Abstract
Avian infectious bronchitis virus (IBV) continues to cause serious economic losses in global chicken production. Concurrent circulation of both classic and variant IBVs have been identified in most parts of the world, raising major challenges to global prevention and control efforts. Therefore, immunopathogenesis, particularly early host responses, needs to be better understood for effective control of diseases caused by different strains of IBVs. We investigated differing immunopathogenesis in chickens following infection with IS/885/00-like (885), QX-like (QX) and M41 IBV strains. We confirmed that the histopathological changes, proinflammatory and innate immune gene responses were induced to different magnitudes, depending on the IBV strain. Results indicated that upregulation of proinflammatory cytokines (such as IL-6 and IL-1β) and lipopolysaccharide-induced tumor necrosis factor-alpha factor (LITAF) expression is induced by IBV M41 in the trachea and by IBV 885 and QX in the kidney, which mainly coincides with tracheal and renal histopathological lesions respectively caused by these strains. In addition, elevated levels of TLR3, MDA5 and IFN-β expression occurred concurrently with greater lesion severity in IBV infected trachea and kidney tissues. Overall, this study reports marked differences in the activation of early host responses by pathogenic IBV strains.
Keywords: Infectious bronchitis virus, Chickens, Differential immunopathogenesis, Innate immune response
Highlights
-
•
IBV M41, QX and 885 strains induces different magnitude of early immune responses.
-
•
Levels of TLR3, MDA5 and IFN-β mRNA is associated with microscopic changes.
-
•
Pro-inflammatory gene responses are associated with kidney and tracheal lesions.
1. Introduction
Infectious bronchitis (IB) continues to cause serious economic losses in chicken production globally. IB virus (IBV) replicates in upper respiratory tract tissues, with the resulting bronchi infection causing severe disease in young birds. Certain IBV strains cause greater systemic infections by replicating in different tissues, including the kidney (causing nephritis), the oviduct (causing decreased egg production), and the intestinal tract (Ganapathy et al., 2012). Vaccination has been considered the most reliable approach for controlling IBV infection (Meeusen et al., 2007). However, continuous emergence of new variant viruses may lead to inadequate protection and control of IBV (De Wit, 2000; de Wit et al., 2011). Concurrent circulation of both classic and variant IBVs have been identified in most parts of the world, raising major challenges to global prevention and control efforts. During the past few decades, there have been reports of new virulent field isolates that represent variant strains, which can cause wide tissue tropism and high pathogenicity (Gelb et al., 1991; Jackwood, 2012; Shaw et al., 1996; Zanella et al., 2000). Though the mechanisms behind chickens’ varying susceptibility to different IBV infections have been studied, little to no data is available on comparative immunopathogenesis on recently emerged IBVs.
Our previous in vitro work concluded that greater levels of apoptosis, and elevated expression of TLR3, MDA5 and IFN-β, correlated to increased pathogenicity of three distinct IBV genotypes in CEK cells and TOCs (Chhabra et al., 2016). Based on these findings, we hypothesized that characteristic host innate immune responses could aid the production of a predictive prognosis for the tissue tropism of novel IBV strains in chickens.
There are few studies regarding the local immunological mechanisms involved during IBV pathogenesis. In one study, different immune responses of chickens were reported from two different IBV genotypes, KIIa and ChVI (Jang et al., 2013; Okino et al., 2014). In chickens infected with the KIIa genotype, simultaneous peaks in the viral copy number, and upregulation of mRNA levels of pro-inflammatory cytokines (IL-6 and IL-1β) and lipopolysaccharide-induced tumor necrosis factor (TNF)-α factor (LITAF), were observed at 7 dpi in the trachea and 9 dpi in the kidney. This appeared to contribute to the intensity of pathophysiological effects in the chickens. In contrast, chickens infected with the ChVI genotype showed comparatively mild upregulation in pro-inflammatory cytokines mRNA expression.
Okino et al. (2014) described that infection with IBV-M41 caused an early (3 dpi) upregulation of IL 6 and IL-1β, followed by a later induction (7 dpi) of CD8αα and Granzyme homolog A mRNA. Both instances were witnessed at the same time point as microscopic lesions and a high viral load were noted in the trachea, suggesting the contributory role of both these cytotoxic enzymes and virus load in the development of tracheal lesions (Okino et al., 2014).
Recently, Okino et al. (2017) reported that challenge with two Brazilian IBV field isolates (A and B) of the same genotype, resulted in only the IBV B isolate causing a down-regulation of TLR7, leading to insufficient pro-inflammatory responses and lower cell mediated immune response that had an association with severe renal lesions and increased capacity of IBV replication (Okino et al., 2017). The work concluded that the differences in the immune responses, triggered by IBV strains with distinct genotypes and with differing pathogenicity for respiratory, urinary and other tissues, require further investigation.
Pattern recognition receptors (PRRs) are present on mucosal surfaces of immune cells. These cells rapidly recognize infectious agents through PRRs, such as Toll-like receptors (TLR), RIG-I like receptors (RLRs), Melanoma differentiation-associated protein 5 (MDA5) and NOD-like receptors (NLRs). Prominent among these are TLRs. An upregulation of TLR2, TLR3, TLR6 and TLR7 mRNA expression has been reported in tracheal epithelial cells after attenuated IBV-M41 intranasal inoculation (Guo et al., 2008; Wang et al., 2006). Its function in viral immunology is well established (Le Goffic et al., 2007; Liu et al., 2007). MDA5 expression levels were reported to be significantly increased in chicken kidney tissue after nephropathogenic IBV infection, suggesting a role for chicken MDA5 limiting IBV infection (Cong et al., 2013). In a more recent study, it has been shown that in vitro virulent IBV infection leads to a significant induction of INFβ transcription through an MDA5-dependent activation of the IFN response (Kint et al., 2015).
The limited published information on the differential immunopathogenesis of IBV stimulated this investigation into innate immune responses in chickens and their association with viral load and pathogenesis of classical and variant IBVs.
2. Material and methods
2.1. Virus
In the present study, we used a classical IBV strain (M41), and two variant strains, IS/885/00-like isolate (885) (Awad et al., 2016; Chhabra et al., 2016) and a UK IBV QX isolate (KG3P) (Ganapathy et al., 2012). IBV 885 causes high mortality, poor weight gain and severe renal damage, while IBV QX causes renal and reproductive problems. Viruses were propagated in specific-pathogen-free (SPF) eggs via the allantoic cavity. To calculate the dose of infection (105.0CD50/bird), viruses were titrated in tracheal organ cultures prepared from 19 to 20-days-old SPF chicken embryos. Confirmation of each genotype was done by RT-PCR (Chen et al., 1996; Worthington et al., 2008), followed by commercial Sanger sequencing. Results showed that each virus had 99% similarity across the part-S1 region, with the published sequences. RT-PCR showed that allantoic fluid was free of Newcastle Disease virus (NDV), avian influenza virus (AIV), avian metapneumovirus (aMPV) and infectious laryngotracheitis virus (ILTV), and culture showed the fluid to be free of mycoplasma, bacterial or fungal contaminations by culture.
2.2. Specific pathogen free (SPF) chicks
The SPF eggs were procured from a commercial source (Lohman, Cuxhaven, Germany). The parental flock was free from all major infectious disease agents, including IBV. Fertile SPF eggs were incubated at 37 °C until hatch. Chicks were kept in an isolation unit (University of Liverpool) throughout the experiment and reared on deep litter, with water and feed provided ad libitum. All procedures were undertaken according to the UK legislation on the use of animals for experiments, as permitted under the project license, which was also approved by the University of Liverpool ethical review committee.
2.3. Experimental design
One hundred SPF chicks were allocated to 4 groups (one control, three IBV infected; n = 25 per group). The chicks in the infected groups were inoculated oculonasally with 105.0 CD50/bird of each virus, and those in the control group were inoculated with TOC medium. Clinical signs were observed daily throughout the experimental period (Grgic et al., 2008). At 1, 3, 7, 9 and 14 days post infection (dpi), five chicks from each group were humanely killed using pentobarbitone injection, and trachea and kidney samples were collected. One portion of each tissue was stored in RNALater (Qiagen, Crawley, UK) and kept at −20 °C until processing for viral load and the expression of virus induced pro-inflammatory cytokines, TLRs and their adaptor molecules via quantitative real time RT-PCR (qRT-PCR). Trachea and kidney portions were also collected in 10% buffered formalin and examined by histopathology.
2.4. Histopathological lesions
For histopathological examination, the upper part of the trachea and anterior kidney tissues were fixed in 10% buffered formalin and embedded in paraffin. Five μm sections were cut for hematoxylin and eosin (H&E) staining. After staining, the sections were scored individually according to the criteria described previously; 0 = no change, 1 = mild, 2 = moderate, 3 = severe (Chen et al., 1996).
2.5. Quantification of viral RNA
All tracheal and renal samples were tested for IBV viral load using qRT-PCR as described previously (Chhabra et al., 2015).
2.6. Host gene expression analysis
At each time point, total RNA was extracted from trachea and kidney samples using the RNeasy Plus Mini Kit (Qiagen) as per manufacturer's instructions. Concentration of extracted RNA was quantified using a NanoDrop® ND-1000. Generation of cDNA was carried out from 1 μg of RNA using the Superscript III First-strand cDNA synthesis system with random primers as recommended by the manufacturer (Invitrogen, UK). Quantification of cDNA was carried out in triplicate using LightCycler 480 SYBR Green I Master mix (Roche, UK) and previously reported primer sequences (Table 1 ) (Chhabra et al., 2016), with data presented as fold difference in viral gene expression versus control samples.
Table 1.
Primer sequences used for host gene expression quantification.
| Gene name | Primer sequences |
|---|---|
| Sense (S) and Anti-sense (AS) | |
| 18S rRNA (18S ribosomal RNA) (Kuchipudi et al., 2012) | (S) TGTGCCGCTAGAGGTGAAATT |
| (AS) TGGCAAATGCTTTCGCTTT | |
| MDA5 (Melanoma differentiation-associated protein 5) | (S) AGGAGGACGACCACGATCTCT |
| (AS) CCACCTGTCTGGTCTGCATGT | |
| TLR3 (Toll like receptor 3) | (S) GCAATTTCTCCTTCACCTTTTCA |
| (AS) CCTTTATGTTTGCTATGTTGTTATTGCT | |
| IFNα (Interferon alpha) (Kuchipudi et al., 2014) | (S) CTTCCTCCAAGACAACGATTACAG |
| (AS) AGGAACCAGGCACGAGCTT | |
| IFNβ (Interferon beta) | (S) TCCAACACCTCTTCAACATGCT |
| (AS) TGGCGTGTGCGGTCAAT | |
| IL-1β (Interleukin 1 beta) | (S) TGCTGGTTTCCATCTCGTATGT |
| (AS) CCCAGAGCGGCTATTCCA | |
| IL-6 (Interleukin 6) (Kuchipudi et al., 2014) | (S) CACGATCCGGCAGATGGT |
| (AS) TGGGCGGCCGAGTCT | |
| LITAF | (S) CCCTTCTGAGGCATTTGGAA |
| (AS) CAGCCTGCAAATTTTGTCTTCTT |
2.7. Statistical analysis
The data from host gene expression was analysed using one-way analysis of variance (ANOVA), followed by the post-hoc LSD multiple comparison test using GraphPad™ Prism version 6.00. Kruskal-Wallis test, followed by Dunn's test, was used for statistical analysis of the viral load by RT-qPCR and histopathological evaluation data. Differences were considered significant at P < 0.05.
3. Results
3.1. Clinical signs and histopathology
No clinical signs or mortalities were observed in the uninfected control chicks during the experiment. The chicks inoculated with IBV 885 virus showed clinical signs from 2 to 11 dpi. These signs included mild tracheal rales, sneezing, coughing, head shaking and eye scratching, and gradually disappeared by 11 dpi. The group inoculated with IBV QX showed similar clinical signs from 3 to 9 dpi. Clinical signs were also observed in the chicks inoculated with IBV M41 from 2 to 10 dpi.
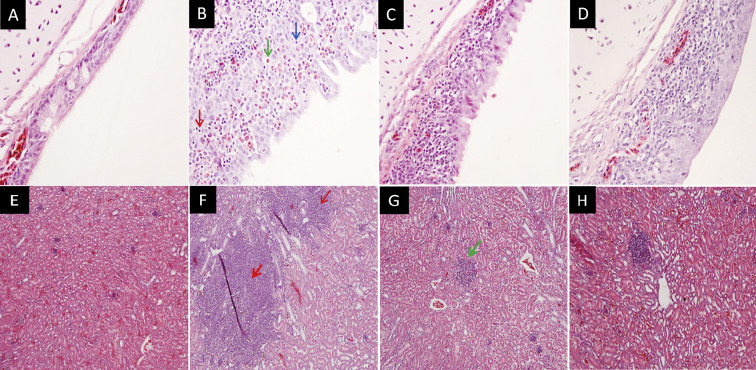
Normal tracheal epithelia, with healthy cilia and goblet cells, were observed in the uninfected control birds (Fig. 1 A). In all tracheas, until 3 dpi, no statistically significant (P < 0.05) differences were found in histopathological lesion score between the groups (Fig. 2 A). Pathological changes were significantly higher in the IBV M41 inoculated group compared to IBVs 885 and QX, which developed and peaked by 7 dpi (Fig. 2 A). The most consistent lesions occurred as loss of cilia, decreased goblet cells, infiltration of plasma cells, heterophils, and lymphocytes (Fig. 1 B–D).
Fig. 1.
Histopathological findings in trachea (A–D) and kidney (E–H) samples, using H&E stains, at 7 dpi from (A, E) Uninfected control (B) 885 infected group showing diffusely hyperplastic epithelium with infiltrate of heterophils, lymphocytes and plasma cells (C) QX infected group showing mild epithelial deciliation, with a moderate infiltrate of lymphocytes and heterophils and (D) M41 infected group showing complete epithelial deciliation and hyperplasia with a severe lymphocyte, plasma cells & heterophil infiltration. (F) 885 infected group showing severe lymphoid infiltration with mild lymphoid nodules and mild heterophil interstitial infiltration (G) QX infected group showing mild to moderate lymphocyte and heterophil interstitial infiltration (H) M41 infected group showing mild lymphocyte and heterophil interstitial infiltration Magnification x 200.
Fig. 2.
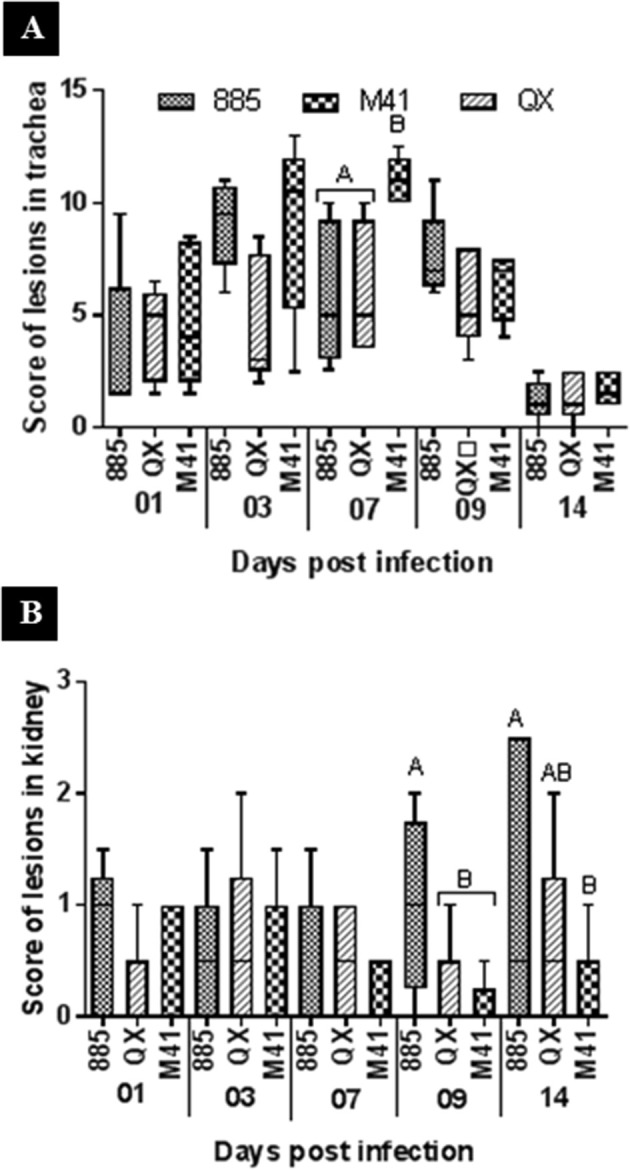
Histopathological score of lesions in (A) Trachea and (B) Kidney samples of chicks. Data represents the median with error bars as standard error. Significantly different (P < 0.05) values are shown with different letters.
The kidneys of the control group showed no obvious lesions (Fig. 1 E). In the infected groups, the main renal histopathological lesions consisted of interstitial lymphoid infiltration with mild lymphoid nodules (Fig. 1 F–H), which were observed throughout the study. No significant (P < 0.05) histological differences were found between the infected groups until 7 dpi (Fig. 2 B). However, on 9 dpi, the IBV 885 infected group showed significantly (P < 0.05) higher histopathological changes than those infected with IBVs QX or M41. These changes became more severe and peaked by 14 dpi where lesions in the IBV 885 infected group were also significantly (P < 0.05) greater when compared to IBVs QX or M41 infected chicks (Fig. 2 B).
3.2. Viral load in trachea and kidney of IBV-infected chickens
Viral RNA loads of birds’ tracheas in the uninfected control group were below the detection limit on all days post mock infection (Fig. 3 A). At 1 dpi, all tracheal samples collected from infected groups were positive. Viral load increased and peaked by 7 dpi, returning to basal level by 14 dpi. No significant difference was determined between the groups at any time point (Fig. 3 A).
Fig. 3.
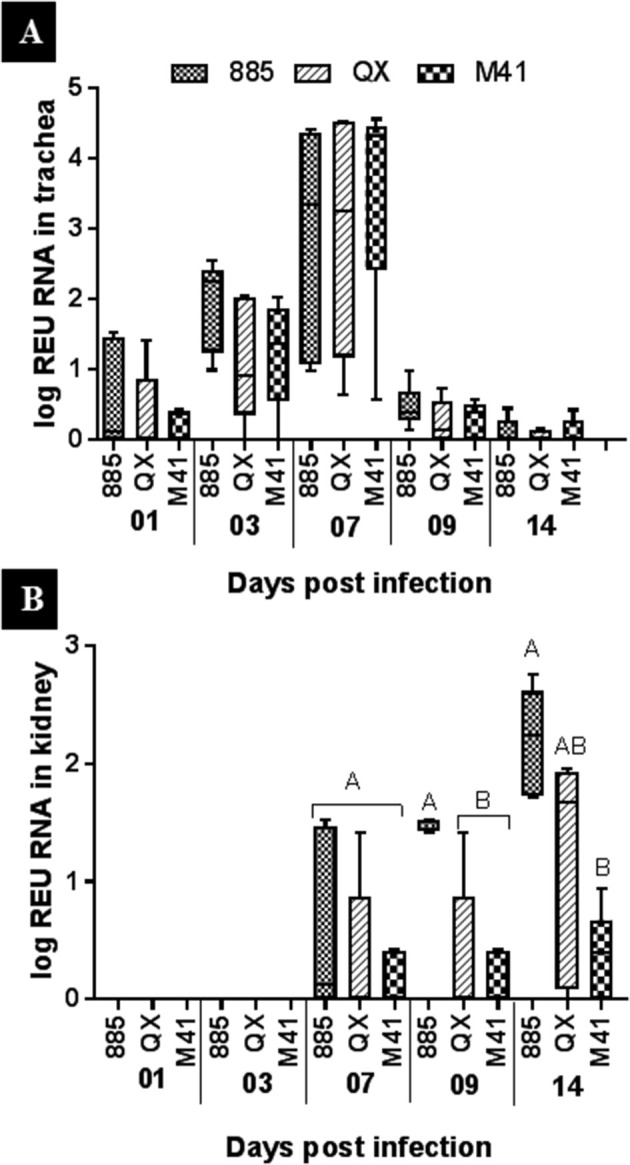
Quantification of viral RNA, expressed as log relative equivalent units (REU) of RNA in (A) Trachea and (B) Kidney samples of chicken. Data represents the median with error bars as standard error. Significantly different (P < 0.05) values are shown with different letters.
Viral RNA load in kidneys was below the detection limit for all sampling points for the control group (Fig. 3 B). Samples from the infected groups were positive from 7 dpi, with no significant difference between the groups. However, at 9 dpi, viral RNA load was significantly higher (P < 0.05) in the IBV 885 infected group compared to those infected with IBV QX or M41. Viral RNA load peaked at 14 dpi, with a significantly higher (P < 0.05) result in the IBV 885 group compared to those infected with IBV QX or M41 (Fig. 3 B).
3.3. Regulation of type I IFN, TLR3 and MDA5 genes in trachea and kidney of IBV-infected chickens
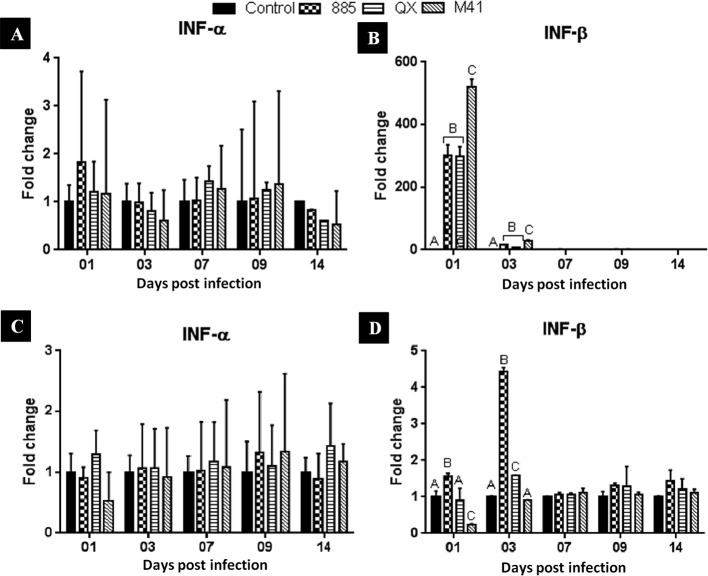
The tracheal mRNA expression data for IFN-α and IFN-β are illustrated in Fig. 4 A and B, respectively. No significant change in the IFN-α mRNA expression levels at any dpi was noticed in the infected groups compared with uninfected control group (Fig. 4 A). Notably, at 1 dpi, tracheal samples of chicks infected with IBV M41 resulted in significantly (P < 0.05) greater IFN-β mRNA expression than those infected with IBV 885 or QX (Fig. 4 B). No significant changes were noted beyond 1 dpi.
Fig. 4.
Relative IFN-α and IFN-β mRNA expression in IBV infected trachea (A, B) and kidney (C, D) of chicks. Data represents the mean with error bars as standard error and are expressed as fold change relative to the uninfected controls group. Significant differences between the groups indicated with different letters (P < 0.05).
The mRNA expression data for IFN-α and IFN-β in the kidney are illustrated in Fig. 4C and D, respectively. No significant (P < 0.05) differences in IFN-α expression levels at any dpi were noticed in any infected group compared with the uninfected control group. At 1 dpi, IFN-β mRNA expression in kidney samples of chicks infected with IBV 885 was up-regulated (P < 0.05), whereas in IBV M41 infected group, it was down-regulated (P < 0.05) compared to the control group. At 3 dpi, expression was significantly (P < 0.05) higher in the IBV 885 infected group compared to the IBV QX infected group. At this time point, no significant change was noticed in IBV M41 infected group compared with uninfected control (Fig. 4 D). No significant changes in IFN-β expression was observed beyond 7 dpi.
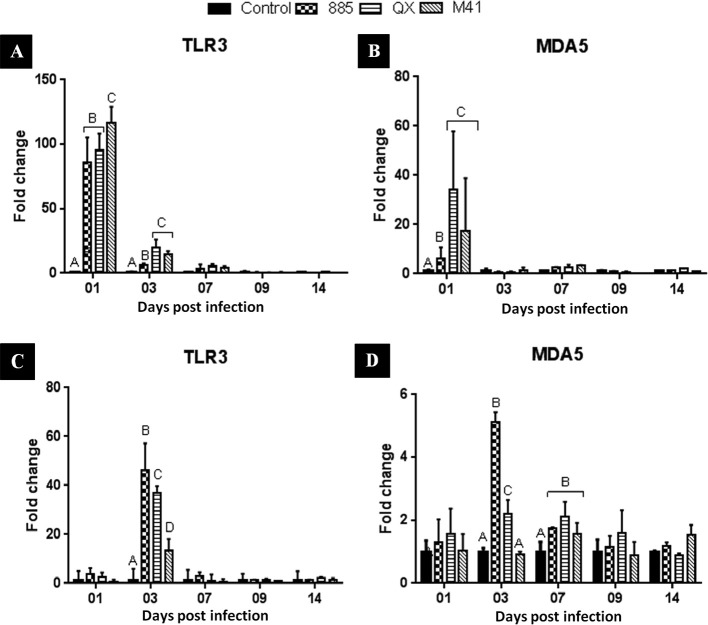
The mRNA expression of TLR3 and MDA5 in tracheal samples are illustrated in Fig. 5 A and B. It was observed that expression of TLR3 (Fig. 5 A) in all infected groups was significantly (P < 0.05) upregulated at 1 dpi compared to the uninfected control group. At this time point, the IBV M41 infected group showed significantly (P < 0.05) higher levels of expression compared to IBV 885 or QX infected groups. At 3 dpi, infection of IBV strains M41 and QX resulted in significantly greater TLR3 mRNA expression in the trachea compared to IBV 885 infection (Fig. 5 A). On subsequent dpi, no significant (P < 0.05) changes in TLR3 mRNA expression levels were detected. Similarly, infection with IBV strains M41 and QX resulted in significantly (P < 0.05) greater MDA5 expression in the trachea at 1 dpi compared to IBV 885 infection (Fig. 5 B). No significant (P < 0.05) changes in the level of MDA5 expression were observed at the other time points (3, 7, 9 or 14 dpi).
Fig. 5.
Transcriptional regulation of innate viral sensing molecules TLR3 and MDA5 in IBV infected trachea (A, B) and kidney (C, D) of chicks. Data represents the mean with error bars as standard error and are expressed as fold change relative to the uninfected controls group. Significant differences between the groups indicated with different letters (P < 0.05).
The mRNA expression data for TLR3 and MDA5 in the kidney are illustrated in Fig. 5 A and 5 D, respectively. At 3 dpi, IBV 885 infection resulted in significantly (P < 0.05) higher mRNA expression in the kidneys for TLR3 (Fig. 5 C), followed by IBVs QX and M41 infection. No significant (P < 0.05) changes in levels of TLR3 mRNA expression were observed at other time points (1, 7, 9 or 14 dpi). At 3 dpi, MDA5 mRNA expression (Fig. 5 D) in kidney samples of chicks infected with IBV 885 was significantly (P < 0.05) up-regulated compared to those infected with IBV QX. No significant (P < 0.05) change was observed in the group infected with IBV M41 compared to the uninfected control group. At 7 dpi, MDA5 mRNA expression level in all infected groups was significantly (P < 0.05) up-regulated compared to the uninfected control group but without any significant difference between the infected groups.
3.4. Differential transcription profile of pro-inflammatory cytokines genes in trachea and kidney
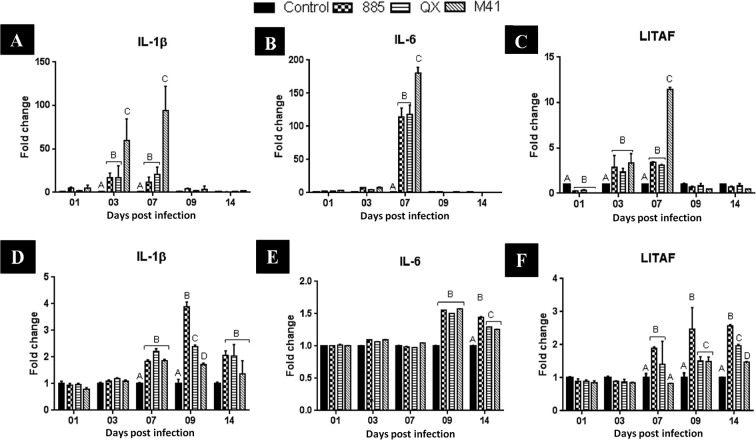
Changes in the levels of three (IL-1β, IL-6, LITAF) pro-inflammatory cytokines in the trachea are illustrated in Fig. 6 . Results indicate that the level of IL-1β mRNA expression peaked at 7 dpi in all infected groups compared with uninfected control. Expression was significantly (P < 0.05) higher at 3 and 7 dpi in chicks infected with IBV M41 compared with IBV 885 or QX. Fig. 6 B shows that at 7 dpi, a significant (P < 0.05) increase in the expression of IL-6 was noticed in chicks infected with IBV M41 compared to IBV 885 or QX. No significant (P < 0.05) changes in the level of IL-6 expression was seen in any infected group at any other time points. At 1 dpi, LITAF transcription levels in all infected groups were significantly (P < 0.05) down-regulated compared with the uninfected control group. At 3 dpi, LITAF mRNA expression levels in all infected groups were significantly (P < 0.05) up-regulated compared with the control group, but without any significant (P < 0.05) difference between infected groups. LITAF mRNA expression was seen to have the same pattern as IL-6 in the trachea. At 7 dpi, the IBV M41 infected group showed significantly (P < 0.05) higher expression levels when compared to IBV 885 or QX (Fig. 6 C).
Fig. 6.
Transcription profile of proinflammatory cytokines IL-1β, IL- 6 and LITAF in IBV infected trachea (A–C) and kidney (D–F) of chicks. Data represents the mean with error bars as standard error and are expressed as fold change relative to the uninfected controls group. Significant differences between the groups indicated with different letters (P < 0.05).
Conversely, in the kidney samples, IL-1β mRNA expression level peaked at 9 dpi in all infected groups and was significantly (P < 0.05) up-regulated in IBV 885 infected group, followed by IBVs QX and M41, when compared to the uninfected control group (Fig. 6 D). At 7 and 14 dpi, IL-1β mRNA expression in all infected groups was significantly (P < 0.05) up-regulated compared to the uninfected control group but without any significant difference between them. The mRNA expression of IL-6 in kidneys from all infected groups peaked at 9 dpi (Fig. 6 E), and was significantly (P < 0.05) up-regulated compared to the uninfected control group but without any significant difference between them. The expression of IL-6 was significantly (P < 0.05) up-regulated at 14 dpi in IBV 885 infected group, followed by IBVs QX and M41, compared to the uninfected control group (Fig. 6 E). The LITAF mRNA expression level was significantly (P < 0.05) up-regulated in all infected groups at 7 dpi and was significantly (P < 0.05) higher in groups infected with IBV 885 and QX compared to M41 (Fig. 6 F). At 9 dpi, a significant (P < 0.05) increase in the LITAF mRNA expression was noticed in chicks infected with IBV 885 rather than IBV QX or M41. Notably, we found that IBV 885 infection resulted in significantly (P < 0.05) higher mRNA expression of LITAF (Fig. 6 F), followed by IBV QX and M41 infection in the kidneys at 14 dpi.
4. Discussion
IBV causes an acute and highly contagious disease in chicks, primarily targeting the trachea and kidney. The investigation of local immunological and cytokine induced mechanisms involved in IBV pathogenesis will help to elucidate the IBV-host interaction. Pathogenic mechanisms of IBV may differ between IBV strains, particularly regarding the kinetics, magnitude, and duration of local inflammatory responses and the immune mechanisms in the target organs. In this study, we investigated the differential immunopathogenesis in chickens infected with three IBV strains used previously for an in vitro study (Chhabra et al., 2016). Infected chicks displayed mild clinical signs like tracheal rales, sneezing, coughing, head shaking and eye scratching, similar to signs which have been observed following infection with other virulent IBVs (Ambali and Jones, 1990; Butcher et al., 1990; Dolz et al., 2012). The onset and duration of these clinical signs differed between IBV infected groups, indicating differences in the susceptibility of chicks to infection by these three IBV strains. The differential modulation of PRRs (TLR and MDA5), type I IFNs, and pro-inflammatory cytokine mRNA expression in chicks, and their association with both the viral load profile and histopathological changes induced by different IBVs were examined.
In the present study, each IBV strain showed clear histopathological changes in the trachea and kidney, similar to those reported previously (Chousalkar et al., 2007; Kotani et al., 2000). Pathological changes in the trachea peaked by 7 dpi in all groups, but were significantly higher in the group infected with IBV M41 compared to IBV 885 or QX. A similar trend was found for viral loads in the trachea, though no significant differences were observed between the groups. At 7 dpi, peak values for histopathological lesions and viral RNA load were found to be associated with peaks expression of inflammatory cytokines, such as IL-6 and IL-1β, and higher LITAF mRNA expression values. Despite the lack of difference in viral load, mRNA expression values for these inflammatory cytokines were significantly higher in IBV M41 compared to IBV 885 or QX. In the kidneys, temporal associations between peak histopathological lesion scores and peak viral load were observed. However, peak values of histopathological changes and viral load were not in accordance with those of the inflammatory cytokines.
The role of the proinflammatory cytokine IL-6 has been investigated for its contribution to nephritis in two genetic lines of chickens (Asif et al., 2007). In this study, IL-6 transcripts were found to be directly related to renal lesions in chicks challenged with a nephropathogenic strain of IBV. In the more susceptible S-line chicks, these IL-6 levels were 20 times higher than those in the more resistant HWL chicks. Concurrent with our findings, it has been reported that chicks expressed differential immune responses to two IBV strains with different genotypes, KIIa and ChVI (Jang et al., 2013). In chicks infected with the KIIa genotype, simultaneous peaks in the histopathological severity, viral copy number and upregulation of mRNA levels of pro-inflammatory cytokines (IL-6 and IL-1β) and LITAF were observed in the trachea at 7 dpi and the kidney at 9 dpi. This has also been supported by previous studies in which upregulation of proinflammatory cytokines such as IL-6 and IL-1β, induced by the M41 strain of IBV, was found to be partially associated with the pathological alterations and/or viral load in trachea of non-immune challenged chicks (Okino et al., 2014, 2017). Interestingly, in our study of tracheal mRNA expression values for these inflammatory cytokines, levels were significantly higher in IBV M41 infected chicks compared to IBVs 885 and QX. In contrast, levels were higher for IBV 885 and QX infected groups in the kidney, compared to IBV M41, suggesting a role for these cytokines in the development of different histopathological lesions caused by different IBVs in these organs.
TLR3 is known for its recognition of RNA virus encoded pathogen associated molecular patterns (PAMPs) and the function of TLR3 in viral immunology is well established (Akira, 2001; Le Goffic et al., 2006). It was observed that IBV strains M41 and QX resulted in significantly higher up-regulation of innate immune sensing gene TLR3 in tracheal samples at 3 dpi when compared to IBV 885 infections. In contrast, upregulation was significantly greater in the IBV 885 infected kidneys than those infected with IBV QX or M41 at 1 dpi. Parallel with our findings, previous studies have also reported a significant increase of TLR3 mRNA expression in the trachea of IBV infected chicks when compared to the uninfected controls (Kameka et al., 2014; Wang et al., 2006). TLR3 also mediates West Nile virus entry into the brain, causing lethal encephalitis (Wang et al., 2004) and contributing to a harmful inflammatory response to influenza virus infection in mice that results in acute pneumonia (Le Goffic et al., 2007).
In this study, MDA5 mRNA expression values at 1 dpi were significantly higher in IBV M41 or QX infected tracheas compared to those infected with IBV 885. In contrast, expression levels were significantly up-regulated in IBV 885 infected kidneys compared to those infected with IBV QX or M41 at 3 dpi. MDA5 mRNA expression levels were reported to be significantly increased in chicken kidney tissue after nephropathogenic IBV infection, suggesting a role of chicken MDA5 against IBV infection (Cong et al., 2013). This has further been supported by more recent work, which showed that in vitro virulent IBV infection leads to a significant induction of INFβ transcription, through an MDA5-dependent activation of the IFN response (Kint et al., 2015). Altogether, we observed that mRNA levels of TLR3 and MDA5 correlated with the pattern of histopathological lesion scores in tracheas and kidneys produced by IBV strains used in this study.
Innate immune responses contribute towards a network of varied antiviral mechanisms, amongst which the type I IFN response is an essential defence. Concurrent with TLR3 and MDA5 activation, we found a significant up-regulation of IFNβ, but not IFNα, mRNA expression after IBV infection at 1 dpi in the trachea and 3 dpi in the kidney. Many previous studies claim that activation of the TLR3 pathway contributes to an up-regulation of IFN-β production in chickens (Kameka et al., 2014; Karpala et al., 2008; Parvizi et al., 2012). The kinetics of IFN response observed in our study is in line with the previous studies. However, a more recent study alternatively showed that MDA5, not TLR3, is involved in the detection of IBV (Kint et al., 2015). This is despite higher mRNA levels of MDA5 and TLR3 correlating with the most pathogenic strains of IBV in trachea and kidney. We also noted that tracheal samples from chicks infected with IBV M41 resulted in significantly greater IFN-β expression when compared to those infected with IBV 885 or QX. In contrast, IBV 885 and QX infection caused higher induction of this gene than IBV M41 in the kidney. Early studies on gamma coronaviruses in chickens also suggested that IBV-induced IFN production is variable and dependent on both the virus strain and cell type (Holmes and Darbyshire, 1978; Otsuki et al., 1987, 1988).
5. Conclusions
In summary, this study reports that the histopathological changes, pro-inflammatory and innate immune gene responses could be induced to a diverse degree, depending on the strain of IBV. Our results indicate that higher upregulation of proinflammatory cytokine expression (such as IL-6 and IL-1β) and LITAF are induced by the IBV M41 strain in the trachea and IBV 885 and QX in the kidney. This result is in conjunction with the severity of respiratory and renal histopathological lesions caused by these viruses. Further studies may employ these findings to understand the underlying mechanisms of host responses to emerging global IBVs or vaccination-challenge studies.
Acknowledgements
Rajesh Chhabra is a Commonwealth Scholar, funded by the UK government.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.dci.2018.04.026.
Disclaimer statement
This document is provided for scientific purposes only. Any reference to a brand or trademark herein is for information purposes only and is not intended for a commercial purpose or to dilute the rights of the respective owner(s) of the brand(s) or trademark(s).
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Akira S. Toll-like receptors and innate immunity. Adv. Immunol. 2001;78:1–56. doi: 10.1016/s0065-2776(01)78001-7. [DOI] [PubMed] [Google Scholar]
- Ambali A.G., Jones R.C. Early pathogenesis in chicks of infection with an enterotropic strain of infectious bronchitis virus. Avian Dis. 1990;34:809–817. [PubMed] [Google Scholar]
- Asif M., Lowenthal J.W., Ford M.E., Schat K.A., Kimpton W.G., Bean A.G. Interleukin-6 expression after infectious bronchitis virus infection in chickens. Viral Immunol. 2007;20:479–486. doi: 10.1089/vim.2006.0109. [DOI] [PubMed] [Google Scholar]
- Awad F., Chhabra R., Forrester A., Chantrey J., Baylis M., Lemiere S., Hussein H.A., Ganapathy K. Experimental infection of IS/885/00-like infectious bronchitis virus in specific pathogen free and commercial broiler chicks. Res. Vet. Sci. 2016;105:15–22. doi: 10.1016/j.rvsc.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher G.D., Winterfield R.W., Shapiro D.P. Pathogenesis of H13 nephropathogenic infectious bronchitis virus. Avian Dis. 1990;34:916–921. [PubMed] [Google Scholar]
- Chen B.Y., Hosi S., Nunoya T., Itakura C. Histopathology and immunohistochemistry of renal lesions due to infectious bronchitis virus in chicks. Avian Pathol. 1996;25:269–283. doi: 10.1080/03079459608419141. [DOI] [PubMed] [Google Scholar]
- Chhabra R., Forrester A., Lemiere S., Awad F., Chantrey J., Ganapathy K. Mucosal, cellular, and humoral immune responses induced by different live infectious bronchitis virus vaccination regimes and protection conferred against infectious bronchitis virus Q1 strain. Clin. Vaccine Immunol. 2015;22:1050–1059. doi: 10.1128/CVI.00368-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra R., Kuchipudi S.V., Chantrey J., Ganapathy K. Pathogenicity and tissue tropism of infectious bronchitis virus is associated with elevated apoptosis and innate immune responses. Virology. 2016;488:232–241. doi: 10.1016/j.virol.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chousalkar K.K., Roberts J.R., Reece R. Comparative histopathology of two serotypes of infectious bronchitis virus (T and n1/88) in laying hens and cockerels. Poult. Sci. 2007;86:50–58. doi: 10.1093/ps/86.1.50. [DOI] [PubMed] [Google Scholar]
- Cong F., Liu X., Han Z., Shao Y., Kong X., Liu S. Transcriptome analysis of chicken kidney tissues following coronavirus avian infectious bronchitis virus infection. BMC Genom. 2013;14:743. doi: 10.1186/1471-2164-14-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit J.J. Detection of infectious bronchitis virus. Avian Pathol. 2000;29:71–93. doi: 10.1080/03079450094108. [DOI] [PubMed] [Google Scholar]
- de Wit J.J., Nieuwenhuisen-van Wilgen J., Hoogkamer A., van de Sande H., Zuidam G.J., Fabri T.H.F. Induction of cystic oviducts and protection against early challenge with infectious bronchitis virus serotype D388 (genotype QX) by maternally derived antibodies and by early vaccination. Avian Pathol. 2011;40:463–471. doi: 10.1080/03079457.2011.599060. [DOI] [PubMed] [Google Scholar]
- Dolz R., Vergara-Alert J., Perez M., Pujols J., Majo N. New insights on infectious bronchitis virus pathogenesis: characterization of Italy 02 serotype in chicks and adult hens. Vet. Microbiol. 2012;156:256–264. doi: 10.1016/j.vetmic.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganapathy K., Wilkins M., Forrester A., Lemiere S., Cserep T., McMullin P., Jones R.C. QX-like infectious bronchitis virus isolated from cases of proventriculitis in commercial broilers in England. Vet. Rec. 2012;171:597. doi: 10.1136/vr.101005. [DOI] [PubMed] [Google Scholar]
- Gelb J., Jr., Lunt R.L., Metz A.L., Fries P.A. Attenuation of avian infectious bronchitis virus by cold-adaptation. Avian Dis. 1991;35:847–853. [PubMed] [Google Scholar]
- Grgic H., Hunter D.B., Hunton P., Nagy E. Pathogenicity of infectious bronchitis virus isolates from Ontario chickens. Can. J. Vet. Res. 2008;72:403–410. [PMC free article] [PubMed] [Google Scholar]
- Guo X., Rosa A.J., Chen D.G., Wang X. Molecular mechanisms of primary and secondary mucosal immunity using avian infectious bronchitis virus as a model system. Vet. Immunol. Immunopathol. 2008;121:332–343. doi: 10.1016/j.vetimm.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes H.C., Darbyshire J.H. Induction of chicken interferon by avian infectious bronchitis virus. Res. Vet. Sci. 1978;25:178–181. [PubMed] [Google Scholar]
- Jackwood M.W. Review of infectious bronchitis virus around the world. Avian Dis. 2012;56:634–641. doi: 10.1637/10227-043012-Review.1. [DOI] [PubMed] [Google Scholar]
- Jang H., Koo B.S., Jeon E.O., Lee H.R., Lee S.M., Mo I.P. Altered pro-inflammatory cytokine mRNA levels in chickens infected with infectious bronchitis virus. Poult. Sci. 2013;92:2290–2298. doi: 10.3382/ps.2013-03116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameka A.M., Haddadi S., Kim D.S., Cork S.C., Abdul-Careem M.F. Induction of innate immune response following infectious bronchitis corona virus infection in the respiratory tract of chickens. Virology. 2014;450–451:114–121. doi: 10.1016/j.virol.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpala A.J., Lowenthal J.W., Bean A.G. Activation of the TLR3 pathway regulates IFNbeta production in chickens. Dev. Comp. Immunol. 2008;32:435–444. doi: 10.1016/j.dci.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Kint J., Fernandez-Gutierrez M., Maier H.J., Britton P., Langereis M.A., Koumans J., Wiegertjes G.F., Forlenza M. Activation of the chicken type I interferon response by infectious bronchitis coronavirus. J. Virol. 2015;89:1156–1167. doi: 10.1128/JVI.02671-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani T., Wada S., Tsukamoto Y., Kuwamura M., Yamate J., Sakuma S. Kinetics of lymphocytic subsets in chicken tracheal lesions infected with infectious bronchitis virus. J. Vet. Med. Sci. 2000;62:397–401. doi: 10.1292/jvms.62.397. [DOI] [PubMed] [Google Scholar]
- Kuchipudi S.V., Tellabati M., Nelli R.K., White G.A., Perez B.B., Sebastian S., Slomka M.J., Brookes S.M., Brown I.H., Dunham S.P., Chang K.C. 18S rRNA is a reliable normalisation gene for real time PCR basedon influenza virus infected cells. Virol. J. 2012;9:230. doi: 10.1186/1743-422X-9-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchipudi S.V., Tellabati M., Sebastian S., Londt B.Z., Jansen C., Vervelde L., Brookes S.M., Brown I.H., Dunham S.P., Chang K.C. Highly pathogenic avian influenza virus infection in chickens but not ducks is associated with elevated host immune and pro-inflammatory responses. Vet. Res. 2014;45:118. doi: 10.1186/s13567-014-0118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goffic R., Balloy V., Lagranderie M., Alexopoulou L., Escriou N., Flavell R., Chignard M., Si-Tahar M. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2006;2:e53. doi: 10.1371/journal.ppat.0020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goffic R., Pothlichet J., Vitour D., Fujita T., Meurs E., Chignard M., Si-Tahar M. Cutting Edge: influenza A virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells. J. Immunol. 2007;178:3368–3372. doi: 10.4049/jimmunol.178.6.3368. [DOI] [PubMed] [Google Scholar]
- Liu S., Han Z., Chen J., Liu X., Shao Y., Kong X., Tong G., Rong J. S1 gene sequence heterogeneity of a pathogenic infectious bronchitis virus strain and its embryo-passaged, attenuated derivatives. Avian Pathol. 2007;36:231–234. doi: 10.1080/03079450701338730. [DOI] [PubMed] [Google Scholar]
- Meeusen E.N., Walker J., Peters A., Pastoret P.P., Jungersen G. Current status of veterinary vaccines. Clin. Microbiol. Rev. 2007;20:489–510. doi: 10.1128/CMR.00005-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okino C.H., dos Santos I.L., Fernando F.S., Alessi A.C., Wang X., Montassier H.J. Inflammatory and cell-mediated immune responses in the respiratory tract of chickens to infection with avian infectious bronchitis virus. Viral Immunol. 2014;27:383–391. doi: 10.1089/vim.2014.0054. [DOI] [PubMed] [Google Scholar]
- Okino C.H., Mores M.A., Trevisol I.M., Coldebella A., Montassier H.J., Brentano L. Early immune responses and development of pathogenesis of avian infectious bronchitis viruses with different virulence profiles. PLoS One. 2017;12 doi: 10.1371/journal.pone.0172275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuki K., Nakamura T., Kawaoka Y., Tsubokura M. Interferon induction by several strains of avian infectious bronchitis virus, a coronavirus, in chickens. Acta Virol. 1988;32:55–59. [PubMed] [Google Scholar]
- Otsuki K., Nakamura T., Kubota N., Kawaoka Y., Tsubokura M. Comparison of two strains of avian infectious bronchitis virus for their interferon induction, viral growth and development of virus-neutralizing antibody in experimentally-infected chickens. Vet. Microbiol. 1987;15:31–40. doi: 10.1016/0378-1135(87)90126-x. [DOI] [PubMed] [Google Scholar]
- Parvizi P., Mallick A.I., Haq K., Schlegel B., Sharif S. A Toll-like receptor 3 agonist (polyI: C) elicits innate host responses in the spleen and lungs of chickens. Can. J. Vet. Res. 2012;76:230–234. [PMC free article] [PubMed] [Google Scholar]
- Shaw K., Britton P., Cavanagh D. Sequence of the spike protein of the Belgian B164S isolate of nephropathogenic infectious bronchitis virus. Avian Pathol. 1996;25:607–611. doi: 10.1080/03079459608419165. [DOI] [PubMed] [Google Scholar]
- Wang T., Town T., Alexopoulou L., Anderson J.F., Fikrig E., Flavell R.A. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat. Med. 2004;10:1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- Wang X., Rosa A.J., Oliverira H.N., Rosa G.J., Guo X., Travnicek M., Girshick T. Transcriptome of local innate and adaptive immunity during early phase of infectious bronchitis viral infection. Viral Immunol. 2006;19:768–774. doi: 10.1089/vim.2006.19.768. [DOI] [PubMed] [Google Scholar]
- Worthington K.J., Currie R.J., Jones R.C. A reverse transcriptase-polymerase chain reaction survey of infectious bronchitis virus genotypes in Western Europe from 2002 to 2006. Avian Pathol. 2008;37:247–257. doi: 10.1080/03079450801986529. [DOI] [PubMed] [Google Scholar]
- Zanella A., Coaro R., Fabris G., Marchi R., Lavazza A. Avian infectious bronchitis virus: isolation of an apparently new variant in Italy. Vet. Rec. 2000;146:191–193. doi: 10.1136/vr.146.7.191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.