Abstract
In healthy adult Homo sapiens, the most frequent circulating γδ T cells (Vγ9Vδ2) respond to pyrophosphomonoesters, alkylamines (together referred to as non-peptidic antigens, NpAgs) and nitrogen-containing bisphosphonates. The seemingly very low toxicity of bisphosphonate and pyrophosphomonoester drugs in vivo, may allow novel approaches to the immunotherapy of viral infections. For example, these drugs can be used to stimulate Vγ9Vδ2 T cells to release antiviral molecules that directly suppress virus replication without destroying the virus-replicating cells. In addition, the soluble molecules released by γδ T cells could boost the antiviral potency of cytotoxic T lymphocytes (CTLs) and promote antigen presentation. The relative therapeutic value of drug-induced direct antiviral and immunoregulatory activities may depend on the infecting virus as well as on the nature of protective immune responses.
Keywords: Antiviral immunity, Vγ9Vδ2 T cells, Immunotherapy, Non-peptidic antigens, Bisphosphonates
1. Introduction
In viral infections, both adaptive and innate immune reactions cooperate to protect the host and, whenever it is possible, to eradicate or control the infection. The early synthesis of soluble factors (cytokines, chemokines) influences substantially the subsequent immune response and may, therefore, affect the course of infection. One of the important effectors of natural immunity are γδ T lymphocytes, which display a broad antiviral activity against different viruses such as retroviruses, flaviviruses, paramyxoviruses, orthomyxoviruses, picornaviruses, coronaviruses, rhabdoviruses, arenaviruses, herpesviruses, hepadnaviruses, and orthopoxviruses (reviewed in [1]). This broad antiviral activity of γδ T cells is likely to play a crucial defensive role, especially considering that their relatively large numbers (e.g., approximately one out of every 30 adult human peripheral blood lymphocytes is a Vγ9Vδ2 T lymphocyte) can respond very quickly (typically, no antigen processing is required for the potent major histocompatibility complex (MHC)-unrestricted activities of Vγ9Vδ2 T cells) and release soluble antiviral factors.
2. Pharmacological stimulation of γδ T cells
Many natural ligands recognized by human Vγ9Vδ2 T cells are known. Probably the most important representatives of this group are intermediates of isoprenoid biosynthesis [2] (e.g., 3-formyl-1-butyl-pyrophosphate and isopentenyl-pyrophosphate) and were first isolated from mycobacteria [3], [4], [5]. Other natural and synthetic phospho-ligands [6], [7], alkylamines [8] and aminobiphosphonates [9] also stimulate Vγ9Vδ2 T cells. Structure-function studies of Vγ9Vδ2-specific ligands suggest that some of these molecules could be readily docked into a putative binding site of the Vγ9Vδ2 TCR [10], [11]. Functionally, mature Vγ9Vδ2 T cells express cell-surface inhibitory receptors for MHC class I molecules (INMRs) that may control TCR-mediated reactivities in the antiviral response [12], [13].
Phosphostim (phosphobromohydrin) is the first drug that was designed to stimulate selectively Vγ9Vδ2 T cells. Currently, this new drug is in Phase I/Phase II clinical trials in cancer patients. Nitrogen-containing bisphosphonates (N-BPs) have been used to prevent bone demineralization in patients with osteoporosis, multiple myeloma, and certain metastatic cancers (e.g., breast and prostate). Recently, N-BPs have been shown to induce activation of Vγ9Vδ2 T cells accompanied by augmented: (a) cytotoxic activities; (b) cytokine/β-chemokine production; and (c) DNA synthetic responses in Vγ9Vδ2 T cells. These unexpected activities of N-BPs have opened new possibilities of therapeutic usage for these drugs.
The mechanism of N-BP action appears to include the inhibition of farnesyl pyrophosphate synthase activity (as one of the key enzymes in the mevalonate pathway, farnesyl pyrophosphate synthase catalyzes the sequential head-to-tail condensation of two molecules of isopentenyl pyrophosphate with dimethylallyl pyrophosphate). This leads to an accumulation of isopentenyl pyrophosphate, an essential metabolite that is directly recognized by Vγ9Vδ2 T cells [14]. Interestingly, the mevalonate pathway is critical for protein prenylation, which may be important for viral assembly (Fig. 1 ). In hepatoma cells exposed to lovastatin (an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, the enzyme that catalyzes the conversion of HMG-CoA to mevalonate), hepatitic C virus (HCV) RNA replication is impaired due to the dissolution of the HCV replication complex [15]. The lovastatin treatment can also block respiratory syncytial virus (RSV) replication and cell-to-cell fusion in vivo and in vitro by inhibiting the isoprenylation of the cellular protein RhoA [16].
Fig. 1.
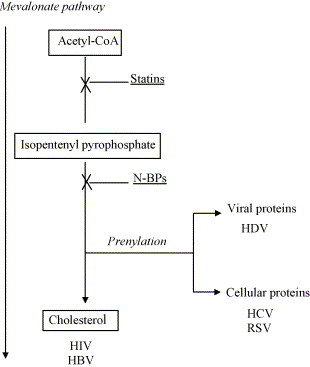
The role of the mevalonate (MVA) pathway in viral assembly. The MVA pathway is critical for protein prenylation and cholesterol biosynthesis. Therefore, the MVA pathway may be important for viral assembly of particles requiring: (a) the prenylation of viral (HDV) or cellular (HCV, RSV) proteins; or (b) the synthesis of cholesterol (HIV, HBV) that is necessary for membrane budding. Blocking this pathway by statins or N-BPs interferes with both prenylation and cholesterol synthesis. However, the ‘pre-IPP’ statin inhibition decreases, whereas the ‘post-IPP’ N-BP block increases the accumulation of IPP, a potent endogenous stimulator of Vγ9Vδ2 T lymphocytes.
Similarly, BZA-5B (an inhibitor of protein prenylation) blocks the production of hepatitis delta virus (HDV) particles in vitro in a dose dependent manner [17]. In particular, the inhibition of large delta antigen prenylation mediated by BZA-5B interferes with the assembly of HDV virions. In addition, the increased concentration of large delta antigen within infected cells may act as a potent trans dominant inhibitor of HDV replication [18].
The block of mevalonate pathway by N-BPs also results in a decreased synthesis of cholesterol. It has been reported that cholesterol is critical for HIV passage through cell membranes and that the ability of Nef protein to increase viral infectivity depends on cholesterol [19]. Specifically, Nef is involved in transporting newly synthesized cholesterol to the site of viral budding and promotes the incorporation of cholesterol into viral particles. These data suggest that an efficient cholesterol synthesis in HIV-infected cells is important for the production of infectious virions [20]. Altogether, these results open a new possibility of using the inhibitors of mevalonate pathway as double-edge swords—that is as antivirals interfering with virion production as well as stimulators of Vγ9Vδ2 T cell cytotoxic and other antiviral effects.
3. Non-cytolytic antiviral immunity mediated by Vγ9Vδ2 T cells
Immune responses to viral infections comprise both cytolytic (i.e., cytotoxicity against virus-infected cells) and non-cytolytic activities of Vγ9Vδ2 T cells. The innate non-cytolytic activity encompasses the production and release of several soluble molecules. As shown in Fig. 2 , peripheral blood mononuclear cells (PBMCs) stimulated with isopentenyl pyrophosphate (IPP, a classical non-peptidic antigen for Vγ9Vδ2 T cells) release an array of cytokines (e.g., IFN-γ, TNF-α, IL-1α, IL-6, GM-CSF, TPO, OSM) and chemokines (e.g., MIP-1α/β, RANTES, SDF-1, MCP-2 MDC, ENA-78 and GRO) with known antiviral and/or immunomodulatory properties. After stimulation and in sharp contrast to many non-γδ TCR-expressing lymphoid cells, single Vγ9Vδ2 T lymphocytes produce more than one of these soluble antiviral/immunoregulatory factors (Fig. 3 ). The innate response is followed by humoral and cellular adaptive immune responses influenced by many of these soluble molecules. In addition, the non-cytolytic antiviral molecules continue to suppress the infectious process in vital organs without destroying important cells and also can boost the antiviral potency of classical αβ cytotoxic T lymphocytes (CTLs) [21].
Fig. 2.
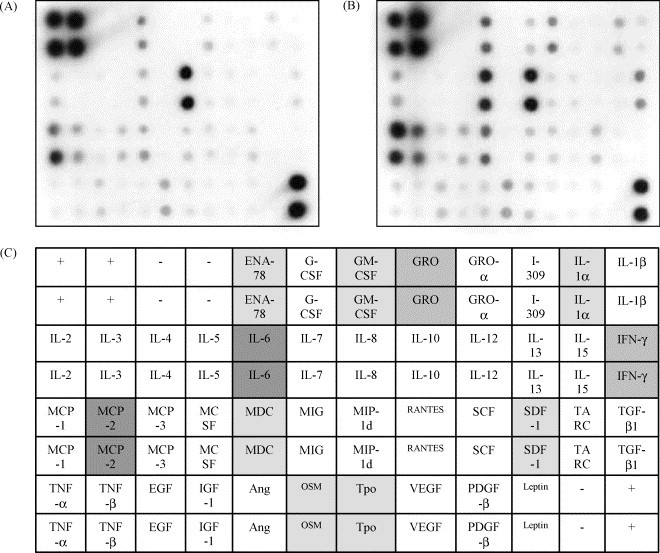
Soluble factors produced by activated Vγ9Vδ2 T cells. PBMC from healthy donors were stimulated for 24 h with IL-2 alone (panel A) or with IL-2 in the presence of IPP (panel B). The production of different soluble molecules was analyzed by TransSignal Human Cytokine Antibody Arrays following the manufacturer instructions (Panomics, Redwood City, CA). The analysis was performed in duplicate and panel C shows the experimental layout. The signal intensity is proportionate to the soluble factor production.
Fig. 3.
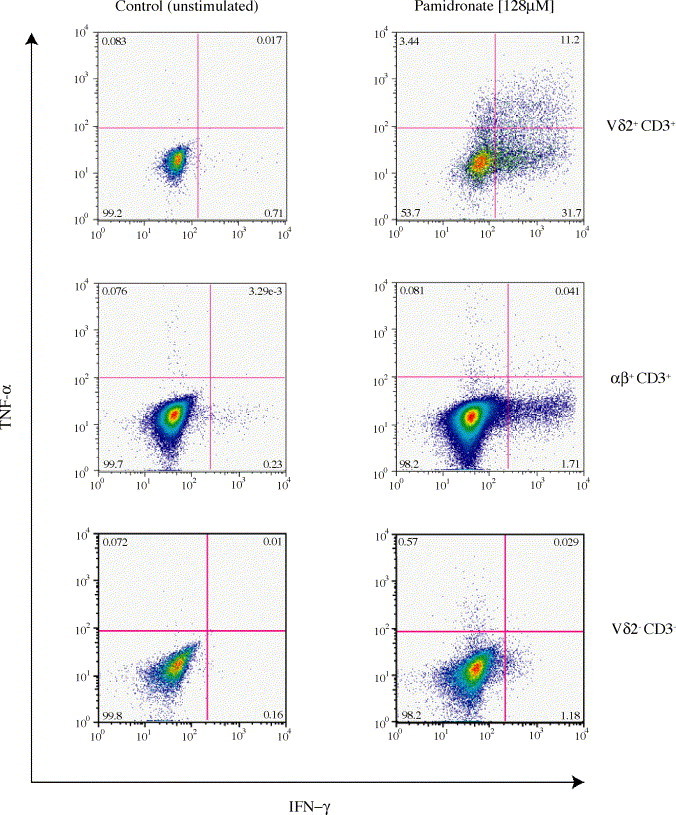
In contrast to αβ T lymphocytes or CD3-negative cells, a substantial proportion of activated Vδ2 T lymphocytes produce multiple cytokines. Human PBMCs were incubated (24 h) in culture medium with of 128 μM sodium pamidronate and the IFN-γ and TNF-α intracellular presence was assessed by flow cytometry. During the last 4 h of incubation, brefeldin A (10 μg/ml) was present to block the cytokine release from cells. Polyclonal stimulation with concanavalin A (not shown) generated a very similar pattern—that is large numbers of Vδ2+ CD3+ double producers (in contrast to predominant single producers in the αβ + CD3+ and Vδ2- CD3-pools) with the exception of substantially larger amounts of single-TNF-α-positive αβ T cells. The cytokine production by the αβ+ CD3+ or Vδ2- CD3-cells in pamidronate-stimulated cultures was inhibited by anti-IFN-γ mAbs suggesting that IFN-γ released by pamidronate-stimulated Vγ9Vδ2 T cells may be an important factor in the induction of cytokine synthesis by the tested non-Vγ9Vδ2 T cell pools.
The main factor with known antiviral activities released by γδ T cells is IFN-γ—a key molecule in recruiting and activating killer T cells, NK cells and macrophages, and triggering intracellular pathways that suppress viral replication without direct cytolytic effects on host cells [22], [23]. In vitro, the non-cytolytic antiviral activity of interferons has been demonstrated in infections with HCV, hepatitis B virus (HBV), herpesviruses, orthopoxviruses, picornaviruses, retroviruses, influenza and other type of viruses (reviewed in [21], [24]). In hepatitis B-transgenic mice, IFN-γ released by CD8 T cells is able to diminish the HBV gene expression and replication and activates hepatocytes to clear the virus through a non-cytolytic pathway [25]. In the acute phase of HCV infection, the ability of T cells to produce IFN-γ is associated with virus clearance [26]. Recently, it has been shown that NS3 peptide-stimulated CD8 T cells release IFN-γ and reduce HCV RNA replication activity [27]. Generally, in chimpanzees (Pan troglodytes), the appearance of CD8-positive CTLs is better correlated with protection against HCV than the antibody response [28]. This is compatible with the idea that the production of IFN-γ by CD8-positive T-lymphocytes may contribute to the resolution of the infection [28]. Despite the potent interferon effects, the majority of patients become persistently infected with HCV. This chronic infection is associated with a drastic impairment of IFN-γ production by T cells [29] and a hepatic expansion of HCV-specific CD8-positive T lymphocytes with a regulatory phenotype [30]. Thus, the induction of IFN-γ production by Vγ9Vδ2 T lymphocytes could represent a new strategy to inhibit viral replication and to support the Th1-type immune response.
Interleukin-1α (IL-1α) produced by activated γδ T cells can have either stimulatory or inhibitory effects on HIV infection [31], [32]. Also, it has been shown that the cytomegalovirus (CMV) infection is reduced in marrow stromal cells that either secrete IL-1 or are treated with exogenous IL-1 [33]. Stimulated γδ T cells also produce interleukin-6 (IL-6), a potent lymphoid cell growth factor, which affects B-lymphocytes, T-lymphocytes or hybridoma cells, and may influence cytotoxic T cells in combination with other factors such as IL-2 and IFN-γ. IL-6 production by skin fibroblasts that support the replication of dengue virus and other flaviviruses in vivo may be an important factor in controlling flavivirus infections [34]. Moreover, IL-6 was shown to strengthen the retinoic acid-mediated suppression of HIV-1 replication in macrophages [32].
The granulocyte-macrophage colony-stimulating factor (GM-CSF) produced by activated γδ T cells is a potent species-specific stimulator of precursors of granulocytes, macrophages and eosinophils. Antiviral effects of GM-CSF have been reported in dengue infections [34]. GM-CSF can boost antiviral humoral immunity to influenza and simian immunodeficiency virus (SIV) [35], increase humoral and cellular immune responses against herpes simplex virus-2 (HSV-2) [36] and improve protection against Epstein–Barr virus (EBV)-induced lymphoproliferative disorders [37]. Also thrombopoietin (stimulates the proliferation and maturation of megakaryocytes) and oncostatin M (growth regulating cytokine) are secreted by activated γδ T cells, but their effect on viral replication remains unclear.
Chemokines, a superfamily of proinflammatory cytokines, are released by many activated γδ T cells. Chemokines act primarily as chemoattractants and activators of specific types of leukocytes. Some chemokines (such as the β-chemokines MIP-1α, MIP-1β and RANTES) influence directly the rate of HIV replication [38]. The stromal-derived factor (SDF-1) that blocks the entry of CXCR4-dependent (T cell-tropic) HIV-1 isolates [39] has been identified as a ligand for LESTR/fusin [40]. RANTES and MCP-3 inhibit T cell-tropic HIV-1 isolates [41] and the macrophage chemotactic protein (MDC, macrophage-derived chemokine) can also block HIV-1 entry [42]. Other CD8 antiviral factors (e.g., CAF) inhibit HIV-1 transcription [43]. The post-integration mode of action makes CAF distinct from β-chemokines and similar to IL-16 [44]. Importantly, antigen-stimulated Vγ9Vδ2 T cells produce the HIV-inhibitory β-chemokines (MIP-1α, MIP-1β, and RANTES) [45], [46] that block both CCR5 co-receptor-dependent (e.g., HIV-1BAL) and CXCR4 co-receptor-dependent (e.g., HIV-1LAI) viral isolates [45]. However, it is likely that additional, not molecularly defined antiviral activities of γδ T cells contribute to the observed antiviral effects.
Antigen-stimulated Vγ9Vδ2 T cells rapidly establish chemotactic gradients that may shape the host inflammatory response against various pathogens including HIV. The activation of Vγ9Vδ2 T cells with IPP induces (within 4–12 h) the production of MIP-1α, MIP-1β, and lymphotactin, but not macrophage chemoattractant protein-1 (MCP-1) [46]. The induction of MIP-1α and MIP-1β is unaffected by IL-4, IL-10, or INF-γ, but IPP plus IL-12-induced release of MIP-1α, but not MIP-1β, is blocked by TGF-β. Vγ9Vδ2 T cells also express many β-chemokine receptors including CCR1, CCR5, and CCR8 that can be downregulated by activation stimuli. Activating signals for γδ T cells also induce the production of macrophage chemoattractant protein-2 (MCP-2) and MDC. MCP-2 plays an important role in the inflammatory response of blood monocytes and tissue macrophages and inhibits the replication of HIV-1 via CCR5 [47]. MDC is chemotactic for monocytes, dendritic cells, activated lymphocytes, and NK cells and exhibits anti-HIV-1 activity [48]. Cumulatively, the high levels of proinflammatory cytokines and chemokines with antiviral properties produced by activated Vγ9Vδ2 T cells may influence the progression of HIV disease.
Other interesting IPP-induced molecules include the epithelial neutrophil activating peptide-78 (ENA-78) that is a chemotactic and activating factor for neutrophils, and human GRO (MGSA) that promotes neutrophil chemotaxis and degranulation. Through these molecules, γδ T cells may influence the behavior of granulocytes—that is the cells that are likely to release of α-defensins and other non-cytolytic antiviral factors. α-defensins are a family of antimicrobial peptides that have been identified in animals (including human and non-human primates) and plants. They play an important role in infections, inflammation, wound repair, and acquired immunity. Based on the disulfide pairing of their characteristic six cysteine residues, they are divided into α-defensins and β-defensins. α-defensins are mainly released by granulocytes and can inhibit HIV infection in vitro [49]. In addition to their anti-HIV activities, α-defensins interfere with the replication of HSV-1 and HSV-2, CMV, vesicular stomatitis virus (VSV), and influenza virus [50]. The expression of α-defensins in γδ T cells has been described [51], but further studies are required to explore the contribution of γδ T cells to the production and action of defensins during viral infections.
Another proinflammatory factor released by activated γδ T cells is TNF-α, a potent lymphoid factor with (unlike the molecules described above) cytotoxic effects on a wide range of target cells. Beneficial effects of TNF-α in cellular immunity to VSV [52], CMV [53], HSV-1 [54], and vaccinia virus [55] have been noted. TNF-α was shown to enhance HIV-1 replication in chronically infected promonocytic and T-lymphoid cell lines by activation of the nuclear factor NF-κB, which stimulates the long terminal repeat (LTR) of the provirus [31]. In contrast, IFN-γ (an important enhancer of TNF-α production by macrophages) inhibits the HIV-1 growth in primary macrophages [56]. While TNF-α has a protective role in activated CD4+ T cells against R5-tropic viruses, it enhances CXCR4 expression of CD4+ T cells and mediates an increased susceptibility to infection with X4-tropic HIV and SIV strains. Therefore, the role of TNF-α in HIV infections is somewhat intricate, and further studies are needed to clarify its effects.
4. Conclusions
Novel synthetic drugs, N-BPs and Phosphostim that can activate Vγ9Vδ2 T cells have been developed recently as well as effective in vivo γδ-stimulatory protocols [57]. Some of these molecules are able not only to activate Vγ9Vδ2 T cells, but also can inhibit protein prenylation or cholesterol biosynthesis that may be required for viral assembly. Thus, N-BPs/Phosphostim-based therapeutic strategies should be considered to enhance the current inadequate armaments for fighting emerging and re-emerging viruses.
References
- 1.Poccia F., Agrati C., Martini F., Capobianchi M.R., Wallace M., Malkovsky M. Antiviral reactivities of γδ T cells. Microbes Infect. 2005;7:518–528. doi: 10.1016/j.micinf.2004.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altincicek B., Moll J., Campos N., Foerster G., Beck E., Hoeffler J.F., Grosdemange-Billiard C., Rodriguez-Concepcion M., Rohmer M., Boronat A., Eberl M., Jomaa H. Cutting edge: human γδ T cells are activated by intermediates of the 2-C-methyl-d-erythritol 4-phosphate pathway of isoprenoid biosynthesis. J Immunol. 2001;166:3655–3658. doi: 10.4049/jimmunol.166.6.3655. [DOI] [PubMed] [Google Scholar]
- 3.Belmant C., Espinosa E., Poupot R., Peyrat M.A., Guiraud M., Poquet Y., Bonneville M., Fournie J.J. 3-Formyl-1-butyl pyrophosphate A novel mycobacterial metabolite-activating human γδ T cells. J Biol Chem. 1999;274:32079–32084. doi: 10.1074/jbc.274.45.32079. [DOI] [PubMed] [Google Scholar]
- 4.Constant P., Davodeau F., Peyrat M.A., Poquet Y., Puzo G., Bonneville M., Fournie J.J. Stimulation of human γδ T cells by non-peptidic mycobacterial ligands. Science. 1994;264:267–270. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka Y., Morita C.T., Tanaka Y., Nieves E., Brenner M.B., Bloom B.R. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature. 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 6.Belmant C., Espinosa E., Halary F., Tang Y., Peyrat M.A., Sicard H., Kozikowski A., Buelow R., Poupot R., Bonneville M., Fournie J.J. A chemical basis for selective recognition of non-peptide antigens by human γδ T cells. FASEB J. 2000;14:1669–1670. doi: 10.1096/fj.99-0909fje. [DOI] [PubMed] [Google Scholar]
- 7.Burk M.R., Mori L., De Libero G. Human Vγ9Vδ2 cells are stimulated in a cross-reactive fashion by a variety of phosphorylated metabolites. Eur J Immunol. 1995;25:2052–2058. doi: 10.1002/eji.1830250737. [DOI] [PubMed] [Google Scholar]
- 8.Bukowski J.F., Morita C.T., Brenner M.B. Human γδ T cells recognize alkylamines derived from microbes, edible plants, and tea: implications for innate immunity. Immunity. 1999;11:57–65. doi: 10.1016/s1074-7613(00)80081-3. [DOI] [PubMed] [Google Scholar]
- 9.Kunzmann V., Bauer E., Wilhelm M. Gamma/delta T-cell stimulation by pamidronate. N Engl J Med. 1999;340:737–738. doi: 10.1056/NEJM199903043400914. [DOI] [PubMed] [Google Scholar]
- 10.Allison T.J., Winter C.C., Fournie J.J., Bonneville M., Garboczi D.N. Structure of a human γδ T-cell antigen receptor. Nature. 2001;411:820–824. doi: 10.1038/35081115. [DOI] [PubMed] [Google Scholar]
- 11.Gossman W., Oldfield E. Quantitative structure—activity relations for γδ T cell activation by phosphoantigens. J Med Chem. 2002;45:4868–4874. doi: 10.1021/jm020224n. [DOI] [PubMed] [Google Scholar]
- 12.Poccia F., Cipriani B., Vendetti S., Colizzi V., Poquet Y., Battistini L., Lopez-Botet M., Fournie J.J., Gougeon M.L. CD94/NKG2 inhibitory receptor complex modulates both anti-viral and anti-tumoral responses of polyclonal phosphoantigen-reactive Vγ9Vδ2 T lymphocytes. J Immunol. 1997;159:6009–6017. [PubMed] [Google Scholar]
- 13.Poccia F., Gougeon M.L., Bonneville M., Lopez-Botet M., Moretta A., Battistini L., Wallace M., Colizzi V., Malkovsky M. Innate T-cell immunity to non-peptidic antigens. Immunol Today. 1998;19:253–256. doi: 10.1016/s0167-5699(98)01266-3. [DOI] [PubMed] [Google Scholar]
- 14.Gober H.J., Kistowska M., Angman L., Jeno P., Mori L., De Libero G. Human T cell receptor γδ cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–168. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye J., Wang C., Sumpter R., Jr., Brown M.S., Goldstein J.L., Gale M., Jr. Disruption of hepatitis C virus RNA replication through inhibition of host protein geranylgeranylation. Proc Natl Acad Sci USA. 2003;100:15865–15870. doi: 10.1073/pnas.2237238100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gower T.L., Graham B.S. Antiviral activity of lovastatin against respiratory syncytial virus in vivo and in vitro. Antimicrob Agents Chemother. 2001;45:1231–1237. doi: 10.1128/AAC.45.4.1231-1237.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glenn J.S., Marsters J.C., Jr., Greenberg H.B. Use of a prenylation inhibitor as a novel antiviral agent. J Virol. 1998;72:9303–9306. doi: 10.1128/jvi.72.11.9303-9306.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glenn J.S., White J.M. Trans-dominant inhibition of human hepatitis delta virus genome replication. J Virol. 1991;65:2357–2361. doi: 10.1128/jvi.65.5.2357-2361.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng Y.H., Plemenitas A., Linnemann T., Fackler O.T., Peterlin B.M. Nef increases infectivity of HIV via lipid rafts. Curr Biol. 2001;11:875–879. doi: 10.1016/s0960-9822(01)00237-8. [DOI] [PubMed] [Google Scholar]
- 20.Zheng Y.H., Plemenitas A., Fielding C.J., Peterlin B.M. Nef increases the synthesis of and transports cholesterol to lipid rafts and HIV-1 progeny virions. Proc Natl Acad Sci USA. 2003;100:8460–8465. doi: 10.1073/pnas.1437453100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeVico A.L., Gallo R.C. Control of HIV-1 infection by soluble factors of the immune response. Nat Rev Microbiol. 2004;2:401–413. doi: 10.1038/nrmicro878. [DOI] [PubMed] [Google Scholar]
- 22.Patterson J.B., Thomis D.C., Hans S.L., Samuel C.E. Mechanism of interferon action: double-stranded RNA-specific adenosine deaminase from human cells is inducible by α and γ interferons. Virology. 1995;210:508–511. doi: 10.1006/viro.1995.1370. [DOI] [PubMed] [Google Scholar]
- 23.Bovolenta C., Lorini A.L., Mantelli B., Camorali L., Novelli F., Biswas P., Poli G. A selective defect of IFN-γ-but not of IFN-α-induced JAK/STAT pathway in a subset of U937 clones prevents the antiretroviral effect of IFN-γ against HIV-1. J Immunol. 1999;162:323–330. [PubMed] [Google Scholar]
- 24.Capobianchi M.R., Abbate I., Cappiello G., Solmone M. HCV and interferon: viral strategies for evading innate defence mechanisms in the virus-host battle. Cell Death Differ. 2003;10(Suppl. 1):S22–S24. doi: 10.1038/sj.cdd.4401142. [DOI] [PubMed] [Google Scholar]
- 25.Guidotti L.G., Rochford R., Chung J., Shapiro M., Purcell R., Chisari F.V. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284:825–829. doi: 10.1126/science.284.5415.825. [DOI] [PubMed] [Google Scholar]
- 26.Lechner F., Wong D.K., Dunbar P.R., Chapman R., Chung R.T., Dohrenwend P., Robbins G., Phillips R., Klenerman P., Walker B.D. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–1512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu C., Zhu H., Tu Z., Xu Y.L., Nelson D.R. CD8+ T-cell interaction with HCV replicon cells: evidence for both cytokine- and cell-mediated antiviral activity. Hepatology. 2003;37:1335–1342. doi: 10.1053/jhep.2003.50207. [DOI] [PubMed] [Google Scholar]
- 28.Cooper S., Erickson A.L., Adams E.J., Kansopon J., Weiner A.J., Chien D.Y., Houghton M., Parham P., Walker C.M. Analysis of a successful immune response against hepatitis C virus. Immunity. 1999;10:439–449. doi: 10.1016/s1074-7613(00)80044-8. [DOI] [PubMed] [Google Scholar]
- 29.Francavilla V., Accapezzato D., De Salvo M., Rawson P., Cosimi O., Lipp M., Cerino A., Cividini A., Mondelli M.U., Barnaba V. Subversion of effector CD8+ T cell differentiation in acute hepatitis C virus infection: exploring the immunological mechanisms. Eur J Immunol. 2004;34:427–437. doi: 10.1002/eji.200324539. [DOI] [PubMed] [Google Scholar]
- 30.Accapezzato D., Francavilla V., Rawson P., Cerino A., Cividini A., Mondelli M.U., Barnaba V. Subversion of effector CD8+ T cell differentiation in acute hepatitis C virus infection: the role of the virus. Eur J Immunol. 2004;34:438–446. doi: 10.1002/eji.200324540. [DOI] [PubMed] [Google Scholar]
- 31.Osborn L., Kunkel S., Nabel G.J. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci USA. 1989;86:2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown X.Q., Hanley T.M., Viglianti G.A. Interleukin-1β and interleukin-6 potentiate retinoic acid-mediated repression of human immunodeficiency virus type 1 replication in macrophages. AIDS Res Hum Retroviruses. 2002;18:649–656. doi: 10.1089/088922202760019347. [DOI] [PubMed] [Google Scholar]
- 33.Randolph-Habecker J., Iwata M., Geballe A.P., Jarrahian S., Torok-Storb B. Interleukin-1-mediated inhibition of cytomegalovirus replication is due to increased IFN-β production. J Interferon Cytokine Res. 2002;22:765–772. doi: 10.1089/107999002320271350. [DOI] [PubMed] [Google Scholar]
- 34.Kurane I., Janus J., Ennis F.A. Dengue virus infection of human skin fibroblasts in vitro production of IFN-β, IL-6 and GM-CSF. Arch Virol. 1992;124:21–30. doi: 10.1007/BF01314622. [DOI] [PubMed] [Google Scholar]
- 35.Lena P., Villinger F., Giavedoni L., Miller C.J., Rhodes G., Luciw P. Co-immunization of rhesus macaques with plasmid vectors expressing IFN-γ, GM-CSF, and SIV antigens enhances anti-viral humoral immunity but does not affect viremia after challenge with highly pathogenic virus. Vaccine. 2002;20(Suppl. 4):A69–A79. doi: 10.1016/s0264-410x(02)00391-2. [DOI] [PubMed] [Google Scholar]
- 36.Sin J.I., Kim J.J., Ugen K.E., Ciccarelli R.B., Higgins T.J., Weiner D.B. Enhancement of protective humoral (Th2) and cell-mediated (Th1) immune responses against herpes simplex virus-2 through co-delivery of granulocyte-macrophage colony-stimulating factor expression cassettes. Eur J Immunol. 1998;28:3530–3540. doi: 10.1002/(SICI)1521-4141(199811)28:11<3530::AID-IMMU3530>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 37.Baiocchi R.A., Ward J.S., Carrodeguas L., Eisenbeis C.F., Peng R., Roychowdhury S., Vourganti S., Sekula T., O’Brien M., Moeschberger M., Caligiuri M.A. GM-CSF and IL-2 induce specific cellular immunity and provide protection against Epstein–Barr virus lymphoproliferative disorder. J Clin Invest. 2001;108:887–894. doi: 10.1172/JCI12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cocchi F., DeVico A.L., Garzino-Demo A., Arya S.K., Gallo R.C., Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 39.Oberlin E., Amara A., Bachelerie F., Bessia C., Virelizier J.L., Arenzana-Seisdedos F., Schwartz O., Heard J.M., Clark-Lewis I., Legler D.F., Loetscher M., Baggiolini M., Moser B. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 40.Bleul C.C., Farzan M., Choe H., Parolin C., Clark-Lewis I., Sodroski J., Springer T.A. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 41.Schols D., Proost P., Van Damme J., De Clercq E. RANTES and MCP-3 inhibit the replication of T-cell-tropic human immunodeficiency virus type 1 strains (SF-2, MN, and HE) J Virol. 1997;71:7300–7304. doi: 10.1128/jvi.71.10.7300-7304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cota M., Mengozzi M., Vicenzi E., Panina-Bordignon P., Sinigaglia F., Transidico P., Sozzani S., Mantovani A., Poli G. Selective inhibition of HIV replication in primary macrophages but not T lymphocytes by macrophage-derived chemokine. Proc Natl Acad Sci USA. 2000;97:9162–9167. doi: 10.1073/pnas.160359197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mackewicz C.E., Patterson B.K., Lee S.A., Levy J.A. CD8(+) cell non-cytotoxic anti-human immunodeficiency virus response inhibits expression of viral RNA but not reverse transcription or provirus integration. J Gen Virol. 2000;81:1261–1264. doi: 10.1099/0022-1317-81-5-1261. [DOI] [PubMed] [Google Scholar]
- 44.Mackewicz C.E., Barker E., Levy J.A. Role of β-chemokines in suppressing HIV replication. Science. 1996;274:1393–1395. doi: 10.1126/science.274.5291.1393. [DOI] [PubMed] [Google Scholar]
- 45.Poccia F., Battistini L., Cipriani B., Mancino G., Martini F., Gougeon M.L., Colizzi V. Phosphoantigen-reactive Vγ9Vδ2 T lymphocytes suppress in vitro human immunodeficiency virus type 1 replication by cell-released antiviral factors including CC chemokines. J Infect Dis. 1999;180:858–861. doi: 10.1086/314925. [DOI] [PubMed] [Google Scholar]
- 46.Cipriani B., Borsellino G., Poccia F., Placido R., Tramonti D., Bach S., Battistini L., Brosnan C.F. Activation of C–C beta-chemokines in human peripheral blood γδ T cells by isopentenyl pyrophosphate and regulation by cytokines. Blood. 2000;95:39–47. [PubMed] [Google Scholar]
- 47.Yang O.O., Garcia-Zepeda E.A., Walker B.D., Luster A.D. Monocyte chemoattractant protein-2 (CC chemokine ligand 8) inhibits replication of human immunodeficiency virus type 1 via CC chemokine receptor 5. J Infect Dis. 2002;185:1174–1178. doi: 10.1086/339678. [DOI] [PubMed] [Google Scholar]
- 48.Struyf S., Proost P., Sozzani S., Mantovani A., Wuyts A., De Clercq E., Schols D., Van Damme J. Enhanced anti-HIV-1 activity and altered chemotactic potency of NH2-terminally processed macrophage-derived chemokine (MDC) imply an additional MDC receptor. J Immunol. 1998;161:2672–2675. [PubMed] [Google Scholar]
- 49.Nakashima H., Yamamoto N., Masuda M., Fujii N. Defensins inhibit HIV replication in vitro. AIDS. 1993;7:1129. doi: 10.1097/00002030-199308000-00019. [DOI] [PubMed] [Google Scholar]
- 50.Daher K.A., Selsted M.E., Lehrer R.I. Direct inactivation of viruses by human granulocyte defensins. J Virol. 1986;60:1068–1074. doi: 10.1128/jvi.60.3.1068-1074.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agerberth B., Charo J., Werr J., Olsson B., Idali F., Lindbom L., Kiessling R., Jornvall H., Wigzell H., Gudmundsson G.H. The human antimicrobial and chemotactic peptides LL-37 and α-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–3093. [PubMed] [Google Scholar]
- 52.Wong G.H., Goeddel D.V. Tumour necrosis factors a and b inhibit virus replication and synergize with interferons. Nature. 1986;323:819–822. doi: 10.1038/323819a0. [DOI] [PubMed] [Google Scholar]
- 53.Pavic I., Polic B., Crnkovic I., Lucin P., Jonjic S., Koszinowski U.H. Participation of endogenous tumour necrosis factor a in host resistance to cytomegalovirus infection. J Gen Virol. 1993;74(Pt 10):2215–2223. doi: 10.1099/0022-1317-74-10-2215. [DOI] [PubMed] [Google Scholar]
- 54.Rossol-Voth R., Rossol S., Schutt K.H., Corridori S., de Cian W., Falke D. In vivo protective effect of tumour necrosis factor α against experimental infection with herpes simplex virus type 1. J Gen Virol. 1991;72(Pt 1):143–147. doi: 10.1099/0022-1317-72-1-143. [DOI] [PubMed] [Google Scholar]
- 55.Sambhi S.K., Kohonen-Corish M.R., Ramshaw I.A. Local production of tumor necrosis factor encoded by recombinant vaccinia virus is effective in controlling viral replication in vivo. Proc Natl Acad Sci USA. 1991;88:4025–4029. doi: 10.1073/pnas.88.9.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meylan P.R., Spina C.A., Richman D.D., Kornbluth R.S. In vitro differentiation of monocytoid THP-1 cells affects their permissiveness for HIV strains: a model system for studying the cellular basis of HIV differential tropism. Virology. 1993;193:256–267. doi: 10.1006/viro.1993.1121. [DOI] [PubMed] [Google Scholar]
- 57.Casetti R., Perretta G., Taglioni A., Mattei M., Colizzi V., Dieli F., D’Offizi G., Malkovsky M., Poccia F. Drug-induced expansion and differentiation of Vγ9Vδ2 T cells in vivo: the role of exogenous interleukin-2. J Immunol. 2005;175:1593–1598. doi: 10.4049/jimmunol.175.3.1593. [DOI] [PubMed] [Google Scholar]


