Abstract
Megachiropteran bats are biologically important both as endangered species and reservoirs for emerging human pathogens. Reliable detection of antibodies to specific pathogens in bats is thus epidemiologically critical. Eight variable flying foxes (Pteropus hypomelanus) were immunized with 2,4-dinitrophenylated bovine serum albumin (DNP-BSA). Each bat received monthly inoculations for 2 months. Affinity-purified IgG was used for production of polyclonal and monoclonal anti-variable flying fox IgG antibodies. ELISA and western blot analysis were used to monitor immune responses and for assessment of polyclonal and monoclonal antibody species cross-reactivity. Protein G, polyclonal antibodies, and monoclonal antibodies detected specific anti-DNP antibody responses in immunized variable flying foxes, with protein G being the most sensitive, followed by monoclonal antibodies and then polyclonal antibodies. While the polyclonal antibody was found to cross-react well against IgG of all bat species tested, some non-specific background was observed. The monoclonal antibody was found to cross-react well against IgG of six other species in the genus Pteropus and to cross-react less strongly against IgG from Eidolon helvum or Phyllostomus hastatus. Protein G distinguished best between vaccinated and unvaccinated bats, and these results validate the use of protein G for detection of bat IgG. Monoclonal antibodies developed in this study recognized immunoglobulins from other members of the genus Pteropus well, and may be useful in applications where specific detection of Pteropus IgG is needed.
Keywords: Bats, Flying fox, Pteropus hypomelanus, Antibody, Protein G, ELISA
Résumé
Les chauves-souris du groupe mégachiroptère sont importantes au point de vue biologique, parce qu’elles représentent un réservoir pour de nouvelles maladies humaines. La possibilité de détecter leurs anticorps contre des pathogènes spécifiques est donc critique au point de vue épidemiologique. Huit rousettes des iles (Pteropus hypomelanus) furent injectées avec de l’albumine de sérum bovin (2,4-dinitrophenylated bovine serum albumin, DNP-BSA) deux fois, à quatre semaines d’intervalle. Les anticorps IgG à affinité purifiée fûrent utilisés pour produire les anticorps mono- et polyclonaux IgG de chauves-souris. La réponse immunitaire des animaux et la réaction croisée entre les anticorps de différentes espèces furent mesurés avec l’ELISA et l’analyse de Western blot.
Au jour 56 apres l’injection, une magnification de la densité optique (optical density, OD405) entre 4 et 17 fois a été mesurée lorsque la protéine G fut utilisée comme agent. Quand des anticorps polyclonaux de lapins souris furent utilisés, la magnification fut de 1.4 a 2, alors que lorsque des anticorps monoclonaux de souris furent utilisés, la magnification fût de 1.7 a 7.
La protéine G, les anticorps mono et polyclonaux ont réussi a détecter une réponse immunitaire contre l’agent DNP chez les roussettes. Malgré que les anticorps polyclonaux aient réussi a faire une réaction croisée avec les IgG des chauves-souris, il y avait aussi beaucoup d’interférences.
Les anticorps monoclonaux ont très bien réagi avec 6 autres espèces du genre Pteropus, mais moins bien avec les epèces Eidolon helvum ou Phyllostomus hastatus. La protéine G fût utile pour distinguer les animaux vaccinés des naïfs, et ces résultats valident l’utilisation de la protéine G pour la détection des anticorps IgG chez les chauves-souris.
Les anticorps monoclonaux developpés dans ce projet a aussi pu détecter les immunoglobulines de d’autres membres de la famille Pteropus, et leur usage pourrait être utile dans certains cas où l’on a besoin de détecter les anticorps IgG.
Mots clés: Chauves-souris, Roussette, Pteropus hypomelanus, Anticorps, Protéine G, ELISA
1. Introduction
Bats are the second largest mammalian order, Chiroptera, with approximately 1100 species [1]. The variable flying fox, Pteropus hypomelanus, is a megachiropteran bat that inhabits Sulawesi and small islands from the Bay of Bengal and the Malay Peninsula to New Guinea and the Solomon Islands. The range of this species is fragmented, and rapid habitat destruction is occurring throughout the range. Since 1989, it has been listed as CITES appendix II. There are seven subspecies of this bat, the most endangered being P. hypomelanus maris, found on the Maldives [2]. The greatest threat to the variable flying fox is human predation and habitat destruction. This bat is heavily hunted and is exported for food in the Philippines. Residents of Guam have imported large numbers of Pteropus spp. from other Pacific islands as a delicacy. Thirty-four percent of megachiropteran bats are at risk of extinction [3], and all members of the genus Pteropus are listed as at least CITES appendix II.
It is not surprising to find significant pathogen diversity in bats. There are over 900 bat species, representing approximately 20% of mammalian diversity [4]. While less than 2% of human pathogens have bats as natural reservoirs, several emerging potentially lethal viral infections of humans have recently been found in wild flying fox populations, attracting significant interest [4]. These include Nipah virus, Menangle virus, Hendra virus, and Australian bat lyssavirus. Evidence suggests that megachiropteran bats are carriers of Ebola virus [5], and Rhinolophid bats, which are closely related to the megachiropterans, are carriers of SARS-like coronaviruses [1], [6], [7]. With the subsequent interest in viruses of bats, Tioman virus has recently been discovered [8], and it is likely that further bat viruses will be discovered in the near future. Evidence for Nipah virus [9], Tioman virus, and Australian bat lyssavirus [10] have been found in wild P. hypomelanus. Currently, serum neutralization assays are the most common tests used to detect the presence of antibodies to specific pathogens in bats, and are accepted as the reference standard [11]. However, serum neutralization assays require days to weeks for results, and require virus culture, which is labor intensive, slow, and involves live virus culture, representing a risk to personnel. IgG assays have also been performed in bats using chimeric protein G, a streptococcal protein, and protein A, a staphylococcal protein, that bind IgG from a number of different species [11], [12], [13]. Assays have also been performed using only protein G [7], [14]. Protein A and protein G do not bind well to immunoglobulins from all species [15]. Protein A has been found not to bind well to immunoglobulins from some bat species [16]. The specificity and avidity of either protein G or chimeric protein G/protein A for bat immunogobulins has not been published.
There is not only a need to rapidly assess wild populations for emerging bat and human pathogens but also a need to establish healthy specific pathogen free captive populations of fruit bats. While transmission studies may result in the death of the animal being challenged, immunization studies are rather benign, having minimal affect on the host other than inducing an immune response. This is an important concern when working with threatened species. This project used an immunization study to develop and validate the reagents needed for developing ELISAs for the variable flying fox. These reagents and the information generated will prove useful for seroepidemiological studies of Pteropus spp.
2. Materials and methods
2.1. Variable flying foxes and immunization
Eight adult (4 male, 4 female) variable flying foxes (P. hypomelanus) housed at the Lubee Bat Conservancy in Gainesville, Florida were used in this study. All were assessed as healthy before inclusion in the study based on physical examination and blood values within reference ranges for this species. This protocol was approved by the University of Florida Institutional Animal Care and Use Committee. Blood samples (2 ml) were collected from the brachial vein and placed into lithium heparin tubes on days 0, 28, and 56. Following blood collection on days 0 and 28, six bats (3 male, 3 female) were immunized by subcutaneous and intramuscular inoculation with 250 μg (500 μl; 1/2 SQ-1/2 IM) conjugated 2,4-dinitrophenylated bovine serum albumin (DNP-BSA) (Molecular Probes, Eugene, OR) in a monophosphoryl lipid A plus synthetic trehalose dimycolate (MPL + TDM) adjuvant system (Sigma Chemical Co., St. Louis, MO). Two bats (1 male, 1 female) were immunized by subcutaneous and intramuscular inoculation with phosphate-buffered saline (PBS) in MPL + TDM adjuvant as negative controls. Plasma was obtained by low speed centrifugation of the tubes at 300 × g for 10 min at room temperature. All plasma samples were stored at −70 °C.
2.2. Cross-reactivity of other anti-immunoglobulin reagents with P. hypomelanus immunoglobulins
To determine the best reagent for purification of P. hypomelanus immunoglobulin, an ELISA looking for binding of anti-IgG reagents to P. hypomelanus plasma was performed. Ninety-six well plates (Nunc Maxisorp, Fisher Scientific, Pittsburgh, PA) were coated with DNP-BSA overnight at 4 °C. Positive control wells were coated with serum appropriate for the secondary antibody. Wells were washed with PBS containing 0.02% sodium azide and 0.05% Tween-20 using a microplate washer (Biotek Instruments, Winooski, VT). A 1:100 dilution of immunized bat serum in PBS containing 0.02% sodium azide (PBS-AZ) was added. Negative control wells were incubated with PBS-AZ only. The plates were incubated at room temperature for 1 h. The plates were washed, and various secondary alkaline phosphatase-conjugated anti-immunoglobulin reagents were added (rabbit anti-mouse IgG [A-1902, Sigma Chemical Co.]; protein G [10-1222, Zymed Laboratories Inc., San Francisco, CA]; goat anti-rat IgG [A-9654, Sigma Chemical Co.]; donkey anti-sheep IgG [A-5187, Sigma Chemical Co.]; rabbit anti-goat IgG [A-4187, Sigma Chemical Co.]; goat anti-human IgG [2010-04, Southern Biotech. Assoc., Birmingham, AL]; goat anti-rabbit IgG [A-8025, Sigma Chemical Co.]; goat anti-guinea pig IgG [A-5062, Sigma Chemical Co.]; rabbit anti-horse IgG [A-6167, Sigma Chemical Co.]; rabbit anti-bovine IgG [A-0705, Sigma Chemical Co.]; mouse anti-black rhino [HL1530, Hybridoma Core Laboratory], and chicken anti-manatee IgG [9892, Hybridoma Core Laboratory]) at dilutions of 1:1000 and 1:2000 in PBS-AZ for each. All wells were done in duplicate. Plates were incubated at room temperature for 1 h. After incubation and washing, each well received 0.1 ml of alkaline phosphatase substrate (1 mg/ml p-nitrophenyl phosphate, Sigma Chemical Co.). Color development was monitored visually, and the absorbance at 405 nm (OD405) was recorded after 30 and 60 min by use of a microplate reader (Spectramax 250, Molecular Devices, Sunnyvale, CA).
2.3. Purification of flying fox immunoglobulin
Purification of P. hypomelanus immunoglobulin was performed using a commercial protein G column (HiTrap Protein G HP 5 ml, GE Healthcare, Piscataway, NJ). The column was prewashed with 50 ml PBS-AZ. A 0.5 ml aliquot of P. hypomelanus plasma was diluted 1:5 in PBS-AZ and circulated over the column for 50 min. The column was washed with 65 ml of PBS-AZ. IgG was eluted from the column using 0.1 M glycine, pH 3.0. Fractions were collected in 3.0 ml aliquots as they eluted from the column. Absorbance at 280 nm of each fraction was measured, and peak fractions were pooled. The eluate was desalted using a centrifugal filter device (Ultrafree, Millipore, Billerca, MA 01821) with a 30 kDa cut-off. The eluted protein was electrophoresed (2100 Bioanalyzer, Agilent Technologies, Palo Alto, CA) and measured.
2.4. Polyclonal antibody production
Purified P. hypomelanus IgG was delivered to a private biological company (Strategic Biosolutions, Newark, DE) for polyclonal antiserum production. Two rabbits were immunized using their standard protocol. Plasma samples were obtained from each rabbit before immunization (preimmune plasma). Rabbits were immunized with 200 μg of P. hypomelanus IgG with complete Freund's adjuvant (first injection), then with incomplete Freund's adjuvant (all subsequent injections). Inoculations were given on days 0, 21, 35, and 49, and plasma samples were obtained on days 42, 57 and 63. Rabbits were anesthetized and exsanguinated on day 89. Serum samples were stored at −70 °C.
2.5. Monoclonal antibody production
Mouse monoclonal antibodies against P. hypomelanus IgG were produced by standard protocols used by the Interdisciplinary Center for Biotechnology Research Hybridoma Core Laboratory at the University of Florida [17]. Briefly, a Balb/c female mouse was immunized subcutaneously with 30 μg of P. hypomelanus IgG in MPL + TDM adjuvant. Immunization was repeated on days 17, 92, and 143. Serum samples were obtained from the tail vein on days 27 and 103. The mouse was euthanatized and the spleen was harvested. Spleen cells from the immunized mice were fused with Sp2/0 mouse myeloma cells at a ratio of 7:1 using 50% polyethylene glycol. Supernatants from the resulting hypoxanthine/aminopterine/thymidine (HAT) resistant hybridoma cells were evaluated for the presence of antibody that bound to P. hypomelanus IgG by ELISA using methods similar to those described above, with P. hypomelanus IgG-coated wells and alkaline phosphatase labeled rabbit anti-mouse IgG (A-1902) as a secondary antibody. Hybridomas from wells with positive antibody results (OD405 3–20× over background) were transferred to 24 well plates and screened by an ELISA a second time. One hybridoma with positive antibody results was selected and cloned. The cloned hybridoma cell line was designated HL1892.
2.6. Detection of the humoral immune response
ELISAs were used to monitor the humoral immune response to DNP-BSA in variable flying foxes. Wells of a high protein binding microplate (Nunc Maxisorp, Fisher Scientific) were coated with 50 μl of DNP-BSA (1.0 μg/ml) and were left to adsorb overnight at 4 °C. After this and each subsequent step, all wells were washed three times with PBS-AZ and 0.05% Tween-20 using an automated microplate washer. After washing, all wells were blocked with PBS-AZ containing 5% nonfat dry milk (NFDM). This and each subsequent step of the ELISA were incubated with gentle agitation for 1 h at approximately 25 °C. Variable flying fox plasma samples were diluted from 1:25 to 1:32,000, and replicate wells were done for each dilution. PBS-AZ was applied to a pair of wells for each plasma sample to serve as duplicate negative control wells. Fifty microliter of preimmune and immune bat plasma dilutions were used to coat the wells. Alkaline phosphatase-conjugated protein G (1:500 in PBS-AZ), rabbit anti-P. hypomelanus IgG polyclonal antibody (1:10,000 in PBS-AZ), and mouse anti-P. hypomelanus IgG monoclonal antibody (undiluted hybridoma supernatant) were added for the detection of bound bat antibodies. Alkaline phosphatase-conjugated goat anti-rabbit IgG (Sigma A-8025, 1:2000 in PBS-AZ) and rabbit anti-mouse IgG (A-1902, 1:1000 in PBS-AZ) were used as secondary antibodies for polyclonal or monoclonal antibodies, respectively. After washing, 0.1 ml of 1 mg/ml p-nitrophenyl phosphate was added for detection. Color development was monitored visually, and the OD405 was recorded after 30 and 60 min by use of a microplate reader. For analysis, the average OD405 of the negative controls were subtracted from the average OD405 readings of all other wells of the corresponding antibody (corrected OD405).
2.7. Western blot analysis of polyclonal and monoclonal antibodies
For further evaluation of the specificity of the polyclonal and monoclonal antibodies, variable flying fox plasma was separated by gel electrophoresis in SDS-PAGE under reducing conditions using pre-cast 10% Bis–Tris gels (Invitrogen, San Diego, CA) and then electrophoretically transferred from the gel to a nitrocellulose membrane (Invitrogen). A Tris–glycine buffer (Invitrogen) in 20% methanol was used as the transfer buffer. The blotting time was 60 min at 30 V. After transfer, the nitrocellulose was immediately blocked overnight with PBS-AZ containing 5% NFDM at room temperature (approximately 25 °C). The nitrocellulose blot was washed three times for 5 min each with PBS solution containing 0.02% sodium azide and 0.05% Tween-20 and placed into a channel divider (Fast Blot Developer, Pierce, Rockford, IL). The rabbit polyclonal antisera was diluted 1:50,000. A 1:50,000 dilution of rabbit preimmune sera was used as a negative control. A total of 900 μl of primary antibody (polyclonal or monoclonal antibodies) was loaded into each channel and incubated on the nitrocellulose for 60 min at room temperature on a rocker. After washing, the nitrocellulose was removed from the manifold and incubated with the appropriate secondary antibody: alkaline phosphatase labeled goat anti-rabbit IgG (diluted 1:2000 in PBS-AZ) or goat anti-mouse IgG (diluted 1:4000 in PBS-AZ) for the polyclonal antibody and monoclonal antibodies, respectively, for 60 min. After washing, the blot was developed with nitroblue tetrazolium 5-bromo-4-chloro-3 inolyphosphate p-toluidine substrate, per manufacturer's instructions (Sigma Chemical Co.).
2.8. Cross-reactivity of rabbit polyclonal and mouse monoclonal anti-P. hypomelanus IgG antibodies
The ability of the rabbit polyclonal antibody and monoclonal antibody HL1892 to cross-react with previously banked plasma from eight bat species, including six other species in the genus Pteropus (P. conspicillatus, P. giganteus, P. poliocephalus, P. pumilus, P. rodricensis, and P. vampyrus), another megachiropteran species (Eidolon helvum), and a phyllostomatid microchiropteran (Phyllostomus hastatus), was evaluated by an ELISA as described above. Wells were coated with 50 μl of a 1:100 dilution of plasma samples from each of the eight bat species. Fifty microliter of a 1:5000 dilution of rabbit polyclonal anti-P. hypomelanus IgG or undiluted mouse monoclonal anti-P. hypomelanus IgG hybridoma supernatant was then evaluated for reactivity on these plasma samples. A 1:5000 dilution of rabbit preimmune sera was used as a negative control for the polyclonal antibody ELISA, and hybridoma culture media was used as a negative control for the monoclonal antibody ELISA. Appropriate secondary antibodies were used as described above. The polyclonal antibody ELISA was read at 30 min and the monoclonal antibody ELISA was read at 60 min. For analysis, the average OD405 of the negative controls were subtracted from the average OD405 readings of all other wells of the corresponding antibody (corrected OD405). Specificity of this reaction was confirmed by western blot analysis as described above using serum from the different bat species.
3. Results
3.1. Cross-reactivity of other anti-immunoglobulin reagents with P. hypomelanus IgG using ELISA
At 60 min, a 33-fold increase in absorbance over the negative control was seen using a 1:1000 dilution of alkaline phosphatase-conjugated protein G. At 60 min, a 10-fold increase over the negative control was seen using a 1:1000 dilution of goat anti-human IgG-lambda chain specific. These wells were detected with a rabbit anti-goat IgG alkaline phosphatase conjugate. Significant increase was not seen with other anti-immunoglobulin antibodies. A protein G column was therefore used for purification of P. hypomelanus IgG.
3.2. Purification of P. hypomelanus immunoglobulin
The protein eluted from the protein G column measured 161 kDa unreduced and had two bands of 58.1 and 28.7 kDa when reduced (Fig. 1 ). This was comparable in size to mouse IgG, which measured 154.9 kDa unreduced and had two bands of 56.4 and 21.5 kDa when reduced on the same equipment. In other reports, bat IgGs were comparably sized [18], [19].
Fig. 1.
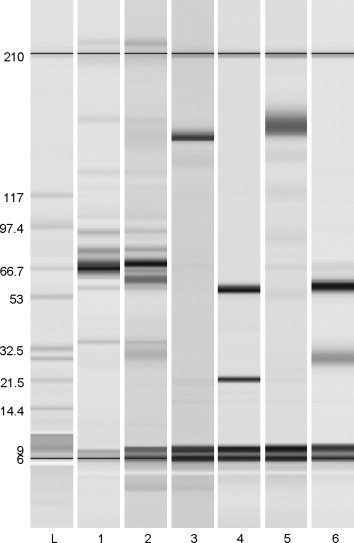
Protein electrophoresis to assess purified P. hypomelanus IgG. L, ladder. Lane 1, unpurified P. hypomelanus plasma. Lane 2, unpurified P. hypomelanus plasma. Lane 3, unreduced mouse IgG. Lane 4, reduced mouse IgG. Lane 5, unreduced P. hypomelanus IgG. Lane 6, reduced P. hypomelanus IgG.
3.3. Detection of the humoral immune response
Immunized variable flying foxes developed an anti-DNP-BSA titer compared with preimmune plasma. Use of protein G in an ELISA showed the greatest increase in antibody titer between pre-vaccination and 56 day post-vaccination variable flying fox plasma with samples diluted 1:2000 in PBS (Fig. 2 ). Detection of the anti-DNP-BSA antibody titer by use of the polyclonal rabbit anti-P. hypomelanus IgG antibody showed the greatest increase in increase in antibody titer between pre-vaccination and 56 day post-vaccination variable flying fox plasma samples diluted 1:800 (Fig. 3 ). Detection of the anti-DNP-BSA antibody titer by use of the monoclonal mouse anti-P. hypomelanus IgG antibody HL1892 showed the greatest increase in antibody titer between pre-vaccination and 56 day post-vaccination variable flying fox plasma samples diluted 1:100 (Fig. 4 ). Bats A and E consistently showed strong antibody responses to DNP-BSA in all assays, whereas bat B showed little to no antibody response to vaccination.
Fig. 2.
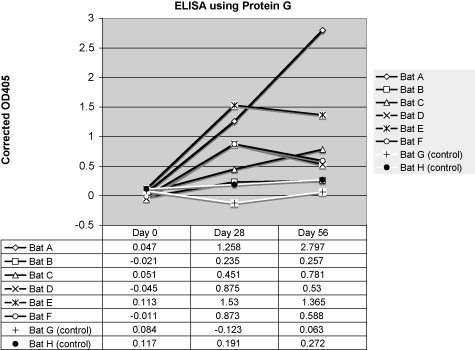
Corrected OD405 values for an ELISA using protein G for detection. Plasma was diluted 1:2000. Vaccinated bats are represented by black lines, and control bats are represented by white lines.
Fig. 3.
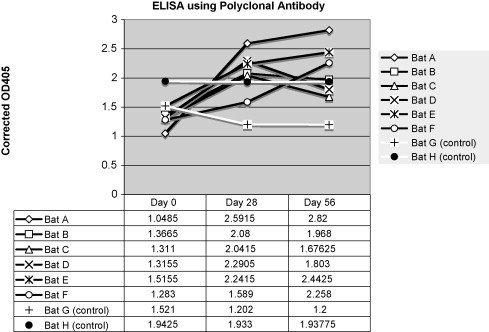
Corrected OD405 values for an ELISA using polyclonal rabbit anti-bat IgG antibody for detection. Plasma was diluted 1:800. Vaccinated bats are represented by black lines, and control bats are represented by white lines.
Fig. 4.
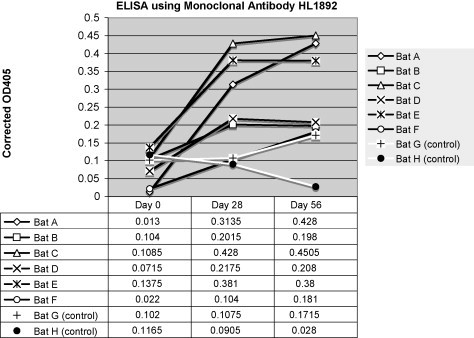
Corrected OD405 values for an ELISA using monoclonal mouse anti-bat antibody HL1892 for detection. Plasma was diluted 1:100. Vaccinated bats are represented by black lines, and control bats are represented by white lines.
3.4. Western blot analysis of polyclonal and monoclonal antibodies
Western blot reactivity of anti-P. hypomelanus IgG's on variable flying fox plasma was determined (Fig. 5 ). The polyclonal antibody (lane 1) reacted with major bands at approximately 58 and 29 kDa, consistent with IgG heavy chain and light chain, respectively. Multiple weaker bands ranging from >191 to 15 kDa were also seen, and were interpreted as non-specific binding. Monoclonal antibody HL1892 (lane 2) reacted with a band at approximately 58 kDa, consistent with IgG heavy chain. Non-specific binding was not seen under the conditions used. No reaction was seen with the negative control (lane 3).
Fig. 5.
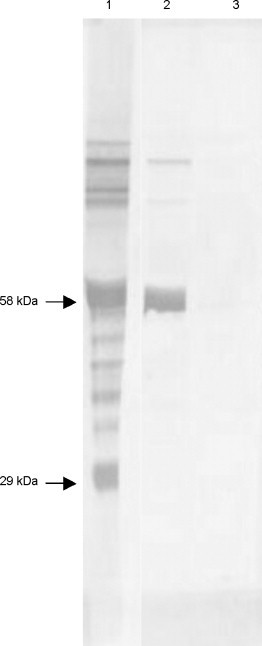
Western blot reactivity of anti-P. hypomelanus IgG's on variable flying fox plasma. Lane 1, polyclonal rabbit anti-bat antibody. Lane 2, monoclonal mouse anti-bat antibody HL1892. Lane 3, preimmune rabbit serum (negative control).
3.5. Cross-reactivity of rabbit polyclonal and mouse monoclonal anti-P. hypomelanus IgG antibodies
The polyclonal anti-P. hypomelanus IgG antibody was found to cross-react well against plasma of all bat species tested by ELISA, although more strongly with bats in the genus Pteropus (Table 1 ). The monoclonal antibody HL1892 was found to cross-react by ELISA equally as well against plasma of P. poliocephalus and P. pumilus as it did against P. hypomelanus plasma. Strong reaction was also seen with plasma from other members of the genus Pteropus tested, with OD405 readings 49–70% of those seen against P. hypomelanus plasma. OD405 readings against plasma from E. helvum or P. hastatus were not significantly different from that seen with negative controls. On western blot using the anti-P. hypomelanus IgG polyclonal antibody for detection, major bands were detected at approximately 58 and 29 kDa with all bat plasma tested, consistent with IgG heavy chain and light chain, respectively (Fig. 6 ). Cross-reaction with some non-specific background similar to that seen with P. hypomelanus plasma was seen for all bat sera tested. Monoclonal antibody HL1892 (Fig. 7 ) reacted with a band at approximately 58 kDa in all bat plasma, consistent with IgG heavy chain. Non-specific binding was not seen under the conditions used. No reaction was seen with the negative control (data not shown).
Table 1.
Corrected OD405 values for ELISAs using polyclonal rabbit anti-P. hypomelanus IgG and monoclonal mouse anti-P. hypomelanus IgG for detection
| Polyclonal | Monoclonal | |
|---|---|---|
| Pteropus hypomelanus | 3.666 | 0.755 |
| Pteropus conspicillatus | 2.84 | 0.3935 |
| Pteropus rodricensis | 2.5865 | 0.3735 |
| Pteropus poliocephalus | 3.3095 | 0.8675 |
| Pteropus giganteus | 2.649 | 0.5355 |
| Pteropus pumilus | 3.234 | 0.7655 |
| Pteropus vampyrus | 3.0525 | 0.4165 |
| Eidolon helvum | 2.025 | 0.0105 |
| Phyllostomus hastatus | 2.0765 | −0.006 |
Six different species in the genus Pteropus are represented. E. helvum is a megachiropteran outside the genus Pteropus. P. hastatus is a microchiropteran.
Fig. 6.
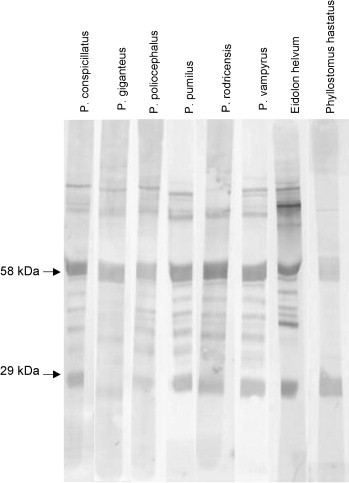
Reactivity of polyclonal rabbit anti-bat antibody against sera from eight different bat species. Six different species in the genus Pteropus are represented. Eidolon helvum is a megachiropteran outside the genus Pteropus. Phyllostomus hastatus is a microchiropteran.
Fig. 7.
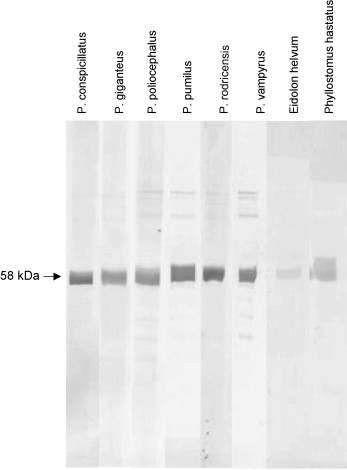
Reactivity of monoclonal mouse anti-bat antibody HL1892 against sera from eight different bat species. Six different species in the genus Pteropus are represented. Eidolon helvum is a megachiropteran outside the genus Pteropus. Phyllostomus hastatus is a microchiropteran.
4. Discussion
The reagents used in this study may be useful for the development of ELISA and western blot tests for antibodies to pathogens found in Pteropus spp. Although neutralization assays are accepted as the reference standard [11], there are several disadvantages to these assays. Serum neutralization requires a period of days to weeks to obtain results, and early diagnosis of infection may be important for epidemiologic control. Serum neutralization assays also require pathogen culture, and as some emerging infections of bats represent serious biohazards, the risk to personnel is significant, and appropriate facilities for culture are limited. Serum neutralization also needs consistent CPE, and some viruses may have limited CPE.
In addition to serum neutralization, several other methods have been used for the detection of antibodies in bats, including hemagglutination inhibition [20], complement fixation [21], and gel immunoprecipitation [22].
Early work showed that in big brown bats (Eptesicus fuscus) experimentally infected with Japanese encephalitis virus, the predominant antibody type shifted from a heavier protein (corresponding to IgM) to a lighter protein (corresponding to IgG) within 20 days [23]. A study using bacteriophage øX174 as an antigen in big brown bats found that vaccinated animals had converted to an IgG response by 8 weeks [24]. Bats had high neutralizing antibody titers 3.5 years after vaccination. Great fruit-eating bats (Artibeus lituratus) were shown to develop strong IgM and IgA responses to experimental Histoplasma capsulatum infection at 3–5 weeks and IgG responses by 8–9 weeks [22]. Within the genus Pteropus, Indian flying foxes (P. giganteus) immunized with sheep erythrocytes had a maximal IgG response at 15–30 days, depending on immunization dose [25]. Vampire bats (Desmodus rotundus) orally vaccinated with a recombinant rabies vaccine showed the protection 18 days post-vaccination, with maximal protection 30 days post-vaccination [26]. Protection decreased at 90 and 120 days post-vaccination. Egyptian fruit bats (Rousettus aegyptiacus) vaccinated with a killed rabies vaccine developed neutralizing antibody titers [27].
Previous ELISA assays have been used in bats. ELISAs using a chimeric protein A/G as conjugate have been used for the detection of antibodies to Nipah and Hendra viruses in bats, and an ELISA using this protocol has been found to have a specificity of 98.4% for Nipah virus in pigs [11]. A similar ELISA was used to screen for lyssavirus in Cambodian bats [12]. In the lyssavirus study, ELISA-positive sera were confirmed using a neutralization assay, and only 27% had neutralizing antibodies. Reasons for this discrepancy may be either low specificity of the lyssavirus ELISA, low sensitivity of the neutralization assay, or detection of antibodies to non-neutralizing epitopes.
ELISA methods that do not require binding of the constant region of immunoglobulins have also been developed. These assays require pathogen-specific rather than host antibody-specific reagents. Competitive ELISA methods have been used to detect antibodies to Nipah virus in lesser short-nosed fruit bats (Cynopterus brachyotis) and common dawn bats (Eonycteris spelaea), two megachiropteran species [28]. A capture ELISA has been used for detection of Nipah and Hendra virus-specific IgM [11].
It is important to remember that antibody assays only measure one aspect of the immune system, and cellular immune responses are likely to be more important for intracellular pathogens such as viruses. In a study on rabies vaccination in vampire bats, 31 of 32 bats were protected from challenge by vaccination, whereas 9 of 10 unvaccinated bats succumbed [29]. Of these 31 protected bats, 9 (29%) did not develop a neutralizing antibody titer, implying a large role for cellular immunity in protection. Absence of antibody does not demonstrate that an animal has not been exposed to a pathogen.
Control strategies for dealing with bat-borne diseases have primarily been centered on elimination of bat populations. This is unacceptable when applied to endangered species, and is likely to be less effective than vaccination strategies [29], [30]. There are currently no active immunization programs in wild bats [31]. Bats are fastidious groomers, and vampire bats have been shown to take up anticoagulants applied to the backs of conspecifics [26]. Oral rabies vaccines have been shown to be effective against challenge in vampire bats [26], [29], [32]. Oral subunit vaccines delivered via transgenic plants have been suggested [31].
Polyclonal antibodies to IgG of another megachiropteran species, the Egyptian fruit bat (Rousettus aegyptiacus), have been made [18]. Based on their western blot results, their polyclonal antibodies appear to have a greater specificity for chiropteran IgG than the more broadly cross-reactive polyclonal antibodies developed in this study. Their antibodies were used to determine phylogeny by examining cross-reactivity with other species. This may be a future use for the antibodies developed in this study. Evidence is mounting that the phylogenetic division between the suborders Microchiroptera and Megachiroptera is a false dichotomy, and that microchiropterans are not monophyletic. Molecular sequence data suggests that Rhinolophoid bats actually cluster with Pteropodid bats, which were considered the sole family in the megachiroptera [1]. These bats have been classified into the Yinpterochiroptera, and the remaining microchiropterans classified into the Yangochiroptera. No Rhinolophoid bat sera were examined, so no conclusions can be drawn regarding cross-reactivity, but this may be a direction for further studies.
In conclusion, protein G, polyclonal anti-P. hypomelanus IgG, and monoclonal anti-P. hypomelanus IgG all detected specific anti-DNP-BSA antibody responses in immunized variable flying foxes. The polyclonal antibody was found to cross-react well against IgG of all bat species tested, but did not show as great a difference between vaccinated and unvaccinated bats. This was likely due to non-specific binding as seen on the western blot, resulting in high backgrounds. The monoclonal antibody was found to cross-react well against IgG of six other species in the genus Pteropus and to cross-react less strongly against IgG from E. helvum or P. hastatus. Protein G distinguished best between vaccinated and unvaccinated bats, and these results validate the use of protein G for detection of bat IgG antibodies. The monoclonal antibodies developed in this study recognized immunoglobulins from other members of the genus Pteropus well, and may be useful in applications where specific detection of Pteropus IgG is needed. Further investigation of these reagents in diagnostic assays is merited.
Acknowledgements
This project was funded by a grant from the Lubee Bat Conservancy, and is published as Lubee Bat Conservancy Publication #130. The authors would like to thank Dana LeBlanc, Pam Thomas, Allyson Walsh, and the rest of the staff at Lubee Bat Conservancy for their assistance. We would also like to thank Maud Lafortune for translation.
References
- 1.Teeling E.C., Springer M.S., Madsen O., Bates P., O’Brien S.J., Murphy W.J. A molecular phylogeny for bats illuminates biogeography and the fossil record. Science. 2005;307(5709):580–584. doi: 10.1126/science.1105113. [DOI] [PubMed] [Google Scholar]
- 2.Jones D.P., Kunz T.H. Pteropus hypomelanus. Mamm Species. 2000;639:1–6. [Google Scholar]
- 3.Jones K.E., Purvis A., Gittleman J.L. Biological correlates of extinction risk in bats. Am Nat. 2003;161(4):601–614. doi: 10.1086/368289. [DOI] [PubMed] [Google Scholar]
- 4.Dobson A.P. What links bats to emerging infectious diseases? Science. 2005;310(5748):628–629. doi: 10.1126/science.1120872. [DOI] [PubMed] [Google Scholar]
- 5.Leroy E.M., Kumulungui B., Pourrut X., Rouquet P., Hassanin A., Yaba P., Délicat A., Paweska J.T., Gonzalez J.-P., Swanepoel R. Fruit bats as reservoirs of Ebola virus. Nature. 2005;438(7068):575–576. doi: 10.1038/438575a. [DOI] [PubMed] [Google Scholar]
- 6.Li W., Shi Z., Yu M., Ren W., Smith C., Epstein J.H., Wang H., Crameri G., Hu Z., Zhang J., McEachern J., Field H., Daszak P., Eaton B.T., Zhang S., Wang L.-F. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310(5748):676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- 7.Lau S.K.P., Woo P.C.Y., Li K.S.M., Tsoi H.-W., Wong B.H.L., Wong S.S.Y., Leung S.-Y., Chan K.-H., Yuen K.-Y. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc Nat Acad Sci. 2005;102(39):14040–14045. doi: 10.1073/pnas.0506735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chua K.B., Wang L.F., Lam S.K., Crameri G., Yu M., Wise T., Boyle D., Hyatt A.D., Eaton B.T. Tioman virus, a novel paramyxovirus isolated from fruit bats in Malaysia. Virology. 2001;283(2):215–229. doi: 10.1006/viro.2000.0882. [DOI] [PubMed] [Google Scholar]
- 9.Chua K.B., Koh C.L., Hooi P.S., Wee K.F., Khong J.H., Chua B.H., Chan Y.P., Lim M.E., Lam S.K. Isolation of Nipah virus from Malaysian Island flying-foxes. Microbes Infect. 2002;4(2):145–151. doi: 10.1016/s1286-4579(01)01522-2. [DOI] [PubMed] [Google Scholar]
- 10.Arguin P.M., Murray-Lillibridge K., Miranda M.E., Smith J.S., Calaor A.B., Rupprecht C.E. Serologic evidence of Lyssavirus infections among bats, the Philippines. Emerg Infect Dis. 2002;8(3):258–262. doi: 10.3201/eid0803.010330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniels P., Ksiazek T., Eaton B.T. Laboratory diagnosis of Nipah and Hendra virus infections. Microbes Infect. 2001;3(4):289–295. doi: 10.1016/s1286-4579(01)01382-x. [DOI] [PubMed] [Google Scholar]
- 12.Reynes J.-M., Molia S., Audry L., Hout S., Ngin S., Walston J., Bourhy H. Serologic evidence of lyssavirus infection in bats, Cambodia. Emerg Infect Dis. 2004;10(12):2231–2234. doi: 10.3201/eid1012.040459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lew A.M., Beck D.J., Thomas L.M. Recombinant fusion proteins of protein A and protein G with glutathione S-transferase as reporter molecules. J Immunol Methods. 1991;136(2):211–219. doi: 10.1016/0022-1759(91)90008-4. [DOI] [PubMed] [Google Scholar]
- 14.Williamson M.M., Hooper P.T., Selleck P.W., Gleeson L.J., Daniels P.W., Westbury H.A., Murray P.K. Transmission studies of Hendra virus (equine morbillivirus) in fruit bats, horses, and cats. Aust Vet J. 1998;76(12):813–818. doi: 10.1111/j.1751-0813.1998.tb12335.x. [DOI] [PubMed] [Google Scholar]
- 15.Åkerstrom B., Brodin T., Reis K., Björck L. Protein G: a powerful tool for binding and detection of monoclonal and polyclonal antibodies. J Immunol. 1985;135(4):2589–2592. [PubMed] [Google Scholar]
- 16.Oelofsen M.J., Smith M.S. Rabies and bats in a rabies-endemic area of southern Africa: application of two commercial test kits for antigen and antibody detection. Onderstepoort J Vet Res. 1993;60(3):257–260. [PubMed] [Google Scholar]
- 17.Simrell C.R., Klein P.A. Antibody responses of tumor-bearing mice to their own tumors captured and perpetuated as hybridomas. J Immunol. 1979;123(5):2386–2394. [PubMed] [Google Scholar]
- 18.Omatsu T., Ishii Y., Kyuwa S., Milanda E.G., Terao K., Yoshikawa Y. Molecular evolution inferred from immunological cross-reactivity of immunoglobulin G among Chiroptera and closely related species. Exp Anim. 2003;52(5):425–428. doi: 10.1538/expanim.52.425. [DOI] [PubMed] [Google Scholar]
- 19.Shankar V., Bowen R.A., Davis A.D., Rupprecht C.E., O'Shea T.J. Rabies in a captive colony of big brown bats (Eptesicus fuscus) J Wildl Dis. 2004;40(3):403–413. doi: 10.7589/0090-3558-40.3.403. [DOI] [PubMed] [Google Scholar]
- 20.Seymour C., Dickerman R.W., Martin M.S. Venezuelan encephalitis virus infection in neotropical bats. II. Experimental infections. Am J Trop Med Hyg. 1978;27(2 (Pt. 1)):297–306. doi: 10.4269/ajtmh.1978.27.297. [DOI] [PubMed] [Google Scholar]
- 21.McMurray D.N., Greer D.L. Immune responses in bats following intranasal infection with Histoplasma capuslatum. Am J Trop Med Hyg. 1979;28(6):1036–1039. doi: 10.4269/ajtmh.1979.28.1036. [DOI] [PubMed] [Google Scholar]
- 22.McMurray D.N., Stroud J., Murphy J.J., Carlomango M.A., Greer D.L. Role of immunoglobulin classes in experimental histoplasmosis in bats. Dev Comp Immunol. 1982;6(3):557–567. doi: 10.1016/s0145-305x(82)80042-6. [DOI] [PubMed] [Google Scholar]
- 23.Leonard L.L., Allen R., Sulkin S.E. Bat immunoglobulins formed in response to experimental Japanese B encephalitis (JBE) virus infection. J Immunol. 1968;101(6):1168–1175. [PubMed] [Google Scholar]
- 24.Hatten B.A., Allen R., Sulkin S.E. Studies on the immune capabilities of chiroptera. I. Quantitative and qualitative nature of the immune responses in bats to bacteriophage øX174. J Immunol. 1970;105(4):872–878. [PubMed] [Google Scholar]
- 25.Chakraborty A.K., Chakravarty A.K. Antibody-mediated immune response in the bat, Pteropus giganteus. Dev Comp Immunol. 1984;8(2):415–423. doi: 10.1016/0145-305x(84)90048-x. [DOI] [PubMed] [Google Scholar]
- 26.Sétien A.A., Brochier B., Tordo N., De Paz O., Desmettre P., Péharpré D., Pastoret P.-P. Experimental rabies vaccination and oral vaccination in vampire bats (Desmodus rotundus) Vaccine. 1998;16(11–12):1122–1126. doi: 10.1016/s0264-410x(98)80108-4. [DOI] [PubMed] [Google Scholar]
- 27.Peters C., Isaza R., Heard D.J., Davis R.D., Moore S.M., Briggs D.J. Vaccination of Egyptian fruit bats (Rousettus aegyptiacus) with monovalent inactivated rabies vaccine. J Zoo Wildl Med. 2004;35(1):55–59. doi: 10.1638/03-027. [DOI] [PubMed] [Google Scholar]
- 28.Kashiwazaki Y., Na Y.N., Tanimura N., Imada T. A solid-phase blocking ELISA for detection of antibodies to Nipah virus. J Virol Methods. 2004;121(2):259–261. doi: 10.1016/j.jviromet.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 29.Aguilar-Sétien A., Campos Y.L., Cruz E.T., Kretschmer R., Brochier B., Pastoret P.-P. Vaccination of vampire bats using recombinant vaccinia-rabies virus. J Wildl Dis. 2002;38(3):539–544. doi: 10.7589/0090-3558-38.3.539. [DOI] [PubMed] [Google Scholar]
- 30.Mayen F. Haematophagous bats in Brazil, their role in rabies transmission, impact on public health, livestock industry, and alternatives to an indiscriminate reduction of bat population. J Vet Med B. 2003;50(10):469–472. doi: 10.1046/j.1439-0450.2003.00713.x. [DOI] [PubMed] [Google Scholar]
- 31.Mackenzie J.S., Field H.E., Guyatt K.J. Managing emerging diseases borne by fruit bats (flying foxes), with particular reference to henipaviruses and Australian bat lyssavirus. J Appl Microbiol. 2003;94(Suppl.):59S–69S. doi: 10.1046/j.1365-2672.94.s1.7.x. [DOI] [PubMed] [Google Scholar]
- 32.Almeida M.F., Martorelli L.F.A., Aires C.C., Sallum P.C., Massad E. Indirect oral immunization of captive vampires, Desmodus rotundus. Virus Res. 2005;111(1):77–82. doi: 10.1016/j.virusres.2005.03.013. [DOI] [PubMed] [Google Scholar]


