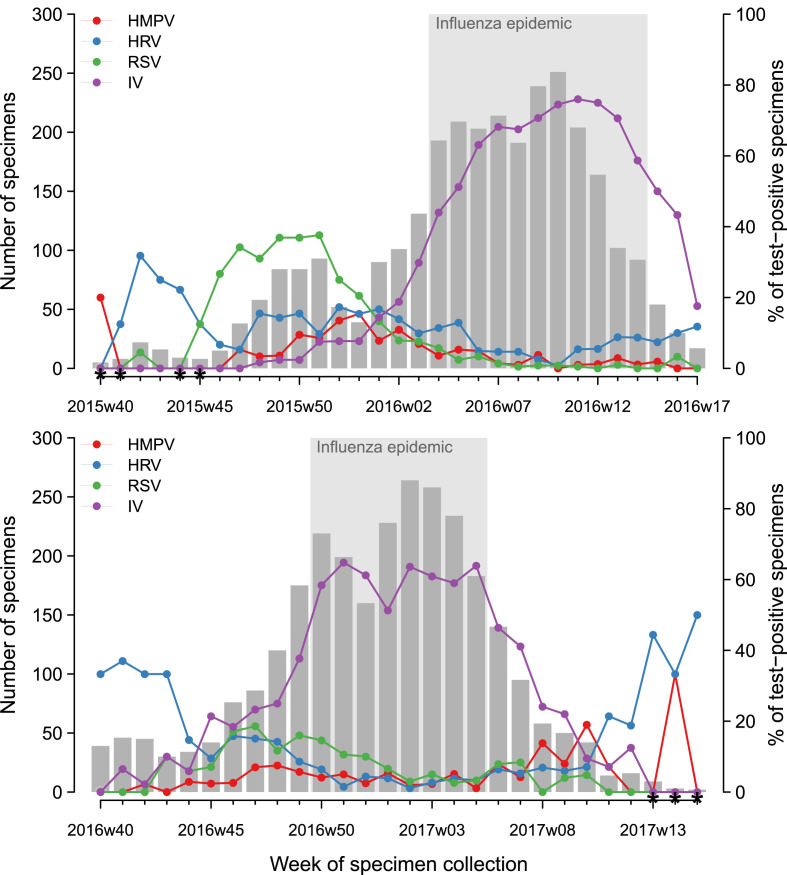
Virological surveillance of respiratory viruses in France involves 250 general practitioners and 100 paediatricians of the French Sentinelles network with a nationwide coverage [9]. The surveillance uses a specific definition of S-ILI for patient recruitment: sudden onset of fever >39°C with myalgia and respiratory signs, diagnosed by the physician. This definition was chosen for its high predictive value for influenza infection [7]. The study period covered two surveillance seasons: 2015/16 (week 40, 2015 to week 17, 2016) and 2016/17 (week 40, 2016 to week 15, 2017) (Fig. 1 ).
Fig. 1.
Size of study population and percentage of patients tested positive for the four virus classes studied: influenza viruses (IV), respiratory syncytial viruses (RSV), human rhinoviruses (HRV) and human metapneumoviruses (HMPV) by week, seasons 2015/16 and 2016/17, France. * Number of samples for the week was less than ten.
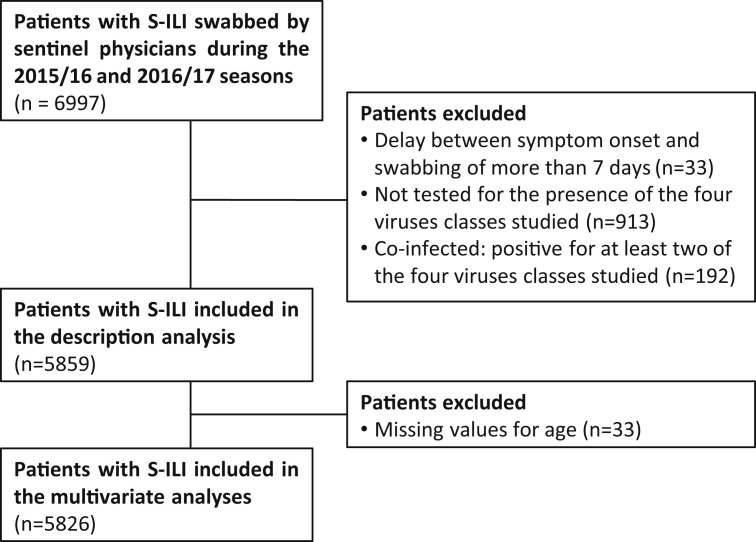
During the virological surveillance period, sentinel physicians collected nasopharyngeal swabs along with clinical data in a systematically selected sample of their patients presenting with S-ILI [9]. The sample consisted of the first two S-ILI patients of the week, unrelated to one another, consulting within <48 h since symptom onset and consenting to provide a nasopharyngeal specimen. Each patient could be included only once a year. During the 2016/17 influenza epidemic, general practitioners were allowed to include one additional patient aged 65 years or older presenting with S-ILI, to improve influenza surveillance among the elderly. The study population consisted of all those S-ILI patients that were swabbed, with exclusion of co-infected patients, patients that were not tested for the presence of the four virus classes and patients swabbed more than 7 days after symptom onset.
Virological analyses were performed by the French National Reference Centre for respiratory viruses (CNR, in Paris and Lyon) and the Laboratory of Virology at the University of Corsica. The three laboratories performed real-time RT-PCR tests for the detection of IV (A and B), RSV, HMPV and HRV.
Outcomes and explanatory variables
For each patient, the outcome corresponded to the virological result: positive for IV, RSV, HMPV and HRV, positive for several virus classes—called ‘co-infected’, or negative for all viruses tested—called ‘negative’. Based on a previous study reporting no differences between IVA and IVB symptoms [2], we did not discriminate IVA and IVB patients.
Explanatory variables studied were those identifiable by the physician during the consultation: age (eight groups: <5, 5–14, 15–29, 30–44, 45–64 and ≥65 years), influenza vaccination for current season (received at least 14 days before onset of symptoms), period of onset of symptoms (before, during or after the influenza epidemic as published by the Sentinelles network for France [8]—reported in Fig. 1), cough, rhinorrhoea, dyspnoea, sore throat, conjunctivitis, headache, malaise, vomiting and diarrhoea.
To identify which of these explanatory variables were associated with the viral infection, we performed the following analyses:
-
-
four independent analyses with the virological result as outcome (IV, RSV, HMPV or HRV), considering the whole study population (i.e. whatever the virological result, positive or negative);
-
-
four independent analyses with the virological result as outcome (IV, RSV, HMPV or HRV), considering independently three age groups belonging to the study population: young children (<5 years), older children (5–14 years) and adults (≥15 years);
-
-
IV detection as outcome, considering a subgroup of the study population with the patients positive for IV or negative for all four virus classes;
-
-
IV detection as outcome, considering a subgroup of the study population with the patients positive for any of the four virus classes; and
-
-
any virus detection as outcome, considering the whole study population.
Statistical analyses
Multivariate logistic regressions were performed to identify factors associated with the outcomes of interest. Starting with all explanatory variables in the model (see above), we used a backward process based on likelihood-ratio test to remove the least significant variables from the model step-by-step (p-value level of 0.05).
Ethical statement
The protocol was conducted in agreement with the Helsinki declaration. We obtained authorization from the French Data Protection Agency (CNIL#471393) and the French ethical research committee (Comité de protection des personnes). All participating physicians consent to publication of the results from the surveillance.
Results
During the 2015/16 and 2016/17 surveillance seasons, sentinel physicians swabbed 6997 patients with S-ILI. Among them, 5859 (83.7%) were included in the study (Fig. 2 ): 3003 collected during the 2015/16 season (from 29 September 2015 to 1 May 2016) and 2856 during the 2016/17 season (from 3 October 2016 to 16 April 2017). Laboratory analysis allowed the identification of 2815 (48.0%) patients positive for IV, 462 (7.9%) positive for HRV, 437 (7.5%) positive for RSV, 242 (4.1%) positive for HMPV and 1903 (32.5%) patients negative for all virus classes tested (Table 1 ). A total of 192 patients were co-infected and were excluded from the analysis (Fig. 2, Table 1). These patients were always positive for IV and for one or two other virus classes (57.3% for HRV, 26.6% for RSV and 18.8% for HMPV).
Fig. 2.
Flowchart of data exclusion of study population, seasons 2015/16 and 2016/17, France.
Table 1.
Description of study population according to viral aetiology, seasons 2015/16 and 2016/17, France
| Influenza virus (n = 2815) | Respiratory syncytial virus (n = 437) | Human metapneumovirus (n = 242) | Human rhinovirus (n = 462) | Co-infected (n = 192) | No virus (n = 1903) | Total included in the studya (n = 5859) | ||
|---|---|---|---|---|---|---|---|---|
| Season | 2015/16 | 1483 (52.7%) | 218 (49.9%) | 115 (47.5%) | 249 (53.9%) | 94 (49%) | 938 (49.3%) | 3003 (51.3%) |
| 2016/17 | 1332 (47.3%) | 219 (50.1%) | 127 (52.5%) | 213 (46.1%) | 98 (51%) | 965 (50.7%) | 2856 (48.7%) | |
| Clinical presentation | ||||||||
| Onset of symptoms | Before influenza epidemic | 238 (8.5%) | 263 (60.2%) | 94 (38.8%) | 238 (51.5%) | 30 (15.6%) | 706 (37.1%) | 1539 (26.3%) |
| During influenza epidemic | 2399 (85.2%) | 150 (34.3%) | 110 (45.5%) | 180 (39%) | 155 (80.7%) | 955 (50.2%) | 3794 (64.8%) | |
| After influenza epidemic | 178 (6.3%) | 24 (5.5%) | 38 (15.7%) | 44 (9.5%) | 7 (3.6%) | 242 (12.7%) | 526 (9%) | |
| Cough | 2477 (88%) | 403 (92.2%) | 220 (90.9%) | 382 (82.7%) | 170 (88.5%) | 1408 (74%) | 4890 (83.5%) | |
| Rhinorrhoea | 2150 (76.4%) | 382 (87.4%) | 191 (78.9%) | 394 (85.3%) | 156 (81.2%) | 1305 (68.6%) | 4422 (75.5%) | |
| Headache | 1881 (66.8%) | 120 (27.5%) | 95 (39.3%) | 218 (47.2%) | 85 (44.3%) | 1157 (60.8%) | 3471 (59.2%) | |
| Sore throat | 1447 (51.4%) | 171 (39.1%) | 104 (43%) | 221 (47.8%) | 87 (45.3%) | 918 (48.2%) | 2861 (48.8%) | |
| Conjunctivitis | 479 (17%) | 65 (14.9%) | 29 (12%) | 75 (16.2%) | 35 (18.2%) | 256 (13.5%) | 904 (15.4%) | |
| Malaise | 366 (13%) | 24 (5.5%) | 15 (6.2%) | 50 (10.8%) | 12 (6.2%) | 252 (13.2%) | 707 (12.1%) | |
| Dyspnoea | 205 (7.3%) | 81 (18.5%) | 27 (11.2%) | 54 (11.7%) | 22 (11.5%) | 184 (9.7%) | 551 (9.4%) | |
| Vomiting | 300 (10.7%) | 52 (11.9%) | 23 (9.5%) | 54 (11.7%) | 24 (12.5%) | 237 (12.5%) | 666 (11.4%) | |
| Diarrhoea | 172 (6.1%) | 40 (9.2%) | 18 (7.4%) | 30 (6.5%) | 15 (7.8%) | 145 (7.6%) | 405 (6.9%) | |
| Baseline characteristics | ||||||||
| Sex | F | 1329 (48.1%) | 214 (50%) | 110 (46.2%) | 222 (49.2%) | 82 (43.2%) | 913 (49.3%) | 2788 (48.7%) |
| M | 1432 (51.9%) | 214 (50%) | 128 (53.8%) | 229 (50.8%) | 108 (56.8%) | 939 (50.7%) | 2942 (51.3%) | |
| Missing valuesb | 54 (1.9%) | 9 (2.1%) | 4 (1.7%) | 11 (2.4%) | 2 (1%) | 51 (2.7%) | 129 (2.2%) | |
| Age groups (years) | 0–4 | 641 (22.9%) | 323 (74.3%) | 125 (52.1%) | 239 (52%) | 107 (55.7%) | 589 (31.1%) | 1917 (32.9%) |
| 5–14 | 675 (24.1%) | 33 (7.6%) | 31 (12.9%) | 44 (9.6%) | 44 (22.9%) | 315 (16.6%) | 1098 (18.8%) | |
| 15–29 | 447 (16%) | 17 (3.9%) | 21 (8.8%) | 52 (11.3%) | 14 (7.3%) | 299 (15.8%) | 836 (14.3%) | |
| 30–44 | 442 (15.8%) | 11 (2.5%) | 14 (5.8%) | 54 (11.7%) | 15 (7.8%) | 335 (17.7%) | 856 (14.7%) | |
| 45–64 | 410 (14.7%) | 37 (8.5%) | 27 (11.2%) | 51 (11.1%) | 7 (3.6%) | 257 (13.6%) | 782 (13.4%) | |
| ≥65 | 182 (6.5%) | 14 (3.2%) | 22 (9.2%) | 20 (4.3%) | 5 (2.6%) | 99 (5.2%) | 337 (5.8%) | |
| Missing valuesb | 18 (0.6%) | 2 (0.5%) | 2 (0.8%) | 2 (0.4%) | 0 (0%) | 9 (0.5%) | 33 (0.6%) | |
| Chronic condition | 316 (11.7%) | 60 (14.3%) | 39 (16.9%) | 60 (13.7%) | 26 (13.8%) | 247 (13.6%) | 722 (12.8%) | |
| Missing valuesb | 104 (3.7%) | 18 (4.1%) | 11 (4.5%) | 25 (5.4%) | 4 (2.1%) | 81 (4.3%) | 239 (4.1%) | |
| Influenza vaccination (for current season) | 178 (6.4%) | 19 (4.4%) | 16 (6.7%) | 21 (4.6%) | 129 (6.9%) | 7 (3.7%) | 129 (6.9%) | |
| Missing valuesb | 39 (1.4%) | 6 (1.4%) | 4 (1.7%) | 6 (1.3%) | 4 (2.1%) | 25 (1.3%) | 80 (1.4%) | |
Excluding co-infected individuals.
Missing values were not included in percentage calculations.
Characteristics of the patients included are reported in Table 1. Among the study population, 32.9% (n = 1917) were aged <5 years, 18.8% (n = 1098) were between 5 and 14 years and 48.2% (n = 2811) were 15 years or older. The median age of the adults (≥15 years) was 39 years (interquartile range 28–53 years). The period of onset of symptoms was during the influenza epidemic in 64.8% of patients (n = 3794), before the epidemic in 26.3% (n = 1539) and after the epidemic in 9.0% (n = 526) (Fig. 1). Among the study population, 12.8% (n = 722) had at least one chronic disease, and 6.3% (n = 363) were vaccinated with the seasonal influenza vaccine.
Symptoms and baseline patient characteristics associated with laboratory confirmation of each of the four virus classes are reported in Table 2 . Results of age-subgroup analyses are reported in the Supplementary material (Table S1).
Table 2.
Clinical characteristics associated with four respiratory virus classes among patients seen by physicians of the Sentinelles network for influenza-like illness, multivariate analyses, seasons 2015/16 and 2016/17, France
| Influenza virus |
Respiratory syncytial virus |
Human metapneumovirus |
Human rhinovirus |
|||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Cough | 2.14 (1.81–2.52) | <10−5 | 2.52 (1.75–3.74) | <10−5 | 2.15 (1.40–3.45) | <10−3 | NS | |
| Headache | 1.19 (1.03–1.37) | 0.016 | 0.60 (0.46–0.78) | <10−3 | 0.62 (0.45–0.85) | 0.003 | NS | |
| Rhinorrhoea | 1.21 (1.05–1.39) | 0.009 | 1.43 (1.06–1.97) | 0.018 | NS | 1.75 (1.34–2.32) | <10−4 | |
| Dyspnoea | 0.56 (0.46–0.70) | <10−5 | 2.33 (1.73–3.12) | <10−5 | NS | NS | ||
| Conjunctivitis | 1.27 (1.08–1.50) | 0.004 | NS | NS | NS | |||
| Period of onset of symptoms | <10−5 | <10−5 | <10−5 | <10−5 | ||||
| Before influenza epidemic | 0.11 (0.09–0.13) | 3.72 (2.98–4.66) | 1.89 (1.41–2.52) | 3.30 (2.68–4.06) | ||||
| During influenza epidemic | — a | — a | — a | — a | ||||
| After influenza epidemic | 0.30 (0.24–0.36) | 1.19 (0.74–1.84) | 2.74 (1.84–3.99) | 1.80 (1.26–2.53) | ||||
| Age group (years) | <10−5 | <10−5 | <10−3 | <10−5 | ||||
| <5 | 0.59 (0.48–0.72) | 10.6 (5.89–21.12) | 3.05 (1.75–5.73) | 1.67 (1.23–2.31) | ||||
| 5–14 | 1.59 (1.29–1.95) | 2.83 (1.46–5.94) | 1.82 (0.98–3.55) | 0.64 (0.42–0.97) | ||||
| 15–29 | 1.05 (0.85–1.30) | 1.69 (0.79–3.77) | 1.61 (0.82–3.27) | 0.98 (0.66–1.46) | ||||
| 30–44 | — a | — a | — a | — a | ||||
| 45–64 | 1.1 (0.88–1.36) | 3.74 (1.95–7.79) | 2.02 (1.07–4.00) | 1.02 (0.68–1.53) | ||||
| ≥65 | 1.34 (0.99–1.82) | 3.1 (1.38–7.14) | 3.78 (1.92–7.70) | 1.00 (0.57–1.68) | ||||
| Influenza vaccination (for current season) | 0.67 (0.51–0.86) | 0.002 | NS | NS | NS | |||
For each virus, an independent multivariate regression analysis was performed considering the whole study population (i.e. whatever the virological result, positive or negative).
Reference group; NS, non significant.
Symptoms associated with detection of IV were cough, absence of dyspnoea, conjunctivitis, headache and rhinorrhoea. Patients were more likely to be IV-positive during the epidemic period. Compared with the middle-aged adults (30–44 years), school-aged children (5–14 years) had a higher risk of being IV-positive, and the youngest (<5 years) had a lower risk. Seasonal influenza vaccination was associated with a lower risk of IV detection. Among the youngest (<5 years), headache was associated with IV detection whereas cough and rhinorrhoea were not. Conjunctivitis was associated with IV detection among children (<15 years). The complementary analysis comparing IV-positive patients with two other groups (all negative patients or patients positive for another respiratory virus) provided results consistent with the primary analysis for IV (Table 2, and see Supplementary material, Table S2).
Detection of RSV was associated with cough, dyspnoea, absence of headache and rhinorrhoea. RSV was more frequently identified before the influenza epidemic period. The youngest individuals (<5 years) had the highest risk of being RSV-positive, the 5–14 years, 45–64 years and ≥65 years (elderly) had an increased risk compared with the middle-aged adults (30–44 years). In age-subgroup analyses, some differences should be noted: cough and dyspnoea were associated with RSV detection in the <5-year group whereas sore throat was identified for adults (≥15 years).
Laboratory confirmation of HMPV was associated with cough and absence of headache. Patients were more likely to be HPMV-positive outside the influenza epidemic period. Compared with the 30–44-year group, the <5, 45–64 and ≥65 year-olds had a higher risk of being HMPV-positive. Concerning age-subgroup analyses, cough was associated with confirmation of HMPV for children (<15 years) and absence of headache was associated with HMPV detection for the <5-year group.
Only rhinorrhoea was associated with HRV detection. Patients were more likely to be HRV-positive outside the influenza epidemic and particularly before this period. The youngest (<5 years old) had a higher risk and the 5–14-year group a lower risk of being HRV-positive, compared with the 30–44-year group. Among age groups, rhinorrhoea was associated with HRV detection for the <5 and the ≥15 year group, whereas only absence of headache was associated with HRV detection for the 5–14-year age group.
Lastly, when comparing patients positive for any of the four virus classes with patients whose findings were negative, we highlighted two symptoms (cough and rhinorrhoea) associated with virus detection along with extreme age groups (<15 years or ≥45 years, compared with the 30–44 years group) (see Supplementary material, Table S2).
Discussion
During the two surveillance seasons analysed, an aetiological agent was detected in more than two-thirds of patients with S-ILI (defined as a sudden onset of fever >39°C with myalgia and respiratory signs) seen by physicians of the Sentinelles network. We found that three baseline characteristics (age, period of symptom onset, seasonal influenza vaccination) and five symptoms (cough, rhinorrhoea, headache, conjunctivitis and dyspnoea) were associated with an increased or decreased risk of detection of at least one of the four virus classes. These factors are identifiable by the physician at the time of S-ILI diagnosis (Table 3 ).
Table 3.
Clinical and epidemiological characteristics associated with different viruses responsible for Sentinelles influenza-like illnesses (defined as a sudden onset of fever >39°C with myalgia and respiratory signs)
| Influenza virus | Respiratory syncytial virus | Human metapneumo-virus | Human rhinovirus | |
|---|---|---|---|---|
| Cough | + + | + + | + + | |
| Headache | + | – | – | |
| Rhinorrhoea | + | + | + | |
| Dyspnoea | – | + + | ||
| Conjunctivitis | + | |||
| Period of onset of symptoms | ||||
| Before influenza epidemic | – – – | +++ | + | +++ |
| During influenza epidemic | a | a | a | a |
| After influenza epidemic | – – – | + + | + | |
| Age group (years) | ||||
| <5 | – | +++ | +++ | + |
| 5–14 | + | + | – | |
| 15–29 | ||||
| 30–44 | a | a | a | a |
| 45–64 | + + + | + + | ||
| ≥65 | + + + | + + + | ||
| Influenza vaccination (for current season) | – |
Adjusted OR significantly >1: ‘+’ for 1 < OR < 2; ‘+ +’ for 2 ≤ OR < 3; ‘+ + +’ for OR ≥ 3. Adjusted OR significantly <1: ‘–’ for 0.5 < OR < 1; ‘– –’ for 0.3 < OR ≤ 0.5; ‘– – –’ for OR ≤ 0.3.
Reference group.
All four respiratory virus classes co-circulated to various extents during the winter period in France (December to March). However, their intensity of circulation varied over the months, consistent with previous studies [4], [10], [11], [12]: HRV circulated mostly during autumn and could reappear in spring, RSV had higher activity in late autumn to early winter, IV predominated mainly during winter. As expected, we showed that the risk of IV detection was higher during the influenza epidemic period, which supports the use of this S-ILI definition and the influenza epidemic detection method [13]. However, the proportion of S-ILI patients positive for IV was 48.0% over the study period and never higher than 76.0% by week (Fig. 1). Laboratory confirmation of virus infection by RT-PCR is costly and is not feasible in real time at the physician's office. Although an accurate aetiological diagnosis will require laboratory analysis, knowledge of combined observable factors predictive of viruses responsible for infection among S-ILI patients, might help to improve the rapid management of these infections. Analyses provided here are comparisons in relative terms, which should not be interpreted as patterns of incidence.
Compared with middle-aged adults (30–44 years), the youngest children (<5 years) had an increased risk to be positive for HRV, HMPV and, especially, RSV, which affects this age group more commonly [14], [15], [16]. Moreover, the 45–64 years and ≥65 years age groups had a higher risk to be positive for RSV and HMPV compared with the 30–44 year age group—in line with a previous study [3]. The clinical signs associated with detection of a virus among patients with S-ILI varied among age groups. However, differences in age-related clinical symptoms should be interpreted cautiously, as the frequency of the four isolated viruses differed markedly among children and adults [4], [16]. Consequently, the limited number of individuals for some age-subgroup analyses could explain in part the age-related differences in clinical signs highlighted.
Cough was the most frequently reported symptom, associated with IV, RSV and HMPV detections, as expected [1], [3], [17]. Presence of dyspnoea was associated with RSV infection and absence of dyspnoea was related to IV detection, as previously reported [16]. This is consistent with the biology of these viruses: typically, IV infections predominantly involve the upper respiratory tract and trachea [18], [19], as human strains of IV primarily target cells of the upper airway [20], unlike RSV infections, which are more commonly associated with lower respiratory tract infections such as bronchiolitis and pneumonia [21], [22]. Headache was associated with an increased risk of IV detection and a lower risk of RSV and HMPV detection, consistent with previous studies [7], [16], [23]. Association between conjunctivitis and IV detection was mainly reported among the youngest patients [7], [19]. HRV detection was associated with one symptom—rhinorrhoea, in line with less severe illness caused by HRV compared with other viruses [4].
Influenza vaccination was associated with a lower risk of being IV-positive, in concordance with the influenza vaccine effectiveness estimated for these two seasons in Europe (33%, 95% CI 10–51 against A (H1N1)pdm09 viruses in 2015/16; 20%, 95% CI –14 to 43 against B viruses in 2015/16; 21%, 95% CI 6–34 against A (H3N2) viruses in 2016/17) [24].
Our study is based on data collected by a longstanding surveillance system with a standardized protocol, allowing the inclusion of a large number of patients during two surveillance seasons. However, some limitations should be noted. First, patients were included using a fever definition of >39°C, resulting in a high proportion of IV-positive patients (48.0%). This did not allow the study of milder respiratory infections nor of the impact of symptoms included in the definition. Second, we highlighted symptoms associated with IV detection without type/sub-type/lineage distinction. This choice was based on previous studies in France [2], although others reported that symptoms may differ with influenza types and subtypes [7]. In France, the 2015/16 influenza season was dominated by influenza type B viruses (among IVs detected 72% were type B and 27% were subtype A (H1N1)pdm09) [9], whereas the 2016/17 season was dominated by subtype A (H3N2) (98%) [25]. Third, only four virus classes were studied—others such as adenoviruses or coronaviruses were not investigated. Fourth, we excluded the 192 co-infected patients (2.7%) from the analyses. As all of them were infected by IV, we compared these co-infected patients with those who were positive for IV only: we found that the co-infected were younger and presented similar symptoms, with slight differences: more dyspnoea, less malaise and less headache. Fifth, data collected allowed the analysis of neither the intensity nor the severity of the infection in terms of duration of symptoms or risk for hospitalization.
This study allowed the identification of symptoms associated with several viral aetiologies in patients presenting with S-ILI in primary care. As patients are not routinely virologically tested, a proper knowledge and understanding of clinical signs of these respiratory illnesses may contribute to improve decision-making and medical care of patients.
Transparency declaration
Dr Lina received travel grants to attend meetings from GSK and Sanofi Pasteur and is a member of the scientific boards of the GII and GHISN. All personal remuneration stopped in September 2010. Dr van der Werf reports grants from Santé publique France, during the conduct of the study; other from ESWI, outside the submitted work. All other authors have no conflicts of interest to declare.
Funding
This project has received funding from the European Union's Horizon 2020 Research and Innovation Programme under grant agreement No 634446 to conduct the study in individuals aged 65 years or older and from Santé publique France, the national public health agency in France.
Acknowledgements
We thank all general practitioners and paediatricians participating in the French Sentinelles network. We also thank the European Centre for Disease Prevention and Control, which contributed to the funding of the study.
Editor: Mical Paul
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2019.01.014.
Author contributions
TH, TB and CS designed the study. SM, CP, CT, LC, AF, BL, SvdW, IB, AMV, MV and SB participated in data collection and analysis. CS performed the statistical analysis. CS, SM and TH drafted the manuscript. All the authors contributed to and approved the final version of the manuscript.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Monto A.S., Gravenstein S., Elliott M., Colopy M., Schweinle J. Clinical signs and symptoms predicting influenza infection. Arch Intern Med. 2000;160:3243–3247. doi: 10.1001/archinte.160.21.3243. [DOI] [PubMed] [Google Scholar]
- 2.Mosnier A., Caini S., Daviaud I., Nauleau E., Bui T.T., Debost E. Clinical characteristics are similar across type A and B influenza virus infections. PLoS One. 2015;10:e0136186. doi: 10.1371/journal.pone.0136186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimmerman R.K., Rinaldo C.R., Nowalk M.P., Balasubramani G.K., Thompson M.G., Moehling K.K. Influenza and other respiratory virus infections in outpatients with medically attended acute respiratory infection during the 2011–12 influenza season. Influenza Other Respir Virus. 2014;8 doi: 10.1111/irv.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monto A.S. Epidemiology of viral respiratory infections. Am J Med. 2002;112(Suppl. 1):4–12. doi: 10.1016/s0002-9343(01)01058-0. [DOI] [PubMed] [Google Scholar]
- 5.Mandell L.A. Etiologies of acute respiratory tract infections. Clin Infect Dis. 2005;41:503–506. doi: 10.1086/432019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanchon T., Geffrier F., Turbelin C., Daviaud I., Laouenan C., Duval X. Use of neuraminidase inhibitors in primary health care during pandemic and seasonal influenza between 2009 and 2013. Antivir Ther. 2015;20:753–761. doi: 10.3851/IMP2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carrat F., Tachet A., Rouzioux C., Housset B., Valleron A.-J. Evaluation of clinical case definitions of influenza: detailed investigation of patients during the 1995–1996 epidemic in France. Clin Infect Dis. 1999;28:283–290. doi: 10.1086/515117. [DOI] [PubMed] [Google Scholar]
- 8.Souty C., Amoros P., Falchi A., Capai L., Bonmarin I., van der Werf S. Influenza epidemics observed in primary care from 1984 to 2017 in France: a decrease of epidemic size over time. Influenza Other Respir Virus. 2018 Nov 14 doi: 10.1111/irv.12620. PMID: 30428158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vilcu A.M., Souty C., Enouf V., Capai L., Turbelin C., Masse S. Estimation of seasonal inflenza vaccine effectiveness using data collected in primary care in France: comparison of the test-negative design and the screening method. Clin Microbiol Infect. 2018;24:431. doi: 10.1016/j.cmi.2017.09.003. e5–.e12. [DOI] [PubMed] [Google Scholar]
- 10.Monto A.S., Cavallaro J.J. The Tecumseh study of respiratory illness. II. Patterns of occurrence of infection with respiratory pathogens, 1965–1969. Am J Epidemiol. 1971;94:280–289. doi: 10.1093/oxfordjournals.aje.a121321. [DOI] [PubMed] [Google Scholar]
- 11.Zimmerman R.K., Rinaldo C.R., Nowalk M.P., Balasubramani G., Moehling K.K., Bullotta A. Viral infections in outpatients with medically attended acute respiratory illness during the 2012–2013 influenza season. BMC Infect Dis. 2015;15:87. doi: 10.1186/s12879-015-0806-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mullins J.A., Erdman D.D., Weinberg G.A., Edwards K., Hall C.B., Walker F.J. Human metapneumovirus infection among children hospitalized with acute respiratory illness. Emerg Infect Dis. 2004;10:700–705. doi: 10.3201/eid1004.030555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costagliola D., Flahault A., Galinec D., Garnerin P., Menares J., Valleron A.J. A routine tool for detection and assessment of epidemics of influenza-like syndromes in France. Am J Public Health. 1991;81:97–99. doi: 10.2105/ajph.81.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zambon M.C., Stockton J.D., Clewley J.P., Fleming D.M. Contribution of influenza and respiratory syncytial virus to community cases of influenza-like illness: an observational study. Lancet. 2001;358:1410–1416. doi: 10.1016/s0140-6736(01)06528-x. [DOI] [PubMed] [Google Scholar]
- 15.Papenburg J., Boivin G. The distinguishing features of human metapneumovirus and respiratory syncytial virus. Rev Med Virol. 2010;20:245–260. doi: 10.1002/rmv.651. [DOI] [PubMed] [Google Scholar]
- 16.Casalegno J.S., Eibach D., Valette M., Enouf V., Daviaud I., Behillil S. Performance of influenza case definitions for influenza community surveillance: based on the French influenza surveillance network GROG, 2009–2014. Euro Surveill. 2017;22 doi: 10.2807/1560-7917.ES.2017.22.14.30504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navarro-Mari J.M., Perez-Ruiz M., Cantudo-Munoz P., Petit-Gancedo C., Jimenez-Valera M., Rosa-Fraile M. Influenza-like illness criteria were poorly related to laboratory-confirmed influenza in a sentinel surveillance study. J Clin Epidemiol. 2005;58:275–279. doi: 10.1016/j.jclinepi.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Taubenberger J.K., Morens D.M. The pathology of influenza virus infections. Annu Rev Pathol. 2008;3:499–522. doi: 10.1146/annurev.pathmechdis.3.121806.154316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cox N.J., Subbarao K. Influenza. Lancet. 1999;354:1277–1282. doi: 10.1016/S0140-6736(99)01241-6. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Parra G., De Ridder F., Huntjens D., Roymans D., Ispas G., Dobrovolny H.M. A comparison of RSV and influenza in vitro kinetic parameters reveals differences in infecting time. PLoS One. 2018;13:e0192645. doi: 10.1371/journal.pone.0192645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X., Li J., Meng L., Zhu W., Liu X., Yang M. Viral etiologies and epidemiology of patients with acute respiratory infections based on sentinel hospitals in Gansu Province, Northwest China, 2011–2015. J Med Virol. 2018;90:828–835. doi: 10.1002/jmv.25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins P.L., Graham B.S. Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol. 2008;82:2040–2055. doi: 10.1128/JVI.01625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall C.B., Long C.E., Schnabel K.C. Respiratory syncytial virus infections in previously healthy working adults. Clin Infect Dis. 2001;33:792–796. doi: 10.1086/322657. [DOI] [PubMed] [Google Scholar]
- 24.Valenciano M., Kissling E., Larrauri A., Nunes B., Pitigoi D., O'Donnell J. Exploring the effect of previous inactivated influenza vaccination on seasonal influenza vaccine effectiveness against medically attended influenza: results of the European I-MOVE multicentre test-negative case-control study, 2011/2012–2016/2017. Influenza Other Respir Virus. 2018;12:567–581. doi: 10.1111/irv.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Souty C., Vilcu A.M., Capai L., van der Werf S., Valette M., Blanchon T. Early estimates of 2016/17 seasonal influenza vaccine effectiveness in primary care in France. J Clin Virol. 2017;95:1–4. doi: 10.1016/j.jcv.2017.08.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.