Abstract
Viral respiratory infections are the most common cause of an acute asthma exacerbation in both children and adults and represent a significant global health burden. An increasing body of evidence supports the hypothesis that these infections cause a greater degree of morbidity in asthmatic subjects than in the healthy population, emphasizing a discrepancy in the antiviral response of asthmatics. In this review we discuss why such a discrepancy might exist, examining the role of the bronchial epithelium as well as the main inflammatory cells, mediators, and molecular pathways that are involved in the immune response. In addition, the potential impact of virus-induced asthma exacerbations on airway remodelling is reviewed and we explore which therapeutic options might be of benefit in preventing the deterioration of asthma control seen following viral infection.
Key words: Asthma, acute exacerbation, virus
Abbreviations used: BAL, Bronchoalveolar lavage; BEC, Bronchial epithelial cell; FGF, Fibroblast growth factor; HRV, Human rhinovirus; ICAM-1, Intercellular adhesion molecule 1; IP-10, Interferon-inducible protein 10; IRF, Interferon regulatory factor; NF-κB, Nuclear factor kappa B; PRR, Pattern-recognition receptor; SOCS1, Suppressor of cytokine signaling 1; TLR, Toll-like receptor; VEGF, Vascular endothelial growth factor
Information for Category 1 CME Credit
Credit can now be obtained, free for a limited time, by reading the review articles in this issue. Please note the following instructions.
Method of Physician Participation in Learning Process: The core material for these activities can be read in this issue of the Journal or online at the JACI Web site: www.jacionline.org. The accompanying tests may only be submitted online at www.jacionline.org. Fax or other copies will not be accepted.
Date of Original Release: June 2010. Credit may be obtained for these courses until May 31, 2012.
Copyright Statement: Copyright © 2010-2012. All rights reserved.
Overall Purpose/Goal: To provide excellent reviews on key aspects of allergic disease to those who research, treat, or manage allergic disease.
Target Audience: Physicians and researchers within the field of allergic disease.
Accreditation/Provider Statements and Credit Designation: The American Academy of Allergy, Asthma & Immunology (AAAAI) is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians. The AAAAI designates these educational activities for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
List of Design Committee Members: Authors: David J. Jackson, MD, and Sebastian L. Johnston, MD
Activity Objectives
-
1.
To understand the importance of acute respiratory tract viral infections as triggers of asthma exacerbations.
-
2.
To understand the mechanisms of airway inflammatory response to respiratory tract viral infections in patients with asthma.
-
3.
To recognize potential therapeutic approaches for asthma exacerbations.
Recognition of Commercial Support: This CME activity has not received external commercial support.
Disclosure of Significant Relationships with Relevant Commercial
Companies/Organizations: D. Jackson has declared that he has no conflicts of interest. S. L. Johnston has consultant arrangements with AstraZeneca, Centocor, Sanofi-Pasteur, Synairgen, GlaxoSmithKline, anc Chiesi; has a share option with Synairgen; receives research support from Sanofi-Pasteur, the Medical Research Council, the University of Leicester, the British Medical Association, Asthma UK, Imperial College Healthcare NHS Trust, EU ERC MoRIAE, Centocor Inc, and the National Institute for Health Research.
Viral respiratory tract infections are one of the most common illnesses in human subjects, with 500 million cases and an economic burden estimated at $40 billion annually in the United States alone.1 Since the early 1970s, viral respiratory tract infections have been reported as triggers for exacerbations of asthma in both adults and children.2, 3 The development of highly sensitive and specific molecular diagnostic and detection techniques in the 1990s led to greatly improved detection of respiratory tract viruses and allowed clear demonstration of the important link between viral infections and asthma exacerbations. When RT-PCR is used to supplement or instead of conventional culture techniques, viruses have been found in approximately 80% of wheezing episodes in school-aged children and in approximately one half to three quarters of the acute wheezing episodes in adults. Of the respiratory tract viruses identified in these circumstances, rhinoviruses are most commonly found and are detected approximately 65% of the time.4, 5, 6, 7
In addition to rhinoviruses, other respiratory tract viruses, such as respiratory syncytial virus (RSV), influenza viruses, coronaviruses, human metapneumoviruses, parainfluenza viruses, adenoviruses, and bocaviruses, have all been detected in subjects with asthma exacerbations. However, in a recent epidemiologic study performed after the discovery of several newer viruses, such as bocavirus, the only virus type significantly associated with asthma exacerbations in children aged 2 to 17 years were rhinoviruses.8
Understanding the mechanisms provoking virus-induced airway inflammation in asthmatic subjects might offer significant opportunities for improved disease management. The reality at present is that current drugs for the treatment of virus-induced exacerbations of asthma are poorly effective, and alternative therapies to modulate viral pathogenesis are desperately needed. Experimental human9 and murine10 models of rhinovirus-induced asthma exacerbations have recently been developed, and these offer great potential in increasing our understanding, with the goal of arriving at novel therapies in the future. In a recent study of this kind, experimental infection of both asthmatic and healthy volunteers with rhinovirus led to increased lower respiratory tract symptoms typical of a mild exacerbation in asthmatic subjects with only minimal lower respiratory tract symptoms in healthy volunteers. Increases in airway hyperresponsiveness and decreases in both peak expiratory flow and FEV1 were demonstrated in asthmatic subjects without any change in these measures in healthy subjects.9 This study, along with several others, supports the idea that respiratory tract viral infections cause a greater degree of morbidity in asthmatic subjects than in the healthy population. In this review we discuss why such a discrepancy in antiviral immune response might exist. In addition, we explore which therapeutic options might be of benefit in preventing the deterioration of asthma control seen after viral infection.
The airway epithelium
The bronchial epithelium provides a barrier between the microbe-rich outside world and the internal parenchyma. It is, however, far more than simply an inert barrier and is immunologically active, playing a pivotal role in both innate and adaptive immunity.
All respiratory tract viruses enter and replicate within airway epithelial cells and can damage both ciliated and nonciliated respiratory epithelial cells, leading to necrosis of the airway epithelium, ciliostasis, loss of cilia, and impairment of mucociliary clearance.11, 12, 13 However, it is likely that the clinical manifestations might be secondary to the release of proinflammatory mediators by damaged bronchial epithelial cells (BECs), as well as a direct cytotoxic effect of the virus.
Rhinoviruses attach to epithelial cells through intercellular adhesion molecule 1 (ICAM-1) for major human rhinovirus (HRV) serotypes or low-density lipoprotein receptor for minor HRV serotypes. The receptor or receptors for the recently identified group C rhinoviruses are yet to be identified. Indeed, rhinovirus infection itself upregulates expression of ICAM-1 both in vivo and in vitro to further the availability of receptors to bind to and infect epithelial cells.14, 15 Infection of BECs with rhinovirus induces the secretion of a wide range of cytokines and chemokines, including IL-1, IL-6, CXCL8/IL-8, GM-CSF, CCL5/RANTES, and CXCL10/interferon-inducible protein 10 (IP-10), as well as many others.16, 17, 18, 19
BEC-derived cytokines and chemokines are able to induce neutrophilic (CXCL8/IL-8), lymphocytic (CXCL10/IP-10), and eosinophilic (CCL5/RANTES) inflammation, as well as airway hyperresponsiveness and airway remodeling. However, they also initiate antiviral immune responses, highlighting the delicate balance that exists between harmful and protective influences in the asthmatic airway.
The innate immune response
Interferons
Corne et al20 demonstrated more severe and prolonged virus-induced symptoms (including lower respiratory tract symptoms) in asthmatic compared with nonasthmatic subjects, suggesting for the first time that there might be some inherent differences in the way that asthmatic subjects respond to respiratory tract viral infections. Wark et al21 subsequently provided a mechanistic insight into why this might be the case with studies involving cultured BECs from adult asthmatic and nonasthmatic subjects obtained by means of bronchoscopy, followed by infection with rhinovirus in vitro. Although rhinovirus induction of the proinflammatory cytokine IL-6 and CCL5 was not different between the 2 groups, asthmatic BECs produced lower levels of the type I IFN-β and also had higher levels of rhinovirus replication. Importantly, deficient IFN-β expression was observed in both steroid-treated and steroid-naive asthmatic subjects. The asthmatic BECs responded to exogenous treatment with IFN-β, exhibiting reduced rhinovirus release and demonstrating that the deficiency in asthmatic cells was associated with production of antiviral IFN-β rather than the actions of IFN-β.
Contoli et al22 provided further evidence of the importance of antiviral interferons using a human experimental infection model of adults with mild-to-moderate asthma. Not only did bronchoalveolar lavage (BAL) cells from asthmatic subjects have lower levels of rhinovirus and LPS-induced type III IFN-λ, but deficient IFN-λ in asthmatic cells was related to the pathogenesis of asthma exacerbations in vivo, with abundance of IFN-λ being negatively correlated with airway symptom scores, changes in lung function, virus load, and markers of inflammation in vivo. In a more recent study, this time in subjects with moderate-to-severe asthma, Bullens et al23 demonstrated that IFNL1, but not IFNL2/3, mRNA levels obtained from sputum cells negatively correlated with asthma symptoms, again highlighting a protective role for IFN-λ in asthma.
At present, the precise mechanism or mechanisms behind deficient interferon production in asthmatic subjects remain unknown. Because asthmatic subjects have deficient IFN-β, IFN-λ, and perhaps some of the IFN-αs,24 it is less likely a result of a polymorphism in one of these genes or their promoters. A polymorphism in a gene encoding a transcription factor or signaling molecule required for the expression of all these interferons is a more likely possibility, however.25 Interestingly, Thomas et al26 recently demonstrated that excess TGF-β present in asthmatic airways mediates enhanced rhinovirus replication, probably through its suppressive actions on host type I interferon responses. Specifically, the effect of TGF-β appeared to be mediated through actions on interferon regulatory factor (IRF) 3 pathways.26
Macrophages
Macrophages are the predominant leukocyte in the airway and are distributed from the upper airways down to the alveoli. They produce inflammatory cytokines to recruit cells of the adaptive immune system, express a number of innate pattern-recognition receptors (PRRs), and play a key role in phagocytosis of bacterial organisms. They also modulate the immune response by inhibiting antigen presentation by dendritic cells, T-cell activation, and B-cell antibody production.27, 28
The interaction between rhinoviruses and macrophages stimulates secretion of proinflammatory cytokines, such as IL-1, IL-8, IFN-γ, and CCL3/macrophage inflammatory protein 1α. In addition, TNF-α, a highly inflammatory cytokine, is produced by macrophages in response to rhinovirus, and this has been shown to require viral replication.29 Rhinovirus infection also induces release of type I interferons from airway macrophages, a process that might limit viral spread by inducing an antiviral state in epithelial cells.29
More recently, Oliver et al30 identified virus-induced impairment of antibacterial host defense in human alveolar macrophages, suggesting viral infection might alter this function to facilitate additional bacterial infection.
Neutrophils
Increased neutrophil counts and levels of IL-8, a potent chemoattractant for neutrophils, are found in the nasal secretions, sputum, and BAL fluid of allergic subjects undergoing experimental rhinovirus infection.9, 31, 32, 33 Teran et al34 provided the first in vivo evidence of an important role for IL-8 in the neutrophil influx during viral-associated asthma exacerbations. Studies have since demonstrated that numbers of neutrophils correlate with levels of IL-8 after rhinovirus infection,33, 35 and IL-8 levels in nasal lavage correlates with the degree of airway responsiveness in asthmatic subjects.36 This is in contrast to healthy subjects, in whom IL-8 levels were not found to be significantly increased.37
Granulocyte colony-stimulating factor levels are also increased in nasal lavage fluid and sputum and later in the circulation after rhinovirus infection. Increased concentrations of circulatory granulocyte colony-stimulating factor could in turn act on the bone marrow to increase circulating neutrophil counts.38, 39
Using a murine model of human rhinovirus infection, Nagarkar et al40 recently showed that CXCR2, the receptor for ELR-positive CXC chemokines, is required for rhinovirus-induced neutrophilic airway inflammation and that neutrophil TNF-α release is required for airway hyperresponsiveness. Products of neutrophil activation could also cause airway obstruction through the production of elastase, which upregulates goblet cell mucus secretion.41
In combination, these data suggest that rhinovirus infection of airway epithelial cells might potentiate pre-existing inflammation by enhancing the production of neutrophil chemoattractants and neutrophilic airway inflammation.
Eosinophils
Viral infections can also trigger increased recruitment and activation of eosinophils in the airway. Eosinophil granule proteins have been detected in nasal secretions of asthmatic children with wheezing illness caused by rhinovirus or RSV.42, 43, 44 In these children levels of CCL5/RANTES, a potent chemotactic cytokine and activator of eosinophils, were also significantly increased.44 After experimental rhinovirus infection, bronchial eosinophil infiltration has been observed in both healthy and asthmatic adults. However, in asthmatic subjects the eosinophil infiltrate was more prolonged and still present 6 to 8 weeks after infection.45 In adults with allergic rhinitis, experimental rhinovirus infection increased allergen-induced eosinophil numbers in BAL fluid,46 and in asthmatic adults eosinophil products were increased in sputum.32 In addition, airway hyperresponsiveness correlated with rhinovirus-induced eosinophils, as reflected by an increase in eosinophilic cationic protein in sputum supernatants.32 In vitro experiments indicate that rhinoviruses do not activate eosinophils directly47 but rather probably through the activity of virus-induced mediators, adhesion molecules,48, 49 or cytokines secreted by T cells, epithelial cells, or other airway cells.50 It remains unclear, however, whether eosinophils contribute to host defense or to the immunopathology observed after viral infection. In a murine model of RSV infection, increased numbers of eosinophils improved viral clearance and reduced airway dysfunction, suggesting that eosinophils might be protective.51
Mucociliary clearance
Mucociliary clearance is a critical innate defense system, and mucus overproduction is one of the major symptoms of asthma exacerbations, significantly contributing to the morbidity and mortality of asthmatic subjects.52 The mucus that occludes the lumen in an asthma exacerbation is quite a complex biological material comprising a mixture of mucins, plasma proteins, and products of cell death. Indeed, a recent proteomic analysis identified 191 different proteins in human induced sputum.53 In asthmatic subjects the major mucin components of airway mucus secretions are MUC5AC and MUC5B, which contribute to the viscoelastic properties of the mucus.54 He et al55 have shown that rhinovirus-16 infection of undifferentiated BECs induces secretion of MUC5AC. Zhu et al56 have recently demonstrated that rhinovirus induces mucin production in epithelial cells and that this depends on viral replication and at least partly on Toll-like receptor (TLR) 3, a PRR that specifically recognizes viral double-stranded RNA. Hewson et al57 have recently shown that rhinovirus induction of MUC5AC in BECs occurred through nuclear factor (NF) κB–dependent induction of matrix metalloproteinase–mediated TGF-α release. This activates an epidermal growth factor receptor–dependent cascade culminating in specificity protein 1 transactivation of the MUC5AC promoter. After experimental infection with rhinovirus-16, Hewson et al57 also demonstrated increased MUC5AC production in healthy and asthmatic volunteers in vivo. This was related to viral load in asthmatic subjects but not healthy volunteers.
Toll-like receptors
TLRs recognize and respond to a variety of pathogen-associated molecular patterns. The first indication of a TLR recognizing a viral pathogen-associated molecular pattern was in the case of TLR4 and RSV fusion protein.58 Since then, several studies have demonstrated that in response to viral infection, TLR3,59, 60, 61, 62 TLR7,63, 64 TLR8,63, 64 and TLR965 are involved in induction of type I interferons. TLR7, TLR8, and TLR3 are particularly relevant in relation to RNA viruses because they recognize and respond to single-stranded viral RNA and double-stranded viral RNA, respectively. TLR3 recognizes rhinovirus and is upregulated on infection. Blocking TLR3 suppresses antiviral responses, and rhinovirus replication and release is increased.59 Roponen et al66 recently demonstrated impaired TLR7 function in adolescents with mild-to-moderate asthma; however, the ability of a TLR3 ligand to induce key antiviral molecules was similar in asthmatic and control groups. Moreover, polymorphisms of TLR7 and TLR8 have been shown to be associated with asthma, identifying TLR7 and TLR8 as novel risk genes in subjects with asthma and related disorders.67
Role of T lymphocytes
Asthma is a disease characterized by type 2 T-cell infiltration with production of IL-4, IL-5, and IL-13. Viral infection, on the other hand, is characterized by type 1 T-cell responses leading to production of IFN-γ. The balance between TH1 and TH2 cytokine production can be crucial to viral clearance. There are data supporting the suggestion that within a pre-existing type 2 cytokine allergic asthmatic microenvironment, the normally effective type 1 antiviral immune response might be inhibited, the immune response is skewed toward type 2 responses, or both. Gern et al68 demonstrated that the IFNG/IL5 sputum mRNA ratio during infection was inversely related to peak cold symptom scores and time to viral clearance, suggesting that a strong TH1 response is key to limiting viral replication.
A recent study involving experimental infection with rhinovirus-16 identified deficient induction of TH1 cytokines and IL-10 and augmented induction of TH2 cytokines in asthmatic subjects. In addition, TH1 cytokines and IL-10 were associated with protection from exacerbation, whereas TH2 cytokines were associated with increased disease severity. These observations support an important role for rhinovirus-induced lower airway inflammation in precipitating asthma exacerbations, perhaps through impaired TH1/IL-10 and augmented TH2 responses.9 Epithelial cells infected with rhinovirus secrete CCL5 and CXCL10, which both promote T-cell chemotaxis.69 Levels of CXCL10, a chemokine that binds to CXCR3, are increased in virus-induced asthma exacerbations. Indeed, it can distinguish virus-induced from non–virus-induced exacerbations.69
A defective TH1 response has also been implicated in RSV-induced bronchiolitis. Children with a deficient TH1 and a relatively increased TH2 immune response after RSV infection had better viral clearance and were more likely to have acute bronchiolitis than those with stronger type 1 responses who had milder illness without bronchiolitis.70 In addition, other studies have demonstrated that those children with a relatively increased TH2 cytokine profile during RSV-induced bronchiolitis were at increased risk of wheezing during follow-up.71
NF-κB
NF-κB is a transcription factor expressed in numerous cell types, playing a key role in the expression of many proinflammatory genes. It leads to the synthesis of cytokines, adhesion molecules, chemokines, respiratory mucins, and growth and angiogenic factors, and an increasingly large body of evidence demonstrates a clear role for NF-κB in both subjects with stable asthma and those with acute exacerbations.
Greater levels of NF-κB p65 and p50 activation are seen in bronchial biopsy specimens, sputum cells, and cultured BECs from stable, untreated asthmatic subjects when compared with those seen in nonasthmatic subjects.72, 73 PPBMCs of adults with moderate and severe asthma, as well as children with moderate asthma, have higher levels of NF-κB p65 protein expression than those of healthy subjects.74, 75
Viral infection causes upregulation of a plethora of proinflammatory molecules in an NF-κB–dependent manner. These include the neutrophil chemokines CXCL8/IL-8,76 CXCL5,77 and CXCL178 and the T-cell chemokines CXCL10/IP-10,79 CCL5/RANTES,80, 81, 82 and CCL11/eotaxin.83
In addition, various cytokines and growth factors are induced, including IL-6,84, 85 GM-CSF,86, 87 IL-11,88 fibroblast growth factor (FGF) 2, and vascular endothelial growth factor (VEGF).89 Adhesion molecules49 and respiratory mucins, including MUC5AC and MUC5B, are also NF-κB dependent.55, 57, 90
Although our understanding of each NF-κB signaling molecule is expanding, which members of this complex signaling cascade represent the best therapeutic target in asthma exacerbations is far from clear. Moreover, there is the fundamental consideration of the beneficial role of NF-κB in host responses to infectious agents because it is essential for interferon induction in response to infection.91 Furthermore, the question of why known NF-κB inhibitors, such as corticosteroids, are only partially effective in controlling exacerbations illustrates the greater level of understanding required.
The importance of genes
The host response to infection is in part genetically programmed. In recent years, a large number of genetic polymorphisms relevant to both infection and asthma have been identified. These include several polymorphisms of PRRs, such as TLRs (see earlier), which have been shown to be associated with both infection severity and asthma. In addition, genes involved in regulating interferon production have been identified. The transcription factor IRF1 is centrally important to regulating IFN-γ production and function. The IRF1 gene is located on chromosome 5q31 in the TH2 cytokine cluster involved in the development of allergic disease, and almost 50 polymorphisms of the IRF1 locus have been analyzed. Eleven are significantly associated with atopy, total IgE levels, or specific IgE levels.92 In addition, suppressor of cytokine signaling 1 (SOCS1) regulates IFN-γ signaling and antiviral responses, and a significant association between a polymorphism in the SOCS1 promoter and adult asthma has been observed. This polymorphism resulted in increased production of SOCS1 protein, which in turn inhibited phosphorylation of signal transducer and activator of transcription 1 in response to IFN-β stimulation.93
In addition to the genes involved in the innate/TH1 response to infection and the associated risk of asthma, single nucleotide polymorphisms in TH2-associated genes are also potentially important. Forton et al94 recently identified a haplotype within the IL13-IL4 gene locus conferring an increased risk of severe primary RSV-induced bronchiolitis in early infancy.94 It is likely that as technology continues to improve, future studies will reveal numerous other polymorphisms playing a key role in the asthmatic immune response to viral infection.
Airway remodeling
The central role that the bronchial epithelium plays in asthma is well appreciated, but what remains less clear is what effect viral infection of the asthmatic airway has on the chronic inflammatory response. Specifically, is there a lasting effect that might lead to airway wall remodeling and worsening of fixed airflow obstruction?
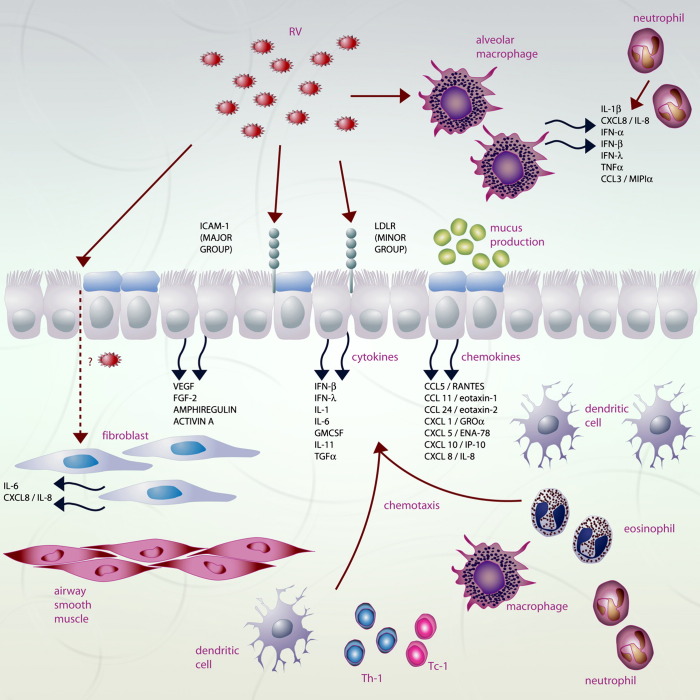
Fig 1.
Epithelial and immune cell responses to rhinovirus infection. Following infection a wide range of mediators are secreted including pro-inflammatory cytokines, chemokines, interferons, and growth factors. This leads to eosinophilic, neutrophilic, and lymphocytic inflammation, as well as mucus hypersecretion and likely airway remodelling. CCL, CC chemokine ligand; CCL5, RANTES (Regulated on Activation Normal T-cell Expressed and Secreted); CCL11, eotaxin; CCL24, eotaxin-2; CXCL, CXC chemokine ligand; CXCL 1, GRO-α (growth-related oncogene α); CXCL-5, ENA-78 (epithelial neutrophil activating protein-78); CXCL10, IP-10 (IFN-γ-inducible protein 10); DC, dendritic cell; FGF-2, fibroblast growth factor-2; GMCSF, granulocyte macrophage-colony stimulating factor; ICAM-1, intercellular adhesion molecule-1; IFN, interferon; IL, interleukin; LDLR, low density lipoprotein receptor; RV, rhinovirus; TC1, cytotoxic CD8+ T lymphocyte type 1; TH1, T-helper 1 CD4+ T lymphocyte; TNF-α, tumour necrosis factor-α; VEGF, vascular endothelial growth factor.
Leigh et al95 recently demonstrated that rhinovirus infection of cultured epithelial cells led to upregulation of amphiregulin, activin A, and VEGF protein levels. These 3 mediators have all been strongly implicated in the remodeling process. Amphiregulin, a member of the epidermal growth factor family, is released in response to airway epithelial injury and engages epidermal growth factor receptors to drive the altered repair process that is characteristic of airway remodeling.96 Activin A is a member of the TGF-β superfamily and has been implicated in the subepithelial fibrosis characteristic of airway remodeling.97, 98 Expression of VEGF and its receptors is increased in asthmatic subjects, and VEGF is the major proangiogenic activator in asthmatic airways.99, 100 Increases in the size and number of airway wall blood vessels are characteristic of airway remodeling, even in subjects with mild asthma,101 and overexpression of VEGF in the airways of mice has been shown to result in both vascular and airway remodeling.102 Moreover, nasal lavage samples from subjects with confirmed natural rhinovirus infections showed significantly higher VEGF protein levels during peak cold symptoms compared with both baseline levels and control levels.95
Although epithelial cells are the main target for rhinovirus infection, studies have indicated that rhinovirus might be detected in the subepithelial layer by means of in situ hybridization103, 104 and that human lung fibroblasts are susceptible to rhinovirus infection.105 It is possible that rhinovirus gains access to underlying fibroblasts through the epithelial disruption that occurs in asthmatic airways. This susceptibility might then allow rhinovirus replication in the lower airways to develop, especially in asthmatic subjects in whom there is reduced protection offered by epithelially derived interferons. Bedke et al106 recently infected normal and asthmatic airway fibroblasts in vitro with rhinovirus-1B, illustrating both the ability of fibroblasts to support viral replication and their vigorous proinflammatory response with induction of IL-6 and IL-8. This latter response compounds the existing airway inflammation to contribute to an exacerbation. Furthermore, this study demonstrated that exogenous IFN-β protects fibroblasts against infection, providing further support for its potential use as a therapy against virus-induced asthma exacerbations.
TGF-β is a key regulator of wound repair and healing, and increased TGF-β and TGFB mRNA levels in BAL fluid and endobronchial biopsy specimens, respectively, have been reported in patients with asthma.107, 108 Thomas et al26 recently demonstrated that rhinovirus replication is enhanced in TGF-β1–treated fibroblasts and fibroblasts from subjects with asthma, and this is linked to deficient type I interferon responses. TGF-β might therefore act as an endogenous immunosuppressant, promoting viral replication during the evolution of virus-induced asthma exacerbations.
Therapeutic options
Several therapeutic strategies have the potential to alter the natural history of virus-induced asthma exacerbations. These include prevention of infection with either vaccines or mAbs; antiviral agents, such as viral attachment inhibitors or viral protease inhibitors; anti-inflammatory treatments, such as corticosteroids; macrolide/ketolide antibiotics; and supplementation of protective antiviral responses, such as treatment with interferon. Whichever intervention is considered, the treatment would need to be taken early in the course of infection to maximize the chances of success, be easy to administer, and be safe.
Prevention
At present, there are no safe and effective human vaccines for RSV or rhinovirus. The antigenic diversity of the more than 100 serotypes of rhinovirus means that creating a truly successful vaccine is an extremely difficult task. Recent attention has focused on live attenuated vaccines and developing subunit vaccines for RSV in combination with TH1-inducing adjuvants, although neither approach is likely to be ready for clinical use for some time.109, 110
Palivizumab, an mAb against the RSV fusion protein, is the only US Food and Drug Administration–approved mAb for RSV. In a murine model it reduces viral load, acute disease, and chronic lung function abnormalities.111 A randomized controlled trial in children resulted in a 55% reduction in RSV-induced hospitalization,112 and a recent prospective case-controlled study of at-risk preterm infants demonstrated protection against recurrent wheezing.113 Motavizumab, a second-generation derivative of palivizumab has a 70-fold higher affinity for the RSV fusion glycoprotein. In a rat model it had 50 to 100 times greater anti-RSV activity in the lower respiratory tract compared with palivizumab.114 In a large phase 3 noninferiority study comparing motavizumab with palivizumab for RSV prevention in high-risk children, motavizumab demonstrated fewer RSV-induced hospitalizations and a reduction in the incidence of RSV-specific outpatient lower respiratory tract infections.115 In addition, motavizumab significantly reduced viral load by day 1 after treatment in children hospitalized with RSV, suggesting a role for both RSV treatment and prevention.116 Motavizumab is currently pending US Food and Drug Administration approval.
Targeting viral attachment and protease inhibitors
Although it is theoretically possible to interfere with every step of the infectious cycle of respiratory tract viruses from viral attachment, viral entry and uncoating, translation, replication, and onward to virus release, with the exception of the neuramidase inhibitors for influenza infection, few approaches have met with success thus far.
The great majority of rhinoviruses use ICAM-1 to enter cells. Boehringer-Ingelheim developed a soluble ICAM-1 receptor called tremacamra, showing marginal benefit in symptoms, viral replication, and development of clinical colds. It has not been tested as a therapy for asthma exacerbations, and the requirement for 6 times daily treatment along with the fact that it was only really effective if used within 12 hours of infection led to its development being halted.117 A long-acting neutralizing antibody (akin to palivizumab for RSV) would likely be a more promising approach. Pleconaril, an antiviral drug against picornavirus infection, is currently being studied in subjects with asthma exacerbations. It blocks uncoating and attachment by binding into a hydrophobic pocket within the capsid. Other similar drugs are also in active development.
Inhibition of the essential viral proteases HRV 2A, 3C, or both has been investigated as an antirhinovirus treatment, and early work seemed promising. These proteases are required for replication through cleavage of the viral polyprotein. Ruprintrivir is an intranasally administered 3C protease inhibitor with good antiviral activity against all rhinovirus serotypes.118, 119 Unfortunately, despite encouraging results from an experimental infection study, a natural infection study had no significant effect on viral load or symptoms, and further clinical development was halted.120 There are several other inhibitors of both 3C and 2A proteases, but none have been tested in clinical trials thus far.121
Inhaled corticosteroid and long-acting β-agonist combination therapy
Glucocorticosteroids are by far the most widely used drugs in subjects with moderate and severe asthma and have potent anti-inflammatory activity. Despite this, patients with asthma continue to have exacerbations. In recent years, an ever-increasing body of work supporting their use in combination with long-acting β-agonists, such as salmeterol and formoterol, highlights the superiority of combination therapy over either drug in isolation. Although these studies on the whole failed to identify the trigger of the asthma exacerbation, it is likely that the majority of these were secondary to viral infections.
Edwards et al122 demonstrated that a combination of salmeterol and fluticasone treatment synergistically suppressed induction of CXCL8 (a neutrophil chemoattractant and activator), CCL5 (a lymphocyte chemokine), and CXCL10 (an activated T-cell chemokine) after rhinovirus infection in vitro. Skevaki et al123 recently demonstrated that the corticosteroid budesonide effectively suppressed rhinovirus-mediated induction of proinflammatory (CCL5, CXCL8, IL-6, and CXCL10) and remodeling-associated (FGF and VEGF) mediators in BECs in a concentration-dependent manner. Formoterol treatment alone also suppressed the production of CXCL8 and FGF by bronchial epithelium; however, the combination of budesonide and formoterol demonstrated a concentration-dependent, additive, or synergistic effect in the suppression of rhinovirus-induced CCL5, CXCL8, CXCL10, and VEGF.
Volonaki et al124 demonstrated that both fluticasone and salmeterol administered individually were able to reduce rhinovirus-induced VEGF production. Addition of both factors, however, inhibited VEGF synergistically. Moreover, FGF-2 production was not inhibited by either fluticasone or salmeterol alone but was significantly reduced when both substances were present. The effective suppression of growth factors highlighted above certainly represents a plausible mechanism through which these drugs might inhibit remodeling and inflammatory processes.
Macrolides and ketolide antibiotics
An increasing body of work highlights the immune-modulatory effects of macrolide antibiotics that are distinct from their antimicrobial actions. Jang et al125 demonstrated reduced expression of ICAM-1, IL-6, and IL-8 after treatment with erythromycin of rhinovirus-infected epithelial cells. Gielen et al126 have recently shown that pretreatment of BECs with azithromycin significantly increased rhinovirus-1B– and rhinovirus-16–induced interferons and interferon-stimulated gene mRNA and protein expression. In addition, azithromycin reduced rhinovirus replication and release.
In infants with RSV-induced bronchiolitis, clarithromycin reduced systemic inflammation acutely and led to fewer wheezing episodes in the following 6 months.127 Although this was a small study, a much larger study of asthmatic adults randomized to receive either telithromycin, a ketolide antibiotic, or placebo resulted in a greater reduction in asthma symptom scores and lung function in the ketolide group.128 Safety concerns have limited the widespread use of ketolides, and further studies are needed to clarify the position of macrolides in the management of asthma exacerbations.129
Interferon supplementation
Wark et al21 observed deficient IFN-β responses in BECs from asthmatic subjects after rhinovirus infection, and phase 2 trials involving inhaled IFN-β after the onset of a respiratory tract viral infection in asthmatic subjects are currently underway. Treatment of corticosteroid-resistant asthmatic subjects with IFN-α in one case series led to improved lung function and a reduction in oral corticosteroid use; however, the majority of subjects experienced side effects, including a “flu-like” illness, nausea, and headaches.130 A number of other trials have assessed the use of intranasally administered IFN-α2 in healthy volunteers undergoing experimental rhinovirus infection. These studies showed a dose-dependent improvement in symptom scores and reduced viral shedding, but side effects were again a problem.131, 132, 133 An approach using interferon aims at boosting host defense rather than targeting specific respiratory viruses. This is a significant advantage over specific antiviral approaches because it would treat all virus-induced asthma attacks rather than just those caused by a specific virus. Despite the problems with side effects in the early trials, there are sufficient in vitro and in vivo data supporting a re-examination of this treatment approach. Similar approaches aiming to boost antiviral activity with TLR ligands are also in development.
Conclusion
Respiratory tract viral infections play a key role in the pathogenesis of asthma exacerbations. Despite this, no specific treatments exist that are able to significantly alter the clinical outcome of infection. Numerous therapeutic strategies have been considered; however, success has been hampered because of problems such as virus specificity, side effects, delivery problems, and the need for early administration after diagnosis of infection. Results of ongoing clinical trials with IFN-β in which many of these problems have been addressed are awaited with interest.
In-depth studies of the molecular pathways underlying virus-induced inflammation are also required to identify new targets for development of novel therapies. The use of gene array analysis to determine whether asthma is associated with a unique pattern of epithelial cell gene expression after viral infection has the potential to radically improve our understanding of the immune pathways involved and has the potential to identify such new therapeutic targets. Although this approach has been performed in vitro on cultured epithelial cells,134 gene expression of either BECs or alveolar macrophages during experimental infection in vivo has yet to be performed. Moreover, future studies will need to sample both asthmatic children and nonatopic asthmatic subjects to gain a more complete picture of the role of viruses in asthma exacerbations. Finally, our understanding of the effect of virus-induced exacerbations on subsequent remodeling and development of severe airflow obstruction requires further study.
Footnotes
Series editors: Donald Y. M. Leung, MD, PhD, and Dennis K. Ledford, MD
References
- 1.Fendrick A.M., Monto A.S., Nightengale B., Sarnes M. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch Intern Med. 2003;163:487–494. doi: 10.1001/archinte.163.4.487. [DOI] [PubMed] [Google Scholar]
- 2.Lambert H.P., Stern H. Infective factors in exacerbations of bronchitis and asthma. BMJ. 1972;3:323–327. doi: 10.1136/bmj.3.5822.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minor T.E., Dick E.C., DeMeo A.N., Ouellette J.J., Cohen M., Reed C.E. Viruses as precipitants of asthmatic attacks in children. JAMA. 1974;227:292–298. [PubMed] [Google Scholar]
- 4.Johnston S.L., Pattemore P.K., Sanderson G., Smith S., Lampe F., Josephs L. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicholson K.G., Kent J., Ireland D.C. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wark P.A., Johnston S.L., Moric I., Simpson J.L., Hensley M.J., Gibson P.G. Neutrophil degranulation and cell lysis is associated with clinical severity in virus-induced asthma. Eur Respir J. 2002;19:68–75. doi: 10.1183/09031936.02.00226302. [DOI] [PubMed] [Google Scholar]
- 7.Grissell T.V., Powell H., Shafren D.R., Boyle M.J., Hensley M.J., Jones P.D. Interleukin-10 gene expression in acute virus-induced asthma. Am J Respir Crit Care Med. 2005;172:433–439. doi: 10.1164/rccm.200412-1621OC. [DOI] [PubMed] [Google Scholar]
- 8.Khetsuriani N., Kazerouni N.N., Erdman D.D., Lu X., Redd S.C., Anderson L.J. Prevalence of viral respiratory tract infections in children with asthma. J Allergy Clin Immunol. 2007;119:314–321. doi: 10.1016/j.jaci.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Message S.D., Laza-Stanca V., Mallia P., Parker H.L., Zhu J., Kebadze T. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci U S A. 2008;105:13562–13567. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartlett N.W., Walton R.P., Edwards M.R., Aniscenko J., Caramori G., Zhu J. Mouse models of rhinovirus-induced disease and exacerbation of allergic airway inflammation. Nat Med. 2008;14:199–204. doi: 10.1038/nm1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Schans C.P. Bronchial mucus transport. Respir Care. 2007;52:1150–1156. [PubMed] [Google Scholar]
- 12.Ahern W., Bird T., Court S. Pathological changes in virus infections of the lower respiratory tract in children. J Clin Invest. 1970;23:7–18. doi: 10.1136/jcp.23.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thornton D.J., Rousseau K., McGuckin M.A. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol. 2008;70:459–486. doi: 10.1146/annurev.physiol.70.113006.100702. [DOI] [PubMed] [Google Scholar]
- 14.Grünberg K., Sharon R.F., Hiltermann T.J., Brahim J.J., Dick E.C., Sterk P.J. Experimental rhinovirus 16 infection increases intercellular adhesion molecule-1 expression in bronchial epithelium of asthmatics regardless of inhaled steroid treatment. Clin Exp Allergy. 2000;30:1015–1023. doi: 10.1046/j.1365-2222.2000.00854.x. [DOI] [PubMed] [Google Scholar]
- 15.Papi A., Papadopoulos N.G., Degitz K., Holgate S.T., Johnston S.L. Corticosteroids inhibit rhinovirus-induced intercellular adhesion molecule-1 up-regulation and promoter activation on respiratory epithelial cells. J Allergy Clin Immunol. 2000;105:318–326. doi: 10.1016/s0091-6749(00)90082-4. [DOI] [PubMed] [Google Scholar]
- 16.Schroth M.K., Grimm E., Frindt P., Galagan D.M., Konno S.I., Love R. Rhinovirus replication causes RANTES production in primary bronchial epithelial cells. Am J Respir Cell Mol Biol. 1999;20:1220–1228. doi: 10.1165/ajrcmb.20.6.3261. [DOI] [PubMed] [Google Scholar]
- 17.Papadopoulos N.G., Papi A., Meyer J., Stanciu L.A., Salvi S., Holgate S.T. Rhinovirus infection up-regulates eotaxin and eotaxin-2 expression in bronchial epithelial cells. Clin Exp Allergy. 2001;31:1060–1066. doi: 10.1046/j.1365-2222.2001.01112.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Z., Tang W., Gwaltney J.M., Jr., Wu Y., Elias J.A. Rhinovirus stimulation of interleukin-8 in vivo and in vitro: role of NF-kappaB. Am J Physiol Lung Cell Mol Physiol. 1997;273:L814–L824. doi: 10.1152/ajplung.1997.273.4.L814. [DOI] [PubMed] [Google Scholar]
- 19.Terajima M., Yamaya M., Sekizawa K., Okinaga S., Suzuki T., Yamada N. Rhinovirus infection of primary cultures of human tracheal epithelium: role of ICAM-1 and IL-1beta. Am J Physiol Lung Cell Mol Physiol. 1997;273:L749–L759. doi: 10.1152/ajplung.1997.273.4.L749. [DOI] [PubMed] [Google Scholar]
- 20.Corne J.M., Marshall C., Smith S., Schreiber J., Sanderson G., Holgate S.J. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet. 2002;359:831–834. doi: 10.1016/S0140-6736(02)07953-9. [DOI] [PubMed] [Google Scholar]
- 21.Wark P.A., Johnston S.L., Bucchieri F., Powell R., Puddicombe S., Laza-Stanca V. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Contoli M., Message S.D., Laza-Stanca V., Edwards M.R., Wark P.A., Bartlett N.W. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 23.Bullens D.M., Decraene A., Dillssen E., Meyts I., De Boeck K., Dupont L.J. Type III IFN-lambda mRNA expression in sputum of adult and school-aged asthmatics. Clin Exp Allergy. 2008;38:1459–1467. doi: 10.1111/j.1365-2222.2008.03045.x. [DOI] [PubMed] [Google Scholar]
- 24.Gehlhar K., Bilitewski C., Reinitz-Rademacher K., Rohde G., Bufe A. Impaired virus-induced interferon-alpha2 release in adult asthmatic patients. Clin Exp Allergy. 2006;36:331–337. doi: 10.1111/j.1365-2222.2006.02450.x. [DOI] [PubMed] [Google Scholar]
- 25.Edwards M.R., Johnston S.L. Deficient IFN in virus-induced asthma exacerbations. Clin Exp Allergy. 2008;38:1416–1418. doi: 10.1111/j.1365-2222.2008.03064.x. [DOI] [PubMed] [Google Scholar]
- 26.Thomas B.J., Lindsay M., Dagher H., Freezer N.J., Li D., Ghildyal R. Transforming growth factor-beta enhances rhinovirus infection by diminishing early innate responses. Am J Respir Cell Mol Biol. 2009;41:339–347. doi: 10.1165/rcmb.2008-0316OC. [DOI] [PubMed] [Google Scholar]
- 27.Holt P.G. Down-regulation of immune responses in the lower respiratory tract: the role of alveolar macrophages. Clin Exp Immunol. 1986;63:261–270. [PMC free article] [PubMed] [Google Scholar]
- 28.Holt P.G., Oliver J., Bilyk N., McMenamin C., McMenamin P.G., Kraal G. Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J Exp Med. 1993;177:397–407. doi: 10.1084/jem.177.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laza-Stanca V., Stanciu L.A., Message S.D., Edwards M.R., Gern J.E., Johnston S.L. Rhinovirus replication in human macrophages induces NF-kappaB-dependent tumor necrosis factor alpha production. J Virol. 2006;80:8248–8258. doi: 10.1128/JVI.00162-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliver B.G., Lim S., Wark P., Laza-Stanca V., King N., Black J.L. Rhinovirus exposure impairs immune responses to bacterial products in human alveolar macrophages. Thorax. 2008;63:519–525. doi: 10.1136/thx.2007.081752. [DOI] [PubMed] [Google Scholar]
- 31.Fleming H., Little F., Schnurr D., Avila P., Wong H., Liu J. Rhinovirus-16 colds in healthy and in asthmatic subjects: similar changes in upper and lower airways. Am J Respir Crit Care Med. 1999;160:100–108. doi: 10.1164/ajrccm.160.1.9808074. [DOI] [PubMed] [Google Scholar]
- 32.Grunberg K., Smits H.H., Timmers M.C., De Klerk E.P.A., Dolhain R.J.E.M., Dick E.C. Experimental rhinovirus 16 infection. Effects on cell differentials and soluble markers in sputum in asthmatic subjects. Am J Respir Crit Care Med. 1997;156:609–616. doi: 10.1164/ajrccm.156.2.9610079. [DOI] [PubMed] [Google Scholar]
- 33.Gern J.E., Vrtis R., Grindle K.A., Swenson C., Busse W.W. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am J Respir Crit Care Med. 2000;162:2226–2231. doi: 10.1164/ajrccm.162.6.2003019. [DOI] [PubMed] [Google Scholar]
- 34.Teran L.M., Johnston S.L., Schröder J.M., Church M.K., Holgate S.T. Role of nasal interleukin-8 in neutrophil recruitment and activation in children with virus-induced asthma. Am J Respir Crit Care Med. 1997;155:1362–1366. doi: 10.1164/ajrccm.155.4.9105080. [DOI] [PubMed] [Google Scholar]
- 35.Pizzichini M.M., Pizzichini E., Efthimiadis A., Chauhan A.J., Johnston S.L., Hussack P. Asthma and natural colds: inflammatory indices in induced sputum: a feasibility study. Am J Respir Crit Care Med. 1998;158:1178–1184. doi: 10.1164/ajrccm.158.4.9712082. [DOI] [PubMed] [Google Scholar]
- 36.Grunberg K., Timmers M.C., Smits H.H., de Klerk E.P., Dick E.C., Spaan W.J. Effect of experimental rhinovirus 16 colds on airway hyperresponsiveness to histamine and interleukin-8 in nasal lavage in asthmatic subjects in vivo. Clin Exp Allergy. 1997;27:36–45. doi: 10.1111/j.1365-2222.1997.tb00670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Kluijver J., Grunberg K., Pons D., de Klerk E.P., Dick C.R., Sterk P.J. Interleukin-1 and interleukin-1ra levels in nasal lavages during experimental rhinovirus infection in asthmatic and non-asthmatic subjects. Clin Exp Allergy. 2003;33:1415–1418. doi: 10.1046/j.1365-2222.2003.01770.x. [DOI] [PubMed] [Google Scholar]
- 38.Gern J.E. Rhinovirus respiratory infections and asthma. Am J Med. 2002;112(suppl 6A):19S–27S. doi: 10.1016/s0002-9343(01)01060-9. [DOI] [PubMed] [Google Scholar]
- 39.Jarjour N.N., Gern J.E., Kelly E.A., Swenson C.A., Dick C.R., Busse W.W. The effect of an experimental rhinovirus 16 infection on bronchial lavage neutrophils. J Allergy Clin Immunol. 2000;105:1169–1177. doi: 10.1067/mai.2000.106376. [DOI] [PubMed] [Google Scholar]
- 40.Nagarkar D.R., Wang Q., Shim J., Zhao Y., Tsai W.C., Lukacs N.W. CXCR2 is required for neutrophilic airway inflammation and hyperresponsiveness in a mouse model of human rhinovirus infection. J Immunol. 2009;183:6698–6707. doi: 10.4049/jimmunol.0900298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cardell L.O., Agusti C., Takeyama K., Stjarne P., Nadel J.A. LTB(4)- induced nasal gland serous cell secretion mediated by neutrophil elastase. Am J Respir Crit Care Med. 1999;160:411–414. doi: 10.1164/ajrccm.160.2.9808117. [DOI] [PubMed] [Google Scholar]
- 42.Garofalo R., Kimpen J.L., Welliver R.C., Ogra P.L. Eosinophil degranulation in the respiratory tract during naturally acquired respiratory syncytial virus infection. J Pediatr. 1992;120:28–32. doi: 10.1016/s0022-3476(05)80592-x. [DOI] [PubMed] [Google Scholar]
- 43.Heymann P., Rakes G., Hogan A., Ingram J., Hoover G., Platts-Mills T. Assessment of eosinophils, viruses and IgE antibody in wheezing infants and children. Int Arch Allergy Immunol. 1995;107:380–382. doi: 10.1159/000237043. [DOI] [PubMed] [Google Scholar]
- 44.Teran L.M., Seminario M.C., Shute J.K., Papi A., Compton S.J., Low J.L. RANTES, macrophage-inhibitory protein 1alpha, and the eosinophil product major basic protein are released into upper respiratory secretions during virus-induced asthma exacerbations in children. J Infect Dis. 1999;179:677–681. doi: 10.1086/314618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fraenkel D.J., Bardin P.G., Sanderson G., Lampe F., Johnston S.L., Holgate S.T. Lower airways inflammation during rhinovirus colds in normal and in asthmatic subjects. Am J Respir Crit Care Med. 1995;151:879–886. doi: 10.1164/ajrccm/151.3_Pt_1.879. [DOI] [PubMed] [Google Scholar]
- 46.Calhoun W., Dick E., Schwartz L., Busse W. A common cold virus, rhinovirus 16, potentiates airway inflammation after segmental antigen bronchoprovocation in allergic subjects. J Clin Invest. 1994;94:2200–2208. doi: 10.1172/JCI117581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Handzel Z.T., Busse W.W., Sedgwick J.B., Vrtis R., Lee W.M., Kelly E.A. Eosinophils bind rhinovirus and activate virus-specific T cells. J Immunol. 1998;160:1279–1284. [PubMed] [Google Scholar]
- 48.Papi A., Johnston S.L. Rhinovirus infection induces expression of its own receptor intercellular adhesion molecule 1 (ICAM-1) via increased NF-kappaB mediated transcription. J Biol Chem. 1999;274:9707–9720. doi: 10.1074/jbc.274.14.9707. [DOI] [PubMed] [Google Scholar]
- 49.Papi A., Johnston S.L. Respiratory epithelial cell expression of vascular cell adhesion molecule-1 and its up-regulation by rhinovirus infection via NFkappaB and GATA transcription factors. J Biol Chem. 1999;274:30041–30051. doi: 10.1074/jbc.274.42.30041. [DOI] [PubMed] [Google Scholar]
- 50.Gern J., Vrtis R., Kelly E., Dick E., Busse W. Rhinovirus produces nonspecific activation of lymphocytes through a monocyte-dependent mechanism. J Immunol. 1996;157:1605–1612. [PubMed] [Google Scholar]
- 51.Phipps S., Lam C.E., Mahalingam S., Newhouse M., Ramirez R., Rosenberg H.F. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood. 2007;110:1578–1586. doi: 10.1182/blood-2007-01-071340. [DOI] [PubMed] [Google Scholar]
- 52.Kuyper L.M., Pare P.D., Hogg J.C., Lambert R.K., Ionescu D., Woods R. Characterization of airway plugging in fatal asthma. Am J Med. 2003;115:6–11. doi: 10.1016/s0002-9343(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 53.Nicholas B., Skipp P., Mould R., Rennard S., Davies D.E., O'Connor C.D. Shotgun proteomic analysis of human-induced sputum. Proteomics. 2006;6:4390–4401. doi: 10.1002/pmic.200600011. [DOI] [PubMed] [Google Scholar]
- 54.Rogers D.F. The airway goblet cell. Int J Biochem Cell Biol. 2003;35:1–6. doi: 10.1016/s1357-2725(02)00083-3. [DOI] [PubMed] [Google Scholar]
- 55.He S., Zheng J., Duan M. Induction of mucin secretion from human bronchial tissue and epithelial cells by rhinovirus and lipopolysaccharide. Acta Pharmacol Sin. 2004;25:1176–1181. [PubMed] [Google Scholar]
- 56.Zhu L., Lee P., Lee W., Zhao Y., Yu D., Chen Y. Rhinovirus-Induced Major Airway Mucin Production Involves a Novel TLR3-EGFR–Dependent Pathway. Am J Respir Cell Mol Biol. 2009;40:610–619. doi: 10.1165/rcmb.2008-0223OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hewson CA, Haas JJ, Bartlett NW, Message SD, Laza-Stanca V, et al. Rhinovirus induces MUC5AC in vivo and in vitro via NFKB and EGFR pathways. ERJ. In press 2010. [DOI] [PubMed]
- 58.Kurt-Jones E.A., Popova L., Kwinn L., Haynes L.M., Jones L.P., Tripp R.A. Pattern recognition receptors TLR4 and CD14 mediate response to RSV. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 59.Hewson C.A., Jardine A., Edwards M.R., Laza-Stanca V., Johnston S.L. Toll-like receptor 3 is induced by and mediates antiviral activity against rhinovirus infection in human bronchial epithelial cells. J Virol. 2005;79:12273–12279. doi: 10.1128/JVI.79.19.12273-12279.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alexopoulou L., Holt A.C., Medzhitov R., Flavell R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 61.Tabeta K., Georgel P., Janssen E., Du X., Hoebe K., Crozat K. Toll-like receptors 9 and 3 as essential components of innate immune defence against mouse cytomegalovirus infection. Proc Natl Acad Sci U S A. 2004;101:3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schulz O., Diebold S.S., Chen M., Naslund T.I., Nolte M.A., Alexopoulo L. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433:887–892. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- 63.Heil F., Hemmi H., Hochrein H., Ampenberger F., Kirschning C., Akira S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 64.Yang K., Puel A., Zhang S., Eidenschenk C., Ku C.L., Casrouge A. Human TLR-7,-8, and -9 mediated induction of IFN-alpha/beta and lambda is IRAK-4 dependent and redundant for protective immunity to viruses. Immunity. 2005;23:465–478. doi: 10.1016/j.immuni.2005.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hochrein H., Schlatter B., O'Keeffe M., Wagner C., Schmitz F., Schiemann M. Herpes simplex virus type-1 induces IFN-alpha production via Toll-like receptor 9-dependent and independent pathways. Proc Natl Acad Sci U S A. 2004;101:11416–11421. doi: 10.1073/pnas.0403555101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roponen M., Yerkovich S.T., Hollams E., Sly P.D., Holt P.G., Upham J.W. Toll-like receptor 7 function is reduced in adolescents with asthma. Eur Respir J. 2010;35:64–71. doi: 10.1183/09031936.00172008. [DOI] [PubMed] [Google Scholar]
- 67.Moller-Larsen S., Nyegaard M., Haagerup A., Vestbo J., Kruse T.A., Borglum A.D. Association analysis identifies TLR7 and TLR8 as novel risk genes in asthma and related disorders. Thorax. 2008;63:1064–1069. doi: 10.1136/thx.2007.094128. [DOI] [PubMed] [Google Scholar]
- 68.Gern J.E., Vrtis R., Grindle K.A., Swenson C., Busse W.W. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am J Respir Crit Care Med. 2000;162:2226–2231. doi: 10.1164/ajrccm.162.6.2003019. [DOI] [PubMed] [Google Scholar]
- 69.Wark P.A., Bucchieri F., Johnston S.L., Gibson P.G., Hamilton L., Mimica J. IFN-gamma-induced protein 10 is a novel biomarker of rhinovirus-induced asthma exacerbations. J Allergy Clin Immunol. 2007;120:586–593. doi: 10.1016/j.jaci.2007.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Legg J., Hussain I., Warner J., Johnston S., Warner J. Type 1 and type 2 cytokine imbalance in acute respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med. 2003;168:633–639. doi: 10.1164/rccm.200210-1148OC. [DOI] [PubMed] [Google Scholar]
- 71.Roman M., Calhoun W.J., Hinton K.L., Avendano L.F., Simon V., Escobar A.M. Respiratory syncytial virus infection in infants is associated with predominant Th-2-like response. Am J Respir Crit Care Med. 1997;156:190–195. doi: 10.1164/ajrccm.156.1.9611050. [DOI] [PubMed] [Google Scholar]
- 72.Hart L.A., Krishnan V.L., Adcock I.M., Barnes P.J., Chung K.F. Activation and localization of transcription factor, nuclear factor-kappaB, in asthma. Am J Respir Crit Care Med. 1998;158:1585–1592. doi: 10.1164/ajrccm.158.5.9706116. [DOI] [PubMed] [Google Scholar]
- 73.Zhao S., Qi Y., Liu X., Jiang Q., Liu S., Jiang Y. Activation of NF-kappa B in bronchial epithelial cells from children with asthma. Chin Med J (Engl) 2001;114:909–911. [PubMed] [Google Scholar]
- 74.Gagliardo R., Chanez P., Mathieu M., Bruno A., Costanzo G., Gougat C. Persistent activation of nuclear factor-kappaB signaling pathway in severe uncontrolled asthma. Am J Respir Crit Care Med. 2003;168:1190–1198. doi: 10.1164/rccm.200205-479OC. [DOI] [PubMed] [Google Scholar]
- 75.La Grutta S., Gagliardo R., Mirabella F., Pajno G.B., Bonsignore G., Bousquet J. Clinical and biological heterogeneity in children with moderate asthma. Am J Respir Crit Care Med. 2003;167:1490–1495. doi: 10.1164/rccm.200206-549OC. [DOI] [PubMed] [Google Scholar]
- 76.Zhu Z., Tang W., Gwaltney J.M., Jr., Wu Y., Elias J.A. Rhinovirus stimulation of interleukin-8 in vivo and in vitro: role of NF-kappaB. Am J Physiol Lung Cell Mol Physiol. 1997;273:L814–L824. doi: 10.1152/ajplung.1997.273.4.L814. [DOI] [PubMed] [Google Scholar]
- 77.Donninger H., Glashoff R., Haitchi H.M., Syce J.A., Ghildyal R., van Rensburg E. Rhinovirus induction of the CXC chemokine epithelial-neutrophil activating peptide-78 in bronchial epithelium. J Infect Dis. 2003;187:1809–1817. doi: 10.1086/375246. [DOI] [PubMed] [Google Scholar]
- 78.Edwards M.R., Johnson M.W., Johnston S.L. Combination therapy: synergistic suppression of virus induced chemokines in airway epithelial cells. Am J Respir Cell Mol Biol. 2006;34:616–624. doi: 10.1165/rcmb.2005-0385OC. [DOI] [PubMed] [Google Scholar]
- 79.Spurrell J.C., Wiehler S., Zaheer R.S., Sanders S.P., Proud D. Human airway epithelial cells produce Ip-10 (Cxcl10) in vitro and in vivo upon rhinovirus infection. Am J Physiol Lung Cell Mol Physiol. 2005;289:85–95. doi: 10.1152/ajplung.00397.2004. [DOI] [PubMed] [Google Scholar]
- 80.Thomas L.H., Friedland J.S., Sharland M., Becker S. Respiratory syncytial virus induced RANTES production from human bronchial epithelial cells is dependent on nuclear factor-kappa B nuclear binding and is inhibited by adenovirus-mediated expression of inhibitor of kappa B alpha. J Immunol. 1998;161:1007–1016. [PubMed] [Google Scholar]
- 81.Rudd B.D., Burstein E., Duckett C.S., Li X., Lukacs N.W. Differential role for TLR3 in respiratory syncytial virus-induced chemokine expression. J Virol. 2005;79:3350–3357. doi: 10.1128/JVI.79.6.3350-3357.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Veckman V., Osterlund P., Fagerlund R., Melen K., Matikainen S., Julkunen I. TNF-alpha and IFN-alpha enhance influenza-A-virus-induced chemokine gene expression in human A549 lung epithelial cells. Virology. 2005;345:96–104. doi: 10.1016/j.virol.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 83.Papadopoulos N.G., Papi A., Meyer J., Stanciu L.A., Salvi S., Holgate S.T. Rhinovirus infection up-regulates eotaxin and eotaxin-2 expression in bronchial epithelial cells. Clin Exp Allergy. 2001;31:1060–1066. doi: 10.1046/j.1365-2222.2001.01112.x. [DOI] [PubMed] [Google Scholar]
- 84.Edwards M.R., Haas J., Panettieri R.A., Jr., Johnson M., Johnston S.L. Corticosteroids and beta2 agonists differentially regulate rhinovirus-induced interleukin-6 via distinct Cis-acting elements. J Biol Chem. 2007;282:15366–15375. doi: 10.1074/jbc.M701325200. [DOI] [PubMed] [Google Scholar]
- 85.Oliver B.G., Johnston S.L., Baraket M., Burgess J.K., King N.J., Roth M. Increased proinflammatory responses from asthmatic human airway smooth muscle cells in response to rhinovirus infection. Respir Res. 2006;7:71. doi: 10.1186/1465-9921-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Funkhouser A.W., Kang J.A., Tan A., Li J., Zhou L., Abe M.K. Rhinovirus 16 3C protease induces interleukin-8 and granulocyte-macrophage colony-stimulating factor expression in human bronchial epithelial cells. Pediatr Res. 2004;55:13–18. doi: 10.1203/01.PDR.0000099801.06360.AB. [DOI] [PubMed] [Google Scholar]
- 87.Kim J., Sanders S.P., Siekierski E.S., Casolaro V., Proud D. Role of NF-kappa B in cytokine production induced from human airway epithelial cells by rhinovirus infection. J Immunol. 2000;165:3384–3392. doi: 10.4049/jimmunol.165.6.3384. [DOI] [PubMed] [Google Scholar]
- 88.Einarsson O., Geba G.P., Zhu Z., Landry M., Elias J.A. Interleukin-11: stimulation in vivo and in vitro by respiratory viruses and induction of airways hyperresponsiveness. J Clin Invest. 1996;97:915–924. doi: 10.1172/JCI118514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Volonaki E., Psarras S., Xepapadaki P., Psomali D., Gourgiotis D., Papadopoulos N.G. Synergistic effects of fluticasone propionate and salmeterol on inhibiting rhinovirus-induced epithelial production of remodelling-associated growth factors. Clin Exp Allergy. 2006;36:1268–1273. doi: 10.1111/j.1365-2222.2006.02566.x. [DOI] [PubMed] [Google Scholar]
- 90.Inoue D., Yamaya M., Kubo H., Sasaki T., Hosoda M., Numasaki M. Mechanisms of mucin production by rhinovirus infection in cultured human airway epithelial cells. Respir Physiol Neurobiol. 2006;154:484–499. doi: 10.1016/j.resp.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 91.Thanos D., Maniatis T. NF-kappa B: a lesson in family values. Cell. 1995;80:529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 92.Schedel M., Pinto L.A., Schaub B., Rosenstiel B., Cherkasov D., Cameron L. IRF-1 gene variations influence IgE regulation and atopy. Am J Respir Crit Care Med. 2008;177:613–621. doi: 10.1164/rccm.200703-373OC. [DOI] [PubMed] [Google Scholar]
- 93.Harada M., Nakashima K., Hirota T., Shimizu M., Doi S., Fujita K. Functional polymorphism in the suppressor of cytokine signaling 1 gene associated with adult asthma. Am J Respir Cell Mol Biol. 2007;36:491–496. doi: 10.1165/rcmb.2006-0090OC. [DOI] [PubMed] [Google Scholar]
- 94.Forton J.T., Rowlands K., Rockett K., Hanchard N., Herbert M., Kwiatkowski D.P. Genetic association study for RSV bronchiolitis in infancy at the 5q31 cytokine cluster. Thorax. 2009;64:345–352. doi: 10.1136/thx.2008.102111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leigh R., Oyelusi W., Wiehler S., Koetzler R., Zaheer R.S., Newton R. Human rhinovirus infection enhances airway epithelial cell production of growth factors involved in airway remodeling. J Allergy Clin Immunol. 2008;121:1238–1245. doi: 10.1016/j.jaci.2008.01.067. [DOI] [PubMed] [Google Scholar]
- 96.Wang S.W., Oh C.K., Cho S.H., Hu G., Martin R., Demissie-Sanders S. Amphiregulin expression in human mast cells and its effect on the primary human lung fibroblasts. J Allergy Clin Immunol. 2005;115:287–294. doi: 10.1016/j.jaci.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 97.Cho S.H., Yao Z., Wang S.W., Alban R.F., Barbers R.G., French S.W. Regulation of activin A expression in mast cells and asthma: its effect on the proliferation of human airway smooth muscle cells. J Immunol. 2003;170:4045–4052. doi: 10.4049/jimmunol.170.8.4045. [DOI] [PubMed] [Google Scholar]
- 98.Le A.V., Cho J.Y., Miller M., McElwain S., Golgotiu K., Broide D.H. Inhibition of allergen-induced airway remodeling in Smad 3-deficient mice. J Immunol. 2007;178:7310–7316. doi: 10.4049/jimmunol.178.11.7310. [DOI] [PubMed] [Google Scholar]
- 99.Feltis B.N., Wignarajah D., Zheng L., Ward C., Reid D., Harding R. Increased vascular endothelial growth factor and receptors: relationship to angiogenesis in asthma. Am J Respir Crit Care Med. 2006;173:1201–1207. doi: 10.1164/rccm.200507-1105OC. [DOI] [PubMed] [Google Scholar]
- 100.Simcock D.E., Kanabar V., Clarke G.W., O'Connor B.J., Lee T.H., Hirst S.J. Proangiogenic activity in bronchoalveolar lavage fluid from patients with asthma. Am J Respir Crit Care Med. 2007;176:146–153. doi: 10.1164/rccm.200701-042OC. [DOI] [PubMed] [Google Scholar]
- 101.Li X., Wilson J.W. Increased vascularity of the bronchial mucosa in mild asthma. Am J Respir Crit Care Med. 1997;156:229–233. doi: 10.1164/ajrccm.156.1.9607066. [DOI] [PubMed] [Google Scholar]
- 102.Lee C.G., Link H., Baluk P., Homer R.J., Chapoval S., Bhandari V. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med. 2004;10:1095–1103. doi: 10.1038/nm1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bardin P.G., Johnston S.L., Sanderson G., Robinson B.S., Pickett M.A., Fraenkel D.J. Detection of rhinovirus infection of the nasal mucosa by oligonucleotide in situ hybridization. Am J Respir Cell Mol Biol. 1994;10:207–213. doi: 10.1165/ajrcmb.10.2.8110476. [DOI] [PubMed] [Google Scholar]
- 104.Papadopoulos N.G., Bates P.J., Bardin P.G., Papi A., Leir S.H., Fraenkel D.J. Rhinoviruses infect the lower airways. J Infect Dis. 2000;181:1875–1884. doi: 10.1086/315513. [DOI] [PubMed] [Google Scholar]
- 105.Ghildyal R., Dagher H., Donninger H., de Silva D., Li X., Freezer N.J. Rhinovirus infects primary human airway fibroblasts and induces a neutrophil chemokine and a permeability factor. J Med Virol. 2005;75:608–615. doi: 10.1002/jmv.20315. [DOI] [PubMed] [Google Scholar]
- 106.Bedke N., Haitchi H.M., Xatzipsalti M., Holgate S.T., Davies D.E. Contribution of bronchial fibroblasts to the antiviral response in asthma. J Immunol. 2009;182:3660–3667. doi: 10.4049/jimmunol.0802471. [DOI] [PubMed] [Google Scholar]
- 107.Redington A.E., Madden J., Frew A.J., Djukanovic R., Roche W.R., Holgate S.T. Transforming growth factor-beta 1 in asthma: measurement in bronchoalveolar lavage fluid. Am J Respir Crit Care Med. 1997;156:642–647. doi: 10.1164/ajrccm.156.2.9605065. [DOI] [PubMed] [Google Scholar]
- 108.Minshall E.M., Leung D.Y., Martin R.J., Song Y.L., Cameron L., Ernst P. Eosinophil-associated TGF-beta1 mRNA expression and airways fibrosis in bronchial asthma. Am J Respir Cell Mol Biol. 1997;17:326–333. doi: 10.1165/ajrcmb.17.3.2733. [DOI] [PubMed] [Google Scholar]
- 109.Meyer G., Deplanche M., Schelcher F. Human and bovine respiratory syncytial virus vaccine research and development. Comp Immunol Microbiol Infect Dis. 2008;31:191–225. doi: 10.1016/j.cimid.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 110.Karron R.A., Wright P.F., Belshe R.B., Thumar B., Casey R., Newman F. Identification of a recombinant live attenuated respiratory syncytial virus vaccine candidate that is highly attenuated in infants. J Infect Dis. 2005;191:1093–1104. doi: 10.1086/427813. [DOI] [PubMed] [Google Scholar]
- 111.Mejias A., Chavez-Bueno S., Jafri H., Ramilo O. Respiratory syncytial virus infections: old challenges and new opportunities. Pediatr Infect Dis J. 2005;24(suppl):S189–S197. doi: 10.1097/01.inf.0000188196.87969.9a. [DOI] [PubMed] [Google Scholar]
- 112.IMPACT study Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics. 1998;102:531–537. [PubMed] [Google Scholar]
- 113.Simoes E.A., Groothuis J.R., Carbonell-Estrany X., Rieger C.H., Mitchell I., Fredrick L.M. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr. 2007;151:34–42. doi: 10.1016/j.jpeds.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 114.Wu H., Pfarr D.S., Johnson S., Brewah Y.A., Woods R.M., Patel N.K. Development of motavizumab, an ultra-potent antibody for the prevention of respiratory syncytial virus infection in the upper and lower respiratory tract. J Mol Biol. 2007;368:652–665. doi: 10.1016/j.jmb.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 115.Carbonell-Estrany X, Losonsky GA, Micki H, Edward C. Phase 3 trial of motavizumab (MEDI-524), an enhanced potency respiratory syncytial virus (RSV) specific monoclonal antibody (Mab) for the prevention of serious RSV disease in high risk infants. Poster presented at: Pediatric American Society Annual Meeting; May 8, 2007; Toronto, Ontario, Canada.
- 116.Lagos R., Devincenzo J.P., Munoz A., Hultquist M., Suzich J., Connor E.M. Safety and antiviral activity of motavizumab, a respiratory syncytial virus (RSV)-specific humanized monoclonal antibody, when administered to rsv-infected children. Pediatr Infect Dis J. 2009;28:835–837. doi: 10.1097/INF.0b013e3181a165e4. [DOI] [PubMed] [Google Scholar]
- 117.Turner R.B., Wecker M.T., Pohl G., Witek T.J., McNally E., St George R. Efficacy of tremacamra, a soluble intercellular adhesion molecule 1, for experimental rhinovirus infection: a randomized clinical trial. JAMA. 1999;281:1797–1804. doi: 10.1001/jama.281.19.1797. [DOI] [PubMed] [Google Scholar]
- 118.Dragovich P.S., Prins T.J., Zhou R., Brown E.L., Maldonado F.C., Fuhrman S.A. Structure-based design, synthesis, and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. 6. Structure–activity studies of orally bioavailable, 2-pyridone-containing peptidomimetics. J Med Chem. 2002;45:1607–1623. doi: 10.1021/jm010469k. [DOI] [PubMed] [Google Scholar]
- 119.Binford S.L., Maldonado F., Brothers M.A., Weady P.T., Zalman L.S., Meador J.W., III Conservation of amino acids in human rhinovirus 3C protease correlates with broad-spectrum antiviral activity of rupintrivir, a novel human rhinovirus 3C protease inhibitor. Antimicrob Agents Chemother. 2005;49:619–626. doi: 10.1128/AAC.49.2.619-626.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Patick A.K., Brothers M.A., Maldonado F., Binford S., Maldonado O., Fuhrman S. In vitro antiviral activity and single-dose pharmacokinetics in humans of a novel, orally bioavailable inhibitor of human rhinovirus 3C protease. Antimicrob Agents Chemother. 2005;49:2267–2275. doi: 10.1128/AAC.49.6.2267-2275.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.De Palma A.M., Vliegen I., De Clercq E., Neyts J. Selective inhibitors of picornavirus replication. Med Res Rev. 2008;28:823–884. doi: 10.1002/med.20125. [DOI] [PubMed] [Google Scholar]
- 122.Edwards M.R., Johnson M.W., Johnston S.L. Combination therapy: synergistic suppression of virus-induced chemokines in airway epithelial cells. Am J Respir Cell Mol Biol. 2006;34:616–624. doi: 10.1165/rcmb.2005-0385OC. [DOI] [PubMed] [Google Scholar]
- 123.Skevaki C.L., Christodoulou I., Spyridaki I.S., Tiniakou I., Georgiou V., Xepapadaki P. Budesonide and formoterol inhibit inflammatory mediator production by bronchial epithelial cells infected with rhinovirus. Clin Exp Allergy. 2009;39:1700–1710. doi: 10.1111/j.1365-2222.2009.03307.x. [DOI] [PubMed] [Google Scholar]
- 124.Volonaki E., Psarras S., Xepapadaki P., Psomali D., Gourgiotis D., Papadopoulos N.G. Synergistic effects of fluticasone propionate and salmeterol on inhibiting rhinovirus-induced epithelial production of remodelling-associated growth factors. Clin Exp Allergy. 2006;36:1268–1273. doi: 10.1111/j.1365-2222.2006.02566.x. [DOI] [PubMed] [Google Scholar]
- 125.Jang Y.J., Kwon H.J., Lee B.J. Effect of clarithromycin on rhinovirus-16 infection in A549 cells. Eur Respir J. 2006;27:12–19. doi: 10.1183/09031936.06.00008005. [DOI] [PubMed] [Google Scholar]
- 126.Gielen V., Johnston S.L., Edwards M.R. Azithromycin induces anti-viral responses in bronchial epithelial cells. Eur Respir J. 2010 doi: 10.1183/09031936.00095809. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 127.Tahan F., Ozcan A., Koc N. Clarithromycin in the treatment of RSV bronchiolitis: A double-blind, randomised, placebo-controlled trial. Eur Respir J. 2007;29:91–97. doi: 10.1183/09031936.00029206. [DOI] [PubMed] [Google Scholar]
- 128.Johnston S.L., Blasi F., Black P.N., Martin R.J., Farrell D.J., Nieman R.B. The effect of telithromycin in acute exacerbations of asthma. N Engl J Med. 2006;354:1589–1600. doi: 10.1056/NEJMoa044080. [DOI] [PubMed] [Google Scholar]
- 129.Johnston S.L. Macrolide antibiotics and asthma treatment. J Allergy Clin Immunol. 2006;117:1233–1236. doi: 10.1016/j.jaci.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 130.Simon H.U., Seelbach H., Ehmann R., Schmitz M. Clinical and immunological effects of low-dose IFN-alpha treatment in patients with corticosteroid-resistant asthma. Allergy. 2003;58:1250–1255. doi: 10.1046/j.1398-9995.2003.00424.x. [DOI] [PubMed] [Google Scholar]
- 131.Scott G.M., Phillpotts R.J., Wallace J., Gauci C.L., Greiner J., Tyrrell D.A. Prevention of rhinovirus colds by human interferon alpha-2 from Escherichia coli. Lancet. 1982;2:186–188. doi: 10.1016/s0140-6736(82)91031-5. [DOI] [PubMed] [Google Scholar]
- 132.Gwaltney J.M., Jr., Buier R.M., Rogers J.L. The influence of signal variation, bias, noise and effect size on statistical significance in treatment studies of the common cold. Antiviral Res. 1996;29:287–295. doi: 10.1016/0166-3542(95)00935-3. [DOI] [PubMed] [Google Scholar]
- 133.Hayden F.G., Gwaltney J.M., Jr. Intranasal interferon-alpha 2 treatment of experimental rhinoviral colds. J Infect Dis. 1984;150:174–180. doi: 10.1093/infdis/150.2.174. [DOI] [PubMed] [Google Scholar]
- 134.Bochkov Y.A., Hanson K.M., Keles S., Brockman-Schneider R.A., Jarjour N.N., Gern J.E. Rhinovirus-induced modulation of gene expression in bronchial epithelial cells from subjects with asthma. Mucosal Immunol. 2010;3:69–80. doi: 10.1038/mi.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]