Highlights
-
•
A 195 nt fragment of the nsP4 non-structural protein gene of alphaviruses was amplified in several frugivorous bats.
-
•
Venezuelan equine encephalitis virus RNA was detected Artibeus planirostris and Sturnira lilium from Cordoba – Colombia.
-
•
Frugivorous bats from the Caribbean area of Colombian may be involved in the Venezuelan equine encephalitis virus enzootic cycle.
Keywords: Tropics, Ecology disease, Alphavirus, Public health
Abstract
Alphavirus infection associated encephalitis is an emerging infectious disease with a high impact on public health in Latin America.
Objective
To study the eco-epidemiology of alphaviruses in bats of departments of Córdoba and Sucre, Colombia.
Methodology
A prospective descriptive cross-sectional study with a non-probabilistic sampling, in 12 localities of Córdoba and Sucre was carried out. Using mist nets capture of the specimens was carried out. The size of the sample was 286 bats, each specimen captured was taxonomically classified. The bats were immobilized with anesthetic and analgesic treatment according to the ethics committee of the University of Córdoba, morphometric measurements and blood samples were taken, later they were necropsied in the field to obtain a collection of tissues which were preserved in liquid N2 −190 °C. The averages of the climatic conditions of the sampling sites were extracted from the WorldClim database (http://www.worldclim.org/). The open source software QGIS (Quantum GIS Development Team.2015) was used to map and visualize bioclimatic regions of Córdoba. We used descriptive and retrospective information about the equine population and reports of foci of equine encephalitis.
Results
In Córdoba and Sucre, 286 bats were captured and 23 species were classified, Artibeus and Phyllostomus discolor were the most frequent captured genus. The geographic ranges of the captured species were variable, some had a wide distribution and others were restricted to some areas. Venezuelan equine encephalitis virus RNA was detected in Artibeus planirostris and Sturnira lilium (2/286 = 0.70%) from Cordoba – Colombia. The univariate descriptive analysis showed no significant association for any of the analyzed variables climatic.
Conclusions
Frugivorous bats from the Caribbean area of Colombia may be involved in the Venezuelan equine encephalitis virus enzootic cycle.
1. Introduction
The Venezuelan equine encephalitis virus (VEEV) belongs to the genus of alphaviruses (Togaviridae) with enzootic and epizootic serotypes. Within this same group are the equine encephalitis viruses of the East and West, Mayaro, Mucambo and Everglades (Zacks and Paessler, 2010; Gardner et al., 2016). VEEV is an emerging infectious disease in Latin America (Aguilar et al., 2011; Vittor et al., 2016). The outbreaks have been reported for decades in countries with enzootic circulation, the implementation of surveillance systems has allowed the detection of additional human cases in countries and areas with previously unknown VEEV activity. The enzootic subtypes of VEEV are frequently detected and isolated in ecological habitats, where they circulate in transmission cycles between rodents and mosquitoes. The main vertebrate reservoirs are rodents of wild species of Oryzomys, Zigodontomys, Heteromys, Peromyscus and Proechimys. These animals become infected in nature and develop viremia that is sufficient to infect the vectors (Johnson and Martin, 1974; Deardorff and Scott, 2010).
Bats, the only flying mammals are abundant and diverse, geographically extended on all continents except at the poles. They provide important eco-systemic services, such as pollination, seed dispersal and insect control among others. However, some bat species are capable of transmitting pathogenic viruses such as rabies and possibly Ebola and coronaviruses. Bats harbor more zoonotic viruses per species than rodents and are now recognized as an important source of zoonotic agents (Luis et al., 2013). Recently some viruses associated with bats have been identified that are closely related to human pathogens, including hepacivirus, pegivirus (Quan et al., 2013), influenza A virus (Tong et al., 2012), hantavirus (Guo et al., 2013) and paramyxoviruses such as mumps and respiratory syncytial virus (Drexler et al., 2012). Arenavirus (Malmlov et al., 2017). Bats are also important reservoirs; different studies have reported VEEV infection which suggests that bats could host arboviruses of public health impact in America (Correa et al., 1972; Seymour et al., 1978; Thompson et al., 2015; Sotomayor-Bonilla et al., 2017). Equines are usually susceptible to the virus and the fatality rate for horses is between 20 to 80% (Gil et al., 2017). The epidemic/epizootic types include the IAB and IC subtypes, both of which are responsible for large outbreaks of disease in humans and equines. The other subtypes, or enzootic viruses such as type ID, cause disease in humans and usually do not affect equines (Forrester et al., 2017; Gupta et al., 2017). Equines can serve as amplifiers of the epizootic form of viruses; and in this way they become the source of infection of thousands of mosquitoes during the febrile period. (Forrester et al., 2017). Culicidae mosquitoes (Diptera: Culicidae) occupy a preferential position due to their obligate hematophagy, maximum adaptability to multiple environments at different latitudes and altitudes, and a great variability of preferred hosts from which they can feed and in those that can spread the viruses. Some efficient vectors can be arthropods of the genera Aedes, Anopheles, Culex, Mansonia Psorophora and probably Ochlerotatus. The objective of the present study was to study the eco-epidemiology of alphaviruses in bats of departments of Córdoba and Sucre, Colombia.
2. Materials and methods
2.1. Type of study and sample size
Between 2015–2017 a prospective descriptive cross-sectional study was carried out. A non-probabilistic sampling was carried out and 286 bats were captured. The geographical areas of Córdoba and Sucre departments of the Colombian Caribbean area were chosen; a total of 12 capture sites were selected, 8 in Córdoba and 4 in Sucre. The sites were chosen taking into account information such as the equine population and the consolidated case reports of the Institute Colombiano Agropecuario (ICA) about epidemiological situation of equine encephalitis and the human cases reported by the Colombian National Institute of Health (Instituto Nacional de Salud, 2017).
2.2. Geographic areas of the study
Two departments of the Colombian Caribbean were chosen, Córdoba and Sucre. In the department of Córdoba, the Sinú River measures 415 km and runs through the department of Córdoba from South to North and flows into the Caribbean Sea, on this geographical features the department is divided into Alto Sinú, Medio Sinú and Bajo Sinú. Based on this territorial division, eight sampling sites were geographically chosen; in the Alto Sinú three municipalities were selected: Montelibano, Buenavista and Tierralta; in the Medio Sinú the municipalities of Montería and San Carlos, in the Bajo Sinú subregion of the Caribbean coast, the municipalities of Canalete and Puerto Escondido. In addition to the Sinú River, in the department of Córdoba there is another river the Rio San Jorge with a length of 368 km; in its main municipality Ayapel the other sampling site was placed. In the department of Sucre, four sampling sites were geographically divided, in the sub-region of the Caribbean coast, the municipality of Coloso; sub-region of the Sincelejo Savannas and in the sub-region of the La Mojana River the municipalities of San Marcos and Majagual. In total there were 12 capture sites, 8 in Córdoba and 4 in Sucre.
2.3. Capture of bats
The mist nets (6 m × 2 m) were placed in places near water sources, forests, wetlands, tree plantations, croplands and pastures, livestock corrals and sites near rural residences. The nets were placed between 18:00–3:00 h were reviewed every 15 min to collect the specimens, the taxonomic identification was carried out by means of standard morphometric data such as: total length, tail length, leg and forearm length (Fernández et al., 1988; Linares, 2002). Biological data were collected such as sex, reproductive status, relative age, weight and presence of ectoparasites. The data was geo-referenced and entered into an excel database.
2.4. Ethical aspects and tissue obtaining
The procedures of capture, manipulation, euthanasia and identification of the biological material were previously approved by the Ethics Committee of the Faculty of Veterinary Medicine of the University of Córdoba (Resolution 029 of June 13th, 2014). The authorization for scientific research in biological diversity was also obtained, which involves activities of collection, capture, hunting, fishing, biological resource manipulation at the University of Córdoba through resolution 00914 of August 4, 2017. To avoid animal suffering, Chiroptera were sedated with acetylpromazine-ketamine (0.02 mg / g-0.05 mg / g), then, an euthanasia with an overdose of sodium pentobarbital (200 mg) at a dose of 0.05 mg/g was performed. The necropsy was carried out at the capture site and sample tissues from brain, heart, lung, spleen, liver and kidneys were obtained. Dissections were made at the capture site with the use of biosecurity equipment and materials compulsory for this type of study (Mills et al., 1995). To preserve the species, specimens in danger of extinction and pregnant or lactating females were released. The tissues and organs were conserved in liquid N2 at −196 °C and transported to the Institute of Biological Research of the Tropics (IIBT) where they were stored at −80 °C until molecular analysis was performed.
2.5. Molecular methods
RNA extraction was done with Trizol™ (Invitrogen) following the manufacturer's protocol (Chomczynski, 1993). The cDNA synthesis was done with the reverse transcriptase enzyme M-MLV ™ (Invitrogen) using random primers. The reverse transcription reaction was performed in a single cycle at 42 °C using the M-MLV RT. An RT-PCR-nested fragment of the nsP4 gene encoding the alpha-virus polymerase was amplified, using the primers Alpha1 +, Alpha1 − and Alpha2 +, Alpha2- (Invitrogen™) proposed by Sánchez-Seco et al. (2001). As a control of species and internal control, complementary primers were used to a sequence of a mitochondrial gene mt DNA from bats (Ramírez et al., 2014), as a positive control the lyophilized vaccine prepared with attenuated virus of equine encephalomyelitis was used. TC83; and as a negative control water molecular grade. The positive samples were reamplified and the amplicons were sequenced by the Sanger method (Sanger et al., 1977).
2.6. Geographic information system (GIS) and climate analysis
The unit of spatial analysis used were the departments of Córdoba and Sucre and the environmental variables were temperature, precipitation and elevation, obtained from the Worldclim world climate data website (Hijmans et al., 2014). An integrating database was constructed to characterize the diversity of vector-host associations in space and time. The averages of the climatic conditions of the sampling sites were extracted from the WorldClim database (http://www.worldclim.org/). The resolution proposed by WorldClim was used as 30 s of arc (1 km). An open source software, QGIS (Quantum GIS Development Team, 2015), was used to produce maps with the bioclimatic regions of Córdoba (Colombia). The statistical analyzes were carried out in the program R version 3 (R Core Team, 2013), as part of the methodology descriptive and retrospective information was used.
3. Results
23 species of bats were captured distributed in 18 genera and 6 families, 69% of the specimens were captured in Córdoba and 31% in Sucre; the number of males was 53% and females 47%. According to the composition of the trophic groups for the localities studied, six clusters were found, in which frugivorous predominated with 69.57%, followed by opportunistic omnivorous with 15.03%, insectivores with 9.8%, nectarivorous with 2.10%, piscivorous with 2.10%, hematophagous with 1.40% and carnivorous 0.35%. Table 1 shows the distribution of bats species, dietary habits and gender
Table 1.
Distribution of bats species, dietary habits and gender.
| Genus and species | Dietary habits | # of captures |
Quantity | % | |
|---|---|---|---|---|---|
| Males | Female | ||||
| Artibeus lituratus | Frugivorous | 19 | 11 | 30 | 10,48 |
| Artibeus planirostris | Frugivorous | 53 | 46 | 99 | 34,61 |
| Carollia brevicauda | Frugivorous | 1 | 0 | 1 | 0,35 |
| Carollia perspicillata | Frugivorous | 26 | 12 | 38 | 13,29 |
| Uroderma bilobatum | Frugivorous | 5 | 6 | 11 | 3,85 |
| Sturnira lilium | Frugivorous | 10 | 10 | 20 | 6,99 |
| Phyllostomus discolor | Omnivorous | 15 | 27 | 42 | 14,68 |
| Trachops cirrhosus | Omnivorous | 0 | 1 | 1 | 0,35 |
| Carollia castanea | Insectivorous | 0 | 1 | 1 | 0,35 |
| Eptesicus brasilensis | Insectivorous | 0 | 1 | 1 | 0,35 |
| Eumops glaucinus | Insectivorous | 1 | 0 | 1 | 0,35 |
| Lasiurus ega | Insectivorous | 1 | 0 | 1 | 0,35 |
| Micronycteris microtis | Insectivorous | 0 | 1 | 1 | 0,35 |
| Molossops temminckii | Insectivorous | 1 | 0 | 1 | 0,35 |
| Molossus molossus | Insectivorous | 10 | 4 | 14 | 4,90 |
| Myotis nigricans | Insectivorous | 1 | 0 | 1 | 0,35 |
| Saccopteryx leptura | Insectivorous | 0 | 1 | 1 | 0,35 |
| Saccopteryx bilineata | Insectivorous | 0 | 4 | 4 | 1,40 |
| Rhogeessa yo | Insectivorous | 1 | 1 | 2 | 0,70 |
| Desmodus rotundus | Hematophagous | 2 | 2 | 4 | 1,40 |
| Glossophaga soricina | Nectarivorous | 3 | 3 | 6 | 2,10 |
| Noctilio albiventris | Piscivorous | 1 | 2 | 3 | 1,05 |
| Noctilio leporinus | Piscivorous | 2 | 1 | 3 | 1,05 |
| 23 | 152 (53,14%) | 134 (46,85%) | 286 | 100,00 | |
Venezuelan equine encephalitis virus RNA was detected in A. planirostris and S. lilium (2/286 = 0.70%) from Cordoba – Colombia. Table 2 shows the different municipalities and the number of specimens captured in the departments of Córdoba and Sucre.
Table 2.
Different municipalities and the number of specimens captured in the departments of Córdoba and Sucre.
| Departments | Municipalities | Captured specimens | Length | Latitude |
|---|---|---|---|---|
| Córdoba | Montería | 25 | 8°34´9´´ | 75°43´6´´ |
| Canalete | 25 | 8°47´26,1´´ | 76°14´16´´ | |
| Tierralta | 25 | 8°03´43,9´´ | 76°9´35,9´´ | |
| Buenavista | 25 | 8°16´32´´ | 75°24’55’’ | |
| Ayapel | 25 | 8°17´53,8´´ | 75°9´20,6´´ | |
| Puerto Escondido | 25 | 9°03´46,9´´ | 76°11´35,8´´ | |
| Momil | 25 | 9°03´44,6´´ | 76°11´27,6´´ | |
| San Carlos | 25 | 8°44´40,7´´ | 75°39´00,9´´ | |
| Sucre | Majagual | 25 | 8°32´35,7´´ | 74°34´31,7´´ |
| Sincelejo | 15 | 9°19´21,9´´ | 75°26´22,9´´ | |
| Coloso | 26 | 9°29´59,2´´ | 75°20´54,3´´ | |
| San Marcos | 21 | 8°42´57,5´´ | 75°16´10,0´´ | |
| Total | 12 sites | 286 | ||
Regarding the analysis of equines and human cases of VEEV, the ICA in 2017 reported two outbreaks in equines in the department of Córdoba, a case of VEEV in Canalete and another in Puerto Libertador (Instituto Nacional de Salud, 2017). On the other hand, the National Health Institute (Instituto Nacional de Salud, 2017) reported 17 human cases of VEEV all in departments close to Venezuelan’s border (Table 3 ).
Table 3.
Human cases of Venezuelan Equine Encephalitis, reported in Colombia by the National Health Institute, 2017.
| Department of origin | Cases | Relative frequency |
|---|---|---|
| Norte de Santander | 12 | 70,5 |
| Santander | 2 | 11,8 |
| Arauca | 1 | 5,9 |
| Guajira | 1 | 5,9 |
| Imported from Venezuela | 1 | 5,9 |
| Total | 17 | 100 |
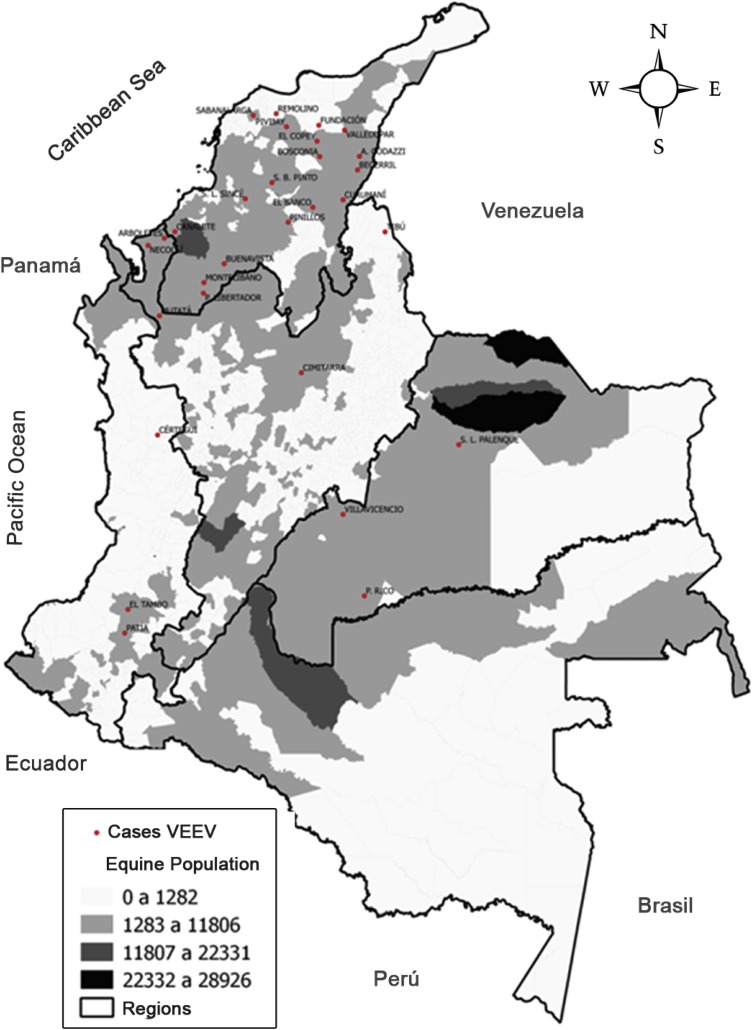
58.8% of cases of VEEV belonged to male sex; 76.5% of the human cases came from rural areas. Regarding age, 50.0% belonged to the group of 5 to 9 years. Fig. 1 shows, the number of cases of equine encephalitis reported by the ICA during the period comprised between 2014-2017 versus the equine population (ICA, 2014–2017).
Fig. 1.
Equine population and cases of Venezuelan equine encephalitis, Colombia 2014–2017. The Quitar regions color densities indicate the equine population ranges.
4. Discussion
To study encephalitis, we must take into account the epidemiological behavior, transmission cycles, etiological agent, transmission mechanisms (wild vertebrates, equids, mosquito vectors and humans), ecological and demographic characteristics. Equines are amplifiers of the virus and for this reason it is an important element to prevent the risk in humans. The department of Córdoba has the second largest equine population in Colombia, this issue is important because the prevention of disease in equines is fundamental through periodic vaccination. The ICA performs surveillance and control of equine encephalitis in susceptible animals through a national official control program and coordinates actions with public health authorities for the prevention of encephalitis in the human population (INS, 2017). However, despite these controls, cases of equine encephalitis occur throughout the Colombian Caribbean region. On the other hand, Colombian biodiversity is recognized worldwide, the country hosts the largest number of bat species in neotropical ecosystems, approximately 13% of all species in the world (Gardner, 2007).
In the Colombian Caribbean, several species of small mammals cohabit with the vectors. The imbalance in associated ecosystems associated with deforestation, human colonization and urbanization of new areas, could contribute to the emergence of new arbovirus outbreaks. The present study shows the first molecular evidences of the circulation in a natural form of Venezuelan equine encephalitis virus in frugivorous non-haematophagous bats A. planirostris and S. lilium that could be hosts of encephalitis virus. A. planisrostris and S. lillium were the first and fourth most abundant species captured in this study, which are considered as species that do not need strict habitat requirements, species of these genera can be found in high-grade ecosystems with anthropic intervention (Soriano, 2000).
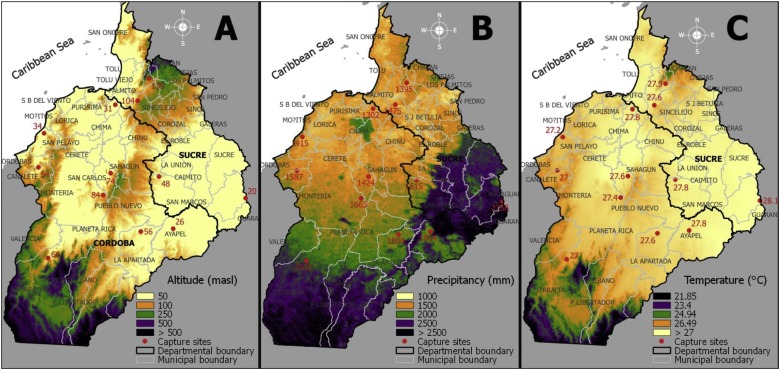
The abundance of frugivorous bats in the study areas is due to the presence of families of cosmopolitan plants that offer food to these species. In this area there are plants with fruits that serve as food to the frugivorous bats, especially of the Piperaceas, Solanaceas and Cecropiaceas groups, which are common species in human interrupted zones and fragments of forests. These families of plants with fruits coincide with other studies of frugivorous bats in Guatemala and Costa Rica (Howell and Burch, 1974, López, 1996, Lou and Yurrita, 2005, Ballesteros et al., 2007), who found that the fruits of the plants of this family are the basis of the diet of the frugivorous bats of Guatemala, Central America and Cordoba, Colombia (Fig. 2 ).
Fig. 2.
Ecological zones and bioclimatic variables of the department of Córdoba. The maps were generated with QGIS Version 2.4 (https://mappinggis.com/2014/07/qgis-2-4-chugiak/).
The 52.17% of frugivorous bats captured were the most abundant trophic group, the variety of families and abundance of specimens, it is because Colombia due to the ecological, climatic and hydric conditions among others, brings as a consequence the mega diversity of species and positions Colombia within the fourteen countries that host the highest index of biodiversity on earth (Andrade, 2011). The dominant abundance of A. planirostris coincides with the characteristic of the species in terms of diet and habitat use (Medellín et al., 2000). Although no association was found between climate variables temperature and precipitation with the presence of natural infection of VEEV and frugivorous bats, ecological work carried out in the study area (Ballesteros and Racero-Casarrubia, 2012) indicated that despite the high degree of human intervention there is abundance of bats.
Bats are considered tolerant to habitats intervened by man and their presence generates discomfort by cohabiting with humans because they could cause public health problems by hosting and possibly amplifying the transmission of VEEV as indicated by the results of the present study.
A study of bats in Madagascar associated to paramyxovirus infection in which a univariate analysis was conducted, found that both abiotic and biotic factors can promote infection by the virus. This study used generalized linear models of infection superimposed on biotic and abiotic variables, demonstrating that the sympatric occurrence of bats is the main factor for the transmission of the virus (Mélade et al., 2016). Although the present study found no association of climatic variables with the presence of the virus in frugivorous bats of the Colombian Caribbean, it is known that these variables influence the vectors.
The Instituto de Investigaciones Biológicas del Trópico conducted an investigation for the surveillance of alphaviruses in mosquitoes in three areas of Córdoba between August 2012 and January 2013, in which Psorofora confinnis were captured, one of the most important species in the epizootic cycle of the VEEV (Ferro et al., 2015), as well as the species Mansonia indubitans, Culex quinquefasciatus, Mansonia titillans, all these species are related to transmission of epizootic strains IAB, VEEV IC (Acha and Szyfres, 2003; Mesa et al., 2005). The species C. nigripalpus, Aedes scapularis, C. vomerifer, C. panocossa, which have been involved in the Americas with the maintenance of enzootic VEEV strains, were also captured during that entomological surveillance. The special importance that Culex (Melanoconion) spp has in the transmission of VEEV has been well documented throughout Latin America. Specifically, C. (Mel.) vomerifer, C. (Mel.) pedroi and C. (Mel.) adamesi have been found infected with the subtype ID in the Magdalena Valley, Colombia (Atasheva et al., 2014; Weaver, & Reisen, 2010; Groot et al., 1996; Dickerman et al., 1986). There is evidence that some species of mosquitoes can feed on bat blood, although the frequency with which this happens in the field is not well understood and difficult to study (Kading and Schountz, 2016).
A study that aimed to determine the source of the blood used as food for two Culiseta mosquito species in New York with respect to the transmission of the eastern equine encephalitis virus, found that Culiseta morsitans and Pipistrellus subflavus mosquitoes they fed on bat’s blood (Molaei et al., 2006). This description comprised less than 1% of the total number of blood tests of this mosquito species. In Papua New Guinea, Anopheles punctulatus mosquitoes were found to be fed blood from two species of frugivorous bats Dobsonia moluccensis and Dobsonia praedatrix (Logue et al., 2016). In Uganda, (Karabatsos, 1985; Kading and Schountz, 2016) identified multiple species of frugivorous bats in C. neavei, C. perfuscus fed bat’s blood. In the species of mosquitoes C. decens, and Coquillettidia fuscopennata, several pathogenic arboviruses were identified in humans, including the Spondweni virus and ZIKAV of C. neavei (Diallo et al., 2014).
On the other hand, climate change and the emergence of infectious diseases are the most important ecological problems of our times (Rohr et al., 2011). In the case of Venezuelan equine encephalitis, mosquitoes are totally dependent on rainfall regimes because they are raised in flooded areas and the size of the population is directly related to the size of the breeding site (Gleiser et al., 2000). With respect to temperature, this can affect the distribution of the vector, the size of the vector, the feeding habits and the extrinsic incubation period (Lifson, 1996). In addition, the temperature has a direct effect on the intrinsic rate of growth since it has been observed that at high temperatures, the growth time is accelerated and the mosquitoes became adults faster (Focks et al., 2000), established a model in which increasing temperature, the females are fed more frequently and the survival rate in the egg stage is reduced. With fewer individuals, there is less larval competition and therefore the females will take better advantage of the resources of the breeding site and may reach a larger size. The main habitats for the principal urban vector species of arboviruses A. aegypti and A. albopictus are propitiated by humans that use water containers for storaging or accumulation of rainwater contribute to population mosquitoes (Morrison et al., 2004). Some local studies show that there is a relationship between precipitation and the abundance of vectors (Scott et al., 2000; Romero-Vivas, & Falconar, 2005), however, we were unable to find it.
5. Conclusions
The detection of Venezuelan equine encephalitis virus in 2 frugivorous bats, make likely the conditions for the appearance of outbreaks of Venezuelan equine encephalitis in Córdoba Colombia.
Formatting of funding sources
This work was supported by the University of Córdoba - Montería. FMVZ-2014 Project.
Acknowledgements
We are grateful to the Universidad de Córdoba (Monteria, Colombia). This study was kindly supported by Special Research Fund for sustainability groups.
References
- Acha P., Szyfres B. Organización Panamericana de la Salud; Washington D.C: 2003. Zoonosis y enfermedades transmisibles comunes al hombre y a los animales: clamidiosis, rickettsiosis y virosis. Volumen II. 3era Edición; p. 425. [Google Scholar]
- Aguilar P.V., Estrada-Franco J.G., Navarro-Lopez R., Ferro C., Haddow A.D., Weaver S.C. Endemic Venezuelan equine encephalitis in the Americas: hidden under the dengue umbrella. Future Virol. 2011;6(6):721–740. doi: 10.2217/FVL.11.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade C.M.G. Estado del conocimiento de la biodiversidad en Colombia y sus amenazas. Consideraciones para fortalecer la interacción ambiente-política. Rev. Acad. Colomb. Cienc. 2011;35(137):491–507. ISNN 0370-3908. [Google Scholar]
- Atasheva S., Kim D.Y., Frolova E.I., Frolov I. Venezuelan equine encephalitis virus variants lacking transcription inhibitory functions demonstrate highly attenuated phenotype. J. Virol. 2014;89:71–82. doi: 10.1128/JVI.02252-14. PMID: 25320296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros J., Racero-Casarrubia J. Murciélagos del área urbana en la ciudad de Montería, Córdoba – Colombia. Rev. MVZ Córdoba. 2012;17(3):3193–3199. [Google Scholar]
- Ballesteros J., Racero J., Núñez M. Diversidad de murciélagos en cuatro localidades de la zona costanera del Departamento de Córdoba-Colombia. Rev. MVZ Córdoba. 2007;12(2):1013–1019. [Google Scholar]
- Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques. 1993;15:532–537. [PubMed] [Google Scholar]
- Correa G.P., Calisher C., Baer G.M. Epidemic strain of Venezuelan equine encephalomyelitis virus from a vampire bat captured in Oxaca Mexico. Science. 1972;175(4021):546–547. doi: 10.1126/science.175.4021.546. [DOI] [PubMed] [Google Scholar]
- Deardorff Eleanor R., Scott C. Weaver vector competence of Culex (Melanoconion) taeniopus for equine-virulent subtype IE strains of Venezuelan equine encephalitis virus. Am. J. Trop. Med. Hyg. 2010;82(6):1047–1052. doi: 10.4269/ajtmh.2010.09-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diallo D., Sall A.A., Diagne C.T., Faye O., Faye O., Ba Y., Hanley K. Zika virus emergence in mosquitoes in southeastern Senegal. PLoS One. 2014;9:e109442. doi: 10.1371/journal.pone.0109442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerman R.W., Cupp E.W., Groot H., Morales Alarcon A., Cura E.N., Dickerman A., Ibagos A.L. 1986. Venezuelan equine encephalitis virus activity in northern Colombia during April and May 1983. PMID: 2879583. [PubMed] [Google Scholar]
- Drexler J.F., Corman V.M., Muller M.A., Maganga G.D., Vallo P., Binger T., Gloza-Rausch F. Bats host major mammalian paramyxoviruses. Nat. Commun. 2012;3:796. doi: 10.1038/ncomms1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández B., Guerrero R., Lord R., Ochoa J., Ulloa G. In: Mamíferos de Venezuela, Lista y claves para su identificación. Museo del Instituto de Zoología Agrícola, editor. Universidad Central de Venezuela; Caracas: 1988. [Google Scholar]
- Ferro C., De las Salas J., González M., Díaz A., Cabrera C., Flórez Z., Duque M.C. Existen condiciones que favorecen la reaparición del virus de la encefalitis equina venezolana en la Alta Guajira colombiana? Biomédica. 2015;35(1):62–72. doi: 10.1590/S0120-41572015000100009. [DOI] [PubMed] [Google Scholar]
- Focks D., Brenner R., Hayes J., Daniels E. Transmission thresholds for dengue in terms of Aedes aegypti pupae per person with discussion of their utility in source reduction efforts. Am. J. Trop. Med. Hyg. 2000;62:11–18. [PubMed] [Google Scholar]
- Forrester N.L., Wertheim J.O., Dugan V.G., Auguste A.J., Lin D. Evolution and spread of Venezuelan equine encephalitis complex alphavirus in the Americas. PLoS Negl. Trop. Dis. 2017;11(8):e0005693. doi: 10.1371/journal.pntd.0005693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner A.L. University of Chicago Press; Chicago: 2007. Mammals of South America, Volume 1. Marsupials, Xenarthrans, Shrews and Bats. [Google Scholar]
- Gardner S.N., McLoughlin K., Be N.A., Allen J., Weaver S.C., Forrester N., Guerbois M. Characterization of genetic variability of Venezuelan equine encephalitis viruses. PLoS One. 2016;11(4) doi: 10.1371/journal.pone.0152604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil P.I., Albrieu-Llinás G., Mlewski E.C., Monetti M., Fozzatti L., Cuffini C., Fernandez Romero J. Pixuna virus modifies host cell cytoskeleton to secure infection. Sci. Rep. 2017:5757. doi: 10.1038/s41598-017-05983-w. www.nature.com/scientificreports Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleiser R., Gorla D., Schelotto G. Population dynamics of Aedes lbifasciatus (Diptera: Culicidae) South of Mar Chiquita Lake, Central Argentina. J. Med. Entomol. 2000;37:21–26. doi: 10.1603/0022-2585-37.1.21. [DOI] [PubMed] [Google Scholar]
- Groot H., Morales A., Romero M., Ferro C., PrõÂas E., Vidales H., Buitrago B. Estudios de arbovirosis en Colombia en la deÂcada de 1970. BiomeÂdica. 1996;16:331. [Google Scholar]
- Guo W.P., Lin X.D., Wang W., Tian J.H., Cong M.L., Zhang H.L., Wang M.R. Phylogeny and origins of hantaviruses harbored by bats, insectivores, and rodents. PLoS Pathog. 2013;9:e1003159. doi: 10.1371/journal.ppat.1003159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P., Sharma A., Han J., Yang A., Bhomia M., Knollmann-Ritschel B., Raj Puri. Differential host gene responses from infection with neurovirulent and partiallyneurovirulent strains of Venezuelan equine encephalitis virus Gupta et al. BMC Infect. Dis. 2017;17:309. doi: 10.1186/s12879-017-2355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijmans R.J., Cameron S., Parra J., Worldclim . University of California; Berkeley: 2014. 2015. Global Climate data.http://www.worldclim.org/ [Internet] Retrieved from. [Google Scholar]
- Howell D., Burch D. Food habits of some Costa Rican bats. Rev. Biol. Trop. 1974;21(2):281–294. [PubMed] [Google Scholar]
- ICA Censo población de equinos. Vigilancia epidemiológ ica, ocurrencia de encefalitis equina, 2014-2017. Retrieved from https://www.ica.gov.cohttp://bit.ly/2pnFooN.
- Instituto Nacional de Salud . 2017. Informe del evento encefalitis equinas hasta el periodo epidemiológico IX, Colombia.https://cruevalle.org/files/PRO-Encefalitis-Equinas.pdf Retrieved from. [Google Scholar]
- Johnson K.M., Martin D.H. Venezuelan equine encephalitis. Classic review providing excellent descriptions of the first documentation of endemic Venezuelan equine encephalitis (VEE), as well as studies of equine virulence and amplification. Adv. Vet. Sci. Comp. Med. 1974;18(0):79–116. PubMed: 4609399. [PubMed] [Google Scholar]
- Kading Rebekah C., Schountz T. Flavivirus infections of bats: potential role in Zika virus ecology review article. Am. J. Trop. Med. Hyg. 2016;95(5):993–996. doi: 10.4269/ajtmh.16-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabatsos N. 3rd edition. vol. 1. American Society of Tropical Medicine and Hygiene; San Antonio, TX: 1985. p. 147. (International Catalogue of Arboviruses). [Google Scholar]
- Lifson A. Mosquitoes, models and dengue. Lancet. 1996;347:1201–1202. doi: 10.1016/s0140-6736(96)90730-8. [DOI] [PubMed] [Google Scholar]
- Linares O. Sociedad Conservacionista Audubon; Venezuela. Venezuela: 2002. Mamíferos de Venezuela. [Google Scholar]
- Logue K., Keven J.B., Cannon M.V., Reimer L., Siba P., Walker E., Zimmerman P.A. Unbiased characterization of Anopheles mosquito blood meals by targeted high-throughput sequencing. PLoS Negl. Trop. Dis. 2016;10:e0004512. doi: 10.1371/journal.pntd.0004512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López J. Universidad de Heredia; Costa Rica: 1996. Hábitos alimentarios de murciélagos frugívoros en la estación biológica “La Selva”, Costa Rica. Tesis de Maestría. [Google Scholar]
- Lou S., Yurrita C. Universidad San Carlos de Guatemala; Guatemala: 2005. Análisis de nicho alimentario en la comunidad de murciélagos frugívoros de Yaxhá, Petén, Guatemala. Escuela de Biología, Facultad de Ciencias Químicas y Farmacia. p. 12. [Google Scholar]
- Luis A.D., Hayman D.T.S., O’Shea T.J., Cryan P.M., Gilbert A.T., Pulliam J.R., Mills J.N. A comparison of bats and rodents as reservoirs of zoonotic viruses: are bats special? Proc. Biol. Sci. 2013;280:20122753. doi: 10.1098/rspb.2012.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmlov A., Seetahal J., Carrington C., Ramkisson V., Foster J., Miazgowicz K.L., Quackenbush S. Serological evidence of arenavirus circulation among fruit bats in Trinidad. PLoS One. 2017;12(9):e0185308. doi: 10.1371/journal.pone.0185308. doi:1371/journal.pone.0185308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medellín R., Equihua M., Amín M. Bat diversity and abundance as indicators of disturbance in neotropical rainforests. Conserv. Biol. 2000;14:1666–1675. doi: 10.1111/j.1523-1739.2000.99068.x. [DOI] [PubMed] [Google Scholar]
- Mélade J., Wieseke N., Ramasindrazana B., Florez O., Lagadec E., Gomard Y., Godman S.M. An eco-epidemiological study of morbilli-related paramyxovirus infection in Madagascar bats reveals host-switching as the dominant macro-evolutionary mechanism. Sci. Rep. 2016;6:23752. doi: 10.1038/srep23752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesa F.A., Cárdenas J.A., Villamil L.C. Universidad Nacional de Colombia Press. Facultad de Medicina Veterinaria y de Zootecnia; 2005. Las encephalitis equinas en la salud pública.http://www.col.ops-oms.org/prevencion/encefalitis/libro_encefalitis.pdf Retrieved from. [Google Scholar]
- Mills J.N., Childs J.E., Ksiazek T.G., Peters C.J. US Department of Health & Human Services, Public Health Service/Centers for Disease Control and Prevention; 1995. Methods for trapping and sampling small mammals for virologic testing. [Google Scholar]
- Molaei G., Oliver J., Andreadis T.G., Armstrong P.M., Howard J.J. Molecular identification of blood meal sources in Culiseta melanura and Culiseta morsitans from an endemic focus of eastern equine encephalitis virus in New York. Am. J. Trop. Med. Hyg. 2006;75:1140–1147. [PubMed] [Google Scholar]
- Morrison A.C., Gray K., Getis A., Astete H., Sihuincha M., Focks D., Watts D. Temporal and geographic patterns of Aedes aegypti (Diptera: Culicidae) production in Iquitos, Peru. J. Med. Entomol. 2004;41:1123–1142. doi: 10.1603/0022-2585-41.6.1123. [DOI] [PubMed] [Google Scholar]
- Quan P.L., Firth C., Conte J.M., Williams S.H., Zambrana-Torrelio C.M., Anthony S.J., Ellison J.A. Bats are a major natural reservoir for hepaciviruses and pegiviruses. Proc. Natl. Acad. Sci. U. S. A. 2013;110:8194–8199. doi: 10.1073/pnas.1303037110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quantum GIS Development Team . 2015. Quantum GIS Geographic Information System. Open Source Geospatial Foundation Project.http://qgis.osgeo.org Retrieved from. [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2013. R: A Language and Environment for Statistical Computing.http://www.R-project.org/ ISBN 3-900051-07-0. Retrieved from. [Google Scholar]
- Ramírez N.N., Alegre E.A., Ruiz R.M., De Biasio M.B., Bastiani C.E. Detección de leptospiras patógenas en tejido renal de murciélagos de Corrientes, Argentina. Rev. Vet. 2014;25(1):16–20. [Google Scholar]
- Rohr J.R., Dobson A.P., Johnson P.T., Kilpatrick A.M., Paull S.H., Raffel T.R. Frontiers in climate change-disease research. Trends Ecol. Evol. 2011;26(6):270–277. doi: 10.1016/j.tree.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Vivas C.M.E., Falconar A.K.I. Investigation of relationships between Aedes aegypti egg, larvae, pupae, and adult density indices where their main breeding sites were located indoors. J. Am. Mosq. Control Assoc. 2005;21:15–21. doi: 10.2987/8756-971X(2005)21[15:IORBAA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Sánchez-Seco M., Rosario D., Quiroz E., Guzmán G., Tenorio A. A generic nested-RT-PCR followed by sequencing for detection and identification of members of the Alphavirus genus. J. Virol. Methods. 2001;95(1–2):153–161. doi: 10.1016/s0166-0934(01)00306-8. [PubMed] [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. U. S. A. 1977;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott T.W., Amerasinghe P.H., Morrison A.C., Lorenz L.H., Clark G., Strickman D., Kittayapong Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: blood feeding frequency. J. Med. Entomol. 2000;37:89–101. doi: 10.1603/0022-2585-37.1.89. [DOI] [PubMed] [Google Scholar]
- Seymour C., Dickeman R.W., Martin M.S. Venezuelan encephalitis virus infection in Neo tropical bats. I. Natural infection in a Guatemala enzootic focus. Am. J. Trop. Med. Hyg. 1978;2(1):290–296. doi: 10.4269/ajtmh.1978.27.290. [DOI] [PubMed] [Google Scholar]
- Soriano P. Functional structure of bat communities in tropical rainforest rainforest and Andean Cloud Forest. Ecotropicos. 2000;13(1):1–20. [Google Scholar]
- Sotomayor-Bonilla J., Abella C., Chaves A., Álvarez P., Rico-Chávez O., Ibáñez S., Rostal M.K. Potential sympatric vectors and mammalian hosts of Venezuelan equine encephalitis virus in Southern Mexico. J. Wildlife Dis. 2017;53(3):657–661. doi: 10.7589/2016-11-249. [DOI] [PubMed] [Google Scholar]
- Thompson N.N., Auguste A.J., Travassos, da Rosa A.P.A., Carrington C.V.F., Blitvich B.J., Chdee D.D., Tesh R.B. Seroepidemiology of Selected Alphaviruses and Flaviviruses in Bats in Trinidad. Zoonoses Publ. Health. 2015;62:53–60. doi: 10.1111/zph.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong S.X., Li Y., Rivailler P., Conrardy C., Castillo D.A.A., Chen L.M., Recuenco S. A distinct lineage of influenza A virus from bats. Proc. Natl. Acad. Sci. U. S. A. 2012;109:4269–4274. doi: 10.1073/pnas.1116200109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittor A.Y., Armien B., Gonzalez P., Carrera J.P., Dominguez C., Valderrama A., Glass G.E. Epidemiology of emergent Madariaga encephalitis in a region with endemic Venezuelan equine encephalitis: initial host studies and human cross sectional study in Darien, Panama. PLoS Negl. Trop. Dis. 2016;10(4) doi: 10.1371/journal.pntd.0004554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver S.C., Reisen W.K. Present and future arboviral threats. Antivir. Res. 2010;85:328–345. doi: 10.1016/j.antiviral.2009.10.008. PMID: 19857523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacks M.A., Paessler S. Encephalitic alphaviruses. Vet. Microbiol. 2010;140:281–286. doi: 10.1016/j.vetmic.2009.08.023. [PubMed: 19775836] [DOI] [PMC free article] [PubMed] [Google Scholar]