Highlights
-
•
RSV F nanoparticle vaccine induced palivizumab competing antibodies (PCA).
-
•
PCA is in excess of serum protective levels than IM injected palivizumab.
-
•
Live virus infection induces little or no PCA.
-
•
Passively administered RSV F antibodies protects cotton rats against RSV virus.
-
•
RSV F vaccine does not induce disease enhancement in comparison to Lot 100 FI-RSV.
Keywords: Respiratory syncytial virus, Vaccine, Baculovirus, Sf9 cells, Vaccine, Palivizumab, Motavizumab, Nanoparticles, Cotton rat
Abstract
Post-infectious immunity to respiratory syncytial virus (RSV) infection results in limited protection as evidenced by the high rate of infant hospitalization in the face of high titer, maternally derived RSV-specific antibodies. By contrast, RSV fusion (F) glycoprotein antigenic site II humanized monoclonal antibodies, palivizumab and motavizumab, have been shown to reduce RSV-related hospitalization in infants. Immunogenicity and efficacy studies were conducted in cotton rats comparing a recombinant RSV F nanoparticle vaccine with palivizumab and controlled with live RSV virus intranasal immunization and, formalin inactivated RSV vaccine. Active immunization with RSV F nanoparticle vaccine containing an alum adjuvant induced serum levels of palivizumab competing antibody (PCA) greater than passive administration of 15 mg/kg palivizumab (human prophylactic dose) in cotton rats and neutralized RSV-A and RSV-B viruses. Immunization prevented detectable RSV replication in the lungs and, unlike passive administration of palivizumab, in the nasal passage of challenged cotton rats. Histology of lung tissues following RSV challenge showed no enhanced disease in the vaccinated groups in contrast to formalin inactivated ‘Lot 100’ vaccine. Passive intramuscular administration of RSV F vaccine-induced immune sera one day prior to challenge of cotton rats reduced viral titers by 2 or more log10 virus per gram of lung and nasal tissue and at doses less than palivizumab. A recombinant RSV F nanoparticle vaccine protected lower and upper respiratory tract against both RSV A and B strain infection and induced polyclonal palivizumab competing antibodies similar to but potentially more broadly protective against RSV than palivizumab.
1. Introduction
Respiratory syncytial virus (RSV) is the leading cause of severe lower respiratory tract disease in infants and young children worldwide [1] and is an important pathogen in elderly and high risk adults [2]. The World Health Organization (WHO) has estimated that the global annual burden of infections and mortality due to human RSV are 64 million and 160,000, respectively [3]. In industrialized countries, nearly all children have been infected with RSV by 2 years of age [4]. Most infected children present with mild upper respiratory tract symptoms, but a subset develops severe lower respiratory tract disease characterized by tachypnea, hyperinflation, crackles, and expiratory wheezing (i.e., bronchiolitis and pneumonia). The most severe disease occurs within the first months of life in largely full term, healthy infants. Data from the United States (US) and Australia suggest that 1.7–2.6% of infants are hospitalized for RSV infection before one year of age [5], [6], [7]. In the US, approximately 75,000–100,000 infants less than 1 year of age [8], [9] and 132,000–172,000 children less than 5 years of age [10] are hospitalized due to RSV disease annually. RSV is an important pathogen of children in daycare, where it accounts for a substantial portion of single-pathogen acute respiratory tract infections, as well as co-infections with rhinoviruses, coronaviruses, or adenoviruses [11]. The clinical manifestations and morbidity of RSV are similar among infants and young children worldwide but mortality is much higher in the lesser developed countries due to availability of medical care [12].
Despite decades of research there is no licensed RSV vaccine [13]. However, two monoclonal antibodies, palivizumab (Synagis®) and motavizumab, both of which bind to the fusion protein of the virus, have been shown to prevent severe disease in premature and term infants by passive immunoprophylaxis [14], [15], [16]. The efficacy is associated with inhibition of viral infection via binding to a 25 amino acid sequence known as “antigenic site II” on the RSV F protein which provides a rationale for an F based RSV vaccine containing this site [17]. Recent clinical trials have indicated that years of natural infection and thus exposure to live virus, induces little or no F specific site II antibodies [18].
There are two major RSV strains that co-circulate in humans, RSV-A and -B. In both strains, two surface glycoproteins, F and G, engage the host cell to establish and propagate infection respectively [19]. The human RSV viral attachment G glycoprotein is genetically diverse [20], compared to the more highly conserved F-fusion glycoprotein [21]. Natural infection is frequent in all age groups and results in significant immune responses to the F and G glycoproteins, but only the highest levels of neutralizing antibodies appear to confer solid protection against reinfection [22], [23], [24].
The RSV F nanoparticle vaccine is a recombinant near-full length F glycoprotein produced in Spodoptera frugiperda (Sf9) insect cells with a recombinant baculovirus [25]. Purified recombinant RSV F oligomers are hatpin-shaped rods, consistent with a post-fusion-like conformation of RSV F [26], [27], [28], [29]. Cotton rats immunized with this vaccine have demonstrated protection against RSV replication [25]. In the current study the production of vaccine-induced palivizumab competing antibodies (PCA) that bind to site II were studied in cotton rats to assess their relative potency, both in active and passive immunization. The studies were also controlled with RSV infection, which has been shown to induce very limited PCA in humans [18]. Finally, Lot 100 formalin inactivated RSV vaccine, used in the 1960's and associated with disease enhancement in children, allowed comparison of relative safety and the induction of functional immunity.
2. Materials and methods
2.1. Vaccine
Briefly, the RSV F protein nanoparticle vaccine was manufactured by infecting Sf9 cells in exponential growth with baculovirus containing the RSV F gene, as previously described [25]. After infection, cells are collected by centrifugation, washed with sterile PBS, and then lysed in the presence of NP9 to release membrane bound RSV F protein. The supernatant containing the RSV F protein is clarified using depth filtration and then purified by ion exchange (trimethylaminoethyl, TMAE) chromatography. The flow-through fraction is affinity purified using lentil lectin washed and eluted from the column with buffer containing methyl-α-d-mannopyranoside (MMP) and polysorbate (PS) 80. The eluted fraction was further purified by cation exchange (sulfate) chromatography. The product was sterile filtered (0.22 μm) and formulated with buffer containing 25 mM sodium phosphate, pH 6.2, 1% histidine, 0.01% PS80. The vaccine was adsorbed to aluminum phosphate (aluminum as phosphate salt in 0.15 M NaCl without buffer) purchased from Brenntag Biosector, Frederikssund, Denmark.
2.2. Animals
Inbred 6–8 weeks Sigmodon hispidus (cotton rats) were obtained from Sigmovir Biosystems, Inc. (Rockville, MD). All studies were conducted in accordance with the NRC Guide for the Care and Use of Laboratory Animals, the Animal Welfare Act and the CDC/NIH Biosafety in Microbiological and Medical Laboratories under applicable laws and guidelines and were approved by the Institutional Animal Care and Use Committee (IACUC).
2.3. FI-RSV virus, RSV virus
Lot 100 formalin-inactivated RSV vaccine (FI-RSV) manufactured by Pfizer in mid-1960s [30], and RSV-A Long and RSV-B 18537 were provided by Sigmovir Inc. The RSV–A viruses were propagated in HEp-2 cells. A pool of virus designated as hRSV-A Long Lot no. 021413 at approximately 2.0 × 107 plaque forming units (pfu)/ml was stored at −80 °C. RSV-B 18537 (RSV-B) (ATCC, Manassas, VA) was propagated in MA-104 cells. A pool of virus designated as hRSV-B Lot no. 12/03, at approximately 2.7 × 106 pfu/ml 10% was stored at −80 °C.
2.4. Immunization and RSV challenge in cotton rats
Cotton rats (n = 8) were immunized intramuscularly (IM) on day 0 and 28 with FI-RSV, RSV-F nanoparticle vaccine with and without adjuvant, RSV A 1 × 105 pfu intranasally and compared to palivizumab 15 mg/kg given IM, one day prior to challenge. Sera were obtained on day 0, 28, 49 and on day 54 post-challenge. RSV challenge was performed on day 49 intranasally with 1 × 105 pfu in 100 μl (50 μl/nare) RSV-A Long strain and lung tissue collected on day 54.
For the dose-descalation active immunization study, cotton rats received two vaccinations of 0.003, 0.03, 0.3, or 3.0 μg RSV F vaccine adjuvanted with aluminum phosphate on Day 0 and Day 21 and compared to palivizumab 5.0, 2.5, 1.25 or 0.625 mg/kg IM on day 41. Sera were obtained on day 0, 21, 42 prior to challenge, on day 46 post-challenge and stored at −20 °C until tested.
A pool of immune sera from RSV F nanoparticle vaccine-immunized cotton rats was prepared and assayed in the PCA ELISA as described below. Cotton rats (n = 5/group) were then passively immunized by IM with 0.6, 1.4 or 5.6 mg/kg of palivizumab-like antibody activity and compared to palivizumab given at 5.0, 2.5, 1.25 or 0.625 mg/kg IM on day 41. RSV challenge was performed on day 42 by intranasal administration of 100 μl (50 μl/nare) live RSV-B 18537 (1 × 105 pfu). Cotton rats were bled on Day 0, 21 and Day 42 following one and two immunizations and on Day 46 (4 days after challenge). Nasal wash, BAL, nose and lung tissues were collected on Day 46.
2.5. RSV F ELISA, palivizumab competitive antibody (PCA) ELISA and neutralization assay
RSV F-specific antibodies in cotton rat sera were measured in an enzyme linked immunosorbent assay (ELISA) as previously described [25]. Competitive inhibition by cotton rat sera of the binding of palivizumab monoclonal antibody (ASD Specialty Heath Care Inc., Chicago IL) was measured by an ELISA method as previously described [25]. Serum RSV virus neutralization titers were determined as described previously [25].
2.6. Lung viral load determination and pulmonary histopathology
Five days after intranasal RSV challenge, cotton rats were sacrificed and the lungs harvested. Lung tissues were homogenized and clarified by centrifugation at 12,000 × g for 10 min. Virus titer in the supernatant was determined by plaque assay as described previously [25]. Lung tissue slides were stained with hematoxylin, eosin (H&E) and observed under a Nikon Eclipse microscope. Slides were evaluated in a blinded fashion using a score of 0–4 (0 = none; 1 = minimal; 2 = mild; 3 = moderate; 4 = maximum inflammation) in order of increasing severity for each of the following 5 parameters: (a) peribronchiolitis; (b) perivasculitis; (c) bronchoiolitis; (d) alveolitis and (e) interstitial pneumonitis as described by Prince et al. [31]. Summary scores for animals in each group were used to generate an overall score/group expressed as the arithmetic mean + SEM of the individual animals.
2.7. Statistical methods
Comparisons between mean scores of each group and non-immune animal challenge scores were analyzed using Student's t-test. The sum of the scores of five parameters per animal was used for analysis of histopathology data. Pair wise t-test was analyzed in EXCEL while the GMT and 95% CI were calculated using Graph Pad Prizm.
3. Results
3.1. RSV F vaccine induced immune responses compared to palivizumab
Immune responses to RSV F nanoparticle vaccine (30 μg) administered IM in the presence or absence of adjuvant were compared to animals that received passively transferred palivizumab at the recommended human dose of 15 mg/kg. As controls, animals were infected with 105 pfu of RSV-A Long and allowed to recover, or vaccinated with FI-RSV (Lot 100 at 1:25 dilution), or treated with placebo. Three weeks after the second vaccine dose the immunization with unadjuvanted RSV F nanoparticle vaccine had induced titers of anti-RSV F serum IgG (Fig. 1A) that were significantly higher (p < 0.001, t-test) than cotton rats immunized with FI-RSV antigen or infected with RSV-A virus (p < 0.001, t-test). Adjuvant enhanced RSV F vaccine antibody titers by about 10-fold after the boost (Fig. 1A). Cotton rats that received palivizumab (IM injection) exhibited lower anti-RSV F IgG serum titers compared to the polyclonal responses obtained following immunization with adjuvanted RSV F (GMT = 1926 vs 1469,084 E.U., respectively Fig. 1A).
Fig. 1.
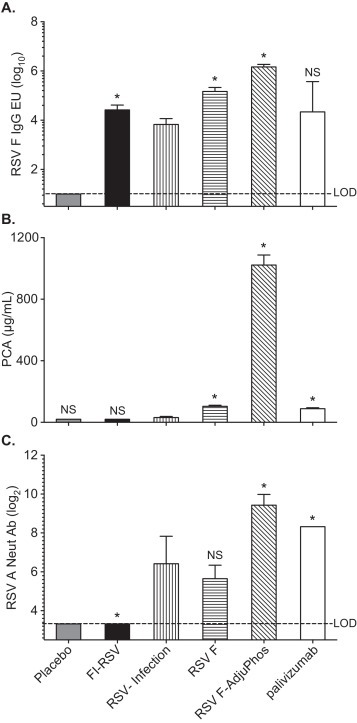
Immune responses to the RSV F nanoparticle vaccine. Cotton rats (n = 8) were immunized on day 0 and 28 with FI-RSV, RSV-A2, or RSV-F nanoparticle vaccine with and without AdjuPhos. An additional group received palivizumab 15 mg/kg intramuscularly on day 48, one day prior to challenge. A placebo group served as a negative control. Sera were obtained from all the groups on day 49, prior to challenge. Panel A. RSV F IgG responses as determined by ELISA; expressed as log10 of the GMT with 95% CI. Panel B. Palivizumab competing antibody (PCA) were reported as the μg/ml (log2) antibodies required for 50% inhibition of binding of palivizumab to RSV F. Panel C. Neutralizing antibody titers against RSV-A2 providing 60% inhibition of CPE (log2). GMT for each group is shown with the 95% CI. *p values <0.01; NS: not significant compared with RSV-infection group by two-tailed Student t-test; LOD is the lower limit of detection.
Antigenic site II on the RSV F polypeptide is the target of palivizumab [32]. A palivizumab competitive ELISA was performed to quantify the relative levels of PCA antibody induced by the vaccine in animal sera. Immunization with 30 μg adjuvanted RSV F nanoparticles elicited significantly higher serum levels of PCA (884 μg/ml) than animals that received 15 mg/kg (human dose) of palivizumab (86 μg/ml). PCA was below the LOD of the assay (<20 μg/ml) in cotton rats immunized with FI-RSV, and naïve control groups, and slightly above LOD in the RSV A intranasal immunization group (Fig. 1B).
Sera from all groups, with the exception of FI-RSV and placebo recipients, had virus neutralizing antibodies (Fig. 1C). Adjuvanted RSV F elicited higher neutralization titers (GMT = 697) than natural infection (GMT = 95) or palivizumab passively immunized cotton rats (GMT = 320) (Fig. 1C). The neutralizing titer differences observed between cotton rats that received adjuvanted RSV F and virus infected cotton rats were statistically significant (p < 0.01) following the same trend observed from analysis of PCA and anti-RSV F ELISA responses.
3.2. In vivo efficacy of RSV F nanoparticle vaccine
The in vivo efficacy of RSV F nanoparticle vaccine was evaluated by measuring inhibition of viral replication in the lungs and nasal passages of immunized cotton rats challenged with RSV. Complete inhibition of virus replication was observed in the lungs of cotton rats immunized with live RSV, RSV F nanoparticles administered with and without adjuvant, as well as palivizumab given passively (Fig. 2A). FI-RSV reduced lung viral load (pfu/g tissue; GMT = 2357) when compared to naïve challenged cotton rats (pfu/g tissue; GMT = 194,237) but failed to confer full protection. When viral replication was evaluated in the nasal compartment, only the RSV F vaccine with adjuvant and RSV infection groups were completely protected (Fig. 2B). Cotton rats that received unadjuvanted RSV F and palivizumab had reduced viral load compared to the naïve animal group but with readily measurable virus titers in nasal tissue following challenge (Fig. 2B).
Fig. 2.
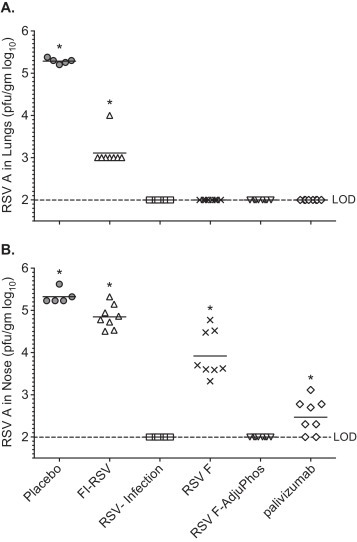
RSV-A Long viral tiers from post challenge lung and nasal tissue of cotton rats. Cotton rats (n = 8) were immunized on day 0 and 28 with FI-RSV, RSV-A Long (IN-infection), or RSV-F nanoparticle vaccine with and without AlP04. An additional group received palivizumab 15 mg/kg via intramuscular injection on day 48 one day prior to challenge. All groups were challenged on day 49. Panel A. Virus, log10 pfu/g lung tissue, GMT shown as a bar. Panel B. Virus log10 pfu/g nasal tissue *p < 0.01 compared to RSV infection and RSV F-alum groups; LOD is the lower limit of detection.
3.3. Comparative histopathology of the RSV F nanoparticle vaccine and FI-RSV vaccine
When Lot 100 FI-RSV vaccine was used in a clinical trial in the late 1960s, vaccinated children developed enhanced respiratory disease (ERD) upon reinfection [33]. Similarly, ERD can be reproduced in the cotton rat model with the same vaccine, known as Lot 100 FI-RSV vaccine [30], [31]. In the current study, Lot 100 FI-RSV induced prominent alveolitis and perivasculitis in the lungs of RSV challenged animals, consistent with ERD. Conversely, significant lung histopathological changes of this magnitude were not observed in cotton rats immunized with the RSV F nanoparticle vaccine administered with or without adjuvant and were similar to the minimal changes seen in placebo and palivizumab animals (Fig. 3A–C).
Fig. 3.
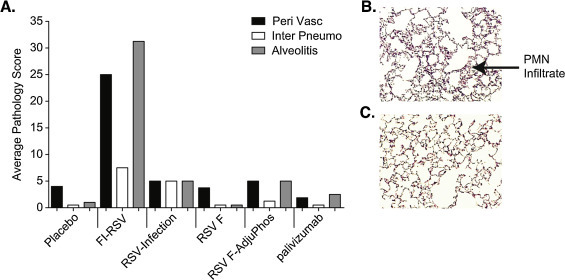
Cotton rat lung histopathology. Cotton rats (n = 8) were immunized on day 0 and 28 with FI-RSV, RSV-A Long (IN-infection), or RSV-F nanoparticle vaccine with and without AlPO4. An additional group received palivizumab 15 mg/kg via intramuscular injection on day 48 a day prior to challenge. All groups were challenged on day 49 with RSV-A Long virus. Lung tissue isolated 5 days after challenge were frozen, sectioned, and stained with hematoxylin and eosin from all the groups. Panel A. Histopathology scores were determined for alveolitis, interstitial pneumonitis and perivasculitis in a blinded fashion. Average pathology scores were plotted. Panel B. FI-RSV induced alveolitis in lung tissue collected on day 54, 5 days post challenge. Panel C. RSV F vaccinated cotton rat lung section on day 54, 5 days post challenge.
3.4. Low doses of RSV F nanoparticle vaccine and protection in cotton rats
The RSV F vaccine was derived from the RSV A long sequence. A dose ranging immunization with the RSV F vaccine was undertaken to compare the protective efficacy of the vaccine against a non-homologous challenge (RSV B) with palivizumab, known to be protective against both RSV A and B [34]. Cotton rats were immunized with 0.003, 0.03, 0.3 or 3.0 μg doses of adjuvanted RSV F vaccine on day 0 and 21. Palivizumab was given at a dose of 0.62, 1.25, 2.5 or 5.0 mg/kg one day prior to challenge.
RSV F nanoparticle vaccine and palivizumab induced serum anti-RSV F IgG titers that were high and dose dependent (GMT = 12,998–310,439, GMTs = 4626–95,441, respectively; Fig. 4A). Similarly the levels of PCA were robust for all groups that received the adjuvanted RSV F vaccine. PCA titers in animals passively transferred with palivizumab were significantly lower and only observed at the 5 and 2.5 mg/kg doses (30, 16 μg/ml). Cotton rats receiving 0.625 and 1.25 mg/kg palivizumab had PCA titers below the level of detection (10 μg/ml) (Fig. 4B). Neutralizing antibodies to RSV-A and RSV-B were induced in a dose-dependent manner (Fig. 4C). Even the lowest dose of 0.003 μg RSV F vaccine induced significant levels of neutralizing antibody against both RSV-A Long and RSV-B 18537. Neutralizing titers in the 5.0 mg/kg palivizumab group were comparable to those induced in animals actively immunized with the lowest dose of 0.003 μg RSV F vaccine (Fig. 4C and D).
Fig. 4.
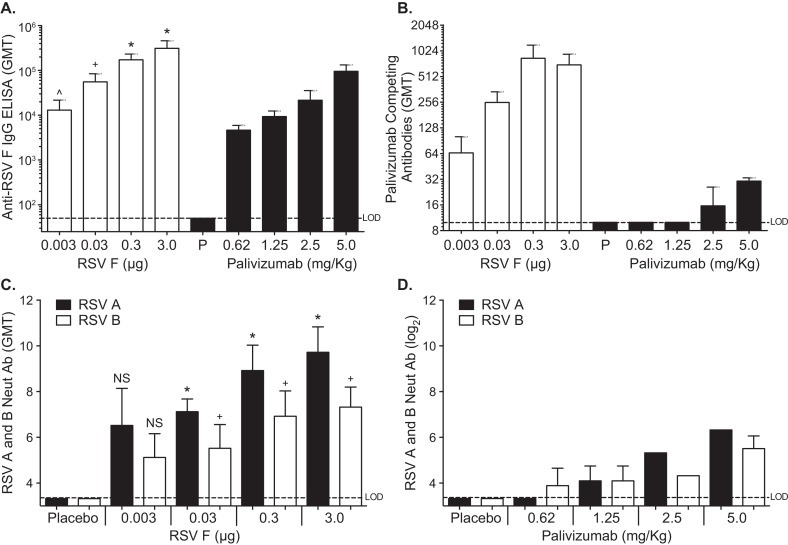
Comparison of RSV-A and -B neutralizing antibodies after active and passive immunization. Female cotton rats, 4–6 weeks old were immunized by intramuscular injection. Anti-RSV F IgG log10 GMT in pre challenge day 42 serum from cotton rats that received Panel A. Adjuvanted RSV F nanoparticle vaccine at 0.003, 0.03, 0.3 or 3.0 μg (filled bars) or Palivizumab monoclonal antibody at 0.625, 1.25, 2.5, or 5 mg/kg (empty bars). Palivizumab competing antibodies log2 GMT in pre challenge day 42 serum from cotton rats that received Panel B. Adjuvanted RSV F nanoparticle vaccine at 0.003, 0.03, 0.3 or 3.0 μg (filled bars) or palivizumab monoclonal antibody at 0.625, 1.25, 2.5, or 5 mg/kg (empty bars). Virus neutralizing antibody log2 GMT in pre challenge day 42 serum from cotton rats that received Panel C. Adjuvanted RSV F nanoparticle vaccine at 0.003, 0.03, 0.3 or 3.0 μg. or Panel D. Palivizumab monoclonal antibody at 0.625, 1.25, 2.5, or 5 mg/kg Animals in the placebo group received PBS. Error bar represent the 95% CI. *p < 0.01 compared to 5 mg/kg palivizumab group. +p < 0.05 compared to 5 mg/kg palivizumab group.
The in vivo protective efficacy of the RSV F nanoparticle vaccine was evaluated in direct comparison to palivizumab by measuring inhibition of viral replication in the lungs and nasal passages of cotton rats challenged with RSV-B 18537. Post-challenge lung virus titers (GMT) were just above the LOD in animals given the lowest dose of RSV F (0.003 μg) and were below the LOD in recipients of higher doses of RSV F vaccine (Fig. 5A). The RSV lung virus titer was 4.5 log10 in the placebo group (Fig. 5A). Palivizumab also reduced lung RSV titers to below the LOD, with more detectable virus in the lowest doses consistent to what has been previously observed [34]. Reduction of RSV titers in the nasal passages was also observed in a dose dependent manner for both the RSV F vaccine and palivizumab, with relatively lower virus levels in the RSV F vaccine group in concert with the levels of neutralizing titers induced (Fig. 5B). Thus, the RSV F vaccine was protective against non-homologous virus challenge in the upper and lower respiratory tract and appears to be a potent immunogen that provided protection via active immunization exceeding that seen with palivizumab, despite the use of very low doses of vaccine.
Fig. 5.
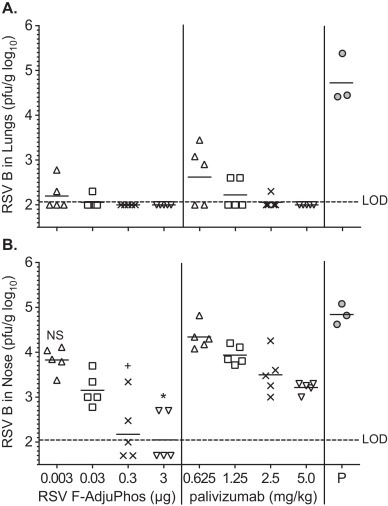
RSV-B 18537 viral titer in lung and nasal tissue from post challenge cotton rats. Female cotton rats, 4–6 weeks old were immunized by intramuscular injection with adjuvanted RSV F nanoparticle vaccine or palivizumab. Animals in the placebo group received PBS. All animals were challenged with RSV-B 18537 on day 42 and sacrificed on day 46. Viral titer is represented in Panel A. RSV-B 18537 log10 pfu/g lung tissue, and Panel B. RSV-B 18537 log10 pfu/g nasal tissue. GMT for each group shown as the bar and *p < 0.01 compared to 3 μg RSV F group, +p < 0.05 compared to 0.3 μg RSV F group. LOD is the lower limit of detection. The limit of detection of this assay was 2.0 log10.
3.5. RSV F vaccine-induced antibody and palivizumab in passive immunoprophylaxis
A passive immunization-virus challenge study was done to compare the relative potency of the vaccine, as measured by the PCA assay, relative to palivizumab. Cotton rats received IM injections of a pooled cotton rat anti-RSV F serum that delivered PCA doses of 5.6, 1.6 or 0.6 mg/kg or a similar range of palivizumab at 5.0, 1.3 or 0.6 mg/kg one day prior to RSV challenge. At 24 h after administration of anti-RSV F antibodies, the levels of RSV F IgG antibodies were high and dose dependent for all the groups with the exception of the group that received normal cotton rat serum (Fig. 6C). The titers were similar for the highest dose of palivizumab and immune anti-sera administered (GMT = 24,166 and 26,636, respectively).
Fig. 6.
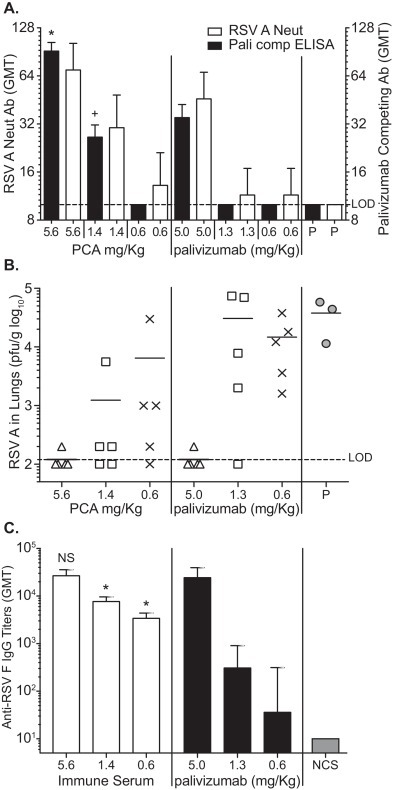
RSV-B 18537 virus Neutralizing antibodies, PCA and protection in passively immunized cotton rats. Cotton rats (n = 5) were passively immunized on day −1 with 5.6; 1.6; 0.6 mg/kg PCA antibodies, or 5; 1.25; 0.625 mg/kg palivizumab antibody. Placebo (Pl) rats received 0.15 ml of pooled pre-immune serum (NCS). Sera from 24 hour post transfer day 0 samples were analyzed and the GMT represented in Panel A. Palivizumab competitive antibody PCA (Filled bars) and RSV-B 18537 virus neutralizing antibody (Empty bars). *p < 0.01 compared with 5.0 mg/kg group and +p < 0.01 compared to 1.3 mg/kg group by two-tailed student t-test. Panel B. The RSV-B 18537 viral titers for each group represented as log10 pfu/g lung tissue. *p < 0.01 compared with placebo group by two-tailed Student t-test. NS: not significant difference with negative control group. LOD is the lower limit of detection.
Panel C. Anti-RSV F IgG log10 GMT for each group are represented with the bar graph shown. *p < 0.01 compared to 1.3 and 0.6 mg/kg palivizumab groups; NS = not significant compared to palivizumab 5 mg/kg group.
One day following passive immunization (day 0), PCA levels were significantly higher for groups that received RSV F anti-sera (p < 0.01) than those given a similar dose of palivizumab, as measured by the PCA assay (Fig. 6A). In palivizumab treated animals, PCA serum titers were at or below the LOD for the assay except at the highest dose, whereas the PCA serum levels in cotton rats passively immunized with anti-RSV F serum were 183 μg/ml and 53 μg/ml at the 5.6 and 1.4 mg/kg dose levels, respectively.
All groups were challenged 24 hours after passive immunization (day 0) with 105 pfu RSV-A Long virus. Lung tissues were collected on day 4 post challenge to determine viral titer by plaque assay on homogenized tissue. The highest doses of anti-RSV F immune sera (5.6 mg/kg) and palivizumab (5.0 mg/kg) conferred apparently complete protection (Fig. 6B), reducing virus replication in the lungs >100-fold relative to the placebo. Virus replication was also significantly reduced in animals given 1.6 and 0.6 mg/kg anti-RSV F immune sera compared to the group that received pre-immune sera (p < 0.01) (Fig. 6B). Palivizumab at 1.3 and 0.6 mg/kg induced a slight reduction in lung virus titers, but were not statistically significant when compared to the group that received pre-immune sera (Fig. 6B).
3.6. Competitive binding of vaccine-induced antibody to other RSV F neutralizing epitopes
Beeler et al. [35] have identified multiple neutralizing epitopes on RSV F protein using competitive binding assays with a panel of RSV F monoclonal antibodies and monoclonal antibody resistant mutant (MARMs) and subsequently, antigenic sites I, II, IV, V and IV were mapped on RSV F [36]. A competitive ELISA was performed using monoclonal antibodies 1107, 1112, 1153, 1243 to identify neutralizing antibodies induced by the RSV F vaccine. Antibodies 1107, 1153 and 1243 map to antigenic sites II and I while the 1112 is more broadly reactive to sites IV, V, and VI (Table 1 ). Polyclonal cotton rat sera raised against RSV F nanoparticle vaccine was competitive against these RSV F monoclonal antibodies (Table 1). Antibodies competitive for antigenic site II monoclonal antibodies 1107 and 1153 were induced by the vaccine without and with adjuvant, respectively while no or minimal site II competitive antibodies were detected in sera from FI-RSV immunized and RSV infected animals (Table 1). The RSV F vaccine also induced polyclonal responses competitive with neutralizing antibodies 1112 and 1243 that recognize RSV F antigenic sites I, IV, V and VI (Table 1).
Table 1.
Competitive binding ELISA titers of RSV neutralizing mAb's binding to RSV F.
| Vaccine groups | 1107 (site II) | 1112 (sites IV, V, VI) | 1153 (site II) | 1243 (site I) |
|---|---|---|---|---|
| FI-RSV | <20a | <20 | 60 | <20 |
| RSV-A long | <20 | 30 | 64 | 29 |
| RSV F | <20 | 164 | 347 | 133 |
| RSV F + AdjuPhos | 292 | 442 | 3205 | 1220 |
<20 denotes lower limit of detection (LOD) of the competition ELISA. mAb competing antibody (MCA) is reported as the polyclonal anti RSV F antibody titers required for 50% inhibition of binding of mAb to RSV F.
4. Discussion
RSV-related lower respiratory tract disease is the most common cause of hospitalization in infants, a common basis for infant and pediatric medical visits and a significant pathogen in the elderly and high-risk adults. Severe RSV infections in young children are clearly associated with ongoing and repeat episodes of wheezing [24], [37], [38]. These populations described above may benefit from vaccination with an RSV F-vaccine and, each population will require a unique vaccination strategy and, clinical development program.
Passive antibody prophylaxis has been shown to effectively reduce serious RSV disease in humans and induction of the immune responses to antigenic site II should be strongly considered in the development of an RSV vaccine. Here we show that the RSV F nanoparticle vaccine induces immune responses that both target site II on the F protein and are associated with functional and protective immunity in the cotton rat. The serially developed RSV prophylactic products, Respigam, palivizumab and motavizumab were first evaluated in cotton rats, a model that reliably predicted the clinical outcomes [16], [34], [39]. Based on these preclinical data, passive prophylaxis studies were advanced using palivizumab and motavizumab and were shown to reduce RSV-related hospitalization by 55–83% in preterm, high risk and term infants [14], [16], [40], [41]. In recent clinical studies, we found that vaccine elicited antibodies to the RSV F nanoparticle vaccine avidly bind to the site II epitope. This is clearly an important observation as it can associate the vaccine-induced immune responses of this novel vaccine with data showing prevention of RSV disease in five randomized clinical trials [14], [16], [40], [41].
In the current study, using an array of antibody assays, we characterized and explored the implications of the production of vaccine-induced PCA in the cotton rat model. The studies use important controls: palivizumab, to assess relative potency of the vaccine, both in active and passive assessments, and the recently available Lot 100 formalin inactivated vaccine, historically associated with clinical disease enhancement. This allowed comparative evaluation of safety, ‘functional’ immunity as measured by PCA and neutralization assays, and protection in this clinically relevant model. The vaccine was shown to be safe, potent, to elicit high levels of neutralizing, PCA, anti-F antibodies and to be protective in both homologous and non-homologous strain viral challenge. The protection seen with active immunization could be reproduced using passively injected immune sera and appeared to be dose for dose, as potent as or more potent than palivizumab. Finally, the RSV F vaccine was also found to elicit antibodies that are known to bind other non-palivizumab F protein binding sites associated with neutralization without evidence of disease enhancement.
The observation that neither adult humans, after decades of RSV infection, nor cotton rats after live virus challenge, elicit PCA in a robust manner is of great interest and warrants further study [18]. The absence of PCA after infection is not absolute and the question of whether the presence of “natural” antibodies confers protection should be the focus of future studies. Fusion activity is critical to the viral pathogenesis and antibody binding to site II disrupts fusion activity [17], together suggesting that selection pressure favors an immunologically cryptic site II. The ‘universal’ nature of the vaccine (protects against homologous and non-homologous virus), the absence of robust natural immunity to an antigen critical for pathogenesis such as site II on the F protein, the genetic stability of the palivizumab binding site [42] as compared to other sites such as antigenic site Ø [43], and the safety and the apparent potency of the vaccine, reinforce the premise that efficacy testing of the vaccine is warranted.
The clinical development of an RSV vaccine may be divided amongst three populations: infants, infants/preschool children and the elderly. Maternal immunization, the active immunization of pregnant women to provide trans-placental transferred antibody for passive protection of the infant, is a priority strategy for protection of young infants against RSV and has been successfully employed for tetanus, pertussis and influenza vaccines [44]. Older infants and toddlers may also benefit from active immunization and many strategies including live viral vaccines and purified subunit vaccines have been employed in early clinical testing [45]. An RSV purified F protein showed clinical promise in children and CF patients, but proved difficult to manufacture and stabilize [22], [46]. The clinical evaluation of a novel vaccine must also take into account the history of the formalin inactivated RSV vaccine (Pfizer Lot 100 vaccine) that unexpectedly caused severe exacerbation of pulmonary disease in children who subsequently acquired RSV infections [33], [47]. Although the precise mechanisms underlying these findings remain open to debate [48], the phenomenon of vaccine-enhanced RSV disease was limited to RSV-naïve infants immunized with FI-RSV and has not been observed either with passive antibody prophylaxis (monoclonal or polyclonal) in clinical trials using purified F protein vaccines in adults or older RSV-seropositive children [22], [46], [49]. Thus, the path forward for development of a vaccine in older infants and children will need to be carefully considered. However, a vaccine that induces high affinity antibodies that exhibit neutralization or fusion inhibition in vitro, largely absent in FI-RSV vaccinated infants [50], and is associated with protection without disease exacerbation in vivo in relevant animal models and finally shows efficacy in another setting such as maternal immunization may be considered in the absence of a licensed vaccine for this population. Finally, the RSV disease burden in elderly and high risk adults and the data indicating an F subunit vaccine is safe along with the absence of historical safety concerns due to enhanced disease in this population suggests further testing of the safety and efficacy as a seasonal respiratory vaccine is warranted. The induction of PCA by the RSV F nanoparticle vaccine provides an important rationale for further clinical evaluation in the relevant susceptible populations.
Acknowledgements
We thank Kwan Ngai for technical support. We are grateful to Dr. Eloi Kpamegan for his statistical analysis of the data. We also thank Sigmovir Inc. for performing the cotton rat animal studies. RSV F specific monoclonal antibodies 1107, 1112, 1153, and 1243 were provided by Dr. Judy Beeler FDA (WHO Repository). Conflict of interest statement
The authors are employees of Novavax.
References
- 1.Hall C.B., Walsh E.E., Long C.E., Schnabel K.C. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163:693–698. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- 2.Falsey A.R., Hennessey P.A., Formica M.A., Cox C., Walsh E.E. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 3.Carbonell-Estrany X., Simoes E.A., Dagan R., Hall C.B., Harris B., Hultquist M. Motavizumab for prophylaxis of respiratory syncytial virus in high-risk children: a noninferiority trial. Pediatrics. 2010;125:e35–e51. doi: 10.1542/peds.2008-1036. [DOI] [PubMed] [Google Scholar]
- 4.Glezen W.P., Taber L.H., Frank A.L., Kasel J.A. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 5.Hall C.B., Weinberg G.A., Iwane M.K., Blumkin A.K., Edwards K.M., Staat M.A. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwane M.K., Edwards K.M., Szilagyi P.G., Walker F.J., Griffin M.R., Weinberg G.A. Population-based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza viruses among young children. Pediatrics. 2004;113:1758–1764. doi: 10.1542/peds.113.6.1758. [DOI] [PubMed] [Google Scholar]
- 7.Ranmuthugala G., Brown L., Lidbury B.A. Respiratory syncytial virus—the unrecognised cause of health and economic burden among young children in Australia. Commun Dis Intell Q Rep. 2011;35:177–184. doi: 10.33321/cdi.2011.35.15. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention RSVFAQ, update October 17, 2008.
- 9.Thompson W.W., Shay D.K., Weintraub E., Brammer L., Cox N., Anderson L.J. Mortality associated with influenza and respiratory syncytial virus in the United States. J Am Med Assoc. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 10.Stockman L.J., Curns A.T., Anderson L.J., Fischer-Langley G. Respiratory syncytial virus-associated hospitalizations among infants and young children in the United States, 1997–2006. Pediatr Infect Dis J. 2012;31:5–9. doi: 10.1097/INF.0b013e31822e68e6. [DOI] [PubMed] [Google Scholar]
- 11.Fairchok M.P., Martin E.T., Chambers S., Kuypers J., Behrens M., Braun L.E. Epidemiology of viral respiratory tract infections in a prospective cohort of infants and toddlers attending daycare. J Clin Virol. 2010;49:16–20. doi: 10.1016/j.jcv.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nair H., Simoes E.A., Rudan I., Gessner B.D., Azziz-Baumgartner E., Zhang J.S. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet. 2013;381:1380–1390. doi: 10.1016/S0140-6736(12)61901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham B.S. Biological challenges and technological opportunities for respiratory syncytial virus vaccine development. Immunol Rev. 2011;239:149–166. doi: 10.1111/j.1600-065X.2010.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groothuis J.R., Hoopes J.M., Hemming V.G. Prevention of serious respiratory syncytial virus-related illness. II: Immunoprophylaxis. Adv Ther. 2011;28:110–125. doi: 10.1007/s12325-010-0101-y. [DOI] [PubMed] [Google Scholar]
- 15.Wu H., Pfarr D.S., Johnson S., Brewah Y.A., Woods R.M., Patel N.K. Development of motavizumab, an ultra-potent antibody for the prevention of respiratory syncytial virus infection in the upper and lower respiratory tract. J Mol Biol. 2007;368:652–665. doi: 10.1016/j.jmb.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 16.2010 MBD. Motavizumab briefing document; 2010. p. 1–147.
- 17.Huang K., Incognito L., Cheng X., Ulbrandt N.D., Wu H. Respiratory syncytial virus-neutralizing monoclonal antibodies motavizumab and palivizumab inhibit fusion. J Virol. 2010;84:8132–8140. doi: 10.1128/JVI.02699-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glenn G.M., Smith G., Fries L., Raghunandan R., Lu H., Zhou B. Safety and immunogenicity of a Sf9 insect cell-derived respiratory syncytial virus fusion protein nanoparticle vaccine. Vaccine. 2013;31:524–532. doi: 10.1016/j.vaccine.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Techaarpornkul S., Barretto N., Peeples M.E. Functional analysis of recombinant respiratory syncytial virus deletion mutants lacking the small hydrophobic and/or attachment glycoprotein gene. J Virol. 2001;75:6825–6834. doi: 10.1128/JVI.75.15.6825-6834.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sullender W.M. Respiratory syncytial virus genetic and antigenic diversity. Clin Microbiol Rev. 2000;13:1–15. doi: 10.1128/cmr.13.1.1-15.2000. [table of contents] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ulbrandt N.D., Ji H., Patel N.K., Barnes A.S., Wilson S., Kiener P.A. Identification of antibody neutralization epitopes on the fusion protein of human metapneumovirus. J Gen Virol. 2008;89:3113–3118. doi: 10.1099/vir.0.2008/005199-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piedra P.A., Cron S.G., Jewell A., Hamblett N., McBride R., Palacio M.A. Immunogenicity of a new purified fusion protein vaccine to respiratory syncytial virus: a multi-center trial in children with cystic fibrosis. Vaccine. 2003;21:2448–2460. doi: 10.1016/s0264-410x(03)00098-7. [DOI] [PubMed] [Google Scholar]
- 23.Luchsinger V., Piedra P.A., Ruiz M., Zunino E., Martinez M.A., Machado C. Role of neutralizing antibodies in adults with community-acquired pneumonia by respiratory syncytial virus. Clin Infect Dis. 2012;54:905–912. doi: 10.1093/cid/cir955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Escobar G.J., Masaquel A.S., Li S.X., Walsh E.M., Kipnis P. Persistent recurring wheezing in the fifth year of life after laboratory-confirmed, medically attended respiratory syncytial virus infection in infancy. BMC Pediatr. 2013;13:97. doi: 10.1186/1471-2431-13-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith G., Raghunandan R., Wu Y., Liu Y., Massare M., Nathan M. Respiratory syncytial virus fusion glycoprotein expressed in insect cells form protein nanoparticles that induce protective immunity in cotton rats. PLOS ONE. 2012;7:e50852. doi: 10.1371/journal.pone.0050852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calder L.J., Gonzalez-Reyes L., Garcia-Barreno B., Wharton S.A., Skehel J.J., Wiley D.C. Electron microscopy of the human respiratory syncytial virus fusion protein and complexes that it forms with monoclonal antibodies. Virology. 2000;271:122–131. doi: 10.1006/viro.2000.0279. [DOI] [PubMed] [Google Scholar]
- 27.Chaiwatpongsakorn S., Epand R.F., Collins P.L., Epand R.M., Peeples M.E. Soluble respiratory syncytial virus fusion protein in the fully cleaved, pretriggered state is triggered by exposure to low-molarity buffer. J Virol. 2011;85:3968–3977. doi: 10.1128/JVI.01813-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLellan J.S., Correia B.E., Chen M., Yang Y., Graham B.S., Schief W.R. Design and characterization of epitope-scaffold immunogens that present the motavizumab epitope from respiratory syncytial virus. J Mol Biol. 2011;409:853–866. doi: 10.1016/j.jmb.2011.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swanson K.A., Settembre E.C., Shaw C.A., Dey A.K., Rappuoli R., Mandl C.W. Structural basis for immunization with postfusion respiratory syncytial virus fusion F glycoprotein (RSV F) to elicit high neutralizing antibody titers. Proc Natl Acad Sci U S A. 2011;108:9619–9624. doi: 10.1073/pnas.1106536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prince G.A., Curtis S.J., Yim K.C., Porter D.D. Vaccine-enhanced respiratory syncytial virus disease in cotton rats following immunization with Lot 100 or a newly prepared reference vaccine. J Gen Virol. 2001;82:2881–2888. doi: 10.1099/0022-1317-82-12-2881. [DOI] [PubMed] [Google Scholar]
- 31.Prince G.A., Jenson A.B., Hemming V.G., Murphy B.R., Walsh E.E., Horswood R.L. Enhancement of respiratory syncytial virus pulmonary pathology in cotton rats by prior intramuscular inoculation of formalin-inactiva ted virus. J Virol. 1986;57:721–728. doi: 10.1128/jvi.57.3.721-728.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arbiza J., Taylor G., Lopez J.A., Furze J., Wyld S., Whyte P. Characterization of two antigenic sites recognized by neutralizing monoclonal antibodies directed against the fusion glycoprotein of human respiratory syncytial virus. J Gen Virol. 1992;73(Pt 9):2225–2234. doi: 10.1099/0022-1317-73-9-2225. [DOI] [PubMed] [Google Scholar]
- 33.Kim H.W., Canchola J.G., Brandt C.D., Pyles G., Chanock R.M., Jensen K. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 34.Johnson S., Oliver C., Prince G.A., Hemming V.G., Pfarr D.S., Wang S.C. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis. 1997;176:1215–1224. doi: 10.1086/514115. [DOI] [PubMed] [Google Scholar]
- 35.Beeler J.A., van Wyke Coelingh K. Neutralization epitopes of the F glycoprotein of respiratory syncytial virus: effect of mutation upon fusion function. J Virol. 1989;63:2941–2950. doi: 10.1128/jvi.63.7.2941-2950.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez J.A., Bustos R., Orvell C., Berois M., Arbiza J., Garcia-Barreno B. Antigenic structure of human respiratory syncytial virus fusion glycoprotein. J Virol. 1998;72:6922–6928. doi: 10.1128/jvi.72.8.6922-6928.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blanken M.O., Rovers M.M., Molenaar J.M., Winkler-Seinstra P.L., Meijer A., Kimpen J.L. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med. 2013;368:1791–1799. doi: 10.1056/NEJMoa1211917. [DOI] [PubMed] [Google Scholar]
- 38.Sigurs N., Aljassim F., Kjellman B., Robinson P.D., Sigurbergsson F., Bjarnason R. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65:1045–1052. doi: 10.1136/thx.2009.121582. [DOI] [PubMed] [Google Scholar]
- 39.Siber G.R., Leombruno D., Leszczynski J., McIver J., Bodkin D., Gonin R. Comparison of antibody concentrations and protective activity of respiratory syncytial virus immune globulin and conventional immune globulin. J Infect Dis. 1994;169:1368–1373. doi: 10.1093/infdis/169.6.1368. [DOI] [PubMed] [Google Scholar]
- 40.Andabaka T., Nickerson J.W., Rojas-Reyes M.X., Rueda J.D., Bacic Vrca V., Barsic B. Monoclonal antibody for reducing the risk of respiratory syncytial virus infection in children. Cochrane Database Syst Rev. 2013;4:CD006602. doi: 10.1002/14651858.CD006602.pub4. [DOI] [PubMed] [Google Scholar]
- 41.Gill M.A., Welliver R.C. Motavizumab for the prevention of respiratory syncytial virus infection in infants. Expert Opin Biol Ther. 2009;9:1335–1345. doi: 10.1517/14712590903287499. [DOI] [PubMed] [Google Scholar]
- 42.McLellan J.S., Chen M., Leung S., Graepel K.W., Du X., Yang Y. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science. 2013;340:1113–1117. doi: 10.1126/science.1234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tapia L.I., Shaw C.A., Aideyan L.O., Jewell A.M., Dawson B.C., Haq T.R. Gene sequence variability of the three surface proteins of human respiratory syncytial virus (HRSV) in Texas. PLOS ONE. 2014;9:e90786. doi: 10.1371/journal.pone.0090786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thwaites C.L., Beeching N.J., Newton C.R. Maternal and neonatal tetanus. Lancet. 2014 doi: 10.1016/S0140-6736(14)60236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guvenel A.K., Chiu C., Openshaw P.J. Current concepts and progress in RSV vaccine development. Expert Rev Vaccines. 2014;13:333–344. doi: 10.1586/14760584.2014.878653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Groothuis J.R., King S.J., Hogerman D.A., Paradiso P.R., Simoes E.A. Safety and immunogenicity of a purified F protein respiratory syncytial virus (PFP-2) vaccine in seropositive children with bronchopulmonary dysplasia. J Infect Dis. 1998;177:467–469. doi: 10.1086/517377. [DOI] [PubMed] [Google Scholar]
- 47.Kapikian A.Z., Mitchell R.H., Chanock R.M., Shvedoff R.A., Stewart C.E. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969;89:405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- 48.Blanco J.C., Boukhvalova M.S., Shirey K.A., Prince G.A., Vogel S.N. New insights for development of a safe and protective RSV vaccine. Hum Vaccin. 2010;6:482–492. doi: 10.4161/hv.6.6.11562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Munoz F.M., Piedra P.A., Glezen W.P. Safety and immunogenicity of respiratory syncytial virus purified fusion protein-2 vaccine in pregnant women. Vaccine. 2003;21:3465–3467. doi: 10.1016/s0264-410x(03)00352-9. [DOI] [PubMed] [Google Scholar]
- 50.Murphy B.R., Walsh E.E. Formalin-inactivated respiratory syncytial virus vaccine induces antibodies to the fusion glycoprotein that are deficient in fusion-inhibiting activity. J Clin Microbiol. 1988;26:1595–1597. doi: 10.1128/jcm.26.8.1595-1597.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


