Abstract
Introduction:
Multimonth dispensing (MMD) of antiretroviral treatment (ART) aims to reduce patient-related barriers to access long-term treatment and improve health system efficiency. However, randomized evidence of its clinical effectiveness is lacking. We compared MMD within community ART refill groups (CARGs) vs. standard-of-care facility-based ART delivery in Zimbabwe.
Methods:
A three-arm, cluster-randomized, pragmatic noninferiority trial was performed. Thirty health care facilities and associated CARGs were allocated to either ART collected three-monthly at facility (3MF, control); ART delivered three-monthly in CARGs (3MC); or ART delivered six-monthly in CARGs (6MC). Stable adults receiving ART ≥six months with baseline viral load (VL) <1000 copies/ml were eligible. Retention in ART care (primary outcome) and viral suppression (VS) 12 months after enrollment were compared, using regression models specified for clustering (ClinicalTrials.gov: NCT03238846).
Results:
4800 participants were recruited, 1919, 1335, and 1546 in arms 3MF, 3MC, and 6MC, respectively. For retention, the prespecified noninferiority limit (-3.25%, risk difference [RD]) was met for comparisons between all arms, 3MC (94.8%) vs. 3MF (93.0%), adjusted RD = 1.1% (95% CI: -0.5% to 2.8%); 6MC (95.5%) vs. 3MF: aRD = 1.2% (95% CI: -1.0% to 3.6%); and 6MC vs. 3MC: aRD = 0.1% (95% CI: -2.4% to 2.6%). VL completion at 12 months was 49%, 45%, and 8% in 3MF, 3MC, and 6MC, respectively. VS in 3MC (99.7%) was high and not different to 3MF (99.1%), relative risk = 1.0 (95% CI: 1.0-1.0). VS was marginally reduced in 6MC (92.9%) vs. 3MF, relative risk = 0.9 (95% CI: 0.9-1.0).
Conclusion:
Retention in CARGs receiving three- and six-monthly MMD was noninferior versus standard-of-care facility-based ART delivery. VS in 3MC was high. VS in six-monthly CARGs requires further evaluation.
Keywords: HIV, antiretroviral treatment, differentiated service delivery, multimonth, community ART refill groups, Zimbabwe
INTRODUCTION
In the “treat all” era of antiretroviral treatment (ART) eligibility, differentiated service delivery (DSD) models are critical to better serve the needs of people living with HIV and reduce unnecessary burdens on the health care system, particularly in sub-Saharan Africa, the region having almost 70% of the people living with HIV globally.1,2 Overburdened health systems need to accommodate substantially increased numbers of people requiring ART at a time when resources for health care are constrained globally and there is a severe shortage of professional health workers in the region.3,4 Multimonth dispensing (MMD) of ART is a DSD model that extends the interval between ART refills for clinically stable patients thus reducing frequent facility visits, long patient waiting times and travel costs, and is expected to reduce facility daily patient loads. Data suggest that ART patients would rather attend clinics less frequently and prefer MMD of ART over other DSD model components.5 MMD is expected to result in cost savings to both service providers and patients.6,7 Clinics that are decongested of stable ART patients may be able to increase the rate of new ART initiations to scale-up ART coverage,8 allow clinic resources to be redirected toward ill patients in need of clinic care, and support ART retention by improving clinic efficiency and reducing unnecessary patient waiting times.
Observational studies have suggested that clinical outcomes of patients receiving MMD of ART in sub-Saharan Africa are favorable.9-12 However, the quality of evidence from observational studies of this intervention is rated as very low to low due to selection bias.13,14 Early results from one trial have shown favorable outcomes of MMD within adherence clubs in South Africa,15 but there is little other evidence from large-scale randomized trials of the safety and clinical effectiveness of MMD. Few studies have yet assessed clinical outcomes of community-based ART delivered as infrequently as twice annually with only annual clinical consultations.10 The optimal interval and delivery method for ART in terms of safety and efficacy has not been defined, and robust data to evaluate DSD models are critically required to inform evidence-based policy decisions.13
Zimbabwe has among the highest HIV prevalences in adults worldwide (13.3%), and 1.3 million people are eligible to receive ART in the country.16,17 Community ART refill groups (CARGs) is a community-based DSD model for stable patients that has recently been implemented to facilitate ART access in the community closer to patients homes, decongest clinics and enable peer support, derived from a model developed in Mozambique.18-21 Zimbabwe thus provides an ideal setting to evaluate community-based MMD models. The MMD of ART in CARGs study is among the first cluster-randomized trials to compare the clinical effectiveness of MMD (at three- and six-monthly intervals) delivered within community-based groups compared with standard-of-care facility-based ART delivery. We report results of the primary outcome and two secondary outcomes from this trial.
METHODS
Study design
A three-arm, parallel, unblinded, pragmatic cluster-randomized noninferiority trial using stratified randomization was conducted. A full description of the trial design is published elsewhere.22 Briefly, each arm consisted of ten clusters (health facilities and associated CARGs) as follows:
Control arm: Participants received ART at three-monthly intervals at the facility (arm 3MF).
Intervention arm 1: Participants received ART at three-monthly intervals in CARGs with annual clinic visits and clinical consultations (arm 3MC).
Intervention arm 2: Participants received ART at six-monthly intervals in CARGs with annual clinic visits and clinical consultations (arm 6MC).
Setting and site selection
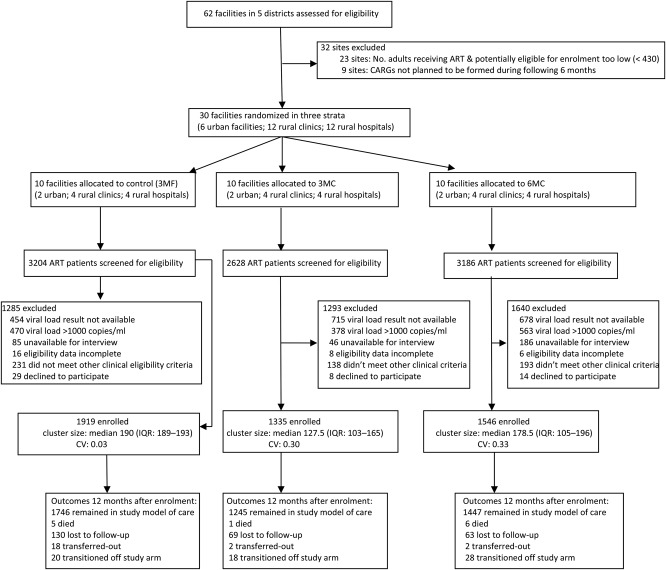
Study facilities were public health facilities in Chitungwiza municipality; Mberengwa district, Midlands province; Masvingo province; and Matebeleland South province. Thirty facilities were selected according to the criteria: supply-chain procedures for implementation of MMD were deemed feasible; implementation of CARGs was deemed feasible or had recently been implemented; routine site data collection systems were adequate; and at least 430 adults were receiving ART (to fulfill site sample size requirements). Of 62 facilities considered, 30 facilities were selected and randomized, all of which completed the study (Figure 1).
Figure 1.
Trial flow diagram. CV, coefficient of variation of cluster size; IQR, interquartile range; CARGs, community ART refill groups; 3MF, participants received three-monthly dispensing of ART at the facility; 3MC, participants received three months’ supply of ART in CARGs; 6MC, participants received 6 months’ supply of ART in CARGs.
Description of interventions
For the study, CARG implementation was aligned to the routine Zimbabwe CARG model,23 with adaptations to allow for extended dispensing intervals. CARGs consisted of 6-12 people, with participants living in a similar geographic location and attending the same health facility. Participants in 3MC and 6MC were recruited from newly formed CARGs (<3 months) in which members had not yet had their first CARG refill meeting. These CARG members received viral load (VL) testing to ascertain stability and eligibility for the study. A CARG leader was nominated, and the CARG met on at least a three-monthly basis at a venue of their choice in the community. Dependent on the individual group need for peer adherence support, CARGs were free to meet more often, but these additional meetings were not compulsory and did not include ART distribution. For the 3MC arm, a single alternating CARG representative collected ART from the facility on a three-monthly basis and distributed the medicines to all other CARG members at the CARG meeting on the same or the following day. Stable 3MC participants were scheduled to receive a clinical consultation and VL test at the facility twelve months after enrollment. All participants from a particular CARG were scheduled to attend this clinic visit on the same day and to collect their ART supply from the clinic at this visit. For the 6MC arm, a single CARG representative collected ART from the facility 6 months after enrollment and distributed to all CARG members at the CARG meeting on the same or the following day. After 12 months, 6MC participants were scheduled to receive a clinical consultation, VL testing and ART supply from the clinic, with all members from a CARG scheduled to attend on the same day. Participants in the control arm (3MF) received clinical consultations and collected their own ART supply at the facility three-monthly. (This schedule differed from national guidelines that recommended only annual clinical consultations for stable facility-based patients).
Cluster allocation
To produce balance in urban and rural facilities as well as hospitals and clinics, a restricted randomization using three strata was conducted,24 i.e., urban facilities, rural hospitals, and rural clinics. Following the stratified randomization, each arm consisted of two urban facilities, four rural hospitals, and four rural clinics. Urban facilities consisted of 5 clinics and one hospital (which was allocated to 6MC).
Outcomes and definitions
The primary outcome was the proportion of participants remaining in ART care 12 months after enrollment by intention-to-treat (ITT). The principal hypothesis was that participant retention in ART care for both intervention arms would be noninferior vs. control (3MF) with a noninferiority limit of -3.25% (risk difference [RD]). An additional hypothesis was that retention in 6MC would be noninferior to 3MC using the same limit. The secondary outcomes reported are the proportions of participants retained in the study arm 12 months after enrollment (retention on the randomized strategy) and the proportions achieving viral suppression (VS) after 12 months.
Retention in ART care was defined as 1-participant attrition, where attrition was defined as either death (all-cause) or loss to follow-up (LTFU). LTFU was defined in all arms as no ART collection for >90 days after the last missed scheduled ART collection date.25,26 Participants not arriving for the scheduled 12-month visit were considered retained if collecting ART within 90 days after the appointment date. For the secondary outcome of retention in the study arm, participants were considered not retained if transitioning off the study arm for any reason including death, LTFU, transfer to another clinic, or required increased ART dispensing frequency. VS was defined as VL <1000 copies/ml.
Participant eligibility criteria and recruitment procedures
The recruitment period was between August 2017 and February 2018. Participant eligibility was aligned to those for stable ART patients in routine settings in Zimbabwe.23 Inclusion criteria were aged ≥18 years; received standard first-line ART for ≥six months; VL <1000 copies/ml at enrollment; weight ≥35 kg; and willing to potentially join a CARG. Exclusion criteria were recent ART tolerability issues; active or suspected tuberculosis; recent, active, or suspected opportunistic infection; received an alternative first-line or second-line ART regimen; active co-morbidities requiring visits to the facility more frequently than six-monthly; confirmed pregnancy; and less than 18 month's postpartum.
Study nurses screened all patients arriving for ART refill visits at study facilities and patients in recently formed CARGs. Potentially eligible patients were invited to participate and to receive VL testing, and eligible patients who provided informed consent were enrolled. All enrolled participants at a particular facility received the same model of care based on the arm to which that facility was allocated.
Sample size estimation
Sample size estimates were calculated for the primary outcome of retention in ART care 12 months after enrollment, for a noninferiority test for the difference in two proportions in a cluster-randomized design. The probability of retention 12 months after enrollment in the control and intervention groups was assumed to be 95%. An intracluster correlation coefficient (ICC) of 0.01 was assumed.27 The noninferiority margin was prespecified as -3.25%. Assuming α = 0.05 and power of 85%, the estimated cluster sample size target was 192 enrolled participants, with 1,920 participants per arm.
Participant follow-up
Defaulter tracking was as per routine site procedures, with no additional tracking for study participants. VL testing was conducted on an annual basis as per routine national guidelines.21 Participants who became pregnant, developed comorbidities, or who had elevated VLs (≥1,000 copies/ml) required clinic follow-up visits more frequently than three-monthly as per national guidelines, thus they transitioned off the study arm but remained under observational follow-up, with CARG participants returning for facility-based follow-up.
Data collection and analysis
Trained study-specific nurses extracted source data from patient files, the routine electronic Patient Monitoring System for HIV patients, and routine CARG data collection forms. Database closure was 15 July 2019. Descriptive measures of participants baseline characteristics were conducted using medians, interquartile ranges, and proportions, as appropriate. Individual-level outcome analyses were conducted by ITT including all enrolled participants in the arm to which they were originally allocated. For retention, risk differences between arms were estimated using binomial population-averaged generalized estimating equations using an exchangeable correlation structure, specifying for clustering by facility and using robust standard errors.28 A small cluster size variance correction was used,29 and randomization strata was included in the model as a fixed-effect parameter. Multivariable analyses were performed adjusting for baseline imbalance between arms. Subgroup analyses of retention were also conducted among participants at rural hospitals and among those newly stable on ART (enrolled 6-18 months after initiating ART).
Two prespecified VS analyses were conducted: (1) ITT analysis including all enrolled participants as allocated and irrespective of whether they had available follow-up VL results or completed the study and (2) an analysis restricted to participants having available VL results 12 months after study enrollment (+-3 months from target date). For the ITT analysis, a three-level outcome variable was generated: VL suppressed; VL unsuppressed; and VL not performed. Using a generalized structural equation framework, a multinomial logit model specified for clustering by facility was constructed to estimate the intervention effect of a suppressed vs. unsuppressed VL, specifying unsuppressed VL as the base category. A modified ITT analysis was also performed on a subset of participants excluding those from 13 sites that had very poor (<10%) VL testing completion (one 3MF site, four 3MC sites, and eight 6MC sites). An additional analysis was conducted excluding all sites allocated to Chitungwiza Municipality (the only district having high VL testing infrastructure).
For the analysis among participants with available VL results, log-binomial regression with generalized estimating equations was used to estimate relative risks (RR) of VS between study arms, specifying for clustering by facility. All regression models were adjusted for randomization stratum. Pooled analyses were also conducted comparing the intervention arms pooled vs. control. Ethical approval was received from the Medical Research Council of Zimbabwe.
RESULTS
During the recruitment period, 9018 ART patients were screened for inclusion (Figure 1). The most common reason for ineligibility was unavailability of VL results (20.5%), followed by having an unsuppressed VL (15.6%). The number of patients excluded due to VL result unavailability was higher at 3MC and 6MC sites than 3MF sites. A total of 1919, 1335, and 1546 participants were enrolled in arms 3MF, 3MC, and 6MC, respectively. The target sample size was not met in 3MC and 6MC as formation of CARGs during the enrollment period was slower than expected (particularly in rural areas), thus fewer than expected ART patients in newly formed CARGs were available for screening.
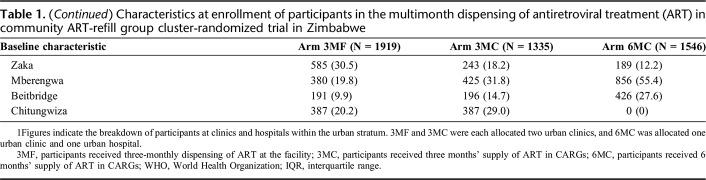
Arms were reasonably balanced with regards to participant’s baseline demographic characteristics excepting that 3MF had a slightly higher proportion of youth (aged 18-24 years) and employment status varied between arms (Table 1). 3MC had a higher proportion from urban facilities. 3MF had a larger proportion living >9 km from the facility. There were also differences in the numbers enrolled by district, with no 6MC sites allocated to Chitungwiza (allocation was not stratified by district).
Table 1.
Characteristics at enrollment of participants in the multimonth dispensing of antiretroviral treatment (ART) in community ART-refill group cluster-randomized trial in Zimbabwe
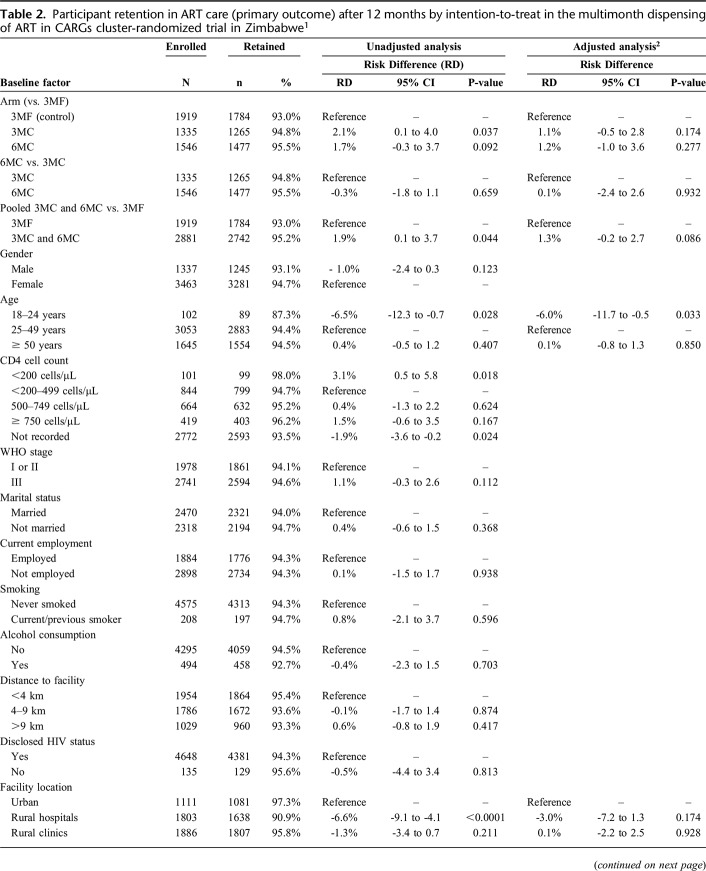
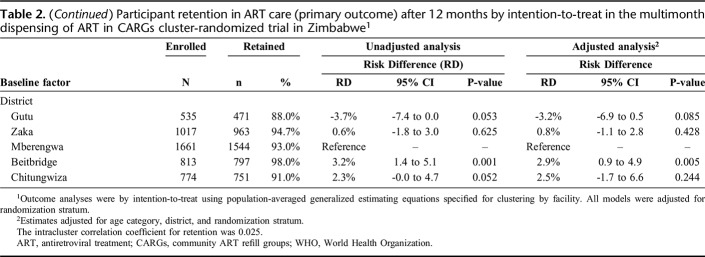
After 12 months, 1784 (93.0%), 1265 (94.8%), and 1477 (95.5%) participants enrolled in 3MF, 3MC, and 6MC remained in ART care, respectively. The ICC for retention was 0.025. Retention was higher in 3MC vs. control in the unadjusted analysis, but there was no difference after adjusting for baseline imbalance (age category and district) (Table 2; Supplementary Figure 1, http://links.lww.com/QAI/B445). Retention in 6MC did not differ vs. either control or 3MC in both adjusted and unadjusted analyses. The noninferiority limit was met for all comparisons of the primary outcome in both unadjusted and adjusted analyses. In the pooled analysis of both intervention arms vs. control, retention was marginally higher in the CARG arms (95.2% vs. 93.0%), adjusted RD = 1.3% (95% CI: -0.2% to 2.7%; P = 0.086). Retention was lower in those aged 18–24 years (87%), reduced at rural hospitals (91%) in unadjusted analyses, and variation by district was apparent (Table 2). Retention among those with baseline CD4 count <200 cells/µL (98%) was high (with 100% retention among fifty-five 3MC and 6MC participants) and was satisfactory among those with WHO stage 3 disease (95%). Little difference in retention between male and female participants was apparent. In the subgroup analysis limited to participants at rural hospitals, retention was slightly better in 6MC (93.9%) vs 3MF (89.5%), adjusted RD = 3.3% (95% CI: -1.0% to 7.6%) and vs. 3MC (89.6%), aRD = 3.7% (95% CI: -1.9% to 9.4%), although was not statistically significantly higher in either comparison (Supplementary Table 1, http://links.lww.com/QAI/B445). Among participants newly stable on ART (6-18 months), comparisons by arm were similar to the main analyses (Supplementary table 2, http://links.lww.com/QAI/B445).
Table 2.
Participant retention in ART care (primary outcome) after 12 months by intention-to-treat in the multimonth dispensing of ART in CARGs cluster-randomized trial in Zimbabwe1
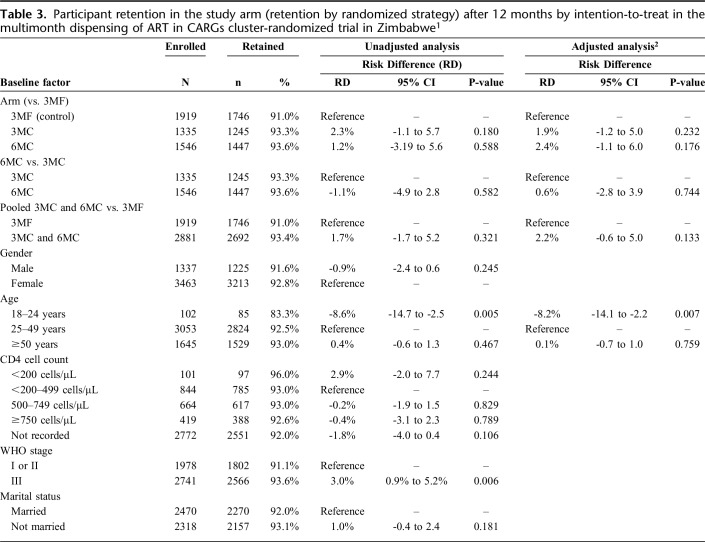
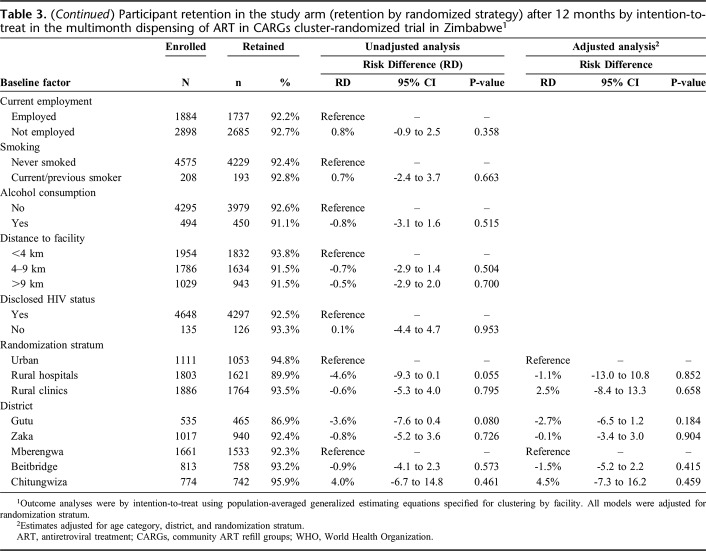
After 12 months, 1746 (91.0%), 1245 (93.3%), and 1447 (93.6%) participants continued receiving ART in arms 3MF, 3MC, and 6MC (retention on the randomized strategy), respectively. The numbers who transitioned off the arms due to requiring ART dispensed more frequently were relatively small and similar between arms, 20 (1.0%), 18 (1.4%), and 28 (1.8%) in 3MF, 3MC, and 6MC, respectively. No differences in retention in the study arm were apparent between any arms in both unadjusted and adjusted analyses (Table 3; Supplementary Figure 2, http://links.lww.com/QAI/B445).
Table 3.
Participant retention in the study arm (retention by randomized strategy) after 12 months by intention-to-treat in the multimonth dispensing of ART in CARGs cluster-randomized trial in Zimbabwe1
Among participants eligible for VL testing at 12 months, 865 (49%), 566 (45%), and 113 (8%) had recorded VL results in 3MF, 3MC, and 6MC, respectively (Table 4).
Table 4.
Viral suppression 12 months after enrollment
VL result availability varied dramatically between facilities (range: 0%–97%) and by district due to variable VL testing infrastructure. VL completion was high in Chitungwiza (96%) but substantially lower in all other districts (range: 14%–32%), particularly in rural areas. As no Chitungwiza facilities were allocated to 6MC, VL completion was lower in this arm.
Among participants with available VL results at 12 months, 857 (99.1%), 564 (99.7%), and 105 (92.9%) achieved VS in arms 3MF, 3MC, and 6MC, respectively. In both ITT analyses and analyses limited to those with available VL results, the probability of VS was not different in 3MC vs control.
VS by ITT was reduced in 6MC vs. control. However, in the modified ITT analysis excluding participants at sites who had very poor VL test completion (<10% of eligible participants tested), VS in 6MC was equivalent to both control and 3MC, RRR = 0.9 (95% CI: 0.2–5.3) and RRR = 0.5 (95% CI: 0.06–4.3), respectively. When considering participants with available VL results only, VS was slightly reduced in 6MC vs. control, RR = 0.9 (95% CI: 0.9–1.0; P = 0.070) and vs. 3MC, RR = 0.9 (95% CI: 0.9–1.0; P = 0.083), with borderline statistical significance for both comparisons. When pooling data from both intervention arms vs. control, VS in the CARG arms was not different to 3MF by ITT nor when including only those with available VL results.
In analyses excluding sites allocated to Chitungiwza, VL completion remained lower in 6MC (7.7%) than 3MF (36.6%) and 3MC (23.0%), and VS by ITT in 6MC remained marginally lower than 3MF; RRR = 0.2 (95% CI: 0.03-1.2; P = 0.083) (Supplementary Table 3, http://links.lww.com/QAI/B445). When considering only participants with available VL results, VS in 6MC in this analysis was similar to the main analysis.
DISCUSSION
In among the first large-scale cluster-randomized trials to evaluate three- and six-monthly dispensing of ART in community-based DSD models in Africa, retention was found to be high and noninferior versus standard-of-care three-monthly facility-based ART delivery, with retention in the pooled CARG arms being marginally greater than control. This suggests that expanded implementation of MMD in community-based groups is an effective way to deliver ART to large numbers of stable ART patients, which can allow decongestion of health care facilities enabling clinics to focus on increasing ART initiation and management of ill and complicated patients. Participants in the CARG arms were scheduled to receive clinical assessments at the clinics only annually (unless clinically unwell), and cost savings may be realized through devolving ART delivery to the community, reducing patient loads at overburdened facilities, improving efficiency of the healthcare system, and reducing patients transport costs and the inconvenience and opportunity costs associated with frequent facility visits.6 Six-monthly MMD may have added benefit for patients associated with rural hospitals, as these are likely to be further from patients' homes and had retention below 90% in the three-monthly arms. Although enrolled numbers were very small, retention among those with baseline CD4 count <200 cells/µL was high, suggesting that these DSD models may possibly be suitable for less stable patients. Recent qualitative work in Zimbabwe has also shown that health care workers and patients overwhelmingly perceive CARGs as beneficial.30
VS in the three-monthly CARG arm was very high (99%) among participants with VL results. VS in the six-monthly CARG arm was marginally reduced; however, accurate estimation of VS in this arm was hindered by low VL result availability. There were substantial differences in VL completion between study districts, with Chitungwiza (the single urban district) having substantially better VL testing infrastructure than other districts. No sites in Chitungwiza were allocated to 6MC, and VL completion was reduced in this arm. Nevertheless, VL completion remained reduced in 6MC when excluding all sites allocated to Chitungwiza, thus it is possible that the 6MC model may be associated with lower VL uptake. Also, VL testing cannot be assumed to be random at facilities with very low VL completion, thus conclusions about VS in this arm cannot be drawn from this data. At facilities with low VL completion, targeted VL testing may have occurred among participants that clinicians were more concerned about having poor adherence. Few studies have measured virological outcomes of patients receiving ART as infrequently as twice annually with annual clinical consulations.10 Further studies are needed to precisely measure VL completion and suppression among six-monthly MMD patients. Where feasible, VL uptake and VS should be closely monitored during routine implementation of six-monthly MMD in CARGs. Facilitating patient-led demand creation to know their annual VL may also be warranted.
In previous observational studies using routine data, Zambian patients with six-monthly return intervals had fewer missed visits and reduced LTFU.10 In rural Malawi, retention was high among patients attending six-monthly clinical consultations with three-monthly fast-track ART refills.11 In a recent South African trial, retention and VS were similar among ART adherence club members (25-30 patients per group) who received ART six-monthly with annual clinical consultations compared with those receiving ART two-monthly in adherence clubs.15 In our study and similar to previous studies, however, retention among youth (aged <25 years) was low in all arms, and DSD models may need to be better tailored to this age group to achieve optimal outcomes.31
The strengths of this study include the robust randomized design which comprised three arms and the distribution of sites in both urban and rural areas in five high HIV-prevalence districts. Study data collection had a minimal impact on routine site clinical procedures; hence, study findings are representative of the realities of routine HIV program settings. Study limitations include that the availability of VL testing varied between sites and districts due to variable expansion of VL testing scale-up. This affected eligibility for the study as only those with suppressed VLs were eligible, and there was differential exclusion between arms due to VL result unavailability. Differential availability of 12-month VL results between arms also affected ITT analyses of VS. VL testing may have been nonrandom at sites with low VL completion, with possible targeted VL testing among higher-risk participants. As formation of CARGs during the enrollment period was slower than anticipated, the enrollment target was not met in 3MC and 6MC due to fewer than anticipated patients in newly formed CARGs being available for screening. The sample of participants aged 18-24 years was very small, thus conclusions regarding this age group cannot be drawn. As the selected sites included only one urban hospital (which was subsequently allocated to 6MC), no 3MF or 3MC participants attended urban hospitals. The study design did not include the element of patient choice regarding DSD model, although choice may often be a feature of DSD implementation in routine settings.32 In addition, participant outcomes were limited to one year after enrollment. Retention and VS may decline over time, and studies are needed to evaluate longer-term outcomes.
CONCLUSIONS
This is one of the first large-scale randomized studies of extended dispensing intervals of ART in community-based groups in sub-Saharan Africa. Retention was found to be high among participants receiving ART at three- and six-monthly intervals in CARGs, being noninferior compared with standard-of-care facility-based three-monthly ART delivery. VS was high in the three-month CARG arm. VS was marginally reduced in the six-monthly CARG arm; however, conclusions could not be drawn due to low VL completion in this arm. Lower VS in this group may indicate slightly lower adherence or targeted VL testing among those participants that clinicians were more concerned about. Close monitoring of VL completion and VS is warranted for this group. Future studies should evaluate clinical outcomes of MMD in community-based groups beyond one year of follow-up, including measuring adherence, precise measurement of VS, cost outcomes, and qualitative experiences. Expanded VL infrastructure will further enhance eligibility for and the monitoring of patients receiving DSD.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge the Zimbabwe MOHCC; Equip Health; the Organization for Public Health Interventions and Development, Zimbabwe; FHI360 Zimbabwe; Population Services International Zimbabwe; and the Data Safety and Monitoring board: Mazvita Sengayi, Richard Chawana, and Tov Manene.
Footnotes
The United States Agency for International Development/President’s Emergency Plan for AIDS Relief, through Equip health (agreement number AID-OAA-A-15-00070).
No conflicts of interest are declared.
REFERENCES
- 1.UNAIDS. UNAIDS Data 2019. 2019. https://www.unaids.org/en/resources/documents/2019/2019-UNAIDS-data. Accessed November 7th, 2019.
- 2.Grimsrud A, Bygrave H, Doherty M, et al. Reimagining HIV service delivery: the role of differentiated care from prevention to suppression. J Int AIDS Soc 2016;19(1):21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kates J, Wexler A, Lief E. Financing the response to HIV in low- and middle-income countries: international assistance from Donor Governments in 2015. 2016. http://www.kff.org/global-health-policy/report/financing-the-response-to-hiv-in-low-and-middle-income-countries-international-assistance-from-donor-governments-in-2015/. Accessed April 15th, 2017.
- 4.Maddison A, Schlech W. Will universal access to antiretroviral therapy ever be possible? The health care worker challenge. Can J Infect Dis Med Microbiol 2010;21(1):e64–e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eshun-Wilson I, Mukumbwa-Mwenechanya M, Kim HY, et al. Differentiated Care Preferences of Stable Patients on Antiretroviral Therapy in Zambia: A Discrete Choice Experiment. J Acquir Immune Defic Syndr 2019;81(5):540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prust ML, Banda CK, Nyirenda R, et al. Multi-month prescriptions, fast-track refills, and community ART groups: results from a process evaluation in Malawi on using differentiated models of care to achieve national HIV treatment goals. J Int AIDS Soc 2017;20(Suppl 4):21650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips A, Shroufi A, Vojnov L, et al. Sustainable HIV treatment in Africa through viral-load-informed differentiated care. Nature 2015;528(7580):S68–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Decroo T, Rasschaert F, Telfer B, Remartinez D, Laga M, Ford N. Community-based antiretroviral therapy programs can overcome barriers to retention of patients and decongest health services in sub-Saharan Africa: a systematic review. International Health 2013;5(3):169–179. [DOI] [PubMed] [Google Scholar]
- 9.Kim MH, Wanless RS, Caviness AC, et al. Multimonth Prescription of Antiretroviral Therapy Among Children and Adolescents: Experiences From the Baylor International Pediatric AIDS Initiative in 6 African Countries. J Acquir Immune Defic Syndr 2018;78(Suppl 2):S71–s80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mody A, Roy M, Sikombe K, et al. Improved Retention With 6-Month Clinic Return Intervals for Stable Human Immunodeficiency Virus-Infected Patients in Zambia. Clin Infect Dis 2018;66(2):237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wringe A, Cawley C, Szumilin E, et al. Retention in care among clinically stable antiretroviral therapy patients following a six-monthly clinical consultation schedule: findings from a cohort study in rural Malawi. J Int AIDS Soc 2018;21(11):e25207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grimsrud A, Patten G, Sharp J, Myer L, Wilkinson L, Bekker LG. Extending dispensing intervals for stable patients on ART. J Acquir Immune Defic Syndr 2014;66(2):e58–60. [DOI] [PubMed] [Google Scholar]
- 13.Mutasa-Apollo T, Ford N, Wiens M, et al. Effect of frequency of clinic visits and medication pick-up on antiretroviral treatment outcomes: a systematic literature review and meta-analysis. J Int AIDS Soc 2017;20(Suppl 4):21647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puttkammer N, Rodriguez T, Robin E, et al. Multi-Month Scripting (MMS) And Retention On HIV Antiretroviral Therapy In Haiti. Paper presented at: Conference on Retroviruses and Opportunistic Infections; 4-7 March, 2018; Boston, MA, USA.
- 15.Lebelo K, Cassidy T, Grimsrud A, et al. Twelve-month retention and viral load outcomes from a noninferiority cluster randomized trial extending adherence club ART refill dispensing intervals to 6-monthly. Paper presented at: 10th IAS Conference on HIV Science; 21-24 July, 2019; Mexico City, Mexico.
- 16.UNAIDS. AIDSInfo. http://aidsinfo.unaids.org/. Accessed November 6th, 2019.
- 17.National AIDS Council; Ministry of Health and Child Care of Zimbabwe; UNAIDS. Global AIDS Response Progress report. 2018. https://www.unaids.org/sites/default/files/country/documents/ZWE_2018_countryreport.pdf. Accessed 06 November, 2019. [Google Scholar]
- 18.Decroo T, Koole O, Remartinez D, et al. Four-year retention and risk factors for attrition among members of community ART groups in Tete, Mozambique. Trop Med Int Health 2014;19(5):514–521. [DOI] [PubMed] [Google Scholar]
- 19.Rasschaert F, Decroo T, Remartinez D, et al. Adapting a community-based ART delivery model to the patients’ needs: a mixed methods research in Tete, Mozambique. BMC public health 2014;14(1):364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rasschaert F, Telfer B, Lessitala F, et al. A qualitative assessment of a community antiretroviral therapy group model in Tete, Mozambique. PLoS One 2014;9(3):e91544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimbabwe Ministry of Health and Child Care. Operational and service delivery manual for the prevention, care and treatment of HIV in Zimbabwe. 2017. http://www.differentiatedcare.org/Portals/0/adam/Content/m2an155byU6RIoHeF4e4FQ/File/Zimbabwe_OSDM_2017.pdf. Accessed 14 August 2017.
- 22.Fatti G, Ngorima-Mabhena N, Chirowa F, et al. The effectiveness and cost-effectiveness of 3- vs. 6-monthly dispensing of antiretroviral treatment (ART) for stable HIV patients in community ART-refill groups in Zimbabwe: study protocol for a pragmatic, cluster-randomized trial. Trials 2018;19(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.FHI 360. Zimbabwe HIV Care and Treatment Standard Operating Procedure for Community ART Refill Groups. 2016. [Google Scholar]
- 24.Ivers NM, Halperin IJ, Barnsley J, et al. Allocation techniques for balance at baseline in cluster randomized trials: a methodological review. Trials 2012;13(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. Operations manual for delivery of HIV prevention, care and treatment at primary health centres in high-prevalence, resource-constrained settings. Edition 1 for field testing and country adaptation. 2008. http://www.who.int/hiv/pub/imai/operations_manual/en/. Accessed 16 Sep, 2016. [PubMed] [Google Scholar]
- 26.Shepherd BE, Blevins M, Vaz LME, et al. Impact of Definitions of Loss to Follow-up on Estimates of Retention, Disease Progression, and Mortality: Application to an HIV Program in Mozambique. American Journal of Epidemiology 2013;178(5):819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fairall L, Bachmann MO, Lombard C, et al. Task shifting of antiretroviral treatment from doctors to primary-care nurses in South Africa (STRETCH): a pragmatic, parallel, cluster-randomised trial. Lancet 2012;380(9845):889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pedroza C, Thanh Truong VT. Performance of models for estimating absolute risk difference in multicenter trials with binary outcome. BMC Med Res Methodol 2016;16(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang S, Fiero MH, Bell ML. Generalized estimating equations in cluster randomized trials with a small number of clusters: Review of practice and simulation study. Clinical Trials 2016;13(4):445–449. [DOI] [PubMed] [Google Scholar]
- 30.Bochner AF, Meacham E, Mhungu N, et al. The rollout of Community ART Refill Groups in Zimbabwe: a qualitative evaluation. J Int AIDS Soc 2019;22(8):e25393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casale M, Carlqvist A, Cluver L. Recent Interventions to Improve Retention in HIV Care and Adherence to Antiretroviral Treatment Among Adolescents and Youth: A Systematic Review. AIDS Patient Care STDS 2019;33(6):237–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geng EH, Holmes CB. Research to improve differentiated HIV service delivery interventions: Learning to learn as we do. PLoS Medicine 2019;16(5):e1002809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.