Coronavirus disease 2019 (COVID-19) was first identified in an outbreak in Wuhan, China, on December 8, 2019. Globally, the number of patients affected with COVID-19 is growing exponentially, with the death toll exceeding 27,300 as of March 27, 2020. Worldwide, there is a limited supply of N95 respirator masks, face shields, ventilator valves, testing kits, and other personal protective equipment (PPE).1 , 2 Thus, adequate production and distribution of PPE is critical during this pandemic. To address these shortages, three-dimensional (3D) printing, a novel and innovative technology used to fabricate complex architectures, is well suited. Three-dimensional printing is an adjustable, robotic platform allowing for tailored deposition of biomaterials using computer-aided design (CAD) systems to formulate layer-by-layer custom designs with controlled architecture and composition.3, 4, 5, 6, 7
Masks
N95 respirators masks have two advantages over surgical, paper or cloth masks: 1) They are >95% efficient at filtering 0.3-µm airborne particles, and 2) they are fit tested to each user to ensure an adequate seal, such that air and small droplets do not enter around the edges of the mask and into the health care worker's breathing zone.4 The Centers for Disease Control and Prevention (CDC) recommends N95 masks for health care workers taking care of patients with COVID-19.
Three-dimensional printing can be used to produce tailored seal designs for improving mask comfort and fit. To customize face mask seals, 3D laser scanning can be implemented to scan exact facial parameters, with a tailored and customized face seal N95 template. Anthropometric data of the chin arc, jawline, face and nose lengths, and nose protrusion measurements can be taken into account with this customized seal. In a study using face seal prototypes with Acrylonitrile Butadiene Styrene plastic using a Fused Deposition Modeling 3D printer, 3 subjects showed improved contact pressure compared with use of 3M 8210 N95 FFR respirator masks.4 Moreover, a personalized mask may account for facial hair length and density for a more precise fit.
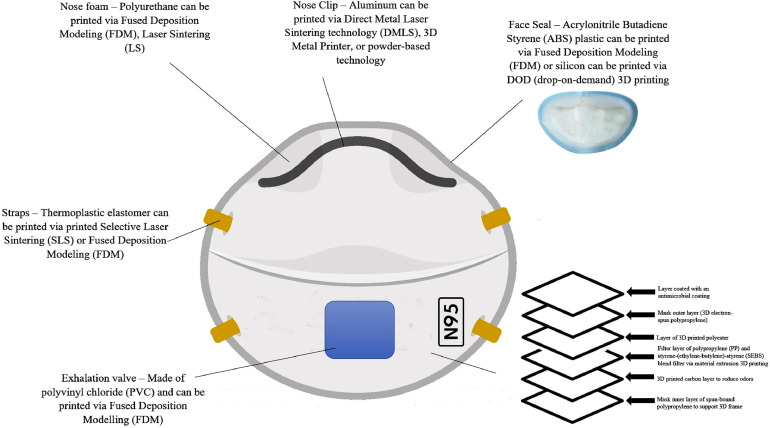
Standard N95 masks consist of filtration material composed of electrostatic nonwoven polypropylene (PP) fibers which are semi-rigid, lightweight, and fatigue resistant. The semi-crystalline structure may cause significant distortion of the 3D printed parts upon cooling, thereby making 3D printing difficult. Material extrusion 3D printing was used to design a 3D printable thermoplastic elastomeric material from a blend of PP and styrene-(ethylene-butylene)-styrene (SEBS).5 This blend provides better printability and flexibility for N95 mask design. PP is commonly used for various industrial applications because of its low cost, processability, printability, recyclability, and mechanical integrity. SEBS is a polymeric elastomer with low processing temperature and low distortion during extrusion.5 Thus, the PP/SEBS combination would improve the processability of 3D printed N95 masks. Moreover, controlling the thermoplastic elastomer ratio allows for tailoring the flexibility and elasticity of the 3D model material for better fitted masks. Three-dimensional melt electrospinning printing can also be used to create PP microfibers with sequential layering to accurately obtain a 3D form.3 , 6 Thus, 3D printing procedures may allow for the creation of stable and biocompatible N95 masks that are comparable to industrial manufacturing brands. Figure 1 displays a potential N95 3D printed mask prototype.
Figure 1.
A potential N95 three-dimensional (3D) printed mask prototype. The biomaterials displayed in this image have been fully characterized in the medical literature, with the same material composition of N95 masks.3, 4, 5, 6 The mask includes several layers thereby ensuring effective filtration of viral particles.
Face Shields
Polycarbonate and polyester, polyvinyl chloride, and other synthetic polymers are commonly used to make surgical face shields.6 These biomaterials are transparent, lightweight, and provide high-optical clarity. The polymers can easily be printed using 3D technology to meet the needs of health care workers treating COVID-19.
COVID-19 Specimen Collection Kit
Creating 3D printed test swabs would help increase COVID-19 testing capacity. Nasopharyngeal and oropharyngeal swabs can be made from a flexible polymer, using polystyrene for the shaft. The tip can be tailored to be micro-fine using computer-aided design software. Thereafter, swab bud lattice fibers can be made from calcium alginate using hydrogels using 3D tissue engineering.3 , 6
Ventilator Valves
Ventilator valves are attachments used to deliver oxygen at fixed concentrations for patients with acute respiratory distress, including patients with COVID-19. Three-dimensional printing technology can be used via a filament extrusion system or a polymer-laser powder bed fusion process to print single-use valve sets, and 3D printers can design the different elements of the valve using biomaterials such as polyamide and polysulfone, polycarbonate, silicone rubber, and stainless steel.6 Furthermore, these disposable valves eliminate time-consuming sterilization.
Medications
Three-dimensional printing techniques, such as fused filament, inkjet, extrusion, and powder extrusion, allow for fabrication of 3D printed pills. Medication-printing technologies typically use a small nozzle to lay thin disc-shaped layers of powders and deposit microscopic droplets of liquid to bind the materials. A coaxial needle extrusion 3D technology was used to print active pharmaceutical ingredients and create combinations of controlled dosing of drugs.7 Although there are no specific antivirals or vaccines for treatment of COVID-19, several well-characterized antiviral drugs are being considered as therapies.2 It may be possible to use 3D medication-printing technology to effectively and rapidly print lopinavir/ritonavir, chloroquine, and hydroxychloroquine pills. Thus, 3D technology has the potential to revolutionize the pharmaceutical industry, making drug research, development, and production applicable to patients with COVID-19.
As the COVID-19 outbreak rapidly evolves, there has been a PPE shortage globally. Three-dimensional printing inventions can be rapidly applied to address these deficiencies.1 , 2 Cost, processing time, testing, and manpower are potential barriers to creating 3D-printed PPE. However, the synthetic polymer biomaterials needed for 3D-printed PPE are exact or similar in composition to the standard manufacturing grade products (ie, N95 masks provides the same fluid barrier and air filtration protection).4, 5, 6 Moreover, these synthetic polymer materials are readily available and cost effective (ie, PP is 12.47 cents per pound). Three-dimensional printer costs vary but are an excellent investment with labor performed via robotics. In conjunction with flattening the curve via social distancing, this pioneering technology can provide adequate PPE for health care workers on the front lines of this pandemic.
Footnotes
Funding: None.
Conflicts of Interest: None.
Authorship: Both authors had access to the data and a role in writing this manuscript.
References
- 1.Ranney, ML, Griffeth V, Jha AK. Critical supply shortages — The need for ventilators and personal protective equipment during the covid-19 pandemic [e-pub head of print]. N Engl J Med, accessed March 25, 2020. DOI: 10.1056/NEJMp2006141 [DOI] [PubMed]
- 2.Marco C, Rajnik M, Cuomo A. StatPearls [Internet]; Treasure Island, FL: 2020 March 20. Features, Evaluation and Treatment Coronavirus (COVID-19) [Google Scholar]
- 3.Ishack S, Lipner SR.A review of 3-dimensional skin bioprinting techniques: applications, approaches, and trends [e-pub ahead of print]. Dermatol Surg, accessed March 25, 2020. doi: 10.1097/DSS.0000000000002378 [DOI] [PubMed]
- 4.Cai M, Li H, Shen S. Customized design and 3D printing of face seal for an N95 filtering facepiece respirator. J Occup Environ Hyg. 2018;15(3):226–234. doi: 10.1080/15459624.2017.1411598. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee SS, Burbine S, Kodihalli N. 3D-printable pp/sebs thermoplastic elastomeric blends: preparation and properties. Polymers (Basel) 2019;11(2):347. doi: 10.3390/polym11020347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bachtiar EO, Erol O, Millrod M. 3D printing and characterization of a soft and biostable elastomer with high flexibility and strength for biomedical applications. J Mech Beh of Biomed Materials. 2020;104 doi: 10.1016/j.jmbbm.2020.103649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu I, Chen RK. A feasibility study of an extrusion-based fabrication process for personalized drugs. J Pers Med. 2020;10(1):16. doi: 10.3390/jpm10010016. [DOI] [PMC free article] [PubMed] [Google Scholar]