Abstract
Cyclic guanosine monophosphate–adenosine monophosphate synthase (cGAS) — stimulator of interferon genes (STING) signaling pathway plays the critical role in the immune response to DNA. Pharmacological modulation of the STING pathway has been well characterized both from structural and functional perspectives, which paves the way for the drug design of small modulators by medicinal chemists. Here, we outline recent progress in studies on the STING pathway, the structure and biological function of STING, the STING related disease, as well as the rationale and progress in the development of STING modulators. Our review demonstrates that STING is a promising drug target, and providing clues for the discovery of novel STING agonists and antagonists for the potential treatment of various disease including microbial infectious diseases, cancer, and autoimmune disease.
Keywords: STING, Innate immune response, Small modulators, Immunotherapy
Graphical abstract
The innate immune response is critical for efficient host defense against microbial invasion. Invading pathogens are identified by pattern recognition receptors (PRRs) in the host cell, which initiate a series of signalling events that lead to the production of type I interferons (IFNs), proinflammatory cytokines and other downstream antimicrobial proteins [1]. In principle, this strategy allows a finite set of receptors to recognize an enormous diversity of potential pathogens. One clear exception to this generalization is the recognition of DNA—the basic building block of “life” [2]. Over the past few years, accumulating evidence indicates that one of the major pathways that mediate the immune response to DNA is governed by the cGAS-STING signaling pathway. Importantly, this pathway is blind to DNA sequence. Such a “universal” sensing mechanism breaks one of the most fundamental rules of the classical pattern recognition dogma, which applies pathogen-specific structural patterns for self-nonself discrimination [3].
Studies have identified STING (also known as TMEM173, MPYS, MITA and ERIS), an endoplasmic reticulum (ER) IFNs stimulator, as a critical signaling molecule in the innate response to cytosolic nucleic-acid ligands [4]. Furthermore, STING has recently emerged as an exciting target for both immunological conditions (i.e. STING inhibition) and oncology (i.e. STING activation) [5]. STING's role in the immune system is consistent with its higher expression in the thymus, heart, spleen, placenta, lung and peripheral leukocytes. While induction of type I IFNs is the major outcome of STING activation in vertebrates, it has recently become clear that STING evolved more than 600 million years ago, predating the evolution of type I IFNs [6,7]. Except the induction of type I IFNs, several studies unraveled that the induction of autophagy process is a primordial function of the cGAS-STING pathway [[8], [9], [10]]. In addition, cGAS and STING are subject to extensive regulation by post-translational modifications and interactions with other proteins and metal ions, indicating the importance of precise regulation of this pathway [6].
The bright prospect in targeting STING to regulate immune response is obviously appealing and the recent clinical achievements have garnered much attention from the scientific community. As an attractive drug target, STING has been subjected to extensive structural and functional studies for the identification and characterization of specific modulators. In this review, we will summarize recent advance progress in studies on the STING pathway, the structure and biological function of STING, the STING related disease, as well as the rationale and progress in the development of STING modulators.
1. cGAS-STING signaling pathway
Human cGAS is identified as a broad-specificity cytosolic DNA sensor which is atypical among innate immune sensors in being both receptor and biosynthetic enzyme [[11], [12], [13]]. cGAS binds dsDNA through its phosphate backbone, therefore making the binding nonsequence dependent [11]. Then the active site of cGAS rearranges and becomes competent to convert adenosine 5′-triphosphate (ATP) and guanosine 5′-triphosphate (GTP) into cyclic guanosine monophosphate–adenosine monophosphate (cGAMP). The produced endogenous cGAMP ligand in turn activate STING on the ER membrane (Fig. 1 ) [[13], [14], [15]]. The ability of a single molecule of cGAS to produce multiple molecules of cGAMP provides a mechanism by which detection of a small amount of cytosolic dsDNA can produce a rapidly amplified antiviral signaling response. Besides cGAMP-mediated activation, STING can be activated directly by bacteria-derived cyclic dinucleotides (CDNs) [16]. Thus, CDNs are both endogenous and pathogen-derived potent activators of the STING pathway, and as such, they function as ubiquitous second messengers in prokaryotic species and eukaryotes [17].
Fig. 1.
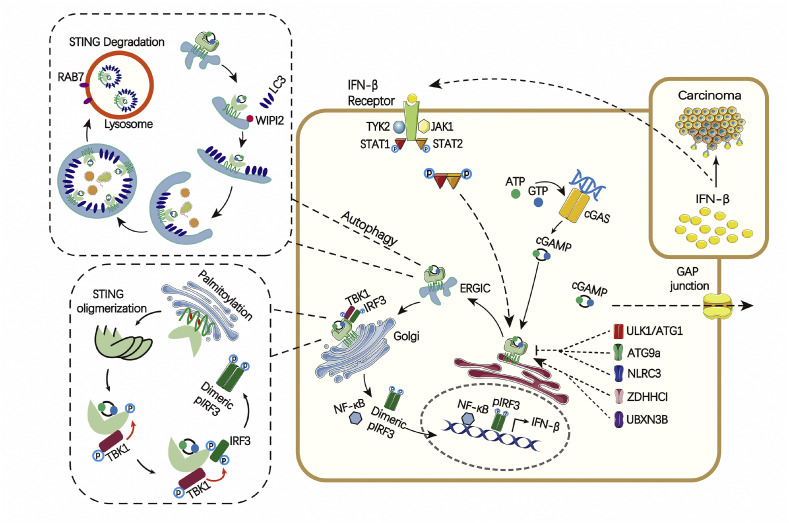
The cGAS-STING pathway of cytosolic DNA sensing. Cytosolic DNA binds to and activates cGAS, which catalyzes the synthesis of 2′3ʹ-cGAMP from ATP and GTP. 2′3ʹ-cGAMP binds to the ER adaptor STING, which traffics to the ER-Golgi intermediate compartment (ERGIC) and the Golgi apparatus. After the tansmembrane domain of STING is palmitoylated, then palmitoylated STING is clustered to produce oligmerization to recruit TBK1. TBK1 phosphorylates STING, which in turn recruits IRF3 for phosphorylation by TBK1. Phosphorylated IRF3 dimerizes and then enters the nucleus, where it functions with NF-kB to turn on the expression of type I interferons and other immunomodulatory molecules. Furthermore, the ERGIC, which contains cGAMP-bound STING, serves as a membrane source for LC3 recruitment and lipidation through a WIPI2-dependent mechanism. LC3-positive membranes target DNA and pathogens to autophagosomes, which are subsequently fused with lysosomes in a process that requires RAB7 GTPase.
Upon cGAMP binding, STING translocates from ER to the Golgi via ER-Golgi intermediate compartment (ERGIC). Tank-binding kinase 1 (TBK1) in turn phosphorylates STING, leading to recruitment of the transcription factor interferon regulatory factor 3 (IRF3). TBK1 then phosphorylates IRF3 in a STING-dependent manner, resulting in the IRF3 dimerization and the translocation to the nucleus, where IRF3 then mediates transcription of IFN-β and other coregulated genes [4,18]. In addition to their direct cytotoxic effects on cancer cells, IFN-β also promotes the maturation and antigen presentation of dendritic cells and thereby links innate immune responses to adaptive immune responses. Meanwhile, the binding of IFN-β to its receptor activates Janus kinases (JAKs), including JAK1 and tyrosine kinase 2 (TYK2), which in turn phosphorylates the receptor. This process allows the binding of the DNA-binding proteins signal transducers and activators of transcription 1 (STAT1) and 2 (STAT2) to the receptor, whereupon they become phosphorylated and dimeric. Then the dimer translocates to the nucleus, where it up-regulates transcription of interferon-response genes, including interferon regulatory factor 7 (IRF7) –dependent transcription of type I IFNs. The synthesis and release of IFNs and their binding to interferon receptor further up-regulate STING and the transcription of other proinflammatory cytokine genes in a positive feedback loop [19]. The cGAS-STING signaling cascade also functions across neighboring cells via gap-junction communication and may connect more distant cell types or tissues through the packaging of cGAMP in viral particles (Fig. 1) [20].
Given that self-DNA can trigger STING-mediated response [21], this pathway requires precise control to avoid excessive immune responses (Fig. 1). LSm14A (protein LSm14 homolog A), a member of the LSm (Sm-like protein) family involved in RNA processing in the processing bodies, plays an important role in maintaining the mature mRNA level of STING in a cell type–specific manner [22]. Meanwhile, after activation and trafficking, STING is phosphorylated by serine/threonine-protein kinase 1/autophagy-related protein 1 (ULK1/ATG1) triggered by cGAS-generated CDNs in order to suppress IRF3 activation. Therefore, CDNs may also initiate a negative-feedback control mechanism to thwart prolonged innate immune gene transcription and to prevent inflammatory disorders [23]. Autophagy-related protein 9a (ATG9a) restricts STING translocation from the Golgi apparatus and regulates the assembly of STING and TBK1 [24]. NOD-like receptor C3 (NLRC3) associates with STING and prevents its trafficking from the ER to Golgi [25], resulting in reducing the response of type I IFNs. While the Zinc finger DHHC domain-containing protein 1 (ZDHHC1), a member of the aspartate-histidine-histidine-cysteine palmitoyl acyltransferase family, associates with STING in the ER and Golgi apparatus after viral DNA stimulation and positively modulates STING activity, leading to increased TBK1-IRF3 engagement and type I IFNs production [26]. UBX domain-containing protein 3B (UBXN3B) complexes with STING and E3 ubiquitin-protein ligase TRIM56 to positively regulate STING-dependent innate immune responses [27]. STING has also been shown to be negatively regulated by E3 ubiquitin transferase RING finger protein 5 (RNF5)-mediated K48-linked ubiquitination, which leads to STING degradation [28]. Interestingly, RING finger protein 26 (RNF26) was shown to attenuate RNF5-directed K48-linked STING ubiquitination at K150, without affecting K63-linked STING ubiquitination, suggesting positive regulation of STING signaling [29]. The mechanism remains to be clarified about how these regulators coordinate with each other in the host cell and make cGAS-STING pathway function as a well-balanced system [29].
Recently, mounting evidence reveals that cGAS–STING pathway can activate autophagy robustly which is an ancient and highly conserved function predating the evolution of type I IFNs (Fig. 1) [8,10,30]. Of note, cGAMP-induced autophagy can clear the cytosolic viruses and DNA effectively, indicating its crucial role in antiviral defence [8]. The ERGIC, which contains cGAMP-bound STING, serves as a membrane source for LC3 recruitment and lipidation. This process is through a WD repeat domain phosphoinositide-interacting protein 2 (WIPI2) dependent mechanism which is important for LC3 lipidation in the conventional autophagy pathway [31]. LC3-positive membranes target DNA and pathogens to autophagosomes, which are subsequently fused with lysosomes in a process that requires Ras-related protein Rab-7a (RAB7) GTPase [8].
2. Structural basis of STING activation
The molecular understanding of the key steps underlying cGAS-STING pathway has only very recently set up. However, the mechanisms of every step are less clear and controversial in some cases. For example, the question about how cGAMP activates STING and how activated STING triggers the recruitment and activation of TBK1 remains to be characterized. Notably, the structure of STING represents a unique fold that is not structurally homologous to any other known protein. Undoubtedly, STING is a mysterious and promising drug target.
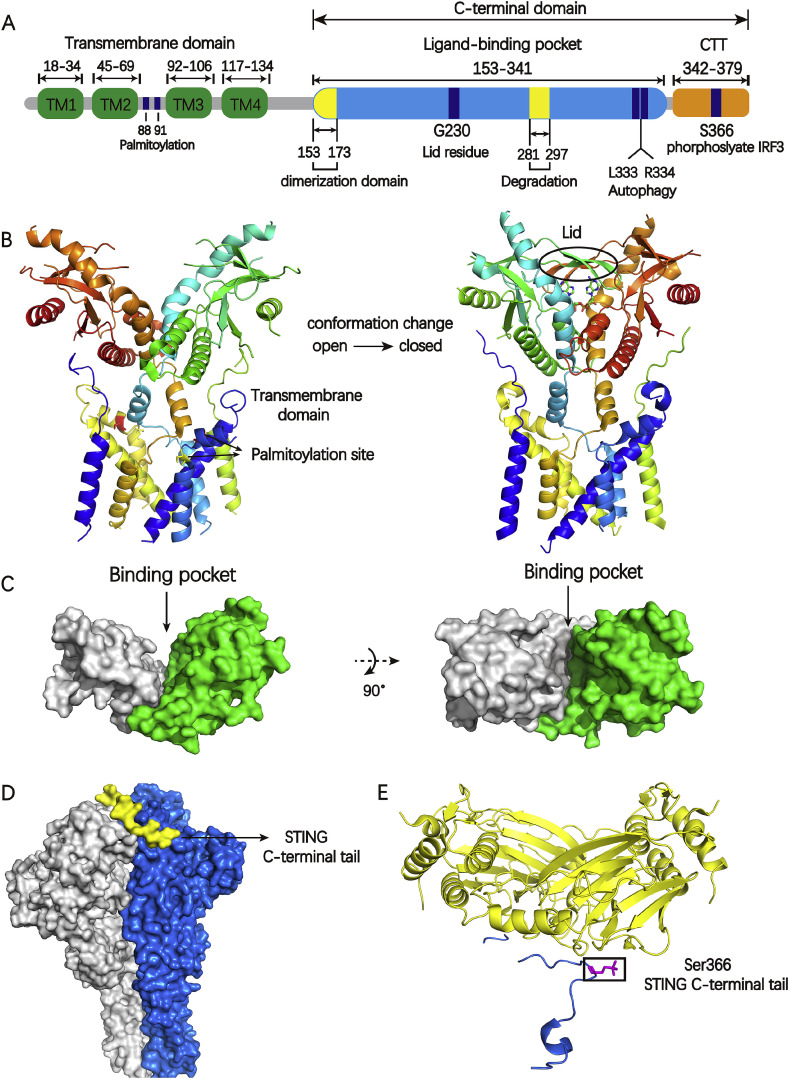
STING is a transmembrane protein consisting of an N-terminal transmembrane domain containing four transmembrane helices (TM1,TM2, TM3, TM4), a globular C-terminal domain (CTD) including the dimerization domain, predicted to be localized in the cytosol (Fig. 2 A) [32]. Based on biophysical and X-ray crystallographic data, the STING CTD exists as a butterfly-shaped dimer and the ligand binding site is located at the dimer interface [[32], [33], [34]]. The cGAMP acts as a “glue” to reinforce the STING homodimer which can induce the conformational change of STING from “open” to “closed” (Fig. 2B). The two monomers of STING shift slightly relative to each other and clamp down onto the ligand binding site, whereas a β-sheet lid region (Fig. 2B) seals the large binding pocket (1400 nm3) (Fig. 2C), thus forming a “closed” conformation [32,35,36]. The CTD of activated STING undergoes extensive conformational alterations, then triggers STING translocation from ER to the Golgi.
Fig. 2.
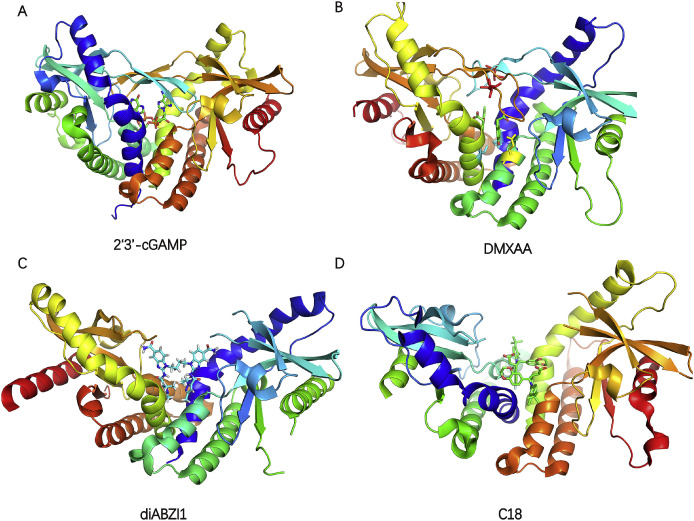
A) The schematic of human STING domain organization; B) The left is the full-length of apo human STING in open conformation (PDB code: 6NT5) and the right is chicken STING with cGAMP in a closed conformation (PDB code: 6NT7). The Cys 88 and Cys 91 in transmembrane domain is palmitoylated which may further promote STING oligomerization and subsequent activation of TBK1, colored green and yellow respectively. The appearance of lid region is the feature of closed conformation; C) The globular C-terminal domain of human STING with a large binding pocket (PDB code: 4F9G); D) The densities for the two protomers of the TBK1 dimer are colored gray or blue. The densities for the two STING C-terminal tails are colored yellow (PDB code: 6NT9); E) Phosphorylated STING C-terminal tail in complex with IRF3, colored blue and yellow, respectively. The residue S366(magenta) in STING C-terminal tail is required for STING to be licensed for the interaction with IRF3 (PDB code:5JEJ). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
After trafficking to the Golgi, STING is palmitoylated at C88 and C91 in transmembrane domain at the Golgi (Fig. 2A,B) [37,38]. This palmitoylation may further promote STING oligomerization and subsequent activation of TBK1. However, TBK1 recruitment can't activate IRF3 alone which is different with most protein kinase whose activation is synonymous with the phosphorylation of its substrates. Rather, the phosphorylation of a conserved consensus pLxIS (p is a hydrophilic residue and x is any residue) motif, presenting in the C-terminal tail of STING (S366), is required for STING to be licensed for the interaction with IRF3 (Fig. 2D,E) [39,40]. Subsequently, phosphorylated IRF3 dimerizes and translocates to the nucleus to drive type I IFNs expression. After initiation of downstream signaling, STING is degraded in endolysosomes. The motif with amino acid 281–297 is required for trafficking mediated STING degradation (Fig. 2A) [41]. L333 and R334 play a crucial role in c-GAMP induced autophagy and phosphorylation of TBK1 and IRF3 (Fig. 2A) [8].
Although most of the human STING proteins can be activated by CDNs robustly, certain single nucleotide polymorphisms (SNPs) within transmembrane protein 173 (TMEM173)—which encodes STING—can differentiate the sensitivity of STING to CDNs. Analysis of SNPs data from the 1000 Genome Project revealed that R71H-G230A-R293Q (STINGHAQ) occurs in 20.4%, R232H in 13.7%, G230A-R293Q (STINGAQ) in 5.2%, and R293Q in 1.5% of human population. Human STING harbouring R232 can be activated by bacterial CDNs as well as endogenous cGAMP, whereas STING variants with a histidine at this position (R232H) are poorly responsive to bacterial CDNs and are only effectively activated by endogenous cGAMP [33]. The R293Q dramatically decreases the sensitivity to all bacterial CDN ligands. However, the STINGAQ and STINGHAQ variants maintain partial abilities to activate the IFN-β signaling in the presence of ligands due primarily to the G230A substitution. Biochemical analysis revealed that the conformation of lid region in STING (G230A) was significantly altered compared with wild type STING [42]. In mice, a T596A STING variant is also defective in type I IFNs induction that is responsive to CDNs [43]. Interestingly, alternative SNPs have been reported to result in a gain-of-function phenotype which are hyperactivated condition without CDNs stimulation. Accordingly, it is likely to contribute to severe inflammatory disorders which are introduced in the following chapter. Taken together, complete understanding of SNPs within TMEM173 may predict susceptibility to pathogen infection and perhaps the response to immunization regimes.
3. STING related disease
3.1. STING and microbial infectious disease
STING's function has been extensively studied during viral infection due to its major role in the induction of type I IFNs, as reviewed recently [29]. Studies with different viruses have shown that viral genome released into the cytosol upon infection can trigger STING-dependent signaling cascade [44,45]. Retrovirus replication leads to accumulation of several DNA containing nucleic acid structures with the capacity to stimulate cGAS-STING axis such as retroviral DNA, retroviral RNA:DNA hybrids, single-stranded DNA (ssDNA) species and the final dsDNA [[46], [47], [48]]. Intriguingly, some studies demonstrated that virus-induced cellular stress and virus-fusion can also trigger the cGAS-STING pathway independently of viral nucleic acids, suggesting a more complex role for STING, possibly through interactions with complexes that govern membrane dynamics [49,50]. However, it remains unresolved whether STING is a genuine signaling molecule governing membrane-reorganization processes, which is supposed to be essential for certain virus-detection pathways.
During the constant ‘arms race’ between pathogens and hosts, most microbes have evolved diverse ways to evade the attack by the immune response of their susceptible hosts [51]. For all microbes, the most common and effective way of avoiding triggering cGAS-STING pathway is by “hiding” their DNA from the cytoplasm. Retroviruses and many DNA viruses keep their DNA in the viral capsid while they traverse the cytoplasm and then deliver their viral DNA into the nucleus [52]. Aside from DNA recognition in the infected cells, the upstream and downstream of cGAS-STING pathway can be damaged to brake antiviral signaling cascade. Kaposi's sarcoma-associated herpesvirus (KSHV) copes with cGAS-STING pathway through various mechanisms including disrupting DNA binding to the upstream DNA sensor of STING such as interferon-γ inducible protein 16 (IFI16) and cGAS [53], dismissing STING binding to TBK1, inhibiting STING phosphorylation [54] and so on. Herpesviruses (HSV) can hydrolyze K27/63 polyubiquitin chain on STING to prevent ubiquitination, then negatively regulate STING-mediated signaling. Similarly, Coronaviruses and Porcine epidemic diarrhea virus (PEDV) can reduce the ubiquitination status of the STING [[55], [56], [57]]. Influenza A virus interacts with STING through its conserved hemagglutinin fusion peptide (FP) to inhibits STING activation in response to membrane fusion [58]. Recently, the NS2B-NS3 protease from several flaviviruses has been shown to cleave STING at the sequence “LRRG” in the N-terminal domain [59,60]. Furthermore, the NS2B of dengue virus can target cGAS to induce its degradation and prevent mitochondrial DNA sensing during infection [61].
Gram-negative and Gram-positive bacteria have also been reported to promote STING signaling including Legionella pneumophila, Francisella tularensis, Chlamydia muridarum, Streptococcus pyogenes, Brucella abortus and Mycobacterium tuberculosis [[62], [63], [64], [65], [66]]. Overall, bacterial DNA recognition by cGAS seems to be the main stimulus for type I IFNs induction, and the effects of bacterial CDNs on STING update the knowledge of the relationship between microorganisms and host [[67], [68], [69]]. Most bacterial CDNs can't induce interferons in the absence of cGAS with the exception of Listeria monocytogenes, which induce interferons in a STING-dependent manner but not cGAS [70]. One possibility is that bacterial and host cells contain enzymes that selectively digest the bacterial CDNs but not the endogenous cGAMP produced by host cells [51,71]. For example, S. agalactiae can degrade CDNs presenting outside the bacteria via a cell-wall anchored ectonucleotidase [72].
3.2. STING and cancer
A major subset of patients with advanced solid tumors show a spontaneous T cell inflamed tumor microenvironment (TME), which has prognostic importance and is associated with clinical response to immunotherapies, while another major subset dose not [73]. Clues gleaned from human cancer gene expression profiling studies reveals an association between type I IFNs signature, T cell-inflamed TME and clinical outcomes. Accumulating evidence suggests that type I IFNs production might be integrally involved with adaptive T cell responses against tumor antigens [[74], [75], [76], [77]]. This has allowed a focus on innate immune sensing pathways known to trigger type I IFN production that is necessary for optimal T cell priming against tumor antigens. It is an important strategy to trigger innate signaling via antigen-presenting cells (APCs) in the TME might facilitate better cross-priming of tumor antigen-specific CD8+ T cells, and augment the chemokine production for the subsequent effector T cell trafficking. The T cell-inflamed TME plays a crucial role in tumor regression and thus yield improved clinical outcome [75].
Defined innate immune mechanisms involving cancer immunotherapy include, but are not limited to antitumor immune responses elicited by recognition of tumor-derived antigens by Toll-like receptors (TLRs), retinoic acid-inducible gene-I (RIG-I)-like Receptors (RLRs), as well as sensation of tumor-derived DNA by STING [[78], [79], [80]]. DNA derived from dying tumor cells can enter the cytosol of dendritic cells as a consequence of TLR9 ligation, phagocytosis, or cell–cell contact, leading to the induction of STING signaling [81]. Meanwhile, RIG-I stimulation coupled with potentiation of the response by STING could impact adaptive immune responses in cancer immunotherapy [82]. Therefore, further insight into the mechanisms of TLRs, RLRs and STING-mediated innate immune signaling in cancer immune evasion, tumorigenesis and cancer development may lead to discovery of novel therapeutic targets for cancer therapy [79,83,84].
More recently, cGAS-STING signaling has shown its importance for response to both radiation therapy and immune checkpoint blockade [[85], [86], [87]]. Radiation can prompt DNA damage in host cells and elicit strong inflammatory triggered by danger-associated molecular patterns (DAMPs). DNA damage leads to nucleosome leakage into the cytosol, then the self-DNA triggers STING-dependent cytokine production [88]. For tumor antigen-specific T cells effectively control the growth of cancer cells in vivo, they must gain access to and function within the TME, thus converting uninflamed “cold” tumors which don't respond to checkpoint inhibitors — into responsive “hot” cancers [5,89]. Accordingly, the STING pathway projects as an essential pathway that can be targeted for cancer therapy which can stimulate adaptive immune response systemically and provide long-lived immunologic memory [90]. Up to now, many studies have discovered that CDNs treatment can increase tumor and immune cell PD-L1 expression and the combinations with STING agonists and PD-1 mAbs have potential to be translated into the clinic, especially for checkpoint blockade resistant case [[91], [92], [93], [94]]. Interestingly, cancerous T cells exhibit an alternate signalling outcome in response to small molecular STING agonists, which manifests in apoptosis rather than the production of type I IFNs [[95], [96], [97]]. This is in sharp contrast to the response elicited by STING agonists in macrophages, dendritic cells or mouse embryonic fibroblasts (MEFs), which were found being largely apoptosis-resistant [97]. It is promising that STING-mediated stimulation of apoptosis should be further explored as a treatment option in the context of T-cell-derived human malignancies [97].
However, the activation of the cGAS–STING pathway can also lead to tolerogenic responses through the induction of indolamine 2,3-dioxygenase (IDO) [98]. Activation of STING promotes the growth of tumors with low antigenicity but not of those with higher antigenicity [99]. Brain-metastatic cancer cells produce cGAMP, which is transferred to neighboring astrocytes to activate the production of inflammatory cytokines, which facilitates metastasis [100]. Consequently, such studies warrant caution in ongoing clinical efforts to enhance cancer immunotherapy by stimulating the STING pathway with cGAMP analogs. Collectively, a good clinical outcome will probably be achieved through an optimal combination of different treatments, including stimulation of the innate immune system, the blockade of immune checkpoints, radiation, targeted cytotoxic agents and even adoptive T cell transfer [51].
3.3. STING and autoimmune disease
Inflammation from almost any causes can lead to the release of cellular debris that includes DNA and RNA. Whereas extracellular nucleases that help to degrade DNA are fairly abundant, self-nucleic acids can still enter into innate immune cells through multiple mechanisms. Although subtle biochemical modifications contained within host or bacterial nucleic acids may alter sensing by human phagocytes, in many cases the sensors respond equally to nucleic acids regardless of their origin and the task of self–nonself discrimination is particularly challenging for innate immune cells [101,102]. Increasing evidence suggests that the mislocalization of nuclear and mitochondrial dsDNA in the cytoplasm combined with the inability of cGAS to differentiate foreign from self dsDNA is a crucial pathogenesis of autoinflammation [[103], [104], [105], [106]].
Type I IFNs and mislocalized dsDNA are hallmarks and key drivers for the pathogenesis of autoimmune diseases such as systemic lupus erythematosus (SLE). Additionally, loss of function mutations in the DNA exonuclease three-prime repair exonuclease 1 (TREX1), which can ensure apoptotic cells remain “immunologically silent” following phagocytosis, lead to the excessive type I IFNs signature found in Aicardi−Goutieres syndrome (AGS) and SLE, implicating the role of self-dsDNA in autoinflammation [[107], [108], [109], [110]]. Furthermore, monogenic Mendelian diseases with STING gain of function mutations such as familial chilblain lupus (FCL) support the role of STING in autoimmune disease [108]. These studies collectively suggest that cGAS-STING pathway might regulate DNA-driven inflammatory diseases [21,36,111]. Mutations in TREX1 cause STING activation through failure to eliminate self-DNA that has leaked into the cytosol. Mutations in STING itself can lead to constitutive activity, then cause autoinflammatory diseases such as STING-associated vasculopathy with onset in infancy (SAVI) [19,62]. In the patients with FCL, STING harbouring G166E mutation induces new intramolecular polar interactions to make the structure of STING dimer tighter, resulting in constitutive type I IFN signalling in the absence of cGAMP ligand [108]. Similarly, patients with vascular and pulmonary syndrome (VAPS) were found to exhibit three mutations in exon 5 of TMEM173 (N154S, V155M and V147L). These variants potently stimulated the type I IFNs promoter, as determined by in vitro expression studies in 293T cells [19]. Speculatively, these mutations may expedite STING trafficking from the endoplasmic reticulum to the perinuclear region or affect STING protein stability, thereby sustaining STING activity [112]. Hiroyasu Konno et al. identified a STING (R284S) as a new gain-of-function mutation which did not require CDNs to augment activity [113]. Taken together, TMEM173 gain-of-function mutations should be screened for as a monogenic cause of this broad spectrum of diseases. STING could represent a new therapeutic target in these disorders as well as other more common inflammatory diseases triggered by cytosolic DNA stimulation of microbial or endogenous origin.
4. The development of STING modulators
4.1. The agonist of STING
Pharmacologic activation of STING-dependent signaling has shown promise in diverse clinically impactful applications including broad-acting antiviral treatments, vaccine adjuvants [[114], [115], [116]] and immunogenic tumor clearance. This has led to academic and commercial efforts to formulate CDNs for pharmaceutical use including their advancement to an ongoing clinical trial. Unfortunately, CDNs may be chemically undesirable for research and clinical work since: 1) They violate Lipinski rules [117] for druglikeness and are not amenable to large structural changes; 2) They are susceptible to phosphodiesterase-mediated degradation [71]; 3) Their size and hydrophilicity render them impermeable to cell membranes [78]. Small molecular STING activators can mitigate these factors, as well-exemplified by the mouse-specific compound 5,6-dimethylxanthenone-4-acetic acid (DMXAA) [[118], [119], [120]]. Identification of novel small molecule STING agonists that are efficacious across species are thus highly sought since they may develop valuable research tools to understand STING-mediated processes. Furthermore, their use in animals enables broad assessment of safety and biological mechanisms.
4.1.1. Cyclic dinucleotides
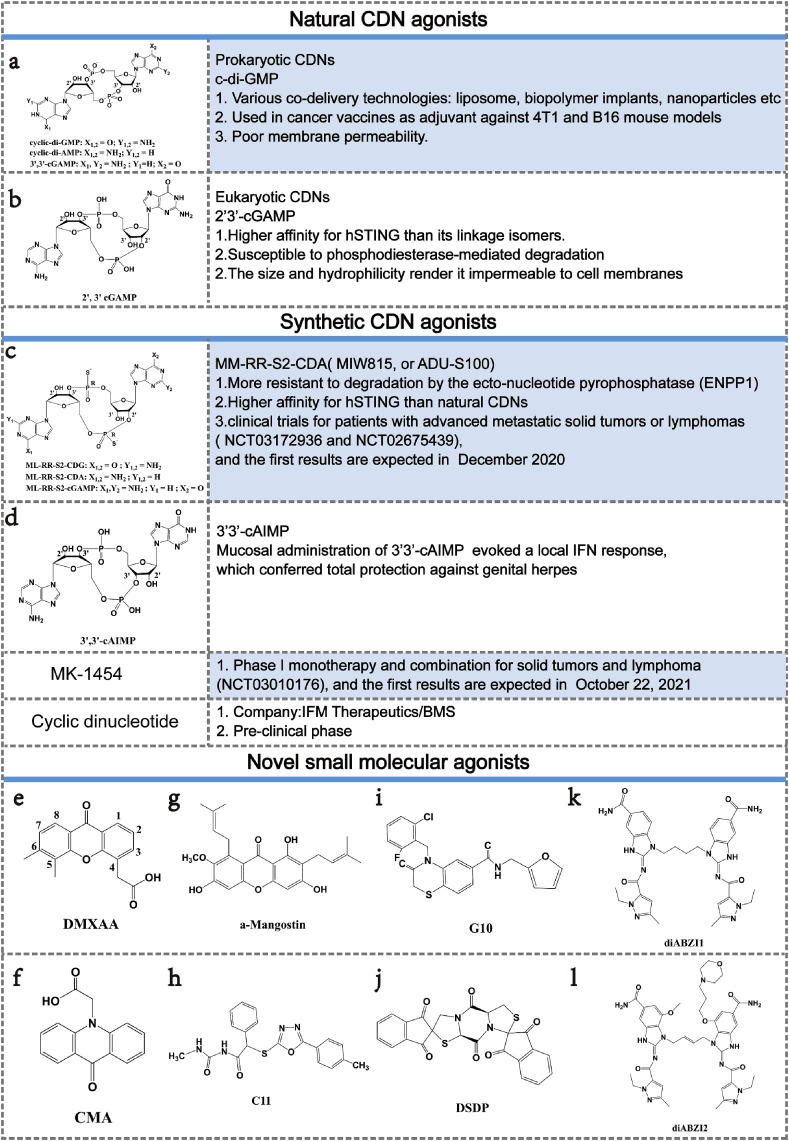
CDNs were first described in bacteria [121] and the known naturally occurring examples consist of two nucleotides(A or G) that are cyclized by canonical phosphodiester bond to form cyclic-di-GMP (c-di-GMP), cyclic-di-AMP (c-di-AMP) or cGAMP (Fig. 3 a) [69]. The endogenous CDNs produced by cGAS is the only known mammalian CDNs, named as 2′3′-cGAMP (Fig. 3b), which is chemically distinct from bacterial CDNs 3′3′-cGAMP (Fig. 3a) by containing mixed phosphodiester bond. Interestingly, relative to 3′3′-cGAMP and other CDNs, 2′3′-cGAMP shows an exquisite high affinity for STING and elicits stronger type I IFNs responses. The Kd of 2′3′-cGAMP was ∼300-fold lower than those of c-di-GMP, 3′2′-cGAMP and 3′3′-cGAMP and ∼75-fold lower than that of 2′2′-cGAMP [13,122,123]. Meanwhile, given that free CDNs alone permeate through cell membrane inefficiently in vitro but require the addition of lipofectamine or another transfection reagent, the different transmembrane capabilities of various CDNs affect the function of type I IFNs at some extent. According to the structural studies, 2′3′-cGAMP adopts an ordered conformation as a free ligand which can form more-extensive interactions inside the STING ligand-binding pocket as compared to 3′3′-cGAMP, thereby stabilizing a specific intermediate state with a readily covered lid (Fig. 4 A) [13,33]. The small-molecule nature of 2′3′-cGAMP and the promise of STING has spurred interest in applying STING agonists for vaccine adjuvants or immunotherapy [[124], [125], [126]]. Given that CDNs has poor druglikeness, technologies for delivering CDNs to the cytosol are required. Intravenous injection of c-di-GMP encapsulated within YSK05-liposomes induced a striking decrease of metastatic lesions in the B16F10 mouse melanoma model with almost 40% of mice showing full protection against tumor rechallenge, suggesting the induction of a memory adaptive immune response [127]. Biopolymer implants were also used to co-deliver c-di-GMP with chimeric antigen receptor T (CAR-T) cells [128]. Meanwhile, STING-activating nanoparticles (STING-NPs)—rationally designed polymersomes for enhanced cytosolic delivery of cGAMP which enhance STING signalling in the TME and sentinel lymph node and convert immunosuppressive tumors to immunogenic, tumoricidal microenvironments [129]. Recently, combination of cytotoxic cationic silica nanoparticles and c-di-GMP has showed dramatically increased expansion of antigen-specific CD8+ T cells and potent tumor growth inhibition in murine melanoma [130]. Juan Fu et al. formulated CDNs ligands with granulocyte-macrophage colony-stimulating factor (GMCSF)–producing cellular cancer vaccines—termed STINGVAX—that demonstrated potent in vivo antitumor efficacy in multiple therapeutic models of established cancer [131]. Taken together, CDNs has high translational potential as a therapeutic intervention strategy to induce activation of the TME in multiple tumor types, with the mechanistic goal of generating effective tumor-initiated CD8+ T cell priming and lasting anti-tumor efficacy [90].
Fig. 3.
The summary of STING agonists.
Fig. 4.
A) Crystal structure of rat STING in complex with 2′3′-cGAMP (PDB code: 5GRM); B) Crystal structure of hSTING(G230I) in complex with DMXAA (PDB code: 4QXP). The DMXAA, S162, G230 and Q266 are colored green, cyan, red and yellow respectively; C) Crystal structure of human STING in complex with diABZI1 (PDB code: 6DXL); D) Crystal structure of human STING (G230A, H232R, R293Q) in complex with C18 (PDB code: 6MXE). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Synthetic CDNs with more favorable properties are in urgent needs and newly designed compounds are now entering trials for patients with advanced metastatic solid tumors or lymphomas (Fig. 3). To increase enzymatic stability, sulfur has been used to replace the nonbridging oxygen from the phosphodiester linkages to make phosphorothioates. The resulting compound, 2′3′-cGsAsMP (Fig. 3c), is more resistant to degradation by the ecto-nucleotide pyrophosphatase (ENPP1) which is identified as the major 2′3′-cGAMP hydrolase, prolonging its systemic half-life while maintaining high affinity for STING [132]. Another ‘dithio’ CDN analog made of two AMP moieties cyclized via 2′3′- and 3′5′-phosphodiester bonds (known as ML-RR-S2-CDA, MIW815 or ADU-S100) (Fig. 3c), shows improved IFN-β responses and tumor regression in established B16 tumors when compared with 2′3′-cGAMP. Antitumor efficacy was also observed after intratumoral injection of ML-RR-S2-CDA in 4T1 and CT26 mouse models with substantial systemic immune responses capable of rejecting distant metastases and providing long-lived immunologic memory [90]. These striking preclinical results provided support for the use of ML-RR-S2-CDA in clinical trials for patients with advanced metastatic solid tumors or lymphomas (clinicaltrials.gov: NCT03172936 and NCT02675439), and the first results are expected in 2020. Merck & Co. launched a trial early in 2017 of a CDN compound MK-1454 alone and in combination with the approved checkpoint PD1 blocker pembrolizumab (clinicaltrials.gov: NCT03010176), while the structure of this molecule has not yet been disclosed. Bristol-Myers Squibb (BMS) acquired the biotech IFM Therapeutics put US$300 million upfront to get their hands on preclinical CDN agonist (Fig. 3) [5]. Mucosal administration of the STING agonist 3′3′-cAIMP (Fig. 3d) evoked a local IFN response, which conferred total protection against genital herpes, even in a highly permissive mouse strain [133,134].
Generally, the efficacy of CDNs is limited by drug delivery barriers, including poor cellular targeting, rapid clearance and inefficient transport to the cytosol where STING is localized. Even so, their nucleotidic and anionic nature warrant the development of molecules with improved drug-like qualities, with simple chemical synthesis and more favorable pharmacokinetic profiles [135].
4.1.2. DMXAA and its analogues
Different with CDNs activating STING universally, DMXAA (also known as ASA404 or vadimezan) (Fig. 3e) was the first small molecule discovered to be a potent agonist of mouse STING (mSTING), regrettably, but not human STING (hSTING), which may explain the failure of DMXAA in clinical trials for human cancer [118,120,136]. 10-carboxymethyl-9-acridanone (CMA) (Fig. 3f) and DMXAA are structurally similar. Like DMXAA, CMA also showed impressive anti-cancer activity in mice but failed in men [120].
According to the structural researches of STING, the modification of DMXAA is likely to produce potent modulator of hSTING [137,138]. Atomic-level understanding of the interaction between DMXAA and hSTING became available by elegant structural, biophysical and cellular assay studies reported by Gao Pu and coworkers. They identified three points substitutions (S162A, G230I and Q266I) of hSTING which synergistically rendered the mutated hSTING highly sensitive to DMXAA (Fig. 4B). This inspiring structural and functional results shed light on strategies to restore an efficient hSTING response to DMXAA based on the binding-pocket S162A or Q266I substitutions [33,138]. Xing Che et al. further discovered E260I mutation together with S162A endowed human STINGAQ with DMXAA sensitivity. The molecular dynamics simulations showed that these two mutations changed the interaction network between DMXAA and STINGAQ. Moreover, a smaller number of water molecules are displaced upon DMXAA binding to STINGAQ than that on binding to its mutants, leading to a larger entropic penalty for the former [139]. Amy Y. Shih et al. unraveled the dynamic structural differences between hSTING and mSTING. Simulations found that an I230 side chain (corresponding residue in hSTING is G) was enough to form a steric barrier that prevents the exit of DMXAA, whereas in wild-type hSTING, the G230 without a side chain formed a porous lid region that allowed DMXAA to exit [35]. Such observations gave us insight into translating STING agonists from mouse active into human active, while also providing avenues to pursue for designing a small-molecule drug targeting human STING [139].
Current efforts to generate reciprocal DMXAA derivatives containing polar groups (OH, OCH3, F, Cl, and NO2) at the C1/C2 (S162A substitution) and C7 (Q266I substitution) positions within the DMXAA ring so as to form additional intermolecular hydrogen bonds, as well as to replace the six-membered aromatic rings with their five-membered counterparts [138,140]. Given that two molecules of DMXAA bind to the STING dimer, ongoing efforts are also being directed toward the generation and evaluation of covalently linked DMXAA dimers. Meanwhile, 7-methyl-XAA and 8-methyl-XAA which involve the redistribution of CH3 substitutions on the DMXAA scaffold, have been identified as weak yet human-active and possibly also human-selective DMXAA analogs [137]. Meanwhile, Yibo Zhang et al. identified α-Mangostin (Fig. 3g) sharing the same xanthone skeleton with DMXAA as a weak agonist of hSTING [141]. These findings provide a critical guide for future rational drug design of DMXAA variants with potential to enhance the interferons production in humans, which are needed for the development of anticancer therapies and vaccine adjuvants.
4.1.3. Novel small molecular agonists
Direct STING agonists have been used widely in preclinical and clinical studies for the treatment of cancer, but most STING agonists are modified CDNs with limited clinical application due to their poor druggability. However, it is a huge challenge to develop small molecular STING agonists that is human-active and suitable for systemic administration. Large binding pocket of STING posts a challenge to drug design because of the demand for ∼700 Da molecular weight ligands, which generally possess undesirable physicochemical properties.
Bryan Gall et al. conducted a high-throughput screening assay and identified N (Methylcarbamoyl)-2-{[5-(4-methylphenyl)-1,3,4-oxadiazol-2-yl]sulfany-2 phenylacetamide (referred to as C11) (Fig. 3h), a novel compound that triggers IRF3/IFN-dependent responses in a STING-dependent manner. The luciferase activity in THF reporter cell lines increased to 50-fold above that for mock treatments at 100 μM C11 [142]. Similarly, Tina M. Sali et al. identified 4-(2-chloro-6-fluorobenzyl)-N-(furan-2-ylmethyl)-3-oxo-3,4-dihydro-2H-benzo[b]thiazine-6-carboxamide (referred to as G10) (Fig. 3i) which selectively induces STING-dependent synthesis and secretion of bioactive interferons [143]. Bowei Liu et al. identified a dispiro diketopiperzine (DSDP) (Fig. 3j) inducing proinflammatory cytokine response in a manner dependent on the expression of functional hSTING selectively. The CC50 values of DSDP were greater than 100 μM in the corresponding reporter cell lines. However, the above small agonists are lacked of the evidence that suggest compounds binding directly to STING [144].
Excitingly, Joshi M. ramanjulu et al. discovered a new class of synthetic small molecular STING agonists suitable for systemic administration, named as dimeric amidobenzimidazole (diABZI). Similar to cGAMP, diABZI1 (Fig. 3k) induced dose-dependent secretion of IFN-β with an apparent effective constant (EC50 app) of 3.1 ± 0.6 μM diABZI1 is around 18-fold more potent than cGAMP, which has an EC50 app of 53.9 ± 5 μM. With further lead optimization, diABZI2 (Fig. 3l) selectively induced dose-dependent activation of STING and secretion of IFN-β with an EC50 app of 130 nM that is more than 400-fold more potent than cGAMP in human peripheral blood mononuclear cells (PBMCs). Different with cGAMP inducing STING to the closed conformation, diABZI can activate STING in “open” conformation (Fig. 4C). Intravenous administration of diABZI to immunocompetent mice with established syngeneic colon tumors elicited strong anti-tumor activity with complete and lasting regression of tumors [145]. The ABZI series of STING agonists bring in a new chemical class with completely different physicochemical properties than the CDN class. This enriches the chemical space for activating STING and provides more options for the clinical development of STING agonists.
4.2. The antagonists of STING
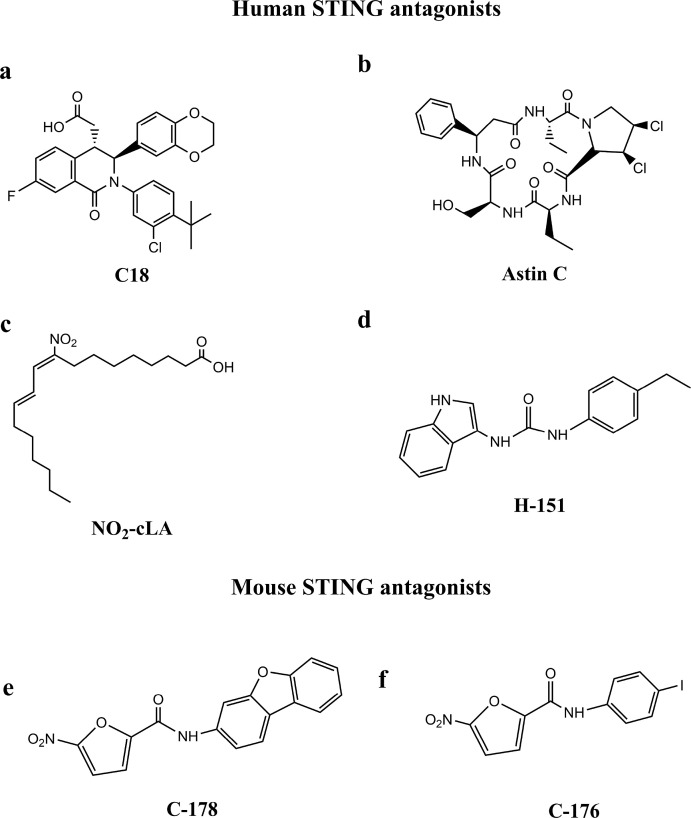
The biological rationale linking STING to inflammatory diseases supports the development of STING antagonists. However, the general lack of biochemical mechanistic understanding of STING activation leads to a significant challenge in the development of binders to the STING protein [36]. It is inspiring that the possibility of utilizing 2:1 binding stochiometry can offset the challenge from a large binding pocket, and thus effectively compete with cGAMP, while still maintaining physiochemical properties compatible with oral drugs. With the help of Automated Ligand Identification System (ALIS), Tony Siu identified a weak antagonist C18 (Fig. 5 a) of STING-mediated signaling which can compete with cGAMP at a 2:1 binding ratio in the inactive “open” conformation (Fig. 4D) [36]. The compound C18 did not stimulate IFN-β production (EC50 ≥ 30 μM), but modestly inhibited cGAMP induced IFN-β production (IC50 = 11 μM), with a >100-fold shift in potency from binding to the functional cell assay. Considering that the assay is stimulated with nonphysiological levels of cGAMP, it is speculated that the cellular IC50 value may not be an accurate representation of antagonist potency [36]. Senlin Li et al. identified Astin C (Fig. 5b), a cyclopeptide isolated from the medicinal plant Aster tataricus, specifically blocks the recruitment of IRF3 onto the STING signalosome through binding to the STING CTD. Astin C showed comparable binding affinity to STING R232 as 2′3′-cGAMP, with a dissociation constant value (Kd) of 53 nM. The inhibitory effect of Astin C on intracellular DNA-induced IFN-β expression was dose dependent, with IC50 values of 3.42 ± 0.13 and 10.83 ± 1.88 μM for MEFs and human fetal lung fibroblasts cells (IMR-90), respectively [146].
Fig. 5.
The structures of STING antagonists.
From now on, it has been largely unknown how the N-terminal region and its four predicted transmembrane helices contribute to STING function. Recently, studies has identified N-terminal cysteine residues at positions 88 and 91 as the palmitoylation site [37]. Palmitoylation at C88/91 was important for STING-dependent phosphorylation of TBK1 in the trans-Golgi network and thus central to STING dependent induction of type I IFNs. Anne Louise Hansen et al. have reported that endogenous nitro-conjugated linoleic acid (NO2-cLA) (Fig. 5c) can covalently modify STING by nitro-alkylation at C88/91 and H16, then lead to a deregulation of STING palmitoylation and inhibit the STING signaling in human cells, like fibroblasts from SAVI patients. Since NO2–FAs have been reported to be a well-tolerated treatment in humans (clinicaltrials.gov: NCT02460146 and NCT02313064), this finding has considerable medical potential [147]. Simone M. Haag et al. performed a cell-based chemical screening and identified two nitrofuran derivatives—C-178 (Fig. 5e) and C-176 (Fig. 5f) —that strongly inhibited mSTING selectively through covalently targeting the C91, then prevented palmitoylation at C91 to prevent STING from interacting with TBK1 [37,38]. Furthermore, they found H151 (Fig. 5d) could efficiently inhibit hSTING selectively through the same mechanism as C-176 and C-178. Meanwhile, it is significant that in vivo studies of H151 and C176 provide proof-of-concept that STING antagonists are efficacious in the treatment of autoinflammatory disease.
5. Summary and future perspectives
Initial successes with therapies targeting the immunostimulatory effects of the cGAS-STING pathway suggest a major clinical impact in areas of cancer immunotherapy, vaccine development and autoimmune disease. However, it is a challenge to design small molecules because the dimer interface of STING CTD was dominated by hydrophobic interactions up to 65.5% with no salt bridges participating in the dimerization. The hydrophobic interface may lead to the discovery of false positive hits. Furthermore, the large ligand binding pocket in STING requires a ligand of ∼700 Da to occupy. Inevitably, ligands with large volume may cause downstream development problems such as toxicity and poor pharmacokinetics [148]. Joshi M. ramanjulu et al. designed a linker between two amidobenzimidazole molecules, then enhance their activities significantly [145]. Similarly, Tony Siu et al. was inspired by DMXAA binding to STING at a 2:1 ratio, they identified a series of antagonists and concluded that maximal buried interaction with STING protein can be achieved while maintaining the ligand physicochemical properties necessary for oral exposure [36]. Up to now, it has been a mainstream that “open” to “closed” conformational change is in charge of STING activation. However, the above two series of compounds can bind to the STING CTD in “open” conformation but produce opposite effect. Undoubtedly, the information from crystal structures is insufficient. Different ligand-protein interactions may have the capacity to alter protein flexibility, dynamics, ultimately, function. It has been recognized that dynamics is important in populating available protein conformational states, rates and probabilities of states redistribution upon ligand binding and proteins shift conformational populations through cooperative motions, while enabling their functions [139]. Consequently, it is in urgent need of molecular dynamics study of STING. Taken together, with more crystal structures of STING available and the advancement of molecular dynamics studies, structure-based and/or ligand-based design may be utilized to design novel small molecular modulators targeting STING with improved selectivity/specificity profiles [148].
Except molecules binding to STING directly, pharmacological interventions aimed at modulating the upstream and downstream signaling may hold similar promise. For example, Mavupharma company has identified MAVU-104 as a first-in-class, orally active, small molecule inhibitor of ENPP1, a phosphodiesterase that hydrolysis 2′3′-cGAMP selectively. Inhibiting ENPP1 activity with MAVU-104 allows for highly-controlled enhancement of STING signaling in all tumors and lymph nodes without any injections. The molecules aimed at antagonizing cGAS or STING functions hold similar promise. For instance, RU.521, PF-06928215 and suramin were found to be high-affinity inhibitors of cGAS [149,150]. Interestingly, a few antimalarial drugs, including quinacrine and chloroquine, were also reported to dismiss the activity of cGAS [151,152]. Moreover, disrupting the degradation and expression of STING may provide new insight into the development of small molecular modulators of STING activity.
Considering that complicated mechanisms to regulate the magnitude of cGAS-STING pathway signaling remain to be clarified, choosing STING as drug target should be conscious of the potential risks. Henrique Lemos et al. identified that STING agonists could promote the growth of tumors characterized by low antigenicity via the activation of IDO which is an immune checkpoint that attenuates tumor immunity [99]. Consequently, STING agonists may not be effective in all tumor settings, particularly those where robust tolerogenic responses to DNA and low tumor antigenicity prevail. Furthermore, hyperactivation of STING is likely to spark a severe inflammatory response called a cytokine storm [153], in which levels of cytokines become abnormally high. The occurrence of cytokine storm results in fever, low blood pressure, heart problems and, in some cases, organ failure and death. Meanwhile, the existence of SNPs in hSTING leads to some patients showing low response to STING agonists. Combination with personalized cancer immunotherapy offers the potential to make the therapy more specific, more effective and safer compared with the cancer immunotherapies that we have available today [154].
It is clear that we now stand at an important crossroad of opportunities to enhance our ability to use the immune system to make cancer a controllable and, in some cases, curable disease. An in-depth understanding of the cGAS-STING pathway, including the careful consideration of possible species-specific differences, will be instrumental for further development of therapeutics targeting the DNA-sensing pathway. If successful, pharmacological agonists and antagonists of cGAS and STING could become effective therapies for many prevalent and emerging diseases, including infectious, autoimmune, inflammatory and cancer. In the end, one should be reminded that honey is never far away from the “STING”.
Acknowledgments
This work was in part supported by the National Key Research and Development Program (SQ2018YFGH000100 ZJM), Nature Science Foundation of China (81672559), CAMS Innovation Fund for Medical Sciences (CAMS-I2M-1-012 ZJM) and National Mega-Project for Significant new drug discovery (2018ZX09711003-002-002,ZJM).
Abbreviations used
- cGAS
cyclic guanosine monophosphate–adenosine monophosphate synthase
- STING
stimulator of interferon genes
- PRRs
pattern recognition receptors
- IFNs
interferons
- ER
endoplasmic reticulum
- ATP
adenosine 5′-triphosphate
- GTP
guanosine 5′-triphosphate
- cGAMP
cyclic guanosine monophosphate–adenosine monophosphate
- CDNs
cyclic dinucleotides
- dsDNA
double-stranded DNA
- ERGIC
ER-Golgi intermediate compartment
- TBK1
tank-binding kinase 1
- IRF3
interferon regulatory factor 3
- IFN-β
interferon β
- JAKs
janus kinases
- TYK2
tyrosine kinase 2
- STAT1
signal transducers and activators of transcription 1
- STAT2
signal transducers and activators of transcription 2
- IRF7
interferon regulatory factor 7
- LSm14A
protein LSm14 homolog A
- LSm
Sm-like protein
- mRNA
messenger RNA
- ULK1/ATG1
serine/threonine-protein kinase 1/autophagy-related protein 1
- ATG9a
autophagy-related protein 9a
- NLRC3
NOD-like receptor C3
- ZDHHC1
zinc finger DHHC domain-containing protein 1
- UBXN3B
UBX domain-containing protein 3B
- RNF5
RING finger protein 5
- RNF26
RING finger protein 26
- K
lysine
- WIPI2
WD repeat domain phosphoinositide-interacting protein 2
- RAB7
Ras-related protein Rab-7a
- CTD
C-terminal domain
- C
cystine
- S
serine
- L
leucine
- R
arginine
- SNPs
single nucleotide polymorphisms
- TMEM173
transmembrane protein 173
- H
histidine
- G
glycine
- A
alanine
- Q
glutamine
- T
threonine
- ssDNA
single-stranded DNA
- HIV
human immunodeficiency virus
- KSHV
Kaposi's sarcoma-associated herpesvirus
- IFI16
interferon-γ inducible protein 16
- HSV
Herpesviruses
- PEDV
Porcine epidemic diarrhea virus
- FP
hemagglutinin fusion peptide
- NS2B-NS3 protease
nonstructural protein 2B-nonstructural 3 protease
- TME
tumor microenvironment
- DAMPs
danger-associated molecular patterns
- PD-L1
programmed cell death-ligand 1
- mAbs
monoclonal antibodies
- MEFs
mouse embryonic fibroblasts
- IDO
indolamine 2,3-dioxygenase
- SLE
systemic lupus erythematosus
- TREX1
three-prime repair exonuclease 1
- AGS
Aicardi−Goutieres syndrome
- FCL
familial chilblain lupus
- SAVI
STING-associated vasculopathy with onset in infancy
- E
glutamic acid
- VAPS
vascular and pulmonary syndrome
- N
asparagine
- V
valine
- M
methionine
- DMXAA
5,6-dimethylxanthenone-4-acetic acid
- c-di-GMP
cyclic-di-GMP
- c-di-AMP
cyclic-di-AMP
- CAR-T cells
chimeric antigen receptor T cells
- STING-NPs
STING-activating nanoparticles
- GMCSF
granulocyte-macrophage colony-stimulating factor
- ENPP1
ecto-nucleotide pyrophosphatase
- ML-RR-S2-CDA
two AMP moieties cyclized via 2′3′- and 3′5′-phosphodiester bonds
- mSTING
mouse STING
- hSTING
human STING
- CMA
10-carboxymethyl-9-acridanone
- I
isoleucine
- DSDP
dispiro diketopiperzine
- diABZI
dimeric amidobenzimidazole
- EC50app
apparent 50% effective constant
- PBMCs
peripheral blood mononuclear cells
- ABZI
amidobenzimidazole
- ALIS
Automated Ligand Identification System
- IC50
50% inhibitory constant
- Kd
dissociation constant
- IMR-90
human fetal lung fibroblasts cells
- NO2-cLA
nitro-conjugated linoleic acid
References
- 1.Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Roers A., Hiller B., Hornung V. Recognition of endogenous nucleic acids by the innate immune system. Immunity. 2016;44:739–754. doi: 10.1016/j.immuni.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Ablasser A., Chen Z.J. cGAS in action: expanding roles in immunity and inflammation. Science. 2019:363. doi: 10.1126/science.aat8657. [DOI] [PubMed] [Google Scholar]
- 4.Abe T., Harashima A., Xia T., Konno H., Konno K., Morales A., Ahn J., Gutman D., Barber G.N. STING recognition of cytoplasmic DNA instigates cellular defense. Mol. Cell. 2013;50:5–15. doi: 10.1016/j.molcel.2013.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mullard A. Can innate immune system targets turn up the heat on 'cold' tumours? Nat. Rev. Drug Discov. 2018;17:3–5. doi: 10.1038/nrd.2017.264. [DOI] [PubMed] [Google Scholar]
- 6.Margolis S.R., Wilson S.C., Vance R.E. Evolutionary origins of cGAS-STING signaling. Trends Immunol. 2017;38:733–743. doi: 10.1016/j.it.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Wu X., Wu F.H., Wang X., Wang L., Siedow J.N., Zhang W., Pei Z.M. Molecular evolutionary and structural analysis of the cytosolic DNA sensor cGAS and STING. Nucleic Acids Res. 2014;42:8243–8257. doi: 10.1093/nar/gku569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gui X., Yang H., Li T., Tan X., Shi P., Li M., Du F., Chen Z.J. Autophagy induction via STING trafficking is a primordial function of the cGAS pathway. Nature. 2019;567:262–266. doi: 10.1038/s41586-019-1006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rasmussen S.B., Horan K.A., Holm C.K., Stranks A.J., Mettenleiter T.C., Simon A.K., Jensen S.B., Rixon F.J., He B., Paludan S.R. Activation of autophagy by alpha-herpesviruses in myeloid cells is mediated by cytoplasmic viral DNA through a mechanism dependent on stimulator of IFN genes. J. Immunol. 2011;187:5268–5276. doi: 10.4049/jimmunol.1100949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y., Gordesky-Gold B., Leney-Greene M., Weinbren N.L., Tudor M., Cherry S. Inflammation-induced, STING-dependent autophagy restricts Zika virus infection in the Drosophila brain. Cell Host Microbe. 2018;24:57–68 e53. doi: 10.1016/j.chom.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lijun S., Jiaxi W., Fenghe D., Xiang C., Chen Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kranzusch P.J., Lee A.S., Berger J.M., Doudna J.A. Structure of human cGAS reveals a conserved family of second-messenger enzymes in innate immunity. Cell Rep. 2013;3:1362–1368. doi: 10.1016/j.celrep.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ablasser A., Goldeck M., Cavlar T., Deimling T., Witte G., Rohl I., Hopfner K.P., Ludwig J., Hornung V. cGAS produces a 2'-5'-linked cyclic dinucleotide second messenger that activates STING. Nature. 2013;498:380–384. doi: 10.1038/nature12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai X., Chiu Y.H., Chen Z.J. The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Mol. Cell. 2014;54:289–296. doi: 10.1016/j.molcel.2014.03.040. [DOI] [PubMed] [Google Scholar]
- 15.Wu J., Sun L., Chen X., Du F., Shi H., Chen C., Chen Z.J. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339:826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin Q., Tian Y., Kabaleeswaran V., Jiang X., Tu D., Eck M.J., Chen Z.J., Wu H. Cyclic di-GMP sensing via the innate immune signaling protein STING. Mol. Cell. 2012;46:735–745. doi: 10.1016/j.molcel.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krasteva P.V., Sondermann H. Versatile modes of cellular regulation via cyclic dinucleotides. Nat. Chem. Biol. 2017;13:350–359. doi: 10.1038/nchembio.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aoyama T., Suzuki K., Tashiro H., Tada Y., Arimoto H., Horiuchi K. Boron penetration in p-channel metal–oxide–semiconductor field-effect transistors enhanced by gate ion-implantation damage. J. Appl. Phys. 2001;89:4570–4574. [Google Scholar]
- 19.Liu Y., Jesus A.A., Marrero B., Yang D., Ramsey S.E., Montealegre Sanchez G.A., Tenbrock K., Wittkowski H., Jones O.Y., Kuehn H.S., Lee C.-C.R., DiMattia M.A., Cowen E.W., Gonzalez B., Palmer I., DiGiovanna J.J., Biancotto A., Kim H., Tsai W.L., Trier A.M., Huang Y., Stone D.L., Hill S., Kim H.J., Hilaire C. St, Gurprasad S., Plass N., Chapelle D., Horkayne-Szakaly I., Foell D., Barysenka A., Candotti F., Holland S.M., Hughes J.D., Mehmet H., Issekutz A.C., Raffeld M., McElwee J., Fontana J.R., Minniti C.P., Moir S., Kastner D.L., Gadina M., Steven A.C., Wingfield P.T., Brooks S.R., Rosenzweig S.D., Fleisher T.A., Deng Z., Boehm M., Paller A.S., Goldbach-Mansky R. Activated STING in a vascular and pulmonary syndrome. N. Engl. J. Med. 2014;371:507–518. doi: 10.1056/NEJMoa1312625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gentili M., Kowal J., Tkach M., Satoh T., Lahaye X., Conrad C., Boyron M., Lombard B., Durand S., Kroemer G., Loew D., Dalod M., Thery C., Manel N. Transmission of innate immune signaling by packaging of cGAMP in viral particles. Science. 2015;349:1232–1236. doi: 10.1126/science.aab3628. [DOI] [PubMed] [Google Scholar]
- 21.Ahn J., Barber G.N. Self-DNA, STING-dependent signaling and the origins of autoinflammatory disease. Curr. Opin. Immunol. 2014;31:121–126. doi: 10.1016/j.coi.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Liu T.T., Yang Q., Li M., Zhong B., Ran Y., Liu L.L., Yang Y., Wang Y.Y., Shu H.B. LSm14A plays a critical role in antiviral immune responses by regulating MITA level in a cell-specific manner. J. Immunol. 2016;196:5101–5111. doi: 10.4049/jimmunol.1600212. [DOI] [PubMed] [Google Scholar]
- 23.Konno H., Konno K., Barber G.N. Cyclic dinucleotides trigger ULK1 (ATG1) phosphorylation of STING to prevent sustained innate immune signaling. Cell. 2013;155:688–698. doi: 10.1016/j.cell.2013.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitzel D.N., Lowry V., Shirali A.C., Liu Y., Stout-Delgado H.W. Age-enhanced endoplasmic reticulum stress contributes to increased Atg9A inhibition of STING-mediated IFN- production during Streptococcus pneumoniae infection. J. Immunol. 2014;192:4273–4283. doi: 10.4049/jimmunol.1303090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mangan M.S., Latz E. NLRC3 puts the brakes on STING. Immunity. 2014;40:305–306. doi: 10.1016/j.immuni.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Q., Lin H., Wang S., Wang S., Ran Y., Liu Y., Ye W., Xiong X., Zhong B., Shu H.B., Wang Y.Y. The ER-associated protein ZDHHC1 is a positive regulator of DNA virus-triggered, MITA/STING-dependent innate immune signaling. Cell Host Microbe. 2014;16:450–461. doi: 10.1016/j.chom.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Yang L., Wang L., Ketkar H., Ma J., Yang G., Cui S., Geng T., Mordue D.G., Fujimoto T., Cheng G., You F., Lin R., Fikrig E., Wang P. UBXN3B positively regulates STING-mediated antiviral immune responses. Nat. Commun. 2018;9:2329. doi: 10.1038/s41467-018-04759-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhong B., Zhang L., Lei C., Li Y., Mao A.P., Yang Y., Wang Y.Y., Zhang X.L., Shu H.B. The ubiquitin ligase RNF5 regulates antiviral responses by mediating degradation of the adaptor protein MITA. Immunity. 2009;30:397–407. doi: 10.1016/j.immuni.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Ma Z., Damania B. The cGAS-STING defense pathway and its counteraction by viruses. Cell Host Microbe. 2016;19:150–158. doi: 10.1016/j.chom.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang Q., Seo G.J., Choi Y.J., Kwak M.J., Ge J., Rodgers M.A., Shi M., Leslie B.J., Hopfner K.P., Ha T., Oh B.H., Jung J.U. Crosstalk between the cGAS DNA sensor and Beclin-1 autophagy protein shapes innate antimicrobial immune responses. Cell Host Microbe. 2014;15:228–238. doi: 10.1016/j.chom.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dooley H.C., Razi M., Polson H.E., Girardin S.E., Wilson M.I., Tooze S.A. WIPI2 links LC3 conjugation with PI3P, autophagosome formation, and pathogen clearance by recruiting Atg12-5-16L1. Mol. Cell. 2014;55:238–252. doi: 10.1016/j.molcel.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ouyang S., Song X., Wang Y., Ru H., Shaw N., Jiang Y., Niu F., Zhu Y., Qiu W., Parvatiyar K., Li Y., Zhang R., Cheng G., Liu Z.J. Structural analysis of the STING adaptor protein reveals a hydrophobic dimer interface and mode of cyclic di-GMP binding. Immunity. 2012;36:1073–1086. doi: 10.1016/j.immuni.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao P., Ascano M., Zillinger T., Wang W., Dai P., Serganov A.A., Gaffney B.L., Shuman S., Jones R.A., Deng L., Hartmann G., Barchet W., Tuschl T., Patel D.J. Structure-function analysis of STING activation by c[G(2',5')pA(3',5')p] and targeting by antiviral DMXAA. Cell. 2013;154:748–762. doi: 10.1016/j.cell.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsuchida T., Zou J., Saitoh T., Kumar H., Abe T., Matsuura Y., Kawai T., Akira S. The ubiquitin ligase TRIM56 regulates innate immune responses to intracellular double-stranded DNA. Immunity. 2010;33:765–776. doi: 10.1016/j.immuni.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 35.Shih A.Y., Damm-Ganamet K.L., Mirzadegan T. Dynamic structural differences between human and mouse STING lead to differing sensitivity to DMXAA. Biophys. J. 2018;114:32–39. doi: 10.1016/j.bpj.2017.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siu T., Altman M.D., Baltus G.A., Childers M., Ellis J.M., Gunaydin H., Hatch H., Ho T., Jewell J., Lacey B.M., Lesburg C.A., Pan B.S., Sauvagnat B., Schroeder G.K., Xu S. Discovery of a novel cGAMP competitive ligand of the inactive form of STING. ACS Med. Chem. Lett. 2019;10:92–97. doi: 10.1021/acsmedchemlett.8b00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukai K., Konno H., Akiba T., Uemura T., Waguri S., Kobayashi T., Barber G.N., Arai H., Taguchi T. Activation of STING requires palmitoylation at the Golgi. Nat. Commun. 2016;7:11932. doi: 10.1038/ncomms11932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haag S.M., Gulen M.F., Reymond L., Gibelin A., Abrami L., Decout A., Heymann M., van der Goot F.G., Turcatti G., Behrendt R., Ablasser A. Targeting STING with covalent small-molecule inhibitors. Nature. 2018;559:269–273. doi: 10.1038/s41586-018-0287-8. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka Y., Chen Z.J. STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci. Signal. 2012;5:ra20. doi: 10.1126/scisignal.2002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu S., Cai X., Wu J., Cong Q., Chen X., Li T., Du F., Ren J., Wu Y.T., Grishin N.V., Chen Z.J. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science. 2015;347:aaa2630. doi: 10.1126/science.aaa2630. [DOI] [PubMed] [Google Scholar]
- 41.Wang Q., Liu X., Cui Y., Tang Y., Chen W., Li S., Yu H., Pan Y., Wang C. The E3 ubiquitin ligase AMFR and INSIG1 bridge the activation of TBK1 kinase by modifying the adaptor STING. Immunity. 2014;41:919–933. doi: 10.1016/j.immuni.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 42.Yi G., Brendel V.P., Shu C., Li P., Palanathan S., Cheng Kao C. Single nucleotide polymorphisms of human STING can affect innate immune response to cyclic dinucleotides. PLoS One. 2013;8 doi: 10.1371/journal.pone.0077846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sauer J.D., Sotelo-Troha K., von Moltke J., Monroe K.M., Rae C.S., Brubaker S.W., Hyodo M., Hayakawa Y., Woodward J.J., Portnoy D.A., Vance R.E. The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of Sting in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides. Infect. Immun. 2011;79:688–694. doi: 10.1128/IAI.00999-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Almine J.F., O'Hare C.A., Dunphy G., Haga I.R., Naik R.J., Atrih A., Connolly D.J., Taylor J., Kelsall I.R., Bowie A.G., Beard P.M., Unterholzner L. IFI16 and cGAS cooperate in the activation of STING during DNA sensing in human keratinocytes. Nat. Commun. 2017;8:14392. doi: 10.1038/ncomms14392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jonsson K.L., Laustsen A., Krapp C., Skipper K.A., Thavachelvam K., Hotter D., Egedal J.H., Kjolby M., Mohammadi P., Prabakaran T., Sorensen L.K., Sun C., Jensen S.B., Holm C.K., Lebbink R.J., Johannsen M., Nyegaard M., Mikkelsen J.G., Kirchhoff F., Paludan S.R., Jakobsen M.R. IFI16 is required for DNA sensing in human macrophages by promoting production and function of cGAMP. Nat. Commun. 2017;8:14391. doi: 10.1038/ncomms14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao D., Wu J., Wu Y.T., Du F., Aroh C., Yan N., Sun L., Chen Z.J. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science. 2013;341:903–906. doi: 10.1126/science.1240933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jakobsen M.R., Olagnier D., Hiscott J. Innate immune sensing of HIV-1 infection. Curr. Opin. HIV AIDS. 2015;10:96–102. doi: 10.1097/COH.0000000000000129. [DOI] [PubMed] [Google Scholar]
- 48.Lahaye X., Satoh T., Gentili M., Cerboni S., Conrad C., Hurbain I., El Marjou A., Lacabaratz C., Lelievre J.D., Manel N. The capsids of HIV-1 and HIV-2 determine immune detection of the viral cDNA by the innate sensor cGAS in dendritic cells. Immunity. 2013;39:1132–1142. doi: 10.1016/j.immuni.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 49.West A.P., Khoury-Hanold W., Staron M., Tal M.C., Pineda C.M., Lang S.M., Bestwick M., Duguay B.A., Raimundo N., MacDuff D.A., Kaech S.M., Smiley J.R., Means R.E., Iwasaki A., Shadel G.S. Mitochondrial DNA stress primes the antiviral innate immune response. Nature. 2015;520:553–557. doi: 10.1038/nature14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holm C.K., Jensen S.B., Jakobsen M.R., Cheshenko N., Horan K.A., Moeller H.B., Gonzalez-Dosal R., Rasmussen S.B., Christensen M.H., Yarovinsky T.O., Rixon F.J., Herold B.C., Fitzgerald K.A., Paludan S.R. Virus-cell fusion as a trigger of innate immunity dependent on the adaptor STING. Nat. Immunol. 2012;13:737–743. doi: 10.1038/ni.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Q., Sun L., Chen Z.J. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat. Immunol. 2016;17:1142–1149. doi: 10.1038/ni.3558. [DOI] [PubMed] [Google Scholar]
- 52.Rasaiyaah J., Tan C.P., Fletcher A.J., Price A.J., Blondeau C., Hilditch L., Jacques D.A., Selwood D.L., James L.C., Noursadeghi M., Towers G.J. HIV-1 evades innate immune recognition through specific cofactor recruitment. Nature. 2013;503:402–405. doi: 10.1038/nature12769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu J.J., Li W., Shao Y., Avey D., Fu B., Gillen J., Hand T., Ma S., Liu X., Miley W., Konrad A., Neipel F., Sturzl M., Whitby D., Li H., Zhu F. Inhibition of cGAS DNA sensing by a herpesvirus virion protein. Cell Host Microbe. 2015;18:333–344. doi: 10.1016/j.chom.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma Z., Jacobs S.R., West J.A., Stopford C., Zhang Z., Davis Z., Barber G.N., Glaunsinger B.A., Dittmer D.P., Damania B. Modulation of the cGAS-STING DNA sensing pathway by gammaherpesviruses. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E4306–E4315. doi: 10.1073/pnas.1503831112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen Y., Wang L., Jin J., Luan Y., Chen C., Li Y., Chu H., Wang X., Liao G., Yu Y., Teng H., Wang Y., Pan W., Fang L., Liao L., Jiang Z., Ge X., Li B., Wang P. p38 inhibition provides anti-DNA virus immunity by regulation of USP21 phosphorylation and STING activation. J. Exp. Med. 2017;214:991–1010. doi: 10.1084/jem.20161387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun L., Xing Y., Chen X., Zheng Y., Yang Y., Nichols D.B., Clementz M.A., Banach B.S., Li K., Baker S.C., Chen Z. Coronavirus papain-like proteases negatively regulate antiviral innate immune response through disruption of STING-mediated signaling. PLoS One. 2012;7 doi: 10.1371/journal.pone.0030802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xing Y., Chen J., Tu J., Zhang B., Chen X., Shi H., Baker S.C., Feng L., Chen Z. The papain-like protease of porcine epidemic diarrhea virus negatively regulates type I interferon pathway by acting as a viral deubiquitinase. J. Gen. Virol. 2013;94:1554–1567. doi: 10.1099/vir.0.051169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holm C.K., Rahbek S.H., Gad H.H., Bak R.O., Jakobsen M.R., Jiang Z., Hansen A.L., Jensen S.K., Sun C., Thomsen M.K., Laustsen A., Nielsen C.G., Severinsen K., Xiong Y., Burdette D.L., Hornung V., Lebbink R.J., Duch M., Fitzgerald K.A., Bahrami S., Mikkelsen J.G., Hartmann R., Paludan S.R. Influenza A virus targets a cGAS-independent STING pathway that controls enveloped RNA viruses. Nat. Commun. 2016;7:10680. doi: 10.1038/ncomms10680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ding Q., Gaska J.M., Douam F., Wei L., Kim D., Balev M., Heller B., Ploss A. Species-specific disruption of STING-dependent antiviral cellular defenses by the Zika virus NS2B3 protease. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E6310–E6318. doi: 10.1073/pnas.1803406115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stabell A.C., Meyerson N.R., Gullberg R.C., Gilchrist A.R., Webb K.J., Old W.M., Perera R., Sawyer S.L. Dengue viruses cleave STING in humans but not in nonhuman primates, their presumed natural reservoir. Elife. 2018;7 doi: 10.7554/eLife.31919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aguirre S., Luthra P., Sanchez-Aparicio M.T., Maestre A.M., Patel J., Lamothe F., Fredericks A.C., Tripathi S., Zhu T., Pintado-Silva J., Webb L.G., Bernal-Rubio D., Solovyov A., Greenbaum B., Simon V., Basler C.F., Mulder L.C., Garcia-Sastre A., Fernandez-Sesma A. Dengue virus NS2B protein targets cGAS for degradation and prevents mitochondrial DNA sensing during infection. Nat. Microbiol. 2017;2:17037. doi: 10.1038/nmicrobiol.2017.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barber G.N. STING: infection, inflammation and cancer. Nat. Rev. Immunol. 2015;15:760–770. doi: 10.1038/nri3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prantner D., Darville T., Nagarajan U.M. Stimulator of IFN gene is critical for induction of IFN-beta during Chlamydia muridarum infection. J. Immunol. 2010;184:2551–2560. doi: 10.4049/jimmunol.0903704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Storek K.M., Gertsvolf N.A., Ohlson M.B., Monack D.M. cGAS and Ifi204 cooperate to produce type I IFNs in response to Francisella infection. J. Immunol. 2015;194:3236–3245. doi: 10.4049/jimmunol.1402764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dey B., Dey R.J., Cheung L.S., Pokkali S., Guo H., Lee J.H., Bishai W.R. A bacterial cyclic dinucleotide activates the cytosolic surveillance pathway and mediates innate resistance to tuberculosis. Nat. Med. 2015;21:401–406. doi: 10.1038/nm.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watson R.O., Manzanillo P.S., Cox J.S. Extracellular M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Cell. 2012;150:803–815. doi: 10.1016/j.cell.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burdette D.L., Monroe K.M., Sotelo-Troha K., Iwig J.S., Eckert B., Hyodo M., Hayakawa Y., Vance R.E. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478:515–518. doi: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tamayo R., Pratt J.T., Camilli A. Roles of cyclic diguanylate in the regulation of bacterial pathogenesis. Annu. Rev. Microbiol. 2007;61:131–148. doi: 10.1146/annurev.micro.61.080706.093426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Romling U., Galperin M.Y., Gomelsky M. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol. Mol. Biol. Rev. 2013;77:1–52. doi: 10.1128/MMBR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Woodward J.J., Iavarone A.T., Portnoy D.A. c-di-AMP Secreted by Intracellular Listeria monocytogenes Activates a Host Type I Interferon Response. Science. 2010;328:1703–1705. doi: 10.1126/science.1189801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gao J., Tao J., Liang W., Zhao M., Du X., Cui S., Duan H., Kan B., Su X., Jiang Z. Identification and characterization of phosphodiesterases that specifically degrade 3'3'-cyclic GMP-AMP. Cell Res. 2015;25:539–550. doi: 10.1038/cr.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Andrade W.A., Firon A., Schmidt T., Hornung V., Fitzgerald K.A., Kurt-Jones E.A., Trieu-Cuot P., Golenbock D.T., Kaminski P.A. Group B Streptococcus Degrades Cyclic-di-AMP to Modulate STING-Dependent Type I Interferon Production. Cell Host Microbe. 2016;20:49–59. doi: 10.1016/j.chom.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Galon J., Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019;18:197–218. doi: 10.1038/s41573-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 74.Fuertes M.B., Kacha A.K., Kline J., Woo S.R., Kranz D.M., Murphy K.M., Gajewski T.F. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J. Exp. Med. 2011;208:2005–2016. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Woo S.R., Corrales L., Gajewski T.F. The STING pathway and the T cell-inflamed tumor microenvironment. Trends Immunol. 2015;36:250–256. doi: 10.1016/j.it.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Woo S.R., Fuertes M.B., Corrales L., Spranger S., Furdyna M.J., Leung M.Y., Duggan R., Wang Y., Barber G.N., Fitzgerald K.A., Alegre M.L., Gajewski T.F. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41:830–842. doi: 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ascierto M.L., Kmieciak M., Idowu M.O., Manjili R., Zhao Y., Grimes M., Dumur C., Wang E., Ramakrishnan V., Wang X.Y., Bear H.D., Marincola F.M., Manjili M.H. A signature of immune function genes associated with recurrence-free survival in breast cancer patients. Breast Canc. Res. Treat. 2012;131:871–880. doi: 10.1007/s10549-011-1470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Corrales L., McWhirter S.M., Dubensky T.W., Jr., Gajewski T.F. The host STING pathway at the interface of cancer and immunity. J. Clin. Investig. 2016;126:2404–2411. doi: 10.1172/JCI86892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li K., Qu S., Chen X., Wu Q., Shi M. Promising targets for cancer immunotherapy: TLRs, RLRs, and STING-mediated innate immune pathways. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cheng Y.S., Xu F. Anticancer function of polyinosinic-polycytidylic acid. Cancer Biol. Ther. 2010;10:1219–1223. doi: 10.4161/cbt.10.12.13450. [DOI] [PubMed] [Google Scholar]
- 81.Klarquist J., Hennies C.M., Lehn M.A., Reboulet R.A., Feau S., Janssen E.M. STING-mediated DNA sensing promotes antitumor and autoimmune responses to dying cells. J. Immunol. 2014;193:6124–6134. doi: 10.4049/jimmunol.1401869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zevini A., Olagnier D., Hiscott J. Crosstalk between cytoplasmic RIG-I and STING sensing pathways. Trends Immunol. 2017;38:194–205. doi: 10.1016/j.it.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Temizoz B., Kuroda E., Ohata K., Jounai N., Ozasa K., Kobiyama K., Aoshi T., Ishii K.J. TLR9 and STING agonists synergistically induce innate and adaptive type-II IFN. Eur. J. Immunol. 2015;45:1159–1169. doi: 10.1002/eji.201445132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Andrade W.A., Agarwal S., Mo S., Shaffer S.A., Dillard J.P., Schmidt T., Hornung V., Fitzgerald K.A., Kurt-Jones E.A., Golenbock D.T. Type I interferon induction by Neisseria gonorrhoeae: dual requirement of cyclic GMP-AMP synthase and toll-like receptor 4. Cell Rep. 2016;15:2438–2448. doi: 10.1016/j.celrep.2016.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Deng L., Liang H., Burnette B., Beckett M., Darga T., Weichselbaum R.R., Fu Y.X. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Investig. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liang Y., Peng H. STING-cytosolic DNA sensing: the backbone for an effective tumor radiation therapy. Ann. Transl. Med. 2016;4:60. doi: 10.3978/j.issn.2305-5839.2015.12.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Burnette B.C., Liang H., Lee Y., Chlewicki L., Khodarev N.N., Weichselbaum R.R., Fu Y.X., Auh S.L. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71:2488–2496. doi: 10.1158/0008-5472.CAN-10-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deng L., Liang H., Xu M., Yang X., Burnette B., Arina A., Li X.D., Mauceri H., Beckett M., Darga T., Huang X., Gajewski T.F., Chen Z.J., Fu Y.X., Weichselbaum R.R. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41:843–852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ng K.W., Marshall E.A., Bell J.C., Lam W.L. cGAS-sting and cancer: dichotomous roles in tumor immunity and development. Trends Immunol. 2018;39:44–54. doi: 10.1016/j.it.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 90.Corrales L., Glickman L.H., McWhirter S.M., Kanne D.B., Sivick K.E., Katibah G.E., Woo S.R., Lemmens E., Banda T., Leong J.J., Metchette K., Dubensky T.W., Jr., Gajewski T.F. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. 2015;11:1018–1030. doi: 10.1016/j.celrep.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moore E., Clavijo P.E., Davis R., Cash H., Van Waes C., Kim Y., Allen C. Established T cell-inflamed tumors rejected after adaptive resistance was reversed by combination STING activation and PD-1 pathway blockade. Cancer Immunol. Res. 2016;4:1061–1071. doi: 10.1158/2326-6066.CIR-16-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ghaffari A., Peterson N., Khalaj K., Vitkin N., Robinson A., Francis J.A., Koti M. STING agonist therapy in combination with PD-1 immune checkpoint blockade enhances response to carboplatin chemotherapy in high-grade serous ovarian cancer. Br. J. Canc. 2018;119:440–449. doi: 10.1038/s41416-018-0188-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Luo M., Wang H., Wang Z., Cai H., Lu Z., Li Y., Du M., Huang G., Wang C., Chen X., Porembka M.R., Lea J., Frankel A.E., Fu Y.X., Chen Z.J., Gao J. A STING-activating nanovaccine for cancer immunotherapy. Nat. Nanotechnol. 2017;12:648–654. doi: 10.1038/nnano.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Foote J.B., Kok M., Leatherman J.M., Armstrong T.D., Marcinkowski B.C., Ojalvo L.S., Kanne D.B., Jaffee E.M., Dubensky T.W., Jr., Emens L.A. A STING agonist given with OX40 receptor and PD-L1 modulators primes immunity and reduces tumor growth in tolerized mice. Cancer Immunol. Res. 2017;5:468–479. doi: 10.1158/2326-6066.CIR-16-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tang C.H., Zundell J.A., Ranatunga S., Lin C., Nefedova Y., Del Valle J.R., Hu C.C. Agonist-mediated activation of STING induces apoptosis in malignant B cells. Cancer Res. 2016;76:2137–2152. doi: 10.1158/0008-5472.CAN-15-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sze A., Belgnaoui S.M., Olagnier D., Lin R., Hiscott J., van Grevenynghe J. Host restriction factor SAMHD1 limits human T cell leukemia virus type 1 infection of monocytes via STING-mediated apoptosis. Cell Host Microbe. 2013;14:422–434. doi: 10.1016/j.chom.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 97.Gulen M.F., Koch U., Haag S.M., Schuler F., Apetoh L., Villunger A., Radtke F., Ablasser A. Signalling strength determines proapoptotic functions of STING. Nat. Commun. 2017;8:427. doi: 10.1038/s41467-017-00573-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huang L., Li L., Lemos H., Chandler P.R., Pacholczyk G., Baban B., Barber G.N., Hayakawa Y., McGaha T.L., Ravishankar B., Munn D.H., Mellor A.L. Cutting edge: DNA sensing via the STING adaptor in myeloid dendritic cells induces potent tolerogenic responses. J. Immunol. 2013;191:3509–3513. doi: 10.4049/jimmunol.1301419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lemos H., Mohamed E., Huang L., Ou R., Pacholczyk G., Arbab A.S., Munn D., Mellor A.L. STING promotes the growth of tumors characterized by low antigenicity via Ido activation. Cancer Res. 2016;76:2076–2081. doi: 10.1158/0008-5472.CAN-15-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen Q., Boire A., Jin X., Valiente M., Er E.E., Lopez-Soto A., Jacob L., Patwa R., Shah H., Xu K., Cross J.R., Massague J. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature. 2016;533:493–498. doi: 10.1038/nature18268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barrat F.J., Elkon K.B., Fitzgerald K.A. Importance of nucleic acid recognition in inflammation and autoimmunity. Annu. Rev. Med. 2016;67:323–336. doi: 10.1146/annurev-med-052814-023338. [DOI] [PubMed] [Google Scholar]
- 102.Krieg A.M. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 103.Ahn J., Gutman D., Saijo S., Barber G.N. STING manifests self DNA-dependent inflammatory disease. Proc. Natl. Acad. Sci. U. S. A. 2012;109:19386–19391. doi: 10.1073/pnas.1215006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ahn J., Ruiz P., Barber G.N. Intrinsic self-DNA triggers inflammatory disease dependent on STING. J. Immunol. 2014;193:4634–4642. doi: 10.4049/jimmunol.1401337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gao D., Li T., Li X.D., Chen X., Li Q.Z., Wight-Carter M., Chen Z.J. Activation of cyclic GMP-AMP synthase by self-DNA causes autoimmune diseases. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E5699–E5705. doi: 10.1073/pnas.1516465112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Oka T., Hikoso S., Yamaguchi O., Taneike M., Takeda T., Tamai T., Oyabu J., Murakawa T., Nakayama H., Nishida K., Akira S., Yamamoto A., Komuro I., Otsu K. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485:251–255. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stetson D.B., Ko J.S., Heidmann T., Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134:587–598. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Konig N., Fiehn C., Wolf C., Schuster M., Cura Costa E., Tungler V., Alvarez H.A., Chara O., Engel K., Goldbach-Mansky R., Gunther C., Lee-Kirsch M.A. Familial chilblain lupus due to a gain-of-function mutation in STING. Ann. Rheum. Dis. 2017;76:468–472. doi: 10.1136/annrheumdis-2016-209841. [DOI] [PubMed] [Google Scholar]
- 109.Martinez Valle F., Balada E., Ordi-Ros J., Vilardell-Tarres M. DNase 1 and systemic lupus erythematosus. Autoimmun. Rev. 2008;7:359–363. doi: 10.1016/j.autrev.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 110.Nagata S., Kawane K. Autoinflammation by endogenous DNA. Adv. Immunol. 2011;110:139–161. doi: 10.1016/B978-0-12-387663-8.00004-1. [DOI] [PubMed] [Google Scholar]
- 111.Janko C., Schorn C., Grossmayer G.E., Frey B., Herrmann M., Gaipl U.S., Munoz L.E. Inflammatory clearance of apoptotic remnants in systemic lupus erythematosus (SLE) Autoimmun. Rev. 2008;8:9–12. doi: 10.1016/j.autrev.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 112.Jeremiah N., Neven B., Gentili M., Callebaut I., Maschalidi S., Stolzenberg M.C., Goudin N., Fremond M.L., Nitschke P., Molina T.J., Blanche S., Picard C., Rice G.I., Crow Y.J., Manel N., Fischer A., Bader-Meunier B., Rieux-Laucat F. Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J. Clin. Investig. 2014;124:5516–5520. doi: 10.1172/JCI79100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Konno H., Chinn I.K., Hong D., Orange J.S., Lupski J.R., Mendoza A., Pedroza L.A., Barber G.N. Pro-inflammation associated with a gain-of-function mutation (R284S) in the innate immune sensor STING. Cell Rep. 2018;23:1112–1123. doi: 10.1016/j.celrep.2018.03.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ebensen T., Schulze K., Riese P., Link C., Morr M., Guzman C.A. The bacterial second messenger cyclic diGMP exhibits potent adjuvant properties. Vaccine. 2007;25:1464–1469. doi: 10.1016/j.vaccine.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 115.Chen W., Kuolee R., Yan H. The potential of 3',5'-cyclic diguanylic acid (c-di-GMP) as an effective vaccine adjuvant. Vaccine. 2010;28:3080–3085. doi: 10.1016/j.vaccine.2010.02.081. [DOI] [PubMed] [Google Scholar]
- 116.Gray P.M., Forrest G., Wisniewski T., Porter G., Freed D.C., DeMartino J.A., Zaller D.M., Guo Z., Leone J., Fu T.M., Vora K.A. Evidence for cyclic diguanylate as a vaccine adjuvant with novel immunostimulatory activities. Cell. Immunol. 2012;278:113–119. doi: 10.1016/j.cellimm.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 117.Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012;64:4–17. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 118.Conlon J., Burdette D.L., Sharma S., Bhat N., Thompson M., Jiang Z., Rathinam V.A., Monks B., Jin T., Xiao T.S., Vogel S.N., Vance R.E., Fitzgerald K.A. Mouse, but not human STING, binds and signals in response to the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid. J. Immunol. 2013;190:5216–5225. doi: 10.4049/jimmunol.1300097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Prantner D., Perkins D.J., Lai W., Williams M.S., Sharma S., Fitzgerald K.A., Vogel S.N. 5,6-Dimethylxanthenone-4-acetic acid (DMXAA) activates stimulator of interferon gene (STING)-dependent innate immune pathways and is regulated by mitochondrial membrane potential. J. Biol. Chem. 2012;287:39776–39788. doi: 10.1074/jbc.M112.382986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim S., Li L., Maliga Z., Yin Q., Wu H., Mitchison T.J. Anticancer flavonoids are mouse-selective STING agonists. ACS Chem. Biol. 2013;8:1396–1401. doi: 10.1021/cb400264n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Karaolis D.K.R. Bacterial c-di-GMP Is an Immunostimulatory Molecule. J. Immunol. 2007;178:2171–2181. doi: 10.4049/jimmunol.178.4.2171. [DOI] [PubMed] [Google Scholar]
- 122.Diner E.J., Burdette D.L., Wilson S.C., Monroe K.M., Kellenberger C.A., Hyodo M., Hayakawa Y., Hammond M.C., Vance R.E. The innate immune DNA sensor cGAS produces a noncanonical cyclic dinucleotide that activates human STING. Cell Rep. 2013;3:1355–1361. doi: 10.1016/j.celrep.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang X., Shi H., Wu J., Zhang X., Sun L., Chen C., Chen Z.J. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol. Cell. 2013;51:226–235. doi: 10.1016/j.molcel.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dubensky T.W., Jr., Kanne D.B., Leong M.L. Rationale, progress and development of vaccines utilizing STING-activating cyclic dinucleotide adjuvants. Ther. Adv. Vaccines. 2013;1:131–143. doi: 10.1177/2051013613501988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Madhun A.S., Haaheim L.R., Nostbakken J.K., Ebensen T., Chichester J., Yusibov V., Guzman C.A., Cox R.J. Intranasal c-di-GMP-adjuvanted plant-derived H5 influenza vaccine induces multifunctional Th1 CD4+ cells and strong mucosal and systemic antibody responses in mice. Vaccine. 2011;29:4973–4982. doi: 10.1016/j.vaccine.2011.04.094. [DOI] [PubMed] [Google Scholar]
- 126.Chandra D., Quispe-Tintaya W., Jahangir A., Asafu-Adjei D., Ramos I., Sintim H.O., Zhou J., Hayakawa Y., Karaolis D.K., Gravekamp C. STING ligand c-di-GMP improves cancer vaccination against metastatic breast cancer. Cancer Immunol. Res. 2014;2:901–910. doi: 10.1158/2326-6066.CIR-13-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nakamura T., Miyabe H., Hyodo M., Sato Y., Hayakawa Y., Harashima H. Liposomes loaded with a STING pathway ligand, cyclic di-GMP, enhance cancer immunotherapy against metastatic melanoma. J. Control. Release. 2015;216:149–157. doi: 10.1016/j.jconrel.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 128.Smith T.T., Moffett H.F., Stephan S.B., Opel C.F., Dumigan A.G., Jiang X., Pillarisetty V.G., Pillai S.P.S., Wittrup K.D., Stephan M.T. Biopolymers codelivering engineered T cells and STING agonists can eliminate heterogeneous tumors. J. Clin. Investig. 2017;127:2176–2191. doi: 10.1172/JCI87624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shae D., Becker K.W., Christov P., Yun D.S., Lytton-Jean A.K.R., Sevimli S., Ascano M., Kelley M., Johnson D.B., Balko J.M., Wilson J.T. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat. Nanotechnol. 2019;14:269–278. doi: 10.1038/s41565-018-0342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.An M., Yu C., Xi J., Reyes J., Mao G., Wei W.Z., Liu H. Induction of necrotic cell death and activation of STING in the tumor microenvironment via cationic silica nanoparticles leading to enhanced antitumor immunity. Nanoscale. 2018;10:9311–9319. doi: 10.1039/c8nr01376d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fu J., Kanne D.B., Leong M., Glickman L.H., McWhirter S.M., Lemmens E., Mechette K., Leong J.J., Lauer P., Liu W., Sivick K.E., Zeng Q., Soares K.C., Zheng L., Portnoy D.A., Woodward J.J., Pardoll D.M., Dubensky T.W., Jr., Kim Y. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci. Transl. Med. 2015;7 doi: 10.1126/scitranslmed.aaa4306. 283ra252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li L., Yin Q., Kuss P., Maliga Z., Millan J.L., Wu H., Mitchison T.J. Hydrolysis of 2'3'-cGAMP by ENPP1 and design of nonhydrolyzable analogs. Nat. Chem. Biol. 2014;10:1043–1048. doi: 10.1038/nchembio.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Skouboe M.K., Knudsen A., Reinert L.S., Boularan C., Lioux T., Perouzel E., Thomsen M.K., Paludan S.R. STING agonists enable antiviral cross-talk between human cells and confer protection against genital herpes in mice. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1006976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lioux T., Mauny M.A., Lamoureux A., Bascoul N., Hays M., Vernejoul F., Baudru A.S., Boularan C., Lopes-Vicente J., Qushair G., Tiraby G. Design, synthesis, and biological evaluation of novel cyclic adenosine-inosine monophosphate (cAIMP) analogs that activate stimulator of interferon genes (STING) J. Med. Chem. 2016;59:10253–10267. doi: 10.1021/acs.jmedchem.6b01300. [DOI] [PubMed] [Google Scholar]
- 135.Berger G., Marloye M., Lawler S.E. Pharmacological modulation of the STING pathway for cancer immunotherapy. Trends Mol. Med. 2019 doi: 10.1016/j.molmed.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 136.Lara P.N., Jr., Douillard J.Y., Nakagawa K., von Pawel J., McKeage M.J., Albert I., Losonczy G., Reck M., Heo D.S., Fan X., Fandi A., Scagliotti G. Randomized phase III placebo-controlled trial of carboplatin and paclitaxel with or without the vascular disrupting agent vadimezan (ASA404) in advanced non-small-cell lung cancer. J. Clin. Oncol. 2011;29:2965–2971. doi: 10.1200/JCO.2011.35.0660. [DOI] [PubMed] [Google Scholar]
- 137.Tijono S.M., Guo K., Henare K., Palmer B.D., Wang L.C., Albelda S.M., Ching L.M. Identification of human-selective analogues of the vascular-disrupting agent 5,6-dimethylxanthenone-4-acetic acid (DMXAA) Br. J. Canc. 2013;108:1306–1315. doi: 10.1038/bjc.2013.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gao P., Zillinger T., Wang W., Ascano M., Dai P., Hartmann G., Tuschl T., Deng L., Barchet W., Patel D.J. Binding-pocket and lid-region substitutions render human STING sensitive to the species-specific drug DMXAA. Cell Rep. 2014;8:1668–1676. doi: 10.1016/j.celrep.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Che X., Du X.X., Cai X., Zhang J., Xie W.J., Long Z., Ye Z.Y., Zhang H., Yang L., Su X.D., Gao Y.Q. Single mutations reshape the structural correlation network of the DMXAA-human STING complex. J. Phys. Chem. B. 2017;121:2073–2082. doi: 10.1021/acs.jpcb.6b12472. [DOI] [PubMed] [Google Scholar]
- 140.Hwang J., Kang T., Lee J., Choi B.S., Han S. Design, synthesis, and biological evaluation of C7-functionalized DMXAA derivatives as potential human-STING agonists. Org. Biomol. Chem. 2019;17:1869–1874. doi: 10.1039/c8ob01798k. [DOI] [PubMed] [Google Scholar]
- 141.Zhang Y., Sun Z., Pei J., Luo Q., Zeng X., Li Q., Yang Z., Quan J. Identification of alpha-mangostin as an agonist of human STING. ChemMedChem. 2018;13:2057–2064. doi: 10.1002/cmdc.201800481. [DOI] [PubMed] [Google Scholar]
- 142.Gall B., Pryke K., Abraham J., Mizuno N., Botto S., Sali T.M., Broeckel R., Haese N., Nilsen A., Placzek A., Morrison T., Heise M., Streblow D., DeFilippis V. Emerging alphaviruses are sensitive to cellular states induced by a novel small-molecule agonist of the STING pathway. J. Virol. 2018;92 doi: 10.1128/JVI.01913-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sali T.M., Pryke K.M., Abraham J., Liu A., Archer I., Broeckel R., Staverosky J.A., Smith J.L., Al-Shammari A., Amsler L., Sheridan K., Nilsen A., Streblow D.N., DeFilippis V.R. Characterization of a novel human-specific STING agonist that elicits antiviral activity against emerging alphaviruses. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1005324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu B., Tang L., Zhang X., Ma J., Sehgal M., Cheng J., Zhang X., Zhou Y., Du Y., Kulp J., Guo J.T., Chang J. A cell-based high throughput screening assay for the discovery of cGAS-STING pathway agonists. Antivir. Res. 2017;147:37–46. doi: 10.1016/j.antiviral.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ramanjulu J.M., Pesiridis G.S., Yang J., Concha N., Singhaus R., Zhang S.Y., Tran J.L., Moore P., Lehmann S., Eberl H.C., Muelbaier M., Schneck J.L., Clemens J., Adam M., Mehlmann J., Romano J., Morales A., Kang J., Leister L., Graybill T.L., Charnley A.K., Ye G., Nevins N., Behnia K., Wolf A.I., Kasparcova V., Nurse K., Wang L., Li Y., Klein M., Hopson C.B., Guss J., Bantscheff M., Bergamini G., Reilly M.A., Lian Y., Duffy K.J., Adams J., Foley K.P., Gough P.J., Marquis R.W., Smothers J., Hoos A., Bertin J. Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature. 2018 doi: 10.1038/s41586-018-0705-y. [DOI] [PubMed] [Google Scholar]
- 146.Li S., Hong Z., Wang Z., Li F., Mei J., Huang L., Lou X., Zhao S., Song L., Chen W., Wang Q., Liu H., Cai Y., Yu H., Xu H., Zeng G., Wang Q., Zhu J., Liu X., Tan N., Wang C. The cyclopeptide Astin C specifically inhibits the innate immune CDN sensor STING. Cell Rep. 2018;25:3405–3421. doi: 10.1016/j.celrep.2018.11.097. e3407. [DOI] [PubMed] [Google Scholar]
- 147.Hansen A.L., Buchan G.J., Ruhl M., Mukai K., Salvatore S.R., Ogawa E., Andersen S.D., Iversen M.B., Thielke A.L., Gunderstofte C., Motwani M., Moller C.T., Jakobsen A.S., Fitzgerald K.A., Roos J., Lin R., Maier T.J., Goldbach-Mansky R., Miner C.A., Qian W., Miner J.J., Rigby R.E., Rehwinkel J., Jakobsen M.R., Arai H., Taguchi T., Schopfer F.J., Olagnier D., Holm C.K. Nitro-fatty acids are formed in response to virus infection and are potent inhibitors of STING palmitoylation and signaling. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E7768–E7775. doi: 10.1073/pnas.1806239115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cheng B., Yuan W.E., Su J., Liu Y., Chen J. Recent advances in small molecule based cancer immunotherapy. Eur. J. Med. Chem. 2018;157:582–598. doi: 10.1016/j.ejmech.2018.08.028. [DOI] [PubMed] [Google Scholar]
- 149.Hall J., Brault A., Vincent F., Weng S., Wang H., Dumlao D., Aulabaugh A., Aivazian D., Castro D., Chen M., Culp J., Dower K., Gardner J., Hawrylik S., Golenbock D., Hepworth D., Horn M., Jones L., Jones P., Latz E., Li J., Lin L.L., Lin W., Lin D., Lovering F., Niljanskul N., Nistler R., Pierce B., Plotnikova O., Schmitt D., Shanker S., Smith J., Snyder W., Subashi T., Trujillo J., Tyminski E., Wang G., Wong J., Lefker B., Dakin L., Leach K. Discovery of PF-06928215 as a high affinity inhibitor of cGAS enabled by a novel fluorescence polarization assay. PLoS One. 2017;12 doi: 10.1371/journal.pone.0184843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vincent J., Adura C., Gao P., Luz A., Lama L., Asano Y., Okamoto R., Imaeda T., Aida J., Rothamel K., Gogakos T., Steinberg J., Reasoner S., Aso K., Tuschl T., Patel D.J., Glickman J.F., Ascano M. Small molecule inhibition of cGAS reduces interferon expression in primary macrophages from autoimmune mice. Nat. Commun. 2017;8:750. doi: 10.1038/s41467-017-00833-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.An J., Woodward J.J., Sasaki T., Minie M., Elkon K.B. Cutting edge: antimalarial drugs inhibit IFN-beta production through blockade of cyclic GMP-AMP synthase-DNA interaction. J. Immunol. 2015;194:4089–4093. doi: 10.4049/jimmunol.1402793. [DOI] [PubMed] [Google Scholar]
- 152.Piscianz E., Cuzzoni E., Sharma R., Tesser A., Sapra P., Tommasini A. Reappraisal of antimalarials in interferonopathies: new perspectives for old drugs. Curr. Med. Chem. 2018;25:2797–2810. doi: 10.2174/0929867324666170911162331. [DOI] [PubMed] [Google Scholar]
- 153.Gangadhar T.C., Vonderheide R.H. Mitigating the toxic effects of anticancer immunotherapy. Nat. Rev. Clin. Oncol. 2014;11:91–99. doi: 10.1038/nrclinonc.2013.245. [DOI] [PubMed] [Google Scholar]
- 154.Gubin M.M., Artyomov M.N., Mardis E.R., Schreiber R.D. Tumor neoantigens: building a framework for personalized cancer immunotherapy. J. Clin. Investig. 2015;125:3413–3421. doi: 10.1172/JCI80008. [DOI] [PMC free article] [PubMed] [Google Scholar]