Abstract
Currently MedImmune manufactures cold-adapted (ca) live, attenuated influenza vaccine (LAIV) from specific-pathogen free (SPF) chicken eggs. Difficulties in production scale-up and potential exposure of chicken flocks to avian influenza viruses especially in the event of a pandemic influenza outbreak have prompted evaluation and development of alternative non-egg based influenza vaccine manufacturing technologies. As part of MedImmune's effort to develop the live attenuated influenza vaccine (LAIV) using cell culture production technologies we have investigated the use of high yielding, cloned MDCK cells as a substrate for vaccine production by assessing host range and virus replication of influenza virus produced from both SPF egg and MDCK cell production technologies. In addition to cloned MDCK cells the indicator cell lines used to evaluate the impact of producing LAIV in cells on host range and replication included two human cell lines: human lung carcinoma (A549) cells and human muco-epidermoid bronchiolar carcinoma (NCI H292) cells. The influenza viruses used to infect the indicators cell lines represented both the egg and cell culture manufacturing processes and included virus strains that composed the 2006–2007 influenza seasonal trivalent vaccine (A/New Caledonia/20/99 (H1N1), A/Wisconsin/67/05 (H3N2) and B/Malaysia/2506/04). Results from this study demonstrate remarkable similarity between influenza viruses representing the current commercial egg produced and developmental MDCK cell produced vaccine production platforms. MedImmune's high yielding cloned MDCK cells used for the cell culture based vaccine production were highly permissive to both egg and cell produced ca attenuated influenza viruses. Both the A549 and NCI H292 cells regardless of production system were less permissive to influenza A and B viruses than the MDCK cells. Irrespective of the indicator cell line used the replication properties were similar between egg and the cell produced influenza viruses. Based on these study results we conclude that the MDCK cell produced and egg produced vaccine strains are highly comparable.
Keywords: Live attenuated influenza vaccine (LAIV), MDCK
1. Introduction
The currently licensed egg-derived influenza vaccines are efficacious, protecting up to 90 percent of vaccine recipients [1], [2], [3], [4]. The recent threat of pandemic influenza outbreaks and anticipated increase in demand for seasonal influenza vaccine owing to expanded vaccination recommendations require evaluation of alternative non-egg based technologies for manufacturing influenza vaccines [5]. The production of the egg-derived vaccine is not easily scalable and is limited by the availability of chicken eggs [6]. The egg-based influenza vaccine manufacturing process is labor intensive requiring long term planning and long annual production cycles [7]. These difficulties in production scale-up and potential exposure of chicken flocks to avian influenza viruses have prompted the evaluation of cell culture vaccine production platforms as alternatives to egg dependent production [8].
MedImmune currently manufactures, a live attenuated virus (LAIV) vaccine indicated for the active immunization of individuals 2–49 years of age against influenza disease caused by influenza virus subtypes A and B [9], [10], [11]. As part of MedImmune's effort to develop LAIV using cell culture production technologies, we have investigated a biologically cloned Madin-Darby canine kidney (MDCK) cell line that produces high titer of influenza virus as a substrate for production [12].
To evaluate the impact of producing LAIV in high yielding cloned MDCK cells, we have assessed in vitro susceptibility and virus replication kinetics of influenza virus produced from these cells or using specific-pathogen free (SPF) eggs. In addition to MedImmune's cloned MDCK cells we used two human cell lines to evaluate and compare infectivity and replication to assess whether the production system had an impact. The human cell lines used for evaluation are human lung carcinoma (A549) cells and human muco-epidermoid bronchiolar carcinoma (NCI H292) cells. These cells previously have shown to have different susceptibilities to influenza viruses. A549 cells were used in this study because they support productive viral replication to levels and kinetics similar to the human primary lung cells [13]. NCI H292 cells have been previously shown to be good for isolation and replication of many human viruses including vaccinia virus, HSV, adenovirus, polyoma BK virus and paramyxoviruses [14]. NCI H292 cells have also been suggested as an alternate to primary rhesus monkey kidney cells for isolation and replication of human paramyxoviruses [14]. Viruses that do not replicate in NCI H292 cells include CMV, VZV, SV-40, respiratory coronaviruses and some influenza A and type B viruses. Additionally, A549 and NCI H292 cells have been studied to evaluate influenza virus replication, proinflammatory cytokine responses and influenza virus induced apoptosis [15], [16].
The viruses used for these studies were the virus strains that were components of the northern hemisphere 2006–2007 influenza seasonal trivalent vaccine: ca A/New Caledonia/20/99 (H1N1), ca A/Wisconsin/67/05 (H3N2) and ca B/Malaysia/2506/04; each strain was produced in both MDCK cultures as well as eggs [17]. Each vaccine strain was used to infect MDCK, A549 and NCI H292 cell lines at a specific MOI. Cell culture supernatants, cell lysates and formalin-fixed cell monolayers were collected 4 through 96 h post-infection. The respective test samples were analyzed by fluorescent focus assay (FFA), real-time qRT-PCR, immunofluorescence (IF), Western blot analysis and hemagglutination assay (HA).
Here we report the evaluation of the influenza virus produced in a high yielding MDCK cell clone and compared it to the virus produced in SPF chicken eggs. The high yielding MDCK cells were very permissive to both egg produced (EP) and cell produced (CP) attenuated vaccine strains. Both the A549 and NCI H292 cells were less permissive to influenza A strains compared to MDCK cells regardless of the production system. Based on these study results we conclude that the cloned MDCK cell produced and egg produced influenza vaccine strains are highly comparable.
2. Materials and methods
2.1. Egg and cell produced virus strains
The three 2006–2007 seasonal vaccine components were produced using the egg and MDCK cell production platforms. The test virus used represented egg and cell produced H1N1, H3N2 and influenza B virus represented by A/New Caledonia/20/99, A/Wisconsin/67/05 and B/Malaysia/2506/04 virus, respectively. The three seasonal strains were produced by a classical reassortment process according to the currently licensed LAIV master virus seed (MVS) process. The cell produced virus for the study was generated from the same virus seeds used to manufacture the egg-derived 2006–2007 viruses. Briefly, the egg produced virus strains were produced in 10- to 11-day-old SPF eggs inoculated with 0.1 mL of 3.1 log10 TCID50/mL each. The inoculated eggs were incubated at 33 ± 1 in the presence of 70% humidity for 48 h for H1N1 and H3N2 virus and 60 h for influenza B virus. The virus containing allantoic fluid was harvested, stabilized with sucrose phosphate buffer and further processed for downstream purification. The final purified bulk was tested for virus concentration and stored at or below 60 °C until further use. The egg produced viruses were subjected to four passages in eggs during the MVS manufacturing process. The MDCK cell produced viruses were propagated in MDCK cells using sufficient quantity of cells for bioreactor inoculation. The cells were allowed to attach to the microcarriers with controlled pH in the presence of CO2. Viral infection of the MDCK cell culture was achieved by allowing the microcarriers to settle and by addition of infection medium and appropriate volume of master virus seed stock. Upon addition of infection medium and virus inoculum the cell culture was maintained at 33 ± 1 °C for 48 h. At the end of infection the cell culture supernatant was collected and further processed through downstream purification. The final purified bulk was tested for virus concentration and stored at or below 60 °C until further use.
2.2. Cell substrates and propagation
The human lung carcinoma (A549) cells ATCC/Catalogue # CC-185 Lot No. 4257591 were cultured in Ham's F-12k media supplemented with 10% fetal bovine serum (FBS) at 37 ± 1 °C in the presence of 5% CO2. The human muco-epidermoid pulmonary carcinoma (NCI H292) cells ATCC/Catalogue # CRL-1848 Lot 4734654 were propagated in RPMI 1640 medium supplemented with 10% FBS. The MDCK cloned cells a proprietary cell line developed in MedImmune were first grown in roller bottles and then seeded in 6-well tissue culture (TC-6) plates prior to infection. A549 and NCI H292 cells were all expanded in T-225 tissue culture flasks prior to infection in TC-6 well plates. The cells were seeded into TC-6 well plates at a seeding density of 1.75 × 106 cells/well with 2 mL complete growth media. The cells were incubated 12–48 h at 37 ± 1 °C and 5% CO2 until confluent.
2.3. Influenza virus infection of cells
The TC-6 well plates with confluent wells were washed once with 2 mL Hanks Balanced Salt Solution (HBSS) prior to infection. Based on the cell count per well and virus titers, the egg produced (EP) and cell produced (CP) viruses were diluted in complete growth medium and then used to infect cells at an MOI of 0.1 except for A549 which was also infected at an MOI of 1.0. The TC-6 well plates were inoculated with 0.5 mL inoculum and incubated at 33 ± 1 °C, 5% CO2 for 60 min. Following virus adsorption the cell monolayer was washed with HBSS and wells replaced with 2 mL complete growth media. Cell culture supernatants and cell lysates were collected at 4, 8, 12, 24, 48, 72, and 96 h post-infection (PI) and stored at ≤−60 °C until testing. The test samples included clarified and stabilized cell culture supernatant, cell lysates for both RNA extraction and Western blot analysis and formalin-fixed cell monolayers.
2.4. Fluorescent focus assay (FFA) for determination of virus potency
FFA, an antibody binding and staining method was used for the potency determination of influenza virus titers. Cell supernatants collected 4 through 96 h PI were each appropriately diluted in virus growth medium. In the case of samples with low virus concentrations the dilution scheme was altered. Briefly, 2-day-old ATCC Madin-Darby Canine Kidney (MDCK) cells in 96-well tissue culture plates were infected by inoculating 100 μL of appropriate virus dilutions and incubating the plates at 33 ± 1 °C in the presence of 5% CO2 for 18 h. Polyclonal chicken antisera against the virus were used as the primary antibody and rabbit anti-chicken IgG conjugated with FITC was used as the secondary antibody. They were used as to detect the infected cells prior to titer determination.
2.5. Total RNA isolation from cells
Total RNA was isolated from each cell line using the RNeasy® Mini kit (Qiagen) according to the manufacturer's instructions, with minor modifications. Briefly, 375 μL of RLT buffer was added to each TC-6 well and allowed to incubate at room temperature for 5 min. The cells were then scraped and total RNA was extracted with an RNeasy® spin column. Purified RNA was eluted into 50 μL of nuclease-free water and stored at ≤−60 °C until use. Uninfected total cell RNA was also extracted and used as control.
2.6. Viral RNA isolation from supernatants
RNA was isolated from 10× sucrose phosphate buffer stabilized cell supernatants using the RNeasy® 96 kit (Qiagen) according to the manufacturer's spin technology instructions, with minor modifications. RNA was extracted with an RNeasy® 96 spin column plate. Purified RNA was eluted into 100 μL of nuclease-free water and stored at ≤−60 °C until use.
2.7. Real-time one-step RT-PCR
Real-time one-step RT-PCR reactions were carried out using the ABI TaqMan® one-step RT-PCR master mix according to the manufacturer's instructions with a total volume of 25 μL. The primers and probes used for amplification of influenza A and B gene segments include A-MF 5′-AAG ACC AAT CCT GTC ACC TCT GA-3′, A-MR 5′-CAA AGC GTC TAC GCT GCA GTC C-3′ and 6FAM TTC ACG CTC ACC GTG CCC AGT G-Tamra; B-MF 5′-GAG ACA CAA TTG CCT ACC TGC TT-3′, B-MR 5′-TTC TTT CCC ACC GAA CCA AC-3′ and 6FAM-AGA AGA TGG AGA AGG CAA AGC AGA ACT AGC-Tamra; A-PB1F 5′-GAC CTT GGG CCA GCA ACA-3′, A-PB1R 5′-GTG GCA CCG GTA CGT ATA TCT G-3′ and 6FAM-CCC AAC TGG CTC TTA ACT ATT CAT CAA AGA CT-Tamra and B-NPF 5′-CTT GCT TCG GAG CTG CCT AT-3′, B-NPR 5′-GGA AAC CCT TGC ACT TTA ATG C-3′ and 6FAM-CCC AAC TGG CTC TTA ACT ATT CAT CAA AGA CT-Tamra. The primers and probe were used at a final concentration of 900 nM and 250 nM, respectively. Sample RNA volume of 5 μL was added to the 20 μL reaction mix. No template control (NTC) reactions contained 5 μL of nuclease-free water. Each sample was tested in duplicate. A 10-fold dilution series of purified influenza A or B RNA with known virus titers (log10 FFU/mL) was performed and 5 μL of each standard dilution was run in triplicate to construct a 7-point standard curve. Thermal cycling was performed in a Fast Real-time PCR System 9700HT (ABI). The RT step involved incubation at 48 °C for 30 min. The PCR cycling conditions included 95 °C for 10 min, 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The quantity, or copy number, for the test samples was calculated based on the standard curve and reported as RT-PCR log10 copies/mL.
2.8. Real-time two-step RT-PCR
Reverse transcription of RNA with random hexamers was performed in a final volume of 20 μL with 1 unit RNase inhibitor and 10 μL of sample or standard RNA using the high capacity cDNA reverse transcription kit (ABI).
2.9. PCR amplification
Multiplex real-time PCR reactions were carried out using the TaqMan® Universal PCR Master Mix according to the manufacturer's instructions with a total volume of 25 μL. Primers and probes for M, PB1, and NP segments for both influenza A and influenza B were obtained from ABI. In each reaction, the custom primers and probe were used at a final concentration of 900 nM and 250 nM, respectively, along with a 1× final concentration of eukaryotic 18s rRNA TaqMan® Endogenous Control, VIC®/MGB (ABI). Each sample was tested in triplicate. Thermal cycling was performed in a Fast Real-time PCR System 9700HT (ABI) in standard mode. The PCR cycling conditions included a UNG incubation of 50 °C for 2 min, denaturation of 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The quantity, or copy number, for the test samples is calculated and reported as log copies/mL.
2.10. Immunofluorescence assay
Following harvesting the supernatants at each of the time points the cells in the TC 6 plates were washed with 1× in PBS and fixed with 1% formalin for 15 min at 4 °C. The plates were washed twice with 2 mL/well of PBS. Sheep anti-A/New Caledonia/20/99, A/Hiroshima/52/05 (cross-reactive with A/Wisconsin/67/05) and B/Malaysia/2506/04 were used as primary antibodies at 1:1000 dilutions. Primary antibody treated plates were incubated at 37 ± 1 °C for 1 h and washed with 2 mL of TPBS (PBS with 0.1% Tween-20) with 5 min interval between washes. 1:300 diluted FITC-conjugated secondary antibodies was added and plates incubated at 37 ± 1 °C for 1 h and washed 3× with 2 mL/well of TPBS. All of the wash fluid was removed and plates were air-dried at room temperature. The plates were screened for fluorescence using a fluorescent microscope and images were captured for analysis.
2.11. Western blot analysis
Proteins in the virus infected cell lysates were separated by electrophoresis using the BioRad pre-cast gels under denaturing and reducing conditions. Ten-well 10–20% SDS–polyacrylamide gels were used to size separate proteins in the samples. Samples were denatured using sample buffer with β-mercaptoethanol and heated at 95 °C for 5 min. Samples were normalized by protein concentration. BioRad Kaleidoscope and Rainbow Marker (Amersham Biosciences) molecular weight marker was used on each gel. Electrophoresis was performed in running buffer at 80–100 V for 1.5–2 h. Following electrophoresis the size-separated proteins were transferred to pre-cut PVDF membranes in a BioRad blotting module using 1× transfer buffer. Proteins were transferred for 1 h at 100 V in an ice bath. PVDF membranes with transferred proteins were blocked overnight in a 5% solution of nonfat dry milk solution containing 0.1% Tween-20. The blocked PVDF membranes were washed 1× in wash buffer and reacted for 1 h on a rocker plate at room temperature with appropriate dilutions of sheep antisera to A/New Caledonia/20/99 (H1N1), A/Hiroshima/52/05 (H3N2), and B/Malaysia/2506/04, respectively. After washing the PVDF membrane were reacted with goat anti-sheep IgG-HRP conjugate for 1 h on the rocker plate at room temperature. The viral proteins were detected using Pierce, SuperSignal West Pico Chemiluminescent substrate on film.
2.12. Hemagglutination (HA) assay
The hemagglutination (HA) assay was performed using freshly prepared 0.5% chicken, turkey and guinea pig RBCs prepared in PBS. The culture supernatants harvested from 4 through 96 h post-infection were tested for hemagglutination activity. Briefly, 50 μl of undiluted cell supernatant was added to the first well of a V-bottom 96-well plates containing 50 μl/well DPBS. Serial two-fold dilutions were performed and 50 μl of 0.5% appropriate red blood cell suspension was added to each well and the 96-well plate was incubated undisturbed at room temperature (RT) for 30–60 min. HA results were recorded and results reported as log2 HA units.
2.13. HA and NA sequence analysis
RNA from supernatants from virus infected wells was extracted using QIAamp® viral RNA mini kit (Qiagen) per manufacturer's instructions and 5 μl of it was used for generation of HA and NA RT-PCR products for sequencing. Following RT-PCR amplification single-tube MicroSpin S-400 spin columns were used to clean up the amplicons. Analysis and PCR product concentration were determined by agarose gel electrophoresis. Sequencing was performed using Prism BigDye® terminator cycle sequencing ready reaction kit v.3.0 (ABI) using standard cycle sequencing conditions. Unincorporated dye terminators were removed using a Performa™ DTR gel filtration block and purified products were collected in a MicroAmp® PCR plate and electrophoresis for sequencing was performed using 3730 DNA analyzer (ABI) and the data files were processed using Sequencher™ software
3. Results
3.1. Replication kinetics of influenza virus in MDCK, A549 and NCI H292 cells
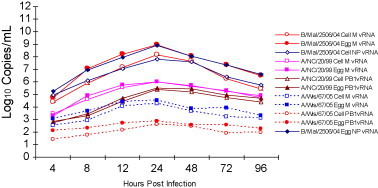
Fluorescent focus assay (FFA) was performed to quantitate infectious virus and compare replication kinetics of EP and CP influenza viruses in MDCK, A549 and NCI H292 cells. The virus titer measured using FFA was found to be comparable for both the EP and CP influenza viruses in all the indicator cells. In MDCK cells infected at 0.1 MOI, a difference of no more than 0.4 log10 FFU/mL in virus peak titer was observed between EP and CP A/New Caledonia/20/99, A/Wisconsin/67/05 and B/Malaysia/2503/04 virus (Fig. 1 ). Results from the replication kinetics suggest MDCK cells to be highly permissive to both CP and EP influenza A and B viruses. The peak virus titers for EP and CP H1N1, H3N2 and influenza B virus were determined to be 8.6 ± 0.0 and 8.5 ± 0.0, 7.5 ± 0.06 and 7.9 ± 0.0 and 7.8 ± 0.10 and 7.9 ± 0.0, respectively. For both A549 and NCI H292 cells the virus titer was below the limit of detection of the FFA (<2.4 log10 FFU/mL) when infected with either EP or CP A/New Caledonia/20/99 and A/Wisconsin/67/05 at MOI of 1.0. Detectable virus titers for EP and CP B/Malaysia/2506/04 virus infected A549 and NCI H292 cells were observed for infection at MOI of 1.0. However, the peak virus titers were <5 log10 FFU/mL and suggest restricted permissiveness of both A549 and NCI H292 cells to influenza virus infection using the study protocol (data not shown). Results demonstrate MedImmune's cloned MDCK cells are highly permissive to influenza virus infection and irrespective of the production platform generate high titers of ca attenuated influenza viruses.
Fig. 1.
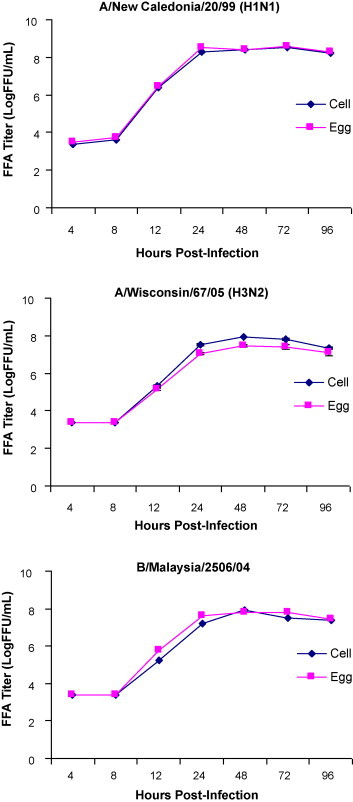
Comparison of replication kinetics of egg and cell produced influenza A and influenza B virus infected MDCK cells. Kinetics of CP and EP cold-adapted A/New Caledonia/20/99, A/Wisconsin/67/05 and B/Malaysia/2506/04 influenza virus replication in MDCK cells measured by FFA (each data point represents average of triplicate samples tested).
3.2. Comparison of viral RNA copy number in MDCK, A549 and NCI H292 cell supernatant
To evaluate the effect of production substrate on replication kinetics of the influenza viruses total viral RNA (log10 copies/mL) was measured in EP and CP infected MDCK, A549 and NCI H292 cells culture supernatants using qRT-PCR. Comparison of EP and CP virus replication kinetics showed both were capable of efficient replication in MDCK cells. The peak viral RNA copy numbers for EP and CP A/New Caledonia/20/99, A/Wisconsin/67/05 and B/Malaysia/2506/04 in MDCK cell supernatant were determined to be 7.8 ± 0.06 and 7.7 ± 0.04, 7.9 ± 0.07 and 7.9 ± 0.06 and 8.7 ± 0.04 and 8.5 ± 0.12, respectively. Similar to MDCK cells, replication of both EP and CP influenza viruses in A549 and NCI H292 cells was found to be comparable. In A549 cells infected at MOI of 1.0 for egg and CP A/New Caledonia/20/99 and B/Malaysia/2056/04 virus the peak viral copy number was determined to be 4.3 ± 0.14 and 4.9 ± 0.08 and 6.1 ± 0.10 and 6.5 ± 0.09, respectively. The EP and CP A/Wisconsin/67/05 virus replicated poorly in A549 cells. In NCI H292 cells virus replication was severely restricted with H1N1 and H3N2 viral RNA not detectable in culture supernatants for both the EP and CP viruses. Similar to replication in A549 cells, replication of B/Malaysia/2056/04 was restricted in NCI H292 cells for both EP and CP viruses (Fig. 2 ). Replication kinetics measured using viral RNA copies like the FFA assay confirm that the virus production platform has minimal impact on replication of cold-adapted influenza viruses. The results also suggest cloned MDCK cell to be highly permissive for both EP and CP influenza viruses.
Fig. 2.
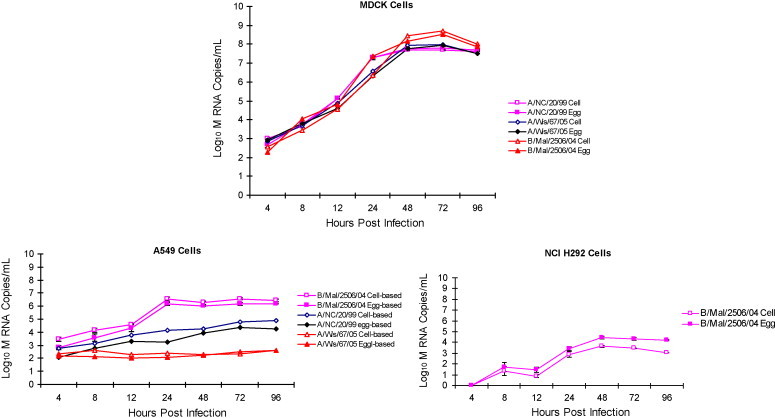
Comparison of viral RNA copy number of egg and cell produced influenza A and B virus infected MDCK, A549 and NCI H292 cells. Kinetics of CP and EP cold-adapted A/New Caledonia/20/99, A/Wisconsin/67/05 and B/Malaysia/2506/04 influenza virus M segment RNA in MDCK, A549 and NCI H292 cells measured by qRT-PCR (each data point represents average of triplicate samples tested). The maximum SD for the triplicates for MDCK, A549 and NCI H292 cells was found to be 0.23, 0.48 and 0.43, respectively.
3.3. Viral RNA in infected MDCK, A549 and NCI H292 cell lysates
Efficiency of intracellular viral replication was also evaluated by measuring PB1, M and NP segment RNA expression kinetics using qRT-PCR in cell lysate that were prepared from EP and CP virus infected cells. Fig. 3, Fig. 4, Fig. 5 summarize the accumulation of viral RNA in cell lysates of infected MDCK, A549 and NCI H292 cells, respectively. The pattern of both EP and CP influenza A PB1 and M segment viral RNA expression kinetics and influenza B NP and M segment viral RNA accumulation in infected whole cell lysates was comparable. Accumulation of PB1, M and NP viral RNA in infected MDCK was higher for all three viruses compared to A549 and NCI H292 cells and confirmed the highly permissive nature of MDCK cells to influenza virus infection (Fig. 3). Results demonstrate intracellular influenza segment RNA expression to be efficient in MDCK cells compared to restricted replication kinetics in A549 and NCI H292 cells. The level of PB1, M and NP segment RNA in MDCK cell lysates was found to be similar for both EP and CP influenza viruses. These results confirm minimal impact of the egg and cell production platforms on intracellular viral expression kinetics.
Fig. 3.
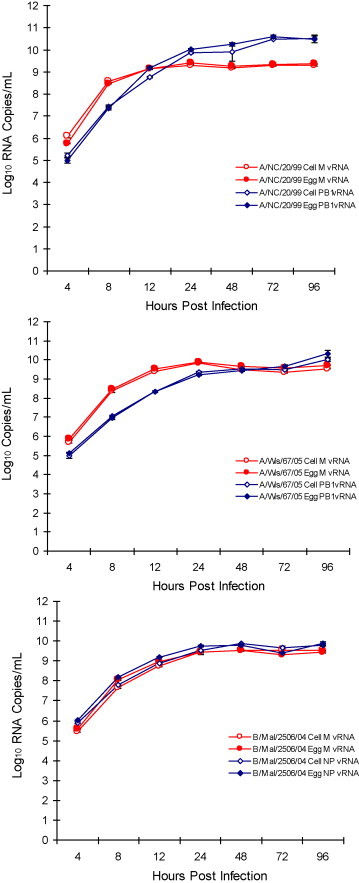
Comparison of influenza A and B viral RNA accumulation in infected MDCK cells. Kinetics of CP and EP cold-adapted A/New Caledonia/20/99 (A/NC), A/Wisconsin/67/05 (A/Wis) and B/Malaysia/2506/04 (B/Mal) influenza virus M, NP and PB1 segment RNA in MDCK cell lysates by qRT-PCR (each data point represents average of triplicate samples tested). The maximum SD for the triplicates for A/NC, A/Wis and B/Mal was found to be 0.13, 0.22 and 0.09, respectively.
Fig. 4.
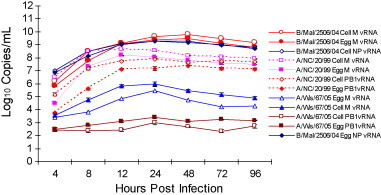
Comparison of influenza A and B viral RNA accumulation in infected A549 cells. Kinetics of CP and EP cold-adapted A/New Caledonia/20/99 (A/NC), A/Wisconsin/67/05 (A/Wis) and B/Malaysia/2506/04 (B/Mal) influenza virus M, NP and PB1 segment RNA in A549 cell lysates by qRT-PCR (each data point represents average of triplicate samples tested). The maximum SD for the triplicates for A/NC, A/Wis and B/Mal was found to be 0.17, 0.10 and 0.09, respectively.
Fig. 5.
Comparison of influenza A and B viral RNA accumulation in infected NCI H292 cells. Kinetics of CP and EP cold-adapted A/New Caledonia/20/99 (A/NC), A/Wisconsin/67/05 (A/Wis) and B/Malaysia/2506/04 (B/Mal) influenza virus M, NP and PB1 segment RNA in NCI H292 cell lysates by qRT-PCR (each data point represents average of triplicate samples tested). The maximum SD for the triplicates for A/NC, A/Wis and B/Mal was found to be 0.17, 0.13 and 0.08, respectively.
3.4. Progression of infection
Spread of influenza virus infection was monitored using IF staining to assess effect of virus production platform on permissivity of MDCK, A549 and NCI H292 cells. Infected MDCK cells were observed as early as 4–8 h post-infection for both CP and EP influenza viruses at 0.1 MOI. At 24 h post-infection all of the cell monolayer was positive for influenza NP protein detected by fluorescence and suggests high permissivity and efficient spread of virus infection in MDCK cells. CPE was more extensive for 48 and 72 h post-infection time points and >90% MDCK cells were in suspension by 96 h post-infection for all three CP and EP viruses (Fig. 6 ). Infection of A549 and NCI H292 cells at a MOI of 0.1 resulted in limited infection and lack of progressive increase in fluorescence positive cells over time for A/New Caledonia/20/99 and B/Malaysia/2506/04. Results demonstrate that the virus production platform had no significant effect on permissivity and spread of virus infection in MDCK cells.
Fig. 6.
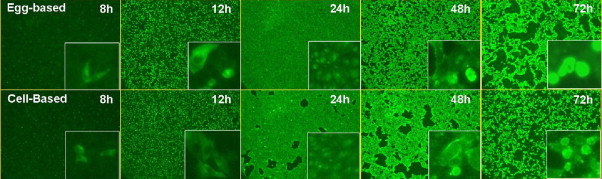
Comparison of progression of infection observed using immunofluorescence in egg and cell produced influenza A and B virus infected MDCK cells (MOI 0.1). Kinetics of CP and EP cold-adapted A/New Caledonia/20/99 influenza virus replication measured by Immunofluorescence assay on MDCK 9B91E4 (magnification 50×).
3.5. Viral protein expression in infected cells
To assess the impact of production platform on influenza virus protein expression Western blotting was performed on cell lysates from CP and EP infected MDCK, A549 and NCI H292 cells. In MDCK cells infected with A/New Caledonia/20/99, A/Wisconsin/67/05 and B/Malaysia/2506/04 virus protein expression detected by Western blot showed three major protein bands corresponding to HA0, HA1 and HA2 as early as 8–12 h post-infection (Fig. 7 ). The profile of HA protein expression was similar for both EP and CP H1N1, H3N2 and B influenza virus, respectively. Similarly viral proteins were also detected in A549 cells infected with A/New Caledonia/20/99 and B/Malaysia/2506/04 virus and in NCI H292 cells infected with both and egg and cell-based B/Malsysia/2506/04 virus (data not shown). The viral protein expression of EP and CP viruses was found to be comparable and confirm minimal impact of production platform on virus replication.
Fig. 7.
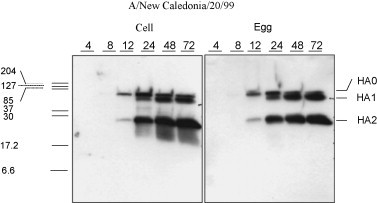
Viral protein expression in MDCK cells infected with cell and egg produced virus. A/New Caledonia/20/99. Kinetics of viral protein expression in CP and EP cold-adapted A/New Caledonia/20/99 MDCK cells detected using sheep antibodies show the major HA proteins including HA0, HA1 and HA2 proteins.
3.6. Hemagglutination activity
To assess the effect of production platform on virus receptor binding properties hemagglutination activity in cell culture supernatants from CP and EP virus infected MDCK, A549 and NCI H292 cells was measured. The HA titers using chicken, turkey and guinea pig red blood cells for the EP and CP influenza viruses from infected MDCK, A549 and NCI H292 cells found to be comparable. For MDCK cells hemagglutination was observed as early as 24 h post-infection for A/New Caledonia/20/99, A/Wisconsin/67/05 and B/Malaysia/2506/04 using all RBCs. No more than two log2 HAU difference in HA titers was observed between EP and CP viruses with the exception of CP A/Wisconsin/67/05 for 48 and 72 h supernatants and for B/Malaysia/2506/04 at 72 and 96 h post-infection using tRBC (Table 1 ). For infected A549 cells HA titers were only observed for B/Malaysia/2506/04 and were similar for both EP and CP viruses (Table 2 ). No detectable hemagglutination was observed for supernatants from both influenza A virus infected A549 cells. Using all three RBCs none of the EP and CP virus infected NCI H292 cells demonstrated hemagglutination activity. Table 1, Table 2 summarize the HA titers both egg and cell-based influenza viruses from MDCK and A549 cells. Absence of hemagglutination in A549 and NCI H292 is consistent with restricted virus replication observed using FFA and qRT-PCR assays. Results from hemagglutination assay confirm efficient virus replication in MDCK cells and demonstrate minimal impact of production substrate on receptor biding property of ca influenza virus.
Table 1.
Comparison of hemagglutination activity in cell and egg produced influenza B virus infected MDCK cells.
| Virus production platform | Time P.I. (h) | cRBC |
tRBC |
gpRBC |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A/N | A/W | B/M | A/N | A/W | B/M | A/N | A/W | B/M | ||
| Egg | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 24 | 256 | 16 | 32 | 128 | 64 | 32 | 32 | 32 | 64 | |
| 48 | 512 | 128 | 64 | 256 | 256 | 128 | 128 | 256 | 128 | |
| 72 | 512 | 128 | 128 | 256 | 256 | 512 | 256 | 256 | 512 | |
| 96 | 512 | 128 | 128 | 256 | 512 | 512 | 128 | 256 | 256 | |
| MDCK | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 24 | 256 | 32 | 8 | 128 | 64 | 4 | 32 | 64 | 64 | |
| 48 | 1024 | 128 | 64 | 512 | 1024 | 64 | 256 | 512 | 256 | |
| 72 | 512 | 128 | 128 | 512 | 1024 | 64 | 512 | 512 | 512 | |
| 96 | 512 | 128 | 64 | 256 | 512 | 64 | 128 | 512 | 256 | |
Abbreviations used: cRBC—chicken red blood cells; tRBC—turkey red blood cells; gpRBC—guinea pig red blood cells; A/N—A/New Caledonia/20/99; A/W—A/Wisconsin/67/05; B/M—B/Malaysia/2506/04.
Table 2.
Comparison of hemagglutination activity in cell and egg produced influenza B virus infected A549 cells.
| Virus production platform | Time P.I. (h) | B/Malaysia/2506/04 |
||
|---|---|---|---|---|
| cRBC | tRBC | gpRBC | ||
| Egg | 4 | 0 | 0 | 0 |
| 8 | 0 | 0 | 0 | |
| 12 | 0 | 0 | 0 | |
| 24 | 16 | 32 | 32 | |
| 48 | 64 | 128 | 128 | |
| 72 | 128 | 128 | 128 | |
| 96 | 128 | 128 | 256 | |
| MDCK | 4 | 0 | 0 | 0 |
| 8 | 0 | 0 | 0 | |
| 12 | 0 | 0 | 0 | |
| 24 | 16 | 32 | 64 | |
| 48 | 64 | 128 | 256 | |
| 72 | 128 | 128 | 256 | |
| 96 | 128 | 128 | 256 | |
Abbreviations used: cRBC—chicken red blood cells; tRBC—turkey red blood cells; gpRBC—guinea pig red blood cells.
3.7. Sequence analysis of MDCK, A549 and NCI H292 derived cold-adapted virus
The HA and NA sequences of MDCK, A549 and NCI H292 cells derived A/New Caledonia/20/99, A/Wisconsin/67/05 and B/Malaysia/2506/04 virus were compared to the sequences of the respective virus used as inoculum. No sequence differences in HA and NA segments were identified in either EP or CP B/Malaysia/2506/04 from MDCK, A549 and NCI H292. The sequence of the HA and NA segments from EP and CP A/Wisconsin/67/05 from MDCK cells was found to be identical to the sequence of the inoculum. Similarly HA and NA segment sequences from EP and only CP NA segment sequence for A/New Caledonia/20/99 from MDCK and A549 cells were found to be identical to the sequence of the inoculum. However, the CP HA segment sequence had a substitution identified to be 505G (141S) compared to the inoculum sequence of 505A (141N) for both MDCK and A549 derived A/New Caledonia/20/99 virus. The nucleotide change resulting in amino acid substitution at position 141 (S to N) for CP A/New Caledonia/20/99 from MDCK and A549 cells of HA occurred in the domain equivalent to the antigenic site A. Interestingly, the G505A leading to A141N amino acid substitution has been previously reported for cold-adapted A/New Caledonia/20/99 (Buonagurio et. al. [18]) when compared to wild-type HA sequence. The hemagglutination-inhibition (HAI) analysis from this previous study comparing virus with and without the A141N substitution revealed no differences between viruses.
4. Discussion
A broad range of cell substrates are available for development of human biologicals. However, only a few including WI-38, Vero, MRC-5, CEF and CHO cells have earned acceptability among the regulatory agencies. Different cell substrates for manufacturing biologicals are being considered for the development of new biologicals. This is especially true in the case of both pandemic and seasonal inactivated influenza vaccines. The cell substrates being evaluated and in use include Vero, suspension and adherent MDCK, PERC6 and insect cells [8], [19], [20], [21], [22], [23], [24], [25], [26], [27]. Transgenic plants have also been shown useful to generate subunit influenza vaccines [28]. These new substrates while useful for vaccine development also raise concerns of safety. Assessing the safety and risks of using these novel substrates takes into account the advances made in manufacturing processes, purification technologies and methodologies to evaluate risks. The high yielding MDCK clone used by MedImmune is well characterized and has been shown to be safe for manufacturing LAIV (data not discussed) [20]. The cloned MDCK cell line was developed from ATCC cell line and a bank was established and later adapted to grow in serum-free growth medium. Briefly, the high yielding MDCK cells were initially cloned in serum containing medium and screened for virus productivity. These clones were later adapted to grow in serum-free conditions for generation of the accession and master cell bank. The cloned MDCK cells have been well characterized and shown to be free of adventitious agents and have been demonstrated to be non-tumorigenic in animal models.
The egg and cell produced influenza viruses showed remarkable similarity in their ability to infect cloned MDCK, A549 and NCI H292 cells. Cloned MDCK cells were highly permissive to infection by all three egg and cell produced influenza virus. A549 and NCI H292 cells in comparison to cloned MDCK cells were not permissive to A/New Caledonia/20/99 and A/Wisconsin/67/05 virus but allowed for limited but restricted replication of B/Malaysis/25060/4 virus representing both egg and cell production processes.
Based on in vitro substrate susceptibility, infection progression, receptor binding ability, replication kinetics, viral RNA and protein expression and HA and NA sequence comparisons, cold-adapted influenza virus generated using the cloned MDCK cell-based production technology were found to be very similar to the LAIV generated using the egg based manufacturing process.
Footnotes
This project is funded in whole or in part with Federal funds from the Office of the Assistant Secretary for Preparedness and Response (ASPR), Biomedical Advanced Research and Development Authority, under Contract Nos. HHSO100200600010C and HHSO100200700036C. The total federal program funding for these contracts is $221,379,570, representing approximately 92% of the total amount of the projects. The remaining 8% of the total amount for the projects is anticipated to be financed by nongovernmental sources.
References
- 1.Belshe R.B., Gruber W.C. Safety, efficacy and effectiveness of cold-adapted, live, attenuated, trivalent intranasal influenza vaccine in adults and children. Philos Trans R Soc Lond Ser B: Biol Sci. 2001;356:1947–1951. doi: 10.1098/rstb.2001.0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belshe R.B., Nichol K.L., Black S.B., Shinefield H., Cordova J., Walker R. Safety, efficacy and effectiveness of live, attenuated, cold-adapted influenza vaccine in an indicated population aged 5–49 years. Clin Infect Dis. 2004;39:920–927. doi: 10.1086/423001. [DOI] [PubMed] [Google Scholar]
- 3.Belshe R.B., Lee M.-S., Walker R.E., Stoddard J., Mendelman P.M. Safety, immunogenicity and efficacy of intranasal, live attenuated influenza vaccine. Expert Rev Vac. 2004;3(6):643–654. doi: 10.1586/14760584.3.6.643. [DOI] [PubMed] [Google Scholar]
- 4.Nichol K.L. The efficacy, effectiveness and cost-effectiveness of inactivated influenza virus vaccines. Vaccine. 2003;21:1769–1775. doi: 10.1016/s0264-410x(03)00070-7. [DOI] [PubMed] [Google Scholar]
- 5.Mathews J. Egg-based influenza vaccine production—30 years of commercial experience and our future expectations for cell culture. National academy of engineering and institute of medicine conference, vaccine production engineering approaches to a pandemic; Washington, DC, April 10–11; 2006. [Google Scholar]
- 6.Fedson D.S. New technologies for meeting the global demand for pandemic influenza vaccines. Biologicals. 2008;36:346–349. doi: 10.1016/j.biologicals.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Gerdil C. The annual production cycle of influenza vaccine. Vaccine. 2003:1776–1779. doi: 10.1016/s0264-410x(03)00071-9. [DOI] [PubMed] [Google Scholar]
- 8.Audsley J.M., Tannock G.A. Cell-based Influenza vaccines: progress to date. Drugs. 2008;68(11):1483–1491. doi: 10.2165/00003495-200868110-00002. [DOI] [PubMed] [Google Scholar]
- 9.Belshe R.B., Edwards K.M., Vesikari T., Black S.V., Walker R.E., Hultquist M. Live attenuated versus inactivated influenza vaccines in infants and young children. N Engl J Med. 2007;3 56(7):685–696. doi: 10.1056/NEJMoa065368. [DOI] [PubMed] [Google Scholar]
- 10.King J.C., Stoddard J., Gaglani M.J., Moore K.A., Magder L., McClure E. Effectiveness of school-based influenza vaccination. N Engl J Med. 2006;355(24):2523–2532. doi: 10.1056/NEJMoa055414. [DOI] [PubMed] [Google Scholar]
- 11.Ambrose C.S., Luke C., Coelingh K. Current status of live attenuated influenza vaccine in the United States for seasonal and pandemic influenza. Influenza Other Respir Viruses. 2008;2:183–192. doi: 10.1111/j.1750-2659.2008.00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J., Shi X., Schwartz R., Kemble G. Use of MDCK cells for production of live attenuated influenza vaccine. Vaccine. 2009;27:6460–6463. doi: 10.1016/j.vaccine.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 13.Turpin E., Luke K., Jones J., Tumpey T., Konan K., Schultz-Cherry S. Influenza virus infection increased p53 activity: role of p53 in cell death and viral replication. J Virol. 2005;79(14):8802–8811. doi: 10.1128/JVI.79.14.8802-8811.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veckman V., Osterlund P., Fagerlund R., Melen K., Matikainen S., Julkunen I. TNF- and IFN-enhance influenza A virus induced chemokine gene expression in human A549 lung epithelial cells. Virology. 2006;345:96–104. doi: 10.1016/j.virol.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 15.Hierholzer J.C., Castells E., Banks G.G., Bryan J.A., McEwen C.T. Sensitivity of NCI-H292 human lung mucoepidermoid cells for respiratory and other human viruses. J Clin Microbiol. 1993;31(6):1504–1510. doi: 10.1128/jcm.31.6.1504-1510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brydon E.W.A., Smith H., Sweet C. Influenza A virus induced apoptosis in bronchiolar epithelial (NCI-H292) cells limits pro-inflammatory cytokine release. J Gen Virol. 2003;84:2389–2400. doi: 10.1099/vir.0.18913-0. [DOI] [PubMed] [Google Scholar]
- 17.Recommended composition of influenza virus vaccines for use in 2006–2007 influenza season. WHO, Weekly Epidemiol Rec. 2006;9(81):81–88. [PubMed] [Google Scholar]
- 18.Buonagurio D.A., Bechert T.M., Yang C.-F., Shutyak L., D’ Arco G.A., Kazachkov Y. Genetic stability of live, cold-adapted influenza virus components of the FluMist®/CAIV-T vaccine throughout the manufacturing process. Vaccine. 2006;24:2151–2160. doi: 10.1016/j.vaccine.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Govorkova E.A., Murti G., Meigner B., De Taisne C., Webster R.G. African green monkey (Vero) cells provide and alternate host cell system for influenza A and B viruses. J Virol. 1996;70(8):5519–5524. doi: 10.1128/jvi.70.8.5519-5524.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J., Mani S., Schwartz R., Richman L., Tabor D.E. Cloning and assessment of tumorigenecity and oncogenicity of a Madin-Darby canine kidney (MDCK) cell line for influenza vaccine production. Vaccine. 2010;28:1285–1293. doi: 10.1016/j.vaccine.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 21.Oh D.Y., Barr I.G., Mosse J.A., Laurie K.L. MDCK-SIAT1 cell show improved isolation rates for recent human influenza viruses compared to conventional MDCK cells. J Clin Microbiol. 2008;46(7):2189–2194. doi: 10.1128/JCM.00398-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tannock G.A., Audsley J.M. The growth of attenuated influenza vaccine donor strains in continuous cell lines. J Virol Methods. 2005;123:187–193. doi: 10.1016/j.jviromet.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Romanova J., Katinger D., Ferko B., Voglauer R., Mochalova L., Bovin N. Distinct host range of influenza H3N2 virus isolates in Vero and MDCK cells is determined by cell specific glycosylation pattern. Virology. 2003;307:90–97. doi: 10.1016/s0042-6822(02)00064-8. [DOI] [PubMed] [Google Scholar]
- 24.Halperin S.A., Smith B., Mabrouk T., Germain M., Trepanier P., Hassell T. Safety and immunogenicity of a trivalent, inactivated, mammalian cell culture-derived influenza vaccine in health adults, seniors and children. Vaccine. 2002;20(78):1240–1247. doi: 10.1016/s0264-410x(01)00428-5. [DOI] [PubMed] [Google Scholar]
- 25.Pau M.G., Ophorst C., Koldijk M.H., Schouten G., Mehtali M., Uydehaag F. The human cell line PER.C6 provides a new manufacturing system for the production of influenza vaccines. Vaccine. 2001;19(17–19):2716–2721. doi: 10.1016/s0264-410x(00)00508-9. [DOI] [PubMed] [Google Scholar]
- 26.Koudstaal W., Hartgroves L., Havenga M., Legastelois I., Ophorst C., Sieuwerts M. Suitability of PER.C6 cells to generate epidemic and pandemic influenza vaccine strains by reverse genetics. Vaccine. 2009;27:2588–2593. doi: 10.1016/j.vaccine.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 27.McPherson C.E. Development of a novel recombinant influenza vaccine in insect cells. Biologicals. 2008:350–353. doi: 10.1016/j.biologicals.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Shoji Y., Chichester J.A., Bi H., Musichuk K., de la Rosa P., Goldschmidt L. Plant expressed HA as a season influenza vaccine candidate. Vaccine. 2008;26:2930–2934. doi: 10.1016/j.vaccine.2008.03.045. [DOI] [PubMed] [Google Scholar]


