Summary
Community-acquired pneumonia causes great mortality and morbidity and high costs worldwide. Empirical selection of antibiotic treatment is the cornerstone of management of patients with pneumonia. To reduce the misuse of antibiotics, antibiotic resistance, and side-effects, an empirical, effective, and individualised antibiotic treatment is needed. Follow-up after the start of antibiotic treatment is also important, and management should include early shifts to oral antibiotics, stewardship according to the microbiological results, and short-duration antibiotic treatment that accounts for the clinical stability criteria. New approaches for fast clinical (lung ultrasound) and microbiological (molecular biology) diagnoses are promising. Community-acquired pneumonia is associated with early and late mortality and increased rates of cardiovascular events. Studies are needed that focus on the long-term management of pneumonia.
Clinical presentation
Community-acquired pneumonia is responsible for great mortality and morbidity and high costs. Community-acquired pneumonia was featured in Seminars in The Lancet in 19981 and 2003.2 In this updated Seminar, we address important topics related to community-acquired pneumonia in immunocompetent adults.
Suspected community-acquired pneumonia is defined by acute symptoms and presence of signs of lower respiratory tract infection (LRTI) without other obvious cause, whereas new pulmonary infiltrate on chest radiograph is needed for definite diagnosis.3, 4, 5, 6 The most common signs and symptoms are dyspnoea, cough, fever, and new focal chest signs (appendix). In subgroups of patients (eg, elderly people), clinical presentation can have less evident symptoms (eg, an altered state of consciousness, gastrointestinal discomfort, and fever can be absent) and diagnosis is frequently delayed. A prolonged time between the onset of symptoms and a medical visit has been described for less severe pneumonia, individuals with alcoholism, and for patients receiving drugs such as corticosteroids, non-steroidal anti-inflammatory drugs, and antibiotics.7 For some pathogens, unusual clinical presentations that involve the gradual onset of symptoms such as dry cough, the absence of fever, and extrapulmonary manifestations are frequent. For example, patients with pneumonia due to Legionella spp can present with headache, confusion, diarrhoea, and clinical manifestations of hyponatraemia.8 Mycoplasma pneumoniae can be associated with upper respiratory involvement (otitis, pharyngitis), skin changes (Stevens-Johnson-like syndrome), and haemolytic anaemia.9 Investigators have clearly shown that differentiation between typical and atypical pneumonia on the basis of patient history and chest radiograph is not reliable in guidance of antibiotic treatment.3, 10 By contrast, the use of validated scores for antibiotic decisions is promising. A 2014 study11 proposed a score that can rule out Legionella spp pneumonia with a negative predictive value of 99%.
Differential diagnosis
Many diseases and syndromes have clinical signs and symptoms that can mimic pneumonia (appendix). When the probability of a differential diagnosis is high, careful assessment is needed because delays in correct diagnoses increase the risks of poor outcomes.12 In patients with not-severe community-acquired pneumonia, the main differential diagnosis is upper respiratory infection. In these cases, clinicians should rely on clinical evaluations (including manifestations of LRTI, focal chest sounds, exclusion of other possible diagnosis) and point-of-care tests (eg, C-reactive protein [CRP]).6
Patients with severe community-acquired pneumonia should be monitored for other life-threatening disorders. Because differentiation of pneumonia from non-infectious disorders such as acute heart failure is occasionally difficult, prompt start of antibiotic treatment is recommended. Biomarkers (eg, procalcitonin [PCT]) can help in the early differentiation from heart failure decompensation, avoiding antibiotic misuse.13 When the diagnosis of pneumonia is excluded, antibiotic treatment must be stopped. Dynamic evaluation of the patient also helps the clinician in terms of management (eg, pulmonary infiltrates that resolve completely after positive pressure ventilation are probably due to heart failure or atelectasis). In patients with recurrent pneumonia, underlying diseases should be suspected such as lung cancer, metastasis, tuberculosis, foreign bodies, hypersensitivity pneumonitis, and unknown immunosuppressed status.
Epidemiology
Worldwide incidence
The Global Burden of Disease Study14 reported that LRTI remains the second biggest cause of deaths and years of life lost in 2013. The age-standardised death rate was 41·7 (95% CI 37·1–44·1) per 100 000 population for LRTI.14 The incidence of pneumonia is estimated to be between 1·5 and 14·0 cases per 1000 person-years.15, 16, 17 This rate varies according to the region, season, and population characteristics. In terms of age, incidence of community-acquired pneumonia is U-shaped—it is common in children younger than 5 years and adults older than 65 years. The incidence is also higher in men and boys than in women and girls. Patients who do not need admission into hospital have a mortality rate of lower than 1%.18, 19 Short-term mortality (in-hospital and 30 day mortality) for hospitalised patients ranges from 4·0% to 18·0%;17, 20, 21 however, for patients in intensive care, this rate can reach 50%.22 Costs related to community-acquired pneumonia are high,23 and few approaches (such as reducing the length-of-stay, adequate use of antibiotics, and the introduction of vaccines) have reduced these costs so far.24, 25
Causative pathogens
Streptococcus pneumoniae is the main pathogen that causes community-acquired pneumonia worldwide, independent of age.23, 26, 27 In Europe, nearly 35% (12–68%)23 of cases are caused by pneumococcal disease; worldwide it is about 27·3% (95% CI 23·9–31·1).28 Other frequent causes include Haemophilus influenzae, which accounts for 12% (2·4–44·9%) of cases23 and the so-called atypical bacteria (including Mycoplasma, Chlamydia, and Legionella spp), which caused 22% of cases in a large worldwide cohort.29
In recent years, the availability of molecular microbiological tests and clinical suspicion has increased isolation of respiratory viruses in community-acquired pneumonia.30 In adults, viruses, particularly influenza, rhinovirus, and coronaviruses, cause a third of cases of pneumonia.30 However, the attribution of the aetiology to respiratory viruses is debatable because it is difficult to define the virus as the causative agent of pneumonia.
Resistance of S pneumoniae to penicillin and macrolides has been nearly stable in recent years.2, 3, 4 The introduction of the conjugated pneumococcal vaccine in children has decreased the incidence of the invasive penicillin-resistant cases; however, infections with serotypes not affected by the vaccine have increased.31 The incidence of Mycoplasma pneumoniae resistant to macrolides varies greatly with geography (eg, with peak of about 69% in China).32, 33
Although the proportion of patients infected with pathogens not covered by standard empirical treatment is low, these pathogens are associated with high mortality and costs. In immunocompetent patients with community-acquired pneumonia, these pathogens are more frequently Pseudomonas aeruginosa, Enterobacteriaceae extended-spectrum β-lactamase (ESBL+) and meticillin-resistant Staphylococcus aureus (MRSA).34, 35
Pathophysiology
In healthy individuals, many microorganisms colonise the nasopharynx and oropharynx. Microaspiration of contaminated secretions can cause infections in the lower airways. The glottal reflexes, the presence of complement proteins and immunoglobulins, the secretion of peptides with antimicrobial activities, and the inhibition of bacteria binding all protect the lower airways.36 The healthy microbiota of the upper airway also exert protection effects by competing with pathogens for nutritional resources and interacting with cellular receptors. The use of broad-spectrum antibiotics can modify the microbiota and predispose to infection.37 The interactions between the virulence of the pathogens, the amount of inoculum, and the innate and adaptive immune responses determine the development of pneumonia.36
Risk factors and genetics
All individuals are at risk for development of pneumonia. However, some individuals are more prone to pneumonia than are others due to intrinsic and extrinsic factors (appendix).38 New findings have revealed individual genetic variability in the predisposition to the development of pneumonia and its clinical presentation.39 For example, specific variants of the FER gene are associated with a reduced risk of death in patients with sepsis due to pneumonia. Thereby, the FER gene might be a potential target for new therapies.40 Misch and colleagues41 showed that TLR6 polymorphism is associated with increased risk of Legionnaires' disease (odds ratio [OR] 5·83, 95% CI 2·21–16·39).
Diagnostic investigations
Laboratory evaluation
In patients who clinicians suspect to have community-acquired pneumonia, blood tests can provide information about the inflammatory state (ie, leucocyte cell number and characteristics [neutrophilia] and CRP), the associated organ damage (ie, acute renal failure), and the severity of the disease. Biomarkers can support clinicians in the differentiation of bacterial pneumonia from other disorders (eg, upper respiratory tract disorders). A meta-analysis suggested that antibiotic exposure can be reduced in suspected LRTI via the use of CRP measurements in primary care (risk ratio [RR] 0·78 [95% CI 0·66–0·92]).42 The 2014 NICE guidelines6 recommend not to offer antibiotics when CRP is lower than 20 mg/L in primary care for patients without a convincing clinical diagnosis of community-acquired pneumonia.
PCT had high sensitivity but moderate specificity to differentiate bacterial and viral infections. For outpatients, patients in emergency departments, and inpatients, an antibiotic is encouraged when PCT concentrations are higher than 0·25 μg/L and is strongly encouraged when PCT concentrations are higher than 0·5 μg/L; whereas they are discouraged when concentrations are lower than 0·10 μg/L.43 In patients admitted to intensive care, antibiotic treatment is always strongly encouraged with PCT concentrations higher than 0·25 μg/L. A meta-analysis reported that the use of PCT to guide antibiotic treatment in pneumonia resulted in a reduction in the exposure to antibiotics from median 8 days [IQR 5–12] to 4 days [0–8], with an adjusted difference of −3·34 days (95% CI −3·79 to −2·88) without increases in mortality or treatment failure.44 Moreover the use of PCT to guide antibiotic treatment reduced costs of treatment.45
Microbiological evaluation
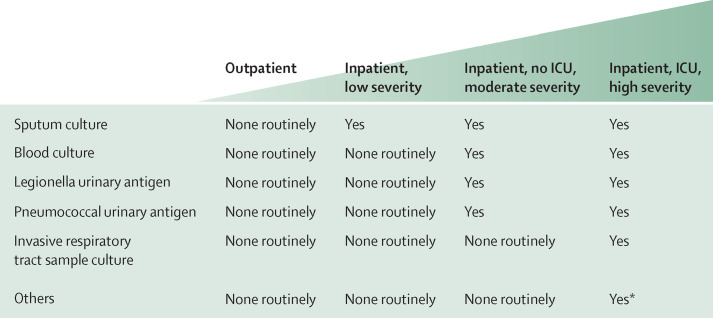
Despite many improvements, the pathogen is not detected in nearly half of pneumonia episodes.3 Microbiological tests are recommended in patients in whom the probability of changing the empirical antibiotic is high: reducing treatment failure and preventing antibiotic overuse. Microbiological evaluations (figure 1 ) are recommended for higher-risk patients such as those with severe community-acquired pneumonia, special disorders (eg, asplenia, immunosuppression, HIV infection, and alcohol abuse), severe sepsis or septic shock, a risk of resistant pathogens, and failure of the initial empirical treatment.3, 4, 5 By contrast, recommendations for microbiological testing remain controversial in less severe pneumonia because such tests are expected to have little effect on antibiotic management due to good responses to empirical treatment.46, 47, 48 However, microbiological evaluations could be valuable for surveillance.
Figure 1.
Microbiological investigations
ICU=intensive care unit. *Others indicates fungal, tuberculosis cultures, PCR, specific serology, lung biopsy.
Although a positive blood (or pleural fluid) culture test definitively identifies the pathogen responsible for pneumonia, a positive respiratory tract sample needs clinical interpretation because the microorganism can be present due to colonisation or be part of the healthy flora. The main difficulties are related to the need for a high-quality sample.3 Furthermore, the collection of any sample after the administration of antibiotics increases the rate of false-negative results. Despite these limitations, in patients in hospital with purulent sputum, a sample collection for Gram stain and culture is recommended.5, 6
Urinary antigens are useful for the detection of all serotypes of S pneumoniae and for serogroup 1 of Legionella pneumophila (responsible for about 90% of legionella cases of community-acquired pneumonia). Advantages of these tests are promptness (<15 min), reasonable accuracy, and the ability to detect the infection while the patient is receiving antibiotic therapy.6 The main drawback is the absence of information about resistance. The urinary antigen for S pneumoniae has a sensitivity of 74·0% (95% CI 66·6–82·3) and a specificity of 97·2% (92·7–99·8).49 For L pneumophila, sensitivity is 74·0% (68·0–81·0) and specificity is 99·1% (98·4–99·7).50 Two randomised controlled trials have tested empirical versus pathogen-directed antibiotic treatment through urinary antigen tests in patients in hospital with stable pneumonia51, 52 and shown no differences in major outcomes, although their conclusions were hampered by methodological issues.
For atypical pathogens, blood serology tests are available for Chlamydia pneumoniae, M pneumoniae, and Legionella spp; however, their clinical usefulness is limited by the delay in the results and difficulty in interpretation. PCR tests are available for bacterial causes related to Mycoplasma, Chlamydia, Streptococcus, and Legionella spp, which have to be done on bronchoalveolar lavage fluid or nasopharyngeal swabs. Real-time and multiplex-panel PCR aim to provide results in a few hours and are promising methods for fast bacterial aetiological diagnoses of community-acquired pneumonia.53 However, their cost-effectiveness is unclear, and there are no data about resistance. PCR tests are available for several respiratory viruses.30 In view of the controversies about the use of antiviral therapy, difficulties related to the diagnosis of viral pneumonia, and cost-effectiveness, clinicians should reserve testing for viruses for special groups of patients and within influenza season.
Imaging
Thoracic images are essential for several aspects of pneumonia management. Chest radiograph has diagnostic accuracies of 75% for alveolar consolidation and 47% for pleural effusion, considering CT as the gold standard technique.54 Performing both posteroanterior and laterolateral projections increases its accuracy. By contrast, chest radiograph has less accuracy in bedridden, obese, and severely immunosuppressed patients and in patients with previous alterations on chest radiograph.
CT is the most accurate imaging technique for the diagnosis of lung condensation54 and provides detailed information about the lung parenchyma and mediastinum and can also reveal alternative diagnoses. However, CT has limitations that include increased cost, radiation exposure, and the impossibility of doing CT at the bedside.54, 55 For these reasons, CT is reserved for specific situations such as excluding the presence of other diagnoses (eg, pulmonary embolism), when the suspicion of a fungal lung infection is present, in patients with unclear chest radiograph (eg, occult pneumonia in chronic obstructive pulmonary disease), and in non-responding pneumonia for the detection of complications (eg, lung abscesses).
Lung ultrasound is a useful method for evaluating respiratory diseases including pneumonia.56 A recent meta-analysis showed a sensitivity of 94·0% (95% CI 92·0–96·0) and a specificity of 96·0% (94·0–97·0) in the diagnosis of pneumonia in adults.57 Compared with previous methods, lung ultrasound has some advantages; it is radiation-free, can be done at the bedside and on pregnant woman, allows for dynamic evaluations, has increased accuracy in the detection of consolidation and pleural effusion compared with chest radiograph, and takes less time.56, 57, 58 Lung ultrasound is limited by its learning curve, repeatability, and operator dependency.59
Acute management
Site of care
Early in the evaluation of patients with community-acquired pneumonia, two questions need to be answered: does the patient need to be admitted in the hospital and should they be treated in intensive care? These decisions need to be made early because it has been widely shown that late admission into intensive care is associated with increased mortality.60 By contrast, the admission of patients who can be treated outside the hospital is associated with increased costs and risk of the development of nosocomial infections.61
Clinical judgment is the main determinant of the site-of-care decision.6 Oxygen saturation (SpO2) and arterial gas analysis can give important information about severity (eg, SpO2 <92% can be considered a safer cutoff than can SpO2 <90% for hospital admission).62 Furthermore, scores and biomarkers can assist the clinical judgment. The Pneumonia Severity Index (PSI)18 and CURB-6563 are the most frequently used scores. PSI is composed of 20 items and classifies patients into five categories of severity that are associated with the risk of mortality. Age and comorbidities are highly weighted in the PSI, and for these reasons, PSI can underestimate the severity of pneumonia in young patients and in those without previous diseases. CURB-65 uses five items and is practical for calculations, although it does not account for comorbidities. Important considerations not included in either score are socioeconomic status and social support, both of which can affect outcomes.64 Both PSI and CURB-65 were not developed to predict complications associated with community-acquired pneumonia; clinical research is needed to develop specific scores to predict these events.
Patients should be admitted to intensive care when they require mechanical ventilation or vasopressors (both of which are major criteria for severe pneumonia in the American Thoracic Society and Infectious Diseases Society of America guidelines).3 In addition to the major criteria, nine minor criteria are included to predict admission into intensive care.3 A meta-analysis proposed a simplification of the American Thoracic Society and Infectious Diseases Society of America minor criteria through removal of three variables (thrombocytopenia, hypothermia, and leucopenia), which had a similar accuracy.65 Other useful scores that are used to predict admission into intensive care are the SMART-COP66 and the REA-ICU for late admission.67 Some biomarkers can increase the performance of some scores to predict ICU admission (eg, proadrenomedullin)68 and can identify severe community-acquired pneumonia (eg, CRP).69 Biomarkers can also identify patients who are first admitted to the ward who might need an admission into intensive care later.70
Selection of antibiotics
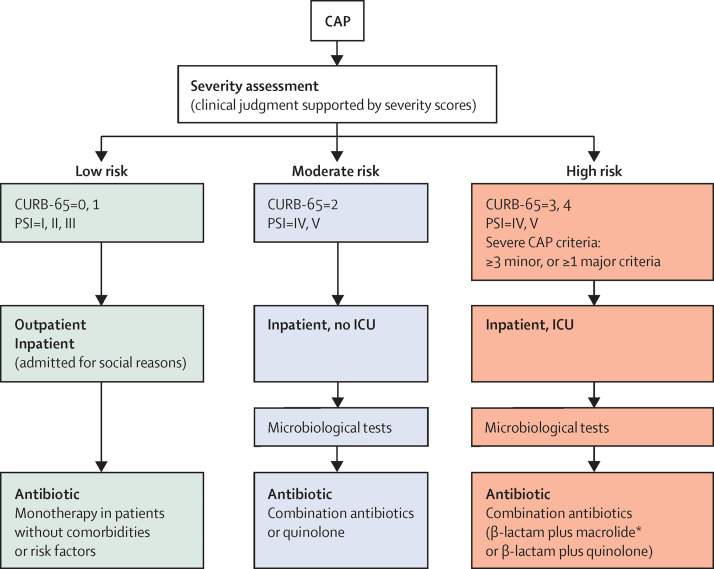
Antibiotic treatment is typically chosen empirically because of the absence of microbiological results upon diagnosis. The choice of the empirical antibiotic depends on the most likely pathogen, individual risk factors, comorbidities, allergies, and cost-effectiveness (appendix). Figure 2 and the table describe the management and antibiotic treatment proposed by community-acquired pneumonia guidelines.3, 4, 5, 6 Several studies have shown reductions in mortality when these guidelines are followed.71, 72 Guidelines suggest the coverage of S pneumoniae and atypical pathogens (eg, combination of a β-lactam plus macrolide or respiratory flouroquinolone).3, 6 However, dual coverage is still debated,6, 73 and three meta-analyses reported different results about mortality.74, 75, 76 Furthermore, concerns exist about side-effects (such as an increased risk of cardiovascular events in patients who receive macrolides)77, 78 and selective pressure for resistance to macrolides and fluoroquinolone.
Figure 2.
Acute management of the community-acquired pneumonia
CAP=community-acquired pneumonia. CURB-65=Confusion Urea Respiratory rate Blood pressure and age ≥65 year old score. PSI=Pneumonia Severity Index. ICU=intensive care unit. *Combination with macrolide is preferred.
Table.
Empirical antibiotics suggested for community-acquired pneumonia
|
American (IDSA/ATS)3 |
British (NICE/BTS)4, 6 |
European5 |
||||
|---|---|---|---|---|---|---|
| Preferred | Alternative | Preferred | Alternative | Preferred | Alternative | |
| Outpatient without comorbidities; low severity | Macrolide | Doxycycline | Amoxicillin | Macrolide or tetracycline | Amoxicillin or tetracycline | Macrolide |
| Outpatient with comorbidities or high rate bacterial resistance | β-lactam plus macrolide | Respiratory fluoroquinolone | Respiratory fluoroquinolone | |||
| Inpatient not in ICU; moderate severity | β-lactam* plus macrolide | Respiratory fluoroquinolone | Amoxicillin plus macrolide | Respiratory fluoroquinolone† | Aminopenicillin with or without macrolide | Respiratory fluoroquinolone |
| Inpatient in ICU; high severity | β-lactam‡ plus macrolide | β-lactam‡ plus respiratory fluoroquinolone | β-lactamase stable β-lactams¶ plus macrolide | Respiratory fluoroquinolone† | Third-generation cephalosporin§ plus macrolide | Respiratory fluoroquinolone with or without a third-generation cephalosporin§ |
Local or adapted guidelines should be used to adapt for different epidemiology. IDS=Infectious Diseases Society of America. ATS=American Thoracic Society. NICE=National Institute for Health and Care Excellence. BTS=British Thoracic Society. ICU=intensive care unit.
Preferred β-lactam drugs include cefotaxime, ceftriaxone, and ampicillin.
Respiratory fluoroquinolone limited to situations in which other options cannot be prescribed or are ineffective (eg, hepatotoxicity, skin reactions, cardiac arrhythmias, and tendon rupture).
Preferred β-lactam drugs include cefotaxime, ceftriaxone, or ampicillin-sulbactam.
β-lactamase-stable β-lactams include co-amoxiclav, cefotaxime, ceftaroline fosamil, ceftriaxone, cefuroxime, and piperacillin-tazobactam.
Third-generation cephalosporin (eg, cefotaxime, ceftriaxone).
Two recent randomised controlled trials provided important results about antibiotic treatment for people admitted into hospital with non-severe community-acquired pneumonia. A cluster-crossover trial assessed the non-inferiority of β-lactam versus β-lactam plus macrolide versus fluoroquinolone regimens with 90 day mortality as the primary outcome. Including 2283 patients with clinically suspected pneumonia treated in non-intensive-care-unit wards, monotherapy with β-lactam was not inferior to the other antibiotic regimens.79 Another non-inferiority, open-label trial randomly assigned 580 patients with moderately severe community-acquired pneumonia to receive β-lactam or β-lactam plus macrolide.80 The study was unable to show non-inferiority for clinical stability after 7 days of treatment. Nevertheless, a non-significant trend for superiority was shown in favour of dual therapy (between-group difference 7·6%, two-sided 95% CI −0·8% to 16·0%). For severe community-acquired pneumonia, coverage of typical and atypical pathogens seems to be protective of mortality and is recommended by major guidelines.3, 4, 5 Macrolides seem to have additional benefits due to their immunomodulatory effects in severe community-acquired pneumonia.81, 82
A small proportion of patients with specific pathogens require a different treatment because they do not respond to the standard empirical treatment.34 For this reason, the 2005 American Thoracic Society and Infectious Diseases Society of America nosocomial pneumonia guidelines introduced a new category of pneumonia called health-care-associated pneumonia to help clinicians to select patients who need an extended-spectrum antibiotic due to a high probability of resistant pathogens.83 This definition has been widely criticised because it has many limitations, and studies have shown it not to be accurate in the detection of at-risk patients.84, 85 Other scores have been developed and have better accuracies; however, they also have some limitations and still need strong external validation.34, 86, 87, 88 A summary of risk factors for resistant pathogens is contained in the appendix. Because resistant pathogens have different treatments, scores based on specific risk factors for each pathogen might be more useful methods compared with general definitions.34, 35, 89 Another concern is related to the treatment of patients who are at risk for resistant pneumococcus, such as elderly patients (age >65 years), those who have received recent therapy with β-lactams, macrolides, or fluoroquinolones; alcohol consumption; and immunosuppression (appendix).3, 90
New antibiotics are urgently needed for infections because of the spread of resistance in some settings. A recent phase 3 trial showed promising results for ceftaroline fosamil, a fifth-generation cephalosporin with activity against MRSA, in the treatment of community-acquired pneumonia with PSI III–IV in Asian patients.91 Among macrolides, solithromycin is a potential new antibiotic with activity against macrolide-resistant bacteria.92
The efficacy of neuraminidase inhibitors to prevent and treat influenza pneumonia is still controversial.93 For patients with influenza A H1N1, a recent meta-analysis showed a reduction in mortality in hospitalised patients who received neuraminidase inhibitors.94
Timing of antibiotic treatment
The first dose of antibiotics should be given as soon as possible after diagnosis of community-acquired pneumonia. The antibiotics should be started preferably within the first 4–8 h of hospital arrival and a shorter time to the first dose of antibiotic can be a marker of quality of care.95 However, a meta-analysis of stable patients with community-acquired pneumonia revealed that administration within 4 h was not associated with lower mortality (OR 0·95, 95% CI 0·73–1·23)96 and the pressure for rapid antibiotic administration was associated with an increased risk of misdiagnosis and an increased risk of adverse effects.97 In unstable patients with severe sepsis or septic shock, the time to the first dose is strongly associated with a reduction in mortality, and administration in the first hour after diagnosis is recommended.82, 98
Care of pneumonia-related sepsis
Pneumonia is the main cause of sepsis worldwide, and for severe sepsis or septic shock, the previous aspects of care are the priority (ie, assessment of pathogens, antibiotics, and whether early intensive care unit admission is needed).82, 98 The Surviving Sepsis Campaign also advocates the measure of lactate concentration at diagnosis and prompt initial expansion with 30 mL/kg of crystalloid for hypotension or lactate concentrations of 4 mmol/L or higher.82 Results related to recommendation of early goal-directed therapy are controversial mainly because of insufficient benefits reported in well designed multicentre randomised controlled trials.99, 100, 101 A major concern about patients with sepsis due to pneumonia are the risks associated with cumulative fluid balance and blood transfusion because of worsening in respiratory function.102, 103
Respiratory support
Patients with acute respiratory failure due to pneumonia must be assessed early for a need for respiratory support, and oxygen saturation is an important marker for outcome.62 Patients with severe pneumonia are candidates for invasive mechanical ventilation, and a delay can lead to an increased mortality.104 Patients with moderately severe disease can be cautiously managed with the use of non-invasive ventilation by trained staff.105 A meta-analysis suggested that the appropriate use of non-invasive ventilation in pneumonia can reduce the need for endotracheal intubation (OR 0·28, 95% CI 0·09–0·88), intensive care unit mortality (0·26, 0·11–0·61), and the length-of-stay in intensive care units (mean −1·00, 95% CI −2·05 to −0·05). However, this meta-analysis included only 151 patients in three randomised trials, and benefits were particularly evident in patients with chronic obstructive pulmonary disease or immunosuppression.106 Non-invasive ventilation can also be considered a palliative treatment in patients with terminal illness.107 For mechanically ventilated patients, protective ventilation is strongly recommended on diagnosis of acute respiratory distress syndrome. For less severe pneumonia, protective ventilation also seems to prevent the progression of lung injury.3, 108
Adjunctive therapy
The use of corticosteroids for community-acquired pneumonia is debated, especially how it affects mortality.109 Meta-analyses110, 111 have reported reduced hospital length-of-stay (mean −1·21 days, 95% CI −2·12 to −0·29) with use of corticosteroids. A multicentre randomised controlled trial112 showed a shorter time to reach clinical stability in patients with pneumonia receiving oral prednisone (50 mg a day for 7 days) in relation to the placebo group (3·0 days vs 4·4 days, hazard ratio 1·33 95% CI 1·15–1·50). Another multicentre randomised controlled trial113 showed that methylprednisolone (0·5 mg/kg per 12 h for 5 days) reduced risk for treatment failure compared with placebo (OR 0·34, 95% CI 0·14–0·87) in patients with severe community-acquired pneumonia with high baseline concentrations of CRP. For mortality, updated meta-analyses110, 111, 114, 115, 116 report no conclusive results for hospitalised patients, although corticosteroids were associated with better survival in the subgroup with severe community-acquired pneumonia.114, 115, 116, 117 However, trials included in the meta-analyses were small, have high heterogeneity, and insufficient power to assess mortality. No definitive data are available for the best type and dose of corticosteroids for patients with community-acquired pneumonia, nor those for whether they should be given continuously or to intermittent and tapering schemes.6 The clinician should be aware of possible steroid-induced side-effects in patients. In controlled settings (eg, randomised controlled trials), only hyperglycaemia was more frequently reported for patients with community-acquired pneumonia receiving a corticosteroid. However, large trials including patients with severe sepsis or septic shock with community-acquired pneumonia as the main source of infection, showed other steroid side-effects such as superinfection.6, 118
Investigators have proposed statins as an adjunctive therapy in pneumonia due to their anti-inflammatory activities and ability to reduce cardiovascular events, but their effects are controversial.119
Long-term management
Evaluation of clinical stability
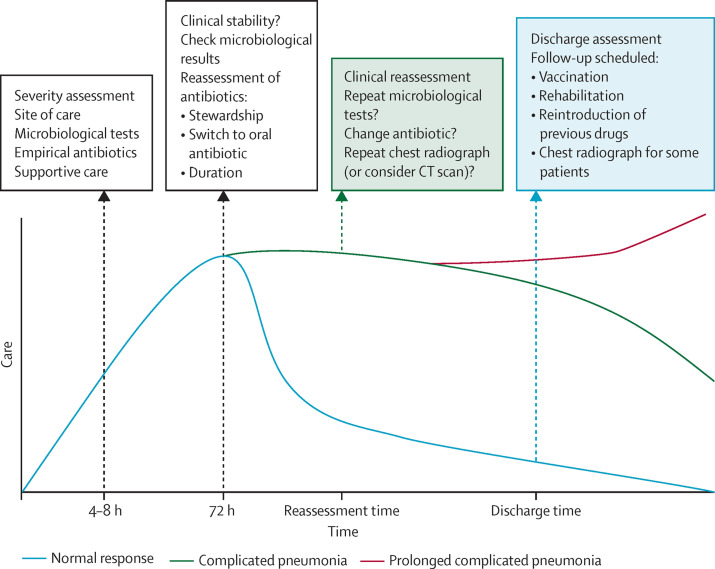
After the initial management of community-acquired pneumonia, the subsequent days are fundamental for good outcomes and high-quality management needs a multidimensional approach (figure 3 ). The evaluation of clinical stability (appendix) is a fundamental aspect of community-acquired pneumonia care.120, 121 Stability criteria offer information about antibiotic treatment (eg, the appropriateness of such treatment, switching to oral medication, and short antibiotic treatment durations) and indications for hospital discharge that reduce hospital length-of-stay.122, 123, 124
Figure 3.
Acute and long-term assessment of community-acquired pneumonia
Stewardship
When microbiological tests become available, it is important to re-evaluate antibiotic treatment. Antibiotics should be adapted according to antibiogram results, narrowed according to the identified pathogen, and discontinued when a diagnosis of pneumonia is unlikely.25 Stewardship is fundamental to avoid the continuation of unnecessary treatment, increasing the selective pressure for resistance, and reducing the risks of unnecessary complications (eg, Clostridium difficile infection).125
Switch to oral therapy
Most patients in hospital with community-acquired pneumonia began treatment with an intravenous antibiotic. A switch to oral therapy should be considered for patients who reach clinical stability. Two randomised controlled trials25, 126 have shown no difference in mortality, but important reductions in the length-of-stay and adverse drug reactions, in patients who switch to oral therapy early.
Duration of therapy
5 days of treatment should be given for low-severity pneumonia with clinical stability after 3 days of treatment, and 7 days should be given for severe pneumonia, which should be adapted depending on the improvements in symptoms and stability.3, 4, 6, 122, 127 Indeed, two meta-analyses reported similar efficacies for short-course (≤7 days) and long-course (>7 days) treatments when patients with severe pneumonia were excluded.127, 128 Additionally, an observational study with robust analyses reported similar outcomes for short-course and long-course antibiotic treatments for patients with severe community-acquired pneumonia.123 Patients with extrapulmonary complications or empyema and pneumonia due to specific pathogens (eg, Legionella spp and MRSA) seem to have benefits from prolonged treatments.
Biomarkers can be used to guide antibiotic duration. One-time PCT values lower than 0·25 μg/mL or a decrease from the peak by 80–90% are a strong indication that antibiotics should be discontinued.43, 44, 45 A randomised controlled trial129 to compare PCT and CRP for antibiotic guidance in patients with severe sepsis and septic shock showed similar outcomes; however, more studies are needed to compare cost-effectiveness among biomarkers.6
Clinical failure
Patients with community-acquired pneumonia can present with deterioration, known as clinical failure, which predicts mortality.130 Therefore, definition of the causes of failure is essential. Early failure (<72 h) seems to be related to the severity of the primary infection (eg, the development of septic shock), whereas the late failures (>72 h) tend to be due to secondary events (eg, nosocomial superinfection, exacerbation of comorbidities). The development of severe sepsis is the primary reason for failure.131 Outpatients also need an early follow-up (after 72 h) to detect development of failure.132 Non-responding pneumonia is a different disorder that comprises the persistence of pulmonary infiltrates 1 month after symptom onset and can be due to many causes, such as the presence of lung cancer or an underlying lung disease.3
Early rehabilitation
Patients in hospital seem to benefit from early mobilisation and rehabilitation.133, 134
Follow-up and outcomes
Readmission rate
Between 7% and 12% of patients who are admitted into hospital for community-acquired pneumonia are readmitted within 30 days.135, 136 In more than half of cases, comorbidities are the cause of readmission (mainly cardiovascular, pulmonary, or neurological diseases), whereas in other patients, a new episode of pneumonia is the cause of readmission. The main risk factors for readmission are initial treatment failure, clinical instability at hospital discharge, older age, comorbidities, and impaired functional status.135, 136
Long-term mortality
Pneumonia causes much short-term and long-term mortality. Mortality for patients with community-acquired pneumonia is higher than for those with other infections and patients who are admitted into hospital for other reasons after adjusting for important variables.137 Several predictors of long-term mortality have been described and include age, comorbidities, frailty, cardiovascular complications, inflammation and the severity of the initial insult.137
Cardiovascular events
Community-acquired pneumonia is associated with an increased risk of cardiovascular complications.138 Some explanatory reasons for this include hypoxaemia, inflammation, prothrombotic status, pathogen-specific factors, and host characteristics.137, 139 A meta-analysis for the incidence of cardiac events within 30 days of hospital admission for community-acquired pneumonia reported a cumulative rate of heart failure of 14% (range 7–33%), an arrhythmia rate of 5% (range 1–11%), and an acute coronary syndrome rate of 5% (range 1–11%).140
Prevention and vaccines
Clinicians should pay attention regarding modifying factors available to decrease the risk of a new episode of community-acquired pneumonia (appendix). Influenza vaccines are robustly associated with a reduced rate of pneumonia and better outcomes.3, 4 A study of 286 000 individuals older than 65 years reported a 30% reduction in the rate of pneumonia and influenza infection that was followed by a reduction in all-cause mortality.141
Two vaccines are available for S pneumoniae: the pneumococcal polysaccharide vaccine and the pneumococcal conjugate vaccine. The pneumococcal polysaccharide vaccine contains polysaccharides for 23 pneumococcal serotypes and the most recent version of pneumococcal conjugate vaccine contains 13 serotypes. By comparison with pneumococcal polysaccharide vaccine, the pneumococcal conjugate vaccine seems to induce a stronger and longer-lasting secondary immune response with booster effect.142 Results from a recent meta-analysis showed strong evidence for the recommendation for pneumococcal polysaccharide vaccine-23 vaccination to prevent invasive pneumococcal disease in adults.143 Nevertheless, there is less clear evidence for its efficacy in the prevention of non-bacteraemic pneumonia,143 in patients with chronic illnesses, and for the reduction of all-cause pneumonia and mortality.143 The pneumococcal conjugate vaccine-13 was approved for clinical use in adults by the US and European agencies. The CAPiTA study144 (a double-blind, randomised, placebo-controlled clinical trial involving nearly 85 000 adults older than 65 years) showed clinical efficacy of pneumococcal conjugate vaccine-13 in the prevention of the first episode of vaccine-serotype pneumococcal community-acquired pneumonia (including non-bacteraemic pneumonia and invasive pneumococcal disease);144 however, the trial excluded immunosuppressed patients and previously vaccinated person. Because a substantial number of cases of pneumonia is caused by serotypes not included in the pneumococcal conjugate vaccine-13 (38% of invasive pneumococcal disease in the US in 2013), the US Centers for Disease Control and Prevention recommended the administration of both pneumococcal conjugate vaccine-13 and pneumococcal polysaccharide vaccine-23 in series to all adults aged 65 years and older (panel 1 ).142 Outstanding research questions remain, which should be addressed in future large trials (panel 2 ).
Panel 1. Controversies and uncertainties.
-
1
The implementation of rapid diagnostic testing using PCR techniques for viruses and bacteria might increase the number of microbiological diagnoses and consequently the number of initial appropriate treatments; although some devices are able to provide rapid diagnoses, well designed studies are needed to investigate major outcomes and cost-effectiveness4, 53
-
2
The real rates of different to treat pathogens in community-acquired pneumonia, such as Pseudomonas aeruginosa, Enterobacteriaceae extended-spectrum β-lactamase, and meticillin-resistant Staphylococcus aureus, differ between continents and countries (eg, the USA and Japan vs Europe); the concept of health-care-associated pneumonia is not accurate and has resulted in the excessive administration of broad-spectrum antibiotics;84 risk factors for these microorganisms have been described recently, but implementation in clinical practice is still lacking34, 35
-
3
Combination antibacterial therapy is a matter of debate; such therapy is recommended for patients with community-acquired pneumonia who are admitted to the intensive care unit,3, 4 and in patients with bacteraemic Streptococcus pneumoniae;82 furthermore, patients with high mortality admitted to the ward might benefit from this treatment strategy80
-
4
Recent data from randomised controlled trials112, 113 showed a reduction of time to clinical stability and of treatment failure in patients with community-acquired pneumonia receiving corticosteroids; however, data are controversial for effect on mortality; a prematurely halted trial of severe community-acquired pneumonia revealed an important decrease in mortality (39% vs 0%)117
-
5
The long-term cardiovascular complications of patients with community-acquired pneumonia are not completely understood; but it seems that residual inflammation might have an important role in triggering procoagulation pathways and leading to cardiovascular complications37, 138
Panel 2. Outstanding research questions.
-
1
Interventional studies are needed of microbiological testing techniques to increase the rate of initial appropriate treatments, which would result in improved outcomes and reduced overuse of antibiotics
-
2
Validation studies that use risk factors for different-to-treat microorganisms to confirm the accuracies of these risk factors for their implementation in clinical practice are also needed
-
3
Interventional studies should be done to assess cost-effectiveness for C-reactive protein and procalcitonin in low-income and middle-income countries and specific settings
-
4
A randomised controlled trial is needed in patients with severe community-acquired pneumonia who are not admitted to the intensive care unit that compares monotherapy with respiratory quinolones and combination therapy (β-lactam plus macrolide)
-
5
Investigators should do a large randomised controlled trial in patients with community-acquired pneumonia who are admitted to intensive care units that assesses the administration of corticosteroids versus placebo powered to address mortality and quality-of-life outcomes
-
6
Studies of severe community-acquired pneumonia are needed, both in animal models and in human beings, to test new coadjutant treatments, such as enriched immunoglobulin M, monoclonal antibodies, and molecules that can block the endotoxins and exotoxins of the microbes
-
7
Prospective observational follow-up studies in patients with community-acquired pneumonia are needed to better describe the clinical and biological risk factors for cardiovascular complications to design a pharmacological randomised controlled trial
Search strategy and selection criteria
We searched Medline, Embase, and the Cochrane Library for papers published from inception to Jan 31, 2015. We used the search terms “community-acquired pneumonia” or “lower respiratory tract infection”, in combination with the terms “epidemiology”, “diagnosis”, “aetiology”, “pathophysiology”, “risk factors”, “management”, “treatment”, “outcomes”, “long-term”, and their variations. We restricted the search strategy to adults. We largely selected publications in the past 5 years and also searched the reference lists of articles identified by this search strategy. We gave more weight to randomised controlled trials and meta-analyses, as suggested by The Lancet. Review articles and book chapters are cited to provide readers with more details and more references. The reference list was modified on the basis of peer-review process.
Contributors
All authors equally contributed in the literature search and data interpretation, conceived, wrote, and approved the final version of the manuscript.
Declaration of interests
AT participated in advisory boards for Merck, Pfizer (vaccines), and Cempra. EP and OTR declare no competing interests.
Supplementary Material
References
- 1.Brown PD, Lerner SA. Community-acquired pneumonia. Lancet. 1998;352:1295–1302. doi: 10.1016/S0140-6736(98)02239-9. [DOI] [PubMed] [Google Scholar]
- 2.File TM. Community-acquired pneumonia. Lancet. 2003;362:1991–2001. doi: 10.1016/S0140-6736(03)15021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandell LA, Wunderink RG, Anzueto A, for the Infectious Diseases Society of America. the American Thoracic Society Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim WS, Baudouin SV, George RC, for the Pneumonia Guidelines Committee of the BTS Standards of Care Committee BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009;64(suppl 3):iii1–iii55. doi: 10.1136/thx.2009.121434. [DOI] [PubMed] [Google Scholar]
- 5.Woodhead M, Blasi F, Ewig S, for the Joint Taskforce of the European Respiratory Society and European Society for Clinical Microbiology and Infectious Diseases Guidelines for the management of adult lower respiratory tract infections–full version. Clin Microbiol Infect. 2011;17(suppl 6):E1–E59. doi: 10.1111/j.1469-0691.2011.03672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institute for Health and Care Excellence Pneumonia: Diagnosis and management of community- and hospital-acquired pneumonia in adults. NICE guidelines. 2014. https://www.nice.org.uk/guidance/cg191 (accessed Jan 15, 2015). [PubMed]
- 7.Sanz F, Restrepo MI, Fernández-Fabrellas E. Does prolonged onset of symptoms have a prognostic significance in community-acquired pneumonia? Respirology. 2014;19:1073–1079. doi: 10.1111/resp.12346. [DOI] [PubMed] [Google Scholar]
- 8.Arancibia F, Cortes CP, Valdés M. Importance of Legionella pneumophila in the etiology of severe community-acquired pneumonia in Santiago, Chile. Chest. 2014;145:290–296. doi: 10.1378/chest.13-0162. [DOI] [PubMed] [Google Scholar]
- 9.Cunha BA. The atypical pneumonias: clinical diagnosis and importance. Clin Microbiol Infect. 2006;12(suppl 3):12–24. doi: 10.1111/j.1469-0691.2006.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bochud PY, Moser F, Erard P. Community-acquired pneumonia. A prospective outpatient study. Medicine (Baltimore) 2001;80:75–87. doi: 10.1097/00005792-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Haubitz S, Hitz F, Graedel L. Ruling out Legionella in community-acquired pneumonia. Am J Med. 2014;127:1010. doi: 10.1016/j.amjmed.2014.03.042. e11–19. [DOI] [PubMed] [Google Scholar]
- 12.Metlay JP, Fine MJ. Testing strategies in the initial management of patients with community-acquired pneumonia. Ann Intern Med. 2003;138:109–118. doi: 10.7326/0003-4819-138-2-200301210-00012. [DOI] [PubMed] [Google Scholar]
- 13.Schuetz P, Kutz A, Grolimund E, for the ProHOSP Study Group Excluding infection through procalcitonin testing improves outcomes of congestive heart failure patients presenting with acute respiratory symptoms: results from the randomized ProHOSP trial. Int J Cardiol. 2014;175:464–472. doi: 10.1016/j.ijcard.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 14.GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Millett ER, Quint JK, Smeeth L, Daniel RM, Thomas SL. Incidence of community-acquired lower respiratory tract infections and pneumonia among older adults in the United Kingdom: a population-based study. PLoS One. 2013;8:e75131. doi: 10.1371/journal.pone.0075131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ochoa-Gondar O, Vila-Córcoles A, de Diego C, for the EVAN-65 Study Group The burden of community-acquired pneumonia in the elderly: the Spanish EVAN-65 study. BMC Public Health. 2008;8:222. doi: 10.1186/1471-2458-8-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.File TM, Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122:130–141. doi: 10.3810/pgm.2010.03.2130. [DOI] [PubMed] [Google Scholar]
- 18.Fine MJ, Auble TE, Yealy DM. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 19.Carratalà J, Fernández-Sabé N, Ortega L. Outpatient care compared with hospitalization for community-acquired pneumonia: a randomized trial in low-risk patients. Ann Intern Med. 2005;142:165–172. doi: 10.7326/0003-4819-142-3-200502010-00006. [DOI] [PubMed] [Google Scholar]
- 20.Ewig S, Birkner N, Strauss R. New perspectives on community-acquired pneumonia in 388 406 patients. Results from a nationwide mandatory performance measurement programme in healthcare quality. Thorax. 2009;64:1062–1069. doi: 10.1136/thx.2008.109785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnold FW, Wiemken TL, Peyrani P, Ramirez JA, Brock GN, for the CAPO authors Mortality differences among hospitalized patients with community-acquired pneumonia in three world regions: results from the Community-Acquired Pneumonia Organization (CAPO) International Cohort Study. Respir Med. 2013;107:1101–1111. doi: 10.1016/j.rmed.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Woodhead M, Welch CA, Harrison DA, Bellingan G, Ayres JG. Community-acquired pneumonia on the intensive care unit: secondary analysis of 17,869 cases in the ICNARC Case Mix Programme Database. Crit Care. 2006;10(suppl 2):S1. doi: 10.1186/cc4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 2012;67:71–79. doi: 10.1136/thx.2009.129502. [DOI] [PubMed] [Google Scholar]
- 24.Johnstone J, Marrie TJ, Eurich DT, Majumdar SR. Effect of pneumococcal vaccination in hospitalized adults with community-acquired pneumonia. Arch Intern Med. 2007;167:1938–1943. doi: 10.1001/archinte.167.18.1938. [DOI] [PubMed] [Google Scholar]
- 25.Carratalà J, Garcia-Vidal C, Ortega L. Effect of a 3-step critical pathway to reduce duration of intravenous antibiotic therapy and length of stay in community-acquired pneumonia: a randomized controlled trial. Arch Intern Med. 2012;172:922–928. doi: 10.1001/archinternmed.2012.1690. [DOI] [PubMed] [Google Scholar]
- 26.Drijkoningen JJ, Rohde GG. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect. 2014;20(suppl 5):45–51. doi: 10.1111/1469-0691.12461. [DOI] [PubMed] [Google Scholar]
- 27.Howard LS, Sillis M, Pasteur MC, Kamath AV, Harrison BD. Microbiological profile of community-acquired pneumonia in adults over the last 20 years. J Infect. 2005;50:107–113. doi: 10.1016/j.jinf.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Said MA, Johnson HL, Nonyane BA, the AGEDD Adult Pneumococcal Burden Study Team Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. PLoS One. 2013;8:e60273. doi: 10.1371/journal.pone.0060273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnold FW, Summersgill JT, Lajoie AS, the Community-Acquired Pneumonia Organization (CAPO) Investigators A worldwide perspective of atypical pathogens in community-acquired pneumonia. Am J Respir Crit Care Med. 2007;175:1086–1093. doi: 10.1164/rccm.200603-350OC. [DOI] [PubMed] [Google Scholar]
- 30.Ruuskanen O, Lahti E, Jennings LC, Murdoch DR. Viral pneumonia. Lancet. 2011;377:1264–1275. doi: 10.1016/S0140-6736(10)61459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kyaw MH, Lynfield R, Schaffner W, the Active Bacterial Core Surveillance of the Emerging Infections Program Network Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006;354:1455–1463. doi: 10.1056/NEJMoa051642. [DOI] [PubMed] [Google Scholar]
- 32.Principi N, Esposito S. Macrolide-resistant Mycoplasma pneumoniae: its role in respiratory infection. J Antimicrob Chemother. 2013;68:506–511. doi: 10.1093/jac/dks457. [DOI] [PubMed] [Google Scholar]
- 33.Cao B, Zhao CJ, Yin YD. High prevalence of macrolide resistance in Mycoplasma pneumoniae isolates from adult and adolescent patients with respiratory tract infection in China. Clin Infect Dis. 2010;51:189–194. doi: 10.1086/653535. [DOI] [PubMed] [Google Scholar]
- 34.Webb BJ, Dascomb K, Stenehjem E, Dean N. Predicting risk of drug-resistant organisms in pneumonia: moving beyond the HCAP model. Respir Med. 2015;109:1–10. doi: 10.1016/j.rmed.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 35.Prina E, Ranzani OT, Polverino E. Risk factors associated with potentially antibiotic-resistant pathogens in community-acquired pneumonia. Ann Am Thorac Soc. 2015;12:153–160. doi: 10.1513/AnnalsATS.201407-305OC. [DOI] [PubMed] [Google Scholar]
- 36.Alcón A, Fàbregas N, Torres A. Pathophysiology of pneumonia. Clin Chest Med. 2005;26:39–46. doi: 10.1016/j.ccm.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 37.Ubeda C, Pamer EG. Antibiotics, microbiota, and immune defense. Trends Immunol. 2012;33:459–466. doi: 10.1016/j.it.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torres A, Peetermans WE, Viegi G, Blasi F. Risk factors for community-acquired pneumonia in adults in Europe: a literature review. Thorax. 2013;68:1057–1065. doi: 10.1136/thoraxjnl-2013-204282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wunderink RG, Waterer GW. Genetics of community-acquired pneumonia. Semin Respir Crit Care Med. 2005;26:553–562. doi: 10.1055/s-2005-925522. [DOI] [PubMed] [Google Scholar]
- 40.Rautanen A, Mills TC, Gordon AC. Genome-wide association study of survival from sepsis due to pneumonia: an observational cohort study. Lancet Respir Med. 2015;3:53–60. doi: 10.1016/S2213-2600(14)70290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Misch EA, Verbon A, Prins JM, Skerrett SJ, Hawn TRA. A TLR6 polymorphism is associated with increased risk of Legionnaires' disease. Genes Immun. 2013;14:420–426. doi: 10.1038/gene.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aabenhus R, Jensen JU, Jørgensen KJ, Hróbjartsson A, Bjerrum L. Biomarkers as point-of-care tests to guide prescription of antibiotics in patients with acute respiratory infections in primary care. Cochrane Database Syst Rev. 2014;11 doi: 10.1002/14651858.CD010130.pub2. CD010130. [DOI] [PubMed] [Google Scholar]
- 43.Christ-Crain M, Stolz D, Bingisser R. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174:84–93. doi: 10.1164/rccm.200512-1922OC. [DOI] [PubMed] [Google Scholar]
- 44.Schuetz P, Müller B, Christ-Crain M. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012;9 doi: 10.1002/14651858.CD007498.pub2. CD007498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuetz P, Balk R, Briel M. Economic evaluation of procalcitonin-guided antibiotic therapy in acute respiratory infections: a US health system perspective. Clin Chem Lab Med. 2015;53:583–592. doi: 10.1515/cclm-2014-1015. [DOI] [PubMed] [Google Scholar]
- 46.Fine MJ, Orloff JJ, Arisumi D. Prognosis of patients hospitalized with community-acquired pneumonia. Am J Med. 1990;88:1N–8N. [PubMed] [Google Scholar]
- 47.Malcolm C, Marrie TJ. Antibiotic therapy for ambulatory patients with community-acquired pneumonia in an emergency department setting. Arch Intern Med. 2003;163:797–802. doi: 10.1001/archinte.163.7.797. [DOI] [PubMed] [Google Scholar]
- 48.Marrie TJ, Lau CY, Wheeler SL, Wong CJ, Vandervoort MK, Feagan BG. A controlled trial of a critical pathway for treatment of community-acquired pneumonia. CAPITAL Study Investigators. Community-Acquired Pneumonia Intervention Trial Assessing Levofloxacin. JAMA. 2000;283:749–755. doi: 10.1001/jama.283.6.749. [DOI] [PubMed] [Google Scholar]
- 49.Sinclair A, Xie X, Teltscher M, Dendukuri N. Systematic review and meta-analysis of a urine-based pneumococcal antigen test for diagnosis of community-acquired pneumonia caused by Streptococcus pneumoniae. J Clin Microbiol. 2013;51:2303–2310. doi: 10.1128/JCM.00137-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimada T, Noguchi Y, Jackson JL. Systematic review and metaanalysis: urinary antigen tests for Legionellosis. Chest. 2009;136:1576–1585. doi: 10.1378/chest.08-2602. [DOI] [PubMed] [Google Scholar]
- 51.van der Eerden MM, Vlaspolder F, de Graaff CS. Comparison between pathogen directed antibiotic treatment and empirical broad spectrum antibiotic treatment in patients with community acquired pneumonia: a prospective randomised study. Thorax. 2005;60:672–678. doi: 10.1136/thx.2004.030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Falguera M, Ruiz-González A, Schoenenberger JA. Prospective, randomised study to compare empirical treatment versus targeted treatment on the basis of the urine antigen results in hospitalised patients with community-acquired pneumonia. Thorax. 2010;65:101–106. doi: 10.1136/thx.2009.118588. [DOI] [PubMed] [Google Scholar]
- 53.Schulte B, Eickmeyer H, Heininger A. Detection of pneumonia associated pathogens using a prototype multiplexed pneumonia test in hospitalized patients with severe pneumonia. PLoS One. 2014;9:e110566. doi: 10.1371/journal.pone.0110566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Syrjälä H, Broas M, Suramo I, Ojala A, Lähde S. High-resolution computed tomography for the diagnosis of community-acquired pneumonia. Clin Infect Dis. 1998;27:358–363. doi: 10.1086/514675. [DOI] [PubMed] [Google Scholar]
- 55.Brenner DJ, Hall EJ. Computed tomography–an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 56.Volpicelli G, Elbarbary M, Blaivas M, the International Liaison Committee on Lung Ultrasound (ILC-LUS) for International Consensus Conference on Lung Ultrasound (ICC-LUS) International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38:577–591. doi: 10.1007/s00134-012-2513-4. [DOI] [PubMed] [Google Scholar]
- 57.Chavez MA, Shams N, Ellington LE. Lung ultrasound for the diagnosis of pneumonia in adults: a systematic review and meta-analysis. Respir Res. 2014;15:50. doi: 10.1186/1465-9921-15-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reissig A, Copetti R, Mathis G. Lung ultrasound in the diagnosis and follow-up of community-acquired pneumonia: a prospective, multicenter, diagnostic accuracy study. Chest. 2012;142:965–972. doi: 10.1378/chest.12-0364. [DOI] [PubMed] [Google Scholar]
- 59.Ramirez P, Torres A. Should ultrasound be included in the initial assessment of respiratory patients? Lancet Respir Med. 2014;2:599–600. doi: 10.1016/S2213-2600(14)70142-0. [DOI] [PubMed] [Google Scholar]
- 60.Restrepo MI, Mortensen EM, Rello J, Brody J, Anzueto A. Late admission to the ICU in patients with community-acquired pneumonia is associated with higher mortality. Chest. 2010;137:552–557. doi: 10.1378/chest.09-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.González-Moraleja J, Sesma P, González C, López ME, García JF, Alvarez-Sala JL. What is the cost of inappropriate admission of pneumonia patients? Arch Bronconeumol. 1999;35:312–316. doi: 10.1016/s0300-2896(15)30067-3. [DOI] [PubMed] [Google Scholar]
- 62.Majumdar SR, Eurich DT, Gamble JM, Senthilselvan A, Marrie TJ. Oxygen saturations less than 92% are associated with major adverse events in outpatients with pneumonia: a population-based cohort study. Clin Infect Dis. 2011;52:325–331. doi: 10.1093/cid/ciq076. [DOI] [PubMed] [Google Scholar]
- 63.Lim WS, van der Eerden MM, Laing R. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jones B, Gundlapalli AV, Jones JP, Brown SM, Dean NC. Admission decisions and outcomes of community-acquired pneumonia in the homeless population: a review of 172 patients in an urban setting. Am J Public Health. 2013;103(suppl 2):S289–S293. doi: 10.2105/AJPH.2013.301342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salih W, Schembri S, Chalmers JD. Simplification of the IDSA/ATS criteria for severe CAP using meta-analysis and observational data. Eur Respir J. 2014;43:842–851. doi: 10.1183/09031936.00089513. [DOI] [PubMed] [Google Scholar]
- 66.Charles PG, Wolfe R, Whitby M, for the Australian Community-Acquired Pneumonia Study Collaboration SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis. 2008;47:375–384. doi: 10.1086/589754. [DOI] [PubMed] [Google Scholar]
- 67.Labarère J, Schuetz P, Renaud B, Claessens YE, Albrich W, Mueller B. Validation of a clinical prediction model for early admission to the intensive care unit of patients with pneumonia. Acad Emerg Med. 2012;19:993–1003. doi: 10.1111/j.1553-2712.2012.01424.x. [DOI] [PubMed] [Google Scholar]
- 68.Renaud B, Schuetz P, Claessens YE, Labarère J, Albrich W, Mueller B. Proadrenomedullin improves Risk of Early Admission to ICU score for predicting early severe community-acquired pneumonia. Chest. 2012;142:1447–1454. doi: 10.1378/chest.11-2574. [DOI] [PubMed] [Google Scholar]
- 69.Chalmers JD, Singanayagam A, Hill AT. C-reactive protein is an independent predictor of severity in community-acquired pneumonia. Am J Med. 2008;121:219–225. doi: 10.1016/j.amjmed.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 70.Ramírez P, Ferrer M, Martí V. Inflammatory biomarkers and prediction for intensive care unit admission in severe community-acquired pneumonia. Crit Care Med. 2011;39:2211–2217. doi: 10.1097/CCM.0b013e3182257445. [DOI] [PubMed] [Google Scholar]
- 71.Dean NC, Silver MP, Bateman KA, James B, Hadlock CJ, Hale D. Decreased mortality after implementation of a treatment guideline for community-acquired pneumonia. Am J Med. 2001;110:451–457. doi: 10.1016/s0002-9343(00)00744-0. [DOI] [PubMed] [Google Scholar]
- 72.Martínez R, Reyes S, Lorenzo MJ, Menéndez R. Impact of guidelines on outcome: the evidence. Semin Respir Crit Care Med. 2009;30:172–178. doi: 10.1055/s-0029-1202936. [DOI] [PubMed] [Google Scholar]
- 73.File TM, Jr, Marrie TJ. Does empiric therapy for atypical pathogens improve outcomes for patients with CAP? Infect Dis Clin North Am. 2013;27:99–114. doi: 10.1016/j.idc.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 74.Nie W, Li B, Xiu Q. β-Lactam/macrolide dual therapy versus β-lactam monotherapy for the treatment of community-acquired pneumonia in adults: a systematic review and meta-analysis. J Antimicrob Chemother. 2014;69:1441–1446. doi: 10.1093/jac/dku033. [DOI] [PubMed] [Google Scholar]
- 75.Asadi L, Sligl WI, Eurich DT. Macrolide-based regimens and mortality in hospitalized patients with community-acquired pneumonia: a systematic review and meta-analysis. Clin Infect Dis. 2012;55:371–380. doi: 10.1093/cid/cis414. [DOI] [PubMed] [Google Scholar]
- 76.Eliakim-Raz N, Robenshtok E, Shefet D. Empiric antibiotic coverage of atypical pathogens for community-acquired pneumonia in hospitalized adults. Cochrane Database Syst Rev. 2012;9 doi: 10.1002/14651858.CD004418.pub4. CD004418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schembri S, Williamson PA, Short PM. Cardiovascular events after clarithromycin use in lower respiratory tract infections: analysis of two prospective cohort studies. BMJ. 2013;346:f1235. doi: 10.1136/bmj.f1235. [DOI] [PubMed] [Google Scholar]
- 78.Mortensen EM, Halm EA, Pugh MJ. Association of azithromycin with mortality and cardiovascular events among older patients hospitalized with pneumonia. JAMA. 2014;311:2199–2208. doi: 10.1001/jama.2014.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Postma DF, van Werkhoven CH, van Elden LJ, for the CAP-START Study Group Antibiotic treatment strategies for community-acquired pneumonia in adults. N Engl J Med. 2015;372:1312–1323. doi: 10.1056/NEJMoa1406330. [DOI] [PubMed] [Google Scholar]
- 80.Garin N, Genné D, Carballo S. β-Lactam monotherapy vs β-lactam-macrolide combination treatment in moderately severe community-acquired pneumonia: a randomized noninferiority trial. JAMA Intern Med. 2014;174:1894–1901. doi: 10.1001/jamainternmed.2014.4887. [DOI] [PubMed] [Google Scholar]
- 81.Sligl WI, Asadi L, Eurich DT, Tjosvold L, Marrie TJ, Majumdar SR. Macrolides and mortality in critically ill patients with community-acquired pneumonia: a systematic review and meta-analysis. Crit Care Med. 2014;42:420–432. doi: 10.1097/CCM.0b013e3182a66b9b. [DOI] [PubMed] [Google Scholar]
- 82.Dellinger RP, Levy MM, Rhodes A, for the Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.American Thoracic Society. the Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 84.Ewig S, Welte T, Chastre J, Torres A. Rethinking the concepts of community-acquired and health-care-associated pneumonia. Lancet Infect Dis. 2010;10:279–287. doi: 10.1016/S1473-3099(10)70032-3. [DOI] [PubMed] [Google Scholar]
- 85.Dobler CC, Waterer G. Healthcare-associated pneumonia: a US disease or relevant to the Asia Pacific, too? Respirology. 2013;18:923–932. doi: 10.1111/resp.12132. [DOI] [PubMed] [Google Scholar]
- 86.Aliberti S, Di Pasquale M, Zanaboni AM. Stratifying risk factors for multidrug-resistant pathogens in hospitalized patients coming from the community with pneumonia. Clin Infect Dis. 2012;54:470–478. doi: 10.1093/cid/cir840. [DOI] [PubMed] [Google Scholar]
- 87.Shorr AF, Zilberberg MD, Reichley R. Validation of a clinical score for assessing the risk of resistant pathogens in patients with pneumonia presenting to the emergency department. Clin Infect Dis. 2012;54:193–198. doi: 10.1093/cid/cir813. [DOI] [PubMed] [Google Scholar]
- 88.Shindo Y, Ito R, Kobayashi D. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2013;188:985–995. doi: 10.1164/rccm.201301-0079OC. [DOI] [PubMed] [Google Scholar]
- 89.Wunderink RG. Community-acquired pneumonia versus healthcare-associated pneumonia. The returning pendulum. Am J Respir Crit Care Med. 2013;188:896–898. doi: 10.1164/rccm.201308-1536ED. [DOI] [PubMed] [Google Scholar]
- 90.Kuster SP, Rudnick W, Shigayeva A, for the Toronto Invasive Bacterial Diseases Network Previous antibiotic exposure and antimicrobial resistance in invasive pneumococcal disease: results from prospective surveillance. Clin Infect Dis. 2014;59:944–952. doi: 10.1093/cid/ciu497. [DOI] [PubMed] [Google Scholar]
- 91.Zhong NS, Sun T, Zhuo C. Ceftaroline fosamil versus ceftriaxone for the treatment of Asian patients with community-acquired pneumonia: a randomised, controlled, double-blind, phase 3, non-inferiority with nested superiority trial. Lancet Infect Dis. 2015;15:161–171. doi: 10.1016/S1473-3099(14)71018-7. [DOI] [PubMed] [Google Scholar]
- 92.Oldach D, Clark K, Schranz J. Randomized, double-blind, multicenter phase 2 study comparing the efficacy and safety of oral solithromycin (CEM-101) to those of oral levofloxacin in the treatment of patients with community-acquired bacterial pneumonia. Antimicrob Agents Chemother. 2013;57:2526–2534. doi: 10.1128/AAC.00197-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jefferson T, Jones MA, Doshi P. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev. 2014;4 doi: 10.1002/14651858.CD001265.pub3. CD008965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Muthuri SG, Venkatesan S, Myles PR, the PRIDE Consortium Investigators Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med. 2014;2:395–404. doi: 10.1016/S2213-2600(14)70041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Barlow G, Nathwani D, Williams F. Reducing door-to-antibiotic time in community-acquired pneumonia: controlled before-and-after evaluation and cost-effectiveness analysis. Thorax. 2007;62:67–74. doi: 10.1136/thx.2005.056689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yu KT, Wyer PC. Evidence-based emergency medicine/critically appraised topic. Evidence behind the 4-hour rule for initiation of antibiotic therapy in community-acquired pneumonia. Ann Emerg Med. 2008;51:651–662. doi: 10.1016/j.annemergmed.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 97.Welker JA, Huston M, McCue JD. Antibiotic timing and errors in diagnosing pneumonia. Arch Intern Med. 2008;168:351–356. doi: 10.1001/archinternmed.2007.84. [DOI] [PubMed] [Google Scholar]
- 98.Noritomi DT, Ranzani OT, Monteiro MB. Implementation of a multifaceted sepsis education program in an emerging country setting: clinical outcomes and cost-effectiveness in a long-term follow-up study. Intensive Care Med. 2014;40:182–191. doi: 10.1007/s00134-013-3131-5. [DOI] [PubMed] [Google Scholar]
- 99.Peake SL, Delaney A, Bailey M, for the ARISE Investigators. the ANZICS Clinical Trials Group Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371:1496–1506. doi: 10.1056/NEJMoa1404380. [DOI] [PubMed] [Google Scholar]
- 100.Yealy DM, Kellum JA, Huang DT, for the ProCESS Investigators A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mouncey PR, Osborn TM, Power GS, for the ProMISe Trial Investigators Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372:1301–1311. doi: 10.1056/NEJMoa1500896. [DOI] [PubMed] [Google Scholar]
- 102.Azevedo LC, Park M, Salluh JI, for the ERICC (Epidemiology of Respiratory Insufficiency in Critical Care) investigators Clinical outcomes of patients requiring ventilatory support in Brazilian intensive care units: a multicenter, prospective, cohort study. Crit Care. 2013;17:R63. doi: 10.1186/cc12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gajic O, Rana R, Winters JL. Transfusion-related acute lung injury in the critically ill: prospective nested case-control study. Am J Respir Crit Care Med. 2007;176:886–891. doi: 10.1164/rccm.200702-271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carrillo A, Gonzalez-Diaz G, Ferrer M. Non-invasive ventilation in community-acquired pneumonia and severe acute respiratory failure. Intensive Care Med. 2012;38:458–466. doi: 10.1007/s00134-012-2475-6. [DOI] [PubMed] [Google Scholar]
- 105.Brambilla AM, Aliberti S, Prina E. Helmet CPAP vs. oxygen therapy in severe hypoxemic respiratory failure due to pneumonia. Intensive Care Med. 2014;40:942–949. doi: 10.1007/s00134-014-3325-5. [DOI] [PubMed] [Google Scholar]
- 106.Zhang Y, Fang C, Dong BR, Wu T, Deng JL. Oxygen therapy for pneumonia in adults. Cochrane Database Syst Rev. 2012;3 doi: 10.1002/14651858.CD006607.pub4. CD006607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nava S, Ferrer M, Esquinas A. Palliative use of non-invasive ventilation in end-of-life patients with solid tumours: a randomised feasibility trial. Lancet Oncol. 2013;14:219–227. doi: 10.1016/S1470-2045(13)70009-3. [DOI] [PubMed] [Google Scholar]
- 108.Gajic O, Frutos-Vivar F, Esteban A, Hubmayr RD, Anzueto A. Ventilator settings as a risk factor for acute respiratory distress syndrome in mechanically ventilated patients. Intensive Care Med. 2005;31:922–926. doi: 10.1007/s00134-005-2625-1. [DOI] [PubMed] [Google Scholar]
- 109.Póvoa P, Salluh JI. What is the role of steroids in pneumonia therapy? Curr Opin Infect Dis. 2012;25:199–204. doi: 10.1097/QCO.0b013e32834f44c7. [DOI] [PubMed] [Google Scholar]
- 110.Chen Y, Li K, Pu H, Wu T. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2011;3 doi: 10.1002/14651858.CD007720.pub2. CD007720. [DOI] [PubMed] [Google Scholar]
- 111.Shafiq M, Mansoor MS, Khan AA, Sohail MR, Murad MH. Adjuvant steroid therapy in community-acquired pneumonia: a systematic review and meta-analysis. J Hosp Med. 2013;8:68–75. doi: 10.1002/jhm.1992. [DOI] [PubMed] [Google Scholar]
- 112.Blum CA, Nigro N, Briel M. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2015;385:1511–1518. doi: 10.1016/S0140-6736(14)62447-8. [DOI] [PubMed] [Google Scholar]
- 113.Torres A, Sibila O, Ferrer M. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA. 2015;313:677–686. doi: 10.1001/jama.2015.88. [DOI] [PubMed] [Google Scholar]
- 114.Nie W, Zhang Y, Cheng J, Xiu Q. Corticosteroids in the treatment of community-acquired pneumonia in adults: a meta-analysis. PLoS One. 2012;7:e47926. doi: 10.1371/journal.pone.0047926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cheng M, Pan ZY, Yang J, Gao YD. Corticosteroid therapy for severe community-acquired pneumonia: a meta-analysis. Respir Care. 2014;59:557–563. doi: 10.4187/respcare.02758. [DOI] [PubMed] [Google Scholar]
- 116.Confalonieri M, Annane D, Antonaglia C, Santagiuliana M, Borriello EM, Meduri GU. Is prolonged low-dose glucocorticoid treatment beneficial in community-acquired pneumonia? Curr Infect Dis Rep. 2013;15:158–166. doi: 10.1007/s11908-013-0322-8. [DOI] [PubMed] [Google Scholar]
- 117.Confalonieri M, Urbino R, Potena A. Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med. 2005;171:242–248. doi: 10.1164/rccm.200406-808OC. [DOI] [PubMed] [Google Scholar]
- 118.Kalil AC, Sun J. Low-dose steroids for septic shock and severe sepsis: the use of Bayesian statistics to resolve clinical trial controversies. Intensive Care Med. 2011;37:420–429. doi: 10.1007/s00134-010-2121-0. [DOI] [PubMed] [Google Scholar]
- 119.Khan AR, Riaz M, Bin Abdulhak AA. The role of statins in prevention and treatment of community acquired pneumonia: a systematic review and meta-analysis. PLoS One. 2013;8:e52929. doi: 10.1371/journal.pone.0052929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Menéndez R, Martinez R, Reyes S. Stability in community-acquired pneumonia: one step forward with markers? Thorax. 2009;64:987–992. doi: 10.1136/thx.2009.118612. [DOI] [PubMed] [Google Scholar]
- 121.Halm EA, Fine MJ, Marrie TJ. Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. JAMA. 1998;279:1452–1457. doi: 10.1001/jama.279.18.1452. [DOI] [PubMed] [Google Scholar]
- 122.el Moussaoui R, de Borgie CA, van den Broek P. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate-severe community acquired pneumonia: randomised, double blind study. BMJ. 2006;332:1355. doi: 10.1136/bmj.332.7554.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Choudhury G, Mandal P, Singanayagam A, Akram AR, Chalmers JD, Hill AT. Seven-day antibiotic courses have similar efficacy to prolonged courses in severe community-acquired pneumonia—a propensity-adjusted analysis. Clin Microbiol Infect. 2011;17:1852–1858. doi: 10.1111/j.1469-0691.2011.03542.x. [DOI] [PubMed] [Google Scholar]
- 124.Blasi F, Ostermann H, Racketa J, Medina J, McBride K, Garau J, for the REACH study group Early versus later response to treatment in patients with community-acquired pneumonia: analysis of the REACH study. Respir Res. 2014;15:6. doi: 10.1186/1465-9921-15-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Polgreen PM, Chen YY, Cavanaugh JE. An outbreak of severe Clostridium difficile-associated disease possibly related to inappropriate antimicrobial therapy for community-acquired pneumonia. Infect Control Hosp Epidemiol. 2007;28:212–214. doi: 10.1086/512174. [DOI] [PubMed] [Google Scholar]
- 126.Oosterheert JJ, Bonten MJ, Schneider MM. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomised trial. BMJ. 2006;333:1193. doi: 10.1136/bmj.38993.560984.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li JZ, Winston LG, Moore DH, Bent S. Efficacy of short-course antibiotic regimens for community-acquired pneumonia: a meta-analysis. Am J Med. 2007;120:783–790. doi: 10.1016/j.amjmed.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 128.Dimopoulos G, Matthaiou DK, Karageorgopoulos DE, Grammatikos AP, Athanassa Z, Falagas ME. Short- versus long-course antibacterial therapy for community-acquired pneumonia: a meta-analysis. Drugs. 2008;68:1841–1854. doi: 10.2165/00003495-200868130-00004. [DOI] [PubMed] [Google Scholar]
- 129.Oliveira CF, Botoni FA, Oliveira CR. Procalcitonin versus C-reactive protein for guiding antibiotic therapy in sepsis: a randomized trial. Crit Care Med. 2013;41:2336–2343. doi: 10.1097/CCM.0b013e31828e969f. [DOI] [PubMed] [Google Scholar]
- 130.Aliberti S, Blasi F. Clinical stability versus clinical failure in patients with community-acquired pneumonia. Semin Respir Crit Care Med. 2012;33:284–291. doi: 10.1055/s-0032-1315640. [DOI] [PubMed] [Google Scholar]
- 131.Menéndez R, Torres A, Zalacaín R, for the Neumofail Group Risk factors of treatment failure in community acquired pneumonia: implications for disease outcome. Thorax. 2004;59:960–965. doi: 10.1136/thx.2003.017756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ott SR, Hauptmeier BM, Ernen C. Treatment failure in pneumonia: impact of antibiotic treatment and cost analysis. Eur Respir J. 2012;39:611–618. doi: 10.1183/09031936.00098411. [DOI] [PubMed] [Google Scholar]
- 133.Mundy LM, Leet TL, Darst K, Schnitzler MA, Dunagan WC. Early mobilization of patients hospitalized with community-acquired pneumonia. Chest. 2003;124:883–889. doi: 10.1378/chest.124.3.883. [DOI] [PubMed] [Google Scholar]
- 134.Stolbrink M, McGowan L, Saman H. The Early Mobility Bundle: a simple enhancement of therapy which may reduce incidence of hospital-acquired pneumonia and length of hospital stay. J Hosp Infect. 2014;88:34–39. doi: 10.1016/j.jhin.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 135.Jasti H, Mortensen EM, Obrosky DS, Kapoor WN, Fine MJ. Causes and risk factors for rehospitalization of patients hospitalized with community-acquired pneumonia. Clin Infect Dis. 2008;46:550–556. doi: 10.1086/526526. [DOI] [PubMed] [Google Scholar]
- 136.Capelastegui A, España Yandiola PP, Quintana JM. Predictors of short-term rehospitalization following discharge of patients hospitalized with community-acquired pneumonia. Chest. 2009;136:1079–1085. doi: 10.1378/chest.08-2950. [DOI] [PubMed] [Google Scholar]
- 137.Restrepo MI, Faverio P, Anzueto A. Long-term prognosis in community-acquired pneumonia. Curr Opin Infect Dis. 2013;26:151–158. doi: 10.1097/QCO.0b013e32835ebc6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Corrales-Medina VF, Alvarez KN, Weissfeld LA. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA. 2015;313:264–274. doi: 10.1001/jama.2014.18229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yende S, D'Angelo G, Kellum JA, the GenIMS Investigators Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am J Respir Crit Care Med. 2008;177:1242–1247. doi: 10.1164/rccm.200712-1777OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Corrales-Medina VF, Suh KN, Rose G. Cardiac complications in patients with community-acquired pneumonia: a systematic review and meta-analysis of observational studies. PLoS Med. 2011;8:e1001048. doi: 10.1371/journal.pmed.1001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nichol KL, Nordin J, Mullooly J, Lask R, Fillbrandt K, Iwane M. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N Engl J Med. 2003;348:1322–1332. doi: 10.1056/NEJMoa025028. [DOI] [PubMed] [Google Scholar]
- 142.Tomczyk S, Bennett NM, Stoecker C, the Centers for Disease Control and Prevention (CDC) Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2014;63:822–825. [PMC free article] [PubMed] [Google Scholar]
- 143.Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013;1 doi: 10.1002/14651858.CD000422.pub3. CD000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bonten MJ, Huijts SM, Bolkenbaas M. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114–1125. doi: 10.1056/NEJMoa1408544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.