Abstract
Pregnant women with epilepsy (PWWE) require continuous anti-epileptic drug (AED) treatment to avoid risk to themselves and fetal risks secondary to maternal seizures, resulting in prolonged AED exposure to the developing embryo and fetus. The objectives of this study were to determine whether high-resolution metabolomics is able to link the metabolite profile of PWWE receiving lamotrigine or levetiracetam for seizure control to associated pharmacodynamic (PD) biological responses. Untargeted metabolomic analysis of plasma obtained from 82 PWWE was completed using high-resolution mass spectrometry. Biological alterations due to lamotrigine or levetiracetam monotherapy were determined by a metabolome-wide association study that compared patients taking either drug to those who did not require AED treatment. Metabolic changes associated with AED use were then evaluated by testing for drug-dose associated metabolic variations and pathway enrichment. AED therapy resulted in drug-associated metabolic profiles recognizable within maternal plasma. Both the parent compounds and major metabolites were detected, and each AED was correlated with other metabolic features and pathways. Changes in metabolites and metabolic pathways important to maternal health and linked to fetal neurodevelopment were detected for both drugs, including changes in one-carbon metabolism, neurotransmitter biosynthesis and steroid metabolism. In addition, decreased levels of 5-methyltetrahydrofolate and tetrahydrofolate were detected in women taking lamotrigine, which is consistent with recent findings showing increased risk of autism spectrum disorder traits in PWWE using AED. These results represent a first step in development of pharmacometabolomic framework with potential to detect adverse AED-related metabolic changes during pregnancy.
Keywords: Epilepsy, Folate, High-resolution mass spectrometry, Lamotrigine, Levetiracetam, Pharmacometabolomics
INTRODUCTION
Anti-epileptic drugs (AEDs) are the most frequently prescribed known teratogens during pregnancy, imparting increased risk for congenital malformations, intra-uterine growth retardation and neurodevelopmental deficits (Harden et al., 2009). One-half million women with epilepsy are of childbearing age in the US, and greater than three births per thousand are to pregnant women with epilepsy (PWWE) (Harden et al., 2009). A far greater number of pregnancies (21.9 per thousand) include exposure at some interval during pregnancy for additional indications other than epilepsy, including headache, chronic pain, obesity, mood disorders, and other psychiatric diagnoses that prescribe AED treatment (Bobo et al., 2012; Pennell, 2016; Voinescu and Pennell, 2017). However, pregnant women with epilepsy (PWWE) require continuous AED treatment throughout all three trimesters to avoid risk to themselves and fetal risks secondary to maternal seizures, resulting in prolonged exposure to the developing embryo and fetus (Pennell, 2005; Chen et al., 2009; Pennell, 2016). While prescriptions of AEDs with elevated risks for adverse outcomes in human studies are discouraged in treatment guidelines, the more commonly used AEDs during pregnancy, lamotrigine (LTG) and levetiracetam (LEV), demonstrate some uncertainty in the limited human studies published to date (Chen et al., 2009; Harden et al., 2009). For example, LTG is one of the most commonly prescribed AEDs to PWWE, yet studies show that LTG is associated with increased risk for major congenital malformations at doses ≥300 mg/day and detailed neurodevelopmental studies have shown relative decreases in verbal compared to non-verbal abilities and increased left-handedness, possibility due to alterations in cerebral lateralization. (Tomson et al., 2011; Hernandez-Diaz et al., 2012; Meador et al., 2013). LEV is another commonly prescribed AED for PWWE and dose-dependent risks have not been studied (Registry, 2014; Meador et al., 2016). In principle, dose-dependent metabolic responses associated with AEDs could be used to improve an individual’s treatment regimen by identifying metabolic responses associated with effective therapy as well as adverse outcomes. Such information could be used to develop strategies to modify dose or therapeutic agent to improve maternal and fetal safety.
The human metabolome represents the functional output of gene expression, environmental factors, diet and the core biochemical processes crucial for life (Wishart et al., 2013; Liu et al., 2016). By linking internal dose to biological response and disease pathology in a single measure, the metabolome provides important insights into drug pharmacology, including pharmacokinetics (PK) and pharmacodynamics (PD). Comparison of metabolic profiles from PWWE who utilize AEDs for seizure control to those not requiring AEDs can be used to assess biological changes associated with AED use during pregnancy, improving the ability to characterize unintended effects of medication and mitigating potential risks during pregnancy.
Untargeted metabolic profiling techniques based upon high-resolution mass spectrometry now make possible highly sensitive and global profiling of up to 20,000 chemical features in biological samples, providing sufficient characterization to support precision medicine research, delineate biological response to drug therapies and inform clinical decision-making when data gaps exist (Jones et al., 2012; Liu et al., 2016; Uppal et al., 2016b). In the current study, we used high-resolution metabolomics (HRM) to assess biological changes associated with AED therapy during pregnancy. Analyses were performed using plasma collected throughout pregnancy from PWWE being treated with LTG or LEV monotherapy and compared to metabolic profiles of PWWE not requiring AEDs. Assessment of AED treatment was then characterized by identifying dose-dependent alterations in drug metabolites and metabolic pathways associated with neurodevelopment, birth outcomes and maternal health. This study represents the first metabolome-wide association study (MWAS) of AED treatment during pregnancy and provides insight into potential mechanisms underlying the effects of these medications on pregnancy outcomes.
MATERIALS AND METHODS
Study design
Plasma samples were obtained from PWWE enrolled in the Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drug (MONEAD) study (ClinicalTrials.gov Identifier: NCT01730170); a multicenter, prospective, observational study focused on establishing the relationship between AED exposure and maternal and child outcomes in PWWE. Protocols for the collection, storage and use of human data and samples were approved by the Emory University Institutional Review Board (IRB) and the IRBs at 19 additional clinical sites (Boston MA, Chicago IL, New York City NY (2 sites), Danville PA, Detroit MI, Baltimore MD, Manhasset NY, Augusta GA, St. Paul MN, Pittsburg PA, Miami FL, Birmingham AL, Tucson AZ, Cincinnati OH, Los Angeles CA, Seattle WA, Winston-Salem NC, Stanford CA). Inclusion criteria for PWWE included ages 14-45 years and ability to maintain a diary and follow-up through 6 years after delivery. Key exclusion criteria included IQ<70, substance abuse, positive syphilis or HIV test, other major medical illnesses, psychogenic non-epileptic seizures, exposure to other known teratogens, detection of major fetal congenital malformations prior to enrollment, switching AEDs during pregnancy and prior to enrollment, and a known genetic disorder in the subject or a first degree relative. Women were enrolled in the MONEAD study up to 20 weeks gestational age (GA) (visit 1). Follow-up included visit 2 at 21-27 weeks GA and visit 3 at 30-36 weeks GA. At each study visit (i.e., 1, 2, and 3), general physical and neurological examinations were performed, electronic seizure and medication adherence diaries were reviewed. AED daily dose was reported by the patient’s physician and verified both by medical records at the time of the visit and review of the medication adherence diaries. Maternal blood was collected via standard venipuncture and centrifuged at 2750 rpm at 3°C for 10 min. Plasma samples were stored in 500 μL aliquots at −80°C. This metabolomics study was limited to patients with samples collected over the course of 2013-2014, with completion of visit 3 by 11/2014. Sample analysis was completed in early 2015, as a result maximum storage time at −80°C was two years. Patient selection was limited to PWWE being treated with the two most common AED regimens, LTG and LEV monotherapy, or no AEDs to serve as controls in this analysis.
High-resolution metabolomics
Untargeted metabolic profiling was completed using liquid chromatography with ultra-high resolution mass spectrometry (LC-HRMS; Q-Exactive HF, Thermo Scientific) following established protocols (Go et al., 2015). Prior to HRM, samples were randomized so that all plasma measures for a given patient were present in the same batch of 25 samples and analysts were blinded to treatment groups. Briefly, plasma proteins were precipitated by diluting plasma samples (50 μL) with 100 μL acetonitrile containing internal standards; the resulting supernatant was then analyzed using five technical replicates. Analyte separation was accomplished using hydrophilic interaction chromatography (HILIC) (Accucore HILIC 50mm x 2.1mm x 2.6μm, Thermo Scientific) and an Ultimate 3000 pump (Thermo Scientific) operated at 0.5mL/min with water, acetonitrile and solution A (5% [v/v] formic acid in water) mobile phases operating under the following gradient: 10% water, 80% acetonitrile, 10% solution A hold for 0.5 min, linear gradient to 80% water, 10% acetonitrile, 10% solution A at 2 min, and hold for 3 min, resulting in a total run time of 5 min. The electrospray ionization source was operated in positive ion mode with spray voltage of 3.5 kV, probe temperature of 300°C, capillary temperature of 300°C, sheath gas flow of 45 (arbitrary units), auxiliary gas flow of 25 (arbitrary units), sweep gas flow of 1 (arbitrary units), and S-lens RF level of 45%. Resolution was 120,000 (FWHM) and the mass-to-charge (m/z) scan range was 85-1275. Samples were analyzed in batches of 25 samples. To evaluate system performance, we used two separate quality control (QC) reference samples. The first QC sample consisted of NIST SRM 1950, Metabolites in Human Plasma and was analyzed twice, once before batch 1 and once after batch 25 (Simon-Manso et al., 2013). The second QC sample (Q-Std) included commercially purchased plasma that was pooled from an unknown number of males and females without demographic information (Equitech-Bio, Inc, Kerrville, Texas) (Go et al., 2015). Q-Std was analyzed at the beginning and end of each batch, providing 50 separate analyses over the course of the study.
After completion of LC-HRMS analysis, mass spectral features with replicate coefficient of variation (CV) ≤ 100% were extracted and aligned using apLCMS with xMSanalyzer; batch effects were then corrected with ComBat (Johnson et al., 2007; Uppal et al., 2013; Yu et al., 2013). Detected chemical signals, represented by accurate mass m/z, retention time and intensity, are referred to as m/z features. Raw data included 16,974 features. Since each sample was analyzed with five injections, we calculated the median CV from all study sample replicates; the mean replicate CV for all features was 39.8%. Prior to statistical analysis, replicate injections were averaged, m/z features not detected in ≥ 75% of the patients in at least one treatment group were removed. The remaining ion intensities were log2 transformed and used for the subsequent regression analyses. The final datasets included 8,829 m/z features for the LTG group, while 9,019 m/z features remained for the LEV comparison. Only 254 and 444 m/z features were unique to LTG and LEV, respectively. Median CV was recalculated for the filtered datasets; mean CV for all features was 31.5%. This CV is consistent with previous HRM studies, and is due to an increase in the CV filtering threshold during data extraction that allows the detection of low abundance chemicals close to the instrument detection limit (Yu et al., 2009; Yu et al., 2013; Uppal et al., 2016c; Walker et al., 2016c). Improved sensitivity is important for measurement of low-level exogenous chemicals and drug metabolites in biological samples, which are often present at four or five orders-of-magnitude lower abundance than endogenous metabolites (Jones, 2016; Walker et al., 2016a). By increasing the number of replicate injections to five, the number of ions that can be reliably quantified in a biological sample is increased and noise reduced when averaged across the multiple replicates (Uppal et al., 2013; Go et al., 2015; Walker et al., 2016b).
AED metabolome-wide association study
Statistical analyses were completed using R, version 3.1.2. To evaluate the relationship between metabolic phenotype and AED treatment, linear mixed-effects regression models were applied for the two AEDs, LTG and LEV during visits 1-3. For each detected m/z feature, fixed effects included AED use, visit number, age, and race. To account for repeat measures, a random intercept for each subject was included. Regression analysis was performed using the R package lme4 and completed separately for each AED by comparing to PWWE who were not on any medications for seizure control (Bates et al., 2015). The likelihood ratio test of the complete model against the null model, which included trimester, age, race and a random intercept for each subject, was used to obtain p for each m/z feature associated with AED treatment. Model fits were evaluated by calculating the conditional and marginal r2 and reported for all m/z features meeting the p threshold. To account for multiple hypothesis testing, a Benjamini-Hochberg false discovery rate (FDR) of 5% was applied (Benjamini and Hochberg, 1995). This represents a stringent threshold that minimizes Type I error (false positives) at the increased risk of Type II error (false negatives). MWAS of phenylalanine metabolism has shown that for initial discovery purposes, raw p values combined with metabolic pathway enrichment improves detection of biological effects; however, since this was a unique clinical population without a proper validation cohort, we biased our findings towards increased stringency (smaller p) and decreased risk of false positives at the expense of rejecting some true effects (Go et al., 2014). The resulting m/z features with p below the FDR threshold (5%) were selected for characterization by hierarchical cluster analysis (HCA) using complete linkage agglomeration, annotation and comparison of metabolic changes associated with LTG and LEV. To determine if LTG or LEV dose and corresponding metabolites detected using HRM were associated with metabolic alterations, we used a network correlation approach that used samples from all patients and timepoints to define the relationship between drug metabolites detected by HRM, patient ingested dose (mg/day) and m/z features meeting the MWAS FDR. Pairwise correlations were determined using MetabNet by Spearman |r| ≥ 0.3 with FDR<5% and correlation networks visualized in Cytoscape (Su et al., 2014; Uppal et al., 2015).
Annotation and metabolic pathway enrichment
HRM provides accurate mass (± 5 parts-per-million; ppm) measures of ion m/z, which can be related to chemical monoisotopic mass, an intrinsic molecular property. Annotation of m/z features associated with each of the AEDs was first matched to a reference database of 75 metabolites previously confirmed by comparing ion dissociation and elution time to reference standards.(Go et al., 2015) Additional m/z features not matching these metabolites were annotated(Uppal et al., 2017) based upon common positive mode adducts using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database and the Human Metabolome Database (HMDB) (Kanehisa et al., 2012; Wishart et al., 2013). Feature identities were assigned using evidence scoring(Uppal et al., 2016a) and a ± 5 ppm mass tolerance (Δmerror /Δmtheoretical × 106). Enriched metabolic pathways were selected using Mummichog based upon a scoring threshold ≤ 0.05 and a requirement that enriched pathways must have three or more metabolites associated with AED use (Li et al., 2013).
RESULTS
Study population
Patient characteristics are provided in Table 1. The participants were primarily white, and the cohort size was insufficient to allow stratification by race. All AED-treated and non-treated PWWE had blood drawn from a minimum of two study visits during pregnancy. Only plasma samples collected during visits 1-3 were considered in this metabolomics study and samples collected during delivery were excluded to allow isolation of AED metabolic associations and minimize potential confounding effects from labor and delivery.
Table 1:
Demographic characteristics and AED treatment distribution for PWWE selected for HRM profiling
| Subjects | PWWE | AED Treated PWWE | |
|---|---|---|---|
| Controls (n= 10) | LTG (n=39) | LEV (n= 33) | |
| Demographic characteristics | |||
| Age, mean (SD) | 30 (5) | 32 (4) | 29 (5) |
| Race, n (%) | |||
| Caucasian | 7 (70%) | 36 (92%) | 27 (82%) |
| African American | 2 (22%) | 1 (2%) | 3 (9%) |
| Asian | 0 | 2 (5%) | 1 (3%) |
| Multiracial | 0 | 0 | 2 (6%) |
| Not answered | 1 (1%) | 0 | 0 |
| AED daily dose at the study visit* (mg), mean ± SD | |||
| Visit 1† | NA | 410 ± 160 | 1770 ± 961 |
| Visit 2‡ | NA | 531 ± 222 | 2052 ± 958 |
| Visit 3§ | NA | 606 ± 244 | 2167 ± 906 |
| Number of samples, n (%) | |||
| Total | 26 | 108 | 88 |
| Visit 1† | 8 (30%) | 38 (35%) | 30 (34%) |
| Visit 2‡ | 9 (35%) | 33 (31%) | 28 (32%) |
| Visit 3§ | 9 (35%) | 37 (34%) | 30 (34%) |
All visits were at least 4 weeks apart
Visit 1: < 20 weeks gestational age
Visit 2: 21-27 weeks gestational age
Visit 3: 30-36 weeks gestational age
High-resolution metabolomics
We divided description of results into four parts in which we: 1) selected m/z features associated with LTG or LEV use, 2) examined these selected features for inclusion of drug metabolites to test for correlation with drug dose and endogenous metabolites 3) examined these selected features for known endogenous metabolites (not derived from drug) and 4) tested the selected features associated with AED use for pathway enrichment. The first three parts are provided for LTG, followed by the first three parts for LEV. The selected metabolic features for both LTG and LEV were combined for the fourth part to examine similarities and differences in metabolic phenotypes of the AEDs.
LTG metabolic phenotype
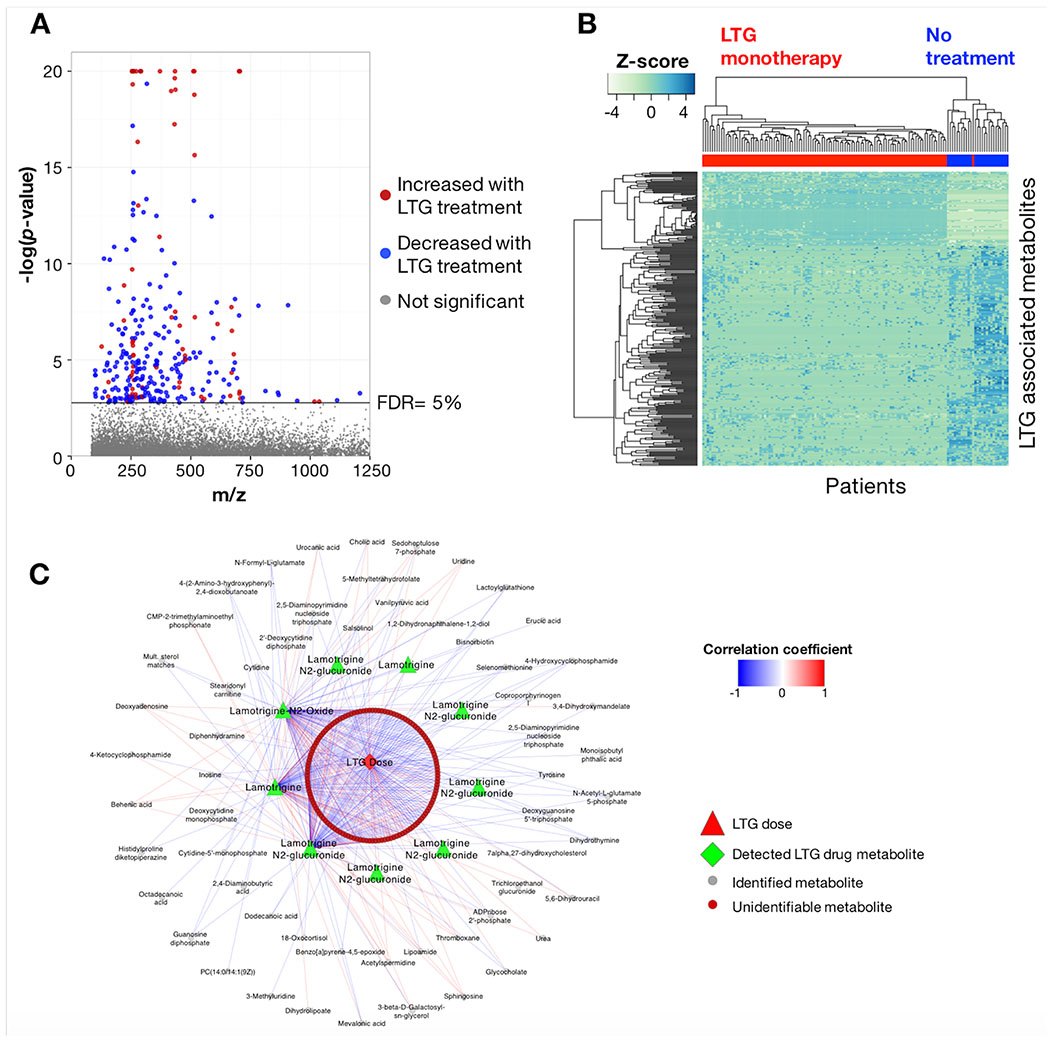
After correction for multiple comparisons, 297 differentially expressed m/z features were associated with LTG at FDR ≤ 5% (p < 0.0017) (Figure 1A). For the selected features, 186 exhibited p ≤ 10−4 with marginal and conditional r2 of 0.095-0.95 and 0.097-0.96, respectively. Regression coefficients (β) for LTG treatment ranged from −22.2 to 28.4, with 221 features decreasing in the LTG treatment group relative to the no treatment group. The m/z features meeting the FDR threshold included 52 unique to LTG, all of which were positively associated with treatment (β= 7.9-28.4). HCA of the 297 m/z features identified nine sample clusters and 39 metabolite clusters (Figure 1B). Additional regression results are provided in Supplementary Table 1.
Figure 1:
Metabolome-wide association study (MWAS) of LTG use during pregnancy. A) Manhattan plot of −log p for m/z features associated with LTG monotherapy in PWWE at FDR threshold of 5%; B) Unsupervised hierarchical clustering of m/z features in 1A clustered in both the metabolite and patient dimensions; clear separation between AED-treated and non-treated PWWE was present; C) Both identified metabolites and unidentified m/z features associated with LTG showed correlation with patient drug dose and detected drug metabolites at Spearman |r| ≥ 0.3 and FDR ≤ 5%.
The m/z features associated with LTG treatment included accurate mass matches to LTG (β = 17.7; p < 0.0001), and LTG-metabolites, including LTG-N2-glucuronide (β = 24.4; p< 0.0001) and LTG-N2-oxide (β = 21.5; p < 0.0001). To test if daily LTG dose (mg/day) was correlated with m/z features detected in the MWAS analysis, we calculated the correlation coefficient between reported patient LTG daily dose and the parent compound detected by HRM, the two LTG metabolites and other m/z features associated with LTG (Figure 1C). The correlation was determined using all timepoints and LTG reported daily dose for the corresponding visit. Using Spearman |r| ≥ 0.3 and FDR <5%, 196 (71%) of the 276 m/z features associated with LTG that were detected in plasma from LTG patients were correlated with LTG dose, LTG and/or the two LTG metabolites. LTG and LTG-metabolites were correlated with dose, including LTG (r = 0.26), LTG-N2-oxide (r = 0.29) and LTG-N2-glucuronide (r = 0.61). LTG was also correlated with LTG-N2-oxide (r = 0.42) and LTG-N2-glucuronide (r = 0.43). LTG-N2-oxide and LTG-N2-glucuronide showed the highest degree of metabolite correlation at r= 0.57. LTG-N2-methyl (β = 7.2; p = 0.02) was also detected but did not meet the FDR criteria.
Accurate mass m/z for 100 of the 297 differentially expressed features associated with LTG treatment matched 89 unique metabolites. A selected list of metabolites which appear likely to be relevant to biologic effects of LTG is provided in Table 2; the complete results are available in Supplementary Table 1. Additional matches to C21-steroids were also detected, including 11-beta,17alpha,21-trihydroxypregnenolone (β = −11.8; p < 0.0001), cortisol (β = −8.1; p = 0.0013), and 18-oxocortisol (β = 9.5; p < 0.0001); however, we could not definitively identify these individual compounds due to the presence of multiple isobaric species. Pathway enrichment analysis identified 9 pathways associated with LTG use (Table 3). These included nucleoside (pyrimidine and purine), co-enzyme (biopterin, folate), thiol antioxidant (methionine and cysteine; selenoamino acid) metabolism, lipid (glycerophospholipid, glycosphingolipid), and complex carbohydrate (sialic acid) metabolism.
Table 2:
Selected metabolites associated with AED monotherapy
| Metabolite annotation | Marginal r2 | Conditional r2 | β coefficient | p-value |
|---|---|---|---|---|
| Lamotrigine | ||||
| Tyrosine | 0.40 | 0.68 | 17.6 | 9.0E-08 |
| 5-Methyltetrahydrofolate | 0.17 | 0.22 | −6.4 | 8.1E-04 |
| Tryptophan | 0.26 | 0.39 | −6.6 | 1.5E-07 |
| 7α,27-dihydroxycholesterol | 0.18 | 0.18 | −6.7 | 8.6E-06 |
| Tetrahydrofolate | 0.12 | 0.22 | −7.1 | 1.0E-03 |
| Methylthioadenosine | 0.36 | 0.44 | −7.2 | 4.1E-07 |
| 5a-Androstane-3b,17b-diol | 0.21 | 0.61 | −9.6 | 1.2E-03 |
| Progesterone | 0.17 | 0.39 | −10.5 | 4.1E-04 |
| Uric acid | 0.17 | 0.23 | −11.8 | 1.3E-05 |
| Thromboxane | 0.64 | 0.79 | −16.4 | 4.4E-14 |
| Levetiracetam | ||||
| Norepinephrine | 0.39 | 0.63 | 14.3 | 1.2E-06 |
| Mevalonate | 0.32 | 0.81 | 13.3 | 1.8E-04 |
| Mevalonate-5-phosphate | 0.31 | 0.51 | 13.2 | 2.8E-06 |
| Dihydrobiopterin | 0.34 | 0.46 | 10.4 | 2.0E-06 |
| Leucine/Isoleucine | 0.11 | 0.14 | 2.6 | 3.8E-04 |
| Kynurenine | 0.36 | 0.73 | 1.0 | 1.2E-05 |
| Tryptophan | 0.25 | 0.39 | −6.2 | 1.6E-06 |
| γ-Aminobutyric acid | 0.27 | 0.28 | −10.1 | 1.5E-05 |
| 3,4-Dihydroxyphenylglycol | 0.27 | 0.52 | −11.0 | 5.1E-05 |
| Dihydrolipoate | 0.32 | 0.57 | −13.0 | 3.2E-05 |
Table 3:
Metabolic pathways associated with LTG or LEV monotherapy
| Pathway | Number overlapping metabolites* | Total number metabolites† | Mummichog score‡ |
|---|---|---|---|
| LTG MWAS pathways | |||
| Pyrimidine metabolism | 10 | 47 | 0.0009 |
| Biopterin metabolism | 5 | 17 | 0.0012 |
| Purine metabolismd | 9 | 52 | 0.0022 |
| Glycerophospholipid metabolism§ | 7 | 45 | 0.0072 |
| Sialic acid metabolism | 4 | 27 | 0.0336 |
| Vitamin B9 (folate) metabolism | 3 | 18 | 0.0397 |
| Glycosphingolipid metabolism | 4 | 28 | 0.0404 |
| Methionine and cysteine metabolism | 6 | 48 | 0.0431 |
| Selenoamino acid metabolism | 3 | 19 | 0.0495 |
| LEV MWAS pathways | |||
| Urea cycle/amino group metabolism | 6 | 47 | 0.0007 |
| Purine metabolismd | 6 | 51 | 0.0008 |
| Butanoate metabolism | 4 | 24 | 0.0009 |
| Aspartate and asparagine metabolism | 6 | 64 | 0.0012 |
| Glycerophospholipid metabolismd | 3 | 47 | 0.0223 |
Number of metabolites associated with AED use from pathway
Total number of pathway metabolites detected
See Li et al. (2013)
Pathways in bold were associated with both LTG and LEV
LEV metabolic phenotype
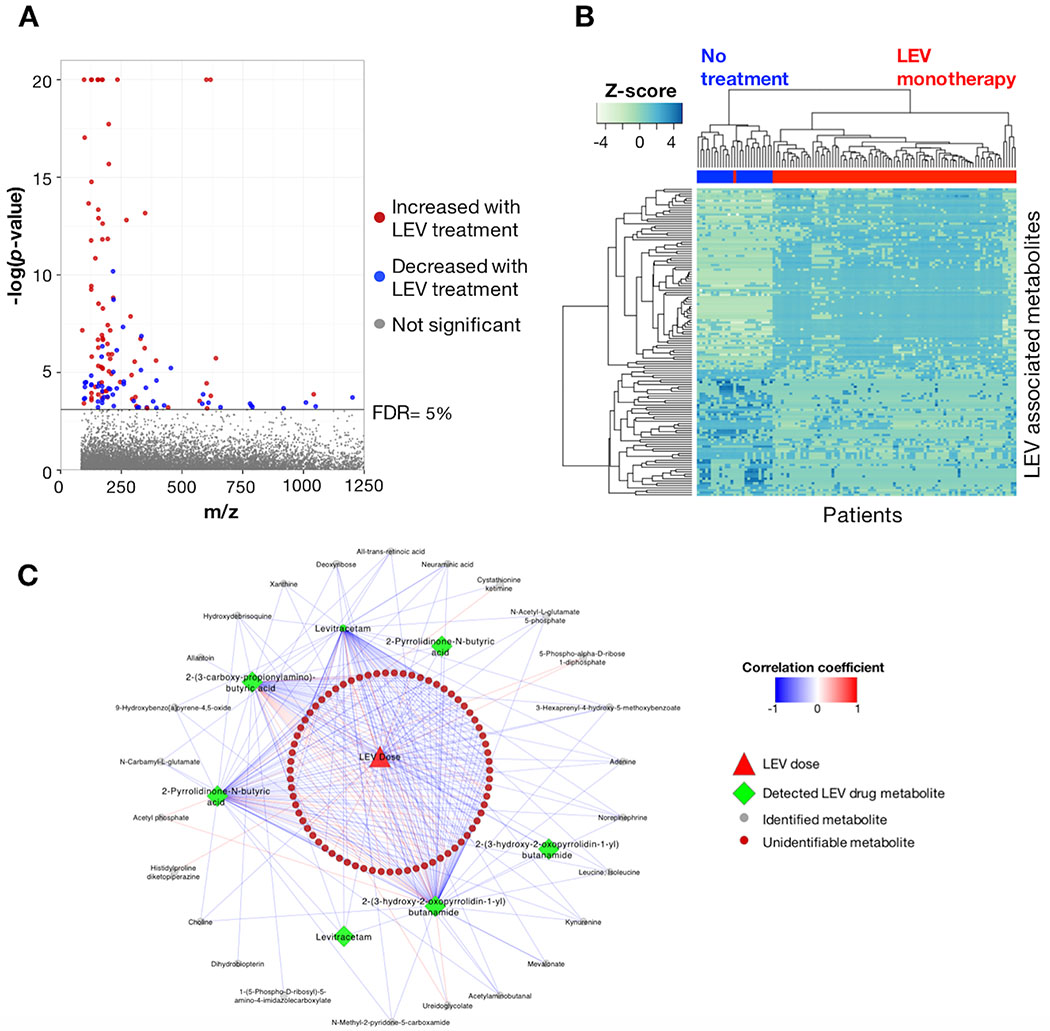
For PWWE taking LEV, 134 m/z features were associated with treatment at FDR ≤ 5% (p < 0.00075) (Figure 2A). For the LEV associated m/z features, 89 exhibited p ≤ 10−4 with marginal and conditional r2 of 0.11-0.95 and 0.11-0.96, respectively. Regression coefficients for LEV treatment ranged from −13.7 to 24.3, with 55 features exhibiting decreased levels in the LEV treatment group relative to the no treatment group. The m/z features meeting the FDR threshold included 48 that were unique to the LEV treatment group and positively associated with treatment (β = 9.3-24.3). HCA of the 134 m/z features identified six sample clusters and 14 metabolite clusters (Figure 2B). Regression results for the 134 features are provided in Supplementary Table 2.
Figure 2:
Metabolome-wide association study (MWAS) of LEV use during pregnancy. A) Manhattan plot of −log p for m/z features associated with LEV monotherapy in PWWE at FDR threshold of 5%. Patterns of m/z features were largely different than those detected in PWWE taking LTG. B) Similar to what was observed for LTG, unsupervised hierarchical clustering of m/z features associated with LEV use clustered in both the metabolite and patient dimensions and showed clear separation between the groups. C) Both identified metabolites and unidentified m/z features showed correlation with patient drug dose and detected drug metabolites at Spearman |r| ≥ 0.3 and FDR ≤ 5%.
Annotated LEV metabolites included the parent compound (β = 22.7; p < 0.0001), 2-pyrrolidone-N-butyric acid (β = 8.1; p < 0.0001), 2-(3-carboxy-propionylamino)-butyric acid (β = 16.0; p < 0.0001) and multiple stereo isomers of 2-(3-hydroxy-2-oxopyrrolidin-1-yl)butanamide (β = 11.9; p < 0.0001). We next evaluated correlation coefficients for daily drug dose at all visits, LEV metabolites detected by HRM and the 129 m/z features identified in the LEV MWAS to evaluate if dose and/or identified LEV metabolites were correlated with metabolomic changes. Using a threshold of Spearman |r| ≥ 0.3 and FDR ≤ 5%, 100 (77%) m/z features were correlated with drug dose, LEV and/or the three LEV metabolites (Figure 2C). LEV and LEV metabolites were positively correlated with dose, including LEV (r = 0.35) and 2-(3-hydroxy-2-oxopyrrolidin-1-yl)butanamide (r = 0.3). Correlations between LEV and LEV metabolites included 2-pyrrolidinone-N-butyric acid (r = 0.44), 2-(3-carboxy-propionylamino)-butyric acid (r= 0.29) and 2-(3-hydroxy-2-oxopyrrolidin-1-yl)butanamide (r = 0.65).
Annotation of m/z features associated with LEV treatment resulted in 41 m/z features that matched 37 metabolites. A list of sixteen relevant metabolites is provided in Table 2; the complete results are available in Supplementary Table 2. Pathway enrichment analysis identified six metabolic pathways that had three or more detected metabolites associated with LEV therapy (Table 3). These included nucleoside (purine), amino acid (urea cycle; aspartate and asparagine), short-chain fatty acid (butanoate), and lipid (glycerophospholipid) metabolism.
Metabolic response to AED treatment
To test for similarity in metabolic response to AEDs in PWWE, we compared the 297 m/z features associated with LTG treatment to the 134 associated with LEV. Including both annotated and unidentifiable m/z features, 25 met the FDR threshold of 5% in both treatment groups. These included dihydrolipoate, tryptophan, histidylproline diketopiperazine, and 1-(5-phospho-D-ribosyl)-5-amino-4-imidazolecarboxylate. Except for the unidentified m/z features 308.0696 and 356.2842, the remaining 23 m/z features associated with both treatment groups were lower relative to the non-treated PWWE.
DISCUSSION
HRM detects metabolites in greater than 80% of human metabolic pathways and enables simultaneous testing for multiple drug metabolites and biological response associated with drug treatments or interventions, providing an integrative framework for pharmacometabolomics. In principle, if metabolic responses were also connected to adverse outcomes, the framework could be extended to include toxic responses. The present study demonstrates an application of HRM to PWWE receiving LTG or LEV monotherapy. MWAS of AED use showed metabolic alterations associated with LTG and LEV, including both drug metabolites and dose-dependent associations with endogenous metabolites. Common endogenous pathways for LTG and LEV included purine and glycerophospholipid metabolism, suggesting that these pathways could be linked to pharmacodynamic (antiepileptic) effects. Other correlated metabolites differed for LTG and LEV and were in pathways important for embryonic development, maternal and post-natal health.
Although a case-control framework was used to identify metabolic features associated with AEDs, testing for dose-dependent relationships indicated 71% and 77% of the metabolic features were correlated with dose and/or drug metabolites of LTG and LEV, respectively. The presence of dose-related variation suggests the observed metabolic changes are due to drug-related biological responses and not confounding factors. Thus, the AED associated metabolic variations identified here may reflect underlying biological mechanisms contributing to AED-related adverse outcomes. While correlation alone does not establish a cause-effect relationship, the principle of inferred causality is useful under these conditions, where testing by randomized clinical trial is not ethically possible (Kleinberg and Hripcsak, 2011). In this regard, the dose-response character, as observed for both LTG and LEV, is particularly important as a criterion for interpretation.
PWWE taking LTG for seizure control showed the greatest metabolic response. While a large number of the m/z features did not match known metabolites, annotation and pathway analysis identified alterations to metabolites that are important for maternal health and neurodevelopmental outcomes. These included decreased levels of two neurosteroids, progesterone and 3β-androstanediol, that are associated with LTG treatment. In studies in the general population, low progesterone concentrations have been identified as a risk factor for miscarriage during the first trimester (Arck et al., 2008). Human studies have demonstrated an increase in seizure frequency during times of elevated estrogen/progesterone ratio and declining or low progesterone levels (Harden and Pennell, 2013). Animal studies have demonstrated proconvulsant effects for estrogen, anticonvulsant effects for progesterone, and evidence that allopregnanolone is likely a modulator for the anti-seizure effects of progesterone (Verrotti Alberto et al., 2012). Circulating neurosteroids have positive neurodevelopmental functions, likely through multiple mechanisms with potential roles in neuronal development and plasticity, as well as the maintenance of the nervous system (Belelli et al., 2006; Mellon, 2007). Many other AEDs alter the sex steroid hormone metabolic pathways in adult men and non-pregnant women; however, previous studies have not addressed this effect during pregnancy (Hill et al., 2011; Harden and Pennell, 2013). While the results from this study support an LTG-associated alteration in steroid metabolism in PWWE, additional studies are needed to confirm identities of specific steroids and determine if abnormal levels contribute to adverse effects.
Changes to neurotransmitter-related pathways were also associated with LTG treatment. Tyrosine, which was elevated in PWWE receiving LTG monotherapy, is a precursor of dopamine, norepinephrine and epinephrine, which have been associated with increased risk of preterm birth (Holzman et al., 2009). While increased levels of catecholamines were not detected in this study, elevated circulating levels of tyrosine affects neurotransmitter synthesis (Rasmussen et al., 1983). Unlike tyrosine, plasma levels of tryptophan were reduced in response to LTG monotherapy. During pregnancy, the placental tryptophan hydroxylase pathway converts maternal tryptophan to 5-hydroxytryptophan (5-HTP), while placental indoleamine 2,3-dioxygenase converts tryptophan to kynurenine (Goeden et al., 2016). Both of these pathways are critical in brain development and protection from the maternal immune system (Munn et al., 1998; Goeden et al., 2016). Biopterin metabolites serve several functions related to neurotransmitter synthesis. Tetrahydrobiopterin (BH4) is an essential cofactor for both tyrosine hydroxylase (conversion of L-tyrosine to L-DOPA) and tryptophan hydroxylase (conversion of L-tryptophan to 5-HTP). Interestingly, LTG shares a high degree of chemical similarity with BH4 (Tanimoto Score 84/100), suggesting competitive inhibition as a possible mechanism for the observed alterations in biopterin and related pathways.
LTG monotherapy was also associated with changes in one-carbon metabolism, with altered pathways including vitamin B9 (folate), purine and sulfur amino acid metabolism. Folate deficiency during pregnancy is linked to poor neurocognitive and birth outcomes, including neural tube defects, low birth weight, and increased risk of pre-term birth (Scholl and Johnson, 2000; Roth et al., 2011). A recent prospective, observational study of folate supplementation during pregnancy found mothers with epilepsy using AEDs had a 5- to 8-fold increased risk of having children with autism spectrum disorder (ASD) traits if supplementation was not used during the periconceptional period (Bjork et al., 2018). Association with ASD traits was independent of AED type, was not observed in untreated PWWE, and blood folate levels showed an inverse association with ASD trait score. In our study, both 5-methyltetrahydrofolate (5-MTHF) and tetrahydrofolate (THF) were decreased with LTG treatment, suggesting a possible AED-induced effect on folate uptake or metabolism that could be improved by maternal supplementation prior to and during pregnancy. 5-MTHF is the most biologically active form of folate and is critical as a methyl-donor at the cellular level for regulation of homocysteine, synthesis of S-adenosylmethionine (SAM), and DNA methylation and synthesis (Crider et al., 2012). THF is a cofactor in many enzymatic reactions requiring transfer of a one-carbon group and is also a transformation product of 5-MTHF during regeneration of methionine from homocysteine by methionine synthase.
Alterations in purine and sulfur amino acid metabolism further support the interpretation that LTG alters folate metabolism. 5′-Methylthioadenosine (MTA), a nucleoside produced from S-adenosylmethionine that is a critical intermediate in both purine and methionine salvage pathways, was decreased with LTG treatment (Avila et al., 2004). Purine metabolites decreased in association with LTG included inosine, uric acid, 1-(5-phospho-D-ribosyl)-5-amino-4-imidazolecarboxylate and deoxyadenosine, while the nucleotide precursor deoxyguanosine 5′-triphosphate was elevated. Taken together, these results show variations in nucleotide salvage pathways consistent with the observed variations in folate metabolites.
LEV treatment also resulted in metabolic variations with important implications for maternal health and fetus development, although with a different pattern than LTG. LEV-associated metabolites included tryptophan, kynurenine, γ-aminobutyric acid (GABA), dihydrobiopterin (BH2), norepinephrine, and 3,4-dihydroxyphenylglycol. While tryptophan was decreased in PWWE taking either LEV or LTG, kynurenine was increased in association with LEV treatment only. Kynurenine has been identified as a pro-convulsant metabolite in animal models and humans (Munoz-Hoyos et al., 1997; Sharma et al., 2015). The association of neurotransmitter products with LEV, rather than the precursors that were observed in LTG treatment, suggest different metabolic effects for the two drugs. Elevated levels of norepinephrine during pregnancy are indicators of excess stressors and increase risk for spontaneous preterm delivery (Holzman et al., 2009). Additionally, women with epilepsy may have higher rates of preterm labor, although differential risks amongst AEDs have not yet been studied (MacDonald et al., 2015). Plasma levels of GABA were also decreased in response to LEV treatment. GABA inhibition has been observed to impair neurogenesis and cell migration in animal models, resulting in cortical dysplasia and decreased brain volume (Manent et al., 2007).
We acknowledge several limitations of this study. First, the patient cohort is limited to PWWE and will need to be expanded before the findings can be generalized to other treatment groups. Second, only a small number of non-AED treated PWWE were available to compare to the LTG- and LEV-treated groups, and an independent cohort was not available to replicate the findings. Third, possible gestation-dependent pharmacokinetics was not considered. Fourth, this study was focused on known LTG and endogenous metabolites; unidentified metabolites were not extensively characterized. Finally, the results of this study are correlative in nature. We could not account for unknown confounders, nor delineate exact mechanism. Despite these limitations, we identified AED-associated changes to metabolic pathways that are critical for proper neurodevelopment and could contribute to risk for adverse outcomes. The results demonstrate that HRM provides a useful platform to study pharmacometabolomics of AED treatment by PWWE. Further research identifying how AED-induced metabolic alterations are related to maternal seizure control, obstetric and neurodevelopmental outcomes is ongoing, in addition to metabolic characterization of one-carbon metabolism and association with risk of ASD traits.
CONCLUSIONS
Management of PWWE requires the treating physician to balance the risk of poor pregnancy outcomes due to maternal seizures and the risk to the developing fetus from AED exposure. Metabolic changes associated with specific AED therapy provide insight into potential risks during the embryonic and fetal development stages, making possible identification of therapeutic targets to mitigate these effects. This HRM study of plasma obtained from PWWE detected metabolic alterations associated with LTG or LEV therapy, two commonly prescribed AEDs. The results demonstrate that metabolic features associated with AED use included both drug and endogenous metabolites, with the majority exhibiting a dose-dependent relationship. Although detected in maternal plasma, the results suggest alterations to pathways potentially relevant to fetal structural and brain development, including steroid metabolism, neurotransmitter biosynthesis and one-carbon metabolism. While this study was limited to PWWE, the metabolic associations represent the first HRM characterization for PD study of AED treatment. More generally, the results establish feasibility to use metabolic phenotyping to assist in characterizing drug mode-of-action and unintended effects in susceptible human populations. Studies with larger cohort size and an expanded number of therapeutically important AEDs and combinations thereof will be important to develop metabolomics for use in management of PWWE requiring AEDs.
Supplementary Material
HIGHLIGHTS.
Metabolic changes were associated with lamotrigine and levetiracetam monotherapy
Metabolic variations included maternal health and fetal development pathways
Associations with one-carbon, neurotransmitter and steroid metabolism were observed
Folate metabolites were decreased in lamotrigine treatment.
The metabolome provides an integrated measure of anti-epileptic drug pharmacology
Acknowledgements:
This work was supported by funds received from the NIH Common Fund Initiative promoting collaborative activities in metabolomics research (3UO1NS038455-13S1), the National Institute of Environmental Health Sciences (ES019776, ES026560, ES012870), National Institute of Mental Health (MH107205) and the National Institute of Neurological Disorders and Stroke and Eunice Kennedy Shriver National Institute of Child Health and Human Development (NS038455, NS050659). Funding sources did not direct the study.
Abbreviations:
- 5-HTTP
5-hydroxytryptophan
- 5-MTHF
5-methyltetrahydrofolate
- AED
Anti-epileptic drug
- ASD
Autism Spectrum Disorder
- BH2
Dihydrobiopterin
- BH4
Tetrahydrobiopterin
- CV
Coefficient of Variation
- FDR
False discovery rate
- GA
Gestational age
- GABA
Aminobutyric acid
- HCA
Hierarchical cluster analysis
- HILIC
Hydrophilic interaction chromatography
- HMDB
Human metabolome data base
- HRM
High-resolution metabolomics
- IRB
Institutional review board
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- LC-HRMS
Liquid chromatography with high resolution mass spectrometry
- LEV
Levetiracetam
- LTG
Lamotrigine
- MTA
5′-Methylthioadenosine
- MWAS
Metabolome-wide association study
- PWWE
Pregnant women with epilepsy
- SAM
S-adenosylmethionine
- THF
Tetrahydrofolate
Footnotes
Potential conflicts of interest: Dr. Meador has received research support from Sunovion Pharmaceuticals and travel support from UCB Pharma. The Epilepsy Study Consortium pays Dr. Meador’s university for his research consultant time related to Eisai, GW Pharmaceuticals, NeuroPace, Novartis, Supernus, Upsher-Smith Laboratories, UCB Pharma, and Vivus Pharmaceuticals. All remaining authors declare no actual or potential conflicts of interests.
REFERENCES
- Arck PC, Rucke M, Rose M, Szekeres-Bartho J, Douglas AJ, Pritsch M, Blois SM, Pincus MK, Barenstrauch N, Dudenhausen JW, Nakamura K, Sheps S, Klapp BF, 2008. Early risk factors for miscarriage: a prospective cohort study in pregnant women. Reprod Biomed Online 17, 101–113. [DOI] [PubMed] [Google Scholar]
- Avila MA, Garcia-Trevijano ER, Lu SC, Corrales FJ, Mato JM, 2004. Methylthioadenosine. The international journal of biochemistry & cell biology 36, 2125–2130. [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S, 2015. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software 67. [Google Scholar]
- Belelli D, Herd MB, Mitchell EA, Peden DR, Vardy AW, Gentet L, Lambert JJ, 2006. Neuroactive steroids and inhibitory neurotransmission: mechanisms of action and physiological relevance. Neuroscience 138, 821–829. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met 57, 289–300. [Google Scholar]
- Bjork M, Riedel B, Spigset O, Veiby G, Kolstad E, Daltveit AK, Gilhus NE, 2018. Association of Folic Acid Supplementation During Pregnancy With the Risk of Autistic Traits in Children Exposed to Antiepileptic Drugs In Utero. JAMA neurology 75, 160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobo WV, Davis RL, Toh S, Li DK, Andrade SE, Cheetham TC, Pawloski P, Dublin S, Pinheiro S, Hammad T, Scott PE, Epstein RA Jr., Arbogast PG, Morrow JA, Dudley JA, Lawrence JM, Avalos LA, Cooper WO, 2012. Trends in the use of antiepileptic drugs among pregnant women in the US, 2001-2007: a medication exposure in pregnancy risk evaluation program study. Paediatr Perinat Epidemiol 26, 578–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Chiou HY, Lin HC, Lin HL, 2009. Affect of seizures during gestation on pregnancy outcomes in women with epilepsy. Arch Neurol 66, 979–984. [DOI] [PubMed] [Google Scholar]
- Crider KS, Yang TP, Berry RJ, Bailey LB, 2012. Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate’s role. Adv Nutr 3, 21–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go YM, Walker DI, Liang Y, Uppal K, Soltow QA, Tran V, Strobel F, Quyyumi AA, Ziegler TR, Pennell KD, Miller GW, Jones DP, 2015. Reference Standardization for Mass Spectrometry and High-resolution Metabolomics Applications to Exposome Research. Toxicological sciences : an official journal of the Society of Toxicology 148, 531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go YM, Walker DI, Soltow QA, Uppal K, Wachtman LM, Strobel FH, Pennell K, Promislow DE, Jones DP, 2014. Metabolome-wide association study of phenylalanine in plasma of common marmosets. Amino acids. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeden N, Velasquez J, Arnold KA, Chan Y, Lund BT, Anderson GM, Bonnin A, 2016. Maternal Inflammation Disrupts Fetal Neurodevelopment via Increased Placental Output of Serotonin to the Fetal Brain. J Neurosci 36, 6041–6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden CL, Meador KJ, Pennell PB, Hauser WA, Gronseth GS, French JA, Wiebe S, Thurman D, Koppel BS, Kaplan PW, Robinson JN, Hopp J, Ting TY, Gidal B, Hovinga CA, Wilner AN, Vazquez B, Holmes L, Krumholz A, Finnell R, Hirtz D, Le Guen C, American Academy of N., American Epilepsy, S., 2009. Practice parameter update: management issues for women with epilepsy--focus on pregnancy (an evidence-based review): teratogenesis and perinatal outcomes: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology 73, 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden CL, Pennell PB, 2013. Neuroendocrine considerations in the treatment of men and women with epilepsy. The Lancet. Neurology 12, 72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Diaz S, Smith CR, Shen A, Mittendorf R, Hauser WA, Yerby M, Holmes LB, North American, A.E.D.P.R., North American, A.E.D.P.R., 2012. Comparative safety of antiepileptic drugs during pregnancy. Neurology 78, 1692–1699. [DOI] [PubMed] [Google Scholar]
- Hill M, Vrbikova J, Zarubova J, Kancheva R, Velikova M, Kancheva L, Kubatova J, Duskova M, Marusic P, Parizek A, Starka L, 2011. The steroid metabolome in lamotrigine-treated women with epilepsy. Steroids 76, 1351–1357. [DOI] [PubMed] [Google Scholar]
- Holzman C, Senagore P, Tian Y, Bullen B, Devos E, Leece C, Zanella A, Fink G, Rahbar MH, Sapkal A, 2009. Maternal catecholamine levels in midpregnancy and risk of preterm delivery. American journal of epidemiology 170, 1014–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WE, Li C, Rabinovic A, 2007. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 8, 118–127. [DOI] [PubMed] [Google Scholar]
- Jones DP, 2016. Sequencing the exposome: A call to action. Toxicology Reports 3, 29–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DP, Park Y, Ziegler TR, 2012. Nutritional metabolomics: progress in addressing complexity in diet and health. Annual review of nutrition 32, 183–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M, 2012. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic acids research 40, D109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinberg S, Hripcsak G, 2011. A review of causal inference for biomedical informatics. Journal of biomedical informatics 44, 1102–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Park Y, Duraisingham S, Strobel FH, Khan N, Soltow QA, Jones DP, Pulendran B, 2013. Predicting network activity from high throughput metabolomics. PLoS computational biology 9, e1003123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KH, Walker DI, Uppal K, Tran V, Rohrbeck P, Mallon TM, Jones D, 2016. High resolution metabolomics assessment of military personnel. Journal of Occupational and Environmental Medicine Submitted; DoD Biomarkers Supplement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald SC, Bateman BT, McElrath TF, Hernandez-Diaz S, 2015. Mortality and Morbidity During Delivery Hospitalization Among Pregnant Women With Epilepsy in the United States. JAMA neurology 72, 981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manent JB, Jorquera I, Mazzucchelli I, Depaulis A, Perucca E, Ben-Ari Y, Represa A, 2007. Fetal exposure to GABA-acting antiepileptic drugs generates hippocampal and cortical dysplasias. Epilepsia 48, 684–693. [DOI] [PubMed] [Google Scholar]
- Meador K, Pennell P, May R, Gerard E, Kalayjian L, Velez-Ruiz N, Penovich P, Cavitt J, French J, Hwang S, Pack A, Sam M, Moore E, Ippolito D, 2016. Antiepileptic Drug Prescribing Patterns in Pregnant Women with Epilepsy: Findings from the MONEAD Study; Abstract #1.211, American Epilepsy Society Annual Meeting https://www.aesnet.org/meetings_events/annual_meeting_abstracts/view/2421479, Houston, TX, pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador KJ, Baker GA, Browning N, Cohen MJ, Bromley RL, Clayton-Smith J, Kalayjian LA, Kanner A, Liporace JD, Pennell PB, Privitera M, Loring DW, Group, N.S., 2013. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. The Lancet. Neurology 12, 244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon SH, 2007. Neurosteroid regulation of central nervous system development. Pharmacol Ther 116, 107–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL, 1998. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281, 1191–1193. [DOI] [PubMed] [Google Scholar]
- Munoz-Hoyos A, Molina-Carballo A, Rodriguez-Cabezas T, Uberos-Fernandez J, Ruiz-Cosano C, Acuna-Castroviejo D, 1997. Relationships between methoxyindole and kynurenine pathway metabolites in plasma and urine in children suffering from febrile and epileptic seizures. Clin Endocrinol (Oxf) 47, 667–677. [DOI] [PubMed] [Google Scholar]
- Pennell PB, 2005. Using current evidence in selecting antiepileptic drugs for use during pregnancy. Epilepsy Curr 5, 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell PB, 2016. Use of Antiepileptic Drugs During Pregnancy: Evolving Concepts. Neurotherapeutics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Ishizuka B, Quigley ME, Yen SS, 1983. Effects of tyrosine and tryptophan ingestion on plasma catecholamine and 3,4-dihydroxyphenylacetic acid concentrations. The Journal of clinical endocrinology and metabolism 57, 760–763. [DOI] [PubMed] [Google Scholar]
- Registry NAAP, 2014. Trends in AED monotherapy use, http://www.aedpregnancyregistry.org/wp-content/uploads/newsletter_fall_2014.pdf, pp.
- Roth C, Magnus P, Schjolberg S, Stoltenberg C, Suren P, McKeague IW, Davey Smith G, Reichborn-Kjennerud T, Susser E, 2011. Folic acid supplements in pregnancy and severe language delay in children. JAMA : the journal of the American Medical Association 306, 1566–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl TO, Johnson WG, 2000. Folic acid: influence on the outcome of pregnancy. Am J Clin Nutr 71, 1295S–1303S. [DOI] [PubMed] [Google Scholar]
- Sharma M, Anand C, Chugani DC, 2015. Role of the Kynurenine Pathway in Epilepsy In Mittal S, (Ed.), Targeting the Broadly Pathogenic Kynurenine Pathway. Springer International Publishing, Cham, pp. 205–213. [Google Scholar]
- Simon-Manso Y, Lowenthal MS, Kilpatrick LE, Sampson ML, Telu KH, Rudnick PA, Mallard WG, Bearden DW, Schock TB, Tchekhovskoi DV, Blonder N, Yan X, Liang Y, Zheng Y, Wallace WE, Neta P, Phinney KW, Remaley AT, Stein SE, 2013. Metabolite profiling of a NIST Standard Reference Material for human plasma (SRM 1950): GC-MS, LC-MS, NMR, and clinical laboratory analyses, libraries, and web-based resources. Analytical chemistry 85, 11725–11731. [DOI] [PubMed] [Google Scholar]
- Su G, Morris JH, Demchak B, Bader GD, 2014. Biological network exploration with cytoscape 3. Current protocols in bioinformatics / editoral board, Baxevanis Andreas D. … [et al. ] 47, 8 13 11-18 13 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomson T, Battino D, Bonizzoni E, Craig J, Lindhout D, Sabers A, Perucca E, Vajda F, group, E.s., 2011. Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. The Lancet. Neurology 10, 609–617. [DOI] [PubMed] [Google Scholar]
- Uppal K, Soltow QA, Promislow DE, Wachtman LM, Quyyumi AA, Jones DP, 2015. MetabNet: An R Package for Metabolic Association Analysis of High-Resolution Metabolomics Data. Frontiers in bioengineering and biotechnology 3, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppal K, Soltow QA, Strobel FH, Pittard WS, Gernert KM, Yu T, Jones DP, 2013. xMSanalyzer: automated pipeline for improved feature detection and downstream analysis of large-scale, non-targeted metabolomics data. BMC bioinformatics 14, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppal K, Walker DI, Jones DP, 2016a. xMSannotator: an R package for network-based annotation of high-resolution metabolomics data. Analytical chemistry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppal K, Walker DI, Jones DP, 2017. xMSannotator: An R Package for Network-Based Annotation of High-Resolution Metabolomics Data. Analytical chemistry 89, 1063–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppal K, Walker DI, Liu K, Li S, Go YM, Jones DP, 2016b. Computational metabolomics: a framework for the million metabolome. Chemical research in toxicology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppal K, Walker DI, Liu K, Li S, Go YM, Jones DP, 2016c. Computational Metabolomics: A Framework for the Million Metabolome. Chemical research in toxicology 29, 1956–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrotti Alberto, A., D’Egidio, Verrotti Agostinelli, Pavone P, 2012. Diagnosis and management of catamenial seizures: a review. International Journal of Women’s Health, 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voinescu PE, Pennell PB, 2017. Delivery of a Personalized Treatment Approach to Women with Epilepsy. Semin Neurol 37, 611–623. [DOI] [PubMed] [Google Scholar]
- Walker DI, Go Y, Liu K, Pennell K, Jones D (Eds.), 2016a. Population Screening for Biological and Environmental Properties of the Human Metabolic Phenotype: Implications for Personalized Medicine. Elsevier. [Google Scholar]
- Walker DI, Mallon CT, Hopke PK, Uppal K, Go YM, Rohrbeck P, Pennell KD, Jones DP, 2016b. Deployment-Associated Exposure Surveillance With High-Resolution Metabolomics. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine 58, S12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DI, Uppal K, Zhang L, Vermeulen R, Smith M, Hu W, Purdue MP, Tang X, Reiss B, Kim S, Li L, Huang H, Pennell KD, Jones DP, Rothman N, Lan Q, 2016c. High-resolution metabolomics of occupational exposure to trichloroethylene. International journal of epidemiology 45, 1517–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu Y, Djoumbou Y, Mandal R, Aziat F, Dong E, Bouatra S, Sinelnikov I, Arndt D, Xia J, Liu P, Yallou F, Bjorndahl T, Perez-Pineiro R, Eisner R, Allen F, Neveu V, Greiner R, Scalbert A, 2013. HMDB 3.0--The Human Metabolome Database in 2013. Nucleic acids research 41, D801–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Park Y, Johnson JM, Jones DP, 2009. apLCMS--adaptive processing of high-resolution LC/MS data. Bioinformatics 25, 1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Park Y, Li S, Jones DP, 2013. Hybrid feature detection and information accumulation using high-resolution LC-MS metabolomics data. Journal of proteome research 12, 1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.