This article reviews the epidemiology of Parkinson's disease (PD). The first section identifies terms and concepts important to the subsequent discussion. The next sections consider disease frequency and proposed risk factors. Etiologic theories resulting from these epidemiologic studies are discussed briefly.
DEFINITIONS
Parkinson's Disease
Parkinson's disease, a disorder of unknown cause, is a distinct clinical and neuropathologic entity, characterized clinically by bradykinesia, resting tremor, cogwheel rigidity, and postural reflex impairment. Loss of pigmented neurons, most prominently in the substantia nigra, and presence of associated characteristic ubiquitin-positive cytoplasmic inclusion bodies (Lewy bodies, clear bodies) are the chief pathologic features. Parkinsonism of known cause and neurodegenerations with multiple system involvement or significant striatal lesions are excluded (such as progressive supranuclear palsy, olivopontocerebellar atrophy, Shy-Drager syndrome, multiple system atrophy, or striatonigral degeneration).77
Epidemiologic Terms
Incidence is the number of new cases of a disorder first developed or diagnosed during a specific time interval within a predefined population at risk. In contrast, prevalence refers to the total number of persons with the disorder at a fixed point in time. Prevalence is a function of both disease incidence and duration. Improved diagnostic capabilities can produce an apparent increase in prevalence. Prolonged survival of persons with disease causes an actual increase in prevalence. Ideally, incidence and prevalence are determined by screening entire populations defined by specific geographic or political boundaries: community- or population-based studies. 127 Estimates of prevalence based on populations identified by other methods, such as participants in a hospital clinic, do not reflect accurately the general population of an area, as cultural, economic, or other factors may influence case selection (selection bias).
Investigations of risk factors for disease may be prospective or cross-sectional.127 Prospective cohort or follow-up studies identify unaffected persons who differ with respect to a specific factor proposed to be related to the disease (an exposure). These individuals are observed over time, and the frequency of new disease in exposed and unexposed persons is determined. PD is an uncommon disorder beginning in late life. The latency from onset of neurodegeneration to onset of clinical signs is unknown. In such a situation, prospective cohort studies are problematic because a large number of unaffected individuals must be followed for decades. Cross-sectional methods, such as case-control studies, are faster and more economical. In case-control studies, individuals already affected with the disease of interest are compared to individuals without the illness, and exposures proposed to relate to the illness are compared. The retrospective nature of case-control studies makes them prone to recall bias, whereby cases have improved or altered recall of past exposures relative to controls, the result of heightened vigilance in association with the diagnosis of a chronic illness. Finally, in some cases (such as toxicant-induced parkinsonism), identification and intensive investigation of an unusually high incidence of disease, either in space or in time (a cluster), may provide important clues to disease etiology.
PROBLEMS ENCOUNTERED IN EPIDEMIOLOGIC STUDIES OF PARKINSON'S DISEASE
There is no antemortem diagnostic test for PD. Consequently, diagnostic accuracy is a challenge in epidemiologic studies of PD. The most reliable antemortem diagnostic method is expert neurologic examination. Although autopsy diagnosis is definitive, this long follow-up has not been possible in any published epidemiologic study of PD. Because differing diagnostic methods can result in differences in the persons studied, these methods should be reviewed critically in all epidemiologic studies of PD. For example, the level of expertise of the diagnosticians can affect diagnostic accuracy dramatically. In some communities, essential tremor accounted for 10% to 40% of the false-positive diagnoses of PD.94, 104 Conversely, bona fide PD may be misdiagnosed as depression or, in the very elderly, “normal” aging. Neurodegenerative disorders, such as progressive supranuclear palsy or multiple system atrophy, may not be distinguished easily from PD early in the course of illness.77
Temporal differences in diagnostic practices also can limit comparability across studies. Many of the neurodegenerative disorders with multiple system involvement and parkinsonism generally were recognized only in the last several decades. These may have been included in earlier studies of PD. Some cases classified as arteriosclerotic parkinsonism in the past today might be considered typical PD.15, 80, 93 In other reports, parkinsonism of known cause (drug-induced parkinsonism and postencephalitic parkinsonism) were grouped along with PD in determining incidence or prevalence rates.15, 80, 116 Because these three types of parkinsonism are distinct, such a grouping could result in erroneous conclusions about disease patterns or risk factors.
FREQUENCY OF PARKINSON'S DISEASE
Incidence and Prevalence
James Parkinson's 1817 report of six cases110 preceded the first community-based investigations of disease frequency by one and one half centuries. In 1958, Kurland80 reported an estimated combined prevalence of 187 per 100,000 for postencephalitic, arteriosclerotic, and nonarteriosclerotic parkinsonism in the population of Rochester, Minnesota. Annual disease incidence was estimated to be 20 per 100,000 persons. Case identification was performed through the records linkage system of the Mayo Clinic. Those residents not seeking medical care would have been missed by this method. Because PD has significant associated disability, however, most cases were likely identified. In 1967, Gudmundsson55 identified PD cases in Iceland by physicians' reports and personal examination, estimating a combined annual incidence of 16 per 100,000 cases for arteriosclerotic and nonarteriosclerotic parkinsonism. Estimated prevalence for these two disorders was 162 per 100,000, an estimate similar to that for Rochester, Minnesota. Numerous subsequent surveys have used variations on these methods with a variety of available health care records (e.g., national health registries; pharmacy rosters; hospital and chronic care facility rosters; questionnaires directed to physicians, nurses, and social workers.) to identify persons with PD. * All of these studies would have missed early cases and cases not receiving medical care.103, 129 Pharmacy drug-sale rosters have been shown to overestimate PD prevalence, especially in women.25 Diagnostic errors, therefore, could have caused increased or decreased inclusion of actual cases. Results from many of these are shown in Table 1 .
Table 1.
INCIDENCE AND CRUDE PREVALENCE OF PARKINSON'S DISEASE IN COMMUNITY-BASED STUDIES
| Location (Reference) | Publication Year | Prevalence (per 100,000) | Annual Incidence (per 100,000) |
|---|---|---|---|
| Rochester, MN (Kurland80) | 1958 | 187.0 | 20.0 |
| Carlisle, England (Brewis et al15) | 1966 | 113.0 | 12.0 |
| Victoria, Australia (Jenkins67) | 1966 | 85.0 | — |
| Iceland (Gudmundsson55) | 1967 | 162.0 | 16.0 |
| Baltimore, MD (Kessler72) | 1972 | 128.0* | — |
| Turku, Finland (Marttila & Rinne94) | 1976 | 120.1 | 15.0 |
| Aberdeen, Scotland (Mutch et al104) | 1986 | 164.2 | — |
| San Marino (D`Alessandro et al28) | 1987 | 152.0 | — |
| Yonago, Japan (Harada et al57) | 1983 | 80.6 | 10.0 |
| Sardinia, Italy (Rosati et al124) | 1980 | 65.6 | 4.9 |
| Northampton, United Kingdom (Sutcliffe et al136) | 1985 | 108.4 | — |
| Benghazi, Libya (Ashok et al6) | 1986 | 31.4 | 4.5 |
| Rochester, MN (Rajput et al116) | 1987 | — | 20.5 |
| Izumo City, Japan (Okada et al107) | 1990 | 82.0 | — |
| Ferrara, Italy (Granieri et al54) | 1991 | 164.7 | 10.0 |
| New York, NY (Mayeux et al98) | 1992 | 99.4 | — |
| Dunedin, New Zealand (Caradoc-Davies et al20) | 1992 | 110.4 | — |
| Alberta, Canada (Svenson et al137) | 1993 | 244.4 | — |
Male patients.
A potentially more accurate method for determining disease frequency in a community involves a door-to-door survey of all households. Because such studies are time- and cost-intensive, often only those groups at higher risk of disease are studied. For example, only persons older than a certain age have been evaluated in surveys of populations interested in PD. Persons identified by a screening method as possibly suffering from a specific disorder then are referred for expert evaluation. Door-to-door surveys are costly, but a number of such surveys have been performed successfully, often in association with a national census.1, 12, 87, 103, 123, 129, 130, 153 Results of these surveys are presented in Table 2 .
Table 2.
PARKINSON'S DISEASE CRUDE PREVALENCE IN DOOR-TO-DOOR SURVEYS
| Location (Reference) | Publication Year | Ages Screened (Years) | Prevalence (per 100,000) |
|---|---|---|---|
| Chinese cities (Li et al87) | 1985 | >50 | 44.0 |
| Copiah Co, MS (Schoenberg et al129) | 1985 | >39 | 347.0 |
| Igbo-ora, Nigeria (Schoenberg et al130) | 1988 | >39 | 58.6 |
| Parsi community, Bombay, India (Bharucha et al12) | 1988 | All | 328.3 |
| Vejer de la Fontera, Cadiz, Spain (Acosta et al1) | 1989 | All | 270.0 |
| Terrasini, Santa Teresa di Riva, Sicily, Italy (Rocca et al123) | 1990 | All | 243.0 |
| Sicily, Italy (Morgante et al103) | 1992 | >12 | 257.2 |
| Kin-Hu, Kinmen, China (Wang et al153) | 1994 | >50 | 170.0 |
Geographic Variation
Estimates of disease prevalence vary widely, from 31 per 100,000 in Libya6 to 328 per 100,000 in the Parsi community in Bombay, India.12 Although methodologic differences and population age distributions may explain some of this variation, even after adjusting for many of these inconsistencies, geographic prevalence differences persist.160 Several North American studies also have found significant regional differences in prevalence, with a suggestion of northwest to southeast gradients in both Canada and the United States.11, 84, 137 It is possible that different distributions of factors related to PD causes across populations may contribute to geographic differences in disease frequency. These factors could include genetic differences in susceptibility to disease, differences in exposure to causative factors, and differences in exposure to protective factors.
Temporal Variation
A number of studies have attempted to look at changes in the frequency of PD over time, searching for temporal patterns that could provide important etiologic clues. For example, an increasing incidence over time that correlates with the industrialization of a region could implicate industrial chemicals or related lifestyle changes as risk factors. Similarly, periodic fluctuation in incidence might suggest an infectious etiology.
A review of Olmstead County data found no significant change in PD incidence over the relatively brief period from 1967 through 1979.117 Zhang et al160 reviewed a number of incidence studies, age adjusting them to a single population, and detected no change in incidence rates over the past 50 years. Changes in disease frequency over long periods, however, are very difficult to measure reliably because of changes in and inconsistent application of diagnostic criteria. Additionally, many studies rely on death record analysis for ascertaining cases, introducing great variability owing to the inconsistent reporting of PD as a cause of death. Their shortcomings acknowledged, these studies show consistent dramatic changes in PD-related mortality. During the past 2 to 3 decades, irrespective of geographic location, death rates have fallen for those younger than age 65 and increased in those older than age 75.24, 26, 64, 83, 146, 150 These observations are compatible with improved survival due to levodopa therapy, with mean age at death increasing approximately 5 years during the past 20 years.83, 149
Changes in PD incidence and mortality (after adjustment for population aging) could reflect improved diagnosis or improved record-keeping, or might reflect relative changes in mortality from other competing diseases, such as stroke and heart disease.121, 122
RISK FACTORS FOR PARKINSON'S DISEASE
Age
Increasing age is the only unequivocal risk factor for PD. PD incidence increases with increasing age throughout the life span.116 This is true in all community-based studies, regardless of the absolute prevalence of disease in the population.20, 57, 80, 94, 98, 103, 104, 124, 136 Some examples of age-specific prevalence in different communities are shown in Figure 1 . The reasons for this relationship are not known. Possible explanations for the near exponential correlation between increasing age and PD prevalence include age-related neuronal vulnerability or a causal mechanism dependent on the passage of time.
Figure 1.
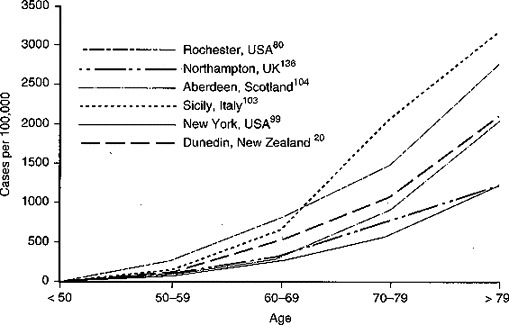
Parkinson's disease age-specific prevalence.
Because of the strength and consistency of this risk factor, comparisons of disease frequency among populations must be adjusted for their differing age distributions.160 One would expect crude (unadjusted) PD incidence and prevalence to be higher in a community with a greater proportion of elderly individuals. Similarly, evaluation of any putative risk factor requires concomitant adjustment for the distribution of that risk factor with respect to age.
Gender
Because of their greater longevity, women constitute an increasing percentage of the population as age increases. For this reason, if risk is equal, one would expect the crude prevalence of PD to be greater in women. Age-adjusted or age-specific prevalences are more useful statistics for understanding potential causes of PD (i.e., at a given age, what is the likelihood that an individual will have PD?). Although by no means a universal finding, an increasing percentage of studies find men to have a modestly increased age-adjusted PD prevalence. * This trend also persists across races,64, 83, 89, 98 particularly in China, where the male-to-female ratio ranges from 2.4 to 3.7.23, 87, 140, 153 Examples of age-adjusted prevalence ratios by gender are shown in Table 3 . Whether the increased prevalence in men is a function of biology or of male-related lifestyle factors is not clear. Further investigation of factors underlying these foci of increased male prevalence may provide important clues to the cause of PD.
Table 3.
PARKINSON'S DISEASE: GENDER RATIO OF AGE-ADJUSTED PREVALENCE
| Location (Reference) | Male-to-Female Ratio |
|---|---|
| San Marino (D'Alessandro et al28) | 1.24 |
| Yonago, Japan (Harada et al57) | 0.86 |
| Chinese cities (Li et al87) | 3.70 |
| Sardinia, Italy (Rosati et al124) | 1.38 |
| Northampton, United Kingdom (Sutcliffe et al136) | 1.30 |
| Finland (Marttila & Rinne94) | 0.98 |
| Benghazi, Libya (Ashok et al6) | 1.04 |
| Rochester, MN (Rajput et al117) | 1.48 |
| Alberta, Canada (Svenson et al137) | 1.20 |
| New York, NY (Mayeux et al98) | 1.73 |
| Denmark (Kurtzke et al83) | 1.79* |
| Japan (Imaizumi & Kaneko64) | 1.29* |
Mortality data.
Race
PD prevalence generally appears to be highest in Europe and North America, whereas rates in Japan, China, and Africa are markedly lower (see Tables 1 and 2). Similarly, one community-based98 and several hospital-based series in the United States and Africa found PD prevalence to be much lower among blacks.71, 108, 109 These observations suggest that there is a greater risk for PD among whites. Other differences among the populations studied, however, such as underlying age distribution and use of medical care, also could cause such differences in prevalence. Exceptions to the above findings are two door-to-door studies—one performed in Copiah County, Mississippi,130 and the other in a Parsi colony in Bombay, India.12 Prevalence in Copiah County was similar between whites and blacks, if cases were chosen using the least stringent diagnostic criteria (i.e., possible Parkinson's disease), although whites continued to have higher prevalence if more rigorous criteria (i.e., probable Parkinson's disease) were used to identify cases. In the Parsi community in Bombay, prevalence was similar to that found in Europe and North America. The Parsis are Persian descendants who migrated to India between the seventh and tenth centuries. They formed a closed community into which conversion is impossible. These facts may account for the high prevalence observed, despite their Asian residence.
Although socioeconomic or environmental factors may explain the prevalence differences cited here, a still-to-be-refuted possibility is that PD is more common in whites, most likely as the result of a common genetic characteristic.
Genetic Predisposition
Heredity is another commonly identified risk factor for PD. First, many examples of familial parkinsonism have been reported. Several kindreds with multiple members with apparent autosomal-dominant parkinsonism have been described,33 but most of these studies are limited by their reliance on family-derived histories for deceased relatives. In addition, the clinical and pathologic features of the majority of described kindreds are not fully consistent with those of typical PD.34, 50, 100, 125, 156
Maraganore et al91 studied the first-degree relatives of 20 British PD patients who reported at least one additional family member with PD. Examination-verified idiopathic PD indistinguishable from sporadic PD was found in 13 of 69 living first-degree relatives, leading the authors to propose an autosomal-dominant pattern of inheritance with reduced penetrance. Payami et al112 studied PD frequency in 586 first-degree relatives of 114 PD patients ascertained from a movement disorder clinic and in a similar number of controls. All affected relatives had been diagnosed by a community physician. Relatives of PD patients were 3.5 times more likely to have PD than relatives of controls, again suggesting an autosomal-dominant pattern. Notwithstanding their possible significance, it must be emphasized that neither of these studies used population-based methods, and it cannot be assumed that these findings are generalizable to the population at large. Additionally, these studies assess the probability of a positive family history of PD given that the proband has PD, when the real question regards the probability of PD given a positive family history.
Other studies support a less prominent role for genetic factors, postulating multifactorial inheritance with symptoms dependent on environmental factors.7, 79, 93, 101 Twin studies further support a less prominent contribution of genetic factors in PD, as concordance rates between monozygotic and dizygotic twins are similar.92, 96, 151, 152, 155, 161 In classic autosomal-dominant disorders, monozygotic twins show much higher concordance than do dizygotic twins. PD, however, is a disorder of late life. If onset age differs significantly between twins, and one twin dies before symptoms are apparent, genetically concordant twins may appear to be discordant. A recent positron emission tomography (PET) scan study by Burn et al18 supports this possibility. They looked at putamenal18 F-dopa uptake in twin pairs discordant for PD clinically and found that 30% to 40% of asymptomatic cotwins had significantly decreased uptake. Similar PET scan results were reported recently by Piccini et al.113 Concordance rates for decreased18 F-dopa uptake, however, did not differ for monozygotic and dizygotic twins, arguing against a classic Mendelian autosomal-dominant cause of this observation. Moreover, the relationship between this radiographic finding and subsequent clinically or pathologically diagnosed PD is unknown. Neither is the population prevalence of decreased putamenal18 F-dopa uptake well understood. Prospective observation of these and other cases will be useful in determining the significance of these interesting findings.
Consistent with multifactorial theories of PD etiology, several genes that code for metabolic enzymes have been identified that may contribute to PD risk. Variant alleles for cytochrome P450 isoenzyme CYP2D6,2, 5, 78, 132, 147 and several specific monoamine oxidase haplotypes61, 82 occur with increased frequency in individuals with PD, although these associations are not reported universally.81 These variants potentially could result in toxic effects from levels of some endogenous or exogenous compounds that might not otherwise be toxic (i.e., gene-environment interaction).
It is hoped that the role of genetic factors in PD etiology will be clarified soon, as the rapid development of molecular genetic technology has focused much attention on this question.
Toxicant Exposure
The idea that exposure to an exogenous agent might cause PD was triggered by the observation of a cluster of parkinsonism caused by the intravenous injection of the compound 1-methyl-1,2,4,6-tetrahydropyridine (MPTP) by narcotics addicts.85 Prior to this discovery, parkinsonism was known to result from numerous chemical injuries,46 but MPTP-induced parkinsonism is remarkable in that it strictly mimics the anatomic and clinical features of PD rather than causing more widespread CNS injury. This observation sparked a search for naturally occurring environmental factors that might be causally related to idiopathic PD. One key aspect to the search for environmental causes of PD, in contrast to MPTP, is that signs of idiopathic PD develop very gradually. Substances that are mildly toxic, or which ingress or accumulate in the brain slowly, are difficult to evaluate, and their significance could be missed.144
International differences in PD prevalence could be explained by international differences in toxicant exposure. PD appears to be less common in countries more recently industrialized. Studies using antiparkinsonian drug sales to estimate prevalence found vegetable farming, wood pulp mills, and steel alloy industries in areas with the highest disease prevalence.4, 7 Very similar results were found in a Michigan ecologic study using county-specific PD mortality rates,126 where significantly higher rates were found in counties with paper-, chemical-, iron-, or copper-related industries.
Rajput et al118 found an association between young age at PD onset and residence in rural Saskatchewan, and Tanner et al141 found a similar association in a Chicago-based clinical series. These observations have been tested in numerous case-control and ecologic studies. * Many of these are summarized in Table 4 . Although both the methods used and the locations of these studies have differed, all show an association between at least one of the proposed exposures—rural residence, farming, well water drinking, or herbicide/pesticide exposure—and an increased risk for developing PD. Hubble et al63 used a multiple logistic regression model to discern that most of the associations they observed were in fact a function of their relationship to pesticide use.
Table 4.
CASE-CONTROL STUDIES TESTING THE ASSOCIATION BETWEEN RURAL LIFE, AGRICULTURAL CHEMICALS, OR WELL-WATER DRINKING AND PARKINSON'S DISEASE
| Location (Reference) | No. Cases/ No. Controls | Rural Home | Farming | Well-water Drinking | Herbicides/ Pesticides |
|---|---|---|---|---|---|
| China (Tanner et al140) | 100/200 | − | − | − | + |
| Quebec (Zayed et al159) | 42/84 | NA | − | + | + |
| Madrid (Jimenez-Jimenez et al69) | 81/162 | NA | NA | + | + |
| Kansas (Koller77) | 150/150 | + | + | + | − |
| Hong Kong (Ho et al60) | 35/105 | + | + | + | + |
| Chicago† (Tanner et al138) | 78/78 | − | + | − | − |
| British Columbia (Hertzman et al58) | 57/122 | NA | NA | − | + |
| New Jersey/Pennsylvania† (Dulaney et al32) | 154/154 | +* | NA | − | − |
| Campania, Italy (Campanella et al19) | 83/83 | NA | NA | + | NA |
| California, 7th Day Adventists (Davanipour et al29) | 49/>34,000 | + | NA | NA | NA |
| Kansas (Wong et al158) | 38/38 | + | − | + | − |
| Calgary (Semchuk et al131) | 130/260 | − | + | − | + |
| Spain (Morano et al102) | 74/148 | + | − | + | +* |
| + = significant positive association; − = no association; NA = not assessed. | |||||
Parkinson's disease onset <51 y.
p = 0.06.
The structural and mechanistic resemblance of some common agricultural chemicals to the toxin MPTP3, 106, 119 is particularly intriguing. The significance of these associations should be weighed cautiously, however. All of the studies previously described are limited by small size, and differing methods prevent direct comparisons. Although reproducibility of the associations over many different studies lends strength to the observations, a cause/effect relationship cannot be assumed. Much further collaborative work between clinicians, epidemiologists, and laboratory scientists is necessary to clarify the import of the association between rural residence or its associated factors and PD.
Infection
The observation that parkinsonism was a common late sequela of encephalitis lethargica, a disorder that was pandemic in the second and third decades of this century, prompted the postulate that all cases of parkinsonism were the result of exposure to that infectious agent.114 The corollary prediction that PD ultimately would disappear now has been proved incorrect as survivors of that epoch died, and few cases of parkinsonism today are thought to be postencephalitic. One study97 suggested that in utero exposure to influenza virus may cause a loss of nigral neurons and consequent increased vulnerability to PD, but this observation was not confirmed.35
Many attempts to identify an infectious agent in PD failed.36, 95, 154 Fazzini et al41 found increased cerebral spinal fluid (CSF) antibody titers to coronaviruses in persons with PD. Specific coronaviruses have an affinity for basal ganglia in some animals, and members of this species commonly affect agricultural animals such as pigs. Hubble et al62 and Kohbata et al75 found increased Nocardia antibody titers in PD patients, and Nocardia can cause a levodopa-responsive movement disorder in mice associated with its specific affinity for substantia nigral neurons.10, 74 Rather than reflecting an exposure to an environmental chemical, the increased risk of developing PD associated with rural residence may reflect environmental exposure to an infectious agent.
Trauma
Retrospective case-control studies often report an association between head trauma and PD.13, 32, 38, 135, 141 Studies comparing prospectively collected information (that is, information collected before the person got PD), however, do not find this association.117, 157 Patients with a chronic illness typically seek explanations for their disease in prior experiences, and those with CNS injuries might be particularly thoughtful about head injuries. A similar pattern of recall is seen in other neurologic diseases, such as Alzheimer's disease, in which prospectively collected information shows no association between head injury and disease, but retrospectively collected information typically suggests that head trauma is associated with Alzheimer's disease.21 It is most likely that the reported association between head trauma and PD reflects biased recall, rather than a cause/effect association. Consistent with this conclusion, Goetz et al47 in 1991 found that PD patients who sustain head trauma have no change in the long-term course of their disease. Unless prospectively collected information shows such an association, trauma should not be considered to increase the risk for PD.
Emotional Stress
Both Charcot22 and Gowers52 cited stress as a possible cause of PD. Laboratory studies suggest that stress-produced changes in central dopamine systems theoretically could contribute to the development of parkinsonism.133, 134 Similarly, persons already affected with PD experience transient worsening of their symptoms during stressful periods.45 Two reports linked the extreme emotional and physical hardship of concentration camp imprisonment with the subsequent development of PD.43, 146 Whether these observations reflect an accelerated nigral injury as the result of stress-related increase in dopamine turnover with resultant increased oxidative injury, nutritional deficiencies of dietary protective agents, or other factors cannot be determined. Evaluation of the relationship of less severe emotional or physical stress to the development of PD poses a methodologic challenge.
PROTECTIVE FACTORS FOR PARKINSON'S DISEASE
Diet
Oxidative mechanisms have been proposed to be involved in the pathogenesis of PD, and, in consequence, intake of antioxidant vitamins has been proposed to protect against the development of PD.17, 27, 40 Patients with vitamin E deficiency show reduced putamenal18 F-dopa uptake in PET scans,30 although no differences have been found in serum42 or in brain tocopherol levels31 in patients with PD. Consumption of foods rich in tocopherol decreased the risk of developing PD in two case-control studies, one comparing PD cases to same-sex siblings and one comparing subjects to spouses.48, 51 The use of supplemental multivitamins, vitamin E, or cod liver oil was associated similarly with a decreased risk for PD,142 although the ability of vitamin E supplements to slow progression of already diagnosed PD is inconclusive.39, 86 No significant differences between cases and controls in dietary intake of vitamins E or C, beta-carotene, protein calories, or total calories were found in a Chinese population,139 but the tocopherol content of many of the foods commonly eaten in China was not available, so this negative result simply could reflect inadequate information.
Although the numbers studied to date are small, these studies suggest that eating foods rich in tocopherol or some associated behavioral or dietary factors may protect against the development of PD in some cases. These observations allow the suggestion that areas with a low prevalence of PD may not be those with a lesser concentration of environmental toxins but rather those in which there is higher dietary intake of protective substances or a lower dietary intake of pro-oxidants. This latter possibility is supported by a recent case-control study that found increased animal fat intake in PD cases relative to controls.90
Cigarette Smoking
A study of US military veterans in the late 1960s resulted in the observation of an inverse association between smoking and PD.70 The low prevalence of cigarette smoking among prevalent cases of PD is an observation confirmed in numerous subsequent case-control studies in the United States and Europe,9, 13, 16, 32, 44, 56, 68, 73, 105, 143, 154 and recently in a large prospective study in Hawaii, where a modest dose–response relationship was observed.53 Most of these studies found an odds ratio of approximately 0.5 for ever-versus-never having smoked. Five studies, however, found no association between smoking and PD.14, 60, 99, 118, 140 Two of these studies, performed in China or Hong Kong, raise questions concerning any protective effect of cigarette smoking. Smoking is extremely rare among Chinese women but relatively common among men, but PD occurs in Chinese men nearly four times more often than in Chinese women.87, 140 If smoking exerted a true biologic protective effect, a higher prevalence of PD would be expected in the nonsmoking Chinese women than in men.
The proposed protective effect of cigarette smoking could be confounded by many factors, including a greater propensity for persons to quit smoking because they are becoming ill even before being diagnosed99 and the possibility that patients with PD who smoke have greater mortality than those patients who do not smoke.37, 120 Rather than reflecting an actual biologic action, decreased smoking in PD simply could reflect the more conservative personality that may accompany PD13, 48, 111 (i.e., smoking behavior could be a reflection of an underlying process, rather than the cause of that process). Nonetheless, given the consistency and strength of the inverse association and the suggestion of a dose–response relationship in a large prospective study, the bulk of epidemiologic evidence supports a potentially protective effect of cigarette smoking on PD risk. Coupled with studies demonstrating protective effects of nicotine on age-, transection-, and MPTP-induced dopaminergic neuronal cell loss in rodent substantia nigra,65, 66, 115 the evidence further supports the possible protective role of smoking on PD risk.
SUMMARY
The epidemiologic studies reviewed here have provided insights into the etiology of PD. Evidence increasingly suggests that, like many other chronic age-related diseases, PD is a multifactorial disorder, with both genes and environment contributing to risk. As the elderly population of the world grows, incidence and prevalence of PD will continue to increase, underscoring the importance of further delineating risk factors. The introduction of levodopa and other pharmacologic therapies over the last 2 decades has postponed disease morbidity and mortality, but morbidity and mortality still are increased markedly relative to unaffected individuals. The development of therapies that may slow disease progression makes early identification and treatment of PD particularly important. Investigations of early markers of PD, or markers of disease susceptibility, are critical areas for future research. These efforts all will be aided by careful collaboration between epidemiologists and laboratory scientists.
Footnotes
Address reprint requests to Caroline M. Tanner, MD, The Parkinson's Institute, 1170 Morse Avenue, Sunnyvale, CA 94089
References 6, 23, 28, 57, 67, 72, 87, 89, 94, 98, 117, 124, 136, 137, 140, 153.
References 6, 23, 28, 57, 67, 72, 87, 89, 94, 98, 117, 124, 136, 137, 140, 153.
References 19, 29, 32, 54, 58–60, 63, 69, 77, 102, 126, 131, 135, 137, 138, 140, 154.
References
- 1.Acosta J., Calderon E., Obeso J.A. Prevalence of Parkinson's disease and essential tremor in a village of south Spain [abstract] Neurology. 1989;39(suppl 1):181. [Google Scholar]
- 2.Agundez J.A., Jimenez-Jimenez F.J., Luengo A. Association between the oxidative polymorphism and early onset of Parkinson's disease. Clin Pharmacol Ther. 1995;57:291–298. doi: 10.1016/0009-9236(95)90154-X. [DOI] [PubMed] [Google Scholar]
- 3.Ansher S.S., Cadet J.L., Jakoby W.B. Role of N-methyltransferases in the neurotoxicity associated with the metabolites of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and other 4-substituted pyridines present in the environment. Biochem Pharmacol. 1986;35:3359–3363. doi: 10.1016/0006-2952(86)90436-3. [DOI] [PubMed] [Google Scholar]
- 4.Aquilonius S.M., Hartvig P. A Swedish county with unexpectedly high utilization of anti-parkinsonian drugs. Acta Neurol Scand. 1986;74:379–382. doi: 10.1111/j.1600-0404.1986.tb03529.x. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong M., Daly A.K., Chalerton S. Mutant debrisoquine hydroxylation genes in Parkinson's disease. Lancet. 1992;339:1017–1018. doi: 10.1016/0140-6736(92)90537-d. [DOI] [PubMed] [Google Scholar]
- 6.Ashok P.P., Radhakrishan K., Sridharan R. Parkinsonism in Benghazi, East Libya. Clin Neurol Neurosurg. 1986;88:109–113. doi: 10.1016/s0303-8467(86)80005-1. [DOI] [PubMed] [Google Scholar]
- 7.Barbeau A., Pourcher E. New data on the genetics of Parkinson's disease. Can J Neurol Sci. 1982;9:53–60. doi: 10.1017/s031716710004364x. [DOI] [PubMed] [Google Scholar]
- 8.Barbeau A., Roy M. Uneven prevalence of Parkinson's disease in the province of Quebec. Can J Neurol Sci. 1985;12:169. [Google Scholar]
- 9.Baumann R.J., Jameson H.D., McKean H.E. Cigarette smoking and Parkinson's disease: I. A comparison of cases with matched neighbors. Neurology. 1980;30:839–843. doi: 10.1212/wnl.30.8.839. [DOI] [PubMed] [Google Scholar]
- 10.Beaman B.L. Nocardia as a pathogen of the brain: Mechanisms of interactions in the murine brain—a review. Gene. 1992;115:213–217. doi: 10.1016/0378-1119(92)90561-3. [DOI] [PubMed] [Google Scholar]
- 11.Betemps E.J., Buncher C.R. Birthplace as a risk factor in motor neurone disease and Parkinson's disease. Int J Epidemiol. 1993;22:898–904. doi: 10.1093/ije/22.5.898. [DOI] [PubMed] [Google Scholar]
- 12.Bharucha N.E., Bharucha E.P., Bharucha A.E. Prevalence of Parkinson's disease in the Parsi community of Bombay, India. Arch Neurol. 1988;45:1321–1323. doi: 10.1001/archneur.1988.00520360039008. [DOI] [PubMed] [Google Scholar]
- 13.Bharucha N.E., Stokes L., Schoenberg B.S. A case-control study of twin pairs discordant for Parkinson's disease: A search for environmental risk factors. Neurology. 1986;36:284–288. doi: 10.1212/wnl.36.2.284. [DOI] [PubMed] [Google Scholar]
- 14.Breteler M.M., Tzourio C., Manubens-Bertran J.M. Risk factors for Parkinson's disease: A population-based case-control study. Neurology. 1995;45(suppl 4):A214. doi: 10.1212/wnl.52.9.1876. [DOI] [PubMed] [Google Scholar]
- 15.Brewis M., Poskanzer D.C., Rolland C. Neurological disease in an English city. Acta Neurol Scand. 1966;42(suppl 24):9–89. [PubMed] [Google Scholar]
- 16.Burch P.R.J. Cigarette smoking and Parkinson's disease. Neurology. 1981;31:500–503. doi: 10.1212/wnl.31.4.500-b. [DOI] [PubMed] [Google Scholar]
- 17.Burkhardt C.R., Weber H.K. Parkinson's disease: A chronic, low-grade antioxidant deficiency? Med Hypotheses. 1994;43:111–114. doi: 10.1016/0306-9877(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 18.Burn D.J., Mark M.H., Playford E.D. Parkinson's disease in twins studied with 18F-dopa and positron emission tomography. Neurology. 1992;42:1894–1900. doi: 10.1212/wnl.42.10.1894. [DOI] [PubMed] [Google Scholar]
- 19.Campanella G., Filla A., De Michele G. Etiology of Parkinson's disease: Results of two case-control studies. Mov Disord. 1990;5(suppl 1):31. [Google Scholar]
- 20.Caradoc-Davies T.H., Weatherall M., Dixon G.S. Is the prevalence of Parkinson's disease in New Zealand really changing? Acta Neurol Scand. 1992;86:40–44. doi: 10.1111/j.1600-0404.1992.tb08051.x. [DOI] [PubMed] [Google Scholar]
- 21.Chandra V., Kokmen E., Schoenberg B. Head trauma with loss of consciousness as a risk factor for Alzheimer's disease. Neurology. 1989;39:1576–1578. doi: 10.1212/wnl.39.12.1576. [DOI] [PubMed] [Google Scholar]
- 22.Charcot J.M. vol 1. The New Sydenham Society; London: 1878. (Lectures on the Diseases of the Nervous System, trans-ed Sigerson G). [Google Scholar]
- 23.Chia L.G., Liu L.H. Parkinson's disease in Taiwan: An analysis of 215 patients. Neuroepidemiology. 1992;11:113–120. doi: 10.1159/000110920. [DOI] [PubMed] [Google Scholar]
- 24.Chio A., Magnani C., Tolardo G. Parkinson's disease mortality in Italy, 1951 through 1987. Arch Neurol. 1993;50:149–153. doi: 10.1001/archneur.1993.00540020027012. [DOI] [PubMed] [Google Scholar]
- 25.Chio A., Mocellini C., Soffietti R. Prevalence of Parkinson's disease in a district on northern Italy: Comparison between tracer and traditional methodologies. Neurology. 1995;45(suppl 4):A327. [Google Scholar]
- 26.Clarke C.E. Mortality from Parkinson's disease in England and Wales 1921–89. J Neurol Neurosurg Psychiatry. 1993;56:690–693. doi: 10.1136/jnnp.56.6.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cross C.E., Halliwell B., Borish E.T. Oxygen radicals and human disease. Ann Intern Med. 1987;107:526–545. doi: 10.7326/0003-4819-107-4-526. [DOI] [PubMed] [Google Scholar]
- 28.D'Alessandro R., Gamberini G., Granieri E. Prevalence of Parkinson's disease in the republic of San Marino. Neurology. 1987;37:1679–1682. doi: 10.1212/wnl.37.10.1679. [DOI] [PubMed] [Google Scholar]
- 29.Davanipour Z., Will A.D. Residential histories in Parkinson's disease patients. Ann Neurol. 1990;28:295. [Google Scholar]
- 30.Dexter D.T., Brooks D.J., Harding A.E. Nigrostriatal function in vitamin E deficiency: Clinical, experimental, and positron emission tomographic studies. Ann Neurol. 1994;35:298–303. doi: 10.1002/ana.410350309. [DOI] [PubMed] [Google Scholar]
- 31.Dexter D.T., Ward R.J., Wells F.R. Alpha-tocopherol levels in brain are not altered in Parkinson's disease. Ann Neurol. 1992;32:591–593. doi: 10.1002/ana.410320420. [DOI] [PubMed] [Google Scholar]
- 32.Dulaney E., Stern M., Hurtig H. The epidemiology of Parkinson's disease: A case-control study of young-onset versus old-onset patients. Mov Disord. 1990;5(suppl):12. doi: 10.1001/archneur.1991.00530210029018. [DOI] [PubMed] [Google Scholar]
- 33.Duvoisin R.C. The genetics of Parkinson's disease. Adv Neurol. 1993;60:306–315. [PubMed] [Google Scholar]
- 34.Dwork A.J., Balmaceda C., Fazzini E.A. Dominantly inherited, early-onset parkinsonism: Neuropathology of a new form. Neurology. 1993;43:69–74. doi: 10.1212/wnl.43.1_part_1.69. [DOI] [PubMed] [Google Scholar]
- 35.Ebmeier K.P., Mutch W.J., Calder S.A. Does idiopathic parkinsonism in Aberdeen follow intrauterine influenza? J Neurol Neurosurg Psychiatry. 1989;52:911–913. doi: 10.1136/jnnp.52.7.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elizan T.S., Casals J. The viral hypothesis in Parkinson's disease in Alzheimer's disease: A critique. In: Kurstak E., Lipowski S.J., Morozov P.V., editors. Viruses, Immunity and Mental Disorders. Plenum Press; New York: 1987. pp. 47–59. [Google Scholar]
- 37.Ellenberg J.H. Differential postmorbidity mortality in observational studies of risk factors for neurologic disorders. Neuroepidemiology. 1994;13:187–194. doi: 10.1159/000110378. [DOI] [PubMed] [Google Scholar]
- 38.Factor S.A., Weiner W.J. Prior history of head trauma in Parkinson's disease. Mov Disord. 1991;6:225–229. doi: 10.1002/mds.870060306. [DOI] [PubMed] [Google Scholar]
- 39.Fahn S. A pilot trial of high-dose alpha-tocopherol and ascorbate in early Parkinson's disease. Ann Neurol. 1992;32(suppl):S128–S132. doi: 10.1002/ana.410320722. [DOI] [PubMed] [Google Scholar]
- 40.Fahn S., Cohen G. The oxidant stress hypothesis in Parkinson's disease: Evidence supporting it. Ann Neurol. 1992;32:804–812. doi: 10.1002/ana.410320616. [DOI] [PubMed] [Google Scholar]
- 41.Fazzini E., Fleming J., Fahn S. Cerebrospinal fluid antibodies to coronaviruses in patients with Parkinson's disease. Neurology. 1990;40(suppl):169. doi: 10.1002/mds.870070210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandez-Calle P., Molina J.A., Jimenez-Jimenez F.J. Serum levels of alpha-tocopherol (vitamin E) in Parkinson's disease. Neurology. 1992;42:1064–1066. doi: 10.1212/wnl.42.5.1064. [DOI] [PubMed] [Google Scholar]
- 43.Gibberd F.B., Simmons J.P. Neurological disease in ex-Far-East prisoners of war. Lancet. 1980;ii:135–138. doi: 10.1016/s0140-6736(80)90015-x. [DOI] [PubMed] [Google Scholar]
- 44.Godwin-Austin R.B., Lee P.N., Marmot M.G. Smoking and Parkinson's disease. J Neurol Neurosurg Psychiatry. 1982;45:577–581. doi: 10.1136/jnnp.45.7.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goetz C.G. Motor vehicle accident and Parkinson's disease disability. Mov Disord. 1990;5(suppl):16. [Google Scholar]
- 46.Goetz C.G. Neurotoxins in Clinical Practice. SP Medical and Scientific Books; New York: 1985. p. xix. [Google Scholar]
- 47.Goetz C.G., Stebbins G.T. Effects of head trauma from motor vehicle accidents on Parkinson's disease. Ann Neurol. 1991;29:191–193. doi: 10.1002/ana.410290213. [DOI] [PubMed] [Google Scholar]
- 48.Golbe L.I., Cody R.A., Duvoisin R.C. Smoking and Parkinson's disease: Search for a dose-response relationship. Arch Neurol. 1986;43:774–778. doi: 10.1001/archneur.1986.00520080022014. [DOI] [PubMed] [Google Scholar]
- 49.Golbe L.I., Farrell T.M., Davis P.H. Case-control study of early life dietary factors in Parkinson's disease. Arch Neurol. 1988;45:350–353. doi: 10.1001/archneur.1988.00520360068014. [DOI] [PubMed] [Google Scholar]
- 50.Golbe L.I., Farrell T.M., Davis P.H. Follow-up study of early life protective and risk factors in Parkinson's disease. Mov Disord. 1990;5:66–70. doi: 10.1002/mds.870050116. [DOI] [PubMed] [Google Scholar]
- 51.Golbe L.I., Di Torio G., Bonavita V. A large kindred with autosomal dominant Parkinson's disease. Ann Neurol. 1990;27:276–282. doi: 10.1002/ana.410270309. [DOI] [PubMed] [Google Scholar]
- 52.Gowers W.R. Diseases of the Nervous System, American ed. P. Blakiston, Son & Co; Philadelphia: 1888. [Google Scholar]
- 53.Grandinetti A., Morens D.M., Reed D. Prospective study of cigarette smoking and the risk of developing idiopathic Parkinson's disease. Am J Epidemiol. 1994;139:1129–1138. doi: 10.1093/oxfordjournals.aje.a116960. [DOI] [PubMed] [Google Scholar]
- 54.Granieri E., Carreras M., Casetta I. Parkinson's disease in Ferrara, Italy, 1967 through 1987. Arch Neurol. 1991;48:854–857. doi: 10.1001/archneur.1991.00530200096026. [DOI] [PubMed] [Google Scholar]
- 55.Gudmundsson K.R. A clinical survey of parkinsonism in Iceland. Acta Neurol Scand. 1967;33:9–61. [PubMed] [Google Scholar]
- 56.Haack D.G., Baumann R.J., McKean H.E. Nicotine exposure and Parkinson's disease. Am J Epidemiol. 1981;114:119–200. doi: 10.1093/oxfordjournals.aje.a113182. [DOI] [PubMed] [Google Scholar]
- 57.Harada H., Nishikawa S., Takahashi K. Epidemiology of Parkinson's disease in a Japanese city. Arch Neurol. 1983;40:151–154. doi: 10.1001/archneur.1983.04050030045008. [DOI] [PubMed] [Google Scholar]
- 58.Hertzman C., Wiens M., Bowering D. Parkinson's disease: A case-control study of occupational and environmental risk factors. Am J Ind Med. 1990;17:349–355. doi: 10.1002/ajim.4700170307. [DOI] [PubMed] [Google Scholar]
- 59.Hertzman C., Wiens M., Snow B. A case-control study of Parkinson's disease in a horticultural region of British Columbia. Mov Disord. 1994;9:69–75. doi: 10.1002/mds.870090111. [DOI] [PubMed] [Google Scholar]
- 60.Ho S.C., Woo J., Lee C.M. Epidemiologic study of Parkinson's disease in Hong Kong. Neurology. 1989;39:1314–1318. doi: 10.1212/wnl.39.10.1314. [DOI] [PubMed] [Google Scholar]
- 61.Hotamisligil G.S., Girmen A.S., Fink J.S. Hereditary variations in monoamine oxidase as a risk factor for Parkinson's disease. Mov Disord. 1994;9:305–310. doi: 10.1002/mds.870090304. [DOI] [PubMed] [Google Scholar]
- 62.Hubble J., Kjelstrom J., Beamann B. Nocardia serology in Parkinson's disease. Mov Disord. 1992;7:292. [Google Scholar]
- 63.Hubble J.P., Cao T., Hassanein R.E. Risk factors for Parkinson's disease. Neurology. 1993;43:1693–1697. doi: 10.1212/wnl.43.9.1693. [DOI] [PubMed] [Google Scholar]
- 64.Imaizumi Y., Kaneko R. Rising mortality from Parkinson's disease in Japan, 1950–1992. Acta Neurol Scand. 1995;91:169–176. doi: 10.1111/j.1600-0404.1995.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 65.Janson A.M., Moller A. Chronic nicotine treatment counteracts nigral cell loss induced by a partial mesodiencephalic hemitransection: An analysis of the total number and mean volume of neurons and glia in substantia nigra of the male rat. Neuroscience. 1993;57:931–941. doi: 10.1016/0306-4522(93)90039-i. [DOI] [PubMed] [Google Scholar]
- 66.Janson A.M., Fuxe K., Goldstein M. Differential effects of acute and chronic nicotine treatment on MPTP-(1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) induced degeneration of nigrostriatal dopamine neurons in the black mouse. Clin Investig. 1992;70:232–238. doi: 10.1007/BF00184656. [DOI] [PubMed] [Google Scholar]
- 67.Jenkins A.C. Epidemiology of parkinsonism in Victoria. Med J Aust. 1966;2:497–502. doi: 10.5694/j.1326-5377.1966.tb97295.x. [DOI] [PubMed] [Google Scholar]
- 68.Jimenez-Jimenez F.J., Mateo D., Gimenez-Roldan S. Premorbid smoking, alcohol consumption, and coffee drinking habits in Parkinson's disease: A case-control study. Mov Disord. 1992;7:339–344. doi: 10.1002/mds.870070407. [DOI] [PubMed] [Google Scholar]
- 69.Jimenez-Jimenez F.J., Gonzales D.M., Gimenez-Roldan S. Proceedings of the Ninth International Symposium on Parkinson's Disease. World Congress of Neurology; 1988. Exposure to well water drinking and pesticides in Parkinson's disease. A case-control study from the southeast area of Madrid; p. 118. [Google Scholar]
- 70.Kahn H.A. National Cancer Institute, Epidemiologic Approaches to the Study of Cancer and Other Diseases. US Government Printing Office; Washington, DC: 1966. The Dorn Study of smoking among US veterans; pp. 1–125. Monograph 19. [Google Scholar]
- 71.Kessler I.I. Epidemiologic studies of Parkinson's disease. II. A hospital-based survey. Am J Epidemiol. 1972;95:308–318. doi: 10.1093/oxfordjournals.aje.a121399. [DOI] [PubMed] [Google Scholar]
- 72.Kessler I.I. Epidemiologic studies of Parkinson's disease. III. A community-based survey. Am J Epidemiol. 1972;96:242–254. doi: 10.1093/oxfordjournals.aje.a121455. [DOI] [PubMed] [Google Scholar]
- 73.Kessler I.I., Diamond E.L. Epidemiologic studies of Parkinson's disease. I. Smoking and Parkinson's disease: A survey and explanatory hypothesis. Am J Epidemiol. 1971;94:16–25. doi: 10.1093/oxfordjournals.aje.a121289. [DOI] [PubMed] [Google Scholar]
- 74.Kohbata S., Beaman B.L. L-dopa-responsive movement disorder caused by Nocardia asteroides localized in the brains of mice. Infect Immun. 1991;59:181–191. doi: 10.1128/iai.59.1.181-191.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kohbata S., Shimokawa K. Circulating antibody to Nocardia in the serum of patients with Parkinson's disease. Adv Neurol. 1993;60:355–357. [PubMed] [Google Scholar]
- 76.Koller W., Vetere-Overfield B., Gray C. Environmental risk factors in Parkinson's disease. Neurology. 1990;40:1218–1221. doi: 10.1212/wnl.40.8.1218. [DOI] [PubMed] [Google Scholar]
- 77.Koller W.C. Classification of Parkinsonism. In: Koller W.C., editor. Handbook of Parkinson's Disease. Marcel Dekker; New York: 1987. pp. 51–80. [Google Scholar]
- 78.Kondo I., Kanazawa I. Debrisoquine hydroxylase and Parkinson's disease. Adv Neurol. 1993;60:338–342. [PubMed] [Google Scholar]
- 79.Kondo K., Kurland L., Schull W. Parkinson's disease: Genetic analysis and evidence of a multifactorial etiology. Mayo Clin Proc. 1973;48:465–475. [PubMed] [Google Scholar]
- 80.Kurland L.T. Epidemiology: Incidence, geographic distribution and genetic considerations. In: Field W., editor. Pathogenesis and Treatment of Parkinsonism. Charles C. Thomas; Springfield, IL: 1958. pp. 5–43. [Google Scholar]
- 81.Kurth J.H., Hubble J.P., Eggers E.A. Lack of association of CYP2D6 and MAO-B alleles with Parkinson's disease in a Kansas cohort. Neurology. 1995;45(suppl 4):A429. [Google Scholar]
- 82.Kurth J.H., Kurth M.C., Poduslo S.E. Association of a monoamine oxidase B allele with Parkinson's disease. Ann Neurol. 1993;33:368–372. doi: 10.1002/ana.410330406. [DOI] [PubMed] [Google Scholar]
- 83.Kurtzke J.F., Murphy F.M. The changing patterns of death rates in parkinsonism. Neurology. 1990;40:42–49. doi: 10.1212/wnl.40.1.42. [DOI] [PubMed] [Google Scholar]
- 84.Kurtzke J.F., Goldberg I.D. Parkinsonism death rates by race, sex, and geography. Neurology. 1988;38:1558–1561. doi: 10.1212/wnl.38.10.1558. [DOI] [PubMed] [Google Scholar]
- 85.Langston J.W., Ballard P.A., Tetrud J.W. Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 86.LeWitt P.A. Clinical trials of neuroprotection in Parkinson's disease: Long-term selegiline and alpha-tocopherol treatment. J Neural Transm Suppl. 1994;43:171–181. [PubMed] [Google Scholar]
- 87.Li S.C., Schoenberg B.S., Wang C.C. A prevalence survey of Parkinson's disease and other movement disorders in the People's Republic of China. Arch Neurol. 1985;42:655–657. doi: 10.1001/archneur.1985.04060070045013. [DOI] [PubMed] [Google Scholar]
- 88.Lilienfeld D.E., Perl D.P. Projected neurodegenerative disease mortality in the United States, 1990–2040. Neuroepidemiology. 1993;12:219–228. doi: 10.1159/000110320. [DOI] [PubMed] [Google Scholar]
- 89.Lilienfeld D.E., Chan E., Ehland J. Two decades of increasing mortality from Parkinson's disease among the US elderly. Arch Neurol. 1990;47:731–734. doi: 10.1001/archneur.1990.00530070019005. [DOI] [PubMed] [Google Scholar]
- 90.Logroscino G., Marder K., Cote L. A case-control study of diet and Parkinson's disease (PD): Calories, lipids and antioxidants. Neurology. 1995;45(suppl 4):A328. doi: 10.1002/ana.410390113. [DOI] [PubMed] [Google Scholar]
- 91.Maraganore D.M., Harding A.E., Marsden C.D. A clinical and genetic study of familial Parkinson's disease. Mov Disord. 1991;6:205–211. doi: 10.1002/mds.870060303. [DOI] [PubMed] [Google Scholar]
- 92.Marsden C.D. Twins and Parkinson's disease. J Neurol Neurosurg Psychiatry. 1987;50:105–106. doi: 10.1136/jnnp.50.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martin W.E., Young W.I., Anderson V.E. Parkinson's disease: A genetic study. Brain. 1973;96:495–506. doi: 10.1093/brain/96.3.495. [DOI] [PubMed] [Google Scholar]
- 94.Marttila R.J., Rinne U.K. Epidemiology of Parkinson's disease in Finland. Acta Neurol Scand. 1976;53:81–102. doi: 10.1111/j.1600-0404.1976.tb04328.x. [DOI] [PubMed] [Google Scholar]
- 95.Marttila R.J., Halonen P., Rinne U.K. Influenza virus antibodies in parkinsonism. Arch Neurol. 1977;34:99–100. doi: 10.1001/archneur.1977.00500140053010. [DOI] [PubMed] [Google Scholar]
- 96.Marttila R.J., Kaprio J., Koshewvuo M. Parkinson's disease in a nationwide twin cohort. Neurology. 1988;38:1217–1219. doi: 10.1212/wnl.38.8.1217. [DOI] [PubMed] [Google Scholar]
- 97.Mattock C., Marmot M., Stern G. Could Parkinson's disease follow intrauterine influenza?: A speculative hypothesis. J Neurol Neurosurg Psychiatry. 1988;51:753–756. doi: 10.1136/jnnp.51.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mayeux R., Denaro J., Hemenegildo N. A population-based investigation of Parkinson's disease with and without dementia. Arch Neurol. 1992;49:492–497. doi: 10.1001/archneur.1992.00530290076015. [DOI] [PubMed] [Google Scholar]
- 99.Mayeux R., Tang M.X., Marder K. Smoking and Parkinson's disease. Mov Disord. 1994;9:207–212. doi: 10.1002/mds.870090215. [DOI] [PubMed] [Google Scholar]
- 100.Mizutani T., Inose T., Kakimi S. Familial parkinsonism and dementia with ballooned neurons. J Neuropathol Exp Neurol. 1991;50:309. [Google Scholar]
- 101.Mjones H. Paralysis agitans: A clinical and genetic study. Acta Psychiatry and Neurology. 1949;54(suppl):1–194. [Google Scholar]
- 102.Morano A., Jimenez-Jimenez F.J., Molina J.A. Risk factors for Parkinson's disease: Case-control study in the province of Caceres, Spain. Acta Neurol Scand. 1994;89:164–170. doi: 10.1111/j.1600-0404.1994.tb01655.x. [DOI] [PubMed] [Google Scholar]
- 103.Morgante L., Rocca W.A., Di Rosa A.E. Prevalence of Parkinson's disease and other types of parkinsonism: A door-to-door survey in three Sicilian municipalities. Neurology. 1992;42:1901–1907. doi: 10.1212/wnl.42.10.1901. [DOI] [PubMed] [Google Scholar]
- 104.Mutch W.J., Dingwall-Fordyce I., Downie A.W. Parkinson's disease in a Scottish city. BMJ. 1986;292:534–536. doi: 10.1136/bmj.292.6519.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nefzinger M.D., Quadfasel F.A., Karl V.C. A retrospective study of smoking and Parkinson's disease. Am J Epidemiol. 1968;88:149–158. doi: 10.1093/oxfordjournals.aje.a120874. [DOI] [PubMed] [Google Scholar]
- 106.Nicklas W.J., Vyas I., Heikkila R.E. Inhibition of NADH-linked oxidation in brain mitochondria by MPP+, a metabolite of the neurotoxin MPTP. Life Sci. 1985;36:2503–2508. doi: 10.1016/0024-3205(85)90146-8. [DOI] [PubMed] [Google Scholar]
- 107.Okada K., Kobayashi S., Tsunematsu T. Prevalence of Parkinson's disease in Izumo City, Japan. Gerontology. 1990;36:340–344. doi: 10.1159/000213219. [DOI] [PubMed] [Google Scholar]
- 108.Osuntokun B.O. The pattern of neurological illness in tropical Africa: Experience at Ibadan, Nigeria. J Neurol Sci. 1971;12:417–442. doi: 10.1016/0022-510x(71)90110-9. [DOI] [PubMed] [Google Scholar]
- 109.Paddison R.M., Griffith R.P. Occurrence of Parkinson's disease in black patients at Charity Hospital in New Orleans. Neurology. 1974;24:688–690. doi: 10.1212/wnl.24.7.688. [DOI] [PubMed] [Google Scholar]
- 110.Parkinson J. An Essay on the Shaking Palsy. Neely and Jones; London, Sherwood: 1817. [Google Scholar]
- 111.Paulson G.W., Dadmehr N. Is there a premorbid personality typical for Parkinson's disease? Neurology. 1991;41(suppl 2):73–76. doi: 10.1212/wnl.41.5_suppl_2.73. [DOI] [PubMed] [Google Scholar]
- 112.Payami H., Larsen K., Bernard S. Increased risk of Parkinson's disease in parents and siblings of patients. Ann Neurol. 1994;36:659–661. doi: 10.1002/ana.410360417. [DOI] [PubMed] [Google Scholar]
- 113.Piccini P., Burn D., Sawle G. Dopaminergic function in relatives of Parkinson's disease patients: A clinical and PET study. Neurology. 1995;45(suppl 4):A203. [Google Scholar]
- 114.Poskanzer D.C., Schwab R.S., Fraser D.W. Further observations on the cohort phenomenon in Parkinson's syndrome. In: Barbeau A., Brunette J.R., editors. Progress in Neurogenetics. Excerpta Medica; Amsterdam: 1969. pp. 497–505. [Google Scholar]
- 115.Prasad C., Ikegami H., Shimizu I. Chronic nicotine intake decelerates aging of nigrostriatal dopaminergic neurons. Life Sci. 1994;54:1169–1184. doi: 10.1016/0024-3205(94)00839-6. [DOI] [PubMed] [Google Scholar]
- 116.Rajput A.H., Offord K.P., Beard C.M. A case-control study of smoking habits, dementia, and other illnesses in idiopathic Parkinson's disease. Neurology. 1987;37:226–232. doi: 10.1212/wnl.37.2.226. [DOI] [PubMed] [Google Scholar]
- 117.Rajput A.H., Offord K.P., Beard M.C. Epidemiology of parkinsonism: Incidence, classification and mortality. Ann Neurol. 1984;16:278–282. doi: 10.1002/ana.410160303. [DOI] [PubMed] [Google Scholar]
- 118.Rajput A.H., Uitti R.J., Stern W. Early onset of Parkinson's disease in Saskatchewan: Environmental considerations for etiology. Can J Neurol Sci. 1986;13:312–316. doi: 10.1017/s0317167100036635. [DOI] [PubMed] [Google Scholar]
- 119.Ramsay R.R., Dadger J., Trevor A. Energy driven uptake of MPP+ by brain mitochondria mediates the neurotoxicity of MPTP. Life Sci. 1986;39:581–588. doi: 10.1016/0024-3205(86)90037-8. [DOI] [PubMed] [Google Scholar]
- 120.Riggs J.E. Cigarette smoking and Parkinson disease: The illusion of a neuroprotective effect. Clin Neuropharmacol. 1992;15:88–99. doi: 10.1097/00002826-199204000-00002. [DOI] [PubMed] [Google Scholar]
- 121.Riggs J.E. The nonenvironmental basis for rising mortality from Parkinson's disease. Arch Neurol. 1993;50:653–656. doi: 10.1001/archneur.1993.00540060083023. [DOI] [PubMed] [Google Scholar]
- 122.Riggs J.E., Schochet S.S. Rising mortality due to Parkinson's disease and amyotrophic lateral sclerosis: A manifestation of the competitive nature of human mortality. J Clin Epidemiol. 1992;45:1007–1012. doi: 10.1016/0895-4356(92)90116-5. [DOI] [PubMed] [Google Scholar]
- 123.Rocca W.A., Morgante M., Grigoletto F. Prevalence of Parkinson's disease and other parkinsonisms: A door-to-door survey in two Sicilian communities. Neurology. 1990;40(suppl):422. [Google Scholar]
- 124.Rosati G., Granieri E., Pinna L. The risk of Parkinson disease in Mediterranean people. Neurology. 1980;30:250–255. doi: 10.1212/wnl.30.3.250. [DOI] [PubMed] [Google Scholar]
- 125.Roy E.P., Riggs J.E., Martin J.D. Familial parkinsonism, apathy, weight loss, and central hypoventilation: Successful long-term management. Neurology. 1988;38:637–639. doi: 10.1212/wnl.38.4.637. [DOI] [PubMed] [Google Scholar]
- 126.Rybicki B.A., Johnson C.C., Uman J. Parkinson's disease mortality and the industrial use of heavy metals in Michigan. Mov Disord. 1993;8:87–92. doi: 10.1002/mds.870080116. [DOI] [PubMed] [Google Scholar]
- 127.Schlesselman J.J. Case-Control Studies: Design, Conduct, Analysis. Oxford University Press; New York: 1982. [Google Scholar]
- 128.Schoenberg B.S. Descriptive epidemiology of Parkinson's disease: Disease distribution and hypothesis formulation. Adv Neurol. 1987;45:277–283. [PubMed] [Google Scholar]
- 129.Schoenberg B.S., Anderson D.W., Haerer A.F. Prevalence of Parkinson's disease in the biracial population of Copiah County, Mississippi. Neurology. 1985;35:841–845. doi: 10.1212/wnl.35.6.841. [DOI] [PubMed] [Google Scholar]
- 130.Schoenberg B.S., Osuntokun B.O., Adeuja A.O.G. Comparison of the prevalence of Parkinson's disease in black populations in the rural US and in rural Nigeria: Door-to-door community studies. Neurology. 1988;38:645–646. doi: 10.1212/wnl.38.4.645. [DOI] [PubMed] [Google Scholar]
- 131.Semchuk K.M., Love E.J., Lee R.G. Parkinson's disease and exposure to agricultural work and pesticide chemicals. Neurology. 1992;42:1328–1335. doi: 10.1212/wnl.42.7.1328. [DOI] [PubMed] [Google Scholar]
- 132.Smith C.A., Gough A.C., Leigh P.N. Debrisoquine hydroxylase gene polymorphism and susceptibility to Parkinson's disease. Lancet. 1992;339:1375–1377. doi: 10.1016/0140-6736(92)91196-f. [DOI] [PubMed] [Google Scholar]
- 133.Snyder A.M., Stricker E.M., Zigmond M.J. Stress-induced neurological impairments in an animal model of parkinsonism. Ann Neurol. 1985;18:554–551. doi: 10.1002/ana.410180506. [DOI] [PubMed] [Google Scholar]
- 134.Spina M.B., Cohen G. Dopamine turnover and glutathione oxidation: Implications for Parkinson's disease. Proc Natl Acad Sci U S A. 1989;86:1398–1400. doi: 10.1073/pnas.86.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stern M., Dulaney E., Gruber S.B. The epidemiology of Parkinson's disease. Arch Neurol. 1991;48:903–907. doi: 10.1001/archneur.1991.00530210029018. [DOI] [PubMed] [Google Scholar]
- 136.Sutcliffe R.L.G., Prior R., Mawby B. Parkinson's disease in the district of the Northampton Health Authority, United Kingdom. Acta Neurol Scand. 1985;72:363–379. doi: 10.1111/j.1600-0404.1985.tb00886.x. [DOI] [PubMed] [Google Scholar]
- 137.Svenson L.W., Platt G.H., Woodhead S.E. Geographic variations in the prevalence rates of Parkinson's disease in Alberta. Can J Neurol Sci. 1993;20:307–311. [PubMed] [Google Scholar]
- 138.Tanner C.M., Grabler P., Goetz C.G. Occupation and the risk of Parkinson's disease: A case-control study in young onset patients. Neurology. 1990;40(suppl):422. [Google Scholar]
- 139.Tanner C.M., Chen B., Cohen J.A. Dietary antioxidant vitamins and the risk of developing Parkinson's disease. Neurology. 1989;39(suppl):181. [Google Scholar]
- 140.Tanner C.M., Chen B., Wang W. Environmental factors and Parkinson's disease: A case-control study in China. Neurology. 1989;39:660–664. doi: 10.1212/wnl.39.5.660. [DOI] [PubMed] [Google Scholar]
- 141.Tanner C.M., Chen B., Wang W. Environmental factors in the etiology of Parkinson's disease. Can J Neurol Sci. 1987;14:419–423. doi: 10.1017/s0317167100037835. [DOI] [PubMed] [Google Scholar]
- 142.Tanner C.M., Cohen J.C., Summerville B.C. Vitamin use and Parkinson's disease. Ann Neurol. 1988;233:182. [Google Scholar]
- 143.Tanner C.M., Koller W.C., Gilley D.C. Cigarette smoking, alcohol drinking and Parkinson's disease: Cross-cultural risk assessment. Mov Disord. 1990;5(suppl):11. [Google Scholar]
- 144.Tipton K.F., McCrodden J.M., Sullivan J.P. Metabolic aspects of the behavior of MPTP and some analogues. Adv Neurol. 1993;60:186–193. [PubMed] [Google Scholar]
- 145.Tison F., Dartigues J.F., Dubes L. Prevalence of Parkinson's disease in the elderly: A population study in Gironde, France. Acta Neurol Scand. 1994;90:111–115. doi: 10.1111/j.1600-0404.1994.tb02689.x. [DOI] [PubMed] [Google Scholar]
- 146.Treves T.A., Rabey J.M., Korczyn A.D. Case-control study, with use of temporal approach, for evaluation of risk factors for Parkinson's disease. Mov Disord. 1990;5(suppl):11. [Google Scholar]
- 147.Tsuneoka Y., Matsuo Y., Iwahashi K. A novel cytochrome P-450IID6 mutant gene associated with Parkinson's disease. J Biochem (Tokyo) 1993;114:263–266. doi: 10.1093/oxfordjournals.jbchem.a124164. [DOI] [PubMed] [Google Scholar]
- 148.Uitti R.J., Rajput A.H., Offord K.P. Parkinsonism survival in the Levodopa era. Neurology. 1991;41(suppl):190. [Google Scholar]
- 149.Uitti R.J., Ahlskog J.E., Maraganore D.M. Levodopa therapy and survival in idiopathic Parkinson's disease: Olmsted County project. Neurology. 1993;43:1918–1926. doi: 10.1212/wnl.43.10.1918. [DOI] [PubMed] [Google Scholar]
- 150.Vanacore N., Bonifati V., Bellatreccia A. Mortality rates for Parkinson's disease and parkinsonism in Italy (1969–1987) Neuroepidemiology. 1992;11:65–73. doi: 10.1159/000110914. [DOI] [PubMed] [Google Scholar]
- 151.Vieregge P., Schiffke K.A., Friedrich H.J. Parkinson's disease in twins. Neurology. 1992;42:1453–1461. doi: 10.1212/wnl.42.8.1453. [DOI] [PubMed] [Google Scholar]
- 152.Vierregge P., Schiffke A., Kompf D. Parkinson's disease in twins. Neurology. 1991;41(suppl):255. [Google Scholar]
- 153.Wang S.J., Fuh J.L., Liu C.Y. Parkinson's disease in Kin-Hu, Kinmen: A community survey by neurologists. Neuroepidemiology. 1994;13:69–74. doi: 10.1159/000110361. [DOI] [PubMed] [Google Scholar]
- 154.Wang W., Fang X., Cheng X. A case-control study on the environmental risk factors of Parkinson's disease in Tianjin, China. Neuroepidemiology. 1993;12:209–218. doi: 10.1159/000110319. [DOI] [PubMed] [Google Scholar]
- 155.Ward C.D., Duvoisin R.C., Ince S.E. Parkinson's disease in 65 pairs of twins and in a set of quadruplets. Neurology. 1983;33:815–824. doi: 10.1212/wnl.33.7.815. [DOI] [PubMed] [Google Scholar]
- 156.Waters C.H., Miller C.A. Autosomal dominant lewy body parkinsonism in a four-generation family. Ann Neurol. 1994;35:59–64. doi: 10.1002/ana.410350110. [DOI] [PubMed] [Google Scholar]
- 157.Williams D.B., Annegers J.F., Kokmen E. Brain injury and neurologic sequelae. Neurology. 1991;41:1554–1557. doi: 10.1212/wnl.41.10.1554. [DOI] [PubMed] [Google Scholar]
- 158.Wong G.F., Gray C.S., Hassanein R.S. Environmental risk factors in siblings with Parkinson's disease. Arch Neurol. 1991;48:287–289. doi: 10.1001/archneur.1991.00530150055018. [DOI] [PubMed] [Google Scholar]
- 159.Zayed J., Ducic S., Campanella G. Facteurs environnementaux dans l'etiologie de la maladie de Parkinson. Can J Neurol Sci. 1990;17:286–291. [PubMed] [Google Scholar]
- 160.Zhang Z.X., Roman G.C. Worldwide occurrence of Parkinson's disease: An updated review. Neuroepidemiology. 1993;12:195–208. doi: 10.1159/000110318. [DOI] [PubMed] [Google Scholar]
- 161.Zimmerman T.R., Bhatt M., Calne D.B. Parkinson's disease in monozygotic twins: A followup. Neurology. 1991;41(suppl):255. [Google Scholar]


