Abstract
Human enteric adenovirus Ad41 is associated with children gastroenteritis. To infect gastrointestinal cells, the invading virus must be acid-stable and resistant to inactivation by bile salts and proteases. In addition, it has to cross the mucus barrier before it infects mucosa cells. We show that Ad41 infectivity is not diminished by acid exposure, a condition limiting the infectivity of the respiratory Ad. This feature can be attributed to a large extent to the global basic charge of enteric Ad virions and to the stability of Ad41 fiber, a viral protein mediating virus attachment. Upon exposure to pH shock, the respiratory Ad2 loses its ability to interact with lipids while enteric Ad41 still binds to the major phospholipids of gastric and intestine mucus. In addition, contrary to respiratory Ad, enteric Ad41 interacts with several sphingolipid components of plasma membranes. These results show that the molecular bases of the Ad41 enteric tropism stem from its particular physicochemical properties.
Keywords: Ad41, Enteric adenovirus, Gut tropism, Basic charge, Acid shock, Virus–lipid interactions, Mucus layer, Sphingolipids
Abbreviations: AD, adenovirus; FFU, focus forming units; DPPC, dipalmitoyl phosphatidylcholine; DPPG, dipalmitoyl phosphatidylglycerol; PE, phosphatidylethanolamine; RT, room temperature
Introduction
The human stomach secretes about 2.5 l of gastric juice daily. The hydrochloric acid synthesized and secreted by gastric parietal cells may reduce the intraluminal pH to below 2.0. A variety of proteases, secreted by gastric and pancreatic cells and bile salts, enter the duodenum from the biliary tract. To protect the mucosa from the gastric juice, both specialized gastric and intestine cells secrete mucus forming a gel-like layer of about 200 nm. The bicarbonate ions trapped in the mucus create a pH gradient from 6–7 at the epithelium surface to 1–2 in the stomach lumen.
Such extreme conditions encountered in the gastrointestinal tract (GI) are highly inhospitable to invading viruses. Indeed, to initiate infection via the GI route, the virus must be acid-stable and resistant to inactivation by bile salts and proteolytic enzymes. It should also be able to withstand the pH jump from 1–2 in stomach to 7.5 in the intestine. Finally, it has to cross the mucus layer before it encounters and infects the cells lining the GI.
Enteric adenoviruses of subgroup F (Ad40 and Ad41) are associated with gastroenteritis in children Jacobsson et al., 1979, Uhnoo et al., 1983, Uhnoo et al., 1984 and are known as an important enteric pathogen second to rotavirus. The molecular bases of their narrow and specific tropism for GI, unique among the Ads, are still not understood. In contrast to enteroviruses or hepatitis A producing systemic illness, these enteric Ads, similarly to rotaviruses or coronaviruses, do not cause disease outside GI, which may be related to the efficient host immune response. They can be isolated in the infectious state from the stools of infected children (Gary et al., 1979), with approximately 1011 physical virus particles/g of stool, which again shows their survival in the human digestive tract de Jong et al., 1983, Uhnoo et al., 1984.
The adenovirus capsid is composed of three major oligomeric proteins. The trimeric hexons form 20 facettes of viral icosahedron which is sealed at each of the 12 vertices by a complex of the pentameric penton base and an outward extending trimeric fiber. These proteins are multifunctional. In addition to its structural role, the fiber protein is involved in virus attachment through binding primary cell receptor. The penton base protein is responsible for virus cell entry through binding to cellular αv integrins (Wickham et al., 1993) and viral release from endosomes on the pathway towards the cell nucleus (Greber et al., 1993). Recently, we have shown that the hexon of respiratory Ad promotes virus entry independently of CAR (primary) and integrin (secondary) receptors through interaction with phospholipids (Balakireva et al., 2002). This might explain the ability of the respiratory Ad2 to cross the physical barrier composed of lipid surfactant before infecting alveolar epithelium.
During cell attachment, the distal C-terminal globular head domain of the fiber protein interacts with the primary receptor, which for some human Ad serotypes is the coxsackievirus and adenovirus receptor (CAR) Bergelson et al., 1997, Roelvink et al., 1998. While the majority of human Ad serotypes have only one kind of fiber, the enteric serotypes 40 and 41 possess two different fibers Kidd et al., 1993, Pieniazek et al., 1990, Yeh et al., 1994 present in the virion in equal ratio (Favier et al., 2002). Similar to fibers of respiratory Ads, the Ad40/41 long fiber interacts with CAR. However, CAR is not recognized by the short fiber (Roelvink et al., 1998). The narrow tropism of enteric Ads cannot result alone from Ad40/41 long fiber interaction with CAR. CAR mRNA is preferentially expressed in the heart, testis, prostate, and pancreas, which are not tissues targeted by enteric Ads infection. Moreover, the internalization of the majority of Ad serotypes is due to their interaction with integrins αvβ3 and αvβ5 via an RGD motif present in the virus penton base protein Bai et al., 1994, Mathias et al., 1994, Wickham et al., 1993. However, Ad40 and Ad41 lack the RGD motif in their penton bases or fibers (Albinsson and Kidd, 1999). Altogether, these data imply that the entry of enteric Ads is clearly different from that of respiratory serotypes.
We feel that Ad40/41 tropism depends to some extent on the short fiber even though studies on several cells lines showed that the short fiber is not involved in virus attachment (Roelvink et al., 1998, Favier, 2002, personal communication; Nakamura et al., 2003). The short fiber might be involved in virus multiplication through interaction with cellular protein partners (Chroboczek et al., 2003) downstream of virus initial interaction with the cell plasma membrane. In this study, we analyzed the physicochemical properties of Ad41 by distinguishing it from the respiratory serotypes. It is reasonable to assume that the tropism of Ad41 is defined by a combination of features permitting to survive in the stomach and to pass through the mucus layer followed by the recognition of specific cellular plasma membrane partners leading to the successful infection of cells lining the GI.
Results
Physicochemical properties of Ad virions
In the first attempt to understand the survival mechanism of the human enteric Ad41 under acid conditions of the stomach, we compared the predicted pI values of external structural proteins of different Ad serotypes (Table 1) . Using the primary sequences available in the databases, we found that Ad40/41 fibers have very basic predicted pI values in contrast to the predicted acidic values of structural proteins of the majority of human adenoviruses. In particular, the difference of more than 3 pH units between the enteric Ad41 short fiber and fibers of respiratory viruses Ad2 and Ad3 is very striking. Ad37 and Ad8 also display a high pI of the fiber and surprisingly high pI of the Ad37 penton base. The differences in charge of head domains of fibers of different serotypes are illustrated by the clusters of basic amino acids shown in the atomic or modeled structures of head domains of fibers of different serotypes (in blue in Fig. 1 ).
Table 1.
Predicted pI values of Ad major capsid proteins
| Tropism | Serotype | Hexon | Penton base | Fiber (head) | Subgenus | Receptor used |
|---|---|---|---|---|---|---|
| Enteric | Ad41,Long | 5.49 | 5.75 | 7.51 (8.64) | F | CAR |
| Short | 9.13 (7.24) | Not CAR | ||||
| Ad40,Long | 6.68 | 5.75 | 7.51 | F | CAR | |
| Short | 7.78 | Not CAR | ||||
| Respiratory | Ad2 | 4.86 | 5.05 | 5.85 (6.35) | C | CAR |
| Ad5 | 5.03 | 5.18 | 5.89 (5.91) | C | CAR | |
| Ad4 | 4.58 | n.d. | 5.25 | E | CAR | |
| Urinary | Ad35 | 5.10 | 5.32 | 4.71 | B | Not CAR |
| Respiratory and ocular | Ad3 | 5.32 | 5.26 | 5.61 | B | Not CAR |
| Enteric | Ad31 | 5.25 | n.d. | 6.11 | A | CAR |
| (c.d.?) | Ad12 | 5.55 | 6.20 | 4.60 | A | CAR |
| Ocular and urogenital | Ad37 | n.d. | 8.76 | 9.56 (9.28) | D | Sialic acid (Not CAR) |
| Ocular |
Ad8 | 5.50 | 5.39 | 9.42 | D | Not CAR |
| Ad30 | n.d. | n.d. | 5.16 | D | Not CAR | |
| Ad9 | 4.72 | n.d. | 5.70 | D | CAR | |
| Ad15 |
4.76 |
n.d. |
5.71 |
D |
CAR |
c.d.?: suggested involvement in coeliac disease (Kagnoff et al., 1987). n.d.: sequence not known. Accession numbers to the sequences used for the calculation of predicted pI values are given in Table 2 (see Materials and methods).
Fig. 1.
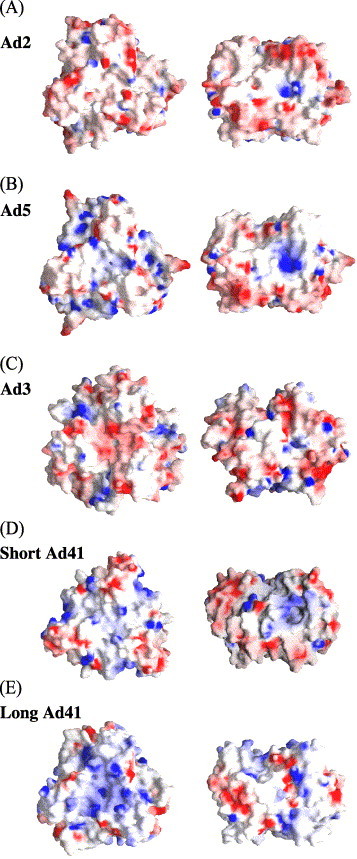
Electrostatic surface potential of fiber knob domains. The molecular surface and its potential have been calculated using GRASP (Nicolls et al., 1991). Colors range from red (potential of −10 kT) to blue (10 kT). Two views are shown, the view on top of the receptor and a side view turned by 90° around x with the top of the fiber head pointing upwards. (A) Ad2 (PDB entry 1 qhv; van Raaij et al., 1999); (B) Ad5 (PDB 1 knb; Xia et al., 1995); (C) Ad3 (PDB 1 h7z; Durmort et al., 2001); (D) theoretical model of Ad41 short fiber. The model has been built using the SWISS-MODEL together with the Swiss-PDB Viewer graphical interface (http://www.expasy.ch/swissmod/SWISS-MODEL.html). It is based mainly on Ad12 (1 nob; Bewley et al., 1999). It has been verified using O (Jones and Miller, 1991) and a badly constructed loop has been corrected manually. (E) Theoretical model of Ad41 long fiber obtained with SWISS-MODEL based mainly on the structure of Ad5.
Approximately 87% of the Ad particle mass consists of proteins (Green and Pina, 1963). It is thus reasonable to assume that the charge difference of some Ad proteins is reflected in the charge of the whole virus particle. Indeed, the particle migration varied when the mobility of different Ad serotypes is compared on native gel (Fig. 2) . While serotypes 2, 3, and 5 migrate towards the anode, Ad41 remained in the wells. The quality of virus preparations was checked by EM, and no aggregation has been noticed (results not shown). Acid treatment did not seem to affect virus mobility (compare a with b in Fig. 2), suggesting that virions were not disassembled. Under non-denaturing conditions, the mobility on native gel depends on the size, the shape (form), and the charge of the migrating entity. The organization and form of Ad virions are quite similar, and the serotypes differ only slightly in their DNA content; for example, Ad2 DNA contains 35 937 bases (Roberts et al., 1984) whereas Ad40 DNA contains 34 214 bases (Davison et al., 1993). It seems therefore that the observed mobility differences stem mainly from the charge difference of the viral proteins and in the case of Ad41 can be attributed to somewhat more basic penton bases, hexons, and significantly more basic fibers (Table 1).
Fig. 2.
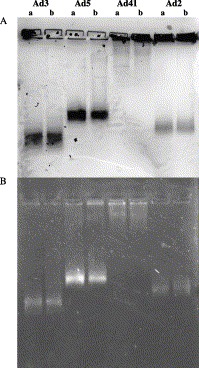
Virus mobility on non-denaturing agarose gel. Ads 3, 5, 41, and 2 were electrophoresed in agarose gel after (a) or before (b) acid treatment as described in Materials and methods. (A) Viral proteins were revealed by Coomassie Brilliant Blue stain. (B) Viral DNA was revealed with ethidium bromide.
Effect of acid exposure on virus viability
Even if it is not known which part of the digestive tract is primarily infected by enteric Ads, it is clear from a mode of dissemination by oral or fecal route that these viruses have to withstand the conditions encountered in the stomach. Using pH of the normal fasting human stomach, we compared the survival of infectious Ads during exposure to acid. We used 293 cells as they are permissive for both Ad41 and Ad2 and conventionally used for Ad41 production. To establish the experimental conditions for the infectivity assessment, at the beginning we obtained a curve of infection using increasing virus quantities for the same amounts of attached cells as described by Favier et al. (2002). From this we estimated the virus amount giving the maximum (saturation) of infection as well as giving 50% of maximum infection. For each virus infection, two sets of condition were used, 50% and nearly 100% of infectivity saturation, and progeny production was measured after viral inoculum has been exposed to buffered HCl. Preliminary time course experiment demonstrated that for Ad2 the largest drop in infectivity occurred after 5 min of acid treatment (results not shown). Therefore, similar to studies on rotavirus acid stability (Weiss and Clark, 1985), short pH exposure was performed. Such exposure, in contrast with long dialysis at pH 6.4–6.8 Pereira and Wrigley, 1974, van Oostrum and Burnett, 1985, does not result in Ad vertex removal. Because acid secretion by gastric parietal cells reduces the intraluminal pH to about 2.0 in adults and to about 4.0 in children (Gryboski and Walker, 1983), both conditions were employed (Fig. 3) . The scatter of results is typical for this kind of experiments; therefore, 33 experiments have been carried out after acid exposure. In about half of the experiments, the Ad41 infectivity was not affected by acid exposure compared to virus diluted in culture medium. Interestingly, in 17 cases out of 33, the infectivity of Ad41 increased. However, at the same time the infectivity of Ad2 was inhibited by acid exposure, with a negligible activation observed in three cases out of 33. On average, the Ad41 infectivity was unchanged, whereas Ad2 infectivity decreased by 20%. No notable difference was observed for pH 2 versus pH 4 or at room temperature (RT) versus 37 °C, in contrast with the results obtained for another enteric virus, rotavirus. For this virus, acid resistance was significantly higher at pH 4 than at pH 2 and at RT than at 37 °C (Weiss and Clark, 1985). These experiments show the remarkable stability of enteric Ad under pH conditions of the human fasting stomach.
Fig. 3.
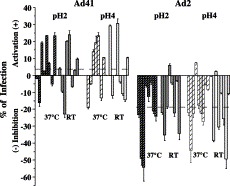
Virus infectivity after acid treatment. 293 cells were infected with Ad2 or Ad41 using two different MOI (see Materials and methods). Virus portions were untreated or exposed to HCl at pH 2 or 4 for 1 min at RT or at 37 °C. Exposure was stopped by dilution in culture medium. Virus progeny was estimated by fluorescence as described in Materials and methods. The results in percent show the activation or inhibition of infection upon HCl treatment (above or below zero line, respectively), where baseline is the infection level for the untreated virus.
Effect of acid exposure on Ad fiber protein
Ad fiber is known to be an extremely sturdy protein, insensitive to different attempts to denature and de-oligomerize it (Devaux et al., 1987). As this protein mediates the initial virus attachment to infected cells, it was of interest to analyze its behavior after acid exposure. Because contrary to respiratory serotypes Ad2 and Ad5, Ad41 (as well as Ad3) fibers cannot be purified from Ad41-infected cells without the penton base (see Favier et al., 2002), therefore Ad41 penton, complex of fiber, and penton base had to be used in these experiments. In the first experiment, the fiber interactions with the primary receptor CAR were compared before or after acid exposure (Fig. 4) . The extracellular domain of CAR was tested to interact with Ad41 short and long fibers contained in pentons (SP41 and LP41), with Ad5 fiber (F5), and with Ad3 fiber contained in Ad3 penton dodecahedra, symmetrical assemblies of 12 Ad3 pentons (P3) (Fender et al., 1997). In agreement with the data of Roelvink et al. (1998), neither Ad3 nor short Ad41 fibers did recognize CAR, whereas both Ad5 and long Ad41 fibers interacted with the receptor. These results were also found after acid exposure, showing that this treatment does not trigger any major structural change of the fiber protein, which would abolish CAR interaction.
Fig. 4.
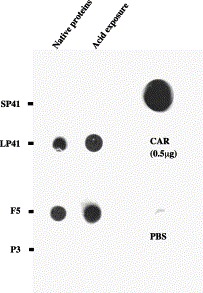
Fiber interaction with CAR. Native purified proteins (0.5 μg) were dotted onto the nitrocellulose membrane before or after acid treatment for 1 min at pH 2.0 then allowed to interact with 1 μg of purified recombinant extracellular domain of human CAR. CAR binding was visualized by Western blot with anti-CAR antibody. Ad41 short and long pentons, Ad5 fiber, and Ad3 penton-dodecahedron (composed of 12 pentons, complexes of base and fiber) are noted as SP41, LP41, F5, and P3, respectively. The positive (CAR) and negative (PBS) controls of anti-CAR antibody are shown on the right.
Proteolytic analysis was employed for further analysis of the eventual changes in the fiber structure upon acid exposure. Because the trimeric and well-folded fiber protein is largely resistant to chymotrypsin (it is cut at a unique N-terminal location only, at the elevated amount of enzyme, Devaux et al., 1987), we used this enzyme to probe fiber protein stability. Native fibers and pentons were purified from cells infected with wild-type viruses. Because we had to use the pentons for Ad41 (see explanation above), the penton was also used for Ad5 (P5). The fibers were revealed with antibody. Initial proper folding of fibers was verified by the presence of the trimeric forms. Under denaturing conditions, Ad5 and long Ad41 fiber monomers run according to their molecular masses of about 62 000 for Ad5 and long Ad41 fibers and about 42 000 for short Ad41 fiber (Fig. 5 , lanes 1). Under non-denaturing conditions, the trimeric long fibers with molecular masses of about 180 000 run above the 175-kDa marker and the trimeric Ad41 short fiber (molecular mass of about 130 000) runs between 82- and 175-kDa markers (Fig. 5, lanes 2). As in the previous experiment, exposure to acid alone did not significantly affect the fiber trimers (lanes 3). Purified Ad5 fiber (F5) was destroyed by the combined action of acid exposure and chymotrypsin (Fig. 5C, lane 5 for F5). Contrary to that, chymotrypsin after prior acid treatment did not result in proteolysis of the Ad41 fibers (Figs. 5A and B, lanes 5). Altogether, these results show that acid exposure followed by pH jump to 7.5 results in such subtle changes in the Ad5 fiber structure that it can still interact with CAR (Fig. 4). However, these changes are sufficient to permit ensuing proteolysis of the respiratory serotype 5 fiber (Fig. 5). On the contrary, both fibers of enteric Ad survive well acid exposure, pH jump to 7.5 and the proteolytic treatment, showing the unusual stability under GI simulating conditions encountered by enteric viruses.
Fig. 5.
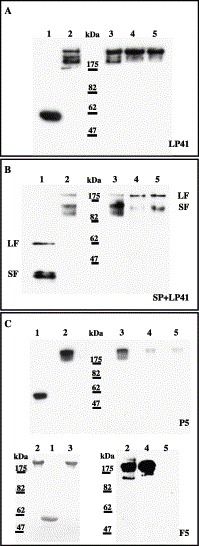
Proteolytic analysis of Ad fibers. (A) Purified wild-type Ad41 long pentons LP41 (complex of penton base and long fiber), 0.2-μg portions. (B) Purified wild-type Ad41 short and long pentons in mixture, SP + LP41, 0.1-μg portions each. (C) Purified wild-type Ad5 pentons (P5, 1-μg portions) or Ad5 fiber (F5, 1-μg portions). The proteins were electrophoresed on a 7.5% or 10% SDS-PAGE gel at 4 °C. Lane 1, boiled fiber; lane 2, native fiber; lane 3, 1-min treatment with HCl, pH 2 at 37 °C; lane 4, fiber digested by chymotrypsin at pH 7.5 with ratio 1/5 enzyme/substrate; lane 5, fiber exposed to HCl for 1 min at pH 2 at 37 °C, neutralized, and digested by chymotrypsin at final pH 7.5 with ratio 1/5 enzyme/substrate. Fibers in pentons were revealed by Western blot with the appropriate antibody. Ad5 fiber was visualized by staining with Coomassie Brilliant Blue (F5, left side) or revealed by Western blot (F5, right side).
Virus–lipids interaction
To understand the mechanism allowing Ad41 to cross the mucus barrier separating the GI lumen from cells lining the GI, we studied Ad41 interaction with lipids and compared it with the respiratory Ad2. Mucosa cells of the stomach contain lamellar bodies that function as lipid storage and secretory organelles, permitting synthesis, excretion, and turnover of the gastric surfactant. Phospholipids exert the greatest impact on the physicochemical properties of gastric mucus. It was found initially that the major phospholipid of the lamellar bodies of the mucosa cells in the stomach is dipalmitoyl phosphatidylcholine (DPPC) (Schmitz and Muller, 1991). It was also thought that this phospholipid would also be predominant in the mucus (gastric surfactant) (Lichtenberger, 1995). However, recent data show that both gastric mucosa and mucus contain significant amounts of unsaturated PC together with phosphatidylethanolamine (PE) Bernhard et al., 1995, Larhed et al., 1998, Nardone et al., 1993.
The Ads interaction with lipids was analyzed using a protein–lipid overlay assay. The assay was performed in the absence or presence of magnesium ions, following the conditions used for the investigation of lipid interaction with pleckstrin homology domains Dowler et al., 1999, Thomas et al., 2001, Thomas et al., 2002. PC, DPPC, PE, and dipalmitoyl phosphatidylglycerol (DPPG) were spotted onto a nitrocellulose membrane and incubated with Ad2 and Ad41 in comparable molar amounts. It should be noted that the assay sensitivity was ensured by the amount of the immobilized lipid being in the pmol range (Thomas et al., 2001). Before acid treatment, at both binding conditions used, Ad2 attached to DPPC, whereas Ad41 attached to DPPC only at pH 6, additionally attaching strongly to DPPG (Fig. 6) . To simulate the abrupt pH jump induced by the passage from stomach to intestine, both viruses were exposed to pH 2 for 1 min at 37 °C, then brought up to pH 7.5 and incubated with immobilized lipids. Contrary to the untreated Ad2 case, no lipid interaction was observed after Ad2 was exposed to a pH shock (Fig. 6). Most interestingly, pH shock of enteric Ad41 confirmed its interaction with DPPC and DPPG and improved its interaction with two other phospholipids. The loss of the lipid interaction by Ad2 can be explained by the changes in virus integrity induced by acid treatment, as it has been illustrated by the decrease in infectivity and in fiber stability Fig. 3, Fig. 5. In the case of Ad41, there is no loss of infectivity upon acid treatment, which would suggest the conservation of virion integrity. However, an increase in lipid interaction observed for Ad41 upon acid shock suggests in addition some capsid transformation improving its ability to recognize different classes of phospholipids.
Fig. 6.
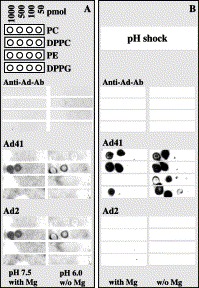
Lipid-binding properties of Ad41 and Ad2. Serial dilutions of PC, DPPC, PE, and DPPG were spotted onto nitrocellulose membranes and interactions with viruses were tested in the presence or absence (w/o) of Mg2+. Bound viruses were detected with specific antibody. (A) Untreated viruses at pH 7.5. (B) Ad41 and Ad2 were treated by pH jump as described in Materials and methods. The control blot with antibodies alone is shown in the upper part.
Mucosal pathogens target sites of infection through specific adherence to host glycoconjugate receptors Mahdavi et al., 2002, Svensson et al., 2003. One class of such receptors is glycoshingolipids, a highly polymorphic class of lipids, which occur in mammalian cells expressed on the cell surface and are predominant in the gastric epithelium (reviewed in Hakomori, 1990). To understand the interaction of enteric Ad with the surface of mucosa cells, the analysis of Ad41's ability to interact with lipids was extended to sphingolipids. Membrane-immobilized sphingolipids were incubated with both viruses (Fig. 7) . Both Ad41 and Ad2 interacted with sulfatide; however, only Ad41 interacted specifically with four additional sphingolipids: sphingosine-1-phosphate, lysophosphatidic acid, mono-sialoganglioside GM1, and di-sialoganglioside GD3, suggesting much higher, possibly multivalent, Ad41 affinity for cell surface. These experiments together show the remarkable lipophilicity of enteric Ad, which most probably plays a role in virus ability to cross the protective mucus barrier and to attach to the surface of cells lining the GI.
Fig. 7.
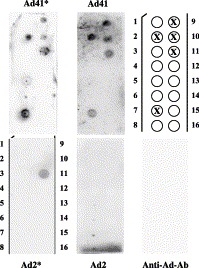
Comparison of glycosphingolipid-binding properties of Ad41 with Ad2. The nitrocellulose membrane containing 100 pmol of the indicated sphingolipids was incubated with the purified viruses as described in Materials and methods. The ligands bound to the membrane-immobilized lipids were detected with specific antibody. Binding was performed at pH 6.0 without Mg2+ (*) or at pH 7.5 in presence of Mg2+. Lipid positions are indicated in the diagram: 1, sphingosine; 2, sphingosine 1-phosphate; 3, phytosphingosine; 4, ceramide; 5, sphingomyeline; 6, sphingosyl-phosphocholine; 7, lysophosphatidic acid; 8, myriocin; 9, monosialoganglioside GM1; 10, disialoganglioside GD3; 11, sulfatide; 12, sphingosylgalactoside (psychosine); 13, cholesterol; 14, phosphatidylcholine; 15, lysophosphophatidylcholine; 16, blank.
Discussion
The goal of this study was to bring us closer to understanding the molecular basis of narrow tropism of enteric adenovirus serotype 41 resulting in the infection of human GI. For this we examined the behavior of Ad41 virions by simulating in vitro four crucial steps leading to successful enteric infection: survival in acidic stomach environment, immunity to proteolytic attack after acid exposure, interaction with phospholipids present in the mucus barrier protecting the GI mucosa, and finally, attachment to the apical surface of GI lining cells.
Comparing the theoretical pI of different Ad capsid proteins, we observed that enteric hexon and penton base proteins are somewhat more basic and enteric fibers are significantly more basic than the appropriate proteins of respiratory serotypes (Table 1). The charge distribution on the head domain of fibers of different serotypes (Fig. 1) shows the presence of clusters of basic amino acids for the Ad41 fibers and the more positive global charge related to the higher pI of these head domains (Table 1) This positive surface potential is particularly pronounced for the Ad41 long fiber. Furthermore, Ad41 mobility observed during electrophoresis under native conditions indicated a much more basic charge of enteric virions than other Ad serotypes (Fig. 2). This feature immediately suggested that upon encountering stomach acidic conditions, the protonation of basic Ad41 particle would be much less damaging to the virion integrity than for the significantly less basic respiratory Ad2 or Ad5. Indeed, acid treatment significantly impaired Ad2 infectivity whereas Ad41 viability was not affected by the acid (Fig. 3). Because all mucosa possess an enzymatic barrier composed primarily of proteolytic enzymes (Zhou and Li Wan Po, 1994), we analyzed the proteolytic resistance of Ad41 fibers, viral proteins mediating cell attachment. Contrary to the fiber of the respiratory serotype, Ad41 fibers were not digested by chymotrypsin after acid exposure (Fig. 5). This tends to show that Ad41 survival in the acid stomach environment stems from the pronounced basic character of the enteric Ad particle, and in particular, from the remarkable stability of its fibers, which under acidic stomach conditions would likely retain, in the presence of proteolytic enzymes, the ability to attach to the host cells. Some viruses (e.g., rotaviruses) have evolved so that proteolytic processing facilitates viral infection. Our results strongly suggest that enteric adenoviruses use another adaptation mechanism, which is the prevalence of basic charge of the virions. It protects the virions against the negative effects of the protonation upon the low pH conditions encountered in the stomach and subsequently against the activity of proteolytic enzymes present in GI.
The human gut harbors many microorganisms, and their acid survival can be linked to the basic character of some of their components. Several types of gut-colonizing bacteria are covered by a semicrystalline layer (S-layer) composed of a single protein or a glycoprotein species. The 43-kDa S-protein of Lactobacillus acidophilus has a predicted pI of 9.4 (Boot et al., 1993). Similarly, a vacuolating cytotoxin VacA secreted by the pathogenic strain of Helicobacter pylori that is able to associate with lipid bilayers (Czajkowsky et al., 1999) has a predicted pI of 9.44. Also the antibacterial defensins, arginine-rich peptides found in the human mucus are cationic and establish electrostatic interaction with the negatively charged membranes (Ganz and Lehrer, 1994).
Interaction of Ad41 with the GI protective mucus barrier was tackled by studying its lipids affinity. One of the potentially important biophysical features of mucus relates to its “unwettability” linked with its hydrophobic character. The hydrophobic properties of mucus as well as its viscosity appear to depend on its lipid constituents and specifically on the presence of a phospholipid surfactant that is synthesized, stored, and secreted by GI mucus cells (reviewed in Lichtenberger, 1995). The mucus layer within the GI tract turns over continuously, and the so-called “soluble mucus” can be found within the GI lumen (Lehr et al., 1991). The phospholipid constituents of the mucus are mainly PC and PE, which together account for more than 60% of total phospholipid (Nardone et al., 1993). Interestingly, the protective effect of the mucosa against gastric juice, damaging agents, and microorganisms can be attributed to the unsaturated phospholipid, DPPC Lichtenberger, 1995, Schmitz and Muller, 1991.
Under close to neutral pH conditions, both Ad2 and Ad41 attached to DPPC, with Ad41 also strongly recognizing DPPG (Fig. 6). Of note, the assay sensitivity is ensured by the amount of the immobilized lipid in the pmol range (Thomas et al., 2001). When using isolated Ads structural proteins in such an assay, we observed that the hexon, the major capsid protein, and the fiber were responsible for both Ads binding to DPPC (data not shown), confirming the data of Balakireva et al. (2002). In addition, Ad2 penton base did not recognize any of the phospholipids used in the assay, whereas binding of enteric Ad to DPPG could be attributed to the penton base protein which attached strongly to DPPG (data not shown). A similar experiment performed with Ad3 dodecahedra gave negative results showing that neither the fiber nor the penton base of Ad3 (respiratory and ocular serotype) has the ability to recognize phospholipids (results not shown).
When Ad2 was exposed to the acid followed by the pH jump to neutral, conditions simulating the passage from stomach to intestine, Ad2 virions lost the capability of lipid interaction (Fig. 6). This can be explained by irreversible changes generated in respiratory virions upon acid exposure due to proteolytic cleavage (Fig. 5). Most remarkably, acid treatment of Ad41 followed by the pH jump to neutral resulted not only in the retention of the lipid interaction observed at the neutral pH but also in the improved interaction with other lipids (Fig. 6). Altogether, these results suggest a scenario in which enteric Ad upon encountering acidic stomach environment is still able to withstand the proteolytic attack and cross the mucus barrier through interaction with its lipid components.
Because the mucosa is primarily lipophilic (Corbo et al., 1990), and mucosal pathogens are known to target sites of infection through adherence to host glycoconjugate receptors Mahdavi et al., 2002, Svensson et al., 2003, the Ad41 aptitude to interact with lipids was extended to a panel of glycosphingolipids, a highly polymorphic class of lipids, the constituents of plasma membrane. High amounts of sphingolipids were particularly found for the apical plasma membrane domains of intestinal cells (Simons and van Meer, 1988). Here again, Ad41 interacted with a significantly larger group of sphingolipids than respiratory Ad (Fig. 7), and these data could explain mechanistically Ad41 interaction with the plasma membrane of epithelial monolayer lining the GI. This interaction is likely to be highly reinforced thanks to the CAR recognition by enteric Ad long fiber.
Interestingly, several sphingolipids are involved in cell binding of enteric pathogens. H. pylori attaches to sulfatides and is able to bind phospholipid PE and gangliosides GM3 at neutral pH, but low pH pulse induces a specific recognition of sulfatides (reviewed in Lingwood, 1999). Similarly, the E. coli enterotoxin b, a basic peptide of pI 9.6, recognizes sulfatide on the pig jejunum brush border epithelial cells (Rousset et al., 1998). In addition, galactosylceramide and sulfatide have attracted attention as the alternative receptors for HIV Bhat et al., 1993, Cook et al., 1994, Ruiz et al., 1994, whereas glycosphingolipid asialo-GM1 is involved in rotavirus cell binding (Willoughby et al., 1990).
Data on the entry and infection mechanism of enteric viruses are scarce. It was observed that the proteolytic cleavage, believed to occur in the lumen of intestine, enhances rotavirus infectivity in vitro (Estes et al., 1981). In addition, observations on membrane permeabilization by rotaviruses led to the hypothesis of a direct virus penetration across the plasma membrane lipid phase (Ruiz et al., 1994). However, not much is known about the virus behavior in the GI lumen as well as about the mechanism allowing the passage through protective mucus layer. To our knowledge, our data on enteric Ad41 are the first allowing the reasonable hypotheses concerning the chain of events starting from the oral route of virus entry, crossing mucus, and attaching to the surface of the epithelial monolayer lining the GI.
A narrow tropism of Ad41, unique among Ads, is the result of a specific adaptation which rendered these viruses very efficacious pathogens of the human GI. The virulence of enteric Ads against human GI has been recognized as being of value for putative applications linked to gastric gene therapy (Croyle et al., 1998a). In addition, the eventual spread of these vectors would be easy to control because their infections are confined to epithelial cells adjacent to the intestinal lumen. However, because of the lack of the recombinant Ads constructed with the backbone of enteric serotypes, all GI gene therapy assays are done with respiratory Ad5-derived vectors (see for example Foreman et al., 1998). Alternatively, Ad5-derived vectors are used in vitro in combination with cyclodextrins, rendering positively charged formulations (Croyle et al., 1998b) or after manipulations allowing ablation of the native (respiratory) tropism (Heideman et al., 2002). In view of our data, it is clear that this kind of recombinant Ads will not survive oral applications in vivo. The construction of GI delivery vectors based on the enteric Ads backbone and further studies on the role of enteric Ads capsid proteins in virus entry will bring us closer to the intelligent use of our fundamental knowledge in human health applications.
Materials and methods
Prediction of protein isoelectrical point (pI)
The theoretical pI of hexon, penton base, and fiber proteins was obtained with the software http://www.up.univ-mrs.fr/∼wabim/d_abim/compo-p.html. Accession numbers to the sequences used for calculation of predicted pI values are given in Table 2 .
Table 2.
Accession numbers of adenovirus proteins sequences
| Serotype | Hexon | Penton base | Fiber |
|---|---|---|---|
| Ad41 | P11820 | Q9QAH8 | Long, P14267; Short, P16883 |
| Ad40 | P11819 | L19443 | Long, P18047; Short, P18048 |
| Ad2 | P03277 | P03276 | P03275 |
| Ad5 | P04133 | P12538 | P11818 |
| Ad4 | Q67814 | n.d. | P36844 |
| Ad35 | Q99174 | AY128640 | Q67733 |
| Ad3 | P36849 | Q65290 | P04501 |
| Ad31 | X74661 | n.d. | P36848 |
| Ad12 | P19900 | P36716 | P36711 |
| Ad37 | n.d. | Q9JEW0 | Q64823 |
| Ad8 | X74663 | Q9IW62 | P36845 |
| Ad30 | n.d. | n.d. | Q8V2D0 |
| Ad9 | X74664 | n.d. | P36846 |
| Ad15 | X74667 | n.d. | P36847 |
n.d.: sequence not known.
Cells and virus
The 293 transformed human embryonic kidney 293 and A549 human lung carcinoma cells were maintained in EMEM supplemented with 2 mM glutamine, 20 U of penicillin–streptomycin, and 10% FBS. Ad3 and Ad5 were propagated in 293 cells. Ad41 Tak strain was propagated in 293 cells as described by Favier et al. (2002) and Ad2 was grown on HeLa cells. All viruses were purified according to Kanegae et al. (1994) and tittered at 9–16 × 108 focus forming units (FFU)/ml, respectively.
Antibodies
For Western blot analysis, polyclonal rabbit antibodies were used at the following dilutions: anti-Ad3 dodecahedron penton at 1:50 000, and anti-Ad41 and anti-Ad2 at 1:10 000 (all made by authors). Antibodies were obtained after two intravenous and one intraperitoneal injections of the purified antigens (boiled Ad3 dodecahedron or a mixture of 50% boiled–50% infectious viruses) to the rabbit (ESD, France). Monoclonal antibody 4D2 recognizing the FNPVYPY epitope conserved in the N-terminal part of Ad fibers (kind gift of J. Engler; Hong and Engler, 1991) was used at 1:50 000. Anti-mouse and anti-rabbit-horseradish peroxidase conjugates were used at 1:5000 (Jackson ImmunoRes.). ECL detection system (Amersham Pharmacia) was used throughout this work.
Adenovirus mobility and acid treatment
Purified Ads virions of serotypes 2, 3, 5, and 41 (approximately 10 μg of Ad3 and Ad5, 5 μg of Ad41 and Ad2) were electrophoresed in 0.8% agarose gel using 0.5% TBE buffer at 50 V. Acid treatment was done by virus incubation with an equal volume of HCl, pH 2, at 37 °C for 1 min. The reaction mixtures were placed in ice-cold bath and 1 M Tris, pH 9.6, was immediately added to reach pH 7.5.
Effect of acid treatment on virus viability
To establish conditions for HCl treatment, the curve of the maximum of infection was obtained for Ad41 and Ad2 on 293 cells as described by Favier et al. (2002). Subsequently, conditions of about 50% or 100% saturation of infection were used as follows. Confluent 293 cells were infected with Ad41 at dilutions of 1/8 and 1/16 (3.37 × 105 and 1.68 × 105 FFU/well of 96-well dish) and with Ad2 at 1/16 and 1/32 dilutions (1 and 0.5 × 105 FFU/well of 96-well dish). For each condition, one mixture was prepared for the five samples containing the virus, yielding five fractions of 5 μl each. Incubation conditions were: 1 min at RT or at 37 °C, with 5 μl of HCl at final pH 2.0 or HCl at pH 4.0 or with culture medium. The reaction was terminated by adding 150 μl of EMEM–0.2% FBS to each sample. Three portions of 293 cells in 96-well dish were infected each with 50 μl of such viral solution. One hour after infection, 100 μl of EMEM–0.2% FBS was added and the viral proteins were estimated by immunofluorescence 24 h later.
Purification of viral proteins
Native fibers of Ad41 and Ad5 were isolated from a CsCl fraction obtained during virus purification. This fraction is localized above the virus band and contains a mixture of free viral proteins. Native Ad41 proteins were obtained from Ad41-infected cells grown in 31 flasks of 175 cm2 Supernatant above the virus band obtained after the first CsCl gradient (Kanegae et al., 1994) was dialyzed against a Q2-Sepharose column followed by an S2-Sepharose with 20 mM MES buffer, pH 6.5. Fibers were visualized by Western blot with the serum recognizing Ad3 penton (complex of penton base and fiber proteins) or with monoclonal antibody 4D2. Dodecahedra made of 12 pentons (P3, complex of penton base and fiber) were purified as described by Fender et al. (1997).
Proteolytic digestions
Fiber samples were treated with an equal volume of HCl, pH 2, at 37 °C and after 1 min the pH was adjusted to 7.5 with 1 M Tris buffer, pH 9.6. Chymotrypsin digestion of fibers was performed with a 1:5 enzyme/substrate ratio for 1 h at 37 °C and pH 7.5. Proteins were electrophoresed under semi-denatured conditions in a 7.5% SDS-polyacrylamide gel at 4 °C as described by Mitraki et al. (1999) followed by Western blot.
Lipid–protein interaction
Freeze-dried lipids were reconstituted in a 1:1 (v/v) mixture of chloroform/methanol at 1 mM concentration. The stock solutions were 2-fold serially diluted in a mixture of chloroform/methanol/water (1:2:0.8, by vol.) and 1 μl of such dilutions (1 pmol to 1 nmol of PC, DPPC, PE, DPPG) was spotted onto the HybondC-extra nitrocellulose membrane (Amersham) and allowed to dry at RT for 1 h. Membranes were blocked for 1 h at RT in buffer A (50 mM MES–NaOH pH 6.0, 150 mM NaCl, and 0.1% Tween 20) or buffer B (10 mM Tris–HCl pH 7.5, 150 mM NaCl, and 0.1% Tween 20) containing 3% fatty acid-free BSA (Sigma). The membrane was then incubated overnight at 4 °C in the same buffers containing 2 μg/ml of viruses. The membranes were washed five times for 10 min in the respective buffer and then incubated for 1 h with the polyclonal anti-Ad41 and anti-Ad2 containing 3% BSA. After three 10-min washes followed by overnight wash at 4 °C without rocking and four 15-min washes at RT, the membranes were incubated for 1 h with anti-rabbit or anti-mouse-horseradish peroxidase conjugate. Finally, after six 30-min washes, the interactions were detected by ECL.
Sphingostrips membranes (Molecular Probes), each containing 100 pmol of 15 different lipids, were blocked for 1 h at RT in buffer A or buffer B. The membrane was then incubated overnight at 4 °C in the same buffers containing 2 μg/ml of viruses. The membranes were washed four times for 15 min in the respective buffer and then incubated for 1 h with the appropriate antibody. After four 15-min washes, membranes were incubated for 1 h with an anti-rabbit or anti-mouse-horseradish peroxidase conjugate. Finally, after four 15-min washes, the interactions were detected by ECL. The overlay reactions were done either in presence or in absence of 10 mM MgCl2.
Acknowledgements
We are indebted to Mark van Raaij for the gift of extracellular CAR domain. We acknowledge the gift of antibodies from Jeff Engler.
References
- Albinsson B, Kidd A.H. Adenovirus type 41 lacks an RGD alpha(v) integrin binding motif on the penton base and undergoes delayed uptake in A549 cells. Virus Res. 1999;64:125–136. doi: 10.1016/s0168-1702(99)00087-8. [DOI] [PubMed] [Google Scholar]
- Bai M, Campisi L, Freimuth P. Vitronectin receptor antibodies inhibit infection of HeLa and A549 cells by adenovirus type 12 but not by adenovirus type 2. J. Virol. 1994;68:5925–5932. doi: 10.1128/jvi.68.9.5925-5932.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakireva L, Schoehn G, Thouvenin E, Chroboczek J. Binding of adenovirus capsid to dipalmitoylphosphatidylcholine provides a novel pathway for virus entry. J. Virol. 2002;77:4858–4866. doi: 10.1128/JVI.77.8.4858-4866.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergelson J.M, Cunningham G.D, Kurt-jones E.A, Krithivas A, Hong A, Horwitz M.S, Crowell R.L, Finberg R.W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- Bernhard W, Postle A.D, Linck M, Sewing K.F. Composition of phospholipid classes and phosphatidylcholine molecular species of gastric mucosa and mucus. Biochim. Biophys. Acta. 1995;1255:99–104. doi: 10.1016/0005-2760(94)00221-j. [DOI] [PubMed] [Google Scholar]
- Bewley M.C, Springer K, Zhang Y.B, Freimuth P, Flanagan J.M. Structural analysis of the mechanism of adenovirus binding to its human cellular receptor, CAR. Science. 1999;286:1579–1583. doi: 10.1126/science.286.5444.1579. [DOI] [PubMed] [Google Scholar]
- Bhat S, Mettus R.V, Reddy E.P, Ugen K.E, Srikanthan V, Williams W.V, Weiner D.B. The galactosyl ceramide/sulfatide receptor binding region of HIV-1 gp120 maps to amino acids 206–275. AIDS Res. Hum. Retroviruses. 1993;9:175–181. doi: 10.1089/aid.1993.9.175. [DOI] [PubMed] [Google Scholar]
- Boot H.J, Kolen C.P, van Noort J.M, Pouwels P.H. S-layer protein of Lactobacillus acidophilus ATCC 4356: purification, expression in Escherichia coli, and nucleotide sequence of the corresponding gene. J. Bacteriol. 1993;175:6089–6096. doi: 10.1128/jb.175.19.6089-6096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chroboczek J, Gout E, Favier A.L, Galinier R. Novel partner proteins of adenovirus penton. Curr. Top. Microbiol. Immunol. 2003;272:37–55. doi: 10.1007/978-3-662-05597-7_2. [DOI] [PubMed] [Google Scholar]
- Cook D.G, Fantini J, Spitalnik S.L, Gonzalez-Scarano F. Binding of human immunodeficiency virus type I (HIV-1) gp120 to galactosylceramide (GalCer): relationship to the V3 loop. Virology. 1994;201:206–214. doi: 10.1006/viro.1994.1287. [DOI] [PubMed] [Google Scholar]
- Corbo D.C, Liu J.C, Chien W.Y. Characterization of the barrier properties of mucosal membranes. J. Pharm. Sci. 1990;79:202–206. doi: 10.1002/jps.2600790304. [DOI] [PubMed] [Google Scholar]
- Croyle M.A, Stone M, Amidon G.L, Roessler B.J. In vitro and in vivo assessment of adenovirus 41 as a vector for gene delivery to the intestine. Gene Ther. 1998;5:645–654. doi: 10.1038/sj.gt.3300645. [DOI] [PubMed] [Google Scholar]
- Croyle M.A, Roessler B.J, Hsu C.P, Sun R, Amidon G.L. Beta cyclodextrins enhance adenoviral-mediated gene delivery to the intestine. Pharm Res. 1998;15:1348–1355. doi: 10.1023/a:1011985101580. [DOI] [PubMed] [Google Scholar]
- Czajkowsky D.M, Iwamoto H, Cover T.L, Shao Z. The vacuolating toxin from Helicobacter pylori forms hexameric pores in lipid bilayers at low pH. Proc. Natl. Acad. Sci. U.S.A. 1999;96:2001–2006. doi: 10.1073/pnas.96.5.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A.J, Telford E.A, Watson M.S, McBride K, Mautner V. The DNA sequence of adenovirus type 40. J. Mol. Biol. 1993;234:1308–1316. doi: 10.1006/jmbi.1993.1687. [DOI] [PubMed] [Google Scholar]
- de Jong J.C, Wigand R, Kidd A.H, Wadell G, Kapsenberg J.G, Muzerie C.J, Wermenbol A.G, Firtzlaff R.G. Candidate adenoviruses 40 and 41: fastidious adenoviruses from human infant stool. J. Med. Virol. 1983;11:215–231. doi: 10.1002/jmv.1890110305. [DOI] [PubMed] [Google Scholar]
- Devaux C, Caillet-Boudin M.L, Jacrot B, Boulanger P. Crystallization, enzymatic cleavage, and the polarity of the adenovirus type 2 fibre. Virology. 1987;161:121–128. doi: 10.1016/0042-6822(87)90177-2. [DOI] [PubMed] [Google Scholar]
- Dowler S, Currie R.A, Downes C.P, Alessi D.R. DAPP1: a dual adaptor for phosphotyrosine and 3-phosphoinositides. Biochem. J. 1999;342:7–12. [PMC free article] [PubMed] [Google Scholar]
- Durmort C, Stehlin C, Schoehn G, Mitraki A, Drouet E, Cusack S, Burmeister W.P. Structure of the fiber head of Ad3, a non-CAR-binding serotype of adenovirus. Virology. 2001;285:302–312. doi: 10.1006/viro.2001.0967. [DOI] [PubMed] [Google Scholar]
- Estes M.K, Graham D.Y, Mason B.B. Proteolytic enhancement of rotavirus infectivity: molecular mechanisms. J. Virol. 1981;39:879–888. doi: 10.1128/jvi.39.3.879-888.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favier, A.L., Ph.D. Dissertation, Interactions moléculaíres entre I'adénovirus enterique de sérotype 41 et la cellule hóle. Grenoble, UJF-2002.
- Favier A.L, Schoehn G, Jaquinod M, Harsi C, Chroboczek J. Structural studies of human enteric adenovirus type 41. Virology. 2002;293:75–85. doi: 10.1006/viro.2001.1235. [DOI] [PubMed] [Google Scholar]
- Fender P, Ruigrok R.W, Gout E, Buffet S, Chroboczek J. Adenovirus dodecahedron, a new vector for human gene transfer. Nat. Biotechnol. 1997;15:52–56. doi: 10.1038/nbt0197-52. [DOI] [PubMed] [Google Scholar]
- Foreman P.K, Wainwright M.J, Alicke B, Kovesdi I, Wickham T.J, Smith J.G, Meier-Davis S, Fix J.A, Daddona P, Gardner P, Huang M.T. Adenovirus-mediated transduction of intestinal cells in vivo. Hum. Gene Ther. 1998;9:1313–1321. doi: 10.1089/hum.1998.9.9-1313. [DOI] [PubMed] [Google Scholar]
- Ganz T, Lehrer R.I. Defensins. Curr. Opin. Immunol. 1994;6:584–589. doi: 10.1016/0952-7915(94)90145-7. [DOI] [PubMed] [Google Scholar]
- Gary G.W, Jr., Hierholzer J.C, Black R.E. Characteristics of noncultivable adenoviruses associated with diarrhea in infants: a new subgroup of human adenoviruses. J. Clin. Microbiol. 1979;10:96–103. doi: 10.1128/jcm.10.1.96-103.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber U.F, Willetts M, Webster P, Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993;5:477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- Green M, Pina M. Biochemical studies on adenovirus multiplication: IV. Isolation, purification and chemical analysis of adenovirus. Virology. 1963;20:199–207. doi: 10.1016/0042-6822(63)90157-0. [DOI] [PubMed] [Google Scholar]
- Gryboski J, Walker W.A. Saunders; Philadelphia: 1983. Gastrointestinal Problems in the Infant. [Google Scholar]
- Hakomori S. Bifunctional role of glycosphingolipids. Modulators for transmembrane signaling and mediators for cellular interactions. J. Biol. Chem. 1990;265:18713–18716. [PubMed] [Google Scholar]
- Heideman D.A, van Beusechem V.W, Offerhaus J.G, Wickham T.J, Roelvink P.W, Craanen M.E, Pinedo H.M, Meijer C.J, Gerritsen W.R. Selective gene transfer into primary human gastric tumors using epithelial cell adhesion molecule-targeted adenoviral vectors with ablated native tropism. Hum. Gene Ther. 2002;13:1677–1685. doi: 10.1089/104303402760293529. [DOI] [PubMed] [Google Scholar]
- Hong J.S, Engler J.A. The amino terminus of the adenovirus fibre protein encodes the nuclear localization signal. Virology. 1991;185:758–767. doi: 10.1016/0042-6822(91)90547-o. [DOI] [PubMed] [Google Scholar]
- Jacobsson P.A, Johansson M.E, Wadell G. Identification of an enteric adenovirus by immunoelectroosmophoresis (IEOP) technique. J. Med. Virol. 1979;3:307–312. doi: 10.1002/jmv.1890030409. [DOI] [PubMed] [Google Scholar]
- Jones E.Y, Miller A. Analysis of structural design features in collagen. J. Mol. Biol. 1991;218:209–219. doi: 10.1016/0022-2836(91)90885-a. [DOI] [PubMed] [Google Scholar]
- Kagnoff M.F, Paterson Y.J, Kumar P.J, Kasarda D.D, Carbone F.R, Unsworth D.J, Austin R.K. Evidence for the role of a human intestinal adenovirus in the pathogenesis of coeliac disease. Gut. 1987;28:995–1001. doi: 10.1136/gut.28.8.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanegae Y, Makimura M, Saito I. A simple and efficient method for purification of infectious recombinant adenovirus. Jpn. J. Med. Sci. Biol. 1994;47:157–166. doi: 10.7883/yoken1952.47.157. [DOI] [PubMed] [Google Scholar]
- Kidd A.H, Chroboczek J, Cusack S, Ruigrok R.W. Adenovirus type 40 virions contain two distinct fibres. Virology. 1993;192:73–84. doi: 10.1006/viro.1993.1009. [DOI] [PubMed] [Google Scholar]
- Larhed A.W, Artursson P, Bjork E. The influence of intestinal mucus components on the diffusion of drugs. Pharm. Res. 1998;15:66–71. doi: 10.1023/a:1011948703571. [DOI] [PubMed] [Google Scholar]
- Lehr C.M, Poelma F.G.J, Junginger H.E, Tukker J.J. An estimate of turnover time of intestinal mucus gel layer in the rat in situ loop. Int. J. Pharm. 1991;70:235–240. [Google Scholar]
- Lichtenberger L.M. The hydrophobic barrier properties of gastrointestinal mucus. Annu. Rev. Physiol. 1995;57:565–583. doi: 10.1146/annurev.ph.57.030195.003025. [DOI] [PubMed] [Google Scholar]
- Lingwood C.A. Glycolipid receptors for verotoxin and Helicobacter pylori: role in pathology. Biochim. Biophys. Acta. 1999;1455:375–386. doi: 10.1016/s0925-4439(99)00062-9. [DOI] [PubMed] [Google Scholar]
- Mahdavi J, Sonden B, Hurtig M, Olfat F.-O, Forsberg L, Roche N, Angstrom J, Larsson T, Teneberg S, Karlsson K.A, Altraja T, Wadstrom T, Kersulyte D, Berg D.E, Dubois A, Petersson C, Magnusson K.E, Norberg T, Lindh F, Lundskog B.B, Arnqvist A, Hammarstrom L, Boren T. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science. 2002;297:573–578. doi: 10.1126/science.1069076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias P, Wickham T, Moore M, Nemerow G. Multiple adenovirus serotypes use alpha v integrins for infection. J. Virol. 1994;168:6811–6814. doi: 10.1128/jvi.68.10.6811-6814.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitraki A, Barge A, Chroboczek J, Andrieu J.P, Gagnon J, Ruigrok R.W. Unfolding studies of human adenovirus type 2 fibre trimers. Evidence for a stable domain. Eur. J. Biochem. 1999;264:599–606. doi: 10.1046/j.1432-1327.1999.00683.x. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Sato K, Hamada H. Reduction of natural adenovirus tropism to the liver by both ablation of fiber-coxsackievirus and adenovirus receptor interaction and use of replaceable short fiber. J. Virol. 2003;77:2512–2521. doi: 10.1128/JVI.77.4.2512-2521.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone G, Laccetti P, Civiletti C, Budillon G. Phospholipid composition of human gastric mucosa: a study of endoscopic biopsy specimens. Gut. 1993;34:456–460. doi: 10.1136/gut.34.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolls A, Sharp K, Horning B. Protein folding and association: insights from the interfacial and thermodynamics properties. Proteins: Struct., Funct., Genet. 1991;11:281–296. doi: 10.1002/prot.340110407. [DOI] [PubMed] [Google Scholar]
- Pereira H.G, Wrigley N.G. In vitro reconstitution, hexon bonding and handedness of incomplete adenovirus capsid. J. Mol. Biol. 1974;85:617–630. doi: 10.1016/0022-2836(74)90319-2. [DOI] [PubMed] [Google Scholar]
- Pieniazek N.J, Slemenda S.B, Pieniazek D, Velarde J, Luftig R.B. Human enteric adenovirus type 41 (Tak) contains a second fibre protein gene. Nucleic Acids Res. 1990;18:1901. doi: 10.1093/nar/18.7.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J.R, O'Neill K.E, Clifford T.Y. DNA sequences from the adenovirus 2 genome. J. Biol. Chem. 1984;259:13968–13975. [PubMed] [Google Scholar]
- Roelvink P.W, Lizonova A, Lee J.G, Li Y, Bergelson J.M, Finberg R.W, Brough D.E, Kovesdi I, Wickham T.J. The coxsackievirus-adenovirus receptor protein can function as protein can function as a cellular protein for adenovirus serotypes from groups A, C, D, E and F. J. Virol. 1998;72:7909–7915. doi: 10.1128/jvi.72.10.7909-7915.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset E, Harel J, Dubreuil J.D. Sulfatide from the pig jejunum brush border epithelial cell surface is involved in binding of Escherichia coli enterotoxin b. Infect. Immun. 1998;66:5650–5658. doi: 10.1128/iai.66.12.5650-5658.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz M.C, Alonso-Torre S.R, Charpilienne A, Vasseur M, Michelangeli F, Cohen J, Alvarado F. Rotavirus interaction with isolated membrane vesicles. J. Virol. 1994;68:4009–4016. doi: 10.1128/jvi.68.6.4009-4016.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz G, Muller G. Structure and function of lamellar bodies, lipid–protein complexes involved in storage and secretion of cellular lipids. J. Lipid Res. 1991;32:1539–1570. [PubMed] [Google Scholar]
- Simons K, van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988;27:6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- Svensson M, Frendeus B, Butters T, Platt F, Dwek R, Svanborg C. Glycolipid depletion in antimicrobial therapy. Mol. Microbiol. 2003;47:453–461. doi: 10.1046/j.1365-2958.2003.03306.x. [DOI] [PubMed] [Google Scholar]
- Thomas C.C, Dowler S, Deak M, Alessi D.R, van Aalten D.M. Crystal structure of the phosphatidylinositol 3,4-bisphosphate-binding pleckstrin homology (PH) domain of tandem PH-domain-containing protein 1 (TAPP1): molecular basis of lipid specificity. Biochem. J. 2001;358:287–294. doi: 10.1042/0264-6021:3580287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C.C, Deak M, Alessi D.R, van Aalten D.M. High-resolution structure of the pleckstrin homology domain of protein kinase b/akt bound to phosphatidylinositol (3,4,5)-trisphosphate. Curr. Biol. 2002;12:1256–1262. doi: 10.1016/s0960-9822(02)00972-7. [DOI] [PubMed] [Google Scholar]
- Uhnoo I, Wadell G, Svensson L, Johansson M.E. Two new serotypes of enteric adenovirus causing infantile diarrhoea. Dev. Biol. Stand. 1983;53:311–318. [PubMed] [Google Scholar]
- Uhnoo I, Wadell G, Svensson L, Johansson M.E. Importance of enteric adenovirus 40 and 41 in acute gastroenteritis in infants and young children. J. Clin. Microbiol. 1984;20:365–372. doi: 10.1128/jcm.20.3.365-372.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oostrum J, Burnett R.M. Molecular composition of the adenovirus type 2 virion. J. Virol. 1985;56:439–448. doi: 10.1128/jvi.56.2.439-448.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Raaij M.J, Louis N, Chroboczek J, Cusack S. Structure of the human adenovirus serotype 2 fiber head domain at 1.5 A resolution. Virology. 1999;262:333–343. doi: 10.1006/viro.1999.9849. [DOI] [PubMed] [Google Scholar]
- Weiss C, Clark H.F. Rapid inactivation of rotaviruses by exposure to acid buffer or acidic gastric juice. J. Gen. Virol. 1985;66:2725–2730. doi: 10.1099/0022-1317-66-12-2725. [DOI] [PubMed] [Google Scholar]
- Wickham T.J, Mathias P, Cheresh D.A, Nemerow G. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- Willoughby R.E, Yolken R.H, Schnaar R.L. Rotaviruses specifically bind to the neutral glycosphingolipid asialo-GM1. J. Virol. 1990;64:4830–4835. doi: 10.1128/jvi.64.10.4830-4835.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia D, Henry L, Gerard R.D, Deisenhofer J. Structure of the receptor binding domain of adenovirus type 5 fiber protein. Curr. Top. Microbiol. Immunol. 1995;199:39–46. doi: 10.1007/978-3-642-79496-4_3. [DOI] [PubMed] [Google Scholar]
- Yeh H.Y, Pieniazek N, Pieniazek D, Gelderblom H, Luftig R.B. Human adenovirus type 41 contains two fibres. Virus Res. 1994;33:179–198. doi: 10.1016/0168-1702(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Zhou X.H, Li Wan Po A. Stability and in vitro absorption of captopril, enalapril and lisinopril across the rat intestine. Biochem. Pharmacol. 1994;47:1121–1126. doi: 10.1016/0006-2952(94)90382-4. [DOI] [PubMed] [Google Scholar]


