Abstract
Introduction:
Exposure to perfluoroalkyl substances (PFAS), synthetic and persistent chemicals used in commercial and industrial processes, are associated with cardiometabolic dysfunction and related risk factors including reduced birth weight, excess adiposity, dyslipidemia, and insulin resistance. Identifying the metabolic changes induced by PFAS exposure could enhance our understanding of biological pathways underlying PFAS toxicity.
Objective:
To identify metabolic alterations associated with serum concentrations of four PFAS in children using a metabolome-wide association study.
Methods:
We performed untargeted metabolomic profiling by liquid chromatography with ultra-high-resolution mass spectrometry, and separately quantified serum concentrations of perfluorooctanoic acid (PFOA), perfluorooctanesulfonic acid (PFOS), perfluorononanoic acid (PFNA), and perfluorohexanesulphonic acid (PFHxS) for 114 8-year old children from Cincinnati, OH. We evaluated associations between each serum PFAS concentration and 16,097 metabolic features using linear regression adjusted for child age, sex, and race with a false discovery rate <20%. We annotated PFAS-associated metabolites and conducted pathway enrichment analyses.
Results:
Serum PFAS concentrations were associated with metabolic features annotated primarily as lipids and dietary factors. Biological pathways associated with all four PFAS included arginine, proline, aspartate, asparagine, and butanoate metabolism.
Conclusions:
In this cross-sectional study, childhood serum PFAS concentrations were correlated with metabolic pathways related to energy production and catabolism. Future studies should determine whether these pathways mediate associations between PFAS exposure and childhood cardiometabolic health.
Introduction
Per- and polyfluoroalkyl substances (PFAS) are synthetic chemicals used as industrial processing aids, in fire-fighting foam, and as oil and water resistant coatings in cookware, food containers, carpets, apparel, and upholstery (Agency for Toxic Substances and Disease Registry, 2015; Buck et al., 2011; DeWitt, 2015; European Food Safety Authority, 2008). Four of these PFAS, perfluorooctanoic acid (PFOA), perfluorooctanesulfonic acid (PFOS), perfluorononanoic acid (PFNA), and perfluorohexanesulphonic acid (PFHxS) have an estimated half-life ranging from 3.8 to 8.5 years in humans, bioaccumulate, and are frequently detected in human serum (Andersen et al., 2013; Jain, 2013; Kingsley et al., 2018; Lee et al., 2013; Olsen et al., 2007; Whitworth et al., 2012; Zhang et al., 2013). Despite reductions in the production of PFOA and PFOS in the United States since the early 2000’s, PFOA and PFOS are environmentally and biologically stable, and human exposure still occurs (Bowman, 2015; Environmental Protection Agency, 2000; Fromme et al., 2009).
PFAS are of particular concern given their biological persistence in humans and the adverse health effects associated with exposure. Early life exposure to PFAS may be particularly important since children are often more susceptible to the effects of environmental chemical exposures (Braun, 2016). Higher serum PFAS concentrations during gestation are associated with cardiometabolic risk factors including reduced birth weight, reduced birth length, and increased adiposity, but not insulin resistance in childhood (Braun et al., 2016; Fleisch et al., 2017; Johnson et al., 2014; Maisonet et al., 2012; Mora et al., 2017). During childhood, higher serum PFAS concentrations are associated with cardiometabolic risk factors, including, increased adiposity, later onset of puberty, dyslipidemia, and alterations in glucose homeostasis (Alderete et al., 2019; Lopez-Espinosa et al., 2011; Nelson et al., 2009; Rappazzo et al., 2017). Several of these risk factors have been associated with alterations in the serum metabolome of both children and adults, but it is not clear whether the effects of PFAS on cardiometabolic endpoints are mediated by metabolome alterations (Guasch-Ferre et al., 2016; Llewellyn et al., 2015; Park et al., 2012; Perng et al., 2014; Ruiz-Canela et al., 2017).
Three human studies have identified metabolic alterations associated with PFAS exposure. Serum or plasma PFAS levels have been associated with metabolomics alterations, including, fatty acid metabolism and the tricarboxylic acid (TCA) cycle pathways among adult men, lipids and amino acids among overweight and obese Hispanic children, and lipids, fatty acid metabolism, and an unnamed metabolite in older adults (Alderete et al., 2019; Salihovic et al., 2018; Wang et al., 2017). An in vitro study found that PFOA altered lipid and amino acid metabolism (Peng et al., 2013). Several experimental studies in animals reported that PFAS exposure caused metabolic changes related to lipid and amino acid pathways (Lankadurai et al., 2013; Lee et al., 2017; Tan et al., 2013; Yu et al., 2016; Zheng et al., 2017).
We are unaware of any studies examining metabolomic responses to PFAS in a general population of children. Thus, we performed a metabolome-wide association study (MWAS) using untargeted ultra-high-resolution mass spectrometry to identify metabolic alterations associated with serum PFAS concentrations in 8-year old children.
Methods
Study Participants
We used data from the Health Outcomes and Measures of the Environment (HOME) Study. Pregnant women enrolled in the HOME Study, a prospective pregnancy and birth cohort based in Cincinnati, Ohio from March 2003 - January 2006. Detailed information about participant recruitment and eligibility criteria has been described previously (Braun et al., 2017). Briefly, 37% of 1,263 eligible women agreed to participant. Of these, 468 women enrolled, and 410 live-born children were delivered and followed-up between birth and age 8 years. For the present study, we randomly selected 115 of the 230 singletons (50%) who returned for the 8-year clinic visit. To ensure an adequate range of PFOA concentrations, we selected children in equal proportions of children from each tercile of age 8-year serum PFOA concentrations.
All women provided written informed consent for themselves and their children before enrolling in the study. The study protocol was approved by the institutional review board (IRB) of Cincinnati Children’s Hospital Medical Center (CCHMC). The Centers for Disease Control and Prevention (CDC) and Brown University deferred to the CCHMC IRB as the IRB of record.
Serum PFAS Measurements
We collected venous blood samples from children at the 8-year clinic visit and measured serum concentrations of PFOA, PFOS, PFNA and PFHxS at the CDC laboratory using a modified version of a previously described analytic chemistry method (Kato et al., 2011; Kato et al., 2014; Kuklenyik et al., 2005). Briefly, online solid phase extraction coupled to high performance liquid chromatography-isotope dilution with tandem mass spectrometry was used to quantify PFAS concentrations. The limits of detection (LOD) are 0.1 ng/mL (PFOA and PFHxS), 0.2 ng/mL (PFOS), and 0.082 ng/mL (PFNA); all serum PFAS concentrations were greater than the LODs in our study participants.
High-Resolution Metabolomics
We conducted untargeted, high-resolution metabolomic profiling of serum collected at age 8 years from 115 participants using established methods (Soltow et al., 2013). Briefly, serum samples were removed from storage at −80°C and thawed on ice. Next, 65 μL of serum was added to 130 μL of acetonitrile containing a mixture of stable isotope standards, vortexed, and allowed to equilibrate for 30 minutes. Samples were analyzed using liquid chromatography and Fourier transform high-resolution mass spectrometry (Dionex Ultimate 3000, Q-Exactive HF, Thermo Scientific) (Johnson et al., 2010). For each sample, 10 μL aliquots were analyzed in triplicate using hydrophilic interaction liquid chromatography (HILIC) with electrospray ionization (ESI) source operated in positive mode and reversed phase chromatography (RPC) with ESI operated in negative mode, which is also referred to as C18-negative mode. This use of complementary chromatography phases and ionization polarity has been shown to improve the detection of endogenous and exogenous chemicals (Liu et al., 2016). The high-resolution mass spectrometer was operated in full scan mode at 120,000 resolution and mass-to-charge ratio (m/z) range 85–1275. Raw data files were extracted and aligned using apLCMS (Yu et al., 2013) with modifications by xMSanalyzer (Uppal et al., 2013). Uniquely detected ions, referred to as m/z features, consisted of m/z, retention time, and ion abundance.
We implemented several quality control measures prior to data analysis. We excluded one sample that did not meet quality control standards resulting in an analytic sample of N=114. All m/z features were batch corrected using ComBat (Johnson et al., 2007). We averaged ion abundances across triplicates and removed those with a coefficient of variation ≥100% or ≥ 20% non-detected intensities for all study participant’s samples. The ion abundances of the remaining 9,899 and 6,198 m/z features for HILIC-positive mode and C18-negative mode, respectively, were log2-transformed for all statistical analyses.
Statistical Analyses
We performed a MWAS using a series of linear regression models to estimate the association between serum PFAS concentration (continuous, ng/mL) and each m/z feature. We adjusted these models for child age (continuous, years), sex (male vs. female), and race (non-Hispanic white vs. Other). We controlled the false discovery rate (FDR<20%) using the Benjamini-Hochberg method (Benjamini and Hochberg, 1995).
We first matched m/z features associated with any of the four serum PFAS concentrations to a library of approximately 500 metabolites with identities previously confirmed by comparison of m/z, retention time and ion dissociation patterns (MS2) to authentic reference standards (Uppal et al., 2016). Additional features not matching these metabolites were annotated with the Human Metabolome Database (HMDB) (Wishart et al., 2018), LipidMaps (Fahy et al., 2007), the Kyoto Encyclopedia of Genes and Genomes (KEGG) (Kanehisa et al., 2017; Kanehisa and Goto, 2000; Kanehisa et al., 2016), and the Norman Network databases PFASTRIER (Trier et al., 2015) and SFISHFLUORO (KEMI Swedish Chemical Agency, 2015). Identities were assigned to m/z features using evidence scoring provided in xMSannotator for matching to commonly detected adducts formed in positive and negative ESI at ±5 ppm (Uppal et al., 2017). Chemical identity confidence was assigned using the Metabolomics Standards Initiative recommendation, where level 1 is a compound identified by comparison to an authentic reference standard, level 2 is annotated compound by matching isotopic patterns (when available) and matching m/z, level 3 is a putatively annotated compound class, and level 4 is an unknown compound (Sumner et al., 2007).
We used a two-step approach to identify biological pathways associated with each PFAS. In the first step, we conducted a network-based metabolome-wide correlation analysis, where we estimated all pairwise Spearman correlation coefficients between the entire set of m/z features (n=29,607) and m/z features identified in the PFAS-MWAS using the R package MetabNet (Uppal et al., 2015). For the second step, m/z features with Spearman r ≥ 0.5 and Benjamini-Hochberg FDR ≤ 20% were tested for metabolic pathway enrichment using Mummichog (Li et al., 2013). We conducted the pathway enrichment analyses with the human_mfn network (Li et al., 2010), mass accuracy of 5, and 10,000 permutations. The two-step approach and pathway enrichment analyses were performed separately for HILIC-positive and C18-negative and for each of the four PFAS. Finally, we calculated the R2 between individual PFAS and metabolites that were associated with each other to evaluate the predictive ability of our models.
Results
On average, children were 8.1 years old (SD=0.6) and had a household income of $76,000 per year (SD=52,000). The children were 57% female and 55% non-Hispanic white. The characteristics of the participants included in this study (N=115) were not materially different than the characteristics of the entire cohort (N=389) at baseline (Supplemental Table 2).
Among study participants, average serum PFOA, PFOS, PFNA, and PFHxS concentrations were 2.6 ng/mL (standard deviation [SD]=1.0), 4.4 (SD=3.2), 0.9 (SD=0.7), and 2.1 (SD=2.7), respectively (Table 1). The median PFAS concentrations were slightly lower than the average concentrations (Supplemental Table 1). Spearman correlations between the four PFAS concentrations were weak to moderate, ranging from 0.2 to 0.6 (Supplemental Table 3).
Table 1:
Characteristics of HOME Study children at age 8 years (n=115)
| Characteristic | Mean ± standard deviation or N (%) |
|---|---|
| Serum PFOA (ng/mL) | 2.6 ± 1.0 |
| Serum PFOS (ng/mL) | 4.4 ± 3.2 |
| Serum PFNA (ng/mL) | 0.9 ± 0.7 |
| Serum PFHxS (ng/mL) | 2.1 ± 2.7 |
| Child age (years) | 8.1 ± 0.6 |
| Child sex (Female) | 66 (57.4) |
| Child race | |
| White, non-Hispanic | 62 (55.4) |
| Other | 50 (44.6) |
| Household Income ($10,000/Yr) | 7.6 ± 5. |
In the C18-negative mode, 17, 63, 47, and 29 m/z features were associated with serum PFOA, PFOS, PFNA, and PFHxS concentrations after covariate adjustment and correction for multiple comparisons, respectively (Figure 1). Annotation of these m/z features indicated that all four PFAS were significantly associated with several lipids and dietary factors (Supplemental Tables 4–7). The most significant features associated with PFOA were other PFAS, including the monoisotopic and 13C isotope for both PFOA and PFOS (Supplemental Table 4). Similarly, serum concentrations of PFOS, PFNA, and PFHxS were significantly associated with PFOS, PFNA, and PFHxS m/z features, respectively. The identity of these metabolites was confirmed by comparison of m/z, retention time, and MSMS to PFOA, PFOS, PFNA, and PFHxS standards. Interestingly, exogenous compounds associated with PFOA, PFOS, and PFHxS included some annotated fungicides and insecticides. In univariate models, R2 values ranged from −0.9% - 65% with a median of −0.5%.
Figure 1:
Manhattan plot of negative log10-transformed p-values for the association between serum m/z features and serum PFAS concentrations in C18-negative mode among HOME Study children at age 8 years
*P-values are adjusted for age, sex, and race. The dotted line is the p-value corresponding to false discover rate threshold of 0.2.
In the HILIC-positive mode, 18, 253, 76, and 39 m/z features were associated with serum PFOA, PFOS, PFNA, and PFHxS concentrations, respectively, after adjusting for covariates and multiple comparisons (Figure 2). Several lipids and dietary factors were associated with all four PFAS concentrations (Supplemental Tables 4–7). Interestingly 10 different annotated pesticides were associated with PFOS, as well as five PFAS, polychlorinated biphenyl-21 (PCB), and a plasticizer (Supplemental Table 5). Additional annotated features associated with PFNA included amino acids and five PFAS (Supplemental Table 6). Serum PFHxS concentrations were also associated with three PFAS, two herbicides, and an insecticide (Supplemental Table 7). In univariate models, R2 values ranged from −0.9% - 49% with a median of −0.5%.
Figure 2:
Manhattan plot of negative log10-transformed p-values for the association between serum m/z features and serum PFAS concentration in HILIC-positive mode among HOME Study children at age 8 years
*P-values are adjusted for age, sex, and race. The dotted line is the p-value corresponding to false discover rate threshold of 0.2.
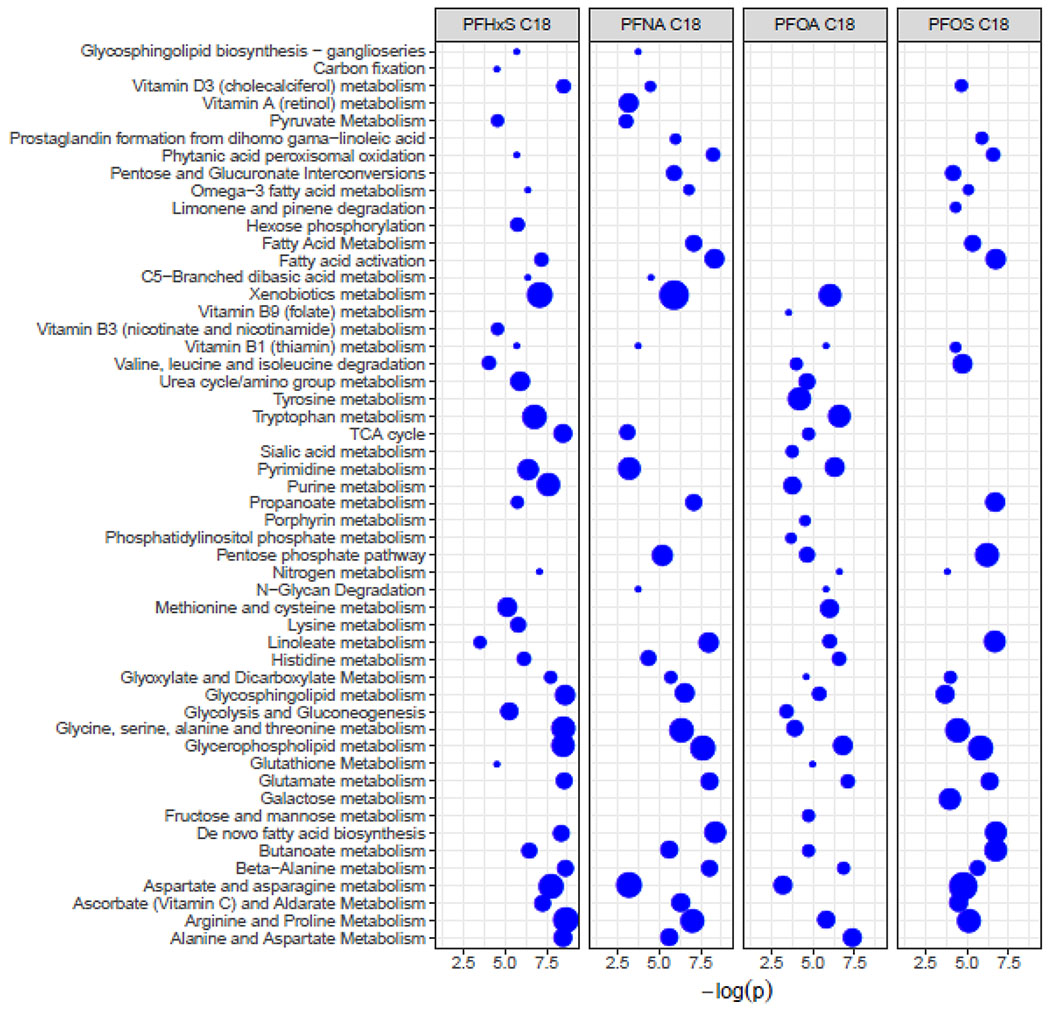
The network-based metabolome-wide correlation analysis identified additional m/z features associated with the significant m/z features from each PFAS-MWAS in C18-negative and HILIC-positive modes. To improve detection of relationships among metabolites, we used all of these m/z features associated with each PFAS for pathway enrichment analyses. Eleven and 9 pathways were enriched for all four PFAS in C18-negative and HILIC-positive modes, respectively (Figure 3 and Figure 4). These included arginine, proline, aspartate, asparagine, butanoate, glycine, serine, alanine, and threonine metabolism (Table 2 and Figures 5 and 6). Some pathways were only associated with one of the four PFAS (Figures 5 and 6). For example, in C18-negative mode tyrosine, galactose, Vitamin A (retinol), and lysine metabolism pathways were enriched for PFOA, PFOS, PFNA, and PFHxS, respectively. De novo fatty acid biosynthesis, TCA cycle, pyrimidine, and purine metabolism pathways were enriched in HILIC-positive mode for PFOA, PFOS, PFNA, and PFHxS, respectively.
Figure 3:
Venn diagram of number of enriched pathways associated with serum PFOA, PFOS, PFHxS, or PFNA concentrations in C18-negative mode
Figure 4:
Venn diagram of number of enriched pathways for m/z features associated with serum PFOA, PFOS, PFHxS, or PFNA concentrations in HILIC-positive mode
Table 2:
List of enriched pathways associated with all four PFAS in C18-negative mode or HILIC-positive mode (Values are permutation p-values).
| C18-negative | HILIC-positive | |||||||
|---|---|---|---|---|---|---|---|---|
| Pathway | PFOA | PFOS | PFNA | PFHxS | PFOA | PFOS | PFNA | PFHxS |
| Arginine and Proline Metabolism | 0.003 | 0.006 | 0.001 | <0.001 | 0.001 | 0.003 | 0.002 | 0.001 |
| Aspartate and asparagine metabolism | 0.040 | 0.008 | 0.041 | <0.001 | 0.001 | 0.003 | 0.002 | 0.041 |
| Beta-Alanine metabolism | 0.001 | 0.004 | <0.001 | <0.001 | ||||
| Butanoate metabolism | 0.009 | 0.001 | 0.004 | 0.002 | 0.040 | 0.004 | 0.029 | 0.016 |
| Glutamate metabolism | 0.001 | 0.002 | <0.001 | <0.001 | ||||
| Glycerophospholipid metabolism | 0.001 | 0.003 | <0.001 | <0.001 | ||||
| Glycine, serine, alanine and threonine metabolism | 0.020 | 0.012 | 0.002 | <0.001 | 0.004 | 0.003 | 0.002 | 0.003 |
| Glycosphingolipid metabolism | 0.005 | 0.025 | 0.001 | <0.001 | ||||
| Glyoxylate and Dicarboxylate Metabolism | 0.010 | 0.018 | 0.003 | <0.001 | ||||
| Histidine metabolism | 0.005 | 0.004 | 0.003 | 0.001 | ||||
| Linoleate metabolism | 0.002 | 0.001 | <0.001 | 0.030 | ||||
| Methionine and cysteine metabolism | 0.001 | 0.003 | 0.003 | 0.001 | ||||
| Tyrosine metabolism | 0.003 | 0.011 | 0.003 | 0.003 | ||||
| Urea cycle/amino group metabolism | 0.001 | 0.003 | 0.002 | 0.001 | ||||
| Vitamin B1 (thiamin) metabolism | 0.003 | 0.013 | 0.024 | 0.003 | ||||
| Vitamin B3 (nicotinate and nicotinamide) metabolism | 0.006 | 0.008 | 0.007 | 0.003 | ||||
Figure 5:
Enriched pathways associated with serum PFOA, PFOS, PFHxS, or PFNA concentrations in C18-negative mode among HOME Study children, Cincinnati, OH
Note: The size of the dot represents the number of overlapping metabolites in the pathway. The position of the dot is determined by the −log p-value obtained from 10,000 permutations.
Figure 6:
Enriched pathways associated with serum PFOA, PFOS, PFHxS, or PFNA concentrations in HILIC-positive mode among HOME Study children, Cincinnati, OH
Note: The size of the dot represents the number of overlapping metabolites in the pathway. The position of the dot is determined by the −log p-value obtained from 10,000 permutations.
Discussion
In this study, we found that serum PFOA, PFOS, PFNA, and PFHxS concentrations were associated with several PFAS, lipids, and dietary factors in the serum of 8-year old children. Pathways associated with these metabolites were related to several aspects of energy production and catabolism. Specifically, the network of metabolites associated with serum PFAS concentrations mapped to several pathways related to amino acid and lipid metabolism.
Despite differences in species, study design, and life-stage, our results are largely consistent with prior studies examining metabolic responses to PFSA exposure. Among overweight and obese Hispanic children from Los Angeles, plasma PFAS concentrations were associated with lipid and amino acid metabolism pathways (Alderete et al., 2019). There was substantial overlap in the enriched pathways associated with PFAS in both the prior and present study, including glycosphingolipids, linoleate, aspartate, asparagine, tyrosine, arginine, proline, glycine, serine, alanine, threonine, histidine, beta-alanine, glutamate, Vitamin B3 (nicotinate and nicotinamide), and urea cycle/amino group metabolism. Our pathway enrichment results were also consistent with a cross-sectional study of PFAS exposure and metabolomic alterations in adult males from China, in which PFOA and PFOS concentrations were associated with fatty acid metabolism, glutathione, ascorbate, purine, and TCA cycle metabolism (Wang et al., 2017). Among 70-year-old adults from Sweden, a group of PFAS was associated with glycerophospholipid, linoleate, and purine metabolism pathways, which is similar to our results (Salihovic et al., 2018). Two other studies in mice reported that PFOA caused alterations in amino acid, fatty acid, and energy metabolism pathways, including, arginine, proline, glutathione, tyrosine, histidine, tryptophan, alanine, aspartate, glutamate, cysteine, methionine, linoleate, glycine, serine, threonine, glutamate, pyruvate, glycolysis, glycerophospholipid, glyoxylate, dicarboxylate, galactose, fructose, and the TCA cycle (Tan et al., 2013; Yu et al., 2016). In human liver cells, PFOA exposure caused changes to amino acid and lipid metabolism pathways (Peng et al., 2013), consistent with our results observing that PFOA was associated with purine, leucine, and tryptophan metabolism pathways.
The results of our study, in addition with prior findings suggest connections between PFAS and alterations in the TCA cycle. Consistent with findings from epidemiologic studies and mouse models, we observed that serum PFAS concentrations were associated with several amino acids involved in the TCA cycle, an important aspect of energy production (Alderete et al., 2019; Tan et al., 2013; Wang et al., 2017; Yu et al., 2016). This complements the findings of previous studies observing cross-sectional associations between PFAS and glucose utilization (Cardenas et al., 2017; Liu et al., 2018).
Findings from our pathway enrichment analyses suggest that lipid metabolism is associated with serum PFAS concentrations. In fact the chemical structure of PFAS is similar to that of fatty acids, suggesting that PFAS could alter lipid metabolism (Fletcher et al., 2013). Moreover, prior epidemiologic studies have consistently reported a positive relationship between serum PFOA and cholesterol concentrations in children, adolescents, and adults (Eriksen et al., 2013; Frisbee et al., 2010; Liu et al., 2018; Steenland et al., 2009).
Our study has several strengths and limitations. We used ultra-high resolution mass spectrometry to identify a wide range of exogenous and endogenous metabolites. Moreover, we used an unbiased and untargeted approach to discover metabolic signals associated with serum concentrations of four different PFAS in children, which is an advantage over targeting specific metabolic pathways (e.g., glucose-insulin homeostasis or specific lipids) or focusing on a single PFAS. Using this approach, we found both endogenous and exogenous metabolites were associated with serum PFAS concentrations. Exogenous compounds do not reflect the biological pathways impacted by PFAS exposure. However, using an untargeted metabolomics approach allowed us to identify potential mixtures of compounds that may be correlated with PFAS exposures. This is particularly important given the growing interest in studying the health effects of chemical mixtures (Braun Joseph et al., 2016).
The most notable limitation of this study is its cross-sectional design, which prevents us from inferring the temporality of these results. Moreover, it is possible that metabolic alterations related to cardiometabolic function could impact PFAS pharmacokinetics and features of the metabolome (Verner et al., 2016). Additional limitations of our study could be improved upon in future studies. The participants included in this pilot study were selected from HOME Study participants who completed the 8-year follow up visit. Thus, selection bias due to loss-to-follow is a potential source of bias. However, participant characteristics were not markedly different between the full HOME Study sample and the participants included in the present study. The modest sample size of 114 limited our statistical power to detect small effect sizes in the face of multiple comparisons and univariate regression models revealed that individual PFAS were generally not good predictors of metabolite intensities. To address these issues, future studies could use longitudinal measures of the metabolome with larger sample sizes and other data dimension reduction methods (e.g., Partial Least Squares Regression) to infer the directionality of these associations, increase statistical power, and better predict metabolite intensities with PFAS concentrations.
The results of this cross-sectional study, which require replication in other populations, provide new insights into the potential biological responses associated with PFAS exposure. Specifically, serum concentrations of four PFAS were associated with a common set of biological pathways related to energy production and catabolism. Future longitudinal cohort studies could provide useful information to determine whether these biological pathways mediate associations between PFAS exposure and adverse childhood cardiometabolic outcomes.
Supplementary Material
Acknowledgements
Funding Support: NIEHS R01 ES025214, R01 ES020349, P01 ES011261, P42 ES013660, U2C ES026561, and P30 ES23515
Footnotes
Conflict of Interest: All authors declare that they have no conflict of interest. JMB was financially compensated for serving as an expert witness for plaintiffs in litigation related to tobacco smoke exposures. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Institutes of Health or the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
Ethics approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in this study.
References
- Agency for Toxic Substances and Disease Registry (2015) Draft Toxicological Profile for Perfluoroalkyls in Agency for Toxic Substances and Disease Registry (Ed). [PubMed] [Google Scholar]
- Alderete TL, Jin R, Walker DI, Valvi D, Chen Z, Jones DP, Peng C, Gilliland FD, Berhane K, Conti DV, Goran MI and Chatzi L (2019) Perfluoroalkyl substances, metabolomic profiling, and alterations in glucose homeostasis among overweight and obese Hispanic children: A proof-of-concept analysis. Environ Int 126, 445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen CS, Fei C, Gamborg M, Nohr EA, Sorensen TI and Olsen J (2013) Prenatal exposures to perfluorinated chemicals and anthropometry at 7 years of age. Am J Epidemiol 178, 921–7. [DOI] [PubMed] [Google Scholar]
- Benjamini Y and Hochberg Y (1995) Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society 57, 289–300. [Google Scholar]
- Bowman L (2015) FluoroCouncil Companies to Phase out Long-Chain Chemicals by Year’s End, American Chemistry.
- Braun JM (2016) Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nature Reviews Endocrinology 13, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Chen A, Romano ME, Calafat AM, Webster GM, Yolton K and Lanphear BP (2016) Prenatal perfluoroalkyl substance exposure and child adiposity at 8 years of age: The HOME study. Obesity (Silver Spring) 24, 231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalloo G, Chen A, Dietrich KN, Liddy-Hicks S, Morgan S, Xu Y, Yolton K and Lanphear BP (2017) Cohort Profile: The Health Outcomes and Measures of the Environment (HOME) study. Int J Epidemiol 46, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun Joseph M, Gennings C, Hauser R and Webster Thomas F (2016) What Can Epidemiological Studies Tell Us about the Impact of Chemical Mixtures on Human Health? Environmental Health Perspectives 124, A6–A9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA and van Leeuwen SP (2011) Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag 7, 513–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas A, Gold DR, Hauser R, Kleinman KP, Hivert MF, Calafat AM, Ye X, Webster TF, Horton ES and Oken E (2017) Plasma Concentrations of Per- and Polyfluoroalkyl Substances at Baseline and Associations with Glycemic Indicators and Diabetes Incidence among High-Risk Adults in the Diabetes Prevention Program Trial. Environ Health Perspect 125, 107001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt JC (2015) Toxicological Effects of Perfluoroalkyl and Polyfluoroalkyl Substances. Humana Press, Springer International Publishing Switzerland; 2015. [Google Scholar]
- Environmental Protection Agency (2000) EPA and 3M Announce Phase out of PFOS.
- Eriksen KT, Raaschou-Nielsen O, McLaughlin JK, Lipworth L, Tjønneland A, Overvad K and Sørensen M (2013) Association between Plasma PFOA and PFOS Levels and Total Cholesterol in a Middle-Aged Danish Population. PLOS ONE 8, e56969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority (2008) Perfluoroctane sulfonate, perfluorooctanoic acid and their salts: Scientific opinion of the panel on contaminants in the food chain. European Food Safety Authority Journal, 653, 1–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahy E, Sud M, Cotter D and Subramaniam S (2007) LIPID MAPS online tools for lipid research. Nucleic Acids Res 35, W606–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisch AF, Rifas-Shiman SL, Mora AM, Calafat AM, Ye X, Luttmann-Gibson H, Gillman MW, Oken E and Sagiv SK (2017) Early-Life Exposure to Perfluoroalkyl Substances and Childhood Metabolic Function. Environ Health Perspect 125, 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher T, Galloway TS, Melzer D, Holcroft P, Cipelli R, Pilling LC, Mondal D, Luster M and Harries LW (2013) Associations between PFOA, PFOS and changes in the expression of genes involved in cholesterol metabolism in humans. Environ Int 57-58, 2–10. [DOI] [PubMed] [Google Scholar]
- Frisbee SJ, Shankar A, Knox SS and et al. (2010) Perfluorooctanoic acid, perfluorooctanesulfonate, and serum lipids in children and adolescents: Results from the c8 health project. Archives of Pediatrics & Adolescent Medicine 164, 860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme H, Tittlemier SA, Volkel W, Wilhelm M and Twardella D (2009) Perfluorinated compounds--exposure assessment for the general population in Western countries. Int J Hyg Environ Health 212, 239–70. [DOI] [PubMed] [Google Scholar]
- Guasch-Ferre M, Hruby A, Toledo E, Clish CB, Martinez-Gonzalez MA, Salas-Salvado J and Hu FB (2016) Metabolomics in Prediabetes and Diabetes: A Systematic Review and Meta-analysis. Diabetes Care 39, 833–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RB (2013) Effect of pregnancy on the levels of selected perfluoroalkyl compounds for females aged 17-39 years: data from National Health and Nutrition Examination Survey 2003-2008. J Toxicol Environ Health A 76, 409–21. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Yu T, Strobel FH and Jones DP (2010) A practical approach to detect unique metabolic patterns for personalized medicine. The Analyst 135, 2864–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PI, Sutton P, Atchley DS, Koustas E, Lam J, Sen S, Robinson KA, Axelrad DA and Woodruff TJ (2014) The Navigation Guide - evidence-based medicine meets environmental health: systematic review of human evidence for PFOA effects on fetal growth. Environ Health Perspect 122, 1028–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WE, Li C and Rabinovic A (2007) Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 8, 118–27. [DOI] [PubMed] [Google Scholar]
- Kanehisa M, Furumichi M, Tanabe M, Sato Y and Morishima K (2017) KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res 45, D353–D361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M and Goto S (2000) KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Research 28, 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Sato Y, Kawashima M, Furumichi M and Tanabe M (2016) KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res 44, D457–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Basden BJ, Needham LL and Calafat AM (2011) Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J Chromatogr A 1218, 2133–7. [DOI] [PubMed] [Google Scholar]
- Kato K, Wong LY, Chen A, Dunbar C, Webster GM, Lanphear BP and Calafat AM (2014) Changes in serum concentrations of maternal poly- and perfluoroalkyl substances over the course of pregnancy and predictors of exposure in a multiethnic cohort of Cincinnati, Ohio pregnant women during 2003-2006. Environ Sci Technol 48, 9600–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEMI Swedish Chemical Agency (2015) Occurrence and use of highly fluorinated substances and alternatives. [Google Scholar]
- Kingsley SL, Eliot MN, Kelsey KT, Calafat AM, Ehrlich S, Lanphear BP, Chen A and Braun JM (2018) Variability and predictors of serum perfluoroalkyl substance concentrations during pregnancy and early childhood. Environ Res 165, 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuklenyik Z, Needham LL and Calafat AM (2005) Measurement of 18 Perfluorinated Organic Acids and Amides in Human Serum Using On-Line Solid-Phase Extraction. Analytical Chemistry 77, 6085–6091. [DOI] [PubMed] [Google Scholar]
- Lankadurai BP, Nagato EG and Simpson MJ (2013) Environmental metabolomics: an emerging approach to study organism responses to environmental stressors. Environmental Reviews 21, 180–205. [Google Scholar]
- Lee JW, Lee JW, Kim K, Shin YJ, Kim J, Kim S, Kim H, Kim P and Park K (2017) PFOA-induced metabolism disturbance and multi-generational reproductive toxicity in Oryzias latipes. J Hazard Mater 340, 231–240. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Kim MK, Bae J and Yang JH (2013) Concentrations of perfluoroalkyl compounds in maternal and umbilical cord sera and birth outcomes in Korea. Chemosphere 90, 1603–9. [DOI] [PubMed] [Google Scholar]
- Li S, Park Y, Duraisingham S, Strobel FH, Khan N, Soltow QA, Jones DP and Pulendran B (2013) Predicting network activity from high throughput metabolomics. PLoS Comput Biol 9, e1003123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Pozhitkov A, Ryan RA, Manning CS, Brown-Peterson N and Brouwer M (2010) Constructing a fish metabolic network model. Genome biology 11, R115–R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HS, Wen LL, Chu PL and Lin CY (2018) Association among total serum isomers of perfluorinated chemicals, glucose homeostasis, lipid profiles, serum protein and metabolic syndrome in adults: NHANES, 2013-2014. Environ Pollut 232, 73–79. [DOI] [PubMed] [Google Scholar]
- Liu KH, Walker DI, Uppal K, Tran V, Rohrbeck P, Mallon TM and Jones DP (2016) High-Resolution Metabolomics Assessment of Military Personnel: Evaluating Analytical Strategies for Chemical Detection. J Occup Environ Med 58, S53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn A, Simmonds M, Owen CG and Woolacott N (2015) Childhood obesity as a predictor of morbidity in adulthood: a systematic review and meta-analysis. Obes Rev. [DOI] [PubMed] [Google Scholar]
- Lopez-Espinosa MJ, Fletcher T, Armstrong B, Genser B, Dhatariya K, Mondal D, Ducatman A and Leonardi G (2011) Association of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) with age of puberty among children living near a chemical plant. Environ Sci Technol 45, 8160–6. [DOI] [PubMed] [Google Scholar]
- Maisonet M, Terrell ML, McGeehin MA, Christensen KY, Holmes A, Calafat AM and Marcus M (2012) Maternal concentrations of polyfluoroalkyl compounds during pregnancy and fetal and postnatal growth in British girls. Environ Health Perspect 120, 1432–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora AM, Oken E, Rifas-Shiman SL, Webster TF, Gillman MW, Calafat AM, Ye X and Sagiv SK (2017) Prenatal Exposure to Perfluoroalkyl Substances and Adiposity in Early and Mid-Childhood. Environ Health Perspect 125, 467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JW, Hatch EE and Webster TF (2009) Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general US population. Environmental health perspectives 118, 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL and Zobel LR (2007) Half-life of serum elimination of perfluorooctanesulfonate,perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115, 1298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MH, Falconer C, Viner RM and Kinra S (2012) The impact of childhood obesity on morbidity and mortality in adulthood: a systematic review. Obes Rev 13, 985–1000. [DOI] [PubMed] [Google Scholar]
- Peng S, Yan L, Zhang J, Wang Z, Tian M and Shen H (2013) An integrated metabonomics and transcriptomics approach to understanding metabolic pathway disturbance induced by perfluorooctanoic acid. J Pharm Biomed Anal 86, 56–64. [DOI] [PubMed] [Google Scholar]
- Perng W, Gillman MW, Fleisch AF, Michalek RD, Watkins SM, Isganaitis E, Patti ME and Oken E (2014) Metabolomic profiles and childhood obesity. Obesity (Silver Spring) 22, 2570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappazzo KM, Coffman E and Hines EP (2017) Exposure to Perfluorinated Alkyl Substances and Health Outcomes in Children: A Systematic Review of the Epidemiologic Literature. Int J Environ Res Public Health 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Canela M, Hruby A, Clish CB, Liang L, Martinez-Gonzalez MA and Hu FB (2017) Comprehensive Metabolomic Profiling and Incident Cardiovascular Disease: A Systematic Review. J Am Heart Assoc 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salihovic S, Fall T, Ganna A, Broeckling CD, Prenni JE, Hyotylainen T, Karrman A, Lind PM, Ingelsson E and Lind L (2018) Identification of metabolic profiles associated with human exposure to perfluoroalkyl substances. J Expo Sci Environ Epidemiol. [DOI] [PubMed] [Google Scholar]
- Soltow QA, Strobel FH, Mansfield KG, Wachtman L, Park Y and Jones DP (2013) High-performance metabolic profiling with dual chromatography-Fourier-transform mass spectrometry (DC-FTMS) for study of the exposome. Metabolomics 9, S132–S143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Tinker S, Frisbee S, Ducatman A and Vaccarino V (2009) Association of Perfluorooctanoic Acid and Perfluorooctane Sulfonate With Serum Lipids Among Adults Living Near a Chemical Plant. American Journal of Epidemiology 170, 1268–1278. [DOI] [PubMed] [Google Scholar]
- Sumner LW, Amberg A, Barrett D, Beale MH, Beger R, Daykin CA, Fan TWM, Fiehn O, Goodacre R, Griffin JL, Hankemeier T, Hardy N, Harnly J, Higashi R, Kopka J, Lane AN, Lindon JC, Marriott P, Nicholls AW, Reily MD, Thaden JJ and Viant MR (2007) Proposed minimum reporting standards for chemical analysis. Metabolomics 3, 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Xie G, Sun X, Li Q, Zhong W, Qiao P, Sun X, Jia W and Zhou Z (2013) High fat diet feeding exaggerates perfluorooctanoic acid-induced liver injury in mice via modulating multiple metabolic pathways. PLoS One 8, e61409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trier X, Lunderberg D, Peaslee G and Wang Z (2015) PFASTRIER.
- Uppal K, Soltow QA, Promislow DE, Wachtman LM, Quyyumi AA and Jones DP (2015) MetabNet: An R Package for Metabolic Association Analysis of High-Resolution Metabolomics Data. Front Bioeng Biotechnol 3, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppal K, Soltow QA, Strobel FH, Pittard WS, Gernert KM, Yu T and Jones DP (2013) xMSanalyzer: automated pipeline for improved feature detection and downstream analysis of large-scale, non-targeted metabolomics data. BMC Bioinformatics 14, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppal K, Walker DI and Jones DP (2017) xMSannotator: An R Package for Network-Based Annotation of High-Resolution Metabolomics Data. Anal Chem 89, 1063–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppal K, Walker DI, Liu K, Li S, Go Y-M and Jones DP (2016) Computational Metabolomics: A Framework for the Million Metabolome. Chemical Research in Toxicology 29, 1956–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verner MA, Ngueta G, Jensen ET, Fromme H, Volkel W, Nygaard UC, Granum B and Longnecker MP (2016) A Simple Pharmacokinetic Model of Prenatal and Postnatal Exposure to Perfluoroalkyl Substances (PFASs). Environ Sci Technol 50, 978–86. [DOI] [PubMed] [Google Scholar]
- Wang X, Liu L, Zhang W, Zhang J, Du X, Huang Q, Tian M and Shen H (2017) Serum metabolome biomarkers associate low-level environmental perfluorinated compound exposure with oxidative /nitrosative stress in humans. Environ Pollut 229, 168–176. [DOI] [PubMed] [Google Scholar]
- Whitworth KW, Haug LS, Baird DD, Becher G, Hoppin JA, Skjaerven R, Thomsen C, Eggesbo M, Travlos G, Wilson R, Cupul-Uicab LA, Brantsaeter AL and Longnecker MP (2012) Perfluorinated compounds in relation to birth weight in the Norwegian Mother and Child Cohort Study. Am J Epidemiol 175, 1209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart DS, Feunang YD, Marcu A, Guo AC, Liang K, Vazquez-Fresno R, Sajed T, Johnson D, Li C, Karu N, Sayeeda Z, Lo E, Assempour N, Berjanskii M, Singhal S, Arndt D, Liang Y, Badran H, Grant J, Serra-Cayuela A, Liu Y, Mandal R, Neveu V, Pon A, Knox C, Wilson M, Manach C and Scalbert A (2018) HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res 46, D608–D617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu N, Wei S, Li M, Yang J, Li K, Jin L, Xie Y, Giesy JP, Zhang X and Yu H (2016) Effects of Perfluorooctanoic Acid on Metabolic Profiles in Brain and Liver of Mouse Revealed by a High-throughput Targeted Metabolomics Approach. Sci Rep 6, 23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Park Y, Li S and Jones DP (2013) Hybrid feature detection and information accumulation using high-resolution LC-MS metabolomics data. J Proteome Res 12, 1419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Beesoon S, Zhu L and Martin JW (2013) Biomonitoring of perfluoroalkyl acids in human urine and estimates of biological half-life. Environ Sci Technol 47, 10619–27. [DOI] [PubMed] [Google Scholar]
- Zheng F, Sheng N, Zhang H, Yan S, Zhang J and Wang J (2017) Perfluorooctanoic acid exposure disturbs glucose metabolism in mouse liver. Toxicol Appl Pharmacol 335, 41–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.